Gender Differences in Pain and Its Relief
Introduction
“Sex matters. Sex, that is, being male or female, is an important basic human variable that should be considered when designing and analyzing studies in all areas and at all levels of biomedical and health-related research … [Furthermore,] the study of sex differences is evolving into a mature science. There is now sufficient knowledge … to allow the generation of hypotheses. The next step is to move from the descriptive to the experimental …” (Wizemann and Pardue 2001). So concluded a committee of the Institute of Medicine of the U.S. National Academy of Sciences in 2000 that had been charged to report on the topic understanding the biology of sex and gender differences. The import of this pronouncement is as compelling now as it was more than a decade ago.
Prior to the mid-1990s, there was only occasional and sporadic interest in the question of whether there are important sex differences related to pain. Several epidemiological studies indicated that some pain conditions were more prevalent in one sex than in the other. In addition, a few studies of experimental pain sensitivity reported greater pain sensitivity in women than in men. However, this topic was not a major one for pain research. This situation began to change after the appearance of several seminal reviews on the topic of sex and gender differences in pain (Fillingim and Maixner 1995, Unruh 1996, Berkley 1997, Riley et al 1998).
Since then, this topic has grown into a field of its own, as indicated by the tremendous growth in publications and activity in this area. This includes a consensus report (Greenspan et al 2007), and two special issue journals devoted to the topic of sex, gender, and pain (Berkley et al 2006, Collett and Berkley 2007). Here, we review what sex and gender differences have been reported in the scientific literature and the mechanisms that are thought to underlie them, as derived from both human and animal studies.
What are the Sex and Gender Differences in Pain?
Epidemiology and Sex Prevalence of Painful Diseases
Clinical and epidemiological studies have shown that many more painful diseases demonstrate a higher female prevalence than a male prevalence (Box 15-1), particularly for pain conditions involving the head and neck, of musculoskeletal or visceral origin, and of autoimmune cause. Furthermore, considering pain of unspecified or uncertain origin, epidemiological studies consistently reveal that women report more severe levels of pain, more frequent pain, pain in more areas of the body, and pain of longer duration than that reported by men (Unruh 1996, Berkley 1997, Dao and LeResche 2000, Isacson and Bingefors 2002).
Some of the higher female prevalence can be accounted for by female-specific problems that occur during a woman’s reproductive years and involve sex-specific organs, as opposed to fewer male-specific disorders. In many other cases, however, the differences are not as straightforward as they might seem. First, the overall prevalence patterns in both sexes for many types of pain (such as those of temporomandibular disorder [TMD], fibromyalgia, migraine, and gastrointestinal, abdominal, back, and joint pain) change across the life span. In some of these cases, the sex prevalence ratio changes with age, sometimes even reversing (LeResche 2000). Other influences that might bias sex difference estimates are differences in the symptoms and signs of some disorders, such as irritable bowel syndrome (IBS), acute appendicitis, migraine, rheumatoid arthritis, and coronary artery disease (Box 15-2; see also Berkley 1997, Weyand et al 1998, Weitzel et al 2001, Chang et al 2006). However, several pain conditions typically do not demonstrate sex differences in pain. One example is cancer pain, for which several studies report no sex differences (Turk and Okifuji 1999, Miaskowski 2004, van den Beuken-van Everdingen et al 2007), whereas fewer reports describe small and inconsistent sex differences in some aspect of cancer pain (Reyes-Gibby et al 2006, Valeberg et al 2008).
Other factors that contribute to higher female prevalence in several chronic pain conditions include gender-related psychological and sociocultural issues. It is recognized that women are more willing to report pain than men are and are more willing to seek health care (Isacson and Bingefors 2002). Thus, the apparent female sex prevalence of some conditions is consistently greater in clinical populations (i.e., among patients seeking health care) than in community ones. For example, the IBS female-to-male prevalence ratio in Western cultures is reduced from as high as 4:1 in patient populations to 2:1 or 1:1 in community-based samples (Hungin et al 2003). A similar reduction from 9:1 to 2:1 has been reported for TMD (LeResche 2000). Cultural differences are also relevant, though less studied. For example, IBS has a greater male than female prevalence in some Asian populations (Chial and Camilleri 2002, Chang et al 2006).
Sex and Gender Differences in Experimental Pain Sensitivity
Well over 100 papers have been published that report on sex or gender differences in experimental pain sensitivity. The majority of studies have reported that women have greater pain sensitivity than men do. However, a sizable number of experimental studies report no significant sex differences. It is not clear why studies differ in their findings in this regard. It has been suggested that sex differences are more likely to be found with certain types of stimuli or certain types of protocols. Two recent reviews suggest some ideas why such differences are normally, but not always, found (Table 15-1; Fillingim et al 2009, Racine et al 2012a). Thermal, pressure, electrical, and algesic chemical stimuli tend to reveal greater pain sensitivity in women. In contrast, studies using ischemic and exercise-induced myalgic stimuli largely fail to show sex differences in pain sensitivity. The few studies that have evaluated sex differences in the temporal summation of pain have reported greater female sensitivity more often than not. Pain thresholds and ratings reveal greater female sensitivity in about half the studies, with less pain tolerance found in approximately 75% of the studies. Thus, these factors of stimulus modality and perceptual level seem to have some relevance to the expression of sex differences in pain but do not fully explain the phenomenon.
Table 15-1
Summary of Studies Evaluating Sex Differences in Experimental Pain Sensitivity
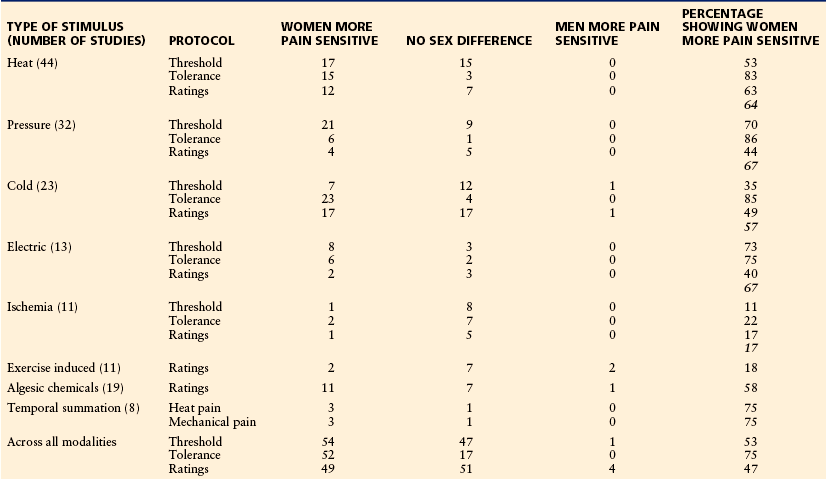
From Holdcroft A, Berkley KJ: Sex and gender differences in pain and its relief. In McMahon S, Koltzenburg M, editors: Wall and Melzack’s textbook of pain, 5th ed, Amsterdam, 2005, Elsevier, pp 1181–1198. Based on reviews by Fillingim RB, King CD, Ribeiro-Dasilva MC, et al 2009 Sex, gender, and pain: a review of recent clinical and experimental findings. Journal of Pain 10:447–485; and Racine M, Tousignant-Laflamme Y, Kloda LA, et al 2012a A systematic literature review of 10 years of research on sex/gender and experimental pain perception—Part 1: are there really differences between women and men? Pain 153:602–618.
An important point is that the differences between men and women in any of these pain measures is relatively small in comparison to the full range of variability found in the population. An apt analogy is the sex difference in height. We all recognize that on average, men are taller than women. At the same time we recognize that the range in heights within each sex provides a large amount of overlap in the populations. A graphic representation of thresholds, as one example, demonstrates this perspective (Fig. 15-1). Even though the mean (and median) pressure pain thresholds is higher in men than in women, the distributions are largely overlapping. One can easily see from such distributions that a particular sampling may or may not actually result in a statistically significant difference.
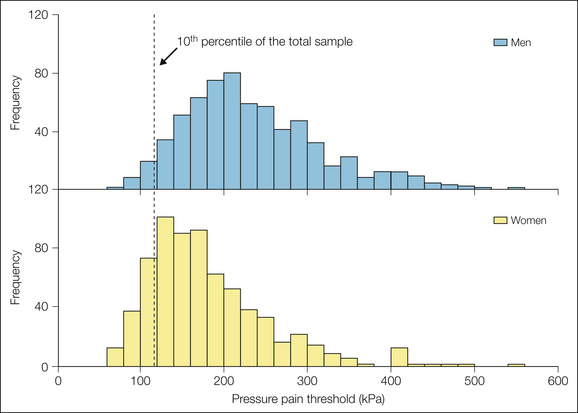
Figure 15-1 Distribution of pressure pain thresholds in a healthy cohort of 697 men and 697 women tested on the masseter muscle.
The vertical line denotes the 10th percentile of the total sample. Among that most sensitive 10%, 83% are women. Data were derived from the OPPERA study (Greenspan JD, Slade GD, Bair E, et al 2011 Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. Journal of Pain 12[11 suppl]:T61–T64).
Related to this issue is that of sample size. Given the distribution of pressure pain thresholds derived from the OPPERA study (Greenspan et al 2011; depicted in Fig. 15-1), a power analysis indicated that a sample size of 49 per group is needed to identify a sex difference at α = 0.05 and a power of 0.80. As elaborated in previous reviews, most studies of sex differences in experimental pain used smaller sample sizes (Fillingim et al 2009, Racine et al 2012a).
Given this modest difference between the sexes, it is reasonable to ask whether this represents a biologically or clinically meaningful difference. One perspective is as follows. The lower end of this distribution represents the most pain-sensitive segments of the population. Considering the lowest 10% of the population’s distribution (Fig. 15-1), we find 4.8 times more women than men. Arguably, this segment of the population is the most susceptible to clinical pain problems by virtue of finding noxious and near-noxious events more painful than the rest of the population does. Consequently, it may be that the tail of this distribution is most relevant to the greater female prevalence of many clinical pain problems despite the large overlap in the populations as a whole.
Sex Differences in Nociception Derived from Animal Studies
Many factors contribute to the expression of sex differences in nociceptive behavior in animals. Biological variables such as genetics or gonadal hormone differences and experimental variables (e.g., type of test and tissue, flexion reflex or complex behavior, hormonal manipulation) all appear to play a role in the expression of sex differences, but not always in a consistent pattern. Consider the type of stimulus and measured end point. Mechanical or thermal cutaneous stimuli evoke a reflex withdrawal or coordinated movement when the stimulus reaches the nociceptive threshold. Radiant heat, a hot plate, or mechanical probing of the hindpaw or tail in normal animals (i.e., in the absence of injury) more often than not reveals sex differences, but the direction of effect varies depending on the strain and species (Mogil et al 2000, Terner et al 2003, Cook and Nickerson 2005, LaCroix-Fralish et al 2005a, Ribeiro et al 2005, Banik et al 2006, Wang et al 2006). In contrast, following inflammation or nerve injury, hyperalgesia in general is more robust (magnitude and/or duration) in females than in males (complete Freund’s adjuvant: Cook and Nickerson 2005, Wang et al 2006; formalin: Kim et al 1999, Gaumond et al 2002, but see Pajot et al 2003; capsaicin: Lu et al 2009b; nerve injury: LaCroix-Fralish et al 2005b, Dominguez et al 2009; temporomandibular joint [TMJ] and muscles of mastication: Cairns et al 2002, Bereiter et al 2005b, Gazerani et al 2010).
