Chapter 44 Thromboangiitis Obliterans (Buerger’s Disease)
Thromboangiitis obliterans (TAO) describes a segmental nonatherosclerotic inflammatory disorder that primarily involves the small and medium arteries, veins, and nerves of the extremities.1–3 Although TAO was initially thought to be a disease confined exclusively to men, recent epidemiological studies demonstrate a growing population of women with the disorder. Also known as Buerger’s disease, TAO has an extremely strong pathophysiological relationship with tobacco use, usually in the form of heavy cigarette smoking.
In 1879, von Winiwarter provided the first description of a patient with TAO. He presented the case of a 57-year-old man who had reported pain in his feet for 12 years. Histopathological examination of an amputation specimen from this patient demonstrated intimal proliferation, luminal thrombosis, and fibrosis; von Winiwarter hypothesized that the endarteritis and endophlebitis observed were distinct from atherosclerosis.4 In his landmark paper 29 years later,5 Leo Buerger published a detailed report of the pathological findings of 11 amputated limbs from patients with the disease and coined the term thromboangiitis obliterans to describe the characteristic observations of endarteritis and endophlebitis. Similar to von Winiwarter, Buerger made a point to distinguish the clinical and pathological findings of TAO from those of atherosclerosis.
In 1928, Allen and Brown described the clinical and pathological characteristics of 200 cases of TAO seen at the Mayo Clinic from 1922 to 1926.6 The majority of cases occurred among Jewish men, and all patients were heavy smokers. The pathological findings in this report were virtually identical to those described in Buerger’s original paper.
Epidemiology
Although it is observed worldwide, TAO is more prevalent in the Middle East and Far East than North America and Western Europe.7,8 Prior to the late 1960s, overdiagnosis of TAO was frequent. Of 205 cases originally diagnosed as TAO at Mount Sinai Hospital from 1933 to 1963, only 33 were later believed to be compatible with the diagnosis, 28 were considered questionable, and 144 were determined incorrect.9
Adoption of stricter diagnostic criteria and a reduction in tobacco use have caused the reported number of new patients diagnosed with TAO in the United States and Europe to decline. Overall incidence of TAO is higher in regions of the world where consumption of tobacco is greater. At the Mayo Clinic over a 40-year period, the prevalence rate of patients with the diagnosis of TAO has decreased from 104 per 100,000 patient registrations in 1947 to 13 per 100,000 patient registrations in 1986.7 The prevalence rate of TAO in patients with peripheral artery disease (PAD) varies across Europe and Asia: 1% to 3% in Switzerland, 0.5% to 5% in West Germany, 1.2% to 5.6% in France, 4% in Belgium, 0.5% in Italy, 0.25% in the United Kingdom, 3.3% in Poland, 6.7% in East Germany, 11.5% in Czechoslovakia, 39% in Yugoslavia, 80% in Israel (among Ashkenazi Jews), 45% to 53% in India, and 16% to 66% in Japan and Korea.10 In Asia, a greater proportion of patients with limb ischemia has been attributed to TAO than in the United States and Europe.
Overall incidence of TAO also appears to be declining in South Asia and Japan.11,12 During the 1990s, the ratio of new patients with TAO to new patients with atherosclerotic PAD was reported to be 1:3 in a vascular outpatient clinic in Japan.13 Since 2000, the ratio has declined to 1:10.13
Thromboangiitis obliterans has been associated with manual labor and lower socioeconomic status in some series. In particular areas of Southeast Asia, including India, TAO has been associated with lower socioeconomic class and smoking unrefined homemade tobacco cigarettes called bidi. In a study of 28 cases of TAO from Korea, 23 patients were farmers or manual laborers and belonged to the lowest socioeconomic class.14 In another analysis of 106 patients with TAO in Java, Indonesia, the majority of patients were from the lowest socioeconomic class.15 However, in a study of 8858 Japanese patients with the disease, there was no significantly greater prevalence among manual laborers.16
Although it has been considered a disease of young men, TAO also occurs in women. Reported incidence was less than 2% in the majority of published case series before 1970. More recent studies have demonstrated a much higher prevalence, ranging from 11% to 23%.17–20 The increasing prevalence of TAO among women has been attributed to rising consumption of tobacco products.
Etiology and Pathogenesis
Thromboangiitis obliterans is a vasculitis characterized by a highly cellular inflammatory thrombus with relative sparing of the vessel wall; its precise etiology remains unknown. Thromboangiitis obliterans is distinct from other vasculitides because levels of acute-phase reactants such as erythrocyte sedimentation rate and C-reactive protein (CRP). Commonly measured autoantibodies are typically normal, but it has been suggested that abnormalities in immunoreactivity and other factors may contribute to the inflammatory process.
Tobacco
Exposure to tobacco is critical to initiation, maintenance, and progression of TAO. Although smoking tobacco is by far the most frequent precipitating factor, chewing tobacco and using snuff21 or marijuana have also been implicated in its development.22,23 The association between heavy tobacco use and TAO is so strong, it is typically considered a sine qua non for the diagnosis.17,24 Patients with TAO have higher tobacco consumption and carboxyhemoglobin levels than those with atherosclerotic vascular disease or healthy controls.25 It has been hypothesized that some patients develop an immunological reaction to a constituent of tobacco that triggers small-vessel occlusive disease.26,27 Because only a small proportion of smokers worldwide eventually develop TAO, other factors are believed to play a contributory role in disease pathogenesis.
As noted earlier, in South Asia, a large proportion of patients diagnosed with TAO belong to the lowest socioeconomic class and smoke bidi. Bidi smoking is believed to account for the higher incidence of TAO in the Indian population. A case-control study from Bangladesh reported that 35% of patients with TAO were cigarette smokers and 65% were bidi smokers, compared with 69.9% and 30.1%, respectively, of controls.28 After adjusting for confounding factors and using 10-per-day smoking frequency as a reference, the study found that smoking 11 to 20 bidi per day was associated with a seven fold increased risk of developing TAO, and smoking over 20 bidi per day led to a 35-fold increased risk. The authors concluded that bidi smoking carried greater risk than cigarette smoking for consequent TAO.28
In addition to its role in disease initiation, tobacco use is a critical factor in disease progression and continued symptoms associated with TAO.2 Although second-hand or passive smoking has not been associated with TAO onset, it may play an important role in continuation of the disease process. In a study of 40 patients with TAO, cotinine, the major metabolite of nicotine, was used as a measure of exposure to tobacco smoke. Urinary cotinine levels were measured to classify them as smokers (cotinine levels >50 ng/mg creatinine), passive smokers (cotinine levels 10-50 ng/mg creatinine), and nonsmokers who did not experience noticeable passive smoking (cotinine levels <10 ng/mg creatinine).29 Using these criteria, 10 patients were classified as smokers, 9 as passive smokers, and 21 as nonsmokers. Seven of the 10 smokers, none of the 9 passive smokers, and 4 of the 21 nonsmokers experienced disease exacerbation. Of the four nonsmokers who experienced disease exacerbation, three had continued to smoke and one had been exposed to second-hand tobacco smoke in the workplace at the time of relapse. Among active smokers, the seven whose conditions had worsened showed significantly higher urinary cotinine levels than the three remaining patients who remained in remission.
Genetic Predisposition
Several studies have suggested there may be a genetic predisposition to developing TAO. Although there appears to be an association between certain human lymphocyte antigen (HLA) haplotypes and development of TAO, no consistent pattern has been identified across patient populations. In the United Kingdom, HLA-A9 and HLA-B5 are particularly prevalent in patients with TAO.30 In a U.S. study, HLA testing was performed in 11 patients with TAO, and no specific pattern could be identified.18 Lack of consistency in HLA haplotype predominance among various populations with TAO is likely due to genetic diversity and methodological differences in each of the studies.31
Polymorphisms of CD14, a main receptor for bacterial lipopolysaccharide, (37.4% vs. 24.2%; P = 0.008; odds ratio [OR] = 1.87; 95% confidence interval [CI], 1.18-2.97), HLA-DRB1 (34.4% vs. 13.2%; P < 0.001; OR = 3.44; 95% CI, 2.06-5.73), and HLA-DPB1 (79.4% vs. 55.1%; P < 0.001; OR = 3.14; 95% CI, 1.93-5.11) have been observed to have a significantly higher frequency in patients with TAO than in controls.32 Stratification analyses of these polymorphisms suggested synergistic roles with ORs that ranged from 4.72 to 12.57 in individuals carrying any two of these three markers. These data suggest that susceptibility to TAO may be controlled in part by genes involved in innate and adaptive immunity.
In a study comparing 21 TAO patients with healthy age-, gender-, and race-matched controls, frequency of mutations associated with arterial vasospasm (stromelysin-1 5A/6A, endothelial nitric oxide synthase [eNOS] T-786 C) was evaluated.33 Homozygosity for 5A/6A stromelysin-1 was present in 7 of 21 (33%) TAO cases, compared with 5 of 21 (24%) controls (risk ratio 1.4; 95% CI, 0.5-3.7). Homozygosity for eNOS T-786 C was present in 3 of 21 (14%) TAO cases, compared with 1 of 21 (5%) controls (risk ratio 3.0; 95% CI, 0.3-26.6).
In another recent study, eNOS 894 G→T and endothelin-1 (ET-1) 8000 T→C polymorphisms were assessed to determine whether either played a role in development of TAO.34 Investigators found that the T allele of the eNOS 894 G→T polymorphism was protective against TAO.
Hypercoagulable States
The role of hypercoagulable states in TAO pathogenesis remains unclear; studies have failed to demonstrate a consistent pattern of association. In a comparison of patients with TAO and healthy controls, levels of urokinase-plasminogen activator (uPA) were twofold higher, and free plasminogen activator inhibitor-1 (PAI-1) were 40% lower, in patients with the disease.35 During venous occlusion, tissue plasminogen activator (tPA) antigen concentrations increased to a greater extent in controls while PAI-1 levels were lower in patients with TAO. Patients with TAO also appear to have an enhanced platelet response to serotonin36 and higher platelet contractile force.37
In one case-control study, the frequencies of factor V Leiden and prothrombin gene 20210A mutations were similar in patients with TAO and healthy subjects.38 However, in another case-control study, OR for the prothrombin 20210 A allele compared with the G allele was 7.98 (95% CI, 2.45-25.93) in patients with TAO.39 Elevated plasma homocysteine levels have been reported in patients with TAO and may be associated with a higher amputation rate.40
Increased levels of anticardiolipin antibodies have been reported in patients with TAO.40–42 In one study, anticardiolipin antibodies were measured in 47 patients with TAO, 48 patients with premature atherosclerosis, and 48 otherwise healthy individuals.42 Prevalence of anticardiolipin antibodies was significantly higher in patients with TAO (36%) compared with those with premature atherosclerosis (8%; P = 0.01) and healthy controls (2%; P < 0.001). Compared with those without detectable autoantibodies, patients with TAO and elevated anticardiolipin antibody titers were younger at age of onset and had increased rates of major amputation. A smaller study, however, did not demonstrate increased amputation rates in TAO patients with elevated anticardiolipin antibodies.40
Immunological Mechanisms
Abnormalities in immunoreactivity are believed to play a critical role in the inflammatory process that characterizes TAO. In a study of 39 patients with TAO, cellular and humoral immune responses to native human collagen type I and type III were evaluated.43 Cell-mediated sensitivity to these collagens as measured by an antigen-sensitive thymidine incorporation assay was significantly higher in patients with TAO than in patients with atherosclerosis or in healthy male controls. Lymphocytes from 77% of the patients with TAO demonstrated cellular sensitivity to type I or type III collagens or both. In 17 of 39 serum samples (44%) from the patients with TAO, a low but significant level of anticollagen antibody activity was detected, whereas no antibody activity was observed in serum samples from controls. Circulating immune complexes found in peripheral arteries of patients with TAO provide further evidence for an immunological basis for this disease.44,45
In a study of nine patients with TAO, immunohistochemical analysis was performed on 33 specimens.46 Architecture of blood vessel walls was well preserved regardless of the stage of disease, but cell infiltration involving the thrombus and intima was observed. Among infiltrating cells, CD3+ T cells greatly outnumbered CD20+ B cells, and CD68+ macrophages or S-100+ dendritic cells were detected in the intima during acute and subacute phases. Deposits of immunoglobulins (Ig)G, IgA, and IgM and complement factors 3d and 4c were noted along the internal elastic lamina. These data indicate that TAO represents an endarteritis characterized by both T cell– (cellular) and B cell–mediated (humoral) immunity in association with activation of antigen-presenting cells in the intima.
