Chapter 46 Acute Arterial Occlusion
Nontraumatic acute occlusion of arterial supply to a limb or organ presents with a constellation of symptoms specific to the tissue suddenly deprived of arterial perfusion. Irrespective of the arterial segment involved, this syndrome represents a vascular emergency. Irreversible organ injury may occur within seconds in the case of acute embolic occlusion of a middle cerebral artery (MCA) or take hours when arterial supply of a lower limb is involved. In everyday clinical practice, acute arterial occlusion is synonymous with acute limb ischemia. Rapid recognition and treatment are required to prevent limb loss and life-threatening morbidity. Acute limb ischemia is thus defined as sudden limb-threatening decrease in arterial perfusion of less than 14 days’ duration. It can occur as a result of embolic occlusion or in situ arterial thrombosis. Over the last several decades, the etiology of acute limb ischemia has varied with changing prevalence of causative conditions. Management of the syndrome has evolved, but the diagnostic skills required to recognize this clinical entity remain unchanged.
Epidemiology of Acute Limb Ischemia
Acute limb ischemia is a rare vascular event, and its incidence eludes exact quantification. It has been influenced by the ever-changing medical landscape. Increasing numbers of patients treated with antiplatelet and antithrombotic therapies, effective therapy for atrial fibrillation, and advances in treatment of valvular and ischemic heart disease have had an impact on the incidence of acute limb ischemia by decreasing the number of embolic events. This may be counterbalanced by increasing numbers of patients undergoing elective surgical and endovascular revascularization therapies, which carry a low but measurable risk of graft or stent thrombosis. An estimate in the 1990s proposed that a vascular center serving a community of 500,000 may expect an annual incidence of 75 patients with acute limb ischemia of the lower extremity.1 Other studies report similar data, estimating the incidence of acute lower-limb ischemia between 13 and 17 cases per 100,000 people per year, with mortality as high as 18% even in the modern era.2,3 Contemporary studies report amputation rates as high as 13% in patients presenting with acute limb ischemia of the lower extremity.4
Acute limb ischemia affects men and women equally. It is infrequent in patients with established peripheral artery disease (PAD), except in those who underwent surgical or endovascular revascularization and developed acute thrombosis of the conduit, graft, or stent. Acute limb ischemia is typically a disease of the middle-aged and older population but can affect younger patients when unusual clinical events such as paradoxical embolism, intracardiac masses and endocarditis, or hypercoagulable syndromes affect the arterial circulation.
Acute nontraumatic ischemia of the upper extremity is even more uncommon. It is less likely to result in limb loss, and thus its importance has been overshadowed by lower-extremity ischemic syndromes. Few published series have been reported, and there are no randomized trials evaluating this clinical syndrome and its treatment. However, the consequences of functional impairment in the upper extremity can be equally devastating to the patient.5 On average, acute arm ischemia accounts for 16.6% of cases of acute ischemia of the extremities and, by extrapolation, occurs with an incidence of 1.2 to 3.5 cases per 100,000 per year.6 However, these estimates are based primarily on surgical series, which usually include only patients who underwent surgical treatment. Surveys of all patients presenting with acute arm ischemia estimate an incidence of 1.13 per 100,000 per year.7 In the absence of more meticulous population studies, the true incidence can only be estimated. Patients with upper-extremity ischemia tend to be older than those with lower-extremity ischemia, with mean ages of 74 and 70 respectively.8
Amputation-free survival is influenced by many modifiable and nonmodifiable factors.9 Among the former, delay in diagnosis stands out as a major factor. Non-Caucasian race, older age, malignancy, congestive heart failure (CHF), and low body weight decrease, whereas systemic atherosclerosis increases the likelihood of amputation-free survival.9,10 Among patients older than 75 years of age, overall 30-day mortality rates approach 42%.11 Survival and functional recovery among patients with acute limb ischemia are directly related to underlying comorbidities and delay in diagnosis and treatment.
Etiology of Acute Limb Ischemia
Acute Upper-Extremity Ischemia
The most common sites of arterial occlusion in the upper extremity are the brachial and axillary arteries, representing 85% of cases of embolic occlusion.6 The subclavian artery is thought to be the most frequent site of occlusion in uncommon cases of in situ thrombosis (Fig. 46-1).
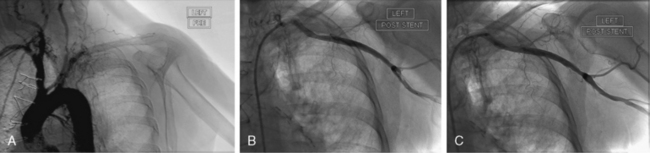
Figure 46-1 In situ thrombosis of subclavian artery in a patient presenting with an inferior ST-segment elevation myocardial infarction (MI) and symptoms of acute left arm and hand ischemia. A, Occlusion of subclavian artery, with angiographic changes suggestive of in situ thrombosis. B, After balloon angioplasty and stenting of subclavian artery, thrombotic component of lesion is seen trapped in filter embolic protection device positioned in axillary artery. C, Patent vessel after filter retrieval.
Iatrogenic causes
In the past, acute ischemia of the arm was primarily caused by cardiac catheterization performed via brachial artery access (Fig. 46-2). In a series from the 1980s reporting on 37 cases of acute arm ischemia treated surgically over a period of 5 years, 56% of cases were caused by this iatrogenic complication, 24% were related to embolic events, and the remainder were due to stab wounds.12 Since brachial artery catheterization fell out of favor, causes of upper-extremity ischemia have changed. In a later series of 65 patients with acute arm ischemia treated surgically over a span of 8 years, a cardioembolic source was identified in 41% of patients, 17% of events were attributed to an arterial source of embolism, and 28% of cases were related to iatrogenic occlusion, mainly a result of cardiac catheterization.13 The resurgence of interest in radial artery access for coronary procedures is unlikely to result in a rise in frequency of upper-extremity ischemia. Occlusion of the radial artery, seen in up to 5% of procedures, is unlikely to compromise perfusion of the hand in a patient with proper preprocedural assessment of a patent palmar arch.
Embolism
Embolic occlusion is the most frequent cause of acute arm ischemia, accounting for 74% to 100% of cases in several reported series.6,7,13–15 Of these, 72% are thought to be cardioembolic in origin, 12% originate from the proximal vessel, and the remainder are of unknown origin.6 Atrial fibrillation and left ventricular (LV) thrombus in patients with ventricular dysfunction are the most frequent causes of cardiac emboli. Common causes of embolization include atrial myxoma16,17 and paradoxical embolism.18 Proximal arteries of the arm can be a source of arterial embolism. Artery-to-artery embolization may cause occlusion of a large- or medium-caliber artery but more commonly presents with digital embolization. Atherosclerotic stenosis of the subclavian artery is a rare cause of embolism but can result in acute hand or arm ischemia.19–21 The rare primary subclavian artery aneurysm or one caused by external compression in a thoracic outlet syndrome (TOS) can result in thromboembolic occlusion of upper-extremity arteries.20,22–24 Aortic arch atheroma has also been implicated as the source of acute arm ischemia.15,25 Other rare arterial sources of embolic events are malignant emboli or paradoxical embolism through intracardiac shunting26,27 (Fig. 46-3).
Thrombosis
Atherosclerotic disease is much less frequent in the upper extremity than the lower limb. Consequently, in situ thrombosis is uncommon and has been estimated to account for 5% of ischemic cases in population studies and 5% to 35% of cases in surgical series.7,14,15,28 Many of the proximal arterial lesions responsible for distal embolization can cause in situ thrombosis. Arteritis, radiation injury, and hypercoagulable syndromes have been reported as rare causes of in situ arterial thrombosis of the upper extremity.20,22,29,30
Acute Lower-Extremity Ischemia
The distinction between embolism and in situ thrombosis should not detract from the need to establish a rapid diagnosis and institute immediate therapy. Nevertheless, embolic etiology is more commonly associated with rapid onset of symptoms, history of cardiac disease, and absence of prior history of PAD. The contralateral limb is likely to have a normal exam, without stigmata of systemic atherosclerosis. Some of the causes of acute limb ischemia are listed in Box 46-1.
In situ thrombosis
In situ thrombosis rather than embolism was responsible for 85% of the acute limb ischemia cases enrolled in the Thrombolysis or Peripheral Arterial Surgery (TOPAS) trial. Rates of embolic cases have been decreasing over the last few decades. In a Greek study evaluating the causes of acute limb ischemia at a referral center between 2000 and 2004, 40% of cases were caused by embolic events, in situ thrombosis was responsible for 50% of cases, and the remaining 10% were due to trauma, iatrogenic injury, vasculitis, or dissection.31 As many as 78% of embolic events were due to a cardiac source; the source of 9% of embolic events could not be determined. Among cases of in situ thrombosis, 30% involved native arteries, and 70% involved thrombosis of vessels after an intervention (65% represented graft thrombosis and 5% iliac or infrainguinal stent thrombosis). Surgical graft thrombosis represented 30% of all cases of acute limb ischemia. Patients with surgical grafts can develop graft thrombosis and symptoms of acute limb ischemia due to graft degeneration or mechanical problems such as anastomotic stenosis or retained valves. Graft compression or kink can also cause its thrombosis. With the advent of stent grafting for aortoiliac aneurysmal disease, acute stentgraft thrombosis has been added as a cause of acute limb ischemia (Fig. 46-4).
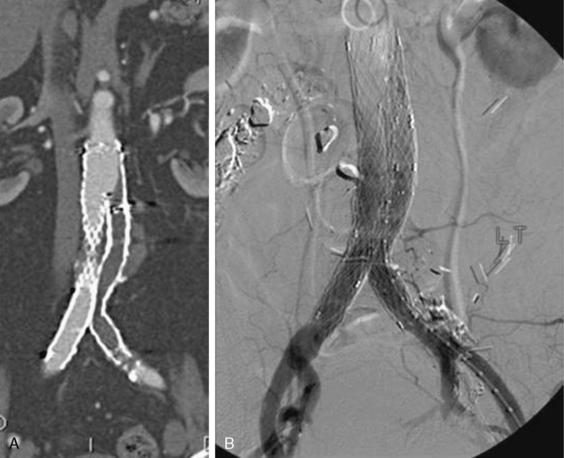
Figure 46-4 A, Acute limb ischemia due to collapse of right iliac endograft limb. B, Treated with ultrasound-accelerated thrombolysis.
In situ thrombosis of a popliteal artery aneurysm usually presents with acute limb ischemia. A review of nearly 900 patients presenting with acute limb ischemia secondary to a thrombosed popliteal aneurysm reported amputation rates of 14%. In this study, catheter-directed thrombolysis prior to surgery did not lower the likelihood of amputation, but it significantly improved the long-term patency of the graft, presumably by maximizing patency of the tibial vessel.32 The decision to perform catheter-directed thrombolysis must depend on the clinical situation and urgency of revascularization. In a Swedish vascular registry, amputation rates for acute thrombosis of the popliteal aneurysm were 17% in patients presenting with acute ischemia and only 1.8% for asymptomatic electively repaired aneurysms.33
Embolism
Acute limb ischemia is often caused by an embolic event, commonly from a cardiac source. The embolus most frequently lodges in the aortoiliac bifurcation, femoral bifurcation, or popliteal trifurcation. Over the last several decades, the etiology of cardioembolic events has evolved. Embolic events caused by rheumatic mitral stenosis with left atrial enlargement have become a rare occurrence because the prevalence of rheumatic valve disease has decreased substantially. Age-related atrial fibrillation and LV dysfunction with apical thrombus formation are the most common causes of cardioembolic events (Fig. 46-5). Less common causes include endocarditis, intracardiac myxoma, or paradoxical embolism due to a patent foramen ovale allowing transit of venous thrombus into the arterial circulation. Acute embolic occlusion related to aortic aneurysmal disease and intramural thrombus is rare.