In contrast, in studies of visceral tissues, in the absence of injury females are more sensitive to noxious stimulation of common viscera (colon: Holdcroft et al 2000, Ji et al 2006; bladder: Ness et al 2001; ureter: Affaitati et al 2011, Aloisi et al 2010). One explanation for the predominantly greater sensitivity of females to visceral stimuli than to somatic stimuli may be the type of stimulation. Organ distention is typically longer in duration (tens of seconds), and test stimuli are usually above the noxious threshold and inescapable. The quantitative metric is not a withdrawal reflex, but rather a pseudo-affective response (e.g., visceromotor response, pressor response) or writhing behavior. The prolonged noxious visceral input could evoke central sensitization similar to mild somatic inflammatory stimuli with greater female sensitivity. Indeed, NMDA receptor signaling contributes to spinal processing of acute visceral, but not somatic, stimuli (Ji and Traub 2001, Traub et al 2002).
Mechanisms Underlying Sex Differences in Pain
To a large extent, mechanistic studies related to sex differences in human pain have involved investigating the role of gonadal hormones. A meta-analysis of seven studies evaluating effects of the menstrual cycle on experimental pain identified some trends (Riley et al 1999). For most stimulus modalities, thresholds were higher during the follicular phase than during later phases of the cycle. Electrical stimulation–evoked pain thresholds, in contrast, were higher during the luteal phase. A subsequent review found that the literature had become more conflicting and concluded that there was little evidence for an effect of the menstrual cycle phase on experimental pain, with the possible exception of electrically evoked pain (Sherman and LeResche 2006). Since publication of these reviews, several other studies have addressed this topic. Many of them failed to find a significant difference in experimental pain measures across the menstrual cycle with the use of pressure (Sherman et al 2005, Vignolo et al 2008), heat (Granot et al 2001, de Leeuw et al 2006, Soderberg et al 2006, Klatzkin et al 2010, Teepker et al 2010), cold (Kowalczyk et al 2006, Klatzkin et al 2010), and ischemic stimuli (Straneva et al 2002, Sherman et al 2005, Klatzkin et al 2010). A smaller number of studies reported significant effects of the menstrual cycle on experimental pain, although the pattern of effects was quite different across studies (Hellstrom and Lundberg 2000, Bajaj et al 2002, Isselée et al 2002, Tassorelli et al 2002, Gazerani et al 2005, Stening et al 2007, Teepker et al 2010). Indeed, it is still not possible to make a general statement on the pattern of menstrual cycle effects that would apply to a majority of these and earlier studies. Even within a given study, significant cycle effects could be found for one measure but not another.
Only a small number of the aforementioned studies measured hormone levels at the time of testing. Of these, two reported no relationship between either estrogens or progesterone and any of their pain measures (Kowalczyk et al 2006, Soderberg et al 2006), some relationships for some measures but not others (Fillingim et al 1997, Teepker et al 2010), or a complex relationship between both estrogens and progesterone on pain measures that varied according to the measure (Stening et al 2007). Taken together, this literature provides little evidence of consistent gonadal hormonal influence on experimental pain sensitivity in the context of normal menstrual fluctuations in healthy women.
Fewer studies have addressed the topic of menstrual cycle and gonadal hormone effects on clinical pain. The severity of symptoms for headache (Arjona et al 2007, Keenan and Lindamer 1992), TMD (LeResche et al 2003), IBS (Heitkemper and Jarrett 1992, Kane et al 1998, Heitkemper et al 2003), and fibromyalgia (Pamuk and Çakir 2005) has been reported to vary significantly across the menstrual cycle. A common finding across these studies is greater pain during the perimenstrual phase. However, findings of no effects of the menstrual cycle on fibromyalgia pain (Alonso et al 2004, Okifuji and Turk 2006) and IBS (Lee et al 2007) have also been reported. Studies of peri- and postmenopausal women have produced variable results with respect to the effects of hormone replacement therapy (HRT). Several studies indicate that HRT is associated with increased TMD pain (LeResche et al 1997) and back pain (Symmons et al 1991, Brynhildsen et al 1998, Musgrave et al 2001). However, discontinuation of HRT has also been associated with increased musculoskeletal pain (Ockene et al 2005) and increased migraine frequency (Somerville 1972, Lichten et al 1996), but a large epidemiological study failed to find a relationship between HRT and musculoskeletal pain (Macfarlane et al 2002). Potentially rapid and large changes in hormone levels—either increases or decreases—could be the key factor by which estrogens and/or progesterone influences clinical pain.
Animal Studies
The mechanisms underlying sex differences in animal pain are complex and involve multiple factors. In contrast to the more limited human research just described, much animal research indicates an important role for gonadal hormones. In adult rodents, circulating levels of estrogens fluctuate in females during the estrous cycle and range from two to three times the level in males, whereas testosterone levels are four to five times greater in males. Accordingly, there has been much research on the role of gonadal hormones in modulating pain, with the majority of studies focusing on the activational effect of hormones. Somatic and visceral nociceptive processing has been reported to fluctuate in parallel with the estrous cycle, thus strongly suggesting hormonal modulation, but nociceptive sensitivity to circulating levels of estrogens and progesterone vary with the tissue and test (estrogens are pro-nociceptive: Sapsed-Byrne et al 1996, Cason et al 2003, Okamoto et al 2003, Martin et al 2007, Ji et al 2008, Lu et al 2009b; estrogens are antinociceptive: Giamberardino et al 1997, Bradshaw et al 1999, Fischer et al 2008, Kramer and Bellinger 2009).
Hormonal depletion by gonadectomy (ovariectomy or orchiectomy) alters nociceptive processing, but no consensus has been reached on the direction of effect. Ovariectomy decreases visceral sensitivity to colorectal distention (Bradesi et al 2003, Ji et al 2003, Fan et al 2009), bladder distention (Robbins et al 2010), and uterine cervix distention (Yan et al 2007) but increases responses to vaginal distention (Bradshaw and Berkley 2002). Hormone replacement with estradiol reverses the effects of ovariectomy.
The pro- and antinociceptive effects of estrogens have been demonstrated with somatic stimuli as well. Ovariectomy decreased (Gaumond et al 2005, Hagiwara et al 2007) or increased (Kuba et al 2006, Fischer et al 2008) behavioral responses to formalin (the direction of change appears to depend on the concentration of formalin). Estradiol replacement reversed the effect of ovariectomy. In one study, ovariectomy increased the formalin response in the lip but not in the hindpaw (Pajot et al 2003). Most, but not all studies using other inflammatory stimuli or nerve injury models support a pro-nociceptive effect of estrogens (decrease in nociceptive behavior following ovariectomy that is reversed by estradiol replacement) (Cairns et al 2002, Bereiter et al 2005b, Cook and Nickerson 2005, Wang et al 2006, Dominguez et al 2009, Lu et al 2009b). However, hyperalgesic priming (prolonged hyperalgesia to a noxious stimulus following a conditioning inflammatory stimulus) is suppressed by estrogens in females and males (Joseph et al 2003). Furthermore, when estrogens or estrogens plus progesterone are elevated in intact rats, either by administration or induced via pseudopregnancy, there is predominantly analgesia (Gintzler and Bohan 1990, Dawson-Basoa and Gintzler 1996, Aloisi et al 2010).
In contrast to the predominantly pro-nociceptive effect of estrogens following injury in female rats, testosterone appears to be protective in male rats. Gonadectomy in males increased the formalin response that was reduced by testosterone replacement (Aloisi et al 2003, Pajot et al 2003, Gaumond et al 2005, Fischer et al 2007). There was a progressive decrease in response to repetitive formalin injections in intact males, but not following gonadectomy (Ceccarelli et al 2003). Antinociceptive mechanisms require testosterone in male rats and are reduced following gonadectomy (Stoffel et al 2005, Borzan and Fuchs 2006, Claiborne et al 2006, Thompson et al 2008). In contrast, estradiol increases the formalin response in males (Aloisi and Ceccarelli 2000).
The effects of gonadal hormones may not be dependent on the absolute concentration of hormones in circulation but reflect changing levels during a normal estrous or menstrual cycle. It has been suggested that estrogen or progesterone withdrawal is more significant in modulating nociceptive sensitivity than is maintenance of high levels of either hormone (Ji et al 2003, Martin et al 2007, Martin 2008, Devall and Lovick 2010, Robbins et al 2010, Puri et al 2011). Furthermore, it is possible that the increase in estradiol-induced nociceptive sensitivity is not due to the facilitatory actions of estradiol but results from decreasing antinociceptive processing. Ovariectomy decreases pain behavior during the interphase of the formalin test, which is thought to reflect an increase in antinociceptive mechanisms (Gaumond et al 2005).
An additional consideration is the effect of estrogens and testosterone on inflammation and subsequent nociceptive processing. Estradiol decreased and testosterone increased plasma extravasation in a model of TMJ inflammation (Flake et al 2006). It is possible that less inflammation may result in inflammatory mediators remaining at the site of injury longer and thereby resulting in greater peripheral sensitization and a longer duration of hyperalgesia. However, estrogens have anti-inflammatory as well as pro-inflammatory effects, depending on multiple factors, including the immune stimulus and response and the cell types affected (for review see Straub 2007).
Primary Afferents
The mechanisms underlying gonadal hormone modulation of nociception occur at many levels of the nervous system. Female craniofacial afferents, including masseter and digastric muscular afferents, were more sensitive to subcutaneous or intramuscular glutamate than were the afferents of male rats, an estradiol-dependent effect. However, there was no sex difference in N-methyl-D-aspartate (NMDA)-evoked discharges in temporalis nociceptors (Cairns et al 2002, Dong et al 2006, Gazerani et al 2010). Alternatively, modulation of transmitter content in the medullary dorsal horn following TMJ inflammation could result from estradiol increasing reuptake mechanisms rather than transmitter release (Bereiter and Benetti 2006).
In visceral tissues, ovariectomy had no effect on the excitability of colonic afferents from non-inflamed rats (Ji et al 2011), but estradiol replacement following ovariectomy increased the excitability of uterine cervix afferents (Liu et al 2005). The excitability of vaginal/uterine afferents fluctuates across the estrous cycle, with excitability being greater during diestrus than during proestrus, although this may be more related to reproduction than to pain (Robbins et al 1992).
