Pain Measurement in Adult Patients
Introduction
Measurement is central to accurate diagnosis and management. Measurement of pain is therefore essential to determine the intensity, perceptual qualities, and time course of the pain so that the differences among pain syndromes can be ascertained and investigated. Furthermore, measurement of these variables provides valuable clues that help in the differential diagnosis of the underlying causes of the pain. They also help determine the most effective treatment necessary to control the pain and are essential to evaluate the relative effectiveness of different therapies. Measurement of pain, then, is important to
Dimensions of the Pain Experience
Research on pain, since the beginning of the 20th century, has been dominated by the concept that pain is purely a sensory experience. Yet pain also has a distinctly unpleasant, affective quality. It becomes overwhelming, demands immediate attention, and disrupts ongoing behavior and thought. It motivates or drives the organism to activity aimed at stopping the pain as quickly as possible. To consider only the sensory features of pain and ignore its motivational–affective properties is to look at only part of the problem. Even the concept of pain as a perception—with full recognition of past experience, attention, and other cognitive influences—still neglects the crucial motivational dimension.
These considerations led Melzack and Casey (1968) to suggest that pain has three major psychological dimensions: sensory–discriminative, motivational–affective, and cognitive–evaluative. They proposed, moreover, that these dimensions of the pain experience are subserved by physiologically specialized systems in the brain.
Melzack and Casey (1968) postulated that these three categories of activity interact with one another to provide perceptual information on the location, magnitude, and spatiotemporal properties of the noxious stimuli; motivational tendency toward escape or attack; and cognitive information based on past experience and probability of the outcome of different response strategies. All three forms of activity could then influence the motor mechanisms responsible for the complex pattern of overt responses that characterize pain.
The Language of Pain
Clinical investigators have long recognized the varieties of the pain experience. Descriptions of the burning qualities of pain after peripheral nerve injury or the stabbing, cramping qualities of visceral pain frequently provide the key to diagnosis and may even suggest the course of therapy. Despite the frequency of such descriptions and the seemingly high agreement that they are valid descriptive words, studies of their use and meaning are relatively recent.
Anyone who has suffered severe pain and tried to describe the experience to a friend or to the doctor often finds herself or himself at a loss for words. The reason for this difficulty in expressing the pain experience actually is not because the words do not exist. As we shall soon see, there are an abundance of descriptive words. Rather, the main reason is that fortunately, they are not words that we have occasion to use often. Another reason is that the words may seem absurd. We may use descriptors such as splitting, shooting, gnawing, wrenching, or stinging as useful metaphors, but there are no external objective references for these words in relation to pain. If we talk about a blue pen or a yellow pencil, we can point to an object and say “that is what I mean by yellow” or “the color of the pen is blue.” However, to what can we point to tell another person precisely what we mean by smarting, tingling, or rasping? A person who experiences terrible pain may say that the pain is burning and add that “it feels as if someone is shoving a red-hot poker through my toes and slowly twisting it around.” These “as if” statements are often essential to convey the qualities of the experience.
If the study of pain in people is to have a scientific foundation, it is essential to measure it. If we want to know how effective a new drug is, we need numbers to say that the pain decreased by some amount. Yet although overall intensity is important information, we also want to know whether the drug specifically decreased the burning quality of the pain or if the especially miserable, tight cramping feeling is gone.
Pain Rating Scales
Traditional methods of pain measurement treat pain as though it were a single unique quality that varies only in intensity (Beecher 1959). These methods include the use of verbal rating scales (VRSs), numerical rating scales (NRSs), and visual analog scales (VASs). These simple methods have all been used effectively and have provided valuable information about pain and analgesia. VRSs, NRSs, and VASs provide simple, efficient, and minimally intrusive measures of pain intensity that have been used widely in clinical and research settings in which a quick index of pain intensity is required and to which a numerical value can be assigned.
VRSs typically consist of a series of verbal pain descriptors ordered from least to most intense (e.g., no pain, mild, moderate, and severe) (Jensen and Karoly 2011). Patients read or are read the list and choose the one word that best describes the intensity of their pain at the moment (or over some time interval such as a day or a week). A score of 0 is assigned to the descriptor with the lowest rank, a score of 1 is assigned to the descriptor with the next lowest rank, and so forth.
NRSs typically consist of a series of numbers ranging from 0–10 or 0–100, with end points intended to represent the extremes of the possible pain experience and labeled “no pain” and “worst possible pain,” respectively. Patients choose the number that best corresponds to the intensity of their pain. VRSs and NRSs are simple to administer and have demonstrated reliability and validity (Williamson and Hoggart 2005).
The most common VAS consists of a 10-cm horizontal or vertical line with the two end points labeled “no pain” and “worst pain ever” (or similar verbal descriptors). Patients are required to place a mark on the 10-cm line at the point that corresponds to the level of pain intensity that they presently feel (or felt over the past day, week, etc.). The distance in centimeters from the low end of the VAS to the patient’s mark is used as a numerical index of the severity of the pain.
VASs for pain affect have been developed in an effort to include domains of measurable pain experience other than the sensory intensity dimension. The patient is asked to rate the unpleasantness of the pain experience (i.e., how disturbing it is). End points are labeled “not bad at all” and “the most unpleasant feeling imaginable” (Price et al 1987).
A major advantage of the VAS as a measure of sensory pain intensity over NRSs and VRSs is its ratio scale properties (Price et al 1983). In contrast to many other pain measurement tools, equality of ratios is implied, which makes it appropriate to speak meaningfully about percent differences between VAS measurements obtained either at multiple points in time or from independent samples of individuals. Despite this advantage, there has been a recent trend away from use of the VAS in clinical and research settings, largely because of its requirement for additional material (e.g., paper and pencil, computer) and empirical data showing that VRSs and NRSs have sound psychometric properties (Williamson and Hoggart 2005).
Standard VASs also have several limitations and disadvantages, including difficulty of administration to patients who have perceptual–motor problems; impractical scoring method in a clinical setting, where immediate measurement of the patient’s response may not be possible; the occasional patient who cannot comprehend the instructions; and problems with use in telephone surveys or with electronic devices that are not equipped with the scale.
Although VRSs, NRSs, and VASs have all been shown to have adequate or better than adequate psychometric properties (i.e., validity and reliability), comparisons of the three scales generally show that VRSs lack sensitivity to detect changes in pain intensity when compared with VASs or NRSs (Breivik et al 2000, 2008). However, despite the advantages associated with unidimensional pain rating scales, they fail to capture the complexity of the pain (Williams et al 2000) and, whenever possible, should be co-administered with a multidimensional measure of pain.
Disadvantages of Pain Rating Scales
The main disadvantage of VASs, NRSs, and VRSs is the assumption that pain is a unidimensional experience that can be measured with a single-item scale (Melzack 1975). Although intensity is without doubt a salient dimension of pain, it is clear that the word pain refers to an endless variety of qualities that are categorized under a single linguistic label, not to a specific, single sensation that varies only in intensity or affect. The development of rating scales to measure pain affect or pain unpleasantness (Price et al 1987) has partially addressed the problem, but the same shortcoming applies within the affective domain. Each pain has unique qualities. Unpleasantness is only one such quality. The pain of a toothache is obviously different from that of a pinprick, just as the pain of coronary occlusion is uniquely different from the pain of a broken leg. To describe pain solely in terms of intensity or affect is like specifying the visual world only in terms of light flux without regard to pattern, color, texture, and the many other dimensions of the visual experience.
The Mcgill Pain Questionnaire
Melzack and Torgerson (1971) developed procedures to specify the qualities of pain. In the first part of their study, physicians and other university graduates were asked to classify 102 words obtained from the clinical literature into small groups that describe distinctly different aspects of the experience of pain. On the basis of the data, the words were categorized into three major classes and 16 subclasses (Fig. 21-1). These classes consist of
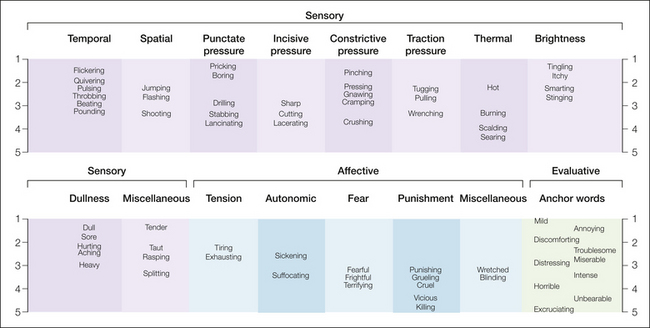
Figure 21-1 Spatial display of pain descriptors based on intensity ratings by patients.
The intensity scale values range from 1 (mild) to 5 (excruciating).
Each subclass, which was given a descriptive label, consists of a group of words that were considered by most subjects to be qualitatively similar. Some of these words are undoubtedly synonyms, others seem to be synonymous but vary in intensity, and many provide subtle differences or nuances (despite their similarities) that may be of importance to a patient who is trying desperately to communicate to a physician.
The second part of the study by Melzack and Torgerson (1971) was an attempt to determine the pain intensity implied by the words within each subclass. Groups of physicians, patients, and students were asked to assign an intensity value to each word by using a numerical scale ranging from least (or mild) pain to worst (or excruciating) pain. When this was done, it was apparent that several words within each subclass had the same relative intensity relationships in all three sets. For example, in the spatial subclass, shooting was found to represent more pain than flashing, which in turn implied more pain than jumping. Although the precise intensity scale values differed for the three groups, all three agreed on the positions of the words relative to each other. Figure 21-1 shows the scale values of the words for patients based on the precise numerical values listed by Melzack and Torgerson (1971).
Because of the high degree of agreement on the intensity relationships among pain descriptors by subjects who have different cultural, socio-economic, and educational backgrounds, a pain questionnaire (Fig. 21-2) was developed as an experimental tool to study the effects of various methods of pain management. In addition to the list of pain descriptors, the questionnaire contains line drawings outlining the body to show the spatial distribution of the pain, words that describe temporal properties of the pain, and descriptors of the overall present pain intensity. The present pain intensity is recorded as a number from 1–5, with each number being associated with the following words: 1, mild; 2, discomforting; 3, distressing; 4, horrible; and 5, excruciating. The mean scale values of these words, which were chosen from the evaluative category, are approximately equally far apart so that they represent equal scale intervals and thereby provide anchors for specification of the overall pain intensity (Melzack and Torgerson 1971).
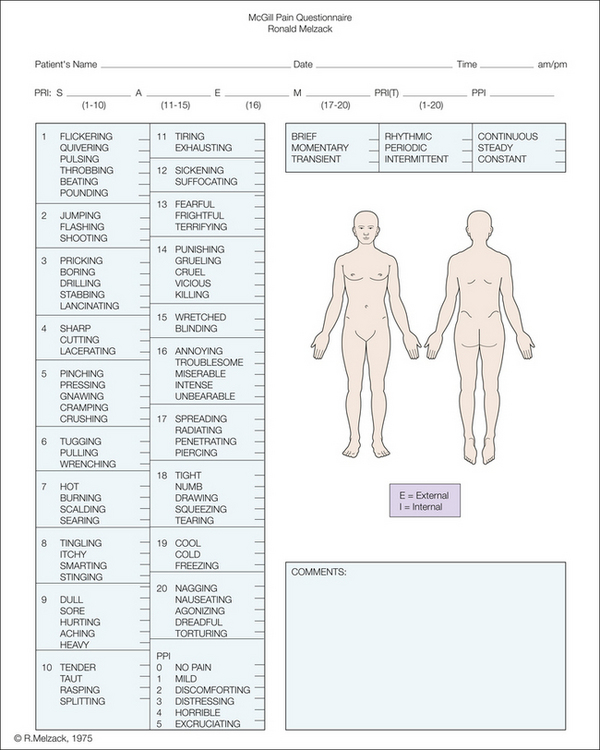
Figure 21-2 McGill Pain Questionnaire.
