Supportive Care for the Cancer Patient
 Section A
Section A
Management of Chronic Cancer Pain
In 1978 Yoxall1 stated: “It is surprising, for instance, how much a dog’s quality of life, observed by the owner, may be improved by the administration of a simple analgesic if the dog is suffering from a tumor, which although painless on palpation, may be causing considerable chronic pain.” Despite this statement and the fact that obvious pain associated with specific tumors such as osteosarcoma (OSA) has been emphasized for a long time as a diagnostic criterion, there is little literature specifically investigating cancer pain in companion animals.2-14 However, it is very encouraging that since the previous edition of this book, there has been an approximately fourfold increase in published studies (from 4 to 17 using a PubMed search). Encouragingly, we have seen the first studies looking at mechanisms in companion animal cancer pain.15,16
This chapter will deal with the treatment of chronic cancer pain in dogs and cats. Given the relative lack of clinical work in dogs and cats, the information in this chapter cannot be based on peer-reviewed investigations. Rather, it is a combination of the authors’ experience and the experience of others who are heavily involved in the treatment of cancer patients. It is also based on considered extrapolations from human medicine and from veterinary research in other chronically painful conditions, such as osteoarthritis. The control of acute perioperative pain in cancer patients is also very important (see section on relationship between cancer and pain), and readers are referred to appropriate texts for information on perioperative pain control.17
How Painful Is Cancer in Animals?
Not all tumors are painful, and the amount of pain is likely to vary considerably from one animal to another, even with similar tumor types. The author’s experience and the experience of others would suggest that, using a conservative estimate, 30% of tumors in dogs and cats are associated with significant pain at the time of diagnosis. Tumors most likely to be associated with pain include those at the following sites: oral cavity, bone, urogenital tract, eyes, nose, nerve roots, gastrointestinal tract, and skin (Table 15-1). The 30% estimate is likely conservative since pain is experienced by 20% to 50% of human patients when the lesion is diagnosed, by nearly half undergoing active treatment, and by up to 90% with far advanced or terminal cancer. An overall average of about 70% of human patients with advanced cancer suffer pain.18
Table 15-1
List of Tumors Most Likely to Be Associated with Pain
| Tumor Category | Notes |
| Tumors involving bone | Primary bone tumors (both of the appendicular and axial skeleton) and metastasis to bone are painful. Just as in humans, sometimes metastasis to bone can be relatively nonpainful; however, this should be considered the exception. |
| Central nervous system tumors | Extradural tumors that expand and put pressure on neural tissue are often associated with pain. Tumors originating from within the neural tissue are often not associated with pain until later on in the course of the disease. In humans with primary brain tumors or metastases to brain, 60%-90% of them suffer from headaches; it should be presumed that animals also suffer such headaches. |
| Gastrointestinal | Especially esophagus, stomach, colon, and rectum. Such pain may be very difficult to localize, and it may manifest as vague signs and behavioral changes. Colonic and rectal pain is often manifested as perineal discomfort. |
| Inflammatory mammary carcinoma | This form of mammary cancer is very painful in humans, and dogs with this form of mammary cancer appear to exhibit obvious signs of pain. |
| Genitourinary tract tumors | Stretching of the renal capsule appears to produce significant pain. Bladder tumors appear to be predictably associated with pain. Tumors of the distal genitourinary tract are often manifested as perineal pain or pain apparently associated with the lower back. |
| Prostate | Prostatic tumors appear to be particularly painful, especially if local metastasis to bone is present, and the pain may be manifested as lower back or abdominal pain. |
| Oral and pharyngeal tumors | Soft tissue tumors that are growing by projecting from the surface (e.g., projecting from the gingival surface) appear to be relatively nonpainful. Tumors involving bone or those growing within the tissues of the maxilla or mandible appear to be significantly more painful. Soft tissue tumors of the pharynx and caudal oral cavity are particularly painful. |
| Intranasal tumors | Pain probably results both from the destruction of turbinates and from the destruction of bone of the nasal cavity. |
| Invasive soft tissue sarcomas | The aggressive vaccine-associated sarcomas in cats are particularly painful—the apparent size of the lesion does not correlate with the degree of pain. Other invasive sarcomas in both species are painful. Also, in both cats and dogs, noninvasive soft tissue sarcomas that are pressing on nerves and other sensitive structures will be painful. One form of soft tissue sarcomas, the peripheral nerve sheath tumor (PNST), is sometimes reported to be painful to the touch. |
| Invasive cutaneous tumors | Especially those that are ulcerative. |
| Liver and biliary tumors | Especially those that are expansile, stretching the liver capsule. Expansile liver tumors are reported to be painful in humans. |
| Disseminated intrathoracic and intraabdominal tumors (e.g., mesothelioma, malignant histiocytosis) | The signs associated with such tumors are particularly vague; however, often, intracavitary analgesia (such as intraabdominal local anesthetic) can markedly improve the animal’s demeanor, and thus, just as in humans, it appears disseminated neoplasia of these cavities is associated with significant pain. |
| Lung tumors | Although significant pain is reported in humans with lung cancer, often animals appear to show few signs of pain. However, even in those animals, the provision of an analgesic can often improve demeanor. |
| Pain following surgical removal of a tumor | Pain well beyond the postoperative period occurs in animals that have undergone surgery and is probably neuropathic in nature. Phantom pain (such as phantom limb pain), a form of neuropathic pain, does appear to exist in animals. If tumor recurrence occurs, significant pain is usually associated with this. |
In addition to pain caused by the tumor itself, pain in cancer patients can also be caused by chemotherapy, radiation therapy, or surgery (perioperative pain, postoperative pain, and conditions such as “phantom limb” associated with some amputations) and by concurrent noncancerous disease, most notably osteoarthritis, gingivitis, and dermatologic conditions.
General Approach to Cancer Pain Management
The incidence of pain in animals caused by cancer is very difficult to estimate, as is the effectiveness of analgesic therapy. Recent surveys have found that significant numbers of animals in the perioperative setting were not receiving analgesic drugs,19-26 although an overview of these studies seems to suggest the situation is improving. Analgesics are even less likely to be used for cancer pain. Glucocorticoids do provide some analgesia, and their use may be more widespread,22 but the specific treatment of cancer pain in animals is still likely to be suboptimal.
Suboptimal treatment of cancer pain in animals probably results from a number of factors such as the following:
• Lack of appreciation that many cancers are associated with significant pain.
• Overly focusing on the cancer treatment, rather than patient comfort.
• Inability to assess pain in cancer patients.
• Lack of knowledge of drugs, drug therapy, and other pain-relieving techniques
• Lack of communication with clients and lack of involvement of clients in the assessment and treatment phases.
• Under-use of nursing staff for assessment and reevaluation of pain in cancer patients.
In large part, these factors associated with suboptimal treatment of cancer pain in animals result from a lack of published, scientifically sound information on the subject. Such information also stimulates presentation and discussion of the topic in continuing education forums. In many respects, continuing education in a topic is facilitated by having Food and Drug Administration (FDA)-approved drugs or proven treatments because industry then has a vested interest in improving knowledge of the subject. Unfortunately, there are no analgesics with FDA approval for cancer pain in animals.
Although drug treatment is the mainstay of cancer pain treatment, effective cancer pain treatment often involves a combination of drug therapy, nondrug therapies, and good communication between all parties involved. A basic approach to cancer pain management is summarized in Table 15-2.
Table 15-2
A Basic Approach to Cancer Pain Management
| A | Assess the patient | Ask for the owner’s perceptions of the pain present or of any compromise of the animal’s quality of life. Assess the patient thoroughly, using palpation and observation. |
| B | Believe the owner | The owner sees the pet every day in its own environment and knows when alterations in behavior occur. They can rarely suggest diagnoses, but they do know when something is wrong and when their pet is in pain, just as a mother knows when something is wrong with her child. |
| C | Choose appropriate therapy | Anything other than mild pain should be treated with more than one class of analgesic, or an analgesic drug combined with nondrug adjunctive therapy. Be aware of potential drug interactions. |
| D | Deliver therapy | Deliver the therapy in a logical coordinated manner and explain carefully to the owner about any possible side effects. |
| E | Empower the client | Empower the clients to participate actively in their pet’s treatment; ask for feedback and updates on how the therapy is working. |
The Importance of Alleviating Pain in the Cancer Patient
The alleviation of pain is important not only from a physiologic and biologic standpoint, but also from an ethical point of view.27 To help in an evaluation of welfare, “five freedoms,” initially proposed by the Brambell Committee in reference to farmed animals,28 have been suggested. They may be applied equally in the context of companion animals27 and are as follows:
• Freedom from hunger and thirst
• Freedom from physical and thermal discomfort
• Freedom from pain, injury, and disease
For each freedom, there should be a consideration of the severity, incidence, and duration. As an example, consider a dog undergoing a maxillectomy. With such a surgery, it is possible that thirst and hunger may result from interference with the dog’s ability to eat and drink. However, the dog could be hand-fed and gradually taught to eat and drink despite the facial alteration. If the dog was never able to eat or drink again on its own, the owners could hand-feed him for the rest of his life or the dog could be fed via a feeding tube. Although this may represent a compromise to his freedom to express normal eating behavior, he would not be hungry or thirsty. Such a surgery may result in a cure for the disease and eliminate pain. However, the surgery may also result in pain and distress. Fear and distress would result because of the unfamiliar surroundings and people during the hospital stay. With appropriate nursing care, the dog should not suffer any physical or thermal discomfort. It can be seen from this example how many factors are interrelated, and all need consideration in the cancer patient, including pain and consideration of the length and severity of pain.
Pain relief may affect survival in cancer patients. Immunosuppression has been shown to depend on the severity of surgery in clinical29-31 and experimental animal studies.32,33 Additionally, the severity of surgery has been shown to influence tumor metastasis.34-37 In 2002, a human clinical study suggested that laparoscopic colectomy was associated with increased survival,38 and although subsequent studies found no difference in long-term survival,39 there is interest in the immunologic and oncologic implications of less-invasive surgery.40 In 2001, Page and coworkers found that the provision of analgesics significantly reduced the tumor-promoting effects of undergoing and recovering from surgery in a rodent model.41 The reduction of the tumor-promoting effects of surgery by analgesics may result from maintenance of natural killer (NK) cell function, but it is likely that other unrecognized factors also play a role.41,42 More recently, it was found that spinal analgesia attenuated the laparotomy-induced suppression of tumoricidal function of liver mononuclear cells and decreased metastasis compared to rodents undergoing laparotomy without spinal analgesia.43 This was considered to be due to preservation of T helper 1/T helper 2 (Th1/Th2) cytokine balance. Thus the provision of adequate perioperative pain management in oncologic surgery may be protective against metastatic sequelae in clinical patients. It is quite possible that chronic pain experienced by animals with cancer may also affect metastasis.
Assessment of Cancer Pain
Assessment of pain in animals, while often difficult, is extremely important. It is likely that the tolerance of pain by an individual animal varies greatly and is further complicated by the innate ability of dogs and cats to mask significant disease and pain. Physiologic variables such as heart rate, respiratory rate, temperature, and pupil size are not reliable measures of acute perioperative pain in dogs44 and are unlikely to be useful in chronic pain states. The mainstay of pain assessment in cats and dogs suffering from cancer is likely to be changes in behavior. Table 15-3 outlines behaviors that are probably indicative in certain situations of pain. In general, if a tumor is considered to be painful in humans, it is appropriate to give an animal with a similar condition the benefit of the doubt and treat it for pain.
Table 15-3
Outline of Behaviors that May Be Seen with Cancer-Associated or Cancer Therapy–Associated Pain in Cats and Dogs
| Behavior | Notes |
| Activity | Less activity than normal; may be very specific activities that are changed; decreased jumping; less playing; less venturing outside; less willing to go on walks (dogs); stiff gait, altered gait, or lameness can be associated with generalized pain but is more often associated with limb or joint pain; slow to rise and get moving after rest (osteoarthritis is often concurrently present). |
| Appetite | Often decreased with chronic pain. |
| Attitude | Any change in behavior can be associated with cancer pain—aggressiveness, dullness, shyness, “clinginess,” increased dependence |
| Facial expression | Head hung low, squinted eyes in cats. Sad expression in dogs, head carried low. |
| Grooming | Failure to groom can be due to either a painful oral lesion or generalized pain. |
| Response to palpation | This is one of the best ways to diagnose and monitor pain. Pain can be elicited by palpation of the affected area, or manipulation of the affected area, which exacerbates the pain present. This is manifested as an aversion response from the animal (i.e., the animal attempts to escape the procedure, or yowls, cries, hisses, or bites). Pain is inferred when this occurs. |
| Respiration | May be elevated with severe cancer pain. |
| Self-trauma | Licking at an area (e.g., joint with osteoarthritis, bone with primary bone cancer, the abdomen with intraabdominal cancer) can indicate pain; scratching can indicate pain (e.g., scratching at cutaneous tumors, scratching and biting at the flank with prostatic or colonic neoplasia). |
| Urinary and bowel elimination | Failure to use litter box (cats); urinating and defecating inside (dogs). |
| Vocalization | Vocalization is rare in response to chronic pain in dogs and cats; however, owners of dogs will often report frequent odd noises (whining, grunting) associated with cancer pain. Occasionally, cats will hiss, utter spontaneous plaintive meows, or purr in association with cancer pain |
One of the most useful ways of determining if a tumor is painful is to palpate the area and evaluate the animal’s response. This may not correlate precisely with the amount of pain the animal spontaneously experiences, but if a tumor is painful on manipulation or palpation, it is highly likely there is spontaneous pain associated with it. As veterinarians, we struggle to measure spontaneous pain. It is perhaps reassuring that there is tremendous debate in the research community on how to measure spontaneous pain in rodent models.
Veterinarians should involve technicians and other staff members in the assessment process because they are usually the ones spending the most time with the patients in the hospital and may have more time to converse in a relaxed and informal way with owners.
The most important people in the assessment process are the owners. The veterinarian must work closely with the owner to capture this information. Often, owners need to be educated as to what signs to look for and that certain behaviors may be indicative of pain. Once very specific changes in behavior can be identified and recorded, these behaviors can be used to monitor the effectiveness of analgesic therapy. Furthermore, these specific activities can be used to create goals and therapy tailored to try to meet these goals. The importance of patient-reported outcomes (PROs) in human medicine is widely recognized.45 These PROs may refer to a large variety of different health data reported by patients, such as symptoms, functional status, quality of life (QOL), and health-related quality of life (HRQOL).45 In humans, QOL is a complex, abstract, multidimensional concept defining an individual’s satisfaction with life in domains he or she considers important. The term HRQOL reflects an attempt to restrict this complex concept to those aspects of life specifically related to the individual’s health and potentially modifiable by healthcare.46 Both QOL and HRQOL have been used in veterinary medicine. Questionnaires have been developed to assess HRQOL in dogs47,48 and cats49 with cancer. Although pain certainly appears to be assessed in these HRQOL tools, it is not known how specific or sensitive these tools are to changing pain status.
The author’s approach to the evaluation of pain at each visit is to evaluate each of the following three aspects:
Follow-up is important, and the assessment of activity and behavioral parameters can be evaluated over the telephone.
Principles of Alleviation of Cancer Pain
Drugs are the mainstay of cancer pain management, although nondrug adjunctive therapies are becoming recognized as increasingly important. The World Health Organization (WHO) has outlined a general approach to the management of cancer pain based on the use of the following groups of analgesics:
• Nonopioid analgesics (e.g., nonsteroidal antiinflammatory drugs [NSAIDs], acetaminophen)
• Weak opioid drugs (e.g., codeine)
• Strong opioid drugs (e.g., morphine)
• Adjuvant drugs (e.g., corticosteroids, tricyclic antidepressants, anticonvulsants, N-methyl d-aspartate [NMDA] antagonists).
The general approach of the WHO ladder is a three-step hierarchy. Initially, pain is treated with a nonopioid (usually an NSAID), ± an adjuvant drug. The next two stages add on a “weak” opioid, then a “strong” opioid. There are several problems with this approach. First, it is naïve because our current understanding of pain indicates that drugs are not “strong” and “weak” but rather “appropriate” and “less appropriate.” Furthermore, there is little information from human medicine and virtually none from veterinary medicine on which drugs are most effective for particular types of cancer pain. It may well be that “third-tier” drugs might be most effective for a particular condition and therefore used upfront.
The second problem is that the approach is not well suited to patients that present initially with significant-to-severe pain. Many veterinary cancer patients present at an advanced stage of disease and thus are already in moderate-to-severe pain. Once pain has been present for a period of time, changes take place in the central nervous system (CNS) that alter the way pain signals are processed. A better understanding of this is helped by understanding the classification of cancer pain.
Classification of Cancer Pain
Pain is a multifactorial experience with sensory (the sensation), affective/emotional (how it makes the subject feel), and functional (can the subject still perform particular functions) components. It can result from obvious causes (e.g., ulcerated skin tumor) and last an expected time. However, in many cases (e.g., postamputation pain) the pain persists after the original painful cancer has been removed or the surgical wound appears to be healed. In the past, pain has often been categorized as acute or chronic based solely on duration—the latter arbitrarily being pain that lasts more than 3 to 6 months. However, it is now accepted that this may not be a helpful classification. It has been suggested that the terms adaptive and maladaptive be adopted; adaptive infers a normal response to tissue damage and involves an inflammatory component (e.g., a surgical incision) and is reversible over an expected, relatively short time. Maladaptive pain results from changes in the spinal cord and brain that result in abnormal sensory processing and is usually persistent. Maladaptive pain can result from poorly treated adaptive pain, and maladaptive pain can occur quickly in some circumstances. A key feature of maladaptive pain is central sensitization—changes in the CNS (anywhere from the dorsal root ganglion rostrally) that are likely initiated by cellular wind-up and result in amplification and facilitation of nociceptive signal generation and transmission. Until proved otherwise, cancer pain should be considered maladaptive, with both peripheral and central changes in the pain processing system contributing to the pain, in addition to noxious input from the cancer or pathologic area.
Ideally, pain would be classified by the underlying mechanism, for example, inflammatory or neuropathic. However, many diseases are associated with overlapping “forms” of pain. Taken one step further, pain occurring in different diseases or conditions would be further classified by the underlying mechanisms in that particular disease and even patient. This could be referred to as the neurobiologic signature of pain in a particular disease or patient. Knowing this would better guide the practitioner in the choice of treatment. For example, a diagnosis of “cancer” pain is not very helpful since the cause could be mechanical compression of a nerve, inflammation from tissue necrosis, mechanical distension of an organ, neuropathic pain, or a combination of these. However, a diagnosis of “transitional cell carcinoma pain of the bladder,” with the associated knowledge of the upregulation of peripheral sodium channel (e.g., NaV1.7) and acid-sensing ion channel (ASIC) receptors and nerve growth factor and central glial cell activity and cyclooxygenase-2 (COX-2) enzyme, is far more informative in terms of guiding clinical treatment. Without this information, treatment is empiric and it may be better termed “hit or miss.”
Early intervention is recommended to limit cellular wind-up and prevent central sensitization. In patients experiencing long-lasting, persistent pain, as is the case with many types of cancer, changes within the peripheral and CNS may occur. These changes (peripheral or central sensitization) may contribute to the further development of pain, separate from the initial inciting cause and should be considered in patients with pain that is unresponsive to first-line or conventional analgesics. Pain syndromes commonly associated with sensitization include prolonged pain or pain experienced outside of the immediate area that is affected, hyperalgesia (increased sensitivity to noxious stimuli), and allodynia (pain after nonnoxious stimuli). The author has found altered sensory processing states to be associated with long-standing osteoarthritis-associated pain, and it is likely many cancers are associated with the same changes.
At the current time, with limited information as to what the “ideal” therapies are for individual cancer pains, the most important aspect to remember in the treatment of cancer pain is that for the majority of situations, multimodal therapy (i.e., concurrent use of more than one class of drug) is required for successful alleviation of the pain.
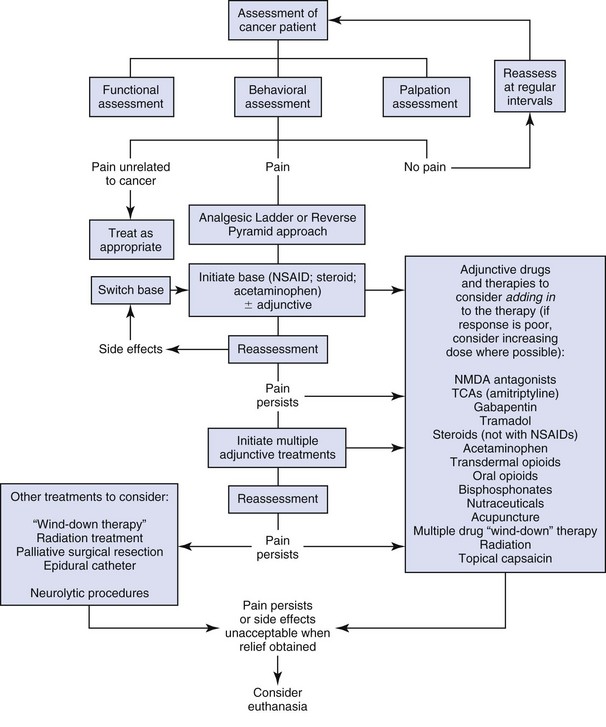
A general outline to approaching cancer pain is given in Figure 15-1. This figure can be used in combination with Tables 15-4 and 15-5. There are many different scenarios that the clinician may face when dealing with cancer pain, and this figure and these tables are only guides. The term wind-down therapy refers to admitting refractory pain patients into the hospital and treating them with multiple intravenous (IV) analgesic medications in the hope that the pain can be controlled, making it easier to subsequently manage the pain at home.
