Chapter 42 Takayasu’s Arteritis
Takayasu’s arteritis is a rare chronic inflammatory large-vessel disease with a female bias. It affects the aorta and its primary branches. Initially thought to be an illness of young Asian women, it is now recognized worldwide. Chronic vascular inflammation leads to vessel stenosis and, less commonly, aneurysm formation.
Epidemiology
Takayasu’s arteritis is a rare disorder that has variable incidence and prevalence depending on the country where it has been studied. In the United States, incidence estimates from Olmstead County, Minnesota, are 2.6 cases/million/yr, whereas in Sweden they are 1.2 cases/million/yr.1,2 Autopsy studies in Japan document a high prevalence, with evidence of Takayasu’s arteritis found in 1 of every 3000 individuals.3 Similar postmortem studies have not been performed elsewhere to provide comparative data.
The peak incidence of Takayasu’s arteritis is in the third decade of life, but not all investigators adhere to guidelines that exclude women beyond reproductive age. Consequently, some series may include women beyond their seventh decade of life.
Pathogenesis
Differences in disease prevalence and characteristics among different racial and ethnic cohorts suggest a genetic predisposition; however, no definite allelic associations have been found consistently across all groups of patients with Takayasu’s arteritis. The well-established association between certain infections and secondary vasculitis has propelled a search for an infectious etiology. Special attention has been directed toward mycobacterial pathogens because of the increased prevalence of Mycobacterium tuberculosis (TB) infection in countries (e.g., India, Korea, Mexico) with a high prevalence of Takayasu’s arteritis, associations of Takayasu’s arteritis with previous mycobacterial infection, and TB skin test positivity in patients with Takayasu’s arteritis. In several small studies, patients with Takayasu’s arteritis were demonstrated to have increased immunological responses to mycobacterial proteins when compared to healthy controls, but these inflammatory responses can be nonspecific and do not prove causality.4,5
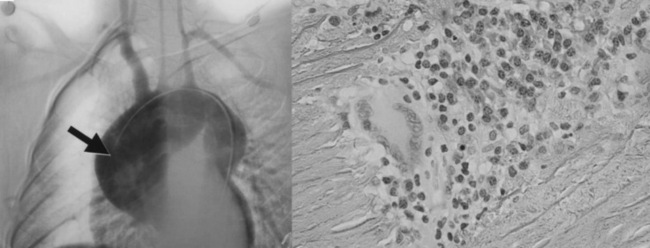
Vessel injury results from the influx and actions of macrophages, cytotoxic T cells, γδT cells, natural killer cells,6 and B cells. Access of leukocytes to the vessel wall is through the vasa vasorum, with subsequent migration toward the large lumen intima. Various cytokines, including perforin, interleukin (IL)-6, RANTES (regulated, upon activation, normal T-cell expressed, and secreted), tumor necrosis factor (TNF)-α,7,8 and B-cell activating factor (BAFF),9 enhance vascular inflammation. Although the most common response to this process is myointimal proliferation that leads to wall thickening and luminal stenosis, destruction of smooth muscle cells (SMCs), elastic fibers, and other matrix proteins may lead to aneurysm formation, especially in the aortic root and arch (Fig. 42-1). Disease progression leads to secondary vessel stiffening associated with atherosclerosis.10
Clinical Manifestations
Systemic symptoms and signs occur in less than half of all patients and include fever, weight loss, malaise, and generalized arthralgias and myalgias. Patients more often present with signs and/or symptoms of tissue ischemia, never having had an associated systemic illness. The most common symptom of Takayasu’s arteritis is upper-extremity claudication, occurring in more than 60% of patients and reflecting disease predilection for aortic arch vessels (≈︀90% of cases)11 (Figs. 42-2 through 42-4). The most frequent clinical findings include blood pressure asymmetry in paired extremities, and bruits found most often over the carotid, subclavian, and aortic vessels.12 Aneurysms most often affect the aortic root, stretching the atrioventricular annulus and producing valvular regurgitation (≈︀20% of patients); valve leaflets are not a site of inflammation. Many within this subset require surgical intervention.
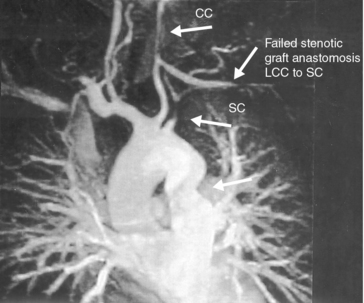
Figure 42-2 Magnetic resonance angiography (MRA) illustrating stenotic lesions involving aorta, left common carotid (CC), and left subclavian (SC) arteries.
