Chapter 22 Managing Fluid and Electrolyte Disorders in Renal Failure
The kidneys are responsible for maintaining homeostasis in the body, and kidney failure may lead to derangements of fluid, electrolyte, and acid-base balance. It is the clinician’s goal in treatment to reverse these derangements and to prevent on-going damage.
Kidney disease is classically compartmentalized into acute and chronic disease, which is a convenient way to view what are very frequently notably different manifestations of kidney disease. Both acute and chronic kidney disease may vary from mild to severe. Many patients with acute kidney injury require hospitalization for optimal management. Patients with chronic kidney disease may present in a decompensated state and need hospitalization, or their fluid and electrolyte management may occur on an outpatient basis. Despite many differences in this diverse field of kidney disease, many of the principles of fluid and electrolyte management are the same despite the cause.
Intrinsic renal failure occurs when damage to the renal parenchyma occurs. The damage may be reversible or irreversible, and includes damage to the glomerulus, tubules, interstitium, or renal vasculature. Hemodynamic-mediated azotemia occurs when blood flow to the kidney is diminished, as may occur with hypovolemia, hypotension, or increased renal vascular resistance. Hemodynamic azotemia is rapidly reversible once the underlying disorder has been controlled. Postrenal azotemia occurs when there is an obstruction to urine flow, from the level of the renal pelvis to the urethra, or when urine leaks into surrounding tissue and is reabsorbed (i.e., ruptured bladder, ureter, or urethra). Postrenal azotemia can also be rapidly reversed by diverting the urine either by a urinary catheter or peritoneal catheter (in cases of an intraabdominal rupture). With both hemodynamic and postrenal causes of azotemia, long-standing problems may progress to intrinsic renal failure. Although significant renal disease can be present without azotemia, fluid therapy is generally not necessary in those situations. In fact, fluid therapy may not be necessary in compensated chronic renal failure with mild to moderate azotemia.
Fluid treatment
Normal fluid losses consist of insensible and sensible losses. Insensible losses are those that are not consciously perceived, such as water lost via respiration, normal stool, or sweating. Sweating is of negligible volume in dogs and cats. There is variation in respiratory losses in dogs, which may lose considerable amounts of fluid by excessive panting, but 22 mL/kg/day is the average. The main sensible fluid loss in the normal patient is urine output. Additional sensible losses include the volume lost from vomiting, diarrhea, body cavity drainage, burns, etc. In healthy animals, these losses are replaced by drinking and the fluid contained in food. In sick animals, who may not be voluntarily consuming food or water, or who may be restricted from consumption due to vomiting, fluid therapy is necessary to replace these losses. With renal disease, urine volume is frequently abnormally high or low, or inappropriate for the situation, and fluid therapy is tailored for the individual patient to maintain fluid balance.
Fluid therapy for hospitalized patients
Although oliguria or anuria are the classic manifestation of acute kidney injury (AKI), AKI may present with polyuria, which frequently portends a less severe renal injury.4,64 AKI may also be a subtle increase in creatinine (>50% of baseline) or urine volume inappropriate for the volume of fluid administered. In this early stage of injury, attempts to lessen further renal damage are warranted. Patients with chronic kidney disease (CKD) may present in a decompensated uremic crisis, which may represent an acute kidney injury superimposed on chronic disease.
Many drugs have been evaluated for their benefit in treating AKI, and some are helpful in certain settings. However, the most effective therapy of AKI is careful management of fluid balance, which involves thoughtful assessment of hydration, a fluid treatment plan personalized for the specific patient, repeated and frequent reassessment of fluid and electrolyte balance, with appropriate changes in the treatment plan in response to the rapidly changing clinical situation of the kidney failure patient.
Assessing Hydration
The key feature to an appropriate fluid plan is accurate determination of hydration status. Blood volume can be measured using indicator dilution techniques, radioactive tracers, bioimpedance spectroscopy, or other methods.54 Unfortunately, readily available accurate measurement of blood volume is not feasible in general practice settings.
Despite a lack of precise objective data, there are many ways to estimate hydration. A deficit of the extravascular fluid compartment (interstitial and intracellular) causes dehydration. A severe deficit may decrease the intravascular compartment, leading to poor perfusion. Dehydration of less than approximately 5% is difficult to detect clinically. A 5% to 6% deficit leads to sticky mucous membranes. Six to eight percent dehydration causes dry mucous membranes and decreased skin elasticity. By 8% to 10% dehydration, the eyes may be sunken, and over 12% dehydration, corneas are dry, mentation is dull, and perfusion is impaired.28 Overhydration may manifest as wet mucous membranes, increased skin elasticity (heavy or gelatinous), shivering, nausea, vomiting, restlessness, serous nasal discharge, chemosis, tachypnea, cough, dyspnea, pulmonary crackles and edema, pleural effusion, ascites, diarrhea, or subcutaneous edema (especially hock joints and intermandibular space).13,36
Difficulties exist in interpreting these physical findings. Uremic patients frequently have xerostomia, causing dry mucous membranes independent of hydration status. Hypoalbuminemia or vasculitis may cause interstitial fluid accumulation despite an intravascular volume deficit. Emaciation or advanced age decrease elasticity of the skin.
Central venous pressure (CVP) measurement through a centrally placed intravenous catheter may provide information about intravascular filling. A volume depleted animal will have a CVP less than 0 cm H20. A CVP over 10 cm H20 is consistent with volume overload or right-sided congestive heart failure.62 However, pleural effusion falsely elevates the CVP.22 An accurate body weight recorded before an illness is an invaluable aid to assessing hydration. Body weight should be measured two to four times a day on the same scale to monitor fluid balance. A sick animal may lose up to 0.5% to 1% of body weight per day due to anorexia; changes in excess of this amount are due to changes in fluid status.10 An increase in blood pressure may indicate a gain of fluid; conversely, a decrease in blood pressure may indicate a net loss of fluid. Because of the high percentage of patients with hypertension (80% of dogs with severe acute uremia and 20% to 30% of dogs and cats with CKD), the trend rather than the absolute value is of more utility in assessing changes in hydration status.13,26,59 Similarly, changes in trends for PCV and total solids may reflect changes in volume, in the absence of bleeding or blood transfusion. Because each parameter is impacted by aspects beyond hydration status, these factors must be viewed in aggregate.
Route of Fluid Administration
In most hospitalized patients, the intravenous route is the most appropriate route of administration. In some situations, such as extremely small patients, including neonates or very young puppies or kittens, IV catheterization may be difficult. Intraosseous fluid administration can be used in that setting. In dehydrated patients, fluids administered into the peritoneal cavity will be readily absorbed, but this method is not reliable for promoting diuresis or in oliguric patients. Fluid administered subcutaneously may not be absorbed rapidly or completely, and it is not possible to administer a large volume by this route, making subcutaneous fluid inappropriate for the hospital setting. It may play a role in outpatient therapy (see later discussion).
Type of Fluid
A balanced polyionic solution (i.e., lactated Ringer’s solution [LRS], Plasmalyte 148, Normosol-R) is an appropriate choice for the initial volume resuscitation fluid and replacement of the dehydration deficits. Physiologic (0.9%) NaCl contains no potassium and is a suitable initial choice for the hyperkalemic patient.
After rehydration, maintenance fluids with a lower sodium concentration are more appropriate (i.e., 0.45% NaCl with 2.5% dextrose, one half strength LRS with 2.5% dextrose).
Dextrose 5% in water (D5W) is rarely appropriate as a sole fluid choice, but may be combined with LRS or 0.9% saline to make one half or three fourths strength sodium solutions (25 mL LRS + 25 mL D5W = 50 mL 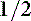 strength LRS + 2.5% dextrose).
strength LRS + 2.5% dextrose).
