Imaging in Oncology
Diagnostic imaging plays an essential role in the management of the cancer patient. The initial diagnosis, staging, surgical and radiation treatment planning and response to therapy all involve imaging to a varying extent. Routine radiographs, ultrasound, nuclear medicine, and cross-sectional imaging in the form of computed tomography (CT) and magnetic resonance imaging (MRI) are routinely used in veterinary oncology. The choice of imaging modality depends on many factors, including the desired outcome. The biologic behavior of the tumor directs the imaging choice in cancer staging, and imaging may play an important role in guiding serial tumor biopsy during the course of therapy. The sophistication of imaging modalities continues to increase exponentially. Each modality has advantages and disadvantages with regard to cost, availability, sensitivity, specificity, and qualities of anatomic versus functional imaging (Table 6-1). Advanced molecular imaging techniques, which measure biologic processes at the cellular level,1-3 are quickly becoming commonplace in physician-based oncology and have the potential to play an important role in the tailoring of cancer therapy in veterinary patients.
Table 6-1
Comparison of Imaging Modalities Used in Veterinary Medicine
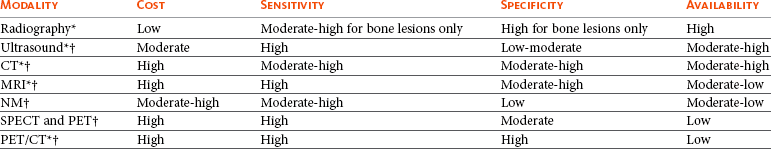
CT, Computed tomography; MRI, magnetic resonance imaging; NM, nuclear medicine; SPECT, single-photon emission computed tomography; PET, positron emission tomography.
*Anatomic imaging.
†Functional/physiologic imaging.
Imaging Modalities
Conventional radiography has been the mainstay of cancer imaging for many years because of its accessibility and low cost. However, it usually is relegated to a screening test and is often followed by other imaging modalities to better differentiate and define tumor extent (Figure 6-1).4,5 Radiographic images are produced by the differential absorption of x-rays as the primary beam passes through the patient. Some x-ray photons are absorbed by the body and some pass through. Absorption depends on the thickness, physical density, and effective atomic number of the tissues of the patient’s body. The x-rays that are not absorbed reach the radiographic film and determine the blackness and gray scale of the image.
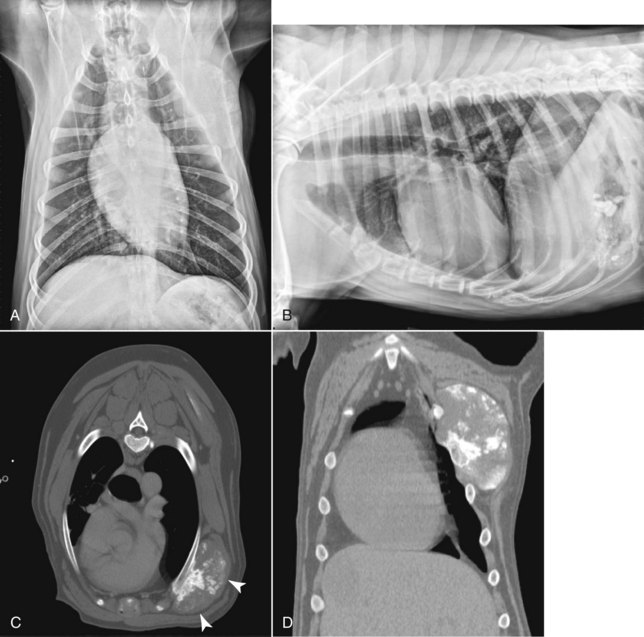
• Figure 6-1 Lateral and ventral dorsal thoracic radiographs (A and B) and corresponding CT images (C, transverse image and, D, dorsal reconstruction) of a dog with a thoracic wall osteosarcoma. Note that the mass is only faintly visible on lateral and ventral dorsal radiographic images, whereas CT provides complete information about tumor margins and extent of rib involvement (white arrows).
Two of the strengths of radiography are the global information it provides and its excellent utility for bone imaging, especially the appendicular skeleton.6 Thoracic and abdominal radiographs are excellent screening tools for feline and canine lymphoma patients to determine thoracic lymph node and pulmonary involvement, as well as liver, spleen, and abdominal lymph node neoplastic spread.7,8 Radiography’s greatest weaknesses are the superimposition of overlying structures and that only a few radiographic opacities are depicted. CT and MRI have replaced radiography for imaging of the head and, in some circumstances, the axial skeleton.9-12
Computed Tomography
As does radiography, CT relies on the physical density differences between tissues to form the image. Unlike radiography, CT portrays slices of the patient without superimposition of structures because the images are computer generated and the gray scale display is superior. Although thoracic radiographs are routinely used as a screening method for evaluation of primary and metastatic tumors of the lung, mediastinum, and ribs, CT provides superior information for characterizing and anatomically localizing thoracic lesions for their diagnosis and treatment (see Figure 6-1). Compared with radiography, CT is more sensitive for identifying pulmonary nodules (Figure 6-2), mediastinal lymphadenopathy, and pleural and other masses.5,13-17 CT should be used to ascertain the full extent of pulmonary nodules from metastatic disease and when a primary lung tumor has been identified to evaluate for intrathoracic metastases and tracheobronchial lymphadenopathy.
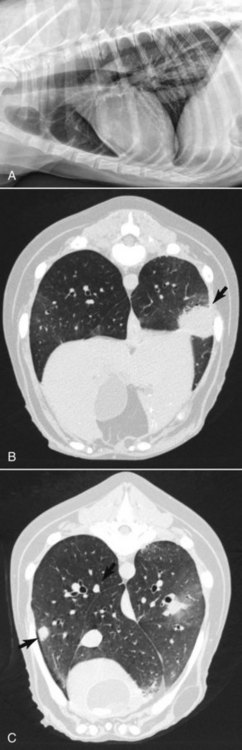
• Figure 6-2 This large pulmonary mass is barely visible on the right lateral thoracic radiograph due to its superimposition over the esophagus (A) but is clearly visible on CT (black arrow, B) along with additional metastatic nodules that were too small to see on radiographs (C).
CT is more sensitive than radiography to osteolysis and osteoproduction associated with neoplasia, and its three-dimensional (3D) information is especially useful for skeletal structures such as the sinonasal region, orbit (Figure 6-3), ear canals, and skeleton (Figure 6-4).9 CT is also useful to determine the origin and extent of abdominal mass lesions, and compared to ultrasonography, CT can better document the relationship of a mass with surrounding anatomic structures (Figure 6-5).18,19 Infiltrative muscular lesions such as infiltrative lipomas and soft tissue sarcomas are routinely imaged with CT for both surgical and radiotherapy planning.12,20 A contrast-enhanced scan is essential during CT to improve visualization of tumor margins, especially for infiltrative tumors such as feline vaccine–associated sarcomas.21
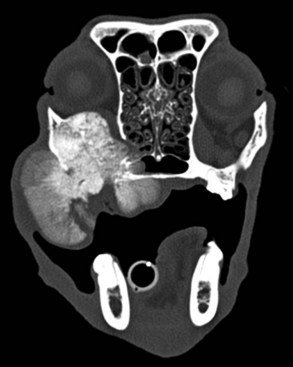
• Figure 6-3 CT transverse image of a dog with a multilobular osteochondrosarcoma; note the nodular bone formations arising from the zygoma and palate that are encroaching on the orbit and displacing the eye.
