Chapter 152 Benign Prostatic Hyperplasia
 General Considerations
General Considerations
Benign prostatic hyperplasia (BPH) is the fourth most common diagnosis in older men.1 More than 50% of men over age 50 years are affected and 90% of men will have an enlarged prostate by the time they are 80 years old. Progression to urinary retention may occur, with an accompanying risk of recurrent urinary tract infections, bladder calculi, and occasionally renal insufficiency. Management options for BPH include exercise, low-fat diet, supplementary medications, minimally invasive therapies, and prostate surgery.
The most common symptoms of BPH are lower urinary tract symptoms (LUTS), such as frequent urination, urgency to urinate, nocturia, weak urinary stream, incomplete bladder emptying, straining to void, and an intermittent stream. A patient may have multiple symptoms but be bothered primarily by one. Prostate cancer, bladder cancer, overactive bladder, urinary tract infections, prostatitis, urethral stricture, and bladder stones can also cause LUTS and must be ruled out before a diagnosis of BPH is made.2
Abdominal obesity and genetic factors appear to be the main risk factors for the development of BPH, which appears to run in families. If one or more first-degree relatives are affected, an individual is at greater risk of developing this disorder.1 Waist size, body mass index, and increases in body weight appear to increase the volume of the prostate gland.3
Hormones and Benign Prostatic Hyperplasia
The role of androgens in the development of prostate enlargement is evident, owing to the fact that BPH does not develop in men who have been castrated before puberty and therefore have greatly depleted levels of circulating androgens. Furthermore, in men with BPH, medical or surgical castration has been shown to lead to a reduction in prostate volume.4
For testosterone to have any effect on the prostate, it must be converted to dihydrotestosterone (DHT) by the enzyme 5-alpha reductase.5 DHT has twice as great an effect on the prostate as testosterone.6
Although testosterone levels decline with age, DHT concentrations remain similar to those in young men in the prostate even if serum levels are low.4 Circulating DHT, by virtue of its low serum plasma concentration and tight binding to plasma proteins, is of diminished importance as a circulating androgen affecting prostate growth.7
Most of the research on BPH and its pharmaceutical treatment has focused on modulating DHT by inhibiting the enzyme 5-alpha reductase. Other intriguing and accumulating research has illustrated the effects of estrogen or, more importantly the estrogen:testosterone ratio in aging men and it effects on BPH.
In animal models, estrogen has shown molecular changes in the prostate of the aging dog.8 The evidence is less clear in humans. Estrogens in the male are predominantly the products of peripheral aromatization of testicular and adrenal androgens.9 Although the testicular and adrenal production of androgens declines with aging, levels of total plasma estradiol do not decline. In fact, estradiol remains relatively constant or even increases with age in men. This increase has been attributed to increased aromatase activity in the aging male, who often also has an increased accumulation of body fat. Fat cells contain aromatase, an enzyme that converts certain androgens to estrogen. The resulting disproportion in the estrogen:testosterone ratio may increase up to 40% in some men.10
By limiting aromatase or inhibiting the binding of estrogen to prostate cells, it may be possible to reduce BPH or slow its progression. More research is needed.
 Diagnostic Considerations
Diagnostic Considerations
International Prostate Symptom Score
A useful subjective assessment tool for BPH patients is the International Prostate Symptom Score (IPSS). The IPSS is a modification of the American Urological Association (AUA) Symptom Index adding a single question assessing the quality of life or bother score based on the patient’s perception of the problem. The IPSS is a questionnaire that assesses the degree of LUTS and the quality of life of patients with BPH.