Estradiol directly increases the excitability of dorsal root ganglion (DRG) neurons in vitro. Estradiol increased the excitability of TMJ afferents, which was further increased following TMJ inflammation (Flake et al 2005). Estradiol increased NMDA- and capsaicin-evoked currents and decreased adenosine triphosphate (ATP)-evoked Ca2+ currents in unidentified DRG cells but increased ATP-evoked currents following colonic inflammation (Chaban and Micevych 2005, McRoberts et al 2007, Fan et al 2009, Lu et al 2009b). Estradiol also altered gene expression in the trigeminal ganglion following TMJ inflammation (Puri et al 2011).
Dorsal Horn Neurons
Sex differences in response to noxious stimulation may be dependent on differential processing of nociceptive stimuli at the level of the spinal cord/medullary dorsal horn as well. Estradiol decreased glycine-evoked currents in dorsal horn neurons (Jiang et al 2009). The response of spinal or medullary dorsal horn neurons to noxious stimulation of peripheral structures (e.g., colon, TMJ) is greater in females than in males and is estrogen dependent Bereiter 2001, You et al 2006). Ovariectomy decreased and estradiol restored the response of dorsal horn neurons to colonic or TMJ stimulation (Ji et al 2003, 2005; Tashiro et al 2009). This increased sensitivity may be due to estradiol increasing NMDA receptor or CREB (cyclic adenosine monophosphate response element–binding protein) phosphorylation in the dorsal horn (Lu et al 2007, Tang et al 2008, Peng et al 2009, Tashiro et al 2009, Peng et al 2010). Sex differences and fluctuating degrees of sensitivity may also result from the differential expression of estrogen receptors. Proestrous rats expressed more estrogen receptor-α in the superficial and deeper layers of the medullary dorsal horn than did males, with mixed expression levels in diestrus females (Bereiter et al 2005a).
Sex Differences in Neural Processing of Pain as Revealed by Human Neuroimaging
Only about a dozen neuroimaging studies have addressed the question of sex differences in human pain processing (Table 15-2). The first of these studies, an 15O-positron emission tomography (PET) study using noxious heat stimuli, reported greater response in women than in men in several brain regions (Paulson et al 1998). However, a fixed-intensity stimulus was used, which evoked more intense pain in the women than in the men, thus leaving open the question of whether this was a sex difference or an intensity difference (Coghill et al 2003). Most subsequent studies applied stimuli that were perceived as being equally painful to the men and women, which often meant that the stimuli were of lesser intensity when applied to women. These studies tended to report greater activation for men than for women in some brain regions; however, there is considerable variability in results across studies. Two studies reported greater activation for men in some brain regions and greater activation for women in other regions, although the specific brain regions were not the same in the two studies (Derbyshire et al 2002, Naliboff et al 2003). One common finding across most of the PET and functional magnetic resonance imaging (fMRI) studies is greater activation in the insula for men than for women. There is no strongly consistent pattern for any other brain region.
Table 15-2
Summary of Neuroimaging Studies Evaluating Sex Differences Related to Pain Processing
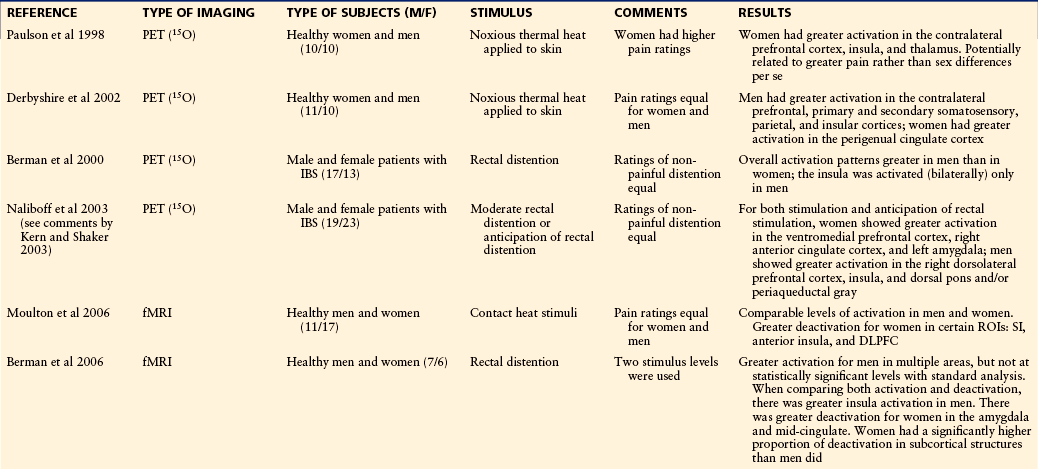
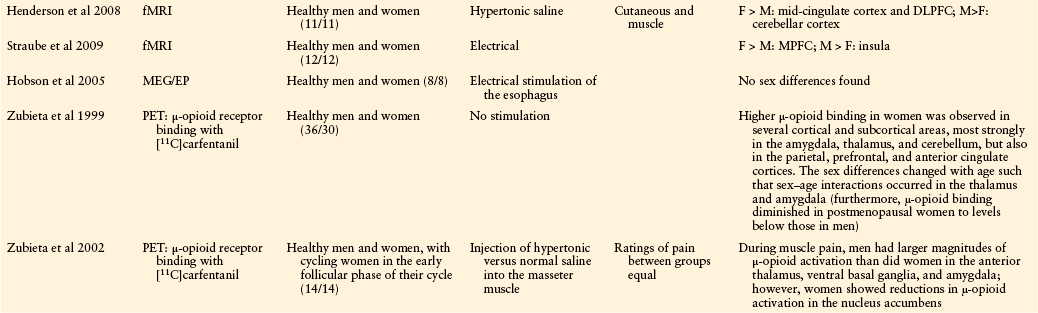
From Holdcroft A, Berkley KJ: Sex and gender differences in pain and its relief. In McMahon SB, Koltzenburg M, editors: Wall and Melzack’s textbook of pain, 5th ed, Amsterdam, 2005, Elsevier, pp 1181–1198.
DLPFC, dorsolateral prefrontal cortex; EP, electrophysiological study; fMRI, functional magnetic resonance imaging; IBS, irritable bowel syndrome; MEG, magnetoencephalography; MPFC, medial prefrontal cortex; PET, positron emission tomography; ROI, region of interest; SI, primary somatosensory cortex.
Two fMRI studies evaluated heat pain–related responses at two different phases of the menstrual cycle (Choi et al 2006, de Leeuw et al 2006). Even though significant differences in pain-related activation were reported in both studies, there was little commonality in results (see Table 15-2).
Psychological, Social, and Cultural Factors Related to Sex and Gender Differences in Pain
Whereas the preceding sections focused on biological mechanisms underlying sex differences in pain, it is quite clear that psychological, social, and cultural factors influence the experience of pain. Consequently, these factors are potentially capable of influencing the sexes differentially.
Gender Roles
One psychosocial phenomenon of interest in this regard is gender role. Although “sex” refers to biological distinctions characterizing male and female, “gender” refers to sex-related roles with which an individual identifies himself or herself. Gender roles have been associated with pain such that masculine gender norms dictate increased tolerance of pain whereas feminine gender norms accept pain as a normal part of life and are more permissive of pain expression (Unruh et al 1999, Myers et al 2003). Using standardized measures of gender roles, several studies have investigated the association of masculinity and femininity with experimental pain responses. Across four studies of experimental pain sensitivity, higher masculinity was associated with lesser pain sensitivity and/or higher femininity was associated with greater pain sensitivity, although these associations were not found for all pain sensitivity measures. In two of these studies, sex differences in pain responses remained significant after statistically controlling for gender roles (Otto and Dougher 1985, Myers et al 2001), whereas gender roles partially mediated the sex difference in experimental pain sensitivity in the other two studies (Sanford et al 2002, Thorn et al 2004).
To explore the topic of gender roles in more detail, Robinson and colleagues (2001) developed a pain-specific gender role measure, the Gender Role Expectations of Pain (GREP). Their research with GREP indicates that both sexes consider women to be more sensitive to pain, less enduring of pain, and more willing to report pain than men are. With respect to experimental pain sensitivity, willingness to report pain was significantly associated with the heat pain threshold and heat pain tolerance. Furthermore, sex differences in pain threshold were not statistically significant after controlling for willingness to report pain, whereas sex differences in pain tolerance remained significant (Wise et al 2002). These investigators also found that the sex differences in temporal summation of heat-induced pain were partially mediated by willingness to report pain (Robinson et al 2004).
A related study found that females viewed overt pain expression as more acceptable than did males, and these beliefs predicted tolerance of cold-related pain, which was lower in females than in males (Nayak et al 2000). Another study found that both men and women agreed that the ideal man should tolerate more pain than the ideal woman, thus further supporting the conception that gender norms are associated with pain tolerance. Furthermore, this study demonstrated that strong identification with the male gender norm was associated with higher electrical pain tolerance in men whereas gender norm identification was not associated with pain tolerance in women (Pool et al 2007).
An associated issue is the effect that the sex of the experimenter has on experimental pain sensitivity. Three studies involving different types of psychophysical protocols reported that male participants provided results indicating less pain sensitivity when tested by a female versus a male experimenter whereas female participants showed no difference (Levine and De Simone 1991, Gijsbers and Nicholson 2005, Aslaksen et al 2007). Another study reported that tolerance of cold-related pain was higher in both men and women when tested by an experimenter of the opposite sex (Kallai et al 2004). In contrast, other investigators have failed to show an effect of experimenter gender on pain responses (Otto and Dougher 1985, Bush et al 1993, Myers et al 2001). It is likely that the significance of this effect is related to various aspects of the interaction between the experimenter and subject, which is difficult to control for or specify completely.
Psychological Distress
Multiple psychological dimensions related to pain demonstrate sex differences, including anxiety, depression, and coping/catastrophizing. Several studies have sought to determine whether sex differences in these psychological domains are related to sex differences in pain.
Among patients with musculoskeletal pain, women reported higher levels of catastrophizing than did men, and higher catastrophizing was associated with poorer perceived health status in women (Jensen et al 1994). In contrast, a telephone survey found no sex differences in catastrophizing despite women reporting more intense pain and using a wider range of coping strategies than men did (Unruh et al 1999). Among osteoarthritis patients, women reported higher levels of pain and disability and exhibited more pain behavior than men did. When statistical adjustments were made for catastrophizing, the sex differences in pain-related outcomes became insignificant (Keefe et al 2000). Another study found that adolescent girls used more social support, positive statements, and internalizing/catastrophizing whereas boys relied more on behavioral distraction. Furthermore, this study reported that internalizing/catastrophizing mediated sex differences in clinical pain (Keogh and Eccleston 2006). Multiple studies have reported higher levels of catastrophizing in healthy women than in men. In one such study, catastrophizing mediated sex differences in reports of daily pain but did not play a role in the sex differences in sensitivity to heat-related pain (Edwards et al 2004). Thus, sex differences in pain coping, particularly catastrophizing, have been reported in multiple studies and have been shown to mediate sex differences in clinical pain in some of these studies.
