Tumors of the Mammary Gland
Mammary Gland Tumors in Dogs
Epidemiology
Mammary gland tumors are common in dogs and represent the most common neoplasms in sexually intact female dogs.1-6 The incidence rates reported vary, depending on the origin of the studies and characteristics of the source population. The current incidence of mammary tumors in the United States is lower than in many other countries due to the common practice of performing ovariohysterectomy (OHE) at a young age. Data from several European national or regional canine cancer registries, including Norway, Denmark, and Italy, provide information regarding tumor incidence in general, as well as details regarding the relative frequency of various tumors according to site, age, and breed. These registries consist of a population of predominantly sexually intact dogs and thus provide insight into the natural or true mammary tumor risk in unaltered dogs. Results from these registries show that mammary tumors are the most common tumors in female dogs and represent 50% to 70% of all tumors in this subset of the population.2,6 In general, open population-based and insurance-based studies may underestimate the true incidence of disease, especially if the diagnosis and subsequent registration require a surgical biopsy. Furthermore, the insured dog population may be skewed toward younger animals because of age restriction and may be void after the tenth year of age, which coincides with the peak incidence age of mammary tumor diagnosis.3,4 Early data from the surveys of Alameda and Contra Costa counties reported an estimated annual incidence rate of 257.7 malignant mammary tumors per 100,000 in intact female dogs.1 A more recent large Swedish study based on 80,000 insured female dogs, most of which were sexually intact, reported a rate of 111 mammary tumors (including both benign and malignant) per 10,000 dog-years at risk.3 This study also reported an increasing risk for tumors with advancing age; 6% of all 8-year-old dogs and 13% of all 10-year-old dogs were diagnosed with at least one mammary tumor. Another large insurance-based study from the United Kingdom reported an annual incidence rate of 205 mammary tumors per 100,000 dogs. This study included all mammary tumors regardless of histology.4
In addition to these open and more heterogeneous population-based studies, closed population studies provide another source of incidence and natural progression data. Longitudinal studies may provide a more accurate estimate of the total lifetime risk of mammary tumors because dogs are monitored closely and all tumors are noted, biopsied, and reported. In a large beagle colony morbidity and mortality study, 71% of female dogs developed at least one mammary gland neoplasm in their lifetime.7 However, this may not accurately represent the incidence in other breeds. Many of the various tumor registries have reported significant breed variations in mammary tumor incidence, suggesting that, in addition to age and hormonal factors, hereditary breed-associated genetic susceptibility also contributes to mammary tumor risk.
Risk Factors
As noted, the incidence of mammary tumors varies, depending on where the studies are performed and the specific characteristics of the population in terms of spay status, age, and breed distribution. Thus, in addition to providing data regarding incidence, the epidemiologic studies also help identify risk factors for mammary tumors. Three main factors have been identified that play important roles in mammary tumor risk: age, hormonal exposure, and breed. To a lesser degree, diet and body weight or obesity may also contribute to risk.
Age
Mammary tumors affect middle-aged and older dogs.1,8-11 Mammary tumors, especially malignant tumors, are extremely rare in dogs younger than 5 years old.1,8,12 The tumor risk increases with age and becomes significant when dogs turn 7 or 8 years old and continues to increase until the age of 11 to 13 years.8,12 Dogs with malignant tumors have been found to be significantly older than dogs with benign tumors: mean age of dogs with malignant tumors is 9 to 11 years and 7 to 9 years with benign tumors.13,14 The peak incidence age also depends on the lifespan of various breeds. In general, larger breeds have a naturally shorter lifespan and therefore tend to be younger than smaller breeds when they are diagnosed. These differences may be further exaggerated in high-risk breeds such as the English Springer spaniels.3,15
Hormonal Exposure
Many mammary tumors in dogs are preventable. Dogs spayed prior to their first estrus have only a 0.5 % risk of developing mammary tumors in their lifetime.16 The protective effect of OHE decreases quickly over the first few estrus cycles, and most studies have not found significant benefit after 4 years of age. According to Schneider’s original study, the risk increases and the benefit diminishes with each estrus cycle, as illustrated by an increasing risk of 8% and 26%, depending on whether the OHE was performed prior to the second or third heat cycle.16 This study found no significant risk reduction in dogs spayed after the second heat cycle, although other researchers have found some modest benefit in dogs spayed later.12,17,18 There is general agreement that the greatest benefit on mammary tumor prevention is seen if the dog is not allowed to go through any heat cycles, suggesting that the pivotal and irreversible effects of ovarian hormones on the mammary glands in terms of cancer risk occur early in life, likely during puberty when the mammary gland develops and matures. These findings may also explain why other factors resulting in physiologic variation in hormonal influence on the mammary tissues such as pseudopregnancy, pregnancy, or parity, which typically occur after a few estrus cycles, have not been found to significantly influence the tumor risk.12,16,19 Exposure to exogenous or pharmacologic doses of hormones (both progestins and estrogens), however, has been found to increase the risk for developing mammary tumors in dogs. Dogs treated with progestins are more likely to develop tumors and are younger when they do. According to the Norwegian Canine Cancer Registry, dogs treated with progestins to prevent estrus had a 2.3 times higher risk for mammary tumors when compared to dogs not receiving such treatment.20 Similarly, a Dutch study found that privately owned dogs with mammary tumors were significantly more likely to have received progestins.18 Numerous studies have investigated the effect of dose, duration, and type of hormones (progestins, estrogens, or combination of both) on mammary tumor development in laboratory dogs. Although some discordance exists, most conclude that low-dose progestins alone increase the risk for predominantly benign tumors, whereas a combination of estrogens and progestins tends to induce malignant tumors.21-25
Breeds and Genetic Susceptibility
In general, mammary tumors tend to be more common in the smaller breeds. Purebred dogs are more commonly affected1; poodles, Chihuahuas, dachshunds, Yorkshire terriers, Maltese, and cocker spaniels are frequently listed as high-risk breeds in the small-breed category.1,2,14,26 However, some of the larger breeds are also at increased risk, including the English Springer spaniel, English setters, Brittany spaniels, German shepherds, Pointers, Dobermans, and Boxers.1-3,14,26 Some noteworthy discrepancies exist, specifically between the U.S. and the European reports. Boxers are noted to have a decreased risk for mammary tumors according to data from the University of Pennsylvania, whereas Scandinavian studies reflect an increased risk in Boxers.2,3,14 A closed population beagle study also has shown that two different lines or families of beagles have very different mammary tumor risk.27 These results collectively support a genetic influence on mammary cancer development. Familial or inherited germline mutations in BRCA1 and BRCA2 account for 5% to 10 % of all human breast cancers and are associated with an 85% cumulative lifetime risk of breast cancer in affected individuals.28-31 Studies of BRCA mutations in canine mammary tumors have so far been limited to tumor gene expression studies and the results have varied; some found underexpression of BRCA1 in malignant tumors and others have documented overexpression of BRCA2 in metastatic tumors.32,33 Germline mutations in both BRCA1 and BRCA2 were found to be associated with significantly increased risk in English Springer spaniels in a large Swedish study.3,15
Other Risk Factors
Body weight, specifically during puberty (9 to 12 months), is found to have a significant effect on later mammary tumor risk; being underweight during this time period provides significant protection against later tumor development.17 This study did not find an increased risk for tumors in dogs fed a high-fat diet or dogs that were obese around the time of tumor detection. However, a subsequent case-control study did document an association between diet and mammary cancer in which obesity early in life and a diet high in red meat were found to increase risk.34 Obesity has also been recognized as a risk factor for developing postmenopausal breast cancer in women.35,36 One of the proposed mechanisms by which diet/obesity may be linked to breast carcinogenesis is via its effect on serum estrogen levels. Obesity is associated with decreased concentration of sex hormone–binding globulin and thus results in elevated serum free estrogen levels.37-41 In addition, adipose tissues may be a source of increased estrogen production via aromatase-mediated conversion of androgens. Interestingly, the mammary cancer–sparing influence of being underweight is most significant during the first year of life when the effects of the endogenous hormones are the greatest.
Tumor Biology: Development, Hormones, Growth Factors, and Clinical Implications
Based on the previous discussion of risk factors, it is clear that exposure to ovarian hormones is important in the development of mammary tumors in dogs. Both estrogens and progesterone are necessary for normal mammary gland development and maturation. The mammary glands undergo distinct clinical and histopathologic changes as hormone levels fluctuate according to the phases of the estrus cycle.42,43 Estrogens and progesterone are mitogens of breast epithelium and induce proliferation of intralobular ductal epithelium and development of ducts and lobules, resulting in expansion of the mammary glands. Historically, the tumorigenic effects of estrogen in human breast cancer were thought to be mediated via their receptor binding and enhanced production of growth factors resulting in increased cellular proliferation.44 However, more recent research shows that estrogen and its metabolites also have direct genotoxic effects by increasing mutations and induction of aneuploidy independent of the estrogen receptors.45-47 The tumorigenic effects of progesterone are in part thought to be mediated via a progesterone-induced increased mammary gland production of growth hormone (GH) and growth hormone receptors (GHR).48-50 GH has direct stimulatory effects on mammary tissues, as well as indirect effects via increasing insulin-like growth factor-1 (IGF-1).51 The GH/IGF-1 axis has been implicated in human breast carcinogenesis. IGF-1 is both a proliferative and a survival factor for breast epithelial cells and regulates the expression of numerous genes involved in breast cancer development.52-57 The complex dysregulation of growth factors and hormones that precedes, initiates, and potentially drives canine mammary tumorigenesis is far from understood; evidence exists indicating that both growth factors and steroid hormones are intrinsically implicated and contribute in an autocrine/paracrine manner. Malignant tumors have significantly higher tissue concentrations of GH, IGF-1, progesterone, and 17β-estradiol than benign tumors, and moreover, levels correspond with important clinicopathologic parameters, such as growth rate, size, and specific histopathologic type.58,59 A complete review of the biologic and molecular aspects of mammary tumor carcinogenesis is beyond the scope of this chapter, but recent reviews provide more complete information.60,61
The entire mammary chains are exposed to growth factors and sex hormones, resulting in a field carcinogenesis effect. Consequently, most dogs develop tumors in multiple glands.7,12,13,62-64 Histologic progression with increasing tumor size is often noted in dogs with multiple tumors and areas of transitions such as carcinoma in situ can be seen in benign tumors.13,62 This provides direct evidence that benign and malignant mammary tumors are not separate entities—instead, they are part of a biologic and histopathologic continuum in which the malignant invasive carcinomas are the endstage of the process. Earlier publications support this hypothesis and document associations between tumors of benign and malignant histology. For instance, dogs with carcinomas often had concurrent benign tumors of the same cell type and dogs with benign mammary tumors were at increased risk for developing subsequent malignant tumors.63 Furthermore, risk was even higher in dogs diagnosed with carcinomas or carcinoma in situ.13,62,64 Evidence of histologic progression has also been reported in which a high incidence of carcinoma in situ and intraepithelial lesions with atypia was noted adjacent to invasive carcinomas.62,65 These studies provide support for the hypothesis that mammary tumors develop initially from benign lesions and progress to invasive malignant lesions as part of a continuum influenced by hormonal field effects on mammary tissues. There are likely regional variations in terms of exposure, resulting in a range of histopathologic and clinical changes. Some tumors may never change and remain small and benign, whereas others progress and become malignant and many develop new tumors in other glands. This suggests that canine mammary tumors provide unique comparative opportunities to study mammary carcinogenesis and progression with direct applications to human breast cancer research.
