6
Topical Anesthetic Agents
Diana Burnham Aboytes, RDH, MS
1. Discuss the purpose of topical anesthetics.
2. Identify ideal properties of topical anesthetics and discuss their mechanism of action.
3. List common forms of topical anesthetics and describe the methods for delivery of topical anesthetic drugs.
4. Identify and describe the common topical anesthetic agents used in dentistry, including classification, available concentrations, onsets of action, duration, considerations, and maximum recommended dosages.
5. Identify and describe topical anesthetic drug combinations used in dentistry.
6. Discuss special considerations when dealing with topical anesthetics in dentistry, including recognizing signs and symptoms of adverse reactions to topical anesthetics.
Introduction
One important component of the local anesthesia armamentarium is topical anesthetic. Topical implies that the anesthetic will be applied to a body surface such as the skin or mucous membrane. In dentistry, topical anesthetics are used routinely to provide pain control before conventional local anesthetic injections. They contribute by minimizing the pain associated with needle insertion. Topical anesthetics are also frequently used for the treatment of minor injuries of the gingiva and oral mucosa, to increase comfort during minor dental and dental hygiene procedures, and to reduce the patient’s gag reflex while taking radiographs and impressions.
There are limitations to topical anesthetics and they should not be used as a substitute for local anesthesia. Topical anesthetics do not provide pulpal anesthesia and will not be effective if root sensitivity is a concern.
Some topical anesthetic agents are available over-the-counter to the public and can be purchased at local food and drug stores (Figure 6-1). People of all ages use them to ease pain from teething, braces, apthous ulcers (canker sores), dentures, or toothaches.
Whether being applied professionally or in the comfort of your home (Tables 6-1 and 6-2), intraoral topical anesthetics should ideally be nonallergenic and produce no damage to the tissue to which it is applied. It should consist of a pain-free application, have an acceptable taste, and be able to remain at the site of application. Also, it must produce reliable, effective anesthesia with a sufficient duration and not induce systemic toxicity (Box 6-1).
Mechanism of Action of Topical Anesthetics
The mechanism of action of topical anesthetics is similar to that of their injectable counterparts. Topical anesthetics, however, do not contain a vasoconstrictor and have a higher concentration in order to diffuse through the mucous membrane. They work by blocking nerve conductions at the surface of the skin or mucous membrane. The permeability of sodium ions to the nerve cell is decreased, resulting in decreased depolarization and an increased excitability threshold that, ultimately, blocks the conduction of the nerve impulse and produces a reversible loss of sensation.
Topical Anesthetic Forms and Methods of Delivery
Topical anesthetic agents are available in a variety of commercial forms, including gels, ointments, sprays (both metered and unmetered), creams, liquids, and lozenges. The type of preparation can affect the efficacy. Depending on the form used, effective concentrations range from 0.2%–20%.1,2 Delivery methods include the use of cotton tip applicators, sprays, brushes, patches, blunted cannulas and/or syringes, and single-dose applicator swabs (Figure 6-2). When professionals apply topical anesthetics, each form and method of delivery is considered on an individual patient basis. Medical and dental histories should always be reviewed before the anesthetic is applied, and manufacturer’s directions should be followed.

FIGURE 6-1 Variety of topical anesthetic products that can be purchased over-the-counter in local food and drug stores.