Table 15-4
Suggested Doses of Analgesics that May Be Used for the Alleviation of Chronic Cancer Pain in the Dog
| Drug | Dose for Dogs | Comments |
| Paracetamol (acetaminophen) | 10-15 mg/kg PO q12 hrs | Associated with fewer GI side effects than regular NSAIDs and has not been noted to be associated with renal toxicity. Toxicity has, however, not been evaluated clinically in dogs. Can be combined with regular NSAIDs in severe cancer pain, but this combination has not been evaluated for toxicity. |
| Paracetamol (acetaminophen) + codeine (30 or 60 mg) | 10-15 mg/kg of acetaminophen | Sedation can be seen as a side effect with doses at or above 2 mg/kg codeine. |
| Amantadine | 4.0-5.0 mg/kg PO q24 hrs | Loose stools and excess GI gas can be seen at higher doses for a few days. Should not be combined with drugs such as selegiline or sertraline until more is known about drug interactions. Should not be used in seizure patients or patients in heart failure. |
| Amitriptyline | 0.5-2.0 mg/kg PO q24 hrs | Has not been evaluated for clinical toxicity in the dog. Should be used cautiously in combination with tramadol. |
| Butorphanol | 0.2-0.5 mg/kg PO up to q8 hrs | May produce sedation at higher doses. Not a very predictable analgesic, especially in the dog, and best when used in combination with other analgesics (e.g., NSAIDs). |
| Codeine | 0.5-2.0 mg/kg PO q8-12 hrs | Sedation can be seen at higher doses. Like all oral opioids, it is subject to significant first-pass effect at the liver, likely limiting its analgesic effect. |
| Fentanyl, transdermal | 2-5 mcg/kg/hr | Can be very useful in the short-term control of cancer pain. For long-term therapy, usefulness is limited due to need to change the patch every 4 to 7 days. |
| Gabapentin | 3-10 mg/kg PO q6-12 hrs | Has not been evaluated in dogs as an analgesic. The most likely side effect is sedation. |
| Glucosamine and chondroitin sulfate | 13-15 mg/kg chondroitin sulfate PO q24 hrs | Can be used in a variety of cancer pains due to its mild antiinflammatory and analgesic effects. |
| Morphine, liquid | 0.2-0.5 mg/kg PO q 6-8 hrs | Can be useful for dosing smaller dogs where the morphine tablets are not suitable. Sedation and particularly constipation are side effects that are seen as the dose is increased. Like all oral opioids, it is subject to significant first-pass effect at the liver, likely limiting its analgesic effect. |
| Morphine, sustained release | 0.5-3.0 mg/kg PO q 8-12 hrs | Doses higher than 0.5-1.0 mg/kg are often associated with unacceptable constipation according to owners, so suggest using 0.5 mg/kg several times a day. Like all oral opioids, it is subject to significant first-pass effect at the liver, likely limiting its analgesic effect. |
| Pamidronate | 1-1.5 mg/kg slowly IV diluted in 4 mL/kg normal saline, administered over 2 hrs; repeat every 4-6 weeks | Inhibits osteoclast activity and thus only provides analgesia in cases suffering from a primary or metastatic bone tumor that is causing osteolysis. Effect may be delayed days-weeks. |
| Prednisolone | 0.25-1 mg/kg PO q12-24 hrs; taper to q48 hrs if possible after 14 days | Do NOT use concurrently with NSAIDs. Can be particularly useful in providing analgesia when there is a significant inflammatory component associated with the tumor and for CNS or nerve tumors. |
| Tramadol | 4-5 mg/kg PO q6-12 hrs | This drug has not been evaluated for efficacy or toxicity in dogs. |
PO, By mouth; GI, gastrointestinal; NSAIDs, nonsteroidal antiinflammatories; IV, intravenous.
None of these drugs have been evaluated for efficacy in the treatment of cancer pain.
None of these drugs are approved or licensed for use in chronic cancer pain. The NSAIDs have not been included in this table. NSAIDs should be used as a first line of pain relief if it is clinically appropriate to use them and should be used at their approved dose.
The doses given come from the author’s experience and the experience of others working in the area of clinical cancer pain control.
Table 15-5
Suggested Doses of Analgesics that May Be Used for the Alleviation of Chronic Cancer Pain in the Cat
| Drug | Cat Dose (mg/kg) | Notes |
| Acetaminophen (Paracetamol) | Contraindicated | Small doses rapidly cause death in cats. |
| Amantadine | 3.0-5.0 mg/kg PO q24 hrs | This drug has not been evaluated for toxicity but is well tolerated in dogs and humans, with occasional side effects of agitation and GI irritation. May be a useful addition to NSAIDs in the treatment of chronic cancer pain conditions. Amantadine powder can be purchased and formulated into appropriately sized capsules. The kinetics have recently been evaluated in cats. |
| Amitriptyline | 0.5-2.0 mg/kg PO q24 hrs | This drug appears to be well tolerated for up to 12 months of daily administration. May be a useful addition to NSAIDs for treatment of chronic pain conditions. |
| Aspirin | 10 mg/kg PO q48 hrs | Can cause significant GI ulceration. |
| Buprenorphine | 0.01-0.02 mg/kg SL q8-12 hrs | SL route is not resented by cats and may be a good way to provide postoperative analgesia at home. Feedback from owners indicates that after 2-3 days dosing at this dose, anorexia develops. Smaller doses (5-10 µg/kg) may be more appropriate for “long-term” administration, especially in combination with other drugs. |
| Butorphanol | 0.2-1.0 mg/kg PO q6 hrs | One study suggests using oral form after surgery may be beneficial. Generally considered to be a poor analgesic in cats except for visceral pain; however, the author has found it to be useful as part of a multimodal approach to cancer pain therapy. |
| Carprofen | Not enough data to enable recommendations for long-term administration. | — |
| Etodolac | Not recommended. | — |
| Firocoxib | — | Has not been reported in clinical cases, but it has a half-life of 8-12 hours in the cat, and at 3 mg/kg provided antipyretic effects in a pyrexia model. |
| Flunixin meglumine | 1 mg/kg PO daily for 7 days | Daily dosing for 7 days results in increased rate of metabolism of the drug but a rise in liver enzymes, suggesting liver toxicity may be a problem with prolonged dosing. |
| Gabapentin | 10 mg/kg q12 hrs | Appears to be particularly effective in chronic pain in cats where an increase in sensitivity has occurred, or where the pain appears to be excessive in comparison to the lesion present. |
| Glucosamine/chondroitin sulphate combinations | Approximately 15 mg/kg chondroitin sulphate PO q12-24 hrs | May be associated with mild analgesic effects. |
| Glucosamine/chondroitin sulphate combination with avocado/soya extracts | Labelled dose | May be associated with mild analgesic effects. |
| Ketoprofen | 1 mg/kg PO q24 hrs | Probably well tolerated as pulse therapy for chronic pain, with a few days “rest” between treatments of approximately 5 days. Has also been used by some at 1 mg/kg every 3 days long term. Another approach has been to use 0.5 mg/kg daily for 5 days (weekdays) followed by no drug over the weekend and repeated. |
| Meloxicam | 0.01 mg/kg PO on day 1, followed by 0.05 mg/kg PO daily for 4 days, then 0.05 mg/kg every other day thereafter. (Approval in the European Union at 0.05mg/kg daily indefinitely for musculoskeletal pain.) | Well received by cats due to its formulation as a honey syrup. Also, the drop formulation makes it very easy to gradually and accurately decrease the dose. A decreasing regimen has not been evaluated for efficacy in cats but has been found to be successful in dogs. Meloxicam should be dosed accurately using syringes. |
| Morphine (oral liquid) | 0.2-0.5 mg/kg PO tid-qid | Best compounded into a palatable, flavored syrup; however, cats usually resent this medication. Morphine may not be as effective in cats as it is in dogs. |
| Morphine (oral sustained release) | Tablets too large for dosing cats. | — |
| Piroxicam | 1 mg/cat PO daily for a maximum of 7 days. If longer term medication is considered, suggest every other day dosing. | Daily dosing for 7 days results in a slight increase in the half-life. |
| Prednisolone | 0.5-1.0 mg/kg PO q24 hrs | Can be very effective. NOT to be combined with concurrent NSAID administration. |
| Polysulfated glycosaminoglycans (PSGAGs; Adequan) | 5 mg/kg SQ twice weekly for 4 weeks; then once weekly for 4 weeks; then once monthly (other suggested regimens call for once weekly injections for 4 weeks, then once monthly). | There is no evidence-based medicine that it provides any analgesic effect, but anecdotal information suggests improvement can be seen after a few injections. |
| Robenacoxib | 1-2 mg/kg q24 hrs | Has varying approvals in different parts of the world (approved for up to 11 days administration in Switzerland). First NSAID that is a COX-2 inhibitor, has a short half-life, and demonstrates tissue selectivity. |
| Tepoxalin | 5-10 mg/kg q24 hrs | The author has used this successfully long-term in cats, likely due to its short half-life (5 hours) and true dual inhibition characteristics. |
| Tolfenamic acid | 4 mg/kg PO q24 hrs for 3 days maximum | Has not been evaluated for chronic pain, but recent objective measurements demonstrate analgesia in the cat when administered perioperatively. |
| Tramadol | 1-2 mg/kg once to twice daily | Main problem is dosing cats—the tablets are very bitter and aversive to cats. |
| Transdermal fentanyl patch | 2-5 µg/kg/hrs | The patches may provide 5-7 days of analgesia in some cases and should be left on for longer than 3 days. Following removal, the decay in plasma levels following patch removal is slow. |
| Vedaprofen | 0.5 mg/kg q 24 hrs for 3 days | Has not been evaluated for chronic pain but was evaluated for controlling pyrexia in upper respiratory infection, and for controlling postoperative pain following ovariohysterectomy. |
PO, By mouth; GI, gastrointestinal; NSAIDs, nonsteroidal antiinflammatories; SL, sublingual; SQ, subcutaneous; tid, three times a day; qid, four times a day; COX-2, cyclooxygenase-2.
None of the drugs have been evaluated for efficacy in the treatment of cancer pain.
None of these drugs are approved or licensed for use in chronic cancer pain. Some drugs are approved for inflammatory or painful conditions in the cat in certain countries, and doses for the control of cancer pain are extrapolated from these. The doses given come from the author’s experience and the experience of others working in the area of clinical cancer pain control.
Drugs and Strategies Used for Management of Pain in Cancer Patients
The drugs that can be used for chronic cancer pain management are outlined in Tables 15-4 and 15-5. The following notes are not a comprehensive appraisal of each class of drug but are suggestions for their use for cancer pain.
Nonsteroidal Antiinflammatory Drugs
NSAIDs have been the mainstay of therapy for chronic pain, especially in osteoarthritis, and they are an excellent first line of treatment in cancer pain. There are several excellent reviews on NSAID use in small animals, and the reader is referred to these.50-54 The choice of NSAIDs available can be bewildering, but a few key points are as follows:
• On a population basis, all NSAIDs are probably equally efficacious in relieving pain, but for a given patient, one drug is often more effective than another.
• Gastrointestinal side effects associated with NSAID use appear to be more common with drugs that preferentially block COX-1 over COX-2.
• There is no difference in renal toxicity between COX-1 selective drugs and COX-2 selective drugs.
• Liver toxicity can occur with any NSAID.
• There are no completely safe NSAIDs, but the approved NSAIDs are significantly safer than the older nonapproved NSAIDs.
• Longer term or continuous NSAID use appears to be more effective than short-term or reactive use,52 but in relatively stable disease states, gradual dose reduction may be possible while maintaining efficacy.55
There are no licensed NSAIDs for long-term administration in cats other than meloxicam, which is approved in the European Union for long-term treatment of musculoskeletal pain. However, a number of these compounds can probably be used safely (see Table 15-5). The key to safe chronic NSAID administration in cats is the use of the smallest effective dose and avoiding use or using decreased doses in cats with renal disease. Choosing drugs with short half-lives is also considered important by the author.
The patient on NSAIDs should be monitored for toxicity by informing the owner of potential toxicity and what signs to watch for (lethargy, depression, vomiting, melena, increased water ingestion), as well as through the regular evaluation of blood work (and urinalysis) to evaluate renal and liver function. A baseline should be obtained when therapy is initiated and parameters monitored on a regular basis thereafter. The author repeats evaluations after 2 to 4 weeks and then at 1- to 4-month intervals as dictated by the individual patient and client.
If pain relief with NSAIDs is inadequate, oral opioid medications such as morphine or tramadol can be administered (see later). Acetaminophen or acetaminophen/codeine combinations can often be used in conjunction with NSAIDs. Transdermal fentanyl can also be used. Fentanyl, morphine, or tramadol can be used for dogs that cannot be given NSAIDs. Other agents that are used to treat chronic pain include: amantadine, an NMDA antagonist; anticonvulsants such as gabapentin; and tricyclic antidepressants such as amitriptyline. These can all be combined with NSAIDs, although we do not know the full extent of side effects. Readers are cautioned that they should not assume that combinations of different adjunctive drugs are without side effects—quite the contrary, there is much to be learned about potential adverse interactions, especially in cancer patients that may be on other therapies.
Acetaminophen
Acetaminophen is a nonacidic NSAID; many authorities do not consider it an NSAID as it probably acts by somewhat different mechanisms.56 With any chronic pain, there are always CNS changes, so for what seems a “peripheral” problem such as many cancers, centrally acting analgesics can be very effective. Although highly toxic in the cat, even in small quantities, it can be effectively used in dogs for pain control in the acute setting.57,58 No studies of toxicity in dogs have been done, but if toxicity is seen, it will probably affect the liver; thus the drug, in common with all NSAIDs and opioids, should be used cautiously in dogs with liver dysfunction. It can be used on its own or in a preparation combined with codeine and is initially dosed at about 10 to 15 mg/kg twice a day (bid). The author often uses it as the first-line analgesic therapy in dogs with renal compromise in which NSAIDs cannot be used or in dogs that appear to be otherwise intolerant to NSAIDs (e.g., vomiting or gastrointestinal ulceration).
Opioids
Opioids can be an effective part of the management of cancer pain, particularly when used as part of a multimodal approach (i.e., including NSAIDs or adjunctive analgesics). Side effects of opioids include diarrhea, vomiting, sedation, and constipation with long-term use. It is often constipation and occasionally the sedation that owners object to most as a side effect in their pet. Oral morphine, transdermal fentanyl, oral butorphanol, sublingual buprenorphine (cats only), and oral codeine are used most often for the alleviation of chronic cancer pain. None of these drugs has been fully evaluated for clinical toxicity when administered long term nor for efficacy against chronic cancer pain. Dosing must be done on an individual basis, and adjustment of the dose to produce effective analgesia without undesirable side effects requires interaction and communication with clients. However, despite these suggestions, recent evidence has indicated that oral opioids may not reach effective plasma concentrations in dogs when dosed at the currently recommended levels.59-62
There is currently no information on the long-term use of oral opioids for chronic pain in the cat. There appears to be individual variation in the level of analgesia obtained with certain opioids, especially morphine and butorphanol, in the acute setting.63,64 Buprenorphine appears to produce predictable analgesia when given sublingually65 and is well accepted by most cats. The small volume required (max 0.066 mL/kg [20 µg/kg]) makes administration simple. Based on clinical feedback from owners, this is an acceptable technique for home use. Inappetence can occur after several days of treatment and sometimes lower doses (5 to 10 µg/kg) can overcome this problem. When administered concurrently with other drugs, less frequent dosing is often required.65
N-Methyl D-Aspartate Antagonists
The NMDA receptor appears to be central to the induction and maintenance of central sensitization,66-68 and the use of NMDA receptor antagonists would appear to offer benefit where central sensitization has become established (i.e., especially chronic pain). Ketamine, tiletamine, dextromethorphan, and amantadine possess NMDA antagonist properties, among other actions.
Ketamine is not obviously useful for the management of chronic pain due to the formulation available and the tendency for dysphoric side effects even at low doses. However, oral ketamine has not been evaluated in dogs or cats for long-term administration. Intraoperative microdose IV ketamine appears to provide beneficial effects for a variety of oncology surgical procedures, including limb amputations,69 and this may decrease the incidence of chronic pain later. Other reports suggest a benefit of using ketamine perioperatively in low doses.70 When used in this manner, ketamine should be administered as a bolus (0.5 mg/kg IV) followed by an infusion (10 µg/kg/min) prior to and during surgical stimulation. A lower infusion rate (2 µg/kg/min) may be beneficial for the first 24 hours postoperatively and an even lower rate (1 µg/kg/min) for the next 24 hours. In the absence of an infusion pump, ketamine can be mixed in a bag of crystalloid solutions for administration during anesthesia. Using anesthesia fluid administration rates of 10 mL/kg/hr, 60 mg (0.6 mL) of ketamine should be added to a 1-liter bag of crystalloid fluids to deliver ketamine at 10 µg/kg/min.
The active metabolite of dextromethorphan may not be produced in dogs, probably negating its use in the species for chronic pain.61
Amantadine has been used for the treatment of neuropathic pain in humans,71 and one report suggests a benefit of adding amantadine to an NSAID in dogs that do not get complete relief from the NSAID alone.72 Suggested dosages are given in Tables 15-4 and 15-5. The toxic side effects have been evaluated in the dog (but not the cat), and the dosages suggested are considered safe.73 Amantadine should be avoided in patients with congestive heart failure, history of seizure, or those on selegiline, sertraline, or tricyclic antidepressants.
Combination Analgesics
Tramadol is a synthetic derivative of codeine and is classified as an opioidergic/monoaminergic drug.74,75 It has been found to be effective in the alleviation of pain associated with osteoarthritis in humans. Tramadol’s analgesic efficacy is a result of complex interactions between opiate, adrenergic, and serotonin receptor systems. Hepatic demethylation of tramadol produces the active metabolite, O-desmethyltramadol (M1). The different metabolites interact with different receptors; thus efficacy in different species is likely to depend on the metabolic characteristics in the particular species. Initial work in dogs suggested that tramadol was absorbed sufficiently, producing levels that would theoretically provide analgesia.76 However, recently there has been some discussion about the actual amounts of the M1 metabolite that are formed in dogs, and the pharmacokinetics of tramadol does not favor an analgesic effect.77-81 There is no published evidence of efficacy for canine pain.
Little is known about the side effects of tramadol in dogs, and almost nothing is known about the side effects seen when tramadol is combined with other drugs in human or canine medicine. In human medicine, a recent study found that for patients hospitalized for peptic ulcer treatment, tramadol use prior to admission was associated with just as high a risk of mortality as was NSAID use prior to admission. Additionally, mortality was 2.02- and 1.41-fold higher in these groups of patients, respectively, than in patients who used neither tramadol nor NSAIDs.82 A recent study evaluating the analgesic effects of various doses of rofecoxib and tramadol alone and in combination found that the most analgesic combination of tramadol and rofecoxib produced gastric injury in rats that was more severe than with rofecoxib or tramadol alone.83 Tramadol is metabolized differently in the cat84,85 and appears to have clinical efficacy when administered intravenously perioperatively86 and has shown antinociceptive activity when administered orally in an experimental setting.87
The dosages given in Tables 15-4 and 15-5 are for the regular (not prolonged release) form of the drug. It has not been thoroughly evaluated for toxicity in the dog or cat.
Anticonvulsant Drugs
Many anticonvulsants such as carbamazepine, phenytoin, baclofen, and more recently gabapentin have been used to treat chronic pain, including neuropathic pain in humans, as well as chemotherapy-induced peripheral neuropathies. Gabapentin and the more recently introduced pregabalin appear to be among the most effective drugs available for neuropathic pain in humans. While the exact mechanism of action of these drugs is unclear, one potential mode by which they exert their analgesic effect is by binding to the α2-δ protein subunit of voltage-gated calcium channels, thereby reducing excitatory neurotransmitter release through channel modulation or channel trafficking. Although there is considerable information on gabapentin disposition in dogs88,89 and some information on its use as an anticonvulsant in dogs,90 there is as yet no information on its use for the control of chronic or long-term pain. However, a recent small study suggested no beneficial effect of perioperative gabapentin.91 Information on the kinetics of gabapentin in cats has become available,92 and one study found a lack of thermal antinociception.93 There are no scientific publications demonstrating its efficacy for long-term pain in this species. While the indications for gabapentin (and pregabalin) are presently unclear in veterinary patients, they do appear to be useful for cancer pain in some patients and are probably particularly effective in cancers that have some neurogenic or nerve destruction component. Suggested dosages are in Tables 15-4 and 15-5.
Tricyclic Antidepressants
Tricyclic antidepressants have been used for many years for the treatment of chronic pain syndromes in people and are becoming widely used for the modulation of behavioral disorders in animals. Within the CNS, there are descending inhibitory serotonergic and noradrenergic pathways that reduce pain transmission in the spinal cord. Tricyclic antidepressants such as amitriptyline, clomipramine, fluoxetine, imipramine, maprotiline, and paroxetine primarily inhibit the reuptake of various monoamines (serotonin for clomipramine, fluoxetine, and paroxetine; noradrenaline for imipramine, amitriptyline, and maprotiline). Tricyclic antidepressants can also interact directly with 5-hydroxytryptamine (5-HT) and peripheral noradrenergic receptors and may also contribute other actions such as voltage-gated sodium channel blockade and reduction in peripheral prostaglandin E2 (PGE2)-like activity or tumor necrosis factor (TNF) production. However, in human medicine, there is a relative lack of controlled, clinical trials specifically evaluating the efficacy of antidepressants in treating cancer pain,94 with the exception of two studies demonstrating a lack of efficacy in the treatment of chemotherapy-induced peripheral neuropathy.95,96 If there are beneficial effects, it could be from modulation of pain or improvement in mood or feeling.
The tricyclic antidepressant amitriptyline appears to be effective in the cat for pain alleviation in interstitial cystitis,97 and many practitioners are reporting efficacy in other chronically painful conditions in the cat, including osteoarthritis. Amitriptyline has been used daily for periods up to 1 year for interstitial cystitis, and few side effects are reported. The author has also used amitriptyline in the cat for cancer pain with some encouraging results. It should probably not be used concurrently with other drugs that modify the serotonergic system such as amantadine or tramadol until more is known about drug interactions.
Sodium Channel Blockade
Alterations in the level of expression, cellular localization, and distribution of sodium channels are seen in many pain states. These aberrantly expressed sodium channels result in hyperexcitability and ectopic activity in peripheral and central nerves that encode nociceptive information. Low doses of lidocaine and other sodium-channel blockers readily block these aberrantly expressed sodium channels, producing pain relief. Low-dose IV lidocaine has proven as effective as other commonly used medications for treatment of neuropathic pain in humans,98 and the author uses such an approach to “downregulate” central sensitization in veterinary cancer patients. There is increasing interest in the use of transdermal lidocaine patches for treatment of cancer pain.99 Much of this interest revolves around using the patch to administer a low systemic level of lidocaine that blocks the aberrantly expressed sodium channels. Studies have been performed evaluating the kinetics of lidocaine absorbed from patches applied to dogs and cats.100-102 Peak plasma concentrations of lidocaine were obtained between 10 and 24 hours postapplication in dogs and at 65 hours postapplication in cats. The results of these studies indicate that, similar to humans, systemic absorption of lidocaine from the patch is minimal. Peak plasma concentrations were over 100 times below the level reported to induce neurologic signs and 10 times below the level reported to result in myocardial depression in dogs and 25 times lower than that observed following an IV injection of lidocaine (2 mg/kg) in cats. Potential systemic toxicity associated with lidocaine administration, including bradycardia, hypotension, cardiac arrest, muscle or facial twitching, tremors, seizures, nausea, and vomiting, were not noted in any study. Dosing guidelines have been suggested,103 although to date there have been no published reports evaluating the analgesic efficacy of lidocaine patches in veterinary cancer patients, but the technique holds promise.