Immunohistochemical and TUNEL (terminal dUTP nick end labeling) studies were conducted on arterial walls obtained from eight patients with TAO to phenotype infiltrating cells with CD4+ (helper T cell), CD8+ (cytotoxic T cell), CD56 (natural killer cell), and CD68 (macrophage) to (1) identify cell activation with vascular cell adhesion molecule-1 (VCAM-1) and inducible nitric oxide synthase (iNOS), (2) determine the presence of cell death with TUNEL analysis, and (3) detect inflammatory cytokines with reverse transcriptase-polymerase chain reaction (RT-PCR).47 T cells were identified mainly in the thrombus, intima, and adventitia. Among infiltrating cells, CD4+ T cells greatly outnumbered CD8+ cells. VCAM-1 and iNOS were expressed in endothelial cells (ECs) around the intima in patent segments or in vaso vasorum in occluded segments. These findings suggest that a T cell–mediated immune response may play an important role in development of TAO.
An immunohistochemistry study compared 58 amputated lower extremities from patients with TAO to 5 autopsy controls.48 In patients with a definite diagnosis of TAO, as determined by clinical criteria, linear arrangement of macrophages, B lymphocytes, and T lymphocytes along vascular elastic fibers was found to be a predictable and specific inflammatory response to the internal elastic lamina of affected vessels. This finding indicates that elastic fibers are important immunogens in TAO pathogenesis.
Endothelial Dysfunction
Abnormalities of endothelial function also appear to contribute to TAO pathogenesis. Although various autoantibodies commonly observed in vasculitides are typically absent, elevations in antiendothelial cell antibody titers have been documented in patients with active TAO.49 In one study, seven patients with active TAO had antiendothelial cell antibody titers of 1857 ± 450 arbitrary units (AU), compared with 461 ± 41 AU in 21 patients in remission (P < 0.01) and 126 ± 15 AU in 30 control subjects (P < 0.001).49 If these findings can be confirmed, measurement of antiendothelial cell antibody titers may serve as a useful tool in following disease activity.
Patients with TAO also demonstrate impairment of endothelium-dependent vasodilation in the peripheral vasculature. Changes in forearm blood flow induced by the endothelium-dependent vasodilator acetylcholine, the endothelium-independent vasodilator sodium nitroprusside, and occlusion-induced reactive hyperemia were measured plethysmographically in the nondiseased limb in eight patients with TAO and in eight healthy controls matched for age and smoking status.50 The increase in forearm blood flow response to intraarterial acetylcholine infusion was diminished in patients with TAO compared with healthy controls (22.9 ± 2.9 vs. 14.1 ± 2.8 mL/min per dL of tissue volume; P < 0.01). In contrast, there was no significant difference in the increase in forearm blood flow response to sodium nitroprusside infusion and reactive hyperemia between the two study groups. These data suggest that peripheral endothelium-dependent vasodilation is impaired even in the nondiseased limbs of patients with TAO.
In a study designed to evaluate the role of circulating progenitor cells in endothelial function in patients with TAO and atherosclerosis obliterans, investigators measured flow-mediated vasodilation, nitroglycerin-induced vasodilation, and circulating progenitor cells in 30 patients with TAO, 30 age- and sex-matched healthy subjects, and 40 patients with atherosclerosis obliterans.51 Flow-mediated vasodilation was decreased in both the TAO group and atherosclerosis obliterans group compared with controls (6.6% ± 2.7%, 5.7% ± 3.3% vs. 9.5 ± 3.1%, P < 0.0001, respectively). However, there was no significant difference in flow-mediated vasodilation between the TAO group and atherosclerosis obliterans group. Nitroglycerin-induced vasodilation was similar in the three groups. The number and migration of circulating progenitor cells were similar in the TAO group and control group, but were significantly decreased in the atherosclerosis obliterans group. There was a significant relationship between the number and migration of circulating progenitor cells and flow-mediated vasodilation (r = 0.43 and r = 0.40, P < 0.0001, respectively). Flow-mediated vasodilation was impaired in patients with TAO and patients with atherosclerosis obliterans compared with control subjects, but the number and function of circulating progenitor cells were not decreased in patients with TAO.
In a study of surgical biopsies obtained from femoral and iliac arteries of patients with TAO, expression of intercellular adhesion molecule-1 (ICAM-1), VCAM-1, and E-selectin was increased on endothelial and inflammatory cells in the thickened intima.52 Immunohistochemistry demonstrated contacts between mononuclear cells and morphologically activated ECs expressing ICAM-1 and E-selectin. These findings provide evidence for EC activation, tumor necrosis factor (TNF)-α secretion by tissue-infiltrating inflammatory cells, ICAM-1, VCAM-1 and E-selectin expression on ECs, and leukocyte adhesion in TAO.
Infection
Chronic anaerobic periodontal infection may represent an additional risk factor for development of TAO.53 Nearly two thirds of patients with TAO have severe periodontal disease. Polymerase chain reaction (PCR) analysis demonstrated deoxyribonucleic acid (DNA) fragments from anaerobic bacteria, in particular Treponema denticola, in both arterial lesions and oral cavities of patients with TAO, but not in arterial samples from healthy control subjects. However, the higher prevalence of periodontal infection in TAO may simply be a marker of lower socioeconomic status in patients who develop the disease, rather than a pathogenic correlate.
Pathology
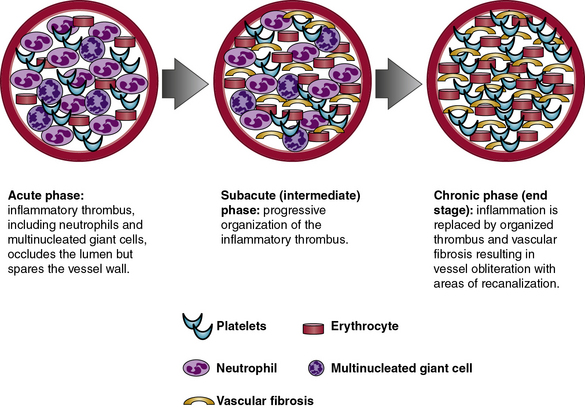
Pathologically, TAO is distinguished by inflammatory thrombus that affects small- and medium-sized arteries and veins. Histopathology of involved blood vessels varies according to the stage at which the tissue sample is obtained. Thromboangiitis obliterans involves three phases: acute, subacute, and chronic (Fig. 44-1). Histopathology is most likely to be diagnostic of TAO in samples obtained during the acute phase of the disease. As the disease progresses from the subacute to chronic phases, the histopathology of TAO becomes virtually indistinguishable from other obstructive vascular diseases that result in fibrosis of the blood vessels in their end stage. Because the histological appearance can vary from patient to patient and depends upon the stage of the disease, a pathological diagnosis of TAO may be challenging in some cases.54 Furthermore, pathological diagnosis may be inconclusive if only amputated specimens or occluded arteries and veins are examined. Subacute and chronic phase lesions have far fewer characteristic features and therefore are rarely diagnostic for TAO.
Acute Phase
The acute phase of TAO consists of an occlusive, highly cellular, inflammatory thrombus. Polymorphonuclear neutrophils, microabscesses, and multinucleated giant cells are often present around the periphery of the thrombus (Fig. 44-2). Presence of multinucleated giant cells is characteristic of but not specific for TAO.55
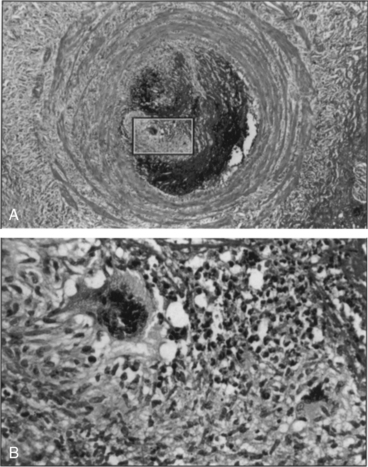
Figure 44-2 A, Typical acute histological lesion in vein obtained from patient with thromboangiitis obliterans (TAO). B, Close-up of boxed area in A, demonstrating a microabscess in the thrombus and two multinucleated giant cells (hematoxylin and eosin, × 64 [A] and × 400 [B]).
(Reproduced with permission from Lie JT: Thromboangiitis obliterans (Buerger’s disease) revisited. Pathol Annu 23:257–291, 1988.)
Inflammatory thrombus is observed with greatest frequency in biopsies of areas demonstrating acute superficial thrombophlebitis taken from patients with TAO. It is unclear whether the vascular lesions of TAO are primarily thrombotic or inflammatory, but the pattern of intense inflammatory cell infiltration and cellular proliferation observed in the acute phase of the disease is particularly distinctive. Acute phlebitis without thrombosis, acute phlebitis with thrombosis, and acute phlebitis with thrombus containing microabscesses and giant cells may coexist in different segments of the same affected vein if it is biopsied at an early stage. These lesions correspond with the clinical finding of thrombophlebitis migrans.56
Subacute (Intermediate) Phase
During the subacute or intermediate phase, progressive organization of the inflammatory thrombus takes place in affected arteries and veins. Although some degree of inflammatory infiltrate remains within the thrombus, the vessel wall is generally spared. Partial recanalization of the vessel and disappearance of microabscesses may also be observed in the subacute phase.46
Chronic (End-Stage) Phase
The chronic phase is characterized by organized thrombus with areas of extensive recanalization, prominent vascularization of the media, and adventitial and perivascular fibrosis. Because they represent the end products of vascular injury and occlusive thrombosis, chronic-phase arterial lesions are the least distinctive of the three morphological stages of TAO. However, focal residual inflammation within the organized thrombus may suggest TAO in an end-stage lesion. Chronic-phase lesions in TAO frequently mimic atherosclerotic vascular disease. In some patients, especially those older than 40 years of age, both TAO and atherosclerotic vascular disease may coexist and thereby create further diagnostic uncertainty.
Additional Histopathological Features
In all three phases of TAO, normal architecture of the vessel wall adjacent to the occlusive thrombus, including the internal elastic lamina, remains intact. This observation distinguishes TAO from atherosclerotic vascular disease and from other systemic vasculitides in which there is typically disruption of the internal elastic lamina and the media. “Skip” areas in which normal vessel segments are observed between diseased ones are common in TAO. In addition, the intensity of the periadventitial inflammatory reaction can be quite variable in different segments of the same vessel.
