Visceral Pain
Basic Mechanisms
Introduction
The response to tissue insult, which generally triggers appropriate reactions that decrease the likelihood of further injury and harm, requires both precise localization and appreciation of the intensity of the stimulus. The ability to localize the source of insult and assess its intensity is best for skin and joints, less for muscle, and very poor for the viscera. Acute visceral stimulation rarely conveys detailed information about localization or intensity. For example, noxious balloon distention of the esophagus (>40 mm Hg) elicits a deep retrosternal pain that may radiate to the neck, shoulder, or jaw and produce symptoms similar to those reported by patients with angina. Moreover, even though balloon distention of the esophagus or other hollow organs may trigger intense pain, it rarely leads to frank tissue injury. In contrast, actual tissue injury, such as cutting or crushing of the intestine, may not be perceived at all. Accordingly, visceral pain differs from somatic (“somatic” is widely used to mean “non-visceral,” although the viscera are of the body—soma, as distinct from the mind) pain in several important ways (see Ness and Gebhart 1990 for a comprehensive review). Visceral pain has the following properties:
The anatomical and functional bases underlying these distinct characteristics of visceral pain sensation are presented in the following sections.
Structural Basis of Visceral Nociception
Peripheral and Central Organization of Visceral Sensory Innervation
Among all tissues in the body, the viscera are unique in that each organ typically receives innervation from two sets of nerves, either vagal and spinal nerves or two anatomically distinct sets of spinal nerves. An older terminology described innervation of the viscera by the thoracolumbar spinal nerve as sympathetic because these afferent axons were anatomically associated with efferent axons of the sympathetic division of the autonomic nervous system; Langley (1921) called them “afferent sympathetic fibers.” Vagus and pelvic nerve afferents were termed parasympathetic because of a similar anatomical association with the parasympathetic division of the autonomic nervous system. This terminology was also related to presumed function. It is still widely assumed that visceral pain is conveyed to the central nervous system (CNS) by afferent sympathetic fibers whereas parasympathetic afferent innervation regulates autonomic control. Contemporary evidence contradicts these assumed functions, and visceral afferent fibers are best described by the nerve’s name to avoid assumed functions as implied by confusing terminology.
Visceral afferent fibers are contained in nerves that terminate in the spinal cord except for those in the vagus and glossopharyngeal nerves, which terminate in the brain stem to provide a supraspinal, cranial component of visceral sensory innervation. The vagus nerve is undoubtedly the most far-reaching sensory nerve in the body. At least 80% of vagal axons are afferent, and most internal organs are innervated by the vagus nerve. The bilateral vagus nerves innervate the larynx, all the thoracic viscera (esophagus, heart, bronchopulmonary system), and most if not all of the abdominal viscera (stomach, small and large intestines, liver, proximal colon, etc.) (Ness and Gebhart 1990, Cervero 1994). The cell bodies of vagal afferent fibers are contained in the nodose ganglion (primarily) and a smaller, more proximally situated jugular ganglion with central terminals located principally in the nucleus of the solitary tract in the dorsal medulla. Not all vagal afferents terminate in the brain stem; about 5% project directly to and terminate in the upper cervical spinal cord (C1–2), where they are believed to contribute to referred sensations, as well as to propriospinal mechanisms of nociceptive modulation (Foreman 1998). In support of the latter, electrical vagal afferent stimulation modulates spinal thoracic and lumbar nociceptive transmission and is analgesic in humans. Despite its widespread innervation of the internal organs, the vagus nerve has long been considered to play no role in the transmission of visceral nociceptive information, a role relegated to spinal afferent nerves, including the pelvic nerves. Growing evidence, however, suggests that vagal afferents are critical for chemonociception (see below) and, importantly, contribute to the affective dimensions and unpleasantness associated with visceral pain.
As illustrated in Figure 51-1, spinal nerve innervation of the viscera is distributed from the cervical to the sacral spinal segments. All spinal nerves have their cell bodies in dorsal root ganglia (DRGs; not illustrated in Fig. 51-1), although unlike spinal somatic nerves, most visceral nerves pass through pre- and paravertebral ganglia en route to the spinal cord (see Fig. 51-2 for detail). In prevertebral (sympathetic) ganglia, axons of visceral nerves often give off collaterals that synapse on secretory or motor neurons contained in the ganglia and thus can influence organ function (e.g., motility). In addition, visceral afferent fibers that access the spinal cord through paravertebral ganglia can travel rostrally or caudally in the sympathetic trunk and enter distant spinal segments. Regardless of their route to the spinal cord, visceral afferents terminate in similar patterns within the spinal cord:
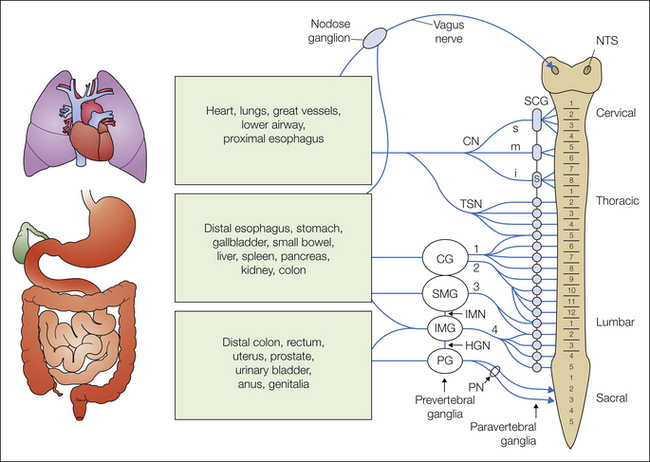
Figure 51-1 Schematic diagram of visceral afferent innervation.
The vagus nerve, with cell bodies in the nodose ganglion and central terminals in the nucleus tractus solitarii (NTS), innervates organs in the thoracic and abdominal cavities. Spinal visceral nerves innervate the same thoracic and abdominal organs, as well as those in the pelvic floor. Most spinal afferents pass through pre- and paravertebral ganglia. Neither dorsal root ganglia nor distribution of afferents between paravertebral ganglia are illustrated (see Fig. 51-2). Prevertebral ganglia: CG, celiac ganglion; IMG and SMG, inferior and superior mesenteric ganglia, respectively; PG, pelvic ganglion. Paravertebral ganglia: s, stellate ganglion, SCG, superior and middle cervical ganglia. Nerves: CN, s, m, and i, superior, middle, and inferior cardiac nerves; HGN, hypogastric nerve; IMN, intermesenteric nerve; PN, pelvic nerve; TSN, thoracic splanchnic nerves; 1, 2, 3, and 4, greater, lesser, least, and lumbar splanchnic nerves, respectively.
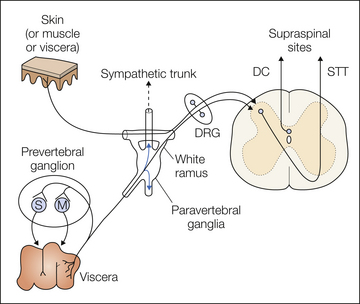
Figure 51-2 Anatomical organization of spinal visceral sensory innervation.
Spinal visceral afferent fibers have their cell bodies in dorsal root ganglia (DRG) and terminate in the spinal dorsal horn, from which visceral sensory information is transmitted rostrally in the contralateral spinothalamic tract (STT) or ipsilateral dorsal column (DC) to supraspinal sites. Many spinal visceral afferents collateralize and terminate on secretory (S) or motor (M) neurons in the prevertebral ganglia and can influence gastrointestinal secretion and motility. Note also that spinal visceral afferents may ascend or descend within the sympathetic trunk and distribute to distant spinal segments. Also illustrated is convergence of cutaneous (or muscle or other visceral) input with visceral input on second-order neurons in the spinal cord.
In addition to this extrinsic sensory innervation, most viscera (e.g., the gastrointestinal [GI] tract, heart) also possess an independent, intrinsic innervation. Best understood is the enteric nervous system of the GI tract, which encodes basic patterns regulating secretion, motility, and blood flow and interacts with the extrinsic innervation of the gut, but in ways that are poorly understood at present.
Visceral Afferent Fibers and Receptive Ending Structures
With the exception of a small number of Aβ fibers associated with pacinian corpuscles in the mesentery, the overwhelming majority of visceral afferent fibers are thinly myelinated Aδ or unmyelinated C fibers. Very little is known about the mechanisms of energy transduction or the structure of visceral afferent peripheral terminals, and it is assumed, as for somatic Aδ and C fibers, that most visceral afferent endings are unencapsulated, “free” nerve endings, but this has little direct experimental support. Most is known about mechanosensitive endings in the viscera, primarily the morphology of vagal afferents that innervate the GI tract. Mechanosensitive afferent endings in hollow organs are assumed to be frequently associated with the muscle layers and to be responsive to tension/stretch (Phillips & Powley, 2000). Best characterized are the intraganglionic laminar endings (IGLEs) and intramuscular arrays (IMAs) associated with vagal afferent fibers that innervate the stomach, both of which have been shown to be mechanosensitive. IMAs differ from IGLEs in morphology, in distribution, and probably in adequate stimulus for activation (Wang & Powley, 2000). As the name implies, IMAs consist of long terminal arrays running within either the circular or longitudinal muscle layers of the organ. However, acute GI discomfort or pain produced by organ distention is not associated with vagal afferent input to the CNS, and the proposed sensitivity of IMAs to stretch (e.g., gastric distention, a noxious stimulus only at high intensity) is uncertain at present, thereby preventing definitive conclusions about their role in nociception.
Complementary morphological information about spinal visceral nerve mechanoreceptor endings in organs is limited (Zagorodnyuk et al 2010). Low-threshold, slowly adapting mechanoreceptors have been described in the rectal innervation of the guinea pig rectum (Lynn et al 2003). Morphologically, these receptors resemble IGLEs; comparable structures have not been described in the spinal afferent innervation of the colon. The distribution of these receptive structures in the proximal and distal portions of the GI tract suggests a potential regulatory role in food intake and defecation. Most studies reveal single, small-sized receptive fields for mechanosensitive afferent fibers, although vagal and spinal mechanosensors in the stomach and colon, as well as afferent fibers innervating the bladder, have occasionally been reported in electrophysiological studies to have multiple receptive fields.
In addition to mechanosensitive endings, chemo- and thermoreceptive endings are present in the viscera. Virtually nothing is known about the morphology of thermoreceptive peripheral terminals, but the architecture of presumptive chemoreceptor endings in the vagal afferent innervation of the rodent pyloric antrum and proximal duodenum has been described. Powley and co-workers (2011) noted the presence of three vagal afferent specializations innervating the mucosa of the proximal part of the GI tract: villus afferents, crypt afferents, and antral gland afferents. Their location suggests roles in chemosensation (response to nutrients, content in chyme, etc.); whether this includes chemonociception remains to be determined. Functional characterization of mechano-, chemo- and thermosensitive endings (discussed below) reveals the presence of receptive endings in the mucosa and serosa, as well as in the muscle layers of hollow organs and mesenteric attachments.
Density and Complexity of Visceral Innervation
The number of axons that innervate the viscera is relatively small in comparison to somatic innervation. It has been estimated that 5–15% of the total afferent input to the spinal cord arises from the viscera, which is disproportionate to the greater than 50% of second-order spinal neurons estimated to respond to visceral afferent input. This apparent discrepancy is explained by the significant arborization and spread of visceral afferent terminals within the spinal cord. Whereas somatic input is commonly restricted to one or a few spinal segments, spinal visceral afferent input has been documented to spread several segments rostral and caudal from the spinal segment of entry and, moreover, to occasionally spread to the contralateral side of the spinal cord (Sugiura and Tonosaki 1995). Similarly, vagal input to the medulla is amplified by the branching and widespread distribution of afferent terminals in the organs of innervation.
