Tumors of the Male Reproductive System
Canine Testicular Tumors
Testicular tumors are the most common tumors of the canine male genitalia and account for approximately 90% of all cancers in the male reproductive tract.1-4 In the intact male dog, the testis is overall the second most common anatomic site for tumor development, with an overall prevalence ranging between 6% and 27%.1,3-7 Many of these reports are case series and involve dogs submitted for routine necropsy and/or castration for cryptorchidism, making comparisons between study prevalence data difficult. However, a recent population-based study conducted in Norway, where elective castration is rare, reported a similar prevalence of 7% for testicular tumors.8
The rate of development of testicular cancer in humans has increased in some populations over time and across successive birth cohorts, and a similar phenomenon has been suggested in dogs.7,9-13 A recent population-based study did not find increased rates of testicular tumors among intact dogs; however, only an 8-year period was evaluated.8 Testicular tumors are most often diagnosed in geriatric male dogs with a median age of approximately 10 years.1,3,4,14,15
The three most common testicular tumors arise from distinct testicular subsets: sustentacular cells of Sertoli, the spermatic germinal epithelium, and the interstitial cells of Leydig, giving rise to Sertoli cell tumors, seminomas, and interstitial cell tumors, respectively (Table 28-1).2 The World Health Organization (WHO) classification of tumors of domestic animals differentiates the major types of testicular tumors in dogs as sex cord stromal tumors (Sertoli cell tumors, interstitial cell tumors), germ cell tumors (seminoma, teratoma), and mixed germ cell sex cord stromal tumors.16 Sertoli cell tumors, interstitial cell tumors, and seminomas have historically developed with equal frequency, although recent studies have suggested that the prevalence of Sertoli cell tumors is lower (8% to 16%).* In one study evaluating lifetime occurrence of neoplasia in German shepherds and Belgian Malinois, seminoma occurred most frequently.17 Human testicular tumors are often divided into seminoma and nonseminoma, with the former further subdivided as classical seminoma (SE), atypical seminoma, and spermatocytic seminoma (SS) according to WHO, and some effort has been made to apply this to canine tumors.9,13,19-23 Sertoli cell tumors and seminomas occur with higher frequency in cryptorchid testes.3,24,25
Rarely, other cell lineages can give rise to testicular tumors such as hemangiomas, granulosa cell tumors, teratomas, sarcomas, embryonal carcinomas, gonadoblastomas, lymphomas, schwannoma, mesothelioma, and rete testis mucinous adenocarcinomas.26-30 Many dogs diagnosed with testicular cancer have more than one primary tumor.3,6,18,31 In three separate studies evaluating relatively large numbers of dogs with testicular tumors, between 4% and 20% of dogs had more than one type of testicular tumor.3,8,32
Risk Factors
Several factors may influence the development of testicular tumors in the dog, including cryptorchidism, age, breed, and carcinogen exposure. There is a significant association between cryptorchidism and the development of Sertoli cell tumors and seminomas but not interstitial cell tumors.4,15,25 An early prospective epidemiologic study compared the incidence of testicular tumors in cryptorchid dogs to age- and breed-matched control dogs.15 None of the control dogs developed testicular tumors during the study, in which the average duration of monitoring was 2 years. The incidence of testicular neoplasia in the cryptorchid dogs was 12.7 per 1000 dog-years at risk, whereas for cryptorchid dogs older than 6 years, the incidence increased to 68.1 per 1000 dog-years at risk. Inguinal cryptorchidism may further increase the risk of testicular tumor development compared to abdominal cryptorchidism (Figure 28-1).4,15,25 In cryptorchid dogs, tumors more frequently develop in the right testicle; however, this is probably due to the fact that the right testicle is more likely to be retained.4,15,32 One study of cryptorchid dogs found that chronologic age was a risk factor for development of a primary testicular tumor and dogs older than 10 years were more likely to develop tumors compared to dogs younger than 6 years.15 A recent study indicated that the detection rate of testicular tumors in dogs younger than 10 years was significantly associated with cryptorchidism, with over 60% of cryptorchid testicular tumors identified in middle-aged dogs (6 to 10 years).4
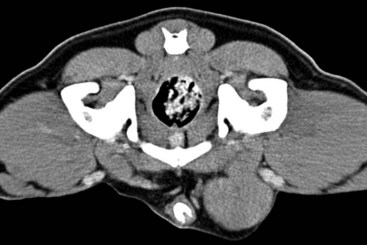
Figure 28-1 Cross-sectional CT image demonstrating an enlarged, minimally rim-enhancing Sertoli cell tumor in a dog with inguinal cryptorchidism. (Courtesy Dr. T. Schwarz, University of Edinburgh.)
Several breeds have been reported to have increased risk of developing primary testicular tumors, including the Boxer, German shepherd, Afghan hound, Weimaraner, Shetland sheepdog, Collie, and Maltese.* The flat-coated retriever, Rottweiler, Bouvier de Flandres, and Leonbergers may have a reduced risk of developing testicular tumors, although low numbers of the latter two breeds were evaluated.8
Two studies evaluating military working dogs suggested evidence of environmental carcinogen exposure during the Vietnam War.35,36 Pathologic changes in the testicles such as hemorrhage, epididymitis, orchitis, sperm granuloma, testicular degeneration, and seminoma were noted, although the causative factor of these could not be definitively determined. These epidemiologic studies postulated that exposure to phenoxy herbicide, dioxin, or tetracycline may have promoted the development of testicular tumors.35,36
Pathology and Pathogenesis
Sertoli cell tumors arise from the sustentacular cells of seminiferous tubules, and seminomas arise from the germinal epithelium of seminiferous tubules. Interstitial cell tumors arise from Leydig cells located between seminiferous tubules. All three tumors have relatively distinct gross appearance but require histopathology for definitive diagnosis. Sertoli cell tumors are firm, lobulated, white-to-gray in appearance, and characterized as “greasy” on palpation.37 Seminomas tend to be homogeneous, soft, and occasionally lobulated and have an ivory appearance when sectioned (Figure 28-2).37 Interstitial cell tumors are soft, expansive, and yellow-to-orange in color when sectioned and often contain cysts with serous or serosanguineous fluid.37
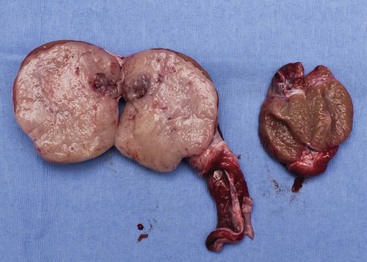
Figure 28-2 Sectioned seminoma in a dog demonstrating its ivory, homogenous appearance in comparison to a mildly atrophied contralateral testicle.
The molecular and cellular biology of primary testicular tumors has been investigated. Proliferation markers (proliferating-cell nuclear antigen [PCNA], Ki67, and argyrophilic nucleolar organizer regions [AgNORs]) and TERT expression (the catalytic reverse transcriptase subunit of telomerase) have been interrogated as indicators of degree of malignancy, local progression, and metastasis with discordant results.38-43 Investigators sought to relate TERT to proliferation indices and p53 expression; however, due to the fact that PCNA and TERT were expressed in all testicular tumors, this limited their prognostic potential.40 However, aggressive testicular tumors did tend to express high levels of all markers examined: TERT, p53, PCNA, and Ki67.40 Proliferative activity in seminomas was assessed using AgNORs in a separate study, in which mean AgNOR scores were higher in invasive or diffuse tumors compared to well-differentiated intraductal seminomas.43 Results suggest that testicular tumors develop a proliferative advantage as they become less differentiated, although larger studies should be performed before proliferative indices can be definitively relied on for prognostication in canine testicular tumors. Cyclins, which are intracellular proteins that form complexes with cyclin-dependent kinases to regulate cell-cycle checkpoints, have also been evaluated in normal and neoplastic testes; however, their significance is yet to be determined.44
The neoplastic and stromal cellular environment plays an important role in tumor invasion and progression, and a few studies have attempted to investigate changes in canine testicular tumors. Laminin is an extracellular matrix protein that plays a role in anchoring cells to the basement membrane. As tumors became more invasive, laminin expression became fragmented or lost in Sertoli cell tumors and seminomas and correlated to increasing proliferative activity as assessed by PCNA scoring, Ki67 index, and mitotic index.38 Connexin 43 is the predominant gap junction protein of the testis that plays a role in phenotypic differentiation, cell pattern formation, and morphogenesis, and altered expression patterns may contribute to tumorigenesis and progression.45-49 Similar to other work, differential alterations in connexin 34 expression occur in canine testicular tumors, and its expression may aid in differentiating neoplastic Sertoli cells from seminomas.50
Mutations of the p53 tumor suppressor gene are a common genetic alteration in both human and canine malignancies, and increased p53 expression has been associated with tumor progression.51-55 A recent evaluation of testicular tumors indicated that nuclear p53 immunoreactivity was detected in 15 of 20 seminomas (75%), 6 of 12 Sertoli cell tumors, and all 3 interstitial cell tumors evaluated.40 Interestingly, expression intensity was stronger in diffuse type Sertoli cell tumors and seminomas.40,54 Results suggest that p53 may be an indicator of tumor aggression; however, further studies should be done to corroborate this.
Similar to p53 and proliferation indices, angiogenesis plays an important role in cancer progression and metastasis. Vascular endothelial growth factor (VEGF) expression and microvessel density (MVD) were higher in seminomas compared to normal testes in one study.56 Additionally, both VEGF expression and MVD were higher in diffuse seminomas compared to more well-differentiated intratubular seminomas, potentially providing a histologic indicator of malignant behavior.56
The KIT protein or CD117 is a transmembrane protein for a tyrosine kinase receptor encoded by the proto-oncogene c-kit, which, when bound to its ligand stem cell factor (SCF), is essential to the development, proliferation, and maturation of several cell types, including germ cells.57-59 Primordial germinal cells express KIT and migrate to interact with Sertoli cells that express SCF to guide the differentiation of the primordial cells into gonocytes.60 Interstitial cells of Leydig also express KIT and, when stimulated by SCF, are stimulated by Sertoli cells to produce testosterone.57,60,61 The expression of KIT is maintained by spermatogonia until differentiation into spermatocytes, making it a useful marker to define primordial germinal cells and early germinal cells.60,62 In human seminomas, immunohistochemical labeling for KIT and placental alkaline phosphatase (PLAP) is used to distinguish SE and SS because SE should express both and SS should be negative.63,64 Normal and neoplastic canine testes were recently characterized for KIT expression, and results were consistent with humans and rodents in that spermatogonia and Leydig cells were KIT positive, whereas Sertoli cells were negative.62 Interestingly, canine testicular tumors in this study maintained the same KIT expression as their cell of origin, with interstitial tumors consistently expressing KIT, similar to human tumors. Similar to human seminomas, canine seminomas appeared to be differentiated into SE and SS on the basis of KIT and PLAP staining.22,62 Further studies investigating the behavior of SE and SS may support the dog as a relevant model for human disease, permitting investigation into additional roles of KIT-inhibitors.65-67
Natural Behavior
Most primary testicular tumors in the dog are characterized by local invasion and rarely metastasize. Regional or distant metastasis occurs in less than 15% of dogs diagnosed with Sertoli cell tumors or seminomas.6,32,68-74 Due to the malignant histologic appearance but low malignant behavior, canine seminoma has been compared to human spermatocytic seminoma, thereby spawning interest in using canine seminoma as a relevant comparative model.21 Recent work has tried to further classify canine seminoma into classic and spermatocytic seminomas in an attempt to help determine metastatic potential, but no clinical conclusions may be drawn as yet.19,21,22 Interstitial cell tumors very rarely metastasize.6 Sites of metastasis may include regional lymph nodes, eyes, brain, lungs, kidney, spleen, liver, adrenal glands, pancreas, skin, and peritoneum.6,32,68-74
Primary testicular tumors can also cause imbalances in hormone levels, regardless of the degree of local invasion and presence or absence of metastasis. Sertoli cell tumors can cause signs of feminization, and over 50% of affected dogs display signs of estrogen production.14,25,32,69 Seminomas and interstitial cell tumors are rarely associated with feminization.75-77 Excess estrogen can cause signs such as bilateral symmetric alopecia, cutaneous hyperpigmentation, epidermal thinning, squamous metaplasia of the prostatic epithelium, gynecomastia, galactorrhea, attraction of other males, preputial atrophy, atrophy of the nonneoplastic testicle, and bone marrow suppression.32 Sertoli cell tumors that develop in retained testicles are more likely to produce signs of hyperestrogenism; however, 17% of dogs with scrotal Sertoli cell tumors developed feminization.14,25,32,69 Plasma hormone concentrations from dogs with primary testicular tumors have been investigated in order to better understand their contribution to tumor type and clinical signs.77-80 Estradiol-17β concentrations were higher in dogs with Sertoli cell tumors compared to normal and were significantly higher in dogs with feminization syndrome secondary to Sertoli cell tumors. Testosterone and testosterone/estradiol ratios are lower in dogs with Sertoli cell tumors when compared to healthy control dogs.76 Plasma estradiol concentrations were lower in seminomas compared to normal dogs in one study but were not significantly altered from normal in another study.76,77 It has been suggested that clinical signs of feminization due to Sertoli cell tumors best correlated to testosterone/estradiol ratio reductions rather than to absolute increases in estradiol.76 Expression of inhibins (inhibins α, β, βα), 3β-hydroxysteroid dehydrogenases, and insulin-like growth factors (IGF-1 and IGF-2) has also been assessed, and further study may shed light on their utility as diagnostic or prognostic markers.77-80 A recent study suggested IGF gene expression was altered in canine testicular tumors compared to normal testicular tissue. A unique pattern of expression, as determined by reverse-transcriptase polymerase chain reaction (RT-PCR) of IGFs, their receptors, and their binding proteins, was observed in interstitial cell tumors compared to Sertoli cell tumors and seminomas.80 The overall changes in gene expression were small, however, and it remains unclear how significant the IGF signaling system is in canine testicular neoplasia.