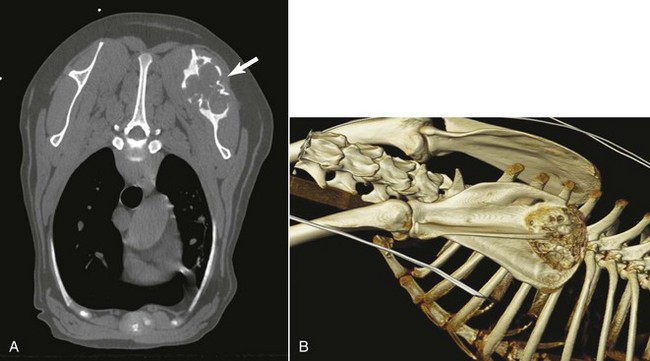
• Figure 6-4 A, CT transverse image (white arrow). B, Three-dimensional reconstructed image of a dog with scapular osteosarcoma, which provides an excellent visual assessment for surgical planning.
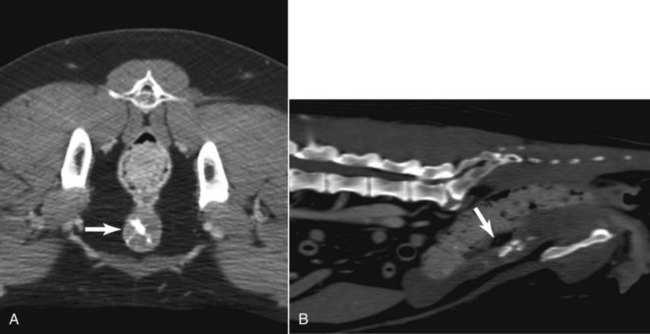
• Figure 6-5 CT images of the urethra of a dog with transitional cell carcinoma (TCC). Note the thickened urethra with multifocal calcifications on the transverse image (A) and sagittal reconstructed image (B).
CT is exceedingly useful for both surgical and radiotherapy planning. Although MRI has better tissue differentiation, CT is used most often for radiotherapy treatment planning because there is no image distortion and the physical tissue density is available for input into treatment planning computers.22,23 It is extremely important to position the radiotherapy patient for the CT scan done for radiation treatment planning in a manner that can reliably be repeated during therapy. This will ensure the treatment is delivered as planned. CT is also amenable to obtaining image-guided biopsy of masses that are not readily obtained with ultrasound guidance, and is particularly helpful for thoracic, brain, spinal, and skeletal lesion biopsy (Figure 6-6).24-28 Contrast-enhanced CT angiography (CTA) is now also becoming more routine due to the increasing availability of multidetector CT scanners in veterinary medicine. CTA allows detection of tumor vascular invasion and can also depict tumor vascular supply for interventional therapies (Figure 6-7). Dynamic multiphase CT can also improve detection of metastases and small tumors such as insulinomas and may distinguish benign from malignant hypervascular primary hepatic tumors.29-32
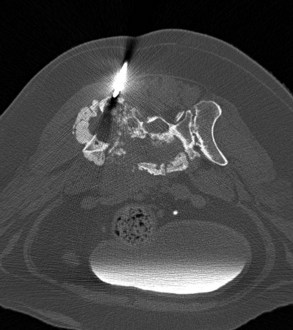
• Figure 6-6 CT transverse image of a biopsy needle being directed into an aggressive osteolytic bone tumor within the ilial wing of the pelvis. The metallic biopsy needle creates a metal streak artifact (black lines above and below the visible portion of the needle) on this image.
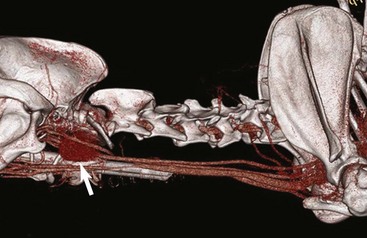
• Figure 6-7 Lateral view of a 3D-reconstructed CT angiogram of a highly vascular thyroid carcinoma (white arrow). This technique can be useful for identifying the larger vascular territory supplying a tumor.
Tumor perfusion, vascular permeability, and tumor blood volume can predict tumor aggressiveness through the use of dynamic contrast-enhanced CT, which estimates tumor wash-in and wash-out contrast kinetics.33,34
Ultrasonography
Ultrasound imaging’s ability to evaluate the internal structure of organs and to image body cavities when effusion is present has made it an essential diagnostic tool that has replaced abdominal radiography as the first-line choice in evaluation of the abdomen. The information provided by ultrasound is based on differences in acoustic impedance. The acoustic impedance of a material is a product of its physical density and the velocity of sound in the material. As sound waves pass from tissue to tissue, the amount of sound reflected (echoes) is determined by the impedance difference between tissues. The reflected echoes are detected by the transducer and processed into an image.
Ultrasound is superior to radiographs in instances of pleural or peritoneal effusion due to the loss of visceral detail that occurs on radiographs as the fluid will efface the margins of organs.35 Ultrasound is also useful in guidance for biopsy or fine-needle aspiration of an observed abnormality. It is more sensitive than survey radiographs for detecting abdominal lymphadenopathy36-38 and size, shape, margins, heterogeneity, perinodal fat appearance, and vascular pattern of lymph nodes are useful in determining malignancy.38-42 However, radiographs are superior for detection of bony invasion that may be associated with malignant medial iliac lymph node enlargement, often seen with tumors of the urinary bladder, prostate,43 and anal glands. Ultrasound is sensitive in the detection of adrenal abnormalities, including vessel invasion, which is usually associated with malignancy (Figure 6-8).19,44 Ultrasound is routinely used to stage and monitor cats and dogs with mast cell disease, which has a varied appearance.45,46 It is used extensively in patients with gastrointestinal disease for initial diagnosis and monitoring of therapy.47-52
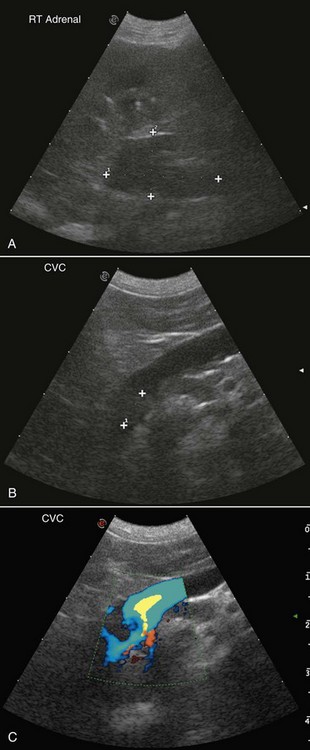
• Figure 6-8 Ultrasound images of a right adrenal gland tumor with invasion into the caudal vena cava and probable tumor thrombus. A, Note the large heterogeneous mass (2.93 × 1.68 cm). B, A tumor thrombus or tumor extension is present. C, Note the turbulent Doppler flow in the caudal vena cava, indicating invasion of the right adrenal tumor into the vessel.