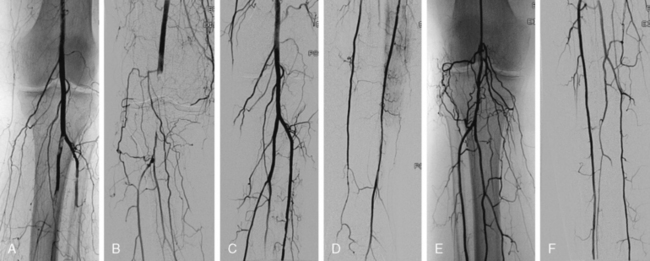
Figure 46-5 Bilateral acute limb ischemia in patient with atrial fibrillation and interrupted anticoagulation. Left anterior tibial artery and tibioperoneal trunk are occluded (A), as is right popliteal artery (B). Mechanical thrombectomy and catheter suction embolectomy restored flow in left (C-D) and right calf (E-F).
Iatrogenic causes
Iatrogenic acute limb ischemia can be caused by arterial access in the common femoral artery (CFA) and injury of the vessel at the access site, be it by deployment of a vascular closure device or direct injury to the common femoral or iliac artery. Similarly, catheter-associated thrombosis and embolism of the popliteal artery can occur.
Other causes
Intense vasospasm, such as can be caused by ergotism34,35 or cocaine ingestion,36 have been reported to cause acute limb ischemia. Aortic dissection can result in occlusion of the distal aorta and iliac vessels when the true lumen is compressed by a pressurized false lumen. Iliofemoral deep vein thrombosis (DVT) with massive swelling of the thigh can compromise arterial inflow to the leg. The syndrome of phlegmasia cerulea dolens requires urgent catheter direct thrombolysis of the venous thrombus to restore venous outflow and thus arterial inflow to the limb.
Pathophysiology of Acute Limb Ischemia
Most emboli lodge in points of arterial branching: aortic, iliac, femoral, or popliteal bifurcations in the leg, and brachial bifurcation in the arm. In situ thrombosis most commonly affects the femoral or popliteal artery, particularly in the setting of an existing arterial bypass, ruptured atherosclerotic plaque, or low-output state. Sudden cessation of arterial flow to the extremity triggers a series of complex pathophysiological processes. Malperfused tissues shift from aerobic to anaerobic metabolism. The shift in lactate-to-pyruvate ratio further increases lactate production, increases the concentration of hydrogen ions, and induces acidosis. Progressive ischemia results in cell dysfunction and eventual cell death. Muscle hypoxia depletes intracellular adenosine triphosphate (ATP) stores, and the consequent dysfunction of the sodium/potassium-ATPase and calcium/sodium pumps causes leakage of intracellular calcium into myocytes.37 Intracellular free calcium levels rise and interact with actin, myosin, and proteases, leading to necrosis of muscle fibers. As the cellular membranes and microvascular integrity fail, intracellular potassium, phosphate, creatinine (Cr) kinase, and myoglobin leak into the systemic circulation. Reperfusion further amplifies these cellular changes.
Nerve and muscle tissue are quite susceptible to ischemic injury, so presence or absence of neuromotor deficit is of paramount importance in assessing the severity of acute limb ischemia. Irreversible muscle damage begins after 3 hours of ischemia and is nearly complete after 6 hours.38 In addition to myocyte injury, progressive microvascular damage follows skeletal muscle injury. The more severe the cellular damage, the greater the microvascular changes. In the setting of muscle necrosis, microvascular flow stops within a few hours. Traditionally, a window of 6 hours has been assumed before irreversible functional injury occurs. This time window may be longer in a “preconditioned” limb with collateral pathways.
Ischemic insult sets the stage for reperfusion injury, a process triggered by restoration of perfusion and mediated by a complex cascade of cytokines, reactive oxygen species (ROS), and neutrophils. Reactive oxygen species (e.g., superoxide anion, hydrogen peroxide, hydroxyl radicals, peroxynitrite) are produced by activated neutrophils and xanthine oxidase, an enzyme located on microvascular endothelial cells (ECs) of skeletal muscle and activated during ischemic conditions.39 Under normal conditions, xanthine dehydrogenase uses nicotinamide adenine dinucleotide to oxidize hypoxanthine to xanthine. Xanthine dehydrogenase is converted to xanthine oxidase after 2 hours of ischemia.40 During ischemia, ATP is degraded to hypoxanthine, but xanthine oxidase requires oxygen to convert hypoxanthine to xanthine. Thus, hypoxanthine accumulates during ischemia. When oxygen is reintroduced during reperfusion, xanthine dehydrogenase isoform becomes active again. Conversion of massive amounts of hypoxanthine generates reactive oxygen species.41
The essential substrate for production of these radicals, molecular oxygen, is provided by reperfusion. Xanthine oxidase–derived oxidants mediate the increased vascular permeability in postischemic muscle. The importance of elemental oxygen and the role of oxygen radicals in reperfusion injury is underscored by studies showing that reperfusion initially with deoxygenated autologous blood prevents increase in permeability after ischemia. Changing the perfusate to oxygenated blood during reperfusion mimicked the microvascular injury response seen after normoxic reperfusion.42 Similarly, gradual reintroduction of oxygen early in reperfusion decreases postischemic injury.42 Additional supplementation with free radical scavengers and reduced oxygen delivery further reduces injury of postischemic necrosis.43
Activated neutrophils are the principal agents responsible for local and systemic damage caused by reperfusion. Leukocytes play an equally important role in reperfusion injury. Activated neutrophils accumulate in the reperfusing muscle and produce reactive oxygen metabolites, release cytotoxic enzymes, and occlude microcirculation pathways.44 Leukocyte depletion has been shown to reduce the ischemia-reperfusion injury. Reperfusion with oxygenated blood depleted of leukocytes by the use of filters completely prevents development of vascular permeability in canine skeletal muscle.45,46 Interestingly, inducing neutropenia before ischemia in rats restores transmembrane potential and contractile function in postischemic rat muscle.47,48
Skeletal muscle ischemia and reperfusion triggers a number of additional inflammatory cascades that include complement activation, increased expression of adhesion molecules, cytokine release, eicosanoid synthesis, free radical formation, cytoskeletal alterations, adenine nucleotide depletion, alterations in calcium and phospholipid metabolism, leukocyte activation, and endothelial dysfunction.40 Interleukin (IL)-1β and tumor necrosis factor (TNF)-α are detected soon after reperfusion and induce adhesion molecules on the surface of endothelial cells, increase capillary leak, and stimulate production of IL-6 and IL-8, which further increase endothelial permeability, destroy endothelial integrity, and activate leukocytes.49–53
The clinical impact of these cellular responses to reperfusion results in tissue swelling, a catastrophic event in the closed spaces of the forearm, thigh, calf, and buttock. Elevated compartment pressures within fascial boundaries cause a compartment syndrome: elevated compartment pressures that reduce the perfusion gradient and capillary blood flow below the metabolic requirement, resulting in further ischemia and necrosis. Release of myoglobin can result in renal injury. Increased endothelial permeability can lead to acute lung injury, a process attenuated in animal models by chemically induced neutropenia, suggesting that activation and transmigration of neutrophils and loss of endothelial integrity are critical in acute lung injury in reperfusion injury.54 Thus, noncardiogenic pulmonary edema can develop after reperfusion of lower limbs, a process that can be prevented by granulocyte depletion.54,55
The reperfusion syndrome consists of two components. The local response to reperfusion triggers tissue swelling, while the systemic response can result in multiorgan failure and death. It is the latter that mitigates intervention in advanced and irreversible limb ischemia. The degree of inflammatory response following reperfusion is variable. There is little inflammatory response when muscle necrosis is uniform. The degree of ischemic damage, however, will vary depending on proximity of the tissue to the occlusion and efficiency of the collateral supply. The magnitude of the inflammatory response will be determined by the extent of the ischemic, but not completely necrotic, zone. Thus, reperfusion of large muscle groups with advanced ischemic injury and tissue necrosis will result in release of large amounts of toxic inflammatory mediators into the systemic circulation. This detrimental effect of reperfusion favors amputation in patients with irreversible ischemic injury.
Diagnosis of Acute Limb Ischemia
The diagnosis of acute limb ischemia may be elusive, especially in patients who present with sensory and motor deficits that direct attention toward neurological evaluation. Clinical signs and symptoms of acute limb ischemia manifest as a spectrum of findings directly related to the severity of ischemia and duration of arterial malperfusion. Diagnosis of acute limb ischemia is made on the basis of physical examination. Confirmatory imaging with computed tomographic angiography (CTA) or magnetic resonance angiography (MRA) introduces a potentially costly delay in therapeutic intervention. Bedside duplex ultrasonography can be performed rapidly and can add information about the level of occlusion and the arterial access strategy for an endovascular procedure. A careful physical examination, including Doppler evaluation of arterial and venous signals, is usually sufficient for obtaining this information. A good physical examination can determine the level of arterial occlusion and obviate the need for additional imaging.
The classic symptoms and physical examination findings of an acutely ischemic limb are commonly known as the six Ps: pulselessness, pallor, pain, poikilothermia, paralysis, and paresthesia. Pain is the most common symptom and progresses with ischemia. Pallor is an early finding in an ischemic extremity and is caused by complete emptying and vasospasm of the arteries (Fig. 46-6). Subsequent stagnation of microvascular circulation will cause mottling of the skin, which initially blanches with pressure. As ischemia continues, paresthesia develops, and numbness replaces pain, often falsely reassuring both patient and physician. In the final stages of ischemic injury, paralysis sets in, and skin mottling is fixed and nonblanching. Loss of motor function and marble-like appearance of the skin herald irreversible ischemic injury.
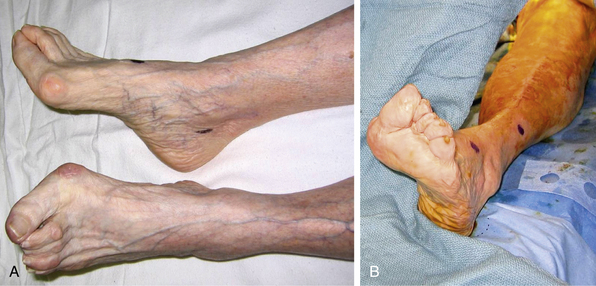
Figure 46-6 Acute limb ischemia due to left common femoral artery (CFA) embolism. Marked pallor of left foot (A) resolves after surgical embolectomy (B).
(Image courtesy Dr. Edwin Gravereaux.)
A careful physical examination can determine the level of occlusion by detecting a temperature gradient along the limb and a deficit in pulses either on palpation or by arterial Doppler exam. The cutaneous changes of pallor and temperature are detected one level below the occluded arterial segment. Physical examination must also include a search for potential sources of acute limb ischemia. Recognition of atrial fibrillation, a cardiac murmur of valvular disease, or symptoms of CHF may implicate a cardioembolic cause of the event. Systemic symptoms of fevers, night sweats, and chills may hint at endocarditis as the etiology of cardiac embolism. Stigmata of PAD in the contralateral limb or signs of prior surgical revascularization point to an in situ arterial thrombosis, whereas chest pain, hypertension, and asymmetry in arterial pulses of the upper extremity may require additional imaging to exclude an aortic dissection.
