Chapter 78 Citicoline (CDP-Choline)
 Introduction
Introduction
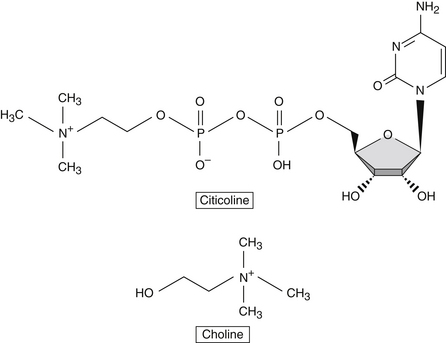
Citicoline (cytidine diphosphocholine) is a mononucleotide composed of ribose, cytosine, pyrophosphate, and choline. As an endogenous compound, citicoline is an essential intermediate in the synthesis of cell membrane structural phospholipids, and its formation is the rate-limiting step in phosphatidylcholine synthesis.
Citicoline is also an exogenous source for acetylcholine synthesis, a key neurotransmitter, and a member of the group of molecules that play important roles in cellular metabolism known as nucleotides.1
First identified in 1955 by Kennedy et al,2 and synthesized in 1956, citicoline has been studied in Europe, Japan, and the United States for several decades. It is widely available as an approved drug for the treatment of neurologic disorders in many countries and is sold as a dietary supplement in the United States.
Citicoline Versus Choline
Choline is a component of the diet and is produced in the brain, albeit in small amounts. Due to its low endogenous production, it is considered an essential nutrient and classified with the B vitamin complex. It plays several essential roles in human physiology, including enhancement of structural integrity and signaling for cell membranes, supporting acetylcholine synthesis, and synthesis of betaine, a methyl donor.3
When taken orally, citicoline is hydrolyzed in the intestinal tract and in circulation to form choline and cytidine, which is the nucleoside of cytosine. Citicoline provides the brain with a source of choline and cytidine, which are efficiently used in the Kennedy cycle to generate phospholipids. Although choline on its own is preferentially used for the synthesis of acetylcholine, cytidine is highly efficiently used in the brain for the synthesis of various nucleotides (Figure 78-1). Studies in neuronal cell lines showed that cytidine administration increased the incorporation of choline into membrane phosphatidylcholine.4
In terms of safety, choline is a substance with a low level of toxicologic concern. Administering choline with cytidine, in the form of citicoline, lowers the toxicity index by twenty-fold.5 Furthermore, citicoline administration is significantly different than administration of choline in cases of cerebral ischemia caused by stroke and other conditions. Citicoline’s therapeutic effects in such conditions stem from its ability to: (1) increase phosphatidylcholine synthesis, the primary component of neuronal membranes; (2) enhance acetylcholine synthesis, ameliorating the symptoms resulting from ischemic loss of cholinergic neurons; (3) promote the synthesis of several other membrane phospholipids, including phosphatidylethanolamine and phosphatidylserine, leading to repair and regeneration of axons and synapses; and (4) prevent the accumulation of free fatty acids and the generation of free radicals at the site of ischemia, thereby preventing the initiation of a proinflammatory cascade of events.5
 Bioavailability/Pharmacokinetics
Bioavailability/Pharmacokinetics
The pharmacokinetics of an oral dose of 14C-labeled citicoline has been studied in humans. Administration of a single 300 mg dose to healthy adults was shown to have nearly complete absorption, with less than 1% of the labeled compound found in feces after a 5-day collection period. Absorption of citicoline gave rise to two chromatographic peaks in concentrations of radioactivity in plasma, the first at 1 hour, and the second, larger peak at 24 hours after dosing. The main route of excretion was found to be via respiratory carbon dioxide, with significant excretion also occurring through urine (Figure 78-2). After 5 days, 16% of the administered dose was recovered, suggesting that the remainder was incorporated into tissues or was available for biosynthetic and biodegradative pathways.6

FIGURE 78-2 Major metabolic pathways for citicoline.
Modified from Weiss GB. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995;56(9):637-60.
A pharmacokinetic study in rats using 14C-methyl-labeled citicoline confirmed almost complete absorption with oral administration, with calculated oral bioavailability being approximately 92% of that obtained from intravenous (IV) dosing. The absorption was categorized as slow and complete with sustained blood levels, the highest being at around 5.5 hours after administration. Radioactive labeling found citicoline and its metabolites widely distributed throughout tissues, including distribution of metabolites to the brain, confirming their ability to participate in the synthesis of phospholipids.7
A confirmatory study, again using radio labeled citicoline in rats, found 62.8% of total radioactivity was distributed in brain tissue as phospholipids, including phosphatidylcholine and sphingomyelin. These results suggested that metabolites of orally administered citicoline were available in the brain for resynthesis of endogenous citicoline.8 Although only a small percentage of the total citicoline dose crosses the blood–brain barrier as choline and cytidine, the utilization of these precursors in brain tissue for phospholipid biosynthesis is extremely efficient.4
 Mechanism of Action
Mechanism of Action
Citicoline has several important mechanisms of action, leading to a broad range of beneficial effects on neurologic function. In cerebral ischemia, citicoline primarily acts by increasing the synthesis of phosphatidylcholine, the primary neuronal membrane phospholipid, and enhancing the production of acetylcholine. Oral citicoline administration increases plasma levels of choline and cytidine, which are building blocks used to restore neuronal membrane integrity.5
Interestingly, citicoline seems to have differential effects on phosphatidylcholine synthesis in younger versus older adults. Phosphatidylcholine is an essential compound for cell membrane integrity and repair. It is normally reduced in brain cell membranes as a result of aging. A study using protein magnetic resonance spectroscopy to measure brain concentrations of cytosolic choline-containing compounds before and after a single oral dose of citicoline found that the choline resonance in the brain of younger individuals increased, whereas it decreased in older subjects. It was presumed that the cytidine component of citicoline enhanced the incorporation of brain choline into neural cell membrane phosphatidylcholine in older subjects, resulting in the decrease.9
The ability of citicoline to stimulate brain phospholipid synthesis in humans was further supported by studies showing that healthy subjects consuming 500 mg/day orally for 6 weeks (administered as Cognizin citicoline) had increased levels of phosphodiesters in brain tissue, such as glycerophosphocholine and glycerophosphoethanolamine, as assessed by phosphorus magnetic resonance spectroscopy. These findings supported citicoline’s ability to increase phosphatidylcholine synthesis.10 Findings from a study of healthy middle-aged adults confirmed these results, but suggested that the increase in phosphorus metabolites attributed to citicoline intake was regionally specific, with the frontal lobe being the preferred site of deposition, ultimately enhancing frontal lobe energetics and improving phospholipid membrane turnover. This area of the brain contributes to memory function by supporting vigilance, attention, and working memory capacity, and by reducing mental fatigue. Because citicoline’s effect was most prominent in this brain region, this is a likely explanation for its clinical benefit of improved cognitive function.11
Citicoline may further benefit patients experiencing ischemia by decreasing the accumulation of free fatty acids at the site of the lesion, which occurs as a result of neuronal cell damage and death. Soon after initiation of ischemia, there is a significant increase in proinflammatory arachidonic acid, glycerols, and free fatty acids, caused by the breakdown of neuronal membranes. Toxic metabolites as well as prostaglandins, thromboxanes, and free radicals can accumulate, leading to further damage. Animal studies showed that intracerebral administration of citicoline before induction of ischemia reduced the increase in free fatty acids, arachidonic acid, and other toxic metabolites, attenuating free radical damage and restoring membrane function.5