Colloidal solutions (i.e., hydroxyethyl starch, 6% dextran) may be appropriate if hypoalbuminemia is present. Hypoalbuminemia may be present with protein-losing nephropathy, diseases associated with vasculitis, or severe gastrointestinal losses or bleeding. The recommended dose is 20 mL/kg/day, and may be used to replace the insensible portion when using the “ins-and-outs” method (see later discussion). Higher doses may be associated with coagulopathy. Despite initial concerns that colloidal solutions may cause acute kidney injury (specifically, osmotic injury), there is no evidence that colloids are directly nephrotoxic.46 An alternative to synthetic colloids is human albumin, but this product carries a risk of anaphylaxis.11,21 Canine and feline albumin have recently become commercially available and can be used for colloidal support.
Treatment of the patient with an acute uremic crisis from a protein-losing nephropathy with severe hypoalbuminemia presents additional considerations. An increase in the intravascular volume and hydrostatic pressure from crystalloid infusion is not opposed by colloid oncotic pressure in the plasma, enhancing interstitial edema in the periphery. Even with administration of a colloidal solution, aggressive diuresis with a crystalloid may not be possible without creating peripheral edema. Loss of antithrombin III in urine causes a hypercoagulable state, which may cause complications associated with intravenous catheterization.
Anemia may be present in both acute and chronic renal failure. Red cell survival is shorter in the uremic environment, blood sampling may create substantial losses, and erythropoietin production is generally suppressed. Gastrointestinal bleeding can acutely cause anemia, and if bleeding is brisk, hypotension and hypovolemia may occur and require rapid infusion of crystalloid or synthetic colloid solutions. Red blood cell transfusion may be indicated if symptomatic anemia is present. Intensive diuresis may exacerbate high output heart failure in cats with anemia. Conversely, rapid blood transfusion may cause congestive heart failure. In patients with compromised cardiovascular function or patients with incipient volume overload, red cell transfusion may need to be given more slowly than usual.
A sometimes overlooked fluid choice is water given enterally. Because vomiting is a common problem with uremia, enteral food or water is frequently contraindicated, and many uremic patients will not voluntarily consume water. However, water administered through a feeding tube should be included in water calculations.
Ultimately, the fluid choice must be guided by monitoring the patient’s fluid and electrolyte balance. A major determining factor in the appropriate fluid choice is the sodium concentration because the degree of free-water loss relative to sodium loss varies greatly in patients with AKI. The guiding principal in treating a sodium disorder is to reverse it at the same rate at which it developed because rapid increases or decreases in sodium concentration may cause CNS dysfunction (see next section).
Volume and Rate
Some patients may present in hypovolemic shock, which is manifest as dull mentation, hypotension (systolic blood pressure <80 mm Hg), poor perfusion of the periphery (cold extremities, pale/gray mucous membranes with slow capillary refill time), hypothermia, or tachycardia.62 Immediate correction of shock is necessary to prevent further and irreversible organ damage. The standard dose of crystalloids is 60 to 90 mL/kg for dogs and 45 to 60 mL/kg for cats, of which one fourth is given over 5 to 15 minutes.41 If hemodynamic parameters do not improve sufficiently with the first one fourth dose, a second dose should be given. Resuscitation efforts are continued until the patient is hemodynamically sound. If the patient remains hypotensive and there are concerns about volume overload, central venous pressure monitoring may be helpful. A CVP less than 0 cm H2O indicates hypovolemia, whereas a CVP more than 10 cm H2O is a contraindication to further fluid therapy. A 10 to 15 mL/kg bolus of crystalloid or 3 to 5 mL/kg of colloid will not change the CVP in hypovolemic patients, but will transiently increase the CVP by 2 to 4 cm H2O in the euvolemic patient, and cause a rise of more than 4 cm H2O in the hypervolemic patient.62 Adequate resuscitation as assessed by achievement of identifiable goals decreases renal morbidity as compared with using standard resuscitation doses in people.30
For patients with dehydration, the dehydration deficit is calculated as body weight (in kilograms) × estimated % dehydration = fluid deficit in L. Because dehydration of less than 5% cannot be detected by clinical examination, a 5% dehydration deficit is presumed in patients with AKI that appear normally hydrated. If a fluid bolus was used for initial resuscitation, that volume is subtracted from the dehydration deficit.
The rate of replacing the dehydration deficit depends on the clinical situation. In patients with AKI, who have presumptively become dehydrated over a short period of time, rapid replacement is prudent. This restores renal perfusion to normal levels and may help prevent further damage to the kidneys. In situations where urine output may be diminished, rapid replacement of dehydration deficits to normalize the fluid status allows the clinician to quickly determine if oliguria is an appropriate response to volume depletion or is a pathologic change from the renal failure. In that setting, replacing the deficit in 2 to 4 hours is recommended. If there is potential compromise of diastolic function of the heart, a rapid fluid bolus may precipitate congestive heart failure, and a more gradual rehydration rate (i.e., over 12 to 24 hours) may be prudent.
In patients with chronic dehydration, a more gradual replacement of the fluid deficit is acceptable to minimize the risk of cardiac problems or too rapid changes in electrolytes, and 24 hours is a commonly selected time frame. In severely dehydrated, chronic debilitated patients, it may take up to 48 hours to rehydrate.
The concept of maintenance fluid rate is based on average fluid losses from insensible (respiration) and sensible (urine output) sources. There are a variety of published values. The most commonly quoted value is 66 mL/kg/day. Ignoring normal individual variation, the presumption with this value is that urine output is normal and there are no other sources of fluid loss, which is rarely the case in patients with renal failure. However, it makes a reasonable starting point for calculating fluid administration volumes. If accurate measurement of urine output and ongoing losses is available, fluid therapy can be adjusted precisely (see “ins-and-outs” method below). If these parameters are not accurately measured, an estimate of the loss should be included in the fluid administration rate. In practical terms, after an initial fluid resuscitation if needed for shock, the volume of fluid to administer is calculated by adding average maintenance fluids (66 mL/kg/day) plus replacement of dehydration (over a selected time frame) plus ongoing losses (estimated volume of polyuria, vomiting).
Because uremic toxins are retained in renal failure, administration of a volume of fluid exceeding “maintenance” can improve excretion of some uremic toxins in animals with the ability to increase urine output in response to a fluid challenge. The volume is varied based on clinical situation and clinician preferences, but generally ranges from 2.5% to 6% of body weight per day, in addition to the maintenance fluid administration rate. In practical terms, twice the maintenance fluid rate is equivalent to a maintenance rate plus a 6% “push” for diuresis (60 mL/kg/day = 6% of body weight). An increase in urine volume does not automatically mean there is an increase in toxin or solute excretion.
If the urine output varies substantially from normal, either oliguria (<0.5 mL/kg/hr) or polyuria (>2 mL/kg/hr), a fluid plan based on these assumptions may be inadequate. Animals with kidney failure may have urine output in a “normal” range (0.5 to 2.0 mL/kg/hr), but if their kidneys are unable to alter the urine volume to excrete a fluid load, the patient has “relative oliguria.” The ins-and-outs method of fluid administration is appropriate in these situations. It should only be used after rehydration is complete and is not appropriate if a patient is still dehydrated.
There are three components of volume calculations in the “ins and outs” method, consisting of (1) insensible loss (fluid lost via respiration and normal stool), which is about 22 mL/kg/day in the average patient; (2) urine volume replacement calculated by actual measurement (see later discussion for measuring techniques); and (3) ongoing losses (i.e., vomiting, diarrhea, body cavity drainage), which are generally estimated.
To write treatment orders for “ins and outs” using two IV catheters, divide the daily insensible loss (22 mL/kg) by 24 to determine the hourly dose of IV fluids to administer through one catheter. You can use this fluid dose to deliver any drugs that need to be given by constant rate infusion (CRI) (metoclopramide, furosemide, mannitol, etc.), being cognizant of drug incompatibilities. Measure urine output to determine the rate of replacement fluid to administer over the next time period. For example, if you are measuring urine output every 6 hours, take that volume and divide by 6 to give the hourly rate of fluid replacement to administer over the next 6 hours. Add to this an estimate of losses during that time period (vomiting and diarrhea). For the starting fluid dose, select a volume based on your estimate of the patient’s needs. If only one IV catheter is available, calculate the amount of medication to be administered by CRI to give over 6 hours. Add this amount to the fluid volume required over the next 6 hours (6 hours of insensible losses + previous 6-hour urine output). Divide the total volume by 6 to get the hourly rate for the CRI.
