Chapter 111 Pancreatic Enzymes
The author wishes to express his deep appreciation in writing these two Enzyme chapters for the cooperation of the following researchers from the United States, Germany, The Czech Republic, and other Eastern European countries: Dr. Barry Ritz, Dr. Winfried Miller, Dr. Claudia Loyall, Dr. Zinovij Masinovsky, Dr. Michaela Lysikova, Dr. Joseph Pizzorno, Dr. Michael Murray, and Ms. Karen Hood.
 Introduction
Introduction
Pancreatic enzymes produced by the body are well known for the integral role they play in the digestion of the foods we eat. Pancreatic juice contains numerous enzymes, including amylase, lipase, cholesterol esterase and phospholipase, and the proenzymes trypsinogen, chymotrypsinogen, and procarboxypolypeptidase, which are converted in the small intestine to their active forms trypsin, chymotrypsin, and carboxypeptidase, respectively.1
Foods in the human diet are composed primarily of protein, carbohydrates, and fats. Protein leaves the stomach predominantly in the form of proteoses, peptones, and large polypeptides.2 Upon reaching the small intestine, these are further digested by the proteolytic enzymes trypsin, chymotrypsin, and carboxypolypeptidase. Protein digestion primarily occurs in the duodenum and jejunum. Carbohydrates are digested by α-amylase in the pancreatic juice, which breaks starches (converting them into maltose and other small glucose polymers), whereas pancreatic lipase, cholesterol esterase, and phospholipase digest fats.1,3
Although critical for proper digestion, pancreatic enzymes also aid in a surprising variety of bodily functions, including detoxification, immunity, aging, blood fluidity, and tissue repair. Unfortunately, an inadequate production of, or an excessive requirement for, pancreatic enzymes can occur for a variety of reasons, including genetics, illness, injury, exercise, aging, and toxins (both endogenous and exogenous). Many authorities believe that a deficiency of pancreatic enzymes, for whatever reason, may be the cause of numerous illnesses and degenerative conditions. When a deficiency occurs, enzymes from an external source may be necessary.
 History
History
Pancreatic enzymes have a long history of clinical use. In the early twentieth century, John Beard, a Scotch embryologist, successfully treated cancer using a pancreatic extract, which he described in his book, The Enzyme Treatment of Cancer and Its Scientific Basis. Beard injected pancreatic juice (freshly extracted from young animals) into cancer patients and, when possible, directly into their tumors. He found that the pancreatic juice could inhibit the growth of cancer cells.
In 1934, Dr. Ernst Freund, a Viennese physician, studied the blood of people who were free from cancer and discovered a substance that had the ability to dissolve cancer cells. Patients with cancer did not have this material, which Freund called “normal substance.” In the early 1930s, Professor Doctor Max Wolf worked with Freund in Vienna and successfully identified “normal substance” as an enzyme that decomposes fatty materials and proteins. For his work in the field of enzymes, Wolf is generally considered the father of modern enzyme therapy. The work of Freund and John Beard sparked Wolf’s interest in the possibilities of treating malignant diseases with enzymes. He subsequently founded the Biological Institute of New York City and, after studying various enzymes and enzyme combinations, developed what he regarded as an optimal preparation for the treatment of various acute and chronic conditions. His preparation was a combination of a fractionated hydrolysate of beef pancreas, calf thymus, Pisum sativum (common pea), Lens esculenta (edible lentil), mannitol, and Carica papaya (papaya, a source of the enzyme, papain).
In the 1960s, Irving Innerfield conducted landmark research in the area of pancreatic enzymes, primarily relating to the clinical use of trypsin, chymotrypsin, and pancreatin as well as streptokinase (a microbial proteolytic enzyme). Professor Heinrich Wrba, who for many years was head of the Austrian Cancer Research Institute at the University of Vienna, believed that enzyme therapy should be considered a highly-effective causal anti-cancer compound. Dr. Wrba’s interest in cancer was piqued when he lost a daughter to leukemia. He devoted his life to educating oncologists in Germany and around the world about enzyme therapy.
It was the late Karl Ransberger, however, who continued and refined Wolf’s research, bringing it to doctors, hospitals, and patients throughout the world. Ransberger encouraged and funded research projects in numerous hospitals and universities in Europe, the Americas, and elsewhere. His research, and that of others, validated enzyme therapy’s effectiveness in treating numerous conditions, including arthritis, cancer, multiple sclerosis, cardiovascular disease, human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS).
 Pancreatic Enzyme Supplements
Pancreatic Enzyme Supplements
The pancreatic enzyme supplements most commonly used in health care are chymotrypsin, trypsin, pancrelipase, and pancreatin. Chymotrypsin and trypsin are proteolytic enzymes that break proteins into peptides. Chymotrypsin liberates the amino acids, L-tyrosine, L-tryptophan, and L-phenylalanine, and other molecules, including several synthetic esters and amides.4 Trypsin hydrolyzes primarily lysyl and arginyl residues. Pancreatin contains amylase (which breaks down starch), lipase (which breaks down fats), and protease (which breaks down proteins). Pancrelipase is similar to pancreatin, but with a higher concentration of lipase.
These enzymes are primarily obtained from hog or ox pancreas, but some (such as lipase) can also be obtained from microbial sources (e.g., Aspergillus niger and Aspergillus oryzae). Nevertheless, only enzymes isolated from animal pancreatic glands can be considered pancreatic enzymes.
According to the U.S. Pharmacopeia (USP), chymotrypsin and trypsin are routinely crystallized from ox pancreas gland extract, and pancreatin from both hog and ox sources, whereas pancrelipase is derived from hog pancreas.5 Porcine pancreas is especially rich in amylase and lipase, and is similar to the human pancreas.4 Bovine pancreas contains considerable amounts of proteolytic enzymes, but substantially lower amounts of lipase and amylase.4 Germany, Japan, England, India, and other countries utilize their own pharmacopeia, and foreign companies may use other sources to formulate their enzyme products.
Enzyme concentration and activity levels can vary depending on the age, sex, and species of pork or ox used to produce the supplement. For example, sow glands (from pork) are high in lipase, whereas butcher hogs (young male hogs, up to 90 kg in weight and 6 months of age) are high in protease. Beef cows and bulls have different enzyme levels from those in steers or heifers. Beef, although it provides all three basic enzyme types, does not exhibit the activity levels of pork (which has an activity level one third to one half higher). Furthermore, the physiology of hogs is more similar to that of humans than to that of any other animal.
Enzymes extracted from animal sources are sensitive to environmental changes, so manufacturers take particular care during extraction to control pH (usually with buffers), temperature (using precooled solutions and apparatus), substrate, and proteolysis (controlled through the use of inhibitors) to render a product that is enzymatically active.6
Enzyme Standardization
In 2010, the Food and Drug Administration (FDA) began requiring manufacturers of pancreatic enzyme products to test them in clinical trials if those products were prescribed to treat individuals with pancreatic diseases. Until that time, there were frequent inconsistencies in enzyme formulation. For example, a study of six different enzyme preparations found that, in some cases, the actual lipase activity in a product was more than twice that listed on the label.7 Another study on nine pancreatic enzyme products found that, although within USP limits, the percentage of lipase activity after dissolution was not always equal.8 These inconsistencies in content and activity level could unexpectedly alter patient enzyme requirements.
The FDA’s ruling ensures that pancreatic enzymes are standardized with consistent activity level. Pancreatic enzymes marketed as dietary supplements, however, do not require testing or approval by the FDA. The requirements regarding activity level apply only to those pancreatic enzyme products prescribed for exocrine pancreatic insufficiency due to cystic fibrosis (CF), chronic pancreatitis, and other conditions.
 Absorption of Proteins
Absorption of Proteins
In the past, it was believed that the intestinal epithelial mucosa was impermeable to large protein molecules.9 However, research over the past several decades has shown that the intestinal epithelium can be crossed by macromolecules, including intact proteins such as proteolytic enzymes.10
These macromolecules normally penetrate the mucosal surface via the transcellular route as, in healthy mucosa, the tight junctions (zonula occludens) between the enterocytes prohibit paracellular passage.11 Binding to the luminal membrane of the enterocyte is followed by phagocytosis.12 Some of the vacuole membrane vesicles formed fuse with lysosomes, and within the resulting phagolysome, the peptides and proteins may be hydrolyzed by lysosomal enzymes.13 Other macromolecules avoid intracellular digestion and are passed from the enterocytes through the basolateral membrane into the interstitial space.14 In the interstitial space, the macromolecules become available to macrophages and lymphoid cells.15 Those molecules not taken up by macrophages or lymphatic cells eventually pass from the interstitial space into the blood or lymph.16
The transport of macromolecular material from the lumen to the interstitium has been extensively studied in the epithelium covering the lymphatic structures, such as Peyer’s patches or isolated follicles.12 In these regions, specialized enterocytes, the follicle-associated epithelium cells17 or M-cells18 (so called because of their occurrence in the microfolds of the luminal surface), transport macromolecular material in both directions.17 The gut-associated immune system is thus supplied with antigenic macromolecules from the intestinal lumen.19 The immunoglobulins produced by the plasma cells in the lamina propria (mainly immunoglobulin-A) are transported transcellularly to the luminal surface.
The exact level of the intestinal absorption of intact molecules or large breakdown products of dietary proteins is not yet totally clear and can vary by individual.20 Although it is generally assumed that, apart from a very small proportion, all protein is hydrolyzed into amino acids or low-molecular-weight peptides before absorption by the mucosa, some research supports the hypothesis that a considerable proportion of dietary protein is taken up in the form of macromolecules and is only then hydrolyzed intercellularly in the peripheral tissue into amino acids (a process called “distributed digestion”).21
Understanding the Absorption of Enzymes
Understanding the intestinal transport of macromolecules is especially important for understanding the functions and absorption of enzymes specifically. Hydrolases such as trypsin or elastase can be transported functionally intact into the bloodstream from the lumen of the gut. These circulating proteinases are bound to antiproteinases, such as alpha2 macroglobulin or alpha1 antiproteinase,22 and can be resorbed from the main bloodstream by pancreatic cells (enteropancreatic circulation as an enzyme conservation process).23 Thus, the intestinal absorption of intact enzymes appears to be important for the balance between hydrolases and antiproteinases in the intracellular space,24 and is an important factor for the establishment and maintenance of the internal stability in the body.
