Complex Regional Pain Syndromes
Introduction
During past decades, complex regional pain syndromes (CRPSs) were recognized as poorly defined pain disorders that mostly confused basic researchers, clinicians, and epidemiologists rather than stimulating their scientific activity. This was mainly due to the fact that diagnostic criteria were defined vaguely, underlying pathophysiological mechanisms were unknown, and therapeutic options limited. No data on incidence, prognosis, and prevention were available; research on mechanisms focused primarily on pain; and controlled treatment studies were absent. However, insight into the pathophysiological mechanisms of CRPS has progressed dramatically during the past few years.
Researchers became aware of the fact that CRPS types 1 and 2 are not just neuropathic pain syndromes. In fact, CRPS 1 is unlikely to only be a neuropathic pain syndrome since no obvious nerve lesion is present and all symptoms occur irrespective of the type of preceding lesion. Based on this notion it has become obvious that multiple different pathophysiological mechanisms may occur in different individual patterns (Baron et al 2002b). Such mechanisms consist of somatosensory changes (including pain) that interact with changes related to the sympathetic nervous system, peripheral inflammatory changes, and changes in the somatomotor system (Jänig and Baron 2003).
Definition of Crps
CRPS is a syndrome characterized by continuing (spontaneous and/or evoked) regional pain that is seemingly disproportionate in time or degree to the usual course of pain after trauma or other lesions. The pain is regional (not in a specific nerve territory or dermatome) and usually has a distal predominance of abnormal sensory, motor, sudomotor, vasomotor edema, and/or trophic findings. The syndrome shows variable progression over time.
In CRPS type 1 (reflex sympathetic dystrophy), minor injuries or fractures of a limb precede the onset of symptoms. CRPS type 2 (causalgia) develops after injury to a major peripheral nerve.
History
The American Civil War physician Weir Mitchell observed that about 10% of patients with traumatic partial peripheral nerve injuries in the distal end of an extremity had a dramatic clinical syndrome that consisted of prominent, distal spontaneous burning pain. In addition, patients reported exquisite hypersensitivity of the skin to light mechanical stimulation. Furthermore, movement, loud noises, or strong emotions could trigger their pain. The distal end of the extremity exhibited considerable swelling, smoothness, mottling, and in some cases, acute arthritis. In most cases the limb was cold and sweaty. Mitchell named this syndrome causalgia. He was emphatic that the sensory and trophic abnormalities spread beyond the innervation territory of the injured peripheral nerve and often occurred remote from the site of injury. The nerve lesions giving rise to this syndrome were always partial; complete transection never caused it. Because of this and the peripheral signs of the disease, he concluded that in addition to pathology in the nerve, some process in the skin or other peripheral tissue was responsible for the pain.
After World War II, Leriche for the first time reported that sympathectomy dramatically relieves causalgia. This notion was supported by several large clinical series, primarily involving wounded soldiers. In 1967 Richards described the clinical features of causalgia and the effect of sympatholytic interventions in hundreds of cases. He repeatedly stressed the dramatic response of causalgia to sympathetic blockade: “One of the outstanding surgical lessons that was learned during World War II was that interruption of the appropriate sympathetic nerve fibers is almost invariably effective in the treatment of causalgia. When the sympathetic chain is blocked by a local anesthetic, complete relief occurs almost immediately if the injection has been correctly placed, and the dramatic change in the patient’s appearance and attitude is remarkable.” The finding that sympatholysis relieves causalgic pain gave rise to the concept of sympathetically maintained pain.
In the years between World Wars I and II, the concept that sympathetic outflow can influence pain was extended to a group of patients without detectable nerve injury. Asymmetrical distal extremity pain and swelling develop in these patients (Fig. 67-1). The disorder had first been described by Paul Sudeck early in the 20th century. Precipitating events include fracture or minor soft tissue trauma, low-grade infection, frostbite or burns, and stroke and myocardial infarction. The swelling and pain often develop at a site remote from the inciting injury, without any obvious local tissue-damaging process at the site of pain and swelling. This syndrome was named reflex sympathetic dystrophy because vasomotor (altered skin color and temperature) and sudomotor (altered sweat production) abnormalities are common, the pain and swelling are often spatially remote from the inciting injury, and patients typically obtain dramatic relief with sympathetic blockade.
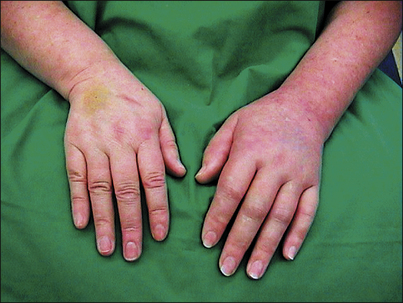
Figure 67-1 Clinical picture of a patient with complex regional pain syndrome type 1 of the upper left extremity following distortion of the left wrist.
In 1994 the terminology changed to CRPS, and thereafter new diagnostic criteria were proposed in 2007 and validated in 2010.
Epidemiology
A population-based study of CRPS 1 in the United States calculated an incidence rate of about 5.5 per 100,000 person-years at risk and a prevalence of around 21 per 100,000. An incidence rate of 0.8 per 100,000 person-years at risk plus a prevalence of about 4 per 100,000 was reported for CRPS 2 (Sandroni et al 2003). A European study using different diagnostic criteria described an incidence of 6.2 per 100,000. CRPS 1 develops more often than CRPS 2. The most common inciting events in CRPS are preceding trauma or surgery. Remarkably, in about 10% of patients CRPS 1 develops after minor trauma and in 5–10% without any known event. The severity of the trauma is not correlated with the clinical characteristics of CRPS (Stanton Hicks 1995). Estimations suggest a 1–37% incidence of CRPS 1 after fractures. The incidence of CRPS 2 after peripheral nerve injury varies from 2–14% in different series, with a mean around 4% (Veldman et al 1993). The arm is affected more often than the leg, and females are affected more often than males, with a female-to-male ratio ranging from 2–4:1. CRPS shows a distribution over all ages, with the mean peak in age being 37–50 years.
Clinical Characteristics
CRPS Type 1 (Reflex Sympathetic Dystrophy)
The most common precipitating event is trauma affecting the distal part of an extremity (~75%), especially fractures, post-surgical conditions, contusions, and strain or sprain. Less common incidents are central nervous system (CNS) lesions such as spinal cord injuries and cerebrovascular accidents.
Asymmetrical distal extremity pain and swelling develop in patients with CRPS 1 without an overt nerve lesion (Fig. 67-1, Table 67-1). These patients often report a burning spontaneous pain in the distal part of the affected extremity. Characteristically, the pain is disproportionate in intensity to the inciting event. The pain usually increases when the extremity is in a dependent position. Stimulus-evoked pain, including mechanical and thermal allodynia and/or hyperalgesia, is a striking clinical feature. These sensory abnormalities often appear early, are most pronounced distally, and have no consistent spatial relationship to individual nerve territories or to the site of the inciting lesion. Typically, pain can be elicited by movement of and pressure on the joints (deep somatic hyperalgesia), even if they are not directly affected by the inciting lesion. Besides symptoms of pain, sensory loss is frequent. A recent study described the coexistence of gain (e.g., pain, hyperalgesia) and loss (e.g., hypoesthesia) in CRPS patients without detecting a distinct sensory pattern.
Table 67-1
Signs and Symptoms of Complex Regional Pain Syndrome
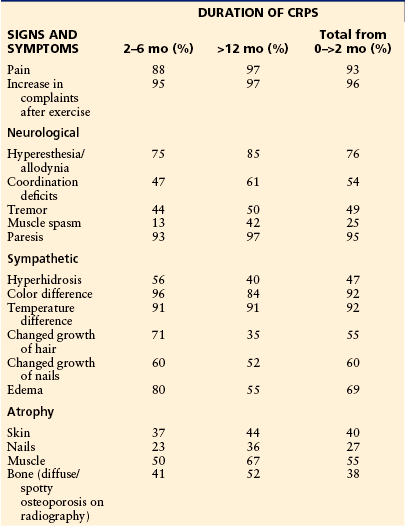
Modified from Veldman PH, Reynen HM, Arntz IE, et al 1993 Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet 342:1012–1016. Copyright 1993, Elsevier Ltd.
Autonomic abnormalities include swelling and changes in sweating and blood flow in the skin. In the acute stages of CRPS 1 the affected limb is often warmer than the contralateral limb. An initially decreased skin temperature (“cold” CRPS type) is thought to be a predictor of an unfavorable course (see Prognosis, later) and is associated with an increased incidence of, for example, dystonia, sensory loss, and cold-induced pain, whereas in “warm” CRPS, mechanical hyperalgesia occurs frequently. Sweating abnormalities—either hypohidrosis or, more frequently, hyperhidrosis—are present in nearly all CRPS 1 patients. The acute distal swelling of the affected limb depends very critically on aggravating stimuli. Since it may diminish after sympathetic blocks, it is likely that it is maintained by sympathetic activity.
Trophic changes such as abnormal nail growth, increased or decreased hair growth, fibrosis, thin glossy skin, and osteoporosis may be present, particularly in the chronic stages. Restriction of passive movement is often observed in long-standing cases and may be related to both functional motor disturbances and trophic changes in joints and tendons.
Weakness of all muscles of the affected distal extremity is frequently present. Small accurate movements are characteristically impaired. Range of motion may be reduced by edema or, later on, by contractures. Passive movements are less affected than voluntary ones. Findings on nerve conduction and electromyography studies are normal, except in patients in very chronic and advanced stages. About half of patients have a postural or action tremor representing an increased physiological tremor. In about 10% of cases, dystonia of the affected hand or foot develops (Harden et al 2001). Additionally, a neglect-like syndrome impairs motor control. (Galer et al 1995).
Importantly, in the course of the disease the symptoms often spread, sometimes also to the contralateral, primarily unaffected extremity.
CRPS Type 2 (Causalgia)
The symptoms of CRPS 2 are similar to those of CRPS 1, the only exception being that a lesion of peripheral nerve structures and subsequent focal deficits are mandatory for the diagnosis. The symptoms and signs spread beyond the innervation territory of the injured peripheral nerve and often occur remote from the site of injury, but restriction to the territory is not in conflict with the current definition.
Spatial Distribution
CRPS occurs predominantly in one extremity. Retrospective studies of large cohorts have shown the ratio of upper and lower extremity involvement to be 1–2:1. In 113 retrospectively reviewed cases the symptoms occurred on the right in 47%, on the left side in 51%, and bilaterally in 2%. Multiple extremities were affected in up to 7% of patients (Veldman et al 1993, Allen et al 1999, Bruehl et al 2002, Sandroni et al 2003).
Time Course
For therapeutic reasons, effort should be undertaken to diagnose CRPS as early as possible. CRPS mostly starts acutely (i.e., the cardinal symptoms may appear within hours or days). At the onset, the main symptoms of CRPS are spontaneous pain, generalized swelling, and systematic between-side difference in skin temperature. These early symptoms already begin to develop in areas and tissues that were not affected by the preceding lesion. Thus, swelling and pain provide valuable information for an early diagnosis of CRPS: before the onset of CRPS, pain is felt inside the area of the preceding lesion; with the onset of CRPS, the pain becomes diffuse and is felt deep inside the distal extremity and the swelling generalizes, yet the initial pain may have already disappeared.
To some extent the tendency of symptoms to generalize may be a physiological phenomenon in post-traumatic states that will disappear without any treatment. Exact differentiation of these physiologically diffuse, post-traumatic reactions and the development of “real“ CRPS is not possible at the present time.