TABLE 6-1
Common Topical Agents Administered Professionally
| Product name | Gel | Spray | Liquid | Ointment | Patch | Lozenge | Cream | Single unit dose | Active ingredient |
| Americaine | X | X | Benzocaine 20% | ||||||
| Anacaine | X | Benzocaine 10% | |||||||
| BeeGentle | X | Benzocaine 20% | |||||||
| Benzo-Jel | X | Benzocaine 20% | |||||||
| CaineTips | X | Benzocaine 20% | |||||||
| Cetacaine | X | X | X | Benzocaine 14%, butamben 2%, tetracaine hydrochloride 2% | |||||
| ComfortCaine | X | Benzocaine 20% | |||||||
| Cora-Caine | X | Benzocaine 16% | |||||||
| Denti-care | X | Benzocaine 20% | |||||||
| Gingicaine | X | X | Benzocaine 20% | ||||||
| HurriCaine | X | X | X | X | Benzocaine 20% | ||||
| HurriPak | X | Benzocaine 20% | |||||||
| Kolorz | X | Benzocaine 20% | |||||||
| Lidocaine | X | Lidocaine 5% | |||||||
| Lollicaine | X | Benzocaine 20% | |||||||
| ProJel-20 | X | Benzocaine 20% | |||||||
| One Touch Advanced | X | Benzocaine 14%, butamben 2%, tetracaine hydrochloride 2% | |||||||
| Oraqix | X | Lidocaine 2.5% and prilocaine 2.5% | |||||||
| Topex | X | X | X | Benzocaine 20% | |||||
| Topex HandiCaine Stix | X | Benzocaine 20% | |||||||
| Topicale | X | X | X | Benzocaine gel and ointment 20%, patch 18% | |||||
| Ultracare | X | Benzocaine 20% |

To provide pain management before the administration of conventional local anesthetic injections, topical gels or ointments are applied with a cotton tip applicator. The site of penetration should first be dried using a 2 × 2 gauze square to increase visibility and accurate placement of the topical anesthetic (Figure 6-3). Only a small amount of gel or ointment on the applicator tip is necessary to achieve the desired result (Figure 6-4). Student dental hygienists and even experienced clinicians can often apply excessive amounts of topical anesthesia, leading to an unnecessary effect on surrounding tissue. This excess mixes with the saliva and may anesthetize areas such as the tongue, soft palate, or pharynx. The topical agent should remain at the site of penetration for 1 to 2 minutes (depending on the concentration of the topical anesthetic) to ensure effectiveness (Figure 6-5). If these steps are followed, anesthesia should be achieved to a depth of approximately 2 to 3 mm into the tissue (Procedure 6-1). This helps provide comfort during the initial penetration of the local anesthetic needle.
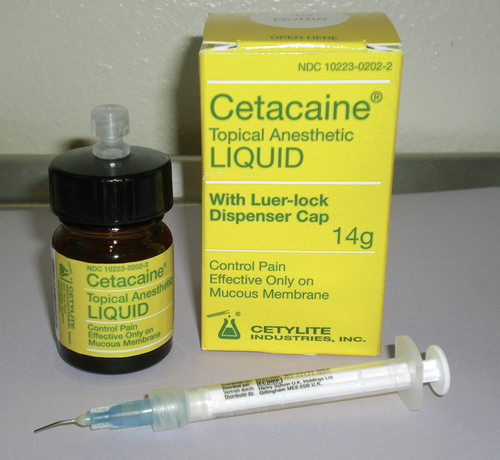
Topical anesthetics in liquid form are also used in dentistry. Liquids provide anesthesia to a wide area. They are especially useful when trying to decrease a patient’s gag reflex, thereby making placement of impression materials or radiograph film more comfortable and tolerable. The use of a liquid for a more site-specific procedure requires an applicator. Cetacaine (Cetylite, Pennsauken, N.J.), which is available only by prescription, is administered by either a cotton pellet or via blunted cannula to deliver the anesthetic subgingivally (Figure 6-6). Another liquid, HurriPak (Beutlich Pharmaceuticals) is designed primarily for the use of subgingival placement by using irrigation syringes with plastic tips (Figure 6-7). These are indicated when attempting to increase comfort during prophylaxis or nonsurgical periodontal therapy procedures.
Gels, ointments, and liquids often come in multidose containers, but some products are packaged in single unit-dose applications. Lollicaine (Centrix), HandiCaine Stix (Topex Sultan Healthcare), and CaineTips (J Morita) are some of the products available through dental suppliers. These single unit–dose applications can also be found in over-the-counter products such as Orajel medicated tooth swabs (Figure 6-8). Individual packaging is less messy, helps prevent possible cross-contamination, and allows monitoring of the dose.