Although there are a number of absorption mechanisms, the primary mechanism for the enteral absorption of enzymes and other macromolecules is pinocytotic transfer by the M-cells of the small intestinal epithelium. The enzymes connect to a receptor in the intestinal wall mucosa and are then absorbed into the wall by pinocytosis, guided through the intestinal cells in vesicles, and finally released into the blood by exocytosis.25
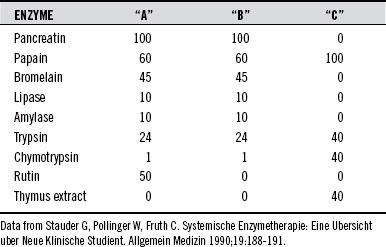
To clarify rate of absorption, Steffen et al26 investigated the absorption of an enzyme mixture “A” (EMA), which contained 100 mg of pancreatin, 60 mg of papain, 10 mg of lipase, 10 mg of amylase, 24 mg of trypsin, 1 mg of chymotrypsin, 45 mg of bromelain, and 50 mg of the bioflavonoid rutin in rabbits. Using electrophoresis, these researchers found that entire enzyme molecules were absorbed. Although enzyme particles were also present, the ratio to the entire amount administered was not measured. EMA was found in both lungs and liver after 1 to 2 hours. After 1 to 4 hours, approximately twice as much EMA was found in the liver as in the lungs. The absorption maximum in all animals occurred approximately 1 hour after administration. After 24 hours, EMA was no longer found in either the lungs or liver.
The absorption rate of individual and combined enzymes can be found in Table 111-1.27,28 The absorption rate of orally-ingested EMA is about 20% within 6 hours.19
TABLE 111-1 Absorption Rate of Individual and Combined Enzymes (within 6 Hours)27,28
| ENZYME | ABSORPTION RATE (%) |
|---|---|
| Amylase | 45 |
| Chymotrypsin | 14-16 |
| Pancreatin | 18-19 |
| Papain | 6 |
| Trypsin | 26-28 |
| Enzyme combination (bromelain, chymotrypsin, pancreatin, papain, and trypsin, with the bioflavonoid rutin) | 22 |
 Factors Affecting Enzyme Activity
Factors Affecting Enzyme Activity
Numerous factors including pH, temperature, substrate (and substrate concentration), cofactors, metal ions, inhibitors, and coating can affect the activity of supplemental enzymes.
Optimal pH Range
Each enzyme has an optimal pH range, depending on such variables as temperature and substrate concentration at which the enzymatic catalytic reaction occurs most rapidly. Chymotrypsin has an optimum pH of 8.0, has reversible denaturation at a pH below 3.0, and becomes inactive at a pH above 9.0.4 Trypsin has an optimum pH between 7.0 and 9.0, is stable at a pH of 3.0 (and at low temperature), and is irreversibly denatured at a pH of 9.0 or higher.4 The pH of the normal human stomach is 1.5 to 3.0,29 low enough to denature or inactivate some or all of a pancreatic enzyme supplement if it is not enterically coated or otherwise treated to protect it from a low-pH environment.
The Effects of Temperature
In general, an increase of 50° F (10° C) in the enzymatic environment approximately doubles the rate of the chemical reaction.30 However, because enzymes are proteins, excessively high temperatures can denature them, thus destroying their activity. Optimum temperature for an enzyme is the temperature at which the catalyzed enzymatic reaction progresses most rapidly without damage to the enzyme. This temperature can vary by enzyme. This is a good rationale for avoiding hot beverages when taking enzyme supplements.
The enzymes in the human body develop high levels of activity at about body temperature, increasing to maximum at about the temperature of a severe fever, that is, 104° F (40° C).
Substrate Concentration
The rate of any reaction is accelerated by raising the substrate concentration until the enzyme is saturated by substrate. At this level, the rate of reaction becomes independent of substrate concentration and is no longer accelerated by the addition of more substrate. This is why it is particularly important to ingest sufficient quantities of supplemental enzymes (which vary according to the condition being treated).
Cofactors
Although all enzymes consist of protein, some are complex proteins, that is, they have a protein component and a “cofactor.” If the cofactor is removed, the protein (no longer active enzymatically) is called the apoenzyme. A cofactor might be a metal (e.g., iron, magnesium, copper, or zinc), a prosthetic group (a moderately-sized organic molecule), or a coenzyme (small organic compound). Prosthetic groups and metals can aid in the catalytic function of the enzyme, whereas coenzymes take part in the enzymatic reaction. Many vitamins, trace elements, and minerals essential to human bodily function are part of enzymatic cofactors. So the physician must ensure that his or her patients take multivitamins and multiminerals to “feed” their enzymes.
Coenzymes are essential for the activity of many enzymes and serve as a type of substrate in certain reactions. In these reactions, the coenzyme is converted to a form no longer active in catalyzing the reaction. The reaction requires a mix that contains one molecule of cofactor for every molecule of substrate to be converted.
Metal Ions
Specific metal ions are required for the activity of many enzymes. Some metal ions increase enzyme activity and others decrease or inhibit it. Calcium, cobalt, copper, iron, magnesium, manganese, molybdenum, potassium, and zinc are the most common enzyme activators in humans. Certain heavy metal ions inhibit enzyme reactions; they are barium, lead, and mercury, and they combine with the sulfhydryl reactive group (−SH) that is part of the active site of many enzymes.
Inhibitors
Ions, atoms, or molecules that terminate or retard enzyme activity are called inhibitors. They are classified as either noncompetitive or competitive. A noncompetitive inhibitor combines with the enzyme at a location other than the active site. The noncompetitive inhibitor retards the conversion of the substrate by the enzyme, although it does not affect the bonding of the substrate of the enzyme. An inhibitor is classified as competitive if it combines with the active site of the enzyme, preventing the substrate from having access to the active site.
Supplement Coating
The pH of the stomach’s hydrochloric acid secretions is about 0.8.31 This low pH inhibits bacterial growth and activates certain enzymes. This acidic nature, however, can destroy pH-sensitive supplemental enzymes. For this reason, many enzyme products are enterically coated. This coating allows the product to reach the small intestine before disintegrating. Other products are encapsulated in “microspheres,” delaying disintegration. For example, pancreatic protease encapsulated with a mixture of cellulose acetate phthalate and maize starch can remain stable in simulated gastric conditions (pH of 3.97) for at least 3 hours.32 This would theoretically provide enough time for the capsule to pass through each part of the gastrointestinal tract. The capsule then disintegrates rapidly under pH 6.82 and temperature of 39.5° C (as would occur in the small intestine).32
Nanotechnology is opening a new field for the delivery of enzymes and other small proteins. Nanotechnology is the study of matter as small as one billionth of a meter. According to the National Nanotechnology Initiative, nanoparticles are being used in timed-release drug delivery.33 Enzymes can be attached to nanoparticles and actually maneuvered to destroy diseased cells. Research on nondegradable nanocapsules showed that proteins can be efficiently transported to individual cells, surviving different pH levels.34 So, it is no wonder that enzymes (which are proteins) can also be attached to nanoparticles and used to treat disease at the cellular level.
 Measuring Enzyme Activity
Measuring Enzyme Activity
When considering enzymes and enzyme applications, the physician must understand the variables affecting their performance. Selection of an enzyme for therapeutic purposes requires more than knowing whether a given product contains amylase, protease, lipase, or other enzymes. The activity levels of the enzymes are critical.
As mentioned previously, the manufacturers of pancreatic enzymes prescribed to treat specific conditions must clearly disclose content and enzyme activity levels. Unfortunately, the same is not true of enzyme products sold as dietary supplements, whose labels may not indicate enzyme activity levels. In addition, even when the activity is stated, the consumer has no way of knowing which enzyme assay the manufacturer used unless the label also indicates that the product conforms to the guidelines of the USP. This is particularly confusing because activity levels are greatly affected by the conditions under which the assay was performed (including temperature, pH, and substrate).
Adding to the confusion, enzyme manufacturers utilize diverse assay methodologies, making direct comparison of competing products difficult, if not impossible. Utilizing a single assay system (such as detailed in the USP) is necessary to directly compare competitive products. Several standardized assay systems are available for enzyme suppliers and are found in the USP (for a definitive assay), the NFIA Laboratory Methods Compendium, and the Food Chemical Codex.
Incomplete labeling and the inconsistent use of standardized assay methodologies make evaluating competitive products extremely challenging. Price could be the first indication of inequities in assay procedures. For example, if company A is selling a product at 1000 U/g for $30 a bottle, and company B is selling a product at 5000 U/g for $10 a bottle, the units are most likely not the same.
For clinical reliability, one must use only appropriately labeled products or obtain the assay procedures from each of the manufacturers. If possible, competitor products should be compared by means of assays performed in an independent laboratory.
 Clinical Applications
Clinical Applications
Historically, enzyme therapy has been used in a wide variety of applications, ranging from oral supplementation to treat pancreatic insufficiency, to the centuries-old external application of enzymes to treat leg ulcers, topical wounds, wrinkles, blemishes, episiotomies, scars, and so on. Usually administered in capsules or tablets, enzymes are also available as lozenges (dissolved in the mouth) or in powder. Topical enzyme ointment is currently used to debride necrotic tissue and other wound debris. Enzymes can also be administered by injection (normally in a hospital setting because of the risk of anaphylactic reaction) or rectally, by retention implant.
Enzymes can be used individually, but are typically more efficacious when used in enzyme mixtures. Enzyme combinations are not simply intensified forms of pancreatin. An enzyme combination has a number of therapeutic advantages over a preparation with only one or two components. Combining enzymes from different sources–animal, plant, and fungi–results in a wider range of optimal pH, synergism of the combined enzymes, greater absorption, higher level of effectiveness, and broader range of application. For example, one German product contains an enzyme extract consisting of proteinases, triacylglycerol lipase, and alpha-glycosidase (amylase); minor amounts of elastase, nuclease, and carboxypeptidase; and calcium ions to boost activity.
Dr. Peter Streichhan, a well-known enzyme researcher, stated that certain enzymatic mixtures have a broader range of action than pancreatin, bromelain, or any other standardized monohydrolytic preparation—this is because certain enzyme mixtures characteristically possess differences in optimal pH and also differences in reactive properties of the proteolytic, lipolytic, and/or amylolytic-acting hydrolases.35
It should be remembered that, at the beginning of therapy, an individual’s symptoms may occasionally become more severe. This is a sign that a therapeutic reaction is occurring and should be evaluated positively. The medication need not be discontinued, although a temporary reduction in dose might be advisable.
Clinical uses of individual enzymes can be found in Boxes 111-1 through 111-5, whereas clinical uses of combinations can be found in Box 111-6. For more information on how enzymes can treat more than 150 conditions, please see the author’s book, The Complete Book of Enzyme Therapy.