History and Clinical Signs
Most dogs with testicular tumors are asymptomatic, and a testicular mass is discovered as an incidental finding. However, clinical signs may be attributable to the primary tumor, to the presence of metastasis, or to paraneoplastic syndromes such as hyperestrogenism. Additionally, breeding dogs may present with fertility problems. Diagnosis is usually made via palpation of an enlarged testicle or a testicular mass during routine physical examination, abdominal ultrasound, or necropsy. Atrophy of the remaining normal testicle is common (Figure 28-3). Tumors in cryptorchid dogs may cause a regional mass effect within the caudal abdominal cavity or inguinal region (Figure 28-4).
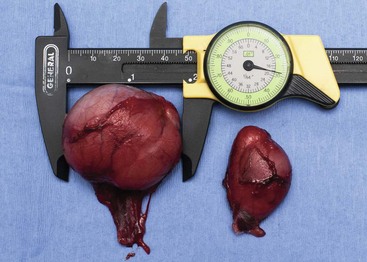
Figure 28-3 Large left seminoma with mild atrophy of the right normal testicle identified as an incidental finding on physical examination.
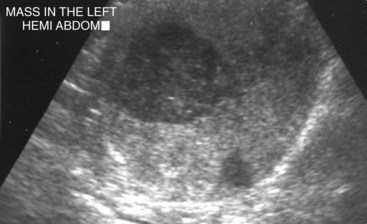
Figure 28-4 Large mixed echogenic and cavitated testicle within the left midabdomen on abdominal ultrasound in a cryptorchid dog. (Courtesy Dr. T. Schwarz, University of Edinburgh.)
Excess estrogen may cause signs of feminization and is the most common paraneoplastic syndrome associated with canine testicular tumors. As stated previously, seminomas and interstitial cell tumors are rarely associated with feminization, whereas over 50% of dogs with Sertoli cell tumors show signs of hyperestrogenism.14,32,69,75-77 Common clinical signs include bilateral symmetric alopecia and hyperpigmentation, pendulous prepuce, gynecomastia, galactorrhea, atrophy of the penis, and squamous metaplasia of the prostate.32 The most deleterious effect of hyperestrogenism is bone marrow suppression, which may be irreversible and life threatening. Early effects of estrogen on the bone marrow include a transient increase in granulopoiesis with peripheral neutrophilia followed by progressive neutropenia, thrombocytopenia, and nonregenerative anemia.75,81 Severe pancytopenia from bone marrow hypoplasia and blood dyscrasias can be fatal, and clinical signs can range from hemorrhage secondary to thrombocytopenia, anemia, and febrile neutropenia.75,81 Less common reported signs associated with testicular neoplasia include lethargy (Sertoli cell tumors), presence of concurrent prostatic cyst or abscess, hematuria, hemoperitoneum, spermatic cord torsion, hypertrophic osteopathy, and perianal gland hyperplasia/adenomas (interstitial cell tumors).3,32,69,82-85 There are several reports of a syndrome in middle-aged miniature Schnauzers of Sertoli cell tumors occurring in cryptorchid male pseudohermaphrodites.86-88 A recent report also described the presence of both a Sertoli cell tumor and an interstitial cell tumor in a mixed breed male pseudohermaphrodite dog.89
Diagnostic Techniques and Staging
Physical examination of intact male dogs, particularly older dogs, should always include palpation of the testicles for masses and/or asymmetry. A thorough rectal palpation should be performed to evaluate the prostate gland, regional lymph nodes, and perianal region. In dogs with clinical signs of hormone imbalance, serum testosterone and estradiol-17β can be measured along with testosterone : estradiol ratio.76,77 It is important to note that not all dogs with signs of feminization have absolute increases in estradiol-17β, and clinical signs may be more closely linked to altered androgen : estrogen ratios.76
Definitive diagnosis is achieved by histopathologic evaluation, although the presence of a testicular mass and cytology may be supportive of testicular neoplasia. Because most dogs with testicular tumors are older and therefore have a high likelihood of another primary tumor (up to 50%) or concurrent diseases, complete staging prior to surgery is generally recommended. Preoperative staging typically includes a complete blood count (CBC) to evaluate for hematologic abnormalities, chemistry profile, urinalysis, abdominal ultrasound, and three-view thoracic radiographs. A coagulation profile may be warranted in dogs with anemia and signs of hemorrhage. Abdominal ultrasound may serve multiple purposes: it can aid in identification of undescended testicles in the abdominal cavity or inguinal canal, assessment of regional lymph nodes, assessment of prostatic changes, and evaluation of common sites of metastasis such as spleen and liver (Figure 28-5). Testicular ultrasonography may aid in differentiating neoplastic processes from orchitis, testicular torsion, and epididymitis; however, changes are not specific enough to identify tumor type.90-92 Ultrasound-guided fine-needle aspiration (FNA) may support a suspicion of neoplasia prior to orchiectomy, particularly in breeding animals.90,93 For owners with financial constraints, minimum staging should consist of a CBC, chemistry profile, and urinalysis. Castration may be performed prior to full staging for some cases, with the decision to do a full work-up following histopathologic evaluation because it is appropriate therapy for most testicular tumors. Histopathologic diagnosis is generally straightforward; however, occasionally, immunohistochemistry (IHC) using vimentin, cytokeratin, desmin, and possibly KIT and inhibin may be indicated to identify the underlying cell of origin.26,78,94-97
Treatment and Prognosis
As most primary canine testicular tumors are characterized by local infiltration with low potential for metastasis, orchiectomy with scrotal ablation is the treatment of choice and is often curative. Bilateral orchiectomy is the treatment of choice for testicular tumors, given that up to 50% of dogs have bilateral tumors and only 12% are clinically detectable in the contralateral testicle.15 In valuable breeding dogs, unilateral orchiectomy can be considered with continued monitoring afterward.98,99 Exploratory laparotomy is indicated in cryptorchid dogs, in which case the regional lymph nodes can be visually assessed and biopsied if indicated. In dogs with signs of hyperestrogenism secondary to the primary tumor, clinical signs typically resolve within 1 to 3 months following castration, unless metastatic lesions provide persistent estrogen release.68,69,74 Recurrence of feminization following castration may be associated with the presence of metastasis.69 Serum hormone levels may be monitored following castration and may correlate to resolution of clinical signs.76,100 Dogs with bone marrow hypoplasia secondary to estrogen toxicity require close monitoring perioperatively and postoperatively for complications requiring medical intervention with blood products and/or antibiotics. These dogs carry a guarded prognosis due to the high morbidity and mortality associated with neutropenia and hemorrhage.75,81 Dogs with aplastic anemia warrant a poor prognosis.75
Primary testicular tumors occasionally metastasize to regional lymph nodes and other distant sites, and therapy other than surgery may be warranted in these dogs. Optimal management employing chemotherapy, radiation therapy (RT), and novel targeted therapies is currently unknown. Cisplatin, actinomycin-D, chlorambucil, mithramycin, and bleomycin have been used; however, too few dogs were treated and evaluated to formulate conclusions regarding efficacy.70,71,73,101 Cisplatin was evaluated in three dogs with aggressive testicular tumors, with survival ranging from 5 months to greater than 31 months.70 RT was successfully used in four dogs with metastatic seminoma confined to the regional lymph nodes using total doses ranging from 17 to 40 Gy with 137Cesium teletherapy.102 In all four cases, tumors regressed and survival times ranged from 6 to 37 months; importantly, none of the dogs died of seminoma. Numbers were small, and further studies are warranted to evaluate the role of external-beam RT in managing metastatic seminomas because seminomas are considered extremely radioresponsive.103
Comparative Aspects
In the United States, testicular cancer is the most common cancer in men 15 to 44 years old but is one of the most curable cancers with early diagnosis.104 Recent studies have shown an increase in testicular cancer over the past 40 years, suggesting that an individual’s risk is a function of the era in which he was born.104,105 Causal factors such as genetic predisposition, maternal estrogen exposure, occupational hazards, dietary factors, smoking habits, and birthplace have been evaluated, but, to date, the most established risk factor remains cryptorchidism.23,104,106-114 Most cancers in men are germ cell tumors and are divided into seminomas and nonseminomas, with the former comprising 50% of tumors in this group.13,20,23 Seminomas are further classified into classical, atypical, or spermatocytic seminoma, although management does not vary considerably between types.20 Standard staging in human seminomas consists of physical examination;, radiographic studies; determination of serum markers, including alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), and lactate dehydrogenase; and histopathologic assessment. Spermatocytic seminoma is a rare variant of germ cell neoplasia that is most commonly seen in older men and carries a low risk of metastasis, suggesting similar behavior to most canine seminomas.21,23 Treatment generally includes surgery for stage I seminomas, and/or RT and chemotherapy for individuals with advanced stage disease.23,103,115-117 Cisplatin-based chemotherapy protocols are generally employed for patients with greater than stage I disease, with cure rates in the range of 70% to 80% despite advanced tumor burdens.23,103,118 The dog has been proposed as a model for studies evaluating the development of testicular tumors.18,21,22 Further interrogation of the molecular pathogenesis, classification system, and behavior of canine tumors may yield further support for use of spontaneously occurring testicular tumors as good comparative models for human disease.
Feline Testicular Tumors
Feline testicular tumors are rare, although Sertoli cell tumor, seminoma, interstitial cell tumor, and teratoma have been reported.34,119-125 The biologic behavior of testicular neoplasia in the cat is unclear due to the sparse literature available. Metastasis of a Sertoli cell tumor to the liver and spleen has been reported, and teratoma metastasis to the omentum was observed in another report.122,125 Optimal therapy other than orchiectomy is not known.