Immunohistochemical Features
Studies focusing on immunohistochemistry have provided limited understanding of the role of the cytoskeleton and other cellular elements in TAO.44–48,52,57 Soon after the inflammatory thrombus has occluded the vessel lumen, spindle cells migrate from the media through fenestrations in the internal elastic lamina into the intima to populate the periphery of the thrombus. These spindle cells express vimentin and α1-actin and originate from smooth muscle cells (SMCs) of the media. Capillaries appear along thrombus margins. Endothelial cells express factor VIII–related antigen and Ulex europaeus agglutinin.
As the thrombus organizes during later disease stages, spindle cells differentiate into fibroblasts and lose their positive staining for α1-actin. Demonstration of the internal elastic lamina by collagen type IV markers confirms that the lamina remains intact in TAO, and that SMCs migrate from the media to the intima via fenestrations. Newly formed capillaries within the thrombus are noted.
Immunohistochemically, the process of thrombus organization in TAO is virtually identical to that of ordinary thrombus, with the exception of the characteristic inflammatory component. However, infiltration of SMCs from the media results in a more hypercellular thrombus and rapid organization.
Clinical Presentation
The typical patient with TAO is a young man with a history of heavy tobacco smoking who presents with onset of ischemic symptoms of the extremities before age 45 years. However, patients should be questioned about chewing tobacco as well as snuff and marijuana use, especially if they deny smoking and present with a history compatible with TAO. Ischemic symptoms result from stenosis or occlusion of the distal small arteries and veins. Thromboangiitis obliterans frequently progresses proximally and involves multiple limbs. Large-artery involvement is atypical and rarely occurs in the absence of small-vessel occlusive disease.58 The most common symptoms result from arterial occlusive disease, secondary vasospasm, and superficial thrombophlebitis (Table 44-1).
Table 44-1 Presenting Symptoms and Signs in 112 patients with Thromboangiitis Obliterans Evaluated at Cleveland Clinic Between 1970 and 1987*
| Clinical Finding | N (%) |
|---|---|
| Intermittent claudication | 70 (63) |
| Rest pain | 91 (81) |
| Ischemic ulcerations: Upper extremity Lower extremity Both |
85 (76) 24 (28) 39 (46) 22 (26) |
| Thrombophlebitis | 43 (38) |
| Raynaud phenomenon | 49 (44) |
| Sensory findings | 77 (69) |
| Abnormal Allen test | 71 (63) |
* Data from Olin JW, Young JR, Graor RA, et al: The changing clinical spectrum of thromboangiitis obliterans (Buerger’s disease). Circulation 82:3–8, 1990.
Arterial Occlusive Disease
Arterial occlusive disease due to TAO most often presents as intermittent claudication of the feet, legs, hands, or arms. In a study of 112 patients with TAO evaluated at the Cleveland Clinic from 1970 to 1987, intermittent claudication occurred in 70 patients (63%).17 In a retrospective study of 344 patients treated surgically for TAO in Turkey, major complaints included foot coldness in 312 (90.6%) patients, color changes in 290 (84.3%), rest pain in 160 (46.5%), claudication in 166 (48.2%), and necrotic ulcers in 185 (53.1%).59 Foot or arch claudication may be a presenting symptom and is frequently attributed to an orthopedic problem, resulting in diagnostic delay. As lower-extremity disease progresses proximally, patients with TAO may report classic calf claudication.
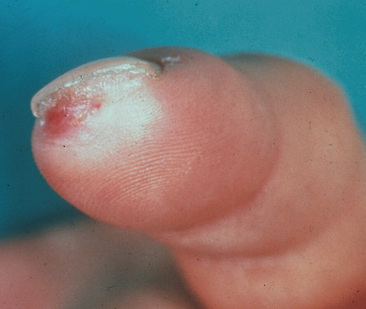
Symptoms and signs of critical limb ischemia, including rest pain, ulcerations, and digital gangrene, occur with advanced arterial occlusive disease. At the time of presentation, 76% of patients have ischemic ulcerations (Fig. 44-3A and Fig. 44-4).17 With early recognition of symptoms and signs of TAO, many patients can be identified and treated before critical limb ischemia develops.
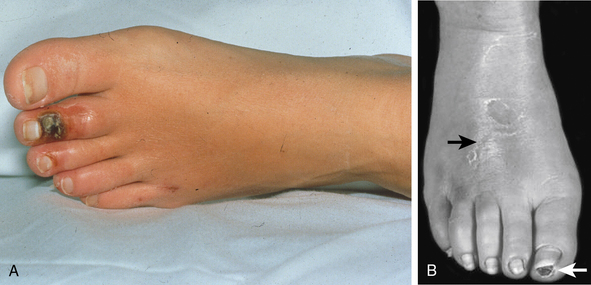
Figure 44-3 A, Ischemic ulceration on second toe in young woman with thromboangiitis obliterans. B, Superficial thrombophlebitis on dorsum of the right foot (black arrow) in patient with TAO. Note ischemic ulceration on distal right great toe.
(Reproduced with permission from Olin JW, Lie JT: Current management of hypertension and vascular disease. In Cooke JP, Frohlich ED, editors: Thromboangiitis obliterans (Buerger’s disease), St. Louis, 1992, Mosby-Yearbook, p 65.)
Although only one limb may be affected clinically, arterial occlusive disease always involves two or more extremities on angiographic evaluation. In one series of patients with TAO, two limbs were affected in 16% of patients, three limbs in 41%, and all four limbs in 43%.60 The Intractable Vasculitis Syndromes Research Group of Japan reported isolated lower limb involvement in 75%, isolated upper limb involvement in 5%, and both upper and lower limb involvement in 20% of patients with TAO.61
Raynaud Phenomenon
A common complaint in TAO, cold sensitivity may represent one of the earliest manifestations of the disease. Indeed, presentations of TAO appear to be more common in the winter.62 Cold sensitivity likely results from ischemia or markedly increased muscle sympathetic nerve activity, which has been demonstrated in TAO patients compared with controls.63 Raynaud phenomenon is present in over 40% of patients with TAO and may be asymmetrical.2 The extremities, particularly the digits, may be characterized by either rubor or cyanosis. This discoloration has been termed Buerger’s color.64
Superficial Thrombophlebitis
Although it may also be observed in Behçet disease, superficial thrombophlebitis differentiates TAO from other vasculitides and atherosclerotic vascular disease (see Fig. 44-3B). Superficial thrombophlebitis occurs in approximately 40% of patients with TAO.17 Superficial thrombophlebitis may predate the onset of ischemic symptoms caused by arterial occlusive disease56 and may parallel disease activity.65 Some patients may describe a migratory pattern of tender nodules that follow a venous distribution (thrombophlebitis migrans).56
Neurological Findings
Sensory abnormalities are common in TAO and were observed in up to 70% of cases in a series from the Cleveland Clinic.17 Sensory findings are most likely due to ischemic neuropathy, a late finding in the course of TAO. Sensory findings may also be due to primary involvement of the nerves themselves, since earlier studies have suggested that inflammatory cell infiltrate may surround the nerves.5
Unusual Presentations
TAO is typically a disease affecting vessels in distal parts of the extremities, but it has also been reported to involve unusual vascular beds: the great vessels and pulmonary, proximal extremity, mesenteric, cerebral, coronary, renal, pelvic, and ophthalmic arteries. Thromboangiitis obliterans in atypical vascular beds is characterized by similar pathological findings as found in the extremities. Of note, reports of TAO in unusual locations should be interpreted with caution because the diagnosis of TAO in such cases may not meet criteria suggested in this chapter.
Thromboangiitis obliterans of large elastic arteries such as the pulmonary66 and iliac arteries58 has been rarely documented. Visceral involvement may present as abdominal pain, nausea, vomiting, diarrhea, melena, hematochezia, weight loss, and anorexia and result in mesenteric ischemia or infarction.67–72 Cerebrovascular involvement may manifest as transient ischemic attack (TIA), ischemic stroke, and psychotic disorders.73–77
Coronary artery involvement may present as myocardial ischemia or infarction.78–82 Thromboangiitis obliterans affecting the intrarenal arterial branches has been reported.83 Rarely, TAO may involve the pelvic vessels, including the pudendal arteries and veins, resulting in erectile dysfunction.84 Thromboangiitis obliterans involving the temporal and ophthalmic arteries may mimic giant cell arteritis (GCA).85,86
Involvement of saphenous vein bypass grafts in patients with TAO is a truly rare occurrence.87
Differential Diagnosis
A clinical diagnosis of TAO requires exclusion of disorders that may mimic the disease (Box 44-1). The most important disorders to exclude are atherosclerotic vascular disease, thromboembolic disease, and autoimmune diseases such as scleroderma or CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia) syndrome. In most cases, the combination of serological testing, echocardiography, and arteriography can exclude these disorders and help establish the diagnosis of TAO.
![]() Box 44-1 Disorders That May Mimic Thromboangiitis Obliterans
Box 44-1 Disorders That May Mimic Thromboangiitis Obliterans
CREST, calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia.
A scleroderma or CREST syndrome diagnosis is typically suggested by clinical presentation, including skin findings. Nailfold capillaroscopy may be performed and is usually quite distinctive in patients with these disorders. However, characteristic findings of capillary loop dropout in scleroderma or CREST syndrome may also be observed in some patients with TAO. Detection of serological markers such as anti-ACL-70 or anticentromere antibodies provides further evidence for scleroderma or CREST syndrome.
Clinicians should evaluate patients for features of other autoimmune diseases such as systemic lupus erythematosus, rheumatoid arthritis, and other vasculitides. Serological markers are often helpful in excluding such disorders. Patients with antiphospholipid antibody syndrome pose a particular diagnostic challenge because they may present with both arterial and venous thrombotic events. Antiphospholipid antibody syndrome is suggested by detection of lupus-type anticoagulants or presence of elevated titers of anticardiolipin antibodies. Of note, lupus anticoagulant and anticardiolipin antibodies may be detected in some patients with TAO, but may also indicate an unrelated thrombophilia.1 Pathological examination can clearly differentiate between the two disorders because antiphospholipid antibody syndrome is characterized by the presence of bland thrombosis, whereas TAO results in an inflammatory thrombus.
Thromboangiitis obliterans is differentiated from other vasculitides in that it results in distal extremity ischemia, whereas patients with Takayasu’s arteritis or GCA present with more proximal arterial involvement. Arteriographic features of TAO are also quite distinctive from those observed in Takayasu’s arteritis or giant cell arteritis. In addition, vasculitides such as Takayasu’s arteritis and GCA are typically associated with elevations in inflammatory markers, including erythrocyte sedimentation rate and C-reactive protein.
Clinicians evaluating patients with suspected TAO should inquire about possible ergotamine or cocaine abuse, in addition to disorders of repetitive mechanical trauma such as vibration-induced vascular injury and hypothenar hammer syndrome. Serum ergotamine levels can be obtained to exclude vascular injury caused by this drug. Because it can mimic TAO, all patients should be questioned about cocaine abuse. A complete toxicology screen is recommended in patients who present with a history and physical compatible with TAO, especially if they deny tobacco use.