Some evidence points to the ability of citicoline to normalize neurotransmitter release patterns. In conditions of cerebral hypoxia, which exist in ischemia, norepinephrine release may decrease, whereas the release of dopamine may increase. In several animal models, citicoline was shown to inhibit the impairment of neurotransmitter release in hypoxic conditions. Furthermore, citicoline administration to rats kept in a chronic hypoxic state reduced behavioral deteriorations and increased survival time. Additional studies found that citicoline was able to increase the dilation of blood vessels in animals with cerebral microcirculation injury, significantly increasing cerebral blood flow.4
Citicoline shows neural restorative effects, presumably via action on the dopaminergic system of the central nervous system. Rats with substantia nigra lesions were shown to regenerate nerve cells after treatment with citicoline, indicating its protective effect in this region. Further studies found that citicoline administration to rats increased striatal dopamine synthesis. Several other investigations in animal models yielded evidence of citicoline’s ability to enhance dopaminergic synthetic pathways.4 This was a result of the activation of tyrosine hydroxylase and inhibition of dopamine reuptake, which is related to citicoline’s activity on phospholipid synthetic pathways. Citicoline is also known to have effects on serotonin and norepinephrine.12 Studies in rats showed that citicoline improved learning and memory capacity and enhanced motor performance and coordination in aged rats. These findings provided further evidence for citicoline’s cholinergic activity.13
Additional mechanisms of citicoline’s neuroprotective effects were highlighted in recent research. Studies suggested that citicoline enhanced the preservation of an inner mitochondrial membrane component known as cardiolipin, which is important for preservation of mitochondrial function. Citicoline facilitated the preservation of sphingomyelin, which promotes signal transduction in nerve cells. Citicoline exhibited direct antioxidant effects, because research showed that it has an ability to stimulate glutathione synthesis and activity of the enzyme glutathione reductase. Furthermore, citicoline attenuates lipid peroxidation. These downstream effects may be attributable to citicoline’s larger function of attenuating the activation of phospholipase A2, thus reducing inflammation in neural tissues and in general.14 Citicoline was shown to have direct free radical suppressive effects, as seen in animal models of transient cerebral ischemia, in which citicoline had a suppressive effect on hydroxyl radical generation.15
Citicoline may significantly impact brain remodeling activity. In an animal model, citicoline treatment significantly increased the length and branch points of dendrites, which led to an increased efficiency of sensory information processing.16 This mechanism of activity could potentially account for a significant portion of citicoline’s neurorestorative functions.
 Clinical Applications
Clinical Applications
Learning and Memory
Experiments in animals and humans provided evidence of citicoline’s ability to promote important cognitive processes, including learning ability and memory functions. Clinical studies evaluating citicoline administration for cognitive enhancement have been conducted for several decades. A review of trials utilizing citicoline as a treatment for senile alterations of memory in 1991 found significant benefits in patients with cerebral insufficiency and chronic cerebrovascular disease.17
A randomized, double-blinded, placebo-controlled study was undertaken to assess the effects of citicoline supplementation on verbal memory function in 95 healthy subjects aged 50 to 85 years (47 women and 48 men) who were administered citicoline (500 mg orally twice daily) or placebo for 3 months. The study subjects were well educated (mean, 14.3 years of education). Baseline testing included a logical memory assessment test, which was used to classify those with relatively inefficient memories. At the end of the initial study, 32 individuals (16 from the citicoline group and 16 from the placebo group) from this pool were recruited to participate in a follow-up crossover study. The initial study found citicoline improved delayed recall for only those with relatively inefficient memory at the beginning of the trial. In the follow-up crossover study, the dose of citicoline was increased to 2000 mg/day. In this subgroup, the higher dose of citicoline improved immediate and delayed logical memory.18
An open-label crossover trial consisting of 24 elderly individuals without dementia, but with demonstrable memory impairment (assessed by comparison with 24 healthy young control subjects), showed that oral citicoline (500, 1000, or 300 mg/day combined with nimodipine [90 mg/day]) significantly improved memory performance compared with the no treatment periods, as evidenced by reduced error scores on word recall tasks, immediate object recall, and delayed object recall.19
Alzheimer’s Disease and Dementia
Citicoline supplementation has been well studied in Alzheimer’s disease and vascular dementia. A study of 19 patients (mean age 66.21 ± 1.48 years) given oral citicoline at a dosage of 1000 mg/day for 30 days found significant improvements in cognitive function in the subgroup of patients with early-onset Alzheimer’s disease and a trend toward increased cognitive function in the overall group, as assessed by brain electrical mapping. Brain spectral data readings provided an indication that the brains of early-onset Alzheimer’s patients showed greater damage than those of late-onset Alzheimer’s patients, whereas both groups had the same degree of cognitive impairment. It was postulated that the therapeutic effects of citicoline might be mediated by an enhancement of cholinergic neural transmission, activation of repair mechanisms to rejuvenate neuronal membranes, a regulatory effect on parameters associated with blood flow and circulation, as well as regulation of several immunologic responses, which, if left unchecked, would lead to potential neuronal dysfunction and cell death.20
In further studies, oral administration of citicoline (1000 mg/day) to 20 patients (age range 57 to 78 years) with early- or late-onset Alzheimer’s disease resulted in improvements in mental function, particularly in the early-onset group. This 1-month treatment with citicoline resulted in an increased blood flow velocity from baseline measures (assessed by transcranial Doppler ultrasound) in the middle cerebral artery, which has been found to decrease with age, possibly resulting in neuropathologic changes. Citicoline’s cholinergic effects and influence on cytokine production might also partially account for its benefits.21
Researchers investigated the regulatory effects of citicoline on blood histamine levels. Alterations in the histamine system are present in Alzheimer’s disease, as high levels have been found in several central nervous system regions, cerebrospinal fluid, and serum. Histamine may also participate in the aging process, with histamine-related changes reported in several different tissues, including the central nervous system. In one study, 14 individuals with Alzheimer’s disease (7 early-onset, 7 late-onset) were administered citicoline (1000 mg/day for 30 days). Blood histamine measurements were taken at baseline, at 2 hours after administration of the first dose, and after 30 days of treatment with citicoline. All participants experienced an acute reduction in blood histamine levels; after 30 days, early-onset Alzheimer’s patients saw a decrease in blood levels of histamine of about 55% compared with baseline, whereas late-onset individuals saw a 45% decrease. Early-onset patients clearly had higher baseline levels of histamine than late-onset patients. Reducing endogenous histamine excesses may support cognitive function, as excessive histamine levels have been implicated in etiopathogenic events in Alzheimer’s disease.22