Visceral afferent input to the spinal cord is also characterized by convergence. That is, virtually all second-order spinal neurons that receive visceral input also receive convergent somatic input from skin and/or muscle (see Fig. 51-2), which provides an explanation for referral of visceral sensations to somatic sites (e.g., deep retrosternal pain that radiates to the neck, shoulder, or jaw with angina). Furthermore, viscerovisceral convergence of input onto second-order spinal neurons is also common (e.g., colon and urinary bladder, gallbladder, and heart). An older concept—dichotomizing visceral afferents—has recently been reinvigorated by reports that some visceral afferent endings innervating adjacent organs arise from a common cell body, thus revealing prespinal “convergence” in DRG somata. Morphological studies in the cat, rat, and mouse using retrogradely transported dyes clearly reveal that dichotomizing afferents innervate the pelvic organs (see Brumovsky and Gebhart 2010 for review). The reported proportion of such afferents among the total organ afferent innervation in rodents ranges widely (3–27%) but is generally a small fraction of the visceral innervation. Their role in cross-organ sensitization (see below) remains to be confirmed as being functionally significant.
Accordingly, the diffuse character and poor localization of visceral pain are contributed to by the widespread intraspinal arborization of visceral afferent terminals and by somatovisceral and viscerovisceral convergence onto second-order spinal neurons, thus challenging patients and physicians alike to easily identify the source or sources of visceral pain.
Neurochemistry of Visceral Primary Afferents
As indicated above, most visceral receptive endings in organs are typically non-encapsulated (“free”) and associated with slowly conducting unmyelinated (C) or thinly myelinated (Aδ) axons of generally small- to medium-diameter somata in the dorsal root and nodose ganglia. It would be convenient and useful if neuronal function could be assigned on the basis of cell size and myelination, but neither feature defines a nociceptor (Gold and Gebhart 2010). Because the somata of visceral nociceptors are generally larger than those of non-visceral nociceptors, they have been largely excluded from studies that focus on small-diameter somata in the DRG. Beyond cell size, cell content has been used to broadly segregate nociceptive afferents into peptidergic and non-peptidergic (Snider and McMahon 1998). Peptidergic nociceptors contain the neuropeptide substance P and/or calcitonin gene–related peptide (CGRP) and express the nerve growth factor (NGF) receptor TrkA (tyrosine kinase receptor A). In contrast, non-peptidergic nociceptors express Ret, the receptor for the glial cell line–derived family of neurotrophic factors, and most also bind isolectin B4 (IB4) and express the purinergic P2X3 receptor. Even this classification, however, obscures differences between nociceptors innervating different tissues. For example, a significantly greater proportion of visceral than cutaneous nociceptors are immunoreactive for CGRP and express TrkA (see Robinson and Gebhart 2008 for an overview). The presence or absence of additional or alternative markers (e.g., the voltage-gated sodium channels Nav1.7 or Nav1.8 and transient receptor potential [TRP] channels A1 and V1) has been suggested as a means of identifying subsets of nociceptors. These additional markers also have limitations, principally that their distribution or presence varies among subpopulations of nociceptors defined by the target of innervation, which moreover changes as the state of the tissue changes (e.g., inflammation). Accordingly, the ability to classify a sensory neuron as nociceptive (which is a functional characterization) by cell content or expression of one or a combination of receptors is not possible at present.
Despite the lack of a definitive marker, it is known that more visceral afferent somata (than sensory neurons innervating skin or muscle) contain substance P and/or CGRP. This fraction increases further during inflammatory processes associated with pain. Similarly, more visceral than somatic sensory neurons express the high-affinity NGF receptor TrkA, with further increases occurring during inflammation. TRP channels have been a recent focus of study because many believe that expression of TRPV1 denotes a neuron as a nociceptor. Indeed, approximately 70–80% of visceral DRG somata are immunoreactive for TRPV1, whereas proportions of skin (35–60%) and muscle (≈40%) DRG somata are much less (Robinson and Gebhart 2008). Not surprisingly, there also are significant differences in cell content markers between the two innervations of the same organ. For example, expression of TRPV1 mRNA in DRG neurons did not differ between the lumbar splanchnic (thoracolumbar [TL] DRG) and pelvic (lumbosacral [LS] DRG) nerve innervations of either colon or urinary bladder somata in TL and LS DRGs (≈45–60% of somata expressed TRPV1 mRNA). In contrast, whereas 60% of bladder TL neurons expressed the TRPA1 gene transcript, virtually no bladder LS neurons expressed this gene transcript; co-expression of TRPV1 and TRPA1 transcripts was high in colon DRG neurons (La et al 2011).
Sensory neurons release peptides (e.g., substance P and other neurokinins, CGRP) and other bioactive mediators from their peripheral and central terminals (e.g., in response to “mucosal noxae” or capsaicin, low pH, and distention of the colon; Holzer 2002). For example, substance P affects epithelial cells in the urinary bladder (urothelium) and GI tract, CGRP contributes to local vasodilatation, and mast cells, which in the viscera are found in close proximity to nerves and express neurokinin 1 (NK1) receptors, degranulate in response to substance P, which causes the release of additional mediators (histamine, tryptases). Mucosal noxae can thus lead to vasodilatation and hyperemia, inflammatory infiltration via chemotaxis, capillary leakage with exudate of plasma and swelling, and activation of smooth muscle cells, as well as increased secretion in epithelial cells, all of which are features generally associated with neurogenic inflammation. Emerging evidence suggests that this mechanism contributes to the pathogenesis of acute and chronic diseases involving the viscera (e.g., Ceppa et al 2010 Neuhaus et al., 2006).
Central Processing of Visceral Nociception
Second-order neurons in the spinal cord that receive visceral afferent input (visceroceptive neurons) are located principally in the superficial spinal laminae, deeper in lamina V (including the intermediolateral cell column in the TL spinal cord and the sacral parasympathetic nucleus), and in the medial LS spinal cord dorsal to the central canal (lamina X). Peripheral electrical, mechanical (e.g., distention), chemical, and ischemic (e.g., coronary artery occlusion) stimuli applied to a variety of visceral organs have been shown to excite and sometimes inhibit visceroceptive spinal neurons in numerous species (see Traub 2007). Responses of visceroceptive spinal neurons to mechanical stimuli (i.e., usually distention of hollow organs) have been studied most extensively and reveal patterns of response consistent with the response properties of mechanosensitive visceral afferent fibers.
Gallbladder, esophageal, urinary bladder, and colon distention typically excites visceroceptive spinal neurons but also can inhibit the ongoing activity of some (e.g., see Fig. 51-3). Most visceroceptive spinal neurons excited by distending stimuli have low thresholds for response in the physiological range (e.g., <5 mm Hg), although a significant proportion have thresholds for response in the noxious range (>20–25 mm Hg), consistent with the functional properties of mechanosensitive visceral afferent fibers (see below). Importantly, visceroceptive spinal neurons excited by distention encode the distending stimulus throughout the range of distending pressures tested (Fig. 51-3), from the physiological to well into the noxious range (Traub 2007). Because these visceroceptive spinal neurons can be antidromically invaded from the thalamus, nucleus gracilis (post-synaptic dorsal column neurons), or rostral spinal cord, the input received is conveyed to supraspinal sites and thus contributes to pseudo-affective reflexes, as well as to conscious appreciation of visceral input. Visceroceptive spinal neurons inhibited by organ distention are probably involved in local or short-loop reflexes (e.g., urinary bladder–colon reflexes) and not conscious perception of visceral events.
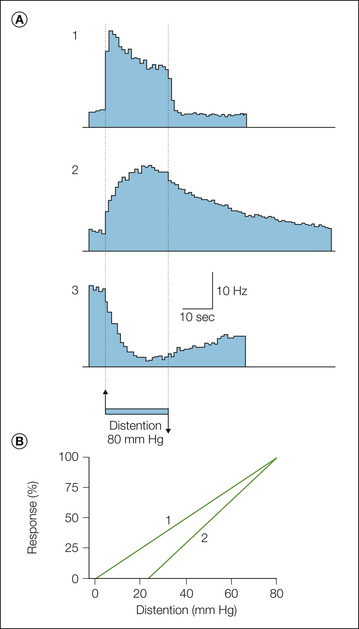
Figure 51-3 A, Mean responses, illustrated as histograms (1-second bin width), of spinal neurons to a noxious intensity of colorectal distention (80 mm Hg). Neuron types 1 and 2 are excited and neuron type 3 is inhibited by distention. All neuron types respond at short latency to distention, but only neuron type 1 is tightly linked to stimulus duration. Neuron type 2 exhibits a sustained afterdischarge, and the inhibitory effect of distention is long lasting as well. Neuron types 1 and 2 are further characterized in B, where normalized stimulus–response functions are illustrated. The mean response threshold for type 1 neurons is within the physiological range (e.g., <5 mm Hg), whereas the response threshold for type 2 neurons is in the noxious range. Type 2 but not type 1 neurons become sensitized after colon inflammation (see Traub 2007). (Reproduced with permission from Ness TJ, Gebhart GF 1987 Characterization of neuronal responses to noxious visceral and somatic stimuli in the medial lumbosacral spinal cord of the rat. Journal of Neurophysiology 57:1867–1892. Copyright 1987 The American Physiological Society.)
As indicated above, convergence of afferent input is characteristic of visceroceptive spinal neurons. Virtually all visceroceptive spinal neurons receive convergent somatic input, if not also convergent input from other viscera (see Fig. 51-2). Consistent with their ability to encode visceral stimulus intensity into the noxious range, adequate excitatory convergent somatic input (commonly from skin) is typically noxious (e.g., pinch). If visceroceptive neurons were to be characterized by their convergent cutaneous input, they would be classified as wide–dynamic range or nociceptive-specific spinal neurons. It is typically the case that visceroceptive spinal neurons with a high threshold for response to distending stimuli are nociceptive specific–like with respect to their cutaneous input. It is uncommon for visceroceptive neurons to respond only to non-noxious input from the convergent cutaneous receptive field. However, after experimental organ inflammation or in patients with a functional GI disorder such as irritable bowel syndrome, the area of convergent somatic input increases and the adequate somatic stimulus need not be noxious. For example, patients with irritable bowel syndrome exhibit increased areas of referred sensation and report discomfort and tenderness on abdominal palpation. Visceral hypersensitivity is considered later in this chapter, but it is relevant here to re-emphasize that experimental organ insult leads both to a significant increase in the number and distribution of visceroceptive neurons excited in the spinal cord and to an increased response magnitude of visceroceptive neurons with thresholds for response greater than 20 mm Hg. Because visceroceptive spinal neurons with thresholds for response greater than 20 mm Hg are predominantly excited by noxious convergent cutaneous input, typically exhibit sustained responses to organ distention that persist after termination of the stimulus, project to supraspinal sites, and become sensitized after organ insult, they appear to be the population of visceroceptive spinal neurons most important for spinal visceral nociceptive processing with respect to both acute visceral pain and visceral hypersensitivity.