The descriptors fall into four major groups: sensory, 1–10; affective, 11–15; evaluative, 16; and miscellaneous, 17–20. The rank value for each descriptor is based on its position in the word set. The sum of the rank values is the pain rating index. The present pain intensity (PPI) is based on a scale of 0–5.
In a preliminary study, the pain questionnaire consisted of the 16 subclasses of descriptors shown in Figure 21-1, as well as additional information deemed necessary for the evaluation of pain. It soon became clear, however, that many of the patients found certain relevant words to be absent. These words were then selected from the original word list used by Melzack and Torgerson (1971), categorized appropriately, and ranked according to their mean scale values. A further set of words—cool, cold, and freezing—were used by patients on rare occasion but were indicated to be essential for an adequate description of some types of pain. Thus, four supplementary, or miscellaneous, subclasses were added to the word lists of the questionnaire (Fig. 21-2). The final classification, then, appeared to represent the most parsimonious and meaningful set of subclasses without at the same time losing subclasses that represent important qualitative properties. The questionnaire, which is known as the McGill Pain Questionnaire (MPQ) (Melzack 1975), has become the most widely used clinical and research tool for measuring pain.
Measures of the Pain Experience
The descriptor lists of the MPQ are read to patients with the explicit instruction that they choose only words that describe their feelings and sensations at that moment. It can also be filled out by the patient in a more leisurely way as a paper-and-pencil test, although the scores are somewhat different (Klepac et al 1981). Three major indices are obtained:
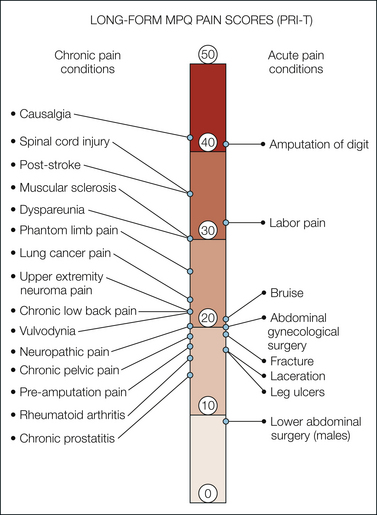
Figure 21-3 Long-form McGill Pain Questionnaire (MPQ) pain rating index scores.
Pain scores are compared in women during labor (Melzack et al 1981), patients in a general hospital pain clinic (Melzack 1975), and patients in an emergency department (Melzack et al 1982). The pain score for causalgic pain is reported by Tahmoush (1981). Other pain ratings come from studies of patients with chronic pain conditions, including lung cancer pain (Wilkie et al 2001), low back pain (Scrimshaw and Maher 2001), upper extremity neuroma pain (Stokvis et al 2010), spinal cord injury pain (Defrin et al 2007), multiple sclerosis–related pain (Douglas et al 2008), dyspareunia (Leclerc et al 2010), vulvodynia (Forth et al 2009), female pelvic pain (Weijenborg et al 2007), chronic prostatectomy (Pontari et al 2010), neuropathic pain (Lynch et al 2003), lower extremity pre-amputation pain (Nikolajsen et al 1997), and rheumatoid arthritis (Roche et al 2003). Also shown are total pain rating index (PRI-T) scores from patients with acute pain arising from venous and arterial leg ulcers (Closs et al 2008) and acute pain after abdominal gynecological surgery (Katz et al 2003) and lower abdominal surgery (Katz et al 1994).
Usefulness of the McGill Pain Questionnaire
The most important requirements of a measure are that it be valid, reliable, consistent, and above all, useful. The MPQ appears to meet all these requirements (Melzack 1975, Chapman et al 1985, Wilkie et al 1990) and provides a relatively rapid way of measuring subjective pain experience (Melzack 1975). When administered to a patient by reading the words in each subclass, it can be completed in about 5 minutes.
Since its introduction in 1975, the MPQ has been used in more than 500 studies of acute, chronic, and laboratory-produced pain. It has been translated into many languages and has also spawned the development of similar pain questionnaires in other languages (Table 21-1), but relatively few data exist on the psychometric properties of these cross-cultural adaptions (Menezes Costa et al 2009).
Table 21-1
Pain Questionnaires in Different Languages Based on the McGill Pain Questionnaire
LANGUAGE |
AUTHORS |
| Amharic (Ethiopia) | Aboud et al 2003 |
| Arabic | Harrison 1988 |
| Chinese | Hui and Chen 1989 |
| Danish | Drewes et al 1993 |
| Dutch (Flemish) | Vanderiet et al 1987 Verkes et al 1989 van Lankveld et al 1992 van der Kloot et al 1995 |
| Finnish | Ketovuori and Pöntinen 1981 |
| French | Boureau et al 1984, 1992 |
| German | Kiss et al 1987 Radvila et al 1987 Stein and Mendl 1988 |
| Greek | Georgoudis et al 2000, 2001 Mystakidou et al 2002 |
| Italian | Maiani and Sanavio 1985 De Benedittis et al 1988 Ferracuti et al 1990 |
| Japanese | Satow et al 1990 Hasegawa et al 2001 Hobara et al 2003 |
| Norwegian | Strand and Wisnes 1991 Kim et al 1995 |
| Polish | Sedlak 1990 |
| Portuguese | Pimenta and Teixeiro 1996 |
| Slovak | Bartko et al 1984 |
| Spanish | Laheurta et al 1982 Bejarano et al 1985 Lázaro et al 1994 Escalante et al 1996 Masedo and Esteve 2000 |
Because pain is a private, personal experience, it is impossible for us to know precisely what someone else’s pain feels like. No man can possibly know what it is like to have menstrual cramps or labor pain. Nor can a psychologically healthy person know what psychotic patients are feeling when they say they have excruciating pain (Veilleux and Melzack 1976). However, the MPQ provides us with an insight into the qualities that are experienced.
Studies indicate that each kind of pain is characterized by a distinctive constellation of words. There is remarkable consistency in the choice of words by patients with the same or similar pain syndromes (Graham et al 1980, Melzack et al 1981, Grushka and Sessle 1984, Katz and Melzack 1991, Katz 1992). For example, in a study of amputees with phantom limb pain (PLP group) or non-painful phantom limb sensation (PLS group), every MPQ descriptor chosen by 33% or more of subjects in the PLS group was also chosen by 33% or more subjects in the PLP group, although other descriptors were endorsed with greater frequency by the latter group (Katz and Melzack 1991). These data indicated that the phantom limb experiences of the two groups have in common a paresthetic quality (e.g., tingling and numb), although painful phantoms consist of more than this shared component.
Reliability and Validity of the McGill Pain Questionnaire
Reading and colleagues (1982) investigated the reliability of the groupings of adjectives in the MPQ by using different methodological and statistical approaches. Subjects sorted each of the 78 words of the MPQ into groups that described similar pain qualities. The mean number of groups was 19 (with a range of 7–31), which is remarkably close to the MPQ’s 20 groups. Moreover, there were distinct subgroups for sensory and affective–evaluative words. Even though the cultural backgrounds of subjects in this study and that of Melzack and Torgerson (1971) were different and the methodology and data analysis were dissimilar, the degree of correspondence was impressive. Gaston-Johansson and colleagues (1990) reported that subjects with diverse ethnic–cultural and educational backgrounds use similar MPQ adjectives to describe commonly used words such as pain, hurt, and ache. Nevertheless, interesting differences between the studies were found, which suggests alternative approaches for future revisions of the MPQ.
Evidence of the stability of pain measures can be difficult to obtain because many types of pain fluctuate over time, resolve spontaneously, or improve as a function of treatment. In cases such as these, repeated administration of the same pain instrument would not be expected to yield similar estimates. Chronic pain conditions that remain relatively constant over time offer the opportunity to evaluate the stability of pain measures. Evidence of the stability of the MPQ comes from a study of patients with chronic low back pain who completed the MPQ on two occasions separated by several days (Love et al 1989). The results showed very strong test–retest reliability coefficients for the MPQ PRIs, as well as for some of the 20 categories. The lower coefficients for the 20 categories may be explained by the suggestion that clinical pain fluctuates in quality over time yet still represents the “same” pain to the person who experiences it. More recently, a study of 120 patients with rheumatoid arthritis showed a stable pattern of MPQ scores across three pain assessments over a 6-year period (Roche et al 2003). The pain remained moderate over the 6-year period in the presence of ongoing disease activity, and the MPQ revealed a consistent choice of descriptors with no significant change in MPQ ratings over time.
Many validity studies of the three-dimensional framework of the MPQ have been conducted. Generally, the distinction between sensory and affective dimensions has held up extremely well, but there is still considerable debate on separation of the affective and evaluative dimensions. Nevertheless, several excellent studies have reported a discrete evaluative factor (Reading 1979, Prieto et al 1980, McCreary et al 1981, Holroyd et al 1992). The different factor analysis procedures that were used undoubtedly account for the reports of four factors (Reading 1979, Holroyd et al 1992), five factors (Crockett et al 1977), six factors (Burckhardt 1984), or seven factors (Leavitt et al 1978). The major source of disagreement, however, seems to be the different patient populations that are used to obtain data for factor analysis. The range includes brief laboratory-induced pain, dysmenorrhea, back pain, and cancer pain. In some studies, relatively few words are chosen, whereas large numbers are selected in others. It is not surprising, then, that factor analysis studies based on such diverse populations have confused rather than clarified some of the issues.
Turk and co-workers (1985) examined the internal structure of the MPQ by using techniques that avoided the problems of most earlier studies and confirmed the three (sensory, affective, and evaluative) dimensions. Lowe’s group (1991) confirmed the three-factor structure of the MPQ by using elegant statistical procedures and a large number of subjects. Finally, a paper by Chen and colleagues (1989) presented data on the remarkable consistency of the MPQ across five studies involving the cold pressor task, and Pearce and Morley (1989) provided further confirmation of the construct validity of the MPQ by using the Stroop color-naming task in chronic pain patients.
Sensitivity of the McGill Pain Questionnaire
Recent studies show that MPQ is sensitive to interventions designed to reduce pain of neuropathic origin (Lynch et al 2003), including PLP (Nikolajsen et al 1996), spinal cord injury pain (Defrin et al 2007), and post-herpetic neuralgia (Dworkin et al 2003). The relative sensitivity of the MPQ to change in postoperative pain following the administration of oral analgesics was evaluated by comparing it with VAS and VRS measures of pain intensity (Jenkinson et al 1995). Although all three measures of pain revealed the same pattern of change over time, effect sizes for the MPQ were consistently related to self-reported, directly assessed change in pain using a VRS. These findings probably underestimate the MPQ’s sensitivity to change because the benchmark for change was a VRS. In support of this, the MPQ appears to provide a more sensitive measure of mild postoperative pain than does a simple VAS, which assesses pain intensity only, because patients can be more precise in describing their experience by selecting appropriate descriptors (Katz et al 1994). This increased ability of the MPQ to detect differences in pain at the low end of the pain continuum is most likely a function of the multidimensional nature of the MPQ and the large number of descriptors from which to choose.