The effects of citicoline administration (1000 mg/day orally for 3 months) were assessed in a trial in patients with senile dementia (Alzheimer’s disease and multi-infarct dementia) to elucidate whether the nutrient was able to restore immune function and improve mental parameters. The study consisted of four groups: control subjects (n = 8), early-onset Alzheimer’s subjects (n = 11), late-onset Alzheimer’s subjects (n = 7), and multi-infarct dementia subjects (n = 10). After 3 months of treatment, citicoline supplementation improved mental performance in all groups (including controls), as assessed by several standard assessment tools (including the Mini-Mental State Examination and the Hamilton Rating Scale for Depression). Early-onset Alzheimer’s patients showed increased levels of interleukin-1β at baseline. Citicoline administration normalized these levels in the early-onset Alzheimer’s group. The researchers concluded that citicoline showed benefit in senile dementia patients as a restorative and palliative treatment, improving vascular risk factors, stabilizing immune function, and improving mental performance.23 Further studies corroborated these effects of citicoline.24
Citicoline was further studied in a double-blinded, placebo-controlled randomized trial in 30 patients with apolipoprotein-E (Apo-E) genotyped Alzheimer’s disease. All 30 participants were categorized as having mild to moderate dementia. Citicoline (1000 mg/day orally) or placebo was administered daily for 12 weeks, and its efficacy was further evaluated on the basis of each of the individuals’ Apo-E genotype. The development of certain symptoms of Alzheimer’s disease is correlated with differing Apo-E genotypes. The results of the study showed that clinical interview-based impression of change scores worsened significantly in the placebo group, whereas a clear trend toward improvement in the citicoline group was observed. In those individuals bearing the ε4 allele of the Apo-E (Apo-E4), citicoline was found to induce significant improvements on the cognitive function subscale of the Alzheimer’s disease assessment scale. Furthermore, statistically significant improvements in Alzheimer’s disease assessment scale scores were found with citicoline administration in the subset of Apo-E4 patients with mild cognitive deterioration (as assessed by Global Deterioration Scale scores less than 5). An overall increase in cerebral blood flow velocity was also seen with citicoline compared with placebo, whereas beneficial changes were further noted in brain bioelectrical activity.25
Parkinson’s Disease
Citicoline may also benefit individuals with Parkinson’s disease. Citicoline was administered by intramuscular (IM) injection to 20 patients (aged 52 to 76 years) at a dosage of 1000 mg/day for 15 days, followed by 500 mg/day for an additional 15-day period. (All of these patients received levodopa alone or in combination with other drugs before and during the trial.) The patients had improved scores on the Columbia rating scale (one of several validated and reliable rating tools for disability in Parkinson’s26) by 7.3%, and also had measurable improvements in rigidity, time to walk 10 meters, time to turn over, and handwriting test scores. Results from a self-assessment revealed that symptomatology was improved in 15 of the 20 patients, including improvements in speech, gait, posture, tremor, agility, and slowness of movements. Five patients showed minor improvements in dyskinesia; however, this was otherwise unaffected by citicoline treatment.27
Additionally, citicoline has a levodopa-sparing effect and an ability to increase dopamine synthesis. A trial of 85 Parkinson’s disease patients were randomly assigned to two groups: patients received either their usual dose of levodopa (mean 381 mg/day) or half their usual dose (mean 196 mg/day). Both groups were simultaneously administered 1200 mg citicoline (400 mg orally three times daily); it was found that the group consuming half of their usual levodopa dose plus citicoline had significant improvements at week 6 (which was the end of the fourth week of citicoline administration) on the Webster Rating Scale, a measure of neurologic and clinical symptoms. This trial indicated that citicoline has the ability to compensate for the reduction of levodopa dosage, potentially contributing to a reduction in side effects associated with long-term levodopa usage.28
A similar trial was performed in 30 individuals with Parkinson’s disease in which the participants were treated with levodopa and concomitantly received 500 mg of citicoline by IM injection daily for 30 days. Significant improvements in neurologic signs were noted (including moderate improvements in facial expression and digital skill, and marked improvements in ability to rise from a seated position, posture, and gait) as well as improvement in certain electrophysiologic parameters. An increase in dyskinesia was noted as a side effect in this group. In the second phase of the trial, the levodopa dosage was reduced by one-third. This decrease restored the incidence of dyskinesia to pretreatment levels, whereas the therapeutic response remained stable.29
An additional double-blinded, placebo-controlled, crossover design trial, in which citicoline (500 mg/day IM) or placebo was administered to 30 Parkinson’s disease patients already on levodopa and a dopa decarboxylase inhibitor, found that citicoline induced improvements in bradykinesia. There was a 26.97% improvement from baseline on the Webster Rating Scale. This improvement was highly statistically significant compared with baseline and compared with placebo. Significant improvements were also noted in rigidity with citicoline treatment, whereas no such improvement occurred with placebo.30
Stroke and Cerebral Ischemia
Multiple trials have shown positive benefits with citicoline administration in stroke and cerebral ischemia. Researchers conducted a multicenter, double-blinded, placebo-controlled trial to evaluate the efficacy of citicoline administration in patients with acute cerebral infarction. The study consisted of 272 Japanese patients with a confirmed diagnosis of cerebral infarction and a mild to moderate impaired level of consciousness. Patients were randomly assigned to receive citicoline (1000 mg/day IV) or placebo for 14 days. When assessed at days 7 and 14, citicoline treatment resulted in significant improvements in level of consciousness and neurologic status in acute stroke patients.31
A second multicenter, randomized trial conducted in the United States (the U.S. Citicoline Stroke Treatment Study) also yielded positive results in acute stroke patients. Initial treatment was begun within 24 hours of stroke onset and continued orally for 6 weeks. Three dose groups of citicoline were established (500, 1000, or 2000 mg/day) in addition to the placebo group. The Barthel Index was used as the primary outcome measure to assess functional improvement. In the 500 mg/day citicoline group, the odds ratio for improvement was 2.0, and in the 2000 mg/day group, the ratio was 2.1, signifying that individuals in these groups were twice as likely to achieve higher Barthel scores than those in the placebo group. Overall, the results showed that citicoline (500 or 2000 mg/day groups) significantly improved functional recovery after 6 weeks of treatment compared with placebo, as assessed at the 12-week follow-up visit. Interestingly, the group taking 1000 mg/day of citicoline did not show a comparable benefit in this study. This was a puzzling result because all baseline characteristics of each group were essentially identical, except for weight, which was higher in this group than in the other treatment groups. The authors postulated this might have played a role in the outcome.32