An anuric patient should receive fluid administration to replace insensible loss only. If the patient is overhydrated, withhold the insensible loss. Overhydration in an anuric patient or inability to induce diuresis in an oliguric or anuric patient is an indication for dialysis, which is the only other effective therapeutic option.
Not withstanding the conventional wisdom that fluid therapy is cornerstone of treatment of kidney failure, evidence of harm from volume overload is mounting. Rapid restoration of renal perfusion may decrease renal damage, but there is no evidence that fluid therapy will reverse established renal injury.44,60 Patients with volume overload (>10%) had decreased survival and impaired renal recovery.6-8 In fact, one study in adult humans found that a 1 L positive fluid balance in 24 hours was associated with a 20% increase in mortality.42
Critically ill patients frequently have increased capillary leakiness, leading to tissue edema as a consequence of aggressive fluid therapy.44 Tissue edema impairs oxygen delivery and metabolite diffusion, distorts tissue architecture, and impairs capillary blood flow and lymphatic drainage.44 The adverse effects of tissue edema may be more predominant in encapsulated organs, such as the kidneys and liver, as the increased tissue volume increases interstitial pressure and decreases organ blood flow. Cardiac dysfunction caused by increased preload and myocardial edema further impairs tissue oxygen delivery and may impair renal recovery.44,53 The lungs are perhaps the most sensitive to volume excess, and the development of pulmonary edema is a common life-threatening condition in oliguric dogs and cats on fluid therapy.
In light of these concerns, restricted fluid administration to avoid fluid retention, and early referral for dialysis if azotemia cannot be controlled with the amount of fluid therapy the patient can tolerate, may prove to be a beneficial therapeutic strategy.
Converting Oliguria to Nonoliguria
A decrease in urine production may be due to hemodynamic, intrinsic renal, or postrenal causes. An appropriate renal response to inadequate renal perfusion from hypovolemia or hypotension includes fluid retention with a concomitant decrease in urine volume. Before determining whether oliguria is pathologic or physiologic, renal perfusion should be optimized by ensuring adequate hydration. A volume of fluid equal to 3% to 5% of body weight should be administered to patients that appear normally hydrated because dehydration of less than 5% cannot be detected clinically. In patients that are volume overexpanded, this fluid administration is not necessary. Healthy kidneys can autoregulate renal blood flow at perfusion pressures between 80 to 180 mm Hg, but renal perfusion may be more linear in damaged kidneys.10,12 The mean arterial pressure should be maintained above 60 to 80 mm Hg, or the systolic pressure above 80 to 100 mm Hg when measured by Doppler technology. Apparent anuria due to obstruction of the urinary tract or leakage into the peritoneal, retroperitoneal, or subcutaneous tissues should be excluded before determining that a lack of urine is due to intrinsic renal damage.
Various values have been used to define oliguria, including less than 0.25 mL/kg/hr, less than 0.5 mL/kg/hr, and less than 1 to 2 mL/kg/hr.13 In a hydrated, well-perfused patient, less than 1.0 mL/kg/hr can be considered absolute oliguria, and urine production between 1 and 2 mL/kg/hr in a patient on fluid therapy is considered relative oliguria.10,13 Anuria is defined as essentially no urine production.13 Urine volume above 2 mL/kg/hr is generally considered polyuria.
If pathologic oliguria or anuria persists despite correcting hemodynamic parameters, most clinicians attempt to convert oliguria to nonoliguria using diuretics. There is no evidence that diuretics improve the outcome of AKI, and some surmise that the ability to respond to diuretics is a marker of less severe renal injury associated with a better prognosis. In people, an increase in urine output with diuretic use delays referral for dialysis, perhaps inappropriately.38 However, in veterinary medicine where dialysis is not as readily available to control fluid status, an increase in urine output from diuretic use may allow an increase in the volume of other medications or nutrition, and may be justified even without improvement in renal function.
Mannitol is an osmotic diuretic that causes extracellular volume expansion, which can improve GFR and inhibit sodium reabsorption in the kidney by inhibiting renin. Mannitol also increases tubular flow, which may relieve intratubular obstruction from casts and debris. Mannitol decreases vascular resistance and cellular swelling; increases renal blood flow, the GFR, and solute excretion; protects from vascular congestion and RBC aggregation; scavenges free radicals; induces intrarenal prostaglandin production and vasodilatation; and induces atrial natriuretic peptide release5,10,13,20 Mannitol may blunt the influx of calcium into mitochondria in sublethally injured renal cells, thus decreasing the risk of sublethal injury progressing to lethal damage. Despite the theoretical advantages, no randomized studies have shown a better clinical response with the use of mannitol and volume expansion than with volume expansion alone in people or healthy cats.20,37
Mannitol is administered as a slow intravenous bolus of 0.25 to 1.0 g/kg. If urine production increases, mannitol may be administered as a constant rate infusion (CRI) of 1 to 2 mg/kg/min IV or 0.25 to 0.5 g/kg q 4 to 6 hr.13 Doses in excess of 2 to 4 g/kg/day may cause ARF. Mannitol should not be given to patients that are dehydrated because it will further exacerbate intracellular dehydration. Conversely, it is also contraindicated if overhydration is present, and may worsen pulmonary edema.
Hypertonic dextrose can be used as an osmotic diuretic, if mannitol is not available. A total daily dose of 22 to 66 mL/kg of a 20% dextrose solution should cause hyperglycemia and glucosuria.47
Loop diuretics such as furosemide can increase urine flow without increasing the GFR.14,20,37,40,61 Despite the increase in urine output, loop diuretics do not improve outcome, suggesting that those who respond have less severe renal failure, resulting in a better outcome for a recovery independent of drug therapy.14,20,40,55,61 For example, in one human study, patients that could be converted from oliguric to nonoliguric renal failure had better APACHE scores (a disease severity scoring system used for people in ICU settings) and higher creatinine clearance before treatment, suggesting that they had less severe renal injury.55 Due to the perception that there is a low complication rate associated with the loop diuretics, they are often used despite lack of proven benefit. Loop diuretics inhibit the Na+-K+-2Cl− pump in the luminal cell membrane of the loop of Henle, decreasing transcellular sodium transport. Basal Na+, K+-ATPase activity becomes unnecessary and the medullary oxygen consumption decreases, which is hypothesized to protect the kidney from further injury.25,55 The amount of structural damage to the thick ascending limb of the loop of Henle is subsequently decreased in isolated perfused kidneys.25 Loop diuretics also have renal vasodilatory effects.45 Despite the theoretical reasons to use loop diuretics, one retrospective study in people showed an increased risk of death or failure of renal recovery in the furosemide treatment group. Potential reasons for this finding include a detrimental effect of the drug, delay in recognizing the severity of renal failure with subsequent delay in starting dialysis, or preferential use of loop diuretics in patients with a more severe course of disease.14,38 Loop diuretics may make fluid management easier in people, without changing the outcome.55 In animals, loop diuretics may play a larger role in management because dialysis is not universally available. Established indications for use of furosemide in veterinary medicine include treatment of overhydration or hyperkalemia.13 Furosemide should not be given to patients with aminoglycoside-induced ARF.10
An increase in urine output should be apparent 20 to 60 minutes after an intravenous dose of furosemide of 2 to 6 mg/kg. Ototoxicity has been reported at high doses in people, and doses of 10 to 50 mg/kg may cause adverse effects in animals (apathy and anorexia in cats; hypotension, apathy, and staggering in dogs).10 If there is no response to high doses of furosemide, therapy should be discontinued. If a response does occur, this dose can be administered every 6 to 8 hours. A continuous rate infusion gives a more sustained diuresis with a lower cumulative dose compared with bolus infusion.14 In people, the time to maximal effect with a CRI without a loading dose is 3 hours, and 1 hour with a loading dose. The dose in people is usually 1 to 9 mg/hr (about 0.01 to 0.15 mg/kg/hr) with some reports using doses as high as 0.75 mg/kg/hr.34 In normal dogs, 0.66 mg/kg/hr resulted in diuresis, and doses of 0.25 to 1 mg/kg/hr have been used in dogs and cats with naturally occurring renal failure.1,2,13 Because electrolyte and fluid balance disorders can develop rapidly if a brisk diuresis ensues, frequent monitoring is necessary.