Stages
The sequential progression of untreated CRPS has repeatedly been described, each stage of which (usually three are proposed) differs in patterns of signs and symptoms. Nevertheless, this concept has come into question in the past few years. In 2002 the clinical validity of this concept was tested in 113 patients by Bruehl and colleagues. Using cluster analysis, three subgroups of patients were identified who could be differentiated by their symptoms and signs regardless of duration of the disease. The sequential concept relies on the course of untreated CRPS; however, thus far all studies performed to test its clinical validity investigated patients already under treatment. Furthermore, vascular disturbances and skin temperature measurements indicated different thermoregulatory types, depending on time.
In conclusion, it is questionable whether staging of CRPS is appropriate. It is much more practical, with a direct implication on therapy, that CRPS be graded according to the intensity of the sensory, autonomic, motor, and trophic changes as being mild, moderate, or severe (see later).
Psychology
Most patients with CRPS exhibit significant psychological distress, most commonly depression and anxiety. Many patients become overwhelmed by the pain and associated symptoms and, without adequate psychosocial support, may develop maladaptive coping skills. Based on these symptoms there is a tendency to ascribe the etiology of CRPS to emotional causes, and it has been proposed that CRPS is a psychiatric illness. In fact, it is sometimes difficult to recognize the organic nature of the symptoms. However, when describing the clinical picture in the 1940s, Livingston was convinced: “The ultimate source of this dysfunction is not known but its organic nature is obvious and no one seems to doubt that these classical pain syndromes are real.” Covington (1996) drew several conclusions on psychological factors in CRPS:
In summary, the author concludes that the widely proposed “CRPS personality” is clearly unsubstantiated.
According to this view, an even distribution of childhood trauma, pain intensity, and psychological distress was confirmed in patients with CRPS as opposed to those with other neuropathic pain and chronic back pain (Ciccone et al 1997). Further studies demonstrated high psychiatric co-morbidity, especially depression, anxiety, and personality disorders, in CRPS patients. These findings are also present in other chronic pain patients and are more likely a result of the long and severe pain and disease (Monti et al 1998). When compared with patients with low back pain, CRPS patients exhibited a higher tendency toward somatization but did not have any other psychological differences (Bruehl et al 1996). In 145 patients, 42% reported stressful life events in close relation to the onset of CRPS, and 41% had a previous history of chronic pain (Birklein et al 2000a). Thus, stressful life events could be risk factors for the development of CRPS. However, a recent review did not identify any psychological predisposition, and a prospective study did not find any psychological factors to be associated with the occurrence of CRPS.
Genetics of CRPS
One of the unsolved features in human pain diseases is the fact that chronic pain develops in only a minority of patients after seemingly identical inciting events. Similarly, in certain nerve lesion animal models, differences in pain susceptibility were found to be due to genetic factors. The clinical importance of genetic factors in CRPS is not clear. Even though familial occurrence has been described, mendelian genetics does not seem to have any impact on the incidence and prevalence of CRPS. However, although the importance of the findings reported later is not clear, they suggest some genetic influence on the development and maintenance of CRPS.
Association of human leukocyte antigen (HLA) with different phenotypes has shown an increase in A3, B7, and DR2 major histocompatibility complex (MHC) antigens in a small group of CRPS patients in whom resistance to treatment correlated with positivity of DR2. Class I and II MHC antigens were typed in a cohort of 52 CRPS patients. The frequency of HLA-DQ1 was found to be significantly increased in comparison to the frequency in controls (Kemler et al 1999). In patients with CRPS who progressed to multifocal or generalized dystonia, an association with HLA-DR13 was reported, and in those with fixed dystonia, an association with HLA-B62 and HLA-DQ8 was noted (van Hilten et al 2000b). Furthermore, a different locus, centromeric in HLA class I, was found to be correlated with the spontaneous development of CRPS, thus suggesting an interaction between trauma severity and genetic factors that influence susceptibility to CRPS (van de Beek et al 2003). So far no associations have been detected for different cytokines, neuropeptides, neuronal endopeptides, dystonia-associated genes (DYT), and the Nav1.7 sodium channel gain-of-function mutation within the SCNA9 gene.
Pathophysiological Mechanisms
Sensory Abnormalities and Pain
Based on numerous animal experimental findings, spontaneous pain and various forms of hyperalgesia at the distal end of the extremity are thought to be generated by the processes of peripheral and central sensitization. Quantitative sensory testing (QST) has revealed heat and cold hyperalgesia in combination with warm and cold hypoesthesia in acute CRPS 1, whereas in chronic CRPS 1, the thermal hyperalgesia declined and thermal hypoesthesia increased. Moreover, similar subtle signs were present in the contralateral extremity. In addition to the positive sensory phenomena of pain, in up to 50% of patients with chronic CRPS 1 hypoesthesia and hypoalgesia develop in the entire half of the body or in the upper quadrant ipsilateral to the affected extremity. Systematic QST has shown that patients with generalized hypoesthesia have increased thresholds to mechanical, cold, warmth, and heat stimuli when compared with the responses generated from the corresponding contralateral healthy side of the body. Patients with these extended sensory deficits have a longer disease duration, greater pain intensity, a higher frequency of mechanical allodynia, and a higher tendency for the development of changes in the somatomotor system than do patients with spatially restricted sensory deficits.
These changed somatosensory perceptions are probably due to functional changes in the central representation of somatosensory sensations. Central sensitization and disinhibition are the two key mechanisms in CRPS that can assist in understanding the sensory changes in CRPS. Accordingly, early positron emission tomography studies demonstrated adaptive changes in the thalamus during the course of the disease (Fukumoto et al 1999). Furthermore, several studies using magnetoencephalography (MEG) and functional magnetic resonance imaging (fMRI) demonstrated a pain network involving nociceptive, motor, and attentional processing that resulted in changed central sensory processing, such as a shortened distance between the little finger and thumb representations in the primary somatosensory (SI) cortex on the painful side (Maihofner et al 2003, 2005; Pleger et al 2004b, 2005). The cerebral responses noted with fMRI and MEG were increased on the affected side, thus indicating processes of central disinhibition (i.e., sensitization; e.g., Juottonen et al 2002) (Fig. 67-2). These findings are in line with the clinical findings of glove-like patterns, hemisensory signs, and referred sensations; they resemble the results of imaging studies demonstrating motor circuit disinhibition.
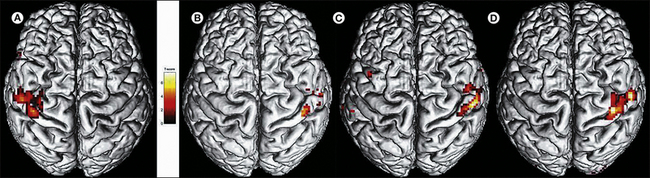
Figure 67-2 Central reorganization of the somatosensory cortex in a patient with complex regional pain syndrome type 1 involving the right hand.
Functional magnetic resonance imaging (fMRI) was performed after mechanical stimulation of digits 1 and 5 on both sides. The hand representation shrinks but recovers after successful treatment. A, Acute stage: nearly complete absence of fMRI signal in the left hemisphere but normal representation on the contralateral side. B–D, Normalization of the representation after 3 (B), 6 (C), and 12 (D) months of treatment.
These central changes depend on continuous nociceptive input from the affected extremity, are associated with spontaneous pain and mechanical hyperalgesia, are not specific to CRPS, and are reversible after successful treatment of the pain (Maihofner et al 2003, 2004; Pleger et al 2005). However, Lebel and co-workers (2008) could show that the altered CNS reactivity to sensory stimuli persisted after recovery from CRPS.
The relevance of structural changes within the peripheral nervous system, such as small-fiber neuropathy or myopathy, is still under debate.
Autonomic Abnormalities
A partial nerve lesion is the important preceding event in the development of CRPS 2. Therefore, it has generally been assumed that abnormalities in skin blood flow within the territory of the lesioned nerve are due to peripheral impairment of sympathetic function and sympathetic denervation. During the first weeks after transection of vasoconstrictor fibers, vasodilatation is present within the denervated area. Later, increased sensitivity to circulating catecholamines may develop in the vasculature, probably because of up-regulation of adrenoceptors.
Central Autonomic Dysregulation
Sympathetic denervation and denervation hypersensitivity cannot completely account for the vasomotor and sudomotor abnormalities in CRPS. First, in CRPS 1 there is no overt nerve lesion, and second, in CRPS 2 the autonomic symptoms spread beyond the territory of the lesioned nerve. In fact, there is direct evidence of reorganization of central autonomic control in these syndromes (Jänig and Baron 2002, 2003).
Hyperhidrosis, for example, is found in many patients with CRPS. Resting sweat output, as well as thermoregulatory and axon reflex sweating, is increased in CRPS 1 patients (Birklein et al 1997). The increased sweat production cannot be explained by a peripheral mechanism only since unlike blood vessels, denervation supersensitivity does not develop in sweat glands. However, the inflammatory release of calcitonin gene–related peptide (CGRP) has been shown to enhance sweat gland activation.
To study cutaneous sympathetic vasoconstrictor innervation in CRPS 1 patients we analyzed central sympathetic reflexes induced by thermoregulatory (whole-body warming, cooling) and respiratory stimuli (Baron and Maier 1996; Wasner et al 1999, 2001). Sympathetic effector organ function (i.e., skin temperature and skin blood flow) was measured bilaterally in the extremities by infrared thermometry and laser Doppler flowmetry. Under normal conditions these reflexes do not show between-side differences.
In CRPS patients three distinct vascular regulation patterns related to the duration of the disorder were identified:
These data support the idea that CRPS 1 is associated with pathological unilateral inhibition of cutaneous sympathetic vasoconstrictor neurons leading to a warmer affected limb in the acute stage (Baron and Maier 1996, Birklein et al 1998). The locus of pathophysiological changes underlying such disturbed reflex activity must be in the CNS. Additional changes in neurovascular transmission may induce disturbed neurovascular transmission with severe vasoconstriction and cold skin in chronic CRPS (Goldstein et al 2000, Haensch et al 2002). Accordingly, α-adrenoceptor density has been reported to be increased in skin biopsy specimens from patients with CRPS 1 (Drummond et al 1996). Furthermore, skin lactate was increased in CRPS patients, indicative of enhanced anaerobic glycolysis, probably as a result of vasoconstriction and chronic tissue hypoxia (Birklein et al 2000b, Koban et al 2003). The few microneurographic studies of small sympathetic nerve fascicles that have been performed thus far in patients with CRPS, however, have not confirmed the presence of reflex abnormalities; the average skin sympathetic activity (i.e., a combination of vasoconstrictor and sudomotor activity) was not different on the two sides (Casale and Elam 1992). Moreover, changes in the endothelium may contribute to the vasomotor symptoms and signs. Dysfunction leads to lower release of nitric oxide and thereby to vasoconstriction and has been shown in patients with cold-type CRPS 1. It is unknown whether these findings are primary or secondary.
Neurogenic Inflammation
Some of the clinical features of CRPS, particularly in its early phase, could be explained by an inflammatory process (van der Laan and Goris 1997, Ludwig and Baron 2004). Consistent with this idea, corticosteroids are often used successfully for acute CRPS (see Treatment, later). Neurogenic inflammation is caused by the release of inflammatory neuropeptides, such as CGRP and substance P, from sensory neuron terminals. Additionally, a nerve injury can sensitize nociceptive neurons and thereby start a vicious cycle of inflammation and pain.