Steroids
Steroids have a mild analgesic action, can produce a state of euphoria, and are often used for these reasons to palliate cancer and cancer pain in cats and dogs. They should not be used concurrently with NSAIDs because the risk of side effects (especially gastrointestinal) is increased dramatically.
Bisphosphonates
Malignant bone disease creates a unique pain state, with a neurobiologic signature distinct from that of inflammatory and neuropathic pain.104-106 Bone cancer–related pain is thought to be initiated and perpetuated by dysregulated osteoclast activity and activation of nociceptors by prostaglandins, cytokines, and H+ ions. Therapies that block osteoclast activity not only have the potential to markedly reduce bone pain but may also mitigate other skeletal complications associated with neoplastic conditions, including pathologic fractures and hypercalcemia of malignancy. Bisphosphonates are synthetic analogs of pyrophosphate whose primary effect is to inhibit osteoclast activity. Bisphosphonates accumulate in bone, and following osteoclast-mediated bone resorption, bisphosphonates are released and disrupt cellular functions, resulting in osteoclast death. Oral absorption of bisphosphonates tends to be poor, and IV dosing is the preferred route of administration. Adverse effects include nephrotoxicity, electrolyte abnormalities, and acute-phase reactions.107,108 In addition to the inhibitory effects of bisphosphonates on osteoclasts, reports suggest that they may also exert direct effects on cancer cells, including canine OSA and fibrosarcoma lines.109,110 Studies have found beneficial effects of IV pamidronate for the treatment of malignant osteolysis associated with primary and secondary bone neoplasms.10,11,108,111 These studies have also suggested analgesic effects; however, the positive assessments have been based on subjective evaluations. In a placebo-controlled study, there was no difference in the pain relief between placebo and bisphosphonates using either subjective or objective (force plate) evaluations.9 Despite these results, it is believed that some patients attain pain relief from bisphosphonate administration, and a better and validated means of assessing cancer pain may help elucidate the degree of pain relief that can be achieved. Pamidronate may be administered at a dose of 1 to 2 mg/kg as a 2 to 4 hour infusion (diluted in saline) and repeated at 3 to 5 week intervals. Other examples of a bisphosphonate that can be used in dogs are clodronate, alendronate, and zoledronate.111-113
Neuronal Ablation or Exhaustion Therapy
Several new approaches to pain treatment revolve around the use of mechanisms to destroy or exhaust neurons involved in pain transmission. One approach is to use targeted neurotoxins to cause neuronal death.114 An example of this is the combination of a neurotoxin (saponin) to substance P. Substance P, when administered, binds to the neurokinin receptor (NKR), and the conjugate is internalized (a normal phenomenon of the receptor-ligand interaction), resulting in cell death due to the neurotoxin.115,116 Because sensory neurons are rich in NKRs, if the conjugate is targeted appropriately (e.g., given intrathecally), sensory neurons are killed. Basic science studies suggest that in models of chronic pain, general sensory function is left intact, whereas hyperalgesia associated with chronic pain is decreased. Some toxicity work has been performed in dogs,117 and clinical trials in dogs with naturally occurring bone OSA are underway, with these being used as a model of human OSA pain. Another approach uses the transient receptor potential channel, vanilloid subfamily member 1 (TRPV1) to target neurons involved in pain. When drugs such as capsaicin and resiniferatoxin bind to TRPV1, the resulting calcium influx that is initiated results in an inability of the neuron to function. If the activation of TRPV1 occurs for long enough or is intense enough, the resulting calcium influx can cause the neurons to degenerate and undergo apoptosis. Capsaicin is used in humans for neuropathic pain, and resiniferatoxin has been evaluated in dogs with naturally occurring bone cancer pain in which it provided prolonged pain relief, despite significant hemodynamic effects.5
Radiation Therapy
Radiation therapy is considered to palliate pain in canine OSA, although the evidence is largely anecdotal. A 0-7-21 palliative regimen, using 8 or 10 Gy fractions, has been reported to result in pain relief lasting about 70 days.4,118 Additionally, two 8 Gy fractions over 2 consecutive days was reported to result in pain relief for a duration of 67 days.13 The difficulty in interpreting the value of radiation therapy for pain relief in these studies is the lack of controls or objective measures. Indeed, the natural course of pain and lameness in dogs with appendicular OSA has not been determined. One study evaluating objective measure of limb use in dogs with OSA undergoing radiation therapy found no significant changes in kinetic parameters after one 8-Gy dose of radiation.2
Samarium is a radioisotope that has been evaluated for use in dogs.119 Although the use of samarium Sm153 lexidronam in veterinary medicine is still limited, results of a noncontrolled clinical study with subjective assessments suggested improvement in lameness scores in 63% of dogs, suggesting that this therapy may be useful in the palliation of pain in dogs with bone tumors in which curative-intent treatment is not pursued.3
Acupuncture
Acupuncture can be provided through simple needle placement or by needle placement combined with electrical stimulation (of high or low frequency, although most types of pain respond to low-frequency stimulation). Results of a study in normal experimental dogs demonstrated a weak analgesic effect of electroacupuncture in anesthetized patients as evaluated by a reduction in the minimum alveolar concentration (MAC) of inhaled anesthetic agent.120 Recent data utilizing a rodent model suggest that electroacupuncture may have beneficial effects in treatment of pain associated with bone cancer.121,122 As yet, there is no evidence that acupuncture provides pain relief in veterinary patients.
The Future: Toward a Mechanistic Understanding of Cancer Pain
Over the last few years, it has become evident that the pain transmission system is plastic (i.e., it alters in response to inputs). This plasticity results in a unique neurobiologic signature within the CNS and peripheral nervous system for each painful disease. Understanding the individual neurobiologic signatures for different disease processes should allow novel, targeted, and more effective treatments to be established.123 This approach should also allow for a more informed choice to be made regarding which of the currently available drugs might be most effective.
The first relevant model of cancer pain has been established in rats—an OSA model.124 Prior to this model, evaluation of mechanisms and treatments were undertaken in chronic pain models such as sciatic nerve ligation or injection of chronic irritants—models that did not involve cancer. The introduction of clinically relevant cancer pain models is allowing tremendous progress to be made in the translation to effective human cancer pain treatment.104 It is likely that spontaneous cancer in veterinary species will play a significant role in the future in the development of new analgesic approaches for humans, with an obvious benefit to canine and feline patients. Indeed, we are starting to see this with an exciting example being the evaluation of resiniferatoxin in dogs with bone cancer.5
References
1. Yoxall, AT. Pain in small animals—its recognition and control. J Small Anim Pract. 1978;19:423–438.
2. Weinstein, JI, Payne, S, Poulson, JM, et al. Use of force plate analysis to evaluate the efficacy of external beam radiation to alleviate osteosarcoma pain. Vet Radiol Ultrasound. 2009;50:673–678.
3. Barnard, SM, Zuber, RM, Moore, AS. Samarium Sm 153 lexidronam for the palliative treatment of dogs with primary bone tumors: 35 cases (1999-2005). J Am Vet Med Assoc. 2007;230:1877–1881.
4. Bateman, KE, Catton, PA, Pennock, PW, et al. 0-7-21 radiation therapy for the palliation of advanced cancer in dogs. J Vet Intern Med. 1994;8:394–399.
5. Brown, DC, Iadarola, MJ, Perkowski, SZ, et al. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. Anesthesiology. 2005;103:1052–1059.
6. Carsten, RE, Hellyer, PW, Bachand, AM, et al. Correlations between acute radiation scores and pain scores in canine radiation patients with cancer of the forelimb. Vet Anaesth Analg. 2008;35:355–362.
7. Coomer, A, Farese, J, Milner, R, et al. Radiation therapy for canine appendicular osteosarcoma. Vet Comp Oncol. 2009;7:15–27.
8. Davis, KM, Hardie, EM, Lascelles, BD, et al. Feline fibrosarcoma: perioperative management. Compend Contin Educ Vet. 2007;29:712–714. [716–720, 722–729 passim].
9. Fan, TM, Charney, SC, de Lorimier, LP, et al. Double-blind placebo-controlled trial of adjuvant pamidronate with palliative radiotherapy and intravenous doxorubicin for canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2009;23:152–160.
10. Fan, TM, de Lorimier, LP, Charney, SC, et al. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med. 2005;19:74–80.
11. Fan, TM, de Lorimier, LP, O’Dell-Anderson, K, et al. Single-agent pamidronate for palliative therapy of canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2007;21:431–439.
12. Karai, L, Brown, DC, Mannes, AJ, et al. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest. 2004;113:1344–1352.
13. Knapp-Hoch, HM, Fidel, JL, Sellon, RK, et al. An expedited palliative radiation protocol for lytic or proliferative lesions of appendicular bone in dogs. J Am Anim Hosp Assoc. 2009;45:24–32.
14. Mueller, F, Poirier, V, Melzer, K, et al. Palliative radiotherapy with electrons of appendicular osteosarcoma in 54 dogs. IN VIVO. 2005;19:713–716.
15. Fan, TM, Barger, AM, Fredrickson, RL, et al. Investigating CXCR4 expression in canine appendicular osteosarcoma. J Vet Intern Med. 2008;22:602–608.
16. Fan, TM, Barger, AM, Sprandel, IT, et al. Investigating TrkA expression in canine appendicular osteosarcoma. J Vet Intern Med. 2008;22:1181–1188.
17. Lascelles, BDX. Surgical pain: Pathophysiology, assessment and treatment strategies. In: Tobias KM, Johnston SA, eds. Veterinary surgery small animal. St. Louis: Elsevier Saunders, 2012.
18. Marcus, DA. Epidemiology of cancer pain. Curr Pain Headache Rep. 2011;15:231–234.
19. Dohoo, SE, Dohoo, IR. Factors influencing the postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can Vet J. 1996;37:552–556.
20. Dohoo, SE, Dohoo, IR. Postoperative use of analgesics in dogs and cats by Canadian veterinarians. Can Vet J. 1996;37:546–551.
21. Dohoo, SE, Dohoo, IR. Attitudes and concerns of Canadian animal health technologists toward postoperative pain management in dogs and cats. Can Vet J. 1998;39:491–496.
22. Watson, AD, Nicholson, A, Church, DB, et al. Use of anti-inflammatory and analgesic drugs in dogs and cats. Aust Vet J. 1996;74:203–210.
23. Williams, VM, Lascelles, BD, Robson, MC. Current attitudes to, and use of, peri-operative analgesia in dogs and cats by veterinarians in New Zealand. N Z Vet J. 2005;53:193–202.
24. Hugonnard, M, Leblond, A, Keroack, S, et al. Attitudes and concerns of French veterinarians towards pain and analgesia in dogs and cats. Vet Anaesth Analg. 2004;31:154–163.
25. Raekallio, M, Heinonen, KM, Kuussaari, J, et al. Pain alleviation in animals: attitudes and practices of Finnish veterinarians. Vet J. 2003;165:131–135.
26. Capner, CA, Lascelles, BD, Waterman-Pearson, AE. Current British veterinary attitudes to perioperative analgesia for dogs. Vet Rec. 1999;145:95–99.
27. Lascelles, BD, Main, DC. Surgical trauma and chronically painful conditions–within our comfort level but beyond theirs? J Am Vet Med Assoc. 2002;221:215–222.
28. Brambell FWR: Report of technical committee to enquire into the welfare of animals kept under intensive husbandry systems (Cmnd 2836). In Vol. London, HM Stationery Office, 1965.
29. Baxevanis, CN, Papilas, K, Dedoussis, GV, et al. Abnormal cytokine serum levels correlate with impaired cellular immune responses after surgery. Clin Immunol Immunopathol. 1994;71:82–88.
30. Lennard, TW, Shenton, BK, Borzotta, A, et al. The influence of surgical operations on components of the human immune system. Br J Surg. 1985;72:771–776.
31. Kutza, J, Gratz, I, Afshar, M, et al. The effects of general anesthesia and surgery on basal and interferon stimulated natural killer cell activity of humans. Anesth Analg. 1997;85:918–923.
32. Oka, M, Hazama, S, Suzuki, M, et al. Depression of cytotoxicity of nonparenchymal cells in the liver after surgery. Surgery. 1994;116:877–882.
33. Sandoval, BA, Robinson, AV, Sulaiman, TT, et al. Open versus laparoscopic surgery: a comparison of natural antitumoral cellular immunity in a small animal model. Am Surg. 1996;62:625–630. [discussion 630–631].
34. Eggermont, AM, Steller, EP, Sugarbaker, PH. Laparotomy enhances intraperitoneal tumor growth and abrogates the antitumor effects of interleukin-2 and lymphokine-activated killer cells. Surgery. 1987;102:71–78.
35. Allendorf, JD, Bessler, M, Horvath, KD, et al. Increased tumor establishment and growth after open vs laparoscopic bowel resection in mice. Surg Endosc. 1998;12:1035–1038.
36. Allendorf, JD, Bessler, M, Horvath, KD, et al. Increased tumor establishment and growth after open vs laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc. 1999;13:233–235.
37. Allendorf, JD, Bessler, M, Kayton, ML, et al. Increased tumor establishment and growth after laparotomy vs laparoscopy in a murine model. Arch Surg. 1995;130:649–653.
38. Lacy, AM, Garcia-Valdecasas, JC, Delgado, S, et al. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229.
39. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059.
40. Lee, SW, Whelan, RL. Immunologic and oncologic implications of laparoscopic surgery: what is the latest? Clin Colon Rectal Surg. 2006;19:5–12.
41. Page, GG, Blakely, WP, Ben-Eliyahu, S. Evidence that postoperative pain is a mediator of the tumor-promoting effects of surgery in rats. Pain. 2001;90:191–199.
42. Lee, SW, Gleason, NR, Southall, JC, et al. A serum-soluble factor(s) stimulates tumor growth following laparotomy in a murine model. Surg Endosc. 2000;14:490–494.
43. Wada, H, Seki, S, Takahashi, T, et al. Combined spinal and general anesthesia attenuates liver metastasis by preserving TH1/TH2 cytokine balance. Anesthesiology. 2007;106:499–506.
44. Conzemius, MG, Hill, CM, Sammarco, JL, et al. Correlation between subjective and objective measures used to determine severity of postoperative pain in dogs. J Am Vet Med Assoc. 1997;210:1619–1622.
45. Arpinelli, F, Bamfi, F. The FDA guidance for industry on PROs: the point of view of a pharmaceutical company. Health Qual Life Outcomes. 2006;4:85.
46. Apolone, G, De Carli, G, Brunetti, M, et al. Health-related quality of life (HR-QOL) and regulatory issues. An assessment of the European Agency for the Evaluation of Medicinal Products (EMEA) recommendations on the use of HR-QOL measures in drug approval. Pharmacoeconomics. 2001;19:187–195.
47. Yazbek, KV, Fantoni, DT. Validity of a health-related quality-of-life scale for dogs with signs of pain secondary to cancer. J Am Vet Med Assoc. 2005;226:1354–1358.
48. Lynch, S, Savary-Bataille, K, Leeuw, B, et al. Development of a questionnaire assessing health-related quality-of-life in dogs and cats with cancer. Vet Comp Oncol. 2011;9:172–182.
49. Tzannes, S, Hammond, MF, Murphy, S, et al. Owners “perception of their cats” quality of life during COP chemotherapy for lymphoma. J Feline Med Surg. 2008;10:73–81.
50. Bergh, MS, Budsberg, SC. The coxib NSAIDs: potential clinical and pharmacologic importance in veterinary medicine. J Vet Intern Med. 2005;19:633–643.
51. Papich, MG. An update on nonsteroidal anti-inflammatory drugs (NSAIDs) in small animals. Vet Clin North Am Small Anim Pract. 2008;38:1243–1266.
52. Innes, JF, Clayton, J, Lascelles, BD. Review of the safety and efficacy of long-term NSAID use in the treatment of canine osteoarthritis. Vet Rec. 2010;166:226–230.
53. Lascelles, BD, Court, MH, Hardie, EM, et al. Nonsteroidal anti-inflammatory drugs in cats: a review. Vet Anaesth Analg. 2007;34:228–250.
54. Kukanich, B, Bidgood, T, Knesl, O. Clinical pharmacology of nonsteroidal anti-inflammatory drugs in dogs. Vet Anaesth Analg. 2012;39:69–90.
55. Wernham, BG, Trumpatori, B, Hash, J, et al. Dose reduction of meloxicam in dogs with osteoarthritis-associated pain and impaired mobility. J Vet Intern Med. 2011;25:1298–1305.
56. Smith, HS. Potential analgesic mechanisms of acetaminophen. Pain Physician. 2009;12:269–280.
57. Mburu, DN. Evaluation of the anti-inflammatory effects of a low dose of acetaminophen following surgery in dogs. J Vet Pharmacol Ther. 1991;14:109–111.
58. Mburu, DN, Mbugua, SW, Skoglund, LA, et al. Effects of paracetamol and acetylsalicylic acid on the post-operative course after experimental orthopaedic surgery in dogs. J Vet Pharmacol Ther. 1988;11:163–170.
59. Kukanich, B, Lascelles, BD, Aman, AM, et al. The effects of inhibiting cytochrome P450 3A, p-glycoprotein, and gastric acid secretion on the oral bioavailability of methadone in dogs. J Vet Pharmacol Ther. 2005;28:461–466.
60. Kukanich, B, Lascelles, BD, Papich, MG. Pharmacokinetics of morphine and plasma concentrations of morphine-6-glucuronide following morphine administration to dogs. J Vet Pharmacol Ther. 2005;28:371–376.
61. Kukanich, B, Papich, MG. Plasma profile and pharmacokinetics of dextromethorphan after intravenous and oral administration in healthy dogs. J Vet Pharmacol Ther. 2004;27:337–341.
62. KuKanich, B. Pharmacokinetics of acetaminophen, codeine, and the codeine metabolites morphine and codeine-6-glucuronide in healthy Greyhound dogs. J Vet Pharmacol Ther. 2010;33:15–21.
63. Lascelles, BD, Robertson, SA. Use of thermal threshold response to evaluate the antinociceptive effects of butorphanol in cats. Am J Vet Res. 2004;65:1085–1089.
64. Robertson, SA, Taylor, PM, Lascelles, BD, et al. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine. Vet Rec. 2003;153:462–465.
65. Lascelles, BD, Robertson, SA, Taylor, PM, et al. Comparison of the pharmacokinetics and thermal antinociceptive pharmacodynamics of 20mcg/kg buprenorphine administered sublingually or intravenously in cats. Vet Anaesth Analg. 2003;30:109. [(Abstr)].
66. Woolf, CJ, Thompson, SWN. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation: implication for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299.
67. Julius, D, Basbaum, AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210.
68. Graven-Nielsen, T, Arendt-Nielsen, L. Peripheral and central sensitization in musculoskeletal pain disorders: an experimental approach. Curr Rheumatol Rep. 2002;4:313–321.
69. Wagner, AE, Walton, JA, Hellyer, PW, et al. Use of low doses of ketamine administered by constant rate infusion as an adjunct for postoperative analgesia in dogs. J Am Vet Med Assoc. 2002;221:72–75.
70. Slingsby, LS, Waterman-Pearson, AE. The post-operative analgesic effects of ketamine after canine ovariohysterectomy–a comparison between pre- or post-operative administration. Res Vet Sci. 2000;69:147–152.
71. Eisenberg, E, Pud, D. Can patients with chronic neuropathic pain be cured by acute administration of the NMDA receptor antagonist amantadine? Pain. 1998;74:337–339.
72. Lascelles, BD, Gaynor, JS, Smith, ES, et al. Amantadine in a multimodal analgesic regimen for alleviation of refractory osteoarthritis pain in dogs. J Vet Intern Med. 2008;22:53–59.
73. Vernier, VG, Harmon, JB, Stump, JM, et al. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol Appl Pharmacol. 1969;15:642–665.
74. Dayer, P, Desmeules, J, Collart, L. Pharmacology of tramadol. Drugs. 1997;53:18–24.
75. Oliva, P, Aurilio, C, Massimo, F, et al. The antinociceptive effect of tramadol in the formalin test is mediated by the serotonergic component. Eur J Pharmacol. 2002;445:179–185.
76. KuKanich, B, Papich, MG. Pharmacokinetics of tramadol and the metabolite O-desmethyltramadol in dogs. J Vet Pharmacol Ther. 2004;27:239–246.
77. Giorgi, M, Del Carlo, S, Saccomanni, G, et al. Pharmacokinetic and urine profile of tramadol and its major metabolites following oral immediate release capsules administration in dogs. Vet Res Commun. 2009.
78. Giorgi, M, Del Carlo, S, Saccomanni, G, et al. Pharmacokinetics of tramadol and its major metabolites following rectal and intravenous administration in dogs. N Z Vet J. 2009;57:146–152.
79. Giorgi, M, Del Carlo, S, Saccomanni, G, et al. Biopharmaceutical profile of tramadol in the dog. Vet Res Commun. 2009;33(Suppl 1):189–192.
80. Giorgi, M, Saccomanni, G, Lebkowska-Wieruszewska, B, et al. Pharmacokinetic evaluation of tramadol and its major metabolites after single oral sustained tablet administration in the dog: a pilot study. Vet J. 2009;180:253–255.
81. McMillan, CJ, Livingston, A, Clark, CR, et al. Pharmacokinetics of intravenous tramadol in dogs. Can J Vet Res. 2008;72:325–331.
82. Torring, ML, Riis, A, Christensen, S, et al. Perforated peptic ulcer and short-term mortality among tramadol users. Br J Clin Pharmacol. 2008;65:565–572.
83. Garcia-Hernandez, L, Deciga-Campos, M, Guevara-Lopez, U, et al. Co-administration of rofecoxib and tramadol results in additive or sub-additive interaction during arthritic nociception in rat. Pharmacol Biochem Behav. 2007;87:331–340.