A multicenter randomized placebo-controlled trial (RCT) in acute stroke patients (National Institute of Health [NIH] Stroke Scale scores higher than 5) given an oral dose of citicoline (500 mg/day) for 6 weeks found no benefits for stroke recovery, unlike earlier studies. However, a significant confounding factor might have impacted the analysis. More participants with milder strokes, as assessed by the NIH criteria, ended up populating the placebo arm of the study, which likely adversely affected the ability to see a treatment effect of citicoline.33
A larger Phase III multicenter RCT assessed the effectiveness of high-dose citicoline (2000 mg/day for 6 weeks) for stroke recovery. This study featured recruitment from 118 stroke centers from which 899 patients were randomized to receive citicoline or placebo. Unlike earlier trials that assessed the efficacy of citicoline in moderate stroke patients, this study recruited participants with NIH Stroke Scale scores of 8 or higher, designating more severe strokes. The major end point of this trial was a comparison of the proportion of individuals with an improvement of 7 or more points on the NIH Stroke Scale as assessed at week 12. Although no significant differences were noted between groups using the NIH Stroke Scale, a benefit was seen for citicoline on the Barthel Index at 6 weeks, as a significantly higher proportion of those in the citicoline group returned to their baseline function.34
Hazama et al35 conducted a double-blinded, placebo-controlled trial in 1980 to assess the impact of citicoline administration as an adjunct to regular rehabilitation in poststroke recovery from hemiplegia. Citicoline was administered once daily for 8 weeks to patients continuing rehabilitation who were assigned to one of three groups: citicoline high-dose group (1000 mg/day IV), citicoline low-dose group (250 mg/day IV), or placebo (isotonic saline). Upper and lower limb joint range of motion was assessed at intervals throughout the study, as were subjective symptoms, neurologic signs, and mental symptoms. Significant improvements were noted in functional recovery in the upper limb in all groups, with dose-dependent improvements noted in both citicoline groups, which were significantly superior to placebo by week 8. No significant differences were noted between groups in the lower limb, although both citicoline groups showed a slightly higher rate of improvement compared with placebo.35
Citicoline was evaluated in a randomized, placebo-controlled study consisting of 92 patients with chronic cerebrovascular conditions. Individuals received citicoline (1000 mg/day IM) or placebo for two treatment cycles of 4 weeks each with a 1-week interval between cycles. The results of the study showed that citicoline significantly improved attention ability by decreasing the number of wrong responses on the Toulouse-Piéron Test for nonverbal stimuli. Furthermore, a constant and progressive improvement was noted with citicoline treatment on memory tests and on emotional and behavioral assessments.36
Citicoline treatment of patients with hemorrhagic, nontraumatic cerebral infarction was found to enhance the recovery of muscular strength associated with recuperation. A double-blinded randomized trial conducted with 32 study subjects divided between those receiving 250 mg of citicoline IV twice daily or a placebo for 14 days showed that, compared with baseline, muscular strength in the citicoline group increased significantly more than in the group receiving placebo.37
An editorial published in the Journal of Neurological Sciences described the conclusions from a meta-analysis (published in the journal Stroke in 2002), which declared citicoline to be the first clinically effective neuroprotective agent in ischemic stroke. This significant editorial recommended that citicoline be the agent of choice in trials of combination therapy for stroke with thrombolytic agents because of its high level of safety and efficacy in promoting recovery from ischemic conditions.38 Furthermore, a review by The Cochrane Collaboration analyzed the outcomes from several trials utilizing citicoline in the treatment of cognitive and behavioral symptoms resulting from chronic cerebral conditions in the elderly. Researchers ultimately included 14 studies in their review. Their analysis of the trials that met the specified inclusion criteria revealed that citicoline showed benefits for improved memory function and behavior in elderly individuals with chronic cerebral disorders.39
Traumatic Head Injuries
Research into the beneficial effects of citicoline for traumatic head injuries and concussions has been ongoing for several years. Injuries to the brain decrease the production of cell membrane phospholipids, resulting in an accumulation of intracellular water, leading to cytotoxic edema and possible deterioration of the hematoencephalic barrier. Citicoline can have therapeutic benefits in these conditions, because it is a precursor for the synthesis of neuronal membrane phospholipids. A single-blinded randomized study assessing the use of IV citicoline (1 g every 6 hours for 2 days, followed by 1 g every 8 hours on the third and fourth days) in addition to conventional therapy in 216 patients with severe or moderate head injuries found citicoline to be superior to conventional therapy alone—as assessed by the Glasgow Outcome Scale 3 months after injury. Citicoline also showed a trend towards shortening the hospital stay for severe head injury patients while improving motor, cognitive, and mental symptoms.40 The authors cited earlier research on citicoline in treating moderate to severe head injuries, which showed that citicoline increased chances of recovery to a nondependent condition (including the ability to walk and perform activities of daily living), improving quality of life. Citicoline also improved levels of consciousness assessed at 60 days after injury in traumatic coma patients and reduced the percentage of patients showing focal neurologic signs at 60 and 90 days postinjury.40
Citicoline therapy may also help alleviate postconcussional symptoms. In one randomized trial with 14 young adults (median age 25 years in the citicoline group and 20 years in the placebo group) with mild to moderate head injuries, participants were administered 1 g of citicoline orally or a placebo. Assessments included tests of memory function, fluency, and attention. Although results at 1 month did not reach statistical significance, the group given citicoline trended towards higher improvement in several categories at the follow-up visit, including improvements in recognition memory, and decreased incidence of headaches, dizziness, and tinnitus.41
A review article examining trials of citicoline for the treatment of traumatic head injuries, including in children as young as age 5, espoused the benefits of citicoline therapy for improving neurologic signs and symptoms, the level of consciousness, and enhancing recuperation and facilitating electroencephalographic improvements. The review highlighted several trials related to traumatic coma patients treated with citicoline, which led to better recovery of motor function and walking ability compared with placebo. The overall results of these trials indicated that citicoline treatment reduced the duration of coma and the incidence and severity of mental and motor deficits associated with traumatic head injuries. Citicoline was also shown to be safe and well tolerated in patient populations of several age ranges and with various types of traumatic head injuries.42
 Eye Health and Visual Function
Eye Health and Visual Function
Several trials with citicoline showed beneficial effects on eye health, specifically in cases of amblyopia and glaucoma. Amblyopia, or lazy eye, is the leading cause of decreased visual acuity in children, resulting in poor depth perception. Glaucoma is a leading cause of blindness in U.S. adults and is a group of conditions resulting in damage to the optic nerve, usually as a result of elevated intraocular pressure.