Canine Prostate Tumors
Prostatic tumors are relatively uncommon in dogs and have a low prevalence at less than 1% (0.2% to 0.6%).126-130 In a collection of over 17,000 confirmed neoplasms of the dog collected from veterinary schools in North America, only 11 prostate carcinomas were identified (0.06%).33 In a separate study that evaluated lifetime occurrence of neoplasia in predominantly intact German shepherd and Belgian Malinois working dogs, over 30% developed at least one cancer; however, only 2 of the 104 primary tumors were prostate adenocarcinomas.17 Despite this low incidence, the dog is one of the few domestic species to develop spontaneous prostate cancer, thus sparking interest in the dog as a comparative model for prostate cancer in men.129,131,132 In three retrospective reviews of dogs with prostatic disease, between 7% and 16% were diagnosed with prostatic adenocarcinoma.133-135 One study of 177 dogs found that prostatic adenocarcinoma was the most common disease in neutered dogs, whereas bacterial prostatitis and prostatic cysts were more common in intact male dogs.134 Elderly dogs are more commonly diagnosed with prostatic carcinoma, with a median age at diagnosis of 10 years.129,135,136 The underlying etiology of canine prostatic cancer is unknown; however, high-grade prostatic intraepithelial neoplasia (PIN or HGPIN), which is believed to be a precursor of human prostate carcinoma, has been detected in dogs without evidence of prostatic disease and in those with existing prostatic carcinoma.137-139 The occurrence of PIN in dogs with concurrent carcinoma varies from 7% to 72%, although in two large studies of dogs without histologic evidence of prostatic carcinoma, the occurrence was low at 0% to 3%.128,137-139
Most tumors of the canine prostate are carcinomas, and the majority are adenocarcinomas. It is believed that prostate tumors in the dog arise from a urothelial or ductular origin rather than acinar because most canine tumors are androgen independent.140-145 Other types of carcinomas, including transitional cell carcinoma (TCC) arising from the prostatic ducts, mixed carcinomas, and squamous cell carcinomas, can occur. Classifying carcinomas on the basis of subtype is somewhat subjective, and there is no standard for definitive diagnosis of canine prostate tumors as there is with humans.139,146,147 Fibrosarcoma, leiomyosarcoma, osteosarcoma, lymphoma, and hemangiosarcoma have also been reported to affect the prostate.128,148-153 Benign tumors of the prostate are rare, although there is a single case report of a dog with a benign prostatic adenoma or nodular hyperplasia.154 TCC of the prostatic urethra will frequently invade the prostate, and it may be difficult to distinguish primary TCC from secondary invasion of a urethral tumor.
Risk Factors
Both intact and castrated dogs develop prostate carcinomas, although multiple studies have suggested there is an increased risk of prostatic adenocarcinoma in castrated male dogs compared to intact male dogs with an odds ratio of approximately 2.3 : 4.3.126,135,136,140,143 More aggressive tumors may develop in castrated males with a higher risk of metastasis.136 The reason for this difference is unclear, although it is possible that castrated dogs live longer than intact dogs and are thus predisposed to developing age-related cancers.135,143 It is also possible that androgens provide a protective effect on prostatic tissue or that, on castration, the relative estrogen effect aids in neoplastic transformation.143,155-157 A recent study observed that prostatic adenocarcinoma may occur relatively more frequently in intact male dogs, further complicating this issue.128,139,143 Although several studies have suggested that androgens may not be required for initiation or progression of adenocarcinoma of the canine prostate, further controlled studies should be done to definitively determine the role of androgens and the impact of early or late castration.140,142-145,158
Breeds that may be at increased risk of developing prostate carcinomas include the Bouvier des Flandres, Doberman Pinscher, Shetland sheepdog, Scottish terrier, beagle, miniature poodle, German shorthaired pointer, Airedale terrier, and Norwegian elkhound.135,143 The Shetland sheepdog and Scottish terrier remained at increased risk even when TCC was excluded in one study.143 Mixed breed dogs have also been reported as at increased risk for prostate carcinoma, regardless of neutering status, suggesting environmental influences may play a significant role in tumor development.143 The American cocker spaniel, miniature poodle, and dachshund may be at decreased risk for developing prostate cancer.143
Natural Behavior
At the time of diagnosis, most canine prostatic tumors are characterized by local invasion with a high propensity for regional and distant metastasis. In one postmortem study that retrospectively evaluated 76 dogs, 80% of those with prostate carcinoma had evidence of measurable metastatic disease, with lung and lymph node being the most common sites.128 Importantly, similar to high-grade prostatic carcinoma in men, canine prostatic carcinomas have a tendency to metastasize to bone and 22% to 42% of canine patients develop skeletal metastasis, predominantly to the lumbar vertebrae and pelvis.128,144,159,160 Younger dogs may be at increased risk for metastasis, although the role of castration status in this group is unclear.128,161 Longitudinal studies in dogs with evidence of PIN are not available, and it is unclear if prostate carcinoma can behave in a slowly progressive fashion in early phases of development. It is presumed that most dogs are diagnosed at an advanced stage of disease due to the high metastatic rate; however, the true behavior from time of onset is not definitively known and prostate carcinoma may behave differently in intact and castrated dogs.135,136
Pathology/Pathogenesis
The underlying cause of prostate tumors is unknown, and it is possible that both genetic and environmental factors contribute to tumor development. As previously mentioned, HGPIN is considered a precursor of human prostate carcinoma and occurs under the influence of androgenic stimulation in those patients at risk for carcinoma.162 Although PIN has been detected in dogs with existing prostatic carcinoma, it has also been detected in dogs without evidence of prostatic disease, making its role in the dog less clear.137-139 HGPIN as a predictor of carcinoma occurrence is likely not as reliable in the dog as it is in men.138,155 It is not known with certainty if low- and intermediate-grade PIN occurs in dogs, although a recent immunohistochemical study suggested the presence of low-grade PIN.163 Investigators evaluated five prostates from middle-aged to older intact dogs containing lesions of PIN and compared nuclear protein p63 (marker of prostatic basal cells), androgen receptor expression, and PCNA to normal prostatic tissue from intact dogs. PIN foci had higher p63 expression, higher PCNA index, and heterogenous androgen receptor expression, suggesting similarities to human low grade PIN.163
The role of hormones in prostate development and tumor progression is also unclear in the dog. Castration does not provide a protective effect and, in fact, may contribute to tumor development and/or progression, although prostatic carcinoma may behave differently in the intact male compared to the neutered male.135,136 Normal prostate development and regulation is androgen dependent in both humans and dogs; however, neoplastic human prostate remains androgen dependent unlike in the dog. Androgen receptor expression within the nuclei can be identified in 90% to 95% of normal noncastrated prostatic secretory epithelial cells and in the majority of acinar basal cells.144,145,164,165 In neutered dogs and dogs with prostatic carcinoma, nuclear androgen expression decreases and is usually lost.144,145 The role of estrogen and progesterone has yet to be fully defined, although nuclear estrogen receptor expression appears to be decreased in prostatic carcinoma tissue compared to normal and hyperplastic prostate tissue.141,165
Chromosomal abnormalities of the neoplastic prostate have only recently been studied, and preliminary data suggest abnormalities such as aneuploidy, centromeric fusions, polysomy of chromosome 13, and hyperdiploidy may occur in the dog.166-168
Because of its aggressive behavior at the time of diagnosis, some investigators have considered mechanisms that may contribute to prostate carcinoma progression and metastasis. The role of cyclooxygenase (COX) has received considerable attention in the human and veterinary literature and inhibition of COX-2 may play a role in the management of prostate tumors. Expression of COX-2 was noted in 75% of prostate carcinomas in one study, whereas none of the normal prostate tissue had expression.169 Two other studies support the notion that COX-2 and its downstream prostaglandin E2 production play a role in prostate carcinomas.170,171 Indeed, a clinical study identified COX-2 protein expression in 88% of 16 prostate carcinomas examined and further showed a survival benefit in dogs treated with either piroxicam or carprofen.172 As stated earlier, prostatic carcinoma has a predilection for bone, which may be mediated in part by transforming growth factor-β (TGF-β), parathyroid hormone–related protein (PTHrP), and endothelin.173-175 PTHrP mediates pathologic bone resorption in many different tumors, including prostate carcinomas, which may encourage release of TGF-β into the microenvironment. In a positive feedback loop, canine prostatic carcinoma cells can increase gene transcription for PTHrP in response to exogenous TGF-β.174 Although PTHrP and TGF-β may be important in establishing skeletal metastases, it is interesting to note that prostate metastases are more commonly osteoblastic in nature. In a rat model, osteoblast activation was increased following incubation with normal canine prostate protein homogenates through an endothelin-dependent pathway, suggesting a possible contribution to bone metastasis formation.175
History and Clinical Signs
Clinical signs in dogs with prostate cancer are variable and may be reflective of local and/or metastatic disease. Common historic and clinical examination signs include hematuria, dysuria, stranguria, dyschezia, tenesmus, bacteriuria, and altered stool shape (flattened or ribbonlike stools).128,136,160,176,177 If complete obstruction of urinary outflow results from prostatic compression or direct tumor extension into the urethra, hydroureter, hydronephrosis, and renal failure may occur. Local invasion into the lumbar vertebrae or nerve roots may cause signs of pain, gait abnormalities, lameness, and/or constipation. Nonspecific systemic illness, including lethargy, exercise intolerance, tachypnea or dyspnea, inappetence, and weight loss, can occur with advanced disease. Dogs with skeletal metastasis may present with signs of severe bone pain, pathologic fracture, or rarely with a palpable mass.128,160,178 Dogs may present with a history of clinical signs that partially improved with empiric therapy for prostatitis.
Diagnostic Techniques and Staging
Dogs with suspected prostatic carcinoma should be fully staged in order to determine extent of disease and to rule out other causes of prostatic disease, such as benign prostatic hypertrophy (BPH), prostatitis, and prostatic cysts or abscesses.177,179,180 Physical examination, including a thorough rectal examination, should be performed on every patient. Rectal palpation often reveals a large, firm, and asymmetric or irregular prostate that may be painful. Sublumbar lymphadenopathy may be detected on rectal or abdominal palpation. A normal-sized prostate on rectal examination in a castrated dog is considered abnormal, even if symmetric and nonpainful. CBC and serum chemistry profile may demonstrate anemia, leukocytosis, hypercalcemia, elevated bone alkaline phosphatase activity, or signs of concurrent disease. Urinalysis and culture may show pyuria, bacteriuria, dysplastic urinary epithelial cells, and secondary urinary bacterial infection. Three-view thoracic radiographs may show evidence of pulmonary metastatic disease, sternal lymphadenopathy, or rarely metastasis to the extrathoracic skeletal structures (ribs, scapula).160 Abdominal radiographs may reveal evidence of an enlarged prostate, with or without evidence of mineralization; periosteal reactions on the vertebrae, femur, or pelvic bones; or sublumbar or retroperitoneal lymphadenopathy (Figure 28-6).136,160,181,182 It is important to note that the presence of mineralization, particularly in intact dogs, is not pathognomonic for neoplasia and can occur in dogs with prostatitis, BPH, or prostatic cysts.182-184 However, neutered dogs with prostatic mineralization are highly likely to have prostatic neoplasia and should undergo further diagnostics.182 If a clinical suspicion of skeletal metastasis exists, survey radiographs or bone scintigraphy may be useful for localization.178 Prostate carcinoma metastases to bone most commonly have an osteoproductive component but may be osteolytic, osteoproductive, or mixed. Contrast studies such as retrograde urethrography may be useful to evaluate irregularities in the prostatic urethra or reflux of contrast into a prostatic mass; however, they are not specific enough to differentiate neoplasia from inflammatory or infectious processes.181,185 Abdominal ultrasound can be useful to further evaluate the prostate, urethra, bladder, locoregional lymph nodes, and cranial abdominal organs. Lymphadenopathy, echogenicity changes, and mineralization may be visualized on ultrasound, although they can also be features of nonneoplastic diseases (Figure 28-7).181,182
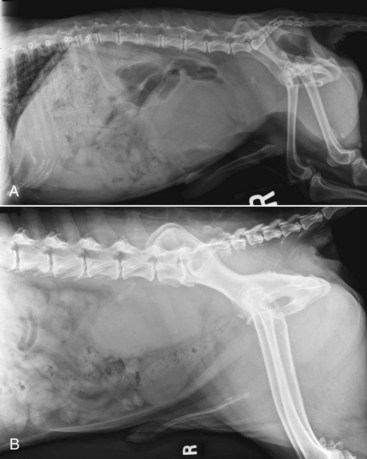
Figure 28-6 A, Right lateral abdominal radiograph demonstrating prostatic mineralization, which may be a feature of benign and malignant disease in intact dogs. B, Right lateral abdominal radiograph of a dog with prostatic carcinoma with metastasis to the sublumbar lymph nodes and local invasion along the sacrum and ventral aspect of the seventh lumbar vertebral body. (A courtesy Dr. S. Holmes, University of Georgia; B courtesy Dr. D. Jimenez, University of Georgia.)