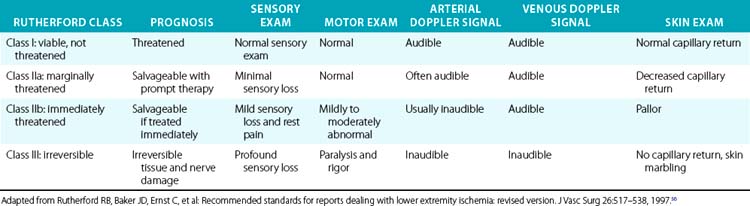
More importantly, it is the physical examination that allows classification of the severity of ischemia, urgency of revascularization, and prognosis after revascularization56 (Table 46-1). These clinical classes are also useful for determining the best intervention strategies. In general, Rutherford class I represents a viable and nonthreatened limb, akin to patients with chronic and noncritical ischemia. Rutherford class II symptoms describe a directly threatened limb. A class IIa limb is characterized by intact sensory and motor examination despite absent arterial Doppler signals in the foot. This limb is marginally threatened. Class IIb includes patients with an immediately threatened limb, characterized by sensory loss, mild motor function impairment, and absent Doppler arterial signals. This limb can be salvaged if treated immediately. Irreversible limb ischemia falls into Rutherford class III, with permanent nerve damage, profound sensory loss and motor paralysis, and absent arterial and venous Doppler signals. Revascularization of such a limb is harmful; amputation is required.
The presence of preexisting arterial occlusive disease may “precondition” the limb by fostering development of collaterals that lessen the severity of tissue malperfusion when acute occlusion occurs. Thus, patients with thrombosis in situ in an atherosclerotic vessel and those with graft failure may tolerate acute ischemia better than patients with no underlying arterial disease who develop acute limb ischemia due to a cardioembolic or an iatrogenic event. Several clinical characteristics may allow differentiation between an embolic event and in situ thrombosis. Patients with the former report a more abrupt onset of pain with clearer demarcation of ischemic temperature change and skin mottling. These patients usually present with symptoms and signs in Rutherford class IIb and III. Patients with in situ arterial thrombosis usually have signs of established PAD and report a more vague onset of symptoms. Physical examination findings are less striking, with a less distinct demarcation of ischemic changes and more cyanosis than pallor. These patients often fall into Rutherford class I and IIa categories.
Treatment of Acute Limb Ischemia
Prompt recognition of acute limb ischemia and rapid restoration of arterial perfusion are cornerstones of therapy. The decision whether revascularization or primary amputation should be undertaken depends largely on the viability of the affected limb. In patients with a salvageable limb, selection of the type of revascularization therapy is equally important. The two major factors affecting morbidity and mortality among patients with acute limb ischemia are the burden of medical comorbidities and the delay in recognition and treatment of the ischemic limb. Other factors associated with lower amputation-free survival are increased age, race, diabetes, and absence of prompt initiation of anticoagulation.57
Surgical intervention has been traditionally associated with high perioperative mortality rates. In a compilation of 3000 patients treated surgically for acute limb ischemia in 30 centers between 1963 and 1978, 30-day mortality rates were as high as 25%.58 Despite rapid advances in surgical and anesthesia techniques, Jivegard reported a 20% mortality rate a decade later.10 Even in the 1990s, 30-day mortality after surgical intervention among selected patients enrolled in the TOPAS, Surgery versus Thrombolysis for Ischemia of the Lower Extremity (STILE), and Rochester randomized trials ranged from 5% to 18%.9,59,60
The high burden of cardiopulmonary disease and high surgical mortality in the population affected by acute limb ischemia provided an impetus for development of less invasive endovascular strategies. Evidence from randomized trials suggests equipoise between endovascular and surgical therapies in selected patients, particularly those with class I and IIa symptoms. The cause of limb ischemia, location of the occlusion, Rutherford class, as well as patient characteristics play a crucial role in selection of appropriate revascularization strategy. The Rochester, STILE, and TOPAS trials form a framework for selection of patients for endovascular therapies.9,59,60 These trials demonstrated that patients with underlying PAD or graft thrombosis and Rutherford class I and IIa thrombolytic-based endovascular therapies do indeed have better outcomes. Patients with cardioembolic events usually present with Rutherford class IIb symptoms and are best treated with prompt surgical embolectomy.4
In modern practice, a rigid division between open surgical and endovascular treatment is artificial. Although many patients can be treated with an entirely endovascular approach, and others require traditional surgical embolectomy, large numbers of patients are treated with hybrid approaches. Indeed, routine use of perioperative angiography suggests a high rate of residual thrombus, necessitating additional combined surgical and endovascular intervention in up to 90% of complex cases.61
In addition to revascularization therapies, the sequelae of acute limb ischemia include ischemia-reperfusion injury, which may range from mild injury without functional or systemic consequences to systemic inflammatory response and multiorgan failure. Treatment of these metabolic consequences of acute limb ischemia is essential to patients’ survival.
Initial Medical Management
Regardless of the revascularization strategy selected, the basic principles of initial therapy are the same: fluid resuscitation, analgesia, and administration of antithrombin and antiplatelet therapy. After decades of clinical experience, heparin therapy has been shown to decrease ischemic injury, reduce thrombus propagation, and improve survival.4,62–64 Some studies dispute the benefit of perioperative anticoagulation, even in patients with a cardiac source of emboli, but the overwhelming amount of data support perioperative anticoagulation with heparin.65 Unfractionated heparin (UFH) should be administered at high doses (100-150 units/kg), with a goal of rapidly achieving a therapeutic level of anticoagulation and a rise in partial thromboplastin time (PTT) by a factor of 2 to 2.5 above baseline. Patients with heparin-induced thrombocytopenia (HIT) should be treated with intravenous (IV) direct thrombin inhibitors (DTI) such as lepirudin or argatroban. Bivalirudin, another DTI commonly used in coronary and endovascular interventions, has a relatively short half-life and is more familiar to most vascular specialists. The decision regarding long-term anticoagulation must be made based on the etiology of the ischemic event, outcome of revascularization, and the balance between bleeding and thrombotic risk.
Correction of laboratory abnormalities and stabilization of the underlying acute medical condition are imperative to achieve best clinical outcomes. Certain laboratory characteristics predict ultimate therapeutic success. Patients presenting with elevated Cr kinase and neutrophil count have a 50% risk of amputation as compared to a 5% risk among those with normal enzyme and neutrophil levels.66 This finding underscores the poor clinical outcomes in patients with advanced ischemic injury of skeletal muscle. In patients who present with irreversible tissue loss, alkalinization of urine may be required to prevent renal injury from myoglobinuria. In some cases, the cause of acute limb ischemia is itself immediately life threatening, such as myocardial infarction (MI) complicated by LV thrombus and cardiogenic shock, or aortic dissection or infective endocarditis with hemodynamic compromise due to valvular incompetence. In such cases, the principle of “life over limb” should guide best therapeutic strategy.
Endovascular Therapy of Acute Limb Ischemia
The basic principle behind endovascular therapy is to restore arterial flow, either by thrombus lysis or unmasking and treating an underlying lesion, thus eliminating the need for surgery or reducing the extent of surgical procedure.
Endovascular therapy for acute limb ischemia became possible when Tillet and Garner discovered the fibrinolytic properties of hemolytic streptococcus in 1933.67 It was not long after the first use of IV streptokinase in healthy volunteers by Tillet et al. in 1955 that Cliffton reported on the therapeutic use of streptokinase to dissolve pathological thrombi in arteries and veins in 1957.68,69 Catheter-based delivery of intraarterial (IA) streptokinase was pioneered by Charles Dotter et al. in 1974.70 Berridge et al. subsequently confirmed that catheter delivery of fibrinolytic agents directly into the affected artery was superior to an IV administered thrombolytic, and improved limb salvage rates (80% vs. 45%) and lowered hemorrhagic complications.71
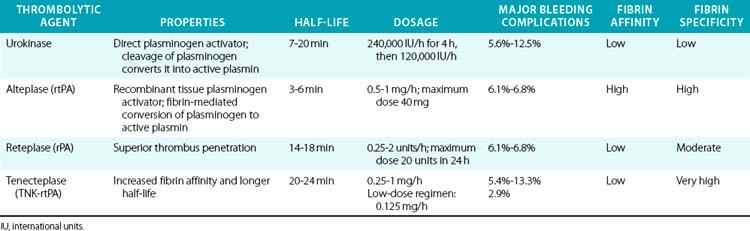
Modern thrombolytic agents work by enhancing the intrinsic fibrinolytic process through activation of plasminogen and its conversion into plasmin, which degrades fibrin (Table 46-2). The conversion of plasminogen into plasmin requires hydrolysis of a lysine-arginine bond, a step catalyzed by tissue-type plasminogen activator (tPA), the model for today’s recombinant plasminogen activators. Technical success of catheter-directed thrombolysis is defined as restoration of antegrade flow and complete or near-complete resolution of thrombus. Clinical success is defined as relief of acute ischemic symptoms or reduction of the level of the subsequent surgical intervention or amputation.72 Enzymatic dissolution of thrombus may be more complete compared to surgical embolectomy, particularly in the distal arterial beds and in cases of distal embolization. Endovascular therapies evolved and became more effective as cumulative experience grew in the 1980s and 1990s. Development of multihole infusion catheters and recognition of the importance of traversing the thrombotic occlusion with the infusion catheter and infusing the drug into the clot rather than above the occlusion have markedly increased the efficacy of these procedures.
Three randomized trials performed in the 1990s compared endovascular therapy to surgical intervention in patients with acute limb ischemia. The Rochester trial randomized 114 patients with limb-threatening ischemia from embolic and thrombotic occlusion of native vessels or grafts to treatment with IA delivery of urokinase or surgery.60 Catheter-directed thrombolysis resulted in resolution of thrombus in 70% of patients. After 1 year, amputation rates were identical in both arms at 18%, but mortality was significantly higher in the surgical arm: 16% vs. 42%, with the majority of deaths in the surgical arm related to cardiopulmonary complications. Thrombolytic therapy was also associated with lower cost.
The larger STILE trial enrolled 393 patients with native vessel or graft thrombosis of less than 6 months duration who were randomized to surgical intervention or thrombolytic therapy.59 The trial was handicapped by inclusion of patients with chronic ischemic symptoms unlikely to respond to thrombolysis. Indeed, 70% of patients in the thrombolytic arm had symptoms of a chronic nature. Technical failure accounted for a large fraction of clinical failures in the fibrinolytic arms. Failure to traverse the occlusive lesion was noted in 28% of patients. In patients who underwent successful catheter placement, patency was restored in 81% of bypass grafts and 69% of native arteries (P = NS). The ability to cross the lesion with a wire was predictive of therapeutic success, a key finding that has guided endovascular therapy for acute limb ischemia ever since.
In the fibrinolytic arm, patients received either recombinant tPA (rtPA) at a dose of 0.05 mg/kg/h for up to 12 hours or urokinase for up to 36 hours. The dose of tPA used in this trial was much larger than usual doses of 1 mg/h used in clinical practice today. The trial was terminated early after the combined endpoint of death, major amputation, and recurrent ischemia occurred in 61.7% and 36.1% of patients, respectively, in the lytic and surgical arms (P <0.001). The 30-day mortality rates were 4.0% in the thrombolysis arm and 4.9% in the surgical arm (P = NS), with amputation rates of 5.2% and 6.3%, respectively (P = NS). The difference in major morbidity of 21% in the thrombolysis arm and 16% in the surgical group stemmed primarily from the hemorrhagic and vascular access complications and recurrent ischemia observed in the former group. Patients in the thrombolysis arm had a reduction in the extent of surgical revascularization.
A post hoc analysis stratified patients according to the duration of symptoms: among patients with symptoms less than 14 days in duration, thrombolytic therapy was associated with a trend toward a lower rate of major amputation compared to surgical intervention (5.7% vs. 17.9%; P = 0.06). Among patients with longer duration of symptoms, 5.3% of those in the thrombolytic arm and 2.1% in the surgical arm underwent amputation (P = NS). Among patients with symptoms for 14 days, the rates of death and amputation at 6 months were 15.3% in the fibrinolytic arm and 37.5% in the surgical arm (P = 0.01). This study firmly established that thrombolytic therapy was not effective in most cases of chronic limb ischemia.
