Chapter 118 Pygeum africanum (Bitter Almond)
Pygeum africanum (family: Rosaceae)
Common names: bitter almond, red stinkwood
 General Description
General Description
Pygeum africanum is an evergreen tree native to Africa that can grow to a height of 120 to 150 ft. It has pendulous branches with thick, oblong, leather-like, mat-colored leaves and creamy white flowers. The fruit (drupe) resembles a cherry when ripe. The dark brown to gray bark of the trunk is the part used for medicinal purposes.
 Chemical Composition
Chemical Composition
The major active components of the bark are as follows:
• Lipid-soluble pentacyclic triterpenes
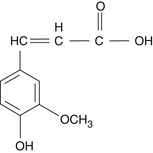
• Esters of ferulic acid (Figure 118-1)
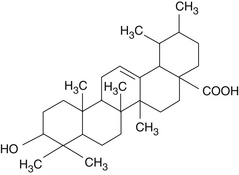
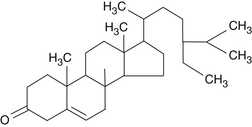
The pentacyclic triterpenic components are ursolic acid (Figure 118-2), oleanolic acid, crataegolic acid, and their derivatives. The sterolic fraction is composed mainly of β-sitosterol and β-sitosterone (Figure 118-3). The fatty acids range from C12 to C24 and the important ferulic acid esters are those bound to n-tetracosanol and n-docosanol.1-4
 History and Folk Use
History and Folk Use
The powdered bark of P. africanum was used by the natives of tropical Africa as a treatment for urinary disorders. It was often given with palm oil or milk. Since about 1970, the bark has been entirely wild-collected, with the major exporters being Cameroon, Madagascar, Equatorial Guinea, and Kenya. Two companies, Groupe Fournier of France and Indena of Italy, produce 86% of the world’s bark extract, both for their own products and for the free market. Worldwide exports of dried bark in 2000 were estimated at 1350 to 1525 metric tons per year, down from its peak of 3225 tons in 1997. Bark extracts (6370 to 7225 kg/year) are worth an estimated $4.36 million U.S. dollars per year. However, wild collection of the bark is no longer sustainable. Since 1995, P. africanum has been declared an endangered species. Alternatives to wild collection to meet future market demand include conservation practices, enrichment plantings, and small- and large-scale production. Currently, the species is at the beginning of a transition from an exclusively wild-collected species to that of a cultivated medicinal tree.5
 Pharmacology
Pharmacology
Pharmacologic screening of various extracts prepared with solvents of differing degrees of polarity indicated that the highest activity was found in lipophilic extracts.1 This finding is interesting in light of pygeum’s historical administration in lipophilic media (palm oil or milk). Virtually all of the pharmacologic research featured a pygeum extract standardized to contain 14% triterpenes, including β-sitosterol and 0.5% n-docosanol. This extract was extensively studied in both experimental animal studies and clinical trials with humans.
The primary target organ for pygeum’s effects in men is the prostate. The three major active components of pygeum appear to exert different, yet complementary, effects in benign prostatic hyperplasia (BPH). In addition, pygeum was shown to enhance the secretions of the prostate and bulbourethral glands, in terms of both quantity and quality.
Ferulic Acid Esters
The esters of ferulic acid act primarily on the endocrine system. Studies in animals showed docosanol reduced levels of luteinizing hormone and testosterone while raising adrenal steroid secretion of both adrenal androgens and corticosteroids.6,7 Docosanol also significantly lowers serum prolactin levels. This reduction of prolactin is quite significant, because prolactin increases both the uptake of testosterone and the synthesis of dihydrotestosterone within the prostate. The accumulation of testosterone within the prostate and its subsequent conversion to the more potent dihydrotestosterone is thought to be the major contributing factor to the hyperplasia of the prostatic cells observed in BPH.8 Although traces of docosanol are present in pygeum, the esterification with ferulic acid results in greater bioavailability and activity.2,4,9
Ferulic acid esters and the sterol fraction of pygeum exert cholesterol-lowering action systemically as well as within the prostate.9 Breakdown products of cholesterol were shown to accumulate in prostate tissue affected with either BPH or cancer.8 These metabolites of cholesterol initiate degeneration of prostatic cells, which can promote prostatic enlargement. Drugs that lower cholesterol levels were shown to have a favorable influence on BPH, preventing the accumulation of cholesterol in prostatic cells and limiting subsequent formation of damaging cholesterol metabolites. The lowering of intraprostatic cholesterol content is an important aspect of the pharmacology of pygeum.