Tumor Hormone Receptors: Prognostic, Clinical, and Therapeutic Implications
Hormonal exposure plays an important role in mammary tumor development, and many tumors, specifically tumors of epithelial origin, express hormone receptors (HRs), suggesting continued hormonal influence and dependence. Benign tumors are more likely than malignant tumors to retain HRs—both estrogen receptors (ER) and progesterone receptors (PR).66-70 The HR status is also influenced by age and hormonal status: dogs that are intact, younger, and in estrus are more likely to have receptor-positive tumors than dogs that are spayed, older, and anestrous.70,71 Furthermore, the HR expression is inversely correlated with tumor size and histopathologic differentiation; larger tumors and undifferentiated or anaplastic tumors are less likely to express receptors than tumors with more differentiated histology, reflecting a biologic drift toward hormone independence and corresponding with aggressive histology and clinical behavior.71-73 HR expression analysis is most commonly performed by immunohistochemistry (IHC). Results from various studies are quite disparate, especially in terms of ER-alpha positivity in malignant tumors, and range from 10% to 92%.66-74 These variations may in part be due to differences between study populations (tumor size, castration status, tumor types) and the fact that IHC methods vary and are neither standardized nor validated. This makes it difficult to consider HR expression results when making treatment decisions. Despite these discrepancies, several studies have documented that tumor expression of ER and PR is associated with a more favorable outcome.71-73 Endocrine therapy is recommended to all women with ER-positive tumors, regardless of intensity of staining, and results in significant improvement in the adjuvant setting.75-80 Currently, there are no published prospective studies on the predictive value of HR status and the effect or benefit of hormonal therapy in dogs with mammary tumors. Until results from such studies are available and found to be predictive, routine IHC for HRs is not likely to influence treatment decisions. In addition to the presence of HRs, the overexpression of human epidermal growth factor receptor-2 (HER2/erb-2), a member of the epidermal growth factor receptor (EGFR) family involved in signal transduction pathways that regulate cell growth and differentiation, may also provide clinical and prognostic insight, as well as therapeutic opportunities in mammary tumors. Overexpression or amplification of HER2 is found in 20% to 25% of all human breast cancer patients and is associated with aggressive behavior, resistance to hormonal therapy, and a poor prognosis.81-83 HER2 overexpression has also been documented in canine mammary tumors using the same HercepTest scoring systems used in human breast cancer and documented positive staining ranging from 17% to 29% in malignant tumors.84,85 HER2 staining was associated with negative histologic features and short survival according to one of these studies,84 but contrary to the human studies, HER2 expression was associated with an increased survival in two other independent studies.85,86
History and Clinical Presentation
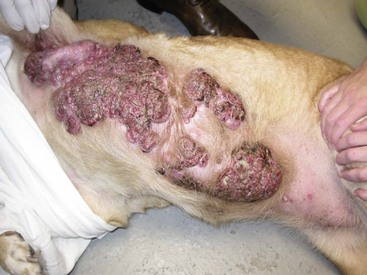
Mammary tumors are usually easy to detect through routine physical examinations. However, high-risk dogs, specifically older intact female dogs, should undergo a thorough examination of the mammary glands. Mammary tumors typically affect the two caudal pairs, where the mammary glands or tissues are naturally larger; thus careful palpation may be necessary to detect small tumors.7,12,63,64 The mammary glands should be palpated again under general anesthesia to ensure that all tumors are found and included in the surgical planning, and both chains should be carefully evaluated. A recent study documented that 70% of intact females had more than one tumor at diagnosis.13 The size of the tumor(s), stage of disease, and presence of systemic signs of illness vary widely. Inflammatory mammary carcinomas represent a rare but clinically important subset of mammary tumors in dogs. Affected dogs may easily be misdiagnosed as having mastitis or severe dermatitis because, rather than presenting with discrete well-circumscribed tumors, the entire mammary chain may appear edematous, swollen, warm, and painful (Figure 27-1).87,88 In addition to the extensive locoregional involvement, most dogs with inflammatory carcinomas have distant metastatic disease and signs of systemic illness.87,88 These dogs are therefore poor surgical candidates. The majority of dogs with mammary tumors are systemically healthy, and the tumors are confined to the mammary glands when they are diagnosed.
Clinical Assessment, Diagnosis, Work-Up, and Staging
Due to the risk of metastasis associated with mammary tumors, staging prior to initiating therapy is strongly recommended, especially if benign disease cannot be histologically confirmed. Minimal staging can include complete blood count (CBC), serum biochemistry, three-view thoracic radiographs, and fine-needle aspiration (FNA) of regional lymph nodes, even if palpably normal. Abdominal ultrasound may be indicated in dogs with suspected regional lymph node involvement or changes on preoperative blood work suggesting tumor-related or non–tumor-related serum biochemistry changes. Even though osseous metaplasia occurs occasionally with mammary adenocarcinoma and the mammary glands are a common site for extraskeletal osteosarcoma, there has been no prognostic value found for serum alkaline phosphatase activity.89,90 There may be value in performing mammary tumor cytology to help rule out nonmammary dermal and subcutaneous tumors (e.g., mast cell tumors, lipomas). Additionally, correlations between cytopathology and ex vivo histopathology have been reported to be between 67.5% and 93%,91,92 and the reported cytologic sensitivity and specificity for a malignant mammary tumor diagnosis were 88% and 96%, respectively.93 Computed tomography (CT) imaging of the thoracic cavity provides more sensitive detection of pulmonary nodules than does thoracic radiography, but this may not be applicable for every patient due to a need for general anesthesia, the increased expense, and decreased availability.93,94 Distant metastatic sites can include lymph nodes, liver, lungs, and bone.95
Lymphatic drainage of normal mammary glands is very complex, with documented drainage occurring to multiple ipsilateral lymph nodes and even to contralateral lymph nodes.96-98 The amount of variation in lymphatic drainage increases in the neoplastic mammary gland.96 Tumor-induced lymphangiogenesis, well documented in human breast cancer, may be responsible for the unpredictable and erratic location of susceptible or “at-risk” lymph nodes in dogs having malignant mammary tumors.99 Thus exclusive anatomic sampling of nearby lymph nodes is not sufficient for accurate lymph node staging and may miss the presence of locoregional disease.100
Staging System
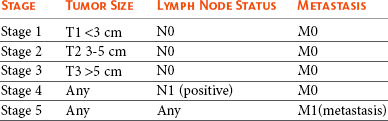
Mammary tumors are staged according to the T (tumor), N (lymph node), and M (metastasis) system. A modified version of the original staging system published by Owens101 is currently used by most oncologists and stage advances from I to II to III as the size of the primary tumor increases from smaller than 3 cm, to between 3 and 5 cm, to larger than 5 cm.102 These size categories capture important changes in prognosis and outcome. Lymph node metastasis represents stage IV disease, regardless of tumor size, and distant metastasis constitutes stage V disease. This staging system should be used for dogs with epithelial tumors (noninflammatory) and not sarcomas (Table 27-1).
Histopathology
The normal mammary gland is a complex branching structure and the histologic and immunohistochemical characteristics are equally complex—the interested reader is referred to a thorough review on the subject.103
Classification Systems
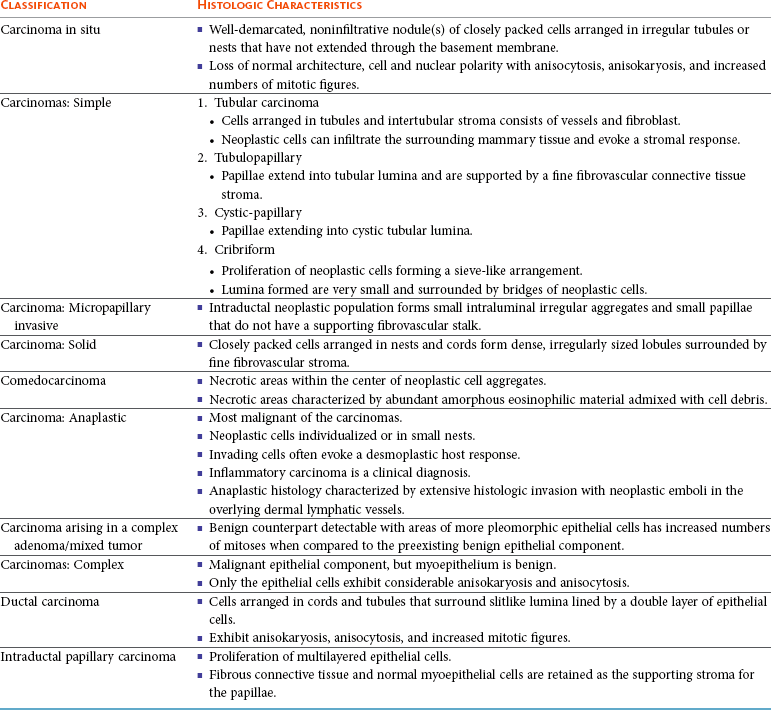
Two early classifications of canine and feline mammary tumors were published in 1974104 and 1999,105 and a revised system for the dog was published in 2011.106 In dogs, although there may be histopathologic evidence of malignancy, only a small percentage of cases will have lymphatic and vascular invasion and metastatic disease, whereas in cats the majority are malignant and metastasis to local lymph nodes is more common. The classification used in this text is based on both morphology106 and prognosis.107 When discussing the classification of mammary neoplasms the terms simple and complex are commonly used. Simple denotes that the neoplasm is composed of one cell type resembling either luminal epithelial cells or myoepithelial cells, whereas complex neoplasms are composed of two cell types, both luminal and myoepithelial cells.106
Canine Mammary Hyperplasia and Dysplasia
Several hyperplastic and dysplastic lesions are considered precursor lesions to the development of mammary neoplasms.106 These include duct ectasis, lobular hyperplasia (with or without secretory activity), lobular hyperplasia with fibrosis, epitheliosis, papillomatosis, fibroses (fibrosclerosis), and fibroadenomatous change (fibroepithelial hyperplasia, fibroepithelial hypertrophy, mammary hypertrophy).
Benign Mammary Neoplasms
Several types of benign mammary neoplasms exist in the dog and include adenoma, intraductal papillary adenoma (duct papilloma), ductal adenoma, fibroadenoma, myoepithelioma, complex adenoma (adenomyoepithelioma), and benign mixed tumors. A histologic description of these various entities is beyond the scope of this chapter, and interested readers are directed to a more thorough review.106
Malignant Canine Mammary Neoplasms
Several types of malignant epithelial tumors (Table 27-2) exist, including carcinoma in situ (well-demarcated, noninfiltrative nodule[s] that have not extended through the basement membrane) and a variety of carcinomas. In addition, several less common subtypes of malignant epithelial neoplasms are squamous cell carcinomas, adenosquamous carcinomas, mucinous carcinomas, lipid-rich carcinomas, and spindle cell carcinomas (including malignant myoepithelioma, squamous cell carcinoma–spindle cell variant, and carcinoma–spindle cell variant).106
Malignant Mesenchymal Neoplasms: Sarcomas
Malignant mesenchymal neoplasms include osteosarcoma, chondrosarcoma, fibrosarcoma, hemangiosarcoma, and carcinosarcoma (malignant mixed mammary tumor).106
Osteosarcoma is the most common mesenchymal neoplasm of the canine mammary gland, and there is often a history of recent rapid growth of a mammary mass that might have been present for some time. A proliferation of cells varies from fusiform to stellate to ovoid, and there is an association with islands of tumor osteoid and/or bone formation. Mitoses are frequently found. Metastasis occurs via the hematogenous route, mainly to the lungs.
Carcinosarcoma (malignant mixed mammary tumor) is composed partly of cells morphologically resembling the epithelial component and partly of cells morphologically resembling connective tissue elements, both types of which are malignant. It is an uncommon mammary neoplasm, but it most often presents as a carcinoma and osteosarcoma (see previous paragraph). The epithelial component metastasizes via lymphatic vessels to regional lymph nodes and the lungs, and the mesenchymal component metastasizes via the hematogenous route to the lungs.
Hyperplasia/Dysplasia/Neoplasia of the Nipple
Ductal adenoma and carcinoma are rare and involve only the nipple, with no neoplastic tissue in the underlying mammary gland. The nipple is enlarged and firm. The histopathology mimics that of ductal adenomas and carcinomas. Carcinoma with epidermal infiltration (Paget-like disease) is a neoplasm occasionally seen in the dog that mimics Paget’s disease of the nipple in women. The carcinoma is present within the mammary gland, and carcinoma cells, either as individual cells or small aggregates, are present within the epidermis of the nipple.
Histopathologic Prognostic Factors and Grading
The epithelial tumors are also graded according to specific histopathologic criteria. Several systems for both canine and feline tumors exist, most of which are based on the Elston and Ellis grading system,108 which incorporates information regarding (1) tubule formation, (2) nuclear pleomorphism, and (3) mitosis per 10 HPF (Table 27-3).109-111 Based on the total score derived from this system, the grade of the tumor will be determined: grade 1 (low score) is a well-differentiated tumor, grade 2 (intermediate score) is moderately differentiated, and grade 3 (high total score) is poorly differentiated (Table 27-4). Tumor grade has been found to provide consistent and reliable prognostic information.71,106-111 In addition to the grading system, information regarding vascular/lymphatic invasion, surrounding stromal invasion, lymph node involvement, and tumor type may also predict behavior.* Sarcomas are typically not graded according to this system, but the majority tend to be biologically aggressive tumors and associated with a very poor long-term survival.111,112
Table 27-3
Criteria Used for Histologic Grading of Malignancy in Feline and Canine Mammary Carcinomas
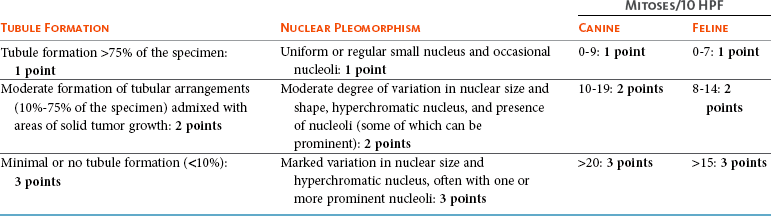
Table 27-4
Tumor Grade Based on Histologic Score in Felines and Canines
| Total Score | Grade of Malignancy |
| 3 to 5 | I (low) Well differentiated |
| 6 to 7 | II (intermediate) Moderately differentiated |
| 8 to 9 | III (high) Poorly differentiated |
Data from Misdorp W: Tumors of the mammary gland. In Meuten DJ, editor: Tumors in domestic animals, ed 4, Ames, Iowa, 2002, Iowa State Press; Clemente M, Perez-Alenza MD, Illera JC, et al: Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer, Vet Pathol 47:265-274, 2010; and Castagnaro M, Casalone C, Bozzetta E, et al: Tumour grading and the one-year post-surgical prognosis in feline mammary carcinomas, J Comp Pathol 119:263-275, 1998.