The TOPAS trial, the third trial comparing surgical intervention to catheter-directed thrombolysis, enrolled only patients with symptoms of less than 14 days’ duration.9 Thrombotic events were the predominant etiology of acute limb ischemia, responsible for 85% of cases, and occurred more frequently in arterial grafts than native arteries. In addition, only 19% of the grafts consisted of autologous vein conduits, a departure from modern practice. The first dose-finding phase of the trial randomized 213 patients to initial infusion of variable doses of urokinase, followed by prolonged low-dose infusion. Complete thrombolysis was achieved in 71% of patients, without statistically significant difference in 12-month limb salvage or mortality rates in the surgical and urokinase arms. Patients treated with urokinase had a prohibitively high rate of intracranial hemorrhage (2.1%), particularly associated with use of a higher urokinase dose. In the second phase of the trial, 542 patients were randomized to surgical intervention or treatment with the safest dose of urokinase infusion. Recanalization occurred in 79.7% of patients and complete thrombolysis in 67.9% of patients. After 1 year, amputation-free survival in the thrombolytic and surgical arms was nearly identical (65% vs. 69.9%; P = NS) but came at a cost of higher rates of intracranial hemorrhage of 1.6% in the thrombolytic arm. Intracranial hemorrhage was associated with concomitant infusion of therapeutic doses of UFH and occurred in as many as 4.8% of patients receiving doses aimed at full systemic anticoagulation, compared to 0.5% of patients who received subtherapeutic doses of heparin.
Major bleeding complications were higher in the thrombolytic arm than in the surgical group (12.5% vs. 5.5%; P = 0.005). At the time of discharge, death occurred in 5.9% of surgical patients and 8.8% of urokinase-treated patients (P = NS). Thrombolytic therapy with urokinase was associated with higher rate of bleeding complications, but effectively reduced the need for surgical interventions without compromising amputation-free survival in patients with primarily thrombotic rather than embolic etiology of acute limb ischemia.
A Cochrane review of five trials of catheter-directed thrombolysis included 1283 patients and reported that there was no significant difference between the two strategies when limb salvage or mortality are compared at 30 days or 1 year. Patients undergoing catheter-directed thrombolysis were more likely to suffer bleeding complications (8.8% vs. 3.3%; 95% confidence interval [CI]: 1.7-4.6) and stroke (1.3% vs. 0%; 95% CI: 1.57, 26.22).73 A “real-world” experience with catheter-directed thrombolysis was reported in the National Audit of Thrombolysis for Acute Leg Ischemia (NATALI) registry of 1133 patients treated with thrombolytic drugs between 1990 and 1999. This study showed amputation-free survival of 75%, with amputation and death rates each at 12% in the first 30 days, and a 7.8% rate of major hemorrhage. It is not clear whether registries of such type included patients in whom thrombolytic therapy was selected because of high perioperative mortality risk.74
Multivariable analysis identified several factors predicting success of thrombolytic therapy.75 Ability to traverse the thrombus and position the thrombolytic infusing catheter directly into the thrombus favored successful fibrinolysis. Similarly, native artery or a prosthetic graft were more responsive to thrombolysis, whereas patients with diabetes were less likely to have successful treatment.
The success of thrombolytic therapy has led to an intense search for the optimal agent and dosing regimen in an ongoing effort to provide maximal thrombolysis effect with minimal bleeding complications. The largest experience in arterial thrombolysis comes from streptokinase, urokinase, and rtPA. Urokinase has been shown to achieve more rapid thrombolysis and fewer bleeding complications than streptokinase.76 Streptokinase use has therefore been abandoned owing to its immunogenic effects, platelet-activating effects, and higher bleeding rates compared to later-generation agents. Urokinase was withdrawn from production in 1999 after concerns about contamination in the production process. Since that time, rtPA agents have become the dominant fibrinolytics used in clinical practice. Three agents are available in this class: alteplase, reteplase, and tenecteplase.
Alteplase and tenecteplase have higher affinity for activation of fibrin-bound plasminogen than urokinase and reteplase, which are less fibrin specific. Reduced fibrin binding of reteplase could allow greater availability of unbound drug for thrombus penetration and faster lysis compared to tPA. Alteplase is commonly used for catheter-directed thrombolysis. Catheter-directed thrombolysis using rtPA has been shown to be superior to streptokinase, achieving better angiographic results and superior 30-day limb salvage rates.71 When compared with urokinase, alteplase was found to have superior efficacy in thrombus resolution but a price of higher incidence of access-site hematoma.77 In the STILE trial, however, there were no differences between urokinase and alteplase. A review of multiple studies evaluating alteplase concluded that the risk of bleeding was directly related to the duration of infusion and overall dose, but did not differ from complications encountered with urokinase.78 Reteplase, a third-generation tPA derivative has a longer half-life of 13 to 16 minutes and has been successfully tested in a small number of patients with acute limb ischemia.79,80 Proliferation of adjunctive endovascular treatments has made direct comparisons between various lytic agents increasingly difficult, but there is no convincing evidence that one rtPA thrombolytic is superior to another in terms of efficacy and safety.
Adjuvant therapy with the glycoprotein (GP) IIb/IIIa inhibitor abciximab was piloted in a small trial of thrombolysis with reteplase. Study results suggested that combined therapy allowed shorter thrombolytic infusion without an increase in bleeding complications.81 Efficacy of combining infusion of fibrinolytics and GP IIb/IIIa inhibitors was further evaluated in the randomized RELAX trial (official title: Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure [RELAX]). In this study, 74 patients with acute occlusion received variable does of reteplase alone or reteplase and abciximab infusion.82 At 90 days, the composite endpoint in patients treated with a tPA dose of 1 mg/h did not differ, whether they received concomitant placebo or abciximab. Interestingly, no instances of intracranial hemorrhage were observed in either arm. Use of these adjuvant agents has not been accepted as standard therapy. Unfractionated heparin, on the other hand, is routinely infused through the catheter’s side arm to achieve a PTT of 40 to 50. Subgroup analysis of the STILE trial suggested that heparin administration during alteplase infusion was associated with reduction in the composite endpoint of death, amputation, major morbidity, and recurrent ischemia. More importantly, adjunctive infusion of heparin in either urokinase or alteplase arms was not associated with increased bleeding.56 Infusion of heparin through the sidearm also lowers the risk of catheter thrombosis.83 Thus, low-dose heparin 400 to 600 units/h should be administered; some authors recommend a lower dose of 100 units/h.
Risk of hemorrhagic complications increases with duration of therapy. It has been estimated that the risk of major complications associated with thrombolytic therapy increases with duration of infusion, from 4% at 8 hours to 34% at 40 hours.84 The optimal duration of thrombolytic infusion is not well defined. There has been a gradual decrease in therapy duration from 48-hour infusions in early trials to 6- to 18-hour infusions used currently in the era of adjunctive techniques. Monitoring of fibrinogen levels during thrombolytic infusion has long been advocated. Fibrinogen levels are checked serially during infusion, and a level below 100 to 150 mg/dL indicates significant dysfibrinogenemia and requires lowering the drug dose or stopping the infusion altogether. Lower fibrinogen levels correlated with bleeding in the STILE trial, but it is not clear whether the fibrinogen level is a reliable predictor of bleeding complications.
One of the drawbacks of catheter-directed thrombolysis are the prolonged infusion times, high costs of fibrinolytic agents, need for repeat angiographic imaging, and monitoring of patients in intensive care units (ICUs). Delays in restoring vessel patency made this therapy unsuitable for patients who require immediate revascularization, so surgical intervention has been recommended for patients with Rutherford class IIb symptoms. The drive to overcome these shortcomings, reduce the dose of thrombolytics required to achieve clinical success, and lower hemorrhagic complications has led to development of several adjunctive techniques and devices designed to achieve more rapid reperfusion of the threatened limb.85–87 Mechanical thrombectomy, pulse-spray thrombectomy, and ultrasound-accelerated thrombolysis are examples of these techniques. In modern practice, endovascular procedures for acute limb ischemia combine catheter-directed thrombolysis with mechanical thrombectomy, pulse-spray thrombectomy, catheter suction embolectomy, ultrasound-assisted thrombolysis, distal embolic protection devices, and angioplasty and stenting. Despite a variety of adjunctive therapies, certain basic principles apply to endovascular thrombolysis: the entire occluded segment must be crossed, and an infusion with multiple side holes positioned across the thrombus to directly infuse the thrombolytic drug into the thrombus must be given. Recombinant tissue plasminogen activator is the most commonly used thrombolytic agent, infused at a rate of 0.5 to 1 mg/h for a minimum of 12 hours.
Mechanical Thrombectomy Devices
The AngioJet Xpeedior rheolytic thrombectomy catheter (Medrad Interventional/Possis, Warrendale, Pa.) is the most commonly used mechanical thrombectomy catheter. This small-caliber catheter uses a system of forced saline jets at its tip to fragment the thrombus, while the vacuum created proximal to the jets by the Venturi effect aids in aspiration of the fragmented debris. A simple modification allows substitution of saline for thrombolytic agents, which can be sprayed into the thrombus without concomitant aspiration. Some 20 to 30 minutes after such pulse-spray treatment, the thrombus laced with fibrinolytic is fragmented and aspirated in standard thrombectomy mode, reducing the thrombotic burden and restoring arterial flow.88 Mechanical thrombectomy can be performed without the pulse spray technique to restore flow in patients intolerant of thrombolytic drugs. In early trials, thrombectomy with the AngioJet catheter in acute limb ischemia of native arteries and bypass grafts reestablished arterial flow in 90% of patients. Clinical improvement was seen in 82% of patients, with distal embolization of thrombus occurring in only 2%.89 Catheter-directed thrombolysis is routinely used with this adjuvant therapy, but the dose and duration of fibrinolytic therapy is reduced.90
Rheolytic thrombectomy was evaluated in a small multicenter registry of patients with mostly class IIa and IIb symptoms who were treated with catheter-directed infusion before or after rheolytic thrombectomy. After adjunctive angioplasty and stenting or elective surgery was performed in 80% of these patients, amputation rates were 7.1% and mortality 4.0% at 30 days.91 Experience with rheolytic thrombectomy suggests that it is particularly effective in cases of in situ thrombosis, irrespective of the conduit type90 (Fig. 46-7). The thrombectomy devices fail to remove organized and adherent thrombus and are best used to treat acute thrombus. Overall technical success rates with the AngioJet range from 56% to 95%, with distal embolization rates of 9.5% and amputation-free survival rates reaching 75% at 2 years. The device can be also used without concomitant thrombolytics, with limb salvage rates reported to be as high as 95%.90,92–94
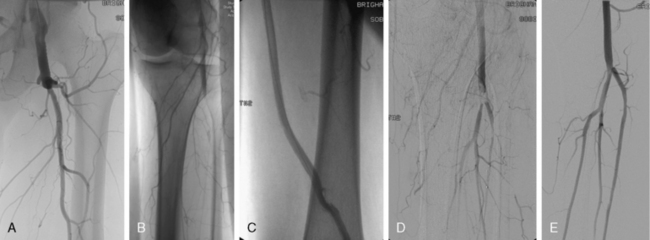
Figure 46-7 Acute limb ischemia caused by thrombosis of left femoral bypass graft (A) and distal embolization to popliteal artery (B). Pulse-spray thrombectomy restored patency of graft (C) and reduced thrombotic burden in popliteal artery (D), allowing catheter-directed thrombolysis to restore patency (E).