The sterolic fraction is also endowed with competitive action against testosterone accumulation within the prostate. In addition, the sterols of pygeum were also shown to reduce inflammation by preventing the intraprostatic formation of inflammatory prostaglandins.9,10
Other Components
Other components of pygeum are also important. For example, the pentacyclic triterpenes exhibit anti-inflammatory effects within the prostatic epithelium and may be responsible for stimulation of the secretory cells of the prostate, seminal vesicles, and bulbourethral glands.9-11 Finally, the fatty acid components are similar to those of Serenoa repens (see Chapter 122) and may exert similar effects as well as improve the oral bioavailability of other components of the lipophilic extract.
 Clinical Applications
Clinical Applications
Prostate Disorders
The pharmacologic actions of the standardized pygeum extract support its use in prostate disorders, BPH in particular. Adding further support are the results of numerous clinical trials in more than 600 patients.12-34 These studies consistently demonstrated that pygeum effectively reduces the symptoms and clinical signs of BPH, especially in early cases. However, it must be pointed out that improvement is largely symptomatic, because the reductions in size of the prostate and in residual urine content of the bladder are modest. The results of the clinical trials on pygeum are given in Table 118-1. A discussion of some of the most important aspects of these studies follows.
TABLE 118-1 Results of the Most Significant Open and Double-Blind Studies of the Last 20 Years on Outpatients Using Pygeum africanum for 1 to 3 Months
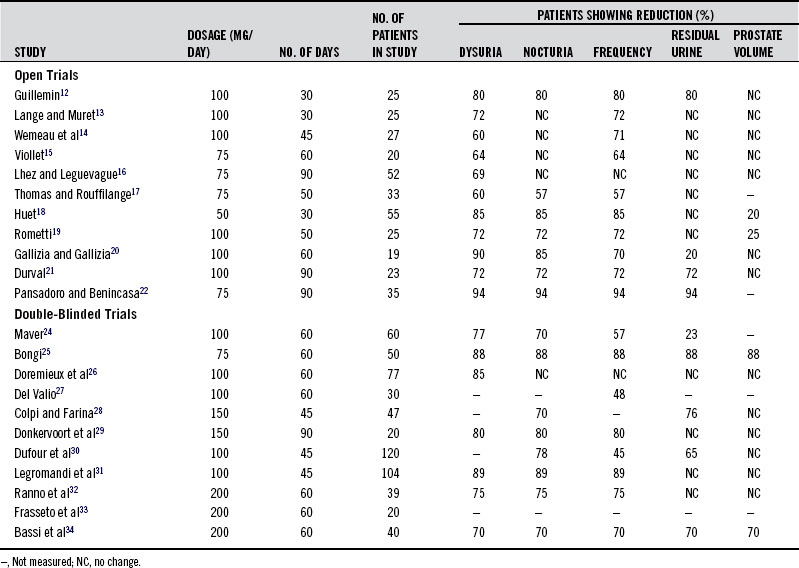
One of the major findings in evaluating the effectiveness of pygeum in BPH was the high rate of response to placebo. This was well-demonstrated in one of the larger double-blind studies.30 As in other double-blind studies, pygeum extract was shown to be statistically superior to placebo in reducing the major symptoms of BPH (nocturnal frequency, difficulty in starting micturition, and incomplete emptying of the bladder). However, there was a high percentage of responses to the placebo (Table 118-2). It seems that simply taking a capsule provides relief to many sufferers.
TABLE 118-2 Patients Showing Response to Placebo and Pygeum
| SYMPTOM | PLACEBO GROUP | PYGEUM GROUP |
|---|---|---|
| Nocturia | 26/52 (50%) | 44/56 (78%) |
| Daytime frequency | 16/50 (33%) | 27/54 (50%) |
| Incomplete voiding | 14/40 (35%) | 21/32 (66%) |
| Dribbling | 15/34 (44%) | 13/33 (39%) |
| Urine flow rate | 11/43 (26%) | 21/38 (55%) |
Data from references 12-22 and 24-34.