Ultrasonography is sensitive for lesion detection, but it is not specific for disease etiology. Many studies have attempted unsuccessfully to differentiate benign from malignant lesions based on sonographic appearance.44,46,50,53-55 Therefore biopsy or fine-needle aspirates of lesions are necessary. Ultrasound-guided sampling of tissue can be performed quickly, accurately, and safely.56-60 A caveat is the potential seeding of tumor cells from ultrasound-guided percutaneous sampling of transitional cell carcinoma.61
Advances in ultrasound equipment and the development of sonographic contrast agents are increasing the specificity of ultrasound.62 Doppler techniques are used to assess tumor vasculature, which is found generally to be tortuous with high velocity as compared to normal tissues.40,42,63,64 Tissue harmonic imaging transmits at one frequency and receives at twice that frequency. This technique has advantages over fundamental imaging.62,65 Harmonic imaging and/or ultrasound contrast agents have been used to differentiate benign from malignant lesions64-76 and continued exploration of this imaging modality is warranted.
Magnetic Resonance Imaging
MRI is an advanced imaging technique that provides superb soft tissue images and sensitive detection of pathology based on properties of hydrogen atoms when placed in magnetic and radiofrequency fields. Images can be acquired directly in any plane with MRI, compared to CT in which sagittal and dorsal images are made by reconstructing transverse image data. MRI is the imaging modality of choice for the evaluation of the central nervous system, and the characteristics of the common canine and feline neurologic tumors are now well described (Figure 6-9).77-82 MRI provides better anatomic detail and more sensitive detection of neuropathology than CT but is less useful for assessing cortical bone because of the number and characteristics of hydrogen protons in bone. Bone landmarks, areas of mineralization, and periosteal bone production are not as obvious with MRI as with CT. Nevertheless, MRI provides excellent anatomic detail and sensitive detection of infiltrative diseases affecting the musculoskeletal soft tissues, including joints, ligaments and tendons, and bone marrow, and is most accurate in determining the extent of canine appendicular osteosarcoma for limb-sparing procedures (Figure 6-10).83
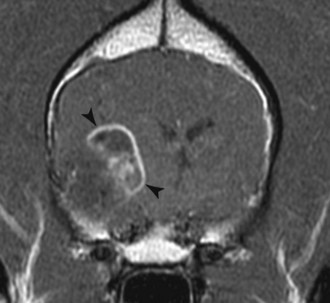
• Figure 6-9 Postcontrast T1-weighted transverse MR image of a canine oligodendroglioma (black arrowheads). Note its intraaxial location and ring enhancement around a hypointense less enhancing center.
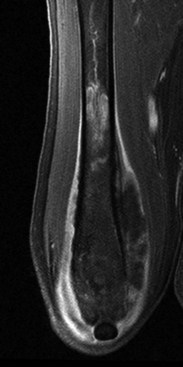
• Figure 6-10 Fat-saturated postcontrast T1-weighted coronal MR image of a canine femur showing contrast-enhancing infiltrative tumor within the bone marrow and extramedullary extension from osteosarcoma.
As advanced MR equipment becomes more common in veterinary medicine, MRI’s use in cancer diagnosis and staging will increase because of its value in determining tumor morphology, margins, characteristics, and composition.32,84 MRI is excellent for tumors of the head, neck, and torso, although special measures are sometimes needed to minimize respiratory motion in certain anatomic sites.85,86 Whole-body MR is under evaluation as a sensitive method for cancer staging that is superior to CT and scintigraphy, and in human clinical trials it can rival the results of positron-emission tomography-CT (PET-CT) without the use of ionizing radiation.87 Whole-body MRI does require ultrafast imaging methods, so it would be limited to veterinary sites having newer and high-field MR instruments.84,87 Although CT scans are most often used for radiation therapy planning, MRI provides advantages in select situations, including the ability to anatomically define small at-risk structures such as the optic chiasm and to contour margins of lesions where beam-hardening artifact is problematic with CT.84 MRI is also quite useful for detecting residual or recurring tumors due to its sensitivity to early disease. New MRI techniques are being developed that allow the study of tumor physiology and metabolism and valuable information about treatment response. Dynamic contrast-enhanced MRI provides information about tumor vascularity, perfusion, and angiogenesis that has been shown to have predictive value for treatment response and outcome (Figure 6-11).88,89 Diffusion MRI evaluates the mobility of tumor water molecules as a function of cell density and tissue architecture, with an increase in tumor diffusion indicating a positive treatment response and cell death.32,88,90,91 In vivo MR spectroscopy for measuring malignant metabolic biomarkers such as choline relative to normal surrounding tissue is moving from the research realm into human clinical use for brain and prostatic cancer and also has future potential for veterinary diagnosis.84,92
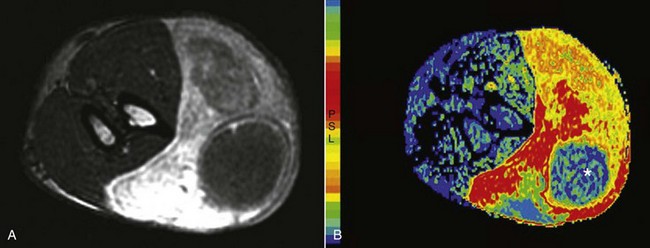
• Figure 6-11 A, Transverse MR images of a soft tissue sarcoma of a canine forelimb. B, A color map of maximum slope of increase indicating the most rapidly contrast-enhancing tumor regions and also showing poor uptake in the necrotic core (*). Compare to the normal musculature and cross-sectional views of the radius and ulna in the left of the image.
Nuclear Medicine
Diagnostic nuclear medicine, or scintigraphy, involves the administration of radiopharmaceuticals that localize to an area of interest in the body by physiologic processes. Images obtained from nuclear medicine studies do not provide the anatomic detail attainable with other imaging techniques; however, the functional dependence on physiologic processes adds important information. Technetium-99m (99mTc) is the most commonly used radionuclide because it has excellent imaging qualities and a short half-life (6 hours) and is easily bound to localizing pharmaceuticals. Bone scintigraphy using 99mTc methylene diphosphonate (99mTc-MDP) is frequently used in veterinary medicine because it is a simple, sensitive, and noninvasive method of evaluating the entire skeleton.93 Other commonly used nuclear imaging studies include renal, thyroid, lung, and liver scintigraphy (Figure 6-12). Parathyroid scintigraphy has been performed using dual-phase imaging94 but due to lack of sensitivity was not recommended for identification of abnormal parathyroid glands in hypercalcemic dogs. At this author’s (LJF) institution, ultrasound is used primarily to locate parathyroid masses in hypercalcemic dogs and cats.95,96
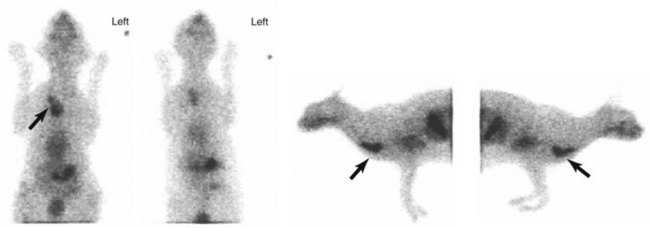
• Figure 6-12 Ventral, dorsal, and left and right lateral scintigraphic images of a cat with ectopic thyroid carcinoma (black arrows). Images were obtained 20 minutes after intravenous administration of technetium-99m (99mTc).
In general, nuclear medicine studies are sensitive for lesion detection but are nonspecific for disease etiology. Benign and malignant lesions can have similar scintigraphic appearances. Bone metastases can be detected earlier with scintigraphy, before clinical and radiographic recognition, and scintigraphy can aid in the selection of the best site for biopsy (Figure 6-13).93,97 Once a lesion is identified, additional imaging (radiography, ultrasonography, CT, MRI) may be necessary.