84. Papich, MG, Bledsoe, DL. Tramadol pharmacokinetics in cats after oral administration of an immediate release tablet. J Vet Intern Med. 2007;21:616. [(abstr.)].
85. Pypendop, BH, Ilkiw, JE. Pharmacokinetics of tramadol, and its metabolite O-desmethyl-tramadol, in cats. J Vet Pharmacol Ther. 2008;31:52–59.
86. Brondani, JT, Luna, SP, Marcello, GC, et al. Perioperative administration of vedaprofen, tramadol or their combination does not interfere with platelet aggregation, bleeding time and biochemical variables in cats. J Feline Med Surg. 2009;11:503–509.
87. Pypendop, BH, Siao, KT, Ilkiw, JE. Effects of tramadol hydrochloride on the thermal threshold in cats. Am J Vet Res. 2009;70:1465–1470.
88. Radulovic, LL, Turck, D, von Hodenberg, A, et al. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1995;23:441–448.
89. Vollmer, KO, von Hodenberg, A, Kolle, EU. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung. 1986;36:830–839.
90. Platt, SR, Adams, V, Garosi, LS, et al. Treatment with gabapentin of 11 dogs with refractory idiopathic epilepsy. Vet Rec. 2006;159:881–884.
91. Wagner, AE, Mich, PM, Uhrig, SR, et al. Clinical evaluation of perioperative administration of gabapentin as an adjunct for postoperative analgesia in dogs undergoing amputation of a forelimb. J Am Vet Med Assoc. 2010;236:751–756.
92. Siao, KT, Pypendop, BH, Ilkiw, JE. Pharmacokinetics of gabapentin in cats. Am J Vet Res. 2010;71:817–821.
93. Pypendop, BH, Siao, KT, Ilkiw, JE. Thermal antinociceptive effect of orally administered gabapentin in healthy cats. Am J Vet Res. 2010;71:1027–1032.
94. Verdu, B, Decosterd, I, Buclin, T, et al. Antidepressants for the treatment of chronic pain. Drugs. 2008;68:2611–2632.
95. Kautio, AL, Haanpaa, M, Leminen, A, et al. Amitriptyline in the prevention of chemotherapy-induced neuropathic symptoms. Anticancer Res. 2009;29:2601–2606.
96. Kautio, AL, Haanpaa, M, Saarto, T, et al. Amitriptyline in the treatment of chemotherapy-induced neuropathic symptoms. J Pain Symptom Manage. 2008;35:31–39.
97. Chew, DJ, Buffington, CA, Kendall, MS, et al. Amitriptyline treatment for severe recurrent idiopathic cystitis in cats. J Am Vet Med Assoc. 1998;213:1282–1286.
98. Challapalli, V, Tremont-Lukats, IW, McNicol, ED, et al. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev. 4, 2005. [CD003345].
99. Fleming, JA, O’Connor, BD. Use of lidocaine patches for neuropathic pain in a comprehensive cancer centre. Pain Res Manag. 2009;14:381–388.
100. Ko, J, Weil, A, Maxwell, L, et al. Plasma concentrations of lidocaine in dogs following lidocaine patch application. J Am Anim Hosp Assoc. 2007;43:280–283.
101. Ko, JC, Maxwell, LK, Abbo, LA, et al. Pharmacokinetics of lidocaine following the application of 5% lidocaine patches to cats. J Vet Pharmacol Ther. 2008;31:359–367.
102. Weiland, L, Croubels, S, Baert, K, et al. Pharmacokinetics of a lidocaine patch 5% in dogs. J Vet Med A Physiol Pathol Clin Med. 2006;53:34–39.
103. Weil, AB, Ko, J, Inoue, T. The use of lidocaine patches. Compend Contin Educ Vet. 2007;29:208–210. [212, 214–206].
104. Jimenez Andrade, JM, Mantyh, P. Cancer pain: From the development of mouse models to human clinical trials. In: Kruger L, Light AR, eds. Translational pain research: from mouse to man. Boca Raton, FL: CRC Press, 2010.
105. Jimenez-Andrade, JM, Mantyh, WG, Bloom, AP, et al. Bone cancer pain. Ann N Y Acad Sci. 2010;1198:173–181.
106. Sabino, MA, Mantyh, PW. Pathophysiology of bone cancer pain. J Support Oncol. 2005;3:15–24.
107. Milner, RJ, Farese, J, Henry, CJ, et al. Bisphosphonates and cancer. J Vet Intern Med. 2004;18:597–604.
108. Fan, TM. Intravenous aminobisphosphonates for managing complications of malignant osteolysis in companion animals. Top Companion Anim Med. 2009;24:151–156.
109. Ashton, JA, Farese, JP, Milner, RJ, et al. Investigation of the effect of pamidronate disodium on the in vitro viability of osteosarcoma cells from dogs. Am J Vet Res. 2005;66:885–891.
110. Farese, JP, Ashton, J, Milner, R, et al. The effect of the bisphosphonate alendronate on viability of canine osteosarcoma cells in vitro. In Vitro Cell Dev Biol Anim. 2004;40:113–117.
111. Fan, TM, de Lorimier, LP, Garrett, LD, et al. The bone biologic effects of zoledronate in healthy dogs and dogs with malignant osteolysis. J Vet Intern Med. 2008;22:380–387.
112. de Lorimier, LP, Fan, TM. Bone metabolic effects of single-dose zoledronate in healthy dogs. J Vet Intern Med. 2005;19:924–927.
113. Tomlin, JL, Sturgeon, C, Pead, MJ, et al. Use of the bisphosphonate drug alendronate for palliative management of osteosarcoma in two dogs. Vet Rec. 2000;147:129–132.
114. Wiley, RG, Lappi, DA. Targeted toxins in pain. Adv Drug Deliv Rev. 2003;55:1043–1054.
115. Wiley, RG. Substance P receptor-expressing dorsal horn neurons: lessons from the targeted cytotoxin, substance P-saporin. Pain. 2008;136:7–10.
116. Wiley, RG, RH, Kline, Vierck, CJ, Jr. Anti-nociceptive effects of selectively destroying substance P receptor-expressing dorsal horn neurons using [Sar9,Met(O2)11]-substance P-saporin: behavioral and anatomical analyses. Neuroscience. 2007;146:1333–1345.
117. Allen, JW, Horais, KA, Tozier, NA, et al. Intrathecal substance P-Saporin selectively lesions NK-1 receptor bearing neurons in dogs. J Pain. 2002;3(suppl 1):51.
118. Ramirez, O, 3rd., Dodge, RK, Page, RL, et al. Palliative radiotherapy of appendicular osteosarcoma in 95 dogs. Vet Radiol Ultrasound. 1999;40:517–522.
119. Milner, RJ, Dormehl, I, Louw, WK, et al. Targeted radiotherapy with Sm-153-EDTMP in nine cases of canine primary bone tumours. J S Afr Vet Assoc. 1998;69:12–17.
120. Culp, LB, Skarda, RT, Muir, WW, 3rd. Comparisons of the effects of acupuncture, electroacupuncture, and transcutaneous cranial electrical stimulation on the minimum alveolar concentration of isoflurane in dogs. Am J Vet Res. 2005;66:1364–1370.
121. Zhang, RX, Li, A, Liu, B, et al. Electroacupuncture attenuates bone-cancer-induced hyperalgesia and inhibits spinal preprodynorphin expression in a rat model. Eur J Pain. 2008;12:870–878.
122. Zhang, RX, Li, A, Liu, B, et al. Electroacupuncture attenuates bone cancer pain and inhibits spinal interleukin-1 beta expression in a rat model. Anesth Analg. 2007;105:1482–1488. [table of contents].
123. Mantyh, PW. Neurobiology of substance P and the NK1 receptor. J Clin Psychiatry. 2002;63(Suppl 11):6–10.
124. Honore, P, Menning, PM, Rogers, SD, et al. Neurochemical plasticity in persistent inflammatory pain. Prog Brain Res. 2000;129:357–363.
 Section B
Section B
Nutritional Management of the Cancer Patient
Over the past 75 years the examination of nutrients and their relationship to cancer control and cancer prevention has led to a better understanding of how nutrition may play a role in the management of neoplastic disease. The paucity of well-controlled studies in companion animals and the extrapolation of data derived from studies conducted in humans investigating tumor types uncommon to companion animals (colon, prostate, pancreas) are frustrating and make general recommendations for nutritional intervention challenging. However, owners often wish to alter their pet’s feeding regimen regardless of proven efficacy. In this context, three areas of nutrition are often discussed with clients, including modification in tumor metabolism, adjustment of nutritional risk factors that may impact outcomes, and nutritional intervention during therapy, all of which will be addressed in this section.
Metabolism of Cancer: Substrate Utilization
Numerous neoplastic cell lines have been successfully propagated in culture allowing examination of cell biology. A fundamental observation is that the majority of neoplastic cells propagate better in a high glucose media, which is likely the result of limited fatty acid metabolism coupled with increases in metabolic pathways that utilize glucose. This has traditionally been termed the Warburg effect after Otto Warburg’s seminal work suggesting that glycolysis is the primary pathway for energy production in neoplastic cells.1,2 Studies in humans have shown that certain cancer patients liberate excessive lactate from solid tumors,3,4 providing evidence that glycolysis and pyruvate production are critical to neoplastic cell metabolism. This has led to the Cori cycle hypothesis of neoplasia, whereby neoplastic tissues, much like skeletal muscle tissue, appear to undergo regeneration of glucose from lactate through hepatic resynthesis of glucose.5 Unfortunately, this regeneration of glucose is an energy costly cycle and is thought to contribute to increases in resting energy requirements.
In veterinary medicine, there is a significant body of work examining metabolism and cancer, often through the application of indirect calorimetry assessments to study whole body metabolism. These studies have interrogated oxygen consumption and carbon dioxide liberation and such data provide an estimate of energy consumption (resting energy expenditure [REE]) and substrate utilization (respiratory quotient [RQ]). Healthy dogs display higher REE than dogs with stage III and IV lymphoma.6 RQ values between the groups were not different, suggesting that all dogs were using similar substrate, and dogs with lymphoma were not preferentially consuming more glucose than their control counterparts.6 In a separate study, dogs with lymphoma fed either a high-fat or high-carbohydrate diet during doxorubicin chemotherapy did not differ in remission times, survival times, or tumor burden, suggesting that dogs with lymphoma under treatment were not sensitive to this basic dietary alteration.7 During this study the REE and RQ assessed during treatment did not change significantly when the tumor burden was eliminated with chemotherapy suggesting no significant changes in energy expenditure or metabolism. These data collectively indicate that there is no fundamental difference observed between normal healthy dogs and dogs with lymphoma and that removal of the tumor burden does not alter the resting energy requirement.
Canine nonhematopoietic malignancies were also examined before and 4 to 6 weeks after excision of their primary tumors, including mammary carcinoma, OSA, high-grade mast cell tumors, and lung carcinoma. As in dogs with lymphoma, REE was not different from control dogs nor was there any difference in REE between preexcision and postexcision of the primary tumor, suggesting no futile cycling of energy in these patients.8 Interestingly, in this study the RQ values were above 0.8 for all control and tumor-bearing dogs, suggesting that the resting energy was not from lipolysis, which was contradictory to a follow-up study performed in dogs with OSA. In that follow-up study, there was an increase observed in the REE in dogs with OSA compared to control dogs but a RQ of 0.7 in both affected and control dogs.9 This increased REE persisted after excision of the primary lesion, suggesting that the modest increase in REE was due to factors other than the primary neoplasia and may be associated with micrometastasis, inflammation associated with neoplastic disease, or heightened pain response associated with the primary tumor and surgical procedure.9 These findings were surprising in light of the previous studies mentioned but were likely more valid considering that the REE calculations in the OSA study were based on lean mass rather than total kilograms of body weight.9 It is well known that fat mass is relatively metabolically inert; therefore REE based on lean body mass is more appropriate. The previous studies in nonhematopoietic malignancies and lymphoma did not adjust for body condition or lean body mass in tumor-bearing or normal populations under study,7,8 and the inability to document differences in REE noted in these two studies may have been confounded by a lack of body condition assessment.
Specific metabolic changes have been reported in dogs with several types of cancer. Alterations in glucose metabolism (potentially higher glucose turnover), increased protein turnover, and urinary protein losses in dogs with OSA have been observed.9 In addition, studies in dogs with lymphoma identified alterations in carbohydrate metabolism such as increased serum lactate and insulin concentrations during glucose tolerance testing that suggest insulin resistance.6,10,11 This may be partially explained by aberrant interleukin-6 (IL-6) and other cytokine influences on glucose metabolism resulting in insulin resistance in dogs with lymphoma.12 Insulin insensitivity and serum lactate did not change once remission was achieved in one of these studies.6 Mild alterations in lipid metabolism in dogs with lymphoma were suggested by higher basal triglyceride and cholesterol concentrations compared to control dogs,13 and treatment with doxorubicin lowered serum cholesterol, perhaps due to hepatic effects of chemotherapy.13 However, the dyslipidemia present was not ameliorated once the primary tumor burden was eliminated, which is logical in light of the insulin resistance observed.
Cancer Anorexia and Cachexia
Anorexia or hyporexia (inadequate food intake) in cancer patients is a common occurrence and can be one of the presenting clinical signs of cancer. Inadequate appetite is often due to an enhanced inflammatory cytokine response (i.e., IL-6 and IL-1) associated with the tumor, resulting in hypothalamic arcuate nucleus suppression of appetite. In the case of gastrointestinal cancer, pain associated with eating, obstruction, or dysfunctional transit mechanisms can lead to anorexia. In patients undergoing treatment, anorexia may be partially explained by adverse events associated with the use of chemotherapy. Chemotherapeutics can cause a variety of alterations in olfactory and taste sensorium. Since dogs and cats rely heavily on olfactory cues, the loss of olfactory bulb stimulus diminishes palatability of foods.13,14 Additionally, the loss or alteration of taste (ageusia or dysgeusia) can further complicate anorexia and may last for several months before neuronal regeneration can take place at the olfactory bulb and tongue.13,14
Cachexia, on the other hand, although identified in many human cancer patients, does not appear to be common in dogs with nonhematopoietic malignancies other than a predisposition toward insulin resistance.6,10,15,16 Evidence in humans and mouse models suggests that the most prominent influence inciting the cachectic phenomenon may be excessive cytokine stimulation, which leads to insulin resistance, extensive lipolysis, and proteolysis of tissue stores.17,18 The three primary cytokines thought to be involved in promoting enhanced proteolysis are TNF-α, IL-1β, and IL-6.17,18 TNF-α and IL-1β have both been directly associated with anorexia and upregulation of mitochondrial uncoupling protein, whereas IL-6 and TNF-α have been observed to increase myofibrillar degradation machinery, all of which may play a role in the anorexia/cachexia syndrome associated with neoplasia.19,20 IL-6 and c-reactive protein, both markers of inflammation, have been observed to increase in canine lymphoma patients.12,21,22 Yet, it does not appear that cachexia is a common occurrence in dogs diagnosed with neoplasia since dogs examined 6 months prior to diagnosis of cancer showed no difference in body weight or body condition than at presentation for various neoplasms.15 This may be partially explained by differences in common tumor types between species. Cachexia in humans is often associated with epithelial cancers such as pancreatic, colon, mammary, and prostate cancer. Additionally, human patients undergo dramatically different and more aggressive treatment protocols over lengthy time periods, which we typically do not encounter in veterinary medicine due to owner financial constraints and QOL considerations.
Cats on the other hand may show a more typical cachectic response involving excessive lean body mass wasting. Recent evidence suggests that approximately 56% of cats with lymphoma and other solid tumors have body condition scores of less than 5 out of 9.23 More intriguing is that the survival time for cats with lymphoma with a body condition score of 5 or greater was 16.9 months, compared to those with lower scores (3.3 months).23 This warrants monitoring of caloric intake and aggressive implementation of nutritional interventions in feline cancer patients.
Epidemiology, Prevention, and Risk Factors
In humans, the two major nutritional factors associated with risk of developing cancer are excess body weight (obesity) and low fruit and vegetable consumption.24-27 Although these parameters may be interrelated, both appear to play a direct role in the risk of cancer development. Convincing data suggest that the westernized diet and lack of fruit and vegetable matter are linked to increased relative risk of nearly all types of neoplasia, including prostate, colon, breast, lymphoma, and leukemia.28-30 Although the evidence is compelling, the data do not support a causal association, and it may be a combination of factors associated with diet, as well as confounding lifestyle differences in humans consuming higher amounts of fruits and vegetables, that may be important in deterring cancer.
In veterinary medicine, there are few studies examining the effects of dietary substrate (protein, fat, carbohydrate) and plant-based dietary intake and cancer incidence. Two epidemiologic studies using validated food frequency questionnaires examined the effect on survival of calories coming from fat, protein, and carbohydrate for 1 year prior to and after diagnosis of mammary carcinoma.31,32 These data were contradictory to human findings, showing that dogs with increased protein intake had increased survival times and that fat and carbohydrate intake did not play a role in progression of disease.31,32 Another study suggested that risk of neoplasia was increased in dogs receiving nontraditional, poorly balanced diets (i.e., table foods as primary consumption).33 Further examination of this group showed no association between blood selenium concentration and mammary carcinoma when compared to healthy age-matched and hospitalized control dogs. Serum retinol (vitamin A) values were also decreased in dogs with mammary carcinoma in this study.33 Whether the lower serum retinol was a reflection of poor retinol intake or a reflection of the disease manifestation was not determined. A diet high in red meat was also identified as a risk factor in this study. In many cases, a high-protein food may be evaluated as higher quality because protein is an expensive ingredient; however, many variables in addition to ingredients are important. Ingredient quality, digestibility, and socioeconomic factors such as veterinary care should be considered. It does suggest that feeding an incomplete diet is associated with increased cancer risk and provides justification for a complete and balanced diet (Association of American Feed Control Officials [AAFCO]-approved commercial dog food) throughout the life of a dog.
Nutritional risk factors examined in Scottish terriers, who have a genetic predisposition to develop transitional cell carcinoma (TCC), showed that the addition of vegetables to a dog’s diet resulted in a lower incidence of the disease.34 However, there are confounding lifestyle factors that cannot be accounted for in this epidemiologic investigation, including better health care, variation in nutrition supplied as commercial food, and other associated environmental exposures. Yet, this study is provocative and suggests further study is warranted.
Specific vitamins and their relationship to cancer development have received significant attention in humans, including retinol, ascorbic acid, vitamin E, selenium, and vitamin D. In veterinary medicine, these nutrients have not been thoroughly studied in relation to cancer risk. Human metaanalyses examining oral supplementation for single nutrients such as ascorbate, selenium, and vitamin E have been inconclusive or negative when examining protective, anticarcinogenic effects.35-37 Other nutrients like β-carotene, the precursor to retinol, is currently thought to be ineffective as a chemopreventive agent and in some instances has proved to be harmful in certain populations (i.e., smoking populations).38-40 Currently, vitamin D (25-hydroxyvitamin [OH] D3) status and supplementation has been an area of vigorous epidemiologic investigation due to an increased relative risk of various neoplastic diseases associated with low serum vitamin D status.41-43 In dogs and cats, unlike humans, vitamin D status is directly dependent on dietary intake since they cannot convert 7-OH-dehydrocholesterol to pre-vitamin D. One would expect that serum vitamin D concentrations would not fluctuate tremendously in dogs being fed commercial dog food.44 A recent investigation suggests that Labrador retrievers with mast cell disease have lower serum vitamin D status than healthy Labrador retrievers, making vitamin D status an interesting area of future investigation.45 Whether this is a direct reflection of dietary intake or a reflection of the biochemical disposition in affected dogs with cancer remains to be determined.
In humans, obesity has been associated with an increased risk of many cancers, including breast, prostate, colon, leukemia, lymphoma, and pancreatic neoplasia.24-27 In companion species, studies examining this association are limited. The largest retrospective study in dogs showed no association between body condition and neoplastic disease,15 whereas other epidemiologic studies suggested that obese cats and dogs may have a slightly higher rate of neoplastic disease.46,47 Two other investigations revealed a more definitive link between obesity in female dogs and the onset of mammary carcinoma.31,33 The risk of mammary carcinoma was greater in obese spayed dogs in one study, although obesity was an increased risk factor but independent of spay status in another.31,33 Both studies suggest an increased risk when obesity is present at 1 year of age, and one suggested that obesity at 1 year before diagnosis also increased risk.47 The question of whether early onset obesity, much like early spaying, epigenetically predisposes mammary glands to an altered risk of cancer remains to be addressed.
Implementing a Nutritional Plan for the Oncology Patient: Nutritional Assessment
To fully assess the cancer patient, information regarding body weight, body condition score, and diet history are crucial. Dietary history, before and during treatment, should be obtained to appropriately assess the kilocalorie intake. This information will allow the practitioner to feed the patient appropriately during hospitalization and, more importantly, to recognize hypophagic behaviors, allowing for proactive interventions. A typical diet history should include the forms of food (wet or dry), amounts fed daily, as well as treats, human table foods, and additional supplements provided. A food diary has been helpful for humans attempting to understand energy and nutrient intake and is likely to also be helpful for dogs in particular as a wide variety of food stuffs are offered to dogs.
Serial assessment of body weight is important, particularly where malnutrition is a consideration. Malnutrition is often associated with cachexia and/or anorexia. Anorexic behavior can be deduced from the diet history and can be treated aggressively with nutritional and/or pharmacologic intervention; however, if cachexia is suspected, then alternative treatments can be sought. The difficulty in clinically differentiating cachexia from anorexia is our inability to measure loss of lean versus fat mass. The loss of fat mass is typical during anorexia, and equal loss of lean and fat mass suggests cachexia. Although this cannot be deciphered efficiently in veterinary practice, overall weight loss guidelines have been offered in the human literature whereby body weight loss of 5% in 1 month or 10% in 3 months without conscientious dieting suggests cancer cachexia.48 Two body condition scoring (BCS) systems have been adopted as a means of nutritional assessment in companion species; however, the 1 to 9 BCS system (Figure 15-2) has been more thoroughly validated in the literature.49,50 Modest differences in BCS between dogs and cats exist due to preferential deposition of body fat along the inguinal and abdominal areas in cats, whereas dogs tend to have no preferential deposition. These differences may justify a muscle condition scoring system in cats (Table 15-6).23
Table 15-6
Description of the Muscle Mass Scoring System (MMS)
| score | muscle Mass |
| 0 | On palpation over the spine, muscle mass is severely wasted. |
| 1 | On palpation over the spine, mass is moderately wasted. |
| 2 | On palpation over the spine, muscle mass is mildly wasted. |
| 3 | On palpation over the spine, muscle mass is normal. |

Figure 15-2 Body condition scoring (BCS) scale (1-9) for a cat. A similar BCS scale exists for dogs. (Reprinted with permission of Nestle Purina PetCare, St. Louis.)