BOX 111-1 Clinical Applications of Chymotrypsin
• In debridement, treatment of abscesses and ulcerations, liquefaction of mucous secretions36
• In ophthalmic cataract surgeries and therapy of eyeball hematomas and ophthalmorrhagias36,37
• Before and after tooth extractions, as well as in operative dentistry38,39
• After episiotomy procedures40
• As an anthelmintic against enterozoic worms41
• In early recognition of tumor cells42
• In histologic gastroenterologic diagnostics43
• In inflammatory conditions (local and systemic) to promote the dispersion of blood extravasates and effusions from fractures37,44-51
• Accidental soft tissue trauma44-4851
• Intervertebral disc lesions52
• In uveitis vitreous hemorrhage, diabetic retinopathy, and asthmatic symptoms53
BOX 111-2 Clinical Applications of Trypsin
• In debridement of necrotizing wounds, ulcerations, abscesses, empyemas, hematomas, fistulas, and decubitus54-57
• To accelerate healing in injuries, inflammations, phlogistic edemas, and traumatic changes44-51
• As an auxiliary agent in meningitis therapy58
• As an ointment or dressing (wet or dry)4
• As a liquid or an aerosol to liquefy sputum in bronchial disorders and in the preparation of sputum for cytologic examination4
• As an anti-inflammatory agent; oily suspensions are injected intramuscularly4
• As an aid in the treatment of intraocular hemorrhage, thrombophlebitis, intestinal obstruction (due to cirrhosis or carcinoma)59
BOX 111-4 Clinical Applications of Lipase
• In pancreatin-containing remedies to increase pancreatic/lipolytic activities (replacement therapy)63-66
• When given with pancreatin (in combined preparations), it reduces fat level in stools67-69
• Synergistically intensifies the activity of lipoprotein-lipase in the blood70 and migration of agranulocytes71
• As a digestive aid69
BOX 111-5 Clinical Applications of Pancreatin
• In pancreatic insufficiency, inadequate secretion of exocrine pancreas, disturbed digestion, and after gastrectomy64,72-84
• In chronic pancreatitis85 and after surgery for chronic pancreatitis86
• In ductal obstruction from neoplasm (e.g., of the pancreas or common bile duct)85
• To treat severe cases of steatorrhea (as found in cystic fibrosis)87-92
BOX 111-6 Clinical Applications of Enzyme Combinations
• Tendonitis97
• Reabsorption of hematomas93,98
• Meniscectomy (pre- and postoperative therapy)107,108
• Traumatology109
• Pancreatitis112
• Lower extremity bypass surgery114
• Operative dentistry115
• Proctology116
• Acute and chronic bronchitis121-123
• Cystitis and lower urinary tract infections117,124,125
• Pelvic inflammatory disease127,128
• Post-thrombotic syndrome129-133
• Pathologic venous processes129,134-138
• Occlusive arterial disease139
• Soft tissue rheumatism (nonarticular rheumatoid syndrome)147,148
• Rheumatoid arthritis (chronic polyarthritis)117,122,131,142,149-164
• Ankylosing spondylitis (Bekhterev’s disease)94,153,154,157
• Degenerative rheumatic joint disease102,117
• Monoarticular, activated osteoarthrosis102,123,165
• Multiple sclerosis121,166-172
• Sepsis178
Most conditions treated by enzymes can be assigned to one or more of the following categories:
Digestive Disorders
Supplementing the diet with pancreatic enzymes can improve food digestion and, therefore, nutrient assimilation. This is especially true if the body’s own pancreatic enzyme production and/or supply is deficient, as can occur with age or because of an underlying health condition, such as pancreatic insufficiency, chronic pancreatitis, steatorrhea, biliary tract disease, celiac disease, or cystic fibrosis (CF). Pancreatic enzymes have a long history of use in treating those conditions, as well as indigestion, heartburn, gas, diarrhea, constipation—the list seems endless.
Patients with CF are routinely prescribed pancreatin or pancrelipase capsules containing enterically-coated beads. A hereditary disease, CF involves a disorder of the exocrine glands and causes the production of thick mucus, which leads to obstructions in various glands and ducts in the body. When the mucus affects the lungs, it leads to severe coughing and repeated lung infections, including bronchitis and pneumonia. When the mucus obstructs the pancreatic duct, it prevents the enzyme-rich pancreatic juice from reaching the intestine, negatively impacting food digestion and nutrient absorption. This can result in malnutrition, weight loss, steatorrhea (fatty stools), or intestinal obstruction. Poor digestion affects approximately 90% of all CF patients.180
Enzyme supplementation can improve the digestion of fats, proteins, and carbohydrates, thus improving nutrient absorption and, therefore, increasing weight gain,181 controlling steatorrhea,182 and improving serum albumin levels (low levels reflect protein calorie malnutrition).181
Inflammatory Diseases and Conditions
The inflammatory process is involved in numerous diseases and conditions, from sports injuries to arthritis to sinusitis to fibrositis. One of the basic concepts in systemic enzyme therapy (that is, using enzymes to treat disorders within the body) is that all kinds of inflammatory processes respond to enzymes. The following steps describe the postulated mechanism of action:
1. Various repair mechanisms are activated in the damaged area, resulting in fibrin surrounding the traumatized region (among other effects).
2. The injured area (after having been partly or completely sealed off by microthrombi and/or a fibrin web) is cut off from the normal circulatory system. This results in stasis, inhibiting the repair process (nutrients cannot get into the damaged area and waste products cannot be removed). Pressure rises in the damaged capillaries, the blood vessels become highly permeable, and fluid is forced out into the surrounding tissue. This causes increased pressure, swelling, and pain.
3. Supplemental enzymes, after absorption, are bound in complexes (e.g., α2-macroglobulin hydrolase) and circulate via the bloodstream to the injured area. Enzymes have been shown to reduce interleukin-2 (IL-2), Il-6, tumor necrosis factor-α (TNF-α), and other proinflammatory cytokines, while increasing anti-inflammatory cytokines such as IL-4, thereby normalizing cytokine homeostasis.183
4. Proteolytic enzymes directly attack the microclots and fibrin formation, lysing the fibrin and breaking open the clogged vessels, thus reestablishing circulation.
The restoration of normal blood flow leads to a faster resolution of postinflammatory pain and edema. Equally important, the essential physiologic inflammatory repair process is not blocked or diminished (as with anti-inflammatory agents), but rather is accelerated and reinforced.
In a Canadian study, postal workers with rotator cuff tendonitis were treated with either naturopathic care (NC), which included dietary counseling, acupuncture, and an enzyme supplement (containing bromelain, trypsin, and rutin), or with physical exercise (PE), which included exercise and placebo.97 Those participants on the NC therapy saw a decrease in their shoulder pain and disability of over 54% (as measured by Shoulder Pain and Disability Index) compared with 18% in the PE group. The NC group also had significant improvement in range of motion compared with the PE group.97
Fractures often require surgery to repair the broken bone. Czech researchers followed the progress of 60 patients after surgery for repair of long bone fractures. Thirty patients received an enzyme mixture (containing trypsin, bromelain, and rutin), whereas another 30 patients were treated by standard antiedema drugs. Both groups received the same pain medicine. The group who received the enzyme mixture had a significantly faster reduction in posttraumatic and postoperative swelling. In addition, those taking enzymes consumed significantly fewer pain killers.184
A similar study in Russia using an enzyme mixture on children with fractured long bones found that swelling and pain subsided twice as fast in the enzyme group as in the group receiving traditional therapy.111
Intense exercise can lead to soft tissue injury and inflammation. Researchers found that supplementing with protease mixtures could help ease muscle soreness and assist muscle healing after exercise.106
Even inflammatory diseases, such as arthritis149 and herpes zoster185 were shown to respond to the systemic application of enzymes. A double-blind, randomized study compared an oral enzyme mixture (containing trypsin, bromelain, and the bioflavonoid, rutin) with diclofenac (a nonsteroidal anti-inflammatory drug frequently prescribed for osteoarthritis and rheumatoid arthritis). Ninety patients received either the enzyme mixture or the diclofenac for a 6-week period. Results indicated that the enzyme mixture was just as effective as diclofenac.186
Individual enzymes and enzyme combinations (particularly those including trypsin, chymotrypsin, pancreatin, amylase, lipase, papain, and bromelain, with rutin) are effective in treating inflammation because they help limit the injury, aid its rectification, and promote new, healthy tissue formation. Enzymes effectively accelerate the inflammatory process (a necessary component of wound healing). This acceleration means, on the one hand, that the work of damage control, damage repair, and new tissue construction is carried out more actively, and thus completed more swiftly. On the other hand, it means that there can be a temporary increase in the visual and sensory effects produced by the inflammation (i.e., more redness, swelling, heat, and pain).
Despite the supportive research, the administration of enzymes in traumatology and injuries is not widely used in the United States, which is in contrast to Europe, where (because the inflammatory reaction is so universal) all types of injuries—sprains, strains, hematomas, dislocations, and even postoperative conditions—are effectively treated with enzymes. Although the inflammatory process is involved in viral and bacterial infections, those disorders are covered in a “Viral and Bacterial Disorders”, separate section within this chapter.
Cancer
Just as Beard researched the effects of enzymes on cancer, so have modern researchers. Dr. William Kelley devised an anticancer program that employed detoxification, pancreatic enzymes (and other nutritional supplements), improved enzyme-rich nutrition, and coffee enemas.187 He initiated his treatment program in response to his own bout with pancreatic cancer.
Standard cancer therapy can often result in serious side effects. For instance, radiation therapy of the head and neck is often accompanied by acute side effects, including difficulty swallowing, mucous membrane inflammation, and skin redness or soreness. Oral enzyme preparations seem to protect against these acute side effects, reducing their severity and duration.188
A study on patients with laryngeal cancer receiving radiation treatment found that those patients receiving an enzyme mixture (containing trypsin, bromelain, and rutin), had no cases of radiomucositis of the second degree and were able to continue on the radiation therapy.189 Researchers also noted that those on enzyme therapy had a mean weight loss of only 5% (compared with 10% in the control group).189
In another study, researchers tested an enzyme combination in patients who underwent radiation therapy for cervical cancer, a therapy that can cause numerous side effects, including bladder irritation, loose bowels, nausea, vomiting, and vaginal dryness. Results indicated that patients who received the enzyme mixture (which contained trypsin, chymotrypsin, and papain) had a significant reduction in vaginal mucosa reactions, genitourinary symptoms, and subcutaneous changes.190
Wobe Mugos E (which contains papain, trypsin, chymotrypsin, and thymic peptides) was found to reduce the incidence of leukopenia, nausea, vomiting, sensory neuropathy, and treatment-related depression in lung cancer patients who underwent chemotherapy.191
Breast Cancer
It is estimated that one of every eight women will be diagnosed with breast cancer sometime during her life.192 Unfortunately, standard treatment, including radiation, chemotherapy, and surgery, often causes serious side effects, including gastrointestinal symptoms, headache, pain, skin disorders, and infections. Researchers in Germany evaluated whether treatment with oral enzymes could reduce some of the typical side effects of breast cancer therapy.193 They found that 74% of the enzyme group experienced a clear reduction in chemotherapy and radiotherapy side effects, and that oral enzyme therapy could improve disease signs and symptoms, reduce side effects, and improve quality of life.193
Lymphedema
Treatment for breast cancer can sometimes lead to lymphedema (swelling caused by a buildup of lymph). This typically occurs after a lymph node is removed or after radiation treatment, and can develop slowly. Symptoms include swelling in the arm, hand, shoulder, chest, or breast, and may be accompanied by changes in skin texture, aching, and restricted mobility.
Typical medical therapy for lymphedema includes massage, exercising, special bandaging, manual lymphatic drainage, and the use of diuretic therapy. One clinical trial compared therapy with enzymes (the product contained pancreatin, papain, bromelain, trypsin, chymotrypsin, and rutosid) with a diuretic.146 After 7 weeks, significantly more of the patients receiving enzymes were pain free compared with the patients receiving diuretics.
