2 Physiologic Factors in Rehabilitation
The effects of immobilization on bone and connective tissue have been widely reported in the literature. The evolution from immobilization to implementation of early motion programs has become accepted practice in the orthopedic community. Proper use of specific exercises can accelerate the healing process, whereas lack of exercise during the early stages of rehabilitation can result in long-term functional impairment. Caution must be observed, however, because exercise that is too vigorous can also result in undesired effects on healing tissues. Immobilization initially results in loss of tissue substrate, with subsequent loss of basic tissue components. The reversibility of these changes appears to depend on the length of immobilization.
To understand the body’s response to immobilization and remobilization, its normal reaction to injury must be addressed. The sequence of events that transpire after trauma to a joint can cause cartilage degradation, chronic joint synovitis, and stretching of the joint capsule as a result of increased effusion.
Reaction to injury
Inflammation is the body’s response to injury, and optimally, it results in healing of tissues by replacement of damaged and destroyed tissue, along with associated restoration of function.1 Repeated injury or microtrauma to a specific region can cause a cumulative effect that results in adverse effects on the joint and its surrounding structures. The inflammatory response is the same, regardless of the location and nature of the injurious agent, and consists of chemical, metabolic, permeability, and vascular changes, followed by some form of repair.2
Figure 2-1 illustrates the primary and secondary injuries affiliated with trauma and the associated inflammation and repair processes. Primary injury is the result of trauma that directly injures the cells themselves. Secondary injury (sometimes referred to as secondary hypoxia) is precipitated by the body’s response to trauma. This response includes decreased blood flow to the traumatized region as a result of vasoconstriction, which decreases the amount of oxygen to the injured area. Thus, additional cells die because of secondary hypoxia; these dead cells organize and ultimately develop into a hematoma.
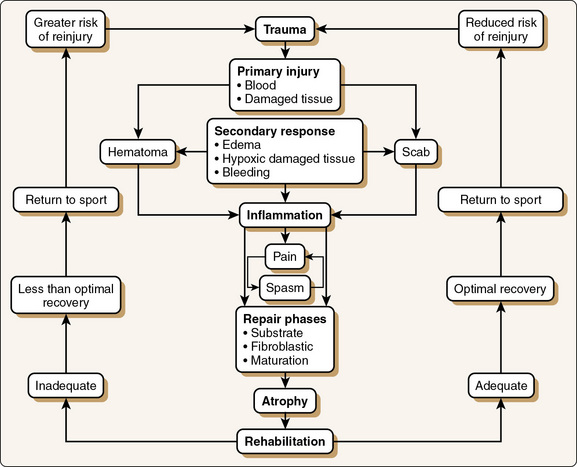
Figure 2-1 Cycle of athletic injury.
(From Booher, J.M., and Thibodeau, G.A. [1989]: Athletic Injury Assessment. St. Louis, Times Mirror/Mosby.)
Cell degeneration or cell death perpetuates the release of potent substances that can induce vascular changes. The most common of these substances is histamine, which increases capillary permeability and allows fluid and blood cells to escape into the interstitial spaces. In the noninjured state, plasma and blood proteins escape from capillaries by osmosis and diffusion into the interstitial spaces but are reabsorbed. This homeostasis is maintained by colloids present within the blood system. However, trauma leads to increased capillary permeability as a result of the release of cell enzymes, which allows blood plasma and proteins to escape into surrounding tissues. Concurrently, the concentration of colloids greatly increases in the surrounding tissues, thus reversing the colloidal effect. Rather than the colloids pulling fluid back into the capillaries, the presence of colloids outside the vessels causes additional fluid to be pulled into the interstitial tissues with resultant swelling and edema.
The body’s reaction after injury is to mobilize and transport the defense components of the blood to the injured area. Initially, blood flow is reduced, which allows white blood cells to migrate to the margins of the blood vessels. These cells adhere to the vessel walls and eventually travel into the interstitial tissues. When in the surrounding tissues, the white cells remove irritating material by the process of phagocytosis. Neutrophils are the first white blood cells to arrive, and they normally destroy bacteria. However, because bacteria are not usually associated with athletic injuries, these neutrophils die.2 Macrophages then appear and phagocytize the dead neutrophils, cellular debris, fibrin, red cells, and other debris that may impede the repair process.2 Unfortunately, destruction of the neutrophils results in the release of active proteolytic enzymes (i.e., enzymes that hasten the hydrolysis of proteins into simpler substances), which can attack joint tissues, into the surrounding inflammatory fluid.3 Although this is the natural response of ridding the body of toxic or foreign material, prolongation of this process can damage surrounding joint structures.
After the inflammatory debris has been removed, repair can begin. Cleanup by macrophages and repair often occur simultaneously. However, for repair to occur, enough of the hematoma must be removed to permit ingrowth of new tissue. Thus, the size of the hematoma or the amount of the exudate is directly related to the total healing time. If the size of the hematoma can be minimized, healing can begin earlier and total healing time is reduced.2
The primary role of the rehabilitation specialist during the acute phase of injury is to decrease inflammation and prevent damaging secondary effects, such as decreased range of motion (ROM), decreased muscle strength, and prolonged edema. The presence of inflammation must be regarded with caution because too much activity can prolong the inflammation and increase pain. Inflammation is controlled with ice, rest, and electrical stimulation, such as electrical galvanic stimulation or transcutaneous electrical stimulation. Secondary effects are prevented with gentle ROM exercises, isometrics, and avoidance of maladaptive postures or gait patterns.
Response of Joint Structures to Injury
As a result of the inflammatory process, each joint structure responds differently to injury (Fig. 2-2). The reaction of the synovial membrane to injury involves the proliferation of surface cells, an increase in vascularity, and gradual fibrosis of subsynovial tissue. Posttraumatic synovitis is not uncommon after most injuries. Continued mechanical irritation can produce chronic synovitis, which results in the reversal of normal synovial cell ratios.3,4 Changes in synovial fluid occur as a result of alterations in the synovial membrane. Cells are destroyed as a consequence of the synovitis; white blood cells ingest the lysosomes and proteolytic enzymes. This ingestion and the subsequent death of white blood cells in the transudate result in the further release of proteolytic enzymes. The overall consequence is spawning of a vicious inflammatory cycle, which can keep reactive synovitis active for some time, even without further trauma (Fig. 2-3).5 As chronic posttraumatic effusions occur, changes in the synovial membrane can continue, with progressing sclerotic alterations as a sequela.6 If conservative treatment consisting of antiinflammatory medications, rest, aspiration, and application of cold does not relieve the symptoms, synovectomy may be necessary.
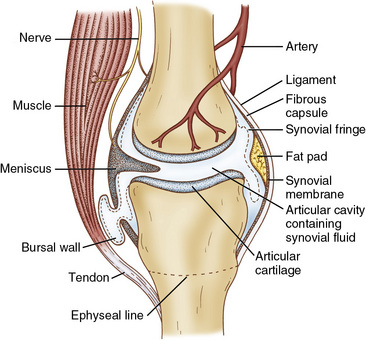
Figure 2-2 Synovial joint structures.
(From Wright, V., Dowson, D., and Kerry, J. [1973]: The structure of joints. Int. Rev. Connect. Tissue Res., 6:105–125.)
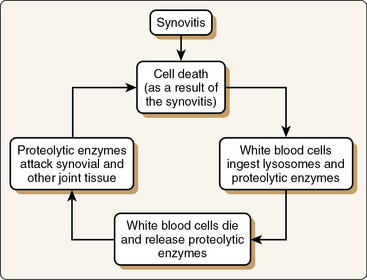
Figure 2-3 Continued mechanical irritation of a joint can result in perpetuation of chronic synovitis in a vicious inflammatory cycle. This keeps the reactive synovitis alive even without further trauma.
Articular cartilage lesions within a synovial joint or meniscal lesions within the knee, whether acute or chronic, are invariably accompanied by increased synovial effusion. Surgical correction is often required to prevent secondary damage to other joint structures as a result of prolonged inflammation. After the problem has been corrected, the synovial irritation usually subsides. If, however, the problem is left uncorrected, tissues not injured by the original trauma can be damaged from the prolonged inflammation, thus resulting in progressive degradation of the synovial membrane.
Fortunately, when the inflammation begins to abate, synovial tissue can regenerate remarkably well, an ability that possibly stems from its excellent blood supply and origin. Synovium regenerates completely within several months into tissue that is indistinguishable from normal tissue.3 Acute and chronic synovitis directly affects the amount and content of synovial fluid produced. Synovitis can result in an increased protein level within the synovial fluid. In addition, chronic synovitis can cause a reduction in the viscosity of synovial fluid and a decrease in the concentration of hyaluronic acid.7 The concentration of hyaluronic acid is directly related to synovial fluid viscosity. Minor joint trauma results in no change in either the concentration or molecular weight of hyaluronic acid.7 As the severity of trauma increases, however, the concentration of hyaluronic acid decreases to levels below normal, and when the inflammatory process becomes sufficiently disruptive, joint-lining cells fail not only to maintain the hyaluronic acid concentration but also to maintain normal polymer weight.7
Hemarthrosis, or bleeding into a joint, can have an effect on joint structures. When a vascular joint structure is damaged, the synovial fluid has a lower sugar concentration, blood clots can be found in the synovial fluid, and fibrinogen can be detected as a result of bleeding into the joint. Although the average time for natural evacuation of a hemarthrosis is approximately 4 days,8 it depends on individual factors, such as the magnitude of injury, the nature of the structures injured, and the individual’s activity level after injury. The presence of blood in a joint has a damaging effect on articular cartilage, with a potentially irreversible decrease in proteoglycan synthesis.9 In addition, younger individuals with hemarthrosis have been shown to have a greater decrease in proteoglycan synthesis and a slower return to normal rates of synthesis.10
The absorption rate of solutions from the joint space is inversely proportional to the size of the solutes: the larger the molecules, the slower the clearance. Clinically, absorption from a joint is increased by active or passive ROM, massage, intraarticular injection of hydrocortisone, or acute inflammation, whereas the effect of external compression is variable.11
The reaction of the joint capsule to injury is similar to that of the synovial membrane. If the inflammatory process continues, the joint capsule eventually becomes a more fibrous tissue, and effusion into the joint cavity can lead to stretching of the capsule and its associated ligaments. The higher the hydrostatic pressure and volume of effusion, the faster the fluid reaccumulates after aspiration.3,12 Conversely, a significant rise in intraarticular hydrostatic pressure contributes to the joint damage by stretching the capsule and associated ligaments.
The load-carrying surfaces of the synovial joint are covered with a thin layer of specialized connective tissue referred to as articular cartilage. The response of articular cartilage to trauma is not unlike that of the other structures within the joint. The mechanical properties of articular cartilage are readily affected by enzymatic degradation of the components of cartilage. This can occur after acute inflammation, synovectomy, immobilization, or other seemingly minor insults.13 When articular cartilage loses its content of proteoglycan (a protein aggregate that helps establish the resiliency and resistance of articular cartilage to deformation), the physical properties of the cartilage are changed; this renders the collagen fibers susceptible to mechanical damage.13 The opposite is also true: if a joint loses collagen in the outer layer of the articular surface, the proteoglycans beneath are subject to damage. As a result of either of these two types of degradation, articular cartilage can erode and leave denuded bone, which results in early, irreversible osteoarthritis or degenerative joint disease (Fig. 2-4).
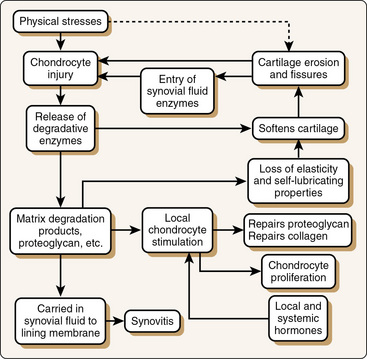
Figure 2-4 Postulated final pathway of cartilage degeneration.
(From Howell, D.S. [1976]: Osteoarthritis—Etiology and pathogenesis. In: American Academy of Orthopaedic Surgeons: Symposium on Osteoarthritis. St. Louis, Mosby.)
Reduction of posttraumatic joint effusion is paramount in the early rehabilitation process and is important for restoration of joint kinematics. Prolonged effusion, if left unchecked, can result in reactive synovitis, damage to the joint capsule, and degradation of articular cartilage. Early use of mobilization techniques, such as continuous passive motion (CPM) and modalities such as cryotherapy and vasopneumatic compression, can aid in reducing joint effusion.
Effects of immobilization
Muscle
One of the first and most obvious changes that occur as a result of immobilization is loss of muscle strength. This correlates with a reduction in muscle size and a decrease in tension per unit of muscle cross-sectional area.14-16 MacDougall et al16 reported that 6 weeks of elbow cast immobilization results in greater than a 40% decrease in muscle strength. This deficit in strength is correlated with loss of fiber cross-sectional area and with an associated decrease in muscle mass. The cross-sectional area of the quadriceps muscle may decrease from 21% to 26% with 4 to 6 weeks of immobilization in an individual without a pathologic condition.17 It is important to note that immobilization atrophy is due to loss of fiber cross-sectional area and not to loss of fibers, as seen in older persons.18
The rate of loss appears to be most rapid during the initial days of immobilization. Structural and metabolic changes in muscle cells have been documented after as little as 2 hours of immobilization.19,20 Lindboe and Platou20 reported that in humans, muscle fiber size is reduced by 14% to 17% after 72 hours of immobilization. After 5 to 7 days of immobilization, the absolute loss in muscle mass appears to slow considerably.21,22 The amount of training before immobilization may dramatically decrease the amount of atrophy during immobilization.21
Both type I (slow-twitch) and type II (fast-twitch) muscle fibers atrophy. It is generally accepted that a selective decrease in type I (redundant) fibers occurs.23,24 However, conflicting evidence is found in the literature.25 After immobilization, the contractile ability of type I fibers is more adversely affected than that of type II fibers.24,26-28 The decreased contractile ability of type I fibers, rather than decreased fiber proportion, may be more clinically relevant, which implies that exercises involving decreased intensity and increased frequency should be performed after immobilization. This is particularly relevant in an athlete who is specifically conditioned to aerobic activity because of the more dramatic decrease in the percent area of type I fibers. These studies evaluated the effect of immobilization only on muscle fiber composition, not the combined effect of injury and immobilization. Muscle fiber atrophy after injury or surgery may be different from that occurring when a healthy muscle is immobilized.