Mammary tumors in dogs represent a wide histologic spectrum with both benign and malignant lesions originating from different tissue types or a combination of tissues. Many dogs present with several different tumors and tumors of different types and can as such represent a rather daunting histopathologic picture; prognosis is determined by the most aggressive tumor, and decisions regarding adjuvant treatments should be based on the largest or the most aggressive histology. In many cases, the most aggressive tumor is the largest.13
Clinical Prognostic Factors
The three prognostic factors that are most consistently reported to be associated with prognosis include tumor size, lymph node involvement, and World Health Organization (WHO) stage (modified and original). These are the only factors that will be discussed here.
Tumor Size
According to MacEwen et al, dogs with tumor volume larger than 40 cc (approximately 3.4 cm in diameter) have a statistically significant worse outcome than smaller tumors, both in terms of remission and survival.114 Other investigators have classified tumors as stage I, smaller than 3 cm; stage II, between 3 cm and 5 cm; and stage III, larger than 5 cm.101,114 Dogs with tumors smaller than 3 cm were reported to have a significantly longer survival.11,71,115 Others, however, have found that a change in prognosis only becomes significant when tumors are larger than 5 cm.26,116 The change in prognosis is likely gradual as tumors increase in size. The modified WHO staging system has incorporated these three size categories representing stage I, stage II, and stage III, respectively.102 These stages are commonly used in mammary tumor staging. Importantly, however, the size of the tumor becomes irrelevant if the local lymph node is involved; according to Kurzman et al, the size of the primary tumor was not significant in dogs with local lymph node involvement.11 A positive lymph node constitutes stage IV disease according to the revised WHO system, attributing a worse prognosis to lymph node involvement rather than tumor size.
Lymph Node
A large retrospective study, including only dogs with carcinomas, all of which had the local or draining lymph node removed and biopsied, found that the status of the local lymph nodes was highly prognostic.11 Others have confirmed these findings.* Therefore information regarding the status of the local lymph node is extremely important when considering the need for adjuvant or systemic therapy in dogs with mammary tumors.
WHO Staging System
Both the original and the revised WHO staging system provide prognostic information. When performing a side-by-side comparison of the two systems, the revised system appears to better reflect the stronger impact of lymph node status on prognosis.103 Nevertheless, the original staging system also provides useful prognostic information as illustrated in two larger separate retrospective studies in which dogs with higher WHO stage disease had a significantly worse prognosis than dogs with lower stage disease.26,117
Therapy
The challenge in preparing surgical recommendations is the lack of uniform, robust prospective clinical trials that clarify the extent or “dose” of surgical excision: simple lumpectomy, mastectomy, regional mastectomy, chain mastectomy, or staged bilateral mastectomies. The goal of the surgery must be defined through staging and counseling with the owner. Is the goal to remove the current tumor(s) with clean margins or remove the current tumor(s) with clean margins and prevent new tumors in the remaining glands? The latter option would require prophylactic mastectomies of clinically normal glands in addition to affected glands.
Several studies have evaluated the effect of surgical dose in canine mammary tumors. A prospective randomized trial of 144 dogs with naïve malignant tumors comparing the overall survival benefit and disease-free interval (DFI) relative to either chain mastectomy or simple mastectomy found no differences.114 Similarly, a retrospective case series of 79 dogs treated at a single institution found no difference in overall survival or DFI comparable to the type of surgical procedure performed, whether lumpectomy, mastectomy, regional mastectomy with en bloc lymph node excision, or chain mastectomy with en bloc lymph node excision.117 However, the relative hazard for death within the first 2 years after surgery was slightly higher for dogs receiving a regional mastectomy over a chain mastectomy.117 Interestingly, in the study by MacEwen et al, the hazard curves for DFI and survival were quite similar, suggesting that most dogs that experienced recurrence developed metastasis and not new tumors. However, the rate of new tumors was not reported in this study.114 A differing study indicates that surgical “dose” is important. In this case series of 99 dogs, all intact female dogs underwent either a regional or chain mastectomy for a single mammary gland tumor with unknown histology.118 Of these, 58% of dogs developed a new tumor in the remaining ipsilateral mammary gland tissue following a regional mastectomy and those whose initial tumor was subsequently determined to be malignant were more likely to develop an ipsilateral tumor. The authors advocated for an initial unilateral chain mastectomy for female intact dogs with a single mammary tumor, although, in their population, 42% of dogs did not develop a subsequent tumor and would have experienced a larger surgical dose than needed.118 Unfortunately, other large useful studies investigating the association between OHE and survival did not report on the completeness or extent of mammary tumor removal.18,119,120 Development of second mammary tumors is well documented and has been reported in over 70% of dogs with malignant mammary tumors following lumpectomy, although the impact of second mammary tumor development on survival is not clear.16,114,118 It seems intuitive that a single standardized guideline for surgical treatment omits consideration of factors such as the dog’s age, tumor size, tumor number, previous mammary tumors, and stage and may not provide the optimal outcome. Future carefully constructed clinical trials may offer more tailored recommendations based on the individual patient’s risk.
Current recommendations based on available data suggest that for dogs with a single mammary tumor of known or unknown histotype, surgical excision wide enough to completely remove the mammary gland tumor is adequate. Incomplete excision or cytoreductive procedures are not endorsed.95 Tumors that are fixed or have skin ulceration and are less than 1 cm in diameter may be sufficiently managed with a mammectomy (Figure 27-2).95 “Wide excision” has not been well defined, but for larger tumors, this may be generalized to a 2-cm lateral margin and modified according to the size of the patient and tumor.95 The deep margin may need to include the abdominal muscular fascia and/or portions of the abdominal wall to be excised en bloc with the mammary tumor, depending on size and fixation.95 If abdominal surgery is to be performed simultaneously for OHE, penetration of the tumor prior to abdominal entry is to be avoided to prevent direct spread of tumor cells; rather, tumor removal should follow abdominal closure. For animals with multiple mammary tumors, more extensive resections such as a regional mastectomy or unilateral chain mastectomy may need to be pursued. As with other tumor resections, surgical margin assessment is critical for malignant mammary tumors, and additional surgery should be pursued if incompletely excised. Elective unilateral or bilateral chain mastectomies may be reasonable for young intact bitches with multiple tumors because there is a suggestion for development of additional tumors (Figure 27-3).95 In spite of the evidence for recurrent mammary gland tumor development, there is no sufficiently compelling evidence at this time for routine recommendation of complete, unilateral or bilateral chain mastectomies.
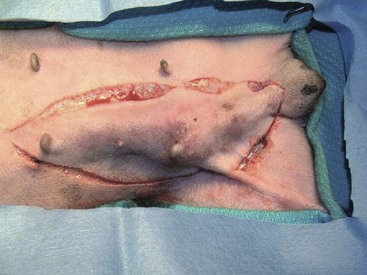
Figure 27-2 Regional mastectomy in a dog. (Courtesy Dr. Julius Liptak, Alta Vista Animal Hospital, Ottawa, Canada.)
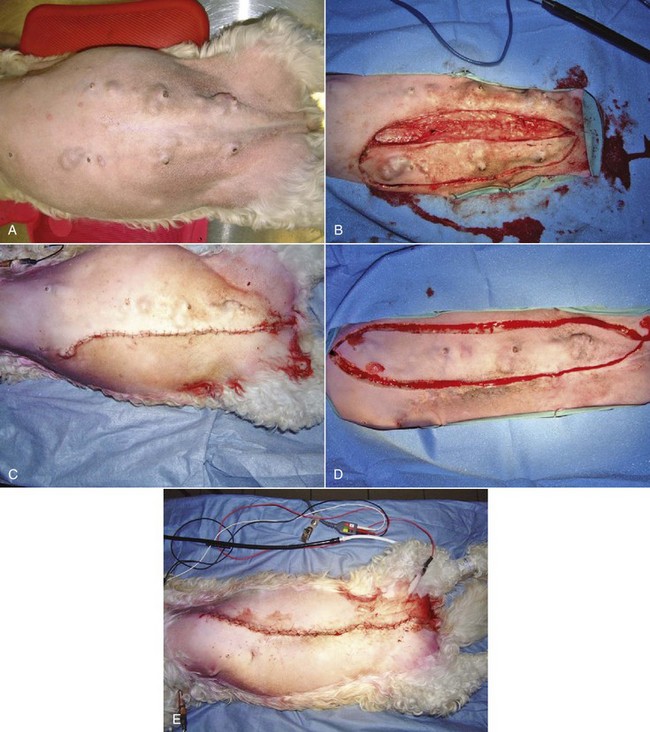
Figure 27-3 A, Multiple bilateral mammary tumors in a dog with taut abdominal tissue. B, A staged left chain mastectomy was performed initially of the side with the larger tumors. C, Immediate postsurgical appearance following the staged unilateral chain mastectomy without undue tension. D, The staged right chain mastectomy was performed 6 weeks later. E, Immediate postsurgical appearance following completed resection of all mammary tumors in this dog. (Courtesy Dr. Julius Liptak, Alta Vista Animal Hospital, Ottawa, Canada.)
Surgical excision is questionable as an adjuvant treatment for dogs presenting with inflammatory carcinoma due to the profound diffuse microscopic extent of cutaneous disease, the significant metastatic rate, and the local tissue coagulopathy that may be present. In 43 dogs with inflammatory carcinoma, only three dogs were considered suitable for unilateral chain mastectomy based on physical examination, yet all three had residual neoplastic cells at the surgical margins.88 Interestingly, two of the dogs also received adjuvant chemotherapy and were among the longer survivors in that study.88
In women, the use of sentinel lymph node mapping has dramatically altered the surgical treatment of breast cancer. As canine mammary carcinoma has been demonstrated as a relevant model for human disease,121,122 incorporation of human lymph node staging techniques should be reconsidered for the dog.100 Sentinel lymph node mapping is a means of detecting which nodes are receiving draining tumor lymph and thus most at risk for lymph node metastasis. Some techniques described for sentinel lymph node mapping in the dog include lymphoscintigraphy using technetium, contrast-enhanced ultrasonography, autogenous hemosiderin, and intraoperative dyes (Figure 27-4).97,100,123-125 The first description of sentinel lymph node mapping in any animal was done in cats 20 years ago.126 Benefits include greater ease in identifying the at-risk lymph nodes intraoperatively with minimal surgical incisions and with efficiency, especially for the rarely assessed axillary nodes. The prognostic value of sentinel lymph node mapping is currently unknown for the dog as is the presence of mammary carcinoma lymph node micrometastasis.127
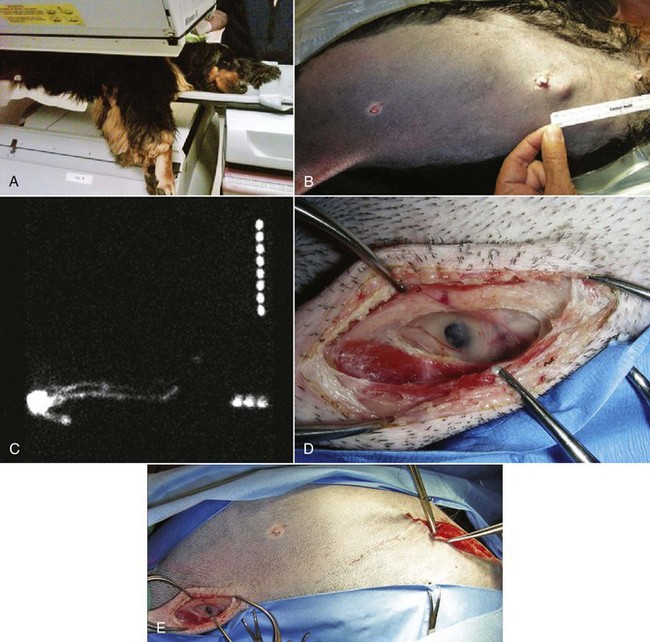
Figure 27-4 A, Regional lymphoscintigraphy being performed in a dog with a single mammary carcinoma. Technetium was injected in four quadrants around the primary tumor in the cranial abdominal mammary gland. B, Gross image of the same tumor in vivo. C, Regional lymphoscintigram of the patient highlighting radiopharmaceutical uptake in the mammary tumor and in the sentinel ipsilateral axillary lymph nodes. D, Close-up surgical appearance of a “hot” and “blue” sentinel accessory axillary lymph node visualized on the lymphoscintigram enhanced with intraoperative methylene blue dye mapping. E, Surgical field highlighting the distance between the mammary tumor and the same sentinel lymph node. (Courtesy Dr. Deanna Worley, Colorado State University, Fort Collins, Colorado.)