Patients can fill out the IPSS form before their examinations, but as little as possible interference from the health care provider and personnel is recommended (Figure 152-1). A score of 7 or less is considered mildly symptomatic, 8 to 19 points as moderate, and a score of 20 to 35 points as severely symptomatic. Both the AUA index and IPSS questionnaire—although not specific for BPH, prostate volume, urinary flow rate, postvoid residual volume, or bladder outlet obstruction—have been validated and are sensitive enough to be to be used in evaluating symptoms and selecting treatment.11
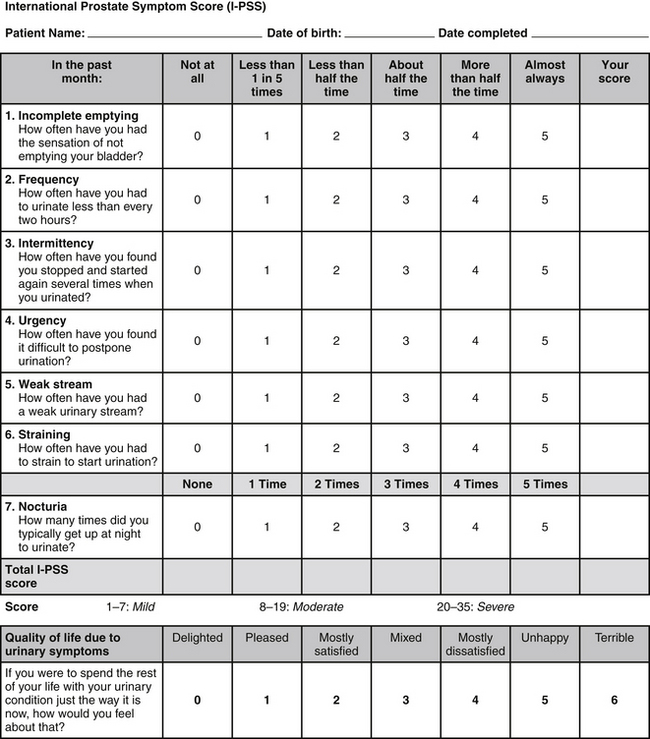
FIGURE 152-1 From Bope E, Kellerman R, Rkel, R. Conn’s current therapy 2011, Saunders 2011. International Prostate Symptom Score (IPSS) questionnaire, available from the urological Sciences Research Foundation at http://www.usrf.org/questionnaires/UA_SymptomScore.html. Accessed September 26, 2011. Used with permission.
A good physical examination should include a digital rectal examination (DRE) to determine the approximate consistency and shape of the patient’s prostate and abnormalities suggestive of prostate cancer, BPH, or prostatitis. The approximate size of the prostate can be determined with a DRE, however, inadvertent misestimations can be made even by physicians who are highly experienced. Prostate size can be determined most accurately by a transrectal ultrasound scan.
Urine and Blood Tests
Patients with suspected BPH should undergo dipstick urinalysis testing or microscopic urinalysis to screen for infection and hematuria, proteinuria, pyuria, glycosuria, and ketonuria. If a urine dipstick is positive, a urine culture may be necessary to determine whether the patient’s LUTS is independent of BPH.
Prostate-Specific Antigen
Because the symptoms of BPH and prostate cancer can be similar, measurement of prostate-specific antigen (PSA) is often used to differentiate BPH from prostate cancer. Recent studies show there are high levels of false-positive and false-negative PSA test results; therefore, the benefits and risks of such test should be discussed with the patient. PSA testing is one of the tools used to assess for prostate cancer and its severity, along with imaging, PSA density, staging, and Gleason score. This remains a controversial test, however, owing to its lack of specificity, which can lead to the overdiagnosis and overtreatment of indolent prostate cancers.12,13 One European study showed that it takes 1 of 48 men treated for prostate cancer to be saved from prostate cancer death over a 10-year period.12
PSA and BPH
PSA is a glycoprotein secreted by the glands of the prostate; it is released in the serum with disruption of normal prostatic tissue due to prostate cancer, inflammation, BPH, or trauma.
Finasteride (5-mg dose) and dutasteride (0.5-mg dose), both of which are 5-alpha reductase inhibitors used for the treatment of BPH and male-pattern baldness (1-mg dose of finasteride), will lower PSA levels by approximately 50% regardless of the dose.14 Ejaculation and DRE have been reported to increase PSA levels, but studies have shown the effects to be variable or insignificant.15,16 Prostate biopsy, however, will usually cause a substantial elevation of PSA levels. Therefore, after biopsy, PSA testing should be postponed for at least 3 to 6 weeks.17 Although an elevated level indicates prostate cancer in about 90% of cases, it must be kept in mind that midrange elevations in PSA can be caused by BPH and that in some instances there may be prostate cancer without an elevation of PSA levels. One approach to distinguish the two conditions when PSA is elevated is to perform a free-to-total PSA ratio: more free PSA than complexed PSA suggests BPH rather than prostate cancer. A ratio of around 20% or greater for free PSA is considered more likely to represent BPH than cancer.18
PSA density (PSAD) can be used to differentiate between prostate cancer and BPH in men with PSAs between 4 and 10 ng/mL and normal DRE results. PSAD should be higher in men with prostate cancer than in those with benign disease, because cancer causes a greater elevation in PSA per prostate volume in comparison with BPH. To determine PSAD, the patient’s PSA can be divided by prostate volume (as determined by a transrectal ultrasound examination). Studies show that a PSAD greater than 0.15 may mean a higher risk of prostate cancer. A lower PSAD would likely indicate the patient has BPH. This method should not conclusively determine whether a patient has BPH or prostate cancer, but it can be one piece of the puzzle.19
 Therapeutic Considerations
Therapeutic Considerations
Left untreated, BPH eventually obstructs the bladder outlet, leading to the retention of urine and eventual kidney damage. Because this situation is potentially life-threatening, proper surgical treatment is crucial. Surgery may also be indicated in patients who have failed medical therapy or have recurrent infections, hematuria, or renal insufficiency. In the past, medical treatment involved a procedure called transurethral resection of the prostate. Because this surgery is associated with the risk of considerable morbidity (sexual dysfunction, incontinence, and bleeding)20 and often makes matters worse, it should be avoided unless absolutely necessary. Surgical procedures that use thermal microwave or laser to reduce hyperplastic tissue are also available. Generally, these newer procedures are less expensive than transurethral resection of the prostate and have fewer complications, although subsequent therapies are often required.21
Lifestyle/Exercise
There is an inverse association between physical activity22 and BPH and a positive one between abdominal obesity and BPH.21
The ingestion of more calories may encourage abdominal obesity and sympathetic nervous system activity, both of which can increase the risk of BPH. Increased sympathetic activity, which is the “fight or flight” arm of the autonomic nervous system, may cause the prostate’s smooth muscle to contract, resulting in a worsening of lower urinary tract symptoms. However, a higher caloric intake does not seem to increase BPH risk when accompanied by increased physical activity.22
It is then possible that physical activity may serve a threefold purpose:
1. It may increase blood flow to the area, allowing the body to remove wastes efficiently.
2. It can decrease sympathetic stress responses, thus relaxing prostatic tissue.
3. It can reduce excess abdominal weight, which increases overall lower body pressure, thus relaxing the prostate/rectal region and improving blood flow into and out of the area.
Dietary and Nutritional Factors
Diet appears to play a critical role in the health of the prostate. Diets high in overall fat (not specific to the type of fat) are associated with an increase risk of BPH, as are diets high in red meat (whether conventional meats were used in comparison with the grass-fed kind in unknown.) Conversely, according to a recent large randomized trial, diets high in protein and vegetables are associated with a decreased risk.21
It is particularly important to avoid pesticides, increase fruit consumption,23 increase the intake of zinc and essential fatty acids, decrease coffee consumption,24 decrease butter consumption, avoid margarine,23 and keep cholesterol levels below 200 mg/dL. A moderate intake of alcohol can possibly benefit BPH but make LUTS worse.25
High-Protein Diet
Research evaluating protein intake and BPH is mixed. Some evidence shows that a high-protein diet (total calories: 44% protein, 35% carbohydrate, 21% fat) can inhibit 5-alpha reductase, whereas a low-protein diet (10% protein, 70% carbohydrate, and 20% fat) may stimulate the enzyme.26 On the other hand, an 8-year study of 3523 men with BPH found that total protein intake is positively associated with BPH, with the association being slightly stronger for animal protein intake than vegetable protein.21 A study of Chinese farmers also revealed a correlation between a higher intake of animal protein and the incidence of BPH (91.1% in those eating diets high in animal protein vs. 11.8% in those not eating animal protein).27
A possible theoretical reason why high protein intake may not be helpful relates to a greater osmolar load, which may influence urinary output and thus impose an undue extra burden on an already taxed system.6 Therefore, we recommend against ingesting excess animal protein as a means to increase total protein intake. Instead, high-quality, plant-derived protein and protein from coldwater fish in moderate amounts is probably a reasonable recommendation until we know more about the relationship between protein intake and BPH.
Zinc
Studies conducted in the 1970s showed that zinc supplementation reduced the size of the prostate and the symptomatology of BPH in the majority of patients.28 A more recent randomized trial comprising 4770 participants indicates a possible protective role of zinc against BPH. In that investigation, BPH was assessed over 7 years and defined in terms of medical or surgical treatment or repeated elevation (above 14) on the IPSS questionnaire. Diet, alcohol, and the use of supplements were assessed via a food frequency questionnaire. Higher zinc intake was associated with a significantly reduced risk for BPH.22 The mechanism probably involves zinc’s ability to inhibit 5-alpha reductase29 and/or by its ability to inhibit prolactin.30 Prolactin has been shown to increase the uptake of testosterone by the prostate, thereby leading to increased levels of DHT by providing additional substrate.31
Alcohol
Although only beer raises prolactin levels, higher alcohol intake may be associated with BPH. In a 17-year study of 6581 men in Hawaii, it was noted that an alcohol intake of at least 25 ounces per month was directly correlated with the diagnosis of BPH.32
The association was most significant for beer, wine, and sake and less so for distilled spirits. Most other recent studies confirm a protective effect of alcohol with regard to BPH but not LUTS.22
Amino Acids
The combination of glycine, alanine, and glutamic acid (in the form of two 6-grain capsules administered three times daily for 2 weeks and one capsule three times daily thereafter) has been shown in several studies to relieve many BPH symptoms. In a controlled study of 45 men, nocturia was relieved or reduced in 95%, urgency reduced in 81%, frequency reduced in 73%, and delayed micturition alleviated in 70%.33 These results have also been reported in other controlled studies.34 The mechanism of action is unknown but is likely related to the amino acids acting as inhibitory neurotransmitters and reducing the feeling of a full bladder. In other words, amino acid therapy is only palliative.