Discriminative Capacity of the McGill Pain Questionnaire
One of the most valuable features of the MPQ is its potential use as an aid in the differential diagnosis between various pain syndromes. The first study to demonstrate the discriminative capacity of the MPQ was carried out by Dubuisson and Melzack (1976), who administered the questionnaire to 95 patients with one of eight known pain syndromes: post-herpetic neuralgia, PLP, metastatic carcinoma, toothache, degenerative disc disease, rheumatoid arthritis or osteoarthritis, labor pain, and menstrual pain. A multiple-group discriminant analysis revealed that each type of pain is characterized by a distinctive constellation of verbal descriptors. Furthermore, when the descriptor set for each patient was classified into one of the eight diagnostic categories, a correct classification was made in 77% of cases. Table 21-2 shows the pain descriptors that are most characteristic of the eight clinical pain syndromes in the study by Dubuisson and Melzack (1976).
Table 21-2
Descriptions Characteristic of Clinical Pain Syndromes*
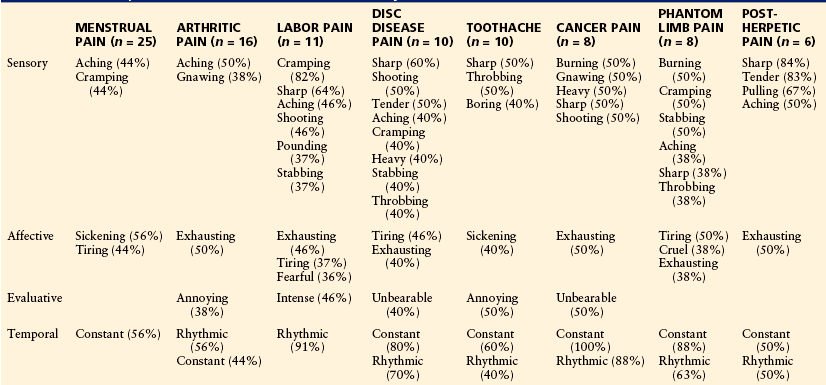
*Only words chosen by more than one-third of the patients are listed, and the percentages of patients who chose each word are shown in parentheses.
Descriptor patterns can also provide the basis for discriminating between low back pain with clear physical causes such as degenerative disc disease and pain for which no physical causes can be found (Leavitt and Garron 1980; Perry et al 1988, 1991). Further evidence of the discriminative capacity of the MPQ was provided by Melzack and co-workers (1986), who differentiated between the pain of trigeminal neuralgia and atypical facial pain.
Specific verbal descriptors of the MPQ have also been shown to discriminate between reversible and irreversible damage to the nerve fibers in a tooth (Grushka and Sessle 1984), between various facial pain disorders (Mongini et al 2000, Mongini and Italiano 2001), and between leg pain caused by diabetic neuropathy and leg pain arising from other causes (Masson et al 1989). Mongini’s group (2003) further showed that the MPQ consistently discriminates between migraine and tension-type headache, thus confirming an earlier report that cluster headache pain is more intense and distressing than other vascular (migraine and mixed) headache pain and is characterized by a distinct constellation of descriptors (Jerome et al 1988). Wilkie and colleagues (2001) compared MPQ descriptors chosen by patients with previously classified nociceptive and neuropathic pain sites secondary to lung cancer. They found that four descriptors (i.e., lacerating, stinging, heavy, and suffocating) were used significantly more frequently to describe nociceptive pain sites than neuropathic pain sites and that 11 other descriptors were used more often to describe the latter than the former pain sites. Using a multivariate regression equation, they showed that 78% of the pain sites were accurately identified as nociceptive (81% sensitivity) or neuropathic (59% sensitivity) by using 10 MPQ descriptors.
It is evident, however, that the discriminative capacity of the MPQ has limits. High levels of anxiety and other psychological disturbance, which may produce high affective scores, may obscure its discriminative capacity (Kremer and Atkinson 1983). Moreover, certain key words that discriminate among specific syndromes may be absent (Reading et al 1982). Nevertheless, it is clear that there are appreciable and quantifiable differences in the way that various types of pain are described and that patients with the same disease or pain syndrome tend to use remarkably similar words to communicate what they feel.
Modifications of the McGill Pain Questionnaire
In general, modifications of the MPQ have involved the development of alternative scoring methods (Hartman and Ainsworth 1980, Charter and Nehemkis 1983, Melzack et al 1985) and efforts to reclassify the original pain descriptors (Clark et al 1995, Fernandez and Towery 1996). Hartman and Ainsworth (1980) proposed transforming the MPQ data into a pain ratio or fraction. Kremer and colleagues (1982) suggested dividing the sum of the obtained ranks within each dimension by the total possible score for a particular dimension, thus making differences between the sensory, affective, evaluative, and miscellaneous dimensions more interpretable.
A final form of computation (Melzack et al 1985) may be useful because it has been argued that the MPQ fails to take into account the true relative intensity of verbal descriptors (Charter and Nehemkis 1983) since the rank order scoring system loses the precise intensity of the scale values obtained by Melzack and Torgerson (1971). For example, Figure 21-1 shows that the affective descriptors generally have higher scale values than the sensory words do. This is clear when we consider the fact that the words throbbing and vicious receive a rank value of 4 but have scale values of 2.68 and 4.26, respectively, thus indicating that the latter descriptor implies considerably more pain intensity than the former. A simple technique was developed (Melzack et al 1985) to convert rank values to weighted rank values that more closely approximate the original scaled values obtained by Melzack and Torgerson (1971). Use of this procedure may provide enhanced sensitivity in some statistical analyses (Melzack et al 1985).
Computer-Administered Versions of the McGill Pain Questionnaire
The general population’s increasing familiarity with computers and the availability of portable computers (laptops, tablets) have led to computer-administered versions of many self-report questionnaires, including the MPQ. The PAINReportIt (Wilkie et al 2003) is a tablet-administered version of the MPQ that has been reported to be acceptable to most patients with a variety of diagnoses, including those who have never before used a computer (Huang et al 2003, Wilkie et al 2009, Jha et al 2010, Page et al 2010). At present, however, research into the psychometric properties (reliability and validity) of the PAINReportIt has not been reported. It is essential that the computer version be compared with the traditional paper-and-pencil version to ensure that the two modes of administration are equivalent. Moreover, published studies of the PAINReportIt do not report the three major indices of the MPQ (i.e., [1] sensory, affective, evaluative, and total PRIs; [2] total number of words chosen; and [3] present pain intensity). Before a computer-administered version of the MPQ is adopted for general use, the proper scoring methods must be calculated and reported.
The Short-Form Mcgill Pain Questionnaire
The SF-MPQ (Melzack 1987; Fig. 21-4) was developed for use in specific research settings when the time to obtain information is limited and when more information is desired than that provided by intensity measures such as the VAS or present pain intensity. The SF-MPQ consists of 15 representative words from the sensory (n = 11) and affective (n = 4) categories of the standard long form. The present pain intensity and a VAS are included to provide indices of overall pain intensity. The 15 descriptors making up the SF-MPQ were selected on the basis of their frequency of endorsement by patients with a variety of acute, intermittent, and chronic pain. An additional word, splitting, was added because it was reported to be a key discriminative word for dental pain (Grushka and Sessle 1984). Each descriptor is ranked by the patient on an intensity scale: 0, none; 1, mild; 2, moderate; and 3, severe. Figure 21-5 shows SF-MPQ scores obtained by patients with a variety of acute and chronic pain conditions.
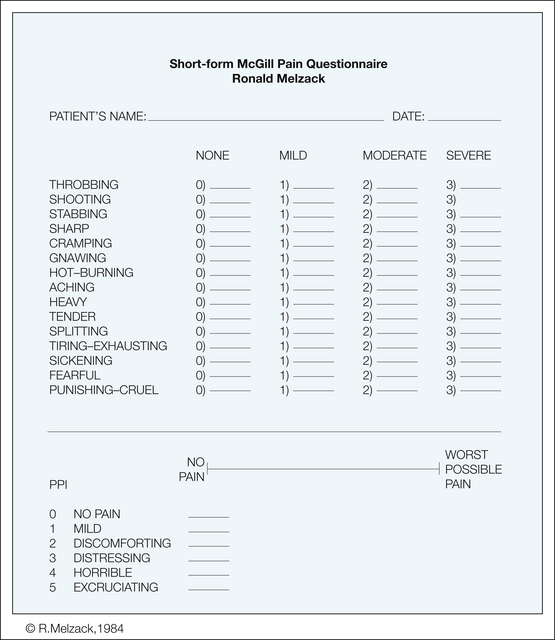
Figure 21-4 Short-form McGill Pain Questionnaire.
Descriptors 1–11 represent the sensory dimension of the pain experience and 12–15 represent the affective dimension. Each descriptor is ranked on an intensity scale of 0, none; 1, mild; 2, moderate; and 3, severe. The rank values associated with the intensity descriptors for each word selected by the patient are summed to obtain a sensory pain rating index (1-11), an affective pain rating index (12-15), and a total pain rating index (1-15). The present pain intensity (PPI) of the standard long-form McGill Pain Questionnaire and the visual analog scale are also included to provide overall pain intensity scores.
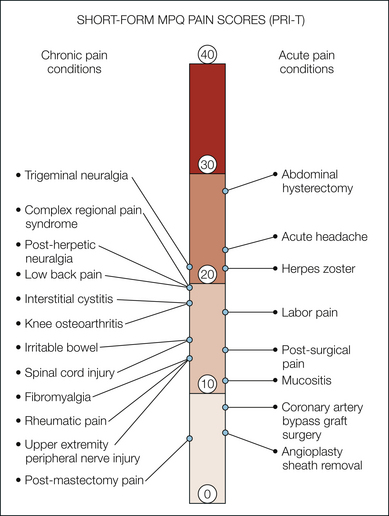
Figure 21-5 Comparison of total pain rating index (PRI-T) scores using the short-form McGill Pain Questionnaire for acute and chronic pain conditions.
References for the various pain conditions are as follows: labor pain and post-surgical pain, Melzack 1987; abdominal hysterectomy, Thomas et al 1995; acute headache, Harden et al 1991; herpes zoster and post-herpetic neuralgia, King 1993; mucositis, McGuire et al 1993; angioplasty sheath removal, Fowlow et al 1995; interstitial cystitis, Nickel et al 2010; trigeminal neuralgia, Perez et al 2009; rheumatic pain, Strand et al 2008; fibromyalgia, Harris et al 2006; knee osteoarthritis, Gandhi et al 2010; irritable bowel, Blanchard et al 2007; upper extremity peripheral nerve injury, Novak et al 2010; post-mastectomy pain, Hack et al 1999; spinal cord injury, Turner et al 2001; complex regional pain syndrome, Bruehl et al 2003; low back pain, Ruoff et al 2003; and coronary artery bypass graft surgery, Watt-Watson et al 2000.
Psychometric Properties of the Short-Form McGill Pain Questionnaire
The SF-MPQ correlates very highly with the major PRIs (sensory, affective, and total) of the long-form MPQ (Melzack 1987, Dudgeon et al 1993). Concurrent validity and test–retest reliability of the SF-MPQ were reported in a study of patients with chronic pain secondary to cancer (Dudgeon et al 1993). On each of three occasions separated by at least a 3-week period, the PRI-S, PRI-A, and PRI-T scores correlated highly with corresponding scores on the original MPQ. Other studies have confirmed the test–retest reliability of the SF-MPQ (Strand et al 2008), with lower intraclass correlation coefficients (ICCs) being associated with longer intervals between testing (Burckhardt and Bjelle 1994) and higher ICCs reported when the interval between test occasions is short and not confounded by treatment (Georgoudis et al 2001, Grafton et al 2005, Yakut et al 2007).