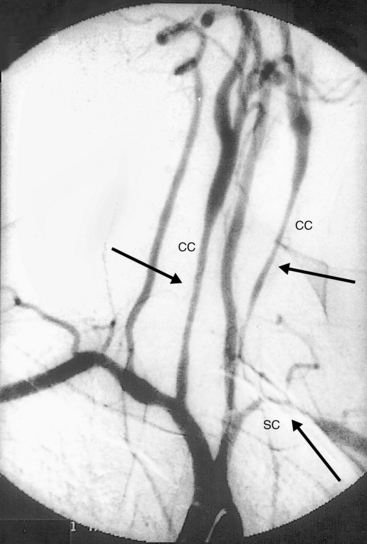
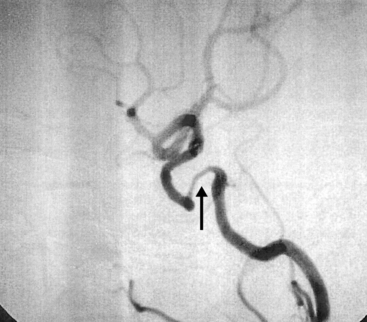
Figure 42-3 Angiography demonstrating stenoses of left subclavian (SC) and bilateral common carotid (CC) arteries.
Hypertension occurs in at least 40% of patients in U.S. cohorts and is even more common in Japanese and Indian cohorts (80%). Hypertension is most frequently due to renal artery stenosis (RAS), but it may also result from suprarenal aortic stenosis or loss of aortic compliance11,13 (Figs. 42-5 through 42-7). The diagnosis of hypertension can easily be missed in patients with disease involving both upper extremities, where peripheral cuff measurements in either arm will be an inaccurate reflection of central aortic pressure. Hypertension with vascular bruits or claudication, especially in younger patients, should lead to a suspicion of Takayasu’s arteritis and to further evaluation of all four extremity pulses and blood pressures for asymmetry. Vascular imaging of the entire aorta and primary branch vessels should then confirm anatomical abnormalities compatible with the diagnosis and delineate the extent of disease and types of lesions.
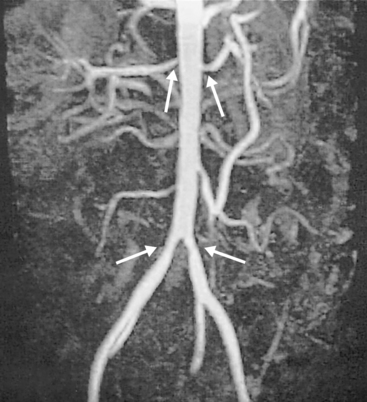
Figure 42-5 Magnetic resonance angiography (MRA) showing bilateral common iliac and renal arterial stenoses.
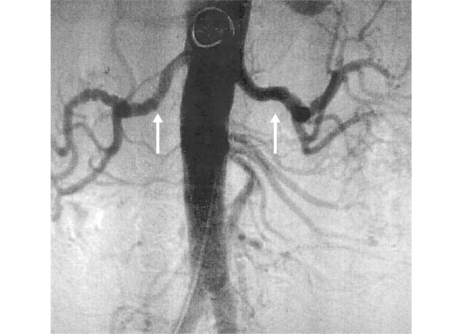
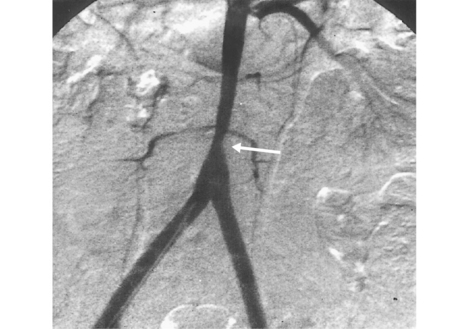
Figure 42-6 Angiography demonstrating bilateral renal artery lesions mimicking fibromuscular dysplasia (FMD).
Coronary arteritis may produce obstructive coronary artery disease (CAD) or coronary artery aneurysms. Patients may develop myocardial ischemia, dysrhythmias, congestive heart failure (CHF), or myocarditis.
Classification of Takayasu’s arteritis is based on sites of arterial involvement. One of the most widely accepted schemes separates patients into the following types (Fig. 42-8):
• Type I: branches of the aortic arch.
• Type IIa: ascending aorta, aortic arch, and its branches.
