Cyclooxygenase Inhibitors
Clinical Use
Introduction
The classic explanation of how non-steroidal anti-inflammatory drugs (NSAIDs) work was that they inhibited the constitutive enzyme cyclooxygenase (Cox), thereby decreasing prostaglandin synthesis, which in turn reduced the sensitizing effect of the prostaglandins. Cox was believed to be expressed at constant levels in individual tissues. This was unlikely to be the complete story because of observations such as an increase in Cox activity in inflammation, the ability of corticosteroids to block this increase, and the analgesic efficacy of the drugs for conditions that do not involve inflammation. Similarly, a solely peripheral site of action does not fit easily with the antipyretic effects of aspirin or its ability to produce tinnitus, which seems likely to be occurring centrally.
The research that followed identification of two isoforms of Cox, Cox-1 and Cox-2, has made some of the mechanisms of action clearer (Hawkey 1999, Patrono and Baigent 2009). The two enzymes are very similar. Both are membrane associated. Arachidonic acid released from neighboring damaged membranes is converted by the enzymes into prostaglandins. The differences between the two enzymes lie in their internal configuration, which dictates which drugs bind to each; in their distribution in different body tissues; and in their relative preponderance in normal conditions (constitutive) and in response to inflammation (induced). The broad distinction between the two systemic isoforms Cox-1 and Cox-2 is that Cox-1 is expressed mainly constitutively and gives rise to prostaglandins that mediate normal cellular processes whereas Cox-2 is generally considered to be an inducible enzyme elicited by inflammation and called up to synthesize more prostanoids. Cox-1, as the constitutive isoform, is necessary for normal functions and is found in most cell types. Cox-2, despite being the inducible isoform, is expressed constitutively (i.e., under normal conditions) in a number of tissues, which probably include brain, testis, and kidney.
It is because these prostanoids play a variety of important roles in the normal physiology and functioning of the gastrointestinal tract, the renal system, and the cardiovascular system that NSAID and coxib therapy, by inhibiting prostaglandin and thromboxane production, can interfere with prostaglandin-mediated maintenance of these systems and result in a range of potential adverse effects.
Paracetamol, Dipyrone, and Nefopam
Nobody knows precisely where paracetamol, dipyrone, or nefopam work. The standard explanation for paracetamol is that it acts as a Cox inhibitor in the brain, which explains both its analgesic and its antipyretic actions. The lack of clinical anti-inflammatory activity may be because paracetamol is not active on peripheral Cox. Similar comments are made for dipyrone. Nefopam, though undoubtedly an analgesic, has neither opioid nor NSAID-like activity. It is neither anti-inflammatory nor antipyretic, and like paracetamol and dipyrone, its site of action is presumed to be in the central nervous system.
Clinical Efficacy
How Well Does the Intervention Work?
Clinicians need to know how well the intervention works: the size of the effect and its clinical significance. Knowing only that an intervention works is much, much less helpful, especially when a range of similar interventions is available. The information on relative efficacy given here uses the number needed to treat (NNT) as the measure of clinical significance, with data being derived from quantitative systematic reviews. The NNT describes the difference between active treatment and control, and in Figures 33-1 to 33-4 this is the difference between active drug and placebo in the proportion of patients who achieve at least 50% pain relief over a 6-hour period following a single postoperative dose of the drug.
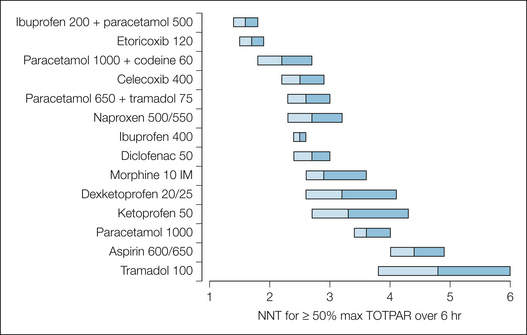
Figure 33-1 Numbers needed to treat (NNTs) for 50% relief of postoperative pain (single dose) for 6 hours after the dose.
The NNT point estimate is at the junction of the two shaded bar segments, which represent the upper and lower 95% confidence intervals. TOTPAR, total pain relief.
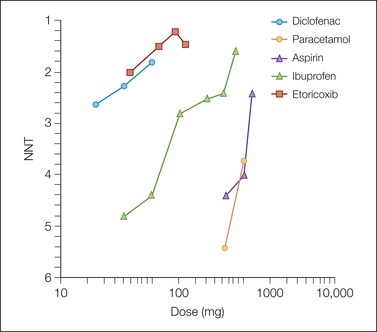
Figure 33-2 Non-steroidal anti-inflammatory drug and coxib 6-hour dose–response relationships for numbers needed to treat (NNTs) for 50% relief of postoperative pain (single dose).
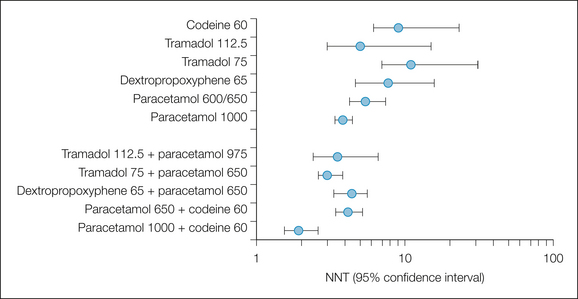
Figure 33-3 Numbers needed to treat (NNTs) to obtain at least 50% pain relief over a 4–6–hour period: comparison of single-dose combination drugs and their components.
Error bars indicate the upper and lower 95% confidence intervals.
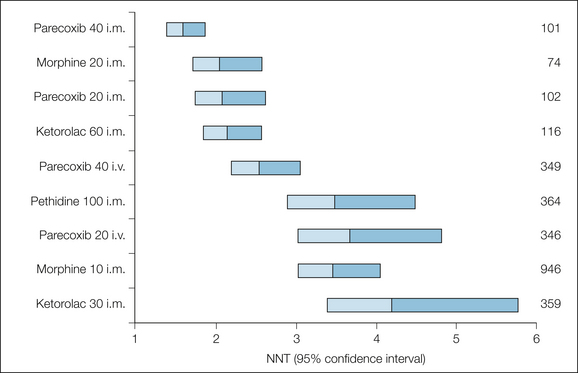
Figure 33-4 Numbers needed to treat (NNTs) to obtain at least 50% pain relief over a 4–6–hour period: comparison of single-dose injected non-steroidal anti-inflammatory drugs, coxibs, and opioids.