Diagnosis
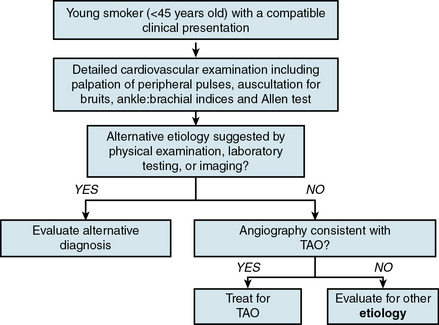
Thromboangiitis obliterans is a clinical diagnosis that requires a compatible history in combination with supportive physical examination findings and vascular abnormalities on imaging studies (Fig. 44-5).
Physical Examination
Physical examination of a patient with suspected TAO should include a detailed vascular evaluation with palpation of peripheral pulses, auscultation for arterial bruits, and measurement of ankle:brachial indices. Extremities should be carefully inspected for superficial thrombophlebitis, which may present as tender superficial venous nodules and cords. Hands and feet should be examined for findings of digital ischemia. Neurological examination may document peripheral nerve involvement in the form of sensory deficits.
Although nonspecific, an abnormal Allen test in a young smoker with digital ischemia is strongly suggestive of TAO because it provides evidence for small-vessel disease (Fig. 44-6). In a series from the Cleveland Clinic, 63% of patients with TAO had an abnormal Allen test.17 Documentation of an abnormal Allen test is helpful because the distal nature of TAO and involvement of both upper and lower extremities distinguishes it from atherosclerotic vascular disease. With the exception of chronic kidney disease patients with diabetes or those who have undergone renal transplantation, atherosclerosis does not involve the hands and rarely occurs distal to the subclavian artery.
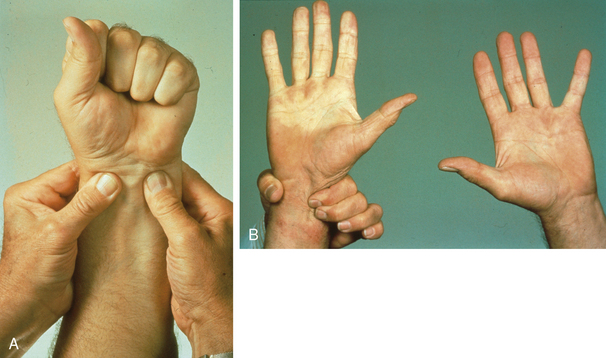
Figure 44-6 A, Allen test with occlusion of radial and ulnar pulses by manual compression. B, When compression of ulnar pulse is released while continuing to compress radial artery, hand does not fill with blood. Pallor of right hand compared with left is consistent with distal arterial occlusive disease of the ulnar artery (right portion of image).
(Reproduced with permission from Olin JW, Lie JT: Current management of hypertension and vascular disease. In Cooke JP, Frohlich ED, editors: Thromboangiitis obliterans (Buerger’s disease), St. Louis, 1992, Mosby-Yearbook, p 65.)
Diagnostic Criteria
Several diagnostic criteria have been proposed for evaluating patients with suspected TAO. The Shionoya criteria require all five of the following to establish the diagnosis of TAO: history of smoking, onset before 50 years of age, infrapopliteal arterial occlusive disease, either upper extremity involvement or superficial thrombophlebitis, and absence of risk factors for atherosclerosis other than smoking.88
Papa and Adar proposed criteria that incorporated various clinical, angiographic, histopathological, and exclusionary elements, and then subsequently devised a point-scoring system for the diagnosis of TAO.89,90
Mills and Porter use a set of major and minor criteria for the diagnosis.91 Commonly used clinical criteria include age younger than 45, current or recent history of tobacco use, distal extremity ischemia confirmed by noninvasive testing, consistent angiographic findings, and exclusion of thrombophilia, autoimmune disease, diabetes mellitus, and a proximal source of emboli2 (Box 44-2).
![]() BOX 44-2 Common Criteria for the Diagnosis of Thromboangiitis Obliterans
BOX 44-2 Common Criteria for the Diagnosis of Thromboangiitis Obliterans
 Age younger than 45 years at disease onset
Age younger than 45 years at disease onset
 Current or recent history of tobacco use
Current or recent history of tobacco use
 Symptoms and signs of distal extremity ischemia with confirmation by noninvasive testing
Symptoms and signs of distal extremity ischemia with confirmation by noninvasive testing
 Consistent angiographic findings
Consistent angiographic findings
 Exclusion of thrombophilia, autoimmune disease, diabetes mellitus, and a proximal source of emboli
Exclusion of thrombophilia, autoimmune disease, diabetes mellitus, and a proximal source of emboli
 Supportive tissue biopsy in patients with unusual presentations such as large-artery involvement or age older than 45-50 years at symptom onset
Supportive tissue biopsy in patients with unusual presentations such as large-artery involvement or age older than 45-50 years at symptom onset
An increasing number of individuals who fulfill clinical criteria for TAO have risk factors for atherosclerotic vascular disease (e.g., hypertension, hyperlipidemia). A subset of these patients may subsequently develop concomitant atherosclerotic vascular disease after the original diagnosis of TAO. Accordingly, if patients meet criteria of distal extremity involvement, tobacco use, and exclusion of a proximal source of emboli, atherosclerosis, and thrombophilia, hyperlipidemia, or hypertension should not exclude the diagnosis of TAO.
Laboratory Evaluation
Although there are no specific blood tests to aid in the diagnosis, laboratory evaluation in patients with suspected TAO is useful for excluding alternative diagnoses. Initial laboratory studies should include a complete blood cell count (CBC), chemistry panel, liver function tests, fasting blood glucose, urinalysis, inflammatory markers such as erythrocyte sedimentation rate and C-reactive protein, cold agglutinins, and cryoglobulins. In addition, serological markers of autoimmune disease, including antinuclear antibody, rheumatoid factor, anticentromere antibody, and anti-SCL-70 antibody, should be obtained and are typically negative in patients with TAO. Evaluation for hypercoagulable states is frequently performed. Of note, antiphospholipid and anticardiolipin antibodies are detected in some patients with TAO but may also indicate an isolated thrombophilia.
Imaging
Imaging in patients with suspected TAO is not only used to establish the diagnosis but also to exclude alternative etiologies for the presentation that may require a radically different therapeutic approach. For example, echocardiography is frequently indicated to exclude a cardiac source of embolism that results in acute arterial occlusion. Likewise, catheter-based angiography provides evidence for TAO, but also excludes proximal sources of artery-to-artery embolism.
Noninvasive vascular laboratory studies such as segmental arterial pressure measurements with pulse volume recordings demonstrating distal abnormalities in the absence of proximal disease suggest a diagnosis of TAO. Digital plethysmography, finger and toe pressures, and transcutaneous oxygen measurement may be useful in documenting distal small-vessel disease in the absence of more proximal upper- or lower-extremity abnormalities in patients with TAO. Arterial duplex scanning can also be used to exclude proximal atherosclerotic lesions and identify distal arterial occlusive disease. “Corkscrew collaterals,” a finding frequently observed in patients with TAO, may also be visualized on arterial duplex scanning.92 Abdominal aortic ultrasonography may be used to exclude abdominal aortic aneurysm or atherosclerosis as a source of distal embolization to the lower extremities.
Computed tomography angiography (CTA), magnetic resonance angiography (MRA), or catheter-based angiography may be performed to exclude a proximal arterial source of embolism and define the anatomy and severity of distal arterial occlusive disease. Although advances in CTA and MRA have shown promise for imaging distal vessels, the majority of patients will require catheter-based angiography to provide the spatial resolution necessary to detect small-artery pathology, especially of the hands and feet. In patients with ischemic ulcerations and in whom secondary infection is a concern, magnetic resonance (MR) may be useful in determining the presence of osteomyelitis.93
Catheter-based angiography plays a critical role in establishing the diagnosis of TAO and excluding proximal arterial pathology that may result in distal arterial occlusive disease.94,95 Although there are no pathognomonic angiographic findings in TAO, angiography helps establish the diagnosis when taken in the context of a compatible clinical presentation. The classic angiographic picture of TAO consists of arterial occlusive disease confined to the distal circulation, most often infrapopliteal and infrabrachial, with proximal arteries free of atheroma, aneurysms, and other sources of emboli (Fig. 44-7). There are often areas of disease interspersed with normal-appearing vessels (skip areas). In the absence of diabetes, isolated arterial occlusive disease distal to the popliteal artery virtually never occurs in atherosclerosis. Commonly, angiographic abnormalities are observed in the digital arteries of the fingers and toes, the palmar and plantar arteries of the hands and feet, and the radial, ulnar, anterior tibial, posterior tibial, and peroneal arteries.96,97
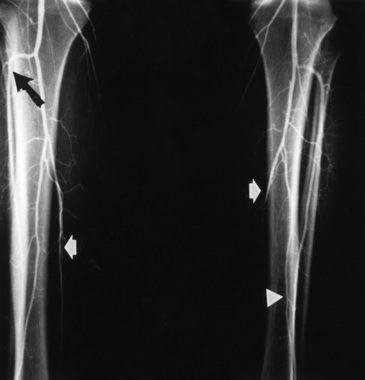
Figure 44-7 Catheter-based angiogram demonstrating severe infrapopliteal arterial occlusive disease in patient with thromboangiitis obliterans (TAO). In right leg, anterior tibial artery (black arrow) occludes just distal to its origin. Posterior tibial artery tapers and then occludes in the mid- to distal calf (white arrow). In left leg, anterior tibial artery is patent, but posterior tibial (white arrow) occludes several centimeters after its origin. Peroneal artery (arrowhead) tapers in mid-calf.
Distal small- to medium-artery involvement, segmental occlusions, and corkscrew-shaped collaterals around areas of occlusions are characteristic angiographic findings in TAO (Fig. 44-8). Corkscrew collaterals are not specific for TAO, however, and may be observed with any disease process that results in small-vessel occlusive disease. In particular, arterial occlusive disease due to scleroderma or CREST syndrome can closely mimic the angiographic appearance of TAO. Findings of arterial wall irregularity, vascular calcification, and proximal artery involvement should call a diagnosis of TAO into question.
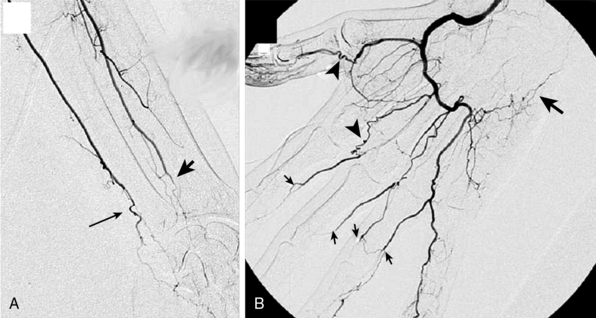
Figure 44-8 A, Catheter-based angiogram of left forearm and hand, demonstrating tapering occlusion of left radial artery (arrowhead) and ulnar artery (arrow) at the wrist. All proximal arteries were normal. B, Right hand of same patient demonstrated an occluded ulnar artery at the wrist (large arrow). A patent radial artery fills the deep palmar arch. Multiple segmental digital artery occlusions are present (small arrows). Numerous “corkscrew collaterals” (arrowheads), which represent collateralization around occluded segments, are visualized.
(Reproduced with permission from Olin JW, Shih A: Thromboangiitis obliterans (Buerger’s disease). Curr Opin Rheumatol 18:18–24, 2006.)