• Type IIb: type IIa plus thoracic descending aorta.
• Type III: thoracic descending aorta, abdominal aorta, or renal arteries, or a combination.
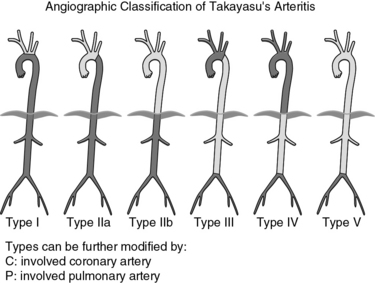
Figure 42-8 Classification of Takayasu’s arteritis based on vessel involvement according to the International Conference on Takayasu Arteritis (1994).
Morbidity results primarily from extremity claudication, less often from cardiac, renal, and central nervous system (CNS) vascular disease. Undetected and/or untreated hypertension is a significant cofactor in these disease sequelae. Mortality estimates range from just 3% at 8 years to 35% at 5 years’ follow-up. Causes of death include stroke, congestive heart failure, sudden death of uncertain cause, and unrecognized or inadequately treated hypertension.14,15
Differential Diagnosis
A thorough and careful investigation is necessary to distinguish Takayasu’s arteritis from its mimics (Box 42-1). Congenital and acquired disorders of tissue matrix may present with aortic root diltation, valvular insufficiency, and aneurysms in other sites; however, they are not generally associated with large-vessel stenoses, the hallmark of Takayasu’s arteritis. In many cases, there are also genetic studies and extravascular features that help identify the syndromic disorders (e.g., Marfan, Loeys-Dietz, Ehlers-Danlos syndromes). Exceptions better known for matrix abnormalities usually leading to stenoses are fibromuscular dysplasia and Grange’s syndrome.16
![]() Box 42-1 Differential Diagnosis of Takayasu’s Arteritis
Box 42-1 Differential Diagnosis of Takayasu’s Arteritis
Autoimmune Disease
* When vasculitis is a complication of these diseases, it most often takes the form of small-vessel disease.
Although other autoimmune disorders can be associated with large-vessel vasculitis, they are most often distinguished by their other associated disease manifestations and age preferences. Aortitis restricted to the aortic arch has emerged as one of numerous examples of single-organ vasculitis. Distinguishing it from Takayasu’s arteritis requires complete evaluation of the large-vessel anatomy and careful follow-up over years to be certain that it is not the first sign of Takayasu’s arteritis. It is important to distinguish isolated aortitis from Takayasu’s arteritis because after surgical intervention, further therapy is usually not required.10,17,18
A significant and often underappreciated overlap exists between Takayasu’s arteritis and giant cell arteritis[GCA], a disease of the elderly (mean age at diagnosis ≈︀70). A recent comparative cohort study of Takayasu’s arteritis and GCA identified numerous shared features of disease, much of this coming to light with the advent and increased use of noninvasive vascular imaging studies.19 Takayasu’s arteritis and GCA may be indistinguishable in patients who present in middle age (45-55 years of age) with large-artery stenoses and less often aneurysms, especially of the aortic root.
Infectious causes of large-vessel aneurysms should always be considered irrespective of age or gender. Stenosis of large vessels is unusual in the setting of infection, where aneurysms dominate.
Diagnosis
Serological tests do not exist to identify Takayasu’s arteritis. Rarely, the diagnosis is first considered after a surgical procedure provides biopsy findings that are compatible with the diagnosis. Most often the diagnosis is based on clinical findings in the presence of compatible vascular imaging abnormalities.20,21
Catheter-directed angiography allows for luminal imaging and pressure measurements and provides opportunities for intervention (e.g., angioplasty), but it provides little direct information about the vessel wall. Computed tomography (CT) or magnetic resonance imaging and angiography (MRI/MRA) provide more information regarding vessel wall thickening and edema, but the clinical implications of these findings, although suggestive of active vascular disease, do not always correlate with disease activity or progression.22 Studies examining the use of combined CT and positron emission tomography (PET) imaging are underway to determine whether more useful information may be derived about the vessel lumen and wall. The presence or absence of increased tagged glucose uptake within large vessels may have improved sensitivity and specificity for identifying active disease in Takayasu’s arteritis.23,24
Although systemic signs and symptoms or elevated acute-phase reactants may be suggestive of active disease, they are inadequately sensitive in patients who only have vascular symptoms or findings. Sequential angiographic evaluations have revealed progression of disease, based on the presence of new vascular abnormalities, in more than 50% of patients with normal erythrocyte sedimentation rates.13 In addition, histopathological proof of vascular inflammation has been documented in 44% of bypass specimens from patients with Takayasu’s arteritis who underwent surgery at a time when their disease was believed to be quiescent.