Evidence is increasing that localized neurogenic inflammation is involved in the generation of reddening, acute edema, and vasodilatation. The mechanisms are thought to be impaired neuropeptide inactivation and increased receptor availability.
Scintigraphic investigations with radiolabeled immunoglobulins have demonstrated extensive plasma extravasation in patients with acute CRPS 1 (Oyen et al 1993). Analysis of joint fluid and synovial biopsy samples in CRPS patients has shown an increase in protein concentration and synovial hypervascularity. Furthermore, synovial effusion is enhanced in affected joints as measured with MRI.
In patients with acute untreated CRPS 1, neurogenic inflammation was elicited by strong transcutaneous electrical stimulation via intradermal microdialysis. Simultaneous assessment of protein extravasation with the microdialysis system revealed that the extravasation was provoked only on the affected extremity. Furthermore, axon reflex vasodilatation was increased significantly. The time course of electrically induced protein extravasation in patients resembled that observed following the application of exogenous substance P (Weber et al 2001). As further support of a neurogenic inflammatory process, systemic tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and IL-8 levels were found to be increased in acute CRPS but not in the chronic stages (Birklein et al 2001). In the fluid of artificially produced skin blisters and in venous blood, significantly higher levels of IL-6 and TNF-α were observed in the involved extremity than in the uninvolved extremity, a finding that changes only marginally in patients with chronic CRPS (Huygen et al 2002). Comparison of serum and systemic levels did show higher TNF-α in tissue, thus pointing to a localized inflammatory response. These findings become clinically relevant since the presence of or increase in pro-inflammatory cytokines is correlated with the presence of mechanical hyperalgesia as a marker of central sensitization (Maihofner et al 2005). Nonetheless, no specific prognostic cytokine profiles have been determined.
Recent studies have indicated a role of the immune system in CRPS. Antibodies against sympathetic and mesenteric neurons and adrenergic and muscarinergic receptors have been described in up to one-third of CRPS patients. Interestingly, injection of IgG antibodies from CRPS patients into mice produced symptoms that partially resembled CRPS. Since CRPS is clinically a localized syndrome, a generalized antibody-related autoimmune disorder seems to be unlikely thus far.
Hence, evidence indicates that inflammatory processes are involved in the pathogenesis of CRPS. One further question is whether the sympathetic nervous system may contribute to the early inflammatory state. De novo expression of adrenoreceptors on macrophages after experimental nerve lesions supports this idea. However, this concept has yet to be proved in patients with CRPS. Figure 67-3 illustrates the possible interactions between sympathetic fibers, afferent fibers, blood vessels, and non-neural cells related to the immune system (e.g., macrophages) that could theoretically lead to the inflammatory changes observed in CRPS patients.
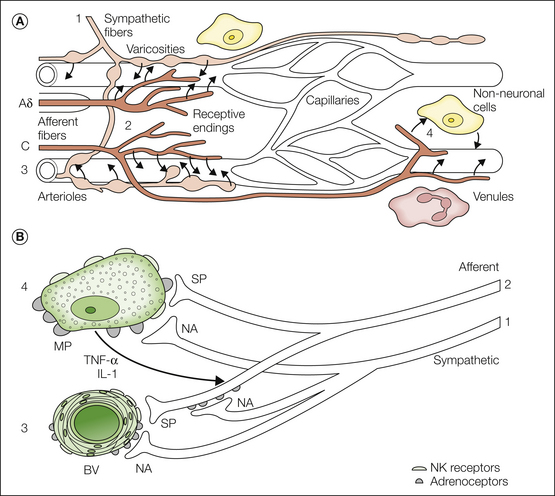
Figure 67-3 A, The micromilieu of nociceptors. The microenvironment of primary afferents is thought to affect the properties of the receptive endings of myelinated (A) and unmyelinated (C) afferent fibers. This has been documented in particular for inflammatory processes, but one may speculate that pathological changes in the direct surroundings of primary afferents may contribute to other pain states as well. The vascular bed consists of arterioles (directly innervated by sympathetic and afferent fibers), capillaries (not innervated and not influenced by nerve fibers), and venules (not directly innervated but influenced by nerve fibers). The micromilieu depends on several interacting components: neural activity in the post-ganglionic noradrenergic fibers (1) supplying blood vessels (3) and release of noradrenaline (NA) and possibly other substances that cause vasoconstriction. Excitation of primary afferents (Aδ and C fibers) (2) causes vasodilatation in precapillary arterioles and plasma extravasation in post-capillary venules (C fibers only) by the release of substance P (SP) and other vasoactive compounds (e.g., calcitonin gene–related peptide [CGRP]). Some of these effects may be mediated by non-neuronal cells such as mast cells and macrophages (4). Other factors that affect control of the microcirculation are the myogenic properties of arterioles (3) and more global environmental influences such as a change in temperature and metabolic state of the tissue. B, Hypothetical relationship between sympathetic noradrenergic nerve fibers (1), peptidergic afferent nerve fibers (2), blood vessels (BV) (3), and macrophages (MP) (4). The activated and sensitized afferent nerve fibers activate macrophages, possibly via release of SP. The immune cells start to release cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1), which further activate afferent fibers. SP (and CGRP) released from afferent nerve fibers reacts with neurokinin 1 (NK1) receptors in blood vessels (arteriolar vasodilatation, venular plasma extravasation, neurogenic inflammation). The sympathetic nerve fibers interact with this system on three levels: (1) via adrenoceptors (mainly α) on the blood vessels (vasoconstriction), (2) via adrenoceptors (mainly β) on macrophages (further release of cytokines), and (3) via adrenoceptors (mainly α) on afferent blood vessels (further sensitization of these fibers). (A, Modified from Jänig W, Koltzenburg M 1991 What is the interaction between the sympathetic terminal and the primary afferent fiber? In Basbaum AI, Besson JM [eds] Towards a new pharmacotherapy of pain. Dahlem Workshop Reports, Wiley, Chichester, pp 331–352; B, modified with permission from Jänig W, Baron R 2003 Complex regional pain syndrome: mystery explained? Lancet Neurology 2:687–697.)
Motor Abnormalities
Motor deficits are common findings in CRPS: about 50% of patients with CRPS have decreased active range of motion, an increased amplitude of physiological tremor, and reduced active motor force of the affected extremity. In about 10% of cases, dystonia of the affected hand or foot develops with a delayed onset. It is unlikely that these motor changes are related to a peripheral process (e.g., influence of the sympathetic nervous system on neuromuscular transmission and/or contractility of skeletal muscle). These somatomotor changes are more likely generated by changes in activity in the motor neurons and maladaptive central neuronal plasticity.
These hypotheses have been confirmed by several functional imaging and behavioral studies: kinematic analysis of target reaching, as well as grip force analysis, to quantitatively assess motor deficits in CRPS patients (Schattschneider et al 2001). These results pointed to abnormalities in cerebral motor processing. Pathological sensorimotor integration in the parietal cortex was found that may induce abnormal central programming and processing of motor tasks, as confirmed by fMRI. Interestingly, motor performance is also slightly impaired on the contralateral unaffected side (Ribbers et al 2002). These early findings have been substantiated by electrophysiological and imaging studies demonstrating widespread central disinhibition of motor processing circuits.
According to this view of central maladaptation, a neglect-like syndrome was clinically described as being responsible for disuse of the extremity and change in body perception (Galer et al 1995). Patients describe their affected limb as being larger, with a change in shape and posture; demonstrate impaired recognition of laterality; and often report an “alien limb,” which explains the occasional desire for limb amputation. However, the neglect-like syndrome in CRPS differs from the neglect after stroke since patients are usually able to report the altered limb perceptions. The complex pathomechanism is the result of an implicit mechanism of pain avoidance and altered central limb representation caused by functional and not structural changes.
In line with these concepts, emerging evidence supports an incongruence between central motor output and sensory input as an underlying mechanism in CRPS. As an example, in the method of mirror visual feedback the visual input from a moving unaffected limb to the brain was able to re-establish the pain-free relationship between sensory feedback and motor execution. After 6 weeks of therapy, pain and function were improved in comparison to the control group (McCabe et al 2003, Moseley 2004). Furthermore, combining laterality recognition training, imagination of movements, and mirror movements was efficacious in reducing pain and restoring function.
Sympathetically Maintained Pain
On the basis of experience and recent clinical studies, the term “sympathetically maintained pain” (SMP) was redefined. Neuropathic pain patients with similar clinical signs and symptoms can clearly be divided into two groups by the negative or positive effect of selective sympathetic blockade or antagonism of α-adrenoceptor mechanisms. The pain component that is relieved by specific sympatholytic procedures is considered to be SMP. Thus, SMP is now defined as a symptom or the underlying mechanism in a subset of patients with neuropathic disorders and not a clinical entity. The positive effect of sympathetic blockade is not essential for the diagnosis. On the other hand, the only way to differentiate between SMP and sympathetically independent pain (SIP) is the efficacy of a correctly applied sympatholytic intervention (Stanton-Hicks et al 1995).
Studies on Patients
Clinical studies of CRPS support the idea that catecholamine sensitivity develops in nociceptors (Baron et al 1999). Intraoperative stimulation of the sympathetic chain induces an increase in spontaneous pain in patients with causalgia (CRPS 2) but not in patients with hyperhidrosis. In CRPS 2 and post-traumatic neuralgias, intracutaneous injection of norepinephrine into a symptomatic area rekindles the spontaneous pain and dynamic mechanical hyperalgesia that had been relieved by sympathetic blockade, thus supporting the idea that noradrenergic sensitivity of human nociceptors is present after partial nerve lesions. Also, intradermal injection of norepinephrine, in physiologically relevant doses, was demonstrated to evoke greater pain in the affected regions of patients with SMP than in the contralateral unaffected limb and in control subjects (Ali et al 2000).
We performed a study of patients with CRPS 1 that involved the use of physiological stimuli of the sympathetic nervous system (Baron et al 2002c). Cutaneous sympathetic vasoconstrictor outflow to the painful extremity was experimentally activated to the highest possible physiological degree by whole-body cooling. During the thermal challenge the affected extremity was clamped at 35°C to avoid thermal effects at the nociceptor level. The intensity as well as the area of spontaneous pain and mechanical hyperalgesia (dynamic and punctate) increased significantly in patients who had been classified as having SMP by positive sympathetic blocks but not in patients with SIP (Fig. 67-4). The experimental setup used in the latter study selectively alters sympathetic cutaneous vasoconstrictor activity without influencing other sympathetic systems innervating the extremities (i.e., piloerector, sudomotor, and muscle vasoconstrictor neurons). Therefore, the interaction of sympathetic and afferent neurons measured here is likely to be located within the skin as predicted by the pain-enhancing effect of intracutaneous norepinephrine injections (Ali et al 2000). Interestingly, relief of spontaneous pain after sympathetic blockade was more pronounced than changes in spontaneous pain that could be induced experimentally by activation of the sympathetic system. One explanation for this discrepancy might be that a complete sympathetic block affects all sympathetic outflow channels projecting to the affected extremity. It is very likely that in addition to coupling in the skin, a sympathetic–afferent interaction may also occur in other tissues, especially in deep somatic domains such as bone, muscle, or joints. Supporting this view, these structures in particular are extremely painful in some patients with CRPS (Baron and Wasner 2001). Furthermore, there may be patients characterized by a selective or predominant sympathetic–afferent interaction in deep somatic tissues with sparing of the skin (Wasner et al 1999).