Dopamine has been shown to make some human oliguric patients nonoliguric, but it does not increase the GFR or improve the outcome in people.20,23,40,50 Because of lack of efficacy and side effects associated with dopamine, it is no longer recommended for treatment of oliguric renal failure, except for pressor control.13,56 Selective dopamine agonists may have better efficacy and fewer adverse effects compared to dopamine. There are two dopaminergic receptors, DA-1 and DA-2. Fenoldopam is a selective DA-1 receptor agonist, and as such, it selectively increases cortical and medullary blood flow, sodium excretion, and urine output while maintaining the GFR in people. It does not have DA-2 or α or β adrenergic activity, so it does not cause vasoconstriction, tachycardia, or arrhythmias as seen with dopamine.20,45 Although no studies have shown a benefit, studies with this drug in people are encouraging and larger clinical trials are needed.45 Although some studies in dogs show an improvement in the GFR with fenoldopam, the GFR may decrease in the first few hours after administration.24,39,57
Calcium channel antagonists have been used to decrease damage after renal transplantation.35 Calcium channel antagonists presumptively reverse renal vasoconstriction by causing predominantly preglomerular vasodilatation, inhibit vasoconstriction induced by tubuloglomerular feedback mechanisms, and cause natriuresis independent of the GFR.35 Although the results of one study using diltiazem in addition to standard care in dogs with AKI from leptospirosis were not statistically significant, there was a trend toward increased urine output and more complete resolution of azotemia.35 Whether this will prove to be a beneficial therapy requires further study.
Atrial natriuretic peptide (ANP) increases tubular excretion of salt and water, and stimulates afferent arteriolar dilation and efferent arteriolar constriction, increasing the GFR. Although ANP reduced the severity of experimental ARF from ischemic but not nephrotoxic causes, it has not been effective in clinical trials thus far.20
Monitoring Fluid Therapy
Monitoring fluid status is an ongoing process that must be repeated throughout the day. Physical examination and body weight should be assessed at least twice daily, and the fluid plan adjusted accordingly. Blood pressure should also be monitored. Urine output and other fluid loss should be monitored and correlated to other findings of volume status.
Determining urine volume can be performed by a variety of methods, including placement of an indwelling urinary catheter with a closed collection system, collection of naturally voided urine, metabolic cage, or weighing cage bedding/litter pans (1 mL of urine = 1 g). An indwelling catheter is usually the most precise method, although technical issues such as urine leakage around the catheter or inadvertent disconnection may cause an artifactually low measurement. The risk of an iatrogenic urinary tract infection from the catheter can be decreased by careful attention to catheter and patient hygiene, including cleaning the external portions of the catheter with antiseptic multiple times daily and changing the collection bag and tubing daily.58
Complete collection of voided urine may be difficult in many patients because of a lack of patient cooperation, or urinary incontinence in obtunded or recumbent patients. An accurate scale is necessary to measure small volumes of urine in cats and small dogs, but weighing cage bedding or litter pans before and after use may provide an adequate assessment of urine volume noninvasively in some patients. Fluid losses from vomiting and diarrhea are usually estimated, and other losses such as body cavity drainage (ascites, pleural effusion) or nasogastric tube suctioning can be measured.
Discontinuing Fluid Therapy
With AKI, once a diuresis has been established, polyuria can be quite profound. Monitoring urine production to prevent inadequate fluid administration is necessary in this setting as well as with oliguria or anuria. Weaning these patients off of IV fluids is a crucial step. When the azotemia has resolved or has reached a plateau, the fluid dose can be decreased by 25% per day. If the urine output diminishes by a corresponding degree and the azotemia does not return, continue tapering over 2 to 3 days. If the urine output does not diminish, the kidneys are unable to regulate fluid balance and further reduction in the fluid administered will lead to dehydration. Attempts to taper the fluid rate can be made again after several days, but generally at an even slower rate (10% to 20% per day). It can take weeks for the kidneys to regain the ability to control fluid volume in rare cases.
With CKD, once the prerenal component of the azotemia has resolved, the serum creatinine concentration will usually decrease by at least 1 mg/dL/day (monitored generally every 48 hr). When the creatinine concentration reaches a baseline value (i.e., no longer decreasing despite IV fluid therapy), fluids should be tapered in preparation for patient discharge. Aggressive diuresis should be tapered gradually over about 2 to 3 days.
Outpatient fluid therapy
Despite widespread use of subcutaneous fluid therapy, its role in managing kidney disease has never rigorously been evaluated. Empirically, chronic dehydration or persistent signs of uremia are rational indications for chronic subcutaneous fluid administration. Dose is empirical, based on subjective assessment of the patient’s well-being and on hydration status. A typical starting dose for cats is 100 to 150 mL daily to every other day. Cats subjectively seem to respond more favorably to subcutaneous fluid therapy compared with dogs. Lactated Ringer’s solution or 0.9% saline are appropriate fluids choices. Dextrose containing fluids increase the risk of abscess formation, and Plasmalyte is reported to sting. Many owners can be taught to administer the fluid dose at home, using a new needle for each administration. An administration tube can be implanted in the subcutaneous space for fluid administration without a needle, but this method increases the risk of infection at the site where the tube exits the skin, and subcutaneous fibrosis with subsequent pain during administration and decreased capacity has been observed.
Nutritional support
Renal failure is a highly catabolic disease. Although it is hard to clearly identify the contribution of nutritional management to outcome, poor nutritional status is a major factor in increasing patients’ morbidity and mortality.63 Early enteral feeding can help preserve gastrointestinal mucosal integrity.32 Although renal diets, characterized by restricted phosphorus and restricted quantities of high quality protein, are indicated for treating chronic kidney disease, the ideal diet for acute renal failure has not been identified.27,48 In the absence of information, enteral diets for critically ill animals or people have been used.10
Anorexia is a common problem in the hospitalized renal failure patient. If the appetite does not return within a few days of therapy, feeding tube placement may allow administration of an appropriate quantity of the desired diet, easy administration of oral medications, and is strongly recommended in animals not voluntarily consuming adequate calories.. If vomiting cannot be controlled, partial or total parenteral nutrition (PPN or TPN) may be necessary.
Whether supplementation is enteral or parenteral, the volume that can be administered may be limited in patients who are anuric or oliguric. Most liquid diets suitable for use in a nasoesophageal or nasogastric tube have a caloric density around 1 kcal/mL.33 Provision of 100% of the basal energy requirements generally will require a volume of about twice the insensible fluid requirements. Common formulas for calculation of total parenteral nutrition will also encompass almost twice the insensible fluid requirements.9 The need for nutritional support is an indication for fluid removal via dialysis in the oliguric patient.