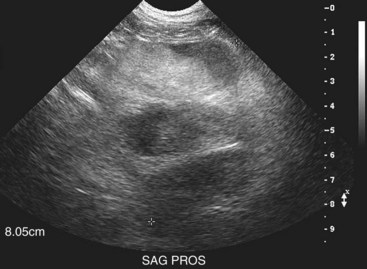
Figure 28-7 Ultrasound image of a symmetrically but severely enlarged prostate of heterogeneous echogenicity in a dog diagnosed with Rocky Mountain spotted fever and bacterial prostatitis. (Courtesy Dr. D. Jimenez, University of Georgia.)
Obtaining tissue samples is considered the gold standard of diagnosis of canine prostatic neoplasia; however, a definitive diagnosis of prostatic neoplasia may be garnered from cytology samples as well.177,186 A number of methods may provide adequate samples for diagnosis, including ejaculation, traumatic catheterization, prostatic massage, prostatic wash, ultrasound-guided FNA cytology, impression smears during surgery, or biopsy via percutaneous, perineal transrectal, or surgical routes. Risks of percutaneous biopsies, ultrasound-guided aspirates or biopsies, and transrectal aspirates may include hemorrhage, urethral trauma, and tumor seeding.179,187-190 Cytology or histology of suspected metastatic lesions (e.g., lymph node) may also aid with diagnosis and offer an easier approach at diagnosis (Figure 28-8). Histologic grading of prostate cancers is not routinely performed because there is no support for its impact on prognostication.128,191 Cytologic evaluation of samples collected via traumatic catheterization or prostatic wash may prove challenging as it can be difficult to differentiate dysplastic epithelial cells from neoplasia.186,192 In one study, discordant results between cytology and histology in prostatic disorders occurred in 20% of cases but were not considered a flaw of aspiration techniques but rather of the pathologic process.186 Multiple techniques were employed, including ultrasound-guided FNA, prostatic massage and wash, and impression smears of biopsies. Other factors such as serum and seminal plasma concentrations of acid phosphatase (AP), prostate-specific antigen (PSA), and canine prostate-specific esterase have not been useful in the definitive diagnosis of prostate carcinoma in the dog.127,193 Although significantly higher serum total AP, prostatic AP, and nonprostatic AP concentrations were noted in dogs with prostatic carcinoma compared to healthy dogs or dogs with BPH, they were neither sensitive nor specific enough to warrant definitive diagnosis.193
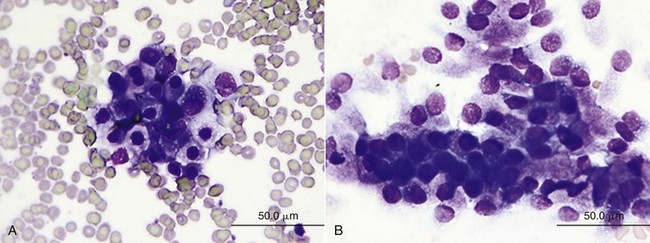
Figure 28-8 A, Prostatic carcinoma showing a cluster of variably sized polygonal epithelial cells with a moderate amount of basophilic cytoplasm, which often contains clear, nonstaining vacuoles. These cells have round-to-ovoid nuclei with coarsely granular chromatin pattern and occasional distinct nucleoli. A single binucleated cell is seen. Nuclear to cytoplasmic (N : C) ratios are moderate, and there is mild-to-moderate anisocytosis and anisokaryosis. B, Normal prostate composed of loosely cohesive clusters of polygonal to columnar epithelial cells with a moderate amount of basophilic cytoplasm that often appears stippled with both eosinophilic granules and tiny, crisp, nonstaining vacuoles. Nuclei are round to ovoid and are often eccentrically located. Chromatin is condensed, and there is minimal anisocytosis and anisokaryosis. (Courtesy Dr. M. Camus, University of Georgia.)
Treatment and Prognosis
Because prostate cancer in dogs is characterized by insidious local progression and a high rate of metastasis, most dogs are diagnosed with advanced disease and the overall prognosis is poor. Median survival times (MSTs) for dogs without therapy are often less than 30 days, and in one report of 76 dogs, most dogs were euthanized at the time of diagnosis.128,172 If therapy is attempted, effort is generally made to control local disease, as well as locoregional and distant metastases, although therapy is considered largely palliative. There is currently no standard-of-care consensus therapy for canine prostate tumors, although use of nonsteroidal antiinflammatory drugs (NSAIDs) is often recommended as minimal therapy.
Therapeutic options for managing local disease include prostatectomy, electrosurgical transurethral resection (TUR), photodynamic therapy (PDT), RT, laser therapy, and medical management. Surgery is generally considered to be a palliative procedure; surgical goals are to minimize clinical signs secondary to the primary tumor while maintaining normal urethral function. Prostatectomy is generally recommended for dogs with early stage disease that is still confined within the capsule (intracapsular disease). Total prostatectomy can be performed, although it is associated with a high rate of postoperative morbidity and a survival benefit has not been definitively demonstrated.194-199 Subtotal intracapsular prostatectomy may be a useful alternative in dogs. In one study that compared 10 dogs that underwent total prostatectomy to 11 dogs that underwent subtotal intracapsular prostatectomy, the latter procedure was associated with longer mean survival time (112 days versus 20 days) and a decreased rate of postoperative complications.196 Importantly, 7 of the 10 dogs that underwent total prostatectomy were euthanized within 2 weeks of surgery compared to only 2 dogs in the subtotal intracapsular group.196 Urinary incontinence is a common sequela to prostatectomy and attempts have been made to reduce trauma to the prostatic urethra, including use of a neodymium : yttrium-aluminum-garnet (Nd : YAG) laser; however, there is still a risk of significant postoperative complications.195,198,199 In one recent report, TUR using an electrocautery cutting loop was performed with or without intraoperative RT in three dogs with prostatic TCC or undifferentiated carcinoma.200 All dogs experienced rapid palliation of dysuria but complications, including urinary tract infection, tumor seeding, and urethral perforation, occurred and survival for all dogs was short.200 Although dogs with prostatic carcinomas may be palliated early with surgical intervention, TUR and total prostatectomy are associated with a high risk of complications and cases should be selected carefully.
In dogs with urethral obstruction due to a prostatic tumor, palliative measures may be attempted to alleviate the obstruction. Placement of a cystostomy tube permits urinary diversion and bladder emptying, but owners should be aware that it generally does not resolve incontinence and stranguria and secondary urinary tract infections are common.201-203 Palliative stenting of the urethra in the obstructed area is a reasonable alternative to cystostomy tubes. The extent and location of the obstruction is determined using fluoroscopy, and stents are typically selected to extend approximately 1 cm proximal and distal to the obstruction.148 Stents can be ordered in various diameters and are recommended to be 10% greater than the diameter of healthy appearing urethra. Of eight dogs with prostatic tumors stented in one study, the severe complication rate was low (two dogs) and the procedure immediately alleviated the obstruction in all dogs.148 Median survival time in this study involving 12 dogs with bladder TCC or prostate tumors was short (20 days) despite stenting and therapy with a COX-2 inhibitor; however, further experience and evaluation of this technique may demonstrate improved quality of life. PDT may be an option, particularly for dogs with minimally invasive prostate cancer. There is one case report describing the resolution of macroscopic hematuria and sanguineous preputial discharge with stable disease for at least 6 months in a dog with prostatic carcinoma.204 PDT remains predominantly investigational, although several recent reports have suggested that the dog provides a good model to investigate novel treatments for prostatic carcinoma that may benefit both dogs and humans.205-208 Challenges in delivering a homogenous dose may limit the utility of PDT in advanced tumors; however, further evaluation may indicate a role in the management of prostatic carcinomas.
RT may be useful in the palliation of clinical signs related to local prostatic carcinoma as well as to palliate painful skeletal metastases, although optimal dose and fractionation are unknown. In one study, 10 dogs with prostatic carcinoma were treated with intraoperative orthovoltage therapy.209 Nine were prescribed 20 to 30 Gy to the prostate, with an MST of 114 days, although the range extended to 750 days.209 It is probable that with advances in 3-dimensional (3D) imaging and intensity-modulated RT (IMRT) planning and delivery, local disease may be better targeted and controlled. However, metastatic disease will remain a challenge unless early prostatic carcinoma can be detected.
The benefit of systemic therapy to manage canine prostatic tumors is unclear, although a pivotal study demonstrated a clear survival benefit in dogs with prostate carcinomas treated with piroxicam or carprofen compared to those untreated (6.9 months versus 0.7 month).172 The role of chemotherapy is less clear, although prospective studies are warranted for further investigation of NSAIDs alone and in combination with chemotherapy.
For dogs with skeletal metastasis, palliative options include systemic analgesics, RT, aminobisphosphonate administration, and 153samarium ethylenediamine-tetramethylene phosphonate (153SM-EDTMP).210,211 Standardized protocols have not been determined, although RT and pamidronate are both widely available options and relatively easy to administer.210,212,213
Comparative Aspects
Prostate cancer is the second most frequently diagnosed cancer and the sixth most common fatal cancer among men worldwide, and its incidence is increasing.214,215 The only well-established and consistent risk factors for prostate cancer in men are race/ethnicity, family history, and age, although other factors such as diet and lifestyle may contribute as well.214-221 As in dogs, most prostatic tumors in men are carcinomas; however, the incidence of disease is considerably higher in men. Geriatric men have a high rate of asymptomatic prostate cancer, in part due to the increased occurrence of PSA-driven biopsies. Serum PSA is a readily detectable serine protease produced by both malignant and benign prostate tissues. The dog is one of the few domestic species to develop spontaneous prostate cancer, resulting in considerable interest in the dog as a model for prostatic carcinoma in men.129,131,132 A similar canine PSA is not detectable with commercially available human antibodies, thus limiting comparisons made between diagnosis of human PSA-driven and canine prostate cancer.127,222 Another important challenge in using the dog as a model is that most early-stage prostate carcinomas in men are highly androgen dependent and disease progression can be manipulated through androgen deprivation.214,223 Prostate carcinoma in men typically initiates as PIN, which is often seen as multifocal premalignant lesions that progress to neoplasia; the role of PIN in the development of canine prostatic diseases in either the intact or castrated dog is unclear. High-grade prostate cancer in men behaves similarly to the disease in dogs, with significant local invasion and a propensity to metastasize to the skeleton. While high-grade aggressive prostate carcinoma is less common in the elderly man, the dog may serve as a model for interventional strategies in this setting. Therapy in humans varies from active surveillance to prostatectomy to RT (external beam and brachytherapy) and is highly dependent on the stage of disease at presentation and partially on risks versus benefits of intervention.214,215,223 Treatment strategies, including chemotherapy, vascular targeting, RT approaches, and management of skeletal metastasis, may be investigated in canine models and ultimately benefit both species.
Feline Prostate Tumors
Prostate tumors in the cat are rare and reports in the veterinary literature are sparse.224-229 Of the few case reports, most tumors are adenocarcinomas and affect older castrated cats. Definitive risk factors have not been identified due to the lack of frequent cases and epidemiologic data. Clinical signs often include lower urinary tract signs, as well as obstipation or constipation, tenesmus, and dyschezia. Rectal palpation can reveal the presence of a mass, which may be further characterized with abdominal ultrasound. There is no standard-of-care therapy, and it is difficult to state overall prognosis. Metastasis appears common, and sites of spread include pancreas, lung, and lymph nodes; most cats died within 3 months of diagnosis.224-228 A recent report described the use of prostatectomy for a low-grade prostatic sarcomatoid carcinoma, with no evidence of local or metastatic disease at 2 years.229 One report described the use of prostatectomy followed by doxorubicin and cyclophosphamide to yield a survival time of 10 months.225
Canine Penile, Preputial, and Scrotal Tumors
Multiple tumor types can affect the soft tissues of the canine penis, prepuce, and scrotum, including transmissible venereal tumor (TVT), squamous cell carcinoma, sebaceous gland adenoma, mesothelioma, papilloma, lymphoma, plasma cell tumor, mast cell tumor, hemangioma, melanoma, and fibrosarcoma.37,230-237 Overall, TVT and squamous cell carcinomas are the most common neoplasms of the canine penis. Ossifying fibroma, benign mesenchymoma, multilobular osteochondrosarcoma, and osteosarcoma can arise from the penile bone (os penis).238-243 Osteosarcoma of the os penis may behave similarly to other axial skeleton sites, with a potential to develop local recurrence following narrow excision and distant metastasis.238,240
Clinical signs are often associated with local disease, and many dogs present with hematuria, stranguria, or dysuria. Occasionally, dogs may present with a visible mass and the absence of urinary signs; this is likely more common in scrotal tumors compared to other tumor sites. It is also possible for clinical signs to be secondary to locoregional or distant metastasis. It is recommended to fully stage dogs with penile tumors prior to definitive therapy. Surgical excision is generally recommended and often involves partial or complete penile amputation and perineal urethrostomy. TVT is an obvious exception because it is a chemoresponsive and radiation-responsive tumor, although surgery may be used for refractory cases.244,245 For dogs with scrotal tumors, castration (if intact) with scrotal ablation is recommended. Depending on the underlying tumor type, adjuvant therapy such as RT or chemotherapy may be indicated. Prognosis is heavily dependent on underlying histology, as well as the ability to obtain adequate local control.