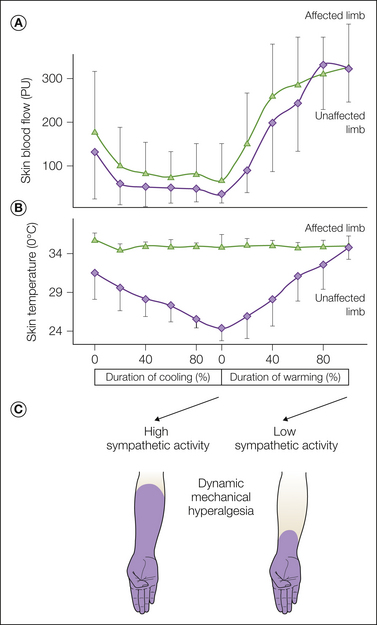
Figure 67-4 Experimental modulation of cutaneous sympathetic vasoconstrictor neurons by physiological thermoregulatory reflex stimuli in 13 patients with complex regional pain syndrome (CRPS).
With the help of a thermal suit, whole-body cooling and warming were performed to alter sympathetic skin nerve activity. The subjects were lying in a suit supplied by tubes in which running water at 12 and 50°C, respectively (inflow temperature), was used to cool or warm the whole body. By these means, sympathetic activity can be switched on and off. A, High sympathetic vasoconstrictor activity during cooling induces a considerable drop in skin blood flow in the affected and unaffected extremities (laser Doppler flowmetry). Measurements were taken at 5-minute intervals at the fingertips (PU, arbitrary perfusion units, mean + SD). B, On the unaffected side a secondary decrease in skin temperature was documented. On the affected side the forearm temperature was clamped at 35°C by a feedback-controlled heat lamp to exclude temperature effects on the sensory receptor level. Measurements were taken at 5-minute intervals (mean + SD). C, Effect of cutaneous sympathetic vasoconstrictor activity on dynamic mechanical hyperalgesia in one CRPS patient with sympathetically maintained pain. Activation of sympathetic neurons (during cooling) leads to a considerable increase in the area of dynamic mechanical hyperalgesia. (Reprinted from Baron R, Schattschneider J, Binder A, et al 2002c Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet 359:1655–1660, with permission from Elsevier. Copyright 2002, Elsevier Ltd.)
Summary of Pathophysiological Mechanisms
In light of the numerous novel studies on the pathophysiological mechanisms in CRPS, we have to recognize that important elements of the pathophysiology of CRPS are obviously located within the CNS and it can therefore be described as a neurological disease (Fig. 67-5) that encompasses the autonomic, sensory, and motor systems, as well as the cortical areas involved in the processing of cognitive and affective information. In addition to these neural abnormalities, the peripheral neuronal inflammatory component appears to be particularly important in the acute phase of the disease.
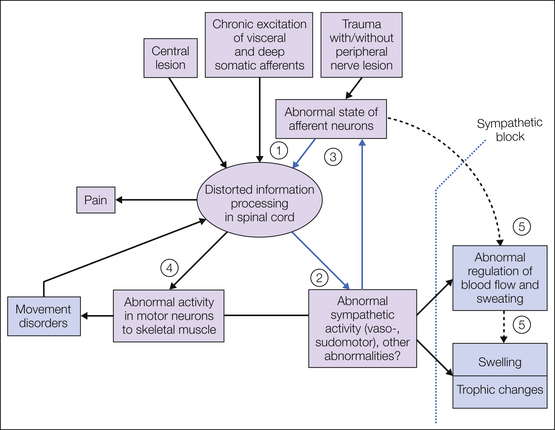
Figure 67-5 Hypothesis about the neural mechanisms of the generation of complex regional pain syndrome (CRPS) types 1 and 2 following peripheral trauma with and without nerve lesions, chronic stimulation of visceral afferents (e.g., myocardial infarction) and deep somatic afferents, and rarely, central trauma.
The clinical findings are summarized in blue boxes. Note the vicious circle (blue arrows). An important component of this circle is the excitatory influence of post-ganglionic sympathetic axons on primary afferent fibers in the periphery. The numbers indicate changes in the activity of peripheral neurons, which have not been measured directly in patients with CRPS but have been postulated on the basis of measurements of effector responses of somatic sensations (including pain): 1, afferent traumatized neurons; 2, sympathetic neurons; 3, sympathetic–afferent coupling; 4, motor neurons; 5 (dashed arrow; hypothetical mechanism), “antidromically” conducted activity in peptidergic afferent C fibers leading to an increase in blood flow (arteriolar vasodilatation) and venular plasma extravasation, both hypothetically contributing to the increase in blood flow and swelling. The dotted line indicates the effect of sympathectomy or sympathetic blocks.
Diagnostic Procedure
The definition of standardized diagnostic criteria for CRPS in 1994 was a major advance in the classification of regional pain disorders associated with vasomotor or sudomotor abnormalities (Stanton-Hicks et al 1995). Based on these criteria, clinical research on mechanisms was performed on a much more homogeneous group of patients and was therefore comparable for the first time. However, these criteria were derived from the consensus opinion of a small group of expert clinicians. Although this was an appropriate first step, it is important to continuously improve the criteria, that is, to validate and, if necessary, modify these initial consensus-based criteria on the basis of results of systematic validation research. Both internal and external validation research suggested that CRPS was overdiagnosed (Bruehl et al 1999, Harden et al 1999). Possibly because of this drawback the new terminology did not replace the former denominations immediately. The inclusion of motor and trophic signs and symptoms, for example, improved specificity to 85% without losing sensitivity (Bruehl et al 2002).
In 2007 the current diagnostic criteria were proposed (“Budapest criteria”), including a set for clinical and a set for research purposes. No less than one of at least three categories and at least one sign of a minimum of two categories must be present. A validation study in 2010 revealed an improved sensitivity of 99% and a specificity of 68%–79%. Clinically and for scientific purposes, one has to realize that use of the different diagnostic criteria leads to high variability in diagnosing CRPS.
In 2010 a severity score for CRPS (CSS) was developed based on assessment of self-reported symptoms and clinical signs. The maximum score of 17 indicates most severe CRPS. The CSS was found to be associated with the dichotomous diagnostic criteria and the burden of disease. The “Budapest criteria” for CRPS can be found in Box 67-1.
Diagnostic Tests That Aid in the Diagnosis of CRPS
For the present, the diagnosis of CRPS is based on the clinical criteria described earlier. However, several tests and procedures are valuable diagnostic tools that can add information to confirm the diagnostic impression about autonomic, sensory, and motor function and dysfunction.
Bone Scintigraphy
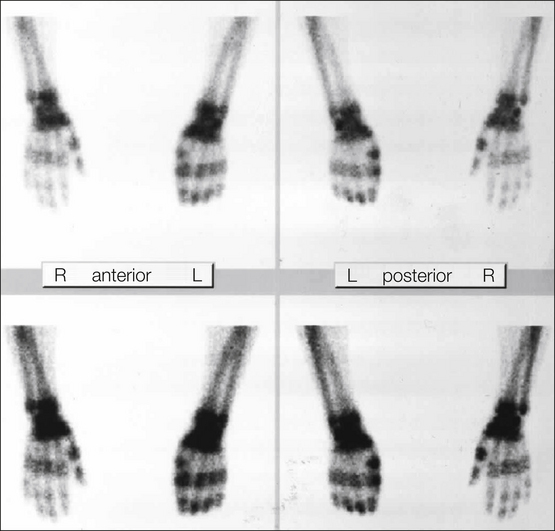
Osseous changes are common in CRPS. Thus, three-phase bone scintigraphy can provide valuable information. Homogeneous unilateral hyperperfusion in the perfusion (30 seconds after injection) and blood pool phases (2 minutes after injection) is characteristic and will help exclude differential diagnoses (e.g., osteoporosis secondary to inactivity). Three hours after injection the mineralization phase will show increased unilateral periarticular tracer uptake (Fig. 67-6). Pathological uptake in the metacarpophalangeal or metacarpal bones is thought to be sensitive and specific. However, a “gold standard” to compare with is, as yet, unknown, but it is useful to rule out pain syndromes of other origin. It should be noted that the incidence of significant changes on bone scintigraphy decreases with disease duration. However, a negative result does not exclude CRPS. Accordingly, specificity is high (75–100%) but sensitivity is low (31–69%), with inter-rater reliability of 0.56.
Plain Radiography and X-ray Bone Densitometry
Endosteal and intracortical excavation, subperiosteal and trabecular bone resorption, spotty and localized bone demineralization, and osteoporosis have been thought to be specific signs of CRPS, but these signs are positive only in chronic stages. A comparison of radiography and three-phase scintigraphy in early post-fracture CRPS showed lower sensitivity and specificity of radiography. MRI is suggested as being more reliable than radiographic examination and probably scintigraphy, but its value needs to be proved in further studies.
Quantitative Sensory Testing
QST can provide information about the sensory symptom profile (function or dysfunction of unmyelinated and myelinated afferent fibers) via psychophysical testing of thermal, pain, and vibratory thresholds. However, no sensory profile is characteristic of CRPS.
Autonomic testing with the quantitative sudomotor axon reflex test (QSART) can provide information about the function of sudomotor reflex loops. Swelling can be quantified by measuring water displacement. Autonomic vascular function can be tested by laser Doppler flowmetry (Birklein et al 1998, Wasner et al 1999, 2001) and infrared thermography.
Skin Temperature Measurements
Skin temperature measurements are an easily performed indication of vascular function and may be particularly helpful in diagnosing CRPS. We performed a study using controlled thermoregulation (whole-body warming, cooling) to change cutaneous sympathetic vasoconstrictor activity (Wasner et al 2002). Skin temperature in the affected and unaffected limbs (infrared thermometry) was measured under resting conditions (before temperature challenge in the office at room temperature) and continuously monitored during controlled modulation of sympathetic activity. Only minor skin temperature asymmetries were present between both limbs under resting conditions in most patients. However, during controlled thermoregulation, differences in temperature between both sides increased dynamically and were most prominent at a high to medium level of vasoconstrictor activity. In patients suffering from painful limbs of other origin and in healthy volunteers (control groups), there were only minor between-side differences in temperature both at rest and during thermoregulatory changes in sympathetic activity. When comparing the diagnostic value of skin temperature asymmetry in CRPS, sensitivity was just 32% under resting conditions but increased up to 76% during controlled alteration of sympathetic activity. Specificity was 100% at rest and 93% during controlled thermoregulation (Fig. 67-7). A new sum score derived from long-term temperature measurements reached a sensitivity and specificity of about 70%.
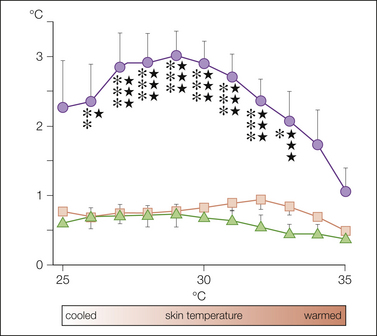
Figure 67-7 Average absolute between-side differences in the skin temperature of the fingers (or toes) of both hands (or feet, as appropriate for the affected extremity) in 25 patients with complex regional pain syndrome (CRPS) (•), in 20 healthy controls (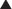 ), and in 15 control patients with extremity pain of other origin (
), and in 15 control patients with extremity pain of other origin ( ) during a controlled thermoregulatory cycle (controlled alteration in cutaneous sympathetic activity).
) during a controlled thermoregulatory cycle (controlled alteration in cutaneous sympathetic activity).