Cholesterol
The association with cholesterol, BPH, and LUTS has been mixed. High levels of serum lipids have been associated with cardiovascular disease and include elevated serum LDL cholesterol, decreased serum HDL cholesterol, and increased serum triglycerides. In the case of BPH, however, as described by Parsons, no positive association has been shown for cholesterol.35
Beta Sitosterol
Beta sitosterol is one of several plant sterols (cholesterol is the main animal sterol) found in almost all plants. High levels are found in rice bran, wheat germ, corn oil, and soybeans. Peanuts and their products—such as peanut oil, peanut butter, and peanut flour—as well as Serenoa repens, avocados, pumpkin seed, Pygeum africanum, and cashew fruit. The ability of beta sitosterol and other phytosterols to lower cholesterol has been well documented.36 Beta sitosterol has also been shown to improve BPH. One double-blind study comprised 200 men receiving beta sitosterol (20 mg) or placebo three times daily.37 The beta sitosterol group experienced an increase in maximum urinary flow rate from a baseline of 9.9 to 15.2 mL/s and a decrease in mean residual urinary volume of 30.4 from 65.8 mL.
In yet another study, 177 patients with benign prostate enlargement were randomized.38 Patients received 130 mg of beta sitosterol each day and were monitored for over 6 months. Measurements of the IPSS, urinary flow, and residual urine in the bladder after voiding were recorded.
On average, urinary flow values increased by 4.5 mL/s whereas residual urinary volumes decreased by a substantial 33.5 mL. The IPSS showed a statistically significant improvement.38
Vitamin D
Krystal and colleagues showed that vitamin D supplementation was associated with a reduced risk of BPH, but the dosage was imprecise. The association in this 4770-subject trial was observed only among men who used both multivitamins and single vitamin D supplements. There were no associations of supplement use with BPH risk with the exception of a trend toward decreased BPH risk with increased intake of supplemental vitamin D.22 Although this study lacked data on the frequency, dose, and duration of vitamin D use, the results are intriguing enough to support further research addressing whether high-dose supplementation will have any benefit for BPH. Numerous studies have shown that vitamin D3 may have protective effects against prostate cancer.39
The mechanism of vitamin D’s favorable effect on BPH is by attaching this molecule to vitamin D receptors on the prostate and bladder and inhibiting prostate growth, lowering excessive contractility, and reducing inflammation.40
Botanical Medicines
Botanical medicines in the treatment of BPH has been a popular recommendation in Europe for decades.43 The chance of clinical success with any of the botanical treatments of BPH appears to be determined by the degree of obstruction, as indicated by the volume of residual urine. For levels less than 50 mL, the results are usually excellent. For levels between 50 and 100 mL, the results are usually quite good. With residual urine levels between 100 and 150 mL, it will be tougher to produce a significant improvement within the customary 4- to 6-week period. If the volume of residual urine is greater than 150 mL, saw palmetto extract and other botanical medicines alone are unlikely to produce any significant improvement.
Serenoa repens (Saw Palmetto)
The liposterolic extract of the fruit of this palm tree (also known as Sabal serrulata), native to Florida, has been shown to significantly improve the signs and symptoms of BPH in numerous clinical studies (see Chapter 122). The mechanism of action is related to inhibition of DHT binding to both the cytosolic and nuclear androgen receptors, inhibition of 5-alpha reductase, and interference with intraprostatic estrogen receptors. Owing to these effects, the results of most randomized trials have been excellent. Systematic literature reviews and meta-analyses of clinical trials report excellent results using Serenoa, comparing quite favorably with those of finasteride (Proscar) and tamsulosin (Flomax) in terms of efficacy but with a better side-effect profile. It appears that the effect of Serenoa extract is most obvious in the early stages of BPH (i.e., in cases of mild to moderate hypertrophy), because the clinical research has clearly shown that roughly 90% of men with mild to moderate BPH experience improvement in symptoms during the first 4 to 6 weeks after starting Serenoa extract (320 mg per day of the liposterolic extract). However, it is likely that Serenoa extract may show little if any clinical benefit in more advanced cases of BPH. Chapter 122 offers a complete discussion of the pharmacology of Serenoa.