Feline Penile, Preputial, and Scrotal Tumors
Little information exists on tumors that affect the feline penis, prepuce, and scrotum. There is a report of fibroma affecting the scrotum of a cat; however, it is probable that clinical signs would be similar to those in dogs.246
Comparative Aspects
Penile tumors in the United States are rare, although they remain a problem in a number of countries in Asia, Africa, and South America, where up to 10% of cancer may arise in the penis.247 The incidence of penile cancer has been declining in part due to increased personal hygiene and circumcision.248-250 Indeed, neonatal circumcision, as practiced by a number of groups, virtually eliminates penile carcinoma from the population.249 Poor local hygiene, phimosis, tobacco, chronic inflammation, lack of circumcision, infection with human papilloma virus, and having multiple sexual partners are associated with the development of malignant penile lesions.249,251-253 Most penile tumors are carcinomas and over 95% of carcinomas are squamous cell in origin. Clinical signs in men are varied and range from a subtle erythematous local lesion to an ulcerated, infected mass lesion. Full staging is important in men who present with penile cancer because nodal status is one of the most significant prognostic variables for survival.247 The prognosis is generally excellent for patients with early stage disease and treatment typically consists of surgery. Occasionally, RT is used for primary treatment of early stage disease, but local recurrence rates are higher than with surgery.247,254-256 In advanced cases, aggressive surgery may include emasculation procedures or hemipelvectomy followed by RT. The role of chemotherapy is still evolving in human penile carcinoma management, but agents such as cisplatin, methotrexate, and bleomycin appear to have modest activity.
References
1. Cotchin, E. Testicular neoplasms in dogs. J Comp Pathol. 1960;70:232.
2. von-Bomhard, D, Pukkavesa, C, Haenichen, T. The ultrastructure of testicular tumours in the dog. I. Germina cells and seminomas. J Comp Pathol. 1978;88:49.
3. Hayes, HM, Pendergrass, TW. Canine testicular tumors: epidemiologic features of 410 dogs. Int J Cancer. 1976;18:482.
4. Liao, AT, Chu, PY, Yeh, LS, et al. A 12-year retrospective study of canine testicular tumors. Theriogenology. 2009;71:919.
5. Cohen, D, Reif, J, Brodey, R, et al. Epidemiologic analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Res. 1974;34:2859.
6. Dow, C. Testicular tumours in the dog. J Comp Pathol. 1962;72:247.
7. Grieco, V, Riccardi, E, Greppi, GF, et al. Canine testicular tumours: a study on 232 dogs. J Comp Pathol. 2008;138:86.
8. Nodtvedt, A, Gamlem, H, Gunnes, G, et al. Breed differences in the proportional morbidity of testicular tumours and distribution of histopathologic types in a population-based canine cancer registry. Vet Comp Oncol. 2011;9:45.
9. Bray, F, Ferlay, J, Devesa, SS, et al. Interpreting the international trends in testicular seminoma and nonseminoma incidence. Nat Clin Pract Urol. 2006;3:532.
10. Chia, VM, Quraishi, SM, Devesa, SS, et al. International trends in the incidence of testicular cancer, 1973-2002. Cancer Epidemiol Biomarkers Prev. 2010;19:1151.
11. Shah, MN, Devesa, SS, McGlynn, KA. Trends in testicular germ cell tumours by ethnic group in the United States. Int J Androl. 2007;30:206.
12. Townsend, JS, Richardson, LC, German, RR. Incidence of testicular cancer in the United States, 1999-2004. Am J Mens Health. 2010;4:353.
13. Bray, F, Richiardi, L, Ekbom, A, et al. Do testicular seminoma and nonseminoma share the same etiology? Evidence from an age-period-cohort analysis of incidence trends in eight European countries. Cancer Epidemiol Biomarkers Prev. 2006;15:652.
14. Weaver, AD. Survey with follow-up of 67 dogs with testicular Sertoli cell tumours. Vet Rec. 1983;113:105.
15. Reif, JS, Maguire, TG, Kenney, RM, et al. A cohort study of canine testicular neoplasia. J Am Vet Med Assoc. 1979;175:719.
16. Kennedy, PC, Cullen, JM, Edwards, JF, et al, Histological classifications of tumors of the genital system of domestic animals. World Health Organization international histological classification of tumors of domestic animals ed 2. Washington, DC: American Registry of Pathology; 1998.
17. Peterson, JR, Frommelt, RA, Dunn, DG. A study of the lifetime occurrence of neoplasia and breed differences in a cohort of German shepherd dogs and Belgian Malinois military working dogs that died in 1992. J Vet Int Med. 2000;14:140.
18. Peters, MA, DeRooij, DG, Teerds, KJ, et al. Spermatogenesis and testicular tumours in ageing dogs. J Reprod Fertil. 2000;120:443.
19. Maiolino, P, Restucci, B, Papparella, S, et al. Correlation of nuclear morphometric features with animal and human World Health Organization international histological classifications of canine spontaneous seminomas. Vet Pathol. 2004;41:608.
20. Mostofi, FK, Sesterhenn, IA, Histologic typing of testis tumors. World Health Organization international histological classification of tumors ed 2. Geneva: Springer; 1998.
21. Kim, JH, Yu, CH, Yhee, JY, et al. Canine classical seminoma: a specific malignant type with human classifications is highly correlated with tumor angiogenesis. BMC Cancer. 2010;10:243.
22. Grieco, V, Riccardi, E, Rondena, M, et al. Classical and spermatocytic seminoma in the dog: histochemical and immunohistochemical findings. J Comp Pathol. 2007;137:41.
23. Bosl, GJ, Bajorin, DF, Sheinfeld, J, et al. Cancer of the testis. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams and Wilkins, 2008.
24. Ortega-Pacheco, A, Rodriguez-Buenfil, JC, Segura-Correa, JC, et al. Pathological conditions of the reproductive organs of male stray dogs in the tropics: prevalence, risk factors, morphological findings and testosterone concentrations. Reprod Domest Anim. 2006;41:429.
25. Reif, JS, Brodey, RS. The relationship between cryptorchidism and canine testicular neoplasia. J Am Vet Med Assoc. 1969;155:2005.
26. Patnaik, AK, Mostofi, FK. A clinicopathologic, histologic, and immunohistochemical study of mixed germ cell-stromal tumors of the testis in 16 dogs. Vet Pathol. 1993;30:287.
27. Radi, ZA, Miller, DL, Hines, ME. Rete testis mucinous adenocarcinoma in a dog. Vet Pathol. 2004;41:75.
28. Turk, JR, Turk, MA, Gallina, AM. A canine testicular tumor resembling gonadoblastoma. Vet Pathol. 1981;18:201.
29. Rothwell, TLW, Papdimitriou, JM, Zu, FN, et al. Schwannoma in the testis of a dog. Vet Pathol. 1986;23:629.
30. Vascellari, M, Carminato, A, Camall, G, et al. Malignant mesothelioma of the tunica vaginalis testis in a dog: histological and immunohistochemical characterization. J Vet Diagn Invest. 2011;23:135.
31. Scully, RE, Coffin, DL. Canine testicular tumors with special reference to their histogenesis, comparative morphology, and endocrinology. Cancer Epidemiol Biomarkers Prev. 1952;5:592.
32. Lipowitz, AJ, Schwartz, A, Wilson, GP, et al. Testicular neoplasms and concomitant clinical changes in the dog. J Am Vet Med Assoc. 1973;163:1364.
33. Priester, WA, McKay, FW. The occurrence of tumors in domestic animals. Natl Cancer Inst Monogr. 1980;54:1.
34. Sapierzynski, R, Malicka, E, Bielecki, W, et al. Tumors of the urogenital system in dogs and cats. Retrospective review of 138 cases. Pol J Vet Sci. 2007;10:97.
35. Hayes, HM, Tarone, RE, Casey, HW. A cohort study of the effects of Vietnam service on testicular pathology of US military working dogs. Mil Med. 1995;160:248.
36. Hayes, HM, Tarone, RE, Casey, HW, et al. Excess of seminomas observed in Vietnam service U.S. military working dogs. J Natl Cancer Inst. 1990;82:1042.
37. McEntee, MC. Reproductive oncology. Clin Tech Small Anim Pract. 2002;17:133.
38. Benazzi, C, Sarli, G, Preziosi, R, et al. Laminin expression in testicular tumours of the dog. J Comp Pathol. 1995;112:141.
39. Sarli, G, Benazzi, C, Preziosi, R, et al. Proliferative activity assessed by anti-PCNA and Ki67 MAbs in canine testicular tumours. J Comp Pathol. 1994;110:357.
40. Papaioannou, N, Psalla, D, Zavlaris, M, et al. Immunohistochemical expression of dog TERT in canine testicular tumours in relation to PCNA, ki67 and p53 expression. Vet Res Commun. 2009;33:905.
41. Nasir, L. Telomeres and telomerase: biological and clinical importance in dogs. Vet J. 2008;175:155.
42. Nasir, L, Devlin, P, McKevitt, T, et al. Telomere lengths and telomerase activity in dog tissues: a potential model system to study human telomere and telomerase biology. Neoplasia. 2001;3:351.
43. DeVico, G, Papparella, S, DiGuardo, G. Number and size of silver-stained nucleoli (Ag-NOR clusters) in canine seminomas: correlation with histological features and tumour behaviour. J Comp Pathol. 1994;110:267.
44. Murakami, Y, Tateyama, S, Uchida, K, et al. Immunohistochemical analysis of cyclins in canine normal testes and testicular tumors. J Vet Med Sci. 2001;63:909.
45. Brehm, R, Ruttinger, C, Fischer, P, et al. Transition from preinvasive carcinoma in situ to seminoma is accompanied by a reduction of connexin 43 expression in Sertoli cells and germ cells. Neoplasia. 2006;8:499.
46. Lin, JH, Takano, T, Cotrina, ML, et al. Connexin 43 enhances the adhesivity and mediates the invasion of malignant glioma cells. J Neurosci. 2002;22:4302.
47. Risley, MS, Tan, IP, Roy, C, et al. Cell-, age-, and stage-dependent distribution of connexin43 gap junctions in testes. J Cell Sci. 1992;103:81.
48. Steger, K, Tetens, F, Bergmann, M. Expression of connexin43 in human testis. Histochem Cell Biol. 1999;112:215.
49. Zhang, W, Nwagwu, C, Le, DM, et al. Increased invasive capacity of connexin43-overexpressing malignant glioma cells. J NeuroSci. 2003;99:1039.
50. Ruttinger, C, Bergmann, M, Fink, L, et al. Expression of connexin 43 in normal canine testes and canine testicular tumors. Histochem Cell Biol. 2008;130:537.
51. Lee, CH, Kim, WH, Lim, JH, et al. Mutation and overexpression of p53 as a prognostic factor in canine mammary tumors. J Vet Sci. 2004;5:63.
52. Lee, CH, Kweon, OK. Mutations of p53 tumor suppressor gene in spontaneous canine mammary tumors. J Vet Sci. 2002;3:321.
53. Queiroga, FL, Raposo, T, Carvalho, MI, et al. Canine mammary tumours as a model to study human breast cancer: most recent findings. In Vivo. 2011;25:455.