The level of overall cutaneous sympathetic vasoconstrictor activity was estimated indirectly by using the skin temperature on the unaffected side (or the right side in healthy controls) as a reference value. A skin temperature on the healthy side of 25°C indicates a high level, a temperature of 30°C an intermediate level, and a temperature of 35°C complete inhibition of sympathetic vasoconstrictor activity in the skin (mean ± SEM). The findings in patients with CRPS versus healthy controls are significantly different from both healthy controls or patients with extremity pain of other origin (*): CRPS versus control patients with extremity pain of other origin ( ): one symbol, P < 0.05; two symbols, P < 0.01; three symbols, P < 0.001. (Reproduced from Wasner G, Schattschneider J, Heckmann K, et al 2001 Vascular abnormalities in reflex sympathetic dystrophy [CRPS I]: mechanisms and diagnostic value. Brain 124:587–599, with permission from Oxford University Press.)
): one symbol, P < 0.05; two symbols, P < 0.01; three symbols, P < 0.001. (Reproduced from Wasner G, Schattschneider J, Heckmann K, et al 2001 Vascular abnormalities in reflex sympathetic dystrophy [CRPS I]: mechanisms and diagnostic value. Brain 124:587–599, with permission from Oxford University Press.)
In conclusion, the degree of unilateral vascular disturbances in CRPS and between-side differences in temperature depend critically on the environmental temperature and spontaneous sympathetic activity. However, the maximal skin temperature difference that occurs during the thermoregulatory cycle distinguishes CRPS from other extremity pain syndromes with high sensitivity and specificity.
Differential Diagnosis
Because of the lack of a gold standard for the diagnosis of CRPS, the risk for overdiagnosis has to be taken into account. To differentiate CRPS from other neuropathic and pain syndromes, a detailed history and physical examination conducted according to the specifications outlined previously are mandatory.
Post-traumatic Neuralgia
It is important to recognize that many patients with post-traumatic neuropathy have pain but do not have the full clinical picture of causalgia (CRPS 2). In these cases, in contrast to patients with causalgia, the pain is located largely within the innervation territory of the injured nerve. Although these patients often describe their pain as burning, they exhibit a less complex clinical picture than do patients with causalgia and do not have marked swelling or progressive spread of symptoms. The cardinal symptoms are spontaneous burning pain, hyperalgesia, and mechanical and especially cold allodynia. These sensory symptoms are confined to the territory of the affected peripheral nerve, although allodynia may extend beyond the border of nerve territories for some centimeters. Spontaneous and evoked pain is felt superficially and not deep inside the extremity, and the intensity of both is not dependent on the position of the extremity. Patients occasionally obtain relief with sympatholytic procedures, though much less often than do those with CRPS.
According to the classification of the International Association for the Study of Pain it is possible to use the term “neuralgia” for this type of neuropathic pain (pain within the innervation territory of a lesioned nerve, e.g., post-traumatic neuralgia). However, the new definition of CRPS 2 includes the statement that symptoms may also be limited to the territory of a single peripheral nerve. Therefore, the term CRPS 2 provides a window to include these localized post-traumatic neuropathies. An inherent weakness of this definition of CRPS 2 is that different syndromes with different underlying mechanisms are obviously included.
Neuropathies (e.g., diabetic polyneuropathy) may also be manifested as spontaneous pain, changes in skin color, and motor deficits but are distinguished by their symmetrical distribution and the patient’s history. Furthermore, all kinds of inflammation or infection (e.g., rheumatism, phlegmon) might induce intense unilateral skin warming. Unilateral arterial or venous occlusive diseases can cause unilateral pain and vascular abnormalities and have to be excluded. Repetitive artificial occlusion of the blood supply to one limb (as in the psychiatric factitious disorders, artifact syndrome) might induce secondary structural changes in blood vessels with consecutive abnormalities in perfusion and therefore mimic CRPS symptoms and signs.
Therapy for CRPS
Lack of understanding of the underlying pathophysiological abnormalities and lack of objective diagnostic criteria result in inherent difficulties in conducting clinical trials with therapeutic modalities. Therefore, only limited evidence-based treatment regimens for CRPS are available thus far; these regimens are summarized in Table 67-2. In fact, literature reviews of outcome studies found discouragingly little consistent information on pharmacological agents and methods of treating CRPS (Kingery 1997, Forouzanfar et al 2002). Moreover, the methodology of the studies available is often of low quality. In the absence of more specific information on the pathophysiological mechanisms and treatment of CRPS one has to rely on outcomes from treatment studies involving other neuropathic pain syndromes. Furthermore, the still hypothetical mechanism-based treatment concept has to be transferred from ideas derived from animal experiments on peripheral nerve lesions to the situation in CRPS patients.
Table 67-2
Pharmacological Treatment of Complex Regional Pain Syndromes (Randomized Controlled Trials)
CPRS, complex regional pain syndrome; DMSO, dimethylsulfoxide; IT, intrathecal; IV, intravenous; NMDA, N-methyl-D-aspartate.
Adapted from Baron R 2011 Complex regional pain syndromes. In: Waldman S (ed) Pain management. Philadelphia, Elsevier.
Pharmacological Therapy
Non-steroidal Anti-inflammatory Agents
Nonsteroidal anti-inflammatory drugs (NSAIDs) have not been investigated in placebo-controlled trials. However, from clinical experience they can control mild to moderate pain.
Opioids
Opioids are clearly effective for postoperative, inflammatory, and cancer pain. The use of opioids for CRPS has not been studied. In other neuropathic pain syndromes, compounds such as tramadol, morphine, oxycodone, and levorphanol are clearly analgesic when compared with placebo. However, there are no long-term studies of oral opioid use for the treatment of neuropathic pain, CRPS included. Even without solid scientific evidence, the expert opinion of pain clinicians is that opioids could and should be used as a part of a comprehensive pain treatment program. Given that some patients with neuropathic pain may obtain considerable pain relief, opioids should be prescribed immediately if other agents do not achieve sufficient analgesia.
Antidepressants
Tricyclic antidepressants have been studied intensely for different neuropathic pain conditions, but not for CRPS. There is solid evidence that reuptake blockers of serotonin and noradrenaline (e.g., amitriptyline, duloxetine) and selective noradrenaline blockers (e.g., desipramine) produce pain relief in patients with diabetic and post-herpetic neuropathy. The effectiveness of selective serotonin reuptake inhibitors in neuropathic pain states is still being discussed. No controlled trial has been performed on CRPS patients.
Sodium Channel–Blocking Agents
Lidocaine (lignocaine) administered intravenously was effective in a small trial of patients with CRPS 1 and 2 in relieving spontaneous and evoked pain (Wallace et al 2000). Carbamazepine or other sodium channel–acting anticonvulsants have not been tested in CRPS.
γ-Aminobutyric Acid Agonists
Intrathecally administered baclofen is effective in the treatment of dystonia in CRPS (van Hilten et al 2000a). Oral baclofen has been effective in treating trigeminal neuralgia. No further trials in CRPS are available, and there is no evidence of an analgesic effect of baclofen, valproic acid, vigabatrin, and benzodiazepines in CRPS or other neuropathic pain conditions.
Gabapentin and Pregabalin
Promising preliminary evidence of an analgesic effect of gabapentin was revealed in early studies on patients with CRPS (Mellick and Mellick 1995, Serpell 2002, van de Vusse et al 2004). Gabapentin and pregabalin are effective in painful diabetic neuropathy and post-herpetic neuralgia.
Steroids and Immunoglobulins
Orally administered prednisone, 30–40 mg daily, has clearly demonstrated efficacy in improving the entire clinical status (up to 75%) of patients with acute CRPS (<13 weeks) (Christensen et al 1982) and in relieving CRPS in stroke patients. A single 60-mg intrathecal methylprednisolone bolus was not effective.
A preliminary trial of immunoglobulin administration, 0.5 g/kg intravenously (IV) for two consecutive days (0.25 g/kg per day), indicated analgesic efficacy (Goebel et al 2010), but further confirmation is needed. No evidence has been obtained with other immune-modulating therapies, such as immunosuppressive drugs.
N-Methyl-D-Aspartate Receptor Blockers
Clinically available compounds that are demonstrated to have N-methyl-D-aspartate receptor–blocking properties include ketamine, dextromethorphan, and memantine.
Two small placebo-controlled trials using intravenous ketamine and one controlled trial using topical ketamine for the treatment of allodynia proved their analgesic efficacy in CRPS 1 patients. However, larger controlled trials are needed to confirm these promising results.
Interestingly, an fMRI study has shown favorable analgesia with a ketamine–morphine combination, along with restoration of somatosensory processing.
Calcium-Regulating Drugs
Calcitonin administered three times daily intranasally provided significant pain reduction in CRPS patients (Gobelet et al 1992). Clodronate, 300 mg daily IV, alendronate, 7.5 mg daily IV or 40 mg orally, and alendronate, 60 mg IV, produced significant improvement in pain and range of movement in acute CRPS (Adami et al 1997, Varenna et al 2000, Robinson et al 2004). The proposed mode of action of CRPS-related bone resorption or its direct analgesic effects are thus far of unknown mechanism. However, other controlled trials showed negative results (Bickerstaff et al 1991, Gobelet et al 1992), and meta-analyses provided conflicting results on the efficacy of calcitonin (Perez et al 2001, Forouzanfar et al 2002).
Free Radical Scavengers
A placebo-controlled trial was performed with the free radical scavengers dimethylsulfoxide (DMSO) 50% topically or N-acetylcysteine (NAC) orally for the treatment of CRPS 1 (Perez et al 2003). Both drugs were found to be equally effective; however, DMSO seemed to be more favorable for “warm” and NAC for “cold” CRPS 1. The results were negatively influenced by a longer disease duration. A previous trial with DMSO showed a small effect in CRPS (Zuurmond et al 1996); however, DMSO has been shown to be more effective than regional blocks with guanethidine in a small population of CRPS patients (Geertzen et al 1994).
α2-Adrenoceptor Agonist
Transdermal application of the α2-adrenoceptor agonist clonidine, which is thought to prevent the release of catecholamines by a presynaptic action, may be helpful when small areas of hyperalgesia are present (Davis et al 1991).
Interventional Therapy at the Sympathetic Nervous System Level
Currently, two therapeutic techniques for blocking sympathetic activity are used:
Many uncontrolled surveys in the literature have reviewed the effect of sympathetic interventions in CRPS. About 70% of patients with CRPS report a full or partial response (Cepeda et al 2002). The efficacy of these procedures, however, is still controversial and has been questioned in the past (Kingery 1997, Schott 1998). In fact, the specificity and the long-term results, as well as the techniques used, have rarely been evaluated adequately.
Sympathetic Ganglion Blocks
One controlled study in patients with CRPS 1 has shown that sympathetic ganglion blocks with local anesthetic have the same immediate effect on pain as does a control injection with saline (Price et al 1998). However, after 24 hours, patients in the local anesthetic group were much better, thus indicating that non-specific effects are important initially and that evaluating the efficacy of sympatholytic interventions is best done after 24 hours. With these data in mind, the uncontrolled studies mentioned earlier must be interpreted cautiously. Only 10 of the 24 studies that we reviewed assessed long-term effects.