Systemic Treatment
Few clinical studies investigate systemic therapy for mammary tumors, and its efficacy has not been evaluated and confirmed according to the highest evidence-based standards. Despite this uncertainty, systemic therapy is routinely recommended and administered in dogs with high-risk tumors. This practice is based on the recognition that dogs with large tumors, positive lymph nodes, and aggressive histology are not treated effectively with surgery alone. The use of hormonal therapy in canine mammary tumors is based on tumor hormone dependence (tumor risk and HRs), as well as the potential to significantly reduce recurrence and prolong survival in HR-positive cancers similar to human hormonal therapy. This can be achieved by surgical means (ovariectomy [OVE] or OHE) or medical means, including specific ER modulators (SERMs) and suppression of estrogen synthesis by aromatase inhibitors or luteinizing hormone-releasing hormone (LHRH) agonists. Tamoxifen, an ER antagonist commonly used in women with ER-positive breast cancer, has been evaluated in dogs both with and without mammary tumors. Due to the side effects, mostly from proestrogenic signs, this strategy may not be tolerable or feasible in dogs.128,129 Surgical ovarian ablation, specifically OVE/OHE, is a more practical solution in the dog. The results are in discordance, however. The majority of earlier publications did not report survival benefit in ovariohysterectomized dogs compared to intact dogs.16,114,115,128,130
Despite some study flaws, it is also possible that hormonal therapy is not effective. Dogs with large primary tumors, lymph node involvement, and undifferentiated histology have a poor prognosis and are less likely to have HR-positive tumors; thus they are less likely to benefit from hormonal ablation. A few studies have found that OHE significantly improves survival in dogs with mammary carcinomas or complex carcinomas. One study found the timing of OHE in relation to tumor surgery was important because only dogs with OHE within 2 years prior to or concurrently with tumor removal benefited. Dogs with OHE more than 2 years prior to surgery had a similar survival to dogs that remained intact.120 Another study found that the benefit of OHE was only significant in dogs with complex carcinomas.117 It is clear that only an adequately powered prospective randomized trial in which the effect of OHE is analyzed in context of HR status can answer questions regarding hormonal therapy in dogs with mammary tumors.
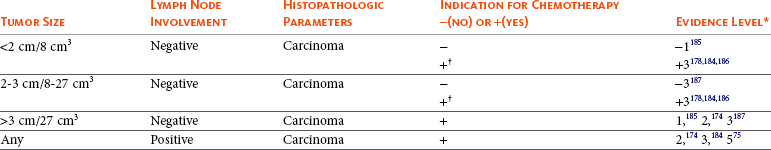
Chemotherapy is often administered to dogs with mammary tumors considered to be at risk for metastasis or recurrence. Most of the evidence regarding the efficacy of adjuvant chemotherapy is weak. A prospective nonrandomized study on dogs with high-risk mammary tumors (stage III or IV) did show a significant survival benefit in dogs receiving a combination of 5-fluorouracil and cyclophosphamide adjuvant to surgery compared to dogs treated with surgery alone.131 Today, anthracycline or taxane combinations are considered part of first-line protocols in human breast cancer therapy in women requiring adjuvant therapy75,132-134; however, only inadequately powered studies on the efficacy of doxorubicin or docetaxel in dogs with mammary tumors exist and they did not establish benefit.135 Similarly underpowered investigations of adjuvant gemcitabine in dogs with advanced stage (IV or V) mammary carcinoma did not establish benefit.136 Adequately powered, randomized prospective trials are needed to determine the future role of adjuvant chemotherapy in dogs with high-risk mammary tumors. Systemic treatment, including nonsteroidal antiinflammatory drugs (NSAIDs), with or without chemotherapy, was found to be effective in prolonging survival in dogs with inflammatory carcinomas according to two independent retrospective case series.88,137 Chemotherapy may also have a role in the treatment of primary mammary gland osteosarcoma. The mammary gland is one of the most common sites for extraskeletal osteosarcoma and according to a small retrospective case series (including primary mammary gland osteosarcoma, as well as other extraskeletal sites), dogs treated with adjuvant chemotherapy were significantly less likely to die from tumor-related causes than dogs treated with surgery alone.138 More recently, a prospective randomized trial documented significant improvement in survival in dogs with histologic grade 2 or 3 carcinoma treated with perioperative desmopressin.139 The antimetastatic properties of desmopressin are not fully understood, but it is hypothesized that they in part are mediated through improving hemostasis and preventing cancer cells from gaining access to the vasculature during surgical manipulation.140,141 The results are intriguing, and further confirmatory studies are warranted. As illustrated previously, there is currently a paucity of high-quality trial evidence from which to draw information and guidance for treating dogs with malignant high-risk mammary tumors. Table 27-5 provides general guidance and treatment consideration/options and the level of supporting evidence.
Table 27-5
Prognostic Factors and Indication for Adjuvant Chemotherapy with Supporting Level of Evidence in Dogs with Malignant Mammary Tumors

*Evidence level 1: Prospective randomized trial (PRT); level 2: Prospective, nonrandomized trial (PT); level 3: Retrospective (RT); level 4: Case report(s) (CR); level 5: Extrapolation from human breast cancer studies (EH).
†Chemotherapy may be considered if unfavorable histology (vascular invasion or high grade).110-113
‡Dogs with stage III disease according to the original WHO staging system were included.131 Stage III disease includes dogs with tumors >5 cm. with or without lymph node metastasis.101
Mammary Tumors in Cats
Epidemiology
There are fewer epidemiologic studies regarding the incidence of mammary neoplasia in cats compared to dogs. Furthermore, due to differences in veterinary care for cats, the available data likely underestimate the true incidence of disease. According to data from one of the largest Swedish insurance companies, approximately 40% to 50 % of all dogs had insurance to cover veterinary expenses, whereas only 20% of cats had such coverage.143,144 Another study from the United States also reported that a significantly lower percentage of cats than dogs receives regular veterinary care.145
The overall mammary tumor incidence is lower in cats than in dogs. According to the California Animal Neoplasia Registry (CANR), mammary tumors represent the third most common tumor type in female cats (after skin tumors and lymphoma), with an annual incidence rate of 25.4/100,000 (compared to 198/100,000 in female dogs), and 12% of tumors in cats regardless of sex.1 Data from an animal tumor registry from two provinces in northern Italy reported that mammary tumors represented 16% of all tumors in cats and 25% in female cats.146 Data from the Swedish insurance company indicate that mammary tumors were the most common cancer, representing 40% of all tumor-related claims in cats.143 It is unclear whether the higher relative incidence of mammary tumors in the latter studies is due to differences in neutering practices or use of progestins in the source population because no information regarding spay status was provided.
Risk Factors
Three main risk factors in cats have been identified: age, breed, and hormonal influence.
Age
As in the dog, mammary neoplasia is a disease seen predominantly in middle-aged to older cats. The mean age of diagnosis is between 10 and 12 years of age.1,147-150 Risk increases incrementally with age but does not become significant until 7 to 9 years, according to the age-specific incidence curves from the CANR, and continues to increase up until 12 to 14 years.1
Breed
Siamese cats are significantly younger when diagnosed with mammary tumors, and risk plateaus at 9 years of age.151 In general, genetic predisposition for a disease is often associated with a younger age of diagnosis. Siamese cats appear overrepresented when compared to other breeds.151,152 However, Siamese cats have an increased risk for many tumor types, not only mammary tumors.153-157 It is therefore possible that the increased incidence in Siamese cats is due to breed-associated germline alterations in common tumor susceptibility genes or defective tumor suppressor gene function that confers increased risk for many different malignancies.
Hormonal Association
Exposure to ovarian hormones is also strongly implicated in mammary tumorigenesis in the cat. According to Dorn et al, sexually intact cats have a sevenfold higher risk than spayed cats.1 The increased risk in intact cats has been confirmed by others.148,151,158 Similar to findings in dogs, exposure from ovarian hormones in cats at an early age appears crucial. The protective effect of OHE diminishes quickly over the first few years; risk reductions of 91%, 86%, and 11% are seen in cats that are ovariohysterectomized before 6 months, between 7 and 12 months, or 13 and 24 months, respectively. No benefit was found after 24 months.158 According to the same study, parity did not influence risk for mammary tumors.
In addition to endogenous ovarian hormonal influence, exposure to exogenous progestins also increases risk. Cats treated with progestins have an overall relative risk of 3.4 compared to those not receiving such treatments, although benign tumors arise more commonly than malignant tumors (relative risk 5.28 versus 2.8).148 Unlike in dogs, progestin-treated cats were not younger than nontreated cats when they developed tumors.148 The tumorigenic effects of oral progestins in cats are supported by reports of male cats with mammary tumors. Mammary tumors are rare in males, but in a report of 22 cases, 8 (36%) had a history of progestin use.159 In a recent case series of three male cats with mammary tumors, all had received multiple injections of a long-lasting (depot) progestin over 5 to 6 years prior to tumor development. All had malignant tumors and all developed subsequent malignant tumors in other glands after initial surgery.160 Shorter duration of treatment or inconsistent administration is less likely to result in malignant tumors but nevertheless induce changes in the mammary glands.161 Fibroepithelial hyperplasia is the most common histopathologic change in cats treated for shorter periods of time and can occur relatively quickly, even after one injection. However, studies show that regular and prolonged administration is needed for malignant tumors to develop.148
Tumor Biology: Development, Hormones, Growth Factors, and Prognostic Implications
The risk for mammary tumor development in cats is determined by exposure to ovarian hormones early in life, but the latency period appears long because most cats are older when diagnosed. In many species, ovarian hormones are necessary for normal mammary gland development and maturation, but few studies have examined hormonal effects on mammary tumorigenesis in cats. The complex interactions between sex hormones, growth hormones, and IGF have been discussed in more detail in the section on canine mammary tumors, but progestin-induced mammary production of growth hormone has been documented in the cat.162,163 It is, however, biologically plausible that the tumorigenic effects on mammary tissues are similar across species and that the same general mechanisms are involved, specifically sex hormones and growth hormones. Despite ER and PR expression being implicated in the initial stages of mammary tumor development, many investigators have reported that most feline mammary carcinomas are ER and PR negative, although slightly more than one-third are PR positive.69,164-167 The percentage of ER/PR expression varies between studies and is likely the result of differences in case selection, methods, and interpretation of the results. The biochemical method, the dextran-coated charcoal (DCC) method, may be more sensitive than IHC when analyzing ER in cats.166 Standardized IHC methods have high concordance with DCC methods; 38.5% of the malignant tumors and 66.7% of the benign lesions expressed PR according to IHC.166 In this particular study, sexually intact cats were more likely to have PR-positive tumors. Lower concordance was found between ER analysis by DCC and IHC, with IHC being less sensitive than DCC; only 20% of the malignant tumors expressed ER according to IHC compared to 44% according to the DCC assay.166 These results are consistent with other publications showing a relatively low ER expression in feline mammary tumors when using IHC.
The low HR-positivity in the tumors is consistent with the higher rate of malignancy and a more aggressive clinical behavior in feline mammary tumors. In contrast to malignant tumors, normal mammary tissue and dysplastic lesions in the mammary gland express both ER and PR.69,164,165 However, this hormone dependence appears to wane with histologic progression from benign to malignant. None of the intermediate- or high-grade ductal carcinomas in situ were ER or PR positive, whereas the normal and hyperplastic adjacent mammary tissue expressed receptors.69,164,165 Fibroepithelial hyperplasia, a progesterone-induced change, has been reported to have high PR expression161 and can be effectively treated by OHE or antiprogesterones (Figure 27-5).168
Figure 27-5 Fibroepithelial hyperplasia in a cat. (Courtesy Dr. Lisa Mestrinho, Faculdade de Medicina Veterinária, Universidade Lusofona de Humanidades e Tecnologias, Lisboa, Portugal.)