Factor analysis studies of the SF-MPQ have generally supported the two-factor structure proposed by Melzack (1987). The presence of sensory and affective factors has been confirmed by using both confirmatory and exploratory analyses and in varied patient populations, including patients with burn injuries (Mason et al 2008), chronic low back pain (Wright et al 2001, Beattie et al 2004), and fibromyalgia and rheumatoid arthritis (Burckhardt and Bjelle 1994). The most methodologically sound study was conducted by Beattie and colleagues (2004), who cross-validated the two-factor solution obtained by using exploratory factor analysis with a subsequent confirmatory factor analysis in a large sample of patients with chronic low back pain. Factor solutions suggesting a structure other than that proposed by Melzack are still consistent with the general distinction between the sensory and affective dimensions. For example, Burckhardt and Bjelle (1994) reported a three-factor solution composed of two sensory factors and one affective factor. As reviewed by Mason and associates (2008), two studies evaluated the cross-cultural validity of the SF-MPQ in African and European American patients with upper and lower back pain (Cassisi et al 2004) and in Asian American cancer patients (Shin et al 2008). Both studies used exploratory factor analysis methods and both failed to find a two-factor solution consistent with the sensory and affective dimensions proposed by Melzack (1987). In one study (Cassisi et al 2004), four- and five-factor solutions emerged, and in the other study (Shin et al 2008), a two-factor solution was found in which both factors contained sensory and affective descriptors. Methodological limitations associated with these studies may, in part, explain the inconsistent findings.
The SF-MPQ is sensitive to changes brought about by various therapies—analgesic drugs (Rice and Maton 2001, Ruoff et al 2003), epidurally or spinally administered agents (Melzack 1987, Harden et al 1991, Serrao et al 1992), transcutaneous electrical nerve stimulation (Melzack 1987), acupuncture (Birch and Jamison 1998, Harris et al 2006), low-power light therapy (Stelian et al 1992), and an intensive 3.5-week multidisciplinary treatment program (Strand et al 2008). It is notable that the SF-MPQ is also capable of detecting clinically significant reductions in various neuropathic pain conditions associated with pharmacological interventions administered in the context of randomized, placebo-controlled trials (Backonja et al 1998, Lesser et al 2004, Gilron et al 2005, Lyrica Study Group 2006).
Voorhies and co-authors (2007) reported the SF-MPQ to be useful in predicting outcome in response to surgical intervention for lumbar radiculopathy. Patients with preoperative SF-MPQ sensory and affective scores of 17 and 7 or higher, respectively (i.e., 50% of the total possible SF-MPQ scores), had between a 42 and 50% chance of obtaining an excellent or good surgical outcome 12 months after surgery.
An important property of the long-form MPQ is that it has been shown to distinguish between different pains. Initial data (Melzack 1987) suggesting that the SF-MPQ may be capable of discriminating among different pain syndromes have been confirmed by Closs and colleagues (2008), who reported that venous leg ulcers were frequently described as “throbbing,” “burning,” and “itchy” whereas arterial ulcers were described as “sharp” and “hurting.” Similarly, modest predictability was reported for distinguishing between pain of neuropathic and musculoskeletal origin in patients with spinal cord injuries (Putzke et al 2002). An established translation institute (Mapi 2003) using forward and backward translation techniques has translated the SF-MPQ into 50 languages.
Short-Form McGill Pain Questionnaire-2
Recent advances in identifying the mechanisms of neuropathic pain (Treede et al 2008) and in improving its management (Dworkin et al 2007) have led to the development of new instruments (Jensen 2006) designed to measure the unique aspects of pain initiated or caused by a primary lesion or dysfunction in the nervous system. Although a neuropathic pain–specific questionnaire has merits, there are also disadvantages. For example, measurement of the various qualities of pain can aid in the process of diagnosis. Use of a neuropathic pain–specific questionnaire will clearly bias diagnosis in that direction and miss potentially important information that might suggest the presence of a non-neuropathic pain problem. In addition, it is not uncommon for patients to be seen clinically with pain that consists of both neuropathic and non-neuropathic components (e.g., nociceptive, inflammatory, musculoskeletal). Neuropathic pain–specific questionnaires provide descriptions of the qualities and other features of the neuropathic component but not the non-neuropathic components. Large-scale, population-based epidemiological studies of chronic pain would be aided by a single, reliable, and valid measure of the many qualities of pain. These factors argue for a single pain questionnaire that is designed to measure the qualities of neuropathic and non-neuropathic pain.
As described earlier, the SF-MPQ has been used successfully in treatment trials of neuropathic pain. However, it does not contain certain descriptors that have been shown to be reliably associated with neuropathic pain conditions. Accordingly, Dworkin and co-workers (2009) developed the SF-MPQ-2, an expanded and revised version of the SF-MPQ designed to measure the qualities of both neuropathic and non-neuropathic pain in research and clinical settings.
The following modifications were involved in development of the SF-MPQ-2 (Fig. 21-6): (1) inclusion of seven new descriptors relevant to neuropathic pain, (2) use of an 11-point NRS for each descriptor, (3) addition of the qualifier “pain” to 13 descriptors, and (4) expansion of the instructions to take into account “different qualities of pain and related symptoms” (Dworkin et al 2009). The SF-MPQ-2 was administered, in a web-based format, to 882 participants with diverse chronic pain conditions and to 226 patients with painful diabetic peripheral neuropathy who were enrolled in a randomized controlled trial. Exploratory and confirmatory factor analysis revealed the presence of the following four factors or subscales (Table 21-3): continuous pain descriptors, intermittent pain descriptors, predominantly neuropathic pain descriptors, and affective descriptors. Subscale scores are computed by taking the arithmetic mean of the ratings for subscale descriptors. Total score is the mean of the ratings for all 22 descriptors (Dworkin et al 2009).
Table 21-3
Short-Form McGill Pain Questionnaire-2 Subscales
SUBSCALE |
ITEM |
| 1. Continuous pain | 1. Throbbing pain 5. Cramping pain 6. Gnawing pain 8. Aching pain 9. Heavy pain 10. Tender |
| 2. Intermittent pain | 2. Shooting pain 3. Stabbing pain 4. Sharp pain 11. Splitting pain 16. Electric shock pain 18. Piercing |
| 3. Predominantly neuropathic pain | 7. Hot–burning pain 17. Cold–freezing pain 19. Pain caused by light touch 20. Itching 21. Tingling or “pins and needles” 22. Numbness |
| 4. Affective | 12. Tiring–exhausting 13. Sickening 14. Fearful 15. Punishing–cruel |
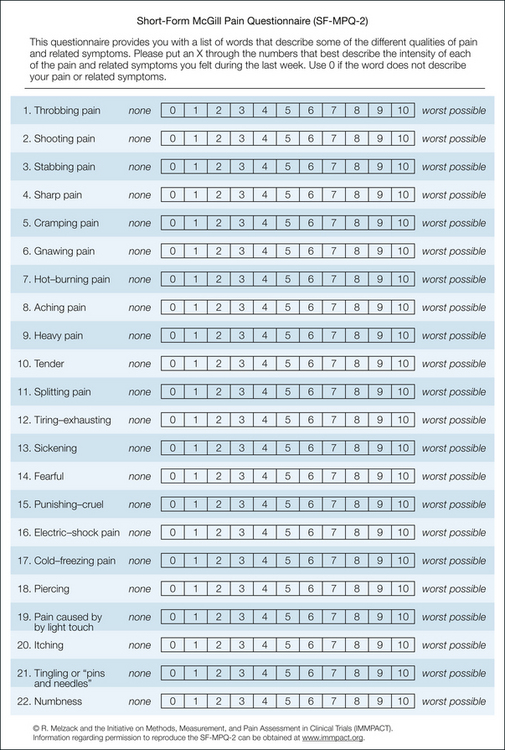
Figure 21-6 Short-form McGill Pain Questionnaire-2.
The 22 descriptors are listed under the following four subscales: continuous pain (items 1, 5, 6, 8–10), intermittent pain (items 2–4, 11, 16, 18), neuropathic pain (items 7, 17, 19–22), and affective descriptors (items 12–15). Each descriptor is rated on an 11-point numerical rating scale ranging from 0 = none to 10 = worst possible. Subscale scores are computed by taking the arithmetical mean of the ratings for subscale descriptors. Total score is the mean of the ratings for all 22 descriptors.
Preliminary analyses indicate that the SF-MPQ-2 has very good to excellent psychometric properties, including adequate to high internal consistency and reliability estimates for the two subscales (0.73–0.87) and the total score (0.91–0.95). Construct validity was demonstrated by correlation with another well-validated measure of pain, the Brief Pain Inventory (Cleeland et al 1996). Consistent with the goal of developing a questionnaire sensitive to both neuropathic and non-neuropathic pain, the SF-MPQ-2 total score and scores on the intermittent pain and neuropathic pain subscales were significantly higher for the web-based participants with neuropathic pain than for the participants with non-neuropathic pain. In contrast, subscale scores for continuous pain and affective descriptors did not differ significantly between the participants with neuropathic and non-neuropathic pain. Finally, the SF-MPQ-2 subscale and total scores showed sensitivity to change in the context of a randomized controlled treatment trial.
A recent study of women with fibromyalgia who had participated in a 75-minute Hatha yoga class twice weekly for 8 weeks demonstrated a significant decrease in the SF-MPQ-2 continuous pain subscale at the end of treatment (Curtis et al 2011). Taken together, these preliminary results suggest that the SF-MPQ-2 is a reliable, valid, and sensitive measure of chronic pain that is capable of discriminating between neuropathic and non-neuropathic pain. Further psychometric evaluation of the SF-MPQ-2 is required to address some of the shortcomings involved in using a web-based sample of participants to validate the questionnaire and to confirm the scale’s ability to discriminate between pain of neuropathic and non-neuropathic origin (Bouhassira and Attal 2009).
Multidimensional Pain Experience
Several groups of researchers have evaluated the theoretical structure of the MPQ by using factor analysis methods (Turk et al 1985, Holroyd et al 1992). Turk’s group concluded that the three-factor structure of the MPQ—sensory, affective, and evaluative—is strongly supported by the analyses; Holroyd’s “most clearly interpretable structure” was provided by a four-factor solution obtained by oblique rotation, in which two sensory factors were identified in addition to an affective and an evaluative factor.
Like most others who have used the MPQ, Turk and colleagues (1985) and Holroyd and associates (1992) have found high intercorrelations among the factors. However, significant intercorrelations among identified factors should not be taken as evidence of the lack of discriminative capacity and clinical utility of the MPQ. There is, in fact, considerable evidence that the MPQ is effective in discriminating among the three factors despite the high intercorrelations.
First, Gracely (1992) has convincingly argued that factor analysis methods may be inappropriate for assessing the factor structure of the MPQ, although they do provide useful information about patient characteristics. Torgerson (1988) distinguished between semantic meaning (how the MPQ descriptors are arranged) and associate meaning (how patients arrange the MPQ descriptors) to emphasize that factor analysis provides a context-dependent structure of the latter—that is, the outcome depends on how specific patient samples make use of the MPQ descriptors. Gracely (1992) elaborated further on the difference between semantic and associative meaning and concluded that factor analysis techniques do not “directly evaluate the semantic structure of the questionnaire.”