TABLE 6-2
Common Topical Anesthetic Agents Self-Administered by Patients
| Product name | Gel | Spray | Liquid | Ointment | Patch | Lozenge | Cream | Single unit dose | Active ingredient |
| Anbesol | X | X | Benzocaine: Baby 7.5%, Jr 10%, max strength 20% | ||||||
| BiZets | X | Benzocaine 15 mg | |||||||
| Cepacol | X | X | Benzocaine-spray 5% Lozenges 7.5 mg to 15 mg | ||||||
| Chloraseptic | X | Benzocaine 6 mg | |||||||
| Dentane | X | Benzocaine 7.5% | |||||||
| Dentapaine | X | Benzocaine 20% | |||||||
| HDA Toothache | X | Benzocaine 6.5% | |||||||
| Kank-A | X | X | X | Benzocaine-gel and liquid 20%, bead 3 mg | |||||
| Orabase∗ | Benzocaine 20% ∗comes in a paste | ||||||||
| Orajel Adult | X | X | X | Benzocaine-regular 10%, ultra and denture 15%, medicated swab 20%, PM max strength 20% | |||||
| Orajel Baby | X | X | X | Benzocaine 7.5%, nighttime 10%, cooling cucumber 10% | |||||
| Ora-Film∗ | Benzocaine 10% ∗comes in a thin film strip | ||||||||
| Red Cross Canker | X | Benzocaine 20% | |||||||
| Sucrets | X | Dyclonine hydrochloride Children 1.2 mg, max strength 3 mg | |||||||
| Tanac | X | Benzocaine 10% | |||||||
| Thorets | X | Benzocaine 10 mg | |||||||
| Trocaine | X | Benzocaine 10 mg | |||||||
| Zilactin-B | X | Benzocaine 10%, max strength 20% |

Sprays are also used to deliver topical anesthesia. Unmetered sprays are not recommended because they do not allow control of the amount of anesthetic dispensed, nor are they easily contained at a specific site. However, the use of a metered spray with a disposable nozzle enables control over the amount of agent being dispensed, thus decreasing the risk for systemic toxicity (Figure 6-9). Both the Institute of Safe Medication Practices (ISMP) and the U.S. Food and Drug Administration (FDA) have released advisory statements informing the public of the association between benzocaine and methemoglobinemia, wherein methemoglobin builds up in the blood, hindering the effective transport of oxygen to body tissues (see Chapter 7).
A new product, Kovacaine mist, has been developed that delivers dental anesthestic by way of nasal spray. The 3% tetracaine HCL with 0.05% oxymetazoline HCL combination is administered via the nasal mucosa and diffuses into the maxillary dental plexus providing anesthesia to both hard and soft tissues of the maxillary arch. Clinical trials have been completed for both pediatric and adult patients, and the U.S. Food and Drug Administration is conducting safety trials for the product. A product that provides anesthesia without a needle offers great promise for the dental profession by giving options to patients who avoid or put off treatment because of the fear associated with the needle injection.

FIGURE 6-3 A 2 × 2 gauze square is used to gently wipe and dry tissue at the site of needle penetration.

FIGURE 6-4 Cotton tip applicator on left shows excessive amount of topical anesthesia. Cotton tip applicator on the right shows correct amount of topical anesthesia to be applied at each penetration site.
Patch delivery of a topical anesthetic is advantageous because the patch can be placed directly on the delivery site and then adheres to the tissue. Transdermal patches are used regularly in medicine on intact skin to administer certain medications or to provide local dermal analgesia. For example, Synera (ZARS Pharma, Salt Lake City, Utah) is an FDA-approved peel-and-stick topical anesthetic patch that numbs intact skin before minor needle procedures and superficial dermatologic procedures.3 Lidoderm (Endo Pharmaceuticals, Chadds Ford, Pa.) is also an FDA-approved adhesive patch that provides pain relief from postherpetic neuralgia, commonly called after-shingles pain.4 These patches are not used intraorally, however.