The NNT point estimate is at the junction of the shaded bars, which represent the upper and lower ends of the 95% confidence intervals.
By deriving the NNT for different analgesics from comparisons with placebo, their relative efficacy can be compared, and such league tables (see Fig. 33-1) are easy to understand. As more trials are reported and systematic reviews are compiled to provide similar data on other analgesics, the league table can be extended and refined and thereby allow drug comparison on a credible evidence base. The league table is legitimate only because it uses information on similar patients with valid inclusion criteria (pain of moderate or severe intensity), similar measurement methods, and similar outcomes, with placebo being used as a common comparator, and in circumstances in which we know that the pain model makes no difference (Barden et al 2004). Although it can be argued that head-to-head comparison between analgesics would be better, the problem is that few such comparisons exist and randomized trials to detect small differences in efficacy between two analgesics would need to be massive to be able to detect differences in direction, let alone in the magnitude of the difference.
This approach depends on having outcomes that are clinically useful, and with acute and chronic pain, a reduction in pain intensity of about 50% is often regarded as clinically useful (Moore et al 2005, Dworkin et al 2008). Moreover, in chronic pain conditions, a reduction in pain by this amount is associated with major benefits in terms of associated symptoms such as sleep-related problems, depression, and fatigue and in terms of major improvements in quality of life (Moore et al 2010c).
Oral Non-opioids
Figure 33-1 shows the NNTs, confidence intervals, and number of patients in the trials at each single oral dose of selected NSAIDs, coxibs, paracetamol, tramadol, and intramuscular morphine. The data are derived from systematic reviews of randomized controlled trials of single doses for postoperative pain. It is clear that the NSAIDs and coxibs do extremely well in this single-dose postoperative comparison. They have NNT values of between 2 and 3, which means that of two or three patients given that drug at that dose, one will achieve at least 50% pain relief, a high hurdle.
The NSAID and coxib NNTs at these doses are lower (i.e., better) than the value achieved by 10 mg of intramuscular morphine, even though the confidence intervals overlap. It has been known for many years from single trials that oral NSAIDs can provide analgesia similar to that achieved with 10 mg of intramuscular morphine, and these data confirm those observations. The limited data that we have on 20 mg of intramuscular morphine give an NNT of less than 2.
Coxibs achieve NNTS that are as good as or better (lower) than NNTs with NSAIDs. The coxibs have been used in postoperative trials at doses several multiples of the dose for chronic pain, whereas the NSAIDs were tested at doses close to the chronic pain dose, so the difference in efficacy between coxibs and NSAIDs in this 6-hour efficacy comparison is largely a function of dose (see Fig. 33-2). There is no reason to expect any greater analgesia from the coxibs than from the NSAIDs—which were after all designed specifically to reduce gastrointestinal hemorrhage rather than because they offered greater efficacy—unless the doses are increased relative to the NSAIDs.
Aspirin, 600 or 650 mg, and paracetamol, 1000 mg, are significantly less effective than 10 mg of intramuscular morphine. The point estimates of the NNT are higher, and there is no overlap of the confidence intervals. The original trial results of Houde and Wallenstein suggested milligram-for-milligram equianalgesic equivalence between aspirin and paracetamol for postoperative pain, and this is borne out by our results. Dipyrone, 500 mg orally, has an NNT of 2.4 (1.9–3.2) from data on only 143 patients (Edwards et al 2001). It too is an effective drug. We have no NNT for nefopam.
These NNTs were derived from single-dose pain studies. It is not possible from these analyses to comment on the speed of onset of analgesia, but we know from single trials that oral normal-release formulations of the original NSAIDs start to work at roughly half an hour, with the peak effect occurring between 60 and 90 minutes. The duration of analgesia with NSAIDs and coxibs is a function of dose (and kinetics). Bigger doses will provide an analgesic effect longer than the 4–6 hours expected with the standard therapeutic dose. The large coxib doses used in these postoperative trials should therefore result in longer duration of analgesia. Most multiple-dosing studies have been performed in patients with arthritis, and the relative efficacy data shown here from single doses seem to tally well with the multiple-dosing studies.
Another old observation from single trials was that the dose–response relationship for analgesia with NSAIDs was flat, meaning that the increase in analgesia as a result of increasing the dose was less marked than that seen with a similar relative increase in dose for, say, morphine. The results from systematic reviews of single doses (see Fig. 33-2) show very similar dose–response relationships for aspirin and paracetamol, a reflection of their milligram-for-milligram equivalence in efficacy, and show the greater potency (more analgesia at a lower milligram dosage) of diclofenac, ibuprofen, and etoricoxib.
The largest doses of etoricoxib, diclofenac, and ibuprofen produce NNTs approaching 1, which is the theoretically perfect NNT (actually, 1.1–1.2 with placebo responses of about 10–15%). Doses are plotted in Figure 33-2 as logarithms. This perhaps emphasizes the clinical perception of the ceiling of non-opioid analgesia. For diclofenac and etoricoxib, the doses shown are on the upper (flatter) part of the sigmoid dose–response curve; further increases in dose may produce little improvement in the NNT. The steeper slopes for aspirin and paracetamol suggest the steep portion of the sigmoid curve; better NNTs could be achieved with larger doses if it were safe to give them.
The analgesic efficacy of the non-opioid analgesics is improved by combining them with weak opioids (see Fig. 33-3) (Edwards et al 2002). The combination of paracetamol, 600 or 650 mg, with codeine or dextropropoxyphene lowers (improves) the NNT of the combination to levels similar to that of 10 mg of intramuscular morphine. At a practical clinical level, combinations of simple analgesics with opioids are considered effective and are often used as one rung in the ladder of analgesic treatments. The clinical need for combinations is based on the fact that a proportion of patients cannot or should not be given NSAIDs or coxibs, usually because of allergy or gastrointestinal problem. In a young group of study patients this proportion was 17% (Merry et al 2004).
The central argument used against combinations is that a combination of A plus B is no better than A alone. Figure 33-3 illustrates that pooling information from individual trials can provide evidence to deal with this argument in a way that individual trials of conventional size cannot. Clearly, the combinations were better than the individual components alone, and the argument that a combination of A plus B is no better than A alone can be rebutted.