The final component of nutritional assessment consists of a routine physical examination, complete blood cell count, and serum chemistry evaluation. Physical examination findings consistent with malnutrition include poor hair coat, chronic gastrointestinal disturbance, seborrhea, lethargy, and pallor. The first signs of chronic nutrient deficiency are often manifested in areas of rapid cellular turnover leading to skin, gastrointestinal, and hematologic signs and should be considered in cases of prolonged anorexia. Chronic malnutrition can result in low hemoglobin and red blood cell counts, as well as hypoproteinemia and hypoalbuminemia. Additionally, with the trend toward nontraditional feeding practices, there is the potential for diets that lack sufficient mineral content (e.g., calcium, iron, and copper) resulting in bone and hematologic manifestations. Many homemade diets lacking supplementation with bone meal can lead to secondary hyperparathyroidism and clinical osteopenia.51,52 Clients using nontraditional diets should be educated through consultation with a veterinary nutritionist.
In dogs, excess body condition (i.e., obesity) may be more of a concern than malnutrition or deficiency. Treatment of obesity is not a priority in many cancer patients, considering metabolic changes that may occur during chemotherapy and the potential for treatment-related eating pattern changes. One study suggests that body condition does not change from 6 months prior to diagnosis to the day of diagnosis,15 whereas another study suggests that over 68% of dogs lose weight during treatment53; however, loss was less than 5% of body weight and nearly 30% of dogs were scored as obese prior to treatment. The occurrence of obesity in dogs with neoplasia appears to follow the national trends in canine obesity.
Feeding the Hospitalized Oncology Patient
Hospitalization of debilitated cancer patients undergoing evaluation or therapy has unique nutritional opportunities and challenges. Hospitalization during some fractionated radiation therapy is common, and the provision of more than the resting energy requirement (RER) during this period is often unnecessary unless extreme circumstances exist where extensive tissue repair is ongoing (e.g., postoperatively, epithelial mucositis). This increased energy requirement is known as the illness energy requirement (IER) and is often considered to be between 1.1 to 2 times the RER, particularly when transudates or exudates are involved with the repair process and protein losses are excessive. Energy requirements postdischarge may also be increased above hospitalization RER due to increased activity and neuter status, which is represented as the maintenance energy requirement (MER). Table 15-7 presents starting calculations for MER based on the linear equation used in veterinary patients taking into account the activity status. Exponential equations are preferred in dogs and cats under 2 kg or over 30 kg to derive a more accurate estimate of the RER:
Table 15-7
Maintenance Energy Requirement Equations for Adult Cats and Dogs
| Animal | MER Equation |
| Neutered adult dog | (70 + 30 [BWkg]) × 1.6 |
| Intact adult dog | (70 + 30 [BWkg]) × 1.8 |
| Obesity-prone adult dog | (70 + 30 [BWkg]) × 1.2 to 1.4 |
| Neutered adult cat | (70 + 30 [BWkg]) × 1.2 to 1.4 |
| Intact adult cat | (70 + 30 [BWkg]) × 1.4 to 1.6 |
| Inactive obesity-prone adult cat | (70 + 30 [BWkg]) × 1.0 |
Evidence suggests that the exponent of the equation may be different for cats (e.g., 0.67).54 Once a patient returns home, its RER typically increases slightly due to increased activity. Therefore clinicians should adjust the energy intake on discharge.
Coax Feeding and Pharmacologic Appetite Stimulation
Ensuring full energy requirement intake enterally may be difficult due to a diminished appetite in canine and feline cancer patients. There are many considerations when trying to promote adequate intake, and these may be different in dogs and cats. Hand-feeding in dogs and cats that enjoy this approach should be considered rather than putting a bowl in the cage and leaving it there. Hand-feeding may be best achieved during owner visits when the animal is most comfortable and often away from the busy atmosphere of most intensive care units or oncology wards.55 For cats, having a quiet place away from distractions that create a fearful environment may be helpful to achieve adequate intake. Making one cage an eating cage that is covered and away from the litter box is ideal as some cats will not eat near the litter box during hospitalization.55
Addition of flavorings may also be helpful. Dogs have salt and sweet receptors, and the addition of sugar, syrups, or other sweeteners can sometimes improve appetite.55 Cats do not have the lingual receptors to appreciate sweet flavors. Therefore salt can be used to entice cats to eat; however, they tend to be more averse to oversalted foods.55 Additional protein added to the diet of both dogs and cats can improve appetite and enhance intake since dogs appear to prefer higher protein diets and cats have increased density of lingual amino acid receptors, making high protein choices logical.55 The supplementation of animal-based or vegetable-based fat may increase palatability but must be monitored as additional fat can dilute the nutrient content of the feed. In the presence of nausea, introducing multiple foods can create long-term aversions limiting choices of form and texture once the nausea has resolved.55 Using one or two foods to coax feed is ideal, rather than an entire array of products from the kitchen.
Pharmacologic approaches to improve enteral support may be attempted; however, many of the purported effects are anecdotal with little evidence of their true utility in veterinary medicine. Human studies suggest several approaches, including pharmacologic alterations in serotonergic stimulation in the brain, decreased cytokine stimulation, and the promotion of hypothalamic satiety center signaling.56,57 Approaches in veterinary medicine have focused on the use of the antiserotonergics (e.g., cyproheptadine and mirtazapine). Unfortunately, no definitive clinical studies documenting the efficacy of these drugs in dogs and cats with cancer exist.55 Of some interest is the use of valium to stimulate appetite in cats and the recommended dosing of 1 mg by mouth (PO) daily has proved useful.58 However, the use of valium does not come without the potential for severe side effects (e.g., hepatic necrosis), making long-term use of valium ill advised.59 In dogs, low-dose propofol is used to briefly stimulate eating behavior to test the integrity of the bowel (i.e., vomiting) when unsure about enteral functional status, although more prolonged use of propofol is not attempted.60
Assisted Enteral Support
In many instances, the use of assisted enteral nutrition should be considered, particularly if the animal is not consuming appropriate kilocalorie requirements. In the hypophagic cancer patient, it may be essential to provide assisted feeding through various techniques, including syringe, nasogastric, esophagostomy, or gastrostomy feeding. Syringe feeding is the easiest and requires the least attention to detail by owners and clinicians. In the nauseous and anosmic patient, this can be difficult to implement due to patient resistance. Nasogastric tubes can be easily placed without anesthesia and can be useful in hospitalized animals but are often problematic to manage at home and are limited to the use of liquid enteral products due to tube diameter. The two most widely accepted means of implementing long-term enteral support is the placement of an esophagostomy or gastrostomy tube. The esophagostomy tube is typically placed under anesthesia; techniques for placement have been described elsewhere.61 Once secured, the tube site is typically wrapped, and the insertion site should be examined every 24 to 48 hours for signs of cellulitis and discharge. These tubes are typically recommended for intermediate to long-term feeding for a period of 2 to 3 weeks up to 2 to 3 months.
Gastrostomy tube placement should be considered when tube placement is required for longer than 6 to 8 weeks.61,62 Advantages include direct gastric delivery of nutrients and prevention of tube eversion if emesis is encountered. Anesthesia is required for either surgical or percutaneous endoscopic placement approaches. The endoscopic approach is generally safe and effective, although associated with a higher risk of complications.62 The author prefers surgical placement in large breed dogs because they may be predisposed to separation of the stomach from the body wall after endoscopic placement. Peritonitis is the most serious complication following tube placement since the peritoneum is disturbed with this approach and leads to a permanent stoma from the stomach to the outside of the body.61,62 After successful gastrostomy tube placement, the tube can be used within 24 hours of placement but should not be removed before 2 weeks, allowing for adhesion and fibrosis of the gastric wall to the abdominal wall. Once a stoma has formed, the original surgically placed tube may be replaced with a low profile or “button” feeding device. Owners should be aware that these low profile devices need replacing every 6 to 8 months and will require mild sedation for replacement.63
Esophagostomy and gastrostomy tubes allow for a diverse number of products to be used for feeding beyond the liquid veterinary diets. Many over-the-counter and veterinary therapeutic diets can be blended for feeding; however, when some products are blended with water, they result in less than 1 kcal/mL. Diets that provide higher caloric density that can be passed through a 7 French or greater diameter catheter are listed in Table 15-8. These products tend to be higher in protein and fat and can be fed at reduced volumes and rates when nausea or food volume is an issue. A typical dog or cat receiving a slurry of food at 1 kcal/mL will also be meeting their fluid requirements.64 Similarly, providing 1 kcal/mL for dogs and cats that are not actively consuming water at home is also advised.
Table 15-8
Selected High Protein/High Calorie Products for Tube Feeding Cancer Patients and the Amount of Water Needed to Make a 1 kcal/mL Mixture to Meet Daily Fluid Requirements
*Dry powdered products—50 cc of water is required for preparation with thorough mixing before administering.
Parenteral Support
If enteral support is not an option, then parenteral nutrition (PN) should be considered. Parenteral support can be either partial PN (PPN) or total PN (TPN). PPN has also been termed peripheral parenteral nutrition considering it is typically delivered through peripheral veins. Prospective studies examining outcome following parenteral support have not been performed in clinical veterinary medicine and only a handful of retrospective investigations characterize complication rates.65-70 In veterinary patients, particularly cats, the metabolic complication most often encountered is hyperglycemia. Mechanical complications are also prevalent (i.e., feeding line problems, inadvertent patient removal). A common misconception is that sepsis is a common complication, but in fact it is quite rare.65-70 Parenteral support should only be considered when enteral support is not an option due to medical complications; enteral support is considered superior as it prevents transmigration of bacteria to the portal blood and improves the immunologic status of patients.71
Parenteral support is not well studied in veterinary medicine, and the relative use and utility of the three main substrates (glucose, amino acid, and lipid) differ, depending on the source of information.72-74 Some advocate using glucose and lipid to meet the energy requirements and then add in amino acids to the formulation based on the protein needs per kilogram of body weight. Others advocate adding just above the minimal protein requirement as amino acids making up part of the REE. The protein requirements for ill cats and dogs are currently unknown, and we can only assume the requirements are similar to those of healthy normal animals. Extrapolation from human data suggests that protein turnover may be higher during catabolic illness, and we often add slightly more protein than required. In an elegantly designed study, it was found that approximately 2.3 g protein/kg body weight is sufficient for IV amino acid solution in normal dogs.75 This suggests that adding 2.5 to 3 g/kg amino acid solution for a dog appears sufficient and 4 g/kg is often used as a starting point for cats. Amino acids come in several different formulations and strengths (e.g., 5.5%, 8.5%, and 10%). Additionally, amino acid solutions come with and without electrolytes. Amino acids with electrolytes typically provide basal sodium, chloride, magnesium, phosphorus, and potassium when used at 1.5 to 2.5 g/kg body weight of protein; however, these are used less often in veterinary species, particularly in cats whose protein requirements are higher. When using amino acids with electrolytes, the electrolytes provided should be considered before supplementing additional electrolytes in fluids. Figure 15-3 describes a typical TPN feeding program for a cat or dog using a 10% amino acid solution without electrolytes. During the first day of TPN, it is recommended to provide only half of the calorie requirement, particularly if there has been a history of anorexia. This recommendation is due to the potential for refeeding syndrome, whereby rapid glucose metabolism can lead to hypophosphatemia, hypokalemia, and hypomagnesemia. This also illustrates the need to assess electrolyte status every 12 to 24 hours for the first 48 to 72 hours when implementing TPN.
PN formulation should be done in a laminar flow hood with appropriate aseptic procedures to prevent contamination of solutions. A sterile catheter should be used, and PN should be administered through its own port in a multilumen catheter with the most distal port reserved for PN. Avoid the addition of other medications or treatments because some medications are not compatible with PN. The typical osmolality and pH of a TPN solution will be far different than plasma osmolality (around 1000 to 1300 mOsmol and pH less than 7). This may be irritating to the vascular endothelium and requires a large vessel for administration.74 Such high osmolar solutions cannot be used in a peripheral vein as they may induce thrombophlebitis, and this is the reason that 5% glucose is used to dilute PPN rather than the 50% glucose solution used in TPN solutions.66,72 Using 5% dextrose creates an osmolality of less than 700 mOsmol, which is a guideline from human medicine that has been adopted by many veterinary nutritionists and internists.76 Figure 15-4 describes guidelines for PPN formulation for dogs and cats.66,72 Addition of B-complex vitamins should also be considered when using TPN and PPN. Most preparations do not include folate, and cobalamin may be insufficient or absent and should be considered separately if long-term support is required Furthermore, if chronic use of TPN is required, the addition of calcium to separate fluids and the use of amino acids with electrolytes and trace mineral additions to the TPN should be considered.
The proportions of glucose and lipids in parenteral solutions is a subject of much debate, particularly in the cancer patient, because of suggestions that neoplastic tissues utilize glucose more readily and the possibility of mild insulin resistance.10,11 However, increasing lipid content to meet energy requirements has also been met with some trepidation due to lipid’s potential to mildly suppress the immune system.77 Lipid has also been incriminated as the cause of microemboli,78 and some suggest placing a 1.5 to 2 µm filter in the line to remove lipid particles and microbes; however, recent evidence suggests that in a typical veterinary-formulated TPN solution the lipid particles remain well emulsified—no bacterial growth was evident for 3 days following formulation when kept refrigerated.79 PPN, with its lower osmolality, is at an increased risk of sequestering microbial growth.
Nutritional Support in the Cancer-Bearing Patient
Based on our present understanding, the use of specific dietary regimens in cancer patients is premature. It has been hypothesized that due to the glycolytic nature of neoplastic cell growth, altering the substrates to hypothetically “starve the tumor” by eliminating some carbohydrates may be indicated.1,2,10,11 This argument falls short for a number of reasons. If carbohydrates are limited, energy sources are replaced with additional fat and/or protein. Added protein will lead to increased transaminase and deaminase activity causing conversion of the protein to glucose and carbon precursors for glucose or fatty acid synthesis, and serum glucose and delivery of glucose to the tumor tissue may still remain constant. If appetite is diminished, choosing a higher protein and higher fat food may enhance palatability and caloric density, making these foods appropriate for long-term management during treatment.55 Previous sections have discussed the discordance of results of studies investigating low-carbohydrate, high-fat, and modified-fat diets.7 One study documented slight increases in remission and survival times when a high polyunsaturated fat diet (high ω-3 fatty acids) and arginine diet was used, suggesting that the type of fats and amino acids may influence remission and survival (see subsequent discussion).12
Cats appear more prone to weight loss during hospitalization. Many cats receive inadequate caloric intake, particularly during radiation treatment when food availability is limited each day due to repeated anesthesia. Many cats will eat 12 to 20 small meals throughout the day and night based on observed feeding patterns.80 The use of higher protein may be worthwhile because recent rodent data show that a high-protein and low-carbohydrate diet decreases tumor growth in a variety of different xenografted tumors.81 In this diet study, calories were met with approximately 50% protein, implying that high protein may be the benefit, rather than low carbohydrate.81 The use of high-protein diets may also have benefits in cats with lean body mass wasting issues.82,83 Although these were small studies, it suggests that in cats, skeletal muscle may respond to higher protein by increasing lean mass to a small extent. With this in mind we often recommend feeding cats higher protein (>35% dry matter) and fat (>20% dry matter). Dogs can be fed similarly, even though many commercial dog foods will have lower protein (typically >30% dry matter is achievable) than cat food.
Amino Acids
The benefits of additional protein to the diet of cancer patients may result from increased circulating amino acids as inhibitory molecules in neoplastic cell proliferation.81 Arginine has received considerable attention since low-millimolar concentrations of arginine can inhibit various neoplastic cell lines by altering cell cycle progression.84-87 Additional xenograft data using human and mouse cell lines show diminished tumor growth when mice are provided foods with high dietary arginine, and a single study in dogs with lymphoma, using an arginine-enhanced diet in combination with enhanced ω-3 fatty acids, documented improved remission and survival times.12 However, the practicality of using an amino acid supplement like arginine leaves much to be desired since the required dose is in excess of 100 mg/kg body weight. Additionally, the bitter taste of arginine and potential for creating amino acid imbalance also prevents its use in long-term feeding regimens. The benefits of glutamine have also been touted due to lean body mass preservation properties and its ability to enhance mucosal barrier function.88,89 However, enterocytes’ ability to utilize glutamine and first-pass hepatic metabolism do not allow glutamine to have any pronounced effects on lean mass. The use of high-protein mixed meals to support enterocyte health and mucosal barrier function is often recommended for general health.
Polyunsaturated Fats
Using fat in diets is helpful in increasing palatability and energy density, but in many instances the fatty acid constituents can influence neoplastic cell growth. Human and rodent studies suggest that consumption of high concentrations of ω-3 fatty acids, namely eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in the form of marine oils may perturb loss of lean body mass and possibly decrease tumor growth rate.90-97 These fatty acids may transform into inert eicosanoids (PGE3, leukotriene B5 [LTB5], 12-hydroxyeicosapentaenoic acid [12-HEPE], 5-HEPE) rather than proinflammatory eicosanoids (PGE2, LTB4, 12-hydroxyeicosatetraenoic acid [12-HETE], and 5-HETE). The pathways and eicosanoids liberated are highly dependent on the enzymatic machinery present in the cells. Although the addition of fatty acids into the cell membrane may affect intracellular signaling events, the intracellular enzymatic machinery that modifies the primary fatty acid into promitogenic or inert eicosanoids may be more important.96-99 Cell signaling events that lead to liberation of arachidonic acid from the cell membrane can be converted to eicosanoids, which when released from the cell can have local or paracrine effects on cell growth through interactions with eicosanoid receptors (Figure 15-5). The two enzymes that have received the most attention are COX and 5-lipoxygenase (5-LOX) due to the promitogenic mechanisms of action observed by their respective eicosanoids, PGE2 (COX) and 5-oxo-ETE/LTB4 (5-LOX).96-99 Although possibly relevant to many types of human cancers, there is a paucity of data in companion animals, with the most intriguing studies documenting the use of COX inhibition in TCC.100,101
Figure 15-5 The liberation of arachidonic acid (AA) from the cell membrane due to cell signaling events leading to nuclear translocation of AA. Cyclooxygenase and lipoxygenase activity coupled with leukotriene synthase or prostaglandin synthase will allow for formation of bioactive eicosanoids that when released can have autocrine or paracrine cell proliferation signaling activities depending on receptor presence. PLA2, Phospholipase A2; LTB4, leukotriene B4; PGE2, prostaglandin E2; 5-LOX, 5-lipoxygenase; COX, cyclooxygenase.
A single study in cancer-bearing dogs using a fish-based ω-3 fatty acid–enhanced diet showed a small improvement in survival times; however, there were multiple changes in the dietary trial, including arginine and energy substrate differences that may have played a role.12 Although the biochemical principle that increased EPA in cells will inhibit promitogenic eicosanoid formation as an autocrine/paracrine signaling molecule is valid,99 it is unclear what tumor types this may apply to. Some neoplastic tissues will utilize the proinflammatory cytokine milieu to promote proliferation or upregulate pathways that may promote metastasis.102 The benefits of fish oils may go beyond mild suppression of tumor cell proliferation because the antiinflammatory effects of fish oil may also quench the inflammatory reactions associated with certain cancers.103-105 Thus there is little downside to increasing ω-3 fatty acid consumption in the diet of cancer patients. The lack of clinical studies in this area precludes an optimal dosing regimen, and recent metaanalysis of human trials using fish oils for QOL issues were inconclusive.106 Additionally, cats seem to be more sensitive to fish oil supplementation than dogs as the result of greater effects on platelet reactivity resulting in alterations of clotting times.107 A safe and tolerable dose for fatty acids in dogs can be extrapolated from studies in cardiac cachexia in dogs,108,109 in which EPA 45 mg and DHA 25 mg/kg body weight (e.g., 1 tsp/20 kg body weight) was used, but higher doses may be needed in dogs with cancer. Furthermore, the type of fish oil can significantly affect the ratio of EPA to DHA. Salmon oils tend to have higher DHA than EPA, and whitefish oils (e.g., herring, menhaden, cod) tend to have higher EPA than DHA; therefore whitefish oils are the preferred source. A safe and potentially effective dose in cats cannot be recommended.
During radiation therapy, the paradigm may be altered because radiation therapy causes irreparable damage to tumor cellular microstructure, resulting in apoptosis of cells and negative effects on surrounding tissues. The use of polyunsaturated fats (the longest, the ω-3 fatty acid DHA, has more double bonds) may be oxidized to a greater extent during radiation treatment, potentially leading to increased membrane compromise and cellular death.110 Surrounding tissues may not exhibit an aggressive inflammatory action due to the hastened eicosanoid response with EPA, and other essential fatty acids quenching this proinflammatory response may lead to less surrounding tissue damage.111 This principle has not been studied in veterinary patients but has been proved to diminish radiation-induced tissue damage in pig models.111
Vitamins and Minerals
Essential vitamin and mineral supplementation has been an interesting area of investigation in human cancer, with nutrients such as vitamin A, vitamin D, and selenium receiving attention.* Much of the research has centered on cancer prevention rather than cancer treatment, which has been addressed earlier in this section. That being said, certain vitamins and minerals are being used in therapeutic clinical trials in humans due to their ability to diminish tumor cell proliferation in preclinical models. Vitamin A, in the form of retinoic acid and synthetic derivatives, has been used to treat certain cancers; however, discordant effects on nuclear signaling occur with different heterodimers.113,114 Some heterodimers drive the proliferative response, whereas others diminish cell proliferation.115 Their use cannot be globally recommended at this time.