Several other devices are used for percutaneous mechanical thrombectomy. The Trellis device consists of a catheter with multiple infusion holes bordered by proximal and distal balloons that when inflated, localize the thrombolytic to the thrombosed segment and potentially limit the systemic effect of these agents. A battery-powered sinusoidal wire rotates around the catheter, effectively mixing the thrombus and thrombolytic agents. Before the balloons are deflated, the debris contained between the balloons is aspirated. The use of this device, more common in venous thrombosis, has been described in a handful of patients with arterial occlusions, but its use was associated with a 11.5% rate of distal embolization.95 The Rotarex device (Straub Medical AG, Wangs, Switzerland) is available in Europe and has been tested to be safe and effective in peripheral arterial thromboembolic disease.96 This over-the-wire catheter is designed for thrombus removal in peripheral vessels. A spiral at the catheter’s tip rotates at 40,000 rpm, and fragments and aspirates particles at 180 mL/min. The catheter is advanced into the thrombus and gently withdrawn during aspiration. The strength of suction can be adjusted to avoid collapse and injury of the vessel around the catheter. The Hydrolyser catheter (Cordis, Warren, N.J.) was originally designed for management of dialysis access thrombosis. This 6 F 0.018-inch guidewire-compatible catheter uses the Venturi effect to create a vacuum when powered by a standard contrast injector filled with saline. It has been reported effective in treatment of graft thrombosis, and in vitro evaluations have found a lower distal embolization rate compared to the AngioJet.97 Technical success rates of 88% in grafts and 73% in native arteries, with amputation rates of 11%, have been reported.98
All thrombectomy devices require frequent use of thrombolysis. None of these devices have been studied rigorously, but they firmly belong in the arsenal of adjunctive devices accelerating reperfusion and decreasing the amount of thrombolytic drug used. Reduction in procedural time and thrombolytic dose is likely counterbalanced by more traumatic effect compared to pharmacotherapy alone. The thrombolytic drug also affects patency of side branches and collateral vessels that are too small to be treated with these devices.
Suction Embolectomy
Percutaneous aspiration thrombectomy may be particularly effective for popliteal and tibial vessels (Figs. 46-8 and 46-9). A large-lumen catheter (6 F-8 F) connected to a 60-mL syringe is advanced into the proximal aspect of the occlusion, vacuum is attached by aspirating the syringe, and the thrombus is aspirated into the catheter and removed from the artery.99,100 Combination catheter suction embolectomy and thrombolysis can result in success rates of up to 90%, with a limb salvage rate of 86% at 4-year follow-up.101
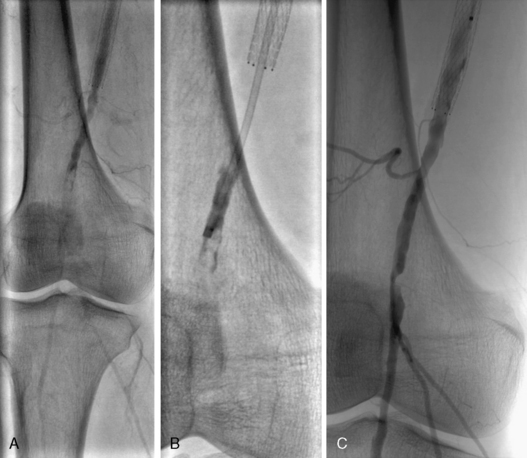
Figure 46-8 Acute limb ischemia due to restenosis and thrombosis of superficial femoral artery (SFA). Embolic occlusion noted distal to stent (A) is engaged with a catheter under suction (B) and retrieved, unmasking additional atherosclerotic disease (C).
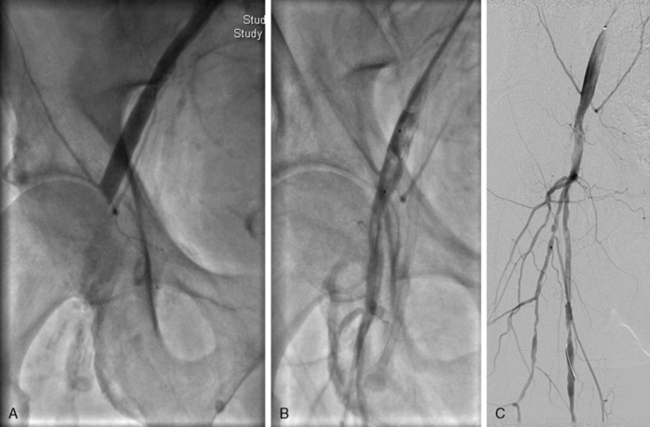
Figure 46-9 Acute limb ischemia after manual compression of right common femoral artery (CFA) access site. A, Thrombotic occlusion of right common femoral artery. B, Thrombus is trapped in a filter embolic protection device and withdrawn from artery toward a sheath. Arterial flow is restored immediately after percutaneous thrombus removal, with evidence of calcified atherosclerotic disease in CFA (C).
Ultrasound-Assisted Thrombolysis
Ultrasound-emitting catheters have been used to assist and accelerate thrombolysis. Administration of high-energy ultrasound can mechanically fragment thrombus,102,103 whereas low-energy ultrasound accelerates enzymatic thrombus lysis by dissociating fibrin strands, exposing more fibrin binding sites, and increasing thrombus permeability and penetration by thrombolytics.104,105 These effects have the potential for accelerating reperfusion and reducing hemorrhagic complications of thrombolytic therapy.
Four small studies investigated ultrasound-assisted thrombolysis for acute limb ischemia. The EKOS EndoWave low-energy system (EKOS Corp., Bothell, Wash.) was tested in 25 patients with acute lower-extremity arterial occlusion. Complete thrombus resolution was noted in 88% of patients after mean therapy time of only 16.9 ± 10.9 hours.106 Another study compared ultrasound accelerated thrombolysis with mechanical thrombectomy using the Rotarex device in 20 patients with acute femoropopliteal graft occlusion.107 Motarjeme used ultrasound-accelerated thrombolysis to treat 24 subacute arterial occlusions, with a technical success rate of 100% and complete thrombus lysis in 96% of cases after a mean treatment period of 16.4 hours (range, 3-25 hours).108 The mean duration of thrombolytic infusion in the ultrasound arm was 15 hours, with a technical success rate of 90%. Another prospective study of 21 patients treated with ultrasound-accelerated thrombolysis showed that complete lysis was achieved in 20 patients, without hemorrhagic complications and 30-day vessel or graft patency of 81%.109 The Dutch DUET study will compare the efficacy of standard catheter-directed thrombolysis and ultrasound-assisted thrombolysis in a randomized trial of acute and chronic thrombosis of native and bypassed infrainguinal vessels with class I and IIa symptoms.94
Gradual dissolution of thrombus may provoke distal embolization of smaller fragments into the distal circulation. This complication can occur in 5% of procedures and is manifested by sudden worsening of pain or loss of distal pulses.2 This complication requires temporary increase in the thrombolytic dose and, if symptoms do not improve in the course of the next 1 to 2 hours, repeat angiography may be warranted.
In modern practice, the distinction between surgical and endovascular techniques is often blurred, and patients with acute ischemic symptoms are often treated with catheter-directed thrombolysis followed by either endovascular, combined, or open procedures.110 In a recent series of 119 patients with acute limb ischemia, 54% of cases involved solely endovascular techniques, 13% open techniques, and 25% combined techniques.110 Femoropopliteal and tibial thrombosis was associated with less favorable outcomes compared to patients who had occlusion of the aortoiliac segment. After 30 days, 82% of patients in this series were alive without limb loss. Complications included access-site hematoma in 11% of patients, transfusion-requiring bleeding in 8%, and compartment syndrome in 4% of patients. Thirty-day mortality was observed in 6% of patients, most of them associated with surgical amputation, while limb salvage and survival at 1 year were 74.6% and 85.7%, respectively.
Surgical Therapy of Acute Limb Ischemia
Modern surgical therapy for acute limb ischemia was introduced in 1963 in a landmark study by Fogarty et al.111 Prior to development of the Fogarty catheter, emboli were retrieved by direct exposure of the occluded artery and its exploration with rigid instruments and suction devices. These methods were not only largely ineffective but also damaging to the artery.112,113 Fogarty’s technique allowed arterial exposure away from the occluded segment, with much lower risk of arterial injury. Physical examination guides the site of surgical exposure; absence of a palpable popliteal pulse requires femoral artery exposure regardless of the presence of a femoral pulse. This approach allows embolectomy of the iliac, superficial femoral, profunda, and popliteal arteries. Physical examination supporting infrapopliteal occlusion will guide popliteal artery exposure and allow cannulation of individual tibial vessels. In cases of upper-extremity acute limb ischemia, the brachial artery is the preferred exposure site. Appropriately sized balloon-tipped embolectomy catheters are advanced into the occluded artery, inflated distally, and pulled back, removing the thrombus (Fig. 46-10). Appropriate technique is essential to avoid arterial dissection and excessive endothelial injury.
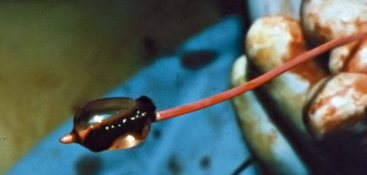
Figure 46-10 Fogarty embolectomy balloon with thrombus removed from popliteal artery.
(Image courtesy Dr. Edwin Gravereaux.)
When embolectomy does not reconstitute pedal perfusion, intraoperative angiography is performed to determine whether adjunctive surgical or endovascular intervention is required to treat residual distal thrombus. Direct exploration of the tibial vessels at the ankle is associated with high rates of rethrombosis, so intraoperative fibrinolytic therapy may be a more effective therapy. Intraoperative angiography should be performed to confirm complete embolectomy. Residual thrombus can be seen in as many as 30% of embolectomy procedures.114,115 Similarly, Doppler examination should accompany completion angiography to document restoration of arterial perfusion, although arterial spasm may attenuate the detected signals. Arterial rupture, perforation, intimal injury, and distal embolization can complicate embolectomy and underscore the importance of performing completion angiography.
In cases of acute limb ischemia caused by embolism, embolectomy is usually sufficient. Removal of the intravascular debris from a healthy vessel restores perfusion without the need for additional intervention. In patients with acute ischemia due to thrombosis, the underlying atherosclerotic disease must be addressed, either by a surgical bypass or hybrid endovascular approach with angioplasty or stent placement. Indeed, as the population presenting with acute limb ischemia has shifted toward elderly patients with preexisting PAD and in situ thrombosis, Fogarty embolectomy has ceased to be a standalone technique. Instead, modern surgical therapy for acute limb ischemia incorporates complex vascular reconstruction, embolectomy, angiography, and hybrid endovascular techniques.114
Treatment of Upper-Extremity Ischemia
Most reported series regarding management of ischemia of the upper extremities come from surgical experience, and therefore carry an inherent bias by underreporting the outcomes of conservative management. The development of simple and well-tolerated embolectomy techniques has increased the frequency of surgical interventions for upper-extremity ischemia.
Before surgical embolectomy techniques gained popularity, conservative management included warming, pharmacological vasodilation, and anticoagulation, with sympathectomy reserved for intractable pain. Baird reported a series of 95 patients treated before the advent of the Fogarty balloon. Among the 78 patients treated conservatively, 68% did not suffer any residual effect, 24% suffered from residual weakness or claudication, and 8% required amputation or had complete loss of function in the extremity. These results and the superior collateral circulation of the upper extremity led to recommendations for conservative treatment, a practice largely abandoned today in favor of surgical embolectomy. Subsequent reports indicated that as many as 50% of patients treated conservatively were left with significant functional impairment, strengthening the argument for more aggressive intervention.116
Prior to the development of balloon embolectomy, surgical interventions involved arteriotomies at multiple sites and removal of the clot by “milking out” the arm or use of corkscrew wires and forceps. Introduction of the Fogarty balloon catheter enabled removal of thrombus under local anesthesia through a single brachial arteriotomy in the antecubital fossa. Modern surgical techniques result in amputation and symptom-free outcomes in 80% to 90% of patients.117,118 A more recent series of 251 patients treated with surgical embolectomy over a period of 2 decades reported amputation rates of 2% and a mortality rate of 5.6% from cardiac and cerebrovascular complications, despite the fact that general anesthesia was used in only 3% of procedures.119 The high perioperative mortality and 40% subsequent mortality underscores the severity of coexisting medical conditions in patients with acute arterial occlusions.