Intravenous Regional Sympatholysis—Open Studies
No improvement in comparison to baseline was found for reserpine (IVRS) and guanethidine (IVRS) (Jadad et al 1995). No differences were found between guanethidine (IVRS) or lidocaine (lignocaine) (IVRS) (Ramamurthy and Hoffman 1995). Guanethidine and pilocarpine showed no differences from placebo after the application of four blocks (Livingstone and Atkins 2002). However, stellate blocks with bupivacaine, as well as regional blocks with guanethidine (IVRS), demonstrated a significant improvement in pain when compared with baseline but no differences between these two therapies (Bonelli et al 1983). One study demonstrated that IVRS with bretylium and lidocaine (lignocaine) produces significantly longer pain relief than does lidocaine (lignocaine) alone (Hord et al 1992). No effect was obtained with droperidol (IVRS) (Kettler and Abram 1988). Hanna and Peat (1989) demonstrated significant improvement in pain with a single (IVRS) bolus of ketanserin. Bounameaux and colleagues (1984) failed to show any significant effect with the same procedure.
There is a desperate need for controlled studies that assess the acute as well as the long-term effect of sympathetic blockade and IVRS on pain and other CRPS symptoms—in particular, motor function. Well-performed sympathetic ganglion blocks should be chosen rather than IVRS (Hord and Oaklander 2003).
Surgical Sympathectomy
Only limited evidence is available on the efficacy of thoracoscopic or surgical sympathectomy, and a meta-analysis could not draw a final conclusion. Four open studies reported partly long-lasting benefits in patients with CRPS 1 and 2 (AbuRahma et al 1994, Schwartzman et al 1997, Bandyk et al 2002, Singh et al 2003). The most important independent factor in determining a positive outcome of sympathectomy is a time interval of less than 12 months, with the highest efficacy seen within 3 months, between the inciting event and sympathectomy (AbuRahma et al 1994, Schwartzman et al 1997). Videoscopic lumbar sympathectomy is as effective as the open surgical intervention (Lacroix et al 1996).
We investigated skin blood flow, sympathetic vasoconstrictor reflexes, and pain after surgical sympathectomy in a small cohort of CRPS patients (Baron and Maier 1996). Postoperatively, no vasoconstriction with deep inspiration (vasoconstrictor reflex) could be elicited in the affected extremity, thus indicating complete sympathetic denervation. Additionally, the skin temperature in the affected hand increased. After 4 weeks, skin temperature decreased without signs of reinnervation. This denervation supersensitivity was associated with recurrence of the pain and is thought to rely on vascular supersensitivity to cold and circulating catecholamines. Only 2 of 12 patients experienced long-term pain relief.
Irreversible sympathectomy may be effective in selected cases. However, because of risk for the development of adaptive supersensitivity, even on nociceptive neurons, and the resulting increase and prolongation of pain, these procedures should not be recommended on a broad indication basis.
Stimulation Techniques and Spinal Drug Application
Transcutaneous electrical nerve stimulation (TENS) may be effective in some cases and has minimal side effects. Epidural spinal cord stimulation has shown efficacy in one randomized study in selected chronic CRPS patients (Kemler et al 2000a). Interestingly, these patients had previously undergone unsuccessful surgical sympathectomy. The pain-relieving effect was not associated with peripheral vasodilatation, thus suggesting that central disinhibition processes are involved. Sensory detection thresholds were not affected by the stimulation, but mechanical allodynia was detected and predicted a negative outcome in patients with chronic CRPS (Kemler et al 2000b). Other stimulation techniques, such as peripheral nerve stimulation with implanted electrodes, repetitive transcranial magnetic stimulation, and deep brain stimulation (sensory thalamus and medial lemniscus, motor cortex), have been reported to be effective in selected cases of CRPS (Hassenbusch et al 1996, Son et al 2003, Pleger et al 2004a).
In selected patients with severe refractory CRPS, epidural application of clonidine resulted in greater pain reduction with higher dosages (700 μg) than with lower dosages (300 μg) (Rauck et al 1993). However, the drug was associated with marked side effects (e.g., sedation and hypotension). Intrathecally administered baclofen is effective in the treatment of dystonia in patients with CRPS (van Hilten et al 2000a).
Physical and Occupational Therapy
Physical and occupational therapy is recommended in patients with CRPS. It should be stressed that clinical experience clearly indicates that physiotherapy is of utmost importance to achieve recovery of function and rehabilitation. Standardized physiotherapy has produced long-term relief of pain and physical dysfunction in children (Sherry et al 1999).
Physical therapy and, to a lesser extent, occupational therapy are able to reduce pain and improve active mobility in CRPS 1 (Oerlemans et al 2000). Lymph drainage provides no benefit when applied together with physiotherapy versus physiotherapy alone (Uher et al 2000). Patients with initially less pain and better motor function are predicted to benefit to a greater degree than others (Kemler et al 2001). Physical therapy for CRPS is both more effective and less costly than either occupational therapy or control treatment (Severens et al 1999).
Mirror visual feedback treatment of patients with CRPS 1 has been shown to reduce pain and improve function. Recent studies have demonstrated that the combination of hand laterality recognition training, imagination of movements, and mirror movements reduces pain and disability in CRPS patients. Using a mirror image of the unaffected limb also enhanced the recovery of tactile acuity. It is important that the order of training—laterality recognition, movement imagination, mirror movements—be followed for success in treatment.
Thus, physical and occupational therapy and attentional training have become important elements for successful therapy in patients with CRPS.
Psychological Therapy
Although there is evidence of a psychological impact on CRPS patients, only one study has addressed the efficacy of psychological treatment. A prospective, randomized, single-blind trial of cognitive–behavioral treatment was conducted together with physical therapy of different intensities in children and adults and showed a long-lasting reduction in all symptoms in both arms (Lee et al 2002).
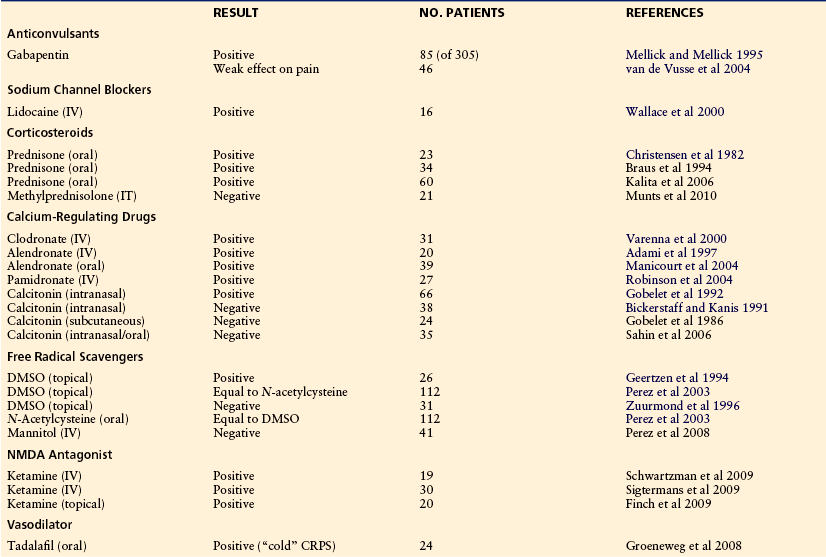
Proposed Treatment Algorithm (Fig. 67-8)
Since the evidence is limited, treatment remains widely empirical. Treatment should be immediate and most importantly be directed toward restoration of full function of the extremity. This objective is best attained in a comprehensive interdisciplinary setting with particular emphasis on pain management and restoration of function (Stanton-Hicks et al 1998, 2002). The pain specialists should include neurologists, anesthesiologists, orthopedic surgeons, physiotherapists, psychologists, and the general practitioner.
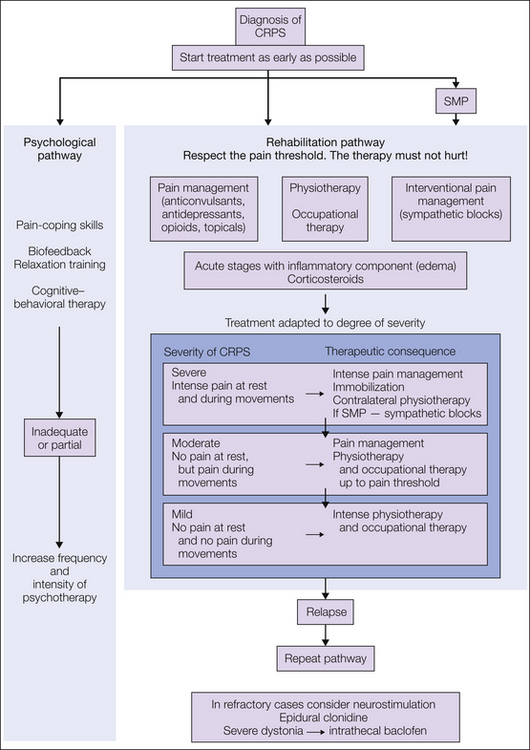
Figure 67-8 Treatment algorithm.
1. At the acute stage of complex regional pain syndrome (CRPS) with severe pain at rest, immobilization and careful contralateral physical therapy should be the treatment of choice. Sympatholytic procedures, preferably sympathetic ganglion blocks, should identify the component of the pain that is maintained by the sympathetic nervous system. 2. If the resting pain subsides, physical therapy should be performed in conjunction with sensory desensitization programs and pain therapy. 3. If the movement-induced pain subsides, physiotherapy and occupational therapy should be intensified. SMP, sympathetically maintained pain. (Modified from Baron R, Binder A, Ulrich W, et al 2002a [Complex regional pain syndrome. Reflex sympathetic dystrophy and causalgia.] Nervenarzt 73:305–318; Stanton-Hicks M, Burton AW, Bruehl SP, et al 2002 An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Practice 2:1–16.)
The severity of the disease determines the therapeutic regimen. Reduction of pain is the precondition to which all other interventions have to comply. All therapeutic approaches must not hurt. At the acute stage of CRPS when the patient still suffers from severe pain at rest and during movement, it is mostly impossible to carry out intensive active therapy. Painful interventions and, in particular, aggressive physical therapy at this stage often lead to deterioration. Therefore, immobilization and careful contralateral physical therapy should be the acute treatment of choice, and intense pain treatment should be initiated immediately. First-line analgesics and co-analgesics are opioids, tricyclic antidepressants, gabapentin, pregabalin, and carbamazepine. Additionally, corticosteroids should be considered if inflammatory signs and symptoms are predominant. Sympatholytic procedures, preferably sympathetic ganglion blocks, should identify the component of the pain that is maintained by the sympathetic nervous system. If effective, a series should be perpetuated.
Calcium-regulating agents should be used in cases of refractory pain. If the resting pain subsides, first passive physical therapy and then later active isometric followed by active isotonic training should be performed in combination with sensory desensitization programs until restitution of motor function. Psychological treatment has to flank the regimen to strengthen coping strategies and discover contributing factors. In refractory cases, spinal cord stimulation and epidural clonidine could be considered. If refractory dystonia develops, intrathecal baclofen application is worth considering.
CRPS in Children
CRPS types 1 and 2 also occur in children. The diagnosis seems to more often be delayed than in adults since less pronounced neurological and sympathetic symptoms are present. The incidence increases with puberty, and females are predominantly affected at a ratio of 4:1. In contrast to adults, the lower limb is affected more often (5.3:1). The mean age was 12.5 years in a cohort of 396 children.
Significant emotional dysfunction was demonstrated in a small number of children with CRPS. A possible association of intensive, parental-forced sports and leisure activities with occurrence of the inciting trauma was discussed. A sign of escape from parents’ excessive demands was hypothesized. Accordingly, a recent study identified higher risk for somatic symptoms and emotional distress in children with CRPS.