Regarding vitamin D, low concentrations of the precursor to active vitamin D (25, OH D3 calcidiol) in people may promote tumorigenesis and treatment with active vitamin D (1,25, OH D3 calcitriol) may cause tumor regression in some cases.41-43,116 Although antiproliferative at high doses, calcitriol can lead to hypercalcemia and its sequelae, including calcification of soft tissues. This is illustrated in a recent trial characterizing dosing in dogs with mast cell tumors in which many patients developed clinical signs of hypercalcemia, inappetence, and vomiting.116,117
Selenium has generated considerable interest in certain human neoplastic diseases, such as lung, squamous cell, and prostatic carcinoma.118-121 Low serum concentrations have been associated with an increased risk of prostatic cancer in humans.118,120 Interestingly, the selenium requirement in most dog food based on the AAFCO is considerably lower than what the National Research Council deems adequate intake in dogs and cats.122 Thus many pet foods may not have optimal concentrations of selenium, and modest supplementation may be considered.118-121 However, recent metaanalysis of human intervention studies suggests no definitive benefits from selenium supplementation in treatment or prevention of neoplastic disease.37
B vitamins of interest include folate and vitamin B12 (cobalamin). The interest once again derives from human literature in which their effects on epigenetic alterations may affect tumor suppressor and oncogene expression over time.122-124 Considering the consistent intake of folate and cobalamin in the pet population caused by commercial dog food consumption, there is a large gap in applying these paradigms of subclinical deficiency to pet populations. Furthermore, the lack of clinical or even canine or feline in-vitro investigation prevents any postulation as to their effects on cancer cells.
Antioxidants/Supplements
Utilization of supplements, most commonly substances termed antioxidants, has grown tremendously in the past 15 years. Studies suggest that approximately 65% of pet owners are using some sort of alternative treatments—over 30% are oral supplements and over 50% say their veterinarian approves of this use.125 In oncology referral centers, the general recommendation to clients is to refrain from using antioxidants or herbal supplements due to the lack of clinical data to support their use.126
It is clear that antioxidant and oxidative balance is altered in tumor tissue. Canine mammary cancer tissue has been shown to have an increased presence of lipid peroxidation coupled with an increase in upregulated antioxidant mechanisms, including glutathione peroxidase, glutathione, superoxide dismutase, and catalase.127 In dogs with lymphoma, reductions in serum antioxidants (tocopherols) and increased lipid peroxidation were observed, whereas total oxygen radical absorption capacity and glutathione peroxidase were increased, suggesting an increase in antioxidant capability.128 Therefore the addition of an antioxidant is unlikely to have a dramatic effect on the overall antioxidant capability of tumor cells when compared to normal tissue.
Further complicating this issue, many substances given as “antioxidants” may be considered pro-oxidants in some environments.129 Many isothiocyanates, flavonoids, and carotenoids that clients use may actually cause alteration of cell signaling or depletion of specific antioxidant systems.129,130 Furthermore, there is increasing evidence that many of these compounds upregulate or downregulate specific or sometimes global cell signaling systems to alter the proliferative cycle from activities such as cell cycle disruption (cyclin-dependent kinase [CDKs], p16, p21), prosurvival signals (nuclear factor-κB [NF-κB], AKT), mitochondrial-induced apoptosis (bcl and bax family proteins), and proliferative signaling pathways (i.e., mitogen-activated protein [MAP] kinase, tyrosine kinase [TK] activity).131-133 Recent primary cancer cell culture data in lymphoma and OSA support these principles. Astaxanthin and lycopene, two carotenoids, showed limited antioxidant capability in canine OSA cell lines, and when coupled with the chemotherapeutic agent doxorubicin or irradiation, there were no protective effects on cell proliferation indices or cell death,134,135 whereas isoflavones appear to induce mitochondrial apoptosis in canine lymphoma cells.136
Even if some of these compounds have little to no detrimental effect on current chemotherapy or radiation protocols, the limiting factor to their effective use is absorption, hepatic metabolism, and the attainment of tissue concentrations that recapitulate what has been used in vitro.131 Pharmacokinetic data have been collected on three nutraceuticals: genistein (an isoflavone) in cats, epigallocatechin gallate (EGCG, a flavone from green tea) in dogs, and lycopene in dogs (carotenoids). All of these nutraceuticals required dosing at very high concentrations, which may preclude their use. There is a tremendous disconnect from what is available and what may be required, as well as a lack of clinical investigation to assess efficacy and safety except for the few examples listed above. Furthermore, metabolism of these compounds may be different in cats and dogs; therefore caution is advised. Doses of over 150 mg/kg of EGCG in dogs caused hepatic necrosis, and the use of lipoic acid (an antioxidant thought to help salvage glutathione) has potential for toxicity and hepatic damage in cats, when used at doses thought safe in dogs and humans.137,138
In conclusion, set nutritional requirements for cancer-bearing companion species do not exist. In part, this is due to the variety of neoplastic diseases involved and the danger in trying to extrapolate data generated in human trials. There remain many aspects to address, including nutritional interventions (during treatment and remission) for anorexia/cachexia and nutrition recommendations based on specific disease processes. Therefore there is no one dietary recommendation for cancer patients; rather each case should be evaluated based on the body condition of the patient, the specific neoplastic process, and the treatment protocol initiated by the oncologist. The topics discussed are merely guidelines for interested clients and clinicians, and the most important factor in nutritional intervention is to supply a complete and balanced ration that meets the energy requirements of the patient to prevent weight loss.
References
1. Koppenol, WH, Bounds, PL, Dang, CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337.
2. Cairns, RA, Harris, IS, Mak, TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95.
3. Walenta, S, Schroeder, T, Mueller-Klieser, W. Lactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncology. Curr Med Chem. 2004;11:2195–2204.
4. Vaupel, P. Metabolic microenvironment of tumor cells: a key factor in malignant progression. Exp Oncol. 2010;32:125–127.
5. Ogilvie, GK, Vail, DM. Nutrition and cancer—recent developments. Vet Clin North Am Small Anim Pract. 1990;20:969–985.
6. Ogilvie, GK, Vail, DM, Wheeler, SL, et al. Effects of chemotherapy and remission on carbohydrate metabolism in dogs with lymphoma. Cancer. 1992;69:233–238.
7. Ogilvie, GK, Walters, LM, Fettman, MJ, et al. Energy expenditure in dogs with lymphoma fed two specialized diets. Cancer. 1993;71:3146–3152.
8. Ogilvie, GK, Walters, LM, Salman, MD, et al. Resting energy expenditure in dogs with nonhematopoietic malignancies before and after excision of tumors. Am J Vet Res. 1996;57:1463–1467.
9. Mazzaferro, EM, Hackett, TB, Stein, TP, et al. Metabolic alterations in dogs with osteosarcoma. Am J Vet Res. 2001;62:1234–1239.
10. Ogilvie, GK, Walters, L, Salman, MD, et al. Alterations in carbohydrate metabolism in dogs with non hematopoietic malignancies. Am J Vet Res. 1997;58:277–281.
11. Vail, DM, Ogilvie, GK, Wheeler, SL, et al. Alterations in carbohydrate metabolism in canine lymphoma. J Vet Int Med. 1990;4:8–11.
12. Ogilvie, GK, Fettman, MJ, Mallinckrodt, CH, et al. Effect of fish oil, arginine, and doxorubicin chemotherapy on remission and survival time for dogs with lymphoma: a double-blind, randomized placebo-controlled study. Cancer. 2000;88:1916–1928.
13. Ogilvie, GK, Ford, RB, Vail, DM, et al. Alterations in lipoprotein profiles in dogs with lymphoma. J Vet Intern Med. 1994;8:62–66.
14. Ackerman, BH, Kasbekar, N. Disturbances of taste and smell induced by drugs. Pharmacotherapy. 1997;17:482–496.
15. Weeth, LP, Fascetti, AJ, Kass, PH, et al. Prevalence of obese dogs in a population of dogs with cancer. Am J Vet Res. 2007;68:389–398.
16. Tisdale, MJ. Are tumoral factors responsible for host tissue wasting in cancer cachexia? Future Oncol. 2010;6:503–513.
17. Penna, F, Minero, VG, Costamagna, D, et al. Anti-cytokine strategies for the treatment of cancer-related anorexia and cachexia. Expert Opin Biol Ther. 2010;10:1241–1250.
18. Seruga, B, Zhang, H, Bernstein, LJ, et al. Cytokines and their relationship to the symptoms and outcome of cancer. Nat Rev Cancer. 2008;8:887–899.
19. Pajak, B, Orzechowska, S, Pijet, B, et al. Crossroads of cytokine signaling–the chase to stop muscle cachexia. J Physiol Pharmacol. 2008;59(Suppl 9):251–264.
20. Fearon, KC. Cancer cachexia and fat-muscle physiology. N Engl J Med. 2011;365:565–567.
21. Merlo, A, Rezende, BC, Franchini, ML, et al. Serum C-reactive protein concentrations in dogs with multicentric lymphoma undergoing chemotherapy. J Am Vet Med Assoc. 2007;230:522–526.
22. Tecles, F, Caldín, M, Zanella, A, et al. Serum acute phase protein concentrations in female dogs with mammary tumors. J Vet Diagn Invest. 2009;21:214–219.
23. Baez, JL, Michel, KE, Sorenmo, K, et al. A prospective investigation of the prevalence and prognostic significance of weight loss and changes in body condition in feline cancer patients. J Feline Med Surg. 2007;9:411–417.
24. Wolin, KY, Carson, K, Colditz, GA, Obesity and cancer. Oncologist 2010;15:556–565.
25. Roberts, DL, Dive, C, Renehan, AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Ann Rev Med. 2010;61:301–316.
26. Calle, EE, Rodriguez, C, Walker-Thurmond, K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638.
27. Lichtman, MA. Obesity and the risk for a hematological malignancy: leukemia, lymphoma, or myeloma. Oncologist. 2010;15:1083–1101.
28. de Boer, EJ, Slimani, N, van ‘t Veer, P, et al. The European Food Consumption Validation Project: conclusions and recommendations. Eur J Clin Nutr. 2011;65(Suppl 1):S102–S107.
29. Jansen, RJ, Robinson, DP, Stolzenberg-Solomon, RZ, et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control. 2011. [] [Epub ahead of print September 14].
30. Magalhães, B, Peleteiro, B, Lunet, N. Dietary patterns and colorectal cancer: systematic review and meta-analysis. Eur J Cancer Prev. 2011. [] [Epub ahead of print September 22].
31. Sonnenschein, EG, Glickman, LT, Goldschmidt, MH, et al. Body conformation, diet, and risk of breast cancer in pet dogs: a case-control study. Am J Epidemiol. 1991;133:694–703.
32. Shofer, FS, Sonnenschein, EG, Goldschmidt, MH, et al. Histopathologic and dietary prognostic factors for canine mammary carcinoma. Breast Cancer Res Treat. 1989;13:49–60.
33. Pérez Alenza, D, Rutteman, GR, Peña, L, et al. Relation between habitual diet and canine mammary tumors in a case-control study. J Vet Intern Med. 1998;12:132–139.
34. Raghavan, M, Knapp, DW, Bonney, PL, et al. Evaluation of the effect of dietary vegetable consumption on reducing risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J Am Vet Med Assoc. 2005;227:94–100.
35. Fulan, H, Changxing, J, Baina, WY, et al. Retinol, vitamins A, C, and E and breast cancer risk: a meta-analysis and meta-regression. Cancer Causes Control. 2011;22:1383–1396.
36. Arain, MA, Abdul Qadeer, A. Systematic review on “vitamin E and prevention of colorectal cancer,”. Pak J Pharm Sci. 2010;23:125–130.
37. Dennart, G, Zwahlen, M, Vinceti, M, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 11(5), 2011. [CD005195].
38. Wu, K, Erdman, JW, Jr., Schwartz, SJ. Plasma and dietary carotenoids, and the risk of prostate cancer: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2004;13:260–269.
39. Bendich, A. From 1989 to 2001: what have we learned about the “biological actions of beta-carotene”? J Nutr. 2004;134:225S–230S.
40. Cooper, DA. Carotenoids in health and disease: recent scientific evaluations, research recommendations and the consumer. J Nutr. 2004;134:221S–224S.
41. Deeb, KK, Trump, DL, Johnson, CS. Vitamin D signaling pathways in cancer: potential for anticancer therapeutics. Nature Rev Canc. 2007;7:684–700.
42. Abbas, S, Linseisen, J, Slanger, T, et al. Serum 25-hydroxyvitamin D and risk of post-menopausal breast cancer—results of a large case-control study. Carcinogenesis. 2007;29:93–99.
43. Yin, L, Grandi, N, Raum, E, et al. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30:113–125.
44. How, KL, Hazewinkel, HA, Mol, JA. Dietary vitamin D dependence of cat and dog due to inadequate cutaneous synthesis of vitamin D. Gen Comp Endocrinol. 1994;96:12–18.
45. Wakshlag, JJ, Rassnick, KM, Malone, EK, et al. Cross sectional study to investigate the association between serum vitamin D and cutaneous mast cell tumours in Labrador retrievers. Br J Nutr. 2011;106:S60–S63.
46. Lund, EM, Armstrong, PJ, Kirk, CA, et al. Prevalence and risk factors for obesity in adult cats from private US veterinary practices. Int J Appl Res Vet Med. 2005;3:88–96.
47. Lund, EM, Armstrong, PJ, Kirk, CA, et al. Prevalence and risk factors for obesity in adult dogs from private US veterinary practices. Int J Appl Res Vet Med. 2006;4:177–186.
48. Inui, A. Cancer anorexia-cachexia syndrome: current issues in research and management. Cancer J Clin. 2002;52:72–91.
49. Laflamme, DP. Development and validation of a body condition score system for cats: a clinical tool. Feline Pract. 1997;25:5–6.
50. Mawby, DI, Bartges, JW, d’Avignon, A, et al. Comparison of various methods for estimating body fat in dogs. J Am Anim Hosp Assoc. 2004;40:109–111.
51. de Fornel-Thibaud, P, Blanchard, G, et al. Unusual case of osteopenia associated with nutritional calcium and vitamin D deficiency in an adult dog. J Am Anim Hosp Assoc. 2007;43:52–60.
52. Taylor, MB, Geiger, DA, Saker, KE, et al. Diffuse osteopenia and myelopathy in a puppy fed a diet composed of an organic premix and raw ground beef. J Am Vet Med Assoc. 2009;234:1041–1048.
53. Michel, KE, Sorenmo, K, Shofer, FS. Evaluation of body condition and weight loss in dogs presented to a veterinary oncology service. J Vet Intern Med. 2004;18:692–695.
54. Kienzle, E. Energy. In: Beitz DC, ed. National Research Council nutrient requirements of dogs and cats. Washington DC: National Academies Press, 2006.
55. Delaney, SJ. Management of anorexia in dogs and cats. Vet Clin North Am Small Anim Pract. 2006;36:1243–1249.
56. Fox, CB, Treadway, AK, Blaszczyk, AT, et al. Megestrol acetate and mirtazapine for the treatment of unplanned weight loss in the elderly. Pharmacotherapy. 2009;29:383–397.
57. Braun, TP, Marks, DL. Pathophysiology and treatment of inflammatory anorexia in chronic disease. J Cachex Sarcopenia Muscle. 2010;1:135–145.
58. Bedford, SW, Godsall, SA. Diazepam for inappetence. Vet Rec. 1988;122:590–591.
59. Center, SA, Elston, TH, Rowland, PH, et al. Fulminant hepatic failure associated with oral administration of diazepam in 11 cats. J Am Vet Med Assoc. 1996;209:618–625.
60. Long, JP, Greco, SC. The effect of propofol administered intravenously on appetite stimulation in dogs. Contemp Top Lab Anim Sci. 2000;39:43–46.
61. Remillard, RL, Saker, KE. Critical care nutrition and enteral-assisted feeding. In Hand MS, Thatcher CD, Remillard RL, et al, eds.: Small animal clinical nutrition, ed 5, Topeka: Mark Morris Institute, 2010.
62. Salinardi, BJ, Harkin, KR, Bulmer, BJ, et al. Comparison of complications of percutaneous endoscopic versus surgically placed gastrostomy tubes in 42 dogs and 52 cats. J Am Anim Hosp Assoc. 2006;42:51–56.
63. Yoshimoto, SK, Marks, SL, Struble, AL, et al. Owner experiences and complications with home use of a replacement low profile gastrostomy device for long-term enteral feeding in dogs. Can Vet J. 2006;47:144–150.
64. Hill, RC. Physical activity and environment. In: Beitz DC, ed. National Research Council nutrient requirements of dogs and cats. Washington DC: National Academy Press, 2006.
65. Chandler, ML, Payne-James, JJ. Prospective evaluation of a peripherally administered three-in-one parenteral nutrition product in dogs. J Am Anim Hosp Assoc. 2006;47:518–523.
66. Chan, DL, Freeman, LM, Labata, MA, et al. Retrospective evaluation of partial parenteral nutrition in dogs and cats. J Vet Int Med. 2002;16:440–445.
67. Pyle, SC, Marks, SL, Kass, PH. Evaluation of complications and prognostic factors associated with administration of total parenteral nutrition in cats: 75 cases (1994-2001). J Am Vet Med Assoc. 2004;225:242–250.
68. Crabb, SE, Freeman, LM, Chan, DL, et al. Retrospective evaluation of total parenteral nutrition in cats: 40 cases (1991-2003). J Vet Emerg Crit Care. 2006;16:S1–S26.
69. Lippert, AC, Fulton, RB, Parr, RB. A retrospective study of the use of total parenteral nutrition in dogs and cats. J Vet Int Med. 1993;7:52–64.
70. Queau, Y, Larsen, JA, Kass, PH, et al. Factors associated with adverse outcomes during parenteral nutrition administration in dogs and cats. J Vet Intern Med. 2011;25:446–452.
71. Qin, HL, Su, ZD, Hu, LG, et al. Effect of early intrajejunal nutrition on pancreatic pathological features and gut barrier function in dogs with acute pancreatitis. Clin Nutr. 2002;21:469–472.
72. Chan, DL. Parenteral nutritional support. In Ettinger SL, Feldman EC, eds.: Textbook of veterinary internal medicine, ed 6, St. Louis: Elsevier Saunders, 2005.
73. Remillard, RL, Saker, KE. Critical care nutrition and enteral-assisted feeding. In Hand MS, Thatcher CD, Remillard RL, et al, eds.: Small animal clinical nutrition, ed 5, Topeka: Mark Morris Institute, 2010.
74. Wakshlag, J, Schoeffler, GL, Russell, DS, et al. Extravasation injury associated with parenteral nutrition in a cat with presumptive gastrinomas. J Vet Emerg Crit Care. 2011;21:375–381.
75. Mauldin, GE, Reynolds, AJ, Mauldin, GN, et al. Nitrogen balance in clinically normal dogs receiving parenteral nutrition solutions. Am J Vet Res. 2011;62:912–920.
76. ASPEN Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. J Parenter Enteral Nutr. 2002;26(1 Suppl):1SA–138SA.
77. Gogos, CA, Kalfarentzos, F. Total parenteral nutrition and immune system activity: a review. Nutrition. 1995;11:339–344.
78. Kitchell, CC, Balogh, K. Pulmonary lipid emboli in association with long-term hyperalimentation. Hum Pathol. 1986;17:83–85.
79. Thomovsky, EJ, Backus, RC, Mann, FA, et al. Effects of temperature and handling conditions on lipid emulsion stability in veterinary parenteral nutrition admixtured during simulated intravenous administration. Am J Vet Res. 2008;69:652–658.
80. Martin, GJ, Rand, JS. Food intake and blood glucose in normal and diabetic cats fed ad libitum. J Feline Med Surg. 1999;1:241–251.
81. Ho, VW, Leung, K, Hsu, A, et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011;71:4484–4493.
82. Nguyen, P, Lerray, V, Dumon, H, et al. High protein intake affects lean body mass but not energy expenditure in nonobese neutered cats. J Nutr. 2004;134:2084S–2086S.
83. Hannah, SS, LaFlamme, DP. Effect of dietary protein on nitrogen balance and lean body mass in cats. Vet Clin Nutr. 1996;3:30.
84. Burns, RA, Milner, JA. Effects of arginine on the carcinogenicity of 7,12-dimethylbenz(a)-anthracene and N-methyl-N-nitrosourea. Carcinogenesis. 1984;5:1539–1542.
85. Brittenden, J, Heys, SD, Ross, J, et al. Natural cytotoxicity in breast cancer patients receiving neoadjuvant chemotherapy: effects of L-arginine supplementation. Eur J Surg Oncol. 1994;20:467–472.
86. Reynolds, JV, Daly, JM, Shou, J, et al. Immunologic effects of arginine supplementation in tumor-bearing and non-tumor-bearing hosts. Ann Surg. 1990;211:202–210.
87. Wakshlag, JJ, Kallfelz, FA, Wakshlag, RR, et al. The effects of branched-chain amino acids on canine neoplastic cell proliferation and death. J Nutr. 2006;136:2007S–2010S.
88. Kaufmann, Y, Kornbluth, J, Feng, Z, et al. Effect of glutamine on the initiation and promotion phases of DMBA-induced mammary tumor development. J Parenter Enteral Nutr. 2003;27:411–418.
89. Yoshida, S, Kaibara, A, Ishibashi, N, et al. Glutamine supplementation in cancer patients. J Nutr. 2001;17:766–768.
90. Wigmore, SJ, Barber, MD, Ross, JA, et al. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr Cancer. 2000;36:177–184.
91. Togni, V, Ota, CC, Folador, A, et al. Cancer cachexia and tumor growth reduction in Walker 256 tumor-bearing rats supplemented with N-3 polyunsaturated fatty acids for one generation. Nutr Cancer. 2003;46:52–58.
92. Fearon, KC, Von Meyenfeldt, MF, Moses, AG, et al. Effect of a protein and energy dense N-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomized double blind trial. Gut. 2003;52:1479–1486.
93. Colas, S, Paon, L, Denis, F, et al. Enhanced radiosensitivity of rat autochthonous mammary tumors by dietary docosahexaenoic acid. Int J Cancer. 2004;109:449–454.
94. Senzaki, H, Iwamoto, S, Ogura, E, et al. Dietary effects of fatty acids on growth and metastasis of KPL-1 human breast cancer cells in vivo and in vitro. Anticancer Res. 1998;18:1621–1627.
95. Noguchi, M, Earashi, M, Minami, M, et al. Effects of eicosapentaenoic and docosahexaenoic acid on cell growth and prostaglandin E and leukotriene B production by a human breast cancer cell line (MDA-MB-231). Oncology. 1995;52:458–464.
96. Hawcroft, G, Loadman, PM, Belluzzi, A, et al. Effect of eicosapentaenoic acid on E-type prostaglandin synthesis and EP4 receptor signaling in human colorectal cancer cells. Neoplasia. 2010;12:618–627.