The mechanical properties of the myotendinous junction are changed as a result of immobilization. The contact area between the muscle cells and the collagen fibers of the tendon is decreased by 50%29; the glycosaminoglycan (GAG) content of the myotendinous junction is also decreased.29 These changes can predispose the myotendinous junction to injury after immobilization. A dramatic increase in activity after immobilization may lead to secondary tendinopathy.
In addition to causing changes in muscle size and volume, immobilization also results in histochemical changes. Such changes include a reduction in levels of adenosine triphosphate (ATP), adenosine diphosphate, creatine, creatine phosphate, and glycogen and a greater increase in lactate concentration with work. Furthermore, the rate of protein synthesis decreases within 6 hours of immobilization.16,30-33
Immobilization also causes an increase in muscle fatigability as a result of decreased oxidative capacity. Reductions occur in maximum oxygen consumption, glycogen levels, and high-energy phosphate levels.14,16,30,34,35 Rifenberick and Max36 reported fewer mitochondria in atrophic muscle and a significant decrease in mitochondrial activity by day 7 after immobilization, which causes a reduction in cell respiration and contributes to decreased muscle endurance (Box 2-1).
It appears that the muscle atrophy that occurs after immobilization is selective. For example, immobilization of the thigh is often associated with selective atrophy of the quadriceps femoris muscle.37 Although the knee is the area traditionally noted for selective atrophy, this phenomenon can also be observed in the triceps brachii of an immobilized elbow. Clinically, one can observe that atrophy of the quadriceps is greater than that of the hamstrings. This finding is supported by evidence on computed tomography that despite significant loss of quadriceps cross-sectional area, no significant difference in hamstring or adductor muscle cross-sectional area is seen after 5 weeks of immobilization.37
The selective atrophy that occurs in the quadriceps femoris and the triceps brachii with immobilization of the knee and elbow, respectively, may be due to their roles as primarily one-joint muscles. Three of the four heads of the quadriceps cross only the knee joint, and two of the three triceps heads cross only the elbow joint. In contrast, all heads of the biceps and the hamstrings cross two joints. The biceps and hamstrings are therefore less immobilized by having all portions contract across one of the two joints that they cross (the hip or the shoulder), which may be the reason that cross-sectional area is preserved in these muscles.28
The volume of muscle in the thigh decreases after immobilization, but the volume of subcutaneous adipose tissue does not change. This can mask the amount of quadriceps atrophy and invalidate girth measurements as a tool for measuring atrophy.38 Girth measurements also do not distinguish between muscle groups and can therefore underestimate the amount of quadriceps atrophy. Girth measurements do not correlate with deficits in strength.
Reflexive inhibition, or arthrogenous muscle wasting, can contribute to selective muscle atrophy, particularly of the quadriceps, after trauma to or surgery on a joint. Pain has traditionally been regarded as the general cause of reflex inhibition. The perception and fear of pain can greatly affect muscular strength. Athletes who fear that muscle contraction will result in pain may be very apprehensive about contracting these particular muscles, but severe inhibition of muscle strength is seen even after the pain subsides.39 At 1 to 2 hours after arthrotomy and meniscectomy, a 62% decrease in quadriceps electromyographic (EMG) activity occurs.40 This decrease is due to reflexive inhibition. A significant amount of anesthetic injected into the knee may decrease (but not eliminate) the inhibition temporarily, but the effects are lost after 4 to 5 hours.40 Ten to 15 days after surgery, quadriceps EMG activity decreases by 35%.40 Whether this reduced activity is due to reflexive inhibition or disuse atrophy is unclear. Pain has been shown to inhibit strength in patients with preoperative pathologic changes in the shoulder, but postoperative reflex inhibition has not been investigated.41-43 In studies investigating arthrogenous muscle wasting, the level of quadriceps activation is typically determined by EMG testing. The degree of unilateral quadriceps inhibition is then judged by the difference in maximum voluntary activation between the two limbs. Because no method is available to directly measure inhibition, it is indirectly quantified by EMG testing. This may not be a valid measure of true reflex inhibition during the first few hours after injury or surgery. After injury, reflex inhibition leads to muscle atrophy. When the inhibition has ceased, muscle weakness from disuse or immobilization atrophy will continue. Caution must be used when one generalizes the findings of studies pertaining to arthrogenous muscle weakness.
The nature of the surgical procedure performed may have an effect on the amount of arthrogenous muscle wasting after surgery. Advances in technology have led to the use of a less invasive arthroscope for many procedures that previously required an arthrotomy. A significant decrease in quadriceps muscle EMG activity is seen after arthrotomy of the knee. Although this decrease in EMG activity is still present after arthroscopy of the knee, the magnitude of the change is much decreased.44 Two-portal arthroscopy produces a lesser decrease in quadriceps strength after surgery than three-portal arthroscopy does.45 Clinically, patients undergoing an arthroscopic procedure would be expected to recover at a more rapid rate, but consideration must be given to the specific surgical procedure. In general, patients who have undergone less invasive procedures (arthroscopy) will initially recover faster than those who have undergone more invasive procedures (arthrotomy), but the long-term outcome is usually the same. This has been demonstrated in the knee46 and shoulder.47
Tourniquet-related ischemia has also been thought to contribute to quadriceps shutdown. When a tourniquet is used to provide a relatively bloodless field during surgery, the pressure required to staunch blood flow is also enough to damage the tissues being compressed. Use of a tourniquet, although necessary, leads to postoperative EMG changes48,49 and increased quadriceps atrophy.48 Nonroutine complications from tourniquet use include compression neurapraxia, wound hematoma, tissue necrosis, vascular injury, and compartment syndrome.50
Research has shown that distension of a knee with plasma can lead to quadriceps shutdown and subsequent quadriceps weakening in normal individuals, even in the absence of pain.51-55 Young et al56 and others53 reported that injection of small volumes of fluid (20 to 30 mL) into normal knees results in 60% quadriceps inhibition, with the inhibition increasing as the infusion increases. Spencer et al54 found the threshold amount of effusion for reflexive shutdown to be between 20 and 30 mL for the vastus medialis and between 50 and 60 mL for the vastus lateralis and rectus femoris. The decreased threshold for the vastus medialis can contribute to patellar tracking problems when the knee is even slightly effused. Inhibition is directly related to the degree of effusion and increases as the amount of effusion increases.57 With a chronically effused joint, aspiration of the effusion does not result in any difference in quadriceps inhibition.58 This may be attributed to concomitant disuse atrophy.
Joint angle has also been shown to have an effect on quadriceps inhibition. In normal knees, the highest quadriceps EMG activity is obtained with the knee in the shortened position, or full extension.59 Stratford60 reported that effusion inhibits quadriceps contraction less when the knee is in 30° of flexion than when it is fully extended. Similar results have been found even after arthrotomy with meniscectomy, with isometric quadriceps contraction being inhibited less in flexion than in extension.18,39,59 This has been postulated to occur because intraarticular pressure is less when the knee is in 30° of flexion than when in full extension.39,52,61-63 Despite higher EMG activity being obtained with the knee in flexion, it is not desirable to perform all exercises in the clinic with a flexed knee position. On the contrary, this information emphasizes to the clinician the importance of training the quadriceps in a fully extended position to overcome the biomechanical disadvantage.
The length at which the muscle is immobilized also affects selective atrophy. Tardieu et al64 suggested that muscle fibers under stretch lengthen by adding sarcomeres in series whereas those immobilized in a shortened position lose sarcomeres. Thus, when a muscle is immobilized in a lengthened position, the length of the muscle fibers increases to accommodate the muscle’s new length, along with other connective tissue changes. A similar adjustment in sarcomere number occurs in muscle that is immobilized in a shortened position; the length of the fibers decreases, and the number of sarcomeres is reduced to achieve the physiologic change.65 Immobilization of a muscle in a shortened position leads to increased connective tissue and reduced muscle extensibility.64 Muscle immobilization in a lengthened position maintains muscle weight and fiber cross-sectional area better than does immobilization in a shortened position.66,67 This theoretically explains the selective atrophy of the quadriceps when the knee is immobilized in full extension. Because the knee is usually immobilized in an extended or slightly flexed position, the hamstrings are placed in a lengthened position and the quadriceps in a shortened position. These positions help preserve muscle cross-sectional area and strength, but the sarcomeres will no longer be able to shorten enough to achieve maximal extension. Clinically, the position of the joint cannot be the only factor that influences muscle atrophy; for example, in a shoulder that is immobilized in internal rotation, the external rotators (the rotator cuff muscles) still demonstrate marked atrophy.
One of the factors that may also influence reflex inhibition is the muscle spindle. When a muscle is immobilized in a shortened or lengthened position, the spindle will assume a new resting length.68 A muscle in the shortened position will then have greater resistance to stretch, and a muscle immobilized in the lengthened position will have decreased contractile ability. This is especially true at the shoulder, which is typically immobilized in internal rotation, and at the elbow, which is typically immobilized in flexion. The shortened position facilitates the shortened muscles (elbow flexors and shoulder internal rotators) and may account for the decreased strength of the shoulder external rotators and elbow extensors. Treatment of these joints should include facilitatory techniques for the lengthened muscles and inhibitory techniques for the shortened muscles. The shortened muscles should be stretched often but gently because quick or aggressive stretching can cause a facilitatory response. Isometric contractions can also preserve tension in the muscle spindle while allowing healing of damaged tissues.
Despite the fact that the knee is typically immobilized in extension, patients tend to hold the knee in a slightly flexed position, either sitting with the hips flexed or supine with the pelvis tilted posteriorly, which shortens the hamstrings. Hamstring stretches to increase the resting length of the spindle are an important part of a knee rehabilitation program after immobilization. Increasing the length of the hamstrings will decrease the amount of resistance that the quadriceps must contract against to achieve full knee extension. Stretching of the posterior capsule may also be required if the knee is held in a flexed position.
Despite the increased preservation of quadriceps muscle cross-sectional area and increased EMG activity in flexion, this is not the preferential position for immobilization of the knee after injury or surgery. If the knee is immobilized in flexion, full passive extension must be maintained by passively extending the knee to 0° several times per day (barring medical or surgical precautions.) This ensures that the quadriceps will maintain proper length to allow shortening of the muscle to full knee extension.
Active quadriceps exercises should be done in full extension for several reasons. Full active knee extension is needed for a proper gait pattern during initial contact (heel strike). A quadriceps contraction in full extension allows maximal superior glide of the patella in the trochlear groove, thereby preventing patella infera. If patients do not have full active extension, they must be taught to glide the patella superiorly to preserve length of the patellar tendon. If patients do not have full passive knee extension after immobilization, a motion complication program should be initiated.
Periarticular Connective Tissue
The periarticular connective tissue consists of ligaments, tendons, synovial membrane, fascia, and joint capsule. As a consequence of immobilization, injury, or surgery, biochemical and histologic changes occur in the periarticular tissue around synovial joints and may result in arthrofibrosis. Arthrofibrosis has been referred to as ankylosis, joint stiffness, or joint contracture.69 It is a term that describes excessive formation of scar tissue around a joint after a surgical procedure or traumatic injury.70,71 Its characteristic feature is the formation of scar tissue within the joint capsule, the synovium, or the intraarticular spaces.72
The two main components of fibrous connective tissue are the cells and an extracellular matrix. The matrix consists primarily of collagen and elastin fibers and a nonfibrous ground substance. Fibrocytes, located between the collagen fibers in fibrous connective tissue, are the main collagen-producing cells. As collagen fibers mature, intramolecular and intermolecular bonds or cross-links are formed and increase in number, thereby providing tensile strength to the fibers.73 On the basis of the arrangement of its collagen fibers, connective tissue is commonly classified into two types: irregular and regular.74 The irregular type of connective tissue is characterized by fibers running in different directions in the same plane.75 This arrangement is of functional value for capsules, aponeuroses, and sheaths, which are physiologically stressed in many directions.74,75 Conversely, in regularly arranged tissues, collagen fibers run more or less in the same plane and in the same linear direction.75 This arrangement affords great tensile strength to ligaments and tendons, which physiologically undergo primarily unidirectional stress.75
The extracellular matrix is often referred to as ground substance and is composed of GAGs and water. To understand the changes that occur with immobilization it is important to be familiar with GAGs and their effect on connective tissue extensibility. Four major GAGs are found in connective tissue: hyaluronic acid, chondroitin 4-sulfate, chondroitin 6-sulfate, and dermatan sulfate. Generally, GAGs are bound to a protein and are collectively referred to as proteoglycans. In connective tissue, proteoglycans combine with water to form a proteoglycan aggregate.76
Water constitutes 60% to 70% of the total connective tissue content. GAGs have enormous water-binding capacity and are responsible for this large water content. Together, GAGs and water form a semifluid viscous gel in which collagen and fibrocytes are embedded. Hyaluronic acid with water is thought to serve as a lubricant between the collagen fibers.74,77,78 This lubricant maintains a distance between the fibers, thereby permitting free gliding of the fibers past each other and perhaps preventing excessive cross-linking. Such free gliding is essential for normal connective tissue mobility.77
Sliding of collagen fibers across each other, the collagen weave pattern, and their cross-links can all be illustrated by the Chinese finger trap analogy.75 When tension is applied to the Chinese finger trap, the trap lengthens to a certain point as the straw weave patterns of the trap move across one another (Fig. 2-5). If tension continues to increase when the end point of the trap is reached, the straw fibers will begin to fail. This illustration is not unlike how body connective tissue functions.

Figure 2-5 Chinese finger trap. The trap can be used to illustrate the sliding of collagen fibers over each other in normal connective tissue.
Arthrofibrosis is induced primarily by immobilization after trauma to a joint; a significant reduction in GAG content and subsequent water loss take place and contribute to abnormal cross-link formation and joint restriction. In addition, within the joint space and recesses, excessive connective tissue is deposited in the form of fatty fibers, which later mature to form scar tissue that adheres to the intraarticular surfaces and further restricts motion.75
The most significant reduction in GAG content occurs within the matrix. Akeson et al77,79-81 reported a 40% decrease in hyaluronic acid and 30% decreases in chondroitin 4-sulfate and chondroitin 6-sulfate; collagen mass decreases by about 10% and collagen turnover increases, with accelerated degradation and synthesis (Box 2-2).