Pancreatic Cancer
Pancreatic cancer kills over 30,000 people every year.194 In the past, survival rates for this disease were extremely low because treatment options were limited, and diagnosis usually did not occur until the disease was quite advanced. However, treatment improvements and earlier diagnosis have dramatically improved survival rates.195 Standard medical treatment for pancreatic cancer involves surgery, radiation therapy, chemotherapy, and immunotherapy (also called biological therapy).
Pancreatic cancer can block the pancreatic duct and prevent enzymes from reaching the small intestine, resulting in malnutrition and weight loss. It is estimated that 80% to 90% of all pancreatic cancer patients have malabsorption and weight loss.196 Supplementing with pancreatic enzymes is an important supportive step in treating pancreatic cancer because it can improve digestion and, therefore, nutrient absorption.79,182,197
Autoimmune and Immune Complex-Mediated Diseases
In autoimmune disease, tissue-bound immune complexes activate the complement system. Activation of the enzyme cascade results in an intense protein-destroying inflammatory response, leading to significant local tissue destruction. For instance, when immune complexes collect in the kidneys, complement activation causes inflammation, resulting in glomerulonephritis.
Research showed that some enzymes can inhibit immune complex-mediated diseases, such as glomerulonephritis and rheumatoid arthritis, by interrupting the complement cascade. Other disorders with similar mechanisms also respond to supplemental enzymes. Some are conditions such as Crohn’s disease, pulmonary fibrosis, chronic rheumatism, and ankylosing spondylitis, which do not respond well to conventional medical treatments.
Multiple Sclerosis
The National Multiple Sclerosis Society estimates that there are approximately 400,000 people with multiple sclerosis (MS) in the United States alone.198 Although its cause is unknown, it is generally believed to be an autoimmune disease. In MS, antibodies attack the myelin sheath that protects and covers nerve fibers. This leads to inflammation and myelin sheath damage, which in turn interferes with nerve communications.
MS patients typically have higher concentrations of circulating immune complexes (CICs) than healthy individuals. Research showed that enzyme therapy can help reduce those CICs, and thereby reduce inflammation.199
A Ukrainian study on an enzyme combination containing trypsin, bromelain, and rutin (a flavonoid) found that MS patients taking the combination for 1 to 3 years had fewer and less severe complications and longer remissions, and also experienced a slowing of their disease progression.172
Cardiovascular Disorders
Cardiovascular disorders include diseases of the heart and blood vessels (including strokes).
In the United States, heart disease and strokes kill nearly three quarters of a million people every year and account for nearly one third of all deaths.194 However, these conditions are not limited to the elderly, because over 140,000 Americans younger than 65 years old die from these diseases every year.194
In normal circulation, there is a constant dynamic balance between blood clotting and fibrinolysis.200 If fibrinolysis is impaired, clot formation is abnormal. If fibrinolysis increases, a tendency toward excessive bleeding results. Therefore, maintenance of proper equilibrium is extremely important for the circulatory system.
One study examined the effects of various enzyme combinations on fibrinolysis and fibrin formation.121 The researchers induced fibrin deposition with calcium ions or staphylocoagulase in the plasma of centrifuged, acellular citrated blood from humans or rabbits and treated the clot with various concentrations of enzyme suspensions. They found that the higher the enzyme content of the solution, the more rapidly clots were degraded. Inflammation is considered to play a central role in all atherosclerotic processes. Therefore, the use of enzymes is indicated because of their anti-inflammatory effect.
A German study measured the effects of a proteolytic enzyme combination (containing trypsin, bromelain, and rutin) on blood fluidity. Researchers found that even a minimum enzyme concentration resulted in a significant reduction in plasma viscosity and erythrocyte aggregation.201
Systemic enzyme therapy appears to be particularly effective in the treatment of phlebitis and thrombophlebitis,202 as well as the chronic venous insufficiency (also called postphlebitic syndrome) that can occur because of phlebitis. In one study, treatment with systemic enzyme therapy led to a decrease in several symptoms, including pain, edema, and trophic ulcers.203
Viral and Bacterial Disorders
As more and more bacteria become resistant to antibiotics, researchers and physicians are investigating supportive treatments, including systemic enzyme therapy. Enzymes reduce inflammation, improve circulation, stimulate and balance immune system function, and enhance the antibiotic’s ability to penetrate target tissues. Enzymes fortify the action of antibiotics, leading to complete healing and preventing the progression of an acute infection into its chronic form. A good example is urogenital chlamydiosis, which is a sexually transmitted disease and a major cause of infertility. According to the Centers for Disease Control, there were more than one million cases reported in the United States in 2008.204 Standard treatment is with antibiotics, including azithromycin or doxycycline. Although antibiotic treatment appears to be highly efficacious, in some individuals the infection becomes persistent and chronic, leading to long-term disorders in females, including pelvic inflammatory disease, ectopic pregnancy, and sterility.205 A Russian study of 227 patients with urogenital chlamydiosis found that treatment with antibiotics led to complete recovery in only 61.4% of patients, whereas enzyme therapy in conjunction with antibiotics resulted in over 90% treatment success.206
Many viral and bacterial conditions, including abscesses, acne, adenoiditis, adnexitis, bladder infection, boils, conjunctivitis, diarrhea, ear infections, empyemas, epididymitis, gingivitis, kidney disorders, laryngitis, pneumonia, rheumatic fever, sinusitis, staphylococcal infection, tonsillitis, and sepsis can be helped with enzymes.
Sepsis is a particularly dangerous systemic response to infection. Researchers in India found that proteolytic enzyme therapy improved recovery time in children with sepsis.178 Thirty young boys (ages 1 month to 12 years) with sepsis were given proteolytic enzymes. Another group of 30 boys received placebo tablets. Both groups received supportive treatment and the appropriate antibiotics. Fever subsided faster in the enzyme group (3 days) than in the placebo group (4 days), and those in the enzyme group were able to begin oral feeding in 4 days, as opposed to 5 days in the placebo group. In addition, hemodynamic support was only needed for 2 days in the enzyme group (as opposed to 3 days in the placebo group), and the modified Glasgow coma scale normalized for enzyme participants in 3 days (as opposed to 5.5 days in the placebo group).178
Enzyme therapy can also aid in the treatment of viruses. The human body defends against viruses with the help of macrophages or natural killer (NK) cells. Oral enzyme combinations (such as papain, trypsin, and chymotrypsin) synergistically increase the antiviral effects of TNF, macrophages, and NK cells, and the breakdown of CICs. Enzymes can aid in the treatment of numerous viral conditions, including AIDS, chancre sores, chickenpox, colds and coughs, hepatitis, herpes simplex, herpes zoster, influenza, measles, pneumonia (viral), and warts.
Other Conditions
Enzyme therapy may be of value in conditions that do not easily fit into one of the preceding categories. Autism is just one example. No causative agent has been found for this developmental disease, although many theories abound, including vaccinations, a genetic link, nutritional deficiencies, and reactions to chemicals or other environmental factors. Some research indicates that enzyme therapy to improve protein digestion may be of particular benefit in autism as well as many other conditions.207
 Dosage
Dosage
Regardless of the condition being treated, a wide range in daily dosage has historically been used for the following reasons:
• There are individual differences in patient health.
• The level of effective absorption in tablet strength can vary.
For example, pancreatin dosage depends on the condition being treated, as well as on the patient’s diet and digestive requirements. Dosage can vary because of pancreatin’s susceptibility to inactivation in the stomach and duodenum.192
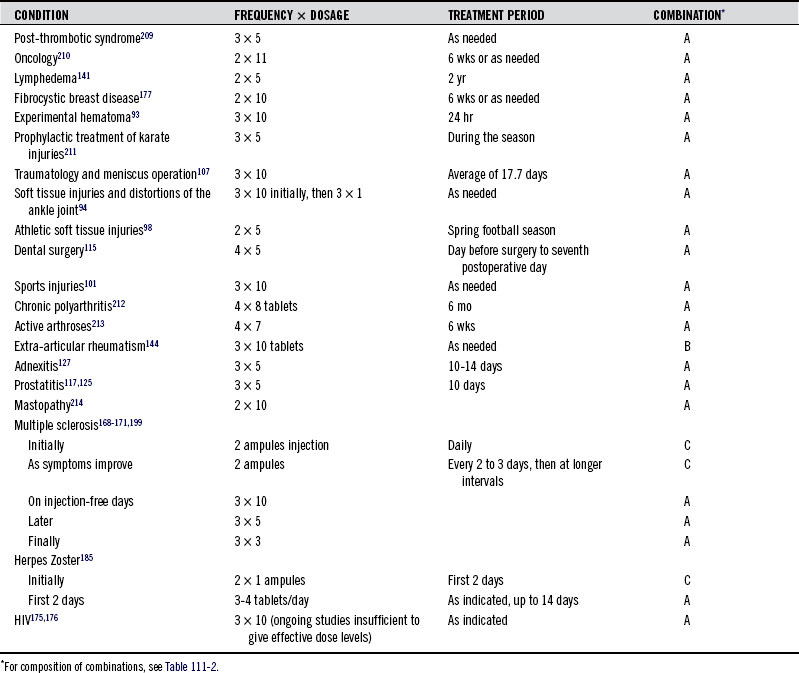
Table 111-2 indicates the composition of three enzyme combinations (A, B, and C),208 whereas Table 111-3 lists some of the conditions effectively treated with these products, the dose required, and the treatment period.
 Toxicology
Toxicology
Oral Enzymes
In general, side effects of orally administered enzymes are few, short-termed, and due primarily to higher dosages or hypersensitivity reactions. According to Wolf and Ransberger, high dosages (70 tablets of 17.5 g each) of a proteolytic enzyme mixture have been given without long-term side effects.215 Studies and literature searches commissioned by regulatory authorities such as the U.S. FDA apparently confirmed that enzyme preparations obtained from suitable sources (e.g., nontoxic, nonpathogenic sources) are safe to consume.216
Potential side effects of pancreatic enzyme therapy include diarrhea, intestinal gas, abdominal pain or cramping, and greasy, pale, or pungent stools. Reducing the dose can usually reduce or eliminate these side effects. Hyperuricosuria (excess uric acid in the urine) and hyperuricemia (excess uric acid in the blood) have been associated with extremely high doses. Sneezing, lacrimation, rash, and other hypersensitivity reactions have been reported in sensitive individuals.
Pancreatin preparations held in the mouth or chewed before swallowing can result in stomatitis, or ulcerations and irritation of the mucosa, particularly in infants, in whom pancreatin may also cause perianal soreness.217 However, some of the capsules containing microencapsulated enzyme beads can be opened and dispensed by the spoonful to infants or mixed into foods such as apple sauce or gelatin.
Enzyme enemas can sometimes cause the rectum or anus to itch or burn. However, these are only minimal side effects in relation to the positive benefits obtained.