Even though the lateral spinothalamic tract (STT) is an important pathway for carrying nociceptive information from skin, joints, and muscle to supraspinal sites, bilateral STT transection does not completely block the behavioral responses to noxious visceral stimulation in rats. Consistent with these findings, neurons within the ventral posterolateral thalamus continue to respond to visceral stimulation after interruption of the STT via bilateral cordotomy. Additional studies have demonstrated that post-synaptic spinal neurons originating primarily in lamina X ascend the dorsal columns to the thalamus through cuneothalamic pathways (see Fig. 51-2). Consistent with the importance of this pathway, transection of the dorsal columns abolished nocifensive behavior in response to noxious visceral stimulation. It is possible that the higher number of visceral sensory neurons projecting through the dorsal columns than through the STT explain these results. However, bilateral lesions of the dorsal columns did not affect autonomic responses to visceral noxae and reflexes mediated within the brain stem, thus suggesting that information contributing to the affective, autonomic, and discriminatory dimensions of visceral pain is carried by different pathways within the spinal cord.
Within the thalamus, the main relay station for somatosensory input, visceral afferent input primarily projects into the ventral posterolateral nucleus, where again most of the neurons receive convergent somatic input. Similarly, viscerovisceral convergence is not uncommon, with some thalamic neurons responding to stimulation from distant visceral sites, such as the esophagus and colon. Studies of brain activation using positron emission tomography or functional magnetic resonance imaging have identified sites of cortical pain processing in humans. Visceral input, whether perceived as innocuous or painful, is part of an interoceptive system that conveys important homeostatic information about the body to the CNS and elicits endocrine, autonomic, and behavioral responses to react to threats and ensure survival (Craig 2009, Mayer et al 2009). Noxious visceral stimuli trigger bilateral activation of thalamic and cortical areas, a pattern that does not differ significantly from that produced by noxious somatic stimulation. The one distinction appears to be the preferential projection of visceral sensory input to the posterior insula, with the anterior and middle sections of the insular cortex receiving input from “somatic” structures (Mayer et al 2009, Tillisch et al 2011). Studies comparing visceral and somatic stimulation reveal subtle differences (Dunckley et al 2005a) that largely correlate with the more significant emotional impact experienced during visceral pain.
Functional Basis of Visceral Nociception
Visceral mechanosensation has been studied most widely, both in behavioral and electrophysiological experiments. Hollow-organ balloon distention is an adequate noxious stimulus and easy to control in duration and intensity. It is relevant for use in non-human animal studies because distention of hollow organs in humans reproduces the distribution of referred sensations from a viscus, as well as the quality and intensity of the visceral sensation (Ness and Gebhart 1990). Chemical and thermal stimuli are less well characterized behaviorally, although visceral chemonociception is a growing area of investigation. With our increasing understanding of nociceptor activation, specific agonists have been used to activate visceral nociceptive pathways. Most of these strategies have focused on activation of TRPV1 by infusing capsaicin into the esophageal, gastric, or small or large intestinal lumen or even the pancreatic duct in animals (Eijkelkamp et al 2007, Qin et al 2008) or proximal GI tract in humans (Hammer et al 2008, Chen et al 2010).
Functional Properties of Visceral Mechanoreceptors (in Vivo)
When distention of hollow organs has been used as the stimulus, two groups of mechanosensitive afferent fibers have been found in spinal visceral nerves. The larger proportion, typically 75–80% of the sample, respond at low distending pressure in the physiological range (e.g., <5 mm Hg) and encode distending pressure well into the noxious range. The smaller proportion, typically 20–25% of the sample, does not respond until the distending pressure is in or approaches the noxious range (i.e., ≈25–30 mm Hg) and then continues to encode distending pressure. Both low- and high-threshold mechanoreceptors are generally slowly adapting (e.g., Fig. 51-3), encode stimulus intensity, and have receptive endings that are probably located in organ muscle layers. High-threshold afferent fibers are present in all organs studied to date (e.g., colon, gallbladder, urinary bladder, stomach, uterus) and in different species (e.g., cat, ferret, opossum, rodents) and are considered to be visceral nociceptors that respond to acute, noxious mechanical stimulation. The view that low- and high-threshold mechanoreceptors in the viscera are the counterpart of somatic non-nociceptors (e.g., low-threshold mechanoreceptors) and nociceptors, respectively, is probably incorrect for several reasons:
Accordingly, it is likely that both low- and high-threshold visceral mechanoreceptors contribute to visceral nociception in persistent visceral pain states. These features of visceral afferent fiber innervation may explain why normally subliminal physiological stimuli are perceived as uncomfortable or painful in persons with functional bowel and bladder disorders (e.g., functional dyspepsia, irritable bowel syndrome, painful bladder syndrome). A third mechanosensitive receptor, a mucosal mechanosensitive ending, has been identified functionally, but mucosal mechanosensors have not been widely studied in vivo and are not commonly encountered in such studies. They are typically rapidly adapting, do not encode stimulus intensity, and tend to respond to initiation and termination of balloon distention.
With respect to mechanosensitive vagal afferent fibers, only a single population of fibers with low thresholds for response to gastric distention has been described in vivo, although response magnitude continues to increase well into the noxious range of distention. Though characterized as mechanoreceptive, both vagal afferent fibers and spinal visceral afferent fibers (Box 51-1) are generally multimodal and respond to thermal and/or chemical stimuli, in addition to balloon distention. Furthermore, exposure to either thermal (heat) or chemical stimuli typically sensitizes subsequent responses to organ distention, as does organ insult. Although not established for all mechanosensitive fibers, it appears that all spinal and vagal mechanosensitive fibers studied in vivo respond to at least two modalities of stimuli.
Functional Properties of Visceral Mechanoreceptors (in Vitro)
More recently, in vitro organ–nerve preparations have permitted broader functional characterization of mechanoreceptive endings in the colon, esophagus, stomach, ureter, urinary bladder, and uterus. Mucosal, tension (muscular), and muscular–mucosal receptors have been characterized in the vagal afferent innervation of the esophagus and stomach, and mesenteric, serosal, mucosal, muscular, and mucosal–muscular receptors have been described in the urinary bladder and colon (Fig. 51-4). Colonic mucosal/bladder urothelial receptors respond to gentle brushing or stroking of the receptive field, as well as to blunt probing, but do not respond to circumferential stretch of the organ. Muscular (tension) receptors respond to circumferential stretch (and encode the magnitude of tension) but do not respond to mucosal/urothelial stroking. Serosal receptors respond in a graded manner to von Frey–like probing of the receptive field but do not respond to mucosal stroking or circumferential stretch. Mucosal–muscular receptors respond to gentle mucosal/urothelial stroking, as well as to circumferential stretch. Mesenteric mechanoreceptors respond in a graded manner to von Frey–like probing along the mesenteric attachment, typically associated with the vasculature but not to stretch. They are distinguished from serosal receptors by their location in the mesentery. Although we tend to focus on endings in organs associated with mucosa, muscle, or serosal layers, axons of visceral sensory neurons on intramural blood vessels may also serve as transduction sites for mechanosensation, including responses to stretch and distention of hollow viscera (e.g., Song et al 2009). Use of the in vitro organ–nerve preparation has established that some vagal and some spinal afferent fibers have multiple receptive fields in the stomach and colon, respectively, but testing typically reveals punctate receptive fields with diameters of 1–2 mm2.
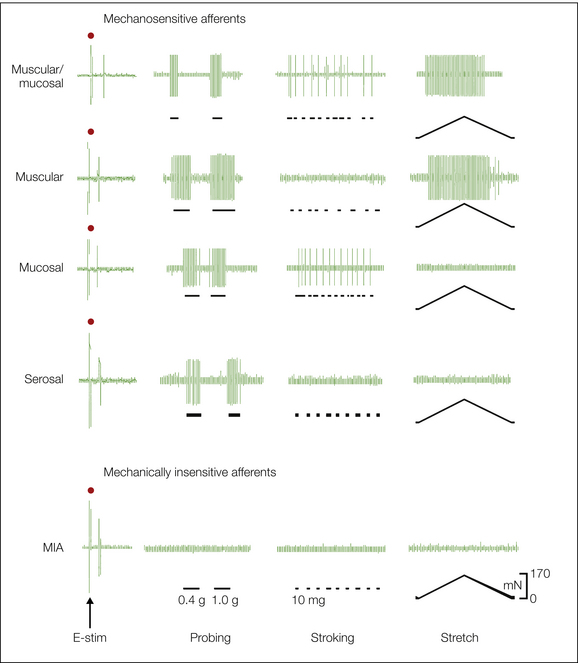
Figure 51-4 Mechanosensitive and mechano-insensitive (MIA) endings in the mouse colon.
In this in vitro colon–pelvic nerve preparation, single colonic afferent fibers were identified by an electrical search stimulus (Feng and Gebhart 2011) and their mechanoreceptive properties characterized. The left most column shows electrical stimulation artifacts (denoted by • above the artifact) and single-fiber action potentials at varying latencies. All mechanosensitive endings respond to blunt probing (1.0 g) and endings characterized as serosal respond only to probing. Other mechanosensitive endings are differentiated by their responses to a ramped, controlled circumferential stretch (0–170 mN, equivalent to 45 mm Hg) and/or stroking (10 mg) of the mucosal surface. Muscular endings respond to circumferential stretch but not to stroking of the mucosa. Mucosal receptors respond to repetitive stroking of the mucosa (lines beneath the record) but not to stretch. Muscular–mucosal endings respond to both stroking of the mucosa and circumferential stretch. Note that the designations are functional, not histological (see Brierley et al 2004, Feng and Gebhart 2011). MIAs do not respond to any of the mechanical stimuli.
It has long been appreciated clinically that the two nerves innervating an internal organ have different functions, but this has not been investigated experimentally until relatively recently (Brierley et al 2004, Xu and Gebhart 2008). The principal mechanosensitive endings in the lumbar splanchnic innervation are serosal and mesenteric in the mouse colon and serosal in the urinary bladder; relatively few muscular (tension) and mucosal receptors have been found in this nerve (Fig. 51-5). In contrast, serosal endings represent a smaller proportion of the pelvic nerve innervation of the colon and urinary bladder, whereas mechanoreceptive endings that respond to circumferential stretch (muscular or muscular–mucosal/urothelial receptors) predominate. Accordingly, colorectal nociceptive mechanosensation is conveyed by the pelvic nerve, consistent with evidence that visceromotor responses to noxious colorectal distention are unaffected after lumbar splanchnic nerve transection but absent after pelvic nerve transection (Kyloh et al 2011). Not only do the proportions of classes of receptive endings differ between the lumbar splanchnic and pelvic nerve innervations of the colon and bladder, but the topographical distribution of these receptive endings also differs significantly (see Fig. 51-5).
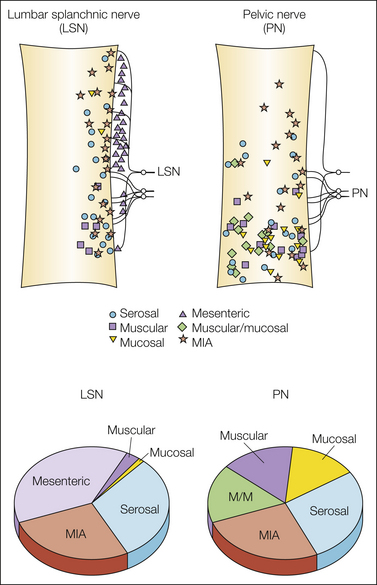
Figure 51-5 Topographical distribution of mechanosensitive and -insensitive receptive endings in the mouse colon and proportions of endings in the lumbar splanchnic (LSN) and pelvic (PN) nerves. Both the distribution of receptive endings and proportions of classes of endings significantly differ between the LSN and PN innervations of the colon. MIA, mechanically insensitive afferent fibers. (Adapted from Brierley SM, Jones RCW 3rd, Gebhart GF, et al 2004 Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127:166–178; Jones RCW 3rd, Xu L, Gebhart GF 2005 The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. Journal of Neuroscience 25:10981–10989; and Feng B, Gebhart GF 2011 Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. American Journal of Physiology 300:G170–G180; see Xu L, Gebhart GF 2008 Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. Journal of Neurophysiology 99:244–253 for mouse urinary bladder.)