Second, high correlation among variables does not necessarily imply a lack of discriminant capacity. Traditional psychophysics has shown repeatedly that in the case of vision, for example, increasing the intensity of light produces increased capacity to discriminate color, contours, texture, and distance (Kling and Riggs 1971). Similarly, in the case of hearing, increases in volume lead to increased discrimination of timbre, pitch, and spatial location (Kling and Riggs 1971). In these cases there are clearly very high intercorrelations among the variables in each modality. However, this does not mean that we should forget about the differences between color and texture or between timbre and pitch just because they intercorrelate highly. This approach would lead to the loss of valuable, meaningful data (Gracely 1992).
Third, many papers have demonstrated the discriminant validity of the MPQ (Melzack and Perry 1975, Reading and Newton 1977, Melzack et al 1981, Reading 1982, Melzack et al 1984). In studies on labor pain, Melzack and colleagues (1981, 1984) found that distinctly different variables correlate with the sensory, affective, and evaluative dimensions. Prepared childbirth training, for example, correlates significantly with the sensory and affective dimensions but not the evaluative one. Menstrual difficulties correlate with the affective but neither the sensory nor evaluative dimension. Physical factors, such as mother’s and infant’s weight, also correlate selectively with one or another dimension.
Similarly, a study of acute pain in emergency department patients has “revealed a normal distribution of sensory scores but very low affective scores compared with patients with chronic pain” (Melzack et al 1982). Finally, Chen and co-workers (1989) have consistently identified a group of pain-sensitive and pain-tolerant subjects in five laboratory studies of tonic (prolonged) pain. When compared with pain-tolerant subjects, pain-sensitive subjects show significantly higher scores on all PRIs except the sensory dimension. Atkinson and colleagues (1982) are undoubtedly right that high affect scores tend to diminish the discriminant capacity of the MPQ such that at high levels of anxiety and depression, some discriminant capacity is lost. However, the MPQ still retains good discriminant function even at high levels of anxiety.
The Descriptor Differential Scale
Simple but sophisticated psychophysical techniques have been applied to the development of pain measurement instruments that have been used to assess clinical and experimentally induced pain (Gracely and Kwilosz 1988). The psychophysical approach uses cross-modality–matching procedures (Gracely et al 1978a) or bimodality stimulus comparison (Doctor et al 1995) to determine the relative magnitudes of the verbal descriptors of pain.
The Descriptor Differential Scale (DDS) (Gracely and Kwilosz 1988) was developed by Gracely’s group (Gracely et al 1978a) to remedy a number of deficiencies associated with existing pain measurement instruments. The DDS was designed to reduce bias, to separately assess the sensory intensity and “unpleasantness” (hedonic) dimensions of pain, and to provide quantification by ratio-scaling procedures (Gracely 1983). The DDS consists of two forms that separately measure the sensory intensity and unpleasantness qualities of pain. Each form consists of 12 verbal descriptors in which each descriptor is centered over a 21-point scale with a minus sign at the low end and a plus sign at the high end. The patients rate the magnitude of the sensory intensity or unpleasantness of the pain that they are experiencing. The magnitude of pain endorsed by the patient in relation to each descriptor is assigned a score of 0 (minus sign) to 20 (plus sign), with a score of 10 representing pain intensity or unpleasantness equal to the magnitude implied by the descriptor. Total mean scores may be obtained for the sensory intensity and unpleasantness dimensions by averaging the patient’s scores on each 12-item form.
The DDS has been demonstrated to be differentially sensitive to pharmacological interventions that alter the sensory or unpleasantness dimensions of pain (Gracely et al 1978b, 1979; Gracely 1992; Atkinson et al 1998, 1999, 2007; Ellis et al 2009). Results point to the importance of using multidimensional measures of pain with clear instructions to separately rate the sensory intensity and unpleasantness aspects of pain as opposed to the painfulness of the experience (Gracely and Dubner 1987). When used in conjunction with cross-modality–matching techniques, the DDS has been shown to be a reliable and valid instrument with ratio scale properties (Gracely et al 1978a, 1978b).
Gracely and Kwilosz (1988) assessed the psychometric properties of the DDS for use as a clinical pain measure in a sample of 91 dental patients after third-molar extraction. Sensory intensity and unpleasantness DDS forms were administered to all patients 1 and 2 hours after surgery. The total scores on both forms showed high test–retest reliability coefficients, as did scores derived from individual items. Correlation coefficients between individual items and the total score revealed a high degree of internal consistency for both forms of the DDS. One of the most useful features of the DDS is the potential to define a measure of scaling consistency that can be used to identify invalid patient profiles obtained by inconsistent responding. Elimination of invalid profiles improved the reliability and internal consistency of the DDS.
The Pain Quality Assessment Scale
The Pain Quality Assessment Scale (PQAS) was developed by Jensen and colleagues (2006) to measure the qualities of both neuropathic and non-neuropathic pain. The PQAS is a modification of the Neuropathic Pain Scale (Galer and Jensen 1997), a self-report measure of neuropathic pain with established reliability and validity (Galer and Jensen 1997, Argoff et al 2004, Jensen et al 2006, Jensen and Karoly 2011). An additional 10 non-neuropathic and neuropathic pain descriptors were added to the Neuropathic Pain Scale to form the PQAS. The PQAS consists of the following 18 items describing various qualities of pain: intense, sharp, hot, dull, cold, sensitive, tender, itchy, shooting, numb, electrical, tingling, cramping, radiating, throbbing, aching, heavy, and unpleasant. The PQAS also includes a measure of the spatial quality of the pain, for which patients rate the intensity of “deep” and “surface” pain. Each item is rated on an 11-point numerical scale ranging from 0–10, with end points corresponding to the extremes of the pain quality being rated (e.g., for item 7, describing how tender the pain is, 0 and 10 correspond to “not tender” and “the most tender sensation imaginable” [“like a bruise”], respectively). The final item assesses the temporal quality of pain as intermittent, variable, or stable.
The PQAS is sensitive to change in response to treatments that are known to reduce pain intensity and quality, including a 5% lidocaine patch, corticosteroid injection, and extended-release oxymorphone (Jensen et al 2006, Victor et al 2008, Gould et al 2009). An exploratory factor analysis study consisting of 368 patients with osteoarthritis of the knee and 455 patients with low back pain revealed three factors representing paroxysmal pain sensations (shooting, sharp, electric, hot, and radiating), superficial pain sensations (itchy, cold, numb, sensitive, and tingling), and deep pain sensations (aching, heavy, dull, cramping, and throbbing) (Victor et al 2008). These three factors were confirmed in a subsequent factor analysis using an independent sample of 138 patients with carpal tunnel syndrome (Victor et al 2008).
A variety of other pain scales have recently been developed to measure neuropathic pain (Jensen 2006, Haanpaa et al 2011), including the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) Pain Scale (Bennett 2001), the S-LANSS Pain Scale for patient self-report (Bennett et al 2005), the Neuropathic Pain Questionnaire (Krause and Backonja 2003), the Neuropathic Pain Questionnaire–short-form (Backonja and Krause 2003), the Neuropathic Pain Symptom Inventory (Bouhassira et al 2004), DN4 (Bouhassira et al 2005), PainDETECT (Freynhagen et al 2006), ID pain (Portenoy 2006), and the Standardized Evaluation of Pain (StEP) (Scholz et al 2009). Although psychometric data on these scales have been accumulating, further validation is required (Haanpaa et al 2011). Comparative studies have not been conducted, and although the various scales share common features, none has been universally accepted (Bennett et al 2007, Haanpaa et al 2011).
Behavioral Approaches to Pain Measurement
Research into the development of behavioral measures of pain has produced a wide array of sophisticated observational techniques and rating scales designed to assess the objective behavior that accompanies the pain experience (Keefe et al 2011). Techniques that have demonstrated high reliability and validity are especially useful for measuring pain in infants and preverbal children who lack language skills and in adults who have a poor command of language or when mental clouding and confusion limit the patient’s ability to communicate meaningfully. Under these circumstances, behavioral measures provide important information that is otherwise unavailable from patient self-report. Moreover, when administered in conjunction with a subjective, patient-rated measure, behavioral measures may provide a more complete picture of the total pain experience. However, behavioral measures of pain should not replace self-rated measures if patients are capable of rating their subjective state and such administration is feasible.
The subjective experiences of pain and pain behavior are, presumably, reflections of the same underlying neural processes. However, the complexity of the human brain indicates that although experience and behavior are usually highly correlated, they are far from identical. One person may be stoic, and thus calm behavior belies the person’s true subjective feelings. Another patient may seek sympathy (or analgesic medication or some other desirable goal) and in so doing exaggerate complaints without also eliciting the behavior that typically accompanies pain complaints of that degree. Concordance between patients’ self-ratings of pain and ratings of the same patients by nurses or other medically trained personnel may be modestly low, but even in the presence of a significant correlation between health care providers’ and patients’ ratings of pain, health care providers often underestimate the degree of pain that patients report experiencing (Prkachin et al 2007). Moreover, when health care providers observe a discordance between non-verbal pain behavior and the patient’s verbal report of pain, the discrepancy is often resolved by disregarding the patient’s self-report (Prkachin et al 2007). These studies point to the importance of obtaining multiple measures of pain and should keep us aware that because pain is a subjective experience, the patient’s self-report is the most valid measure of that experience.
Physiological Approaches to Pain Measurement
Profound physiological changes often accompany the experience of pain, especially if the injury or noxious stimulus is acute (Kehlet and Wilmore 2002). Physiological correlates of pain may serve to elucidate mechanisms that underlie the experience and thus may provide clues that may lead to novel treatments. Physiological correlates of the pain experience that are frequently measured include heart rate, blood pressure, peripheral blood flow, electrodermal activity, electromyographic activity, cortical evoked potentials, and a variety of neuroimaging techniques (Flor and Meyer 2011). Despite high initial correlations between pain onset and changes in these physiological responses, many habituate with time despite the persistence of pain. In addition, these responses are not specific to the experience of pain per se and occur under conditions of general arousal and stress. Studies that have examined the general endocrine–metabolic stress response to a surgical incision indicate that under certain conditions it is possible to dissociate different aspects of the stress response and pain (Kehlet 1986, 1988). Severe injury to a denervated limb produces a significant adrenocortical response (Kehlet 1988), but use of general anesthesia clearly eliminates the conscious experience of pain in response to a surgical incision without altering the subsequent rapid rise in plasma cortisol levels (Brandt et al 1976, Christensen et al 1982). These studies indicate that although many physiological, immune, and endocrine events occur concurrently with the experience of pain, many appear to be general responses to stress and are not unique to pain.
Acknowledgment
This work was supported by a Canada Research Chair in Health Psychology to JK.
The references for this chapter can be found at www.expertconsult.com.
References
Aboud F.E., Hiwot M.G., Arega A., et al. The McGill Pain Questionnaire in Amharic: Zwai Health Center patients’ reports on the experience of pain. Ethiopian Medical Journal. 2003;41:45–61.
Argoff C.E., Galer B.S., Jensen M.P., et al. Effectiveness of the lidocaine patch 5% on pain qualities in three chronic pain states: assessment with the Neuropathic Pain Scale. Current Medical Research and Opinion. 2004;20(Suppl 2):S21–S28.
Atkinson J.H., Kremer E.F., Ignelzi R.J. Diffusion of pain language with affective disturbance confounds differential diagnosis. Pain. 1982;12:375–384.