Furthermore, although Serenoa is often very effective on its own, better results may be achieved by combining Urtica dioca root extract (discussed below) with Serenoa extract. One double-blind study involving 431 patients found this combination to produce clinical benefit equal to that of finasteride.44 In a more recent long-term study, the efficacy and tolerability of this combination was investigated in elderly male patients suffering from lower urinary tract symptoms (LUTS) caused by BPH.45 A total of 257 patients were randomized to treatment with the combination (320 mg of Serenoa and 240 mg of Urtica extracts per day) or placebo. A 24-week double-blind treatment was followed by an open control period of 24 weeks during which all patients were given the Serenoa and Urtica combination. Patients treated with the botanical combination exhibited a substantially greater reduction in their total IPSS scores after 24 weeks of double-blind treatment than did patients of the placebo group. This applied to obstructive as well as to irritative symptoms and to patients with moderate symptoms as well as those with severe symptoms at baseline. Patients randomized to placebo showed a marked improvement in LUTS (as measured by the IPSS) after being switched to the botanical combination. Like the extract of Serenoa, Urtica extract appears to interact with the binding of DHT to cytosolic and nuclear receptors.46 In vitro studies also show that lignans found in Urtica may modulate hormonal effects owing to their affinity for sex hormone–binding globulin.45
Cernilton
Cernilton, an extract of rye-grass flower pollen, has been used to treat prostatitis and BPH in Europe for more than 35 years.47 It has been shown to be quite effective in several double-blind clinical studies in the treatment of BPH,48,49 where the overall success rate is about 70%.50 Patients who respond typically experience less nocturia and diurnal frequency (a reduction of about 70%) as well as significant reductions in residual urine volume.44 The extract has been shown to exert some antiinflammatory action and to produce a contractile effect on the bladder while simultaneously relaxing the urethra. In addition, Cernilton contains a substance that inhibits the growth of prostate cells.46
In one study, the clinical efficacy of Cernilton in the treatment of symptomatic BPH was examined over a 1-year period.48 Seventy-nine men averaging 68 years of age (range 62 to 89) with a mean baseline prostatic volume of 33.2 cm2 were given 63 mg Cernilton pollen extract twice daily for 12 weeks. As a result, their average urinary maximum flow rate increased from 5.1 to 6 mL/s. Average flow rate increased from 9.3 to 11 mL/s. Residual urine volume decreased from 54.2 mL to less than 30 mL. Clinical efficacy, based on symptoms, was as follows:
• Urgency or discomfort: improved by 76.9%
• Incomplete emptying: improved by 66.2%
• Prolonged voiding: improved by 64.1%
• Delayed voiding: improved by 62.2%
Overall, 85% of the test subjects experienced benefit: 11% reporting “excellent,” 39% “good,” 35% “satisfactory,” and 15% “poor” results.
A summary review of two placebo-controlled studies, two comparative trials (both lasting 12 to 24 weeks), and three double-blind studies of 444 men showed that, although Cernilton did not improve urinary flow rates, residual volume, or prostate size compared with placebo or the comparative study agents, it did improve self-rated urinary symptom scores and reduced nocturia compared with placebo and an amino acid mixture.46 Clearly, more long-term studies of Cernilton need to be conducted in order to elucidate the terms of its usefulness as an alternative or adjunct to Serenoa.
Last, a study by Preuss and associates illustrates the potential value of a combination of nutrients used for BPH. In this randomized trial, 144 subjects were randomized to cernitin, saw palmetto, B sitosterol, vitamin E, or placebo. After 3 months, the combination group had significantly less nocturia, frequency, and overall BPH symptoms without any adverse side effects.51
Pygeum africanum
The bark of P. africanum, an evergreen tree native to Africa, has historically been used in the treatment of urinary tract disorders. The major active components of the bark are fat-soluble sterols and fatty acids. Virtually all of the research on Pygeum has featured a Pygeum extract standardized to contain 14% triterpenes, including beta-sitosterol and 0.5% n-docosanol. This extract has been extensively studied in both experimental animals and humans in clinical trials (see Chapter 118). A study on rat prostatic cells suggests that the therapeutic effect of Pygeum may be due in part to the inhibition of growth factors EGF, bFGF, and IGF-I, which are responsible for prostatic overgrowth.52
Numerous clinical trials with more than 600 patients have shown Pygeum extract to be effective in reducing the symptoms and clinical signs of BPH, especially in early cases.53 However, in a double-blind study that compared Pygeum extract with extract of saw palmetto, the latter produced a greater reduction of symptoms and was better tolerated.54 In addition, the effects on objective parameters, especially urine flow rate and residual urine content, were better in the clinical studies with saw palmetto. However, there may be circumstances in which Pygeum is more effective than saw palmetto. For example, saw palmetto has not been shown to produce some of Pygeum’s effects on prostate secretion. Of course the two extracts have somewhat overlapping mechanisms of action and can be used in combination.