Another study highlighted the importance of double-blind studies that featured both objective and subjective findings. In the study, both patients and physicians rated the placebo and pygeum extract to be effective in improving subjective symptoms, which were daytime frequency, nocturia, weak stream, after-dribbling, hesitation, and interruption of flow.29 However, urodynamic variables (flow, frequency, and histogram) clearly demonstrated the superiority of pygeum over placebo.
One of the shortcomings of some of the clinical research on pygeum in BPH was the lack, in many of the studies, of objective measures, such as urine flow rate (milliliters per second), residual urine content, and prostate size. Studies that used objective measurements showed some good results. For example, in one open trial, 30 patients with BPH who were given 100 mg/day of the pygeum extract for 75 days demonstrated significant improvements in objective parameters: maximum urine flow rate increased from 5.43 to 8.20 mL/s, and the residual urine volume dropped from 76 to 33 mL.23
Pygeum may emerge as a significant protector against prostate cancer (PCa), because pygeum extract and its components (e.g., atraric acid and N-butylbenzene-sulfonamide) inhibit the transactivation mediated by the ligand-activated human androgen receptor (AR). Inhibition of human AR by androgen ablation therapy and synthetic antiandrogens is the primary goal in treatment of PCa. Both in vitro and in vivo studies showed pygeum components inhibited the nuclear transport of AR and endogenous prostate-specific antigen expression and efficiently repressed the growth of both the androgen-dependent and some types of androgen-independent prostate cancer cells.35-38 Currently used synthetic AR antagonists bind to the AR, and induce a conformational change that leads to the dissociation of key proteins in the AR, but the antagonist-bound AR is still translocated to the nucleus where it recruits core pressors to block its transcriptional activity. Pygeum components act a little differently, in that they selectively bind to the AR and inhibit the translocation to the nucleus and, in consequence, it functions as an androgen-activated transcription factor. This difference in mechanism may overcome a major shortcoming with antihormone therapy in PCa. Typically, antihormone treatment is effective only for a limited period of about 16 to 24 months, after which PCa becomes androgen independent. Different mechanisms seem to be involved in this process, but AR mutations during PCa progression render therapeutically used antiandrogens into AR agonists. In consequence, those therapeutics then become useless, and even counterproductive, because they can promote PCa progression.
Male Infertility and Impotence
Pygeum may be effective in improving fertility when diminished prostatic secretion plays a significant role in the problem. Pygeum was shown to increase prostatic secretions and improve the composition of seminal fluid.39-41 Specifically, pygeum administration to men with decreased prostatic secretion led to higher levels of total seminal fluid plus increases in alkaline phosphatase and protein levels. Pygeum appears to be most effective in cases in which the level of alkaline phosphatase activity is reduced (i.e., less than 400 IU/cm3), and there is no evidence of inflammation or infection (i.e., absence of white blood cells and immunoglobulin-A [IgA]). The lack of IgA in the semen is a good predictor of clinical success. In one study, the patients with no IgA in the semen demonstrated an alkaline phosphatase increase from 265 to 485 IU/cm339 In contrast, subjects whose semen contained IgA showed only a modest increase, from 213 to 281 IU/cm3.
Pygeum extract also showed an ability to improve the capacity to achieve an erection in patients with BPH or prostatitis, as determined by nocturnal penile tumescence in a double-blind clinical trial.42 BPH and prostatitis are often associated with erectile dysfunction and other sexual disturbances. Presumably by improving the underlying condition, pygeum can improve sexual function.
Pygeum Versus Serenoa
The standardized liposterolic extract of Serenoa repens is another popular botanical treatment for BPH (see Chapter 122). In a double-blind study that compared pygeum extract with extract of serenoa, the serenoa extract produced a greater reduction of symptoms and was better tolerated.43 In addition, the improvement of objective parameters, especially urine flow rate and residual urine content, was better in the clinical studies of serenoa. However, there may be circumstances in which pygeum is more effective than serenoa. For example, serenoa has not been shown to have the effects on prostate secretion that pygeum has, and the anticancer effects of pygeum may be more significant. Although the two extracts have somewhat overlapping mechanisms of actions, they can be used in combination.
 Dosage
Dosage
The dosage of the lipophilic extract of P. africanum standardized to contain 14% triterpenes including β-sitosterol and 0.5% n-docosanol is 100 to 200 mg/day. Typically, the dosage has been divided for twice a day administration; however, results were similar in a double-blind study with 100 mg given once a day and 50 mg twice a day, indicating that taking pygeum once a day is equivalent to using divided doses.44 The crude herb is not used.