Efficacy with Chronic Use
Efficacy comparisons of NSAIDs and coxibs for arthritis are of high quality, large, and of long duration and have eclipsed previous studies of NSAIDs for arthritis (Bandolier 2002). Coxibs and NSAIDs were more effective than placebo, and coxibs were as effective as the maximum daily doses of most standard NSAIDs (diclofenac, 150 mg, and naproxen, 1000 mg daily). Re-analysis of trial data by using outcomes of at least 30% and at least 50% pain relief over a period of 12 weeks and defining responders as having the response without withdrawal emphasizes the good equivalence between coxibs and most NSAIDs, with the possible exception of ibuprofen (Fig. 33-5; Moore et al 2010a). Patients achieved either good pain relief or virtually none, and no drug provided good relief of osteoarthritis pain in more than 60% of the patients. No single coxib or NSAID will work in every patient. For coxibs that level of efficacy is achieved with better gastrointestinal safety.
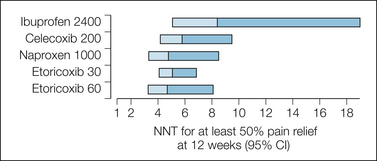
Figure 33-5 Numbers needed to treat (NNTs) to obtain at least 50% pain relief over baseline at 12 weeks with coxibs and non-steroidal anti-inflammatory drugs.
The NNT point estimate is at the junction of the shaded bars, which represent the upper and lower ends of the 95% confidence intervals (CI).
There is limited good-quality evidence from long-duration trials on treatment of chronic low back pain, but the evidence we have is that etoricoxib, 60 or 90 mg, has NNTs of 7 or 8 for at least 50% pain relief over a 12-week period (Moore et al 2010b).
Another way of considering efficacy with chronic use is to look at the proportion of patients who discontinue the drug because of lack of efficacy. In the arthritis trials, 20% of patients discontinued by 6 weeks because of the lack of efficacy of placebo, two-thirds of those who discontinue for any reason. For paracetamol, 4 g/day, the discontinuation rate because of lack of efficacy was similar. For the maximum daily dose of NSAIDs or the trial dosages of coxibs, the lack-of-efficacy discontinuation rate at 6 weeks was around 6%. At 6 months, the rate was about 14% for both NSAIDs and coxibs.
Paracetamol was not very different from placebo in trials of patients with osteoarthritis. The NNT of 16 for overall pain was regarded to be of questionable clinical significance (Towheed et al 2006), and even that result may be an overestimate because of residual bias in some of the trials. This may be confounded because people with adequate relief from paracetamol do not enter the trials.
Other Routes of Administration
Evidence that topical NSAIDs are effective for strains and sprains and for arthritic conditions came from a systematic review of 86 randomized controlled trials involving 10,160 patients (Moore et al 1998). Measures approximating at least 50% pain relief were used, with analysis at 1 week for acute and 2 weeks for chronic conditions. In acute pain conditions, placebo-controlled trials had an NNT of 3.9 (3.4–4.4). Analyzing by drug (at least three trials), ketoprofen (NNT of 2.6), felbinac (3.0), ibuprofen (3.5), and piroxicam (4.2) had significant efficacy. Benzydamine and indomethacin were not distinguished from placebo. In placebo-controlled trials of chronic pain conditions, the NNT for topical NSAIDs was 4.6 (3.8–5.9) (Mason et al 2004). This analgesia was not due to rubbing a cream on the painful area because both placebo and the topical NSAID were applied in the same way. Topical NSAIDs (but not rubefacients) are now recommended first-line treatment of osteoarthritis in many countries because they are effective and relatively safe.
Injected and Rectal
A league table of the relative analgesic efficacy of injected NSAIDs and coxibs for postoperative pain is shown in Figure 33-4, with injected opioid used for comparison. As with the oral doses in Figure 33-1, the best performers have NNT values between 1 and 2.
Injected NSAIDs, coxibs, paracetamol (propacetamol), dipyrone, and nefopam are all effective analgesics, but it is difficult to establish whether they are any more effective than their oral equivalents. Indeed, oral ketorolac, 10 mg, was equivalent to intramuscular ketorolac, 30 mg, in one review (Smith et al 2000). If oral and injected formulations are equally effective, the advantage of the injected route would be restricted to contexts in which patients cannot swallow or, if the injected form was faster in onset of action, to contexts where rapid analgesia was required. To prove that the injected route is better than the oral requires that the same drug be compared at the same bio-available dose across the two routes. A systematic review of randomized controlled trials (2225 analyzed patients) published between 1970 and 1996 in which the difference in analgesic efficacy and adverse effects of NSAIDs given by different routes of administration was examined found 15 trials that compared the same drug by different routes (Tramèr et al 1998). In just nine of them (35% of all trials) was the same drug compared at the same dose.
A simple clinical conclusion is that we lack convincing evidence that the same dose of NSAID is any more effective when given by injection than by mouth. One might then argue that it may make little sense to give that dose by injection instead of by mouth to a patient who can swallow.
Safety
NSAIDs can cause a number of minor adverse effects at recommended doses, but they can also cause major adverse effects at such doses. At recommended doses, paracetamol can also cause minor adverse effects but no major adverse effects. It is principally in overdose that paracetamol is dangerous, with the potential to cause hepatic failure. Dipyrone is not available in many countries because of concerns about agranulocytosis; controversy continues about the incidence of this problem. Dipyrone was (re)withdrawn from the market in Sweden recently after 6 cases occurred in 10,000 patients exposed. This is a much higher incidence than that encountered in other countries where the drug is used widely.
With the NSAIDs it is worth recalling the concept that the slope of the dose–response curve for analgesia may not be as steep as that for morphine (see Fig. 33-2). The slope of the dose–response curves for adverse effects need not be the same as that for analgesia. If the slope of the dose–response curve for an adverse effect is steeper than that for analgesia, an increase in dose to produce greater analgesia may produce a proportionately greater increase in the adverse effect.
NSAID Safety with Acute Use
With acute pain the main concerns with NSAIDs are allergic reaction, renal failure, coagulation problems, and impact on healing processes, particularly of bone. Acute renal failure may be precipitated in patients with pre-existing heart or kidney disease, those taking loop diuretics, or those who have lost more than 10% of blood volume. NSAIDs cause significant lengthening (by about 30%) of the bleeding time, but usually still within the normal range. This can last for days with aspirin but just for hours with non-aspirin NSAIDs. This raises the possibility that NSAIDs can cause a significant increase in blood loss.
A comparison of ketorolac, diclofenac, and ketoprofen in more than 11,000 patients undergoing major surgery and given injected and then oral doses of one of the three NSAIDs resulted in a 1% incidence of increased surgical site bleeding, 0.1% incidence of allergy, 0.1% incidence of acute renal failure, and 0.04% incidence of gastrointestinal bleeding (Forrest et al 2002). There was no difference between the three NSAIDs.