Amblyopia
An open trial and a pilot double-blinded follow-up study were conducted to assess possible benefits of citicoline therapy in patients with amblyopia. The open trial consisted of 50 patients (mean age, 16.6 years) who were administered citicoline (1000 mg/day IM) for 15 days. Visual acuity of both eyes was tested 1 week after the initiation of treatment and continuing at weekly intervals for the first month, and then on a monthly basis for an additional 6 to 18 months. For the double-blinded portion of the study, 10 patients were divided into 2 groups: 1 group received citicoline (1000 mg/day IM), and the other group received placebo. Citicoline improved visual acuity in 92% of the patients in the open study. Improvements were noted in both the sound and amblyopic eyes and were highly statistically significant. In the double-blinded study, significant improvements were noted between groups, with the citicoline group showing enhanced visual acuity.43
Another trial of citicoline took place in 45 children with amblyopia aged 5 to 9 years old. Participants were divided into three treatment groups: group A received 500 mg of citicoline daily via IM injection for 10 days every 6 months; group B received the same dosage of citicoline as group A in combination with 1 hour of occlusion (of the sound eye) per day; and group C received daily occlusion therapy alone. Although visual acuity improved in all groups at the end of the treatment period, treatment with citicoline was found to enhance the effect of occlusion therapy. Visual acuity improved in 73% of participants in group A, 86.6% of group B, and 66.6% of group C.44
An open-label trial with oral citicoline (800 to 1200 mg/day of citicoline according to body weight for 30 days) plus partial occlusion therapy in 61 children (aged 5 to 10 years) found that citicoline contributed to stabilizing the gains obtained during the treatment period when assessed at the 60-day posttreatment follow-up visit. Those receiving occlusion therapy alone showed a decrease in visual acuity gains at the 60-day follow-up, whereas those in the citicoline group maintained the gains achieved with occlusion therapy.45
Citicoline likely influences improvements in visual acuity in amblyopic individuals by stimulating the availability of several neurotransmitters and neuromodulators. It also enhances the activity of endogenous dopamine, while improving vascular aspects of neurologic function.46
Glaucoma
A randomized clinical study evaluating the effects of citicoline in 40 patients with open-angle glaucoma, who received either daily IM injections containing 1000 mg of citicoline or placebo for 60 days, found that citicoline significantly improved visual evoked potential (a measure of bioelectrical activity of the visual cortex in response to visual stimuli) and the pattern-electroretinographic (used to evaluate the functional integrity of the innermost retinal layers) parameters compared with placebo. Patients in the citicoline group were then divided into two age-matched groups after a 120-day washout period. In one of these groups, the washout period was extended for an additional 120 days, whereas the second group received a further 60-day treatment of citicoline. This second group showed further improvements in visual evoked potential and pattern-electroretinographic parameters, indicating citicoline’s ability to enhance retinal function and visual cortical response in glaucoma patients.47
A double-blinded RCT assessing the benefits of citicoline in glaucoma patients confirmed that administration of citicoline for 60 days (1000 mg/day IM) was superior to placebo and significantly improved retinal function and cortical bioelectrical responses. This particular trial included an analysis of 8 years of follow-up data.48
A review examining the potential mechanisms through which citicoline exerts its beneficial influence in glaucoma patients theorized that citicoline’s ability to enhance the synthesis of phosphatidylcholine and other cell-membrane phospholipids was a major factor of improvement. Glaucoma is considered a neurodegenerative disease in which the pathology extends to retinal ganglion cells. Death of these cells is likely a result of apoptotic mechanisms. The enhancement of phosphatidylcholine synthesis as a result of citicoline intake countered the neuronal apoptotic mechanisms associated with glaucoma and conferred neuroprotection.49
Ischemic Optic Neuropathy
Nonarteritic ischemic neuropathy is an irreversible ischemic event associated with the intraocular optic nerve. The condition occurs acutely and painlessly, yet induces a loss of visual acuity and visual field. In a pilot study designed to assess citicoline’s effect on this condition, 26 patients with at least a 6-month history of nonarteritic ischemic optic neuropathy were divided into two groups: one received treatment consisting of oral citicoline (1600 mg/day) for 60 days, whereas the second group received no treatment. After the 60-day treatment cycle, there was a washout period of 120 days. After this, a second period of treatment with citicoline for 60 days was instituted in the original citicoline group. A third group of 14 age-matched healthy subjects provided control data. At the end of treatment, statistically significant improvements were noted in visual evoked potential, visual acuity, and pattern-electroretinographic parameters in the citicoline group compared with pretreatment values, whereas no such changes were observed in the untreated group of nonarteritic ischemic neuropathy subjects.50
 Emerging Clinical Applications
Emerging Clinical Applications
Substance Abuse
Citicoline has been considered as an adjunct treatment for cocaine dependence by researchers in the field in recent years. The justification for its use stems from citicoline’s ability to repair neuronal membranes, which are damaged by cocaine use, and its ability to increase central nervous system dopamine levels, attenuating cravings for cocaine and other abused substances.51
A small double-blinded, placebo-controlled trial in 14 subjects with a history of cocaine dependence found that oral citicoline (500 mg twice a day for 14 days) caused no adverse events and also attenuated some measures of cocaine craving and drug use.52
An additional experiment in eight healthy occasional cocaine users investigated the influence of citicoline pretreatment on cocaine-induced cardiovascular and behavioral effects and plasma levels of cocaine. The primary outcome measure was to determine the safety of coadministration of citicoline with cocaine. Because citicoline did not adversely impact cardiovascular end points associated with acute cocaine intake, the use of citicoline in this patient population was presumed to be safe. Although citicoline did not block the acute subjective effects of cocaine use, cocaine users experienced a high incidence of major cerebrovascular events. Thus, the authors speculated that citicoline could play a role in attenuating these undesirable consequences of cocaine use, although further studies are needed to investigate these potential benefits.53
Individuals with bipolar disorder are at an increased risk for substance abuse, with cocaine use being particularly common in this condition. Both the disorder and cocaine use are associated with mood symptoms and cognitive deficits. Given these commonalities, a 12-week double-blinded RCT was conducted to assess the impact of citicoline supplementation on 44 individuals with bipolar disorder and cocaine dependence. Citicoline resulted in an improvement in some aspects of declarative memory. However, no evidence for antidepressant properties was seen with the use of citicoline. There was also a significantly lower probability of a positive urine test for cocaine at the end of the study in the citicoline group.54
Infectious Disease