The results of in vitro studies of visceral afferent fibers, initially in the rat and ferret and, as presented above, increasingly in the mouse, reveal good correspondence with earlier in vivo studies. The in vitro approach permits study of and accordingly reveals a broader range of mechanosensitive endings in the organ, but significant discrepancies from characterization of mechanosensitive afferents in vivo have not been reported to date.
Mechanically Insensitive Afferents
In addition to mechanosensitive afferents, silent (or sleeping) nociceptors, first documented in the innervation of the knee joint of the cat (Schaible and Schmidt 1985), are present in the visceral innervation. Most appropriately termed mechanically insensitive afferent fibers (MIAs), they are characterized by their ability to acquire mechanosensitivity in pathophysiological conditions and can thus contribute significant new peripheral drive to the development of hyperalgesia (Weidner et al 1999, Schmelz et al 2000). Visceral MIAs were initially “discovered” in the electrophysiological record after inflammatory or chemical organ insult. Consequently, initial estimates of the proportion of MIAs in the visceral innervation were both broad and inaccurate. Recently, MIAs in the lumbar splanchnic and pelvic nerve innervations of the mouse colon have been documented as representing approximately 25% of the innervation of the colon (Feng and Gebhart 2011). Consistent with their presumed role in maintenance of organ hypersensitivity, colorectal MIAs acquire a time-limited mechanosensitivity after brief exposure to a soup of inflammatory mediators, and their proportion is significantly reduced in the presence of long-lasting colorectal hypersensitivity (Feng et al 2012b), thus supporting their potential contribution to organ hypersensitivity.
Chemosensitivity and Thermosensitivity
Studies on chemosensitivity are limited, but most fibers in the colonic innervation in the rat, whether serosal, muscular, or mucosal, also respond to chemical stimuli (e.g., hypertonic saline, bile, HCl, 5-hydroxytryptamine [5-HT], capsaicin; Brierley et al 2005, Coldwell et al 2007). Interestingly, excitation of mechanosensitive endings by 5-HT was found to be mediated by 5-HT3 as well as by non–5-HT3 receptors. Mechanosensitive vagal afferents have also been shown to respond to capsaicin, γ-aminobutyric acid, and purinergic agonists, as well as to inhalational toxins (e.g., Page et al 2002, Christianson et al 2009). Limited information is available at present regarding whether some or all mechanosensitive endings described are also thermosensitive. When tested, pelvic nerve mechanosensitive endings in the colon were found to be heat and/or cold sensitive, and vagal mechanosensitive fibers innervating the stomach respond to heat. In addition, there may be specialized thermoreceptors that do not respond to other stimulus modalities.
As has been established in standard in vivo teased fiber preparations, mechanosensitive muscular receptors in vitro are sensitized by exposure to a mixture of inflammatory mediators (histamine, serotonin, prostaglandin E2, and bradykinin at varying pH). Similarly, local application of an inflammatory soup to muscular or muscular–mucosal receptive endings in the mouse colon also sensitizes responses to subsequent mechanical stimulation (stretch) and thereby produces a leftward shift in the mechanical stimulus–response function.
Whereas mechanical stimuli trigger pain and discomfort in the GI and urinary tracts, cardiac and lower airway pain is primarily initiated by chemical rather than mechanical stimuli. In view of the importance of ischemia in patients with cardiac chest pain, experimental interest has focused on mechanisms activating ischemia-sensitive cardiac afferents. Occlusion of coronary artery blood flow triggers a rapid decrease in pH in the myocardium to about 6.9, which is associated with an increase in the action potential generation of ischemia-sensitive fibers (Fu and Longhurst 2009). Tissue hypoxia leads to an accumulation of lactate, which eventually lowers pH within cells and the interstitial space. Consistent with a role of this metabolic consequence of ischemia, experimental acidification of the myocardium to comparable proton concentrations mimics the effects of ischemia. Interestingly, local application of lactic acid is more potent than similar changes in pH triggered by other acids (Benson and McCleskey 2007). It is unlikely, however, that lactic acid alone causes ischemic cardiac pain because the change in pH is quite small; adenosine triphosphate (ATP) is probably involved as well (Wang et al 2008). ATP is also released from ischemic muscle and has been reported to work together with acid by increasing the pH sensitivity of acid-sensing ion channel 3 (ASIC3), which normally detects lactic acidosis (Birdsong et al 2010). These results suggest that ATP, the endogenous ligand for P2X and some P2Y receptors, forms a molecular complex with ASICs.
Protons and ATP are not the only chemicals that trigger action potentials in ischemia-sensitive cardiac afferent fibers. Production and release of bradykinin and prostaglandins also contribute to their activation, and platelet activation during arterial occlusion releases serotonin, which similarly stimulates cardiac afferent fibers (Fu and Longhurst 2002). Many of these cardiac afferent fibers also have mechanosensitive receptive fields on the myocardium. Accordingly, like other mechanosensitive visceral afferent fibers mentioned above, many cardiac afferent fibers are multimodal and can be activated by other stimulus modalities.
As is common for most viscera, pain and discomfort are the principal conscious sensations that arise from the lower airways, and either mechanical or irritant chemical stimuli can be adequate noxious stimuli. The lower airways are innervated by vagal and spinal nerves, but their functional characterization has only recently been expanded and principally for vagal afferents and their role in chemonociception. Vagal sensory ganglia include the larger nodose and a smaller, superior jugular ganglion. Sensory neuron somata in the nodose ganglion are derived from the epibranchial placodes; the neural crest gives rise to somata in the jugular ganglion. In the mouse these ganglia are contained in a single structure where neural crest–derived neurons are located rostrally and non-neural crest (nodose) neurons are located centrally and caudally. Undem’s group (Nassenstein et al 2010) has phenotyped and characterized vagal sensory neurons: placode-derived cells are non-peptidergic and express the P2X2 and P2X3 purinoceptors, TrkB, GDNF (glilal cell-line derived neurotrophic factor) family receptor (GFR)-α1, and RET, whereas neural crest–derived cells do not express P2X2 and include both peptidergic and non-peptidergic groups. Peptidergic neural crest cells express TrkB and GFR-α1, and non-peptidergic cells express TrkA and GFR-α3. Bronchopulmonary vagal afferents also respond to environmental toxicants (e.g., ozone; Taylor-Clark and Undem 2010), endogenous activators of TRPV1 and TRPA1, and protease-activated receptors (PARs), as well as kinins and protons, and undergo changes after airway inflammation (e.g., Christianson et al 2009, Kwong et al 2010, Nassenstein et al 2010). Bronchopulmonary vagal afferent fibers are also mechanosensitive and include both rapidly and slowly adapting stretch receptors, which are principally Aδ and C fibers (Christianson et al 2009).
The mucosa all along the GI tract is exposed to acid, digestive enzymes, nutrients, and other substances, and afferent fibers respond to changes in composition of the luminal contents. Although such chemosensitive fibers are important in the regulation of absorption, secretion, motility, and blood flow, they probably never contribute to nociception, and their activity is rarely perceived. This differs from the effects of potentially noxious chemical stimuli, termed “mucosal noxae” by Holzer (2002), such as acid and bile. Exposure of the esophagus, stomach, or duodenum to acid triggers pain and discomfort in patients with dyspeptic symptoms, and acid-sensitive afferents have been identified in the esophagus, stomach, and duodenum (Holzer 2011a). Consistent with a possible role of these sensory pathways in chemonociception, exposure of the gastric mucosa to noxious concentrations of HCl triggers aversive responses in rats (Lamb et al 2003) associated with increased transcription of the immediate–early gene c-fos in the nucleus of the solitary tract, but not in the spinal cord, thus confirming that vagal rather than spinal pathways are involved in chemonociception (see Holzer 2002 Holzer 2011a). In further support, vagotomy, but not splanchnic nerve resection, blunts the visceromotor response to intragastric acid instillation under control conditions and in animals with mild gastritis or experimentally induced gastric ulcers (Lamb et al 2003). Another noxious mucosal stimulus, exposure to bile as a result of duodenogastric and duodenogastroesophageal reflux, has long been implicated in the pathogenesis of dyspeptic symptoms. Interestingly, in vitro electrophysiological experiments have shown that bile activates mechanosensitive vagal afferent fibers with receptive fields in the stomach or distal esophagus. Similarly, gastric luminal exposure to the bile acid glycocholic acid increased the resting activity of gastric vagal mechanosensitive afferents and, when combined with HCl to lower the pH of the glycocholic acid solution to 1.2, sensitized responses to gastric distention as well (Kang et al 2004). However, it remains unclear whether mucosal exposure to bile acids triggers nocifensive behavior in vivo. Despite these unresolved questions, elimination or reduction of such noxae by acid suppression has been used successfully in the treatment of patients with functional diseases of the esophagus and stomach.
Excitability of Visceral Afferent Neurons
Visceral afferent neuron excitability has been studied in isolated cell somata. Because the density of visceral innervation is low in comparison to that in skin, muscle, or joints and because the number of visceral sensory neurons is accordingly low as well in the DRG, retrograde labels injected into target tissues are necessary to identify visceral afferent somata for study. Study of sensory neuron somata is based on the assumption that the cell body, dendrites, and axons are sufficiently similar. The selective targeting of many membrane proteins to specialized areas within cells raises questions about this assumption. Nonetheless, this experimental strategy allows comparisons between somatic and visceral sensory neurons, as well as studies of the effects of injury or inflammation on neuron properties (see Beyak 2010).
Voltage-Gated Ion Channels
Voltage-sensitive ion channels form the basis for the generation of action potentials. Thus, the expression, properties, and density of these membrane proteins determine neuron excitability. Voltage-sensitive sodium channels (Nav) are responsible for rapid upstroke of the action potential. At least six of the known sodium channels have been identified in primary afferent neurons, including Nav channels 1.7, 1.8, and 1.9, which are primarily found in peripheral sensory neurons. Nav1.8 and Nav1.9 channels are not blocked by the neurotoxin tetrodotoxin (TTX) and can thus be pharmacologically isolated as a TTX-resistant sodium current. In the DRG, message for Nav1.8 is primarily found in small-diameter neurons with unmyelinated axons, which has led to identifying any neuron expressing Nav1.8 as a nociceptor. Although essentially all visceral sensory neurons have unmyelinated or thinly myelinated axons, they do not consistently exhibit a differential distribution of TTX-resistant sodium currents based on cell size. This may be due to the fact that at least in the GI tract, many DRG neurons are medium size in diameter. Prostaglandin E2 and serotonin, mediators implicated in the rapid development of peripheral sensitization, increase the peak amplitude of TTX-resistant sodium currents in DRG neurons through activation of protein kinases and phosphorylation of sodium channels (Beyak 2010; Fig. 51-6D). Interestingly, inflammatory models of visceral hypersensitivity are associated with an increase in excitability as evidenced by a lower threshold for generation of action potentials and a higher number of action potentials during prolonged stimulation (Fig. 51-6B). This is at least in part due to changes in sodium currents, primarily the TTX-resistant sodium current, thus supporting a role of these channels in visceral nociception.