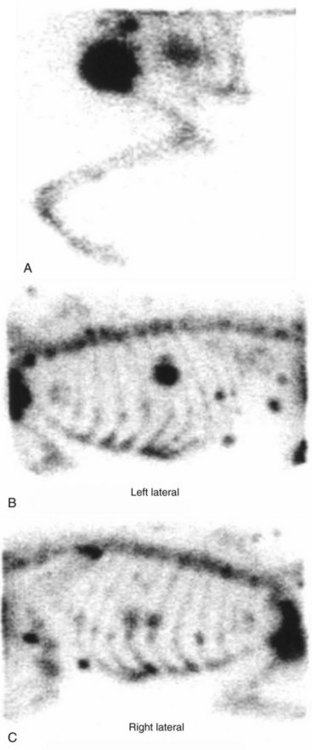
• Figure 6-13 Bone scan images of a dog with primary osteosarcoma of the left proximal humerus (A) with subcutaneous and rib metastases (B and C). Images were obtained 2 hours after intravenous administration of technetium-99m methylene diphosphonate (99mTc MDP). Note the large mass with associated radiopharmaceutical uptake in the left proximal humerus (A) and the focal increased uptake in multiple ribs and in subcutaneous tissue overlying the thoracic spine and dorsal to the scapula (B and C).
Single-Photon Emission Computed Tomography and Positron Emission Tomography
Two advanced radionuclide imaging techniques used extensively in human medicine are single-photon emission computed tomography (SPECT) and positron emission tomography (PET). SPECT utilizes traditional gamma ray–emitting radionuclides, often with a rotating gamma camera or stationary ring, which reconstructs the images in cross-sections. SPECT provides better lesion localization as compared to planar scintigraphy, and hybrid instruments coupling functional SPECT information with CT for anatomy are now in use for oncology.98 PET detectors also register gamma rays in a cross-section to reconstruct a 3D image, but these gamma rays are produced during annihilation of another emitted particle, the positron.99 Positron-emitting radionuclides are closer in identity to physiologic atoms than gamma ray–emitting radionuclides, allowing synthesis of compounds of more biologic relevance. Dual-modality hybrid PET/CT and, most recently, PET/MRI scanners are now commercially available with widespread use in oncology. The systems are housed in the same instrument allowing for almost simultaneous acquisition of both images and their fusion, providing both anatomic and physiologic information. The complementary data provided by the two imaging procedures provide the most accurate cancer staging for oncology patients.100
Compared with normal cells, tumor cells have increased glucose utilization through glycolysis; this makes glucose an appropriate molecule for modification and labeling with a positron-emitting radionuclide. The increased energy demand of tumors is met through upregulation of the hexose monophosphate pathway, cell membrane glucose transporter proteins, and hexokinase. A glucose analog, 18F-fluorodeoxyglucose (FDG) acts as a glucose molecule and is transported by membrane proteins and becomes phosphorylated by hexokinase in the cell. However, phosphorylated FDG is not used in the glycolytic pathway and remains trapped intracellularly, as tumor cells are deficient in glucose-6-phosphatase. The differential uptake of FDG by tumor cells forms the basis for contrast in images.
Whole-body FDG PET/CT imaging has been utilized extensively for human cancer imaging for cancer staging and detecting metastases and is also proving useful for target definition for radiation therapy.3,99,101 Its use has been demonstrated not only for staging and follow-up of human lymphoma but also for prognostication and response evaluation.102,103 FDG PET/CT is now also available and being actively studied for use in veterinary cancer patients (Figure 6-14).104-107 Its utility for cancer staging of canine mast cell tumor and lymphoma has already been demonstrated.108 However, there are limitations to the interpretation of FDG PET images. The uptake of FDG in normal tissues must be understood relative to veterinary species to avoid false-positive diagnoses because some normal tissues are also highly metabolic.109-112 Normal gray matter of the brain can confound interpretation of intracranial tumor images because of its high glucose utilization normally. Inflammatory processes also have increased FDG uptake, and postradiation tissue reaction can be difficult to distinguish from tumor recurrence during the early posttherapy time period.113 Also, certain low-grade or poorly differentiated tumors do not show sufficient FDG uptake to be distinguished from background. Although FDG is still the most commonly used commercial PET agent for cancer imaging, limitations of FDG PET have led to exploration of other novel PET probes.101
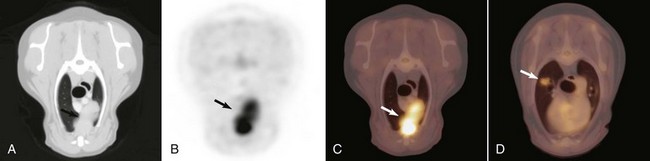
• Figure 6-14 Transverse CT (A), PET (B), and fused PET/CT image (C) of a thymoma in the cranial mediastinum of a dog (arrows). D, A small hypermetabolic pulmonary adenocarcinoma (white arrow), which was not visible radiographically, was found incidentally on fused PET/CT image and also surgically removed.
Thymidine, the only nucleoside used solely in construction of DNA and not in RNA, provides an accurate measurement of increased cellular proliferation in a tumor both before and after treatment.105,114,115 Two fluorinated compounds that have been proved stable to degradation and useful in tumor imaging are 2′-fluoro-5-methyldeoxyuracil (FMAU) and 3′-deoxy-3′-fluorothymidine (FLT). Both compounds are phosphorylated by thymidine kinase (TK) intracellularly and incorporated into DNA to variable degrees. Although FLT acts as a chain terminator in DNA synthesis and has limited incorporation into DNA, for imaging purposes it remains trapped intracellularly by TK and indirectly reflects cellular proliferation. Early studies show normal FLT distribution within the bone marrow, liver, and urinary system as a result of excretion. Research using dogs with spontaneous tumors continues to look at the utility of FLT as a marker of tumor proliferation.114 Figure 6-15 is a sagittal reconstruction PET FLT scan of a dog with a nasal adenocarcinoma. PET radiotracers for noninvasive imaging of tumor hypoxia have been found in human cancer trials to have value in prognostication, predicting response, and better defining hypoxic tumor volumes for boost radiation therapy.99,116 Hypoxic radiotracers include several in the nitroimidazole class, including 18F-misonidazole, the more hydrophilic 18F-fluoroazomycin (FAZA), and the more lipophilic version 2-(2-nitro-1H-imidazol-1-yl)-N-(2,2,3,3,3pentafluoropropyl)-acetamide (18F-EF5). 60Cu-labeled diacetyl-bis(N4-methylthiosemicarbazone (60Cu-ATSM) is a non-nitroimidazole tracer that is also being evaluated as a hypoxic tracer.
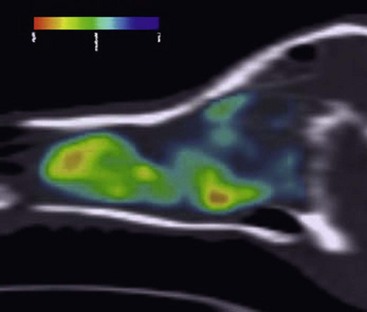
• Figure 6-15 3′-Deoxy-3′-fluorothymidine (FLT) sagittal reconstruction of dog with nasal adenocarcinoma.
18F-sodium fluoride (18F-NaF) is a PET tracer that is now commercially available for bone scans. 18F-NaF localizes to areas of high bone turnover similar to 99mTc-MDP that is used for bone scintigraphy. PET imaging with this tracer provides 3D, highly detailed whole-body skeletal images that are superior to bone scintigraphy and SPECT imaging for bone metastases.117-119 Its use is being evaluated in veterinary patients for detection of skeletal metastases and other bone disease processes (Figure 6-16).