Prolonged skin contact with proteolytic enzymes (i.e., in ointment form) may cause skin irritation. Care should be exercised when using powdered enzymes or enzyme capsules to avoid inhaling dust particles, because irritation of the mucous membranes of the throat, nose, and eyes may result.216 In addition, inhalation may trigger an asthma attack.218
It is not known whether pancreatin or pancrelipase given to pregnant women can harm the fetus, because animal reproductive studies have not been conducted. Therefore, pancreatin should probably not be used during pregnancy.217 Also, it is not known if pancreatin or pancrelipase is distributed into mother’s milk. Therefore, caution should be exercised with use of this substance in nursing women.217
Enzymes should not be used by hemophiliacs or those on blood thinners (unless under a physician’s care), nor immediately before or after surgical procedures with an increased risk of bleeding. Enzymes should also be avoided by those with pulmonary emphysema or thrombocytopenia. Pancrelipase (and any other enzyme derived from pork sources) is contraindicated in those who are hypersensitive or allergic to pork products.
There is some indication that patients with CF who are undergoing long-term high-dose pancreatic enzyme therapy may demonstrate colonic wall thickening, a condition called fibrosing colonopathy (FCP). It was once believed that the copolymer coating on the tablets might be the culprit,219 and there have been instances of FCP in CF patients not taking any type of enzyme preparation.220 However, it is now generally believed that FCP occurs because of prolonged treatment with very high doses of pancreatic enzymes. Many of the individuals who were first diagnosed with FCP were taking tens of thousands of units in excess of label recommendations.221 To reduce the risk of FCP, it is extremely important that patients closely follow their physicians’ recommendations regarding dosage.
A note about supplement safety: During the last few decades, there has been increased concern regarding mad cow disease (bovine spongiform encephalopathy [BSE]). Called Creutzfeldt-Jakob disease (CJD) in humans, it can be caused by eating beef that is infected with BSE, but can also be transmitted through corneal transplant tissue, from hormone extracts (such as human growth hormone), and possibly from ingesting contaminated glandular products. Unfortunately, it may take as long as 20 years after infection for the characteristic symptoms of CJD to manifest. Since 1995, the FDA has required that dietary supplement manufacturers ensure that any “high risk” animal products used in their supplements do not originate in BSE countries, in countries with inadequate surveillance, or with requirements less strict than those that would be acceptable for importation into the United States. The list is constantly updated; for the most current list of BSE countries, refer to the FDA website: www.FDA.gov. Fortunately, bovine (cow) pancreas (the source of some pancreatic enzymes) is not considered a “high risk,” but rather a category III or low infectivity tissue. In addition, many manufacturers use pork sources for pancreatic enzymes. Unfortunately, there is no test to see whether a supplement is infected with BSE. Contact the supplement manufacturer for concerns about pancreatic supplement safety or to see what measures they employ to ensure BSE-free products.
 Drug Interactions
Drug Interactions
In addition to lipases and amylases, pancreatic enzymes contain proteases, so are subject to some of the same drug interaction warnings as is bromelain (see Chapter 71 on Bromelain). As with bromelain, caution should be exercised when pancreatic enzymes are used with anticoagulants (blood thinners), including Coumadin, Jantoven, Lovenox, and warfarin, as well as over-the-counter nonsteroidal anti-inflammatory drugs, including aspirin and ibuprofen. In addition, certain botanicals, including garlic, gingko biloba, and ginseng, may increase bleeding risk, and so should be monitored when used in conjunction with pancreatic enzymes.
Certain diabetes drugs such as acarbose (brand names Glyset and Precose) function as alpha-glucosidase inhibitors, lowering blood sugar levels by delaying the digestion of dietary carbohydrates. This, in turn, limits the rise of blood sugar levels. However, the amylase in pancreatic enzymes has the potential to reduce the effectiveness of alpha-glucosidase inhibitors, because amylase improves carbohydrate digestion. Therefore, pancreatic enzymes should not be taken concomitantly with acarbose and other alpha-glucosidase inhibitors.
Similarly, drugs that function as lipase inhibitors (such as Orlistat) should be avoided while taking pancreatic enzymes. Orlistat is available in prescription (as Xenical) and nonprescription (as Alli) forms. It functions by inhibiting lipase in both the lumen of the stomach and the small intestine. Although there are no known studies on Orlistat given concomitantly with pancreatic enzymes, it would seem counterproductive to take a drug designed to inhibit lipase activity while also taking a drug containing lipase. In addition, because Orlistat inhibits the activity of lipase, it may interfere with the action of pancreatic enzymes.
Any foods that contain enzyme inhibitors have the potential to decrease the activity of pancreatic enzymes. Heat inactivates enzyme inhibitors (as is the case in potatoes, legumes, and soybeans, which are rarely eaten raw).
 Conclusion
Conclusion
As we can see, the use of pancreatic enzymes has been found to be extremely effective in treating a wide variety of conditions, from digestive diseases to inflammation, cancer, autoimmune diseases, viral and bacterial conditions, and more. It is my firm belief that future research will not only continue to validate, but will also reveal new and exciting applications for systemic enzyme therapy.
This chapter is dedicated to the memory of Dr. Max Wolf, the father of systemic enzyme therapy, as well as Dr. Karl Ransberger, who validated and marketed systemic enzyme therapy, making it a highly accepted worldwide treatment for a wide variety of systemic diseases and injuries.
1. Guyton G.C., Hall J.E. Textbook of Medical Physiology, 11th ed. Philadelphia: WB Saunders, 2006. 799
2. Guyton G.C., Hall J.E. Textbook of Medical Physiology, 11th ed. Philadelphia: WB Saunders, 2006. 810
3. Guyton G.C., Hall J.E. Textbook of Medical Physiology, 11th ed. Philadelphia: WB Saunders, 2006. 809
4. Ruyssen R., Lauwers A. Pharmaceutical enzymes: properties and assay methods. Gent: Story-Scientia. 1978:34–35. 45, 57-58, 70
5. US Pharmacopeia XXII. Rockville, MD: United States Pharmacopeial Convention; 1990.
6. Eisenthal R., Danson M.J. Enzyme assays: a practical approach. Oxford: IRL Press; 1992. 263-264
7. O’Hare M.M., McMaster C., Dodge J.A. Stated versus actual lipase activity in pancreatic enzyme supplements; implications for clinical use. J Pediatr Gastr Nutr. 1995;21:59–63.
8. Case C.L., Henniges F., Barkin J.S. Enzyme content and acid stability of enteric-coated pancreatic enzyme products in vitro. Pancreas. 2005;30:180–183.
9. Gardner M.L. Intestinal assimilation of intact peptides and proteins from the diet—a neglected field? Biol Rev Camb Philos. 1984;59:289–331.
10. Brambell F.W.R. The Transmission of Passive Immunity from Mother to Young, Vol. 18. Amsterdam: North-Holland Publishing. 1970.
11. Bjarnason I., Peters T.J. Helping the mucosa make sense of macromolecules. Gut. 1987;28:1057–1061.
12. Adibi S.A., Mercer D.W. Protein digestion in human intestine as reflected in luminal, mucosal, and plasma amino acid concentrations after meals. J Clin Invest. 1973;52:1586–1594.
13. Georgopoulou U., Dabrowski M.F., Vernier J.M. Absorption of intact proteins by the intestinal epithelium of trout, Salmo gairdneri. Cell Tissue Res. 1988;251:145–152.
14. Borgstrom B., Dahlqvist A., Lundh G., et al. Studies of intestinal digestion and absorption in humans. J Clin Invest. 1957;36:1521–1536.
15. Seifert J., Sellschopp C.H., Sass W. Werden Makromolekule resorbiert und welchen Einfluss hat dabei die Immunitatslage? Z Hautkrankheiten. 1987;62:55–59.
16. Chung Y.C., Kim Y.S., Shadchehr A., et al. Protein digestion and absorption in human small intestine. Gastroenterol. 1979;76:1415–1421.
17. Bockman D.E., Boydston W.R., Beezhold D.H. The role of epithelial cells in gut-associated immune reactivity. Ann N-Y Acad Sci. 1983;409:129–143.
18. Pabst R. Anatomische grundlagen fur verdauung und immunfunktion im gastrointestinaltrakt. Z Hautkrankheiten. 1987;62:39–44.
19. Streichhan P. Resorption enteraler makromolekule. Verdauungs-krankheiten. 1989;7:28–38.
20. Hemmings W.A., Williams E.W. Transport of large breakdown products of dietary protein through the gut wall. Gut. 1978;19:715–723.
21. Hemmings W.A. Distributed digestion. Med Hypotheses. 1980;6:1209–1213.
22. LeFevre M.E., Joel D.D. Intestinal absorption of particulate matter. Life Sci. 1977;21:1403–1408.
23. Lake-Bakaar G., Smith-Laing G., Summerfield J.A. Origin of circulating serum immunoreactive trypsin in man. Dig Dis Sci. 1982;27:143–148.
24. Seifert J., Ganser R., Brendel W. Die resorption eines proteolytischen enzyms pflanzlichen ursprungs aus dem magen-darm-trakt in das blut und die lymphe von erwachsenen ratten. Z Gastroenterol. 1979;17:1–8.
25. Gebert G. Physiologie. Stuttgart: Schattauer; 1987.
26. Steffen C., Menzel J., Smolen H. Untersuchungen uber intestinale resorption mit 3H-markiertem enzymgemisch (Wobenzym). Acta Med Aust. 1979;6:13–18.
27. Seifert J., Siebrecht P., Lange J.P. Quantitative untersuchungen zur resorption von trypsin, chymotrypsin, amylase, papain und pankreatin aus dem magen-darm-trakt nach oraler application. Allgemein Medizin Springer-Verlag. 1990;19:132–137.
28. Papp M., Feher S., Folly G., et al. Absorption of pancreatic lipase from the duodenum into lymphatics. Experienta. 1977;33:1191–1192.
29. Burtis G., Davis J., Martin S. Applied Nutrition and Diet Therapy. Philadelphia: WB Saunders; 1988.
30. Hasselberger F.X. Uses of Enzymes and Immobilized Enzymes. Chicago: Nelson-Hall; 1978.
31. Guyton G.C., Hall J.E. Textbook of Medical Physiology, 11th ed., Philadelphia: WB Saunders; 2006:796.
32. Lin C.W., Lee T.G. A note on the micro-encapsulation of pancreatic protease for protection against gastric digestion. Anim Prod. 1993;56:413–417.
33. Small wonders, endless frontiers: A review of the National Nanotechnology Initiative. National Academy of Science, 36, 2002. http://www.nap.edu/openbook.php?record_id=10395&page=36. Accessed 11/01/2011
34. Yan M., Du J., Gu Z., et al. A novel intracellular protein delivery platform based on single-protein nanocapsules. Nat Nanotechnol. 2010;5:48–53.
35. Wobenzym Streichan P. An orally administered combination preparation consisting of hydrolytic enzymes and rutin acting in circulating body fluids. Inventory text Part A, Preclinical results. Geretsried: Mucos Pharma GmbH & Co; 1993.