Atkinson J.H., Slater M.A., Capparelli E.V., et al. Efficacy of noradrenergic and serotonergic antidepressants in chronic back pain: a preliminary concentration-controlled trial. Journal of Clinical Psychopharmacology. 2007;27:135–142.
Atkinson J.H., Slater M.A., Wahlgren D.R., et al. Effects of noradrenergic and serotonergic antidepressants on chronic low back pain intensity. Pain. 1999;83:137–145.
Atkinson J.H., Slater M.A., Williams R.A., et al. A placebo-controlled randomized clinical trial of nortriptyline for chronic low back pain. Pain. 1998;76:287–296.
Backonja M., Beydoun A., Edwards K.R., et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. Journal of the American Medical Association. 1998;280:1831–1836.
Backonja M.M., Krause S.J. Neuropathic pain questionnaire—short form. Clinical Journal of Pain. 2003;19:315–316.
Bartko D., Kondos M., Jansco S. Slovak version of the McGill-Melzack’s Questionnaire on pain. Ceskoslovenska Neurologie a Neurochirurgie. 1984;47:113–121.
Beattie P.F., Dowda M., Feuerstein M. Differentiating sensory and affective-sensory pain descriptions in patients undergoing magnetic resonance imaging for persistent low back pain. Pain. 2004;110:189–196.
Beecher H.K. Measurement of subjective responses. New York: Oxford University Press; 1959.
Bejarano P.F., Noriego R.D., Rodriguez M.L., et al. Evaluación del dolor: adaptatión del cuestionario del McGill [Evaluation of pain: adaptation of the McGill Pain Questionnaire]. Revista Columbia Anesesia. 1985;13:321–351.
Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157.
Bennett M.I., Attal N., Backonja M.M., et al. Using screening tools to identify neuropathic pain. Pain. 2007;127:199–203.
Bennett M.I., Smith B.H., Torrance N., et al. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. Journal of Pain. 2005;6:149–158.
Birch S., Jamison R.N. Controlled trial of Japanese acupuncture for chronic myofascial neck pain: assessment of specific and nonspecific effects of treatment. Clinical Journal of Pain. 1998;14:248–255.
Blanchard E.B., Lackner J.M., Sanders K., et al. A controlled evaluation of group cognitive therapy in the treatment of irritable bowel syndrome. Behaviour Research and Therapy. 2007;45:633–648.
Bouhassira D., Attal N. All in one: is it possible to assess all dimensions of any pain with a simple questionnaire? Pain. 2009;144:7–8.
Bouhassira D., Attal N., Alchaar H., et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114:29–36.
Bouhassira D., Attal N., Fermanian J., et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–257.
Boureau F., Luu M., Doubrère J.F., et al. Elaboration d’un questionnaire d’auto-évaluation de la douleur par liste de qualicatifs [Development of a self-evaluation questionnaire comprising pain descriptors]. Thérapie. 1984;39:119–129.
Boureau F., Luu M., Doubrère J.F. Comparative study of the validity of four French McGill Pain Questionnaire (MPQ) versions. Pain. 1992;50:59–65.
Brandt M.R., Kehlet H., Binder C., et al. Effect of epidural analgesia on the glucoregulatory endocrine response to surgery. Clinical Endocrinology. 1976;5:107–114.
Breivik E.K., Bjornsson G.A., Skovlund E. A comparison of pain rating scales by sampling from clinical trial data. Clinical Journal of Pain. 2000;16:22–28.
Breivik H., Borchgrevink P.C., Allen S.M., et al. Assessment of pain. British Journal of Anaesthia. 2008;101:17–24.
Bruehl S., Chung O.Y., Burns J.W. Differential effects of expressive anger regulation on chronic pain intensity in CRPS and non-CRPS limb pain patients. Pain. 2003;104:647–654.
Burckhardt C.S. The use of the McGill Pain Questionnaire in assessing arthritis pain. Pain. 1984;19:305–314.
Burckhardt C.S., Bjelle A. A Swedish version of the short-form McGill Pain Questionnaire. Scandinavian Journal of Rheumatology. 1994;23:77–81.
Cassisi J.E., Umeda M., Deisinger J.A., et al. Patterns of pain descriptor usage in African Americans and European Americans with chronic pain. Cultural Diversity & Ethnic Minority Psychology. 2004;10:81–89.
Chapman C.R., Casey K.L., Dubner R., et al. Pain measurement: an overview. Pain. 1985;22:1–31.
Charter R.A., Nehemkis A.M. The language of pain intensity and complexity: new methods of scoring the McGill Pain Questionnaire. Perceptual and Motor Skills. 1983;56:519–537.
Chen A.C.N., Dworkin S.F., Haug J., et al. Human pain responsivity in a tonic pain model: psychological determinants. Pain. 1989;37:143–160.
Christensen P., Brandt M.R., Rem J., et al. Influence of extradural morphine on the adrenocortical and hyperglycaemic response to surgery. British Journal of Anaesthesia. 1982;54:23–27.
Clark W.C., Fletcher J.D., Janal M.N., et al. Hierarchical clustering of pain and emotion descriptors: toward a revision of the McGill Pain Questionnaire. In: Bromm B., Desmedt J.E., eds. Advances in pain research and therapy. New York: Raven Press; 1995:319–330.
Cleeland C.S., Nakamura Y., Mendoza T.R., et al. Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67:267–273.
Closs S.J., Nelson E.A., Briggs M. Can venous and arterial leg ulcers be differentiated by the characteristics of the pain they produce? Journal of Clinical Nursing. 2008;17:637–645.
Crockett D.J., Prkachin K.M., Craig K.D. Factors of the language of pain in patients and normal volunteer groups. Pain. 1977;4:175–182.
Curtis K., Osadchuk A., Katz J. An eight-week yoga intervention is associated with improvements in pain, psychological functioning and mindfulness, and changes in cortisol levels in women with fibromyalgia. Journal of Pain Research. 2011;4:189–201.
De Benedittis G., Massei R., Nobili R., et al. The Italian pain questionnaire. Pain. 1988;33:53–62.
Defrin R., Grunhaus L., Zamir D., et al. The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Archives of Physical Medicine and Rehabilitation. 2007;88:1574–1580.
Doctor J.N., Slater M.A., Atkinson J.H. The Descriptor Differential Scale of Pain Intensity: an evaluation of item and scale properties. Pain. 1995;61:251–260.
Douglas C., Wollin J.A., Windsor C. Illness and demographic correlates of chronic pain among a community-based sample of people with multiple sclerosis. Archives of Physical Medicine and Rehabilitation. 2008;89:1923–1932.
Drewes A.M., Helweg-Larsen S., Petersen P., et al. McGill Pain Questionnaire translated into Danish: experimental and clinical findings. Clinical Journal of Pain. 1993;9:80–87.
Dubuisson D., Melzack R. Classification of clinical pain descriptors by multiple group discriminant analysis. Experimental Neurology. 1976;51:480–487.
Dudgeon D., Ranbertas R.F., Rosenthal S. The Short-Form McGill Pain Questionnaire in chronic cancer pain. Journal of Pain and Symptom Management. 1993;8:191–195.
Dworkin R.H., Corbin A.E., Young J.P., Jr., et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2003;60:1274–1283.
Dworkin R.H., O’Connor A.B., Backonja M., et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–251.
Dworkin R.H., Turk D.C., Revicki D.A., et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35–42.
Ellis R.J., Toperoff W., Vaida F., et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–680.
Escalante A., Lichtenstein M.J., Rios N., et al. Measuring chronic rheumatic pain in Mexican Americans: cross-cultural adaptation of the McGill Pain Questionnaire. Journal of Clinical Epidemiology. 1996;49:1389–1399.
Fernandez E., Towery S. A parsimonious set of verbal descriptors of pain sensation derived from the McGill Pain Questionnaire [published erratum appears in Pain 1996;68:437]. Pain. 1996;66:31–37.
Ferracuti S., Romeo G., Leardi M.G., et al. New Italian adaptation and standardization of the McGill Pain Questionnaire. Pain. 1990;41(Suppl 5):S300.
Flor H., Meyer P. Psychophysical and neuroimaging measures in the assessment of patients with chronic pain. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. ed 3. New York: Guilford Press; 2011:151–175.
Forth H.L., Cramp M.C., Drechsler W.I. Does physiotherapy treatment improve the self-reported pain levels and quality of life of women with vulvodynia? A pilot study. Journal of Obstetrics and Gynaecology. 2009;29:423–429.
Fowlow B., Price P., Fung T. Ambulation after sheath removal: a comparison of 6 and 8 hours of bedrest after sheath removal in patients following a PTCA procedure. Heart & Lung. 1995;24:28–37.
Freynhagen R., Baron R., Gockel U., et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Current Medical Research and Opinion. 2006;22:1911–1920.
Galer B.S., Jensen M.P. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332–338.
Gandhi R., Tsvetkov D., Dhottar H., et al. Quantifying the pain experience in hip and knee osteoarthritis. Pain Research & Management. 2010;15:224–228.
Gaston-Johansson F., Albert M., Fagan E., et al. Similarities in pain descriptors of four different ethnic-culture groups. Journal of Pain and Symptom Management. 1990;5:94–100.
Georgoudis G., Oldham J.A., Watson P.J. Reliability and sensitivity measures of the Greek version of the short form of the McGill Pain Questionnaire. European Journal of Pain. 2001;5:109–118.
Georgoudis G., Watson P.J., Oldham J.A. The development and validation of a Greek version of the short-form McGill Pain Questionnaire. European Journal of Pain. 2000;4:275–281.
Gilron I., Bailey J.M., Tu D., et al. Morphine, gabapentin, or their combination for neuropathic pain. New England Journal of Medicine. 2005;352:1324–1334.
Gould E.M., Jensen M.P., Victor T.W., et al. The pain quality response profile of oxymorphone extended release in the treatment of low back pain. Clinical Journal of Pain. 2009;25:116–122.
Gracely R.H. Pain language and ideal pain assessment. In: Melzack R., ed. Pain measurement and assessment. New York: Raven Press; 1983:71–78.
Gracely R.H. Evaluation of multi-dimensional pain scales. Pain. 1992;48:297–300.
Gracely R.H., Dubner R. Reliability and validity of verbal descriptor scales of painfulness. Pain. 1987;29:175–185.
Gracely R.H., Kwilosz D.M. The Descriptor Differential Scale: applying psychophysical principles to clinical pain assessment. Pain. 1988;35:279–288.
Gracely R.H., McGrath P.A., Dubner R. Ratio scales of sensory and affective verbal pain descriptors. Pain. 1978;5:5–18.
Gracely R.H., McGrath P.A., Dubner R. Validity and sensitivity of ratio scales of sensory and affective verbal pain descriptors: manipulation of affect by diazepam. Pain. 1978;5:19–29.
Gracely R.H., McGrath P.A., Dubner R. Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science. 1979;203:1361–1379.
Grafton K.V., Foster N.E., Wright C.C. Test-retest reliability of the Short-Form McGill Pain Questionnaire: assessment of intraclass correlation coefficients and limits of agreement in patients with osteoarthritis. Clinical Journal of Pain. 2005;21:73–82.
Graham C., Bond S.S., Gerkovitch M.M., et al. Use of the McGill Pain Questionnaire in the assessment of cancer pain: replicability and consistency. Pain. 1980;8:377–387.
Grushka M., Sessle B.J. Applicability of the McGill Pain Questionnaire to the differentiation of “toothache” pain. Pain. 1984;19:49–57.