The paper does not tell us what would happen in the absence of NSAIDs, so these estimates are in a sense the worst case, with relatively large doses of injected and oral NSAIDs being used. The paper shows that the postoperative risk for acute renal failure with NSAIDs is greater than the risk for gastrointestinal bleeding. No clinical renal failure was seen in the tenoxicam study (750 patients) (Merry et al 2004). The risk will be context dependent, and the incidence reported will depend on the definition, biochemical or clinical. Older dehydrated patients will be at greater risk than young fit adults undergoing third molar removal. There would not appear to be any biological reason why the incidence would be any lower with coxibs than with NSAIDs. If higher doses of coxibs are used, the incidence may be greater, although studies of long-duration use of high doses of coxibs have not yet reported a high incidence of renal problems, even in older cohorts.
Surgical site bleeding with NSAIDs after tonsillectomy has been the subject of two systematic reviews (Marret et al 2003, Møiniche et al 2003). The number needed to harm (NNH) for reoperation after NSAID versus placebo use was about 60. One patient in 60 given an NSAID will require a reoperation who would not have needed that reoperation if an NSAID had not been given. A very similar reoperation NNH with aspirin versus non-aspirin was reported for coronary artery bypass grafting (Ferraris et al 2002). We do not yet know whether the clinically relevant incidence of surgical site bleeding will be lower with coxibs than with NSAIDs.
The questions of whether NSAIDs delay healing to a clinically relevant extent and whether coxibs differ from NSAIDs remain contentious, particularly with bone injury. NSAIDs inhibit Coxs, which are essential for prostaglandin production. Prostaglandins mediate inflammation, influence the balance of bone formation and resorption, and are essential for bone repair. Some animal studies have shown inhibition of fracture healing by NSAIDs. There is no good clinical evidence that NSAIDs or coxibs inhibit bone healing in humans. NSAID and coxib use appears to increase bone density and does not increase the risk for fractures. Smoking reduces bone density, significantly impairs healing after surgery or trauma, and is likely to be an important confounder in studies of the effects of NSAIDs and coxibs on tissue healing.
Minor Problems
Adverse effects in single-dose oral acute pain studies have been examined systematically for paracetamol, ibuprofen, and aspirin (Edwards et al 1999). Common adverse effects such as nausea, dizziness, or drowsiness were reported more often when diaries were used, and drowsiness was reported more often in dental than in other pain models. The incidence of any adverse effect with any single dose of analgesic was low; however, for paracetamol and ibuprofen, but not aspirin, the incidence was statistically greater than that with placebo. Gastric irritation was two to three times more common with aspirin than with placebo, with an NNH of 22 (95% confidence interval, 22–174) (Edwards et al 2000). For injected and rectal NSAIDs, commonly reported adverse effects independent of the route of administration were nausea, vomiting, dizziness, drowsiness, sedation, anxiety, dyspepsia, indigestion, and dry mouth (Tramèr et al 1998). Two studies reported changes in bleeding times. In 12 patients with rheumatoid arthritis treated with indomethacin, 100–150 mg orally and rectally, in a crossover design for 2 weeks, endoscopically diagnosed gastric mucosal damage was independent of the route of administration.
The risk for postoperative bleeding is higher with NSAIDs than with non-NSAIDs (Møiniche et al 2003), but there is probably no increase in risk with coxibs.
Adverse effects related to the route of administration were most often reported for intramuscular and rectal regimens. Discomfort at the site of injection was the most frequent complaint in relation to intramuscular injections. After rectal administration, diarrhea, rectal irritation, and non-retention of suppositories were reported.
For topical NSAID treatment of both acute and chronic pain, the incidence of local and systemic adverse events and drug-related study withdrawal was low and no different from that with placebo. Longer-duration use was not associated with gastrointestinal bleeding.
NSAID Safety with Chronic Use
Harm from coxibs and NSAIDs as a result of upper gastrointestinal bleeding, cardiovascular events, mortality, and heart failure is being investigated in a large individual-patient meta-analysis carried out by the Coxib and NSAID Trialists Collaboration, which is due to report in 2013. This meta-analysis is likely to provide more detailed information than presently available, but it is unlikely to change the broad results already known (Table 33-1).
Table 33-1
NSAID Use and History of Heart Disease: Effect on Risk for Congestive Heart Failure
HEART DISEASE |
NSAID USE |
ODDS RATIO (95% CONFIDENCE INTERVAL) |
| No history | Non-user | 1 |
| No history | User | 1.6 (0.7–3.7) |
| History | Non-user | 2.5 (1.4–4.3) |
| History | User | 26 (6–119) |
Gastrointestinal Adverse Effects
Oral NSAIDs cause ulcers in some people. In some of those who do have ulcers, some also have symptoms, including bleeding ulcers. In some of those who have bleeding ulcers, the bleeding is sufficiently severe to result in hospital admission and may cause death (in about 1 in 13 overall, but as high as about 1 in 5 in those taking NSAIDs; Straube et al 2009). The variables are drug and dose, duration of exposure, and patient characteristics. The total burden is large, with approximately 106,000 NSAID-related hospital admissions and 16,500 deaths occurring every year in the United States (Singh 1998). Age and sex are the major risk factors for serious gastrointestinal complications with NSAIDs, although a history of previous ulcers and heart disease is also important. Of the different NSAIDs, some are implicated more than others, although case–control and cohort studies give somewhat different estimates. Both types of study indicate that ibuprofen is among the safest of the NSAIDs.
Gastrointestinal emergencies associated with oral NSAID use are a big problem. Two U.K. studies, each involving about 1% of the U.K. population, indicated, first, that 1.9% of NSAID users might be admitted to the hospital each year with upper gastrointestinal emergencies (Blower et al 1997) and, second, that one episode of bleeding ulcer in the elderly will be expected for each 2823 prescriptions (Hawkey et al 1997), and this was confirmed by a Canadian study (Mamdani et al 2002). Another way of stating this is that if oral NSAIDs are taken for at least 2 months, the risk for an endoscopically confirmed ulcer is 1 in 5; for a symptomatic ulcer, about 1 in 70; for a bleeding ulcer, about 1 in 150; and for death from a bleeding ulcer, about 1 in 1300 (Tramèr et al 2000). None of these risks are associated with topical NSAIDs, which have much lower plasma concentrations.