Electrolyte abnormalities
Sodium and chloride
The serum sodium concentration may be normal, elevated, or decreased with renal failure. Hypernatremia before fluid therapy indicates excessive free-water loss. Administration of sodium bicarbonate or hypertonic saline may cause hypernatremia. Hyponatremia may indicate excess sodium loss associated with vomiting or pancreatitis, or transient dilutional hyponatremia after administration of mannitol, hypertonic dextrose, or colloid solutions. Hyponatremic solutions (5% dextrose, total parenteral nutrition, enteral formulations) may cause hyponatremia. In many situations, initial dehydration is caused by isonatremic fluid loss with a normal sodium concentration.10,13
The initial fluid deficit should be replaced by an isonatremic solution such as lactated Ringer’s solution, 0.9% saline, or Plasmalyte 148. Continued administration of these solutions over several days may lead to hypernatremia. A lower sodium fluid, such as one half strength LRS or 0.45% saline, with 2.5% dextrose to maintain isotonicity, is a more appropriate fluid choice after the initial rehydration phase. The serum sodium concentration should be monitored regularly and the fluid choice adjusted as needed.
Clinical signs of sodium disorders are unlikely unless rapid changes occur in the sodium concentration, and the signs are generally related to neurologic dysfunction. The rate of sodium change should not be more than 1 mEq/L/hr.17 Chloride changes tend to parallel sodium changes.
Potassium
Hypokalemia
Hypokalemia is more likely to be present in chronic kidney disease compared to acute kidney injury, and is more likely in cats compared with dogs. In cats with CKD, 20% to 30% of cats are hypokalemic.16,19,31 Multiple mechanisms may contribute to the development of hypokalemia, including excessive renal wasting associated with polyuria. Alkalemia worsens hypokalemia as potassium shifts intracellularly in response to translocation of hydrogen ions out of the cells. Vomiting and loop diuretics cause further potassium loss. Decreased oral intake alone generally does not cause hypokalemia, but prolonged anorexia exacerbates hypokalemia. Hypokalemia may be present at admission, particularly with polyuric CKD, or it may develop during hospitalization, particularly in the diuretic phase of recovery from acute kidney injury or with effective diuretic therapy. Hypokalemia is both a cause and effect of renal dysfunction; hypokalemia interferes with urinary concentrating ability, but the renal dysfunction is generally reversible with normalization of the potassium concentration.43
Signs of hypokalemia include muscle weakness (stiff stilted gait in hind legs, cervical ventroflexion, respiratory muscle paralysis). Cardiac changes occur inconsistently but may include ventricular and supraventricular arrhythmias. Rarely are U waves noted on the electrocardiogram. Other signs include fatigue, vomiting, anorexia, and gastrointestinal ileus.13,49 Clinical signs of hypokalemia are likely when the concentration is less than 2.5 mEq/L; a concentration of less than 2.0 may be life threatening.10,13
Hypokalemia is diagnosed by a low serum potassium concentration. Evaluation of the fractional excretion of potassium may help distinguish renal potassium loss (fractional excretion >4%) from nonrenal loss (fractional excretion <4%).15,18
Because excretion of potassium may be impaired with renal failure, treatment in this setting requires judicious supplementation with careful monitoring. However, as normalization of hypokalemia can improve renal function and decrease clinical signs, treatment of hypokalemia should not be overlooked.43 In the hospitalized patient unable to tolerate oral medications, potassium chloride may be added to the intravenous fluids. The rate of supplementation is based on the patient serum potassium concentration, based on an empirically derived scale (Table 22-1). The rate of potassium supplementation should not exceed 0.5 mEq/kg/hr. The serum potassium concentration might decrease during initial fluid therapy despite supplementation because of extracellular fluid volume expansion, increased distal tubular flow, and cellular uptake, especially if administered with dextrose.
Table 22-1 Sliding Scale of Potassium Supplementation
| Serum Potassium Concentration (mEq/L) | Potassium Concentration in Fluids (mEq/L) |
|---|---|
| 3.5-4.5 | 20 |
| 3-3.5 | 30 |
| 2.5-3 | 40 |
| 2-2.5 | 60 |
| <2 | 80 |
In situations with an immediately life-threatening hypokalemic emergency (i.e., respiratory muscle weakness with hypoventilation, hypokalemic cardiac arrhythmias), some recommend administering an intravenous bolus of KCl. This should only be undertaken with constant EKG monitoring because a rapid potassium bolus could potentially cause a fatal arrhythmia. To calculate the amount to administer, subtract the patient [K] from the desired [K] of 3 mEq/L. Calculate the blood volume (8% of body weight in kilograms in dogs, 6% in cats), and multiply the blood volume by 60% to estimate the plasma volume. Multiply the plasma volume by the difference between the measured and desired potassium concentration to determine the number of milliequivalents of KCl to administer as an IV bolus over 1 to 5 minutes through a central vein. Check the serum potassium 5 minutes later. A second bolus, calculated from the new [K], can be administered, but use caution and administer more slowly as the serum [K] approaches 3 mEq/L.49
Once oral intake is possible, potassium gluconate can be administered. A dose of 5 to 10 mEq per day divided into 2 to 3 doses is used to replenish potassium, followed by 2 to 4 mEq/day for maintenance.18 Potassium citrate (40 to 60 mg/kg/day divided into 2 to 3 doses) is an alternative to potassium gluconate that also helps to correct acidosis. Potassium chloride can be added to subcutaneous fluids up to a concentration of 35 mEq/L.
For patients on intravenous potassium supplementation, frequent monitoring (once to multiple times daily) is recommended. During potassium repletion on an outpatient basis, monitoring every 7 to 14 days until a stable maintenance dose is reached is recommended.43 If hypokalemia remains refractory to standard supplementation, hypomagnesemia may be present, and magnesium supplementation may be necessary.
Hyperkalemia
Renal excretion is the major mechanism for removing potassium from the body, and chronic hyperkalemia is unlikely to occur with normal renal function. Hyperkalemia is more likely to develop in oliguric or anuric acute renal failure, and usually does not occur in chronic kidney disease unless oliguria or severe metabolic acidosis are present.10 Metabolic acidosis from mineral acids (e.g., NH4Cl, HCl) but not organic acids (e.g., lactic acid, ketoacids) causes translocation of potassium out of cells as hydrogen ions enter the cells. CKD patients may have a reduced ability to tolerate an acute potassium load and may take 1 to 3 days to reestablish external potassium balance after a potassium load.15 Mild hyperkalemia seems relatively common in stable patients on angiotensin converting enzyme inhibitor therapy; my experience is that most do not exceed 6.5 mEq/L, but clinical significance of this is uncertain. Hyperkalemia and azotemia are common with hypoadrenocorticism and acute tumor lysis syndrome.18
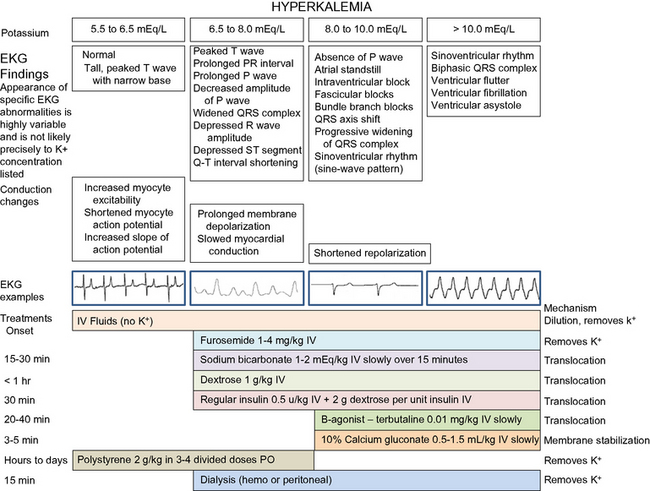
Hyperkalemia can be an immediately life-threatening electrolyte disorder. The increase in extracellular potassium changes the electrical potential of excitable cells. The myocardium is relatively resistant compared to the conduction cells. Typical EKG changes include bradycardia; tall, spiked T waves; shortened QT interval; wide QRS complex; and a small, wide, or absent P wave (Figure 22-1). Severe hyperkalemia can lead to a sinoventricular rhythm, ventricular fibrillation, or standstill. Muscle weakness may be present with a serum potassium concentration above 8 mEq/L.18 Characteristic EKG changes may require emergency therapy before results of serum potassium concentration are available. Pseudohyperkalemia may occur ex vivo if the red cell potassium content is high, as in Akita dogs.