Box 2-2 Summary of the Effects of Immobilization on Connective Tissue
The pathophysiology of arthrofibrosis appears to be a reduction in the semifluid gel as a result of the loss of GAGs and water, which causes a decrease in the critical fiber distance between collagen fibers.75 Friction is created between fibers, thus reducing collagen extensibility. It has been suggested that arthrofibrosis may be the result of an increase in the expression of collagen IV, which forms a fibrous network between collagen I and III fibrils. Collagen IV forms irregular cross-links between the collagen fibrils that decrease the ability of the fibrils to slide (Fig. 2-6).55 Evidence suggests that arthrofibrosis may be the result of an autoimmune reaction.82
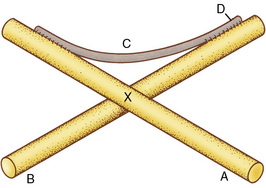
Figure 2-6 Idealized model of the interaction of collagen cross-links at the molecular level. A and B, Preexisting fibers; C, newly synthesized fibril; D, cross-links created as the fibril becomes incorporated into the fiber; X, point at which adjacent fibers are normally freely movable past each other.
(From Akeson, W.H., Armiel, D., and Woo, S. [1980]: Immobility effects of synovial joints: The pathomechanics of joint contracture. Biorheology, 17:95, with permission from Elsevier Science Ltd, The Boulevard, Langford Lane, Kidlington 0X5 1 GB, UK.)
Currently, arthrofibrosis most commonly affects the knee joint, particularly as a complication after anterior cruciate ligament (ACL) reconstructive surgery in which the operative knee develops a thicker joint capsule and a secondary flexion contracture. Arthrofibrosis of the knee joint is characterized by a lack of flexion, as well as extension (the most commonly involved motion is extension). Prolonged joint immobilization is the most recognized risk factor for the development of arthrofibrosis. Microscopic examination of a knee with arthrofibrosis shows a proliferation of fibroblasts and an associated accumulation of extracellular matrix. The principal component of the matrix is type I collagen, which is specifically found as an unorganized network of fibers.83 Although these findings are well recognized, the cause underlying formation of the exuberant scar tissue is less clear.
With a lack of experimental studies and suitable animal models, the pathophysiology of arthrofibrosis remains poorly understood. However, several theories have been proposed to explain and prevent this condition. Several authors84,85 have recommended delaying reconstructive surgery on an acutely injured ACL until the knee joint recovers from the initial trauma. It is theorized that the synovitis that occurs after the initial injury, which is then compounded by the synovitis developing after surgical reconstruction, may predispose the joint to arthrofibrosis. The authors suggest that surgery be delayed until the hemarthrosis and synovitis have decreased, ROM is restored, and the patient has active control of the quadriceps. ACL reconstruction is not an emergency surgical procedure, and better outcomes are seen after preoperative rehabilitation to decrease the initial response to injury.
Fibrosis results from increased numbers of collagen-synthesizing cells (from proliferation and recruitment), increased synthesis by existing cells, or deficient collagen degradation with continued collagen synthesis.86 Injury-induced inflammation precedes the repair process; therefore, precise regulation and control of the inflammatory response will have a direct impact on the timing and amount of fibrosis developing during healing. It has been suggested that an immune response is the cause of the capsulitis that leads to excessive connective tissue proliferation.34 This supports the theory that the excessive intraarticular deposition of connective tissue is a result of consecutive inflammatory phases after trauma to the joint. This has clinical implications; if the rehabilitation is overly aggressive, it can cause increased inflammation and potentially worsen this process. Long-duration, low-load techniques should be used to increase ROM and decrease the potential for an inflammatory response.87 The location of the fibrous lesions has a relationship to the amount of motion lost in terms of knee flexion. The presence of fibrous connective tissue bands in the suprapatellar pouch is the primary reason for limitation of flexion.87 Flexion can also be limited by patella infera or by shortening of the patellar tendon. This complication can be prevented by superior mobilization of the patella on a daily basis.88 Extension of the knee is limited by shortening of the posterior capsule.
It has been shown that arthrofibrotic tissue matures over time and that maturation is complete by approximately 6 months after onset. However, although the tissue matures over time, progressive loss of ROM has not been seen.87 Attempts to lengthen the tissues will be less successful as time passes because of maturation and decreased remodeling capability of the tissues.89 Efforts to treat arthrofibrosis conservatively are likely to fail after 6 months has passed.87 Figure 2-7 illustrates the sequelae leading to arthrofibrosis or return to sport.
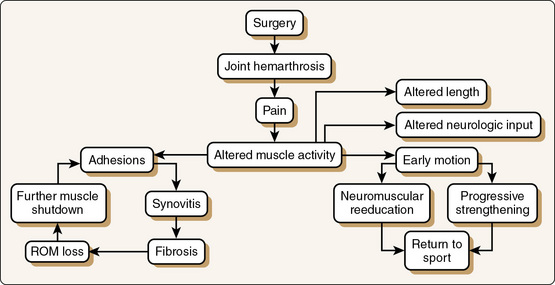
Figure 2-7 Arthrofibrotic loop. A key to avoiding arthrofibrosis is early motion and muscle “turn-on“ through neuromuscular reeducation and progressive muscle strengthening. ROM, Range of motion.
Early recognition of arthrofibrosis is important, and its prevention is paramount. Arthrofibrosis has been associated with Dupuytren disease and diabetes. Patients with these conditions, as well as patients who appear to have an excessive amount of fibrous tissue at other joints, should be viewed as being at risk for the development of arthrofibrosis. It is a useful clinical test to examine joint play at another synovial joint, such as a metacarpophalangeal joint, to determine whether the patient is systemically hypermobile or hypomobile. Clinically, arthrofibrosis is rarely found to develop in hypermobile individuals.
Joint motion is essential for the prevention of contractures and formation of adhesions within joints. Physical forces and motion modulate the synthesis of proteoglycans and collagen in normal joints. Stress and motion also influence the deposition of newly synthesized collagen fibers by allowing proper orientation of collagen to resist tensile stress. Motion appears to inhibit periarticular tissue contractures by the following mechanisms90:
The matrix changes associated with immobilization (noted earlier) are relatively uniform in ligaments, capsules, tendons, and fasciae. These changes involve loss of extracellular water and depletion of GAGs, along with changes in collagen cross-linkage.
Articular Cartilage
Articular cartilage is a thin covering on the ends of bones that creates the moving surfaces of synovial joints.91 It varies from 1 to 7 mm in thickness, with the cartilage covering larger weight-bearing joints (e.g., hip and knee joints) being thicker than that covering smaller, non–weight-bearing joints.91,92 Articular cartilage consists of fibers, ground substance, and cells. The fibers are composed primarily of type II collagen and make up 57% to 75% of the dry weight of cartilage.93 Collagen provides the tensile strength of articular cartilage and aids in the gliding of opposing articular surfaces. The ground substance is similar to that of periarticular tissue and consists of water (70% to 80%) and proteoglycans (15% to 30%).3,94,95 Proteoglycans have a unique bond with water that allows articular cartilage to resist and distribute compressive forces. The quantity of proteoglycans in articular cartilage depends on joint location, with weight-bearing joints having a higher proteoglycan content than non–weight-bearing joints.96 Both collagen and proteoglycans are produced by chondrocytes, the cells residing in articular cartilage.
As a result of immobilization, articular cartilage undergoes structural, biochemical, and physiologic changes at the cellular and ultrastructural levels.91 Consistently reported changes include the following: fibrillation, fraying, cyst formation, varying degrees of chondrocyte degeneration, atrophy in weight-bearing areas, sclerosis, and resorption of cartilage. The proteoglycan GAG content is also decreased, which reduces the ability of cartilage to resist compressive forces. These changes are generally permanent, but the length of immobilization is important in determining whether the articular changes are irreversible.
Articular cartilage is avascular, with its nutritional requirements being met by diffusion and osmosis. Diffusion occurs through a hydraulic pressure gradient, and this pressure is increased by weight bearing or joint movement. Low hydraulic pressure has no effect, whereas constant pressure interferes with nutrition.91 High intermittent pressure loading does not contribute much to the diffusion rate. Joint motion, however, increases the diffusion rate to three to four times the static level.97 This implies that in the absence of weight bearing, motion must be present to preserve the integrity of articular cartilage. The opposite (weight bearing without ROM) is not desirable, however, because compressive forces on only the weight-bearing aspects of the immobilized joint can cause severe articular damage.98
The effects of immobilization depend on the position in which the joint is immobilized. A flexed knee position produces greater chondrocyte necrosis and degeneration of the articular cartilage than does an extended position.26 This result may be due to the increased compression and intraarticular pressure in the fully flexed position. The position of immobilization is not as critical in the upper extremities because they contain primarily non–weight-bearing joints.
The effects of immobilization on articular cartilage can be separated into contact and noncontact effects. In contact areas, the seriousness of the changes depends mainly on the degree of compression. In noncontact areas, it depends on the ingrowth of connective tissue into the articular surface.99 Constant compression of articular cartilage decreases the diffusion rate of synovial fluid and leads to pressure necrosis and chondrocyte death.100,101 Whether the lesions are reversible depends directly on the duration of continuous compression.102 In addition, loss of contact between opposed articular surfaces in weight-bearing and non–weight-bearing joints appears to lead to degenerative changes, thus suggesting a functional relationship between joint motion and normal articular cartilage surface contact101,103 (Box 2-3).
Intermittent joint loading appears to have a critical role in maintaining healthy articular cartilage. The formation and circulation of synovial and interstitial fluids are stimulated by intermittent joint loading and retarded in its absence. Because synovial fluid is important for nourishment and lubrication of cartilage, intermittent pressure can facilitate chondrocyte nourishment and is vital for cell function.104-106 Conversely, joint immobilization in which the joint is constantly loaded or unloaded can compromise the metabolic exchange necessary for proper structure and function and eventually result in cartilage degradation and eburnation.33,107-109 Immobilization of the knees in extension leads to irreversible and progressive osteoarthritis. The compression between articular surfaces increases in an immobilized knee and, after 4 weeks of immobilization, reaches a level that is three times greater than the initial level.101
Roth et al110 found that prolonged knee immobilization after ACL reconstruction leads to significant patellofemoral chondromalacia and that immediate mobilization prevents patellofemoral degeneration. It may be assumed that immobilization after other types of surgery leads to articular changes as well. Although it is important to begin motion and gentle strengthening exercises after surgery, it is essential for the clinician to remember that excessive activity has the potential to compromise the articular surfaces. Moderate activity after immobilization has been shown to stimulate regeneration of the articular surface, but strenuous activity can reduce the proteoglycan content of articular cartilage.111 Do not “overrehabilitate“ the articular surfaces with too much activity too soon.
Ligaments
Ligaments undergo the same changes in structure as do other elements in periarticular tissue. However, because of the function of ligaments and because of the bone-ligament interface, additional factors must be considered to understand the response of ligaments to immobilization.
Like bone, a ligament appears to remodel in response to the mechanical demands placed on it. Stress results in a stiffer, stronger ligament, whereas inactivity yields a weaker, more compliant structure.112 These structural changes appear to be caused more by an alteration in the mechanical properties of ligaments113 and by subperiosteal resorption at the bone-ligament junction than by actual ligament atrophy.114,115 With immobilization, the bone-ligament junction rather than the midsubstance of the ligament is at increased risk for injury. The alterations in ligament collagen lead to a decrease in tensile strength of the ligament and thus reduce the ability of ligaments to provide joint stability (Box 2-4).22,79,116-120
Box 2-4 Summary of the Response of Ligaments to Immobilization
Immobilization of the ligaments of the knee has been widely studied. It is well established in the literature that the amount of time that the ligament is immobilized is much shorter than the amount of remobilization time necessary for the ligament to reach its preimmobilization strength.90,121 The reconditioning process must provide progressive stress that overloads the ligament enough to stimulate regeneration, but the stress must be controlled to prevent cumulative microtrauma. The effects of immobilization on ligament structure and function may be specific to each particular ligament.
With immobilization, the medial collateral ligament (MCL) decreases in cross-sectional area and ultimate stress load.121 These effects are assumed to depend on the exact position of the knee joint. The MCL is taut in full extension, so the assumption is that if the knee is immobilized with the MCL in a shortened position, a tighter ligament would be provided. Research has shown122-124 that a reconstructed MCL has increased creep, or lengthening per unit stress, with immobilization rather than with full motion. This has implications for rehabilitation after MCL injury or reconstruction. After MCL injury or reconstruction, the knee should be placed in a position of slight flexion so that extension is preserved but the ligament is not under stress. Progressive motion should be allowed several times per day, however, to prevent creep and loss of cross-sectional area. Although this research involves only the MCL, it is likely that the same biomechanical principles may be applied to the lateral collateral ligament.
The effects of immobilization on the ACL have been widely studied. The specific effects are difficult to measure consistently because of the complex fiber orientation of the ligament. For the rehabilitation specialist, it is enough to note that the ACL is at increased risk for damage after immobilization and that the amount of reconditioning necessary to restore the ligament is much greater than the duration of the immobilization. A discussion of the effects of immobilization after reconstruction is not warranted because of the widespread use of early aggressive ROM exercises and rehabilitation after reconstruction.
The menisci also undergo degenerative changes with immobilization, and this degeneration is directly related to the amount of time that the joint was immobilized.125 In an animal model, blood flow to the menisci after injury was increased fivefold. However, there was no increase in blood flow when the joint was immobilized.126 Currently, healing is thought to depend on an adequate blood supply, and immobilization may therefore delay healing of the meniscus. Knee joint immobilization decreases the proteoglycan and water content of the meniscus,127 which changes its ability to distribute compressive force. In the absence of weight bearing, active joint motion has been shown to decrease the loss of ligament and meniscal mass.128
When ligament reconstruction (not a primary repair) is performed, the graft undergoes neovascularization and ligamentization. This process begins at approximately 3 to 7 weeks postoperatively for a patellar tendon autograft.129-131 This is the time when the graft is the weakest, and the rehabilitation specialist must take care to not overstress the graft during this period. Bone-to-bone healing occurs faster than bone-to-tendon healing, which implies that rehabilitation should be less aggressive after ACL reconstruction with a hamstring autograft than with a patellar tendon autograft.132 At 6 months postoperatively the patellar tendon autograft is indistinguishable from a normal ACL,133 and the mechanical properties are not different. These findings imply that return to sport may occur at this time, but other factors, such as strength, dynamic control, balance, and psychologic readiness, must be considered.