Epidemiological studies published in the 1990s associating NSAID use and upper gastrointestinal problems have been reviewed and the data pooled to give a much clearer picture of the risks (Hernández-Díaz and Rodríguez 2000). To be included, studies had to be case–control or cohort studies on non-aspirin NSAIDs and have data on bleeding, perforation, or other serious upper gastrointestinal tract events resulting in hospital admission or referral to a specialist, as well as data for calculating relative risk. Eighteen studies were found, all of which had specific definitions of exposure and outcome and similar ascertainment for comparison groups. All but two attempted to control for potential confounding factors such as age, sex, history of ulcer, or concomitant medications.
The main results are summarized in Figures 33-6 and 33-7. When compared with non-users, NSAID users had a higher risk for upper gastrointestinal bleeding when they were current NSAID users and took a higher dose. The duration of use was unimportant, but different NSAIDs had different risks, with ibuprofen (especially doses below 2400 mg/day) being the least harmful.
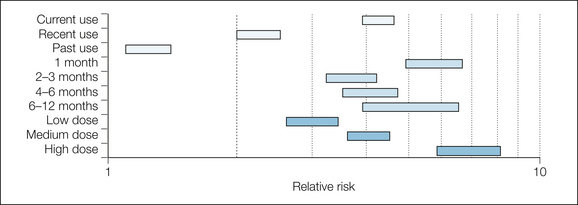
Figure 33-6 Risk for upper gastrointestinal bleed or perforation in NSAID users versus non-users.
Bars represent 95% confidence intervals of relative risk.
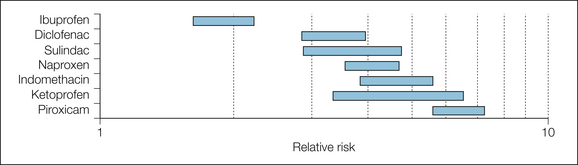
Figure 33-7 Risk for upper gastrointestinal bleeding or perforation for particular non-steroidal anti-inflammatory drug users versus risk in non-users.
Bars represent 95% confidence intervals of relative risk.
The effect of ulcer history and age is shown in Figures 33-8 and 33-9. People with a history of ulcers or with previous bleeding who took NSAIDs were at much greater risk than those with no history of ulcer who took NSAIDs. Older patients who took NSAIDs were at greater risk than under-50s who took NSAIDs.
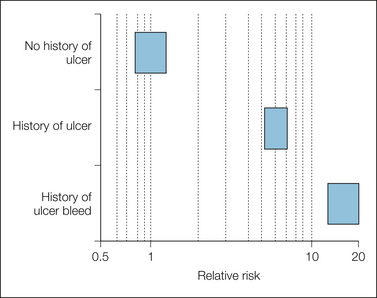
Figure 33-8 Effect of history of ulcer in users of non-steroidal anti-inflammatory drugs.
Bars represent 95% confidence intervals of relative risk.
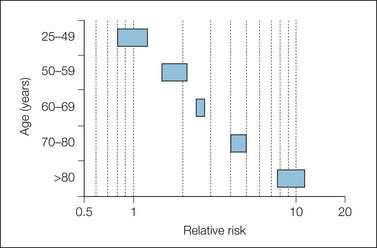
Figure 33-9 Effect of age in users of non-steroidal anti-inflammatory drugs.
Bars represent 95% confidence intervals of relative risk.
The advantage of coxibs over NSAIDs is their reduced potential for causing serious gastrointestinal bleeding. Evidence for this advantage, from both trials (Bombadier et al 2000) and meta-analyses (Goldstein et al 2000, Langman et al 1999), is that serious bleeding is less frequent with coxibs than with NSAIDs. The cumulative incidence of perforation, ulcers, and bleeding over a 12-month period is about half that with the classic NSAIDs. The limited evidence available also suggests that there are fewer dyspeptic symptoms with coxibs than with NSAIDs. A rule of thumb is that for most gastrointestinal adverse events, the ratio for no drug–coxib–NSAID is on the order of 1:2:4.
NSAIDs and NSAIDs plus a proton pump inhibitor, but not coxibs, are also associated with bleeding from the lower gastrointestinal tract, some of which is serious. The evidence we have is that this is not uncommon (Fortun and Hawkey 2007). Fecal blood loss is typically appreciable with NSAIDs, but not with coxibs (Moore et al 2008), and this may be linked with anemia, which is itself associated with serious morbidity and mortality in older persons.
Cardiovascular Adverse Effects
The first signal that there may be a cardiovascular problem with coxibs and/or NSAIDs came in the VIGOR study, in which there was a numerical excess of largely non-fatal events for rofecoxib versus naproxen (Bombardier et al 2000). The best current estimate from randomized trials is that both coxibs and NSAIDs carry a similar small excess in risk over placebo, with the exception of naproxen, 500 mg twice daily, for which there is a protective effect (Kearney et al 2006, Patrono and Baigent 2009).
There is no significant difference between coxibs and non-naproxen NSAIDs (mainly ibuprofen and diclofenac), with serious vascular events occurring in 0.9% and 1.0%, respectively (Patrono and Baigent 2009). With naproxen, significantly fewer events (0.8%) occurred than with the coxibs (1.1%).
A meta-analysis of observational studies in which the use of coxibs or NSAIDs was compared with non-use broadly confirms that there is no major increase in risk for myocardial infarction, although the protective effect of naproxen was not seen outside the context of randomized trials (Hernández-Díaz et al 2006).
There does not appear to be any difference in vascular death between coxibs and NSAIDs.
Hypertensive Effects of Analgesics
NSAIDs and coxibs raise blood pressure in some individuals, but to a variable extent between individuals. For example, in patients who had 24-hour systolic blood pressure readings taken before and after starting 6 weeks of treatment with celecoxib, rofecoxib, or naproxen, some had rises or falls in blood pressure in excess of 20 mm Hg; most had very small effects (Sowers et al 2005). Hypertensive patients taking coxibs or NSAIDs are more susceptible than normotensive patients to increases in blood pressure, and the mean increase in blood pressure tends to be slightly higher in untreated hypertensive patients than in normotensive patients. Hypertensive patients receiving antihypertensive therapy experienced a greater mean increase in supine blood pressure as a result of NSAID therapy than did uncontrolled hypertensive patients (4.7 versus 1.8 mm Hg), and increases in blood pressure were greater in patients receiving β-blockers than in those receiving vasodilators and diuretics (Johnson et al 1994).