Role for Biopsy
Tissue biopsy is rarely required for the diagnosis of TAO unless the clinical presentation involves an unusual vascular territory or a patient older than 45 or 50 years of age at the onset of symptoms. Biopsy is most likely to be diagnostic when obtained from a vein with superficial thrombophlebitis during the acute phase of the disease. A highly inflammatory thrombus, relative sparing of the blood vessel wall, and preservation of the internal elastic lamina in arterial biopsies are characteristic histological findings in TAO.
Prognosis
Prognosis for patients with TAO greatly depends on their ability to discontinue tobacco use. In a retrospective series of 110 patients with TAO (106 men, 4 women; mean age, 40 years), the natural history of the disease was compared in those who quit smoking and those who did not.98 Seven of 110 study patients (6%) died during a mean follow-up of 10.6 years. Forty-seven patients (43%) underwent 108 amputation procedures, including either major amputation (13 patients) or minor amputation (34 patients) of an upper or lower extremity. Of 69 patients who continued smoking, 13 patients (19%) underwent major amputation. None of those who stopped smoking underwent amputation. Continued smoking correlated with the risk of limb amputation (P = 0.007).
Quality of life is substantially diminished in patients with TAO.98,99 In the retrospective analysis referenced above, 11 of 13 patients (85%) who underwent major lower-limb amputation lost their jobs, compared to only 9 of 97 patients (9%) without major amputation.98 Limb amputation strongly correlated with job loss (P < 0.0001).
Management
Although various options exist for TAO management, discontinuation of tobacco use is the definitive and most effective therapy for the disease (Box 44-3). Efficacy of alternative therapies in TAO is profoundly limited in the setting of ongoing tobacco use.
![]() BOX 44-3 Therapeutic Options for the Management of Thromboangiitis Obliterans
BOX 44-3 Therapeutic Options for the Management of Thromboangiitis Obliterans
Tobacco Cessation
Again, the cornerstone of therapy for TAO is total discontinuation of any tobacco use (Box 44-4). Even a few cigarettes a day may drive disease progression and culminate in amputation.1,17,100,101 Patients should be counseled to abstain from using smokeless tobacco as well as smoking marijuana; both have been associated with TAO.21–23 Patient education on the role of tobacco exposure in initiation, maintenance, and progression of TAO is of paramount importance. Adjunctive measures to assist in discontinuing tobacco use (e.g., pharmacotherapy, smoking-cessation groups) should be made available to all patients with TAO. However, nicotine replacement therapy should be avoided in these patients because it may contribute to disease activity. Agents such as bupropion and varenicline may be preferred as smoking-cessation aids in patients with TAO. Although it remains unclear whether passive smoke exposure can cause TAO, patients with active disease should be advised to avoid second-hand smoke as much as possible.
![]() Box 44-4 Pearls for Tobacco Cessation in Patients with Thromboangiitis Obliterans
Box 44-4 Pearls for Tobacco Cessation in Patients with Thromboangiitis Obliterans
 Educate patients on the critical role of tobacco in the initiation, maintenance, and progression of thromboangiitis obliterans (TAO).
Educate patients on the critical role of tobacco in the initiation, maintenance, and progression of thromboangiitis obliterans (TAO).
 Counsel patients and members of their households about the role of secondhand smoke exposure in perpetuating the disease process.
Counsel patients and members of their households about the role of secondhand smoke exposure in perpetuating the disease process.
 Ask the patient at every office visit if they have been successful in tobacco cessation. This approach lets the patient know that tobacco cessation is the single most important aspect in treating this disease.
Ask the patient at every office visit if they have been successful in tobacco cessation. This approach lets the patient know that tobacco cessation is the single most important aspect in treating this disease.
 Explain to patients the limited efficacy of alternative therapies for TAO in the absence of complete and continued tobacco cessation.
Explain to patients the limited efficacy of alternative therapies for TAO in the absence of complete and continued tobacco cessation.
 Measure urinary nicotine, cotinine, and cannabis in patients who continue to have signs and symptoms consistent with active disease, despite claims of tobacco cessation.
Measure urinary nicotine, cotinine, and cannabis in patients who continue to have signs and symptoms consistent with active disease, despite claims of tobacco cessation.
 Offer adjunctive therapies such as pharmacotherapy and smoking-cessation groups to assist with discontinuation of tobacco use.
Offer adjunctive therapies such as pharmacotherapy and smoking-cessation groups to assist with discontinuation of tobacco use.
 Prescribe bupropion and varenicline as the preferred pharmacological adjuncts to assist in tobacco cessation because nicotine replacement therapy may contribute to continued disease activity.
Prescribe bupropion and varenicline as the preferred pharmacological adjuncts to assist in tobacco cessation because nicotine replacement therapy may contribute to continued disease activity.
It has been stated in the past that patients with TAO have a greater degree of tobacco dependence than those with atherosclerotic cardiovascular disease, but this is not an accurate assumption. Patients with TAO may have a higher frequency of tobacco cessation than those with atherosclerotic vascular disease. In a study of 112 patients with TAO followed over a mean of 92 months, 43 (48%) patients stopped smoking for a mean of 80 months.17 A case-control study compared 103 patients with TAO confirmed on angiography, biopsy, or noninvasive testing with 273 patients with coronary artery disease (CAD) confirmed by angiography to determine patterns of tobacco dependence.102 Degree of tobacco dependence in each group was ascertained by questionnaire. Kaplan-Meier curves demonstrated no significant difference in time to tobacco cessation after initial diagnosis. Patients with TAO smoked fewer cigarettes per day than those with CAD (22.3 ± 10.7 vs. 27.7 ± 15.3 cigarettes/day, P = 0.003). Among 170 current smokers in the analysis, patients with TAO smoked fewer cigarettes/day (20.2 ± 8.2 vs. 24.6 ± 12.7, P = 0.03) and were more likely to have made a serious attempt to quit smoking (97% vs. 90%, P = 0.03). Based on these data, the study investigators concluded that patients with TAO did not appear to have greater tobacco dependence than CAD patients.
Patients with TAO should be reassured that if they are able to discontinue tobacco use, the disease will become quiescent and the risk of amputation will greatly diminish, provided critical limb ischemia is not present. If significant arterial occlusive disease has developed, symptoms of intermittent claudication and secondary vasospasm (Raynaud phenomenon) may continue but should not progress. Alternative therapies such as vasodilators may help reduce symptoms in such patients.
Vasodilators
The use of vasodilators in patients with TAO is largely palliative. The most extensive clinical experience with vasodilators in TAO comes from trials evaluating the prostacyclin analog iloprost. In a prospective randomized double-blind trial, 133 patients with TAO and critical limb ischemia were randomly allocated to receive iloprost or low-dose aspirin for 28 days.103 Lower-extremity ischemic ulcerations were present in 98 patients. At 1-month follow-up, 58 (85%) of 68 iloprost-treated patients showed ulcer healing or relief of rest pain, compared with 11 (17%) of 65 in the aspirin-treated group. Compared with 18 (28%) on aspirin, 43 (63%) treated with iloprost had complete relief of pain. Ulcers healed completely in 18 of 52 (35%) treated with iloprost, compared with 6 of 46 (13%) who received aspirin. At 6-month follow-up, the response rate was 45 of 51 (88%) patients treated with iloprost, compared with 12 of 44 (21%) patients treated with aspirin.
A pharmacokinetic study demonstrated that an oral extended-release preparation of iloprost had pharmacological equivalence to the intravenous formulation in patients with TAO.104 Based on these findings, a double-blind randomized trial comparing oral iloprost with placebo was conducted in 319 TAO patients with rest pain, trophic lesions, or both.105 The primary study end point was total healing of the most important lesion; a secondary end point was relief of rest pain without need of analgesic medications. A combined end point included amputation- free survival, absence of trophic lesions and rest pain, and need for analgesic medications. Total healing of trophic lesions was not significantly different between study groups at any time point. Low-dose oral iloprost was significantly more effective than placebo at end of follow-up in relieving rest pain, without the need for analgesic medications, and improving the benefit over placebo. Based on these studies, iloprost may be considered for patients with TAO who have critical limb ischemia and require symptomatic relief early in the treatment period while they discontinue tobacco use.
Phosphodiesterase (PDE) inhibitors with vasodilator properties have the potential to play a role in the management of TAO, but require evaluation in prospective trials. Although not specifically described in patients with TAO, cilostazol has been reported to aid in healing ischemic ulcerations in patients who were ineligible for revascularization.106,107 Although it is helpful in treating claudication due to atherosclerotic peripheral vascular disease, clinical experience with cilostazol for this indication in patients with TAO is limited. Sildenafil may represent another option in this drug class for patients with TAO, but requires investigation.
Other vasodilators such as α-adrenergic receptor antagonists, calcium channel antagonists, and transdermal nitrates may be helpful in patients who experience vasospasm, but these agents have not been studied in prospective clinical trials.
Periarterial Sympathectomy and Sympathetic Blockade
Peripheral periarterial sympathectomy may be considered for patients with refractory pain and digital ischemia due to TAO but remains controversial. Sympathectomy has anecdotally been reported to occasionally assist the healing of ischemic ulcerations, but a series from the Cleveland Clinic demonstrated no difference in amputation rate in patients undergoing the procedure compared to those who did not.17 In a single case report, intravenous regional sympathetic blockade (Bier block) with guanethidine and lidocaine increased finger blood flow and resulted in complete disappearance of fingertip ischemic ulcerations and rest pain in a patient with advanced TAO.108
Spinal Cord Stimulation
Epidural spinal cord stimulation has been evaluated in a limited number of patients to decrease ischemic pain and avoid amputation when revascularization is not feasible and other therapeutic interventions have not been effective.109–112 In a retrospective study, 29 patients were evaluated to determine the effect of epidural spinal cord stimulation in the treatment of TAO.113 The regional perfusion index (ratio between foot and chest transcutaneous oxygen pressure) at baseline was 0.27 ± 0.25. Three months after spinal cord stimulation implantation, the regional perfusion index increased to 0.41 ± 0.22. During the 1- and 3-year follow-up period, sustained improvement in microcirculation was recorded. The most pronounced improvement in regional perfusion index values was observed in the subgroup of 13 patients with trophic lesions. In this group, the regional perfusion index increased significantly from 0.17 ± 0.21 to 0.4 ± 0.18 (P < 0.02) after a mean follow-up of 5.7 years. Limb survival rate was 93.1%.
Intermittent Pneumatic Compression
Intermittent pneumatic compression of the foot and calves has been used to augment perfusion to the lower extremities in patients with severe claudication or critical limb ischemia who are not candidates for revascularization because of advanced distal arterial occlusive disease, including those with TAO. In a retrospective study at the Mayo Clinic, the effect of intermittent pneumatic compression on nonhealing wounds was evaluated in 101 patients with critical limb ischemia and lower-extremity ulcerations.114 Of all ulcerations, 64% were multifactorial in etiology, and 60% had associated transcutaneous oxygen tension levels below 20 mmHg. Patients were instructed to use the intermittent compression device on the affected limbs for 6 hours daily. Complete wound healing with limb preservation was achieved in 40% of patients with transcutaneous oxygen tension levels below 20 mmHg, 48% with osteomyelitis or active wound infection, 46% with insulin-requiring diabetes mellitus, and 28% with a previous amputation. Intermittent pneumatic compression appears to be most beneficial for patients with distal arterial occlusive disease and in whom revascularization is not feasible.