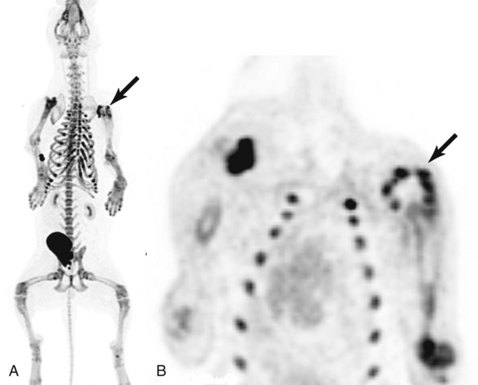
• Figure 6-16 A, 18F-sodium fluoride (18F-NaF) PET bone scan 3D maximum intensity projection (MIP) image of a dog with a proximal humeral osteosarcoma (black arrow) 6-months poststereotactic radiation therapy. Note the excellent skeletal image with multifocal uptake at the costochondral junctions, the injection catheter, the opposite shoulder joint, and the urinary bladder due to excretion. B, The irradiated tumor (black arrow) on the dorsal plane PET image exhibits a photopenic center consistent with inactive bone remodeling and/or necrosis.
Advances
The state of the art in human radiotherapy is represented by conformal radiotherapy and intensity-modulated radiotherapy (IMRT). In the former technique, the treatment beam is conformed to the tumor volume. In the latter technique, in addition to shaping the treatment beam to the tumor, beam intensity is modulated to maximize the tumor dose and minimize the dose to normal tissue.120,121 These advanced treatment modalities require imaging periodically through the radiation course.
In particular, IMRT requires precise and accurate tumor and normal tissue localization due to its high-dose gradients and maximal sparing of surrounding normal structures. This is accomplished through image-guided radiation therapy (IGRT) via a range of on-board imaging incorporated with IMRT linear accelerators.122 In addition to traditional portal image options, IGRT also includes the ability to use kV x-ray and cone-beam CT (CBCT) systems to image the patient at the time of setup so that slight movements can be made with the therapy table to perfectly match bone and soft tissue landmarks on the digitally reconstructed radiographic images and 3D–planning CT scan. These advances in technology make it possible to perform adaptive radiotherapy by taking into consideration changes in size and shape of the target volumes during a treatment course. Tumor motion can be a problem for many sites, especially as oncologists attempt to balance increased tumor dose with reduced normal tissue complications while attempting to improve overall survival. To this end, 4-dimensional (4D) CT imaging, in which respiratory motion is taken into account in the treatment planning process and delivery, is being implemented in human radiation oncology centers.120,123-125
Another advance is helical tomotherapy, which integrates a linear accelerator with a helical CT scanner, allowing IGRT.126 Each day before treatment, a CT scan is obtained with the linear accelerator; this scan is fused with the planning CT image, and the patient is moved accordingly to ensure precise treatment delivery.127 CT imaging is an integral part of radiotherapy planning and delivery.
Advances are also being made in the area of molecular imaging.1-3,101,104,106 These techniques can be used to characterize and measure biologic processes, assess molecular targets, and monitor treatment at the cellular level.1,128 Molecular imaging can be used to assess gene delivery and identify marker proteins for apoptosis, angiogenesis, hypoxia, and other growth factors.1,3,101,116,129 Moreover, treatment of cancer using molecular targets holds great promise for individualized cancer therapy.116,130-132
References
1. Higgins, LJ, Pomper, MG. The evolution of imaging in cancer: current state and future challenges. Semin Oncol. 2011;38:3–15.
2. Smith, RA, Guleryuz, S, Manning, HC. Molecular imaging metrics to evaluate response to preclinical therapeutic regimens. Front Biosci. 2011;16:393–410.
3. Zhu, A, Lee, D, Shim, H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol. 2011;38:55–69.
4. Prather, AB, Berry, CR, Thrall, DE. Use of radiography in combination with computed tomography for the assessment of noncardiac thoracic disease in the dog and cat. Vet Radiol Ultrasound. 2005;46:114–121.
5. Nemanic, S, London, CA, Wisner, ER. Comparison of thoracic radiographs and single breath-hold helical CT for detection of pulmonary nodules in dogs with metastatic neoplasia. J Vet Intern Med. 2006;20:508–515.
6. Schultz, RM, Puchalski, SM, Kent, M, et al. Skeletal lesions of histiocytic sarcoma in nineteen dogs. Vet Radiol Ultrasound. 2007;48:539–543.
7. Blackwood, L, Sullivan, M, Lawson, H. Radiographic abnormalities in canine multicentric lymphoma: a review of 84 cases. J Small Anim Pract. 1997;38:62–69.
8. Geyer, NE, Reichle, JK, Valdes-Martinez, A, et al. Radiographic appearance of confirmed pulmonary lymphoma in cats and dogs. Vet Radiol Ultrasound. 2010;51:386–390.
9. Forrest, LJ. The head: excluding the brain and orbit. Clin Tech Small Anim Pract. 1999;14:170–176.
10. Kraft, SL, Gavin, PR. Intracranial neoplasia. Clin Tech Small Anim Pract. 1999;14:112–123.
11. Thomas, WB. Nonneoplastic disorders of the brain. Clin Tech Small Anim Pract. 1999;14:125–147.
12. Rudich, SR, Feeney, DA, Anderson, KL, et al. Computed tomography of masses of the brachial plexus and contributing nerve roots in dogs. Vet Radiol Ultrasound. 2004;45:46–50.
13. Swensen, SJ, Jett, JR, Hartman, TE, et al. CT Screening for lung cancer: Five-year prospective experience. Radiology. 2005;235(1):259–265.
14. Yoon, J, Feeney, DA, Cronk, DE, et al. Computed tomographic evaluation of canine and feline mediastinal masses in 14 patients. Vet Radiol Ultrasound. 2004;45:542–546.
15. Li, F, Sone, S, Abe, H, et al. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology. 2004;233:793–798.
16. Ballegeer, EA, Adams, WM, Dubielzig, RR, et al. Computed tomography characteristics of canine tracheobronchial lymph node metastasis. Vet Radiol Ultrasound. 2010;51:397–403.
17. Eberle, N, Fork, M, von Babo, V, et al. Comparison of examination of thoracic radiographs and thoracic computed tomography in dogs with appendicular osteosarcoma. Vet Comp Oncol. 2010;9:131–140.
18. Fife, WD, Samii, VF, Drost, WT, et al. Comparison between malignant and nonmalignant splenic masses in dogs using contrast-enhanced computed tomography. Vet Radiol Ultrasound. 2004;45:289–297.
19. Rosenstein, DS. Diagnostic imaging in canine pheochromocytoma. Vet Radiol Ultrasound. 2000;41:499–506.
20. McEntee, MC, Thrall, DE. Computed tomographic imaging of infiltrative lipoma in 22 dogs. Vet Radiol Ultrasound. 2001;42:221–225.
21. McEntee, MC, Page, RL. Feline vaccine-associated sarcomas. J Vet Intern Med. 2001;1:176–182.
22. Peters, TM, Slomka, PJ, Fenster, A. Imaging for radiation therapy planning (MRI, nuclear medicine, ultrasound. In: Van Dyk J, ed. The modern technology of radiation oncology. Madison, WI: Medical Physics Publishing, 1999.