Congestive Heart Failure
Another major problem is congestive heart failure in older people (Page and Henry 2000), with as many hospital admissions resulting from NSAID-induced congestive heart failure as from gastrointestinal bleeding.
A study at two hospitals in New South Wales (caring for a population of about 450,000) enrolled as subjects consecutive patients between 1993 and 1995 when the medical officer admitting the patient and the attending physician agreed that the primary reason for admission was congestive heart failure (Page and Henry 2000). Patients admitted for other reasons with incidental congestive heart failure were not included. Study nurses ensured that all the patients included met the Framingham criteria for congestive heart failure. Control subjects (target of two per patient) were patients of the same sex and within 5 years of age admitted to the same hospital but with no clinical or radiological signs of congestive heart failure.
A total of 365 patients and 658 control subjects with a mean age of 76 years were enrolled. Most patients had moderate or severe congestive heart failure. Non-aspirin NSAIDs were used by 17% of the patients in the week before admission versus 12% of controls. The adjusted odds ratio was 2.1 (95% confidence interval, 1.2–3.3) for all patients and 2.8 (1.5–5.1) for the 272 patients with a first admission for congestive heart failure (Table 33-2).
Table 33-2
Summary of Risks* Associated with NSAIDs and Coxibs
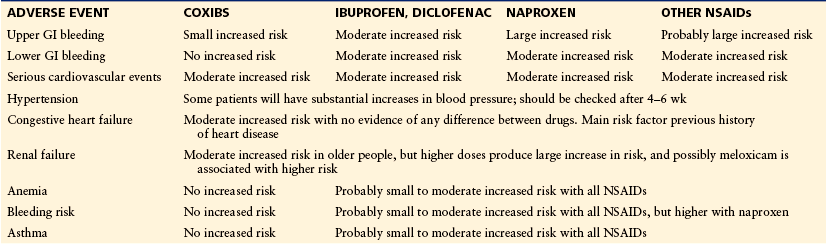
GI, gastrointestinal; NSAID, non-steroidal anti-inflammatory drug.
*Small increased risk: relative risk typically below 1.5 but above 1.0. Moderate increased risk: relative risk typically between 1.5 and 3.0. Large increased risk: relative risk typically above 3.0.
Congestive heart failure was far more likely in patients with a previous history of heart disease, for which the odds ratio was 26 (5.8–119). Complicated statistical analysis confirmed the effect of pre-existing heart disease and suggested that NSAIDs with longer half-lives (naproxen, piroxicam, and tenoxicam) had a much higher risk than those with short half-lives (ibuprofen and diclofenac, for instance), although with small numbers in subgroup analysis.
The importance of precipitation of congestive heart failure by NSAIDs was substantiated in a large study from Sweden (Merlo et al 2001). Ecological line regression established an increased relative risk of 1.26 (1.23–1.28) for hospitalized heart failure with outpatient NSAID use. Starting NSAID therapy doubles the incidence of heart failure in susceptible individuals: those with renal failure, diabetes, or hypertension (García Rodríguez and Hernández-Díaz 2003).
Renal Failure
NSAIDs can cause acute renal failure with chronic use, as they can with acute use. In renal dysfunction, the kidneys may depend on extra prostaglandin production just to maintain function. Taking NSAIDs, which reduce the extra prostaglandin production, will then impair function. Risk factors, as with acute NSAID use, include pre-existing heart or kidney disease, use of loop diuretics, or loss of more than 10% of blood volume. An estimate of the risk came from a study of all members of the Tennessee Medicaid program who were 65 years or older in 1987–1991 and enrolled for at least 1 year (Griffin et al 2000). Those with a first admission to the hospital for acute renal failure (admission creatinine level of 180 μmol/L or greater) were patients with community-acquired acute renal failure. Controls were randomly selected for all persons in the study population. Exclusions were people with end-stage renal disease and those with hospital-acquired acute renal failure. NSAID exposure was ascertained from prescriptions filled in the year before the index date.
There were 1799 patients with an annual incidence of community-acquired acute renal failure of 4.5 admissions per 1000. The median hospital stay was 8 days. Thirty-six percent of patients died within 30 days. Forty-two percent were classified as having new renal disease. The remainder were classified as having chronic renal failure with acute exacerbation based on a prior creatinine level higher than 122 μmol/L, a documented history of chronic renal failure, or imaging studies compatible with chronic renal disease. A total of 9899 control subjects were included. Controls were less likely to be nursing home residents or 85 years or older.
Use of NSAIDs was higher (18%) in the patients with acute renal failure than in control subjects (11%). For current NSAID use, the odds ratio was 1.6 (95% confidence interval, 1.3–1.9). Those who had stopped taking NSAIDs within the past 30 days had no increased risk for renal failure. With certain NSAIDs for which there was sufficient information, ibuprofen and indomethacin, there was a dose-related response for risk. For individual NSAIDs, ibuprofen, piroxicam, fenoprofen, and indomethacin had the greatest increased risk, with odds ratios of about 2.
A previous detailed study, though with smaller numbers, indicated that prior renal disease or gout—but in particular a combined history of gout and previous renal disease—was a major risk factor for renal failure with NSAIDs (Henry et al 1997). A subsequent large study confirmed the association between NSAID use and renal failure, with a relative risk of about 3, but possibly higher on switching NSAIDs or with meloxicam, although the number of events was too low for confidence in these conclusions (Huerta et al 2005).
There seems to be no biological reason why the risk for renal problems should be lower with coxibs than with the NSAIDs.
Comment
The four major risks associated with NSAIDs—gastrointestinal bleeding, serious cardiovascular events, congestive heart failure, and renal failure—are important, particularly since increased age is an important factor for all four of them; each increases with increasing age, and with increasing age comes the greater probability that coxibs or NSAIDs will be required to treat chronic pain. If all this is put into the perspective of an average primary care grouping of 100,000 (Bandolier 2000), in this population (3500 over-65s taking NSAIDs) there would be 18 hospital admissions every year for upper gastrointestinal bleeding, 10 for acute renal failure, and 22 for congestive heart failure. The majority of the renal and heart failure cases would occur in those 75 years and older. For both renal failure and congestive heart failure, NSAIDs uncover existing disease problems, and for both there are plausible mechanisms, dose–response relationships, and particular association with NSAIDs that have longer half-lives.