Patches available for intraoral topical anesthesia are limited. The first FDA-approved transoral anesthetic patch, DentiPatch, was introduced in 1996 by Noven Pharmaceuticals, but the manufacturer discontinued the product. Currently the only patch available for intraoral use is Topicale GelPatch (Premier Dental Co.) (Figure 6-10).
Regardless of the form and method of delivery, there is more than one option for delivering topical anesthetics to dental patients. Furthermore, dental hygienists must be aware that patients often self-medicate using these same drugs in over-the-counter formulations and should query their patients before administering any anesthetic agents.
Common Topical Agents Used in Dentistry
Benzocaine
Benzocaine is one of the more common and widely used topical anesthetics. It is available in gel, cream, ointment, lozenge, liquid solution, spray, and patch (Figure 6-11). It exists almost entirely in its base form, making absorption into circulation slow and therefore having a very low potential for systemic toxicity in healthy individuals.
• Classification: Benzocaine is an ester.
• Available concentration: The most commonly used concentration used in dentistry is 20%, although it is available in concentrations ranging from 6% to 20%.
• Onset of action: Rapid. Onset can occur as early as 30 seconds and have its peak effect at 2 minutes.
• Duration: 5 to 15 minutes.
• Maximum recommended dose: There are no published maximum dosage recommendations.5
• Metabolism/excretion: Benzocaine is metabolized via hydrolysis in the plasma and to a lesser extent in the liver by cholinesterase. Excretion occurs primarily through kidneys with only a small portion remaining unchanged in the urine.
• Pregnancy/lactation: FDA Category C/Excretion in breast milk unknown, use with caution.
• Special considerations: Methemoglobinemia has been reported following topical anesthesia use of benzocaine, particularly with higher concentrations of 14% to 20% spray applications applied to the mouth and mucous membrane. Always apply as directed. Benzocaine should not be used on children younger than 2 years of age.
Lidocaine
Lidocaine is a good alternative if a patient has sensitivity to esters. The most common topical preparation is an ointment (Figure 6-12), but it can be found as a patch, as a spray, and in a solution. It is available in two forms, as a base or a hydrochloride salt. The base form is poorly soluble in water and has poor penetration and absorption abilities. The hydrochloride salt form on the other hand is water soluble and can easily penetrate and be absorbed in the tissues, significantly increasing the risk of toxicity. The base form is preferred for application to mucous membranes and for covering large areas.
• Classification: Lidocaine is an amide.
• Available concentration: Most common in 2% or 5% preparations.
• Onset of action: Between 2 and 10 minutes.
• Duration: Depends on method of application; approximately 15 to 45 minutes.
• Maximum recommended dose: 200 mg (300 mg manufacturer recommendation).
• Metabolism/excretion: Metabolized in the liver and excreted via the kidney with less than 10% remaining unchanged.
• Pregnancy/lactation: FDA Category B/Enters breast milk in small amounts, use with caution.
• Special considerations: Always follow manufacturer’s application directions and ask questions of physician staff if necessary.
Dyclonine Hydrochloride
Dyclonine hydrochloride has a unique classification in that it is neither an ester nor an amide agent, but rather a ketone. This unique property is beneficial for patients with sensitivities to traditional topical anesthetics. As a topical anesthetic, dyclonine hydrochloride is available by prescription, and to patients it is available over-the-counter in Sucrets lozenges (Figure 6-13).
• Classification: Ketone.
• Available concentration: Formulated for use in dentistry as a 0.5% or 1% solution.
• Onset of action: Slow; may take up to 10 minutes to become effective.
• Duration: Average duration of 30 minutes; however, effects may last up to 1 hour.
• Maximum recommended dose: 200 mg (40 mL of 0.5% solution or 20 mL of a 1% solution).
• Metabolism/excretion: No information is available on the metabolism and excretion of dyclonine hydrochloride.