23. Van Dyk, J, Barnett, RB, Battista, JJ. Computerized radiation treatment planning systems. In: Van Dyk J, ed. The modern technology of radiation oncology. Madison, Wisconsin: Medical Physics Publishing, 1999.
24. Giroux, A, Jones, JC, Bohn, JH, et al. A new device for stereotactic CT-guided biopsy of the canine brain: design, construction, and needle placement accuracy. Vet Radiol Ultrasound. 2002;43:229–236.
25. Tidwell, AS, Johnson, KL. Computed tomography-guided percutaneous biopsy in the dog and cat: description of technique and preliminary evaluation in 14 patients. Vet Radiol Ultrasound. 1994;35:445–456.
26. Tidwell, AS, Johnson, KL. Computed tomography-guided percutaneous biopsy: criteria for accurate needle tip identification. Vet Radiol Ultrasound. 1994;35:440–444.
27. Troxel, MT, Vite, CH. CT-guided stereotactic brain biopsy using the Kopf stereotactic system. Vet Radiol Ultrasound. 2008;49:438–443.
28. Zekas, LJ, Crawford, JT, O’Brien, RT. Computed tomography-guided fine-needle aspirate and tissue-core biopsy of intrathoracic lesions in thirty dogs and cats. Vet Radiol Ultrasound. 2005;46:200–204.
29. Robben, JH, Pollak, YWEA, Kirpensteijn, J, et al. Comparison of ultrasonography, computed tomography, and single-photon emission computed tomography for the detection and localization of canine insulinoma. J Vet Intern Med. 2005;19:15–22.
30. Taniura, T, Marukawa, K, Yamada, K, et al. Differential diagnosis of hepatic tumor-like lesions in dog by using dynamic CT scanning. Hiroshima J Med Sci. 2009;58:17–24.
31. Mai, W, Caceres, AV. Dual-phase computed tomographic angiography in three dogs with pancreatic insulinoma. Vet Radiol Ultrasound. 2008;49:141–148.
32. Kanematsu, M, Kondo, H, Goshima, S, et al. Imaging liver metastases: Review and update. Euro J Radiol. 2006;58:217–228.
33. Pollard, RE, Garcia, TC, Stieger, SS, et al. Quantitative evaluation of perfusion and permeability of peripheral tumors using contrast-enhanced computed tomography. Invest Radiol. 2004;39:340–349.
34. Van Camp, S, Fisher, P, Thrall, DE. Dynamic CT measurement of contrast medium washin kinetics in canine nasal tumors. Vet Radiol Ultrasound. 2000;41:403–408.
35. Monteiro, CB, O’Brien, RT. A retrospective study on the sonographic findings of abdominal carcinomatosis in 14 cats. Vet Radiol Ultrasound. 2004;45:559–564.
36. Llabres-Diaz, FJ. Ultrasonography of the medial iliac lymph nodes in the dog. Vet Radiol Ultrasound. 2004;45:156–165.
37. Nyman, HT, Kristensen, AT, Flagstad, A, et al. A review of the sonographic assessment of tumor metastases in liver and superficial lymph nodes. Vet Radiol Ultrasound. 2004;45:438–448.
38. Nyman, HT, O’Brien, RT. The sonographic evaluation of lymph nodes. Clin Tech Small Anim Pract. 2007;22:128–137.
39. Kinns, J, Mai, W. Association between malignancy and sonographic heterogeneity in canine and feline abdominal lymph nodes. Vet Radiol Ultrasound. 2007;48:565–569.
40. Prieto, S, Gomez-Ochoa, P, De Blas, I, et al. Pathologic correlation of resistive and pulsatility indices in canine abdominal lymph nodes. Vet Radiol Ultrasound. 2009;50:525–529.
41. de Swarte, M, Alexander, K, Rannou, B, et al. Comparison of sonographic features of benign and neoplastic deep lymph nodes in dogs. Vet Radiol Ultrasound. 2011.
42. Della Santa, D, Gaschen, L, Doherr, MG, et al. Spectral waveform analysis of intranodal arterial blood flow in abnormally large superficial lymph nodes in dogs. Am J Vet Res. 2008;69:478–485.
43. Bradbury, CA, Westropp, JL, Pollard, RE. Relationship between prostatomegaly, prostatic mineralization, and cytologic diagnosis. Vet Radiol Ultrasound. 2009;50:167–171.
44. Besso, JG, Penninck, DG, Gliatto, JM. Retrospective ultrasonographic evaluation of adrenal lesions in 26 dogs. Vet Radiol Ultrasound. 1997;38:448–455.
45. Sato, AF, Solano, M. Ultrasonographic findings in abdominal mast cell disease: a retrospective study of 19 patients. Vet Radiol Ultrasound. 2004;45:51–57.
46. Hanson, JA, Papageorges, M, Girard, E, et al. Ultrasonographic appearance of splenic disease in 101 cats. Vet Radiol Ultrasound. 2001;42:441–445.
47. Beck, C, Slocombe, RF, O’Neill, T, et al. The use of ultrasound in the investigation of gastric carcinoma in a dog. Aust Vet J. 2001;79:332–334.
48. Paoloni, MC, Penninck, DG, Moore, AS. Ultrasonographic and clinicopathologic findings in 21 dogs with intestinal adenocarcinoma. Vet Radiol Ultrasound. 2002;43:562–567.
49. Penninck, DG, Moore, AS, Gliatto, J. Ultrasonography of canine gastric epithelial neoplasia. Vet Radiol Ultrasound. 1998;39:342–348.
50. Penninck, DG, Smyers, B, Webster, CR, et al. Diagnostic value of ultrasonography in differentiating enteritis from intestinal neoplasia in dogs. Vet Radiol Ultrasound. 2003;44:570–575.
51. Rivers, BJ, Walter, PA, Johnston, GR, et al. Canine gastric neoplasia: utility of ultrasonography in diagnosis. J Am Anim Hosp Assoc. 1997;33:144–155.
52. Gaschen, L. Ultrasonography of small intestinal inflammatory and neoplastic diseases in dogs and cats. Vet Clin North Am Small Anim Pract. 2011;41:329–344.
53. Cruz-Arambulo, R, Wrigley, R, Powers, B. Sonographic features of histiocytic neoplasms in the canine abdomen. Vet Radiol Ultrasound. 2004;45:554–558.
54. Cuccovillo, A, Lamb, CR. Cellular features of sonographic target lesions of the liver and spleen in 21 dogs and a cat. Vet Radiol Ultrasound. 2002;43:275–278.
55. Ramirez, S, Douglass, JP, Robertson, ID. Ultrasonographic features of canine abdominal malignant histiocytosis. Vet Radiol Ultrasound. 2002;43:167–170.
56. Crystal, MA, Penninck, DG, Matz, ME, et al. Use of ultrasound-guided fine-needle aspiration biopsy and automated core biopsy for the diagnosis of gastrointestinal diseases in small animals. Vet Radiol Ultrasound. 1993;34:438–444.
57. Penninck, DG, Crystal, MA, Matz, ME, et al. The technique of percutaneous ultrasound guided fine-needle aspiration biopsy and automated microcore biopsy in small animal gastrointestinal diseases. Vet Radiol Ultrasound. 1993;34:433–436.
58. Wang, KY, Panciera, DL, Al-Rukibat, RK, et al. Accuracy of ultrasound-guided fine-needle aspiration of the liver and cytologic findings in dogs and cats: 97 cases (1990-2000). J Am Vet Med Assoc. 2004;224:75–78.