Higher levels of anxiety have been associated with increased clinical pain and heightened experimental pain sensitivity (Rhudy and Williams 2005). Sex differences in anxiety have been reported, with women tending to report higher levels of anxiety and being at increased risk for many anxiety disorders (Bekker and van Mens-Verhulst 2007). Additionally, anxiety has been suggested as a potential mediator of sex differences in pain sensitivity (Rollman 1995). However, other evidence suggests that anxiety may be more strongly associated with pain responses in males than in females. Multiple studies using experimental pain have reported that anxiety is positively associated with pain sensitivity in men but not in women (Fillingim et al 1996, Jones et al 2003). Among patients with chronic low back pain, anxiety was more strongly related to both ongoing clinical pain and pain induced via low back exercise in men than in women (Robinson et al 2005). Another study similarly reported that anxiety was more strongly related to clinical pain severity in male than in female patients with chronic pain (Edwards et al 2000). That same group subsequently reported that higher pretreatment anxiety predicted greater reductions in pain after interventional therapy for men but not for women (Edwards et al 2003). Thus, anxiety appears to be more strongly related to experimental and clinical pain and to treatment-related pain reductions in men.
Of related interest, women report higher levels of anxiety sensitivity, which refers to the fear of anxiety-related body sensations. Furthermore, anxiety sensitivity has been associated with both clinical and experimental pain responses (Keogh et al 2004, 2006; Stewart and Asmundson 2006). In one study, anxiety sensitivity was more strongly related to pain in women than in men with chest pain (Keogh et al 2004). In another study, higher anxiety sensitivity predicted lower cold pressor pain threshold and tolerance only in men, whereas higher anxiety sensitivity was associated with greater sensory and affective pain ratings in women (Keogh et al 2006). Though few in number, these findings suggest that anxiety sensitivity may contribute differently to pain responses in women and men.
Sex and Gender Differences in Analgesia and Their Underlying Mechanisms
Sex differences in response to analgesic medications have been explored in several studies (as reviewed by Kest et al 2000, Craft 2003, Fillingim and Gear 2004, Niesters et al 2010, Rasakham and Liu-Chen 2011). Though not a direct measure of analgesic response, studies of self-administration of opioids using patient-controlled analgesia (PCA) have been used to investigate sex differences in opiate analgesia. Several early studies revealed lower postoperative opioid consumption in women than in men (Miaskowski and Levine 1999). Along with the most direct interpretation of these results—greater analgesic efficacy in women—this lower opioid consumption in women could be driven by other factors, such as increased adverse effects, which have been documented in women (Fillingim et al 2005). Subsequent studies have provided a mixed picture of sex differences in opioid analgesia (Joels et al 2003, Gagliese et al 2008).
Recently, a systematic review of the literature on this topic considered 25 clinical studies on μ-opioids and found no significant sex–analgesia association (Niesters et al 2010). Restricting analysis to PCA studies identified greater analgesia in women (n = 15, effect size = 0.22, 95% confidence interval [CI] = 0.02–0.42, P = 0.028). Further restricting the analysis to morphine PCA studies yielded an even greater effect in women (n = 11, effect size = 0.36, 95% CI = 0.17–0.56, P = 0.003). A further analysis indicated that the longer the duration of PCA, the greater the difference between the sexes.
Others have investigated analgesic responses to mixed-action opioid agonist–antagonists in women relative to men. In several studies of pain after oral surgery, women have shown more robust and longer-lasting analgesic responses than have men in response to κ-opioid agonists such as pentazocine, nalbuphine, and butorphanol (Gear et al 1999, 2003). After endodontic surgery, women showed significantly greater pain relief with a pentazocine-naloxone combination than did men (Ryan et al 2008). In contrast, no sex differences in butorphanol analgesia were observed in patients treated in the emergency department for trauma-related pain (Miller and Ernst 2004).
Human Experimental Studies
Only a handful of studies have examined sex differences in analgesic responses using experimental pain models. As with clinical pain, most of these studies examined μ-opioid–mediated analgesia. A recent systematic review of this topic reported that women had greater antinociception from opioids (n = 11, effect size = 0.35, 95% CI = 0.01–0.69, P = 0.047), which was predominantly derived from six morphine studies (Niesters et al 2010). A previous review found little evidence of sex differences in opiate hypoalgesia for experimental pain and warned against interpreting data without reference to placebo effects (Fillingim et al 2009). One of the studies showing significantly greater hypoalgesia in women’s response to morphine also found a significantly greater hypoalgesic response to placebo. Adjusting for the placebo response eliminated the sex difference in morphine response (Pud et al 2006).
Three studies have reported on sex differences in the hypoalgesic effects of non-opioid pain medications in response to experimental pain. Two studies using non-steroidal anti-inflammatory drugs produced mixed results, with one showing greater effects of ibuprofen for electrical stimuli in men than in women (Walker and Carmody 1998) and another study showing no sex differences in effects on tolerance of cold pain (Compton et al 2003). In this latter study, men showed substantially increased tolerance in response to both placebo and ketorolac, whereas females showed no placebo response and a very modest increase in response to ketorolac. A third study reported that lidocaine iontophoresis produced greater cutaneous anesthesia to pressure pain in men than in women (Robinson et al 1998). These few and very different studies prevent any general statement at this time.
Another aspect of experimental pain sensitivity that has been evaluated with respect to sex differences is the ability to evoke endogenous analgesia. This has been evaluated most often in the context of conditioned pain modulation (CPM; previously termed diffuse noxious inhibitory control [DNIC]) (Yarnitsky et al 2010), in which one painful stimulus is administered to evaluate its effect on the pain evoked by another painful stimulus. In other studies, stressors are administered to evaluate their effects on experimental pain perception. Based on recent reviews of this literature (Fillingim et al 2009, Popescu et al 2010, Racine et al 2012b), 9 of 19 CPM studies showed a significantly greater analgesic effect in men, and all but 1 of the other studies showed no sex difference. In contrast, four of six studies evaluating the effects of stress on experimental pain found a significantly greater analgesic effect in women, whereas two studies reported no sex difference (Table 15-3). Given the multiple systems involved in these types of studies (attentional, stress related, cognitive), it is easy to envision that results can vary from study to study, even when using the same protocol. For example, one study reported no sex difference in the degree of endogenous analgesia provoked in a CPM protocol, yet the relationship between the self-reported stressfulness of the protocol and the degree of analgesia was very different for men and women (Quiton and Greenspan 2007). In another instance, a sex difference in CPM analgesia could be eliminated if the results were statistically corrected for catastrophizing (Weissman-Fogel et al 2008). Both these studies exemplify the important role that psychological factors play in pain assessment, even in the context of a controlled experimental environment.
Table 15-3
Summary of Studies Evaluating Sex Differences in Endogenous Analgesia Mediated by Either Conditioned Pain Modulation (CPM) Protocols or Stressful Manipulations

Based on reviews by Fillingim RB, King CD, Ribeiro-Dasilva MC, et al 2009 Sex, gender, and pain: a review of recent clinical and experimental findings. Journal of Pain 10:447–485; Popescu A, LeResche L, Truelove EL, et al 2010 Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain 150:309–318; and Racine M, Tousignant-Laflamme Y, Kloda LA, et al 2012b A systematic literature review of 10 years of research on sex/gender and experimental pain perception—Part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain 153:619–635.
One research group used PET with radiolabeled carfentanil (a μ-opioid receptor agonist) to evaluate the cerebral mechanisms underlying endogenous analgesic mechanisms. In the absence of pain, μ-opioid–binding measures were found to be higher for women than for men in several brain regions. Interestingly, this difference decreased with age, with levels tending to be reduced in older women (Zubieta et al 1999). In the presence of pain, men showed greater activation of the μ-opioid system than did women in regions such as the thalamus, amygdala, and basal ganglia, and women showed a reduction in μ-opioid activation in the nucleus accumbens (Zubieta et al 2002). These results suggest greater engagement of the endogenous opioid system in men than in women when experiencing pain. A third study evaluated the endogenous opioid system in response to painful stimulation when women were in the follicular phase of their cycle, either with or without estradiol supplementation (Smith et al 2006). When tested in the high-estradiol condition, women reported lower pain ratings and showed greater μ-opioid activation in the thalamus, nucleus accumbens, and amygdala. Perhaps counterintuitively, the high-estradiol condition resulted in a response profile that was more similar to the male response than the low-estradiol condition was. Nonetheless, the explicit manipulation of estradiol levels, rather than relying on menstrual cycle effects, allowed the authors to conclude that estradiol functioned in an antinociceptive manner that involved engagement of components of the endogenous opioid system.
Animal Studies
The effects of sex, hormones, and genotype on analgesic mechanisms in rodents, especially opioids, have been extensively reviewed (Fillingim and Ness 2000, Kest et al 2000, Craft 2003, Craft et al 2004, Fillingim and Gear 2004, Dahan et al 2008, Hurley and Adams 2008, Loyd and Murphy 2009, Bodnar and Kest 2010). One main point is that sex differences and hormonal effects are inversely correlated with opioid efficacy and intensity of the noxious stimulus: the more effective the opioid or the more intense the stimulus, the less obvious are differences based on sex or hormonal milieu. When sex differences are found, they tend to indicate that μ- and κ-opioid agonists produce greater antinociception/analgesia in males than in females. Several factors contribute to this finding. Males express more μ-opioid receptor protein in the spinal cord and midbrain, especially when compared with females in proestrus (Kren et al 2008, Loyd et al 2008, Murphy et al 2009). The greater antinociception in males is also due in part to the activational effects of testosterone; Craft and colleagues (2004) reported on 16 studies in rodents and found greater (7/16, 44%) or equal (6/16, 37%) morphine antinociception in intact males and gonadectomized males with testosterone replacement than in gonadectomized males without replacement. In females, estrogen appears to decrease morphine analgesia; ovariectomy increased morphine’s effect (more pronounced at shorter times following ovariectomy), which was reversed by estradiol replacement (Craft et al 2004, Stoffel et al 2005, Ji et al 2007). However, increasing or decreasing morphine antinociception may be dose and time dependent (Craft et al 2008). Other opioids follow a similar pattern of testosterone increasing antinociception and estradiol decreasing antinociception (Stoffel et al 2005, Claiborne et al 2006).
Genetic Factors
Sex differences in nociception and antinociceptive mechanisms also depend on genetic factors, independent of hormonal influences (extensively reviewed by Mogil and Bailey 2010). For example, the direction of sex difference in thermal sensitivity and morphine antinociception varies by mouse or rat strain and is differentially modulated by stress (Kest et al 1999, Mogil et al 2000). Non-opioid stress-induced analgesia is dependent on the NMDA receptor in males, but not in females, although ovariectomy switches females to the male phenotype (Mogil et al 1993, 1997). κ-Opioid receptor antinociception is modulated by variants of the MC1R gene (melanocortin receptor, which also influences hair color) in females but not in males. This genetic link was demonstrated for mice and human beings and was further shown to account for the female mechanism underlying one form of stress-induced analgesia (Mogil et al 1993, Kavaliers and Choleris 1997, Mogil et al 2003). In a model of neuropathic pain, sex differences are dependent on the strain of rat, and there are sex differences in injury-induced changes in gene expression (LaCroix-Fralish et al 2005a, 2006).