36. Reynolds J.E.P., ed. Martindale—the Extra Pharmacopoeia. London: Pharmaceutical Press, 1982.
37 Konotey-Ahulu F.I.D. Enzyme treatment of vitreous haemorrhage. Lancet. 1972;2:714–715.
38. Sowray J.H. An assessment of the value of lyophilised chymotrypsin in the reduction of post-operative swelling following the removal of impacted wisdom teeth. Brit Dent J. 1961;110:30–133.
39. Wrba F., Messer E. Enzyme treatment of traumatic swelling in oral and maxillofacial surgery. Clin Med. 1967;74:29–31.
40. Bumgardner H.D., Zatuchni G.I. Prevention of episiotomy pain with oral chymotrypsin. Am J Obstet Gynecol. 1965;92:514–517.
41. Fiel R.A. Tratamiento experimental de la trichuriasis masiva infantil con quimotripsina. Trop Dis Bull Bd. 1968;65:917.
42. Takahashi M., Hashimoto K., Osada H. Parenteral administration of chymotrypsin for the early detection of cancer cells in sputum. Acta Cytol Bd. 1967;11:61–63.
43. Brandborg L.L., Tankersley C.B., Uyeda F. “Low” versus “high” concentration chymotrypsin in gastric exfoliative cytology. Gastroenterol. 1969;57:500–505.
44. Soule S.D., Wasserman H.C., Burstein R. Oral proteolytic enzyme therapy (Chymoral) in episiotomy patients. Am J Obstet Gynecol. 1966;95:820–833.
45. Boyne P.S., Medhurst H. Oral anti-inflammatory enzyme therapy in injuries in professional footballers. Practitioner. 1967;198:543–546.
46. Blonstein J.L. Oral enzyme tablets in the treatment of boxing injuries. Practitioner. 1967;198:547–548.
47. Buck J.E., Phillips N. Trial of Chymoral in professional footballers. Brit J Clin Pract. 1970;24(9):375–377.
48. Rathgeber W.F. The use of proteolytic enzymes (Chymoral) in sporting injuries. S Afr Med J. 1971;45:181–183.
49. De N’Yeurt A. The use of Chymoral in vasectomy. J R Coll Gen Pract. 1972;22:633–637.
50. Winsor T. Inhibition of the response to thermal injury by oral proteolytic enzymes. J Clin Pharmacol N. 1972;12:325–330.
51. Rathgeber W.F. The use of proteolytic enzymes in tenosynovitis. Clin Med. 1973;80:39–41.
52. Gibson T., Dilke T.F., Grahame R. Chymoral in the treatment of lumbar disc prolapse. Rheumatol Rehabil. 1975;14:186–190.
53. Transcript from the American Academy Opthalmology and Otolaryngology. Chicago:1959:October 11-16.
54. Wrba H. Trypsin und chymotrypsin—klinisches sachverstan-digengutachten zur systemischen wirkung. Inst f angewante u exptl Onkologie d Univ Wien. Working Paper. 1988.
55. Stille G, Tuluwett K. Pharmakologisch-toxikologisches sach-verstandigengutachten zut trypsin/chymotrypsin. Working Paper. 1988.
56. Gordon B. The use of topical proteolytic enzymes in the treatment of post-thrombotic leg ulcers. Brit J Clin Pract. 1975;29:143–146.
57. Sather M.R., Weber C.E., Jr., George J. Pressure sores and the spinal cord injury patient. Drug Intel Clin Phar. 1977;11:154–169.
58. Marquez O.H.D., Segur F.G. The intrathecal use of proteolytic enzymes in tuberculous meningoencephalitis. Preliminary Communication. Abstr Wld Med. 1968;42:800–801.
59. Lichtman A.L. Traumatic injury in athletes. In Rec Med. 1957;170:322–325.
60. Dixon M., Webb E. Enzymes, 3rd ed., New York: Academic Press; 1979:28–29. 40, 860-861
61. Windholz M., ed. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals. Rahway, NJ: Merck, 1983.
62. Auterhoff H., Knage J. Lehrouch der pharmazueutischen chemie. Stuttgart: Verlagsgesellschaft Wissenschafel; 1983.
63. Graham D.Y. Enzyme replacement therapy of exocrine pancreatic insufficiency in man. N Engl J Med. 1977;296:1314–1317.
64. Meyer J.H. The ins and outs of oral pancreatic enzymes. N Engl J Med. 1977;296:1347–1348.
65. Yeh T.L., Rubin M.L. Potency of pancreatic enzyme preparations. N Engl J Med. 1977;297:615–616.
66. Kirshen R. Letter to the editor. N Engl J Med. 1977;297:616.
67. Mackie R.D., Levine A.S., Levitt M.D. Malabsorption of starch in pancreatic insufficiency. Gastroenterol. 1981;80:1220.
68. Lankisch P.G., Creutzfeldt W. Therapy of exocrine and endocrine pancreatic insufficiency. Clin Gastroenterol. 1984;13:985–999.
69. Schneider M.U., Knoll-Ruzicka M.L., Domschke S., et al. Pancreatic enzyme replacement therapy. Comparative effects of conventional and enteric-coated microspheric pancreatin and acid-stable fungal enzyme preparations on steatorrhoea in chronic pancreatitis. Hepato-gastroenterol. 1985;32:97–102.
70. Hall D.A., Zajac A.R., Cox R., et al. The effect of enzyme therapy on plasma lipid levels in the elderly. Artherosclerosis. 1982;43:209–215.
71. Tylewska S., Tyski S., Hrynie-Wicz W. Effect of Staphylococcus aureus lipase on granulocyte chemotaxis. Med Dosw Mikrobiol. 1983;35:171–174. [Polish]
72. LeBauer E., Smith K., Greenberger N.J. Pancreatic influence and vitamin B12 malabsorption. Arch Intern Med. 1968;122:423–425.
73. Kataria M.S., Bhaskarrao D. A clinical double-blind trial with a broad spectrum digestive enzyme product (Combinzym) in geriatric practice. Brit J Clin Pract. 1969;23:15–17.
74. Karani S., Kataria M.S., Barber A.E. A double-blind clinical trial with a digestive enzyme product. Brit J Pract. 1971;25:375–377.
75. Knill-Jones R.P., Pearce H., Batten H., et al. Comparative trial of Nutrizym in chronic pancreatic insufficiency. BMJ. 1970;4:21–24.
76. DiMagno E.P., Go V.L.W., Summerskill W.H.J. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med. 1973;288:813–815.
77. Saunders J.H.B., Wormsley K.G. Progress report. Pancreatic extracts in the treatment of pancreatic exocrine insufficiency. Gut. 1975;16:157–162.
78. Bank S., Marks I.N., Barbezat G.O. Treatment of acute and chronic pancreatitis. Drugs. 1977;13:373–381.
79. DiMagno E.P., Malagelada J.R., Go V.L.W., et al. Fate of orally ingested enzymes in pancreatic insufficiency; comparison of two dosage schedules. N Engl J Med. 1977;296:1318–1322.
80. Saunders J.H.B., Drummond S., Wormsley K.G. Inhibition of gastric secretion in treatment of pancreatic insufficiency. BMJ. 1977;1:418–419.
81. Anonymous. Pancreatic extracts. Lancet. 1977;2:73–74.
82. Regan P.T., Malagelada J.R., DiMagno E.P., et al. Comparative effects of antacids, cimetidine and enteric coating on the therapeutic response to oral enzymes in severe pancreatic insufficiency. N Engl J Med. 1977;297:854–858.
83. Regan P.T., Malagelada J.R., Dimagno E.P., et al. Rationale for the use of cimetidine in pancreatic insufficiency. Mayo Clin Proc. 1978;53:79–88.
84. Austad W.J. Pancreatitis; the use of pancreatic supplements. Drugs. 1979;17:480–487.
85. Physicians’ Desk Reference, Vol. 47. Montvale, NJ: Medical Economics. 1993. 1426
86. Van Hoozen C.M., Peeke P.G., Taubeneck M., et al. Efficacy of enzyme supplementation after surgery for chronic pancreatitis. Pancreas. 1997;14:174–180.
87. Anonymous. Pancreatic extracts. BMJ. 1970;2:161–163.
88. Anderson C.M. Pancreatic enzyme replacement in the treatment of cystic fibrosis. Prescribers J. 1972;12:45–49.
89. Roy C.C., Weber A.M., Morin C.L., et al. Abnormal biliary lipid composition in cystic fibrosis. N Engl J Med. 1977;197:1301–1305.
90. Smalley A.C., Brown G.A., Parkes M.E., et al. Reduction of bile acid loss in cystic fibrosis by dietary means. Arch Dis Child. 1978;53:477–482.
91. Goodschild M.C., Sagaro E., Brown G.A., et al. Comparative trial of Pancrex V. forte and Nutrizym in treatment of malabsorption in cystic fibrosis. BMJ. 1974;3:712–714.
92. Nakamura T., Takebe K., Kuoh K., et al. Effects of pancreatic digestive enzymes, sodium bicarbonate, and a proton pump inhibitor on steatorrhoea caused by pancreatic disease. J Int Med Res. 1995;23:37–47.
93. Kleine M.-W., Pabst H. Die wirkung einer oralen enzymtherapie auf experimentell erzeugte hamatoma. Forum des Prakt und Allgemeinarztes. 1988;27:42–48.
94. Baumüller M. Der einsatz von hydrolytischen enzymen bei steumpfen weichteilverletzungen und sprunggelenksdistorsionen. Allgemeinmedizin. 1990;19:178–182.
95. Baumüller M. Enzyme zur wiederherstellung nach sprunggelenkdistorsionen. Z Allg Med. 1992;68:61–65.
96. Baumüller M. Therapy of ankle joint distortions with hydrolytic enzymes—Results from a double blind clinical trial. In: Hermans G.P.H., Mosterd W.L. Sports, Medicine and Health. Amsterdam: Excerpta Medica; 1990:1137.
97. Szczurko O., Cooley K., Mills E.J., et al. Naturopathic treatment of rotator cuff tendonitis among Canadian postal workers: a randomized controlled trial. Arthritis Rheum. 2009;61:1037–1045.
98. Cichoke A.J., Marty L. The use of proteolytic enzymes with soft tissue athletic injuries. Am Chiro. 1981:32–33.
99. Helpap B. Leitfaden der Allgemeinen Entzundungslehre. Berlin: Springer; 1987.
100. Kleine M- W. Systemische enzymtherapie in der sportmedizin. Deut Z Sportmed. 1990;41:126–134.
101. Müller-Hepburn W. Anwendung von Enzymen in der Sportmedizin. Forum d Prakt Arztes. 1970;18:7–10.
102. Niethard F.U., Pfeil J. Orthopadie. Stuttgart: Hippokrates Verlag; 1989.
103. Hiss WF. Enzyme in der sport- und unfallmedizin. Z Naturheilmethoden: Contincationars;2:1.
104. Doenicke A., Hoernecke R. Wirksame behandlung von traumen mit schwellung und/oder hamatom im eishockeysport durch enzymtherapie. Deut Z Sportmed. 1993;5:214–219.
105. Kleine M.-W. Introduction to systemic enzyme therapy and results of experimental trials. In: Hermans G.P.H., Mostered W.L. Sports, Medicine and Health. Amsterdam: Excerpta Medica; 1990:1131.