Diagnostic bone scintigraphy in children seems to be of minor value in comparison to adults because it shows higher variability and, interestingly, often decreased diffuse uptake. To minimize exposure to radiation, it should not be performed in children on a routine basis.
Limited attention has been paid to differences in response to therapy in children. Thus far, conservative strategies such as TENS and cognitive and behavioral pain management, as well as physical therapy, are effective in treating childhood CRPS and preventing prolongation of symptoms. Between 50 and 90% of children with CRPS 1 showed good long-term resolution of all symptoms (Lee et al 2002). In 2009 a retrospective follow-up study demonstrated that the course of the disease was comparable to that in adults.
Prevention Studies
Only two reliable randomized, placebo-controlled prevention studies have been conducted to date. Preoperatively administered guanethidine (20 mg, IVRS (intravenous regional sympatholysis)) did not prevent CRPS in patients undergoing fasciotomy for Dupuytren’s disease (Gschwind et al 1995).
Prognosis
The duration of disease is variable and it may persist for decades. In rare cases, a causal therapy (e.g., decompression of an entrapment syndrome) may lead to complete recovery. A 5.5-year follow-up study showed that 62% of patients were still limited in their activities of daily living, with pain and motor impairment being the most important factors (Geertzen et al 1994). In more than 60% of patients with CRPS 2 the complaints remained unchanged even after 1 year of intensive therapy (Karstetter and Sherman 1991). In contrast, a retrospective study reported resolution of symptoms in up to 30–74% of patients with CRPS (Sandroni et al 2003). In a 13-month follow-up study in a small cohort, nearly all patients still suffered from functional impairment of the affected extremity, although most of the other clinical features of CRPS had resolved (Zyluk 1998). Spontaneous remission of CRPS seems to be rare.
The severity rather than the etiology seems to determine the course of the disease. Age, sex, and affected side are not associated with the outcome (Sandroni et al 2003), but females have higher risk for the development of severe CRPS (van der Laan 1998). Fractures may be associated with a higher resolution rate (91%) than sprain (78%) or other inciting events (55%) (Sandroni et al 2003). In contrast, spontaneous CRPS has been linked to a longer duration of disease.
In 1183 patients (Veldman and Goris 1996) the incidence of recurrence was 1.8% per year. Patients with recurrent CRPS were significantly younger but did not differ in gender or primary localization. Recurrence of CRPS is more often manifested by few symptoms and signs and by spontaneous onset. A low skin temperature at the onset of the disease may predict an unfavorable course and outcome (Veldman et al 1993, van der Laan 1998). A retrospective analysis of 1006 CRPS cases showed the incidence of severe complications to be about 7%. Such complications consisted of infection, ulceration, chronic edema, dystonia, and/or myoclonus. Mostly female and younger patients with CRPS of the lower limb were affected (van der Laan et al 1998).
Acknowledgments
The author’s work was supported by the German Ministry of Research and Education, German Research Network on Neuropathic Pain (BMBF, 01EM05/04).
The references for this chapter can be found at www.expertconsult.com.
References
AbuRahma A.F., Robinson P.A., Powell M., et al. Sympathectomy for reflex sympathetic dystrophy: factors affecting outcome. Annals of Vascular Surgery. 1994;8:372–379.
Adami S., Fossaluzza V., Gatti D., et al. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Annals of the Rheumatic Diseases. 1997;56:201–204.
Ali Z., Raja S.N., Wesselmann U., et al. Intradermal injection of norepinephrine evokes pain in patients with sympathetically maintained pain. Pain. 2000;88:161–168.
Allen G., Galer B.S., Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999;80:539–544.
Bandyk D.F., Johnson B.L., Kirkpatrick A.F., et al. Surgical sympathectomy for reflex sympathetic dystrophy syndromes. Journal of Vascular Surgery. 2002;35:269–277.
Baron R., Binder A., Ulrich W., et al. Complex regional pain syndrome. Reflex sympathetic dystrophy and causalgia. Nervenarzt. 2002;73:305–318.
Baron R., Fields H.L., Jänig W., et al. National Institutes of Health Workshop: reflex sympathetic dystrophy/complex regional pain syndromes—state-of-the-science. Anesthesia and Analgesia. 2002;95:1812–1816.
Baron R., Levine J.D., Fields H.L. Causalgia and reflex sympathetic dystrophy: does the sympathetic nervous system contribute to the generation of pain? Muscle & Nerve. 1999;22:678–695.
Baron R., Maier C. Reflex sympathetic dystrophy: skin blood flow, sympathetic vasoconstrictor reflexes and pain before and after surgical sympathectomy. Pain. 1996;67:317–326.
Baron R., Schattschneider J., Binder A., et al. Relation between sympathetic vasoconstrictor activity and pain and hyperalgesia in complex regional pain syndromes: a case-control study. Lancet. 2002;359:1655–1660.
Baron R., Wasner G. Complex regional pain syndromes. Current Pain and Headache Reports. 2001;5:114–123.
Bickerstaff D.R., Kanis J.A. The use of nasal calcitonin in the treatment of post-traumatic algodystrophy. British Journal of Rheumatology. 1991;30:291–294.
Birklein F., Riedl B., Neundörfer B., et al. Sympathetic vasoconstrictor reflex pattern in patients with complex regional pain syndrome. Pain. 1998;75:93–100.
Birklein F., Riedl B., Sieweke N., et al. Neurological findings in complex regional pain syndromes—analysis of 145 cases. Acta Neurologica Scandinavica. 2000;101:262–269.
Birklein F., Sittle R., Spitzer A., et al. Sudomotor function in sympathetic reflex dystrophy. Pain. 1997;69:49–54.
Birklein F., Schmelz M., Schifter S., et al. The important role of neuropeptides in complex regional pain syndrome. Neurology. 2001;57:2179–2184.
Birklein F., Weber M., Neundorfer B. Increased skin lactate in complex regional pain syndrome: evidence for tissue hypoxia? Neurology. 2000;55:1213–1215.
Bonelli S., Conoscente F., Movilia P.G., et al. Regional intravenous guanethidine vs. stellate ganglion block in reflex sympathetic dystrophies: a randomized trial. Pain. 1983;16:297–307.
Bounameaux H.M., Hellemans H., Verhaeghe R. Ketanserin in chronic sympathetic dystrophy. An acute controlled trial. Clinical Rheumatology. 1984;3:556–557.
Bruehl S., Harden R.N., Galer B.S., et al. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. Pain. 1999;81:147–154.
Bruehl S., Harden R.N., Galer B.S., et al. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95:119–124.
Bruehl S., Husfeldt B., Lubenow T.R., et al. Psychological differences between reflex sympathetic dystrophy and non-RSD chronic pain patients. Pain. 1996;67:107–114.
Casale R., Elam M. Normal sympathetic nerve activity in a reflex sympathetic dystrophy with marked skin vasoconstriction. Journal of the Autonomic Nervous System. 1992;41:215–219.
Cepeda M.S., Lau J., Carr D.B. Defining the therapeutic role of local anesthetic sympathetic blockade in complex regional pain syndrome: a narrative and systematic review. Clinical Journal of Pain. 2002;18:216–233.
Christensen K., Jensen E.M., Noer I. The reflex dystrophy syndrome response to treatment with systemic corticosteroids. Acta Chirurgica Scandinavica. 1982;148:653–655.
Ciccone D.S., Bandilla E.B., Wu W. Psychological dysfunction in patients with reflex sympathetic dystrophy. Pain. 1997;71:323–333.
Covington E.C., Psychological issues in reflex sympathetic dystrophy. Stanton-Hicks J.W., Stanton-Hicks M., eds. Reflex sympathetic dystrophy: a reappraisal. Progress in pain research and management, vol 6. Seattle: IASP Press; 1996:192–216.
Davis K.D., Treede R.D., Raja S.N., et al. Topical application of clonidine relieves hyperalgesia in patients with sympathetically maintained pain. Pain. 1991;47:309–317.
Drummond P.D., Skipworth S., Finch P.M. Alpha 1-adrenoceptors in normal and hyperalgesic human skin. Clinical Science. 1996;91:73–77.
Forouzanfar T., Koke A.J., van Kleef M., et al. Treatment of complex regional pain syndrome type I. European Journal of Pain. 2002;6:105–122.
Fukumoto M., Ushida T., Zinchuk V.S., et al. Contralateral thalamic perfusion in patients with reflex sympathetic dystrophy syndrome. Lancet. 1999;354:1790–1791.
Galer B.S., Butler S., Jensen M.P. Case reports and hypothesis: a neglect-like syndrome may be responsible for the motor disturbance in reflex sympathetic dystrophy (complex regional pain syndrome-1). Journal of Pain and Symptom Management. 1995;10:385–391.
Geertzen J.H., de Bruijn H., de Bruijn-Kofman A.T., et al. Reflex sympathetic dystrophy: early treatment and psychological aspects. Archives of Physical and Medical Rehabilitation. 1994;75:442–446.
Gobelet C., Waldburger M., Meier J.L. The effect of adding calcitonin to physical treatment on reflex sympathetic dystrophy. Pain. 1992;48:171–175.
Goebel A., Baranowski A., Maurer K., Ghiai A., McCabe C., Ambler G., et al. Intravenous immunoglobulin treatment of the complex regional pain syndrome: a randomized trial. Ann Intern Med. 2010;152(3):152–158.
Goldstein D.S., Tack C., Li S.T. Sympathetic innervation and function in reflex sympathetic dystrophy. Annals of Neurology. 2000;48:49–59.
Gschwind C., Fricker R., Lacher G., et al. Does peri-operative guanethidine prevent reflex sympathetic dystrophy? Journal of Hand Surgery, British Volume. 1995;20:773–775.
Haensch C.A., Jorg J., Lerch H. I-123–metaiodobenzylguanidine uptake of the forearm shows dysfunction in peripheral sympathetic mediated neurovascular transmission in complex regional pain syndrome type I (CRPS I). Journal of Neurology. 2002;249:1742–1743.
Hanna M.H., Peat S.J. Ketanserin in reflex sympathetic dystrophy. A double-blind placebo controlled cross-over trial. Pain. 1989;38:145–150.
Harden R.N., Baron R., Jänig W., Complex regional pain syndrome, vol 22. Seattle: IASP Press; 2001.
Harden R.N., Bruehl S., Galer B.S., et al. Complex regional pain syndrome: are the IASP diagnostic criteria valid and sufficiently comprehensive? Pain. 1999;83:211–219.
Harden R.N., Duc T.A., Williams T.R., et al. Norepinephrine and epinephrine levels in affected versus unaffected limbs in sympathetically maintained pain. Clinical Journal of Pain. 1994;10:324–330.
Hassenbusch S.J., Stanton-Hicks M., Schoppa D., et al. Long-term results of peripheral nerve stimulation for reflex sympathetic dystrophy. Journal of Neurosurgery. 1996;84:415–423.
Hord E.D., Oaklander A.L. Complex regional pain syndrome: a review of evidence-supported treatment options. Current Pain and Headache Reports. 2003;7:188–196.
Hord A.H., Rooks M.D., Stephens B.O., et al. Intravenous regional bretylium and lidocaine for treatment of reflex sympathetic dystrophy: a randomized, double-blind study. Anesthesia and Analgesia. 1992;74:818–821.
Huygen F.J., De Bruijn A.G., De Bruin M.T., et al. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators of Inflammation. 2002;11:47–51.
Jadad A.R., Carroll D., Glynn C.J., et al. Intravenous regional sympathetic blockade for pain relief in reflex sympathetic dystrophy: a systematic review and a randomized, double-blind crossover study. Journal of Pain and Symptom Management. 1995;10:13–20.