Haanpaa M., Attal N., Backonja M., et al. NeuPSIG guidelines on neuropathic pain assessment. Pain. 2011;152:14–27.
Hack T.F., Cohen L., Katz J., et al. Physical and psychological morbidity after axillary lymph node dissection for breast cancer. Journal of Clinical Oncology. 1999;17:143–149.
Harden R.N., Carter T.D., Gilman C.S., et al. Ketorolac in acute headache management. Headache. 1991;31:463–464.
Harris R.E., Gracely R.H., McLean S.A., et al. Comparison of clinical and evoked pain measures in fibromyalgia. Journal of Pain. 2006;7:521–527.
Harrison A. Arabic pain words. Pain. 1988;32:239–250.
Hartman L.M., Ainsworth K.D. Self-regulation of chronic pain. Canadian Journal of Psychiatry. 1980;25:38–43.
Hasegawa M., Hattori S., Mishima M., et al. The McGill Pain Questionnaire, Japanese version, reconsidered: confirming the theoretical structure. Pain Research & Management. 2001;6:173–180.
Hobara M., Fujiwara H., Clark W.C., et al. A translation of the Multidimensional Affect and Pain Survey (MAPS) from English to Japanese. Gan To Kagaku Ryoho. 2003;30:721–729.
Holroyd K.A., Holm J.E., Keefe F.J., et al. A multi-center evaluation of the McGill Pain Questionnaire: results from more than 1700 chronic pain patients. Pain. 1992;48:301–311.
Huang H.Y., Wilkie D.J., Zong S.P., et al. Developing a computerized data collection and decision support system for cancer pain management. Computers, Informatics. Nursing. 2003;21:206–217.
Hui Y.L., Chen A.C. Analysis of headache in a Chinese patient population. Ma Tsui Hsueh Tsa Chi. 1989;27:13–18.
Jenkinson C., Carroll D., Egerton M., et al. Comparison of the sensitivity to change of long and short form pain measures. Quality of Life Research. 1995;4:353–357.
Jensen M.P. Review of measures of neuropathic pain. Current Pain and Headache Reports. 2006;10:159–166.
Jensen M.P., Friedman M., Bonzo D., et al. The validity of the neuropathic pain scale for assessing diabetic neuropathic pain in a clinical trial. Clinical Journal of Pain. 2006;22:97–103.
Jensen M.P., Gammaitoni A.R., Olaleye D.O., et al. The pain quality assessment scale: assessment of pain quality in carpal tunnel syndrome. Journal of Pain. 2006;7:823–832.
Jensen M.P., Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. ed 3. New York: Guilford Press; 2011:19–44.
Jerome A., Holroyd K.A., Theofanous A.G., et al. Cluster headache pain vs. other vascular headache pain: differences revealed with two approaches to the McGill Pain Questionnaire. Pain. 1988;34:35–42.
Jha A., Suarez M.L., Ferrans C.E., et al. Cognitive testing of PAINReportIt in adult African Americans with sickle cell disease. Computers, Informatics. Nursing. 2010;28:141–150.
Katz J. Psychophysical correlates of phantom limb experience. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55:811–821.
Katz J., Clairoux M., Kavanagh B.P., et al. Pre-emptive lumbar epidural anaesthesia reduces postoperative pain and patient-controlled morphine consumption after lower abdominal surgery. Pain. 1994;59:395–403.
Katz J., Cohen L., Schmid R., et al. Postoperative morphine use and hyperalgesia are reduced by preoperative but not intraoperative epidural analgesia: implications for preemptive analgesia and the prevention of central sensitization. Anesthesiology. 2003;98:1449–1460.
Katz J., Melzack R. Auricular TENS reduces phantom limb pain. Journal of Pain and Symptom Management. 1991;6:73–83.
Keefe F.J., Somers T.J., Williams D.A., et al. Assessment of pain behaviors. In: Turk D.C., Melzack R., eds. Handbook of pain assessment. ed 3. New York: Guilford Press; 2011:134–150.
Kehlet H. Pain relief and modification of the stress response. In: Cousins M.J., Phillips G.D., eds. Acute pain management. New York: Churchill Livingstone; 1986:49–75.
Kehlet H. Modification of responses to surgery by neural blockade: clinical implications. In: Cousins M.J., Bridenbaugh P.O., eds. Neural blockade in clinical anesthesia and management of pain. ed 2. Philadelphia: Lippincott; 1988:145–188.
Kehlet H., Wilmore D.W. Multimodal strategies to improve surgical outcome. American Journal of Surgery. 2002;183:630–641.
Ketovuori H., Pöntinen P.J. A pain vocabulary in Finnish—the Finnish pain questionnaire. Pain. 1981;11:247–253.
Kim H.S., Schwartz-Barcott D., Holter I.M., et al. Developing a translation of the McGill Pain Questionnaire for cross-cultural comparison: an example from Norway. Journal of Advanced Nursing. 1995;21:421–426.
King R.B. Topical aspirin in chloroform and the relief of pain due to herpes zoster and postherpetic neuralgia. Archives of Neurology. 1993;50:1046–1053.
Kiss I., Müller H., Abel M. The McGill Pain Questionnaire—German version. A study on cancer pain. Pain. 1987;29:195–207.
Klepac R.K., Dowling J., Rokke P., et al. Interview vs. paper-and-pencil administration of the McGill Pain Questionnaire. Pain. 1981;11:241–246.
Kling J.W., Riggs L.A. Experimental psychology. New York: Holt, Rinehart, & Winston; 1971.
Krause S.J., Backonja M.M. Development of a neuropathic pain questionnaire. Clinical Journal of Pain. 2003;19:306–314.
Kremer E., Atkinson J.H. Pain language as a measure of effect in chronic pain patients. In: Melzack R., ed. Pain measurement and assessment. New York: Raven Press; 1983:119–127.
Kremer E., Atkinson J.H., Ignelzi R.J. Pain measurement: the affective dimensional measure of the McGill Pain Questionnaire with a cancer pain population. Pain. 1982;12:153–163.
Lahuerta J., Smith B.A., Martinez-Lage J.L. An adaptation of the McGill Pain Questionnaire to the Spanish language. Schmerz. 1982;3:132–134.
Lázaro C., Bosch F., Torrubia R., et al. The development of a Spanish questionnaire for assessing pain: preliminary data concerning reliability and validity. European Journal of Psychological Assessment. 1994;10:145–151.
Leavitt F., Garron D.C. Validity of a back pain classification scale for detecting psychological disturbance as measured by the MMPI. Journal of Clinical Psychology. 1980;36:186–189.
Leavitt F., Garron D.C., Whisler W.W., et al. Affective and sensory dimensions of pain. Pain. 1978;4:273–281.
Leclerc B., Bergeron S., Binik Y.M., et al. History of sexual and physical abuse in women with dyspareunia: association with pain, psychosocial adjustment, and sexual functioning. Journal of Sex Medicine. 2010;7:971–980.
Lesser H., Sharma U., LaMoreaux L., et al. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63:2104–2110.
Love A., Leboeuf D.C., Crisp T.C. Chiropractic chronic low back pain sufferers and self-report assessment methods. Part I. A reliability study of the Visual Analogue Scale, the pain drawing and the McGill Pain Questionnaire. Journal of Manipulative and Physiological Therapeutics. 1989;12:21–25.
Lowe N.K., Walker S.N., McCallum R.C. Confirming the theoretical structure of the McGill Pain Questionnaire in acute clinical pain. Pain. 1991;46:53–60.
Lynch M.E., Clark A.J., Sawynok J. Intravenous adenosine alleviates neuropathic pain: a double blind placebo controlled crossover trial using an enriched enrollment design. Pain. 2003;103:111–117.
Lyrica Study Group. Pregabalin for peripheral neuropathic pain: results of a multicenter, non-comparative, open-label study in Indian patients. International Journal of Clinical Practice. 2006;60:1060–1067.
Maiani G., Sanavio E. Semantics of pain in Italy: the Italian version of the McGill Pain Questionnaire. Pain. 1985;22:399–405.
Mapi R.I., Quality of life instruments data base. 2003. Available at, http://www.qolid.org
Masedo A.I., Esteve R. Some empirical evidence regarding the validity of the Spanish version of the McGill Pain Questionnaire (MPQ-SV). Pain. 2000;85:451–456.
Mason S.T., Arceneaux L.L., Abouhassan W., et al. Confirmatory factor analysis of the Short Form McGill Pain Questionnaire with burn patients. Eplasty. 2008;8:e54.
Masson E.A., Hunt L., Gem J.M., et al. A novel approach to the diagnosis and assessment of symptomatic diabetic neuropathy. Pain. 1989;38:25–28.
McCreary C., Turner J., Dawson E. Principal dimensions of the pain experience and psychological disturbance in chronic low back pain patients. Pain. 1981;11:85–92.
McGuire D.B., Altomonte V., Peterson D.E., et al. Patterns of mucositis and pain in patients receiving preparative chemotherapy and bone marrow transplantation. Oncology Nursing Forum. 1993;20:1493–1502.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197.
Melzack R., Casey K.L. Sensory, motivational, and central control determinants of pain: a new conceptual model. In: Kenshalo D., ed. The skin senses. Springfield, IL: Thomas; 1968:423–443.
Melzack R., Katz J., Jeans M.E. The role of compensation in chronic pain: analysis using a new method of scoring the McGill Pain Questionnaire. Pain. 1985;23:101–112.
Melzack R., Kinch R., Dobkin P., et al. Severity of labour pain: influence of physical as well as psychologic variables. Canadian Medical Association Journal. 1984;130:579–584.
Melzack R., Perry C. Self-regulation of pain: the use of alpha-feedback and hypnotic training for the control of chronic pain. Experimental Neurology. 1975;46:452–469.
Melzack R., Taenzer P., Feldman P., et al. Labour is still painful after prepared childbirth training. Canadian Medical Association Journal. 1981;125:357–363.
Melzack R., Terrence C., Fromm G., et al. Trigeminal neuralgia and atypical facial pain: use of the McGill Pain Questionnaire for discrimination and diagnosis. Pain. 1986;27:297–302.
Melzack R., Torgerson W.S. On the language of pain. Anesthesiology. 1971;34:50–59.
Melzack R., Wall P.D., Ty T.C. Acute pain in an emergency clinic: latency of onset and description patterns related to different injuries. Pain. 1982;14:33–43.
Menezes Costa Lda C., Maher C.G., McAuley J.H., et al. Systematic review of cross-cultural adaptations of McGill Pain Questionnaire reveals a paucity of clinimetric testing. Journal of Clinical Epidemiology. 2009;62:934–943.
Mongini F., Deregibus A., Raviola F., et al. Confirmation of the distinction between chronic migraine and chronic tension-type headache by the McGill Pain Questionnaire. Headache. 2003;43:867–877.
Mongini F., Italiano M. TMJ disorders and myogenic facial pain: a discriminative analysis using the McGill Pain Questionnaire. Pain. 2001;91:323–330.
Mongini F., Italiano M., Raviola F., et al. The McGill Pain Questionnaire in patients with TMJ pain and with facial pain as a somatoform disorder. Cranio. 2000;18:249–256.
Mystakidou K., Parpa E., Tsilika E., et al. Greek McGill Pain Questionnaire: validation and utility in cancer patients. Journal of Pain and Symptom Management. 2002;24:379–387.