Future Directions
The presence of sex differences in pain and analgesia in people and in animals has clearly been established in a wide array of studies, only some of which are described here. What still needs to be elucidated are the conditions that either exaggerate or minimize these sex and gender differences and the biological and psychological factors that underlie expression of these differences.
Under the heading of biological mechanisms, preclinical studies are starting to examine the role of different estrogen receptors in nociceptive processing. Several groups are investigating the specific roles of different isoforms of the classic estrogen receptors α and β (Peng et al 2009, Coulombe et al 2011, Ji et al 2011) and the G protein–coupled receptor GPER1 (previously called GPR30) (Fehrenbacher et al 2009, Liverman et al 2009, Lu et al 2009a). The contribution of sex chromosomes independent of gonadal expression is being examined with the four-core genotype model in the mouse (Gioiosa et al 2008). Local synthesis of estradiol from testosterone by neurons and glia can alter nociceptive processing on a time scale on the order of seconds or faster, thus indicating a role for both neurosteroids and rapid estrogenic signaling in the modulation of pain (Evrard 2006).
In the realm of human experimental and clinical research, greater understanding of sex and gender differences in pain and analgesia will require multifactorial studies capable of capturing and analyzing information across multiple domains of interest—biological, psychological, clinical, and situational. In this way, understanding the nature of sex and gender differences in pain and analgesia is a microcosm of understanding the nature of pain in general. As this and other recent reviews have made clear, the assembly of information from multiple small studies can provide only a suggestive and sometimes conflicting picture. The multitude of factors that influence pain and the multidimensional nature of pain ultimately require examination of combinations of factors to understand human pain and its modulation.
The references for this chapter can be found at www.expertconsult.com.
References
Affaitati G., Ceccarelli I., Fiorenzani P., et al. Sex differences in the analgesic effects of ICI 182,780 and flutamide on ureteral calculosis in rats. Hormones and Behavior. 2011;59:9–13.
Aloisi A.M., Affaitati G., Ceccarelli I., et al. Estradiol and testosterone differently affect visceral pain–related behavioural responses in male and female rats. European Journal of Pain. 2010;14:602–607.
Aloisi A.M., Ceccarelli I. Role of gonadal hormones in formalin-induced pain responses of male rats: modulation by estradiol and naloxone administration. Neuroscience. 2000;95:559–566.
Aloisi A.M., Ceccarelli I., Fiorenzani P. Gonadectomy affects hormonal and behavioral responses to repetitive nociceptive stimulation in male rats. Annals of the New York Academy of Sciences. 2003;1007:232–237.
Alonso C., Loevinger B.L., Muller D., et al. Menstrual cycle influences on pain and emotion in women with fibromyalgia. Journal of Psychosomatic Research. 2004;57:451–458.
Arjona A., Rubi-Callejon J., Guardado-Santervas P., et al. Menstrual tension-type headache: evidence for its existence. Headache. 2007;47:100–103.
Aslaksen P.M., Myrbakk I.N., Hoifodt R.S., et al. The effect of experimenter gender on autonomic and subjective responses to pain stimuli. Pain. 2007;129:260–268.
Bajaj P., Bajaj P., Madsen H., et al. A comparison of modality-specific somatosensory changes during menstruation in dysmenorrheic and nondysmenorrheic women. Clinical Journal of Pain. 2002;18:180–190.
Banik R.K., Woo Y.C., Park S.S., et al. Strain and sex influence on pain sensitivity after plantar incision in the mouse. Anesthesiology. 2006;105:1246–1253.
Bekker M.H., van Mens-Verhulst J. Anxiety disorders: sex differences in prevalence, degree, and background, but gender-neutral treatment. Gender Medicine. 2007;4(Suppl B):S178–S193.
Bereiter D.A. Sex differences in brainstem neural activation after injury to the TMJ region. Cells, Tissues, Organs. 2001;169:226–237.
Bereiter D.A., Benetti A.P. Amino acid release at the spinomedullary junction after inflammation of the TMJ region in male and female rats. Pain. 2006;126:175–183.
Bereiter D.A., Cioffi J.L., Bereiter D.F. Oestrogen receptor–immunoreactive neurons in the trigeminal sensory system of male and cycling female rats. Archives of Oral Biology. 2005;50:971–979.
Bereiter D.A., Okamoto K., Bereiter D.F. Effect of persistent monoarthritis of the temporomandibular joint region on acute mustard oil–induced excitation of trigeminal subnucleus caudalis neurons in male and female rats. Pain. 2005;117:58–67.
Berkley K.J. Sex differences in pain. Behavioral and Brain Sciences. 1997;20:371–380.
Berkley K.J., Zalcman S.S., Simon V.R. Sex and gender differences in pain and inflammation: a rapidly maturing field. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R241–R244.
Berman S.M., Naliboff B.D., Suyenobu B., Labus J.S., Stains J., Bueller J.A., et al. Sex differences in regional brain response to aversive pelvic visceral stimuli. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R268–R276.
Bodnar R.J., Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Hormones and Behavior. 2010;58:72–81.
Borzan J., Fuchs P.N. Organizational and activational effects of testosterone on carrageenan-induced inflammatory pain and morphine analgesia. Neuroscience. 2006;143:885–893.
Bradesi S., Eutamene H., Garcia-Villar R., et al. Stress-induced visceral hypersensitivity in female rats is estrogen-dependent and involves tachykinin NK1 receptors. Pain. 2003;102:227–234.
Bradshaw H.B., Berkley K.J. Estrogen replacement reverses ovariectomy-induced vaginal hyperalgesia in the rat. Maturitas. 2002;41:157–165.
Bradshaw H.B., Temple J.L., Wood E., et al. Estrous variations in behavioral responses to vaginal and uterine distention in the rat. Pain. 1999;82:187–197.
Brynhildsen J.O., Bjors E., Skarsgard C., et al. Is hormone replacement therapy a risk factor for low back pain among postmenopausal women? Spine. 1998;23:809–813.
Bush F.M., Harkins S.W., Harrington W.G., et al. Analysis of gender effects on pain perception and symptom presentation in temporomandibular pain. Pain. 1993;53:73–80.
Cairns B.E., Sim Y., Bereiter D.A., et al. Influence of sex on reflex jaw muscle activity evoked from the rat temporomandibular joint. Brain Research. 2002;957:338–344.
Cason A.M., Samuelsen C.L., Berkley K.J. Estrous changes in vaginal nociception in a rat model of endometriosis. Hormones and Behavior. 2003;44:123–131.
Ceccarelli I., Scaramuzzino A., Massafra C., et al. The behavioral and neuronal effects induced by repetitive nociceptive stimulation are affected by gonadal hormones in male rats. Pain. 2003;104:35–47.
Chaban V.V., Micevych P.E. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca(2+) signaling in mouse dorsal root ganglion neurons. Journal of Neuroscience Research. 2005;81:31–37.
Chang L., Toner B.B., Fukudo S., et al. Gender, age, society, culture, and the patient’s perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446.
Chial H.J., Camilleri M. Gender differences in irritable bowel syndrome. Journal of Gender Specific Medicine. 2002;5:37–45.
Choi J.C., Park S.K., Kim Y.H., et al. Different brain activation patterns to pain and pain-related unpleasantness during the menstrual cycle. Anesthesiology. 2006;105:120–127.
Claiborne J., Nag S., Mokha S.S. Activation of opioid receptor like-1 receptor in the spinal cord produces sex-specific antinociception in the rat: estrogen attenuates antinociception in the female, whereas testosterone is required for the expression of antinociception in the male. Journal of Neuroscience. 2006;26:13048–13053.
Coghill R.C., McHaffie J.G., Yen Y.F. Neural correlates of interindividual differences in the subjective experience of pain. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8538–8542.
Collett B.J., Berkley K. The IASP Global Year Against Pain in Women. Pain. 2007;132:S1–S2.
Compton P., Charuvastra V.C., Ling W. Effect of oral ketorolac and gender on human cold pressor pain tolerance. Clinical and Experimental Pharmacology & Physiology. 2003;30:759–763.
Cook C.D., Nickerson M.D. Nociceptive sensitivity and opioid antinociception and antihyperalgesia in Freund’s adjuvant–induced arthritic male and female rats. Journal of Pharmacology and Experimental Therapeutics. 2005;313:449–459.
Coulombe M.A., Spooner M.F., Gaumond I., et al. Estrogen receptors beta and alpha have specific pro- and anti-nociceptive actions. Neuroscience. 2011;184:172–182.
Craft R.M. Sex differences in opioid analgesia: “from mouse to man,”. Clinical Journal of Pain. 2003;19:175–186.
Craft R.M., Mogil J.S., Aloisi A.M. Sex differences in pain and analgesia: the role of gonadal hormones. European Journal of Pain. 2004;8:397–411.
Craft R.M., Ulibarri C., Leitl M.D., et al. Dose- and time-dependent estradiol modulation of morphine antinociception in adult female rats. European Journal of Pain. 2008;12:472–479.
Dahan A., Kest B., Waxman A.R., et al. Sex-specific responses to opiates: animal and human studies. Anesthesia and Analgesia. 2008;107:83–95.
Dao T.T.T., LeResche L. Gender differences in pain. J Orofacial Pain. 2000;14:169–184.
Dawson-Basoa M.E., Gintzler A.R. Estrogen and progesterone activate spinal kappa-opiate receptor analgesic mechanisms. Pain. 1996;64:608–615.
de Leeuw R., Albuquerque R.J.C., Andersen A.H., et al. Influence of estrogen on brain activation during stimulation with painful heat. Journal of Oral and Maxillofacial Surgery. 2006;64:158–166.
Derbyshire S.W.G., Nichols T.E., Firestone L., et al. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. Journal of Pain. 2002;3:401–411.
Devall A.J., Lovick T.A. Differential activation of the periaqueductal gray by mild anxiogenic stress at different stages of the estrous cycle in female rats. Neuropsychopharmacology. 2010;35:1174–1185.
Dominguez C.A., Kouya P.F., Wu W.P., et al. Sex differences in the development of localized and spread mechanical hypersensitivity in rats after injury to the infraorbital or sciatic nerves to create a model for neuropathic pain. Gender Medicine. 2009;6(Suppl 2):225–234.
Dong X.D., Mann M.K., Sessle B.J., et al. Sensitivity of rat temporalis muscle afferent fibers to peripheral N-methyl-D-aspartate receptor activation. Neuroscience. 2006;141:939–945.
Edwards R.R., Augustson E., Fillingim R.B. Sex-specific effects of pain-related anxiety on adjustment to chronic pain. Clinical Journal of Pain. 2000;16:46–53.
Edwards R.R., Augustson E., Fillingim R.B. Differential relationships between anxiety and treatment-associated pain reduction among male and female chronic pain patients. Clinical Journal of Pain. 2003;19:208–216.
Edwards R.R., Haythornthwaite J.A., Sullivan M.J., et al. Catastrophizing as a mediator of sex differences in pain: differential effects for daily pain versus laboratory-induced pain. Pain. 2004;111:335–341.