• Pregnancy/lactation: FDA Category C/Caution is recommended during lactation.
Tetracaine Hydrochloride
Tetracaine hydrochloride is considered the most potent of the topical anesthetics. It is not made available for injection and with topical preparations is typically combined with other drugs.
• Classification: Tetracaine hydrochloride is an ester.
• Available concentration: 2% in topical preparations.
• Onset of action: Slow; peak effects may take up to 20 minutes.
• Duration: Approximately 45 minutes.
• Maximum recommended dose: 20 mg for topical administration; 1 mL of a 2% solution.
• Metabolism/excretion: Metabolized by plasma pseudocholinesterase/excreted in the kidneys.
• Pregnancy/lactation: FDA Category C/Caution is recommended during lactation.
• Special considerations: Highly soluble in lipids, making absorption into local tissues very rapid.
Combinations of Topical Drugs
Topical anesthetic agents are also mixed and used in combinations to increase the anesthetic effect.
Benzocaine, Butamben, and Tetracaine
Cetacaine (Cetylite Industries), which contains the triple-action formula of benzocaine, butamben, and tetracaine, is the brand most often used in the dental office. The benzocaine provides a quick onset while the properties of tetracaine allow deeper penetration of the agent, thus contributing to an increase in the duration of action. It is available by prescription only in spray, liquid, and gel forms (Figure 6-14).
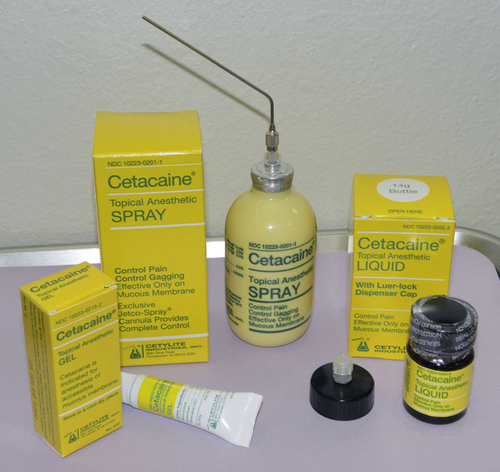
FIGURE 6-14 Benzocaine, butamben, and tetracaine products available. Cetacaine (Cetylite Industries).
• Classification: Benzocaine, butamben, and tetracaine are all esters.
• Available concentration: Triple-active formula of benzocaine 14%, butamben 2%, and tetracaine hydrochloride 2%.
• Onset of action: Rapid; approximately 30 seconds.
• Duration: Typically 30 to 60 minutes.
• Maximum recommended dose: Spray administered for 1 second. Gel and liquid 200 mg.
• Metabolism/excretion: Hydrolysis via cholinesterase.
• Pregnancy/lactation: FDA Category C/Use caution while nursing.
• Special considerations: Tetracaine is highly lipid-soluble, making absorption into local tissues very rapid. Not suitable for injection. (Source: Cetacaine prescribing information.)
Eutectic Mixtures
A eutectic mixture is a mixture of two elements that together have a lower melting temperature than any of the individual components. This increases the concentration and enhances the drug’s properties, resulting in a faster, more penetrating, longer-acting agent.
EMLA: 2.5% Lidocaine/2.5% Prilocaine Cream
The composition of 2.5% lidocaine and 2.5% prilocaine as an oil-in water emulsion is referred to as an EMLA: eutectic mixtures of local anesthetics. EMLA is available commercially as a cream or disc and represents the first major breakthrough for surface anesthesia on intact skin. It should only be applied to intact skin and often requires an occlusive dressing to allow the release of the drug into the epidermal and dermal layers. The onset, depth, and duration of dermal anesthesia depend primarily on the contact time during application. Satisfactory results should be achieved 1 hour after application, reach a maximum at 2 to 3 hours, and last for 1 to 2 hours after removal.6 The combined use of lidocaine/prilocaine has been well recognized in the medical community, and has been used to provide efficacious topical anesthesia for a variety of medical procedures, such as venipuncture, circumcision, and minor gynecologic procedures.7,8 When used as directed, the risk of systemic toxicity remains low; however, if not used as prescribed, the systemic absorption of lidocaine and prilocaine can also become a side effect of the desired effect because the amount of drug absorbed depends on the surface area and the duration of the application. Although EMLA is FDA approved, the FDA released a Public Health Advisory in 2007 expressing concerns and warning the public of potential danger associated with the use of the topical anesthetic drug (Box 6-2).