54. Inoue, M, Wada, N. Immunohistochemical detection of p53 and p21 proteins in canine testicular tumours. Vet Rec. 2000;146:370.
55. Vitellozzi, G, Mariotti, F, Ricci, G. Immunohistochemical expression of the p53 protein in testicular tumours in the dog. Eur J Vet Pathol. 1998;4:61.
56. Restucci, B, Maiolino, P, Paciello, O, et al. Evaluation of angiogenesis in canine seminomas by quantitative immunohistochemistry. J Comp Pathol. 2003;128:252.
57. Yoshinaga, K, Nishikawa, S, Ogawa, M, et al. Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development. 1991;113:689.
58. Sattler, M, Salgia, R. Targeting c-kit mutation: basic science to novel therapies. Leuk Res. 2004;28S1:S11.
59. Goddard, NC, McIntyre, A, Summersgill, B, et al. KIT and RAS signalling pathways in testicular germ cell tumours: new data and a review of the literature. Int J Androl. 2007;30:337.
60. Mauduit, C, Hamamah, S, Benahmed, M. Stem cell factor/c-kit system in spermatogenesis. Hum Reprod Update. 1999;5:535.
61. Rothschild, G, Sottas, CM, Kissel, H, et al. A role for kit receptor signaling in Leydig cell steroidogenesis. Biol Reprod. 2003;69:925.
62. Grieco, V, Banco, B, Giudice, C, et al. Immunohistochemical expression of the KIT protein (CD117) in normal and neoplastic canine testes. J Comp Pathol. 2010;142:213.
63. Stoop, H, Honecker, F, vandeGeijn, GJ, et al. Stem cell factor as a novel diagnostic marker for early malignant germ cells. J Pathol. 2008;216:43.
64. Cummings, OW, Ulbright, TM, Eble, JN, et al. Spermatocytic seminoma: an immunohistochemical study. Hum Pathol. 1994;25:54.
65. Nurmio, M, Toppari, J, Zaman, F, et al. Inhibition of tyrosine kinases PDGFR and c-kit by imatinib mesylate interferes with postnatal testicular development in the rat. Int J Androl. 2007;30:366.
66. Nurmio, M, Kallio, J, Toppari, J, et al. Adult reproductive functions after early postnatal inhibition of the two receptor tyrosine kinases, c-kit and PDGFR, in the rat testis. Reprod Toxicol. 2008;25:442.
67. Basciani, S, Brama, M, Mariani, S, et al. Imatinib mesylate inhibits Leydig cell tumor growth: evidence for in vitro and in vivo activity. Cancer Res. 2005;65:1897.
68. Hogenesch, H, Whitely, HE, Vicini, DS, et al. Seminoma with metastases in the eyes and the brain in a dog. Vet Pathol. 1987;24:278.
69. Brodey, RS, Martin, JE. Sertoli cell neoplasms in the dog: the clinicopathological and endocrinological findings in thirty-seven dogs. J Am Vet Med Assoc. 1958;133:249.
70. Dhaliwal, RS, Kitchell, BE, Knight, BL, et al. Treatment of aggressive testicular tumors in four dogs. J Am Anim Hosp Assoc. 1999;35:311.
71. Spugnini, EP, Bartolazzi, A, Ruslander, D. Seminoma with cutaneous metastases in a dog. J Am Anim Hosp Assoc. 2000;36:253.
72. Takiguchi, M, Iida, T, Kudo, T, et al. Malignant seminoma with systemic metastases in a dog. J Small Anim Pract. 2001;42:360.
73. Weller, RE, Palmer, B. Metastatic seminoma in a dog. Mod Vet Pract. 1983;64:275.
74. Gopinath, D, Draffan, D, Philbey, AW, et al. Use of intralesional oestradiol concentration to identify a functional pulmonary metastasis of canine Sertoli cell tumor. J Small Anim Pract. 2009;50:198.
75. Morgan, RV. Blood dyscrasias associated with testicular tumors in the dog. J Am Anim Hosp Assoc. 1982;18:970.
76. Mischke, R, Meurer, D, Hoppen, HO, et al. Blood plasma concentrations of oestradiol-17B, testosterone and testosterone/oestradiol ratio in dogs with neoplastic and degenerative testicular diseases. Res Vet Sci. 2002;73:267.
77. Peters, MAJ, Jong, FS, Teerds, KJ, et al. Ageing, testicular tumours and the pituitary-testis axis in dogs. J Endocrinol. 2000;166:153.
78. Taniyama, H, Hirayama, K, Nakada, K, et al. Immunohistochemical detection of inhibin-alpha, -beta B, and –beta A chains and 3beta-hydroxysteroid dehydrogenase in canine testicular tumors and normal testes. Vet Pathol. 2001;38:661.
79. Grootenhuis, AJ, vanSluijs, FJ, Klaij, IA, et al. Inhibin, gonadotrophins and sex steroids in dogs with Sertoli cell tumours. J Endocrinol. 1990;127:235.
80. Peters, MA, Mol, JA, vanWolferen, ME, et al. Expression of the insulin-like growth factor (IGF) system and steroidogenic enzymes in canine testis tumors. Reprod Biol Endocrinol. 2003;14:22.
81. Sherding, RG, Wilson, GP, Kociba, GJ. Bone marrow hypoplasia in eight dogs with Sertoli cell tumor. J Am Vet Med Assoc. 1981;178:497.
82. Scott, DW, Reimers, TJ. Tail gland and perianal gland hyperplasia associated with testicular neoplasia and hypertestosteronemia in a dog. Canine Pract. 1986;13:15.
83. Laing, EJ, Harari, J, Smith, CW. Spermatic cord torsion and Sertoli cell tumor in a dog. J Am Vet Med Assoc. 1983;183:879.
84. Spackman, CJ, Roth, L. Prostatic cyst and concurrent Sertoli cell tumor in a dog. J Am Vet Med Assoc. 1988;192:1096.
85. Barrand, KR, Scudamore, CL. Canine hypertrophic osteoarthropathy associated with a malignant Sertoli cell tumour. J Small Anim Pract. 2001;42:143.
86. Brown, TT, Burek, JD, McEntee, K. Male pseudohermaphroditism, cryptorchism, and Sertoli cell neoplasia in three miniature Schnauzers. J Am Vet Med Assoc. 1976;169:821.
87. Norrdin, RW, Baum, AC. A male pseudohermaphrodite dog with a Sertoli’s cell tumor, mucometra, and vaginal glands. J Am Vet Med Assoc. 1970;156:204.
88. Frey, DC, Tyler, DE, Ramsey, FK. Pyometra associated with bilateral cryptorchidism and Sertoli’s cell tumor in a male pseudohermaphroditic dog. J Am Vet Med Assoc. 1965;146:723.
89. Bigliardi, E, Parma, P, Peressotti, P, et al. Clinical, genetic, and pathological features of male pseudohermaphroditism in dog. Reprod Biol Endocrinol. 2011;9:12.
90. Johnston, GR, Feeney, DA, Johnston, SD, et al. Ultrasonographic features of testicular neoplasia in dogs: 16 cases (1980-1988). J Am Vet Med Assoc. 1991;198:1779.
91. Pugh, CR, Konde, LJ. Sonographic evaluation of canine testicular and scrotal abnormalities: a review of 26 case histories. Vet Radiol. 1991;32:243.
92. Eilts, BE, Pechman, RD, Hedlund, CS, et al. Use of ultrasonography to diagnose Sertoli cell neoplasia and cryptorchidism in a dog. J Am Vet Med Assoc. 1988;192:533.
93. Masserdotti, C, DeLorenzi, D, Gasparotto, L. Cytologic detection of Call-Exner bodies in Sertoli cell tumors from 2 dogs. Vet Clin Pathol. 2008;37:112.
94. Banco, B, Giudice, C, Veronesi, MC, et al. An immunohistochemical study of normal and neoplastic canine Sertoli cells. J Comp Pathol. 2010;143:239.
95. Peters, MA, Teerds, KJ, vanderGaag, I, et al. Use of antibodies against LH receptor, 3beta-hydroxysteroid dehydrogenase and vimentin to characterize different types of testicular tumour in dogs. Reproduction. 2001;121:287.
96. Yu, CH, Hwang, DN, Kim, JH, et al. Comparative immunohistochemical characterization of canine seminomas and Sertoli cell tumors. J Vet Sci. 2009;10:1.
97. Doxsee, AL, Yager, JA, Best, SJ, et al. Extratesticular interstitial and Sertoli cell tumors in previously neutered dogs and cats: a report of 17 cases. Can Vet J. 2006;47:763.
98. Archbald, LI, Waldow, D, Gelatt, K. Interstitial cell tumor. J Am Vet Med Assoc. 1997;210:1423.
99. England, GC. Ultrasonographic diagnosis of non-palpable Sertoli cell tumours in infertile dogs. J Small Anim Pract. 1995;36:476.
100. Metzger, FL, Hattel, AL. Hematuria, hyperestrogenemia, and hyperprogesteronemia due to a Sertoli-cell tumor in a bilaterally cryptorchid dog. Canine Pract. 1993;18:32.
101. Madewell, BR, Theilen, GH. Tumors of the genital tract. In: Theilen GH, Madewell BR, eds. Veterinary cancer medicine. Philadelphia: Lea & Febiger, 1987.
102. McDonald, RK, Walker, M, Legendre, A, et al. Radiotherapy of metastatic seminoma in the dog. Case reports. J Vet Intern Med. 1988;2:103.
103. Albers, P, Albrecht, W, Algaba, F, et al. EAU Guidelines on testicular cancer: 2011 update. Eur Urol. 2011;60:304.
104. Rosen, A, Jayram, G, Drazer, M, et al. Global trends in testicular cancer incidence and mortality. Eur Urol. 2011;60:374.
105. Jacobsen, R, Moller, H, Thoresen, S, et al. Trends in testicular cancer incidence in the Nordic countries, focusing on the recent decrease in Denmark. Int J Androl. 2006;29:199.
106. Myrup, C, Wohlfahrt, J, Oudin, A, et al. Risk of testicular cancer according to birthplace and birth cohort in Denmark. Int J Cancer. 2010;126:217.
107. Henderson, BE, Benton, B, Jing, J, et al. Risk factors for cancer of the testis in young men. Int J Cancer. 1979;23:598.
108. Weir, HK, Marrett, LD, Kreiger, N, et al. Pre-natal and peri-natal exposures and risk of testicular germ-cell cancer. Int J Cancer. 2000;87:438.
109. Garner, MJ, Birkett, NJ, Johnson, KC, et al. Dietary risk factors for testicular carcinoma. Int J Cancer. 2003;106:934.
110. Hu, J, LaVecchia, C, Morrison, H, et al. Salt, processed meat and the risk of cancer. Eur J Cancer Prev. 2011;20:132.
111. Kratz, CP, Mai, PL, Greene, MH. Familial testicular germ cell tumours. Best Pract Res Clin Endocrinol Metab. 2010;24:503.
112. VandenEeden, SK, Weiss, NS, Strader, CH, et al. Occupation and the occurrence of testicular cancer. Am J Ind Med. 1991;19:327.
113. Garner, MJ, Turner, MC, Ghadirian, P, et al. Epidemiology of testicular cancer: an overview. Int J Cancer. 2005;116:331.
114. Pinczowski, D, McLaughlin, JK, Lackgren, G, et al. Occurrence of testicular cancer in patients operated on for cryptorchidism and inguinal hernia. J Urol. 1991;146:1291.
115. Tandstad, T, Smaaland, R, Solberg, A, et al. Management of seminomatous testicular cancer: a binational prospective population-based study from the Swedish Norwegian testicular cancer study group. J Clin Oncol. 2011;29:719.
116. Aparicio, J, delMuro, G, Maroto, P, et al. Multicenter study evaluating a dual policy of postorchiectomy surveillance and selective adjuvant single-agent carboplatin for patients with clinical stage I seminoma. Ann Oncol. 2003;14:867.
117. Oliver, RT, Mead, GM, Rustin, GJ, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214). J Clin Oncol. 2011;29:957.
118. Motzer, RJ, Nichols, CJ, Margolin, KA, et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol. 2007;25:247.
119. Rosen, DK, Carpenter, JL. Functional ectopic interstitial cell tumor in a castrated male cat. J Am Vet Med Assoc. 1993;202:1865.