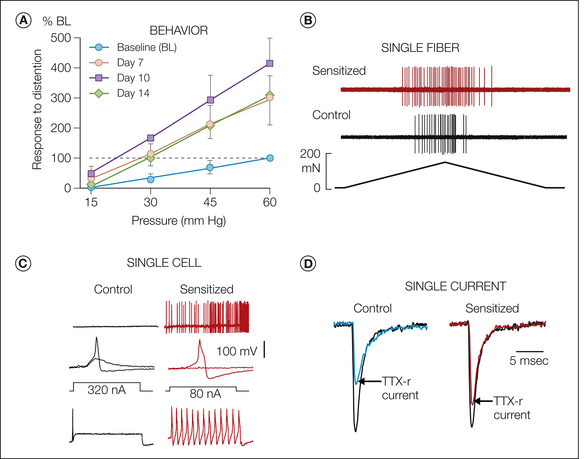
Figure 51-6 Sensitization.
A, Responses in the unanesthetized mouse to graded intensities of colorectal distention before (baseline) and in the same mice 7, 10, and 14 days after intracolonic instillation of zymosan (responses to distention are illustrated relative to baseline). Colorectal hypersensitivity can be produced with a variety of intracolonic treatments (e.g., acetic acid, trinitrobenzene sulfonic acid). B, Acute sensitization of a stretch-sensitive muscular ending (ramped stretch from 0–170 mN over a 35-second period) in the mouse colon before (control) and after (sensitized) local exposure of the ending to an inflammatory soup (5-hydroxytryptamine, bradykinin, histamine, and prostaglandin E2 at pH 6.0). As illustrated here, the response threshold is typically reduced and the response magnitude (number of action potentials) is increased. The long-lasting colorectal hypersensitivity in A is associated with sensitization of muscular and muscular–mucosal afferent endings taken from mice with established colorectal hypersensitivity (see Jones et al 2005, Feng et al 2011), from which these illustrations were adapted). C, Effects of visceral insult (urinary bladder inflammation) on neuron excitability (whole-cell patch clamp recordings). In bladder neurons taken from control animals (top to bottom), there is no spontaneous activity, the rheobase is 320 nA (maximum injected current before generation of an action potential), and only one action potential is generated at 1.5 × rheobase. In bladder neurons taken from mice with inflamed bladders, about one-third of the neurons exhibit spontaneous activity, the rheobase is significantly reduced, and current injection at 1.5 × rheobase generates a train of action potentials. D, Effects of visceral insult (gastric ulceration) on voltage-gated ion channels. In this example, gastric ulceration leads to a significant increase in the tetrodotoxin (TTX)-resistant contribution to the peak sodium current, which has been normalized to illustrate the differential contribution in the control as compared with the sensitized (gastric ulceration) state (see Beyak 2010 for discussion and citations). (C, Adapted from Dang K, Bielefeldt K, Gebhart GF 2005 Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons and capsaicin. Journal of Neuroscience 25:3973–3984.)
Because no highly selective Nav channel blockers are available, several investigators have examined the role of TTX-resistant sodium currents by using knockout or knockdown strategies. Functional deletion of the Nav1.8 gene in mice causes a significant reduction in the behavior produced by intracolonic administration of capsaicin or mustard oil and in the referred hypersensitivity (Laird et al 2002) and prevention of increased DRG neuron excitability that normally develops following jejunal inflammation (Hillsley et al 2006). Other sodium channels also probably contribute to changes in excitability and the development of peripheral and/or central sensitization. Induction of gastric ulcers in rats was associated with accelerated recovery from inactivation of TTX-sensitive sodium currents, which may be due to expression of Nav1.3, a sodium channel that is typically expressed in DRG neurons only during embryonic development but is up-regulated after injury to peripheral axons.
Visceral sensory neurons, like somatic sensory neurons, express both high- and low-threshold calcium currents. Because of their slower activation kinetics and lower current density than sodium currents, opening of calcium channels contributes relatively little to the depolarizing phase of the action potential. However, influx of calcium activates important secondary processes within cells. At the synapse, a rise in the intracellular calcium concentration triggers the release of transmitters (exocytosis), thus allowing transfer of information from one neuron to the next. In addition, calcium influx activates calcium-dependent potassium channels, which are responsible for the slow after-hyperpolarization and fatigue during bursts of action potentials. Finally, changes in intraneuronal calcium concentration regulate the activity of protein kinases and phosphatases, which may in turn interact with various target proteins, including ion channels, and hence potentially change membrane excitability.
Activation of voltage-sensitive potassium currents underlies the repolarizing phase of the action potential. The high potassium concentration within the cell in comparison to the extracellular compartment favors efflux of this ion, which is the major contributor to the resting membrane potential. Thus, longer-lasting activation of potassium channels hyperpolarizes the cell and decreases excitability. Several distinct potassium currents have been described in visceral sensory neurons. Even though their properties are similar to those observed in other sensory neurons, visceral sensory neurons express transient potassium currents (A current) that differ in voltage dependence and kinetics from the typical A currents found in somatic sensory neurons. Interestingly, this slowly as well as a more rapidly inactivating potassium current decreases significantly after GI inflammation associated with visceral hypersensitivity, thus demonstrating the importance of potassium channel expression and modulation in determining action potential duration and firing patterns (Beyak 2010). Other potassium channels have a role in regulating neuronal excitability by setting the resting membrane potential and shaping the action potential. These two-pore domain potassium (K2P) channels, including TWIG-related arachidonic acid-stimulated K+ channel (TREK) and TWIK-related K+ channel (TRAAK), can be activated by stimuli such as membrane stretch, intracellular acidosis (TREK-1 and -2), or alkalosis (TRAAK) and lipids, which implicates them in mechano- and/or chemosensation. All three K2P channels are present in nodose (Zhao et al 2010), urinary bladder, and colon (La and Gebhart 2011) afferent somata. A recent study found their expression and function to be decreased in colon DRG somata in a mouse model of colitis, thus implicating them in the increased colon mechanosensitivity that develops in this model (La and Gebhart 2011).
Ligand-Gated Ion Channels
Visceral afferent neurons also express ion channels gated by ligands, temperature, or protons. Two distinct ligand-gated ion channels appear to play important roles in visceral sensation and/or nociception: the serotonin (5-HT3) receptor and purinergic P2X receptors. Within the GI tract, mechanical and chemical stimuli trigger the release of 5-HT from enteroendocrine cells in the epithelial layer, which constitutes the largest reservoir of 5-HT in the body. GI inflammation is associated with increases in the number of enteroendocrine cells and basal serotonin release; similar changes may be found in some patients with irritable bowel syndrome (Gershon and Tack 2007). Because of its abundance and location in the GI tract and ability to activate intrinsic neurons within the enteric plexuses, as well as extrinsic (primary) afferents when released, serotonin (and its receptors and transporters) has been widely studied with regard to mediation of GI motility, nausea and emesis, and pain. About 30% of colon sensory neurons respond to selective 5-HT3 receptor agonists or show immunoreactivity for the 5-HT3 receptor (Hicks et al 2002), which prompted the development of 5-HT3 receptor antagonists as a useful means of managing the discomfort and pain associated with irritable bowel syndrome, but their efficacy for this purpose is limited. In addition to enteroendocrine cells, activated mast cells and platelets also release serotonin, which may contribute to the development of symptoms. However, mucosal stimulation in the GI tract activates primary afferent neurons even after inhibition of serotonin receptors, hence pointing to the presence of other signaling systems.
ATP, the physiological ligand of P2X receptors, is released from epithelial cells by mechanical stimulation, including bladder urothelial and colon epithelial cells during organ distention (Burnstock 2009). Visceral sensory neurons express P2X receptors, which typically form homo- or heteromultimeric complexes consisting of three subunits. Activation of these ligand-gated receptors causes an inward current, thus depolarizing the cell and potentially triggering action potentials. Deletion of one of the seven known P2X receptors, the P2X3 receptor, alters the micturition reflex and thereby results in bladder hyporeflexia (Cockayne et al 2000) and attenuates responses to colorectal distention, as well as sensitization of these responses after colonic inflammation (Shinoda et al 2009). Purinergic signaling is well established as being involved in visceral nociception (Burnstock 2009). For example, instillation of ATP into the bladder lowers the response threshold of high-threshold mechanosensitive fibers (i.e., fibers activated by bladder pressure in the noxious range) (Rong et al 2002). Similarly, colonic application of the P2X3 receptor agonist α,β-methylene ATP activates pelvic nerve afferents and enhances fiber responses to colon distention (Wynn et al 2003); α,β-methylene ATP also increases the responses of vagal esophageal afferents to mechanical stimulation after induction of esophagitis (Page et al 2000). Conversely, genetic deletion of P2X3 receptors blunts the responses of vagal afferents innervating the mouse esophagus or stomach (McIlwrath et al 2009). Bladder inflammation in rodents is associated with apparent changes in the subunit composition of P2X receptors, which moreover, differs between the lumbar splanchnic and pelvic nerve innervations of the bladder (Dang et al 2005, Chen and Gebhart 2010). The importance of purinergic signaling goes beyond the direct activation of P2X receptors inasmuch as ATP and its metabolites also interact with P2Y receptors, which may significantly alter visceral neuron excitability (e.g., Chen et al 2010).
Ion channels gated by acid (see Holzer 2011a for review) or temperature have also been implicated in visceral nociception. Four ASICs are members of the degenerin/epithelial sodium channel (DEG/ENaC) superfamily of voltage-insensitive Na+ channels. ASIC channels 1–3 are expressed, typically as heteromers, in sensory neurons and have been reported to be involved in visceral ischemic and inflammatory pain. When compared with cutaneous and muscle afferents, cardiac sensory neurons express a higher density of acid-sensitive currents, thus pointing at a potential role of these channels in cardiac pain during ischemia (Benson and McCleskey 2007). Similarly, gastric ulceration increases pH sensitivity and the density of acid-sensitive currents in rat nodose and DRG neurons (e.g., Sugiura et al 2005). Studies examining the role of ASIC family members by genetic deletion have revealed that deletion of ASIC3 blunts responses to colorectal distention and mechanical stimulation of gastroesophageal afferents in mice, which suggests a role in visceral mechanosensation (Jones et al 2005, Page et al 2005). In contrast, deletion of ASIC1a increased the mechanosensitivity of gastroesophageal and colonic afferents without affecting cutaneous afferents. Finally, deletion of ASIC2 attenuated the responses of gastroesophageal but enhanced those of mesenteric afferents in the distal colon (Page et al 2005), hence revealing a complex picture of the roles of different ASIC channels in visceral mechanosensation. Chronic inflammation in the human intestinal tract increases the expression of ASICs (Yiangou et al 2001). Similar results were obtained in experimentally induced inflammation associated with hyperalgesia (Voilley et al 2001). Consistent with these findings, the peak amplitude of acid-sensitive currents increases and pH sensitivity shifts to lower acid concentrations in DRG neurons obtained from animals with gastric ulcerations (Sugiura et al 2005).