Calcium gluconate 10% (0.5 to 1.0 mL/kg IV to effect, given slowly) can be used in critical situations to restore cardiac membrane excitability, but it does not decrease potassium concentration. During infusion the ECG must be monitored, and the infusion slowed or stopped if the arrhythmia worsens. The cardiac effects should be apparent within minutes. Despite a rapid onset of action, the duration of its effect is less than 1 hour.15 Calcium administration increases the risk of soft tissue mineralization if hyperphosphatemia is present.
Several methods can be used to translocate potassium intracellularly. Regular insulin (0.5 units/kg IV) has an effect within 20 to 30 minutes. Dextrose (1 to 2 g/unit of insulin as an IV bolus, then 1 to 2 g/unit of insulin in intravenous fluids administered over the next 4 to 6 hours) is necessary to prevent hypoglycemia when insulin is used. Dextrose induces endogenous insulin release in nondiabetic patients and can be used to control mild to moderate hyperkalemia without concurrent insulin administration at a dose of 0.25 to 0.5 g/kg IV.
Metabolic acidosis from mineral acids causes an extracellular shift of K+ as H+ increases intracellularly. Correction of metabolic acidosis with bicarbonate allows an intracellular shift of K+ as the H+ is combined with 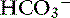 and removed. The dose of sodium bicarbonate used to treat hyperkalemia is based on the base deficit, or 1 to 2 mEq/kg IV over 10 to 20 minutes. Sodium bicarbonate is contraindicated if partial pressure of carbon dioxide (Pco2) is elevated or metabolic alkalosis is present, and it may cause hypernatremia or paradoxical CNS acidosis. If the ionized calcium concentration is low, dextrose is preferred to bicarbonate because alkalemia exacerbates hypocalcemia.10
and removed. The dose of sodium bicarbonate used to treat hyperkalemia is based on the base deficit, or 1 to 2 mEq/kg IV over 10 to 20 minutes. Sodium bicarbonate is contraindicated if partial pressure of carbon dioxide (Pco2) is elevated or metabolic alkalosis is present, and it may cause hypernatremia or paradoxical CNS acidosis. If the ionized calcium concentration is low, dextrose is preferred to bicarbonate because alkalemia exacerbates hypocalcemia.10
The β-agonist albuterol has been to treat hyperkalemia in people because it causes an intracellular shift of potassium.10 The cation exchange resin sodium polystyrene sulfonate (Kayexalate, Kionex) can be administered orally or by enema at a dose of 2 g/kg in 3 to 4 divided doses as a suspension in 20% sorbitol.13 This substance binds potassium in the GI tract and releases sodium. It takes several hours to work, and side effects include hypernatremia and constipation.
The potassium lowering effects of these drugs, with the exception of polystyrene sulfonate, are temporary. Serum potassium concentrations gradually rise again within several hours after administration unless urine production is induced. Once even minimal urine production resumes, serum potassium concentrations usually decrease. Peritoneal or hemodialysis may be necessary to ultimately control potassium if oliguria or anuria persist.
Certain drugs that contribute to hyperkalemia should be avoided, and these drugs include nonspecific β-blockers, digoxin, angiotensin converting enzyme inhibitors, angiotensin receptor antagonists, nonsteroidal antiinflammatory drugs, potassium-sparing diuretics (spironolactone, amiloride, triamterene), high doses of trimethoprim, cyclosporine, and total parenteral nutrition.48
Calcium
Most of the body calcium is found in the skeleton as hydroxyapatite. The extracellular calcium occurs in three fractions: ionized calcium (55%), which is the active form; protein bound (35%), a storage form generally bound to albumin; and complexed calcium (10%), which is bound to substances such as citrate, lactate, bicarbonate, or phosphate. Total calcium (including all three fractions) is the most common measure of calcium, although ionized calcium measurement is becoming more readily available in practice settings.
There are multiple reasons for calcium disorders in patients with renal failure. An acute decrease in glomerular filtration may lead to an abrupt increase in phosphorus, causing a decrease in calcium by the law of mass action. The decrease in calcium stimulates parathyroid hormone synthesis and release, which works toward increasing the calcium back to normal. On the other hand, chronic kidney failure may cause parathyroid hyperplasia, which rarely leads to hypercalcemia. Metabolic acidosis increases the ionized calcium fraction, although over half of dogs with CKD and metabolic acidosis were hypocalcemic.29
Based on ionized calcium, 36% to 56% of dogs with CKD are hypocalcemic, 20% to 55 % are normocalcemic, and 9% to 24% are hypercalcemic.29,52 Based on total calcium, 8% to 19% are hypocalcemic, 60% to 76% are normocalcemic, and 16% to 22% are hypercalcemic. The concordance between ionized calcium and total calcium is poor, especially in dogs with CKD.29,52
Symptomatic hypocalcemia (tetany) occurs infrequently in renal disease. Hypocalcemia may be more severe with antifreeze induced acute renal failure because antifreeze contains phosphate that can cause severe hyperphosphatemia, and the ethylene glycol is converted to oxalate, which complexes calcium. Treatment with calcium increases the risk of soft-tissue mineralization in hyperphosphatemic patients. The minimal dose of calcium gluconate that controls clinical signs should be used when therapy is needed. Calcium gluconate 10% can be used at a dose of 0.5 to 1.5 mL/kg IV over 20 to 30 minutes. As when treating hyperkalemia, monitor the EKG during infusion.
Hypercalcemia based on total calcium is usually mild and associated with normal ionized calcium; no specific treatment is necessary. If the ionized calcium is elevated, treatment is warranted. Hypercalcemia may respond to fluid therapy, although calcium containing fluids (such as LRS) should be avoided. Saline (0.9% NaCl) is an ideal fluid choice, as the sodium content increases calciuresis. Furosemide also promotes urinary calcium loss. Sodium bicarbonate therapy decreases ionized calcium as more calcium binds to serum proteins. Hypercalcemia from renal failure is not likely to be glucocorticoid-responsive.51 Calcitonin or bisphosphonates could be considered if the hypercalcemia is severe, although bisphosphonates can induce renal failure.51
Magnesium
Magnesium concentrations may be elevated in severe renal failure because the kidneys are the major route of excretion of magnesium, but specific therapy is generally not necessary. Supplemental magnesium, such as that found in some phosphate binders, should be avoided in those situations. Hypomagnesemia may occur with polyuric renal failure. Hypokalemia may be refractory to therapy if concurrent hypomagnesemia is present. In those cases, correction of the magnesium deficit may be necessary to correct the hypokalemia. Magnesium sulfate or magnesium chloride can be used for intravenous supplementation, and various forms are available for oral supplementation.3
Phosphorus
Dietary phosphorus is readily absorbed from the gastrointestinal tract and excreted by the kidneys. Decreased excretion commonly leads to hyperphosphatemia in both acute and chronic renal failure. Intravenous fluid therapy may partially control phosphorus concentration by addressing the hemodynamic component and improving renal blood flow. There are no other specific treatments to decrease serum phosphorus in the acute stage. A phosphate-restricted diet is recommended for long-term control of hyperphosphatemia. Because protein is phosphate-rich, this necessitates a protein-restricted diet. While diet may be sufficient to control phosphorus concentration in mild to moderate cases, diet alone is generally not sufficient as the renal disease worsens.