In human breast cancer, an inverse relationship between the HR status and HER-2/neu expression is documented. HER-2/neu expression tends to be higher in cats than in dogs and humans; however, a wide range (5.5% to 90%) of HER-2/neu-positive tumors is reported.169-172 Although the clinical and histologic association with HER-2/neu expression varies, cats with spontaneous mammary carcinomas may be good models for HER-2/neu-positive, hormone refractory breast cancer in women.
History and Clinical Presentation
Cats with mammary tumors are often older and may be sexually intact or spayed after they were 2 years old. Tumors are easy to detect on physical examination and appear as firm discrete mass(es) in the mammary gland(s). One study reported that all glands are equally susceptible to tumor development, but a later study showed that the anterior glands were less commonly affected.173,174 Multiple tumors are common; 60% of cats had more than one tumor at diagnosis in one report.149 Careful examination of the remaining mammary glands is important when evaluating a cat with a prior history of mammary tumors, especially if treated with simple mastectomy, because new primary tumors are common. Tumor(s) size at diagnosis depends on how early it is detected and how aggressive the tumor behaves. Larger tumors may become ulcerated, inflamed, and infected. Local lymph nodes may or may not appear enlarged. Inflammatory mammary carcinomas are rare in cats, and the clinical picture and outcome are similar to those described in the dog.175
Clinical Assessment, Diagnosis, Work-Up, and Staging
Cats with mammary masses tend to be older and their tumors are commonly malignant; therefore, a thorough work-up is recommended to ascertain any comorbidity as well as advanced disease. This may include at a minimum a CBC, serum biochemistry, serum T4 concentration, three-view thoracic radiographs, abdominal ultrasound, and urinalysis, in addition to FNA of any mammary masses and any palpable (including normal-sized) regional lymph nodes.
Staging System
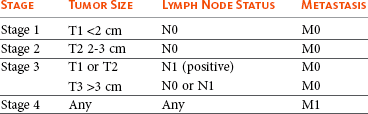
Feline mammary tumors are staged similar to canine tumors using a modification of the original system published by Owens.101,176 In the modified system, stage advances from I to II to III as the size increases from smaller than 2 cm, to between 2 and 3 cm, to larger than 3 cm.102 Unlike the canine system, stage III disease also includes T1 or T2 tumors with concurrent lymph node metastasis and lymph node metastasis does not need to be present with T3 tumors. Stage IV disease is any tumor with any lymph node metastasis and distant metastasis.176 This staging system should not be used with mammary gland sarcomas (Table 27-6).
Histopathology
The vast majority of feline mammary tumors are malignant (85% to 95%), with an aggressive biologic behavior, and lymphatic invasion and lymph node metastasis are more common at the time of initial diagnosis than in dogs. Early classifications of feline mammary tumors were simpler than that used for canine tumors105; however, several new tumor types have subsequently been reported.
Hyperplasia and Dysplasia105
The various hyperplastic and dysplastic lesions included in this group include duct ectasia, lobular hyperplasia, lobular hyperplasia with secretory (lactational) activity, lobular hyperplasia with fibrosis (interlobular fibrous connective tissue), epitheliosis (intralobular ducts), papillomatosis (interlobular ducts), and fibroadenomatous change.177 Fibroadenomatous change (fibroepithelial hyperplasia, fibroepithelial hypertrophy, mammary hypertrophy) is more common in the cat than the dog and is characterized by the proliferation of interlobular ducts and periductal stromal cells. The stroma is often edematous or myxomatous, and both the ductal and stromal cell nuclei exhibit some pleomorphism with mitoses. This lesion is hormonally induced and occurs in progestin-treated female and male cats, as well as being associated with pregnancy. Most cases regress at the end of pregnancy or cessation of progestin treatment.
Benign Feline Mammary Neoplasms105
Benign tumors in cats are uncommon and include adenoma, ductal adenoma, fibroadenoma, and intraductal papillary adenoma (duct papilloma).
Malignant Feline Mammary Neoplasms105,172,175,177-183
The predominant malignant tumor types in cats are simple and epithelial in origin and as such represent carcinomas of various types. Adenocarcinomas, tubular carcinomas, or a combination of tubular, papillary, and solid carcinomas are most common. Other variants include cystic papillary carcinoma, cribriform carcinoma, micropapillary invasive carcinoma, comedocarcinoma, and less commonly, squamous cell carcinoma, mucinous carcinoma, and lipid-rich carcinoma.
Histopathologic Prognostic Factors and Grading
Grading was initially thought not to be prognostic in cats; therefore the classification of mammary tumors was based on morphologic criteria only. More recently, grading, using a system similar to that in dogs (based on the Elston and Ellis scoring system; see Tables 27-3 and 27-4), has been shown to be prognostic in cats. In addition to grade, lymphovascular invasion and lymph node metastasis are independent prognostic factors.178,184 Thus the histopathologic criteria used in dogs (i.e., grade, vascular invasion, lymph node status) can be used in cats when assessing risk for metastasis and prognosis and should be incorporated into decisions regarding the need for systemic treatment in cats with mammary tumors (Table 27-7).
Table 27-7
Prognostic Factors and Indications for Adjuvant Chemotherapy with Level of Supporting Evidence in Cats with Malignant Mammary Tumors
*Evidence level 1: Prospective randomized trial (PRT); level 2: Prospective, nonrandomized trial (PT); level 3: Retrospective (RT); level 5: Extrapolation from human breast cancer studies (EH).
†Vascular invasion and high grade were found to be independent negative prognostic factors in multivariate analysis.
Clinical Prognostic Factors
Few studies reporting prognostic factors in cats with mammary tumors are prospective, only one is randomized, and most are underpowered or not stratified according to treatment. Therefore the results vary and may be significantly affected by bias. Tumor size has, however, consistently been reported to have prognostic significance, including the results of two large prospective studies.
Tumor Size and Prognosis
Three size categories have shown prognostic significance: (1) smaller than 8 cm3 or smaller than 2 cm diameter; (2) larger than 8 to 27 cm3 or larger than 2 to 3 cm diameter; and (3) larger than 27 cm3 or larger than 3 cm diameter. Cats with small tumors (<2 cm) can be effectively treated with surgery alone with a median survival time (MST) of more than 3 years, whereas cats with tumors larger than 3 cm have a MST of only 6 months according to a large, high-quality retrospective study.187 Cats with 2- to 3-cm diameter tumors survived an average of 2 years. Several other publications have confirmed this association between survival and tumor size.150,152,174,185,186 Tumor size is one of the most important factors when assessing risk for metastasis and the need for adjuvant treatments.
Lymph Nodes and Prognosis
Surprisingly few studies have evaluated lymph node status and its prognostic significance in cats with mammary tumors. In a large prospective study of 202 cats, those with lymph node metastasis had a significantly shorter survival than cats with negative lymph nodes.174 A recent retrospective study with 92 cats supported these findings; all cats with lymph node metastasis died within the first 9 months of diagnosis.184
Breed and Prognosis
Domestic shorthair cats had significantly better outcomes than purebred cats in a prospective randomized trial of cats with mammary carcinomas.185 A recent retrospective case series reported that Siamese cats had a worse prognosis than domestic shorthairs.188 These studies may in fact complement each other; however, the first study did not provide information regarding how many Siamese cats were included in the purebred group. Several other studies have not found breed to be prognostic when adjusted for other factors.
Age
The results regarding age and prognosis are conflicting. Several studies report that older cats have a worse prognosis; however, bias due to differences in treatments or differences in tumor size and stage may exist. Importantly, a prospective randomized trial found no difference according to age when comparing cats that were younger or older than 10 years.185
Surgical Treatment
The surgical dose recommended for treating feline mammary tumors is much clearer than in the dog. A chain mastectomy (unilateral for cats possessing a single tumor or a 2-staged bilateral chain mastectomy for cats with bilateral tumors) resulted in a favorable statistically significant DFI, as opposed to cats receiving conservative tumor excision in a series of 100 cats.187 In that same case series, there was a trend for improved survival times for cats receiving chain mastectomies.187 In a retrospective case series of 53 cats, while no significant relation was found between the type of surgical procedure performed, cats experienced longer DFIs following either unilateral or bilateral chain mastectomies.152 In a multiinstitutional retrospective case series comparing patients receiving surgery versus surgery with adjuvant doxorubicin-based chemotherapy, the subset of cats having unilateral chain mastectomies followed with chemotherapy had significantly longer MSTs than cats having unilateral chain mastectomies performed without chemotherapy (1998 versus 414 days).176 In that series, local recurrence developed in 50% of cats and, although not statistically significant, appears to support the use of chain mastectomies for feline mammary carcinomas.176 In a report of male cats diagnosed with mammary carcinomas, a trend toward more frequent local recurrence correlated with more conservative resections.159 Thus, for cats, a unilateral or staged bilateral chain mastectomy is recommended for treatment of mammary carcinoma. For some cats with excessively loose mammary tissue, a bilateral chain mastectomy can be performed during a single surgical session if minimal postsurgical tension can be achieved, but subjectively these cats may have a more difficult recovery (Figure 27-6). For tumors that are fixed, muscular fascia or portions of the body wall should be included with en bloc resections.
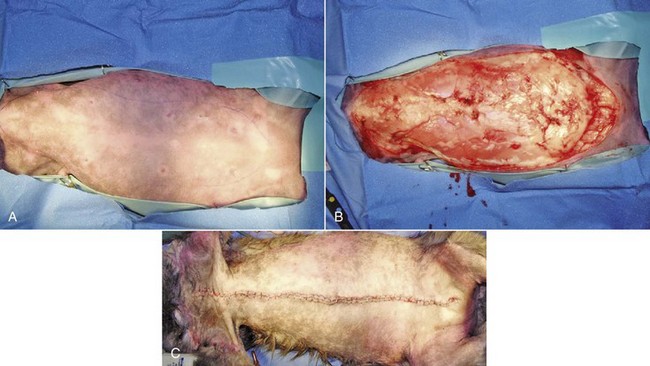
Figure 27-6 A, Cat having mammary adenocarcinoma prepared for bilateral simultaneous chain mastectomies. B, Intraoperative view following excision of all mammary tissue. C, Immediate postsurgical appearance following the bilateral chain mastectomies. (Courtesy Dr. Julius Liptak, Alta Vista Animal Hospital, Ottawa, Canada.)
The high malignancy rate of mammary carcinoma and the poor prognosis associated with lymph node metastasis supports aggressive lymph node assessment. This could include ultrasound-guided FNA of difficult to palpate regional nodes and inguinal lymphadenectomy concurrent with chain mastectomy. Sentinel lymph node mapping has been described in the cat and was the original model for the procedure, which is common for human breast cancer patients.126,189 Published techniques in the cat include CT evaluation following intramammary injection of iopamidol and radiographic imaging following intramammary ethiodized oil injections.189 There are no clinical reports utilizing nuclear lymphoscintigraphy in the cat. Use of blue dyes for node visualization is not recommended because their use may cause Heinz body anemia and methemoglobinemia in this species.
Fibroepithelial hyperplasia has a classic appearance that is very difficult to mistake for malignant mammary tumors. This condition is typically treated with either OHE or medical hormone therapy management. Inflammatory mammary carcinoma has very rarely been reported in cats; in a sole case series of three cats, the disease was described as occurring secondary to postsurgical mastectomy with nonhealing incisions, edema, and suture rejections.175
Systemic Treatment
Early detection and aggressive surgery (including prophylactic chain mastectomy) can result in long-term survival in cats with early stage mammary tumors. However, cats with delayed diagnosis, large primary tumors, or metastatic local lymph nodes are not treated effectively with surgery alone. The incidence of distant metastasis, primarily to the lungs and pleura, is high, although other organs are also frequently involved.174 Despite the high rate of metastasis after surgery, very few advances have been made in identifying effective adjuvant systemic treatments.
Due to low HR expression in feline mammary carcinoma, hormonal therapy is not likely to be effective; however, randomized trials have not been performed to confirm this.