A recently published article highlighted the role citicoline might play as an adjunctive therapeutic agent for the treatment of disease arising from an infectious etiology. The pathology of infectious disease involves dysregulation of the host immune response. Although currently available treatments target the infectious agent, they do little to address the concurrent neurologic abnormalities. These consequences, if unaddressed, lead to eventual mortality in a high percentage of cases. In the case of cerebral malaria, studies highlighted cytokine-induced endothelial inflammation and the compromised blood–brain barrier as major pathologic factors leading to the associated neurologic signs and symptoms. Ischemia is a large underlying mechanism for damage in sepsis as well as cerebral malaria. Because these underlying issues are similar to conditions such as stroke and myocardial infarction, it is likely that citicoline would benefit these patients as an adjunct treatment for sequelae of sepsis and cerebral malaria.2
Appetite Control and Satiety
Given citicoline’s ability to enhance cognitive function, confer neuroprotection, and support neuroregenerative effects, and its action to support dopaminergic activity in the brain, researchers investigated the potential of citicoline administration for controlling appetite and promoting satiety. In this particular study, the effect of oral citicoline supplementation (as Cognizin citicoline, 2000 mg/day) for 6 weeks was assessed by functional magnetic resonance imaging to elucidate cortico-limbic responses to images of various foods, along with subjective ratings of appetite and measures of weight. Sixteen healthy adults (age range of 40 to 57 years, and a body mass index range of 20.1 to 38.6 kg/m2) were included in the study. Eight participants were assigned to a low-dose citicoline group (500 mg/day), and the other eight were assigned to the high-dose group (2000 mg/day). The results of the study indicated that self-assessment ratings for appetite declined significantly between visits in both groups, with the magnitude of decline in the high-dose group reaching statistical significance. No significant between-group differences were noted for the magnitude of weight change. The high-dose citicoline group also showed higher activation within the right lateral orbitofrontal cortex and left amygdala during visual perception of high-calorie foods than the low-dose group when assessed by functional magnetic resonance imaging. This might indicate that high-dose citicoline leads to appetite suppression and feelings of satiety by increasing the responsiveness of these regions to images of calorie-rich foods.55
Mental Health
Citicoline’s influence on cognitive capacity and neurologic health, including modulatory activity on neurotransmitter production and function, make it a logical choice for supporting mental health. A small trial evaluated the effect of citicoline administration in eight depressed patients (500 mg/day IM, as 300 mg at 8 AM and 200 mg at 5 PM) for 21 days or longer. On the day preceding the beginning of treatment, plasma growth hormone was measured, levels of which indicated reduced growth hormone secretion in these patients. Significant improvements were noted in seven of eight participants (treatment was discontinued in one participant because of a noted suicidal tendency) when assessed using the Hamilton Rating Scale for depression. Although the study was small, the results indicated the potential for significant benefit from citicoline therapy in depressed individuals.56
A preliminary study considered combination treatment of citicoline with galantamine for schizophrenic patients. Evidence suggested that α7 nicotinic choline receptors have decreased functionality in schizophrenia. Citicoline provides choline, which is a known α7 nicotinic choline receptor agonist. Galantamine is a modulator of α7 nicotinic choline receptor function and was used to enhance the efficiency of choline binding to these receptors. Six schizophrenic patients consuming 2 g of citicoline in combination with 24 mg of galantamine daily participated in this 12-week open-label pilot study. The combination was well-tolerated by all of the participants, with transient side effects occurring (all of which resolved within a few days), including gastrointestinal symptoms, restlessness, and syncope. No cardiovascular events or symptoms occurred, with five of the six participants having lower than baseline diastolic blood pressure readings at the end of the study. Improvements were noted in five of six patients on the Clinical Global Impressions inventory and the Positive and Negative Syndrome Scale. Total Positive and Negative Syndrome Scale scores and Clinical Global Impressions severity scale scores decreased over the study period, providing encouraging results, suggesting that the combination therapy was potentially effective.57
 Safety and Dosing
Safety and Dosing
Clinical investigations using citicoline revealed a favorable safety profile with few reports of any major adverse events. The most common adverse reports were related to digestive disturbances. Citicoline has also been found to have a lack of significant adverse events in children, as evidenced by its use in clinical trials with pediatric subjects with amblyopia44 and children with traumatic head injuries.42
Citicoline has undergone several toxicologic evaluations in multiple animal species and has proven to have a high level of safety. Single-dose acute oral toxicity studies were performed in mice and rats, with a median lethal dose of 27.14 g/kg in mice and 18.5 g/kg in rats. Chronic oral toxicity tests in dogs (1.5 g/kg per day for 6 months) and subchronic intraperitoneal dosing studies in rats (1 g/kg per day for 12 weeks) showed no abnormal signs.12
An acute 14-day study and a 90-day subchronic toxicity evaluation of Cognizin citicoline in rats revealed that the supplement was well tolerated. In the 14-day study, a single dose of 2000 mg/kg showed no abnormalities, and in the 90-day repeated oral dosing study, doses of 100, 350, and 1000 mg/kg per day resulted in no mortality in the animals. In male rats, slight increases in serum creatinine were noted in the two highest dose groups, whereas in female rats, a dose-related increase in renal tubular mineralization was noted and attributed to an increase in phosphorus intake as a result of high citicoline consumption. Mineralization in female laboratory rats of all universally used strains is a common incidental finding as a result of a decreased calcium/phosphorus ratio in the diet. Because citicoline yields a significant amount of phosphorus—thus influencing the calcium/phosphorus ratio—this finding was not unexpected.58
A drug surveillance study was recently published examining the efficacy and safety of oral citicoline intake in acute ischemic stroke. The study of 4191 Korean patients confirmed a high level of safety for citicoline (500 to 4000 mg/day for 6 weeks or longer) with an incidence of 37 adverse events in 31 patients (only 0.73% of patients experienced adverse events). Adverse events in all but 1 of the 31 patients showed no relationship to citicoline administration. Thirty-two of the 37 (nearly 84%) patients reported events resolved over a mean of 9 days after onset. Furthermore, no dose-related effects of citicoline on the occurrence of adverse events were noted. The most frequent side effects included minor nervous system-related complaints (n = 8) (numbness, headache, tingling sensations) followed by gastrointestinal symptoms (n = 5) (abdominal discomfort, diarrhea).59
Effective dosing of citicoline based on data from clinical trials ranges from 500 to 2000 mg/day. Based on evidence from trials in children as young as 5 years of age,42,44 citicoline is safe for use in pediatric and adult populations. Oral doses of up to 1200 mg/day have been used in children.45 Studies using oral, IM, and IV dosing of citicoline in children and adults, with minimal occurrence of adverse events, affirmed its high level of safety.