Therapeutic Angiogenesis and Cell-Based Therapy
A limited number of options for patients with severe distal arterial occlusive disease and critical limb ischemia due to TAO has driven a growing interest in therapeutic angiogenesis. In a study of seven limbs in six patients with TAO and critical limb ischemia, direct intramuscular injection of naked plasmid DNA-encoding vascular endothelial growth factor (VEGF) resulted in complete healing of ischemic ulcerations that were nonhealing for more than 1 month in three of five limbs.115 Nocturnal rest pain was relieved in the remaining two patients with ulcerations. Evidence of improved perfusion to the distal ischemic limb included an increase of more than 0.1 in the ankle:brachial index in three limbs, improved flow shown by MR imaging in all seven limbs studied, and newly formed collateral vessels demonstrated on serial catheter-based angiography in all seven limbs studied. Two patients with advanced distal-extremity gangrene ultimately required below-knee amputation despite evidence of improved perfusion. The efficacy of therapeutic angiogenesis with VEGF gene transfer for patients with TAO requires confirmation in a prospective controlled trial.
Several studies have evaluated autologous bone marrow mononuclear cell implantation for patients with critical limb ischemia due to TAO.116–119 Although short-term results with autologous bone marrow mononuclear cell implantation have been promising, long-term safety and efficacy remain to be demonstrated.120 Autologous whole bone marrow stem cell transplantation may represent another promising avenue for therapeutic angiogenesis in patients with TAO.121,122
Revascularization Strategies
Endovascular therapy
Endovascular therapy for arterial revascularization in patients with TAO remains controversial. Selective intraarterial infusion of fibrinolytic therapy has been reported as an adjunctive treatment in these patients.123–126 In one series, selective low-dose intraarterial streptokinase (10,000 unit bolus followed by 5000 units/h infusion) was administered to 11 patients with TAO of the lower limbs that caused variable degrees of gangrene or pregangrene of the toes or feet, and who had no other possible therapeutic options except major amputation.125 The investigators noted the overall success rate (defined as an altered or avoided amputation) to be 58.3%. Notably, bleeding complications were observed in 16.6% of the total at-risk limbs included in the study.
The efficacy of intraarterial fibrinolysis for TAO may not be as high as initially reported. From a pathological standpoint, the highly inflammatory thrombus observed in TAO is quickly invaded by fibroblasts and subsequently organized, making it quite resistant to fibrinolysis. In patients facing amputation and in whom no other alternatives for revascularization exist, a short trial of intraarterial fibrinolysis may be reasonable to avoid amputation in the absence of contraindications.
Other percutaneous techniques, including angioplasty and stent placement, have a very limited role in TAO treatment because of the distal and small-vessel nature of the disease.
Surgical revascularization
Surgical revascularization is usually not possible in patients with TAO because of the distal and diffuse nature of the disease, with extremely poor runoff. In addition, there is rarely a suitable distal target vessel for bypass. Short- and long-term patency rates are poor. Superficial thrombophlebitis of the lower extremities frequently limits the number and quality of venous conduits available for bypass surgery. However, surgical bypass using autologous vein may be considered in selected patients with severe ischemia, suitable distal target vessels, and good-quality venous conduits. In a series of 236 patients with TAO, only 11 (4.6%) had occlusive lesions that were amenable to surgery.127 In a retrospective study of 101 patients with TAO who were followed for a mean of 10.6 years, outcomes after surgical bypass were often suboptimal, with primary patency rates of 41%, 32%, and 30% and secondary patency rates of 54%, 47%, and 39% at 1, 5, and 10 years, respectively.98 Graft patency rates are nearly 50% lower in TAO patients who continue to smoke after surgical revascularization.128 For reasons already mentioned, lower-extremity bypass surgery in patients with TAO is rarely carried out in the United States.1–3,17–19
Long-term patency of surgical bypass grafts is limited, but short-term patency may be sufficient to allow healing of ischemic ulcerations due to TAO and preservation of the at-risk limb. In a study of 94 patients with TAO, 27 of 36 (81%) patients who were eligible for surgery underwent revascularization.129 During 36-month follow-up, patency rates at 12, 24, and 36 months were 59.2%, 48%, and 33.3%, respectively. Despite these low patency rates, limb salvage rate was 92.5%.
Another surgical option for patients with TAO consists of omental transfer.130–134 In a study of 50 patients with TAO who underwent omental transfer, all had intermittent claudication, and 40 had evidence of critical limb ischemia such as rest pain, non-healing ulcers, or gangrene.134 All patients demonstrated improved skin temperature, 36 reported improved rest pain, and 48 noted increased claudication-free walking distance. Ischemic ulcerations healed in 32 of 36 patients. Despite these data, omental transfer has not been adopted by most major centers. The reason for this remains unclear but may be due to the lack of published data from centers outside of India where the technique was pioneered.
Unfortunately for a subset of patients with TAO, amputation is necessary to treat refractory rest pain or prevent progression of local infection, including osteomyelitis. In a registry of 111 patients with TAO followed for a mean of 15.6 years at the Mayo Clinic, risk of any amputation was reported to be 25% at 5 years, 38% at 10 years, and 46% at 20 years.99 Risk of major amputation was observed to be 11% at 5 years, 21% at 10 years, and 23% at 20 years. Amputation rate was substantially reduced among patients who discontinued tobacco use compared with those who did not. The authors noted that the increased risk of amputation in former smokers was eliminated by 8 years after tobacco cessation.
Local Wound Care
For patients with areas of frank or threatened ischemic ulceration due to TAO, local wound care is of paramount importance. Consultation with wound care specialists can provide recommendations for dressings and other local interventions to aid wound healing. In addition, wound care specialists can educate patients about daily care and warning signs of progression or infection. In patients with more advanced ischemic ulcerations or gangrene, local débridement and appropriate antibiotic therapy may be required. Vacuum-assisted wound closure may be promising in patients with TAO and ischemic ulcerations, but requires further investigation.135
Supportive Care
Supportive care in patients with TAO and ischemic rest pain or ulcerations is identical to that for patients with critical limb ischemia due to any other arterial occlusive disease. A reverse Trendelenburg position should be used in patients who have severe ischemic rest pain. Adequate analgesia, with narcotics if required, should be used to manage periods of severe ischemic pain. Maintenance of central and peripheral warmth is crucial to reduce cold-induced vasospasm. Meticulous skin care of the hands and feet is important to prevent new ulcerations.
Unproven Therapies
Other alternative therapies that remain unproven in the treatment of patients with TAO include antiplatelet agents, anticoagulants, and hyperbaric oxygen therapy. Although they may be prescribed on an individual basis, the role of antiplatelet agents such as aspirin and clopidogrel has not been established in TAO. Likewise, therapeutic anticoagulation has never been shown to be effective in TAO treatment. Despite this, some clinicians have used anticoagulation in an effort to delay amputation and improve collateral flow in severe critical limb ischemia. A short 30- to 45-day course of anticoagulation may also be used in patients with severe symptoms due to superficial thrombophlebitis.136,137 Pentoxifylline increases red blood cell membrane flexibility and has been used with limited benefit in patients with claudication due to atherosclerotic peripheral vascular disease of the lower extremities. Its role, if any, in TAO remains to be defined. Hyperbaric oxygen therapy has shown promise in the healing of cutaneous wounds due to a variety of disorders but has not been evaluated in treating TAO patients. Although they have pleomorphic effects that include modulation of inflammatory pathways, which may benefit patients with TAO, the role of statins in managing this disorder is unclear.
Overall Therapeutic Algorithm
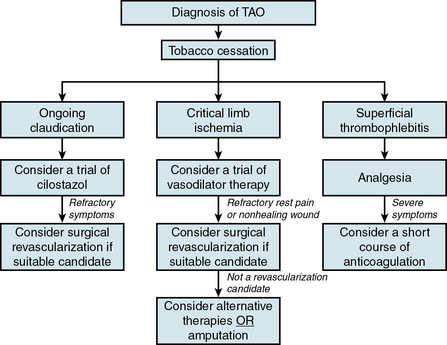
An overall therapeutic algorithm for patients with TAO emphasizes tobacco cessation and then addresses symptoms based on extent of arterial and venous occlusive disease (Fig. 44-9).
Future Perspectives
Although the pathophysiology of TAO is not completely understood, the role of tobacco use in disease initiation and activity is indisputable. Continued population- and individual-based efforts to decrease the frequency of tobacco use will not only prevent TAO but also other smoking-related illnesses such as lung cancer, chronic obstructive pulmonary disease, and atherosclerotic cardiovascular disease.
For patients who are unable to discontinue tobacco use or for those who require palliative therapy to help them get through an episode of critical limb ischemia, more effective therapeutic alternatives would be beneficial. Gene therapy or cell-based therapy holds the greatest promise in this regard. Prospective randomized trials will have to be conducted to determine whether therapeutic angiogenesis is in fact a useful treatment strategy for patients with TAO. Since TAO is a vasculitis characterized by inflammatory thrombus, improved understanding of the mechanisms of inflammation in this disease as well as medications that modulate vascular inflammation may result in more effective therapy for this disorder.
1 Piazza G., Creager M.A. Thromboangiitis obliterans. Circulation. 2010;121:1858–1861.
2 Olin J.W. Thromboangiitis obliterans (Buerger’s disease). N Engl J Med. 2000;343:864–869.
3 Mills J.L.Sr. Buerger’s disease in the 21st century: diagnosis, clinical features, and therapy. Semin Vasc Surg. 2003;16:179–189.
4 von Winiwarter F. Ueber eine eigenthumliche form von endarteritis und endophlebitis mit gangran des fusses. Arch Klin Chir. 1879;23:202–225.
5 Buerger L. Thrombo-angiitis obliterans: a study of the vascular lesions leading to presenile spontaneous gangrene. Am J Med Sci. 1908;136:567–580.
6 Allen E.V., Brown G.E. Thrombo-angiitis obliterans: a clinical study of 200 cases. Ann Intern Med. 1928;1:535–549.
7 Lie J.T. The rise and fall and resurgence of thromboangiitis obliterans (Buerger’s disease). Acta Pathol Jpn. 1989;39:153–158.
8 Lie J.T. Thromboangiitis obliterans (Buerger’s disease) revisited. Pathol Annu. 1988;23(Pt 2):257–291.
9 Herman B.E. Buerger’s syndrome. Angiology. 1975;26:713–716.
10 Cachovan M. Epidemiologie und geographisches verteilungsmuster der thromboangiitis obliterans. In: Heidrich J., ed. Thromboangiitis obliterans morbus Winiwarter-Buerger. Stuttgart, New York: George Thieme; 1988:31–36.
11 Matsushita M., Nishikimi N., Sakurai T., et al. Decrease in prevalence of Buerger’s disease in Japan. Surgery. 1998;124:498–502.
12 Laohapensang K., Rerkasem K., Kattipattanapong V. Decrease in the incidence of Buerger’s disease recurrence in northern Thailand. Surg Today. 2005;35:1060–1065.
13 Kobayashi M., Nishikimi N., Komori K. Current pathological and clinical aspects of Buerger’s disease in Japan. Ann Vasc Surg. 2006;20:148–156.
14 McKusick V.A., Harris W.S. The Buerger syndrome in the Orient. Bull Johns Hopkins Hosp; 1961.
15 Hill G.L., Moeliono J., Tumewu F., et al. The Buerger syndrome in Java A description of the clinical syndrome and some aspects of its aetiology. Br J Surg. 1973;60:606–613.
16 Nishikimi N., Shionoya S., Mizuno S. Result of national epidemiological study of Buerger’s disease. J Jpn Coll Angiol. 1987;27:1125–1130.