In addition to members of the ASIC family, TRPV1 can also be activated by protons, but unphysiological changes (in most tissues) at a pH of 5 or less are required to activate this ion channel, at least in normal conditions. TRPV1 is also activated by capsaicin and endogenous lipid products of the lipoxygenase pathway (hydroxyeicosatetraenoic acid [HETE] and hydroperoxyeicosatetraenoic acid [HPETE]) (Nilius et al 2007), as well as by temperatures in the noxious range. In addition, TRPV1 has been implicated in nociception and inflammatory sensitization in the urinary bladder, small intestine, colon, and pancreas (Birder 2007, Liddle 2007, Boesmans et al 2011, Brierley et al 2010, Holzer 2011b). As indicated previously, the presence and expression of TRPV1 are greater in visceral than in somatic afferents. About 80% of nodose ganglion neurons express message for TRPV1, as do a high proportion of urinary bladder, colonic, and pancreatic afferents. Consistent with these observations in animals, TRPV1 is up-regulated in patients with inflammatory bowel syndrome (Akbar et al 2008), painful bladder syndrome (Mukerji et al 2006), and vulvodynia (Tympanidis et al 2004). In addition, experimental activation of TRPV1 in humans produces abdominal pain (see Holzer 2011b). The endogenous lipid products of the lipoxygenase pathway may be produced during organ inflammation, a time when concomitant decreases in tissue pH facilitate gating of the TRPV1 receptor. In the 5-HT–rich GI tract, activation of TRPV1 can be enhanced by 5-HT (Sugiura et al 2004). Exposure of mouse colon sensory neurons (S1 dorsal root ganglion) to 5-HT significantly enhances both capsaicin- and proton-gated inward currents and significantly reduces the threshold temperature for heat activation to close to resting intracolonic temperature. This arises through interaction with metabotropic serotonergic receptors, thus revealing yet another potential mechanism by which GI sensory neurons can be sensitized by an endogenous mediator commonly present in the gut.
Genetic deletion of TRP channels suggests roles for the V1, V4, and A1 channels of TRP in visceral organ mechanotransduction and/or hypersensitivity (e.g., Blackshaw et al 2010, Cattaruzza et al 2010, Holzer 2011b). TRPV1 knockout mice exhibit a significant reduction in their behavioral (visceromotor) response to colorectal distention and also in responses of colonic afferent fibers to stretch. Similarly, urinary bladder and jejunal afferent subpopulations exhibit reduced mechanosensitivity. TRPV1 also plays a role in hypersensitivity to mechanical stimulation in models of colon, bladder (interstitial cystitis), and pancreatic hypersensitivity (Schwartz et al 2011). The TRPV4 receptor, like TRPV1, has been implicated in visceral mechanosensation and hypersensitivity. TRPV4 receptor mRNA content is significantly greater in colonic DRG afferent somata than in gastric or non-visceral afferents (Brierley et al 2008). As with TRPV1, behavioral responses to colorectal distention and the mechanical responses of colon afferents are reduced when TRPV4 is knocked out genetically or significantly reduced by a small interfering RNA strategy (Cenac et al 2008). In support, intracolonic administration of a TRPV4 receptor–selective agonist dose-dependently increases responses of mice to colorectal distention, thus suggesting that TRPV4 activation contributes to colon hypersensitivity (Cenac et al 2008). Like TRPV1, TRPV4 signaling can be potentiated by 5-HT, as well as by histamine (Cenac et al 2010), and TRPA1 mediates PAR-2–induced colorectal hypersensitivity (Cattaruzza et al 2010). Accordingly, modulation of TRP channels by endogenous mediators appears to contribute to TRP channel mechanotransduction in the colon.
Recently, TRPV1 and TRPA1 mRNA expression and function in pancreatic nodose and DRG neurons were documented in a model of cerulein-induced acute pancreatitis (Schwartz et al 2011). Similarly, TRPV1 is up-regulated in pancreatic DRG neurons during chronic pancreatitis. Pharmacologically, antagonism of TRPV1 attenuates cerulein-induced pancreatitis in both mice and rats (Romac et al 2008, Schwartz et al 2011), but cerulein-induced pancreatitis is unaffected by genetic deletion of TRPV1 (Liddle 2007), which was interpreted to reflect compensation by TRPA1 in this model. In support, pancreatic inflammation was attenuated by either TRPV1 or TRPA1 antagonist treatment, and combining the two antagonists produced a greater than additive effect (Schwartz et al 2011). Pancreatitis-related pain behavior was also reversed by combined TRPV1 and TRPA1 receptor antagonist treatment, thus supporting the notion of an interaction between these two TRP channels that may exist in other viscera but has yet to be examined. The observation that TRPV1 and TRPA1 contribute in a greater than additive fashion suggests that interactions between the two receptors may have important implications for the management of pancreatic inflammation and pain.
Visceral Hypersensitivity
Sensitization (an increase in response magnitude, typically accompanied by a reduction in response threshold) has been considered in parts of preceding sections of this chapter. Experimental organ irritation or inflammation has been shown in vivo to cause increased responses of visceral afferent fibers to hollow-organ distention, often associated with an increase in resting activity and, for visceral afferent fibers with high thresholds for response, a decrease in response threshold (Fig. 51-6B). As indicated above, most mechanosensitive visceral afferent fibers are also chemo- and/or thermo-sensitive (i.e., are multimodal), and sensitization of responses to hollow-organ distention is evident even after acute, non-injurious thermal or chemical stimulation. For example, exposure of the descending colon to hot (50°C) or cold (15°C) solutions increased or decreased, respectively, subsequent responses of pelvic nerve afferent fibers to colon distention. Similarly, exposure of the gastric lumen to hot (46°C) or acidic (HCl, bile acid) solutions that produced no damage to the mucosa significantly affected the resting activity of gastric vagal afferents and subsequent responses to gastric distention (Kang et al 2004). Thus, the visceral innervation is highly malleable and able to undergo rapid and reversible changes in excitability in the absence of injury or inflammation.
As discussed previously, activation of most receptive endings in the viscera is not associated with the conscious appreciation of any sensation. Functional visceral disorders, however, are characterized by discomfort and pain in the absence of either a biochemical or structural explanation for the altered sensations. These disorders, which include functional or non-ulcer dyspepsia, non-cardiac chest pain, painful bladder syndrome, irritable bowel syndrome, and chronic pelvic pain syndrome, represent a state of visceral hypersensitivity because previously non-sensed stimuli now lead to discomfort and/or pain. Like somatic hypersensitivity (hyperalgesia), both peripheral and central mechanisms contribute to visceral hypersensitivity, and like persistent somatic and neuropathic pain states, the initial and often continuing driving force is peripheral. Thus, in most cases, changes reported in CNS processing and/or central modulation of pain are preceded by changes in primary afferent function, and growing evidence reveals that persistent afferent drive contributes significantly to the recurrent, unexplained pain and hypersensitivity that characterize these disorders (e.g., Ortiz Luca, 2010a&b; Ohman & Simren, 2010; Christianson et al., 2010). In the genitourinary literature, the term “afferent neurourology” has been advanced to describe sensory processing disorders from the genitourinary tract, including painful bladder syndrome, chronic prostatitis, pelvic pain syndrome, overactive bladder, and dyspareunia (Clemens 2010). Beyond the viscera, afferent drive has long been understood to be key to neuropathic pain (Gracely et al 1992) and, recently, also important in fibromyalgia (Staud et al 2009, Xu et al 2010). With respect to painful bladder and irritable bowel syndromes, evidence supporting peripheral drive includes the following:
The latter points underscore the importance of local mediators and immune-competent cells in contributing to the persistent visceral hypersensitivity in functional disorders (and support hypotheses that functional visceral disorders are characterized by low-grade inflammation) (see Feng et al 2012a Ohman & Simren, 2010 for review). Clearly, the persistent afferent drive contributed to by sensitized visceral afferents and perhaps MIAs that have acquired mechanosensitivity by exposure to inflammatory or sensitizing mediators leads to changes in the CNS—“central sensitization”—that can amplify afferent input.
Alternatively, it has been argued that central mechanisms are largely responsible for the discomfort, pain, and hypersensitivity that characterize functional visceral disorders. Emotional, attentional, and other cognitive contributions unquestionably modulate interoceptive visceral input, which in functional visceral disorders is considered to reflect dysregulation of bidirectional brain–gut interactions (e.g., Dunckley et al 2005b, 2007; Chang et al 2009; Mayer and Tillisch 2011). Physiologically, dysregulation has been suggested to arise from altered descending modulation of afferent input (e.g., decreased inhibitory or increased facilitatory modulation), alterations in the hypothalamic–pituitary–adrenal axis, and/or disruption of the normal homeostasis of the brain–gut axis (e.g., autonomic nervous system output in response to visceral input is exacerbated; Mayer and Tillisch 2011). Central sites implicated in the above include those having roles in cognitive appraisal and emotion, supported by analysis of brain regions activated by visceral stimulation (e.g., Dunckley et al 2005b, 2007; Mayer and Tillisch 2011), and brain areas associated with nociceptive processing related to stress, anxiety, and attention (Fig. 51-7).
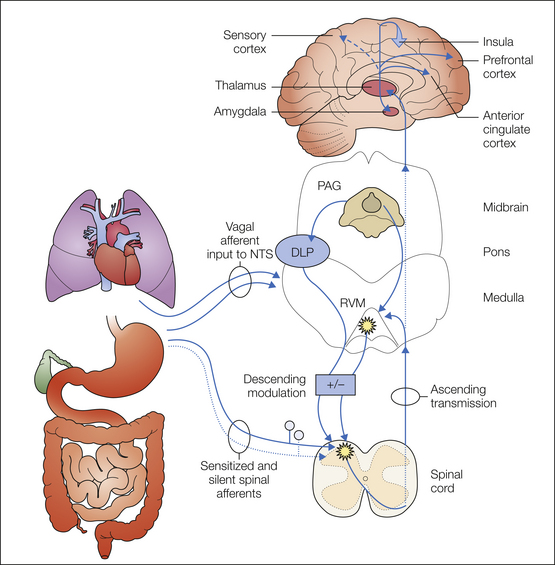
Figure 51-7 Schematic diagram of the supraspinal distribution of visceral nociceptive input and modulation of spinal visceral nociceptive transmission.
Visceral input is relayed through the thalamus and diverges to several targets. Discriminatory projections of visceral nociceptive input to the somatosensory cortex are relatively less (and thus is illustrated by a broken line) than somatic input. Visceral nociceptive input is widely distributed, including distribution to the prefrontal cortex, the perigeniculate area of the anterior cingulate cortex, the amygdala, and laterally to the insula (as illustrated projecting out of the plane of the figure). Descending influences from the midbrain periaqueductal gray (PAG) via the dorsolateral pons (DLP) and rostral ventral medial medulla (RVM) modulate spinal visceral nociceptive transmission. This modulation can be either facilitatory (+) or inhibitory (−). Neurons in both the spinal dorsal horn and RVM are illustrated as sensitized, which represents central sensitization. NTS, nucleus tractus solitarii.