Evrard H.C. Estrogen synthesis in the spinal dorsal horn: a new central mechanism for the hormonal regulation of pain. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R291–R299.
Fan J., Yu L.H., Zhang Y., et al. Estrogen altered visceromotor reflex and P2X3 mRNA expression in a rat model of colitis. Steroids. 2009;74:956–962.
Fehrenbacher J.C., Loverme J., Clarke W., et al. Rapid pain modulation with nuclear receptor ligands. Brain Research Reviews. 2009;60:114–124.
Fillingim R.B., Gear R.W. Sex differences in opioid analgesia: clinical and experimental findings. European Journal of Pain. 2004;8:413–425.
Fillingim R.B., Keefe F.J., Light K.C., et al. The influence of gender and psychological factors on pain perception. J Gender Culture Health. 1996;1:21–36.
Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., et al. Sex, gender, and pain: a review of recent clinical and experimental findings. Journal of Pain. 2009;10:447–485.
Fillingim R.B., Maixner W. Gender differences in the response to noxious stimuli. Pain Forum. 1995;4:209–221.
Fillingim R.B., Maixner W., Girdler S.S., et al. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosomatic Medicine. 1997;59:512–520.
Fillingim R.B., Ness T.J. Sex-related hormonal influences on pain and analgesic responses. Neuroscience and Biobehavioral Reviews. 2000;24:485–501.
Fillingim R.B., Ness T.J., Glover T.L., et al. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. Journal of Pain. 2005;6:116–124.
Fischer L., Clemente J., Tambeli C. The protective role of testosterone in the development of temporomandibular joint pain. Journal of Pain. 2007;8:437–442.
Fischer L., Torres-Chavez K.E., Clemente-Napimoga J.T., et al. The influence of sex and ovarian hormones on temporomandibular joint nociception in rats. Journal of Pain. 2008;9:630–638.
Flake N.M., Bonebreak D.B., Gold M.S. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. Journal of Neurophysiology. 2005;93:1585–1597.
Flake N.M., Hermanstyne T.O., Gold M.S. Testosterone and estrogen have opposing actions on inflammation-induced plasma extravasation in the rat temporomandibular joint. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R343–R348.
Gagliese L., Gauthier L.R., Macpherson A.K., et al. Correlates of postoperative pain and intravenous patient-controlled analgesia use in younger and older surgical patients. Pain Medicine. 2008;9:299–314.
Gaumond I., Arsenault P., Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Research. 2002;958:139–145.
Gaumond I., Arsenault P., Marchand S. Specificity of female and male sex hormones on excitatory and inhibitory phases of formalin-induced nociceptive responses. Brain Research. 2005;1052:105–111.
Gazerani P., Dong X., Wang M., et al. Sensitization of rat facial cutaneous mechanoreceptors by activation of peripheral N-methyl-D-aspartate receptors. Brain Research. 2010;1319:70–82.
Gazerani P., Kaeseler Andersen O., et al. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain. 2005;118:155–163.
Gear R.W., Gordon N.C., Miaskowski C., et al. Sexual dimorphism in very low dose nalbuphine postoperative analgesia. Neuroscience Letters. 2003;339:1–4.
Gear R.W., Miaskowski C., Gordon N.C., et al. The kappa opioid nalbuphine produces gender- and dose-dependent analgesia and antianalgesia in patients with postoperative pain. Pain. 1999;83:339–345.
Giamberardino M.A., Affaitati G., Valente R., et al. Changes in visceral pain reactivity as a function of estrous cycle in female rats with artificial ureteral calculosis. Brain Research. 1997;774:234–238.
Gijsbers K., Nicholson F. Experimental pain thresholds influenced by sex of experimenter. Perceptual and Motor Skills. 2005;101:803–807.
Gintzler A.R., Bohan M.C. Pain thresholds are elevated during pseudopregnancy. Brain Research. 1990;507:312–316.
Gioiosa L., Chen X., Watkins R., et al. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Hormones and Behavior. 2008;53:124–130.
Granot M., Yarnitsky D., Itskovitz-Eldor J., et al. Pain perception in women with dysmenorrhea. Obstetrics and Gynecology. 2001;98:407–411.
Greenspan J.D., Craft R.M., LeResche L., et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–S45.
Greenspan J.D., Slade G.D., Bair E., et al. Pain sensitivity risk factors for chronic TMD: descriptive data and empirically identified domains from the OPPERA case control study. Journal of Pain. 2011;12(Suppl 11):T61–T64.
Hagiwara H., Funabashi T., Mitsushima D., et al. Effects of neonatal testosterone treatment on sex differences in formalin-induced nociceptive behavior in rats. Neuroscience Letters. 2007;412:264–267.
Heitkemper M.M., Cain K.C., Jarrett M.E., et al. Symptoms across the menstrual cycle in women with irritable bowel syndrome. American Journal of Gastroenterology. 2003;98:420–430.
Heitkemper M.M., Jarrett M. Pattern of gastrointestinal and somatic symptoms across the menstrual cycle. Gastroenterology. 1992;102:505–513.
Hellstrom B., Lundberg U. Pain perception to the cold pressor test during the menstrual cycle in relation to estrogen levels and a comparison with men. Integrative Physiology & Behavioral Science. 2000;35:132–141.
Henderson L.A., Gandevia S.C., Macefield V.G. Gender differences in brain activity evoked by muscle and cutaneous pain: a retrospective study of single-trial fMRI data. NeuroImage. 2008;39:1867–1876.
Hobson A.R., Furlong P.L., Worthen S.F., Hillebrand A., Barnes G.R., Singh K.D., et al. Real-time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterology. 2005;128:610–619.
Holdcroft A., Berkley K.J. Sex and gender differences in pain and its relief. In: McMahon S.B., Koltzenburg M., eds. Wall and Melzack’s textbook of pain. 5th ed. Amsterdam: Elsevier; 2005:1181–1198.
Holdcroft A., Sapsed-Byrne S., Ma D., et al. Sex and oestrous cycle differences in visceromotor responses and vasopressin release in response to colonic distention in male and female rats anesthetized with halothane. British Journal of Anaesthia. 2000;85:907–910.
Hungin A.P., Whorwell P.J., Tack J., et al. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Alimentary Pharmacology & Therapeutics. 2003;17:643–650.
Hurley R.W., Adams M.C. Sex, gender, and pain: an overview of a complex field. Anesthesia and Analgesia. 2008;107:309–317.
Isacson D., Bingefors K. Epidemiology of analgesic use: a gender perspective. European Journal of Anaesthesiology. 2002;26:5–15.
Isselée H., De Laat A., De Mot B., et al. Pressure-pain threshold variation in temporomandibular disorder myalgia over the course of the menstrual cycle. Journal of Orofacial Pain. 2002;16:105–117.
Jensen I., Nygren A.L., Gamberale F., et al. Coping with long-term musculoskeletal pain and its consequences: is gender a factor? Pain. 1994;57:167–172.
Ji Y., Murphy A.Z., Traub R.J. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. Journal of Neuroscience. 2003;23:3908–3915.
Ji Y., Murphy A.Z., Traub R.J. Sex differences in morphine induced analgesia of visceral pain are supraspinally and peripherally mediated. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R307–R314.
Ji Y., Murphy A.Z., Traub R.J. Estrogen modulation of morphine analgesia of visceral pain in female rats is supraspinally and peripherally mediated. Journal of Pain. 2007;8:494–502.
Ji Y., Tang B., Traub R.J. Estrogen increases and progesterone decreases behavioral and neuronal responses to colorectal distention following colonic inflammation in the rat. Pain. 2005;117:433–442.
Ji Y., Tang B., Traub R.J. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience. 2008;154:1562–1567.
Ji Y., Tang B., Traub R.J. Spinal estrogen receptor alpha mediates estradiol-induced pronociception in a visceral pain model in the rat. Pain. 2011;152:1182–1191.
Ji Y., Traub R.J. Spinal NMDA receptors contribute to neuronal processing of acute noxious and nonnoxious colorectal stimulation in the rat. Journal of Neurophysiology. 2001;86:1783–1791.
Jiang P., Kong Y., Zhang X.B., et al. Glycine receptor in rat hippocampal and spinal cord neurons as a molecular target for rapid actions of 17-beta-estradiol. Molecular Pain. 2009;5:2.
Joels C.S., Mostafa G., Matthews B.D., et al. Factors affecting intravenous analgesic requirements after colectomy. Journal of the American College of Surgeons. 2003;197:780–785.
Jones A., Zachariae R., Arendt-Nielsen L. Dispositional anxiety and the experience of pain: gender-specific effects. European Journal of Pain. 2003;7:387–395.
Joseph E.K., Parada C.A., Levine J.D. Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain. 2003;105:143–150.
Kallai I., Barke A., Voss U. The effects of experimenter characteristics on pain reports in women and men. Pain. 2004;112:142–147.
Kane S.V., Sable K., Hanauer S.B. The menstrual cycle and its effect on inflammatory bowel disease and irritable bowel syndrome: a prevalence study. American Journal of Gastroenterology. 1998;93:1867–1872.
Kavaliers M., Choleris E. Sex differences in N-methyl-D-aspartate involvement in kappa opioid and non-opioid predator-induced analgesia in mice. Brain Research. 1997;768:30–36.
Keefe F.J., Lefebvre J.C., Egert J.R., et al. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87:325–334.
Keenan P.A., Lindamer L.A. Non-migraine headache across the menstrual cycle in women with and without premenstrual syndrome. Cephalalgia. 1992;12:356–359.
Keogh E., Barlow C., Mounce C., et al. Assessing the relationship between cold pressor pain responses and dimensions of the anxiety sensitivity profile in healthy men and women. Cognitive Behavioural Therapy. 2006;35:198–206.
Keogh E., Eccleston C. Sex differences in adolescent chronic pain and pain-related coping. Pain. 2006;123:275–284.
Keogh E., Hamid R., Hamid S., et al. Investigating the effect of anxiety sensitivity, gender and negative interpretative bias on the perception of chest pain. Pain. 2004;111:209–217.
Kern M., Shaker R. Further characterization of human brain processing of viscero-sensation: the role of gender and a word of caution. Gastroenterology. 2003;124:1975–1977.
Kest B., Sarton E., Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93:539–547.
Kest B., Wilson S.G., Mogil J.S. Sex differences in supraspinal morphine analgesia are dependent on genotype. Journal of Pharmacology and Experimental Therapeutics. 1999;289:1370–1375.
Kim S.J., Calejesan A.A., Li P., et al. Sex differences in late behavioral response to subcutaneous formalin injection in mice. Brain Research. 1999;829:185–189.
Klatzkin R.R., Mechlin B., Girdler S.S. Menstrual cycle phase does not influence gender differences in experimental pain sensitivity. European Journal of Pain. 2010;14:77–82.
Kowalczyk W.J., Evans S.M., Bisaga A.M., et al. Sex differences and hormonal influences on response to cold pressor pain in humans. Journal of Pain. 2006;7:151–160.