97. Hayashi, T, Nishiyama, K, Shirahama, T. Inhibition of 5-lipoxygenase pathway suppresses the growth of bladder cancer cells. Int J Urol. 2006;13:1086–1091.
98. Schley, PD, Brindley, DN, Field, CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137:548–553.
99. Furstenberger, G, Krieg, P, Muller-Decker, K, et al. What are cyclooxygenases and lipoxygenases doing in the driver’s seat of carcinogenesis. Int J Cancer. 2006;119:2247–2254.
100. Mohammed, SI, Bennett, PF, Craig, BA, et al. Effects of the cyclooxygenase inhibitor, piroxicam, on tumor response, apoptosis and angiogenesis in a canine model of human invasive urinary bladder cancer. Cancer Res. 2002;62:356–358.
101. McMillan, SK, Boria, P, Moore, GE, et al. Antitumor effects of deracoxib treatment in 26 dogs with transitional cell carcinoma of the urinary bladder. J Am Vet Med Assoc. 2011;239:1084–1089.
102. Hanahan, D, Weinberg, RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
103. Weylandt, KH, Krause, LF, Gomolka, B, et al. Suppressed liver tumorigenesis in fat-1 mice with elevated omega-3 fatty acids is associated with increased omega-3 derived lipid mediators and reduced TNF-α. Carcinogenesis. 2011;32:897–903.
104. Endres, S, Ghorbani, R, Kelley, VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265–271.
105. Purasiri, P, Murray, A, Richardson, S, et al. Modulation of cytokine production in vivo by dietary essential fatty acids in patients with colorectal cancer. Clin Sci (Lond). 1994;87:711–717.
106. Dewey, A, Baughan, C, Dean, T, et al. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev. 24(1), 2007. [CD004597].
107. Saker, KE, Eddy, AL, Thatcher, CD, et al. Manipulation of dietary (n-6) and (n-3) fatty acids alters platelet function in cats. J Nutr. 1998;128:2645s–2647s.
108. Freeman, LM, Rush, JE, Kehayias, JJ, et al. Nutritional alterations and the effect of fish oil supplementation in dogs with heart failure. J Vet Intern Med. 1998;12:440–448.
109. Freeman, LM, Rush, JE. Cardiovascular diseases: nutritional modulation. In: Pibot P, Elliot D, Biourge V, eds. Encyclopedia of canine clinical nutrition. Paris: Aniwa SAS, 2006.
110. Kikawa, KD, Herrick, JS, Tateo, RE, et al. Induced oxidative stress and cell death in the A549 lung adenocarcinoma cell line by ionizing radiation is enhanced by supplementation with docosahexaenoic acid. Nutr Cancer. 2010;62:1017–1024.
111. Hopewell, JW, van den Aardweg, GJ, et al. Amelioration of both early and late radiation-induced damage to pig skin by essential fatty acids. Int J Radiat Oncol Biol Phys. 1994;30:1119–1125.
112. Paik, J, Blaner, WS, Sommer, KM, et al. Retinoids, retinoic acid receptors, and breast cancer. Cancer Invest. 2003;21:304–312.
113. Tang, XH, Gudas, LJ. Retinoids, retinoic acid receptors, and cancer. Annu Rev Pathol. 2011;6:345–364.
114. Bushue, N, Wan, YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285–1298.
115. Hayes, KC. Nutritional problems in cats: taurine deficiency and vitamin A excess. Can Vet J. 1982;23:2–5.
116. Rassnick, KM, Muindi, JR, Johnson, CS, et al. Oral bioavailability of DN101, a concentrated formulation of calcitriol, in tumor-bearing dogs. Cancer Chemother Pharmacol. 2011;67:165–171.
117. Malone, EK, Rassnick, KM, Wakshlag, JJ, et al. Calcitriol enhances mast cell tumour chemotherapy and receptor tyrosine kinase inhibitor activity in-vitro and has single agent activity against spontaneously occurring canine mast cell tumours. Vet Comp Oncol. 2010;8:209–220.
118. Nelson, MA, Porterfield, BW, Jacobs, ET, et al. Selenium and prostate cancer prevention. Semin Urol Oncol. 1999;17:91–96.
119. Reid, ME, Duffield-Lillico, AJ, Garland, L, et al. Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev. 2002;11:1285–1291.
120. Duffield-Lillico, AJ, Dalkin, BL, Reid, ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–612.
121. Clark, LC, Comb, GF, Jr., Turnbull, BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963.
122. Xiao, SD, Meng, XJ, Shi, Y, et al. Interventional study of high dose folic acid in gastric carcinogenesis in beagles. Gut. 2002;50:61–64.
123. Jhaveri, MS, Wagner, C, Trepel, JB. Impacts of extracellular folate levels on global gene expression. Mol Pharmacol. 2001;60:1288–1295.
124. Friso, S, Choi, SW. The potential cocarcinogenic effect of vitamin B12 deficiency. Clin Chem Lab Med. 2005;43:1158–1163.
125. Lana, SE, Kogan, LR, Crump, KA, et al. The use of complementary and alternative therapies in dogs and cats with cancer. J Am Anim Hosp Assoc. 2006;42:361–365.
126. Seifried, HE, McDonald, SS, Anderson, DE, et al. The antioxidant conundrum in cancer. Cancer Res. 2003;63:4295–4298.
127. Szczubial, M, Kankofer, M, Lopuszynski, W, et al. Oxidative stress parameters in bitches with mammary gland tumors. J Vet Med. 2004;51:336–340.
128. Winter, JL, Barber, LG, Freeman, TM, et al. Antioxidant status and biomarkers of oxidative stress in dogs with lymphoma. J Vet Int Med. 2009;23:311–316.
129. Chandhok, D, Saha, T. Redox regulation in cancer: a double-edged sword with therapeutic potential. Oxid Med Cell Longev. 2010;3:23–34.
130. Zhao, CR, Gao, ZH, Qu, XJ. Nrf2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol. 2010;34:523–533.
131. Crozier, A, Jaganath, IB, Clifford, MN. Dietary phenolics: chemistry, bioavailability and the effects on health. Nat Prod Rep. 2009;26:1001–1043.
132. Khan, N, Afaq, F, Mukhtar, H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxidants Redox Signal. 2008;10:1–36.
133. Shanmugam, MK, Kannaiyan, R, Sethi, G. Targeting cell signaling and apoptotic pathways by dietary agents: role in the prevention and treatment of cancer. Nutr Cancer. 2011;63:161–173.
134. Wakshlag, JJ, Balkman, CA, Morgan, SK, et al. Evaluation of the protective effects of all-trans-astaxanthin on canine osteosarcoma cell lines. Am J Vet Res. 2010;71:89–96.
135. Wakshlag, JJ, Balkman, CE. Effects of lycopene on proliferation and death of canine osteosarcoma cells. Am J Vet Res. 2010;71:1362–1370.
136. Jamadar-Shroff, V, Papich, MG, Suter, SE. Soy-derived isoflavones inhibit the growth of canine lymphoid cell lines. Clin Cancer Res. 2009;15:1269–1276.
137. Serisier, S, Leray, V, Poudroux, W, et al. Effects of green tea on insulin sensitivity, lipid profile and expression of PPAR alpha and PPAR gamma and their target genes in dogs. Br J Nutr. 2008;99:1208–1216.
138. Kapetanovic, IM, Crowell, JA, Krishnaraj, R, et al. Exposure and toxicity of green tea polyphenols in fasted and non-fasted dogs. Toxicology. 2009;260:28–36.
 Section C
Section C
Relationship-Centered Approach to Cancer Communication
The increasing recognition of the relationships that people develop with their companion animals1,2 brings an awareness of the impact of animal illness on pet caregivers and the veterinary team.3 Rising acknowledgment of pets as family members has also been associated with increasing expectations of pet owners for the highest quality medical care for their companion animals, as well as compassionate care and respectful communication for themselves.1,4,5 The human-animal bond is particularly stressed and fragile when an animal is sick and even more so with a cancer diagnosis. Appreciating the impact of animal companionship on the health and well-being of humans creates a new dimension in public health. The responsibilities of veterinary professionals have expanded to include the mental health and well being of their clients, as well as their clients’ pets.4
Cancer communication presents challenges for both oncologists and clients. From the veterinarian’s perspective, a number of factors6,7 may contribute to discomfort, including lack of training, being short of time, practice culture, feeling responsible for the patient’s illness, perceptions of failure, unease with death and dying, lack of comfort with uncertainty, impact on the veterinarian-client-patient relationship, worry about the patient’s QOL, concerns about the client’s emotional response, and their own emotional response to the circumstances. Some of these same reasons may account for client anxiety during difficult conversations. These include self-blame, unease with death and dying, anticipatory grief, effect on the human-animal bond, impact on the veterinarian-client-patient relationship, pet’s QOL, and concerns about their emotional response to the situation. Research6-9 in human medicine indicates that end-of-life discussions are often suboptimal due to many of these barriers and a lack of specific training in communication.
The content, duration, and methods of communication training in veterinary curricula are highly diverse and variable. Many practitioners have not received formal communication training and may feel unprepared to engage in difficult conversations.10,11 The veterinary profession has identified a skills gap between the content of the veterinary school curriculum and the actual skills required to be a successful veterinarian.12 Using experiential techniques, defining key skills, and creating opportunities to practice them enhance effective communication.13-15 In accreditation standards, the American Veterinary Medical Association Council on Education recognizes communication as a core clinical competency for success.
Several aspects of cancer care make it a unique communication context.16 The diagnosis is frequently made by the primary care veterinarian who may refer the client and patient to an oncologist. Therefore the first visit with the oncologist often occurs after the patient has been diagnosed and the focus of the conversation is on confirming the diagnosis, treatment information, and decision making. In this setting, tough conversations occur on the back of a newly formed veterinarian-client-patient relationship. Cancer is an emotionally laden diagnosis, and clients often present with high levels of uncertainty, anxiety, fear, frustration, and guilt, which heightens the stakes for both parties. Fortunately, today we can offer clients a menu of sophisticated diagnostic and therapeutic options to treat their companion animal’s cancer. The challenge is navigating the complexity of the information and the decision-making process of making the “right choice” for their pet without overwhelming the client. It may require as much listening as talking to hear what is most important to your clients to address these challenges.
There are six functions of relationship-centered communication in cancer care: exchanging information, making decisions, fostering healing relationships, enabling clients to provide patient care, managing uncertainty, and responding to emotions.17 Cancer communication is a process that occurs over time, starting with delivering the diagnosis (i.e., often delivering bad news), discussing prognosis, making decisions about treatment options, assessing QOL, transitioning to palliative or supportive care, and ending with preparing families for euthanasia, dying, and death.18 These difficult conversations are spread throughout multiple visits, as the relationship grows and a partnership develops with the client in caring for the patient.
The purpose of this section is to present best practices for cancer communication. There are limited empiric studies in the veterinary literature concerning veterinarian-client-patient communication, and information pertaining specifically to oncology is based largely on clinical experience. In contrast, the literature on human medical communication contains a large number of empiric studies; however, in relation to cancer communication, what is available is based on expert opinion, case studies, reviews, and predominantly descriptive studies.16,18 The objectives of this section are to describe relationship-centered care, define core cancer communication skills, and highlight communication approaches to difficult discussions. The medical cancer communication literature7,9,18-20 and clinical experience provide the foundation for communication techniques presented here.
A Paradigm Shift: Paternalism to Partnership
Recent societal changes have caused a paradigm shift in the veterinarian-client-patient relationship. Growing client expectations, the strong attachment between people and their pets, and increasing consumer knowledge demand a shift in communication style from the traditional paternalistic approach to a collaborative partnership.5,21,22 Many clients are no longer content with taking a passive role in their animal’s healthcare, preferring to take an active role in the decision-making process.5
Paternalism is characterized as a relationship in which the oncologist sets the agenda for the appointment, assumes that the client’s values are the same as the veterinarian’s, and takes on the role of a guardian for the patient.23,24 Traditionally, paternalism is the most common approach to medical and veterinary visits. In general practice, the veterinarian used a paternalistic approach in 31% of wellness visits and 85% of problem visits.21 The topic of conversation is primarily biomedical in nature, focusing on the medical condition, diagnosis, treatment, and prognosis.21
In a paternalistic relationship, the oncologist does most of the talking and the client plays a passive role. This approach is often referred to as the data dump and symbolized by a shot-put.25 Throwing a shot-put is unidirectional, the intent is on the delivery, the information to be delivered is large in mass and scale, and it is challenging to receive the message. Intuitively, it seems like this directive approach enhances efficiency and promotes time management. The challenge is that the agenda and subsequent diagnostic or treatment plan may not be shared between the oncologist and client, compromising the ability to reach agreement, move forward, and achieve full compliance. This could result in a roadblock and the need to take steps backward to recover and regain client understanding, commitment, and trust.
In contrast, partnership or relationship-centered care represents a balance of power between veterinarian and client and is based on mutuality.23,24,26 In the relationship-centered model, the relationship between oncologist and client is characterized by negotiation between partners, resulting in creation of a joint venture, with the veterinarian taking on the role of advisor for the client. Respect for the client’s perspective and values and recognition of the role the animal plays in the life of the client are incorporated into all aspects of care. In general practice, 69% of wellness visits and 15% of problem visits were characterized as relationship-centered.21
The conversation content of relationship-centered visits is broad, including biomedical topics, lifestyle discussion of the pet’s daily activities (e.g., exercise regimen, environment, travel, diet, and sleeping habits) and social interactions (e.g., personality or temperament, behavior, human-animal interaction, and animal-animal interactions) that are key indicators of patient quality of life.21 In addition, a relationship-centered approach encompasses building rapport, establishing a partnership, and encouraging client participation in the animal’s care, all of which have the potential to enhance clinical outcomes.
This collaborative relationship is symbolized by a Frisbee.25 In playing Frisbee, the interaction is reciprocal; the intent is on dialog; the delivery is airy, light, and free; small pieces of information are delivered at a time; and the deliverer and receiver adjust their message to stay on target. The emphasis of the Frisbee analogy is on eliciting client feedback to assess how the client perceives, processes, and understands the information presented.
Relationship-Centered Care
Combining several frameworks, Mead and Bower27 identified the following five distinct dimensions of relationship or patient-centered care in the human medical setting:
1. The biopsychosocial perspective—a perspective on illness that includes the social and psychologic, as well as biomedical factors.
2. The “patient as a person”—understand the personal meaning of the illness for each individual patient.
3. Sharing power and responsibility—sensitivity to patients’ preferences for information and shared decision making.
4. The therapeutic alliance—developing common therapeutic goals and enhancing the physician-patient relationship.
5. The “doctor as person”—awareness of the influence of the subjectivity of the doctor on the practice of medicine.
These principles translate readily to the veterinary context.21,22 Expanding data gathering to explore the broader lifestyle of the client and pet enhances the understanding of the animal’s illness. Discussing unique details such as financial resources, the role of the primary caregiver, feasibility of implementing the plan, and recent life events (i.e., new birth, death, new job, or moving) promotes compliance. With increased recognition of the human-animal bond, it is important to assess the level of attachment and the impact of the animal’s illness on the family. Eliciting information on the client’s expectations, thoughts, feelings, and fears about the pet’s illness fosters client participation and satisfaction and promotes shared decision making.
Based on medical communication studies, the following principles of relationship-centered care are associated with significant clinical outcomes:
1. Broadening the explanatory perspective of disease beyond the biomedical to include lifestyle and social factors is related to expanding the field of inquiry and improved diagnostic reasoning and accuracy.25
2. Building a strong relationship is associated with increased accuracy of data gathering,25 patient satisfaction,28-30 and physician satisfaction.31,32
3. Encouraging participation, negotiation, and shared decision making promotes patient satisfaction,28-30 adherence,33 and improved health.34
In veterinary medicine, a study investigating the use of patient-centered communication in euthanasia discussions with undisclosed standardized clients identified that veterinarians did not fully explore client feelings, ideas, and expectations.22 In these visits, veterinarians did not involve clients in defining the problem and identifying treatment goals. Shared decision making is a key component of relationship-centered care, in which there is two-way exchange between the veterinarian and client, identifying preferences and working toward consensus to achieve significant clinical outcomes for the veterinarian, client, and patient.
Core Communication Skills for Cancer Communication
The Calgary-Cambridge Guide25 is an evidence-based communication model that provides structure to the clinical interview, describing the tasks and identifying key communication skills to help veterinarians achieve clinical outcomes. Defining and demonstrating specific skills and behaviors are instrumental first steps to enhancing communication approaches.14,15 The communication tools described next were identified as core communication skills15 in human cancer communication literature7,13,18,20 and are highly applicable to veterinary oncologist-client-patient interactions.
Gathering Information
Identify the Client’s Full Agenda
Eliciting the client’s full agenda through open-ended inquiry promotes early detection of the problem(s) and sets a plan for the rest of the visit.35 An open-ended question is designed to draw out a full response from the client rather than a brief one and usually begins with “how,” “what,” or “tell me or describe for me.”36
“What brings you and Mandy in today?” [open-ended question]
“What other questions do you have about Mandy’s cancer?” [open-ended question].
“Anything else you would like to discuss?” [open-ended question]
This process of questioning may seem redundant, but clients often bring a laundry list of concerns, questions, or topics that they would like to discuss with their oncologist. Given the overwhelming nature of cancer conversations, these steps help identify the key questions and information sought by the client. Helping to generate the client’s list of concerns and melding it with your agenda will set the structure for the remainder of the appointment and optimizes use of the visit time.
Elicit the Client’s Perspective
Invite the clients to share their thoughts, ideas, feelings, and perceptions.22 How the client perceives the patient’s illness can have a major impact on the decision-making process and compliance. Many clients have had previous experiences with cancer, and it is helpful to hear these stories to address client concerns, provide reassurance, and identify misconceptions or barriers to patient care. Pick up on client verbal cues (“I am not sure how she will do with chemotherapy.” or “I am really concerned about her loss of appetite.” or “My big fear is that we won’t get quality time”). Knowing the client’s expectations enables you to get on the same page and customize the message to the client’s concerns and meet their needs.
Explaining and Planning
Assess the Client’s Knowledge Level
Assessing the client’s prior knowledge and experiences allows you to evaluate the client’s understanding and determine what level to pitch the information.25 An equally important goal is to ascertain the type and kind of information the client desires because not all clients may want the same degree of information. Client preferences for information may change over time; initially, overwhelmed clients may want just the big picture and as they absorb and process the information, they may produce a list of detailed questions for follow-up discussions.
“What have you heard or read about osteosarcoma?”
“I am wondering what your veterinarian told you about Mandy’s cancer.”
“What other questions would you like me to address today?”
“What additional information will be helpful to you?”
“Some clients prefer the big picture and for others it is important to get into the details. What is your preference?”
Give Information in Manageable Chunks and Checks
Chunks and checks (chunk-n-check) consists of giving information in small pieces (i.e., chunks), followed by checking for understanding before proceeding further (i.e., check)—the Frisbee approach in action.25 Sharing small pieces of information, one to three sentences at a time, allows your client time to absorb the news, and checking-in encourages client participation in the discussion and ensures that the client stays with you to achieve shared understanding. This approach to information giving avoids lecturing to the client and aims to increase recall, understanding, and commitment to plans. In this manner, the information-giving process is responsive to the client’s needs and provides an opportunity for the client to participate in the conversation, provide feedback, or ask for clarification. The check can take on various forms such as taking a pause, encouraging the client to contribute to the conversation (“What questions do you have at this point?”), picking up on client cues (“You seem a little hesitant about surgery.”), asking for client suggestions (“What options have you and your husband discussed?”), and checking for the client’s understanding (“What part of the plan will be most difficult for you and Mandy?”).
Building Relationships
Offer Partnership
Partnership is inclusive language (i.e., let’s, we, together, our, or us), which reflects that you and the client are working as a team toward mutual goals. Offering partnership informs the client that they are not alone and that they have a working partner in their oncologist, who will guide and advise them at each stage. Often, clients may arrive at their oncology appointment on their own and it may be helpful to assess their support system and offer to include other key decision makers in the conversations.
Ask Permission
Asking permission is a gentle approach to assess the client’s readiness to take the next step. This act of respect allows the client to ready their minds, be receptive to what you have to say, and pace the conversation with you. Asking permission is a method of structuring the conversation by proposing a transition to the client and to determine whether they would like to move on.
Express Empathy
The stress of cancer can result in intense emotions: sadness, fear, anxiety, uncertainty, and guilt, and acknowledging these emotions reduces client distress. Empathy is an affective response resulting from perceiving the situation of another, vicariously experiencing what it might be like, and paying deep attention to another person’s emotions. As a result, there are three tasks to expressing empathy.37 The first is to appreciate the client’s situation, perspective, and feelings and their attached meanings. The second is to communicate that understanding back to the client and check its accuracy. The third is to move forward in the clinical interview and act on that understanding with the client and patient in a helpful way. Simply, empathy is putting yourself in the client’s shoes and communicating that you understand where they are coming from. Expressing empathy acknowledges, validates, and normalizes the client’s emotional response and is essential to establishing a trusting veterinarian-client-patient relationship.36
Demonstrate Appropriate Nonverbal Behavior
Expression of all of the verbal core communication skills is strengthened when accompanied by complementary nonverbal communication.36 As much as 80% of communication is nonverbal in nature, whereas 20% is based on verbal content.25 When verbal and nonverbal communication are incongruent with each other, the nonverbal behaviors reveal the truth. There are two areas of focus for nonverbal communication: the first is to increase your sensitivity to picking up on client cues and the second is enhanced awareness of the nonverbal messages you are sending out. Tune in closely to client nonverbal behaviors such as breaking eye contact, nervous body movements, or tone of voice because client nonverbal behaviors often reflect their true underlying feelings and responses. Out of respect for their relationship with their oncologist, clients often express hesitation indirectly through their nonverbal behaviors and may not feel comfortable with directly verbalizing their concerns. It is important to pick up on these client clues and follow-up on them with the client to explore the concerns (“I sensed some hesitation when I mentioned chemotherapy as a treatment option.” or “You seem worried about taking Mandy to surgery, what are you most scared about?”).