59. Wood, EF, O’Brien, RT, Young, K. Ultrasound-guided fine-needle aspiration of focal parenchymal lesions of the lung in dogs and cats. J Vet Intern Med. 1998;12:338–342.
60. Samii, VF, Nyland, TG, Werner, LL, et al. Ultrasound-guided fine-needle aspiration biopsy of bone lesions: a preliminary report. Vet Radiol Ultrasound. 1999;40:82–86.
61. Nyland, TG, Wallack, ST, Wisner, ER. Needle-tract implantation following ultrasound-guided fine-needle aspiration biopsy of transitional cell carcinoma of the bladder, urethra, and prostate. Vet Radiol Ultrasound. 2002;43:50–53.
62. Wisner, ER, Pollard, RE. Trends in veterinary cancer imaging. Vet Comparative Oncology. 2004;2:49–74.
63. Nyman, HT, Kristensen, AT, Lee, MH, et al. Characterization of canine superficial tumors using gray-scale B mode, color flow mapping, and spectral Doppler ultrasonography–a multivariate study. Vet Radiol Ultrasound. 2006;47:192–198.
64. Salwei, RM, O’Brien, RT, Matheson, JS. Characterization of lymphomatous lymph nodes in dogs using contrast harmonic and power Doppler ultrasound. Vet Radiol Ultrasound. 2005;46:411–416.
65. Ziegler, LE, O’Brien, RT. Harmonic ultrasound: a review. Vet Radiol Ultrasound. 2002;43:501–509.
66. Bahr, A, Wrigley, R, Salman, M. Quantitative evaluation of Imagent as an abdominal ultrasound contrast medium in dogs. Vet Radiol Ultrasound. 2000;41:50–55.
67. Haers, H, Vignoli, M, Paes, G, et al. Contrast harmonic ultrasonographic appearance of focal space-occupying renal lesions. Vet Radiol Ultrasound. 2010;51:516–522.
68. Kanemoto, H, Ohno, K, Nakashima, K, et al. Characterization of canine focal liver lesions with contrast-enhanced ultrasound using a novel contrast agent—Sonazoid. Vet Radiol Ultrasound. 2009;50:188–194.
69. Matheson, JS, O’Brien, RT, Delaney, F. Tissue harmonic ultrasound for imaging normal abdominal organs in dogs and cats. Vet Radiol Ultrasound. 2003;44:205–208.
70. Nakamura, K, Sasaki, N, Murakami, M, et al. Contrast-enhanced ultrasonography for characterization of focal splenic lesions in dogs. J Vet Intern Med. 2010;24:1290–1297.
71. Nakamura, K, Takagi, S, Sasaki, N, et al. Contrast-enhanced ultrasonography for characterization of canine focal liver lesions. Vet Radiol Ultrasound. 2010;51:79–85.
72. O’Brien, RT. Improved detection of metastatic hepatic hemangiosarcoma nodules with contrast ultrasound in three dogs. Vet Radiol Ultrasound. 2007;48:146–148.
73. O’Brien, RT, Iani, M, Matheson, J, et al. Contrast harmonic ultrasound of spontaneous liver nodules in 32 dogs. Vet Radiol Ultrasound. 2004;45:547–553.
74. Szatmari, V, Harkanyi, Z, Voros, K. A review of nonconventional ultrasound techniques and contrast-enhanced ultrasonography of noncardiac canine disorders. Vet Radiol Ultrasound. 2003;44:380–391.
75. Taeymans, O, Penninck, D. Contrast enhanced sonographic assessment of feeding vessels as a discriminator between malignant vs. benign focal splenic lesions. Vet Radiol Ultrasound. 2011;52(4):457–461.
76. Ziegler, LE, O’Brien, RT, Waller, KR, et al. Quantitative contrast harmonic ultrasound imaging of normal canine liver. Vet Radiol Ultrasound. 2003;44:451–454.
77. Pollard, RE, Reilly, CM, Uerling, MR, et al. Cross-sectional imaging characteristics of pituitary adenomas, invasive adenomas and adenocarcinomas in dogs: 33 cases (1988-2006). J Vet Intern Med. 2010;24:160–165.
78. Westworth, DR, Dickinson, PJ, Vernau, W, et al. Choroid plexus tumors in 56 dogs (1985-2007). J Vet Intern Med. 2008;22:1157–1165.
79. Wisner, ER, Dickinson, PJ, Higgins, RJ. Magnetic resonance imaging features of canine intracranial neoplasia. Vet Radiol Ultrasound. 2011;52:S52–S61.
80. Sturges, BK, Dickinson, PJ, Bollen, AW, et al. Magnetic resonance imaging and histological classification of intracranial meningiomas in 112 dogs. J Vet Intern Med. 2008;22:586–595.
81. Kraft, S, Ehrhart, EJ, Gall, D, et al. Magnetic resonance imaging characteristics of peripheral nerve sheath tumors of the canine brachial plexus in 18 dogs. Vet Radiol Ultrasound. 2007;48:1–7.
82. Petersen, SA, Sturges, BK, Dickinson, PJ, et al. Canine intraspinal meningiomas: Imaging features, histopathologic classification, and long-term outcome in 34 dogs. J Vet Intern Med. 2008;22:946–953.
83. Wallack, ST, Wisner, ER, Werner, JA, et al. Accuracy of magnetic resonance imaging for estimating intramedullary osteosarcoma extent in preoperative planning of canine limb-salvage procedures. Vet Radiol Ultrasound. 2002;43:432–441.
84. Kraft, SL. Cancer imaging. In: Gavin, PR, Bagley, RS, eds. Practical small animal MRI, Vol 1. Ames: Wiley-Blackwell; 2009:333–358.
85. Clifford, CA, Pretorius, ES, Weisse, C, et al. Magnetic resonance imaging of focal splenic and hepatic lesions in the dog. J Vet Intern Med. 2004;18:330–338.
86. Yasuda, D, Fujita, M, Yasuda, S, et al. Usefulness of MRI compared with CT for diagnosis of mesenteric lymphoma in a dog. J Vet Med Sci. 2004;66:1447–1451.
87. Schmidt, GP, Kramer, H, Reiser, MF, et al. Whole-body magnetic resonance imaging and positron emission tomography-computed tomography in oncology. Top Magn Reson Imagining. 2007;18:193–202.
88. Yankeelov, TE, Arlinghaus, LR, Li, X, et al. The role of magnetic resonance imaging biomarkers in clinical trials of treatment response in cancer. Semin Oncol. 2011;38:16–25.
89. Zhao, Q, Lee, S, Kent, M, et al. Dynamic contrast-enhanced magnetic resonance imaging of canine brain tumors. Vet Radiol Ultrasound. 2010;51:122–129.
90. Thamm, DH, Kurzman, ID, Clark, MA, et al. Preclinical investigation of PEGylated tumor necrosis factor alpha in dogs with spontaneous tumors: Phase I evaluation. Clin Cancer Res. 2010;16:1498–1508.
91. Lin, C, Luciani, A, El-Gnaoui, IE, et al. Whole-body diffusion-weighted magnetic resonance imaging with apparent diffusion coefficient mapping for staging patients with diffuse large B-cell lymphoma. Eur Radiol. 2010;20:2027–2038.
92. Glunde, K, Bhujwalla, ZM. Metabolic tumor imaging using magnetic resonance spectroscopy. Semin Oncol. 2011;38:26–41.
93. Forrest, LJ, Thrall, DE. Bone scintigraphy for metastasis detection in canine osteosarcoma. Vet Radiol Ultrasound. 1994;35:124–130.