Several studies, all retrospective, have evaluated the use of chemotherapy in cats with mammary cancer. Two case series of cats with macroscopic primary and/or metastatic tumors documented objective responses in 40% to 50% of the cats treated with a combination of doxorubicin and cyclophosphamide.190,191 The relatively high response rate in the macroscopic setting suggests that this may be an effective protocol in patients with microscopic minimal residual disease (i.e., following surgical cytoreduction). However, results from adjuvant studies do not reflect this, albeit none of the studies were prospective or randomized. A multiinstitutional retrospective study describing outcome in cats treated with adjuvant single-agent doxorubicin reported a median survival of 450 days in cats with tumors less than 2 cm in diameter.176 This is shorter than survival times reported in cats treated with surgery alone (3 years). Interestingly, another study evaluating the combination of a doxorubicin-based chemotherapy protocol and an NSAID (meloxicam) reported similar MSTs of 460 days.188 No control arm consisting of cats treated with surgery alone was included; thus the additive benefit of chemotherapy was not possible to determine. A more recent retrospective, nonrandomized study also compared outcome in cats treated with surgery alone to cats treated with surgery and adjuvant chemotherapy.176 No significant differences in terms of surgical procedure, tumor size, stage, or histopathologic parameters existed between the groups. No difference in overall survival was found between treatment groups. Further subgroup analysis failed to reveal differences in survival between the groups when comparing cats with tumors less than 2 cm (611 days with surgery alone versus 729 with surgery and chemotherapy). However, cats treated with chain mastectomy and adjuvant chemotherapy survived significantly longer than cats treated with chain mastectomy alone (1998 days versus 414 days). These results support the use of adjuvant chemotherapy in this setting. It is interesting that none of these retrospective studies reported survival times as long as earlier studies using surgery alone, especially in the subset of cats with small tumors. This further illustrates the difficulty in comparing outcomes between retrospective studies, especially noncontemporaneous ones. Ultimately, prospective, randomized trials will be necessary to determine the appropriate use of chemotherapy in cats with mammary tumors. Despite the lack of quality evidence in the literature to support it, veterinary oncologists continue to make decisions regarding the use of chemotherapy in cats with mammary tumors. Table 27-7 summarizes the most important prognostic factors, general guidelines for systemic treatments, and the strength of supporting evidence.
References
1. Dorn, CR, Taylor, DO, Schneider, R, et al. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968;40:307–318.
2. Moe, L. Population-based incidence of mammary tumours in some dog breeds. J Reprod Fertil Suppl. 2001;57:439–443.
3. Egenvall, A, Bonnett, BN, Ohagen, P, et al. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev Vet Med. 2005;69:109–127.
4. Dobson, JM, Samuel, S, Milstein, H, et al. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002;43:240–246.
5. Bronden, LB, Nielsen, SS, Toft, N, et al. Data from the Danish veterinary cancer registry on the occurrence and distribution of neoplasms in dogs in Denmark. Vet Rec. 2010;166:586–590.
6. Merlo, DF, Rossi, L, Pellegrino, C, et al. Cancer incidence in pet dogs: findings of the Animal Tumor Registry of Genoa, Italy. J Vet Intern Med. 2008;22:976–984.
7. Benjamin, SA, Lee, AC, Saunders, WJ. Classification and behavior of canine mammary epithelial neoplasms based on life-span observations in beagles. Vet Pathol. 1999;36:423–436.
8. Schneider, R. Comparison of age, sex, and incidence rates in human and canine breast cancer. Cancer. 1970;26:419–426.
9. Priester, WA, Mantel, N. Occurrence of tumors in domestic animals: data from 12 United States and Canadian colleges of veterinary medicine. J Natl Cancer Inst. 1971;47:1333–1344.
10. Brodey, RS, Goldschmidt, MH, Roszel, JR. Canine mammary gland neoplasms. J Am Anim Hosp Assoc. 1983;19:61–90.
11. Kurzman, ID, Gilbertson, SR. Prognostic factors in canine mammary tumors. Semin Vet Med Surg (Small Anim). 1986;1:25–32.
12. Taylor, GN, Shabestari, L, Williams, J, et al. Mammary neoplasia in a closed beagle colony. Cancer Res. 1976;36:2740–2743.
13. Sorenmo, KU, Kristiansen, VM, Cofone, MA, et al. Canine mammary gland tumours: a histological continuum from benign to malignant; clinical and histopathological evidence. Vet Comp Oncol. 2009;7:162–172.
14. Goldschmidt, M, Shofer, FS, Smelstoys, JA. Neoplastic lesions of the mammary gland. In: Mohr U, Carlton WW, Dungworth DL, et al, eds. Pathobiology of the aging dog. Ames, Iowa: Iowa State University Press, 2001.
15. Rivera, P, Melin, M, Biagi, T, et al. Mammary tumor development in dogs is associated with BRCA1 and BRCA2. Cancer Res. 2009;69:8770–8774.
16. Schneider, R, Dorn, CR, Taylor, DO. Factors influencing canine mammary cancer development and postsurgical survival. J Natl Cancer Inst. 1969;43:1249–1261.
17. Sonnenschein, EG, Glickman, LT, Goldschmidt, MH, et al. Body conformation, diet, and risk of breast cancer in pet dogs: a case-control study. Am J Epidemiol. 1991;133:694–703.
18. Misdorp, W. Canine mammary tumours: protective effect of late ovariectomy and stimulating effect of progestins. Vet Q. 1988;10:26–33.
19. Brodey, RS, Fidler, IJ, Howson, AE. The relationship of estrous irregularity, pseudopregnancy, and pregnancy to the development of canine mammary neoplasms. J Am Vet Med Assoc. 1966;149:1047–1049.
20. Stovring, M, Moe, L, Glattre, E. A population-based case-control study of canine mammary tumours and clinical use of medroxyprogesterone acetate. APMIS. 1997;105:590–596.
21. Concannon, PW, Spraker, TR, Casey, HW, et al. Gross and histopathologic effects of medroxyprogesterone acetate and progesterone on the mammary glands of adult beagle bitches. Fertil Steril. 1981;36:373–387.
22. Giles, RC, Kwapien, RP, Geil, RG, et al. Mammary nodules in beagle dogs administered investigational oral contraceptive steroids. J Natl Cancer Inst. 1978;60:1351–1364.
23. Kwapien, RP, Giles, RC, Geil, RG, et al. Malignant mammary tumors in beagle dogs dosed with investigational oral contraceptive steroids. J Natl Cancer Inst. 1980;65:137–144.
24. Selman, PJ, van Garderen, E, Mol, JA, et al. Comparison of the histological changes in the dog after treatment with the progestins medroxyprogesterone acetate and proligestone. Vet Q. 1995;17:128–133.
25. Geil, RG, Lamar, JK. FDA studies of estrogen, progestogens, and estrogen/progestogen combinations in the dog and monkey. J Toxicol Environ Health. 1977;3:179–193.
26. Yamagami, T, Kobayashi, T, Takahashi, K, et al. Prognosis for canine malignant mammary tumors based on TNM and histologic classification. J Vet Med Sci. 1996;58:1079–1083.
27. Schafer, KA, Kelly, G, Schrader, R, et al. A canine model of familial mammary gland neoplasia. Vet Pathol. 1998;35:168–177.
28. Ford, D, Easton, DF, Stratton, M, et al. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689.
29. King, MC, Marks, JH, Mandell, JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302:643–646.
30. Easton, DF, Ford, D, Bishop, DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–271.
31. Fackenthal, JD, Olopade, OI. Breast cancer risk associated with BRCA1 and BRCA2 in diverse populations. Nat Rev Cancer. 2007;7:937–948.
32. Klopfleisch, R, Gruber, AD. Increased expression of BRCA2 and RAD51 in lymph node metastases of canine mammary adenocarcinomas. Vet Pathol. 2009;46:416–422.
33. Nieto, A, Perez-Alenza, MD, Del Castillo, N, et al. BRCA1 expression in canine mammary dysplasias and tumours: relationship with prognostic variables. J Comp Pathol. 2003;128:260–268.
34. Perez Alenza, D, Rutteman, GR, Pena, L, et al. Relation between habitual diet and canine mammary tumors in a case-control study. J Vet Intern Med. 1998;12:132–139.
35. Calle, EE, Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591.
36. Carmichael, AR, Bates, T. Obesity and breast cancer: a review of the literature. Breast. 2004;13:85–92.
37. Tymchuk, CN, Tessler, SB, Barnard, RJ. Changes in sex hormone-binding globulin, insulin, and serum lipids in postmenopausal women on a low-fat, high-fiber diet combined with exercise. Nutr Cancer. 2000;38:158–162.
38. Wu, AH, Pike, MC, Stram, DO. Meta-analysis: dietary fat intake, serum estrogen levels, and the risk of breast cancer. J Natl Cancer Inst. 1999;91:529–534.
39. Hankinson, SE, Willett, WC, Manson, JE, et al. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J Natl Cancer Inst. 1998;90:1292–1299.
40. Cleary, MP, Grossmann, ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542.
41. Cleary, MP, Grossmann, ME, Ray, A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47:202–213.
42. Rehm, S, Stanislaus, DJ, Williams, AM. Estrous cycle-dependent histology and review of sex steroid receptor expression in dog reproductive tissues and mammary gland and associated hormone levels. Birth Defects Res B Dev Reprod Toxicol. 2007;80:233–245.
43. Santos, M, Marcos, R, Faustino, AM. Histological study of canine mammary gland during the oestrous cycle. Reprod Domest Anim. 2010;45:e146–e154.
44. Pike, MC, Spicer, DV, Dahmoush, L, et al. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol Rev. 1993;15:17–35.
45. Russo, J, Russo, IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102:89–96.
46. Okoh, V, Deoraj, A, Roy, D. Estrogen-induced reactive oxygen species-mediated signalings contribute to breast cancer. Biochim Biophys Acta. 2011;1815:115–133.
47. Dickson, RB, Lippman, ME, Slamon, D. UCLA colloquium. New insights into breast cancer: the molecular biochemical and cellular biology of breast cancer. Cancer Res. 1990;50:4446–4447.
48. Mol, JA, Lantinga-van Leeuwen, IS, van Garderen, E, et al. Mammary growth hormone and tumorigenesis–lessons from the dog. Vet Q. 1999;21:111–115.
49. Selman, PJ, Mol, JA, Rutteman, GR, et al. Progestin-induced growth hormone excess in the dog originates in the mammary gland. Endocrinology. 1994;134:287–292.
50. van Garderen, E, Schalken, JA. Morphogenic and tumorigenic potentials of the mammary growth hormone/growth hormone receptor system. Mol Cell Endocrinol. 2002;197:153–165.
51. Mol, JA, Selman, PJ, Sprang, EP, et al. The role of progestins, insulin-like growth factor (IGF) and IGF-binding proteins in the normal and neoplastic mammary gland of the bitch: a review. J Reprod Fertil Suppl. 1997;51:339–344.
52. Hamelers, IH, van Schaik, RF, van Teeffelen, HA, et al. Synergistic proliferative action of insulin-like growth factor I and 17 beta-estradiol in MCF-7S breast tumor cells. Exp Cell Res. 2002;273:107–117.
53. Thorne, C, Lee, AV. Cross talk between estrogen receptor and IGF signaling in normal mammary gland development and breast cancer. Breast Dis. 2003;17:105–114.
54. Laban, C, Bustin, SA, Jenkins, PJ. The GH-IGF-I axis and breast cancer. Trends Endocrinol Metab. 2003;14:28–34.
55. van der Burg, B, Rutteman, GR, Blankenstein, MA, et al. Mitogenic stimulation of human breast cancer cells in a growth factor-defined medium: synergistic action of insulin and estrogen. J Cell Physiol. 1988;134:101–108.
56. Osborne, CK, Clemmons, DR, Arteaga, CL. Regulation of breast cancer growth by insulin-like growth factors. J Steroid Biochem Mol Biol. 1990;37:805–809.
57. Dupont, J, Le Roith, D. Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells: new insights into their synergistic effects. Mol Pathol. 2001;54:149–154.
58. Queiroga, FL, Perez-Alenza, D, Silvan, G, et al. Serum and intratumoural GH and IGF-I concentrations: prognostic factors in the outcome of canine mammary cancer. Res Vet Sci. 2010;89:396–403.
59. Queiroga, FL, Perez-Alenza, MD, Silvan, G, et al. Crosstalk between GH/IGF-I axis and steroid hormones (progesterone, 17beta-estradiol) in canine mammary tumours. J Steroid Biochem Mol Biol. 2008;110:76–82.
60. Klopfleisch, R, von Euler, H, Sarli, G, et al. Molecular carcinogenesis of canine mammary tumors: news from an old disease. Vet Pathol. 2011;48:98–116.
61. Rivera, P, von Euler, H. Molecular biological aspects on canine and human mammary tumors. Vet Pathol. 2011;48:132–146.