Treatment
Essential to effective treatment is accurate determination of disease activity, a goal that may not always be possible. Kerr et al.13 define active disease as any two or more of the following. New or worsening:
Although these features are helpful when present, their absence does not guarantee disease remission. These criteria combined with sequential imaging are currently the best, albeit imperfect, means of monitoring disease activity.
Glucocorticoid therapy induces improvement in nearly all patients and initial disease remission in about 50%, but in 96% of patients, relapses occur during the course of tapering medication (with a mean of 2.8 relapses per patient in one study).13,19 Therapy with other immunosuppressive agents may aid in achieving lower glucocorticoid requirements in such individuals or those with glucocorticoid-responsive and relapsing disease. Low-dose methotrexate was determined to be efficacious in diminishing glucocorticoid requirements in 13 of 16 patients in a National Institutes of Health (NIH) study.25 Shelhamer et al.26 demonstrated that cyclophosphamide administered with glucocorticoid induced remission in four of six patients with relapsing disease on glucocorticoid treatment alone. The authors recommend cyclophosphamide only for patients with severe active disease. Patients generally achieve remission within 3 to 6 months, at which point cyclophosphamide should be changed to maintenance therapy with another immunosuppressive agent, such as methotrexate or azathioprine, to minimize the risk of cyclophosphamide toxicity. More recently, Molloy et al.27 treated 25 Takayasu’s arteritis patients who had refractory disease with anti–tumor necrosis factor therapy (principally infliximab) and found that 60% of them were able to achieve glucocorticoid-free disease remission, and an additional 28% achieved remission requiring less than 10 mg/day of prednisone. Randomized controlled trials are needed to assess the impact of such therapy in a larger cohort, with particular attention paid to the risk of reactivation of tuberculosis.
About 80% of patients require chronic immunosuppressive therapy over extended periods. Only over the past 15 years have investigators recognized the chronic and relapsing nature of Takayasu’s arteritis. Studies that have incorporated sequential angiography have demonstrated that the majority of patients continue to develop new lesions in new vascular territories, even if they appear clinically to be in remission.11,13,15,19 Because clinical symptoms and serological tests are often unreliable in monitoring disease activity, serial imaging studies are routinely used for assessment of disease progression.
Hypertension is a major source of morbidity and mortality in Takayasu’s arteritis. Delay in the diagnosis of hypertension is common because of the high frequency of subclavian and innominate artery involvement, which may result in underestimation of central aortic pressure. Stenotic lesions also may be present in lower-extremity vessels, leaving some patients without any extremity capable of providing cuff blood pressure measurements that reliably represent those within the aortic root (Figs. 42-9 and 42-10). This emphasizes the need for complete vascular imaging at the time of diagnosis and during extended follow-up. Imaging should include the entire aorta and its primary branches. If stenoses of extremity vessels and/or the abdominal aorta preclude accurate determination of central aortic pressure by using an extremity blood pressure cuff, invasive angiography should be considered to obtain direct central aortic pressure measurements and gradient determinations. Effective blood pressure management is crucial and can be complex in Takayasu’s arteritis. Even with reliable peripheral recordings, determining and attaining a target pressure range without causing compromised perfusion in the setting of arterial stenotic lesions affecting major organs (e.g., cerebral or coronary stenoses) can be challenging. For this reason, careful monitoring is essential when intervention for blood pressure control is necessary. In addition, angiotensin-converting enzyme inhibitors (ACEIs) should be used with caution in the setting of renal artery stenosis, because this therapy blunts the renal autoregulatory response to decreased glomerular pressure. Decrease in glomerular filtration rate (GFR) results with lowering of blood pressure and may lead to a clinically significant decline in renal function.
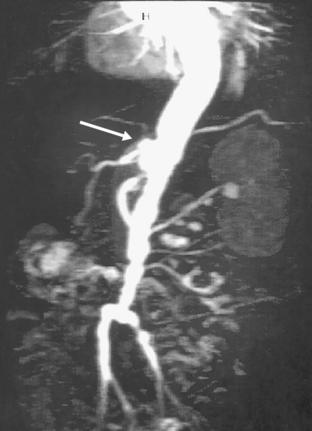
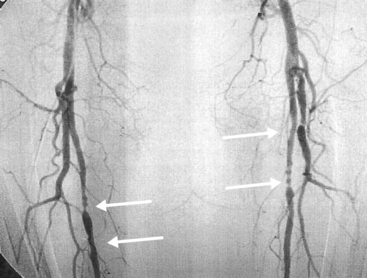
Figure 42-9 Angiography demonstrating diffuse ectasia of abdominal aorta and bilateral common iliac arteries, significant stenosis of left iliac artery, and dilation of origin of celiac artery (arrow).