 Toxicology
Toxicology
Tests for acute and long-term toxicity in the rat and mouse showed that the standardized extract of P. africanum bark is nontoxic. Raising the dosage from 1 to 6 g/kg in the mouse and from 1 to 8 g/kg in the rat caused no deaths within 48 hours. In long-term toxicity studies, giving the animals from 60 to 600 mg/kg for 11 months did not produce any negative effects.
Pygeum extract also demonstrated no significant toxicity in human clinical trials. The most common side effect is gastrointestinal irritation, resulting in symptoms ranging from nausea to severe stomach pains; however, the presence of these side effects rarely leads to discontinuation of therapy.
1. Longo R., Tira S. Steroidal and other components of Pygeum africanum bark. Farmaco. 1983;38:288–292.
2. Martinelli E.M., Seraglia R., Pifferi G. Characterization of Pygeum africanum bark extracts by HRGC with computer assistance. J High Resolut Chrom. 1986;9:106–110.
3. Pierini N., Citti F., Di Marzio S., et al. Identification and determination of n-docosanol in the bark extract of Pygeum africanum and in patent medicines containing it. Boll Chim Farm. 1982;121:27–34.
4. Uberti E., Martinelli E.M., Pifferi G., et al. HPLC analysis of n-docosyl ferulate in Pygeum africanum extracts and pharmaceutical formulations. Fitoterapia. 1990;61:342–347.
5. Stewart K.M. The African cherry (Prunus africana): can lessons be learned from an over-exploited medicinal tree? J Ethnopharmacol. 2003;89:3–13.
6. Muntzing J., Eneroth P., Gustafsson J.A., et al. Direct and indirect effects of docosanol (IK.2), the active principle in Tadenan, on the rat prostate. Invest Urol. 1979;17:176–180.
7. Thieblot L. Preventive and curative action of a bark extract of an African plant, Pygeum africanum: experimental prostatic adenoma in the rat. Therapie. 1971;26:575–580.
8. Hinman F. Benign Prostatic Hyperplasia. New York: Springer-Verlag; 1983.
9. Bombardelli E. Methods, composition and compounds for the treatment of prostatic adenoma. EP Appl 8330491.3. June 10, 1985.
10. Marcoli M. Anti-inflammatory and antiedemigenic activity of extract of Pygeum africanum in the rat. New Trends Androl Sci. 1985;1:89–93.
11. Latalski M. The ultrastructure of the epithelium of bulbourethral glands after administration of the Tadenan preparation. Folia Morphol. 1979;1:193–201.
12. Guillemin P. Clinical trials of V1326, or Tadenan, in prostatic adenoma. Med Prat. 1970;386:75–76.
13. Lange J., Muret P. Clinical trial of V1326 in prostatic disease. Med. 1970;11:2807–2811.
14. Wemeau L., Delmay J., Blankaert J. Tadenan in prostatic adenoma. Vie Medicale. 1970;Jan:585–588.
15. Viollet G. Clinical experimentation of a new drug from prostatic adenoma. Vie Medicale. 1970;June:3457–3458.
16. Lhez A., Leguevague G. Clinical trials of a new lipid-sterolic complex of vegetal origin in the treatment of prostatic adenoma. Vie Medicale. 1970;Dec:5399–5404.
17. Thomas J.P., Rouffilange F. The action of Tadenan in prostatic adenoma. Rev Int Serv. 1970;43:43–45. [French]
18. Huet J.A. Prostatic disease in old age. Med Intern. 1970;5:405–408.
19. Rometti A. Medical treatment of prostatic adenoma. Prov Med. 1970;38:49–51.
20. Gallizia F., Gallizia G. Medical treatment of benign prostatic hypertrophy with a new phytotherapeutic principle. Recent Med. 1972;9:461–468.
21. Durval A. On the use of a new drug in the therapy of prostatic adenoma: Tadenan. Clinical considerations on 23 cases. Minerva Urol. 1970;22:106–111. [Italian]
22. Pansadoro V., Benincasa A. Prostatic hypertrophy. Results obtained with Pygeum africanum extract. Minerva Med. 1972;11:119–144.
23. Zurita I.E., Pecorini M., Cuzzoni G. Treatment of prostatic hypertrophy with Pygeum africanum extract. Rev Bras Med. 1984;41:364–366.