Bone
The effects of immobilization on bone are similar to those on other connective tissues. A consistent finding in response to diminished weight bearing and muscle contraction is bone loss. Changes in bone can be detected as early as 2 weeks after immobilization.87,96,134 Although the pathogenesis of immobilization-associated osteoporosis is unclear, animal studies have shown decreased bone formation and increased bone resorption.135-138
Bone hardness decreases steadily with the duration of immobilization, with hardness dropping to 55% to 60% of normal by 12 weeks.139 A decline in elastic resistance also occurs—the bone becomes more brittle and thus more susceptible to fracture.
It appears that mechanical strain influences osteoblastic and osteoclastic activity on the bone surface.140 Bone loss from disuse atrophy occurs at a rate 5 to 20 times greater than that resulting from metabolic disorders affecting bone.141 The primary cause of this immobilization osteoporosis appears to be mechanical unloading, which may be responsible for the inhibition of bone formation during immobilization.142 Therefore, non–weight-bearing immobilization of an extremity should be limited to as short a period as possible.
Continuous passive motion
In 1970, Salter143 originated the biologic concept of CPM of synovial joints to stimulate healing, regenerate articular tissue, and avoid the harmful effects of immobilization.144 In 1978, Salter and Saringer (who was an engineer) collaborated to develop the first CPM device for humans.143 A CPM machine is an electrical, motor-driven device that helps support the injured limb. It is used to move a joint at variable rates through progressively increasing ROM; no muscular exertion is required of the patient.
Salter et al102,143 provided the first histologic evidence in support of CPM. They reported143,145 that CPM significantly stimulates healing of articular tissues, including cartilage, tendons, and ligaments; prevents adhesions and joint stiffness; does not interfere with healing of incisions over the moving joint; and influences the regeneration of articular cartilage through neochondrogenesis.
When compared with immobilization of tendons, CPM has proved effective in increasing linear and maximum stress, linear load, and ultimate strength in tendons.144 Salter and Minster146 reported the preliminary results of semitendinous tenodesis for MCL reconstruction in experimental animals, in which increased strength was reported after the use of CPM. The application of early tensile force appears to facilitate proper alignment of collagen fibers during the initial healing process. In addition, decreases in medication requests and in wound edema and effusion in operative knees were reported in patients undergoing CPM.147 The greatest benefit of CPM appears to be the prevention of articular cartilage degradation. Salter143 reported that healing of cartilage defects in rabbits appears to be more rapid and complete when CPM is used. More recently, a 2007 study reported that the use of CPM stimulates chondrocyte PRG4 metabolism, which benefits cartilage and joint health.148
CPM has received widespread attention for use in treating pathologic conditions of the knee. However, CPM machines have been developed for the shoulder as well. Raab et al149 found increased ROM and decreased pain with the use of CPM after rotator cuff repair, although no difference in combined outcome measures was seen 3 months postoperatively. Lastayo et al150 found no difference in outcomes between subjects who used a CPM machine and those who had a friend or relative perform manual ROM exercises, thus indicating that the presence of passive motion is more important than the mode of delivery.
It is well established in the literature that the use of CPM is not associated with a significant difference in outcome measures at approximately 4 weeks postoperatively.151-153 However, when the short-term effects of CPM are examined, subjects undergoing CPM regain their motion faster and with less pain than do those who are not undergoing CPM.154,155 Although there may be no long-term difference, CPM appears to be beneficial to patients in the short term (Box 2-5).115,154,156-158
Use of CPM units is considered an acceptable practice after most orthopedic procedures. Although initially designed for the lower extremities, CPM units are available for the upper extremities as well. CPM has helped counteract the deleterious effects of immobilization by allowing early protected ROM. Some indications for the use of CPM include ligament reconstruction or repair, total joint replacement, release of joint contractures, tendon repair, open reduction of fractures, and articular cartilage defects.
Effects of remobilization
Physical forces provide important stimuli to tissues for the development and maintenance of homeostasis.116 Lack or denial of mobilization results in deleterious effects on bone, muscle, connective tissue, and articular cartilage. The advent of CPM in the late 1970s and early 1980s provided an impetus for the initiation of early motion to repair tissues and for using early electrical stimulation of muscle to decrease atrophy and promote early muscle reeducation. In addition, the emergence of hinged braces, which allow early protected motion, has helped foster early mobilization.
Early motion and loading and unloading of joints through partial weight bearing promote the diffusion of synovial fluid to nourish articular cartilage, menisci, and ligaments. Moreover, research has shown that motion enhances transsynovial flow of nutrients.63,97,159 Regardless of the cell-stimulating mechanism, it is clear that fibroblasts and chondrocytes respond to physical forces by increasing their rate of synthesis, and the extracellular degradation of matrix components is similarly controlled.79
Immobilization is still used, however, in the treatment of many ligamentous reconstructions and fractures. It is not known whether the deleterious effects of prolonged immobilization can be reversed with remobilization techniques. These structural changes generally appear to depend on the duration and angle of immobilization and on weight-bearing status.
Rehabilitation protocols for specific injuries and surgical procedures are popular and commonly used. These protocols must be viewed as guidelines and not as rules. Each patient’s condition must be taken into account when determining appropriate progression of activities. Objective and subjective findings are used to determine the patient’s tolerance of a new activity. Signs of intolerance include increased effusion, pain, erythema, or an inability to perform a task correctly. These are signs that the activity should be modified or delayed.
Muscle
Many researchers have investigated the process of remobilization after immobilization. Extrapolation of the results of these studies to an injured or postoperative patient population must be done with caution, however, because injury may compound the effects of immobilization. It is critical to consider how the specific injury or surgical procedure and the length of immobilization will affect the rate of return of muscle strength.
To achieve gains in muscle strength, the principle of overload must be used.160 Overload involves the application of a stimulus that is greater than the stimulus to which the muscle is accustomed. This principle must be used with caution in an injured population, however, because excessive overload can be detrimental to healing tissues.
Return of quadriceps strength after knee surgery has been widely investigated. With ACL reconstruction, loss of quadriceps strength appears to be greater with bone–patellar tendon–bone autografts than with semitendinosus-gracilis autografts.161,162 Despite quadriceps weakness being associated with patellar tendon–bone autografts, only a slight amount of hamstring weakness is associated with semitendinosus-gracilis autografts.163 This may be due to the previously discussed reasons for predisposition of the quadriceps to atrophy. After ACL reconstruction with patellar tendon–bone autografts, quadriceps strength has been found to be less than 50% of that on the contralateral side 3 months postoperatively156 and 72% to 78% of that on the contralateral side from 6 to 12 months postoperatively.156,161 Six months after ACL reconstruction with a semitendinosus-gracilis autograft, quadriceps strength has been found to be 88% of that on the contralateral side, and hamstring strength has been found to be 90% of that on the contralateral side.163 In both a human164 and an animal model,55 performance of this type of reconstruction in a female patient156,165 and older age are factors associated with increased risk for prolonged muscle weakness after surgery.
Shoulder strength after rotator cuff injury has been studied. In shoulders with a rotator cuff tear, strength is decreased by one third to two thirds with respect to abduction, flexion, and external rotation.42,43 Strength is decreased by one third 6 months after repair and becomes comparable to that on the contralateral side at 1 year after repair.166 Shoulder strength is positively correlated with the size of the rotator cuff tear.166
It has been theorized that electrical muscle stimulation (EMS) can provide enough muscle activity to deter atrophy and the deleterious effects of immobilization on muscle. EMS has been investigated primarily as a tool to preserve muscle strength and cross-sectional area after knee surgery, particularly with ACL reconstruction. EMS has been shown to preserve quadriceps cross-sectional area and protein synthesis in an immobilized injured knee167,168 when combined with traditional rehabilitation exercises.162,169,170 EMS has also been shown to be associated with a more normalized gait pattern postoperatively.171 It may be particularly effective in women.165
Some reports in the literature do not support the effectiveness of EMS in preserving muscle strength after injury or surgery.172 This lack of support may be due to the type and individual parameters of the electrical stimulation used. EMS is comparable to voluntary exercise only when the exercises are required to be performed at the same intensity as the EMS.173 In addition, EMS is effective only when used at a level strong enough to produce a contraction in a shortened position that is greater than what the patient can produce voluntarily. Biofeedback training has been shown to be as effective as EMS in recovery of quadriceps strength.174
EMS can provide clinical benefit when a patient does not have active full knee extension. It can be especially helpful if the patient is immobilized in a flexed position. The patient must apply EMS on a regular basis (three to five times a day at home) with the knee in full extension during each use to preserve full ROM.
Many clinicians use EMS for the quadriceps after knee surgery or injury. However, EMS can help preserve motion and allow early neuromuscular reeducation at other joints. Functional electrical stimulation is used on the rotator cuff of patients who have a subluxated shoulder after a cerebral injury.
Articular Cartilage
The effects of remobilization on articular cartilage seem to depend on time. Many studies have examined the effects of remobilization on articular cartilage after a period of immobilization. The period of remobilization required to restore articular cartilage structure and function is significantly longer than the immobilization period required to cause those changes.175-177 Kiviranta et al111,175 found that 50 weeks of remobilization after 11 weeks of immobilization is not sufficient to restore GAG content. Haapala et al178 used a similar protocol to demonstrate the inability of 50 weeks of remobilization to reverse cartilage softening in the cartilage of immature beagles. This indicates that younger individuals may sustain long-term damage to their articular surfaces as a result of immobilization. Evans et al179 reported alterations in cartilage, such as matrix fibrillation, cleft formation, and ulceration, that are not reversible in rats after immobilization for up to 90 days. They noted, however, that the soft tissue changes are reversible if the period of immobilization does not exceed 30 days. Clinically, it is rare that an extremity would be immobilized for longer than the 30 days that is required to cause irreversible damage.
The remobilization process after immobilization must consist of controlled stress. Although moderate activity after a period of immobilization causes increases in cartilage thickness and proteoglycan content, strenuous activity can cause damage to the articular structures.111 It is important to watch for signs of intolerance of a new activity. Signs of intolerance include increased effusion or edema, erythema, pain, or inability to complete a task correctly.
Bone
Immobilization results in disuse osteoporosis, which may not be reversible on remobilization of the limb. Reversibility is related to the severity of changes and to the length of immobilization. Permanent osseous changes appear to occur with an immobilization period exceeding 12 weeks.55 Even though bone lost in the first 12 weeks is regained, the period of recovery is at least as long as and may be many times longer than the immobilization period.76 The most effective means of modifying osteoporosis caused by reduced skeletal loads appears to be through exercise. Isotonic and isometric exercises decreased bone loss in subjects who were exposed to prolonged periods of weightlessness and bed rest.180,181 Activity increases bone formation in these situations and can hasten recovery after return to a normal loading environment. If an appropriate environment can be maintained during immobilization of a limb, the deleterious effects of disuse on bone can be partially prevented, and rehabilitation can be accelerated.76
Ligaments
Remobilization after immobilization of ligaments occurs in an asynchronous fashion. It appears that the bone-ligament junction recovers at a much slower rate than do the mechanical or midsubstance properties of the ligament.182,183 Cabaud et al112 reported that ligament strength and stiffness in rat ACLs can increase with endurance-type exercises. Others have noted similar results.99,184 Moreover, not only does the ligament injury result in weaker mechanical properties at midsubstance and at the bone-ligament complex, but nontraumatized ligaments also become weaker as a result of immobilization. These weakened mechanical properties of ligaments must be considered when a rehabilitation program is being planned.
Recovery from immobilization depends on the duration of immobilization. Woo et al182 noted that 1 year of remobilization is required before the architectural components of the MCL-tibia junction return to normal after 12 weeks of immobilization. Noyes117 reported that after 5 months of remobilization following total body immobilization in primates, ligament strength recovers only partially, although ligament stiffness and compliance parameters return to control values. It was reported that 12 months is required for complete recovery of ligament strength parameters.117 Tipton et al185 observed recovery of 50% of normal strength in a healing ligament by 6 months, 80% after 1 year, and 100% after 1 to 3 years, depending on the type of stress placed on the ligament and on prevention of repeated injury.
It appears that the properties of ligaments return to normal with remobilization, but this depends on the duration of the immobilization, with the bone-ligament junction taking longer to return to normal after immobilization.
Connective Tissue
Few studies have documented the effects of remobilization after immobilization on the formation of cross-links.75 Movement maintains lubrication and critical fiber distance within the matrix and ensures an orderly deposition of collagen fibrils, thereby preventing abnormal cross-link formation.75 Frequently, for ROM to be restored, forceful manipulation to break the intracapsular fibrofatty adhesions may need to be performed.75 Although ROM is restored, it has been speculated that fibrofatty tissue is peeled from the ends of bones, with ragged edges of adhesions remaining in the joint.106,179 Increased joint inflammation also occurs as a result of the manipulation and enhances the potential for chronic synovitis.
Tissue healing with platelet-rich plasma therapy
As sports medicine has developed, clinicians have searched for ways to create a competitive advantage for their athletes. Musculoskeletal injury has often resulted in loss of playing time and continues to increase in active populations.186,187 Returning these athletes to sports participation can be the difference between winning and losing. New science involving tissue regeneration techniques along with other ways of speeding the recovery process has been developed, including the use of platelet-rich plasma (PRP) therapy. An understanding of the physiology of injury leads to a better understanding of the healing process and effective use of PRP. Originally, PRP was shown to enhance bone formation and antiinflammatory function after oral and maxillofacial applications.188,189 Today, more information is being presented on the various beneficial ways in which autologous platelet therapy can benefit a broader patient population, specifically athletes for the purposes of this book.