Urtica dioica (Stinging Nettle)
Extracts of the root of U. dioica have also been shown to be effective in the treatment of BPH. Three double-blind studies have shown it to be more effective than placebo.55,56 The most recent study, conducted in Iran, was a 6-month double-blind placebo-controlled randomized partial crossover comparative trial of Urtica with placebo in 620 patients.57 Both groups continued the medication up to 18 months and only those who continued the therapy, had favorable results. No side effects were identified in either group.
 Therapeutic Approach
Therapeutic Approach
Therapeutic goals for BPH are aimed at doing the following:
• Normalize prostate nutrient levels
• Restore steroid hormones to normal levels
• Inhibit excessive conversion of testosterone to DHT
• Inhibit DHT-receptor binding
• Eliminate excess estradiol production
• Limit promoters of the hyperplastic process (e.g., prolactin)
Severe BPH resulting in significant acute urinary retention may require catheterization for relief; a sufficiently advanced case may not respond rapidly enough to therapy and may require the short-term use of an alpha1 antagonist by itself or combined with a 5-alpha reductase medication or surgical intervention.
Lifestyle
Patients should exercise more, between 4 to 6 times a week, and properly stretch the prostate/urogenital area to increase blood flow.
Diet
Initially, the diet should be higher in vegetable protein, low in carbohydrate, low in animal fats, and high in unsaturated oils. After the patient responds, a less strict whole-foods, balanced approach can be used. The patient should limit alcohol and coffee intake, avoid drug-, pesticide- and hormone-contaminated foods, and limit cholesterol-rich foods. Soy foods should be used regularly.
Botanicals
• Liposterolic extract of saw palmetto (S. repens) (standardized at 85% to 95% fatty acids and sterols): 160 mg two times a day
• Flower pollen extract (e.g., Cernilton): 63 mg two to three times a day
• Pygeum africanum extract (14% triterpene content): 50 to 100 mg/day
1. Bushman W. Etiology, epidemiology, and natural history of benign prostatic hyperplasia. Urol Clin North Am. 2009 Nov;36(4):403–415.
2. Sanda M.G., Beaty T.H., Strutzman R.E., et al. Genetic susceptibility of benign prostatic hyperplasia. J Urol. 1994;152:115–119.
3. Dahle S.E., Chokkalingam A.P., Gao Y.T., et al. Body size and serum levels of insulin and leptin in relation to the risk of benign prostatic hyperplasia. J Urol. 2002;168:599–604.
4. Bartsch G., Rittmaster R.S., Klocker H. Dihydrotestosterone and the concept of 5alpha-reductase inhibition in human benign prostatic hyperplasia. World J Urol. 2002;19:413–425.
5. Imperato-McGinley J., Zhu Y.S. Androgens and male physiology the syndrome of 5alpha-reductase-2 deficiency. Mol Cell Endocrinol. 2002;198:51–59.
6. Wright A.S., Thomas L.N., Douglas R.C., et al. Relative potency of testosterone and dihydrotestosterone in preventing atrophy and apoptosis in the prostate of the castrated rat. J Clin Invest. 1996;98:2558–2563.
7. Partin A.W., Rodriguez R. The molecular biology, endocrinology, and physiology of the prostate and seminal vesicles. In: Walsh P.C., ed. Campbell’s urology. Philadelphia: Saunders; 2002:1237–1296.
8. Barrack E.R., Berry S.J. DNA synthesis in the canine prostate; effects of androgen induction and estrogen treatment. Prostate. 1987;10:45–56.
9. Gooren L.J., Toorians A.W. Significance of oestrogens in male (patho)physiology. Ann Endocrinol (Paris). 2003;64:126–135.
10. Prezioso D., Denis L.J., Klocker H., et al. Estrogens and aspects of prostate disease. Int J Urol. 2007 Jan;14(1):1–16.
11. Barry M.J. Evaluation of symptoms and quality of life in men with benign prostatic hyperplasia. Urology. 2001;58:25–32.
12. Schroder F.H., Hugosson J., Roobol M.J., et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:11320–11328.
13. Woolf S.H. Screening for prostate cancer with prostate-specific antigen: an examination of the evidence. N Engl J Med. 1995;333:1401–1405.
14. Andriole G.L., Guess H.A., Epstein J.I., et al. Treatment with finasteride preserves usefulness of prostate-specific antigen in the detection or prostate cancer: results of a randomized, double-blind, placebo-controlled clinical trial: PLESS Study Group ProscarLong-Term Efficacy and Safety Study. Urology. 1998;52:195–201.
15. Zisman A., Soffer Y., Siegel Y.I., et al. Postejaculation serum prostate-specific antigen level. Eur Urol. 1997;32:54–57.