62. Gilbertson, SR, Kurzman, ID, Zachrau, RE, et al. Canine mammary epithelial neoplasms: biologic implications of morphologic characteristics assessed in 232 dogs. Vet Pathol. 1983;20:127–142.
63. Moulton, JE, Rosenblatt, LS, Goldman, M. Mammary tumors in a colony of beagle dogs. Vet Pathol. 1986;23:741–749.
64. Bender, AP, Dorn, CR, Schneider, R. An epidemiologic study of canine multiple primary neoplasma involving the female and male reproductive systems. Prev Vet Med. 1984;2:715–731.
65. Antuofermo, E, Miller, MA, Pirino, S, et al. Spontaneous mammary intraepithelial lesions in dogs—a model of breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2247–2256.
66. MacEwen, EG, Patnaik, AK, Harvey, HJ, et al. Estrogen receptors in canine mammary tumors. Cancer Res. 1982;42:2255–2259.
67. Rutteman, GR, Misdorp, W, Blankenstein, MA, et al. Oestrogen (ER) and progestin receptors (PR) in mammary tissue of the female dog: different receptor profile in non-malignant and malignant states. Br J Cancer. 1988;58:594–599.
68. Illera, JC, Perez-Alenza, MD, Nieto, A, et al. Steroids and receptors in canine mammary cancer. Steroids. 2006;71:541–548.
69. Millanta, F, Calandrella, M, Bari, G, et al. Comparison of steroid receptor expression in normal, dysplastic, and neoplastic canine and feline mammary tissues. Res Vet Sci. 2005;79:225–232.
70. Donnay, I, Rauis, J, Devleeschouwer, N, et al. Comparison of estrogen and progesterone receptor expression in normal and tumor mammary tissues from dogs. Am J Vet Res. 1995;56:1188–1194.
71. de Las Mulas, JM, Millan, Y, Dios, R. A prospective analysis of immunohistochemically determined estrogen receptor alpha and progesterone receptor expression and host and tumor factors as predictors of disease-free period in mammary tumors of the dog. Vet Pathol. 2005;42:200–212.
72. Chang, CC, Tsai, MH, Liao, JW, et al. Evaluation of hormone receptor expression for use in predicting survival of female dogs with malignant mammary gland tumors. J Am Vet Med Assoc. 2009;235:391–396.
73. Nieto, A, Pena, L, Perez-Alenza, MD, et al. Immunohistologic detection of estrogen receptor alpha in canine mammary tumors: clinical and pathologic associations and prognostic significance. Vet Pathol. 2000;37:239–247.
74. Geraldes, M, Gartner, F, Schmitt, F. Immunohistochemical study of hormonal receptors and cell proliferation in normal canine mammary glands and spontaneous mammary tumours. Vet Rec. 2000;146:403–406.
75. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717.
76. Winer, EP, Hudis, C, Burstein, HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629.
77. Network, NCC. Clinical practice guidelines in oncology-version 2.2006. National Comprehensive Cancer Network, Inc; 2005.
78. Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists’ Collaborative Group. Lancet. 1998;351:1451–1467.
79. Thuerlimann, B, Koeberle, D, Senn, HJ. Guidelines for the adjuvant treatment of postmenopausal women with endocrine-responsive breast cancer: past, present and future recommendations. Eur J Cancer. 2007;43:46–52.
80. Fisher, B, Costantino, JP, Wickerham, DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388.
81. Sjogren, S, Inganas, M, Lindgren, A, et al. Prognostic and predictive value of c-erbB-2 overexpression in primary breast cancer, alone and in combination with other prognostic markers. J Clin Oncol. 1998;16:462–469.
82. Slamon, DJ, Clark, GM, Wong, SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182.
83. Slamon, DJ, Leyland-Jones, B, Shak, S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792.
84. Martin de las Mulas, J, Ordas, J, Millan, Y, et al. Oncogene HER-2 in canine mammary gland carcinomas: an immunohistochemical and chromogenic in situ hybridization study. Breast Cancer Res Treat. 2003;80:363–367.
85. Hsu, WL, Huang, HM, Liao, JW, et al. Increased survival in dogs with malignant mammary tumours overexpressing HER-2 protein and detection of a silent single nucleotide polymorphism in the canine HER-2 gene. Vet J. 2009;180:116–123.
86. Gama, A, Alves, A, Schmitt, F. Identification of molecular phenotypes in canine mammary carcinomas with clinical implications: application of the human classification. Virchows Arch. 2008;453:123–132.
87. Perez Alenza, MD, Tabanera, E, Pena, L. Inflammatory mammary carcinoma in dogs: 33 cases (1995-1999). J Am Vet Med Assoc. 2001;219:1110–1114.
88. Marconato, L, Romanelli, G, Stefanello, D, et al. Prognostic factors for dogs with mammary inflammatory carcinoma: 43 cases (2003-2008). J Am Vet Med Assoc. 2009;235:967–972.
89. Karayannopoulou, M, Koutinas, AF, Polizopoulou, ZS, et al. Total serum alkaline phosphatase activity in dogs with mammary neoplasms: a prospective study on 79 natural cases. J Vet Med A Physiol Pathol Clin Med. 2003;50:501–505.
90. Karayannopoulou, M, Polizopoulou, ZS, Koutinas, AF, et al. Serum alkaline phosphatase isoenzyme activities in canine malignant mammary neoplasms with and without osseous transformation. Vet Clin Pathol. 2006;35:287–290.
91. Cassali, GD, Gobbi, H, Malm, C, et al. Evaluation of accuracy of fine needle aspiration cytology for diagnosis of canine mammary tumours: comparative features with human tumours. Cytopathology. 2007;18:191–196.
92. Simon, D, Schoenrock, D, Nolte, I, et al. Cytologic examination of fine-needle aspirates from mammary gland tumors in the dog: diagnostic accuracy with comparison to histopathology and association with postoperative outcome. Vet Clin Pathol. 2009;38:521–528.
93. Eberle, N, Fork, M, von Babo, V, et al. Comparison of examination of thoracic radiographs and thoracic computed tomography in dogs with appendicular osteosarcoma. Vet Comp Oncol. 2011;9:131–140.
94. Otoni, CC, Rahal, SC, Vulcano, LC, et al. Survey radiography and computerized tomography imaging of the thorax in female dogs with mammary tumors. Acta Vet Scand. 2010;52:20.
95. Lana, SE, Rutteman, GR, Withrow, SJ. Tumors of the mammary gland. In Withrow SJ, Vail DM, eds.: Withrow & MacEwen’s small animal clinical oncology, ed 4, St. Louis: Saunders Elsevier, 2007.
96. Pereira, CT, Rahal, SC, de Carvalho Balieiro, JC, et al. Lymphatic drainage on healthy and neoplastic mammary glands in female dogs: can it really be altered? Anat Histol Embryol. 2003;32:282–290.
97. Pereira, CT, Luiz Navarro Marques, F, Williams, J, et al. 99mTc-labeled dextran for mammary lymphoscintigraphy in dogs. Vet Radiol Ultrasound. 2008;49:487–491.
98. Patsikas, MN, Dessiris, A. The lymph drainage of the mammary glands in the bitch: a lymphographic study. Part II: The 3rd mammary gland. Anat Histol Embryol. 1996;25:139–143.
99. Ran, S, Volk, L, Hall, K, et al. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2009;17(4):229–251.
100. Tuohy, JL, Milgram, J, Worley, DR, et al. A review of sentinel lymph node evaluation and the need for its incorporation into veterinary oncology. Vet Comp Oncol. 2009;7:81–91.
101. Owens, L. Classification of tumors in domestic animals. Geneva: World Health Organization; 1980.
102. Rutteman, G, Withrow, S, MacEwen, E. Tumors of the mammary gland. In Withrow S, MacEwen E, eds.: Small animal clinical oncology, ed 3, Philadelphia: WB Saunders, 2001.
103. Sorenmo, KU, Rasotto, R, Zappulli, V, et al. Development, anatomy, histology, lymphatic drainage, clinical features, and cell differentiation markers of canine mammary gland neoplasms. Vet Pathol. 2011;48:85–97.
104. Hampe, JF, Misdorp, W. Tumours and dysplasias of the mammary gland. Bull World Health Organ. 1974;50:111–133.
105. Misdorp, W, Else, R, Hellmen, E, et al. Histological classification of mammary tumors of the dog and the cat. Washington, DC: American Registry of Pathology; 1999.
106. Goldschmidt, M, Pena, L, Rasotto, R, et al. Classification and grading of canine mammary tumors. Vet Pathol. 2011;48:117–131.
107. Rasotto, R, Zappulli, V, Castagnaro, M, et al. A retrospective study of those histopathologic parameters predictive of invasion of the lymphatic system by canine mammary carcinomas. Vet Pathol. 2012;49(2):330–340.
108. Elston, CW, Ellis, IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410.
109. Misdorp, W. Tumors of the mammary gland. In Meuten DJ, ed.: Tumors in domestic animals, ed 4, Ames, Iowa: Iowa State Press, 2002.
110. Clemente, M, Perez-Alenza, MD, Illera, JC, et al. Histological, immunohistological, and ultrastructural description of vasculogenic mimicry in canine mammary cancer. Vet Pathol. 2010;47:265–274.
111. Karayannopoulou, M, Kaldrymidou, E, Constantinidis, TC, et al. Histological grading and prognosis in dogs with mammary carcinomas: application of a human grading method. J Comp Pathol. 2005;133:246–252.
112. Hellmen, E, Bergstrom, R, Holmberg, L, et al. Prognostic factors in canine mammary tumors: a multivariate study of 202 consecutive cases. Vet Pathol. 1993;30:20–27.
113. Perez Alenza, MD, Pena, L, Nieto, AI, et al. Clinical and pathological prognostic factors in canine mammary tumors. Ann Ist Super Sanita. 1997;33:581–585.
114. MacEwen, EG, Harvey, HJ, Patnaik, AK, et al. Evaluation of effects of levamisole and surgery on canine mammary cancer. J Biol Response Mod. 1985;4:418–426.
115. Philibert, JC, Snyder, PW, Glickman, N, et al. Influence of host factors on survival in dogs with malignant mammary gland tumors. J Vet Intern Med. 2003;17:102–106.
116. Morris, JS, Dobson, JM, Bostock, DE. Use of tamoxifen in the control of canine mammary neoplasia. Vet Rec. 1993;133:539–542.
117. Chang, SC, Chang, CC, Chang, TJ, et al. Prognostic factors associated with survival two years after surgery in dogs with malignant mammary tumors: 79 cases (1998-2002). J Am Vet Med Assoc. 2005;227:1625–1629.
118. Stratmann, N, Failing, K, Richter, A, et al. Mammary tumor recurrence in bitches after regional mastectomy. Vet Surg. 2008;37:82–86.
119. Morris, JS, Dobson, JM, Bostock, DE, et al. Effect of ovariohysterectomy in bitches with mammary neoplasms. Vet Rec. 1998;142:656–658.
120. Sorenmo, KU, Shofer, FS, Goldschmidt, MH. Effect of spaying and timing of spaying on survival of dogs with mammary carcinoma. J Vet Intern Med. 2000;14:266–270.
121. MacEwen, EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990;9:125–136.
122. Vail, DM, MacEwen, EG. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 2000;18:781–792.
123. Gelb, HR, Freeman, LJ, Rohleder, JJ, et al. Feasibility of contrast-enhanced ultrasound-guided biopsy of sentinel lymph nodes in dogs. Vet Radiol Ultrasound. 2010;51:628–633.
124. Pinheiro, LG, Oliveira Filho, RS, Vasques, PH, et al. Hemosiderin: a new marker for sentinel lymph node identification. Acta Cir Bras. 2009;24:432–436.
125. Balogh, L, Thuroczy, J, Andocs, G, et al. Sentinel lymph node detection in canine oncological patients. Nucl Med Rev Cent East Eur. 2002;5:139–144.
126. Wong, JH, Cagle, LA, Morton, DL. Lymphatic drainage of skin to a sentinel lymph node in a feline model. Ann Surg. 1991;214:637–641.
127. Szczubiał, M, Łopuszynski, W. Prognostic value of regional lymph node status in canine mammary carcinomas. Vet Comp Oncol. 2011;9:296–303.
128. Allen, S, Mahaffey, E. Canine mammary neoplasia: prognostic indicators and response to surgical therapy. J Amer Anim Hosp Assoc. 1989;25:504–546.
129. Tavares, WL, Lavalle, GE, Figueiredo, MS, et al. Evaluation of adverse effects in tamoxifen exposed healthy female dogs. Acta Vet Scand. 2010;52:67.