24. Maver A. Medical treatment of fibroadenomatous hypertrophy of the prostate with a new plant substance. Minerva Med. 1972;63:2126–2136.
25. Bongi G. Tadenan in the treatment of prostatic adenoma: anatomo-clinical study. Minerva Urol. 1972;24:129–139.
26. Doremieux J., Masson J.C., Bollack C. Prostatic hypertrophy, clinical effects and histological changes produced by a lipid complex extracted from Pygeum africanum. J Med Strasbourg. 1973;4:253–257. [French]
27. Del Valio B. Use of a new drug in the treatment of chronic prostatitis. Minerva Urol. 1974;26:81–94.
28. Colpi G., Farina U. Study of the activity of chloroformic extract of Pygeum africanum bark in the treatment of urethral obstructive syndrome caused by non-cancerous prostapathy. Urologia. 1976;43:441–448.
29. Donkervoort T., Sterling J., van Ness J., et al. A clinical and urodynamic study of Tadenan in the treatment of benign prostatic hypertrophy. Eur Urol. 1977;8:218–225.
30. Dufour B., Choquenet C., Revol M., et al. Controlled study of the effects of Pygeum africanum extract on the functional symptoms of prostatic adenoma. Ann Urol. 1984;18:193–195.
31. Legramandi C., Ricci-Barbini V., Fonte A. The importance of Pygeum africanum in the treatment of chronic prostatitis void of bacteria. Gazz Med Ital. 1984;143:73–76. [Italian]
32. Ranno S., Minaldi G., Viscusi G., et al. Efficacy and tolerability in the treatment of prostatic adenoma with Tadenan 50. Progr Med. 1986;42:165–169.
33. Frasseto G., Bertoglio S., Mancuso S., et al. Study of the efficacy and tolerability of Tadenan 50 in patients with prostatic hypertrophy. Progr Med. 1986;42:49–52.
34. Bassi P., Artibani W., De Luca V., et al. Standardized extract of Pygeum africanum in the treatment of benign prostatic hypertrophy: controlled clinical study vs. placebo. Minerva Urol. 1987;39:45–50.
35. Shenouda N.S., Sakla M.S., Newton L.G., et al. Phytosterol Pygeum africanum regulates prostate cancer in vitro and in vivo. Endocrine. 2007;31:72–81.
36. Papaioannou M., Schleich S., Prade I., et al. The natural compound atraric acid is an antagonist of the human androgen receptor inhibiting cellular invasiveness and prostate cancer cell growth. J Cell Mol Med. 2009;13:2210–2223.
37. Papaioannou M., Schleich S., Roell D., et al. NBBS isolated from Pygeum africanum bark exhibits androgen antagonistic activity, inhibits AR nuclear translocation and prostate cancer cell growth. Invest New Drugs. 2010;28:729–743.
38. Quiles M.T., Arbós M.A., Fraga A., et al. Antiproliferative and apoptotic effects of the herbal agent Pygeum africanum on cultured prostate stromal cells from patients with benign prostatic hyperplasia (BPH). Prostate. 2010;70:1044–1053.
39. Lucchetta G., Weill A., Becker N., et al. Reactivation of the secretion from the prostatic gland in cases of reduced fertility: biological study of seminal fluid modifications. Urol Int. 1984;39:222–224.
40. Menchini-Fabris G.F., Giorgi P., Andreini F., et al. New perspectives on the use of Pygeum africanum in prostato-bladder pathology. Arch Int Urol Nefrol Androl. 1988;60:313–322.
41. Clavert A., Cranz C., Riffaud J.P., et al. Effects of an extract of the bark of Pygeum africanum (V.1326) on prostatic secretions in the rat and man. Ann Urol. 1986;20:341–343.
42. Carani C., Salvioli C., Scuteri A., et al. Urological and sexual evaluation of treatment of benign prostatic disease using Pygeum africanum at high doses. Arch Ital Urol Nefrol Androl. 1991;63:341–345.
43. Duvia R., Radice G.P., Galdini R. Advances in the phytotherapy of prostatic hypertrophy. Med Praxis. 1983;4:143–148.
44. Chatelain C., Autet W., Brackman F. Comparison of once and twice daily dosage forms of Pygeum africanum extract in patients with benign prostatic hyperplasia: a randomized, double-blind study, with long-term open label extension. Urology. 1999;54:473–478.