106. Miller P.C., Bailey S.P., Barnes M.E., et al. The effects of protease supplementation on skeletal muscle function and DOMS following downhill running. J Sports Sci. 2004;22:365–372.
107. Rahn H.-D., Kilic M. Die wirksamkeit hydrolytischer enzyme in der traumatologie. Ergebnise nach 2 prospektiven randomisierten doppelblindstudien. Allgemeinarzt. 1990;19:183–187.
108. Rahn H-D. Enzyme verkurzen rekonvaleszenz. Lecture given at 13th Systemische Enzymtherapie Symposium, Lindau, 1990 May 27-June 1.
109. Carillo A.R. Klinische untersuchung eines enzymatischen entzundungshemmers in der unfallchirurgie. Arztl Praxis. 1972;24:2307–2308.
110. Kamenicek V., Holan P., Franek P. Systemic enzyme therapy in the treatment and prevention of post-traumatic and postoperative swelling. Acta Chir Orthop TrCech. 2001;68:45–49. [Czech]
111. Isaeva A.V., Minaev S.V., Sternin IuI., et al. Modern approach to the rehabilitation of children with fractured long tubular bones. Vopr Kurortol Fizioter Lech Fiz Kult. 2009;3:29–31. [Russian]
112. Chappa-Alvarez R. Pankreatitisbehandlung mit Wobenzym. Working paper, 1992.
113. Guggenbichler J.P. Einfluss hydrolytischer enzyme auf thrombusbildung und thrombolyse. Med Welt. 1988;39:277–280.
114. Rahn H-D. Wobenzymnach Gefassbypassoperationen am bein. Lecture given at 17th Systemische Enzymtherapie Symposium, Vienna, 1991.
115. Vinzenz K. Ödembehandlung bei zahnchirurgischen eingriffen mit hydrolytischen enzymen. Die Quintessenz. 1991;42(7):1053–1064.
116. Werk W. Ein Polyenzympraparat zur Beschleunigung der Narbenbildung. Proktologie. 1979;3:28–29.
117. Riede N.U., Schaefer H.E., Wehner H. Allgemeine und Spezielle Pathologie. Stuttgart: Thieme Verlag; 1989.
118. Ryan R.E. A double-blind clinical evaluation of bromelain in the treatment of acute sinusitis. Headache. 1967;7:13–17.
119. Zollner N., ed. Innere Medizin. Heidelberg: Springer, 1991.
120. Wohlrab R. Enzymkombinationspraparat zur therapie der sinusitis acuta. Der Allgemeinarzt. 1993;15:104–114.
121. Inderst R. Enzymtherapie—grundlagen und anwendungsmoglichkeiten. Natur-und Ganzheitsmedizin. 1991:3.
122. Zech R., Domagk G. Enzyme—biochemie, pathobiochemie, klinik, therapie. Weinheim: VCH Verlagsgesellschaft mbH; 1986.
123. Grimminger A. Enzymtherapie bei thoraxerkrankungen. Erfahrungsheilkunde. 1971;1:18.
124. Barsom S., Sasse-Rollenhagen K., Bettermann A. Zur behandlung von zystitden und zystopyelitiden mit hydrolytischen enzymen. Acta Medica Empirica. 1983;32:125–129.
125. Barsom S., Sasse-Rollenhagen K., Bettermann A. Erfolgreiche prostatitisbehandlung mit hundrolytischen enzymen. Erfarungsheilkunde. 1982;31:2.
126. Rugendorff E.W., Burghele A., Schneider H.-J. Behandlung der chronischen abakteriellen prostatitis mit hydrolytischen enzymen. Der Kassenarzt. 1986;14:43–51.
127. Dittmar F.-W., Weissenbacher E.R. Therapie der adnexitis—unterstutzung der antibiotischen basisbehandlung durch hydrolytische enzyme. Int J Exper Clin Chemother. 1992;5:73–82.
128. Dittmar F.-W. Enzymtherapie in der gynakologie. Allgemeinmedizin. 1990;19:158–159.
129. Rahn H-D. Wirksamkeit von enzymen bei gefasserkrankungen. Lecture given at 2nd Systemische Enzymtherapie Symposium, Dusseldorf, Germany, 1987.
130. Ernst E., Matrei A. Orale therapie mit proteolytischen enzymen modifiziert die blutrheologie. Klin Wsch. 1987;65:994.
131. Klein K. Proteolytisches enzympraparat erfolgreich. Therapiewoche Osterreich. 1989;39:448.
132. Mörl H. Behandlung des postthrombotischen syndroms mit einem enzymgemisch. Therapiewoche. 1986;36:2443.
133. Mörl H. Therapie und prophylze des postthrombotischen syndroms mit Wobenzym. Lecture given at 17th Systemische Enzymtherapie Symposium, Vienna, 1991.
134. Valls-Serra J. Proteolytische enzyme in der behandlung von thrombophlebitis. Med Clin. 1967.
135. Mahr H. Zur enzymtherapie entzundlicher venenerkrankungen der tiefen beinvenenthrombose und des postthrombotischen syndromes. Erfahrungsheilkunde. 1983:117.
136. Maehder K. Enzymtherapie venoser gefasserkrankungen. Die Arztpraxis. 1972:2.
137. Kluken N. Venose krankheiten in klinik und praxis, systemische enzymtherapie, medizinische woche Baden-Baden, 1990. Natur-und Ganzheitsmedizin. 1991;2:8–13.
138. Vogler W. Enzymtherapie. Hessen: Hausarzt. 1989;4:116–119.
139. Rokitansky O. Ozontherapie und enzyme bei der chronisch-arteriellen verschlusskrankeit. Systemische Enzymtherapie am 31.10.90. Medizinische Woche, Baden-Baden, 1990. Natur-und Ganzheitsmedizin, 1991;Suppl 1:14–16.
140. Keim H., Al-Yousef S., Wachter K. Methode zur linderung der lymphstauung am arm nach behandlung des mammakarzinoms. Rontgenberichte. 1, 1972. 45-41
141. Scheef W., Pischnamazadeh M. Proteolytische enzyme als einfache und sichere methode zur verhutung des lymphodems nach ablatio mammae. Med Welt. 1984;35:1032–1033.
142. Streichhan P., Inderst R. Konventionelle und enzymtherapeutische massnahmen bei der behandlung brustkrebsbedingter armlymphodeme. Der Prakt Arzt. 1991;13:37–38.
143. Ransberger K., Stauder G., Streichhan P. Wissenschaftliche monographie zur praklinik Wobenzym N, Mulsal N, Phlogenzym. Forum Medizin. 1991.
144. König W. Erfahrungen der Robert-Janker-Klinik, Bonn, mit systemischer enzymtherapie und emulgierten vitaminen. Acta Medica Empirica. 1988;37:11–17.
145. König W. Proteolytische enzyme verhindern lymphodem. In: Medizinische Enzym-Forschungesgesellschaft e.V., ed. Systemische Enzymtherapie, Symposium Munich, 1986; Medizin Aktuell (Enzyme Series). Informed International Congress Report 53, 1986.
146. Korpan M.I., Fialka V. Wobenzyme and diuretic therapy in lymphedema after breast operation. Wien Med Wochenschr. 1996;146:67–72. [German]
147. Uffelmann K., Vogler W., Fruth C. Der eisatz hydrolytischer enzyme beim extraartikularen rheumatismus. Allgemeinmedizin. 1990;19:151–153.
148. Vogler W. Der stellenwert der enzymtherapie bei entzundlich-rheumatischen erkrankungen. Systemische Enzymtherapie, Medizinische Woche, Baden-Baden, 1990. Natur-und Ganzheitsmedizin. 1991;2:23.
149. Miehlke K. Enzymtherapie bei rheumatoider arthritis. Natur-und Ganzheits-medizin. 1988:108–111.
150. Inderst R. Enzymtherapie. Erfahrungsheilkunde. 1989;38:305.
151. Hörger I., Moro V., van Schaik W. Zirkulierende Immunkomplexe bei polyarthritis-patienten. Natur-und Ganzheitsmedizin. 1988;1:117–122.
152. Steffen C., Smolen J., Miehlke K., et al. Enzymtherapie im vergleich mit immunkiomplexbestimmungen bei chonischler polyarthritis. Z Rheumatol. 1985;44:51–56.
153. Reinbold H. Die biologische alternative in der therapie entzundlicher rheumatischer erkrankungen. Z Allgemeinmed. 1981:34.
154. Reinbold H. Die therapie des morbus bechterew mit enzymen. Erfahrungsheilkunde. 1980:10.
155. Ekerot L., Ohlsson K., Necking L. Elimination of protease-inhibitor complexes from the arthritic joint. Int J Tissue React. 1985:391–395.
156. Ballachi G. Hydrolytische enzyme bei aktivierten polyarthrosen. Rheuma. 1988:8.
157. Goebel KM. Enzymtherapie bei spondylitis ankylosans. Lecture given at 17th Systemische Enzymtherapie Symposium, Vienna, 1991.
158. Hörger I. Enzymtherapie bei einem rheumakollektiv. Therapiewoche. 1983;33:3948–3957.
159. Panijel M. Entzundlich-rheumatische erkrankungen. Z Allgemeinmedizin. 1985;61:1305–1307.
160. Steffen C., Zeitlhofer J., Menzel J., et al. Die antigen-induzierte experimentelle arthritis als prufverfahren fur entzundungshemmung durch oral appliqierte substanzen. Z Rheumatol. 1979;38:264–278.
161. Miehlke K. Enzymtherapie bei chronischer polyarthritis. Der Kassenarzt. 1989:46.
162. Miehlke K. Rheumabahandlung mit enzymen ist mehr als nur antiphlogistische therapie. Lecture given at 17th Systemische Enzymtherapie Symposium, Munich, 1991.
163. Klein G., Schwann H., Kullich W. Enzymtherapie bei chronischer polyarthritis. Natur-und Ganzheitsmedizin. 1988;1:112–116.
164. Klein K. Behandlung der rheumatoiden arthritis mit Wobenzym im vergleich zur basistherapie mit gold. Lecture given at 17th Systemische Enzymtherapie u Vienna 1991.
165. Singer F. Aktivierte arthrosen knorpelschonend behandeln. Lecture given at 10th Systemische Enzymtherapie Symposium, Frankfurt, 1990.
166. Tilwe G.H., Beria S., Tutrakhia N.H., et al. Efficacy and tolerability of oral enzyme therapy as compared to diclofenac in active osteoarthritis of knee joint: an open randomized controlled clinical trial. J Assoc Physicians I. 2001;49:617–621.
167. Masuhr K.F. Neurologie. Stuttgart: Hippokrates; 1989.
168. Neuhofer Ch, van Schaik W., Stauder G., et al. Pathogenetic immune complexes in MS: their elimination by hydrolytic enzymes. A therapeutic approach. International Multiple Sclerosis Conference. Rome. Bologna: Monduzzi Editore SPA; September 14-17, 1988.