Visceral Organ Cross-Sensitization
An additional consequence of visceral afferent sensitization and organ hypersensitivity is organ cross-sensitization. Although viscerosomatic convergence and referral have been well documented clinically and widely investigated, viscerovisceral convergence and sensitization (termed cross-organ sensitization) have only recently received attention as being important in visceral disease states (e.g., Berkley 2005, Malykhina 2007, Brumovsky and Gebhart 2010). Second-order spinal neurons receive convergent input from multiple viscera (e.g., the esophagus, heart, lower airways, stomach, pancreas, duodenum, gallbladder, colon, rectum, ureter, urinary bladder, pelvic urethra, uterus, and prostate). Cross-organ sensitization between the lower gut and pelvic urinary or gynecological organs is recognized to be relatively common and clinically troublesome with respect to management of symptoms. For example, patients with irritable bowel syndrome often exhibit signs of urinary bladder hypersensitivity: nocturia, frequency and micturition urgency, incomplete bladder emptying, back pain, and in women, dyspareunia. In rodents, colon irritation produced “colon-to-bladder” sensitization as evidenced by increased frequency of bladder contractions, reduced intercontraction intervals, and altered micturition reflexes. Conversely, bladder inflammation produces “bladder-to-colon” cross-sensitization as evidenced by hypersensitivity to colon distention and sensitization of colon afferent fibers (see Brumovsky and Gebhart 2010 for citations).
The references for this chapter can be found at www.expertconsult.com.
References
Akbar A., Yiangou Y., Facer P., et al. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929.
Barbara G., Stanghellini V., De Giorgio R., et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702.
Barbara G., Wang B., Stanghellini V., et al. Mast cell–dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37.
Benson C.J., McCleskey E.W. ASICs function as lactic acid sensors during cardiac ischemia. In: Wang D.H., ed. Molecular sensors for cardiovascular homeostasis. New York: Springer; 2007:32–50.
Berkley K.J. A life of pelvic pain. Physiology & Behavior. 2005;86:272–280.
Beyak M.J. Visceral afferents—determinants and modulation of excitability. Autonomic Neuroscience: Basic & Clinical. 2010;153:69–78.
Birder L.A. TRPs in bladder diseases. Biochimica et Biophysica Acta. 2007;1772:879–884.
Birdsong W.T., Fierro L., Williams F.G., et al. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749.
Blackshaw L.A., Brierley S.M., Hughes P.A. TRP channels: new targets for visceral pain. Gut. 2010;59:126–135.
Boesmans W., Owsianik G., Tack J., et al. TRP channels in neurogastroenterology: opportunities for therapeutic intervention. British Journal of Pharmacology. 2011;162:18–37.
Brierley S.M., Hughes P.A., Harrington A.M., et al. Identifying the ion channels responsible for signaling gastro-intestinal based pain. Pharmaceuticals. 2010;3:2768–2798.
Brierley S.M., Jones R.C.W., 3rd., Gebhart G.F., et al. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–178.
Brierley S.M., Jones R.C.W., 3rd., Xu L., et al. Differential chemosensory function and receptor expression of splanchnic and pelvic colonic afferents in mice. Journal of Physiology. 2005;567:267–281.
Brierley S.M., Page A.J., Hughes P.A., et al. Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology. 2008;134:2059–2069.
Brumovsky P.R., Gebhart G.F. Visceral organ cross-sensitization—an integrated perspective. Autonomic Neuroscience: Basic & Clinical. 2010;153:106–115.
Burnstock G: Purinergic receptors and pain. Current Pharmaceutical Design 15:1717–1735
Cattaruzza F., Spreadbury I., Miranda-Morales M., et al. Transient receptor potential ankyrin-1 has a major role in mediating visceral pain in mice. American Journal of Physiology. 2010;298:G81–G91.
Cenac N., Altier C., Chapman K., et al. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946.
Cenac N., Altier C., Motta J.P., et al. Potentiation of TRPV4 signalling by histamine and serotonin: an important mechanism for visceral hypersensitivity. Gut. 2010;59:481–488.
Ceppa E., Cattaruzza F., Lyo V., et al. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. American Journal of Physiology. 2010;299:G556–G571.
Cervero F. Sensory innervation of the viscera: peripheral basis of viscera. Physiological Reviews. 1994;74:95–138.
Chang L., Sundaresh S., Elliott J., et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterology and Motility. 2009;21:149–159.
Chen C.L., Liu T.T., Yi C.H., et al. Effects of capsaicin-containing red pepper sauce suspension on esophageal secondary peristalsis in humans. Neurogastroenterology and Motility. 2010;22:1177–1182.
Chen X., Gebhart G.F. Differential purinergic signaling in bladder sensory neurons of naïve and bladder inflamed mice. Pain. 2010;148:462–472.
Chen X., Molliver D.C., Gebhart G.F. The P2Y2 receptor sensitizes mouse bladder sensory neurons and facilitates purinergic currents. Journal of Neuroscience. 2010;30:2365–2372.
Christianson J.A., Bielefeldt K., Altier C., et al. Development, plasticity and modulation of visceral afferents. Brain Research Reviews. 2009;60:171–186.
Christianson J.A., Bielefeldt K., Malin S.A., et al. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–549.
Clemens J.Q. Afferent neurourology: a novel paradigm. Journal of Neurourology. 2010;29:S29–S31.
Cockayne D.A., Hamilton S.G., Zhu Q.M., et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015.
Coldwell J.R., Phillis B.D., Sutherland K., et al. Increased responsiveness of rat colonic splanchnic afferents to 5-HT after inflammation and recovery. Journal of Physiology. 2007;579:203–213.
Craig A.D. How do you feel—now? The anterior insula and human awareness. Nature Neuroscience. 2009;10:59–70.
Dang K., Bielefeldt K., Gebhart G.F. Differential responses of bladder lumbosacral and thoracolumbar dorsal root ganglion neurons to purinergic agonists, protons and capsaicin. Journal of Neuroscience. 2005;25:3973–3984.
Dunckley P., Aziz Q., Wise R.G., et al. Attentional modulation of visceral and somatic pain. Neurogastroenterology and Motility. 2007;19:569–577.
Dunckley P., Wise R.G., Fairhurst M., et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25:7333–7341.
Dunckley P., Wise R.G., Fairhurst M., et al. Processing in the human brainstem using functional magnetic resonance imaging. Journal of Neuroscience. 2005;25:7333–7341.
Eijkelkamp N., Kavelaars A., Eisenbruch S., et al. Increased visceral sensitivity to capsaicin after DSS-induced colitis in mice: spinal cord c-Fos expression and behavior. American Journal of Physiology. 2007;293:G749–G757.
Feng B., Gebhart G.F. Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. American Journal of Physiology. 2011;300:G170–G180.
Feng B., La J.-H., Schwartz E.S., et al. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Neural and neuro-immune mechanisms of visceral hypersensitivity in irritable bowel syndrome. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;302(10):G1085–1098.
Feng B., La J.-H., Schwartz E.S., et al. Long-term sensitization of mechano-sensitive and -insensitive afferents in mice with persistent colorectal hypersensitivity. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2012;302:G676–G683.
Foreman R. Mechanisms of cardiac pain. Annual Review of Physiology. 1998;61:143–167.
Forrest J.B., Moldwin R. Diagnostic options for early identification and management of interstitial cystitis/painful bladder syndrome. International Journal of Clinical Practice. 2008;62:1926–1934.
Fu L.W., Longhurst J.C. Activated platelets contribute to stimulation of cardiac afferents during ischaemia in cats: role of 5-HT3 receptors. Journal of Physiology. 2002;544:897–912.
Fu L.W., Longhurst J.C. Regulation of cardiac afferent excitability in ischemia. Handbook of Experimental Pharmacology. 2009;194:185–225.
Gershon M.D., Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414.
Gold M.S., Gebhart G.F. Nociceptor sensitization in pain pathogenesis. Nature Medicine. 2010;16:14–23.
Gracely R.H., Lynch S.A., Bennett G.J. Painful neuropathy: altered central processing maintained dynamically by peripheral input. Pain. 1992;51:175–194.
Hamilton M.J., Sinnamon M.J., Lyng G.D., et al. Essential role for mast cell tryptase in acute experimental colitis. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:290–295.
Hammer J., Führer M., Matiasek M. Hypersensitivity for capsaicin in patients with functional dyspepsia. Neurogastroenterology and Motility. 2008;20:125–133.
Hicks G.A., Coldwell J.R., Schindler M., et al. Excitation of rat colonic afferent fibres by 5-HT3 receptors. Journal of Physiology. 2002;544:861–869.
Hillsley K., Lin J.H., Stanisz A., et al. Dissecting the role of sodium currents in visceral sensory neurons in a model of chronic hyperexcitability using Nav1.8 and Nav1.9 null mice. Journal of Physiology. 2006;576:257–267.
Holzer P. Sensory neuron responses to mucosal noxae in the upper gut: relevance to mucosal integrity and gastrointestinal pain. Neurogastroenterology and Motility. 2002;14:459–475.
Holzer P. Acid sensing by visceral afferent neurones. Acta Physiologica. 2011;201:63–75.
Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacology & Therapeutics. 2011;131:142–170.
Jones R.C.W., 3rd., Xu L., Gebhart G.F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. Journal of Neuroscience. 2005;25:10981–10989.
Kajander K., Myllyluoma E., Kyronpalo S., et al. Elevated pro-inflammatory and lipotoxic mucosal lipids characterise irritable bowel syndrome. World Journal of Gastroenterology. 2009;15:6068–6074.
Kang Y.M., Bielefeldt K., Gebhart G.F. Sensitization of mechanosensitive gastric vagal afferent fibers by thermal and chemical stimuli and gastric ulcers. Journal of Neurophysiology. 2004;91:1981–1989.
Kwong K., Nassenstein C., de Garavilla L., et al. Thrombin and trypsin directly activate vagal C-fibres in mouse lung via protease-activated receptor-1. Journal of Physiology. 2010;588:1171–1177.
Kyloh M., Nicholas S., Zagorodnyuk V.P., et al. Identification of the visceral pain pathway activated by noxious colorectal distension in mice. Frontiers in Neuroscience. 2011;5:16.
La J-H, Gebhart GF: 201 Colitis decreases mechanosensitive K2P channel expression and function in mouse colon sensory neurons. American Journal of Physiology 301:G91–G99
La J.-H., Schwartz E.S., Gebhart G.F. Differences in the expression of TRPV1, TRPA1 and mechanosensitive K2P channels between the lumbar splanchnic and pelvic nerve innervations of mouse urinary bladder and colon. Neuroscience. 2011;86:179–187.
Laird J.M., Souslova V., Wood J.N., et al. Deficits in visceral pain and referred hyperalgesia in Nav1.8 (SNS/PN3)-null mice. Journal of Neuroscience. 2002;22:8352–8356.
Lamb K., Kang Y.M., Gebhart G.F., et al. Gastric inflammation triggers hypersensitivity to acid in awake rats. Gastroenterology. 2003;125:1410–1418.
Langley J.N. The autonomic nervous system. Cambridge: Cambridge University Press; 1921.
Liddle R.A. The role of transient receptor potential vanilloid 1 (TRPV1) channels in pancreatitis. Biochimica et Biophysica Acta. 2007;1772:869–878.
Lynn P.A., Olsson C.A., Zagorodnyuk V.A., et al. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology. 2003;125:786–794.
Malykhina A.P. Neural mechanisms of pelvic organ cross-sensitization. Neuroscience. 2007;149:660–672.
Mayer E.A., Aziz Q., Coen S., et al. Brain imaging approaches to the study of functional GI disorders: a Rome working group team report. Neurogastroenterology and Motility. 2009;21:579–596.
Mayer E.A., Tillisch K. The brain-gut axis in abdominal pain syndromes. Annual Review of Medicine. 2011;62:381–396.