Phosphate binders prevent absorption of phosphorus in ingested food in the gastrointestinal tract. Aluminum containing phosphate binders are commonly used in veterinary medicine. They are rarely used in people because of the potential for complications from long-term exposure to aluminum, including anemia and neurologic disorders. These effects are rarely noted in animals unless receiving chronic hemodialysis. Aluminum hydroxide or aluminum carbonate are administered at 30 to 90 mg/kg/day divided with meals. Calcium acetate and calcium carbonate are alternatives to aluminum containing binders. They may cause hypercalcemia, and should be avoided in patients with an elevated calcium concentration. Calcium carbonate combined with chitosan is a veterinary specific product for binding phosphorus. Several newer phosphate binders such as sevelamer hydrochloride or lanthanum carbonate are available for people, but there is limited veterinary experience with them yet. With all phosphate binders, dose is adjusted by serial determination of serum phosphorus concentration. Because of their binding properties, they can interfere with absorption of orally administered medications, especially antibiotics.
Metabolic acidosis
Metabolic acidosis is a common acid-base disturbance in kidney failure. Daily H+ load is excreted with NH3 as 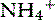 or with phosphate as
or with phosphate as 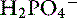 . With kidney failure, the kidneys are unable to excrete H+ and cannot reabsorb
. With kidney failure, the kidneys are unable to excrete H+ and cannot reabsorb 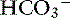 . There may be some contribution from lactic acidosis from dehydration and poor perfusion. If acidosis persists after correcting dehydration and perfusion (and thus any lactic acidosis component), intravenous sodium bicarbonate therapy can be considered. Sodium bicarbonate therapy is usually reserved for patients with a pH less than 7.2 or HCO3 less than 12 mEq/L. Treatment with sodium bicarbonate is geared toward causing acid (H+) to combine with bicarbonate (
. There may be some contribution from lactic acidosis from dehydration and poor perfusion. If acidosis persists after correcting dehydration and perfusion (and thus any lactic acidosis component), intravenous sodium bicarbonate therapy can be considered. Sodium bicarbonate therapy is usually reserved for patients with a pH less than 7.2 or HCO3 less than 12 mEq/L. Treatment with sodium bicarbonate is geared toward causing acid (H+) to combine with bicarbonate (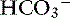 ) to form H2CO3, which dissociates to H2O and CO2. If the lungs are unable to eliminate the CO2, the reaction does not proceed. Bicarbonate administration in this situation can increase the Pco2 and can lead to paradoxical CNS acidosis due to the ability of CO2 to diffuse into the CNS where it can be converted back to acid (H+). Sodium bicarbonate treatment is also contraindicated with hypernatremia. The bicarbonate dose can be calculated from the formula: 0.3 × body weight (kg) × base deficit, where the base deficit = 24 − patient HCO3. Give
) to form H2CO3, which dissociates to H2O and CO2. If the lungs are unable to eliminate the CO2, the reaction does not proceed. Bicarbonate administration in this situation can increase the Pco2 and can lead to paradoxical CNS acidosis due to the ability of CO2 to diffuse into the CNS where it can be converted back to acid (H+). Sodium bicarbonate treatment is also contraindicated with hypernatremia. The bicarbonate dose can be calculated from the formula: 0.3 × body weight (kg) × base deficit, where the base deficit = 24 − patient HCO3. Give 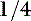 to
to 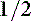 dose IV and an additional
dose IV and an additional 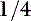 to
to 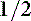 dose in the IV fluids over the next 2 to 6 hours. Adjust any subsequent doses based on serial evaluation of blood gas determinations.
dose in the IV fluids over the next 2 to 6 hours. Adjust any subsequent doses based on serial evaluation of blood gas determinations.
Oral alkalinizing agents can be used for treatment of chronic acidosis. Potassium citrate (40 to 75 mg/kg PO q12h) simultaneously addresses metabolic acidosis and hypokalemia. Oral sodium bicarbonate (8 to 12 mg/kg PO q12h) is more palatable in tablet form compared with powder. Doses should be adjusted based on the individual patient response.
Conclusions
Careful fluid therapy is the most important aspect of treating a uremic crisis, and involves astute assessment of hydration status with frequent reassessment, appropriate fluid type and rate, and flexibility to respond to changes in the patient’s clinical status. Electrolyte and acid-base disturbances are common with renal failure, and frequently require specific therapy.
1 Adin D.B., Hill R.C., Scott K.C. Short-term compatibility of furosemide with crystalloid solutions. J Vet Intern Med. 2003;17(5):724-726.
2 Adin D.B., Taylor A.W., Hill R.C., Scott K.C., Martin F.G. Intermittent bolus injection versus continuous infusion of furosemide in normal adult greyhound dogs. J Vet Intern Med. 2003;17(5):632-636.
3 Bateman S. Disorders of magnesium: magnesium deficit and excess. In: DiBartola S.P., editor. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd ed. St. Louis: Saunders Elsevier; 2006:210-226.
4 Behrend E., Grauer G.F., Mani I., Groman R., Salman M., Greco D. Hospital-acquired acute renal failure in dogs: 29 cases (1983–1992). JAMA. 1996;208(4):537-541.
5 Better O.S., Rubinstein I., Winaver J.M., Knochel J.P. Mannitol therapy revisited (1940–1997). Kidney Int. 1997;51:866-894.
6 Bouchard J., Mehta R.L. Fluid accumulation and acute kidney injury: consequence or cause. Curr Opin Crit Care. 2009;15(6):509-513.
7 Bouchard J., Soroko S.B., Chertow G.M., et al. Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int. 2009;76(4):422-427.
8 Cerda J., Sheinfeld G., Ronco C. Fluid overload in critically ill patients with acute kidney injury. Blood Purif. 2010;29(4):11-18.
9 Chan D. Parenteral nutritional support. Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine. St. Louis: Saunders Elsevier. 2005;vol. 1:586-591.
10 Chew D.J., Gieg J.A. Fluid therapy during intrinsic renal failure. In: DiBartola S.P., editor. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd ed. St. Louis: Saunders Elsevier; 2006:518-540.
11 Cohn L.A., Kerl M.E., Lenox C.E., Livingston R.S., Dodam J.R. Response of healthy dogs to infusions of human serum albumin. Am J Vet Res. 2007;68(6):657-663.
12 Conger J.D. Vascular alterations in acute renal failure: roles in initiation and maintenance. In: Molitoris B.A., Finn W.F., editors. Acute renal failure: a companion to Brenner & Rector’s the kidney. Philadelphia: W.B. Saunders; 2001:13-29.
13 Cowgill L.D., Francey T. Acute uremia. Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine, 6th ed, vol. 2. Philadelphia: Elsevier Saunders, 2005;1731-1751.
14 De Vriese A.S. Prevention and treatment of acute renal failure in sepsis. J Am Soc Nephrol. 2003;14:792-805.
15 DiBartola S.P., de Morais H.A. Disorders of potassium: hypokalemia and hyperkalemia. In: DiBartola S.P., editor. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd ed. St. Louis: Saunders Elsevier; 2006:91-121.
16 DiBartola S.P., Rutgers H.C., Zack P.M., Tarr M.J. Clinicopathologic findings associated with chronic renal disease in cats: 74 cases (1973–1984). J Am Vet Med Assoc. 1987;190(9):1196-1202.
17 DiBartola S.P. Disorders of sodium and water: hypernatremia and hyponatremia. In: DiBartola S.P., editor. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd ed. St. Louis: Saunders Elsevier; 2006:47-79.
18 DiBartola S.P. Management of hypokalemia and hyperkalemia. J Feline Med Surg. 2001;3:181-183.
19 Elliott J., Barber P.J. Feline chronic renal failure: clinical findings in 80 cases diagnosed between 1992 and 1995. J. Small Anim Pract. 1998;39:78-85.
20 Finn W.F. Recovery from acute renal failure. In: Molitoris B.A., Finn W.F., editors. Acute renal failure: a companion to Brenner & Rector’s the kidney. Philadelphia: W.B. Saunders; 2001:425-450.
21 Francis A.H., Martin L.G., Haldorson G.J., et al. Adverse reactions suggestive of type III hypersensitivity in six healthy dogs given human albumin. J Am Vet Med Assoc. 2007;230(6):873-879.