Veterinarian nonverbal cues include attentive body posture, appropriate distance from the client, turning your body toward the client, sitting at the same level, maintaining good eye contact, and complementary gestures. Display your compassion through nonverbal cues such as sitting close to your client; using a gentle, calm tone and soft volume; slowing your pace of speech; and leaning forward and reaching out through touch. Use silence to create time for the client to examine his/her thoughts and feelings. It can be difficult at times to find the right words to say and simply being a caring presence can provide just as much comfort to the client as any spoken words. Being mindful of the messages sent is important because when veterinarians are triggered or feeling judgmental, these sentiments can be leaked to the client through nonverbal behaviors.
Providing Structure
Summarize
Summarizing is an explicit review of the information that has been discovered and discussed with the client. Therefore there are multiple opportunities to present a summary: reflect back what you heard and learned at several stages during information gathering, then take time to repeat the key aspects of the diagnostic and treatment plan, and finally provide a full and complete summary at the end of the clinical interview. Summarizing helps structure the conversation by reviewing what has been discussed, identifying data that needs further clarification, providing an opportunity for reflection on where the interview should go next, and managing effective use of time during the visit.25 The skill of summary creates a window to inform the client that they have been heard and time for the clinician to gather their thoughts, synthesize and integrate the data, and work through the diagnostic reasoning process.
“So, if I understand it correctly, your referring veterinarian felt the large lymph nodes, took a sample, and diagnosed lymphoma. You were sent here for further testing to determine if the lymphoma has spread to other organs. Is that correct?”
“What we talked about doing today, is requesting a second opinion from our pathologist on the tumor sample and conducting an ultrasound exam to look at the abdominal lymph nodes, liver, and spleen for spread of the tumor.”
“I Don’t Have Time for This…”
Before moving on to how to use these skills in crucial cancer conversations, one of the most common concerns expressed in communication training is that there is not enough time in the clinical interview. It seems like the facilitative approach (i.e., Frisbee) of relationship-centered care takes more time; however, it was found in veterinary general practice visits that relationship-centered care appointments were shorter in length because the veterinarian and the client achieved common ground.21 In human medicine, when patients are left to tell their story uninterrupted, their average talking time was 92 seconds, sharing key clues to the diagnosis.25 Empathy can be expressed as well without prolonging the appointment time; in one study, as little as 40 seconds of empathy decreased the patient’s anxiety level.38,39 Although counterintuitive, evidence suggests that using the core communication skills actually saves time and allows for a more efficient veterinarian-client-patient interaction. In addition, spending time to build a relationship at the beginning of the evaluation process creates trust, which will pay dividends as the management process progresses through the diagnostic and treatment phases.
Approaches to Cancer Conversations
As presented in the introduction, cancer communication is a series of conversations over time, starting with delivering the diagnosis (i.e., delivering bad news), discussing prognosis, making decisions about treatment options, assessing QOL, transitioning to palliative or supportive care, and ending with preparing families for euthanasia, dying, and death.18 These difficult conversations are spread throughout multiple visits. This step-by-step approach is guided by the veterinarian’s expertise, the client’s agenda and perspective, and the patient’s condition, response to treatment, and QOL.
Delivering Bad News
Bad news is defined as any news that drastically and negatively alters the person’s views of her or his future with their pet such as a cancer diagnosis.7 Clients interpret bad news on an individual basis and their response is related to their relationship with their companion animal, severity of the diagnosis, past experiences, other stressors in their lives, and their support system. Grief often accompanies change, and clients may express a wide range of emotions that are largely unpredictable. One useful model for delivering bad news is the SPIKES six-step model developed by Buckman7 and employed in many medical school curricula. The SPIKES model7 (setting, perception, invitation, knowledge, empathize, and summarize) provides guidelines on how to present information, structure the conversation, and create a supportive environment. Communication techniques for delivering bad news in the veterinary setting were previously published.40,41
Discussing Prognosis
Three different approaches have been described in the medical communication literature for presenting prognostic information—realism, optimism, and avoidance.9 The challenge with realism in human medicine is that approximately 20% of patients do not want full information about their prognosis,42-45 and unfortunately such studies of client perceptions are lacking in veterinary medicine. The drawback of optimism is that clients may lose opportunities to fulfill last wishes, prepare themselves and their family, and spend quality time with their pet. Finally, the shortcoming of avoidance is appearing evasive or dishonest, risking the trust that has been built between veterinarian and client potentially compromising the care of the pet.
Based on recommendations in human medicine,9,18,19 be mindful of making assumptions about what the client wants to know and instead explicitly ask if and how the client wants to talk about prognosis (“How much would you like to know about the course of Mandy’s cancer?” or “Some clients would like all the details and others would like the big picture. What works best for you?”). It is effective to break the information into small pieces (i.e., chunk) and then to check for client understanding and for how the prognostic information is impacting the client (“This is hard to talk about.” or “I am wondering if this is the kind of information you need.”). Asking permission is a key skill in this conversation to assess the client’s readiness to hear more information (“What questions do you have at this point?” or “Would you like me to continue?”), along with reading the client’s nonverbal cues to assess how they are processing the information (“I notice that you seem hesitant when I was talking about survival time with chemotherapy. Could you tell me more about this?”). To balance sustaining hope and maintaining reality, it may be helpful to frame the prognosis, using both positive and negative language (“Median survival time means that half of the patients live longer than 2 years and half the patients live less than 2 years.”).
Given the overwhelming nature of this discussion, take time to acknowledge the client’s emotional reactions (“This is a lot of information to take in. How are you doing?” or “This is really difficult to talk about and we can take it one step at a time.” or “I can see how sad this is for you.”). Allowing for silence or offering to take a break creates space for clients to work through their emotions and process the information shared with them. It can be difficult for clients to recall and understand all of the data presented, so you may want them to invite friends or family to take part (“Who else plays a role in caring for Mandy? I am wondering if they might want to be part of this discussion.”). As this is an emotionally laden conversation loaded with complex information and associated with decision-making, it is helpful to compose and center yourself beforehand, pace yourself with your client, and offer some time for reflection.
Assessing Quality of Life
In human medicine, a spectrum of hopes has been described from the initial cancer diagnosis to preparing for death.46 With a cancer diagnosis, a client’s initial hopes may center around curing the cancer and the pet living longer and then move toward spending special time with loved ones, finding meaning, and then seeking a peaceful death. This reflects the transition that many veterinary clients go through in caring for their companion animal with cancer. This breakpoint discussion is a crucial conversation that signals the transition from fighting for quantity of time to fighting for QOL.47 It can be challenging for clients who have been working so hard to fight the cancer to shift their energy to living the fullest life with their pet right now and preparing to let go (“It can be difficult to switch gears from fighting the cancer to preserving Mandy’s quality of life.” or “It seems like it may be helpful to focus on what time Mandy has left with you.” or “Just because we can do something does not mean that we should.”).
Eliciting the client and patient goals may help in moving the conversation forward (“Can we create a plan together to ensure Mandy’s quality of life?” or “Let’s focus on what we can do to help Mandy now.” or “What is most important to you in caring for Mandy at the end of life?”). A supportive way to acknowledge the client’s desire to do more is through expressions of “I hope” or “I wish” statements (“I wish there were something we could do to cure Mandy’s cancer.” or “I hope that Mandy has many good weeks ahead.”).48 At this stage, it is equally important to reflect on the oncologist’s conversational emphasis and what influence the presentation of information may have on client decision making49 such as how much time is spent talking about anticancer therapy compared to QOL, supportive care, or euthanasia. Inadvertently, the veterinarian can influence the client’s decisions because of the prioritization placed on the options for care.49
As a source of validation and support, clients may also need to hear from their oncologist that they did everything they could for their pet (“You have given Mandy every chance possible.”).50 Words of reassurance and partnership can be highly supportive such as “All along you have made your decisions with Mandy’s best interests in mind.” or “We will do this together, just as we have done everything that got us to this point.”).50 Clients are often overwhelmed and feel alone in the enormity of the decision to euthanize their pet, and it is comforting to know that their veterinarian will guide, advise, and inform them through the process.
Transitioning to Palliative Care
Fortunately, there is much that can be done for patient comfort, despite the inability to effect a cure, including symptom management, supportive care, and pain management to ease suffering. Depending on the resources in your region, it may be appropriate to refer the client and patient to a veterinary hospice service.51,52 Veterinary hospice is the patient care provided after a terminal diagnosis of weeks to months has been given and includes providing palliative treatment for the animal and emotional support for the family to prepare for the imminent death of the animal and focus on spending quality time together. At-home patient care entails administering medications, assessing and monitoring pain management, evaluating proper hydration and nutrition, and educating families about euthanasia, the grief process, and death and dying.51 Today, statements such as “there is nothing more we can do” can be replaced with words of encouragement and offers of partnership to comfort clients (“There is still much that we can do to make sure that Mandy is content and comfortable.”).49
Client and patient abandonment may be a concern that arises during this stage of care. The value placed on the client’s relationship with the oncologist may increase as the patient’s cancer progresses, as the desire for information lessens and the need for support grows.53 With the change in focus on the care provided from cancer treatment to palliative care, the client may perceive that the oncologist’s relationship with the client and patient has ended. Offering partnership helps create a sense of support for the client (“We will work through these decisions together.” or “I will be here to help you and Mandy whatever your decisions may be.”) Clients may want to hear explicitly that the oncologist will still be taking care of their pet, even if they decide to discontinue treatment. Depending on the client’s relationship with the oncologist and the primary care clinician, it may be critical to determine the client’s expectations and offer to maintain the relationship to provide end-of-life care. Caring for clients and patients at the end of life can be a source of meaning and fulfillment for the oncologist as well and an opportunity to recognize the special relationships formed during this difficult time.
Preparing Families for Euthanasia
Clients may be waiting for the oncologist to raise the option of euthanasia to give them permission to consider euthanasia as a valid and supported option (“One of the options that is important to discuss is hospice care and euthanasia”). Clients may be worried that the oncologist may perceive them as “giving up” if they bring up the option of euthanasia and therefore clients may need your validation (“It is a valid and caring decision to consider euthanasia at this time” or “Euthanasia is a humane option for Mandy given how the cancer has spread.”). Client anxiety results from the uncertainty that lies ahead, and a large part of these conversations is helping clients cope.47 Previously, clients had a clear plan for how to treat the cancer, and it may be helpful to have a designated path for how to care for their animal at the end of life. Discussing end-of-life wishes for the patient is crucial to preparing the client for euthanasia decision making, and creating a euthanasia plan often eases the client’s discomfort.41 Once completed, it can be put on the shelf until it is needed and the client can focus their energies on being present with their pet during these final precious days, weeks, or months. Being prepared ensures that the client’s needs are met and minimizes regrets during this difficult time of grief. Communication techniques for euthanasia decision making were previously published and provide guidelines on how to walk a client through making a euthanasia decision, discussing the procedure, and presenting options for location, body care, memorializing, and family presence.41,54
Providing Support for Grief and Loss
Research indicates that 70% of clients are affected emotionally by the death of their pet and that as many as 30% of clients experience severe grief in anticipation of or following the death of their pet.3 In addition, approximately 50% of clients studied reported feeling guilty about their decision to euthanize their pet. One of the factors contributing to client grief was the perception of the professional support provided by the veterinarian. The manner in which the veterinarian provides care for a client whose pet has died has the potential to alleviate or aggravate grief. A thorough description of client grief responses and techniques for providing emotional support were outlined in the previous edition of this textbook.54
Caring for Yourself
Compassion fatigue is deep physical, emotional, and spiritual exhaustion that can result from working day to day in an intense caregiving environment.55,56 The natural response to this downward spiral is to work harder until there is nothing left to give, which is counter to the adaptive response of taking a break. The symptoms are the same as those of chronic stress and are a consequence of caring for the needs of others before caring for your own needs.54 Compassion fatigue results from a lack of daily self-care practices that create opportunities to reflect, refuel, and rejuvenate. The good news is that feeling compassion fatigue results from being a deeply caring person. When oncologists care for themselves, they can care for others from a place of abundance not scarcity. With development of healthy self-care routines, practitioners can continue to successfully provide compassionate care to others. Recognizing the signs of compassion fatigue is the first step toward positive change, and the second step is making a daily firm commitment to choices that lead to resiliency. A thorough description of caregiver stress and stress management strategies was provided in the previous edition of this textbook.54
Conclusion
Given the growing expectations of clients, the strength of the human-animal relationship, and the resultant emotional impact of cancer communication on pet caregivers and the oncology team, relationship-centered care is integral to providing quality cancer care.5,21,22 Extrapolating from evidence in human medicine, compassionate cancer communication is related to significant clinical outcomes for the oncologist, client, and the patient, including enhancing client28-30 and veterinarian satisfaction,31,32 improving adherence to recommendations,33 working through emotions,38,39 and promoting patient health.34 Effective techniques for cancer communication can be taught and are a series of learned skills.15,25 Through supportive approaches, cancer communication can be made less distressing to the client, fostering client relationships and optimizing patient care while promoting professional fulfillment for the veterinarian.
References
1. Brown, JP, Silverman, JD. The current and future market for veterinarians and veterinary medical services in the United States. J Am Vet Med Assoc. 2004;225:161–183.
2. Lue, TW, Patenburg, DB, Crawford, PM. Impact of the owner-pet and client-veterinarian bond on the care that pets receive. J Vet Med Assoc. 2008;232:531–540.
3. Adams, CL, Bonnett, BN, Meek, AH. Predictors of owner response to companion animal death in 177 clients from 14 practices in Ontario. J Vet Med Assoc. 2000;217:1303–1309.
4. Blackwell, MJ. The 2001 Iverson Bell Symposium keynote address: beyond philosophical differences: the future training of veterinarians. J Vet Med Educ. 2001;28:148–152.
5. Coe, JB, Adams, CL, Bonnett, BN. A focus group study of veterinarians’ and pet owners’ perceptions of veterinarian-client communication in companion animal practice. J Vet Med Assoc. 2008;233:1072–1080.
6. Gorman, TE, Ahern, SP, Wiseman, J, et al. Residents’ end-of-life decision making with adult hospitalized patients: a review of the literature. Acad Med. 2005;80:622–633.
7. Buckman, R. Practical plans for difficult conversations in medicine: Strategies that work in breaking bad news. Baltimore: Johns Hopkins University Press; 2010.
8. Girgis, A, Sanson-Fisher, RW. Breaking bad news: current best advice for clinicians. Behav Med. 1998;24:53–60.
9. Back, AL, Arnold, RM. Discussing prognosis: “How much do you want to know?” Talking to patients who are prepared for explicit information. J Clin Oncol. 2006;24:4209–4213.
10. Tinga, CE, Adams, CL, Bonnett, BN, et al. Survey of veterinary technical and professional skills in students and recent graduates of a veterinary college. J Am Vet Med Assoc. 2001;219:924–931.
11. Butler, C, William, S, Koll, S. Perceptions of fourth-year veterinary students regarding emotional support of clients in veterinary practice and in veterinary college curriculum. J Am Vet Med Assoc. 2002;221:360–363.
12. North American Veterinary Medical Education Consortium, Roadmap for veterinary medical education in the 21st century: responsive, collaborative, flexible. American Association of Veterinary Medical Colleges 2010. [Draft Report].
13. Bylund, CL, Brown, R, Gueguen, JA, et al. The implementation and assessment of a comprehensive communication skills training curriculum for oncologists. Psycho-Onc. 2010;19:583–593.
14. Shaw, JR, Barley, GE, Hill, AE, et al. Communication skills education onsite in a veterinary practice. Patient Educ Couns. 2010;80:337–344.
15. Kurtz, SM, Silverman, J, Draper, J. Teaching and learning communication skills in medicine. Abingdon UK: Radcliffe Medical Press; 2005.
16. Venetis, MK, Robinson, JD, LaPlant Turkiewics, K, et al. An evidence base for patient-centered cancer care: a meta-analysis of studies of observed communication between cancer specialists and their patients. Patient Educ Couns. 2009;77:379–383.
17. Epstein, RM, Street, RL. Patient-centered communication in cancer care: Promoting healing and reducing suffering. Publication No. 07-6225. Bethesda, MD: National Institutes of Health; 2007.
18. Back, AL, Anderson, WG, Bunch, L, et al. Communication about cancer near the end of life. Cancer. 2008;113:1897–1910.
19. Back, AL, Arnold, RM. Discussing prognosis: “How much do you want to know?” Talking to patients who do not want information or who are ambivalent. J Clin Oncol. 2006;24:4214–4217.
20. Roter, DL, Larson, S, Rischer, GS, et al. Experts practice what they preach: A descriptive study of best and normative practices in end-of-life discussions. Arch Intern Med. 2000;160:3477–3485.
21. Shaw, JR, Bonnett, BN, Adams, CL, et al. Veterinarian-client-patient communication patterns used during clinical appointments in companion animal practice. J Am Vet Med Assoc. 2006;228:714.
22. Nogueira Borden, LJ, Adams, CL, Bonnett, BN, et al. Use of the measure of patient-centered communication to analyze euthanasia discussions in companion animal practice. J Am Vet Med Assoc. 2010;237:1275–1286.
23. Emanual, EJ, Emanual, LG. Four models of the physician-patient relationship. JAMA. 1992;267:2221–2226.
24. Roter, DL. The enduring and evolving nature of the patient-physician relationship. Patient Educ Couns. 2000;39:5–15.
25. Silverman, J, Kurtz, SM, Draper, J. Skills for communicating with patients. Abingdon UK: Radcliffe Medical Press; 2005.
26. Tresolini C: Pew-Fetzer Task Force. Health professional education and relationship-centered care. San Francisco: The Pew-Fetzer Task Force on Advancing Psychosocial Health Education, 1994, Pew Health Professions Commission on the Fetzer Institute.
27. Mead, N, Bower, P. Patient-centredness: a conceptual framework and review of the empirical literature. Soc Sci Med. 2000;51:1087–1110.
28. Bertakis, KD, Roter, DL, Putnam, SM. The relationship of physician medical interview style to patient satisfaction. J Fam Pract. 1991;32:175–181.
29. Buller, MK, Buller, DB. Physicians’ communication style and patient satisfaction. J Health Soc Behav. 1987;28:375–388.
30. Hall, JA, Dornan, MC. Meta-analyses of satisfaction with medical care: description of research domain and analysis of overall satisfaction levels. Soc Sci Med. 1988;27:637–644.
31. Levinson, W, Stiles, WB, Inui, TS, et al. Physician frustration in communicating with patients. Med Care. 1993;31:285–295.
32. Roter, DL, Stewart, M, Putnam, SM, et al. Communication patterns of primary care physicians. JAMA. 1997;277:350–356.
33. DiMatteo, MR, Sherbourne, CD, Hays, RD. Physicians’ characteristics influence patient’s adherence to medical treatments: results from the medical outcomes study. Health Psychology. 1993;12:93–102.
34. Stewart, MA. Effective physician-patient communication and health outcomes: a review. Can Med Assoc J. 1995;152:1423–1433.
35. Dysart, LM, Coe, JB, Adams, CL. Analysis of solicitation of client concerns in companion animal practice. J Am Vet Med Assoc. 2011;238:1609–1615.
36. Shaw, JR. Four core communication skills of highly effective practitioners. Vet Clinic Small Anim. 2006;36:385–396.
37. Neumann, M, Bensing, J, Mercer, S, et al. Analyzing the “nature” and “specific effectiveness” of clinical empathy: A theoretical overview and contribution towards a theory-based research agenda. Patient Educ Couns. 2009;74:339–346.
38. Fogarty, LA, Curbow, BA, Wingard, JR, et al. Can 40 seconds of compassion reduce patient anxiety? J Clin Oncol. 1999;17:371.
39. Roter, DL, Hall, JA, Kern, DE, et al. Improving physicians’ interviewing skills and reducing patients’ emotional distress. A randomized clinical trial. Arch Intern Med. 1995;155:1877.
40. Allen, E, Shaw, JS. Delivering bad news: a crucial conversation. Except Vet Team. 2010;2:17–19.
41. Shaw, JR, Lagoni, L. End-of- life communication in veterinary medicine: delivering bad news and euthanasia decision making. Vet Clin Small Anim Pract. 2007;37:95.
42. Fried, TR, Bradley, EH, O’Leary, J. Prognosis communication in serious illness: Perceptions of older patients, caregivers and clinicians. J Am Geriatr Soc. 2003;51:1398–1403.
43. Leydon, GM, Boulton, M, Moynihan, C, et al. Faith, hope and charity: An in-depth interview study of cancer patients’ information needs and information-seeking behavior. West J Med. 2000;173:26–31.
44. Jenkins, V, Fallowfield, L, Poole, K. Information needs of patients with cancer: Results from a large study in UK Cancer Centres. Br J Cancer. 2001;84:322–331.
45. Cassileth, BR, Zupkis, RV, Sutton-Smith, K, et al. Information and participation preferences among cancer patients. Ann Intern Med. 1980;92:832–836.
46. Clayton, JM, Butow, PN, Arnold, RM, et al. Fostering coping and nurturing hope when discussing the future with terminally ill cancer patients and their caregivers. Cancer. 2005;103:164.
47. Gawande, A. Letting go: What should medicine do when it can’t save your life? The New Yorker. 2010. [August 2].
48. Pantilat, SZ. Communication with seriously ill patients: Better words to say. JAMA. 2009;301:1279–1281.
49. Yeates, JW, Main, DC. The ethics of influencing clients. J Vet Med Assoc. 2010;237:263–267.
50. Harpham, WS. View from the other side of the stethoscope: “It’s okay”. Oncology Times. 40, 2011. [February 25].
51. Bishop, GA, Long, CC, Carlsten, KS, et al. The Colorado State University pet hospice program: end-of-life care for pets and their families. J Vet Med Educ. 2008;35:525–531.
52. Johnson, CL, Patterson-Kane, E, Lamison, A, et al. Elements of and factors important in veterinary hospice. J Vet Med Assoc. 2011;238:148–150.
53. Graugaard, PK, Holgersen, K, Eide, H, et al. Changes in physician-patient communication from initial to return visits: a prospective study in a haematology outpatient clinic. Patient Educ Couns. 2005;57:22.
54. Lagoni, L. Bond-centered cancer care: an applied approach to euthanasia and grief support for your clients, your staff, and yourself. In Withrow SJ, Vail DM, eds.: Withrow and McEwen’s small animal clinical oncology, ed 4, St. Louis: Saunders Elsevier, 2007.
55. Pfifferling, JH, Gilley, K. Overcoming compassion fatigue. Fam Prac Mngmt. 2000;April:39–44.
56. Figley, CR, Roop, RG. Compassion fatigue in the animal-care community. Washington, DC: Humane Society Press; 2006.