Jänig W., Baron R. Complex regional pain syndrome is a disease of the central nervous system. Clinical Autonomic Research. 2002;12:150–164.
Jänig W., Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurology. 2003;2:687–697.
Jänig W., Koltzenburg M. What is the interaction between the sympathetic terminal and the primary afferent fiber? In: Basbaum A.I., Besson J.M., eds. Towards a new pharmacotherapy of pain. Dahlem Workshop Reports. Chichester: Wiley; 1991:331–352.
Juottonen K., Gockel M., Silen T., et al. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain. 2002;98:315–323.
Karstetter K., Sherman R.A. Use of thermography for initial detection of early reflex sympathetic dystrophy. Journal of the American Podiatric Medical Association. 1991;81:437–443.
Kemler M.A., Barendse G.A., van Kleef M., et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. New England Journal of Medicine. 2000;343:618–624.
Kemler M.A., Barendse G.A., van Kleef M., et al. Pain relief in complex regional pain syndrome due to spinal cord stimulation does not depend on vasodilation. Anesthesiology. 2000;92:1653–1660.
Kemler M.A., Rijks C.P., de Vet H.C. Which patients with chronic reflex sympathetic dystrophy are most likely to benefit from physical therapy? Journal of Manipulative and Physiological Therapeutics. 2001;24:272–278.
Kemler M.A., van de Vusse A.C., van den Berg-Loonen E.M., et al. HLA-DQ1 associated with reflex sympathetic dystrophy. Neurology. 1999;53:1350–1351.
Kettler R.E., Abram S.E. Intravenous regional droperidol in the management of reflex sympathetic dystrophy: a double-blind, placebo-controlled, crossover study. Anesthesiology. 1998;69:933–936.
Kingery W.S. A critical review of controlled clinical trials for peripheral neuropathic pain and complex regional pain syndromes. Pain. 1997;73:123–139.
Koban M., Leis S., Schultze-Mosgau S., et al. Tissue hypoxia in complex regional pain syndrome. Pain. 2003;104:149–157.
Lacroix H., Vander Velpen G., Penninckx F., et al. Technique and early results of videoscopic lumbar sympathectomy. Acta Chirurgica Belgica. 1996;96:11–14.
Lee B.H., Scharff L., Sethna N.F., et al. Physical therapy and cognitive–behavioral treatment for complex regional pain syndromes. Journal of Pediatrics. 2002;141:135–140.
Livingstone J.A., Atkins R.M. Intravenous regional guanethidine blockade in the treatment of post-traumatic complex regional pain syndrome type 1 (algodystrophy) of the hand. Journal of Bone and Joint Surgery. British Volume. 2002;84:380–386.
Ludwig J., Baron R. Complex regional pain syndrome type: an inflammatory pain condition? Drug Discoveries Today: Disease Mechanisms. 2004;1:449–455.
Maihofner C., Handwerker H.O., Neundorfer B., et al. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61:1707–1715.
Maihofner C., Handwerker H.O., Neundorfer B., et al. Cortical reorganization during recovery from complex regional pain syndrome. Neurology. 2004;24:693–701.
Manicourt D.H., Brasseur J.P., Boutsen Y., et al. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis and Rheumatism. 2004;50:3690–3697.
McCabe C.S., Haigh R.C., Ring E.F., et al. A controlled pilot study of the utility of mirror visual feedback in the treatment of complex regional pain syndrome (type 1). Rheumatology. 2003;42:97–101.
Mellick G.A., Mellick L.B. Gabapentin in the management of reflex sympathetic dystrophy. Journal of Pain and Symptom Management. 1995;10:265–266.
Monti D.A., Herring C.L., Schwartzman R.J., et al. Personality assessment of patients with complex regional pain syndrome type I. Clinical Journal of Pain. 1998;14:295–302.
Moseley G.L. Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain. 2004;108:192–198.
Oerlemans H.M., Oostendorp R.A., de Boo T., et al. Adjuvant physical therapy versus occupational therapy in patients with reflex sympathetic dystrophy/complex regional pain syndrome type I. Archives of Physical and Medical Rehabilitation. 2000;81:49–56.
Oyen W.J., Arntz I.E., Claessens R.M., et al. Reflex sympathetic dystrophy of the hand: an excessive inflammatory response? Pain. 1993;55:151–157.
Perez R.S., Kwakkel G., Zuurmond W.W., et al. Treatment of reflex sympathetic dystrophy (CRPS type 1): a research synthesis of 21 randomized clinical trials. Journal of Pain and Symptom Management. 2001;21:511–526.
Perez R.S., Zuurmond W.W., Bezemer P.D., et al. The treatment of complex regional pain syndrome type I with free radical scavengers: a randomized controlled study. Pain. 2003;102:297–307.
Pleger B., Janssen F., Schwenkreis P., et al. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neuroscience Letters. 2004;356:87–90.
Pleger B., Tegenthoff M., Ragert P., et al. Sensorimotor returning in complex regional pain syndrome parallels pain reduction. Annals of Neurology. 2005;57:425–429.
Pleger B., Tegenthoff M., Schwenkreis P., et al. Mean sustained pain levels are linked to hemispherical side-to-side differences of primary somatosensory cortex in the complex regional pain syndrome I. Experimental Brain Research. 2004;155:115–119.
Price D.D., Long S., Wilsey B., et al. Analysis of peak magnitude and duration of analgesia produced by local anesthetics injected into sympathetic ganglia of complex regional pain syndrome patients. Clinical Journal of Pain. 1998;14:216–226.
Ramamurthy S., Hoffman J. Intravenous regional guanethidine in the treatment of reflex sympathetic dystrophy/causalgia: a randomized, double-blind study. Guanethidine Study Group. Anesthesia and Analgesia. 1995;81:718–723.
Rauck R.L., Eisenach J.C., Jackson K., et al. Epidural clonidine treatment for refractory reflex sympathetic dystrophy. Anesthesiology. 1993;79:1163–1169.
Ribbers G.M., Mulder T., Geurts A.C. Reflex sympathetic dystrophy of the left hand and motor impairments of the unaffected right hand: impaired central motor processing? Archives of Physical and Medical Rehabilitation. 2002;83:81–85.
Robinson J.N., Sandom J., Chapman P.T. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Medicine. 2004;5:276–280.
Sandroni P., Benrud-Larson L.M., McClelland R.L., et al. Complex regional pain syndrome type I: incidence and prevalence in Olmsted County, a population-based study. Pain. 2003;103:199–207.
Schattschneider J., Wenzelburger R., Deuschl G., et al, Kinematic analysis of the upper extremity in CRPS. Harden R.N., Baron R., Jänig W., eds. Complex regional pain syndrome, vol 22. Seattle: IASP Press; 2001:119–128.
Schott G.D. Interrupting the sympathetic outflow in causalgia and reflex sympathetic dystrophy [editorial]. British Medical Journal. 1998;316:792–793.
Serpell M.G. Gabapentin in neuropathic pain syndromes: a randomised, double-blind, placebo-controlled trial. Pain. 2002;99:557–566.
Severens J.L., Oerlemans H.M., Weegels A.J., et al. Cost-effectiveness analysis of adjuvant physical or occupational therapy for patients with reflex sympathetic dystrophy. Archives of Physical and Medical Rehabilitation. 1999;80:1038–1043.
Sherry D.D., Wallace C.A., Kelley C., et al. Short- and long-term outcomes of children with complex regional pain syndrome type I treated with exercise therapy. Clinical Journal of Pain. 1999;15:218–223.
Singh B., Moodley J., Shaik A.S., et al. Sympathectomy for complex regional pain syndrome. Journal of Vascular Surgery. 2003;37:508–511.
Son U.C., Kim M.C., Moon D.E., et al. Motor cortex stimulation in a patient with intractable complex regional pain syndrome type II with hemibody involvement. Case report. Journal of Neurosurgery. 2003;98:175–179.
Stanton-Hicks M., Baron R., Boas R., et al. Complex regional pain syndromes: guidelines for therapy. Clinical Journal of Pain. 1998;14:155–166.
Stanton-Hicks M., Burton A.W., Bruehl S.P., et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Practice. 2002;2:1–16.
Stanton-Hicks M., Jänig W., Hassenbusch S., et al. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127–133.
Uher E.M., Vacariu G., Schneider B., et al. Comparison of manual lymph drainage with physical therapy in complex regional pain syndrome, type I. A comparative randomized controlled therapy study. Wiener Klinische Wochenschrift. 2000;112:133–137.
van de Beek W.J., Roep B.O., van der Slik A.R., et al. Susceptibility loci for complex regional pain syndrome. Pain. 2003;103:93–97.
van der Laan L., Goris R.J. Reflex sympathetic dystrophy. An exaggerated regional inflammatory response? Hand Clinics. 1997;13:373–385.
van der Laan L., Veldman P.H., Goris R.J. Severe complications of reflex sympathetic dystrophy: infection, ulcers, chronic edema, dystonia, and myoclonus. Archives of Physical and Medical Rehabilitation. 1998;79:424–429.
van de Vusse A.C., Stomp-van den Berg S.G., Kessels A.H., et al. Randomised controlled trial of gabapentin in complex regional pain syndrome type 1. BMC Neurology. 2004;4:13.
van Hilten B.J., van de Beek W.J., Hoff J.I., et al. Intrathecal baclofen for the treatment of dystonia in patients with reflex sympathetic dystrophy. New England Journal of Medicine. 2000;343:625–630.
van Hilten J.J., van de Beek W.J., Roep B.O. Multifocal or generalized tonic dystonia of complex regional pain syndrome: a distinct clinical entity associated with HLA-DR13. Annals of Neurology. 2000;48:113–116.
Varenna M., Zucchi F., Ghiringhelli D., et al. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. Journal of Rheumatology. 2000;27:1477–1483.
Veldman P.H., Goris R.J. Multiple reflex sympathetic dystrophy. Which patients are at risk for developing a recurrence of reflex sympathetic dystrophy in the same or another limb. Pain. 1996;64:463–466.
Veldman P.H., Reynen H.M., Arntz I.E., et al. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012–1016.
Wallace M.S., Ridgeway B.M., Leung A.Y., et al. Concentration–effect relationship of intravenous lidocaine on the allodynia of complex regional pain syndrome types I and II. Anesthesiology. 2000;92:75–83.
Wasner G., Heckmann K., Maier C., et al. Vascular abnormalities in acute reflex sympathetic dystrophy (CRPS I)—complete inhibition of sympathetic nerve activity with recovery. Archives of Neurology. 1999;56:613–620.
Wasner G., Schattschneider J., Baron R. Skin temperature side differences—a diagnostic tool for CRPS? Pain. 2002;98:19–26.
Wasner G., Schattschneider J., Heckmann K., et al. Vascular abnormalities in reflex sympathetic dystrophy (CRPS I): mechanisms and diagnostic value. Brain. 2001;124:587–599.
Weber M., Birklein F., Neundorfer B., et al. Facilitated neurogenic inflammation in complex regional pain syndrome. Pain. 2001;91:251–257.
Zuurmond W.W., Langendijk P.N., Bezemer P.D., et al. Treatment of acute reflex sympathetic dystrophy with DMSO 50% in a fatty cream. Acta Anaesthesiologica Scandinavica. 1996;40:364–367.
Zyluk A. The natural history of post-traumatic reflex sympathetic dystrophy. Journal of Hand Surgery, British Volume. 1998;23:20–23.