22 Gookin J.L., Atkins C.E. Evaluation of the effect of pleural effusion on central venous pressure in cats. J Vet Intern Med. 1999;13(6):561-563.
23 Group AaNZICSCT. Low-dose dopamine in patients with early renal dysfunction: a placebo-controlled randomised trial. Lancet. 2000;356:2139-2143.
24 Halpenny M., Markos F., Snow H.M., et al. Effects of prophylactic fenoldopam infusion on renal blood flow and renal tubular function during acute hypovolemia in anesthetized dogs. Crit Care Med. 2001;29(4):855-860.
25 Heyman S.N., Rosen S., Epstein F.H., Spokes K., Brezis M.L. Loop diuretics reduce hypoxic damage to proximal tubules of the isolated perfused rat kidney. Kidney Int. 1994;45:981-985.
26 Jacob F., Polzin D.J., Osborne C.A., et al. Association between initial systolic blood pressure and risk of developing a uremic crisis or of dying in dogs with chronic renal failure. J Am Vet Med Assoc. 2003;222(3):322-329.
27 Jacob F., Polzin D.J., Osborne C.A., et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs. J Am Vet Med Assoc. 2002;220(8):1163-1170.
28 Kirby R., Rudloff E. Crystalloid and colloid fluid therapy. Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine, 6th ed, vol. 1. St. Louis: Elsevier Saunders, 2005;412-424.
29 Kogika M.M., Lustoza M.D., Notomi M.K., Wirthl V.A.B.F., Mirandola R.M.S., Hagiwara M.K. Serum ionized calcium in dogs with chronic renal failure and metabolic acidosis. Vet Clin Pathol. 2006;35:441-445.
30 Lin S.M., Huang C.D., Lin H.C., Liu C.Y., Wang C.H., Kuo H.P. A modified goal-directed protocol improves clinical outcomes in intensive care unit patients with septic shock: a randomized controlled trial. Shock. 2006;26(6):551-557.
31 Lulich J.P., Osborne C.A., O’Brien T.D., Polzin D.J. Feline renal failure: questions, answers, questions. Compendium Continuing Educ Practicing Vet. 1992;14(2):127-153.
32 Macintire D.K. Bacterial translocation: clinical implications and prevention. In: Bonagura J.D., editor. Kirk’s current veterinary therapy XIII: small animal practice. Philadelphia: W.B. Saunders; 2000:201-203.
33 Marks S.L. The principles and implementation of enteral nutrition. Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine, 6th ed, vol. 1. St. Louis: Saunders Elsevier, 2005;596-598.
34 Martin S.J., Danziger L.H. Continuous infusion of loop diuretics in the critically ill: A review of the literature. Crit Care Med. 1994;22(8):1323-1329.
35 Mathews K.A., Monteith G. Evaluation of adding diltiazem therapy to standard treatment of acute renal failure caused by leptospirosis: 18 dogs (1998–2001). J Vet Emerg Crit Care. 2007;17(2):149-158.
36 Mathews K.A. Monitoring fluid therapy and complications of fluid therapy. In: DiBartola S.P., editor. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd ed. St. Louis: Saunders Elsevier; 2006:377-391.
37 McClellan J.M., Goldstein R.E., Erb H.N., Dykes N.L., Cowgill L.D. Effects of administration of fluids and diuretics on glomerular filtration rate, renal blood flow, and urine output in healthy awake cats. Am J Vet Res. 2006;67:715-722.
38 Mehta R.L., Pascual M.T., Soroko S., Chertow G. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288(20):2547-2553.
39 Murray C., Markos F., Snow H.M. Effects of fenoldopam on renal blood flow and its function in a canine model of rhabdomyolysis. Eur J Anaesthesiol. 2003;20:711-718.
40 Nolan C.R., Anderson R.J. Hospital-Acquired acute renal failure. J Am Soc Nephrol.. 1998;9(4):710-718.
41 Otto C.M. Shock. Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine, 6th ed, vol. 1. St. Louis: Saunders Elsevier, 2005;455-457.
42 Payen D., de Pont A.C., Sakr Y., Spies C., Reinhart K., Vincent J.L. A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care. 2008;12(3):R74.
43 Polzin D.J., Osborne C.A., Ross S. Chronic kidney disease. Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine, 6th ed, vol. II. St. Louis: Elsevier Saunders, 2005;1756-1785.
44 Prowle J.R., Echeverri J.E., Ligabo E.V., Ronco C., Bellomo R. Fluid balance and acute kidney injury. Nat Rev Nephrol. 2010;6(2):107-115.
45 Pruchnicki M.C., Dasta J.F. Acute renal failure in hospitalized patients: Part II. Ann Pharmacother. 2002;36:1430-1442.
46 Roche A.M., James M.F. Colloids and crystalloids: does it matter to the kidney? Curr Opin Crit Care. 2009;15(6):520-524.
47 Ross L.R. Fluid therapy for acute and chronic renal failure. Vet Clin North Am Small Anim Pract. 1989;19:343-359.
48 Ross S., OSborne C.A., Polzin D.J., Lowry S.R., Kirk C.A., Koehler L. Clinical evaluation of effects of dietary modification in cats with spontaneous chronic renal failure (abstract). J Vet Intern Med. 2005.
49 Rubin S.I., LeClerc S.M. A practical guide to recognizing and treating hypokalemia. Vet Med. 2001;96:462-476.
50 Rudis M.I. Low-dose dopamine in the intensive care unit: DNR or DNRx. Crit Care Med. 2001;29:1638-1639.
51 Schenck P.A., Chew D.J., Nagode L.A., Rosol T.J. Disorders of calcium: hypercalcemia and hypocalcemia. In: DiBartola S.P., editor. Fluid, electrolyte, and acid-base disorders in small animal practice. 3rd ed. St. Louis: Saunders Elsevier; 2006:122-194.
52 Schenck P.A., Chew D.J. Prediction of serum ionized calcium concentration by use of serum total calcium concentration in dogs. Am J Vet Res. 2005;66:1330-1336.
53 Schrier RW. Fluid administration in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol Feb 18
54 Segev G. Methods of measuring water and body composition. Paper presented at: Advanced Renal Therapies Symposium. New York, 2008.
55 Shilliday I.R., Quinn K.J., Allison M.E.M. Loop diuretics in the management of acute renal failure: a prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant. 1997;12:2592-2596.
56 Sigrist N.E. Use of dopamine in acute renal failure. J Vet Emerg Crit Care. 2007;17(2):117-126.
57 Simmons J.P., Wohl J.S., Schwartz D.D., Edwards H.G., Wright J.C. Diuretic effects of fenoldopam in healthy cats. J Vet Emerg Crit Care. 2006;16(2):96-103.
58 Smarick S.D., Haskins S.C., Aldrich J., et al. Incidence of catheter-associated urinary tract infection among dogs in a small animal intensive care unit. J Am Vet Med Assoc. 2004;224(12):1936-1940.
59 Syme H.M., Barber P.J., Markwell P.J., Elliott J. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc. 2002;220(12):1799-1804.
60 Townsend D.R., Bagshaw S.M. New insights on intravenous fluids, diuretics, and acute kidney injury. Nephron Clin Pract. 2008;109:206-216.
61 Vijayan A., Miller S.B. Acute renal failure: prevention and nondialytic therapy. Semin Nephrol. 1998;18(5):523-532.
62 Waddell L.S. Hypotension. Ettinger S.J., Feldman E.C., editors. Textbook of veterinary internal medicine, 6th ed, vol. 1. St. Louis: Elsevier Saunders, 2005;480-483.
63 Wooley J.A., Btaiche I.F., Good K.L. Metabolic and nutritional aspects of acute renal failure in critically ill patients requiring continuous renal replacement therapy. Nutr Clin Pract. 2005;20(2):176-191.
64 Worwag S., Langston C.E. Feline acute intrinsic renal failure: 32 Cats (1997–2004). J Am Vet Med Assoc. 2008;232(5):728-732.