94. Matwichuk, CL, Taylor, SM, Daniel, GB, et al. Double-phase parathyroid scintigraphy in dogs using technetium-99M-sestamibi. Vet Radiol Ultrasound. 2000;41:461–469.
95. Sueda, MT, Stephanacci, JD. Ultrasound evaluation of the parathyroid glands in two hypercalcemic cats. Vet Radiol Ultrasound. 2000;41:448–451.
96. Wisner, ER, Penninck, DG, Biller, DS, et al. High-resolution parathyroid sonography. Vet Radiol Ultrasound. 1997;38:462–466.
97. Head, LL, Daniel, GB. Scintigraphic diagnosis-an unusual presentation of metastatic pheochromocytoma in a dog. Vet Radiol Ultrasound. 2004;45:574–576.
98. Brandon, D, Alazraki, A, Halkar, RK, et al. The role of single-photon emission computed tomography and SPECT/computed tomography in oncologic imaging. Semin Oncol. 2011;38:87–108.
99. Nestle, U, Weber, W, Hentschel, M, et al. Biological imaging in radiation therapy: role of positron emission tomography. Phys Med Biol. 2009;54:R1–R25.
100. Bar-Shalom, R, Valdiva, AY, Blaufox, MD. PET imaging in oncology. Semin Nucl Med. 2010;30:150–185.
101. Chen, K, Chen, X. Positron emission tomography imaging of cancer biology: current status and future prospects. Semin Oncol. 2011;38:70–86.
102. Terasawa, T, Lau, J, Bardet, S, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for interim response assessment of advanced-stage Hodgkin’s lymphoma and diffuse large B-cell lymphoma: a systematic review. J Clin Oncol. 2009;27:1906–1914.
103. Wahl, RL, Jacene, H, Kasamon, Y, et al. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S.
104. Lawrence, J, Rohren, E, Provenzale, J. PET/CT today and tomorrow in veterinary cancer diagnosis and monitoring: fundamentals, early results and future perspectives. Vet Comp Oncol. 2010;8:163–187.
105. Ballegeer, EA, Forrest, LJ, Jeraj, R, et al. PET/CT for primary lung tumor in a dog. Vet Radiol Ultrasound. 2006;47:228–233.
106. Hansen, AE, McEvoy, F, Engelholm, SA, et al. FDG PET/CT imaging in canine cancer patients. Vet Radiol Ultrasound. 2011;52:201–206.
107. Kang, B-Y, Park, C, Yoo, J-H, et al. 18F-fluorodeoxyglucose positron emission tomography and magnetic resonance imaging findings of primary intracranial histiocytic sarcoma in a dog. J Vet Med Sci. 2009;71:1397–1401.
108. LeBlanc, AK, Jakoby, BW, Townsend, DW, et al. 18FDG-PET imaging in canine lymphoma and cutaneous mast cell tumor. Vet Radiol Ultrasound. 2009;50:215–223.
109. LeBlanc, AK, Wall, JS, Morandi, F, et al. Normal thoracic and abdominal distribution of 2-deoxy-2-[18F]fluoro-D-glucose (18FDG) in adult cats. Vet Radiol Ultrasound. 2009;50:436–441.
110. LeBlanc, AK, Jakoby, B, Townsend, DW, et al. Thoracic and abdominal organ uptake of 2-deoxy-2-[18F]Fluoro-D-Glucose (18FDG) with positron emission tomography in the normal dog. Vet Radiol Ultrasound. 2008;49:182–188.
111. Lee, AR, Lee, MS, Jung, IS, et al. Imaging diagnosis—FDG-PET/CT of a canine splenic plasma cell tumor. Vet Radiol Ultrasound. 2010;51:145–147.
112. Lee, MS, Lee, AR, Jung, MA, et al. Characterization of physiologic 18F-FDG uptake with PET-CT in dogs. Vet Radiol Ultrasound. 2010;51:670–673.
113. Greven, KM. Positron-emission tomography for head and neck cancer. Semin Radiat Oncol. 2004;14:121–129.
114. Lawrence, J, Vanderhoek, M, Barbee, D, et al. Use of 3′-deoxy-3′-[18F]fluorothymidine PET/CT for evaluating response to cytotoxic chemotherapy in dogs with non-Hodgkin’s lymphoma. Vet Radiol Ultrasound. 2009;50:660–668.
115. Barwick, T, Bencherif, B, Mountz, JM, et al. Molecular PET and PET/CT imaging of tumour cell proliferation using F-18 fluoro-L-thymidine: a comprehensive evaluation. Nucl Med Commun. 2009;30:908–917.
116. Chitneni, SK, Palmer, GM, Zalutsky, MR, et al. Molecular imaging of hypoxia. J Nucl Med. 2011;52:165–168.
117. Grant, FD, Fahey, FH, Packard, AB, et al. Skeletal PET with 18F-fluoride: applying new technology to an old tracer. J Nucl Med. 2008;49:68–78.
118. Even-Sapir, E. PET/CT in malignant bone disease. Semin Musculoskelet Radiol. 2007;11:312–321.
119. Bridges, RL, Wiley, CR, Christian, JC, et al. An introduction to Na(18)F bone scintigraphy: basic principles, advanced imaging concepts, and case examples. J Nucl Med Technol. 2007;35:64–76. [quiz 78–79].
120. Keall, P. 4-Dimensional computed tomography imaging and treatment planning. Semin Radiat Oncol. 2004;14:81–90.
121. Staffurth, J. A review of the clinical evidence for intensity-modulated radiotherapy. Clin Oncol. 2010;22:643–657.
122. Bhide, SA, Nutting, CM. Recent advances in radiotherapy. BMC Med. 2010;8:25.
123. Underberg, RW, Lagerwaard, FJ, Cuijpers, JP, et al. Four-dimensional CT scans for treatment planning in stereotactic radiotherapy for stage I lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:1283–1290.
124. Chang, JY, Cox, JD. Improving radiation conformality in the treatment of non-small-cell lung cancer. Semin Radiat Oncol. 2010;20:171–177.
125. Jiang, SB. Radiotherapy of mobile tumors. Semin Radiat Oncol. 2008;16:239–248.
126. Mackie, TR, Balog, J, Ruchala, K, et al. Tomotherapy. Semin Radiat Oncol. 1999;9:108–117.
127. Forrest, LJ, Mackie, TR, Ruchala, K, et al. The utility of megavoltage computed tomography images from a helical tomotherapy system for setup verification purposes. Int J Radiat Oncol Biol Phys. 2004;60:1639–1644.
128. Zaidi, H, Vees, H, Wissmeyer, M. Molecular PET/CT imaging-guided radiation therapy treatment planning. Acad Radiol. 2009;16:1108–1133.
129. Bogdanov, A, Jr., Mazzanti, ML. Molecular magnetic resonance contrast agents for the detection of cancer: past and present. Semin Oncol. 2011;38:42–54.
130. Bentzen, SM. Dose painting and theragnostic imaging: towards the prescription, planning and delivery of biologically targeted dose distributions in external beam radiation oncology. Cancer Treat Res. 2008;139:41–62.
131. Thorwarth, D, Geets, X, Paiusco, M. Physical radiotherapy treatment planning based on functional PET/CT data. Radiother Oncol. 2010;96:317–324.
132. Thorwarth, D, Alber, M. Implementation of hypoxia imaging into treatment planning and delivery. Radiother Oncol. 2010;97:172–175.