Kramer P.R., Bellinger L.L. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint–induced nociception. Endocrinology. 2009;150:3680–3689.
Kren M.C., Haller V.L., Welch S.P. The role of gonadal hormones on opioid receptor protein density in arthritic rats. European Journal of Pharmacology. 2008;578:177–184.
Kuba T., Wu H.B., Nazarian A., et al. Estradiol and progesterone differentially regulate formalin-induced nociception in ovariectomized female rats. Hormones and Behavior. 2006;49:441–449.
LaCroix-Fralish M.L., Rutkowski M.D., Weinstein J.N., et al. The magnitude of mechanical allodynia in a rodent model of lumbar radiculopathy is dependent on strain and sex. Spine. 2005;30:1821–1827.
LaCroix-Fralish M.L., Tawfik V.L., DeLeo J.A. The organizational and activational effects of sex hormones on tactile and thermal hypersensitivity following lumbar nerve root injury in male and female rats. Pain. 2005;114:71–80.
LaCroix-Fralish M.L., Tawfik V.L., Spratt K.F., et al. Sex differences in lumbar spinal cord gene expression following experimental lumbar radiculopathy. Journal of Molecular Neuroscience. 2006;30:283–295.
Lee S.Y., Kim J.H., Sung I.K., et al. Irritable bowel syndrome is more common in women regardless of the menstrual phase: a Rome II–based survey. Journal of Korean Medical Science. 2007;22:851–854.
LeResche L. Epidemiologic perspectives on sex differences in pain. In: Fillingim R.B., ed. Sex, gender, and pain. Seattle: IASP Press; 2000:233–249.
LeResche L., Mancl L., Sherman J.J., et al. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261.
LeResche L., Saunders K., Von Korff M.R., et al. Use of exogenous hormones and risk of temporomandibular disorder pain. Pain. 1997;69:153–160.
Levine F.M., De Simone L.L. The effects of experimenter gender on pain report in male and female subjects. Pain. 1991;44:69–72.
Lichten E.M., Lichten J.B., Whitty A., et al. The confirmation of a biochemical marker for women’s hormonal migraine: the depo-estradiol challenge test. Headache. 1996;36:367–371.
Liu B., Eisenach J.C., Tong C. Chronic estrogen sensitizes a subset of mechanosensitive afferents innervating the uterine cervix. Journal of Neurophysiology. 2005;93:2167–2173.
Liverman C.S., Brown J.W., Sandhir R., et al. Role of the oestrogen receptors GPR30 and ERalpha in peripheral sensitization: relevance to trigeminal pain disorders in women. Cephalalgia. 2009;29:729–741.
Loyd D.R., Murphy A.Z. The role of the periaqueductal gray in the modulation of pain in males and females: are the anatomy and physiology really that different? Neural Plasticity. 2009;2009:462879.
Loyd D.R., Wang X., Murphy A.Z. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. Journal of Neuroscience. 2008;28:14007–14017.
Lu C.L., Hsieh J.C., Dun N.J., et al. Estrogen rapidly modulates 5-hydroxytrytophan–induced visceral hypersensitivity via GPR30 in rats. Gastroenterology. 2009;137:1040–1050.
Lu C.L., Hsieh J.C., Tsaur M.L., et al. Estrogen rapidly modulates mustard oil–induced visceral hypersensitivity in conscious female rats: a role of CREB phosphorylation in spinal dorsal horn neurons. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2007;292:G438–G446.
Lu Y.C., Chen C.W., Wang S.Y., et al. 17Beta-estradiol mediates the sex difference in capsaicin-induced nociception in rats. Journal of Pharmacology and Experimental Therapeutics. 2009;331:1104–1110.
Macfarlane T.V., Blinkhorn A., Worthington H.V., et al. Sex hormonal factors and chronic widespread pain: a population study among women. Rheumatology (Oxford). 2002;41:454–457.
Martin V.T. New theories in the pathogenesis of menstrual migraine. Current Pain and Headache Reports. 2008;12:453–462.
Martin V.T., Lee J., Behbehani M.M. Sensitization of the trigeminal sensory system during different stages of the rat estrous cycle: implications for menstrual migraine. Headache. 2007;47:552–563.
McRoberts J.A., Li J., Ennes H.S., et al. Sex-dependent differences in the activity and modulation of N-methyl-D-aspartic acid receptors in rat dorsal root ganglia neurons. Neuroscience. 2007;148:1015–1020.
Miaskowski C. Gender differences in pain, fatigue, and depression in patients with cancer. Journal of the National Cancer Institute. Monographs. 2004;32:139–143.
Miaskowski C., Levine J.D. Does opioid analgesia show a gender preference for females? Pain Forum. 1999;8:34–44.
Miller P.L., Ernst A.A. Sex differences in analgesia: a randomized trial of mu versus kappa opioid agonists. Southern Medical Journal. 2004;97:35–41.
Mogil J.S., Bailey A.L. Sex and gender differences in pain and analgesia. Progress in Brain Research. 2010;186:141–157.
Mogil J.S., Chesler E.J., Wilson S.G., et al. Sex differences in thermal nociception and morphine antinociception in rodents depend on genotype. Neuroscience and Biobehavioral Reviews. 2000;24:375–389.
Mogil J.S., Richards S.P., O’Toole L.A., et al. Identification of a sex-specific quantitative trait locus mediating nonopioid stress-induced analgesia in female mice. Journal of Neuroscience. 1997;17:7995–8002.
Mogil J.S., Sternberg W.F., Kest B., et al. Sex differences in the antagonism of swim stress–induced analgesia: effects of gonadectomy and estrogen replacement. Pain. 1993;53:17–25.
Mogil J.S., Wilson S.G., Chesler E.J., et al. The melanocortin-1 receptor gene mediates female-specific mechanisms of analgesia in mice and humans. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:4867–4872.
Moulton E.A., Keaser M.L., Gullapalli R.P., Maitra R., Greenspan J.D. Sex differences in the cerebral BOLD signal response to painful heat stimuli. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R257–R267.
Murphy A.Z., Suckow S.K., Johns M., et al. Sex differences in the activation of the spinoparabrachial circuit by visceral pain. Physiology & Behavior. 2009;97:205–212.
Musgrave D.S., Vogt M.T., Nevitt M.C., et al. Back problems among postmenopausal women taking estrogen replacement therapy: the study of osteoporotic fractures. Spine. 2001;26:1606–1612.
Myers C.D., Riley J.L., Robinson M.E. Psychosocial contributions to sex-correlated differences in pain. Clinical Journal of Pain. 2003;19:225–232.
Myers C.D., Robinson M.E., Riley J.L., III., et al. Sex, gender, and blood pressure: contributions to experimental pain report. Psychosomatic Medicine. 2001;63:545–550.
Naliboff B., Berman S., Chang L., et al. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747.
Nayak S., Shiflett S.C., Eshun S., et al. Culture and gender effects in pain beliefs and the prediction of pain tolerance: cross-cultural research. Journal of Comparative Social Sciences. 2000;34:135–151.
Ness T.J., Lewis-Sides A., Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distention in rats: sources of variability and effect of analgesics. Journal of Urology. 2001;165:968–974.
Niesters M., Dahan A., Kest B., et al. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain. 2010;151:61–68.
Ockene J.K., Barad D.H., Cochrane B.B., et al. Symptom experience after discontinuing use of estrogen plus progestin. JAMA. 2005;294:183–193.
Okamoto K., Hirata H., Takeshita S., et al. Response properties of TMJ units in superficial laminae at the spinomedullary junction of female rats vary over the estrous cycle. Journal of Neurophysiology. 2003;89:1467–1477.
Okifuji A., Turk D.C. Sex hormones and pain in regularly menstruating women with fibromyalgia syndrome. Journal of Pain. 2006;7:851–859.
Otto M.W., Dougher M.J. Sex differences and personality factors in the responsivity to pain. Perceptual and Motor Skills. 1985;61:383–390.
Pajot J., Ressot C., Ngom I., et al. Gonadectomy induces site-specific differences in nociception in rats. Pain. 2003;104:367–373.
Pamuk Ö N., Çakir N. The variation in chronic widespread pain and other symptoms in fibromyalgia patients. The effects of menses and menopause. Clinical and Experimental Rheumatology. 2005;23:778–782.
Paulson P.E., Minoshima S., Morrow T.J., et al. Gender differences in pain perception and patterns of cerebral activation during noxious heat stimulation in humans. Pain. 1998;76:223–229.
Peng H.Y., Chen G.D., Lai C.Y., et al. PI3K modulates estrogen-dependent facilitation of colon-to-urethra cross-organ reflex sensitization in ovariectomized female rats. Journal of Neurochemistry. 2010;113:54–66.
Peng H.Y., Chen G.D., Tung K.C., et al. Estrogen-dependent facilitation on spinal reflex potentiation involves the Cdk5/ERK1/2/NR2B cascade in anesthetized rats. American Journal of Physiology. Endocrinology and Metabolism. 2009;297:E416–E426.
Pool G.J., Schwegler A.F., Theodore B.R., et al. Role of gender norms and group identification on hypothetical and experimental pain tolerance. Pain. 2007;129:122–129.
Popescu A., LeResche L., Truelove E.L., et al. Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain. 2010;150:309–318.
Pud D., Yarnitsky D., Sprecher E., et al. Can personality traits and gender predict the response to morphine? An experimental cold pain study. European Journal of Pain. 2006;10:103–112.
Puri J., Bellinger L.L., Kramer P.R. Estrogen in cycling rats alters gene expression in the temporomandibular joint, trigeminal ganglia and trigeminal subnucleus caudalis/upper cervical cord junction. Journal of Cellular Physiology. 2011;226:3169–3180.
Quiton R.L., Greenspan J.D. Sex differences in endogenous pain modulation by distracting and painful conditioning stimulation. Pain. 2007;132(Suppl 1):S134–S149.
Racine M., Tousignant-Laflamme Y., Kloda L.A., et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception—Part 1: are there really differences between women and men? Pain. 2012;153:602–618.
Racine M., Tousignant-Laflamme Y., Kloda L.A., et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception—Part 2: do biopsychosocial factors alter pain sensitivity differently in women and men? Pain. 2012;153:619–635.
Rasakham K., Liu-Chen L.Y. Sex differences in kappa opioid pharmacology. Life Sciences. 2011;88:2–16.
Reyes-Gibby C.C., Aday L.A., Anderson K.O., et al. Pain, depression, and fatigue in community-dwelling adults with and without a history of cancer. Journal of Pain and Symptom Management. 2006;32:118–128.
Rhudy J.L., Williams A.E. Gender differences in pain: do emotions play a role? Gender Medicine. 2005;2:208–226.
Ribeiro S., Yang P., Reyes-Vazquez C., et al. Sex differences in tail-flick latency of non-stressed and stressed rats. International Journal of Neuroscience. 2005;115:1383–1395.
Riley J.L., Robinson M.E., Wise E.A., et al. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187.