Catheter-directed thrombolysis has not been widely used in the treatment of acute arm ischemia. Upper-limb salvage rates are much higher than in lower-limb arterial occlusion, and the risk of bleeding associated with thrombolytic therapy may thus be more justified in lower-extremity interventions. Nevertheless, initial reports of catheter-directed thrombolysis have been successful. Coulon et al. described a series of 13 patients with acute occlusion of the axillary and brachial arteries largely due to atrial fibrillation. Catheter-directed thrombolysis resulted in complete thrombus resolution in 8 patients, full recovery in 11, and no limb loss.120 Others have reported similar results in small groups of patients.121,122 Thrombolytic therapy may be particularly useful in cases of digital vessel thrombosis.
Compartment Syndrome
Compartment syndrome follows intracranial hemorrhage as the most feared complication of revascularization procedures in patients with acute limb ischemia. Post-reperfusion compartment syndrome most frequently occurs in patients with surgically treated class IIb and III symptoms, but can also occur in patients with less severe ischemia undergoing endovascular therapies. Ischemic reperfusion injury can occur even after only an hour of ischemia.123 Mortality from this syndrome ranged from 7.5% to 41% in the 1960s and 1970s and remains high today.124 The likelihood of developing compartment syndrome is directly related to the duration of ischemia; the longer the ischemic period, the higher the likelihood of reperfusion syndrome and worse clinical outcome. Reperfusion within 12 hours of ischemia onset has been associated with mortality and limb salvage rates of 12% and 93%, respectively. Reperfusion after more than 12 hours of ischemia carries a much worse prognosis: mortality rates can be as high as 31%, with limb salvage rates of 78%.125
Compartment syndrome is caused by tissue swelling following restoration of blood flow and reperfusion injury.126 The tissue injury initiated during the ischemic period is continued by reperfusion with oxygenated blood, with introduction of oxygen free radicals and inflammatory cells. Free radicals peroxidate the lipid component of cell membranes, thus enhancing capillary permeability and muscle edema.123,127 Compartment syndrome occurs when high pressure in a confined fascial space reduces capillary perfusion below a level needed to maintain tissue viability.128 The resulting pressure decreases venous drainage from swollen muscle groups encased in firm fascial layers. Pressure in the limb compartment increases to further decrease venous and capillary flow, and eventually overcomes arterial pressure and stops arterial perfusion. Unless rapidly decompressed, the compartment pressures will result in irreversible neuromuscular damage.
Clinical signs and symptoms of the syndrome include rapidly progressive pain out of proportion to the clinical situation. Clinical examination is characterized by pain on passive stretch of the muscle in the affected compartment, paresthesias of the muscles in the compartment, and hypoesthesia in the distribution of the nerve traversing the affected compartment. Limb examination is notable for a pale and painful swollen calf, thigh, or forearm. Distal pulses may become undetectable if the pressure becomes severe enough, but their presence does not exclude the syndrome.129 Timely recognition of this complication is crucial because compartment pressure exceeding 30 mmHg for 6 to 8 hours leads to irreversible limb injury and limb loss. Some reports indicate that untreated compartment syndrome results in muscle necrosis within 3 hours.130 Diagnosis of the syndrome is established by physical examination, but compartment pressure measurement can help confirm the diagnosis in some cases. The compartment pressure criteria used to guide the decision for urgent fasciotomy vary from 30 mmHg, 45 mmHg, or any pressure exceeding the diastolic arterial pressure by 10 to 30 mmHg.131–135
Once recognized, urgent fasciotomy of the three compartments in the thigh or four compartments in the calf (anterior, lateral, deep posterior, and superficial posterior) is recommended. Delay in therapy results in limb loss, rhabdomyolysis, tissue necrosis, renal failure, and death.136,137 Even after successful fasciotomy, amputation rates can be as high as 11% to 21%.138,139 Among patients undergoing fasciotomy for reperfusion injury, even successful limb salvage leaves 36% of the limbs with some degree of dysfunction.139
The frequency of fasciotomies after revascularization has been reported to be as high as 16% to 22%, although many of these procedures are performed prophylactically to prevent compartment syndrome.140,141 Patients undergoing thrombolysis usually have less severe ischemia, and reperfusion is more gradual. Consequently, compartment syndrome rates in patients treated with endovascular therapies occurs in 2% of procedures. Some increase in compartment pressure is routinely seen after revascularization of an ischemic limb, but the pressure rarely reaches levels high enough to cause a clinical syndrome.142,143 Experimental evidence suggests that prophylactic fasciotomies performed at the time of reperfusion reduce the amount of muscle injury, compared to fasciotomies performed several hours later. Some authors recommend prophylactic fasciotomies in cases when ischemia exceeds 6 hours, the patients are young, reperfusion is incomplete, and tissue swelling develops immediately upon or even before reperfusion.129,140,144
Adjunctive Medical Therapy
In addition to the underlying medical comorbidities, reperfusion injury is the principal cause of mortality and morbidity after revascularization. To reduce ischemic reperfusion injury, gradual reperfusion with modified reperfusate has been evaluated in experimental animal models. Hypothermia and low initial flow rates have been shown to decrease the severity of reperfusion injury in animal skeletal muscle.145 Controlled reperfusion consists of a 30-minute infusion of crystalloid reperfusion solution mixed with oxygenated blood directly into the revascularized artery and muscle bed.146 Controlled reperfusion does not eliminate reperfusion injury, but may significantly attenuate it with a decrease in tissue edema and preservation of muscle viability and contraction force.147,148 Other strategies have been proposed over the years, but none have penetrated into clinical practice. Administration of free radical scavengers and antiinflammatory agents has been shown to mitigate the deleterious effects of reperfusion.43 Controlled reperfusion with blood mixed with crystalloid to obtain an alkalotic, hypocalcemic, and substrate-rich perfusate has been shown to successfully reduce the degree of reperfusion injury.146,149–151 Patients undergoing controlled reperfusion have superior functional recovery and a lower rate of amputation.152
Iloprost, a synthetic analog of prostacyclin, has been investigated as adjunctive therapy to reduce limb-related complications by improving microcirculation. In a randomized study of 300 patients with acute limb ischemia, patients treated with IA and IV infusion of iloprost had a statistically significant lower 90-day mortality rate compared to patients in the placebo arm.153 There was, however, no difference in the rate of amputation. None of these investigational therapies have reached the mainstream of modern clinical practice.
1 Earnshaw J.J. Demography and etiology of acute leg ischemia. Semin Vasc Surg. 2001;14:86–92.
2 Davies B., Braithwaite B.D., Birch P.A., et al. Acute leg ischaemia in Gloucestershire. Br J Surg. 1997;84:504–508.
3 Bergqvist D., Troeng T., Elfstrom J., et al. Auditing surgical outcome: ten years with the Swedish Vascular Registry--Swedvasc. The Steering Committee of Swedvasc. Eur J Surg Suppl. 1998:3–8.
4 Eliason J.L., Wainess R.M., Proctor M.C., et al. A national and single institutional experience in the contemporary treatment of acute lower extremity ischemia. Ann Surg. 2003;238:382–389. discussion 9–90
5 Williams N., Bell P.R. Acute ischaemia of the upper limb. Br J Hosp Med. 1993;50:579–582.
6 Eyers P., Earnshaw J.J. Acute non-traumatic arm ischaemia. Br J Surg. 1998;85:1340–1346.
7 Dryjski M., Swedenborg J. Acute ischemia of the extremities in a metropolitan area during one year. J Cardiovasc Surg (Torino). 1984;25:518–522.
8 Stonebridge P.A., Clason A.E., Duncan A.J., et al. Acute ischaemia of the upper limb compared with acute lower limb ischaemia; a 5-year review. Br J Surg. 1989;76:515–516.
9 Ouriel K., Veith F.J., Sasahara A.A. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–1111.
10 Jivegard L., Holm J., Schersten T. Acute limb ischemia due to arterial embolism or thrombosis: influence of limb ischemia versus pre-existing cardiac disease on postoperative mortality rate. J Cardiovasc Surg (Torino). 1988;29:32–36.
11 Braithwaite B.D., Davies B., Birch P.A., et al. Management of acute leg ischaemia in the elderly. Br J Surg. 1998;85:217–220.
12 Lambert M., Ball C., Hancock B. Management of acute brachial artery occlusion due to trauma or emboli. Br J Surg. 1983;70:639–640.
13 Katz S.G., Kohl R.D. Direct revascularization for the treatment of forearm and hand ischemia. Am J Surg. 1993;165:312–316.
14 Wirsing P., Andriopoulos A., Botticher R. Arterial embolectomies in the upper extremity after acute occlusion. Report on 79 cases. J Cardiovasc Surg (Torino). 1983;24:40–42.
15 James E.C., Khuri N.T., Fedde C.W., et al. Upper limb ischemia resulting from arterial thromboembolism. Am J Surg. 1979;137:739–744.
16 Darling R.C., Austen W.G., Linton R.R. Arterial embolism. Surg Gynecol Obstet. 1967;124:106–114.
17 Kaar G., Broe P.J., Bouchier-Hayes D.J. Upper limb emboli. A review of 55 patients managed surgically. J Cardiovasc Surg (Torino). 1989;30:165–168.
18 Gazzaniga A.B., Dalen J.E. Paradoxical embolism: its pathophysiology and clinical recognition. Ann Surg. 1970;171:137–142.
19 Bryan A.J., Hicks E., Lewis M.H. Unilateral digital ischaemia secondary to embolisation from subclavian atheroma. Ann R Coll Surg Engl. 1989;71:140–142.
20 Rapp J.H., Reilly L.M., Goldstone J., et al. Ischemia of the upper extremity: significance of proximal arterial disease. Am J Surg. 1986;152:122–126.
21 Keen R.R., McCarthy W.J., Shireman P.K., et al. Surgical management of atheroembolization. J Vasc Surg. 1995;21:773–780. discussion 80–1
22 Ricotta J.J., Scudder P.A., McAndrew J.A., et al. Management of acute ischemia of the upper extremity. Am J Surg. 1983;145:661–666.
23 Banis J.C.Jr, Rich N., Whelan T.J.Jr. Ischemia of the upper extremity due to noncardiac emboli. Am J Surg. 1977;134:131–139.
24 Nehler M.R., Taylor L.M.Jr, Moneta G.L., et al. Upper extremity ischemia from subclavian artery aneurysm caused by bony abnormalities of the thoracic outlet. Arch Surg. 1997;132:527–532.
25 Sachatello C.R., Ernst C.B., Griffen W.O.Jr. The acutely ischemic upper extremity: selective management. Surgery. 1974;76:1002–1009.
26 Prioleau P.G., Katzenstein A.L. Major peripheral arterial occlusion due to malignant tumor embolism: histologic recognition and surgical management. Cancer. 1978;42:2009–2014.
27 Lorentzen J.E., Roder O.C., Hansen H.J. Peripheral arterial embolism. A follow-up of 130 consecutive patients submitted to embolectomy. Acta Chir Scand Suppl. 1980;502:111–116.