130. Yamagami, T, Kobayashi, T, Takahashi, K, et al. Influence of ovariectomy at the time of mastectomy on the prognosis for canine malignant mammary tumours. J Small Anim Pract. 1996;37:462–464.
131. Karayannopoulou, M, Kaldrymidou, E, Constantinidis, TC, et al. Adjuvant post-operative chemotherapy in bitches with mammary cancer. J Vet Med A Physiol Pathol Clin Med. 2001;48:85–96.
132. Nabholtz, JM, Senn, HJ, Bezwoda, WR, et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. 304 Study Group. J Clin Oncol. 1999;17:1413–1424.
133. Sjostrom, J, Blomqvist, C, Mouridsen, H, et al. Docetaxel compared with sequential methotrexate and 5-fluorouracil in patients with advanced breast cancer after anthracycline failure: a randomised phase III study with crossover on progression by the Scandinavian Breast Group. Eur J Cancer. 1999;35:1194–1201.
134. Morabito, A, Piccirillo, MC, Monaco, K, et al. First-line chemotherapy for HER-2 negative metastatic breast cancer patients who received anthracyclines as adjuvant treatment. Oncologist. 2007;12:1288–1298.
135. Simon, D, Schoenrock, D, Baumgartner, W, et al. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J Vet Intern Med. 2006;20:1184–1190.
136. Marconato, L, Lorenzo, RM, Abramo, F, et al. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet Comp Oncol. 2008;6:90–101.
137. de, MSCH, Toledo-Piza, E, Amorin, R, et al. Inflammatory mammary carcinoma in 12 dogs: clinical features, cyclooxygenase-2 expression, and response to piroxicam treatment. Can Vet J. 2009;50:506–510.
138. Kuntz, CA, Dernell, WS, Powers, BE, et al. Extraskeletal osteosarcomas in dogs: 14 cases. J Am Anim Hosp Assoc. 1998;34:26–30.
139. Hermo, GA, Turic, E, Angelico, D, et al. Effect of adjuvant perioperative desmopressin in locally advanced canine mammary carcinoma and its relation to histologic grade. J Am Anim Hosp Assoc. 2011;47:21–27.
140. Terraube, V, Marx, I, Denis, CV. Role of von Willebrand factor in tumor metastasis. Thromb Res. 2007;120(Suppl 2):S64–S70.
141. Ripoll, GV, Giron, S, Krzymuski, MJ, et al. Antitumor effects of desmopressin in combination with chemotherapeutic agents in a mouse model of breast cancer. Anticancer Res. 2008;28:2607–2611.
142. Hahn, K, Richardson, R, Knapp, D. Canine malignant mammary neoplasia: biological behavior, diagnosis, and treatment alternatives. J Am Anim Hosp Assoc. 1992;28:251–256.
143. Egenvall, A, Bonnett, BN, Haggstrom, J, et al. Morbidity of insured Swedish cats during 1999-2006 by age, breed, sex, and diagnosis. J Feline Med Surg. 2010;12:948–959.
144. Bonnett, BN, Egenvall, A. Age patterns of disease and death in insured Swedish dogs, cats and horses. J Comp Pathol. 2010;142(Suppl 1):S33–S38.
145. Teclaw, R, Mendlein, J, Garbe, P, et al. Characteristics of pet populations and households in the Purdue Comparative Oncology Program catchment area, 1988. J Am Vet Med Assoc. 1992;201:1725–1729.
146. Vascellari, M, Baioni, E, Ru, G, et al. Animal tumour registry of two provinces in northern Italy: incidence of spontaneous tumours in dogs and cats. BMC Vet Res. 2009;5:39.
147. Hayden, DW, Nielsen, SW. Feline mammary tumours. J Small Anim Pract. 1971;12:687–698.
148. Misdorp, W, Romijn, A, Hart, AA. Feline mammary tumors: a case-control study of hormonal factors. Anticancer Res. 1991;11:1793–1797.
149. Hayes, AA, Mooney, S. Feline mammary tumors. Vet Clin North Am Small Anim Pract. 1985;15:513–520.
150. Weijer, K, Head, KW, Misdorp, W, et al. Feline malignant mammary tumors. I. Morphology and biology: some comparisons with human and canine mammary carcinomas. J Natl Cancer Inst. 1972;49:1697–1704.
151. Hayes, HM, Jr., Milne, KL, Mandell, CP. Epidemiological features of feline mammary carcinoma. Vet Rec. 1981;108:476–479.
152. Ito, T, Kadosawa, T, Mochizuki, M, et al. Prognosis of malignant mammary tumor in 53 cats. J Vet Med Sci. 1996;58:723–726.
153. Patnaik, AK, Liu, SK, Hurvitz, AI, et al. Nonhematopoietic neoplasms in cats. J Natl Cancer Inst. 1975;54:855–860.
154. Rissetto, K, Villamil, JA, Selting, KA, et al. Recent trends in feline intestinal neoplasia: an epidemiologic study of 1,129 cases in the veterinary medical database from 1964 to 2004. J Am Anim Hosp Assoc. 2011;47:28–36.
155. Louwerens, M, London, CA, Pedersen, NC, et al. Feline lymphoma in the post-feline leukemia virus era. J Vet Intern Med. 2005;19:329–335.
156. Gabor, LJ, Malik, R, Canfield, PJ. Clinical and anatomical features of lymphosarcoma in 118 cats. Aust Vet J. 1998;76:725–732.
157. Miller, MA, Nelson, SL, Turk, JR, et al. Cutaneous neoplasia in 340 cats. Vet Pathol. 1991;28:389–395.
158. Overley, B, Shofer, FS, Goldschmidt, MH, et al. Association between ovarihysterectomy and feline mammary carcinoma. J Vet Intern Med. 2005;19:560–563.
159. Skorupski, KA, Overley, B, Shofer, FS, et al. Clinical characteristics of mammary carcinoma in male cats. J Vet Intern Med. 2005;19:52–55.
160. Jacobs, TM, Hoppe, BR, Poehlmann, CE, et al. Mammary adenocarcinomas in three male cats exposed to medroxyprogesterone acetate (1990-2006). J Feline Med Surg. 2010;12:169–174.
161. Loretti, AP, Ilha, MR, Ordas, J, et al. Clinical, pathological and immunohistochemical study of feline mammary fibroepithelial hyperplasia following a single injection of depot medroxyprogesterone acetate. J Feline Med Surg. 2005;7:43–52.
162. Mol, JA, van Garderen, E, Rutteman, GR, et al. New insights in the molecular mechanism of progestin-induced proliferation of mammary epithelium: induction of the local biosynthesis of growth hormone (GH) in the mammary glands of dogs, cats and humans. J Steroid Biochem Mol Biol. 1996;57:67–71.
163. Mol, JA, van Garderen, E, Selman, PJ, et al. Growth hormone mRNA in mammary gland tumors of dogs and cats. J Clin Invest. 1995;95:2028–2034.
164. Burrai, GP, Mohammed, SI, Miller, MA, et al. Spontaneous feline mammary intraepithelial lesions as a model for human estrogen receptor- and progesterone receptor-negative breast lesions. BMC Cancer. 2010;10:156.
165. Millanta, F, Calandrella, M, Vannozzi, I, et al. Steroid hormone receptors in normal, dysplastic and neoplastic feline mammary tissues and their prognostic significance. Vet Rec. 2006;158:821–824.
166. de las Mulas, JM, van Niel, M, Millan, Y, et al. Immunohistochemical analysis of estrogen receptors in feline mammary gland benign and malignant lesions: comparison with biochemical assay. Domest Anim Endocrinol. 2000;18:111–125.
167. Martin de las Mulas, J, Van Niel, M, Millan, Y, et al. Progesterone receptors in normal, dysplastic and tumourous feline mammary glands. Comparison with oestrogen receptors status. Res Vet Sci. 2002;72:153–161.
168. Meisl, D, Hubler, M, Arnold, S. [Treatment of fibroepithelial hyperplasia (FEH) of the mammary gland in the cat with the progesterone antagonist Aglepristone (Alizine)]. Schweiz Arch Tierheilkd. 2003;145:130–136.
169. Ordas, J, Millan, Y, Dios, R, et al. Proto-oncogene HER-2 in normal, dysplastic and tumorous feline mammary glands: an immunohistochemical and chromogenic in situ hybridization study. BMC Cancer. 2007;7:179.
170. Millanta, F, Calandrella, M, Citi, S, et al. Overexpression of HER-2 in feline invasive mammary carcinomas: an immunohistochemical survey and evaluation of its prognostic potential. Vet Pathol. 2005;42:30–34.
171. Winston, J, Craft, DM, Scase, TJ, et al. Immunohistochemical detection of HER-2/neu expression in spontaneous feline mammary tumours. Vet Comp Oncol. 2005;3:8–15.
172. Rasotto, R, Caliari, D, Castagnaro, M, et al. An immunohistochemical study of HER-2 expression in feline mammary tumours. J Comp Pathol. 2011;144:170–179.
173. Brodey, RS. Canine and feline neoplasms. Adv Vet Sci. 1957;24:434.
174. Weijer, K, Hart, AA. Prognostic factors in feline mammary carcinoma. J Natl Cancer Inst. 1983;70:709–716.
175. Perez-Alenza, MD, Jimenez, A, Nieto, AI, et al. First description of feline inflammatory mammary carcinoma: clinicopathological and immunohistochemical characteristics of three cases. Breast Cancer Res. 2004;6:R300–R307.
176. McNeill, CJ, Sorenmo, KU, Shofer, FS, et al. Evaluation of adjuvant doxorubicin-based chemotherapy for the treatment of feline mammary carcinoma. J Vet Intern Med. 2009;23:123–129.
177. Hayden, DW, Barnes, DM, Johnson, KH. Morphologic changes in the mammary gland of megestrol acetate-treated and untreated cats: a retrospective study. Vet Pathol. 1989;26:104–113.
178. Castagnaro, M, Casalone, C, Bozzetta, E, et al. Tumour grading and the one-year post-surgical prognosis in feline mammary carcinomas. J Comp Pathol. 1998;119:263–275.
179. Seixas, F, Palmeira, C, Pires, MA, et al. Mammary invasive micropapillary carcinoma in cats: clinicopathologic features and nuclear DNA content. Vet Pathol. 2007;44:842–848.
180. Seixas, F, Pires, MA, Lopes, CA. Complex carcinomas of the mammary gland in cats: pathological and immunohistochemical features. Vet J. 2008;176:210–215.
181. Sarli, G, Brunetti, B, Benazzi, C. Mammary mucinous carcinoma in the cat. Vet Pathol. 2006;43:667–673.
182. Kamstock, DA, Fredrickson, R, Ehrhart, EJ. Lipid-rich carcinoma of the mammary gland in a cat. Vet Pathol. 2005;42:360–362.
183. Matsuda, K, Kobayashi, S, Yamashita, M, et al. Tubulopapillary carcinoma with spindle cell metaplasia of the mammary gland in a cat. J Vet Med Sci. 2008;70:479–481.
184. Seixas, F, Palmeira, C, Pires, MA, et al. Grade is an independent prognostic factor for feline mammary carcinomas: a clinicopathological and survival analysis. Vet J. 2011;187:65–71.
185. MacEwen, EG, Hayes, AA, Mooney, S, et al. Evaluation of effect of levamisole on feline mammary cancer. J Biol Response Mod. 1984;3:541–546.
186. Viste, JR, Myers, SL, Singh, B, et al. Feline mammary adenocarcinoma: tumor size as a prognostic indicator. Can Vet J. 2002;43:33–37.
187. MacEwen, EG, Hayes, AA, Harvey, HJ, et al. Prognostic factors for feline mammary tumors. J Am Vet Med Assoc. 1984;185:201–204.
188. Borrego, JF, Cartagena, JC, Engel, J. Treatment of feline mammary tumours using chemotherapy, surgery and a COX-2 inhibitor drug (meloxicam): a retrospective study of 23 cases (2002-2007)*. Vet Comp Oncol. 2009;7:213–221.
189. Patsikas, MN, Papadopoulou, PL, Charitanti, A, et al. Computed tomography and radiographic indirect lymphography for visualization of mammary lymphatic vessels and the sentinel lymph node in normal cats. Vet Radiol Ultrasound. 2010;51:299–304.
190. Jeglum, KA, deGuzman, E, Young, KM. Chemotherapy of advanced mammary adenocarcinoma in 14 cats. J Am Vet Med Assoc. 1985;187:157–160.
191. Mauldin, GN, Matus, RE, Patnaik, AK, et al. Efficacy and toxicity of doxorubicin and cyclophosphamide used in the treatment of selected malignant tumors in 23 cats. J Vet Intern Med. 1988;2:60–65.