17 Olin J.W., Young J.R., Graor R.A., et al. The changing clinical spectrum of thromboangiitis obliterans (Buerger’s disease). Circulation. 1990;82:IV3–IV8.
18 Mills J.L., Taylor L.M.Jr, Porter J.M. Buerger’s disease in the modern era. Am J Surg. 1987;154:123–129.
19 Lie J.T. Thromboangiitis obliterans (Buerger’s disease) in women. Medicine (Baltimore). 1987;66:65–72.
20 Leu H.J. Buerger’s thromboangiitis obliterans. Pathologico-anatomical analysis of 53 cases. Schweiz Med Wochenschr. 1985;115:1080–1086.
21 Lie J.T. Thromboangiitis obliterans (Buerger’s disease) and smokeless tobacco. Arthritis Rheum. 1988;31:812–813.
22 Disdier P., Granel B., Serratrice J., et al. Cannabis arteritis revisited–ten new case reports. Angiology. 2001;52:1–5.
23 Combemale P., Consort T., Denis-Thelis L., et al. Cannabis arteritis. Br J Dermatol. 2005;152:166–169.
24 Olin J.W., Shih A. Thromboangiitis obliterans (Buerger’s disease). Curr Opin Rheumatol. 2006;18:18–24.
25 Kjeldsen K., Mozes M. Buerger’s disease in Israel. Investigations on carboxyhemoglobin and serum cholesterol levels after smoking. Acta Chir Scand. 1969;135:495–498.
26 Westcott F.N., Wright I.S. Tobacco allergy and thromboangiitis obliterans. J Allergy. 1938;9:555–564.
27 Harkavy J. Tobacco sensitivities in thromboangiitis obliterans, migratory phlebitis, and coronary artery disease. Bull N Y Acad Med. 1933;9:318–322.
28 Rahman M., Chowdhury A.S., Fukui T., et al. Association of thromboangiitis obliterans with cigarette and bidi smoking in Bangladesh: a case-control study. Int J Epidemiol. 2000;29:266–270.
29 Matsushita M., Shionoya S., Matsumoto T. Urinary cotinine measurement in patients with Buerger’s disease–effects of active and passive smoking on the disease process. J Vasc Surg. 1991;14:53–58.
30 McLoughlin G.A., Helsby C.R., Evans C.C., et al. Association of HLA-A9 and HLA-B5 with Buerger’s disease. BMJ. 1976;2:1165–1166.
31 Papa M., Bass A., Adar R., et al. Autoimmune mechanisms in thromboangiitis obliterans (Buerger’s disease): the role of tobacco antigen and the major histocompatibility complex. Surgery. 1992;111:527–531.
32 Chen Z., Takahashi M., Naruse T., et al. Synergistic contribution of CD14 and HLA loci in the susceptibility to Buerger disease. Hum Genet. 2007;122:367–372.
33 Glueck C.J., Haque M., Winarska M., et al. Stromelysin-1 5A/6A and eNOS T-786C polymorphisms, MTHFR C677T and A1298C mutations, and cigarette-cannabis smoking: a pilot, hypothesis-generating study of gene-environment pathophysiological associations with Buerger’s disease. Clin Appl Thromb Hemost. 2006;12:427–439.
34 Adiguzel Y., Yilmaz E., Akar N. Effect of eNOS and ET-1 polymorphisms in thromboangiitis obliterans. Clin Appl Thromb Hemost. 2010;16:103–106.
35 Choudhury N.A., Pietraszek M.H., Hachiya T., et al. Plasminogen activators and plasminogen activator inhibitor 1 before and after venous occlusion of the upper limb in thromboangiitis obliterans (Buerger’s disease). Thromb Res. 1992;66:321–329.
36 Pietraszek M.H., Choudhury N.A., Koyano K., et al. Enhanced platelet response to serotonin in Buerger’s disease. Thromb Res. 1990;60:241–246.
37 Carr M.E.Jr. Hackney MH, Hines SJ, et al: Enhanced platelet force development despite drug-induced inhibition of platelet aggregation in patients with thromboangiitis obliterans–two case reports. Vasc Endovascular Surg. 2002;36:473–480.
38 Brodmann M., Renner W., Stark G., et al. Prothrombotic risk factors in patients with thrombangitis obliterans. Thromb Res. 2000;99:483–486.
39 Avcu F., Akar E., Demirkilic U., et al. The role of prothrombotic mutations in patients with Buerger’s disease. Thromb Res. 2000;100:143–147.
40 Olin J.W., Childs M.B., Bartholomew J.R., et al. Anticardiolipin antibodies and homocysteine levels in patients with thromboangiitis obliterans. Arthritis Rheum. 1996;39:S-47.
41 Olin J.W. Are anticardiolipin antibodies really important in thromboangiitis obliterans (Buerger’s disease)? Vasc Med. 2002;7:257–258.
42 Maslowski L., McBane R., Alexewicz P., et al. Antiphospholipid antibodies in thromboangiitis obliterans. Vasc Med. 2002;7:259–264.
43 Adar R., Papa M.Z., Halpern Z., et al. Cellular sensitivity to collagen in thromboangiitis obliterans. N Engl J Med. 1983;308:1113–1116.
44 Roncon de Albuquerque R., Delgado L., Correia P., et al. Circulating immune complexes in Buerger’s disease. Endarteritis obliterans in young men. J Cardiovasc Surg (Torino). 1989;30:821–825.
45 Gulati S.M., Saha K., Kant L., et al. Significance of circulatory immune complexes in thromboangiitis obliterans (Buerger’s disease). Angiology. 1984;35:276–281.
46 Kobayashi M., Ito M., Nakagawa A., et al. Immunohistochemical analysis of arterial wall cellular infiltration in Buerger’s disease (endarteritis obliterans). J Vasc Surg. 1999;29:451–458.
47 Lee T., Seo J.W., Sumpio B.E., et al. Immunobiologic analysis of arterial tissue in Buerger’s disease. Eur J Vasc Endovasc Surg. 2003;25:451–457.
48 Kurata A., Machinami R., Schulz A., et al. Different immunophenotypes in Buerger’s disease. Pathol Int. 2003;53:608–615.
49 Eichhorn J., Sima D., Lindschau C., et al. Antiendothelial cell antibodies in thromboangiitis obliterans. Am J Med Sci. 1998;315:17–23.
50 Makita S., Nakamura M., Murakami H., et al. Impaired endothelium-dependent vasorelaxation in peripheral vasculature of patients with thromboangiitis obliterans (Buerger’s disease). Circulation. 1996;94:II211–II215.
51 Idei N., Nishioka K., Soga J., et al. Vascular function and circulating progenitor cells in thromboangiitis obliterans (Buerger’s disease) and atherosclerosis obliterans. Hypertension. 2011;57:70–78.
52 Halacheva K., Gulubova M.V., Manolova I., et al. Expression of ICAM-1, VCAM-1, E-selectin and TNF-alpha on the endothelium of femoral and iliac arteries in thromboangiitis obliterans. Acta Histochem. 2002;104:177–184.
53 Iwai T., Inoue Y., Umeda M., et al. Oral bacteria in the occluded arteries of patients with Buerger disease. J Vasc Surg. 2005;42:107–115.
54 Dible J.H. The pathology of limb ischemia. Edinburgh: Oliver & Boyd; 1966.
55 Leu H.J., Bollinger A. Migrating phlebitis. Vasa. 1978;7:440–442.
56 Fazeli B., Modaghegh H., Ravrai H., et al. Thrombophlebitis migrans as a footprint of Buerger’s disease: a prospective-descriptive study in north-east of Iran. Clin Rheumatol. 2008;27:55–57.
57 Wang M.H., Jin X., Wu X.J., et al. Phosphatidylcholine-specific phospholipase C activity and level increase evidently in thromboangiitis obliterans. Biofactors. 2010;36:196–200.
58 Shionoya S., Ban I., Nakata Y., et al. Involvement of the iliac artery in Buerger’s disease (pathogenesis and arterial reconstruction). J Cardiovasc Surg (Torino). 1978;19:69–76.
59 Ates A., Yekeler I., Ceviz M., et al. One of the most frequent vascular diseases in northeastern of Turkey: thromboangiitis obliterans or Buerger’s disease (experience with 344 cases). Int J Cardiol. 2006;111:147–153.
60 Shionoya S. Buerger’s disease (thromboangiitis obliterans). In: Rutherford R.B., ed. Vascular surgery. ed 3. Philadelphia: WB Saunders; 1989:207–217.
61 Sasaki S., Sakuma M., Kunihara T., et al. Distribution of arterial involvement in thromboangiitis obliterans (Buerger’s disease): results of a study conducted by the Intractable Vasculitis Syndromes Research Group in Japan. Surg Today. 2000;30:600–605.
62 Tavakoli H., Rezaii J., Esfandiari K., et al. Buerger’s disease: a 10-year experience in Tehran, Iran. Clin Rheumatol. 2008;27:369–371.
63 Yamamoto K., Iwase S., Mano T., et al. Muscle sympathetic outflow in Buerger’s disease. J Auton Nerv Syst. 1993;44:67–75.
64 Kimura T., Yoshizaki S., Tsushima N., et al. Buerger’s colour. Br J Surg. 1990;77:1299–1301.
65 Papa M.Z., Adar R. A critical look at thromboangiitis obliterans (Buerger’s disease). Vasc Surg. 1992;5:1–21.
66 Alpaslan M., Akgun G., Doven O., et al. Thrombus in the main pulmonary artery of a patient with thromboangiitis obliterans: observation by transthoracic echocardiography. Eur J Echocardiogr. 2001;2:139–140.
67 Siddiqui M.Z., Reis E.D., Soundararajan K., et al. Buerger’s disease affecting mesenteric arteries: a rare cause of intestinal ischemia–a case report. Vasc Surg. 2001;35:235–238.
68 Leung D.K., Haskal Z.J. SIR 2006 film panel case: mesenteric involvement and bowel infarction due to Buerger disease. J Vasc Interv Radiol. 2006;17:1087–1089.
69 Kobayashi M., Kurose K., Kobata T., et al. Ischemic intestinal involvement in a patient with Buerger disease: case report and literature review. J Vasc Surg. 2003;38:170–174.
70 Hassoun Z., Lacrosse M., De Ronde T. Intestinal involvement in Buerger’s disease. J Clin Gastroenterol. 2001;32:85–89.
71 Cho Y.P., Kwon Y.M., Kwon T.W., et al. Mesenteric Buerger’s disease. Ann Vasc Surg. 2003;17:221–223.
72 Cho Y.P., Kang G.H., Han M.S., et al. Mesenteric involvement of acute-stage Buerger’s disease as the initial clinical manifestation: report of a case. Surg Today. 2005;35:499–501.
73 Zulch K.J. The cerebral form of von Winiwarter-Buerger’s disease: does it exist? Angiology. 1969;20:61–69.
74 No Y.J., Lee E.M., Lee D.H., et al. Cerebral angiographic findings in thromboangiitis obliterans. Neuroradiology. 2005;47:912–915.
75 Lippmann H.I. Cerebrovascular thrombosis in patients with Buerger’s disease. Circulation. 1952;5:680–692.
76 Bozikas V.P., Vlaikidis N., Petrikis P., et al. Schizophrenic-like symptoms in a patient with thrombo-angiitis obliterans (Winiwarter-Buerger’s disease). Int J Psychiatry Med. 2001;31:341–346.