28 Jivegard L.E., Arfvidsson B., Holm J., et al. Selective conservative and routine early operative treatment in acute limb ischaemia. Br J Surg. 1987;74:798–801.
29 Vohra R., Lieberman D.P. Arterial emboli to the arm. J R Coll Surg Edinb. 1991;36:83–85.
30 Quraishy M.S., Cawthorn S.J., Giddings A.E. Critical ischaemia of the upper limb. J R Soc Med. 1992;85:269–273.
31 Klonaris C., Georgopoulos S., Katsargyris A., et al. Changing patterns in the etiology of acute lower limb ischemia. Int Angiol. 2007;26:49–52.
32 Kropman R.H., Schrijver A.M., Kelder J.C., et al. Clinical outcome of acute leg ischaemia due to thrombosed popliteal artery aneurysm: systematic review of 895 cases. Eur J Vasc Endovasc Surg. 2010;39:452–457.
33 Ravn H., Bergqvist D., Bjorck M. Nationwide study of the outcome of popliteal artery aneurysms treated surgically. Br J Surg. 2007;94:970–977.
34 Marine L., Castro P., Enriquez A., et al. Four-limb acute ischemia induced by ergotamine in an AIDS patient treated with protease inhibitors. Circulation. 2011;124:1395–1397.
35 Zavaleta E.G., Fernandez B.B., Grove M.K., et al. St. Anthony’s fire (ergotamine induced leg ischemia)--a case report and review of the literature. Angiology. 2001;52:349–356.
36 Mirzayan R., Hanks S.E., Weaver F.A. Cocaine-induced thrombosis of common iliac and popliteal arteries. Ann Vasc Surg. 1998;12:476–481.
37 Knochel J.P. Mechanisms of rhabdomyolysis. Curr Opin Rheumatol. 1993;5:725–731.
38 Blaisdell F.W. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–630.
39 Gillani S., Cao J., Suzuki T., et al. The effect of ischemia reperfusion injury on skeletal muscle. Injury. 2011. epub ahead of print; http://dx.doi.org/10.1016/j.injury.2011.03.008
40 Rubin B.B., Romaschin A., Walker P.M., et al. Mechanisms of postischemic injury in skeletal muscle: intervention strategies. J Appl Physiol. 1996;80:369–387.
41 Collard C.D., Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133–1138.
42 Korthuis R.J., Smith J.K., Carden D.L. Hypoxic reperfusion attenuates postischemic microvascular injury. Am J Physiol. 1989;256:H315–H319.
43 Walker P.M., Lindsay T.F., Labbe R., et al. Salvage of skeletal muscle with free radical scavengers. J Vasc Surg. 1987;5:68–75.
44 Jerome S.N., Akimitsu T., Korthuis R.J. Leukocyte adhesion, edema, and development of postischemic capillary no-reflow. Am J Physiol. 1994;267:H1329–H1336.
45 Korthuis R.J., Grisham M.B., Granger D.N. Leukocyte depletion attenuates vascular injury in postischemic skeletal muscle. Am J Physiol. 1988;254:H823–H827.
46 Carden D.L., Smith J.K., Korthuis R.J. Neutrophil-mediated microvascular dysfunction in postischemic canine skeletal muscle. Role of granulocyte adherence. Circ Res. 1990;66:1436–1444.
47 Walden D.L., McCutchan H.J., Enquist E.G., et al. Neutrophils accumulate and contribute to skeletal muscle dysfunction after ischemia-reperfusion. Am J Physiol. 1990;259:H1809–H1812.
48 Yokota J., Minei J.P., Fantini G.A., et al. Role of leukocytes in reperfusion injury of skeletal muscle after partial ischemia. Am J Physiol. 1989;257:H1068–H1075.
49 Welbourn R., Goldman G., O’Riordain M., et al. Role for tumor necrosis factor as mediator of lung injury following lower torso ischemia. J Appl Physiol. 1991;70:2645–2649.
50 Yassin M.M., Harkin D.W., Barros D’Sa A.A., et al. Lower limb ischemia-reperfusion injury triggers a systemic inflammatory response and multiple organ dysfunction. World J Surg. 2002;26:115–121.
51 Ascer E., Mohan C., Gennaro M., et al. Interleukin-1 and thromboxane release after skeletal muscle ischemia and reperfusion. Ann Vasc Surg. 1992;6:69–73.
52 Hashimoto M., Shingu M., Ezaki I., et al. Production of soluble ICAM-1 from human endothelial cells induced by IL-1 beta and TNF-alpha. Inflammation. 1994;18:163–173.
53 Sato N., Goto T., Haranaka K., et al. Actions of tumor necrosis factor on cultured vascular endothelial cells: morphologic modulation, growth inhibition, and cytotoxicity. J Natl Cancer Inst. 1986;76:1113–1121.
54 Klausner J.M., Anner H., Paterson I.S., et al. Lower torso ischemia-induced lung injury is leukocyte dependent. Ann Surg. 1988;208:761–767.
55 Welbourn C.R., Goldman G., Paterson I.S., et al. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg. 1991;78:651–655.
56 Rutherford R.B., Baker J.D., Ernst C., et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–538.
57 Henke P.K. Contemporary management of acute limb ischemia: factors associated with amputation and in-hospital mortality. Semin Vasc Surg. 2009;22:34–40.
58 Blaisdell F.W., Steele M., Allen R.E. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84:822–834.
59 Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:251–266. discussion 66–8
60 Ouriel K., Shortell C.K., DeWeese J.A., et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–1030.
61 Zaraca F., Stringari C., Ebner J.A., et al. Routine versus selective use of intraoperative angiography during thromboembolectomy for acute lower limb ischemia: analysis of outcomes. Ann Vasc Surg. 2010;24:621–627.
62 Tawes R.L.Jr, Harris E.J., Brown W.H., et al. Arterial thromboembolism. A 20-year perspective. Arch Surg. 1985;120:595–599.
63 Hobson R.W.2nd, Neville R., Watanabe B., et al. Role of heparin in reducing skeletal muscle infarction in ischemia-reperfusion. Microcirc Endothelium Lymphatics. 1989;5:259–276.
64 Wright J.G., Kerr J.C., Valeri C.R., et al. Heparin decreases ischemia-reperfusion injury in isolated canine gracilis model. Arch Surg. 1988;123:470–472.
65 Jivegard L., Holm J., Bergqvist D., et al. Acute lower limb ischemia: failure of anticoagulant treatment to improve one-month results of arterial thromboembolectomy. A prospective randomized multi-center study. Surgery. 1991;109:610–616.
66 Currie I.S., Wakelin S.J., Lee A.J., et al. Plasma creatine kinase indicates major amputation or limb preservation in acute lower limb ischemia. J Vasc Surg. 2007;45:733–739.
67 Tillet W.S. The fibrinolytic activity of hemolytic streptococci. J Exp Med. 1933;58:485–502.
68 Tillett W.S., Johnson A.J., McCarty W.R. The intravenous infusion of the streptococcal fibrinolytic principle (streptokinase) into patients. J Clin Invest. 1955;34:169–185.
69 Clifton E.E. The use of plasmin in humans. Ann N Y Acad Sci. 1957;68:209–229.
70 Dotter C.T., Rosch J., Seaman A.J. Selective clot lysis with low-dose streptokinase. Radiology. 1974;111:31–37.
71 Berridge D.C., Gregson R.H., Hopkinson B.R., et al. Randomized trial of intra-arterial recombinant tissue plasminogen activator, intravenous recombinant tissue plasminogen activator and intra-arterial streptokinase in peripheral arterial thrombolysis. Br J Surg. 1991;78:988–995.
72 Karnabatidis D., Spiliopoulos S., Tsetis D., et al. Quality improvement guidelines for percutaneous catheter-directed intra-arterial thrombolysis and mechanical thrombectomy for acute lower-limb ischemia. Cardiovasc Intervent Radiol. 2011;34:1123–1136.
73 Berridge D.C., Kessel D., Robertson I. Surgery versus thrombolysis for acute limb ischaemia: initial management. Cochrane Database Syst Rev. 2002. CD002784
74 Earnshaw J.J., Whitman B., Foy C. National Audit of Thrombolysis for Acute Leg Ischemia (NATALI): clinical factors associated with early outcome. J Vasc Surg. 2004;39:1018–1025.
75 Ouriel K., Shortell C.K., Azodo M.V., et al. Acute peripheral arterial occlusion: predictors of success in catheter-directed thrombolytic therapy. Radiology. 1994;193:561–566.
76 Olin J.W., Graor R.A. Thrombolytic therapy in the treatment of peripheral arterial occlusions. Ann Emerg Med. 1988;17:1210–1215.
77 Schweizer J., Altmann E., Stosslein F., et al. Comparison of tissue plasminogen activator and urokinase in the local infiltration thrombolysis of peripheral arterial occlusions. Eur J Radiol. 1996;22:129–132.
78 Semba C.P., Murphy T.P., Bakal C.W., et al. Thrombolytic therapy with use of alteplase (rt-PA) in peripheral arterial occlusive disease: review of the clinical literature. The Advisory Panel. J Vasc Interv Radiol. 2000;11:149–161.
79 Hanover T.M., Kalbaugh C.A., Gray B.H., et al. Safety and efficacy of reteplase for the treatment of acute arterial occlusion: complexity of underlying lesion predicts outcome. Ann Vasc Surg. 2005;19:817–822.
80 Laird J.R., Dangas G., Jaff M., et al. Intra-arterial reteplase for the treatment of acute limb ischemia. J Invasive Cardiol. 1999;11:757–762.
81 Drescher P., Crain M.R., Rilling W.S. Initial experience with the combination of reteplase and abciximab for thrombolytic therapy in peripheral arterial occlusive disease: a pilot study. J Vasc Interv Radiol. 2002;13:37–43.
82 Ouriel K., Castaneda F., McNamara T., et al. Reteplase monotherapy and reteplase/abciximab combination therapy in peripheral arterial occlusive disease: results from the RELAX trial. J Vasc Interv Radiol. 2004;15:229–238.
83 McNamara T.O., Fischer J.R. Thrombolysis of peripheral arterial and graft occlusions: improved results using high-dose urokinase. AJR Am J Roentgenol. 1985;144:769–775.
84 Sullivan K.L., Gardiner G.A.Jr, Shapiro M.J., et al. Acceleration of thrombolysis with a high-dose transthrombus bolus technique. Radiology. 1989;173:805–808.
85 Ritchie J.L., Hansen D.D., Vracko R., et al. Mechanical thrombolysis: a new rotational catheter approach for acute thrombi. Circulation. 1986;73:1006–1012.
86 Drasler W.J., Jenson M.L., Wilson G.J., et al. Rheolytic catheter for percutaneous removal of thrombus. Radiology. 1992;182:263–267.
87 Schmitz-Rode T., Gunther R.W., Muller-Leisse C. US-assisted aspiration thrombectomy: in vitro investigations. Radiology. 1991;178:677–679.
88 Allie D.E., Hebert C.J., Lirtzman M.D., et al. Novel simultaneous combination chemical thrombolysis/rheolytic thrombectomy therapy for acute critical limb ischemia: the power-pulse spray technique. Catheter Cardiovasc Interv. 2004;63:512–522.
89 Mathie A.G., Bell S.D., Saibil E.A. Mechanical thromboembolectomy in acute embolic peripheral arterial occlusions with use of the AngioJet Rapid Thrombectomy System. J Vasc Interv Radiol. 1999;10:583–590.
90 Kasirajan K., Gray B., Beavers F.P., et al. Rheolytic thrombectomy in the management of acute and subacute limb-threatening ischemia. J Vasc Interv Radiol. 2001;12:413–421.
91 Ansel G.M., George B.S., Botti C.F., et al. Rheolytic thrombectomy in the management of limb ischemia: 30-day results from a multicenter registry. J Endovasc Ther. 2002;9:395–402.