The first documented use of lidocaine/prilocaine used in the oral cavity was done in 1985, by Holst and Evers.9 Since then, many more studies have been conducted that show great promise for use of EMLA intraorally.10-14 However, EMLA is currently approved by the FDA only for use on intact, nonmucosal skin.
• Classification: Lidocaine and prilocaine are both amides.
• Available concentration: 2.5% lidocaine and 2.5% prilocaine.
• None available for intraoral use.
• Onset of action: Satisfactory results achieved in 1 hour, with exception of genital mucosa which is 10 to 15 minutes. None reported for intraoral use.
• Duration: No information reported for intraoral use.
• Maximum recommended dose: No information for intraoral use.
• Metabolism/excretion: Primarily in the liver.
• Pregnancy/lactation: As a eutectic mixture, FDA Category B. Caution should be taken during lactation.
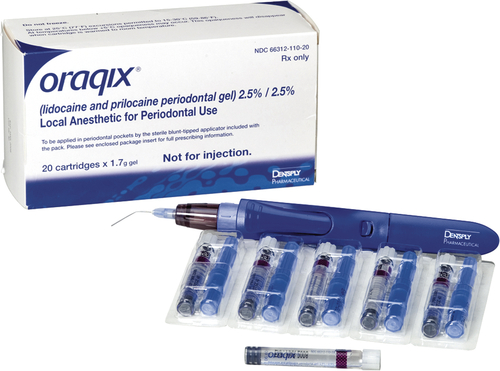
Oraqix: 2.5% Lidocaine/2.5% Prilocaine Gel
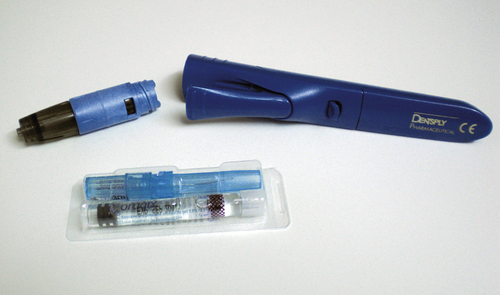
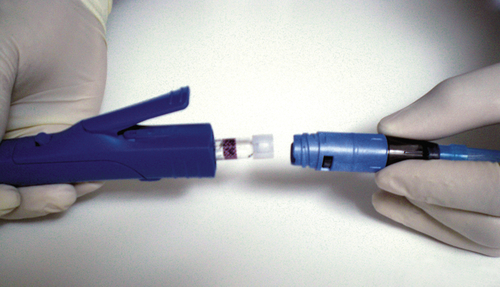
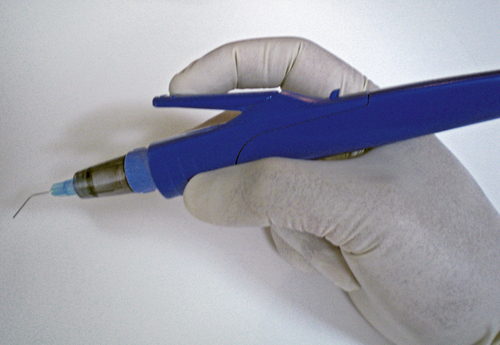
Even though studies do exist on the effectiveness of EMLA cream used intraorally, EMLA cream is not approved by the FDA for intraoral use. However, there is a prescription eutectic mixture available for use in the oral cavity that consists of a 2.5% lidocaine and 2.5% prilocaine gel called Oraqix (Dentsply Pharmaceuticals, York, Pa.). Oraqix is a microemulsion in which the oil phase is a eutectic mixture in a ratio of 1:1 weight. Oraqix can only be administered by means of a special applicator and does not work with standard dental syringes (Figure 6-15). Although Oraqix remains in liquid form at room temperature in the cartridge, it begins to thicken into a gel upon application into the periodontal pocket and reaching body temperature. Even though pulpal anesthesia is not achieved, Oraqix is indicated to provide comfort to the gingival tissues during prophylaxis, periodontal assessment, and nonsurgical periodontal therapy. Assembly of the dispenser is quick and easy, making it available for use within seconds (Procedure 6-2).