77 Berlit P., Kessler C., Reuther R., et al. New aspects of thromboangiitis obliterans (von Winiwarter-Buerger’s disease). Eur Neurol. 1984;23:394–399.
78 Tamura A., Aso N., Kadota J. Corkscrew appearance in the right coronary artery in a patient with Buerger’s disease. Heart. 2006;92:944.
79 Hsu P.C., Lin T.H., Su H.M., et al. Frequent accelerated idioventricular rhythm in a young male of Buerger’s disease with acute myocardial infarction. Int J Cardiol. 2008;127:e64–e66.
80 Hoppe B., Lu J.T., Thistlewaite P., et al. Beyond peripheral arteries in Buerger’s disease: angiographic considerations in thromboangiitis obliterans. Catheter Cardiovasc Interv. 2002;57:363–366.
81 Hong T.E., Faxon D.P. Coronary artery disease in patients with Buerger’s disease. Rev Cardiovasc Med. 2005;6:222–226.
82 Abe M., Kimura T., Furukawa Y., et al. Coronary Buerger’s disease with a peripheral arterial aneurysm. Eur Heart J. 2007;28:928.
83 Goktas S., Bedir S., Bozlar U., et al. Intrarenal arterial stenosis in a patient with thromboangiitis obliterans. Int J Urol. 2006;13:1243–1244.
84 Yavas U.S., Calisir C., Kaya T. Vasculogenic impotence as a symptom in late-onset Buerger’s disease. J Clin Ultrasound. 2007;35:469–472.
85 Lie J.T., Michet C.J.Jr. Thromboangiitis obliterans with eosinophilia (Buerger’s disease) of the temporal arteries. Hum Pathol. 1988;19:598–602.
86 Flammer J., Pache M., Resink T. Vasospasm, its role in the pathogenesis of diseases with particular reference to the eye. Prog Retin Eye Res. 2001;20:319–349.
87 Lie J.T. Thromboangiitis obliterans (Buerger’s disease) in a saphenous vein arterial graft. Hum Pathol. 1987;18:402–404.
88 Shionoya S. What is Buerger’s disease? World J Surg. 1983;7:544–551.
89 Papa M.Z., Rabi I., Adar R. A point scoring system for the clinical diagnosis of Buerger’s disease. Eur J Vasc Endovasc Surg. 1996;11:335–339.
90 Adar R., Papa M.Z., Schneiderman J. Thromboangiitis obliterans: an old disease in need of a new look. Int J Cardiol. 2000;75(Suppl 1):S167–S170. discussion S171–S163
91 Mills J.L., Porter J.M. Buerger’s disease: a review and update. Semin Vasc Surg. 1993;6:14–23.
92 Fujii Y., Nishioka K., Yoshizumi M., et al. Images in cardiovascular medicine. Corkscrew collaterals in thromboangiitis obliterans (Buerger’s disease). Circulation. 2007;116:e539–e540.
93 Jaccard Y., Walther S., Anderson S., et al. Influence of secondary infection on amputation in chronic critical limb ischemia. Eur J Vasc Endovasc Surg. 2007;33:605–609.
94 Olin J.W., Lie J.T. Thromboangiitis obliterans (Buerger’s disease). In: Cooke J.P., Frohlich E.D. Current management of hypertension and vascular disease. St. Louis: Mosby-Yearbook; 1992:265–271.
95 Lambeth J.T., Yong N.K. Arteriographic findings in thromboangiitis obliterans with emphasis on femoropopliteal involvement. Am J Roentgenol Radium Ther Nucl Med. 1970;109:553–562.
96 McKusick V.A., Harris W.S., Ottsen O.E. Buerger’s disease: a distinct clinical and pathologic entity. JAMA. 1962;181:93–100.
97 McKusick V.A., Harris W.S., Ottsen O.E. The Buerger’s syndrome in the United States: arteriographic observations with special reference to involvement of the upper extremities and the differentiation from atherosclerosis and embolism. Bull Johns Hopkins Hosp. 1962;110:145–176.
98 Ohta T., Ishioashi H., Hosaka M., et al. Clinical and social consequences of Buerger disease. J Vasc Surg. 2004;39:176–180.
99 Cooper L.T., Tse T.S., Mikhail M.A., et al. Long-term survival and amputation risk in thromboangiitis obliterans (Buerger’s disease). J Am Coll Cardiol. 2004;44:2410–2411.
100 Gifford R.W.Jr, EA H.ines.Jr. Complete clinical remission in thromboangiitis obliterans during abstinence from tobacco: report of case. Proc Staff Meet Mayo Clin. 1951;26:241–245.
101 Corelli F. Buerger’s disease: cigarette smoker disease may always be cured by medical therapy alone. Uselessness of operative treatment. J Cardiovasc Surg (Torino). 1973;14:28–36.
102 Cooper L.T., Henderson S.S., Ballman K.V., et al. A prospective, case-control study of tobacco dependence in thromboangiitis obliterans (Buerger’s disease). Angiology. 2006;57:73–78.
103 Fiessinger J.N., Schafer M. Trial of iloprost versus aspirin treatment for critical limb ischaemia of thromboangiitis obliterans. The TAO Study. Lancet. 1990;335:555–557.
104 Hildebrand M. Pharmacokinetics and tolerability of oral iloprost in thromboangiitis obliterans patients. Eur J Clin Pharmacol. 1997;53:51–56.
105 The European TAO Study Group. Oral iloprost in the treatment of thromboangiitis obliterans (Buerger’s disease): a double-blind, randomised, placebo-controlled trial. Eur J Vasc Endovasc Surg. 1998;15:300–307.
106 Dean S.M., Vaccaro P.S. Successful pharmacologic treatment of lower extremity ulcerations in 5 patients with chronic critical limb ischemia. J Am Board Fam Pract. 2002;15:55–62.
107 Dean S.M., Satiani B. Three cases of digital ischemia successfully treated with cilostazol. Vasc Med. 2001;6:245–248.
108 Paraskevas K.I., Trigka A.A., Samara M. Successful intravenous regional sympathetic blockade (Bier’s Block) with guanethidine and lidocaine in a patient with advanced Buerger’s disease (thromboangiitis obliterans)–a case report. Angiology. 2005;56:493–496.
109 Swigris J.J., Olin J.W., Mekhail N.A. Implantable spinal cord stimulator to treat the ischemic manifestations of thromboangiitis obliterans (Buerger’s disease). J Vasc Surg. 1999;29:928–935.
110 Pace A.V., Saratzis N., Karokis D., et al. Spinal cord stimulation in Buerger’s disease. Ann Rheum Dis. 2002;61:1114.
111 Chierichetti F., Mambrini S., Bagliani A., et al. Treatment of Buerger’s disease with electrical spinal cord stimulation–review of three cases. Angiology. 2002;53:341–347.
112 Boari B., Salmi R., Manfredini R. Buerger’s disease: spinal cord stimulation may represent a useful tool for delaying amputation in young patients. Eur J Intern Med. 2007;18:259.
113 Donas K.P., Schulte S., Ktenidis K., et al. The role of epidural spinal cord stimulation in the treatment of Buerger’s disease. J Vasc Surg. 2005;41:830–836.
114 Montori V.M., Kavros S.J., Walsh E.E., et al. Intermittent compression pump for nonhealing wounds in patients with limb ischemia. The Mayo Clinic experience (1998-2000). Int Angiol. 2002;21:360–366.
115 Isner J.M., Baumgartner I., Rauh G., et al. Treatment of thromboangiitis obliterans (Buerger’s disease) by intramuscular gene transfer of vascular endothelial growth factor: preliminary clinical results. J Vasc Surg. 1998;28:964–973. discussion 973–965
116 Saito Y., Sasaki K., Katsuda Y., et al. Effect of autologous bone-marrow cell transplantation on ischemic ulcer in patients with Buerger’s disease. Circ J. 2007;71:1187–1192.
117 Saito S., Nishikawa K., Obata H., et al. Autologous bone marrow transplantation and hyperbaric oxygen therapy for patients with thromboangiitis obliterans. Angiology. 2007;58:429–434.
118 Matoba S., Tatsumi T., Murohara T., et al. Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156:1010–1018.
119 Durdu S., Akar A.R., Arat M., et al. Autologous bone-marrow mononuclear cell implantation for patients with Rutherford grade II-III thromboangiitis obliterans. J Vasc Surg. 2006;44:732–739.
120 Miyamoto K., Nishigami K., Nagaya N., et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114:2679–2684.
121 Kim S.W., Han H., Chae G.T., et al. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells. 2006;24:1620–1626.
122 Kim D.I., Kim M.J., Joh J.H., et al. Angiogenesis facilitated by autologous whole bone marrow stem cell transplantation for Buerger’s disease. Stem Cells. 2006;24:1194–1200.
123 Lang E.V., Bookstein J.J. Accelerated thrombolysis and angioplasty for hand ischemia in Buerger’s disease. Cardiovasc Intervent Radiol. 1989;12:95–97.
124 Kubota Y., Kichikawa K., Uchida H., et al. Superselective urokinase infusion therapy for dorsalis pedis artery occlusion in Buerger’s disease. Cardiovasc Intervent Radiol. 1997;20:380–382.
125 Hussein E.A., A e.l Dorri. Intra-arterial streptokinase as adjuvant therapy for complicated Buerger’s disease: early trials. Int Surg. 1993;78:54–58.
126 Hodgson T.J., Gaines P.A., Beard J.D. Thrombolysis and angioplasty for acute lower limb ischemia in Buerger’s disease. Cardiovasc Intervent Radiol. 1994;17:333–335.
127 Inada K., Iwashima Y., Okada A., et al. Nonatherosclerotic segmental arterial occlusion of the extremity. Arch Surg. 1974;108:663–667.
128 Sasajima T., Kubo Y., Inaba M., et al. Role of infrainguinal bypass in Buerger’s disease: an eighteen-year experience. Eur J Vasc Endovasc Surg. 1997;13:186–192.
129 Dilege S., Aksoy M., Kayabali M., et al. Vascular reconstruction in Buerger’s disease: is it feasible? Surg Today. 2002;32:1042–1047.
130 Talwar S., Prasad P. Single-stage lumbar sympathectomy and omentopexy: a new surgical approach towards patients with Buerger’s disease. Trop Doct. 2001;31:73–75.
131 Talwar S., Jain S., Porwal R., et al. Free versus pedicled omental grafts for limb salvage in Buerger’s disease. Aust N Z J Surg. 1998;68:38–40.
132 Talwar S., Jain S., Porwal R., et al. Pedicled omental transfer for limb salvage in Buerger’s disease. Int J Cardiol. 2000;72:127–132.
133 Talwar S., Choudhary S.K. Omentopexy for limb salvage in Buerger’s disease: indications, technique and results. J Postgrad Med. 2001;47:137–142.
134 Singh I., Ramteke V.K. The role of omental transfer in Buerger’s disease: New Delhi’s experience. Aust N Z J Surg. 1996;66:372–376.
135 Canter H.I., Isci E., Erk Y. Vacuum-assisted wound closure for the management of a foot ulcer due to Buerger’s disease. J Plast Reconstr Aesthet Surg. 2009;62:250–253.
136 Geerts W.H., Bergqvist D., Pineo G.F., et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition). Chest. 2008;133:381S–453S.
137 Decousus H., Prandoni P., Mismetti P., et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363:1222–1232.