Blood is made up of many different components. Plasma, the liquid form of blood, is made mostly of water and transports all other components of blood. Red blood cells transport oxygen to the body, and white blood cells fight off infection and act as a defense for the body. Platelets are responsible for homeostasis, revascularization, and construction of new connective tissue. Human blood is composed of 93% red blood cells, 6% platelets, and 1% white blood cells. For many years, platelets were believed to have an effect only on clotting. However, new evidence has shown that they release multiple growth factors that may enhance tissue regeneration and healing.190 Injection of whole blood has been shown to decrease subjective pain scores in humans with tendon injury, but it lacks the healing properties of other injection treatment options.191 PRP is useful as an activator of circulation-derived cells for enhancement of the initial healing process.192 The rationale for the use of PRP is to reverse the blood ratio by decreasing red blood cells, which are less useful in healing, to 5% and increase the platelet ratio to 94%.193 This will stimulate a supraphysiologic release of growth factors in an attempt to jump-start healing.190
The normal human concentration of platelets is 200,000 platelets/μL. Injection of concentrated autologous PRP can increase the platelet concentration by four to eight times the normal value.193,194 The PRP injection procedure is relatively quick and can be done in the doctor’s office, athletic training room, or outpatient clinic. It begins by drawing 30 to 60 mL of blood from the patient, usually the arm. The blood is then placed in a microwave-size centrifuge and spun down to extract the platelets from the other components of blood. Depending on the unit, this process can take 5 to 30 minutes. After separation, 3 to 6 mL of isolated PRP is removed and secured in a separate sterile syringe. After the injury site has been prepared, the injection can be completed. The use of diagnostic ultrasound or magnetic resonance imaging is recommended for accurate injection into the specific damaged tissue. At rest, platelets require a trigger to become active in healing and homeostasis.195 Thrombin will trigger release of the pool of growth factors over the injured tissue. Peerbooms et al196 described a peppering technique for the injection of platelets that naturally causes release of thrombin. A normal platelet’s life span is 7 to 10 days. During this time it is recommended that the use of antiinflammatory drugs, which can kill the injected platelets, be avoided. After injection, rest, ice, compression, and elevation (RICE), as well as acetaminophen or prescription pain medication in extreme cases, have been used to negate pain. Depending on the sensitivity of the injection site, an immediate, short-term increase in pain may be expected by the patient as a normal biologic response to the injection. An important benefit of this procedure is the very low risk for immunogenic reactions or transfer of disease secondary to the autologous nature of the procedure. When compared with injection of isolated growth factors, PRP offers multiple healing components in one treatment. Growth factors act solely on cell membranes with no effect on the cell nucleus, thereby decreasing the chance of hyperplasia, carcinogenesis, or tumor growth.190,197 Sampson et al190 found minimal contraindications, including the presence of tumor, metastasis, active infection, and low platelet or hemoglobin count.
The value of concentrated platelets lies in their ability to release numerous growth factors that promote specific components of the healing process.198,199 Neutrophils and macrophages naturally respond to injury by releasing growth factors, including platelet-derived growth factor, transforming growth factor, vascular endothelial growth factor, and epithelial growth factor. Following injury, administration of concentrated platelets will accelerate the process of releasing growth factors. In tendon injury, this leads to an earlier increase in tendon breaking strength, arguably the most important tendon-healing parameter.200,201 Table 2-1 summarizes the various growth factors that are released as a result of injury.195,202-205 Many other growth factors are also found that will aid in stimulating angiogenesis, regulating cell migration and proliferation, activating satellite cells, and supporting other growth factors.202,206
Table 2-1 Various Growth Factors Released as a Result of Injury
| Growth Factor | Purpose |
|---|---|
| PDGF | Helps promote tissue remodeling and stimulates the production of other growth factors. It is hypothesized that PDGF is the first growth factor found in injured tissue and initiates healing.195 |
| TGF | Promotes the extracellular matrix and regulation of cell replication.202 |
| VEGF | Stimulates angiogenesis, which is instrumental in accelerating tendon cell proliferation and stimulating type I collagen synthesis.203 |
| IGF-I | Stimulates the proliferation of myoblasts and improves skeletal muscle regeneration.204 |
| FGF-2 | Enhances the number and diameter of regenerating muscle fibers.205 |
FGF-2, Fibroblast growth factor-2; IGF-I, insulin-like growth factor I; PDGF, platelet-derived growth factor; TGF, transforming growth factor; VEGF, vascular endothelial growth factor.
To date, much of the literature on PRP is anecdotal and derived from small sample case studies. However, many have attributed decreased pain and decreased loss of function to the use of PRP injection. Early research has shown PRP injection to be effective in treating Achilles tendinopathy after failed physical therapy, with patients reporting significantly reduced or eliminated clinical symptoms.207 A study by Peerbooms et al196 is one of a limited number of randomized controlled studies that compared PRP and corticosteroid injections for the treatment of lateral epicondylitis. Corticosteroid injection has long been considered the “gold standard” treatment, but it has been shown to have limited long-term effects and requires multiple treatments.208 The results demonstrated initial improvement in the corticosteroid group, but a rapid decline and return to preinjection complaints. PRP-injected subjects showed gradual improvement with decreased subjective pain reports and improved function without relapse at 26-week and 1-year followup. Using the DASH (Disabilities of the Arm, Shoulder, and Hand) Outcome Measure, 73% of patients injected with PRP demonstrated improvement versus only a 51% success rate in steroid-injected patients.196 In this case it can be hypothesized that PRP administration will decrease the need for multiple injections. Barrett and Erredge209 reported almost 78% complete resolution of symptoms and return to previous levels of function in patients with plantar fasciitis at 1-year followup after a single PRP injection. Even though this was a small sample pilot study, the conclusions were intriguing, but it should be kept in mind that treatment of plantar fasciitis with multiple injections has been shown to be a factor contributing to eventual rupture of the tendon.210-213 Research is also favorable for surgical patients. Berghoff et al214 compared 137 patients undergoing total knee arthroscopy, 71 treated with PRP and 66 controls, and found increased hemoglobin, fewer transfusions, shorter hospital stay, and quicker return of ROM in those treated with PRP. Similar results were found in the same population by Gardner et al.215 and Everts et al.197 Studies involving animal models have shown increased mechanical properties after cartilage defects,216 as well as support for chondrogenesis and healing of meniscal defects with use of PRP.217,218
More specific uses of PRP have been reported in other studies. Hammond et al206 studied the use of PRP for muscle injuries in rats. Using the anterior tibialis muscles, the authors created two different protocols. One involved inducing a single, large strain injury and compared it with an injury caused by multiple small strain stretching contractions. Interestingly, the specimens with a large, single injury showed improvement only on day 3 with a significant increase in generation of muscle force. Conversely, the multiple small strain–injured specimens improved at several different time points and showed greater overall healing and return to function. This was attributed to the need for myogenesis to take place for complete healing in the multiple-injury group. The authors concluded that PRP was useful for muscle injuries only when myogenesis is required for recovery.206
More examples of the use of PRP are being presented, with increasing use in the field of sports medicine.219 The efficacy of this treatment continues to improve with continued positive results. Combining PRP therapy with appropriate mechanical loading properties can lead to speedier recovery and faster return to sport.220 When treating elite, high-level athletes, clinicians should be aware of concerns expressed by the World Anti-Doping Agency about the use of PRP to create an unfair advantage against competition rules. Care should be taken before treatment to receive an exemption for therapeutic reasons, if necessary.
In the future, PRP treatments can be improved with a standardized method of application, including the optimal time frame after injury for injection. Little is known about tendon healing time after injection or any deleterious effects with rapid return to sport. The literature also lacks a postinjection rehabilitation protocol, neither standard nor injection site specific. No concrete evidence has been presented for the appropriate time to resume antiinflammatory or oral steroid use. Other possible indications for the use of PRP have yet to be studied, including limiting inflammation, which could speed return to sport. Further information must be presented by clinicians with specific results for the increasingly wide use of PRP injection. Injuries, including osteoarthritis, bursitis, acute ligament injuries versus overuse injury, and postoperative joint reconstruction, can be experimented with to create possible future uses of this increasingly popular tool.
Dynamic flexibility
Preexercise routines have long been practiced as a way to prepare for activity. Historically, various stretches and movements to “loosen“ or “warm up“ the body have been implemented despite limited evidence of effectiveness. Warm-up should be designed to increase muscle/tendon suppleness, stimulate blood flow to the periphery, increase body temperature, and enhance coordinated movement.221 The objectives of a warm-up routine developed at West Point also include increasing body temperature, as well as promoting the responsiveness of nerves and muscles, in preparation for activity.222 Traditionally, the main component of these routines has been static stretching. Many different stretching options and methods of coaching an athlete can be used to properly prepare for sports activity (Table 2-2).
Table 2-2 Summary of Common Stretching Methods
| Type of Stretch | Definition |
|---|---|
| Static stretching | Involves the inhibition of tension receptors in muscles. When done properly, static stretching slightly lessens the sensitivity of tension receptors, which allows the muscle to relax and be stretched to greater length. Often referred to as the “reach and hold” technique, an athlete will stretch an isolated muscle to end ROM in an elongated position and typically hold it for 10-30 seconds at a time. Note the difference between static stretching and passive stretching. |
| Passive stretching | Involves no active movement by the athlete. Passive stretching usually requires an outside force to stretch the muscle, for example, another person or a stretch band. |
| Dynamic flexibility | Active motion of a body part through full ROM in a functional movement to increase tissue temperature, improve neuromuscular control and speed, and prepare for physically demanding activity. Properly done, it involves slow, controlled movements with a slow increase in speed and ROM. |
| Ballistic stretching | Sometimes known as the “bouncing technique,” ballistic stretching uses body momentum to passively or dynamically move into an extended ROM unable to be reached with a static approach. |
| Proprioceptive neuromuscular facilitation | Normally found in rehabilitation protocols but can also be used in preparation for exercise. Proprioceptive neuromuscular techniques include agonist contraction, hold-relax, or rhythmic stabilization. Perceived benefits include enhanced neuromuscular control, greater increased ROM than with other techniques, and improved joint stability. |
| Myofascial release | Creates breaking of adhesions between muscle, fascia, skin, and bone to decrease pain and increase tissue extensibility. Manually disturbing the fascia promotes reorganization of connective tissue in a pattern of greater functional extensibility. |
| Eccentric training | Eccentric training begins with the muscle to be stretched in a shortened position, followed by active elongation to end ROM to create an eccentric contraction. |
ROM, Range of motion.
Over time, each has fallen in and out of popularity, and health care professionals and strength and conditioning coaches still debate the most effective way to put together a warm-up routine. For years the gold standard included numerous static stretches that isolate specific muscle groups, such as the hamstrings, quadriceps, triceps, and pectorals, among others. Current research has proposed that a static stretching program may no longer be the most appropriate. Movement toward dynamic flexibility warm-up has gained recognition as a more effective method of preparation. Others note that a specific combination of stretching options in a controlled order will create a well-prepared athlete.
A 2010 literature review by McHugh and Cosgrave223 examined the results of studies reporting the effects of static and dynamic stretching. Nineteen papers were reviewed that used a strength measurement after static stretching. All 19 reported a loss in strength after using static stretch to warm up. Losses ranged from 2% to 28% of function. Eighteen studies chose power as their outcome measure, with 14 of these studies showing that athletes have an average decreased power output of 4.5% after the implementation of preexercise static stretching.222 Other authors have compared static versus dynamic flexibility. In a study measuring knee flexor strength after a 6-minute stretch time, athletes who used static stretching demonstrated a 14% loss of isometric strength as compared with just a 4% drop in the dynamic group.224 Manoel et al225 performed a similar study in 2008 on the knee extensors, but isokinetic tests were used as an outcome measure. Again, the static group had a 4% decrease in power. Comparatively, the dynamic group was shown to have gained 9% in power. In 2009, Sekir et al226 statically stretched the knee extensors and dynamically stretched the knee flexors via an isokinetic test to measure outcomes. Interestingly, the statically stretched knee extensors lost 14% of their strength, whereas the dynamically stretched knee flexors gained 15% in strength. In a 2006 study, Yamaguchi et al227 concluded that a quadriceps statically stretched to the point of discomfort decreased in power by 12%. A year later this group measured quadriceps power after dynamic stretching and reported a 9% increase in power output.118 Although both groups have been shown to exhibit some deleterious effect on strength, statically stretched tissue consistently exhibited greater loss. In terms of power, many times the dynamically stretched athlete shows an increase in output.
With continuing laboratory evidence that static stretching can inhibit performance, Nelson et al228 examined carryover to sport-specific trials. In measuring 20-m dash times, statically stretched athletes were consistently slower on average by 0.04 second. The dynamic groups had better results than the static groups in high-speed performance activity,229 agility testing, upper extremity power, and lower extremity power.222
Dynamic warm-up leads to a lesser increase in ROM than static stretching does.230 This provides a possible explanation for better performance in high-speed activity. Conversely, the inhibited function after static stretch has been attributed to muscle having less resistance to passive stretch. This has been referred to as “stress relaxation,“ or loss of tension after stretch at a constant length.231 The increased muscle compliance resulting from stretching is suggested as a possible explanation for the decreased muscle performance after static stretching. Improved tolerance of stretching, not mechanical change, causes inhibition of muscle, tendon, or joint receptors (nociceptors) and decreases the body’s natural protection of commonly injured structures. A period of delayed neuromuscular response is also reported following stretching exercise. It is possible that these results combined with overwhelming evidence of decreased contracture strength can lead to an increased rate of injury.
To rationalize the loss of strength performance after static stretching it was hypothesized that an alteration in viscoelastic properties has an effect on the length-tendon relationship of the muscle. However, there is no evidence of permanent modification of muscle length at 90 minutes after acute stretching, nor at 24 hours after a 3-week stretch program.232 Strains as little as 20% beyond resting fiber length can cause muscle damage and subsequent decreased force.233 Walking alone can cause a 20% strain in sarcomeres.234 It is a relative certainty that greater than 20% strain occurs with normal stretching routines.
Although most recent research has swung in the direction of dynamic warm-up, this does not mean complete avoidance of static stretching. Static stretching is recommended only after appropriate warm-up and facilitation of the tissue at all times. Recommended use of static stretching includes equalizing any bilateral differences in ROM that can lead to pathology. A popular static stretch is the “sleeper“ stretch for the rotator cuff and capsule stretching in overhead athletes.235 Such stretching has been shown to be effective in increasing ROM in individuals exhibiting posterior shoulder tightness. As with any static stretch, the sleeper stretch should never be implemented on cold tissue.
It is important to understand the difference between preexercise training and flexibility training. Preexercise dynamic warm-up specifically prepares the athlete for optimal performance in competition. Flexibility training is aimed at changing the resting state of available ROM with little regard for immediate high-level functional performance in competition. Flexibility training is specific to individual needs, whereas dynamic warm-up is specific to the activity. A large, randomized controlled trial in 2005 concluded that dynamic, functional warm-up also reduced rates of injury.236 Athletes should be sweating after warm-up. If the athlete complains of being tired, the level of activity may be too high. Too large an energy consumption before activity could cause decreased performance secondary to early fatigue. Despite this possibility, dynamic warm-up appears to be the best option to acutely prepare for sports activity.
Conclusion
Effects of Immobilization
Connective Tissue
Muscle
Periarticular Tissue
Articular Cartilage
Continuous Passive Motion
Effects of Remobilization
Tissue Healing with Platelet-Rich Plasma Therapy
1 Golden A. Reaction to injury in the musculoskeletal system. In: Rosse C., Clawson D.K., editors. The Musculoskeletal System in Health and Disease. New York: Harper & Row; 1980:89-93.