120. Cotchin, E. Neoplasia. In: Wilkinson GT, ed. Diseases of the cat and their management. Oxford: Blackwell, 1984.
121. Miller, MA, Hartnett, SE, Ramos-Vara, JA. Interstitial cell tumor and Sertoli cell tumor in the testis of a cat. Vet Pathol. 2007;44:394.
122. Miyoshi, N, Yasuda, N, Kamimura, Y, et al. Teratoma in a feline unilateral cryptorchid testis. Vet Pathol. 2001;38:729.
123. Ferreira-da-Silva, J. Teratoma in a feline unilateral cryptorchid testis (letter to the editor). Vet Pathol. 2002;39:516.
124. Benazzi, C, Sarli, G, Brunetti, B. Sertoli cell tumour in a cat. J Vet Med A Physiol Pathol Clin Med. 2004;51:124.
125. Meier, H. Sertoli-cell tumor in the cat: report of two cases. North Am Vet. 1956;37:979.
126. Obradovich, J, Walshaw, R, Goullaud, E. The influence of castration on the development of prostatic carcinoma in the dog: 43 cases. J Vet Intern Med. 1987;1:183.
127. Bell, FW, Klausner, JS, Hayden, DW, et al. Evaluation of serum and seminal plasma markers in the diagnosis of canine prostatic disorders. J Vet Intern Med. 1995;9:149.
128. Cornell, KK, Bostwick, DG, Cooley, DM, et al. Clinical and pathological aspects of spontaneous canine prostate carcinoma: a retrospective analysis of 76 cases. Prostate. 2000;45:173.
129. Waters, DJ, Sakr, WA, Hayden, DW, et al. Workgroup 5: spontaneous prostate cancer in dogs and non-human primates. Prostate. 1998;36:64.
130. Weaver, AD. Fifteen cases of prostatic carcinoma in the dog. Vet Rec. 1981;109:71.
131. Waters, DJ, Shen, S, Glickman, LT, et al. Prostate cancer risk and DNA damage: translational significance of selenium supplementation in a canine model. Carcinogenesis. 2005;26:1256.
132. Maini, A, Archer, C, Wang, CY, et al. Comparative pathology of benign prostatic hyperplasia and prostate cancer. In Vivo. 1997;11:293.
133. Hornbuckle, WE, MacCoy, DM, Allan, GS, et al. Prostatic disease in the dog. Cornell Vet. 1978;68:284.
134. Krawiec, DR, Heflin, D. Study of prostatic disease in dogs: 177 cases (1981-1986). J Am Vet Med Assoc. 1992;200:1119.
135. Teske, E, Naan, EC, vanDijk, EM, et al. Canine prostate carcinoma: epidemiological evidence of an increased risk in castrated dogs. Mol Cell Endocrinol. 2002;197:251.
136. Bell, FW, Klausner, JS, Hayden, DW, et al. Clinical and pathologic features of prostatic adenocarcinoma in sexually intact and castrated dogs: 31 cases (1970-1987). J Am Vet Med Assoc. 1991;199:623.
137. Waters, DJ, Bostwick, DG. Prostatic intraepithelial neoplasia occurs spontaneously in the canine prostate. J Urol. 1997;157(2):713–716.
138. Madewell, BR, Gandour-Edwards, R, DeVere-White, RW. Canine prostatic intraepithelial neoplasia: is the comparative model relevant? Prostate. 2004;58:314.
139. Aquilina, JW, McKinney, L, Pacelli, A, et al. High grade prostatic intraepithelial neoplasia in military working dogs with and without prostate cancer. Prostate. 1998;36:189.
140. Sorenmo, KU, Goldschmidt, M, Shofer, F, et al. Immunohistochemical characterization of canine prostatic carcinoma and correlation with castration status and castration time. Vet Comp Oncol. 2003;1:48.
141. Grieco, V, Riccardi, E, Rondena, M, et al. The distribution of oestrogen receptors in normal, hyperplastic and neoplastic canine prostate, as demonstrated immunohistochemically. J Comp Pathol. 2006;135:11.
142. LeRoy, BE, Nadella, MV, Toribio, RE, et al. Canine prostate carcinomas express markers of urothelial and prostatic differentiation. Vet Pathol. 2004;41:131.
143. Bryan, JN, Keeler, MR, Henry, CJ, et al. A population study of neutering status as a risk factor for canine prostate cancer. Prostate. 2007;67:1174.
144. Leav, I, Schelling, KH, Adams, JY, et al. Role of canine basal cells in postnatal prostatic development, induction of hyperplasia, and sex hormone-stimulated growth; and the ductal origin of carcinoma. Prostate. 2001;48:210.
145. Lai, CL, vandenHam, R, Mol, J, et al. Immunostaining of the androgen receptor and sequence analysis of its DNA-binding domain in canine prostate cancer. Vet J. 2009;181:256.
146. Humphrey, PA. Gleason grading and prognostic factors in carcinoma of the prostate. Modern Pathol. 2004;17:292.
147. Young, RH, Srigley, JR, Amin, MB, et al. Tumors of the prostate gland, seminal vesicles, male urethra, and penis. Atlas of Tumor Pathology: Third Series, Fascicle 28. Washington, DC: Armed Forces Institute of Pathology; 2000.
148. Weisse, C, Berent, A, Todd, K, et al. Evaluation of palliative stenting for management of malignant urethral obstructions in dogs. J Am Vet Med Assoc. 2006;229:226.
149. Mainwaring, CJ. Primary lymphoma of the prostate in a dog. J Small Anim Pract. 1990;31:617.
150. Winter, MD, Locke, JE, Penninck, DG. Imaging diagnosis: urinary obstruction secondary to prostatic lymphoma in a young dog. Vet Radiol Ultrasound. 2006;47:597.
151. Hayden, DW, Klausner, JS, Waters, DJ. Prostatic leiomyosarcoma in a dog. J Vet Diagn Invest. 1999;11:283.
152. Bacci, B, Vignoli, M, Rossi, F, et al. Primary prostatic leiomyosarcoma with pulmonary metastases in a dog. J Am Anim Hosp Assoc. 2010;46:103.
153. Hayden, DW, Bartges, JW, Bell, FW, et al. Prostatic hemangiosarcoma in a dog: clinical and pathologic findings. J Vet Diagn Invest. 1992;4:2009.
154. Gilson, SD, Miller, RT, Hardie, EM, et al. Unusual prostatic mass in a dog. J Am Vet Med Assoc. 1992;200:702.
155. LeRoy, BE, Northrup, N. Prostate cancer in dogs: comparative and clinical aspects. Vet J. 2009;180:149.
156. Dore, M, Chevalier, S, Sirois, J. Estrogen-dependent induction of cyclooxygenase-2 in the canine prostate in vivo. Vet Pathol. 2005;42:100.
157. Shidaifat, F, Daradka, M, Al-Omari, R. Effect of androgen ablation on prostatic cell differentiation in dogs. Endocr Res. 2004;30:327.
158. Grieco, V, Patton, V, Romussi, S, et al. Cytokeratin and vimentin expression in normal and neoplastic canine prostate. J Comp Pathol. 2003;129:78.
159. Cooley, DM, Waters, DJ. Skeletal metastasis as the initial clinical manifestation of metastatic carcinoma in 19 dogs. J Vet Intern Med. 1998;12:288.
160. Durham, SK, Dietze, AE. Prostatic adenocarcinoma with and without metastasis to bone in dogs. J Am Vet Med Assoc. 1986;188:1432.
161. Waters, DJ, Cooley, DM, Allen, DK, et al. Host age influences the biological behavior of cancer: studies in pet dogs with naturally occurring malignancies. Gerontologist. 1998;38:110.
162. Ross, RK, Pike, MC, Coetzee, GA, et al. Androgen metabolism and prostate cancer: establishing a model of genetic susceptibility. Cancer Res. 1998;58:4497.
163. Matsuzaki, P, Cogliati, B, Sanches, DS, et al. Immunohistochemical characterization of canine prostatic intraepithelial neoplasia. J Comp Pathol. 2010;142:84.
164. Gallardo, F, Lloreta, J, Garcia, F, et al. Immunolocalization of androgen receptors, estrogen alpha receptors, and estrogen beta receptors in experimentally induced canine prostatic hyperplasia. J Androl. 2009;30:240.
165. Gallardo, F, Mogas, T, Baro, T, et al. Expression of androgen, oestrogen alpha and beta, and progesterone receptors in the canine prostate: differences between normal, inflamed hyperplastic and neoplastic glands. J Comp Pathol. 2007;136:1.
166. Winkler, S, Reimann-Berg, N, Murua-Escobar, H, et al. Polysomy 13 in a canine prostate carcinoma underlining its significance in the development of prostate cancer. Cancer Genet Cytogenet. 2006;169:154.
167. Winkler, S, Murua-Escobar, H, Eberle, N, et al. Establishment of a cell line derived from a canine prostate carcinoma with a highly rearranged karyotype. J Hered. 2005;96:782.
168. Madewell, BR, Deitch, AD, Higgins, RJ, et al. DNA flow cytometric study of the hyperplastic and neoplastic canine prostate. Prostate. 1991;18:173.
169. Tremblay, C, Dore, M, Bochsler, PN, et al. Induction of prostaglandin G/H synthase-2 in a canine model of spontaneous prostatic adenocarcinoma. J Natl Cancer Inst. 1999;91:1398.
170. Mohammed, SI, Coffman, K, Glickman, NW, et al. Prostaglandin E2 concentrations in naturally occurring canine cancer. Prostaglandins Leukot Essent Fatty Acids. 2001;64:1.
171. Mohammed, SI, Khan, KN, Sellers, RS, et al. Expression of cyclooxygenase-1 and 2 in naturally-occurring canine cancer. Prostaglandins Leukot Essent Fatty Acids. 2004;70:479.
172. Sorenmo, KU, Goldschmidt, MH, Shofer, FS, et al. Evaluation of cyclooxygenase-1 and cyclooxygenase-2 expression and the effect of cyclooxygenase inhibitors in canine prostatic carcinoma. Vet Comp Oncol. 2004;2:13.
173. Keller, ET, Zhang, J, Cooper, CR, et al. Prostate carcinoma skeletal metastases: cross-talk between tumor and bone. Cancer Metastasis Rev. 2001;20:333.
174. Sellers, RS, LeRoy, BE, Blomme, EA, et al. Effects of transforming growth factor-beta1 on parathyroid hormone-related protein mRNA expression and protein secretion in canine prostate epithelial, stromal, and carcinoma cells. Prostate. 2004;58:366.
175. LeRoy, BE, Sellers, RS, Rosol, TJ. Canine prostate stimulates osteoblast function using the endothelin receptors. Prostate. 2004;59:148.
176. Leav, I, Ling, GV. Adenocarcinoma of the canine prostate. Cancer. 1968;22:1329.
177. Smith, J. Canine prostatic disease: a review of anatomy, pathology, diagnosis, and treatment. Theriogenology. 2008;70:375.
178. Lee-Parritz, DE, Lamb, CR. Prostatic adenocarcinoma with osseous metastases in a dog. J Am Vet Med Assoc. 1988;192:1569.
179. Barsanti, JA, Finco, DR. Canine prostatic disease. Vet Clin North Am. 1986;16:587.
180. Johnston, SD, Kamolpatana, K, Root-Kustritz, MV, et al. Prostatic disorders in the dog. Anim Reprod Sci. 2000;61:405.
181. Feeney, DA, Johnston, GR, Klausner, JS, et al. Canine prostatic disease–comparison of radiographic appearance with morphologic and microbiologic findings: 30 cases (1981-1985). J Am Vet Med Assoc. 1987;190:1018.
182. Bradbury, CA, Westropp, JL, Pollard, RE. Relationship between prostatomegaly, prostatic mineralization, and cytologic diagnosis. Vet Radiol Ultrasound. 2009;50:167.
183. Head, LL, Francis, DA. Mineralized paraprostatic cyst as a potential contributing factor in the development of perineal hernias in a dog. J Am Vet Med Assoc. 2002;221:533.
184. Zekas, LJ, Forrest, LJ, Swainson, S, et al. Radiographic diagnosis: mineralized paraprostatic cyst in a dog. Vet Radiol Ultrasound. 2004;45:310.