Riley J.L., Robinson M.E., Wise E.A., et al. A meta-analytic review of pain perception across the menstrual cycle. Pain. 1999;81:225–235.
Robbins A., Berkley K.J., Sato Y. Estrous cycle variation of afferent fibers supplying reproductive organs in the female rat. Brain Research. 1992;596:352–356.
Robbins M.T., Mebane H., Ball C.L., et al. Effect of estrogen on bladder nociception in rats. Journal of Urology. 2010;183:1201–1205.
Robinson M., Wise E., Gagnon C., et al. Influences of gender role and anxiety on sex differences in temporal summation of pain. Journal of Pain. 2004;5:77–82.
Robinson M.E., Dannecker E.A., George S.Z., et al. Sex differences in the associations among psychological factors and pain report: a novel psychophysical study of patients with chronic low back pain. Journal of Pain. 2005;6:463–470.
Robinson M.E., Riley J.L., III., Brown F.F., et al. Sex differences in response to cutaneous anesthesia: a double blind randomized study. Pain. 1998;77:143–149.
Robinson M.E., Riley J.L., Myers C.D., et al. Gender role expectations of pain: relationship to sex differences in pain. Journal of Pain. 2001;2:251–257.
Rollman G.B. Gender differences in pain: role of anxiety. Pain Forum. 1995;4:231–234.
Ryan J.L., Jureidini B., Hodges J.S., et al. Gender differences in analgesia for endodontic pain. Journal of Endodontics. 2008;34:552–556.
Sanford S.D., Kersh B.C., Thorn B.E., et al. Psychosocial mediators of sex differences in pain responsivity. Journal of Pain. 2002;3:58–64.
Sanoja R., Cervero F. Estrogen-dependent abdominal hyperalgesia induced by ovariectomy in adult mice: a model of functional abdominal pain. Pain. 2005;118:243–253.
Sapsed-Byrne S., Ma D., Ridout D., et al. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Research. 1996;742:10–16.
Sherman J.J., LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R245–R256.
Sherman J.J., LeResche L., Mancl L.A., et al. Cyclic effects on experimental pain response in women with temporomandibular disorders. Journal of Orofacial Pain. 2005;19:133–143.
Smith Y.R., Stohler C.S., Nichols T.E., et al. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. Journal of Neuroscience. 2006;26:5777–5785.
Soderberg K., Sundstrom P.I., Nyberg S., et al. Psychophysically determined thresholds for thermal perception and pain perception in healthy women across the menstrual cycle. Clinical Journal of Pain. 2006;22:610–616.
Somerville B.W. The influence of progesterone and estradiol upon migraine. Headache. 1972;12:93–102.
Stening K., Eriksson O., Wahren L., et al. Pain sensations to the cold pressor test in normally menstruating women: comparison with men and relation to menstrual phase and serum sex steroid levels. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2007;293:R1711–R1716.
Stewart S.H., Asmundson G.J. Anxiety sensitivity and its impact on pain experiences and conditions: a state of the art. Cognitive Behavioural Therapy. 2006;35:185–188.
Stoffel E.C., Ulibarri C.M., Folk J.E., et al. Gonadal hormone modulation of mu, kappa, and delta opioid antinociception in male and female rats. Journal of Pain. 2005;6:261–274.
Straneva P.A., Maixner W., Light K.C., et al. Menstrual cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychology. 2002;21:358–367.
Straub R.H. The complex role of estrogens in inflammation. Endocrine Reviews. 2007;28:521–574.
Straube T., Schmidt S., Weiss T., Mentzel H.J., Miltner W.H.R. Sex differences in brain activation to anticipated and experienced pain in the medial prefrontal cortex. Human Brain Mapping. 2009;30:689–698.
Symmons D.P., van Hemert A.M., Vandenbroucke J.P., et al. A longitudinal study of back pain and radiological changes in the lumbar spines of middle aged women. I. Clinical findings. Annals of the Rheumatic Diseases. 1991;50:158–161.
Tang B., Ji Y., Traub R.J. Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat. Pain. 2008;137:540–549.
Tashiro A., Okamoto K., Bereiter D.A. NMDA receptor blockade reduces temporomandibular joint–evoked activity of trigeminal subnucleus caudalis neurons in an estrogen-dependent manner. Neuroscience. 2009;164:1805–1812.
Tassorelli C., Sandrini G., Cecchini A.P., et al. Changes in nociceptive flexion reflex threshold across the menstrual cycle in healthy women. Psychosomatic Medicine. 2002;64:621–626.
Teepker M., Peters M., Vedder H., et al. Menstrual variation in experimental pain: correlation with gonadal hormones. Neuropsychobiology. 2010;61:131–140.
Terner J.M., Barrett A.C., Cook C.D., et al. Sex differences in (−)-pentazocine antinociception: comparison to morphine and spiradoline in four rat strains using a thermal nociceptive assay. Behavioural Pharmacology. 2003;14:77–85.
Thompson A.D., Angelotti T., Nag S., et al. Sex-specific modulation of spinal nociception by alpha(2)-adrenoceptors: differential regulation by estrogen and testosterone. Neuroscience. 2008;153:1268–1277.
Thorn B.E., Clements K.L., Ward L.C., et al. Personality factors in the explanation of sex differences in pain catastrophizing and response to experimental pain. Clinical Journal of Pain. 2004;20:275–282.
Traub R.J., Zhai Q.Z., Ji Y., et al. NMDA receptor antagonists attenuate noxious and nonnoxious colorectal distention–induced Fos expression and the visceromotor reflex. Neuroscience. 2002;113:205–211.
Turk D.C., Okifuji A. Does sex make a difference in the prescription of treatments and the adaptation to chronic pain by cancer and non-cancer patients? Pain. 1999;82:139–148.
Unruh A.M. Gender variations in clinical pain experience. Pain. 1996;65:123–167.
Unruh A.M., Ritchie J., Merskey H. Does gender affect appraisal of pain and pain coping strategies? Clinical Journal of Pain. 1999;15:31–40.
Valeberg B.T., Rustoen T., Bjordal K., et al. Self-reported prevalence, etiology, and characteristics of pain in oncology outpatients. European Journal of Pain. 2008;12:582–590.
van den Beuken-van Everdingen M.H., de Rijke J.M., Kessels A.G., et al. High prevalence of pain in patients with cancer in a large population-based study in The Netherlands. Pain. 2007;132:312–320.
Vignolo V., Vedolin G.M., de Araujo C.R., et al. Influence of the menstrual cycle on the pressure pain threshold of masticatory muscles in patients with masticatory myofascial pain, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology. and Endodontics. 2008;105:308–315.
Walker J.S., Carmody J.J. Experimental pain in healthy human subjects: gender differences in nociception and in response to ibuprofen. Anesthesia and Analgesia. 1998;86:1257–1262.
Wang X., Traub R.J., Murphy A.Z. Persistent pain model reveals sex difference in morphine potency. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R300–R306.
Weissman-Fogel I., Sprecher E., Pud D. Effects of catastrophizing on pain perception and pain modulation. Experimental Brain Research. 2008;186:79–85.
Weitzel K.W., Strickland J.M., Smith K.M., et al. Gender-specific issues in the treatment of migraine. Journal of Gender Specific Medicine. 2001;4:64–74.
Weyand C.M., Schmidt D., Wagner U., et al. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis and Rheumatism. 1998;41:817–822.
Wise E.A., Price D.D., Myers C.D., et al. Gender role expectations of pain: relationship to experimental pain perception. Pain. 2002;96:335–342.
Wizemann T.M., Pardue M- L. Exploring the biological contributions to human health: does sex matter?. Washington DC: National Academies Press; 2001.
Yan T., Liu B., Du D., et al. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesthesia and Analgesia. 2007;104:1246–1250.
Yarnitsky D., Arendt-Nielsen L., Bouhassira D., et al. Recommendations on terminology and practice of psychophysical DNIC testing. European Journal of Pain. 2010;14:339.
You H.J., Cao D.Y., Yuan B., et al. Sex differences in the responses of spinal wide–dynamic range neurons to subcutaneous formalin and in the effects of different frequencies of conditioning electrical stimulation. Neuroscience. 2006;138:1299–1307.
Zubieta J.K., Dannals R.F., Frost J.J. Gender and age influences on human brain mu-opioid receptor binding measured by PET. American Journal of Psychiatry. 1999;156:842–848.
Zubieta J.K., Smith Y.R., Bueller J.A., et al. μ-Opioid receptor–mediated antinociceptive responses differ in men and women. Journal of Neuroscience. 2002;22:5100–5107.
Berkley K.J. Sex differences in pain. Behavioral and Brain Sciences. 1997;20:371–380.
Berkley K.J., Zalcman S.S., Simon V.R. Sex and gender differences in pain and inflammation: a rapidly maturing field. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R241–R244.
Bodnar R.J., Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: central mechanisms of action and roles of gonadal hormones. Hormones and Behavior. 2010;58:72–81.
Craft R.M. Sex differences in opioid analgesia: “from mouse to man.”. Clinical Journal of Pain. 2003;19:175–186.
Craft R.M., Mogil J.S., Aloisi A.M. Sex differences in pain and analgesia: the role of gonadal hormones. European Journal of Pain. 2004;8:397–411.
Dahan A., Kest B., Waxman A.R., et al. Sex-specific responses to opiates: animal and human studies. Anesthesia and Analgesia. 2008;107:83–95.
Fillingim R.B., Gear R.W. Sex differences in opioid analgesia: clinical and experimental findings. European Journal of Pain. 2004;8:413–425.
Fillingim R.B., King C.D., Ribeiro-Dasilva M.C., et al. Sex, gender, and pain: a review of recent clinical and experimental findings. Journal of Pain. 2009;10:447–485.
Fillingim R.B., Ness T.J. Sex-related hormonal influences on pain and analgesic responses. Neuroscience and Biobehavioral Reviews. 2000;24:485–501.
Greenspan J.D., Craft R.M., LeResche L., et al. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–S45.
Hurley R.W., Adams M.C. Sex, gender, and pain: an overview of a complex field. Anesthesia and Analgesia. 2008;107:309–317.
Kest B., Sarton E., Dahan A. Gender differences in opioid-mediated analgesia: animal and human studies. Anesthesiology. 2000;93:539–547.
Mogil J.S., Bailey A.L. Sex and gender differences in pain and analgesia. Progress in Brain Research. 2010;186:141–157.
Popescu A., LeResche L., Truelove E.L., et al. Gender differences in pain modulation by diffuse noxious inhibitory controls: a systematic review. Pain. 2010;150:309–318.
Sherman J.J., LeResche L. Does experimental pain response vary across the menstrual cycle? A methodological review. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2006;291:R245–R256.
Stewart S.H., Asmundson G.J. Anxiety sensitivity and its impact on pain experiences and conditions: a state of the art. Cognitive Behaviour Therapy. 2006;35:185–188.
Zubieta J.K., Smith Y.R., Bueller J.A., et al. μ-Opioid receptor–mediated antinociceptive responses differ in men and women. Journal of Neuroscience. 2002;22:5100–5107.