 Drug Interactions
Drug Interactions
Citicoline has a levodopa-sparing effect, potentiating its benefits and reducing certain side effects associated with long-term levodopa use.28-30
 Conclusions
Conclusions
Citicoline is a novel compound with a very broad spectrum of benefits in conditions associated with symptoms of neurologic dysfunction. Citicoline acts at multiple levels to support and maintain neural health and optimal cognitive function. Citicoline promotes cholinergic and dopaminergic functions, and supports phospholipid synthesis and incorporation into cell membranes. Citicoline enhances antioxidant mechanisms in the body, while suppressing the damaging effects of free radicals on neural tissue. It also promotes antiinflammatory activities and optimizes patterns associated with the release of neurotransmitters. Given its widespread activity on neural tissue, citicoline should be considered a comprehensive therapeutic agent for supporting brain health.
1. Secades J.J., Lorenzo J.L. Citicoline: pharmacological and clinical review, 2006 update. Methods Find Exp Clin Pharmacol. 2006;28(Suppl B):1–56.
2. Jambou R., El-Assaad F., Combes V., et al. Citicoline (CDP-choline): what role in the treatment of complications of infectious diseases. Int J Biochem Cell Biol. 2009;41:1467–1470.
3. Higdon J., Drake V.J. Linus Pauling Institute at Oregon State University. Choline. http://lpi.oregonstate.edu/infocenter/othernuts/choline. Accessed 4/6/2010
4. Weiss G.B. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995;56:637–660.
5. D’Orlando K.J., Sandage B.W. Citicoline (CDP-choline): mechanisms of action and effects in ischemic brain injury. Neurol Res. 1995;17:281–284.
6. Dinsdale J.R., Griffiths G.K., Rowlands C., et al. Pharmacokinetics of 14C CDP-choline. Arzneimittelforschung. 1983;33:1066–1070.
7. Agut J., Font E., Sacristán A., et al. Bioavailability of methyl-14C CDP-choline by oral route. Arzneimittelforschung. 1983;33:1045–1047.
8. Agut J., Font E., Sacristán A., et al. Radioactivity incorporation into different cerebral phospholipids after oral administration of 14C methyl CDP-choline. Arzneimittelforschung. 1983;33:1048–1050.
9. Babb S.M., Appelmans K.E., Renshaw P.F., et al. Differential effect of CDP-choline on brain cytosolic choline levels in younger and older subjects as measured by proton magnetic resonance spectroscopy. Psychopharmacology (Berl.). 1996;127:88–94.
10. Babb S.M., Wald L.L., Cohen B.M., et al. Chronic citicoline increases phosphodiesters in the brains of healthy older subjects: an in vivo phosphorus magnetic resonance spectroscopy study. Psychopharmacology (Berl.). 2002;161:248–254.
11. Silveri M.M., Dikan J., Ross A.J., et al. Citicoline enhances frontal lobe bioenergetics as measured by phosphorus magnetic resonance spectroscopy. NMR Biomed. 2008;21:1066–1075.
12. Secades J.J., Frontera G. CDP-choline: pharmacological and clinical review. Methods Find Exp Clin Pharmacol. 1995;17(Suppl B):1–54.
13. Drago F., Mauceri F., Nardo L., et al. Effects of cytidine-diphosphocholine on acetylcholine-mediated behaviors in the rat. Brain Res Bull. 1993;31:485–489.
14. Adibhatla R.M., Hatcher J.F., Dempsey R.J. Citicoline: neuroprotective mechanisms in cerebral ischemia. J Neurochem. 2002;80:12–23.
15. Adibhatla R.M., Hatcher J.F. Citicoline decreases phospholipase A2 stimulation and hydroxyl radical generation in transient cerebral ischemia. J Neurosci Res. 2003;73:308–315.
16. Rema V., Bali K.K., Ramachandra R., et al. Cytidine-5-diphosphocholine supplement in early life induces stable increase in dendritic complexity of neurons in the somatosensory cortex of adult rats. Neuroscience. 2008;155:556–564.
17. De la Morena E. Efficacy of CDP-choline in the treatment of senile alterations in memory. Ann N Y Acad Sci. 1991;640:233–236.
18. Spiers P.A., Myers D., Hochanadel G.S., et al. Citicoline improves verbal memory in aging. Arch Neurol. 1996;53:441–448.
19. Alvarez X.A., Laredo M., Corzo D., et al. Citicoline improves memory performance in elderly subjects. Methods Find Exp Clin Pharmacol. 1997;19:201–210.
20. Franco-Maside A., Caamaño J., Gómez M.J., et al. Brain mapping activity and mental performance after chronic treatment with CDP-choline in Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 1994;16:597–607.
21. Caamaño J., Gómez M.J., Franco A., et al. Effects of CDP-choline on cognition and cerebral hemodynamics in patients with Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 1994;16:211–218.
22. Fernández-Novoa L., Alvarez X.A., Franco-Maside A., et al. CDP-choline-induced blood histamine changes in Alzheimer’s disease. Methods Find Exp Clin Pharmacol. 1994;16:279–284.
23. Cacabelos R., Alvarez X.A., Franco-Maside A., et al. Effect of CDP-choline on cognition and immune function in Alzheimer’s disease and multi-infarct dementia. Ann N Y Acad Sci. 1993;695:321–323.
24. Cacabelos R., Caamaño J., Gómez M.J., et al. Therapeutic effects of CDP-choline in Alzheimer’s disease. Cognition, brain mapping, cerebrovascular hemodynamics, and immune factors. Ann N Y Acad Sci. 1996;777:399–403.
25. Alvarez X.A., Mouzo R., Pichel V., et al. Double-blind placebo-controlled study with citicoline in APOE genotyped Alzheimer’s disease patients. Effects on cognitive performance, brain bioelectrical activity and cerebral perfusion. Methods Find Exp Clin Pharmacol. 1999;21:633–644.
26. Ramaker C., Marinus J., Stiggelbout A.M., et al. Systematic evaluation of rating scales for impairment and disability in Parkinson’s disease. Mov Disord. 2002;17:867–876.