16. The Internal Medicine Clinic Research Consortium. Effect of digital rectal examination on serum prostate-specific antigen in a primary care setting. Arch Intern Med. 1995;155:389–392.
17. Oesterling J.E., Rice D.C., Glenski W.J., et al. Effect of cystoscopy, prostate biopsy, and transurethral resection of prostate on serum prostate-specific antigen concentration. Urol. 1993;42:276–282.
18. Prestigiacomo A.F., Stamey T.A. Clinical usefulness of free and complexed psa. Scand J Clin Lab Invest Suppl. 1995;221:32–34.
19. Allan R.W., Sanderson H. Epstein JI. Correlation of minute (0.5 MM or less) focus of prostate adenocarcinoma on needle biopsy with radical prostatectomy specimen: role of prostate specific antigen density. J Urol. 2003 Aug;170(2 Pt 1):370–372.
20. Dull P., Reagan R.W., Jr., Bahnson R.R. Managing benign prostatic hyperplasia. Am Fam Physician. 2002;66:77–84.
21. Suzuki S., Platz E.A., Kawachi I., et al. Intakes of energy and macronutrients and the risk of benign prostatic hyperplasia. Am J Clin Nutr. 2002;75:689–697.
22. Kristal A.R., Arnold K.B., Schenk J.M., et al. Dietary patterns, supplement use, and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. Am J Epidemiol. 2008 Apr 15;167(8):925–934.
23. Lagiou P., Wuu J., Trichopoulou A., et al. Diet and benign prostatic hyperplasia: a study in Greece. Urology. 1999;54:284–290.
24. Gass R. Benign prostatic hyperplasia: the opposite effects of alcohol and coffee intake. BJU Int. 2002;90:649–654.
25. Parsons J.K., Kashefi C. Physical activity, benign prostatic hyperplasia, and lower urinary tract symptoms. Eur Urol. 2008;53:1228–1235.
26. Kappas A., Anderson K.E., Conney A.H., et al. Nutrition-endocrine interactions. Induction of reciprocal changes in the delta-5-alpha- reduction of testosterone and the cytochrome P-450-dependent oxidation of estradiol by dietary macronutrients in man. Proc Natl Acad Sci U S A. 1983;80:7646–7649.
27. Zhang S.X., Yu B., Guo S.L., et al. Comparison of incidence of BPH and related factors between urban and rural inhabitants in district of Wannan. Zhonghua Nan Ke Xue. 2003;9:45–47.
28. Fahim M., Fahim Z., Der R., et al. Zinc treatment for the reduction of hyperplasia of the prostate. Fed Proc. 1976;35:361.
29. Om A.S., Chung K.W. Dietary zinc deficiency alters 5 alpha-reduction and aromatization of testosterone and androgen and estrogen receptors in rat liver. J Nutr. 1996 Apr;126(4):842–848.
30. Login I.S., Thorner M.O., MacLeod R.M. Zinc may have a physiological role in regulating pituitary prolactin secretion. Neuroendocrinology. 1983;37:317–320.
31. Farnsworth W.E., Slaunwhite W.R., Sharma M., et al. Interaction of prolactin and testosterone in the human prostate. Urol Res. 1981;9:79–88.
32. Chyou P.H., Nomura A.M., Stemmermann G.N., et al. A prospective study of alcohol, diet, and other lifestyle factors in relation to obstructive uropathy. Prostate. 1993;22:253–264.
33. Damrau F. Benign prostatic hypertrophy: amino acid therapy for symptomatic relief. J Am Geriatr Soc. 1962;10:426–430.
34. Feinblatt H.M., Gant J.C. Palliative treatment of benign prostatic hypertrophy: value of glycine-alanine-glutamic acid combination. J Maine Med Assoc. 1958;49:99–101.
35. Parsons J.K. Lifestyle factors, benign prostatic hyperplasia, and lower urinary tract symptoms. Curr Opin Urol. 2011 Jan;21(1):1–4.
36. Tilvis R.S., Miettinen T.A. Serum plant sterols and their relation to cholesterol absorption. Am J Clin Nutr. 1986;43:92–97.
37. Berges R.R., Windeler J., Tramisch H.J., et al. Randomised, placebo-controlled, double-blind clinical trial of beta-sitosterol in patients with benign prostatic hyperplasia. Beta-sitosterol Study Group. Lancet. 1995;345:1529–1532.
38. Klippel K.F., Hiltl D.M., Schipp B. A multicentric, placebo-controlled, double-blind clinical trial of beta-sitosterol (phytosterol) for the treatment of benign prostatic hyperplasia. German BPH-Phyto Study group. Br J Urol. 1997 Sep;80(3):427–432.