Arterial stenoses may affect any organ system. Intervention to correct lesions should be considered in the setting of severely impaired daily function or evidence of significant organ ischemia. Multiple studies demonstrate impressive initial patency rates of 80% to 100% with percutaneous angioplasty.28–30 However, vascular restenosis occurs in 20% to 71% of cases followed up to 45 months post procedure. Case reports of drug-eluting stents (DES) in Takayasu’s arteritis are encouraging and may increase patency duration. However, long-term studies in large numbers of patients will have to be performed before their use can be endorsed.
Bypass procedures also may be complicated by vessel restenosis, but rates of sustained patency are higher than with angioplasty (65%-88%; mean follow-up period 44-60 months).30,31 One postoperative observational study of 29 patients demonstrated that disease activity at the time of surgery is a significant factor in bypass outcomes. In this study, patients with active disease at the time of operative intervention had 53% bypass graft patency rate at 5 years, whereas patients who were operated on when disease was quiescent had an 88% patency rate over the same period.32 Thus, when interventions are planned, it is preferred that they occur in the setting of disease remission. Tissue should be obtained from the origin or insertion of grafts, since histopathological examination can provide critical information in assessing disease activity and later assist in medical management.
Patients with Takayasu’s arteritis are generally young, but disease-related complications may increase their surgical risk. A retrospective review of 106 consecutive patients with the disease noted 12 perioperative deaths (11.3%) following operative intervention.33 Most deaths were due to cardiovascular complications, but other series document perioperative mortality as low as 0% to 3%.13,34 Surgical outcomes can be significantly affected by the experience of the surgical team and medical center in caring for patients with Takayasu’s arteritis.
A multidisciplinary approach to the care of Takayasu’s arteritis patients is essential for optimizing patient outcomes. The team should include a rheumatologist, cardiovascular physician, imaging specialist, and in the setting of critical stenoses or aneurysms, vascular and cardiothoracic surgeons.
1 Hall S., Barr W., Lie J.T., et al. Takayasu arteritis: a study of 32 North American patients. Medicine. 1985;64:89.
2 Waern A.U., Anderson P., Hemmingsson A. Takayasu’s arteritis: a hospital-region based study on occurrence, treatment, and prognosis. Angiology. 1983;34:311.
3 Nasu T. Takayasu’s truncoarteritis in Japan: a statistical observation of 76 autopsy cases. Pathol Microbiol (Basel). 1975;43:140.
4 Chauhan S.K., Tripathy N.K., Sinha N., et al. Cellular and humoral immune responses to mycobacterial heat shock protein-65 and its human homologue in Takayasu’s arteritis. Clin Exp Immunol. 2004;138:547.
5 Chauhan S.K., Tripathy N.K., Nityanand S. Antigenic targets and pathogenicity of anti-aortic endothelial cell antibodies in Takayasu’s arteritis. Arthritis Rheum. 2006;54:2326.
6 Seko Y., Minota S., Kawasaki A., et al. Perforin-secreting killer cell infiltration and expression of a 65 kD heat shock protein in aortic tissue of patients with Takayasu’s arteritis. J Clin Invest. 1994;93:750.
7 Inder S.J., Bobryshev Y.V., Cherian S.M., et al. Immunophenotypic analysis of the aortic wall in Takayasu’s arteritis: involvement of lymphocytes, dendritic cells, and granulocytes in immuno-inflammatory reactions. Cardiovasc Surg. 2000;8:141.
8 Noris M., Daina E., Gamba S., et al. Interleukin-6 and RANTES in Takayasu arteritis: a guide for therapeutic decisions? Circulation. 1999;100:55.
9 Nishino Y., Tamai M., Kawakami A., et al. Serum levels of BAFF for assessing the disease activity of Takayasu arteritis. Clin Exp Rheumatol. 2010;28(1 Suppl 57):14.
10 Hoffman G.S. Large-vessel vasculitis: unresolved issues. Arthritis Rheum. 2003;48:2406.
11 Numano F. Takayasu’s arteritis: clinical aspects. In: Hoffman G.S., Weyand C.M. Inflammatory diseases of blood vessels. New York: Marcel Dekker; 2002:455.