27. Martí Massó J.F., Urtasun M. Citicoline in the treatment of Parkinson’s disease. Clin Ther. 1991;13:239–242.
28. Eberhardt R., Birbamer G., Gerstenbrand F., et al. Citicoline in the treatment of Parkinson’s disease. Clin Ther. 1990;12:489–495.
29. Cubells J.M., Hernando C. Clinical trial on the use of cytidine diphosphate choline in Parkinson’s disease. Clin Ther. 1988;10:664–671.
30. Agnoli A., Ruggieri S., Denaro A., et al. New strategies in the management of Parkinson’s disease: a biological approach using a phospholipid precursor (CDP-choline). Neuropsychobiol. 1982;8:289–296.
31. Tazaki Y., Sakai F., Otomo E., et al. Treatment of acute cerebral infarction with a choline precursor in a multicenter double-blind placebo-controlled study. Stroke. 1988;19:211–216.
32. Clark W.M., Warach S.J., Pettigrew L.C., et al. A randomized dose-response trial of citicoline in acute ischemic stroke patients. Citicoline Stroke Study Group. Neurology. 1997;49:671–678.
33. Clark W.M., Williams B.J., Selzer K.A., et al. A randomized efficacy trial of citicoline in patients with acute ischemic stroke. Stroke. 1999;30:2592–2597.
34. Clark W.M., Wechsler L.R., Sabounjian L.A., et al. A phase III randomized efficacy trial of 2000 mg citicoline in acute ischemic stroke patients. Neurology. 2001;57:1595–1602.
35. Hazama T., Hasegawa T., Ueda S., et al. Evaluation of the effect of CDP-choline on poststroke hemiplegia employing a double-blind controlled trial. Assessed by a new rating scale for recovery in hemiplegia. Int J Neurosci. 1980;11:211–225.
36. Piccoli F., Battistini N., Carbonin P., et al. CDP-choline in the treatment of chronic cerebrovasculopathies. Arch Gerontol Geriatr. 1994;18:161–168.
37. Iranmanesh F., Vakilian A. Efficiency of citicoline in increasing muscular strength of patients with nontraumatic cerebral hemorrhage: a double-blind randomized clinical trial. J Stroke Cerebrovasc Dis. 2008;17:153–155.
38. Overgaard K., Meden P. Citicoline-the first effective neuroprotectant to be combined with thrombolysis in acute ischemic stroke? J Neurol Sci. 2006;247:119–120.
39. Fioravanti M., Yanagi M. Cytidinediphosphocholine (CDP-choline) for cognitive and behavioural disturbances associated with chronic cerebral disorders in the elderly. Cochrane Database Syst Rev, 2005;2:CD000269.
40. Calatayud Maldonado V., Calatayud Pérez J.B., Aso Escario J. Effects of CDP-choline on the recovery of patients with head injury. J Neurol Sci. 1991;103(Suppl):S15–18.
41. Levin H.S. Treatment of postconcussional symptoms with CDP-choline. J Neurol Sci. 1991;103(Suppl):S39–42.
42. Lozano R. CDP-choline in the treatment of cranio-encephalic traumata. J Neurol Sci. 1991;103(Suppl):S43–47.
43. Campos E.C., Schiavi C., Benedetti P., et al. Effect of citicoline on visual acuity in amblyopia: preliminary results. Graefes Arch Clin Exp Ophthalmol. 1995;233:307–312.
44. Campos E.C., Bolzani R., Schiavi C., et al. Cytidin-5’-diphosphocholine enhances the effect of part-time occlusion in amblyopia. Doc Ophthalmol. 1997;93:247–263.
45. Fresina M., Dickmann A., Salerni A., et al. Effect of oral CDP-choline on visual function in young amblyopic patients. Graefes Arch Clin Exp Ophthalmol. 2008;246:143–150.
46. Campos E.C., Fresina M. Medical treatment of amblyopia: present state and perspectives. Strabismus. 2006;14:71–73.
47. Parisi V., Manni G., Colacino G., et al. Cytidine-5’-diphosphocholine (citicoline) improves retinal and cortical responses in patients with glaucoma. Ophthalmology. 1999;106:1126–1134.
48. Parisi V. Electrophysiological assessment of glaucomatous visual dysfunction during treatment with cytidine-5’-diphosphocholine (citicoline): a study of 8 years of follow-up. Doc Ophthalmol. 2005;110:91–102.
49. Grieb P., Rejdak R. Pharmacodynamics of citicoline relevant to the treatment of glaucoma. J Neurosci Res. 2002;67:143–148.
50. Parisi V., Coppola G., Ziccardi L., et al. Cytidine-5’-diphosphocholine (Citicoline): a pilot study in patients with non-arteritic ischaemic optic neuropathy. Eur J Neurol. 2008;15:465–474.
51. O’Leary G., Weiss R.D. Pharmacotherapies for cocaine dependence. Curr Psychiatry Rep. 2000;2:508–513.
52. Renshaw P.F., Daniels S., Lundahl L.H., et al. Short-term treatment with citicoline (CDP-choline) attenuates some measures of craving in cocaine-dependent subjects: a preliminary report. Psychopharmacology (Berl.). 1999;142:132–138.
53. Lukas S.E., Kouri E.M., Rhee C., et al. Effects of short-term citicoline treatment on acute cocaine intoxication and cardiovascular effects. Psychopharmacology (Berl.). 2001;157:163–167.
54. Brown E.S., Gorman A.R., Hynan L.S. A randomized, placebo-controlled trial of citicoline add-on therapy in outpatients with bipolar disorder and cocaine dependence. J Clin Psychopharmacol. 2007;27:498–502.
55. Killgore W.D.S., Ross A.J., Kamiya T., et al. Citicoline affects appetite and cortico-limbic responses to images of high-calorie foods. Int J Eat Disord. 2010;43:6–13.
56. Salvadorini F., Galeone F., Nicotera M., et al. Clinical evaluation of CDP-choline (Nicholin): efficacy as antidepressant treatment. Curr Ther Res Clin Exp. 1975;18:513–520.
57. Deutsch S.I., Schwartz B.L., Schooler N.R., et al. First administration of cytidine diphosphocholine and galantamine in schizophrenia: a sustained alpha7 nicotinic agonist strategy. Clin Neuropharmacol. 2008;31:34–39.
58. Schauss A.G., Somfai-Relle S., Financsek I., et al. Single- and repeated-dose oral toxicity studies of citicoline free-base (choline cytidine 5’-pyrophosphate) in Sprague-Dawley rats. Int J Toxicol. 2009;28:479–487.
59. Cho H.-J., Kim Y.J. Efficacy and safety of oral citicoline in acute ischemic stroke: drug surveillance study in 4,191 cases. Methods Find Exp Clin Pharmacol. 2009;31:171–176.