• Classification: Lidocaine and prilocaine are amides.
• Onset of action: Occurs by 30 seconds. Longer wait time does not enhance the anesthetic effect.
• Duration: Approximately 20 minutes (average, 14 to 31 minutes)
• Maximum recommended dose: Five cartridges at one treatment session.
• Metabolism/excretion: Mainly metabolized in the liver.
• Pregnancy/lactation: FDA Category B/Caution should be taken if administered to nursing mothers.
• Special considerations: Do not inject. (Source: Oraqix prescribing information. Dentsply Pharmaceuticals, York, Pa. www.oraqix.com.)
Special Considerations
The concentration of topical anesthetic agents is higher than that of their injectable counterparts. This is necessary to facilitate diffusion of the agent through the mucous membranes. In addition, topical anesthetics do not contain a vasoconstrictor. With these higher concentrations and the lack of vasoconstriction abilities, the risk of local and systemic absorption increases, thus increasing the risk of toxicity (see Chapter 4).
High plasma concentrations of topical anesthetics can produce adverse effects and results when patients are exposed to excessive amounts of the drugs. Children, elderly, and medically compromised individuals are more susceptible to the adverse reactions of topical anesthetics. Methemoglobinemia, a rare, but serious condition can occur resulting in a decrease in the amount of oxygen being carried through the blood stream. Signs and symptoms of methemoglobinemia include pale, gray or blue colored skin, lips and/or nailbeds, fatigue, shortness of breath, headache and lightheadedness. These signs and symptoms can occur within minutes to hours after topical benzocaine administration. Most of the cases reported are in children under two years of age, prompting the FDA to release a safety announcement in 2011 regarding OTC benzocaine teething preparations and more recently another in 2014 regarding prescription viscous lidocaine use for soothing gums in children (Box 6-3). Adult patients with breathing problems such as asthma, emphysema, and COPD, those with heart disease, and smokers are also at a greater risk for complications related to methemoglobinemia.
Possible localized adverse effects include irritation, stinging or burning at the site of application, sloughing, tissue discoloration, and temporary alteration in taste perception. The most prominent of the systemic effects of topical anesthetics are in the central nervous system and cardiovascular system. Excitatory effects of the central nervous system are often displayed at the initial signs and symptoms of overdose. Some of these signs and symptoms include dizziness, visual disturbances, tinnitus, disorientation, unusual nervousness or apprehension, and localized involuntary muscular activity. The excitatory manifestations may be very brief or not occur at all, in which case the first manifestation would be a depressant response, such as slurred speech, drowsiness, and respiratory impairment. Toxic overdoses result in seizures, unconsciousness, and respiratory arrest. In the cardiovascular system, patients may experience bradycardia and hypotension, leading to rare cases of cardiac arrest (see Chapter 15).
Allergic reactions associated with topical anesthetics are rare. Benzocaine and tetracaine are both esters, which increases their potential for an allergic reaction; however the risks are still low. Anaphylaxis is very rare with topical anesthetics. Any reaction would likely be delayed and not present itself until after the patient has left the dental office. Those mild allergic reactions can include swelling and raised welts on the skin, itching, or burning. Some allergic reactions occur up to 2 days after the anesthetic is given.
Although these adverse reactions are rare, the potential does exist. It is important to take all precautions necessary to minimize or avoid or prevent them (Box 6-4)
.