McIlwrath S.L., Davis B.M., Bielefeldt K. Deletion of P2X3 receptors blunts gastro-oesophageal sensation in mice. Neurogastroenterology and Motility. 2009;21:890-e66.
Mourtzoukou E.G., Iavazzo C., Falagas M.E. Resiniferatoxin in the treatment of interstitial cystitis: a systematic review. International Urogynecology Journal. 2008;19:1571–1576.
Mukerji G., Yiangou Y., Mukerji G., et al. Transient receptor potential vanilloid receptor subtype 1 in painful bladder syndrome and its correlation with pain. Journal of Urology. 2006;176:797–801.
Nassenstein C., Taylor-Clark T.E., Myers A.C., et al. Phenotypic distinctions between neural crest and placodal derived vagal C-fibres in mouse lungs. Journal of Physiology. 2010;588:4769–4783.
Ness T.J., Gebhart G.F. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234.
Neuhaus J., Weimann A., Stolzenburg J.U., et al. Histamine receptors in human detrusor smooth muscle cells: physiological properties and immunohistochemical representation of subtypes. World Journal of Urology. 2006;24:202–209.
Nickel J.C., Moldwin R., Lee S., et al. Intravesical alkalinized lidocaine [PSD597] offers sustained relief from symptoms of interstitial cystitis and painful bladder syndrome. BJU International. 2009;103:1080–1084.
Nilius B., Mahieu F., Karashima Y., et al. Regulation of TRP channels: a voltage-lipid connection. Biochemical Society Transactions. 2007;35:105–108.
Ohman L., Simren M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nature Reviews. Gastroenterology and Hepatology. 2010;7:163–173.
Ortiz-Lucas M., Saz-Peiro P., Sebastian-Domingo J.J. Irritable bowel syndrome immune hypothesis. Part one: the role of lymphocytes and mast cells. Revista Espanola de Enfermedades Digestivas. 2010;102:637–647.
Ortiz-Lucas M., Saz-Peiro P., Sebastian-Domingo J.J. Irritable bowel syndrome immune hypothesis. Part two: the role of cytokines. Revista Espanola de Enfermedades Digestivas. 2010;102:711–717.
Page A.J., Brierley S.M., Martin C.M., et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415.
Page A.J., Martin C.M., Blackshaw L.A. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. Journal of Neurophysiology. 2002;87:2095–3201.
Page A.J., O’Donnell T.A., Blackshaw L.A. P2X purinoceptor–induced sensitization of ferret vagal mechanoreceptors in oesophageal inflammation. Journal of Physiology. 2000;523:403–411.
Park J.H., Rhee P.L., Kim Y.H., et al. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterology and Motility. 2006;18:539–546.
Phillips R.J., Powley T.L. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluation of vagal mechanoreceptor electrophysiology. Brain Research Reviews. 2000;34:1–26.
Powley T.L., Spaulding R.A., Haglof S.A. Vagal afferent innervation of the proximal gastrointestinal tract mucosa: chemoreceptor and mechanoreceptor architecture. Journal of Comparative Neurology. 2011;519:644–660.
Price D.D., Craggs J.G., Zhou O., et al. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. NeuroImage. 2009;47:995–1001.
Qin C., Farber J.P., Foreman R.D. Intraesophageal chemicals enhance responsiveness of upper thoracic spinal neurons to mechanical stimulation of esophagus in rats. American Journal of Physiology. 2008;294:G708–G716.
Robinson D.R., Gebhart G.F. Inside information—the unique features of visceral sensation. Molecular Interventions. 2008;8:242–253.
Romac J.M., McCall S.J., Humphrey J.E., et al. Pharmacologic disruption of TRPV1-expressing primary sensory neurons but not genetic deletion of TRPV1 protects mice against pancreatitis. Pancreas. 2008;36:394–401.
Rong W., Spyer K.M., Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. Journal of Physiology. 2002;541:591–600.
Schaible H.G., Schmidt R.F. Effects of an experimental arthritis on the sensory properties of fine articular afferent units. Journal of Neurophysiology. 1985;54:1109–1122.
Schmelz M., Schmid R., Handwerker H.O., et al. Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain. 2000;123:560–571.
Schwartz E.S., Christianson J.A., Chen X., et al. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140:1283–1291.
Shinoda M., Feng B., Gebhart G.F. Peripheral and central P2X3 receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology. 2009;137:2096–2104.
Snider W.D., McMahon S.B. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632.
Song X., Chen B.N., Zagorodnyuk V.P., et al. Identification of medium/high-threshold extrinsic mechanosensitive afferent nerves to the gastrointestinal tract. Gastroenterology. 2009;137:274–284.
Staud R., Nagel S., Robinson M.E. Enhanced central pain processing of fibromyalgia patients is maintained by muscle afferent input: a randomized, double-blind, placebo-controlled study. Pain. 2009;145:96–104.
Sugiura T., Bielefeldt K., Gebhart G.F. TRPV1 function in mouse colon sensory neurons is enhanced by 5-HT receptor activation. Journal of Neuroscience. 2004;24:9521–9530.
Sugiura T., Dang K., Lamb K., et al. Acid sensing ion currents in rat nodose and dorsal root ganglia from normal and ulcerated stomach. Journal of Neuroscience. 2005;25:2617–2627.
Sugiura Y., Tonosaki Y. Spinal organization of unmyelinated visceral afferent fibers in comparison with somatic afferent fibers. In: Gebhart G.F., ed. Progress in pain research and management. Seattle: IASP Press; 1995:41–59.
Taylor-Clark T.E., Undem B.J. Ozone activates airway nerves via the selective stimulation of TRPA1 ion channels. Journal of Physiology. 2010;588:423–433.
Theoharides T.C., Kempuraj D., Sant G.R. Massive extracellular tryptase from activated bladder mast cells in interstitial cystitis. Urology. 2001;58:605–606.
Tillisch K., Mayer E.A., Labus J.S. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100.
Tirumuru S., Al-Kurdi D., Latthe P. Intravesical botulinum toxin A injections in the treatment of painful bladder syndrome/interstitial cystitis: a systematic review. International Urogynecology Journal. 2010;21:1285–1300.
Traub R.J. Spinal mechanisms of visceral pain and sensitization. In: Pasricha P.J., Willis W.D., Gebhart G.F., eds. Chronic abdominal and visceral pain theory and practice. New York: Informa Healthcare; 2007:85–105.
Tympanidis P., Casula M.A., Yiangou Y., et al. Increased vanilloid receptor VR1 innervation in vulvodynia. European Journal of Pain. 2004;8:129–133.
Verne G.N., Robinson M.E., Vase L., et al. Reversal of visceral and cutaneous hyper-algesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2010;105:223–230.
Voilley N., de Weille J., Mamet J., et al. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. Journal of Neuroscience. 2001;21:8026–8033.
Wang F.B., Powley T.L. Topographic inventories of vagal afferents in gastrointestinal muscle. Journal of Comparative Neurology. 2000;421:302–324.
Wang Y.L.G., Liang S., Zhang A., et al. Role of P2X3 receptor in myocardial ischemia injury and nociceptive sensory transmission. Autonomic Neuroscience: Basic & Clinical. 2008;139:30–37.
Wang Z.Y., Wang P., Bjorling D.E. Role of mast cells and protease-activated receptors-2 in cyclooxygenase-2 expression in urothelial cells. American Journal of Physiology. 2009;297:R1127–R1135.
Weidner C., Schmelz M., Schmidt R., et al. Functional attributes discriminating mechano-insensitive and mechano-responsive C nociceptors in human skin. Journal of Neuroscience. 1999;19:10184–10190.
Wynn G., Rong W., Xiang Z., et al. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–1409.
Xu L., Gebhart G.F. Characterization of mouse lumbar splanchnic and pelvic nerve urinary bladder mechanosensory afferents. Journal of Neurophysiology. 2008;99:244–253.
Xu Y.M., Ge H.Y., Arendt-Nielsen L. Sustained nociceptive mechanical stimulation of latent myofascial trigger point induces central sensitization in healthy subjects. Journal of Pain. 2010;11:1348–1355.
Yiangou Y., Facer P., Smith J.A., et al. Increased acid-sensing ion channel ASIC-3 in inflamed human intestine. European Journal of Gastroenterology & Hepatology. 2001;13:891–896.
Zagorodnyuk V.P., Simon J.H., Brookes S.J., et al. Structure-function relationship of sensory endings in the gut and bladder. Autonomic Neuroscience: Basic & Clinical. 2010;153:3–11.
Zhao H., Sprunger L.K., Simasko S.M. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. American Journal of Physiology. 2010;298:G212–G221.
Benson C.J., McCleskey E.W. ASICs function as lactic acid sensors during cardiac ischemia. In: Wang D.H., ed. Molecular sensors for cardiovascular homeostasis. New York: Springer; 2007:32–50.
Beyak M.J. Visceral afferents—determinants and modulation of excitability. Autonomic Neuroscience: Basic & Clinical. 2010;153:69–78.
Birder L.A. TRPs in bladder diseases. Biochimica et Biophysica Acta. 2007;1772:879–884.
Blackshaw L.A., Brierley S.M., Hughes P.A. TRP channels: new targets for visceral pain. Gut. 2010;59:126–135.
Brumovsky P.R., Gebhart G.F. Visceral organ cross-sensitization—an integrated perspective. Autonomic Neuroscience: Basic & Clinical. 2010;153:106–115.
Burnstock G: Purinergic receptors and pain, Current Pharmaceutical Design 15:1717–1735
Cervero F. Sensory innervation of the viscera: peripheral basis of viscera. Physiological Reviews. 1994;74:95–138.
Christianson J.A., Bielefeldt K., Altier C., et al. Development, plasticity and modulation of visceral afferents. Brain Research Reviews. 2009;60:171–186.
Gershon M.D., Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. 2007;132:397–414.
Holzer P. Acid sensing by visceral afferent neurones. Acta Physiologica. 2011;201:63–75.
Holzer P. Transient receptor potential (TRP) channels as drug targets for diseases of the digestive system. Pharmacology & Therapeutics. 2011;131:142–170.
Liddle R.A. The role of transient receptor potential vanilloid 1 (TRPV1) channels in pancreatitis, Biochimica et Biophysica Acta. 1997;1772:869–878.
Mayer E.A., Aziz Q., Coen S., et al. Brain imaging approaches to the study of functional GI disorders: a Rome working group team report. Neurogastroenterology and Motility. 2009;21:579–596.
Mayer E.A., Tillisch K. The brain-gut axis in abdominal pain syndromes. Annual Review of Medicine. 2011;62:381–396.
Ness T.J., Gebhart G.F. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234.
Price D.D., Craggs J.G., Zhou O., et al. Widespread hyperalgesia in irritable bowel syndrome is dynamically maintained by tonic visceral impulse input and placebo/nocebo factors: evidence from human psychophysics, animal models, and neuroimaging. NeuroImage. 2009;47:995–1001.
Traub R.J. Spinal mechanisms of visceral pain and sensitization. In: Pasricha P.J., Willis W.D., Gebhart G.F., eds. Chronic abdominal and visceral pain theory and practice. New York: Informa Healthcare; 2007:85–105.
Zagorodnyuk V.P., Simon J.H., Brookes S.J., et al. Structure-function relationship of sensory endings in the gut and bladder. Autonomic Neuroscience: Basic & Clinical. 2010;153:3–11.