12 Hotchi M. Pathologic studies on Takayasu arteritis. Heart Vessels Suppl. 1992;7:11.
13 Kerr G., Hallahan C., Giordano J., et al. Takayasu arteritis. Ann Intern Med. 1994;120:919.
14 Lupi-Herrara E., Sanchez-Torrez G., Marchushamer J., et al. Takayasu arteritis: clinical study of 107 cases. Am Heart J. 1977;93:94.
15 Numano F., Kobayashi Y. Takayasu arteritis—beyond pulselessness. Intern Med. 1999;38:226.
16 Grange D.K., Balfour I.C., Chen S.C., et al. Familial syndrome of progressive arterial occlusive disease consistent with fibromuscular dysplasia, hypertension, congenital cardiac defects, bone fragility, brachysyndactyly, and learning disabilities. Am J Med Genet. 1998;75:469.
17 Rojo-Leyva F., Ratliff N., Cosgrove D.M., et al. Study of 52 patients with idiopathic aortitis from a cohort of 1,204 surgical cases. Arthritis Rheum. 2000;43:901.
18 Hernandez-Rodriguez J., Molloy E.S., Hoffman G.S. Single organ vasculitis. Curr Opin Rheumatol. 2008;20:40.
19 Maksimowicz-McKinnon, Clark T.M., Hoffman G.S. Takayasu arteritis and giant cell arteritis: a spectrum within the same disease? Medicine (Baltimore). 2009;88:221.
20 Arnaud L., Haroche J., Limal N., et al. Takayasu arteritis in France: a single-center, retrospective study of 82 cases comparing white, North African, and black patients. Medicine. 2010;89:1.
21 Bicakcigil M., Aksu K., Ozbalkan Z., et al. Takayasu’s arteritis in Turkey-clinical and angiographic features of 248 patients. Clin Exp Rheumatol. 2009;27(Suppl 52):S59.
22 Tso E., Flamm S.D., White R.D., et al. Takayasu arteritis. Utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum. 2002;46:1634.
23 Webb M., Chambers A., Al-Nahhas A., et al. The role of 18-FDG PET in characterizing disease activity in Takayasu arteritis. Eur J Nucl Med. 2004;31:627.
24 Walter M.A., Melzer R.A., Schindler C., et al. The value of F-18 FDG-PET in the diagnosis of large-vessel vasculitis and the assessment of activity and extent of disease. Eur J Nucl Med Mol Imaging. 2005;32:674.
25 Hoffman G.S., Leavitt R.Y., Kerr G.S., et al. Treatment of glucocorticoid-resistant or relapsing Takayasu arteritis with methotrexate. Arthritis Rheum. 1994;37:578.
26 Shelhamer J.H., Volkman D.J., Parrillo J.E., et al. Takayasu’s arteritis and its therapy. Ann Intern Med. 1985;103:121.
27 Molloy E.S., Langford C.A., Clark T.M., et al. Anti-tumour necrosis factor therapy in patients with refractory Takayasu arteritis: long-term follow-up. Ann Rheum Dis. 2008;67:1567.
28 Sharma B.K., Jain S., Bali H.K., et al. A follow-up study of balloon angioplasty and de-novo stenting in Takayasu arteritis. Int J Cardiol. 2000;75:S147.
29 Tyagi S., Gambhir D.S., Kaul U.A., et al. A decade of subclavian angioplasty: aortoarteritis versus atherosclerosis. Indian Heart J. 1996;48:667.
30 Bali H.K., Bhargava M., Jain A.K., et al. De novo stenting of descending thoracic aorta in Takayasu arteritis: intermediate-term follow-up results. J Invasive Cardiol. 2000;12:612.
31 Liang P., Tan-Ong M., Hoffman G.S. Takayasu’s arteritis: vascular interventions and outcomes. J Rheumatol. 2004;31:102.
32 Pajari R., Hekali P., Harjola P.T. Treatment of Takayasu’s arteritis: an analysis of 29 operated patients. Thorac Cardiovasc Surg. 1986;34:176.
33 Miyata T., Sato O., Koyama H., et al. Long-term survival after surgical treatment of patients with Takayasu’s arteritis. Circulation. 2003;108:1474.
34 Lagneau P., Michel J.B., Vuong P.N. Surgical treatment of Takayasu’s disease. Ann Surg. 1987;205:157.