2 Knight K. The effects of hypothermia on inflammation and swelling. Athl. Train.. 1976;11:7-10.
3 Hettinga D.L. I. Normal joint structures and their reaction to injury. J. Orthop. Sports Phys. Ther.. 1979;1:16-22.
4 Roy S., Ghadially F.N., Crane W.A.J. Synovial membrane and traumatic effusion: Ultrastructure and autoradiography with tritiated leucine. Ann. Rheumatol. Dis.. 1966;25:259-271.
5 Bozdech Z. Posttraumatic synovitis. Acta Chir. Orthop. Traumatol. Cech.. 1976;43:244-247.
6 Soren A., Rosenbauer K.A., Klein W., Hugh F. Morphological examinations of so-called posttraumatic synovitis. Beitr. Pathol.. 1973;1950:11-30.
7 Castor C.W., Prince R.K., Hazelton M.J. Hyaluronic acid in human synovial effusions: A sensitive indicator of altered connective tissue cell function during inflammation. Arthritis Rheum.. 1966;9:783-794.
8 Roosendaal G., Vianen M.E., Marx J.J. Blood-induced joint damage: A human in vitro study. Arthritis Rheum.. 1999;42:1025-1032.
9 Roosendaal G., Vianen M.E., van der Berg H.M. Cartilage damage as a result of hemarthrosis in a human in vitro model. J. Rheumatol.. 1997;24:1350-1354.
10 Roosendaal G., Tekoppele J.M., Vianen M.E. Articular cartilage is more susceptible to blood induced damage at young than at old age. J. Rheumatol.. 2000;27:1740-1744.
11 Stravino V.D. The synovial system. Am. J. Phys. Med.. 1972;51:312-320.
12 Hettinga D.L. II. Normal joint structures and their reaction to injury. J. Orthop. Sports Phys. Ther.. 1979;1:83-88.
13 Sledge C.B. Structure, development, and function of joints. Orthop. Clin. North Am.. 1975;6:619-628.
14 Booth F.W., Kelso J.R. Effect of hindlimb immobilization on contractile and histochemical properties of skeletal muscle. Pflugers Arch.. 1973;342:231-238.
15 MacDougall J.D., Elder G.C.B., Sale D.G. Effects of strength training and immobilization on human muscle fibers. Eur. J. Appl. Physiol.. 1980;43:25-34.
16 MacDougall J.D., Ward G.R., Sale D.G., Sutton J.R. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. J. Appl. Physiol.. 1977;43:700-703.
17 Venn M.F. Chemical composition of human femoral and head cartilage: Influence of topographical position and fibrillation. Ann. Rheum. Dis.. 1979;38:57-62.
18 Stokes M., Young A. The contribution of reflex inhibition to arthrogenous muscle weakness. Clin. Sci.. 1984;67:7-14.
19 Leivo I., Kauhanen S., Michelsson J.E. Abnormal mitochondria and sarcoplasmic changes in rabbit skeletal muscle induced by immobilization. APMIS. 1998;106:1113-1123.
20 Lindboe C.F., Platou C.S. Effects of immobilization of short duration on muscle fiber size. Clin. Physiol.. 1984;4:183-188.
21 Appell H.J. Morphology of immobilized skeletal muscle and the effects of a pre- and postimmobilization training program. Int. J. Sports Med.. 1986;7:6-12.
22 Binkley J.M., Peat M. The effects of immobilization on the ultrastructure and mechanical properties of the medial collateral ligament of rats. Clin. Orthop. Relat. Res.. 1986;203:301-308.
23 Haggmark T., Eriksson E., Jansson E. Muscle fiber type changes in human skeletal muscle after injuries and immobilization. Orthopedics. 1986;9:181-185.
24 Haggmark T., Jansson E., Eriksson E. Fiber type area and metabolic potential of the thigh muscle in man after knee surgery and immobilization. Int. J. Sports Med.. 1981;2:12-17.
25 Labarque V.L., Op’t Eijnde B., Van Leemputte M. Effect of immobilization and retraining on torque-velocity relationship of human knee flexor and extensor muscles. Eur. J. Appl. Physiol.. 2002;86:251-257.
26 Hayashi K. Biomechanical studies of the remodeling of knee joint tendons and ligaments. J. Biomech.. 1996;29:707-716.
27 Wolf J. Das Gesetz der Transformation der Knochen 1982 Berlin, A. Hirschwald
28 Young D.R., Niklowitz W.J., Steele C.R. Tibial changes in experimental disuse osteoporosis in the monkey. Calcif. Tissue Int.. 1983;35:304-308.
29 Kannus P., Jozsa L., Kvist M. The effect of immobilization on myotendinous junction: An ultrastructural, histochemical and immunohistochemical study. Acta Physiol. Scand.. 1992;144:387-394.
30 Booth F.W. Physiological and biochemical effects of immobilization on muscle. Clin. Orthop. Relat. Res.. 1987;219:15-20.
31 Booth F.W., Seider M.J. Recovery of skeletal muscle after 3 months of hindlimb immobilization in rats. J. Appl. Physiol.. 1979;47:435-439.
32 Maier A., Crockett J.L., Simpson D.R. Properties of immobilized guinea pig hindlimb muscles. Am. J. Physiol.. 1976;231:1520-1526.
33 Trias A. Effects of persistent pressure on articular cartilage. J. Bone Joint Surg. Am.. 1961;43:376-386.
34 Cooper R.R. Alternatives during immobilization and regeneration of skeletal muscle in cats. J. Bone Joint Surg. Am.. 1972;54:919-953.
35 Max S.R. Disuse atrophy of skeletal muscle: Loss of functional activity of mitochondria. Biochem. Biophys. Res. Commun.. 1972;46:1394-1398.
36 Rifenberick D.H., Max S.R. Substrate utilization by disused rat skeletal muscles. Am. J. Physiol.. 1974;226:295-297.
37 Ingemann-Hansen T., Halkjaer-Kristensen J. Computerized tomographic determination of human thigh components. The effects of immobilization in plaster and subsequent physical training. Scand. J. Rehabil. Med.. 1980;12:27-31.
38 Ingemann-Hansen T., Halkjaer-Kristensen J. Lean and fat composition of the human thigh. The effects of immobilization in plaster and subsequent physical training. J. Rehabil. Med.. 1977;9:67-72.
39 Shakespeare D.T., Stokes M., Sherman K.P., Young A. The effect of knee flexion on quadriceps inhibition after meniscectomy. Clin. Sci.. 1983;65:64P-65P.
40 Shakespeare D.T., Stokes M., Sherman K.P., Young A. Reflex inhibition of the quadriceps after meniscectomy: Lack of association with pain. Clin. Physiol.. 1985;5:137-144.
41 Ben-Yishay A., Zuckerman J.D., Gallagher M., Cuomo F. Pain inhibition of shoulder strength in patients with impingement syndrome. Orthopedics. 1994;17:685-688.
42 Itoi E., Minagawa H., Solo T. Isokinetic strength after tears of the supraspinatus tendon. J. Bone Joint Surg. Br.. 1997;79:77-82.
43 Kirschenbaum D., Coyle M.P., Leddy L.P. Shoulder strength with rotator cuff tears: Pre- and postoperative analysis. Clin. Orthop. Relat. Res.. 1993;288:174-178.
44 Hess T., Gleitz M., Hopf T. Changes in muscular activity after knee arthrotomy and arthroscopy. Int. Orthop.. 1995;19:94-97.
45 Stetson W.B., Templin K. Two versus three portal technique for routine knee arthroscopy. Am. J. Sports Med.. 2002;30:108-111.
46 Rabb D.J., Fischer D.A., Smith J.P. Comparison of arthroscopic and open reconstruction of the anterior cruciate ligament. Early results. Am. J. Sports Med.. 1993;21:680-683.
47 T’Jonck L., Lysens R., De Smet L. Open versus arthroscopic subacromial decompression: Analysis of one-year results. Physiother. Res. Int.. 1997;2:46-61.
48 Arciero R.A., Scoville C.R., Hayda R.A., Snyder R.J. The effect of tourniquet use in anterior cruciate ligament reconstruction. A prospective, randomized study Am. J Sports Med. 24 1996 758-764
49 Saunders K.C., Louis D.L., Weingarden S.I., Waylonis G.W. Effect of tourniquet time on postoperative quadriceps function. Clin. Orthop. Relat. Res.. 1979;143:194-199.
50 Walsh S., Frank C., Shrive N., Hart D. Knee immobilization inhibits biomechanical maturation of the rabbit medial collateral ligament. Clin. Orthop. Relat. Res.. 1993;297:253-261.
51 DeAndrade J.R., Grant C., Dixon A. Joint distension and reflex inhibition in the knee. J. Bone Joint Surg. Am.. 1965;47:313-322.
52 Jayson M.I.V., Dixon A. Intra-articular pressure in rheumatoid arthritis of the knee. III. Pressure changes during joint use. Ann. Rheum. Dis.. 1970;29:401-408.
53 Kennedy J.C., Alexander I.J., Hayes K.C. Nerve supply of the human knee and its functional importance. Am. J. Sports Med.. 1982;10:329-335.
54 Spencer J.D., Hayes K.C., Alexander I.J. Knee joint effusion and quadriceps inhibition in man. Arch. Phys. Med. Rehabil.. 1984;65:171-177.
55 Ziechen J., van Griensven M., Albers I. Immunohistochemical localization of collagen VI in arthrofibrosis. Arch. Orthop. Trauma Surg.. 1999;119:315-318.
56 Young A., Stokes M., Iles J.F. Effects of joint pathology on muscle. Clin. Orthop. Relat. Res.. 1987;219:21-27.
57 Geborek P., Moritz U., Wollheim F.A. Joint capsular stiffness in knee arthritis. Relationship to intraarticular volume, hydrostatic pressures, and extensor muscle function. J. Rheumatol. 1989;16:1351-1358.
58 Jones D.W., Jones D.A., Newham D.J. Chronic knee effusion and aspiration: The effect on quadriceps inhibition. Br. J. Rheumatol.. 1987;26:370-374.
59 Krebs D.E., Staples W.H., Cuttita D., Zickel R.E. Knee joint angle: Its relationship to quadriceps femoris activity in normal and post-arthrotomy limbs. Arch. Phys. Med. Rehabil.. 1983;64:441-447.
60 Stratford P. Electromyography of the quadriceps femoris muscles in subjects with normal knees and acutely effused knees. Phys. Ther.. 1981;62:279-289.
61 Eyring E.J., Murray W.R. The effect of joint position on the pressure of intra-articular effusion. J. Bone Joint Surg. Am.. 1964;46:1235-1241.
62 Levick R.J. Joint pressure-volume studies: Their importance, design and interpretation. J. Rheumatol.. 1983;10:353-357.
63 Levick R.J. Synovial fluid dynamics: The regulation of volume and pressure. In: Holborrow E.J., Maroudas V., editors. Studies in Joint Disease. London: Pitman Medical; 1983:153-240.
64 Tardieu C., Tabary J.C., Tabary C., Tardieu G. Adaptation of connective tissue length in immobilization in the lengthened and shortened positions in cat soleus muscle. J. Physiol.. 1982;78:214-217.
65 Witzmann F.A., Kim D.H., Fitts R.H. Hindlimb immobilization: Length-tension and contractile properties of skeletal muscle. J. Appl. Physiol.. 1982;53:335-345.
66 Jarvinen M.J., Einola S.A., Virtanen E.O. Effect of the position of immobilization upon tensile properties of rat gastrocnemius muscle. Arch. Phys. Med. Rehabil.. 1992;73:253-257.
67 Jokl P., Konstadt S. The effect of limb immobilization on muscle function and protein composition. Clin. Orthop. Relat. Res.. 1983;174:222-229.
68 Edin B.B., Vallbo A.B. Stretch sensitization of human muscle spindles. J. Physiol.. 1988;400:101-111.
69 Hugheston J.C. Complications of anterior cruciate ligament surgery. Orthop. Clin. North Am.. 1985;16:237-240.
70 Paulos L., Rosenberg T., Drawbert J. Infrapatellar contracture syndrome: An unrecognized cause of knee stiffness with patella entrapment and patella infera. Am. J. Sports Med.. 1987;15:331-342.
71 Sprangue N.F., O’Conner R.L., Fox J.M. Arthroscopic treatment of postoperative knee fibroarthrosis. Clin. Orthop. Relat. Res.. 1982;166:165-172.
72 Jackson D.W., Shafer R.K. Cyclops syndrome: Loss of extension following intra-articular anterior cruciate ligament reconstruction. Arthroscopy. 1987;6:171-178.
73 Fujimoto D., Moriquichi T., Ishida T., Hayashi H. The structure of pyridinoline, a collagen cross link. Biochem. Biophys. Res. Commun.. 1978;84:52-57.
74 Ham A.C., Cormack D. Histology Vol. 8 1979 Lippincott Philadelphia
75 Donatelli R., Owens-Burkhart A. Effects of immobilization on the extensibility of periarticular connective tissue. J. Orthop. Sports Phys. Ther.. 1981;3:67-72.
76 Burr D.B., Frederickson R.G., Pavlinch C. Intracast muscle stimulation prevents bone and cartilage deterioration in cast-immobilized rabbits. Clin. Orthop. Relat. Res.. 1984;189:264-278.
77 Akeson W.H., Amiel D., Woo S. Immobility effects of synovial joints: The pathomechanics of joint contracture. Biorheology. 1980;17:95-110.
78 Swann D., Radin E., Nazimiec M. Role of hyaluronic acid on joint lubrication. Ann. Rheum. Dis.. 1976;33:318-326.
79 Akeson W.H., Amiel D., Abel M.F. Effects of immobilization on joints. Clin. Orthop. Relat. Res.. 1987;219:28-37.
80 Akeson W.H., Amiel D., LaViolette D. The connective tissue response to immobility: A study of the chondroitin-4- and 6-sulfate and dermatan sulfate changes in periarticular connective tissue of control and immobilized knee of dogs. Clin. Orthop. Relat. Res.. 1967;51:183-197.
81 Akeson W.H., Woo S.L.Y., Amiel D. The chemical basis of tissue repair. In: Hunter L.Y., Funk F.J., editors. Rehabilitation of the Injured Knee. St. Louis: Mosby; 1984:93-148.