92 Wagner H.J., Muller-Hulsbeck S., Pitton M.B., et al. Rapid thrombectomy with a hydrodynamic catheter: results from a prospective, multicenter trial. Radiology. 1997;205:675–681.
93 Silva J.A., Ramee S.R., Collins T.J., et al. Rheolytic thrombectomy in the treatment of acute limb-threatening ischemia: immediate results and six-month follow-up of the multicenter AngioJet registry. Possis Peripheral AngioJet Study AngioJet Investigators. Cathet Cardiovasc Diagn. 1998;45:386–393.
94 Muller-Hulsbeck S., Kalinowski M., Heller M., et al. Rheolytic hydrodynamic thrombectomy for percutaneous treatment of acutely occluded infra-aortic native arteries and bypass grafts: midterm follow-up results. Invest Radiol. 2000;35:131–140.
95 Sarac T.P., Hilleman D., Arko F.R., et al. Clinical and economic evaluation of the trellis thrombectomy device for arterial occlusions: preliminary analysis. J Vasc Surg. 2004;39:556–559.
96 Stanek F., Ouhrabkova R., Prochazka D. Mechanical thrombectomy using the Rotarex catheter--safe and effective method in the treatment of peripheral arterial thromboembolic occlusions. Vasa. 2010;39:334–340.
97 Bucker A., Schmitz-Rode T., Vorwerk D., et al. Comparative in vitro study of two percutaneous hydrodynamic thrombectomy systems. J Vasc Interv Radiol. 1996;7:445–449.
98 Reekers J.A., Kromhout J.G., Spithoven H.G., et al. Arterial thrombosis below the inguinal ligament: percutaneous treatment with a thrombosuction catheter. Radiology. 1996;198:49–53.
99 Wagner H.J., Starck E.E. Acute embolic occlusions of the infrainguinal arteries: percutaneous aspiration embolectomy in 102 patients. Radiology. 1992;182:403–407.
100 Zehnder T., Birrer M., Do D.D., et al. Percutaneous catheter thrombus aspiration for acute or subacute arterial occlusion of the legs: how much thrombolysis is needed? Eur J Vasc Endovasc Surg. 2000;20:41–46.
101 Wagner H.J., Starck E.E., Reuter P. Long-term results of percutaneous aspiration embolectomy. Cardiovasc Intervent Radiol. 1994;17:241–246.
102 Siegel R.J., Fishbein M.C., Forrester J., et al. Ultrasonic plaque ablation. A new method for recanalization of partially or totally occluded arteries. Circulation. 1988;78:1443–1448.
103 Steffen W., Fishbein M.C., Luo H., et al. High intensity, low frequency catheter-delivered ultrasound dissolution of occlusive coronary artery thrombi: an in vitro and in vivo study. J Am Coll Cardiol. 1994;24:1571–1579.
104 Braaten J.V., Goss R.A., Francis C.W. Ultrasound reversibly disaggregates fibrin fibers. Thromb Haemost. 1997;78:1063–1068.
105 Francis C.W., Blinc A., Lee S., et al. Ultrasound accelerates transport of recombinant tissue plasminogen activator into clots. Ultrasound Med Biol. 1995;21:419–424.
106 Wissgott C., Richter A., Kamusella P., et al. Treatment of critical limb ischemia using ultrasound-enhanced thrombolysis (PARES Trial): final results. J Endovasc Ther. 2007;14:438–443.
107 Wissgott C., Kamusella P., Richter A., et al. Treatment of acute femoropopliteal bypass graft occlusion: comparison of mechanical rotational thrombectomy with ultrasound-enhanced lysis. Rofo. 2008;180:547–552.
108 Motarjeme A. Ultrasound-enhanced thrombolysis. J Endovasc Ther. 2007;14:251–256.
109 Schrijver A.M., Reijnen M.M., van Oostayen J.A., et al. Initial results of catheter-directed ultrasound-accelerated thrombolysis for thromboembolic obstructions of the aortofemoral arteries: a feasibility study. Cardiovasc Intervent Radiol. 2012;35:279–285.
110 Kashyap V.S., Gilani R., Bena J.F., et al. Endovascular therapy for acute limb ischemia. J Vasc Surg. 2011;53:340–346.
111 Fogarty T.J., Cranley J.J., Krause R.J., et al. A method for extraction of arterial emboli and thrombi. Surg Gynecol Obstet. 1963;116:241–244.
112 Dale W.A. Endovascular suction catheters for thrombectomy and embolectomy. J Thorac Cardiovasc Surg. 1962;44:557–558.
113 Green R.M., DeWeese J.A., Rob C.G. Arterial embolectomy before and after the Fogarty catheter. Surgery. 1975;77:24–33.
114 Hill S.L., Donato A.T. The simple Fogarty embolectomy: an operation of the past? Am Surg. 1994;60:907–911.
115 Plecha F.R., Pories W.J. Intraoperative angiography in the immediate assessment of arterial reconstruction. Arch Surg. 1972;105:902–907.
116 Galbraith K., Collin J., Morris P.J., et al. Recent experience with arterial embolism of the limbs in a vascular unit. Ann R Coll Surg Engl. 1985;67:30–33.
117 Davies M.G., O’Malley K., Feeley M., et al. Upper limb embolus: a timely diagnosis. Ann Vasc Surg. 1991;5:85–87.
118 Pentti J., Salenius J.P., Kuukasjarvi P., et al. Outcome of surgical treatment in acute upper limb ischaemia. Ann Chir Gynaecol. 1995;84:25–28.
119 Hernandez-Richter T., Angele M.K., Helmberger T., et al. Acute ischemia of the upper extremity: long-term results following thrombembolectomy with the Fogarty catheter. Langenbecks Arch Surg. 2001;386:261–266.
120 Coulon M., Goffette P., Dondelinger R.F. Local thrombolytic infusion in arterial ischemia of the upper limb: mid-term results. Cardiovasc Intervent Radiol. 1994;17:81–86.
121 Michaels J.A., Torrie E.P., Galland R.B. The treatment of upper limb vascular occlusions using intraarterial thrombolysis. Eur J Vasc Surg. 1993;7:744–746.
122 Widlus D.M., Venbrux A.C., Benenati J.F., et al. Fibrinolytic therapy for upper-extremity arterial occlusions. Radiology. 1990;175:393–399.
123 Beyersdorf F. Protection of the ischemic skeletal muscle. Thorac Cardiovasc Surg. 1991;39:19–28.
124 Dormandy J., Heeck L., Vig S. Acute limb ischemia. Semin Vasc Surg. 1999;12:148–153.
125 Abbott W.M., Maloney R.D., McCabe C.C., et al. Arterial embolism: a 44 year perspective. Am J Surg. 1982;143:460–464.
126 Perry M.O. Compartment syndromes and reperfusion injury. Surg Clin North Am. 1988;68:853–864.
127 McCord J.M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163.
128 Mubarak S.J., Hargens A.R. Acute compartment syndromes. Surg Clin North Am. 1983;63:539–565.
129 Hyde G.L., Peck D., Powell D.C. Compartment syndromes. Early diagnosis and a bedside operation. Am Surg. 1983;49:563–568.
130 Vaillancourt C., Shrier I., Vandal A., et al. Acute compartment syndrome: how long before muscle necrosis occurs? CJEM. 2004;6:147–154.
131 Mubarak S.J., Owen C.A., Hargens A.R., et al. Acute compartment syndromes: diagnosis and treatment with the aid of the wick catheter. J Bone Joint Surg Am. 1978;60:1091–1095.
132 Rorabeck C.H. The treatment of compartment syndromes of the leg. J Bone Joint Surg Br. 1984;66:93–97.
133 Matsen F.A.3rd, Winquist R.A., Krugmire R.B.Jr. Diagnosis and management of compartmental syndromes. J Bone Joint Surg Am. 1980;62:286–291.
134 Whitesides T.E., Haney T.C., Morimoto K., et al. Tissue pressure measurements as a determinant for the need of fasciotomy. Clin Orthop Relat Res. 1975:43–51.
135 Frink M., Hildebrand F., Krettek C., et al. Compartment syndrome of the lower leg and foot. Clin Orthop Relat Res. 2010;468:940–950.
136 Rush D.S., Frame S.B., Bell R.M., et al. Does open fasciotomy contribute to morbidity and mortality after acute lower extremity ischemia and revascularization? J Vasc Surg. 1989;10:343–350.
137 Jensen S.L., Sandermann J. Compartment syndrome and fasciotomy in vascular surgery. A review of 57 cases. Eur J Vasc Endovasc Surg. 1997;13:48–53.
138 Finkelstein J.A., Hunter G.A., Hu R.W. Lower limb compartment syndrome: course after delayed fasciotomy. J Trauma. 1996;40:342–344.
139 Heemskerk J., Kitslaar P. Acute compartment syndrome of the lower leg: retrospective study on prevalence, technique, and outcome of fasciotomies. World J Surg. 2003;27:744–747.
140 Papalambros E.L., Panayiotopoulos Y.P., Bastounis E., et al. Prophylactic fasciotomy of the legs following acute arterial occlusion procedures. Int Angiol. 1989;8:120–124.
141 Allenberg J.R., Meybier H. The compartment syndrome from the vascular surgery viewpoint. Chirurg. 1988;59:722–727.
142 Qvarfordt P., Christenson J.T., Eklof B., et al. Intramuscular pressure after revascularization of the popliteal artery in severe ischaemia. Br J Surg. 1983;70:539–541.
143 Melberg P.E., Styf J., Biber B., et al. Muscular compartment pressure following reconstructive arterial surgery of the lower limbs. Acta Chir Scand. 1984;150:129–133.
144 Patman R.D. Compartmental syndromes in peripheral vascular surgery. Clin Orthop Relat Res. 1975:103–110.
145 Wright J.G., Belkin M., Hobson R.W.2nd. Hypothermia and controlled reperfusion: two non-pharmacologic methods which diminish ischemia-reperfusion injury in skeletal muscle. Microcirc Endothelium Lymphatics. 1989;5:315–334.
146 Beyersdorf F., Schlensak C. Controlled reperfusion after acute and persistent limb ischemia. Semin Vasc Surg. 2009;22:52–57.
147 Dick F., Li J., Giraud M.N., et al. Basic control of reperfusion effectively protects against reperfusion injury in a realistic rodent model of acute limb ischemia. Circulation. 2008;118:1920–1928.
148 Anderson R.J., Cambria R., Kerr J., et al. Sustained benefit of temporary limited reperfusion in skeletal muscle following ischemia. J Surg Res. 1990;49:271–275.
149 Defraigne J.O., Pincemail J., Laroche C., et al. Successful controlled limb reperfusion after severe prolonged ischemia. J Vasc Surg. 1997;26:346–350.
150 Walker P.M., Romaschin A.D., Davis S., et al. Lower limb ischemia: phase 1 results of salvage perfusion. J Surg Res. 1999;84:193–198.
151 Mowlavi A., Neumeister M.W., Wilhelmi B.J., et al. Local hypothermia during early reperfusion protects skeletal muscle from ischemia-reperfusion injury. Plast Reconstr Surg. 2003;111:242–250.
152 Wilhelm M.P., Schlensak C., Hoh A., et al. Controlled reperfusion using a simplified perfusion system preserves function after acute and persistent limb ischemia: a preliminary study. J Vasc Surg. 2005;42:690–694.
153 de Donato G., Gussoni G., de Donato G., et al. The ILAILL study: iloprost as adjuvant to surgery for acute ischemia of lower limbs: a randomized, placebo-controlled, double-blind study by the Italian Society for Vascular and Endovascular Surgery. Ann Surg. 2006;244:185–193.