169. Ransberger K., van Schaik W. Enzymtherapie bei multipler sklerose. Der Kassenarzt. 1986;41:42–45.
170. Neuhofer C.H. Enzymtherapie bei multipler sklerose. Hufeland J. 1986:47–50.
171. Neuhofer C.H. Systemische enzymtherapie bei encephalomyelitis disseminata. Der Prakt Arzt. 1991:702.
172. Mialovyts’ka O.A. Effect of phlogenzym in long-term treatment of patients with multiple sclerosis. Lik Sprava, 2003;3–4, Apr-Jun:109–113. [Ukrainian]
173. Diringer H. Unkonventionelle viruserkrankungen. Bundesgesundhbl. 1990;33:187–194.
174. Inderst R., Ransberger K., Brand G. Fortschritte in der therapie der erworbenen immunschwache durch naturheilkundliche methoden. Naturheilpraxis. 1998;41:1050.
175. Jäger H., Popescu M., Samtleben W., et al. Hydrolytic enzymes as biological response modifiers (BRM) in HIV-infection. San Marino Conferences—highlights in medical virology, immunology and oncology, Vol. 1. Oxford: Pergamon Press. 1988.
176. Jäger H. Hydrolytische enzyme in der therapie der HIV-erkrankung. Z Allgemeinmedizin. 1990;19:160–164.
177. Ransberger K. Naturheilkundliche therapie von AIDS mit enzympraparaten. Forum des Prakt und Allgemeinarztes. 1988. Heft 4.27, Jahrgang, April 1988
178. Shahid S.K., Turakhia N.J., Kunda M., et al. Efficacy and safety of phlogenzym—a protease formulation, in sepsis in children. J Assoc Physicians I. 2002;50:527–531.
179. Scheef W. Gutartige veranderungen der weiblichen brust. Therapiewoche. 1985;35:5909–5912.
180. Munck A., Duhamel J.F., Lamireau T., et al. Pancreatic enzyme replacement therapy for young cystic fibrosis patients. J Cyst Fibros. 2009;8:14–18.
181. Trolli P.A., Conwell D.L., Zuccaro G., Jr. Pancreatic enzyme therapy and nutritional status of outpatients with chronic pancreatitis. Gastroenterol Nurs. 2001;24:84–87.
182. Safdi M., Bekal P.K., Martin S., et al. The effects of oral pancreatic enzymes (Creon 10 capsule) on steatorrhea: a multicenter, placebo-controlled, parallel group trial in subjects with chronic pancreatitis. Pancreas. 2006;33:156–162.
183. Minaev S.V., Nemilova T.K., Knorring G.Iu. Polyenzymatic therapy in prevention of adhesive processes in the abdominal cavity in children. Vestn Khir ImGrekov. 2006;165:49–54. [Russian]
184. Kamenícek V., Holán P., Franěk P. Systemic enzyme therapy in the treatment and prevention of post-traumatic and postoperative swelling. Acta Chir Orthop. 2001;68:45–49. [Czech]
185. Kleine M.W. Therapie des herpes zoster mit proteolytischen. enzymen. Therapiewoche. 1987;37:1108–1112.
186. Klein G., Kullich W., Schnitker J., et al. Efficacy and tolerance of an oral enzyme combination in painful osteoarthritis of the hip. A double-blind, randomized study comparing oral enzymes with non-steroidal anti-inflammatory drugs. Clin Exp Rheumatol. 2006;24:25–30.
187. Kelley W.D. One answer to cancer. Ontario, OR: The Kelley Foundation; 1974.
188. Gujral M.S., Patnaik P.M., Kaul R., et al. Efficacy of hydrolytic enzymes in preventing radiation therapy-induced side effects in patients with head and neck cancers. Cancer Chemoth Pharm. 2001;47(Suppl):S23–S28.
189. Pluzhnikov M.S., Ryabova M.A., Karpiscenko S.A. The effect of systemic enzyme therapy (Phlogenzym) in the treatment of radiomucositis in patients with laryngeal cancer. Folia Otorhinolaryngologiae et Pathologiae Respiratoriae. 1999;5:73–75.
190. Dale P.S., Tamhankar C.P., George D., et al. Co-medication with hydrolytic enzymes in radiation therapy of uterine cervix: evidence of the reduction of acute side effects. Cancer Chemoth Pharm. 2001;4(Suppl):S29–S34.
191. Bahl A., Chander S., Julka P.K., et al. Micronuclei evaluation of reduction in neoadjuvant chemotherapy related acute toxicity in locally advanced lung cancer: an Indian experience. J Assoc Physician I. 2006;54:191–195.
192. National Cancer Institute. Surveillance Epidemiology and End Results (SEER). SEER Stat Fact Sheets: Breast. http://www.Seer.cancer.gov/statfacts/html/breast.html. Accessed 2/10/2010
193. Bleuth J., Ost B., Pakdaman A., et al. Impact of complementary oral enzyme application on the postoperative treatment results of breast cancer patients-results of an epidemiological multicentre retrolective cohort study. Cancer Chemoth Pharm. 2001;47(Suppl):S45–S54.
194. Heron M., Hoyert D.L., Murphy S.L., et al. National vital statistics reports. CDC. Vol 57, April 17, 2009. Number 14 http://www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_14.pdf Accessed 2/18/2010
195. American Cancer Society. Cancer Facts & Figures 2009. Atlanta: American Cancer Society; 2009.
196. Ihse I., Arnesjö B., Kugelberg C., et al. Intestinal activities of trypsin, lipase, and phospholipase after a test meal. An evaluation of 474 examinations. Scand J Gastroentero. 1977;12:663–668.
197. Bruno M.J., Haverkort E.B., Tijssen G.P., et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut. 1998;42:92–96.
198. National Multiple Sclerosis Society. www.nationalmssociety.org/about-multiple-sclerosis/what-we-know-about-ms/who-gets-ms/index.aspx. Accessed 2/5/2010
199. Neuhofer C.H. Autoimmunerkrankungen: Multiple sklerose, amyotrophe lateralsklerose, colitis ulcerosa. Erfahrungsheilkunde. 1988;38:451–454.
200. Haid-Fischer F., Haid H. Venenerkrankungen. Stuttgart: Thieme; 1985.
201. Saradeth T., Quittan M., Ghanem A.H., et al. Improvement in blood fluidity characteristics via Phlogenzym—An in vitro pilot study. Perfusion. 1995;8:196–198.
202. Koshkin V.M., Kirienko A.I., Leontjev S.G., et al. Systemic enzyme therapy of lower limb postphlebitis syndrome. Angiol Vasc Surg. 2000;6:61–64.
203. Kopadze T.Sh., Natsvlishvili G.A., Tvaladze M.G., et al. Application of Wobenzym and Phlogenzym to the angiology and vascular surgery. Georg Med News. 2001;2:27–29.
204. Chlamydia –CDC Fact Sheet, Department of Health and Human Services, Centers for Disease Control and Prevention. http://www.cdc.gov/std/chlamydia/STDFact-Chlamydia.htm. Accessed 7/27/2010
205. Askienazy-Elbhar M., Henry-Suchet J. Persistent “Silent” Chlamydia trachomatis female genital tract infections. Infect Dis Obst Gyn. 1999;7:31–34.
206. Sukhikh G.T., Loginova N.S., Faizullin L.Z., et al. The use of Wobenzym to facilitate interferon synthesis in the treatment of chronic urogenital chlamydiosis. Int J Immunotherapy. 1997:131–133. XIII
207. Brudnak M.A., Rimland B., Kerry R.E., et al. Enzyme-based therapy for autism spectrum disorders—is it worth another look? Med Hypotheses. 2002;58:422–428.
208. Stauder G., Pollinger W., Fruth C. Systemische Enzymetherapie: Eine Ubersicht uber Neue Klinische Studient. Allgemein Medizin. 1990;19:188–191.
209. Inderst R. Enzymtherapie bei Gefasserkrankungen [Enzyme therapy in vascular diseases]. Allgemein Medizin. 1990;19:154–157.
210. Wrba H. Kombinierte Tumortherapie. Stuttgart: Hippokrates; 1992.
211. Worschhauser S. Konservative Therapie der Sportverletzungen. Enzympraparate fur Therapie und Prophylaxe [Conservative therapy for sports injuries. Enzyme preparations for therapy and prophylaxis]. Allgemein Medizin. 1990;19:173–177.
212. Klein G., Pollmann G., Kullich W. Klinische Erfahrungen mit der Enzymtherapie bei Patienten mit Chronischer Polyarthritis im Vergleich Zur Oralen Goldtherapie [Clinical experience with enzyme therapy in patients with rheumatoid arthritis in comparison with oral gold]. Allgemein Medizin. 1990;19:144–147.
213. Gallacchi G. Der Einsatz Hydrolytischer Enzyme bei der Aktivierten Arthrose [The use of hydrolytic enzymes in activated arthrosis]. Allgemein Medizin. 1990;19:148–150.
214. Dittmar F-W, Luh W, Phillipp E. Wobenzym zur Behandlung der Mastopathie. Working paper, 1993.
215. Wolf M., Ransberger K. Enzyme Therapy. Los Angeles: Regent House; 1972.
216. Sears A., Walsh G. Biotechnology in the Feed Industry: Proceedings of Alltech’s Ninth Annual Symposium. Nicholasville, KY: Alltech Technical Publ; 1993.
217. McEvoy G.K., ed. AHFS ‘94 Drug Information, American Hospital Formulary Service. Bethesda, MD: American Society of Hospital Pharmacists, 1994.
218. Park H.S., Kim H.Y., Suh Y.J., et al. Alpha amylase is a major allergenic component in occupational asthma patients caused by porcine pancreatic extract. J Asthma. 2002;39:511–516.
219. Ramsden W.H., Moya E.F., Littlewood J.M. Colonic wall thickness, pancreatic enzyme dose and type of preparation in cystic fibrosis. Arch Dis Child. 1998;79:339–343.
220. Serban D.E., Florescu P., Miu N. Fibrosing colonopathy revealing cystic fibrosis in a neonate before any pancreatic enzyme supplementation. J Pediatr Gastr Nutr. 2002;35:356–359.
221. Dodge J.A. Fibrosing colonopathy: recent advances. J Roy Soc Med Suppl. 1996;89(Suppl 27):19–23.
Cichoke A.J. A New Look at Chronic Disorders and Enzyme Therapy. Portland, OR: Seven C’s Publishing; 1993.
Cichoke A.J. A New Look at Enzyme Therapy. Portland, OR: Seven C’s Publishing; 1993.
Cichoke A.J. Acute Trauma and Systemic Enzyme Therapy. Portland, OR: Seven C’s Publishing; 1993.
Cichoke A.J. Bromelain. New Canaan, CT: Keats; 1998.
Cichoke A.J. Enzymes and Enzyme Therapy: How to Jump Start Your Way to Lifelong Good Health, 2nd ed., New Canaan, CT: Keats, 2000.
Cichoke A.J. The Complete Book of Enzyme Therapy. New York: Avery Publishing; 1999.