82 Bosch U., Ziechen J., Skutek M. Arthrofibrosis in the result of a T-cell mediated immune response. Knee Surg. Sports Trauma Arthrosc.. 2001;9:282-289.
83 Akeson W.H., Woo S.L.Y., Amiel D. The connective tissue response to immobility: Biochemical changes in periarticular connective tissue of the immobilized rabbit knee. Clin. Orthop. Relat. Res.. 1973;93:356-362.
84 Hunter R.E., Mastrangelo J., Freeman J.R. The impact of surgical timing on postoperative motion and stability following anterior cruciate ligament reconstruction. Arthroscopy. 1996;12:667-674.
85 Shelborne K.D., Wilchkens J.H., Mollabashy A., DeCarlo M. Arthrofibrosis in acute anterior cruciate ligament reconstruction: The effect of timing on reconstruction and rehabilitation. Am. J. Sports Med.. 1991;19:332-336.
86 Wakai A., Winter D.C., Street J.T., Redmond P.H. Pneumatic tourniquets in extremity surgery. J. Am. Acad. Orthop. Surg.. 2001;9:345-351.
87 Mariani P.P., Santori N., Rovere P. Histological and structural study of the adhesive tissue in knee fibroarthrosis: A clinical-pathological correlation. Arthroscopy. 1997;13:13-18.
88 Tamberello M., Mangine R.E., Personius W. Patella hypomobility as a cause of extensor lag Presented at Total Care of the Knee: Before and After Injury (Cybex Conference) 1982 KS Overland Park May 17-19, 1985
89 Arem A.J., Madden J.W. Effects of stress on healing wounds: Intermittent noncyclical tension. J. Surg. Res.. 1976;20:93-102.
90 Wright V., Dowson D., Kerr J. The structure of joints. Int. Rev. Connect. Tissue Res.. 1973;6:105-125.
91 Williams P.E., Goldspink G. Changes in sarcomere length and physiological properties in immobilized muscle. J. Anat.. 1978;127:459-468.
92 Wronski T., Morey E.R. Skeletal abnormalities in rats induced by simulated weightlessness. Metab. Bone Dis.. 1982;4:69-74.
93 Videman T. Changes of compression and distances between tibial and femoral condyles during immobilization of rabbit knee. Arch. Orthop. Trauma Surg.. 1981;98:289-294.
94 Elliot R.J., Gardner D.L. Changes with age of the glycosaminoglycans of human cartilage. Ann. Rheum. Dis.. 1979;38:371-377.
95 Westers B.M. Review of the repair of defects in articular cartilage: Part I. J. Orthop. Sports Phys. Ther.. 1982;3:186-192.
96 Uhthoff H.K., Jaworski Z.F.G. Bone loss in response to long-term immobilization. J. Bone Joint Surg. Br.. 1978;60:420-429.
97 Maroudes A., Bullough P., Swanson S., Freemna M. The permeability of articular cartilage. J. Bone Joint Surg. Br.. 1968;50:166-177.
98 Jozsa L., Jarvinen M., Kannus P., Reffy A. Fine structural changes in the articular cartilage of the rat’s knee following short-term immobilization in various positions: A scanning electron microscopical study. Int. Orthop.. 1987;11:129-133.
99 Jurvelin J., Kiviranta I., Tammi M., Helminen J.H. Softening of canine articular cartilage after immobilization of the knee joint. Clin. Orthop. Relat. Res.. 1986;207:246-252.
100 Radin E.L., Paul I.L., Pollock D. Animal joint behavior under excessive loading. Nature. 1970;266:554-555.
101 Virchenko O., Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop.. 2006;77:806-812.
102 Salter R.B., Field P. The effects of continuous compression on living articular cartilage. J. Bone Joint Surg. Am.. 1960;42:31-49.
103 Hall M.C. Cartilage changes after experimental relief of contact in the knee of the mature rat. J. Bone Joint Surg. Am.. 1963;45:36-44.
104 Broom N.D., Myers D.B. A study of the structural response of wet hyaline cartilage to various loading situations. Connect. Tissue Res.. 1980;7:227-237.
105 Ekholm R. Nutrition of articular cartilage: A radioautographic study. Acta Anat.. 1955;24:329-338.
106 Enneking W.F., Horowitz M. The intra-articular effects of immobilization on the human knee. J. Bone Joint Surg. Am.. 1972;54:973-985.
107 Eronin I., Videman T., Friman C., Michelsson J.E. Glycosaminoglycan metabolism in experimental osteoarthritis caused by immobilization. Acta Orthop. Scand.. 1978;49:329-334.
108 Langenskiold A., Michelsson J.E., Videman T. Osteoarthritis of the knee in the rabbit produced by immobilization: Attempts to achieve a reproducible model for studies on pathogenesis and therapy. Acta Orthop. Scand.. 1979;50:1-14.
109 Sherman K.P., Young A., Stokes M., Shakespeare D.T. Joint injury and muscle weakness. Lancet. 1984;2:646-651.
110 Roth J.H., Mendenhall H.V., McPherson G.K. The effect of immobilization on goat knees following reconstruction of the anterior cruciate ligament. Clin. Orthop. Relat. Res.. 1988;229:278-282.
111 Kiviranta I., Tammi M., Jurvelin J. Articular cartilage thickness and glycosaminoglycan distribution in the canine knee joint after strenuous running exercise. Clin. Orthop. Relat. Res.. 1992;283:302-308.
112 Cabaud H.E., Chatty A., Gildengorin V. Exercise effects on the strength of the rat anterior cruciate ligament. Am. J. Sports Med.. 1980;8:79-86.
113 Amiel D., Woo S.L.Y., Harwood F.L., Akeson W.H. The effect of immobilization on collagen turnover in connective tissue: A biochemical-biochemical correlation. Acta Orthop. Scand.. 1982;53:325-332.
114 Noyes F.R., Mangine R.E., Barber S. Biomechanics of ligament failure. II. An analysis of immobilization, exercise, and reconditioning effects in primates. J. Bone Joint Surg. Am.. 1974;56:1406-1418.
115 Noyes F.R., Mangine R.E., Barber S. Early knee motion after open and arthroscopic anterior cruciate ligament reconstruction. Am. J. Sports Med.. 1987;15:149-160.
116 Woo S., Gomez M.A., Sites T.J. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J. Bone Joint Surg. Am.. 1987;69:1200-1211.
117 Noyes F.R. Functional properties of knee ligaments and alterations induced by immobilization. Clin. Orthop. Relat. Res.. 1977;123:210-242.
118 Yamaguchi T., Ishii K., Yamanaka M., et al. Acute effects of static stretching on power output during concentric dynamic constant external resistance leg extension. J. Strength Cond. Res.. 2006;20:804-810.
119 Gamble J.G., Edwards C.C., Max S.R. Enzymatic adaptation in ligaments during immobilization. Am. J. Sports Med.. 1984;12:221-228.
120 Newton P.O., Woo S.L., Kitabayashi L.R. Ultrastructural changes in knee ligaments following immobilization. Matrix. 1990;10:314-319.
121 Yasuda K., Ohkoshi Y., Tanabe Y., Kaneda K. Quantitative evaluation of knee instability and muscle strength after anterior cruciate ligament reconstruction using patellar tendon and quadriceps tendon. Am. J. Sports Med.. 1992;20:471-475.
122 Boorman R.S., Shrive N.G., Frank C.B. Immobilization increases the vulnerability of rabbit medial collateral ligament autografts to creep. J. Orthop. Res.. 1998;16:682-689.
123 Thornton G.M., Boorman R.S., Shrive N.G., Frank C.B. Medial collateral ligament autografts have increased creep response for at least two years and early immobilization makes this worse. J. Orthop. Res.. 2002;20:346-352.
124 Weiss C. Normal and osteoarthritic articular cartilage. Orthop. Clin. North Am.. 1979;10:175-189.
125 Ochi M., Kanda T., Sumen Y., Ikuta Y. Changes in the permeability and histologic findings of rabbit menisci after immobilization. Clin. Orthop. Relat. Res.. 1997;334:305-315.
126 Bray R.C., Smith J.A., Eng M.K. Vascular response of the meniscus to injury: Effects of immobilization. J. Orthop. Res.. 2001;19:384-390.
127 Djurasovic M., Aldridge J.W., Grumbles R. Knee joint immobilization decreases aggrecan gene expression in the meniscus. Am. J. Sports Med.. 1998;26:460-466.
128 Klein L., Heiple K.G., Torzilli P.A. Prevention of ligament and meniscus atrophy by active joint motion in a non–weight-bearing model. J. Orthop. Res.. 1989;7:80-85.
129 Butler D.L., Grood E.S., Noyes F.R. Mechanical properties of primate vascularized versus nonvascularized patellar tendon grafts, changes over time. J. Orthop. Res.. 1989;7:68-79.
130 Falconiero R.P., DiStefano V.J., Cook T.M. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14:197-205.
131 Rougraff B.T., Shelbourne K.D. Early histologic appearance of human patellar tendon autografts used for anterior cruciate ligament reconstruction. Knee Surg. Sports Trauma Arthrosc.. 1999;7:9-14.
132 Papageorgiou C.D., Benjamin C., Abramowitch S.D. Multidisciplinary study of the healing of an intraarticular anterior cruciate ligament in a goat model. Am. J. Sports Med.. 2001;29:620-626.
133 Scranton P.E., Lanzer W.L., Ferguson M.S. Mechanism of anterior cruciate neovascularization and ligamentization. Arthroscopy. 1998;14:702-716.
134 Hardt A.B. Early metabolic responses of bone to immobilization. J. Bone Joint Surg. Am.. 1972;54:119-124.
135 Burdeaux B.D., Hutchinson W.J. Etiology of traumatic osteoporosis. J. Bone Joint Surg. Am.. 1953;35:479-488.
136 Fleisch H., Russell R.G., Simpson B., Muhlbauer R.C. Prevention by a diphosphonate of immobilization osteoporosis in rats. Nature. 1969;223:211-212.
137 Geiser M., Trueta J. Muscle action, bone rarefaction, and bone formation. J. Bone Joint Surg. Br.. 1985;40:282-311.
138 Landry M., Fleisch H. The influence of immobilization on bone formation as evaluated by osseous incorporation of tetracyclines. J. Bone Joint Surg. Br.. 1964;46:764-771.
139 Steinberg F.U. The Immobilized Patient: Functional Pathology and Management. New York: Plenum Press; 1980.
140 Epker B.N., Frost H.M. Correlation of bone resorption and formation behavior of loaded bone. J. Dent. Res.. 1965;44:33-41.
141 Mazess R.B., Whedon G.D. Immobilization and bone. Calcif. Tissue Int.. 1983;35:265-267.
142 Wrotniak M., Bielecki T., Gazdzik T. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Orthop. Traumatol. Rehabil.. 2007;9:227-238.
143 Salter R.B. The biologic concept of continuous passive motion of synovial joints. Clin. Orthop. Relat. Res.. 1989;242:12-25.
144 Loitz B.J., Zernicke R.F., Vailas A.C. Effects of short-term immobilization versus continuous passive motion on the biomechanical and biochemical properties of the rabbit tendon. Clin. Orthop. Relat. Res.. 1989;244:265-271.
145 Salter R.B., Simmonds D.F., Malcolm B.W. The effect of continuous passive motion on the healing of articular cartilage defects: An experimental investigation in rabbits [Abstract]. J. Bone Joint Surg. Am.. 1975;57:570.
146 Salter R.B., Minster R.R. The effect of continuous passive motion on a semitendinous tenodesis in the rabbit knee [Abstract]. Orthop. Trans.. 1982;6:292.
147 McCarthy M.R., Buxton B.P., Yates C.K. Effects of continuous passive motion on anterior laxity following ACL reconstruction with autogenous patellar tendon grafts. J. Sport Rehabil.. 1993;2:171-178.
148 Nugent-Derfus G., Takara T., O’Neill J.K., et al. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis Cartilage. 2007;15:566-574.
149 Raab M.G., Rzeszutko D., O’Conner W., Greatting M.D. Early results of continuous passive motion after rotator cuff repair: A prospective, randomized, blinded, controlled study. Am. J. Orthop.. 1996;25:214-220.
150 Lastayo P.C., Wright T., Jaffe R., Hartzel J. Continuous passive motion after repair of the rotator cuff: A prospective outcome study. J. Bone Joint Surg. Am.. 1998;80:1002-1111.
151 Engstrom B., Sperber A., Wredmark T. Continuous passive motion in rehabilitation after anterior cruciate ligament reconstruction. Knee Surg. Sports Trauma Arthrosc.. 1995;3:18-20.
152 Gasper L., Farkas C., Szepesi K., Csernatony Z. Therapeutic value of continuous passive motion after anterior cruciate replacement. Acta Chir. Hung.. 1997;36:104-105.
153 Rosen M.A., Jackson D.W., Artwell E.A. The efficacy of continuous passive motion in the rehabilitation of anterior cruciate ligament reconstruction. Am. J. Sports Med.. 1992;20:122-127.
154 McCarthy M.R., Yates C.K., Anderson M.A., Yates-McCarthy J.L. The effects of immediate continuous passive motion on pain during the inflammatory phase of soft tissue healing following anterior cruciate reconstruction. J. Orthop. Sports Phys. Ther.. 1993;17:96-101.
155 Lenssen T., van Steyn M.J., Crijns Y.H., et al. Effectiveness of prolonged use of continuous passive motion (CPM) as an adjunct to physiotherapy, after total knee arthroplasty. BMC Musculoskel. Disord.. 2008;9:1-11.
156 Yates C.K., McCarthy M.R., Hirsch H.S., Pascale M.S. Effects of continuous passive motion following ACL reconstruction with autogenous patellar tendon grafts. J. Sport Rehabil.. 1992;1:121-131.
157 Dehert W.J., O’Driscoll S.W., van Royen B.J., Salter R.B. Effects of immobilization and continuous passive motion on postoperative muscle atrophy in mature rabbits. Can. J. Surg.. 1988;31:185-188.
158 Gebhard J.S., Kabo J.M., Meals R.A. Passive motion: The dose effects on joint stiffness, muscle mass, bone density, and regional swelling. A study in an experimental model following intra-articular injury. J. Bone Joint Surg. Am.. 1993;75:1636-1647.
159 Renzoni S.A., Amiel D., Harwood F.L., Akeson W.H. Synovial nutrition of knee ligaments. Trans. Orthop. Res. Soc.. 1984;9:277-283.