What can be done to minimize these risks? The risk for gastrointestinal bleeding with NSAIDs may be reduced by the co-administration of proton pump inhibitors or misoprostol. Gastrointestinal adverse effects limit the tolerability of misoprostol. For gastrointestinal bleeding, the coxibs reduce but do not eliminate the risk. The respective rates of complicated confirmed events (perforation, obstruction, and severe upper gastrointestinal bleeding) were 0.6 per 100 patient-years and 1.4 per 100 patient-years (relative risk, 0.4; 95% confidence interval, 0.2–0.8). There is also some evidence that long-term use of high-dose proton pump inhibitors increases the risk for osteoporotic fractures, which are associated with major mortality and morbidity in the elderly, but the combination of proton pump inhibitor plus a coxib can significantly reduce the risk for upper gastrointestinal bleeding in very high-risk patients.
Coxibs, however, do not reduce the risk for serious cardiovascular events, renal failure, or congestive heart failure, and for these risks there has to be a clinical balance between the analgesia provided by an NSAID or coxib and the risk for complications. Over half the patients in the Celecoxib Long-term Arthritis Safety Study (CLASS) of celecoxib versus NSAIDs for arthritis were receiving hypertensive treatment, a reminder of the awkward fact that patients with pain will often be hypertensive and hence at risk. There is no evidence that the risk for congestive heart failure is higher with coxibs than with NSAIDs, but for both coxibs and NSAIDs there is a slight increase in risk for serious vascular events. Equally, there is no evidence that the risk for renal failure is higher with coxibs than with NSAIDs. The risks can be minimized only by sensible assessment and selection, starting at a low dose, and titration.
A major issue is that we know about harm from coxibs, ibuprofen at doses above those typically used, diclofenac, and naproxen. For most NSAIDs we have almost no usable information about either their efficacy or their harm.
Contraindications
A history of gastrointestinal bleeding, particularly in the past year, and co-administration of steroids, which also increase the risk for gastrointestinal bleeding, are potential contraindications to NSAID use, as would be the presence of moderate or severe renal problems or congestive heart failure. With mild renal dysfunction, dose reduction and the use of drugs with a shorter half-life may reduce the risk.
Asthma and Allergy
The NSAIDs may make asthma worse, and NSAIDs should be avoided in any patient who has had an exacerbation of asthma, angioedema, urticaria, or rhinitis while taking aspirin or any other NSAID (Jenkins et al 2004). The advice to use paracetamol as the alternative in this circumstance seems sound. Recent publications claiming that paracetamol causes asthma appear to suffer from confounding by indication. Patients with asthma are told not to take NSAIDs but to take paracetamol. Therefore, many people taking paracetamol have asthma, but the paracetamol is not causal.
Drug Interactions
In a case–control study of patients attending an anticoagulant therapy unit (2000 patients) over a single year who had been taking warfarin for at least 1 month, had a target international normalized ratio (INR) of 2.0–3.0, but whose actual INR was higher than 6.0, paracetamol was a risk factor (Hylek et al 1998). The more that was taken in the week before the test, the greater the chance of an increased INR (Fig. 33-10). More than nine 500-mg tablets a week gave an odds ratio of 7, and more than 18 tablets a week gave an odds ratio of 10.
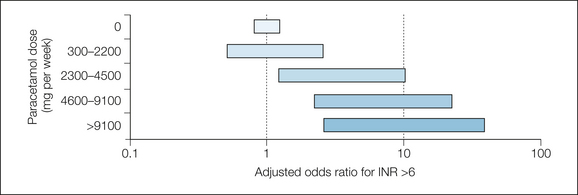
Figure 33-10 Effect of paracetamol dose on risk for the international normalized ratio (INR) being above 6.0.
Several randomized studies have subsequently confirmed that use of paracetamol in the range of 2–4 g daily increases the INR by a significant amount on average and by quite large amounts in some patients (Mahé et al 2006, Parra et al 2007).
The references for this chapter can be found at www.expertconsult.com.
References
Bandolier, More on NSAID adverse effects, 2000. Online. Available at, http://www.medicine.ox.ac.uk/bandolier/ band79/b79–6.html
Bandolier, Coxibs in arthritis: update July, 2002. Online. Available at, http://www.medicine.ox.ac.uk/ bandolier/booth/Arthritis/coxib702.html
Barden J., Edwards J.E., McQuay H.J., et al. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain. 2004;107:86–90.
Blower A.L., Brooks A., Penn G.C., et al. Emergency admissions for upper gastrointestinal disease and their relation to NSAID use. Alimentary Pharmacology and Therapeutics. 1997;11:283–291.
Bombardier C., Laine L., Reicin A., et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. New England Journal of Medicine. 2000;343:1520–1528.
Dworkin R.H., Turk D.C., Wyrwich K.W., et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain. 2008;9:105–121.
Edwards J.E., McQuay H.J., Moore R.A. Combination analgesic efficacy: individual patient data meta-analysis of single-dose oral tramadol plus acetaminophen in acute postoperative pain. Journal of Pain and Symptom Management. 2002;23:121–130.
Edwards J.E., McQuay H.J., Moore R.A., et al. Reporting of adverse effects in clinical trials should be improved. Lessons from acute postoperative pain. Journal of Pain and Symptom Management. 1999;81:427–437.
Edwards J.E., Meseguer F., Faura C.C., et al. Single-dose dipyrone for acute postoperative pain (Cochrane review). Cochrane Database of Systematic Reviews. 2001;3:CD003227.
Edwards J.E., Oldman A., Smith L., et al. Single dose oral aspirin for acute pain. Cochrane Database of Systematic Reviews. 2000;2:CD002067.
Ferraris V.A., Ferraris S.P., Joseph O., et al. Aspirin and postoperative bleeding after coronary artery bypass grafting. Annals of Surgery. 2002;235:820–827.
Forrest J.B., Camu F., Greer I.A., et al. Ketorolac, diclofenac, and ketoprofen are equally safe for pain relief after major surgery. British Journal of Anaesthesia. 2002;88:227–233.
Fortun P.J., Hawkey C.J. Nonsteroidal antiinflammatory drugs and the small intestine. Current Opinions in Gastroenterology. 2007;23:134–141.
García Rodríguez L.A., Hernández-Díaz S. Nonsteroidal antiinflammatory drugs as a trigger of clinical heart failure. Epidemiology. 2003;14:240–246.
Goldstein J.L., Silverstein F.E., Agrawal N.M., et al. Reduced risk of upper gastrointestinal ulcer complications with celecoxib, a novel COX-2 inhibitor. American Journal of Gastroenterology. 2000;95:1681–1690.