Nickel J.C., Tripp D.A., Pontari M., et al. Psychosocial phenotyping in women with interstitial cystitis/painful bladder syndrome: a case control study. Journal of Urology. 2010;183:167–172.
Nikolajsen L., Hansen C.L., Nielsen J., et al. The effect of ketamine on phantom pain: a central neuropathic disorder maintained by peripheral input. Pain. 1996;67:69–77.
Nikolajsen L., Ilkjaer S., Kroner K., et al. The influence of preamputation pain on postamputation stump and phantom pain. Pain. 1997;72:393–405.
Novak C.B., Anastakis D.J., Beaton D.E., et al. Relationships among pain disability, pain intensity, illness intrusiveness, and upper extremity disability in patients with traumatic peripheral nerve injury. Journal of Hand Surgery. 2010;35:1633–1639.
Page D.B., Weaver F., Wilkie D.J., et al. A computerized survey of pain in Parkinson’s disease patients: a pilot feasibility study. Parkinsonism & Related Disorders. 2010;16:139–141.
Pearce J., Morley S. An experimental investigation of the construct validity of the McGill Pain Questionnaire. Pain. 1989;39:115–121.
Perez C., Saldana M.T., Navarro A., et al. Trigeminal neuralgia treated with pregabalin in family medicine settings: its effect on pain alleviation and cost reduction. Journal of Clinical Pharmacology. 2009;49:582–590.
Perry F., Heller P.H., Levine J.D. Differing correlations between pain measures in syndromes with or without explicable organic pathology. Pain. 1988;34:185–189.
Perry F., Heller P.H., Levine J.D. A possible indicator of functional pain: poor pain scale correlation. Pain. 1991;46:191–193.
Pimenta C.A., Teixeiro M.J. Proposal to adapt the McGill Pain Questionnaire into Portuguese. Revista Da Escola de Enfermagem Da USP. 1996;30:473–483.
Pontari M.A., Krieger J.N., Litwin M.S., et al. Pregabalin for the treatment of men with chronic prostatitis/chronic pelvic pain syndrome: a randomized controlled trial. Archives of Internal Medicine. 2010;170:1586–1593.
Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID pain. Current Medical Research and Opinion. 2006;22:1555–1565.
Price D.D., Harkins S.W., Baker C. Sensory-affective relationships among different types of clinical and experimental pain. Pain. 1978;28:297–307.
Price D.D., McGrath P.A., Rafii A., et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56.
Prieto E.J., Hopson L., Bradley L.A., et al. The language of low back pain: factor structure of the McGill Pain Questionnaire. Pain. 1980;8:11–19.
Prkachin K.M., Solomon P.E., Ross J. Underestimation of pain by health-care providers: towards a model of the process of inferring pain in others. Canadian Journal of Nursing Research. 2007;39:88–106.
Putzke J.D., Richards J.S., Hicken B.L., et al. Pain classification following spinal cord injury: the utility of verbal descriptors. Spinal Cord. 2002;40:118–127.
Radvila A., Adler R.H., Galeazzi R.L., et al. The development of a German language (Berne) pain questionnaire and its application in a situation causing acute pain. Pain. 1987;28:185–195.
Reading A.E. The internal structure of the McGill Pain Questionnaire in dysmenorrhea patients. Pain. 1979;7:353–358.
Reading A.E., Everitt B.S., Sledmere C.M. The McGill Pain Questionnaire: a replication of its construction. British Journal of Clinical Psychology. 1982;21:339–349.
Reading A.E., Newton J.R. On a comparison of dysmenorrhea and intrauterine device related pain. Pain. 1977;3:265–276.
Reading A.L. An analysis of the language of pain in chronic and acute patient groups. Pain. 1982;13:185–192.
Rice A.S., Maton S. Gabapentin in postherpetic neuralgia: a randomised, double blind, placebo controlled study. Pain. 2001;94:215–224.
Roche P.A., Klestov A.C., Heim H.M. Description of stable pain in rheumatoid arthritis: a 6 year study. Journal of Rheumatology. 2003;30:1733–1738.
Ruoff G.E., Rosenthal N., Jordan D., et al. Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient study. Clinical Therapeutics. 2003;25:1123–1141.
Satow A., Nakatani K., Taniguchi S., et al. Perceptual characteristics of electrocutaneous pain estimated by the 30-word list and Visual Analog Scale. Japanese Psychological Review. 1990;32:155–164.
Scholz J., Mannion R.J., Hord D.E., et al. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 2009;6:e1000047.
Scrimshaw S.V., Maher C. Responsiveness of visual analogue and McGill pain scale measures. Journal of Manipulative and Physiological Therapy. 2001;24:501–504.
Sedlak K. A Polish version of the McGill Pain Questionnaire. Pain. 1990;41(Suppl 5):S308.
Serrao J.M., Marks R.L., Morley S.J., et al. Intrathecal midazolam for the treatment of chronic mechanical low back pain: a controlled comparison with epidural steroid in a pilot study. Pain. 1992;48:5–12.
Shin H., Kim K., Young Hee K., et al. A comparison of two pain measures for Asian American cancer patients. Western Journal of Nursing Research. 2008;30:181–196.
Stein C., Mendl G. The German counterpart to McGill Pain Questionnaire. Pain. 1988;32:251–255.
Stelian J., Gil I., Habot B., et al. Improvement of pain and disability in elderly patients with degenerative osteoarthritis of the knee treated with narrow-band light therapy. Journal of the American Geriatrics Society. 1992;40:23–26.
Stokvis A., van der Avoort D.J., van Neck J.W., et al. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151:862–869.
Strand L.I., Ljunggren A.E., Bogen B., et al. The Short-Form McGill Pain Questionnaire as an outcome measure: test-retest reliability and responsiveness to change. European Journal of Pain. 2008;12:917–925.
Strand L.I., Wisnes A.R. The development of a Norwegian pain questionnaire. Pain. 1991;46:61–66.
Tahmoush A.J. Causalgia: redefinition as a clinical pain syndrome. Pain. 1981;10:187–197.
Thomas V., Heath M., Rose D., et al. Psychological characteristics and the effectiveness of patient-controlled analgesia. British Journal of Anaesthesia. 1995;74:271–276.
Torgerson W.S. Critical issues in verbal pain assessment: multidimensional and multivariate issues. Washington, DC: American Pain Society Abstracts; 1988.
Treede R.D., Jensen T.S., Campbell J.N., et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635.
Turk D.C., Rudy T.E., Salovey P. The McGill Pain Questionnaire reconsidered: confirming the factor structures and examining appropriate uses. Pain. 1985;21:385–397.
Turner J.A., Cardenas D.D., Warms C.A., et al. Chronic pain associated with spinal cord injuries: a community survey. Archives of Physical Medicine and Rehabilitation. 2001;82:501–509.
Vanderiet K., Adriaensen H., Carton H., et al. The McGill Pain Questionnaire constructed for the Dutch language (MPQ-DV). Preliminary data concerning reliability and validity. Pain. 1987;30:395–408.
van der Kloot W.A., Oostendorp R.A., van der Meij J. The Dutch version of the McGill pain questionnaire: a reliable pain questionnaire. Nederlands Tijdschrift voor Geneeskunde. 1995;139:669–673.
van Lankveld W., van’t Pad Bosch P., van de Putte L., et al. Pain in rheumatoid arthritis measured with the visual analogue scale and the Dutch version of the McGill Pain Questionnaire. Nederlands Tijdschrift voor Geneeskunde. 1992;136:1166–1170.
Veilleux S., Melzack R. Pain in psychotic patients. Experimental Neurology. 1976;52:535–563.
Verkes R.J., Van der Kloot W.A., Van der Meij J. The perceived structure of 176 pain descriptive words. Pain. 1989;38:219–229.
Victor T.W., Jensen M.P., Gammaitoni A.R., et al. The dimensions of pain quality: factor analysis of the Pain Quality Assessment Scale. Clinical Journal of Pain. 2008;24:550–555.
Voorhies R.M., Jiang X., Thomas N. Predicting outcome in the surgical treatment of lumbar radiculopathy using the Pain Drawing Score, McGill Short Form Pain Questionnaire, and risk factors including psychosocial issues and axial joint pain. Spine Journal. 2007;7:516–524.
Watt-Watson J., Stevens B., Costello J., et al. Impact of preoperative education on pain management outcomes after coronary artery bypass graft surgery: a pilot. Canadian Journal of Nursing Research. 2000;31:41–56.
Weijenborg P.T., Greeven A., Dekker F.W., et al. Clinical course of chronic pelvic pain in women. Pain. 2007;132(Suppl 1):S117–S123.
Wilkie D.J., Huang H.Y., Reilly N., et al. Nociceptive and neuropathic pain in patients with lung cancer: a comparison of pain quality descriptors. Journal of Pain and Symptom Management. 2001;22:899–910.
Wilkie D.J., Judge M.K., Berry D.L., et al. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. Journal of Pain and Symptom Management. 2003;25:213–224.
Wilkie D.J., Kim Y.O., Suarez M.L., et al. Extending computer technology to hospice research: interactive pentablet measurement of symptoms by hospice cancer patients in their homes. Journal of Palliative Medicine. 2009;12:599–602.
Wilkie D.J., Savedra M.C., Holzemier W.L., et al. Use of the McGill Pain Questionnaire to measure pain: a meta-analysis. Nursing Research. 1990;39:36–41.
Williams A.C.D.C., Davies H.T., Chadury Y. Simple pain rating scales hide complex idiosyncratic meanings. Pain. 2000;85:457–463.
Williamson A., Hoggart B. Pain: a review of three commonly used pain rating scales. Journal of Clinical Nursing. 2005;14:798–804.
Wright K.D., Asmundson G.J., McCreary D.R. Factorial validity of the short-form McGill pain questionnaire (SF-MPQ). European Journal of Pain. 2001;5:279–284.
Yakut Y., Yakut E., Bayar K., et al. Reliability and validity of the Turkish version short-form McGill Pain Questionnaire in patients with rheumatoid arthritis. Clinical Rheumatology. 2007;26:1083–1087.
Backonja M.M., Krause S.J. Neuropathic pain questionnaire—short form. Clinical Journal of Pain. 2003;19:315–316.
Bennett M.I., Smith B.H., Torrance N., et al. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. Journal of Pain. 2005;6:149–158.
Bouhassira D., Attal N., Fermanian J., et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–257.
Dworkin R.H., Turk D.C., Revicki D.A., et al. Development and initial validation of an expanded and revised version of the short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144:35–42.
Freynhagen R., Baron R., Gockel U., et al. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Current Medical Research and Opinion. 2006;22:1911–1920.
Galer B.S., Jensen M.P. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332–338.
Gracely R.H., Kwilosz D.M. The Descriptor Differential Scale: applying psychophysical principles to clinical pain assessment. Pain. 1988;35:279–288.
Jensen M.P., Gammaitoni A.R., Olaleye D.O., et al. The pain quality assessment scale: assessment of pain quality in carpal tunnel syndrome. Journal of Pain. 2006;7:823–832.
Krause S.J., Backonja M.M. Development of a neuropathic pain questionnaire. Clinical Journal of Pain. 2003;19:306–314.
Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299.
Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197.
Melzack R., Torgerson W.S. On the language of pain. Anesthesiology. 1971;34:50–59.
Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID pain. Current Medical Research and Opinion. 2006;22:1555–1565.
Scholz J., Mannion R.J., Hord D.E., et al. A novel tool for the assessment of pain: validation in low back pain. PLoS Med. 6, 2009. e1000047