160 Kannus P., Jozsa L., Jarvinen T.L. Free mobilization and low- to high-intensity exercise in immobilization-induced muscle atrophy. J. Appl. Physiol.. 1998;84:1418-1424.
161 Keays S.L., Bullock-Saxton J., Keays A.C. Strength and function before and after anterior cruciate reconstruction. Clin. Orthop. Relat. Res.. 2000;373:174-183.
162 Snyder-Mackler L., Delitto A., Bailey S.L., Stralka S.W. Strength of the quadriceps femoris muscles and functional recovery after reconstruction of the anterior cruciate ligament: A prospective, randomized clinical trial of electrical stimulation. J. Bone Joint Surg. Am.. 1995;77:1166-1173.
163 Keays S.L., Bullock-Saxton J., Keays A.C., Newcombe P. Muscle strength and function before and after anterior cruciate ligament reconstruction using semitendinosis and gracilis. Knee. 2001;8:229-234.
164 Osteras H., Augestad L.B., Tondel S. Isokinetic muscle strength after anterior cruciate ligament reconstruction. Scand. J. Med. Sci. Sports. 1998;8:279-282.
165 Arvidsson I., Arvidsson H., Eriksson E., Jansson E. Prevention of quadriceps wasting after immobilization: An evaluation of the effect of electrical muscle stimulation. Orthopedics. 1986;9:1519-1528.
166 Rokito A.S., Zuckerman J.D., Gallagher M.A., Cuomo F. Strength after surgical repair of the rotator cuff. J. Shoulder Elbow Surg.. 1996;5:12-17.
167 Gibson J.N., Smith K., Rennie M.J. Prevention of disuse muscle atrophy by means of electrical muscle stimulation: Maintenance of protein synthesis. Lancet. 1988;7:767-770.
168 Morrissey M.C., Brewster C.E., Shields C.L., Brown M. The effects of electrical stimulation on the quadriceps during postoperative knee immobilization. Am. J. Sports Med.. 1985;13:40-45.
169 Delitto A., Rose S.J., McKowen J.M. Electrical stimulation versus voluntary exercise in strengthening thigh musculature after anterior cruciate ligament surgery. Phys. Ther.. 1988;68:660-663.
170 Eriksson E., Haggmark T. Comparison of isometric muscle training and electrical stimulation supplementing isometric muscle training in the recovery after major knee ligament surgery. Am. J. Sports Med.. 1979;7:169-171.
171 Snyder-Mackler L., Ladin Z., Schepsis A.A., Young J.C. Electrical stimulation of the thigh muscles after reconstruction of the anterior cruciate ligament: Effects of electrically elicited contraction of the quadriceps femoris and hamstring muscles on gait and on strength of the thigh muscles. J. Bone Joint Surg. Am.. 1991;73:1025-1036.
172 Paternostro-Sluga T., Fialka C., Alacamliogliu Y. Neuromuscular electrical stimulation after anterior cruciate ligament surgery. Clin. Orthop. Relat. Res.. 1999;368:166-175.
173 Lieber R.L., Silva P.D., Daniel D.M. Equal effectiveness of electrical and volitional strength training for the quadriceps femoris muscles after anterior cruciate ligament surgery. J. Orthop. Res.. 1996;14:131-138.
174 Draper V., Ballard L. Electrical stimulation versus electromyographic biofeedback in the recovery of quadriceps femoris muscle function following anterior cruciate ligament surgery. Phys. Ther.. 1991;71:455-461.
175 Kiviranta I., Tammi M., Jurvelin J. Articular cartilage thickness and glycosaminoglycan distribution in the young canine knee joint after remobilization of the immobilized limb. J. Orthop. Res.. 1994;12:161-167.
176 Van H., Lillich J.D., Kawcak C.E. Clinical evaluation of the effects of immobilization followed by remobilization and exercise on the metacarpophalangeal joint in horses. Am. J. Vet. Res.. 2002;63:282-288.
177 Veldhuizen J.W., Verstappen F.T., Vroemen J.P. Functional and morphological adaptations following four weeks of knee immobilization. Int. J. Sports Med.. 1993;14:283-287.
178 Haapala J., Arokoski J., Pirttimaki J. Incomplete restoration of immobilization induced softening of young beagle knee articular cartilage after 50-week remobilization. Int. J. Sports Med.. 2000;21:76-81.
179 Evans E.B., Egger G.W.N., Butler M., Blumel J. Experimental immobilization and remobilization of rat knee joints. J. Bone Joint Surg. Am.. 1960;42:737-758.
180 Lynch T.N., Jensen R.L., Stevens D.M. Metabolic effects of prolonged bed rest: Their modification by simulated altitude. Aerosp. Med.. 1967;38:10-20.
181 Wahl S., Renstrom R. Fibrosis in soft-tissue injuries. In: Leadbetter W., Buckwalter J., Gordon S., editors. Sports-Induced Inflammation: Clinical and Basic Science Concepts.. Park Ridge, IL: American Academy of Orthopaedic Surgeons; 1991:63-82.
182 Woo S., Inoue D.M., McGurk-Burleson E., Gomez M.A. Treatment of the medial collateral ligament injury. II: Structure and function of canine knees in response to differing treatment regimes. Am. J. Sports Med.. 1987;15:22-29.
183 Woo S., Matthew J.V., Akeson W.H. Connective tissue response to immobility. Arthritis Rheum.. 1975;18:257-264.
184 Jurvelin J., Helminen H.J., Laurisalo S. Influences of joint immobilization and running exercise on articular cartilage surfaces of young rabbits. Acta Anat.. 1985;122:62-68.
185 Tipton C.M., James S.L., Mergner W. Influence of exercise on strength of medial collateral knee ligament of dogs. Am. J. Physiol.. 1970;218:894-902.
186 Cassel E.P., Finch C.F., Stathakis V.Z. Epidemiology of medically treated sport and active recreation injuries in the Latrobe Valley, Victoria. Australia. Br. J. Sports Med.. 2003;37:405-409.
187 Timpka T., Ekstrand J., Svanstrom L. From sports injury prevention to safety promotion in sports. Sports Med.. 2006;36:733-745.
188 Anitiua E. Plasma-rich in growth factors: preliminary results of the use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implants. 1999;14:529-535.
189 Marx R.E., Carlson E.R., Eichstaedt R.N., et al. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod.. 1998;85:638-646.
190 Sampson S., Gerhardt M., Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: A review. Curr. Rev. Musculoskel. Med.. 2008;1:165-174.
191 Edwards S.G., Calandruccio J.H. Autologous blood injections for refractory lateral epicondylitis. Am. J. Hand Surg.. 2003;28:272-278.
192 Kajikawa Y., Morihara T., Sakamoto H., et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. J. Cell. Physiol.. 2007;215:837-845.
193 Marx R., Garg A. Dental and Craniofacial Applications of Platelet-Rich Plasma. Hanover Park, IL: Quintessence Publishing; 2005.
194 Creaney L., Hamilton B. Growth factor delivery methods in the management of sports injury: The state of play. Br. J. Sports Med.. 2008;42:314-320.
195 Everts P., Knape J., Weirich G., et al. Platelet-rich plasma and platelet gel: a review. J. ECT. 2006;38:174-187.
196 Peerbooms J.C., Sluimer J., Bruijn D.J., Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double blind randomized controlled trial; platelet-rich plasma versus corticosteroid injection with a 1-year follow up. Am. J. Sports Med.. 2010;38:255-262.
197 Everts P., Deville R., Mahoney C., et al. Platelet gel and fibrin sealant reduce allogenic blood transfusions in total knee arthroplasty. Acta Anaesthesiol. Scand.. 2006;50:593-599.
198 Yamaguchi T., Ishii K., Yamanaka M., Yasuda K. Acute effects of static stretching on power output during concentric dynamic constant external resistance leg extension. J. Strength Cond. Res.. 2006;20:804-810.
199 Everts P.A., Overdevest E.P., Jakimowicz J.J., et al. The use of autologous platelet-leukocyte gels in enhancing the healing process in surgery: A review. Surg. Endosc.. 2007;21:2063-2068.
200 Aspenberg P., Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop. Scand.. 2004;75:93-99.
201 Vogt F.B., Mack P.B., Beasley W.G. The effect of bed rest on various parameters of physiological function. Part XII. The effect of bed rest on bone mass and calcium balance. Washington, DC: National Aeronautics and Space Administration; 1965.
202 Molloy T., Wang Y., Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med.. 2003;33:381-394.
203 Anitiua E., Andia I., Sanchez M., et al. Autologous preparations rich in growth factors promote proliferation and induce VEGF and HGF productions by human tendon cells in culture. J. Orthop. Res.. 2005;23:281-286.
204 Mentry J., Kasemkijwattana C., Day C.S., et al. Growth factors improve muscle healing in vivo. J. Bone Joint Surg. Br.. 2000;82:131-137.
205 Lefaucheur J.P., Sebille A. Muscle regeneration following injury can be modified in vivo by immune neutralization of basic fibroblast growth factor, transforming growth factor beta 1 or insulin-like growth factor 1. J. Neuroimmunol.. 1995;57:85-91.
206 Hammond J.W., Hinton R.Y., Curl L.A., et al. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am. J. Sports Med.. 2009;37:1135-1142.
207 Gaweda K., Tarczynska M., Krzyzanowski W. Treatment of Achilles tendonopathy with platelet-rich plasma. Int. J. Sports Med.. 2010;31:577-583.
208 Smidt N., van der Windt D.A., Assendelft W.J., et al. Corticosteroid injections, physiotherapy or a wait-and-see policy for lateral epicondylitis: A randomized controlled trial. Lancet. 2002;359:657-662.
209 Barrett S., Erredge S. Growth factors for chronic plantar fasciitis. Podiatry Today. 2004;17:37-42.
210 Cole C., Seto C., Gazewood J. Plantar fasciitis: Evidence-based review of diagnosis and therapy. Am. Fam. Physician. 2005;72:2237-2242.
211 Acevedo J., Beskin J. Complications of plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int.. 1998;19:91-97.
212 Sellman J. Plantar fascia rupture associated with corticosteroid injection. Foot Ankle Int.. 1994;15:376-381.
213 Leach R., Jones R., Silva T. Rupture of the plantar fascia in athletes. J. Bone Joint Surg. Am.. 1978;60:537-539.
214 Berghoff W., Pietzak W., Rhodes R. Platelet-rich plasma application during closure following total knee arthroscopy. Orthopedics. 2006;29:590-598.
215 Gardner M.J., Demetrakopoulos D., Klepchick P., Mooar P. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty: An analysis of the hemoglobin, narcotic requirement and range of motion. Int. Orthop.. 2006;31:309-313.
216 Sanchez M., Azofra J., Aizpurua B., et al. Plasma rich in growth factors to treat articular cartilage avulsion: A case report. Med. Sci. Sports Exerc.. 2003;35:1648-1652.
217 Akeda K., An H.S., Okuma M., et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2007;14:1272-1280.
218 Ishida K., Kuroda R., Miwa M., et al. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng.. 2007;13:1103-1112.
219 Sanchez M., Anitua E., Orive G., et al. Platelet-rich therapies in the treatment of orthopaedic sports injuries. Sports Med.. 2009;39:345-354.
220 Aspenberg P. Stimulation of tendon repair: mechanical loading. GDFs and platelets. A mini-review. Acta Orthop. Scand.. 2007;31:783-789.
221 Smith C.A. The warm-up procedure: to stretch or not to stretch. A brief review. J. Orthop. Sports Phys. Ther. 1994;19:12-17.
222 McMillian D.J., Moore J.H., Hatler B.S., Taylor D.C. Dynamic vs. static-stretching warm up: The effect on power and agility performance. Strength Cond. Res. 2006;20:492-499.
223 McHugh M.P., Cosgrave C.H. To stretch or not to stretch: the role of stretching in injury prevention and performance. Scand. J. Med. Sci. Sports. 2010;20:169-181.
224 Herda T.J., Cramer J.T., Ryan E.D., et al. Acute effects of static versus dynamic stretching on isometric peak torque, electromyography, and mechanomyography of the biceps femoris muscle. J. Strength Cond. Res.. 2008;22:809-817.
225 Manoel M.E., Harris-Love M.O., Danoff J.V., Miller T.A. Acute effects of static, dynamic, and proprioceptive neuromuscular facilitation stretching on muscle power in women. J. Strength Cond. Res.. 2008;22:1528-1534.
226 Sekir U., Arabaci R., Akova B., Kadagan S.M. Acute effects of static and dynamic stretching on leg flexor and extensor isokinetic strength in elite women athletes. Scand. J. Med. Sci. Sports. 2009;20:268-281.
227 Yamaguchi T., Ishii K., Yamanaka M., Yasuda K. Acute effects of dynamic stretching exercise on power output during concentric dynamic constant external resistance leg extension. J Strength Cond. Res.. 2007;21:1238-1244.
228 Nelson A.G., Driscoll N.M., Landin D.K., et al. Acute effects of passive muscle stretching on spring performance. J. Sports Sci.. 2005;23:449-454.
229 Little T., Williams A.J. Effects of differential stretching protocols during warm-ups on high speed motor capacities in professional footballers. J. Strength Cond. Res.. 2006;20:203-207.
230 Bandy W.D., Irion J.M., Briggler M. The effect of static stretch and dynamic range of motion training on the flexibility of the hamstring muscles. J. Orthop. Sports Phys. Ther.. 1998;27:295-300.
231 McHugh M.P., Magnusson S.P., Gleim G.W., et al. Viscoelastic stress relaxation in human skeletal muscle. Med. Sci. Sports Exerc.. 1992;24:1375-1382.
232 Toft E., Sinkjaer T., Kalund S., et al. Biomechanical properties of the human ankle in relation to passive stretch. J. Biomech.. 1989;22:1129-1132.
233 Shrier I. Does stretching improve muscle performance? A systematic and critical review of the literature. Clin. J. Sports Med.. 2004;14:267-273.
234 Macpherson P.C.D., Schork M.A., Faulkner J.A. Contraction-induced injury to single fiber segments from fast to slow twitch muscles of rats by single stretches. Am. J. Physiol.. 1996;271:C1438-C1446.
235 Laudner K.G., Sipes R.C., Wilson J.T. The acute effects of sleeper stretch on shoulder range of motion. J. Athl. Train.. 2008;43:359-363.
236 Olsen O.E., Myklebust G., Engebretsen L., et al. Exercises to prevent lower limb injuries in youth sports: Cluster randomized control trial. BMJ. 2005;330:449.