185. Ackerman, N. Prostatic reflux during positive contrast retrograde urethrography in the dog. Vet Radiol. 1983;24:251–259.
186. Powe, JR, Canfield, PJ, Martin, PA. Evaluation of the cytologic diagnosis of canine prostatic disorders. Vet Clin Pathol. 2004;33:150.
187. Barsanti, JA, Shotts, EB, Prasse, K, et al. Evaluation of diagnostic techniques for canine prostatic diseases. J Am Vet Med Assoc. 1980;177:160.
188. Barsanti, JA, Finco, DR. Evaluation of techniques for diagnosis of canine prostatic diseases. J Am Vet Med Assoc. 1984;185:198.
189. Leeds, EB, Leav, I. Perineal punch biopsy of the canine prostate gland. J Am Vet Med Assoc. 1969;154:925.
190. Nyland, TG, Wallack, ST, Wisner, ER. Needle-tract implantation following US-guided fine-needle aspiration biopsy of transitional cell carcinoma of the bladder, urethra, and prostate. Vet Radiol Ultrasound. 2002;43:50.
191. MacLachan, NJ, Kennedy, PC. Tumors of the genital systems. In: Mueten DJ, ed. Tumors in domestic animals. Ames, Iowa: Iowa State University Press, 2002.
192. Thrall, MA, Olsen, PN, Freemyer, FG. Cytologic diagnosis of canine prostatic disease. J Am Anim Hosp Assoc. 1985;21:95.
193. Corazza, M, Guidi, G, Romagnoli, S, et al. Serum total prostatic and non-prostatic acid phosphatases in healthy dogs and in dogs with prostatic diseases. J Small Anim Pract. 1994;35:307.
194. Hardie, EM, Barsanti, JA, Rawlings, CA. Complications of prostatic surgery. J Am Anim Hosp Assoc. 1984;20:50.
195. Hardie, EM, Stone, EA, Spaulding, KA, et al. Subtotal canine prostatectomy with the neodymium:yttrium-aluminum-garnet laser. Vet Surg. 1990;19:348.
196. Vlasin, M, Rauser, P, Fichtel, T, et al. Subtotal intracapsular prostatectomy as a useful treatment for advanced-stage prostatic malignancies. J Small Anim Pract. 2006;47:512.
197. Basinger, RR, Rawlings, CA, Barsanti, JA, et al. Urodynamic alterations associated with clinical prostatic diseases and prostate surgery in 23 dogs. J Am Anim Hosp Assoc. 1989;25:385.
198. Goldsmid, SE, Bellenger, CR. Urinary incontinence after prostatectomy in dogs. Vet Surg. 1991;20:253.
199. L’Epplattenier, HF, Klem, B, Teske, E, et al. Partial prostatectomy using Nd:YAG laser for management of canine prostate carcinoma. Vet Surg. 2006;35:406.
200. Liptak, JM, Brutscher, SP, Monnet, E, et al. Transurethral resection in the management of urethral and prostatic neoplasia in 6 dogs. Vet Surg. 2004;33:505.
201. Williams, JM, White, RAS. Tube cystostomy in the dog and cat. J Small Anim Pract. 2007;32:598.
202. Smith, JD, Stone, EA, Gilson, SD. Placement of a permanent cystostomy catheter to relieve urine outflow obstruction in dogs with transitional cell carcinoma. J Am Vet Med Assoc. 1995;206:496.
203. Mann, FA, Barrett, RJ, Henderson, RA. Use of a retained urethral catheter in three dogs with prostatic neoplasia. Vet Surg. 1992;21:342.
204. Lucroy, MD, Bowles, MH, Higbee, RG, et al. Photodynamic therapy for prostatic carcinoma in a dog. J Vet Intern Med. 2003;19:235.
205. Chevalier, S, Anidjar, M, Scarlata, E, et al. Preclinical study of the novel vascular occluding agent, WST11, for photodynamic therapy of the canine prostate. J Urol. 2011;186:302.
206. Xiao, Z, Owen, RJ, Liu, W, et al. Lipophilic photosensitizer administration via the prostate arteries for photodynamic therapy of the canine prostate. Photodiagnosis Photodyn Ther. 2010;7:106.
207. Du, KL, Mick, R, Busch, TM, et al. Preliminary results of interstitial motexafin lutetium-mediated PDT for prostate cancer. Lasers Surg Med. 2006;38:427.
208. Huang, Z, Chen, Q, Luck, D, et al. Studies of a vascular-acting photosensitizer, Pd-bacteriopheophorbide (Tookad), in normal canine prostate and spontaneous canine prostate cancer. Lasers Surg Med. 2005;36:390.
209. Turrel, JM. Intraoperative radiotherapy of carcinoma of the prostate gland in ten dogs. J Am Vet Med Assoc. 1987;190:48.
210. Fan, TM, deLorimier, LP, Charney, SC, et al. Evaluation of intravenous pamidronate administration in 33 cancer-bearing dogs with primary or secondary bone involvement. J Vet Intern Med. 2005;19:74.
211. Lattimer, JC, Jr., Corwin, LA, Stapleton, J, et al. Clinical and clinicopathologic response of canine bone tumor patients to treatment with samarium-153-EDTMP. J Nucl Med. 1990;31:1316.
212. Fan, TM, Charney, SC, deLorimier, LP, et al. Double-blind placebo-controlled trial of adjuvant pamidronate with palliative radiotherapy and intravenous doxorubicin for canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2009;23:152.
213. Klausner, JS, Johnston, SD, Bell, FW. Canine prostatic diseases. In: Kirk RW, ed. Current veterinary therapy XII. Philadelphia: WB Saunders, 1995.
214. Zelefsky, MJ, Eastham, JA, Sartor, OA, et al. Cancer of the prostate. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams and Wilkins, 2008.
215. Damber, JE, Aus, G. Prostate cancer. Lancet. 2008;371:1710.
216. Walsh, PC, Partin, AW. Family history facilitates the early diagnosis of prostate carcinoma. Cancer. 1997;80:1871.
217. Lichtenstein, P, Holm, NV, Verkasalo, PK, et al. Environmental and heritable factors in the causation of cancer: analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78.
218. Boyle, P, Severi, G, Giles, GG. The epidemiology of prostate cancer. Urol Clin North Am. 2003;30:209.
219. Severi, G, Morris, HA, MacInnis, RJ, et al. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarker Prev. 2006;15:86.
220. Wolk, A. Diet, lifestyle and risk of prostate cancer. Acta Oncol. 2005;44:277.
221. Gronberg, H. Prostate cancer epidemiology. Lancet. 2003;361(9360):859–864.
222. Anidjar, M, Villette, JM, Devauchelle, P, et al. In vivo model mimicking natural history of dog prostate cancer using DPC-1, a new canine prostate carcinoma cell line. Prostate. 2001;46:2.
223. Lin, AM, Small, EJ. Prostate cancer update: 2007. Curr Opin Oncol. 2007;20:294.
224. Hawe, JS. What is your diagnosis? Prostatic adenocarcinoma in a cat. J Am Vet Med Assoc. 1983;182:1257.
225. Hubbard, BS, Vulgamott, JC, Liska, WD. Prostatic adenocarcinoma in a cat. J Am Vet Med Assoc. 1990;197:1493.
226. Caney, SM, Hold, PE, Day, MJ, et al. Prostatic carcinoma in two cats. J Small Anim Pract. 1998;39:140.
227. LeRoy, BE, Lech, ME. Prostatic carcinoma causing urethral obstruction and obstipation in a cat. J Feline Med Surg. 2004;6:397.
228. Tursi, M, Costa, T, Valenza, F, et al. Adenocarcinoma of the disseminated prostate in a cat. J Feline Med Surg. 2008;10:600.
229. Zambelli, D, Cunto, M, Raccagni, R, et al. Successful surgical treatment of a prostatic biphasic tumour (sarcomatoid carcinoma) in a cat. J Feline Med Surg. 2010;12:161.
230. Michels, GM, Knapp, DW, David, M, et al. Penile prolapse and urethral obstruction secondary to lymphosarcoma of the penis in a dog. J Am Anim Hosp Assoc. 2001;37:474.
231. Cornegliani, L, Vercelli, A, Abramo, F. Idiopathic mucosal penile squamous papillomas in dogs. Vet Dermatol. 2007;18:439.
232. Ndiritu, CG. Lesions of the canine penis and prepuce. Mod Vet Pract. 1979;60:712.
233. Patnaik, AK, Matthiesen, DT, Zawie, DA. Two cases of canine penile neoplasm: squamous cell carcinoma and mesenchymal chondrosarcoma. J Am Anim Hosp Assoc. 1988;24:403.
234. Hall, WC, Nielsen, SW, McEntee, K. Tumours of the prostate and penis. Bull World Health Organ. 1976;53:247.
235. Bloom, F. Pathology of the dog and cat: the genitourinary system with clinical considerations. Evanston: American Veterinary Publications; 1954.
236. Vascellari, M, Carminato, A, Camali, G, et al. Malignant mesothelioma of the tunica vaginalis testis in a dog: histological and immunohistochemical characterization. J Vet Diagn Invest. 2011;23:135.
237. Cihak, RW, Roen, DR, Klaassen, J. Malignant mesothelioma of the tunica vaginalis in a dog. J Comp Pathol. 1986;96:459.
238. Peppler, C, Weissert, D, Kappe, E, et al. Osteosarcoma of the penile bone (os penis) in a dog. Aust Vet J. 2009;87:52.
239. Webb, JA, Liptak, JM, Hewitt, SA, et al. Multilobular osteochondrosarcoma of the os penis in a dog. Can Vet J. 2009;50:81.
240. Bleier, T, Lewitschek, HP, Reinacher, M. Canine osteosarcoma of the penile bone. J Vet Med A Physiol Pathol Clin Med. 2003;50:397.
241. Mirkovic, TK, Shmon, CL, Allen, AL. Urinary obstruction secondary to an ossifying fibroma of the os penis in a dog. J Am Anim Hosp Assoc. 2004;40:152.
242. Root-Kustritz, MV, Fick, JL, American College of Theriogenologists. Theriogenology question of the month: neoplasia of the os penis. J Am Vet Med Assoc. 2007;230:197.
243. Patnaik, AK. Canine extraskeletal osteosarcoma and chondrosarcoma: a clinicopathologic study of 14 cases. Vet Pathol. 1990;27:46.
244. Rogers, KS, Walker, MA, Dillon, HB. Transmissible venereal tumor: a retrospective study of 29 cases. J Am Anim Hosp Assoc. 1998;34:463.
245. Thrall, DE. Orthovoltage radiotherapy of canine transmissible venereal tumors. Vet Radiol. 1982;23:217.
246. Milks, HJ. Some diseases of the genito-urinary system. Cornell Vet. 1939;29:105.
247. Trabulsi, EJ, Gomella, LG. Cancer of the urethra and penis. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia: Lippincott Williams and Wilkins, 2008.
248. Yeole, BB, Jussawalla, DJ. Descriptive epidemiology of the cancers of male genital organs in greater Bombay. Indian J Cancer. 1997;34:30.
249. Maden, C, Sherman, KJ, Beckman, AM, et al. History of circumcision, medical conditions, and sexual activity and risk of penile cancer. J Natl Cancer Inst. 1993;85:19.
250. Hanash, KA, Furlow, WL, Utz, DC, et al. Carcinoma of the penis: a clinicopathologic study. J Urol. 1970;104:291.
251. Shabbir, M, Minhas, S, Muneer, A. Diagnosis and management of premalignant penile lesions. Ther Adv Urol. 2011;3:151.
252. Harish, K, Ravi, R. The role of tobacco in penile carcinoma. Br J Urol. 1995;75:375.
253. Backes, DM, Kurman, RJ, Pimenta, JM, et al. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20:449.
254. Shapiro, D, Shasha, D, Tareen, M, et al. Contemporary management of localized penile cancer. Expert Rev Anticancer Ther. 2011;11:29.
255. Stancik, I, Holtl, W. Penile cancer: review of the recent literature. Curr Opin Urol. 2003;13:467.
256. Lawindy, SM, Rodriguez, AR, Horenblas, S, et al. Current and future strategies in the diagnosis and management of penile cancer. Adv Urol. 2011. [Epub 30 May].