39. Li H., Stampfer M.J., Hollis J.B., et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med, 2007 Mar;4(3):e103. http://doi.dx.org/10.1371/journal.pmed.0040103.
40. Adorini L., Penna G., Fibbi B., et al. Vitamin D receptor agonists target static, dynamic, and inflammatory components of benign prostatic hyperplasia. Ann N Y Acad Sci. 2010 Apr;1193:146–152. Review
41. Lahtonen R. Zinc and cadmium concentrations in whole tissue and in separated epithelium and stroma from human benign prostatic hypertrophic glands. Prostate. 1985;6:177–183.
42. Sinquin G., Morfin R.F., Charles J.F., et al. Testosterone metabolism by homogenates of human prostates with benign hyperplasia: effects of zinc, cadmium, and other bivalent cations. J Steroid Biochem. 1984;20:733–780.
43. Buck A.C. Phytotherapy for the prostate. Br J Urol. 1996;78:325–336.
44. Habib F.K., Ross M., Lawenstein A. Identification of a prostate inhibitory substance in a pollen extract. Prostate. 1995;26:133–139.
45. Lopatkin N., Sivkov A., Schläfke S., et al. Efficacy and safety of a combination of Sabal and Urtica extract in lower urinary tract symptoms: long-term follow-up of a placebo-controlled, double-blind, multicenter trial. Int Urol Nephrol. 2007;39(4):1137–1146.
46. MacDonald R., Ishani A., Rutks I., et al. A systematic review of Cernilton for the treatment of benign prostatic hyperplasia. BJU Int. 2000;85:836–841.
47. Wilt T., Ishani A., Mac Donald R. Serenoa repens for benign prostatic hyperplasia. Cochrane Database Syst Rev. 3, 2002. CD001423
48. Yasumoto R., Kawanishi H., Tsujino, et al. Clinical evaluation of long-term treatment using Cernitin pollen extract in patients with benign prostatic hyperplasia. Clin Ther. 1995;17:82–86.
49. Buck A.C., Cox R., Rees R.W., et al. Treatment of outflow tract obstruction due to benign prostatic hyperplasia with the pollen extract, Cernilton: a double-blind, placebo-controlled study. Br J Urol. 1990;66:398–404.
50. Dutkiewicz S. Usefulness of Cernilton in the treatment of benign prostatic hyperplasia. Int Urol Nephrol. 1996;28:49–53.
51. Preuss H.G., Marcusen C., Regan J., et al. Randomized trial of a combination of natural products (cernitin, saw palmetto, B-sitosterol, vitamin E) on symptoms of benign prostatic hyperplasia (BPH). Int Urol Nephrol. 2001;33(2):217–225.
52. Yablonsky F., Nicolas V., Riffaud J.P., et al. Antiproliferative effect of Pygeum africanum extract on rat prostatic fibroblasts. J Urol. 1997;157:2381–2387.
53. Duvia R., Radice G.P., Galdini R. Advances in the phytotherapy of prostatic hypertrophy. Med Praxis. 1983;4:143–148.
54. Belaiche P., Lievoux O. Clinical studies on the palliative treatment of prostatic adenoma with extract of Urtica root. Phytother Res. 1991;5:267–269.
55. Romics I. Observations with Bazoton in the management of prostatic hyperplasia. Int Urol Nephrol. 1987;19:293–297.
56. Sokeland J. Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. BJU Int. 2000;86:439–442.
57. Safarinejad M.R. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5(4):1–11.
Bent S., Kane C., Shinohara K., et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med. 2006 Feb 9;354(6):557–566.
Gerber G.S. Phytotherapy for benign prostatic hyperplasia. Curr Urol Rep. 2002 Aug;3(4):285–291.
Hartmann R.W., Mark M., Soldati F. Inhibition of 5-alpha-reductase and aromatase by PHL-0081, a combination of PY102 (Pygeum africanum) and UR102 (Urtica dioica) extracts. Phytomedicine. 1996;3(2):121–128.
Lopatkin N., Sivkov A., Walther C., et al. Long-term efficacy and safety of a combination of sabal and urtica extract for lower urinary tract symptoms: a placebo-controlled, double-blind, multicenter trial. World J Urol. 2005 Jun;23(2):139–146.
Maar K. Regression of the symptoms of prostatic adenomas: results of 6 months’ conservative treatment using ERU capsules. Fortschr Med. 1987 Jan 10;105(1):18–20.
Wilt T.J., Ishani A., Stark G., et al. Saw palmetto extracts for treatment of benign prostatic hyperplasia: a systematic review. JAMA. 1998;280:1604–1609.
Yalla S.V., Sullivan M.P., Lecamwasam H.S., et al. Correlation of American urological association symptom index with obstructive and nonobstructive prostatism. J Urol. 1995;153:674–679.