Griffin M.R., Yared A., Ray W.A. Nonsteroidal antiinflammatory drugs and acute renal failure in elderly persons. American Journal of Epidemiology. 2000;151:488–496.
Hawkey C.J. COX-2 inhibitors. Lancet. 1999;353:307–314.
Hawkey C.J., Cullen D.J., Greenwood D.C., et al. Prescribing of nonsteroidal anti-inflammatory drugs in general practice: determinants and consequences. Alimentary Pharmacology and Therapeutics. 1997;11:293–298.
Henry D., Page J., Whyte I., et al. Consumption of non-steroidal anti-inflammatory drugs and the development of functional renal impairment in elderly subjects. Results of a case–control study. British Journal of Clinical Pharmacology. 1997;44:85–90.
Hernández-Díaz S., Rodríguez L.A.G. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding/perforation. Archives of Internal Medicine. 2000;160:2093–2099.
Hernández-Díaz S., Varas-Lorenzo C., García Rodríguez L.A. Non-steroidal antiinflammatory drugs and the risk of acute myocardial infarction. Basic Clinical Pharmacology and Toxicology. 2006;98:266–274.
Houde R.W., Wallenstein S.L., Beaver W.T., et al. Clinical measurement of pain. In: De Stevens G., ed. Analgetics. New York: Academic Press; 1965:75–122.
Huerta C., Castellsague J., Varas-Lorenzo C., et al. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. American Journal of Kidney Diseases. 2005;45:531–539.
Hylek E.M., Heiman H., Skates S.J., et al. Acetaminophen and other risk factors for excessive warfarin anticoagulation. JAMA: Journal of the American Medical Association. 1998;279:657–662.
Jenkins C., Costello J., Hodge L. Systematic review of prevalence of aspirin induced asthma and its implications for clinical practice. British Medical Journal. 2004;328:434.
Johnson A.G., Nguyen T.V., Day R.O. Do nonsteroidal anti-inflammatory drugs affect blood pressure? Annals of Internal Medicine. 1994;121:289–300.
Kearney P.M., Baigent C., Godwin J., et al. Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. British Medical Journal. 2006;332:1302–1308.
Langman M.J., Jensen D.M., Watson D.J., et al. Adverse upper gastrointestinal effects of rofecoxib compared with NSAIDs. JAMA: Journal of the American Medical Association. 1999;282:1929–1933.
Mahé I., Bertrand N., Drouet L., et al. Interaction between paracetamol and warfarin in patients: a double-blind, placebo-controlled, randomized study. Haematologica. 2006;91:1621–1627.
Mamdani M., Rochon P.A., Juurlink D.N., et al. Observational study of upper gastrointestinal haemorrhage in elderly patients given selective cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs. British Medical Journal. 2002;325:624.
Marret E., Flahault A., Samama C.M., et al. Effects of postoperative, nonsteroidal, antiinflammatory drugs on bleeding risk after tonsillectomy: meta-analysis of randomized, controlled trials. Anesthesiology. 2003;98:1497–1502.
Mason L., Moore R.A., Edwards J.E., et al. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskeletal Disorders. 2004;5:28.
Merlo J., Broms K., Lindblad U., et al. Association of outpatient utilisation of non-steroidal anti-inflammatory drugs and hospitalised heart failure in the entire Swedish population. European Journal of Clinical Pharmacology. 2001;57:71–75.
Merry A.F., Webster C.S., Holland R.L., et al. Clinical tolerability of perioperative tenoxicam in 1001 patients—a prospective, controlled, double-blind, multi-centre study. Pain. 2004;111:313–322.
Møiniche S., Rømsing J., Dahl J.B., et al. Nonsteroidal antiinflammatory drugs and the risk of operative site bleeding after tonsillectomy: a quantitative systematic review. Anesthesia and Analgesia. 2003;96:68–77.
Moore R.A., Derry S., McQuay H.J. Faecal blood loss with aspirin, nonsteroidal anti-inflammatory drugs and cyclo-oxygenase-2 selective inhibitors: systematic review of randomized trials using autologous chromium-labelled erythrocytes. Arthritis Research and Therapy. 2008;10:R7.
Moore R.A., Edwards J.E., McQuay H.J. Acute pain: individual patient meta-analysis shows the impact of different ways of analysing and presenting results. Pain. 2005;116:322–331.
Moore R.A., Moore O.A., Derry S., et al. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases. 2010;69:374–379.
Moore R.A., Straube S., Derry S., et al. Chronic low back pain analgesic studies—a methodological minefield. Pain. 2010;149:431–434.
Moore R.A., Straube S., Paine J., et al. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain. 2010;149:360–364.
Moore R.A., Tramèr M.R., Carroll D., et al. Quantitative systematic review of topically-applied non-steroidal anti-inflammatory drugs. British Medical Journal. 1998;316:333–338.
Page J., Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients. Archives of Internal Medicine. 2000;160:777–784.
Parra D., Beckey N.P., Stevens G.R. The effect of acetaminophen on the international normalized ratio in patients stabilized on warfarin therapy. Pharmacotherapy. 2007;27:675–683.
Patrono C., Baigent C. Low-dose aspirin, coxibs, and other NSAIDS: a clinical mosaic emerges. Molecular Interventions. 2009;9:31–39.
Singh G. Recent considerations in nonsteroidal anti-inflammatory drug gastropathy. American Journal of Medicine. 1998;105:31S–38S.
Smith L.A., Carroll D., Edwards J.E., et al. Single dose ketorolac and pethidine in acute postoperative pain: a systematic review with meta-analysis. British Journal of Anaesthesia. 2000;84:48–58.
Sowers J.R., White W.B., Pitt B., et al. The effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Archives of Internal Medicine. 2005;165:161–168.
Straube S., Tramer M., Moore R.A., et al. Mortality with upper gastrointestinal bleeding and perforation: effects of time and NSAID use. BMC Gastroenterology. 2009;8:18.
Towheed T.E., Maxwell L., Judd M.G., et al. Acetaminophen for osteoarthritis. Cochrane Database of Systematic Reviews. 2006;1:CD004257.
Tramèr M.R., Moore R.A., Reynolds D.J., et al. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain. 2000;85:169–182.
Tramèr M.R., Williams J.E., Carroll D., et al. Comparing analgesic efficacy of non-steroidal anti-inflammatory drugs given by different routes in acute and chronic pain: a qualitative systematic review. Acta Anaesthesiologica Scandinavica. 1998;42:71–79.