Chapter 81 Defects in Metabolism of Carbohydrates
Carbohydrate synthesis and degradation provide the energy required for most metabolic processes. The important carbohydrates include 3 monosaccharides—glucose, galactose, and fructose—and a polysaccharide, glycogen. The relevant biochemical pathways of these carbohydrates are shown in Figure 81-1. Glucose is the principal substrate of energy metabolism. A continuous source of glucose from dietary intake, gluconeogenesis, and glycogenolysis of glycogen maintains normal blood glucose levels. Metabolism of glucose generates adenosine triphosphate (ATP) via glycolysis (conversion of glucose or glycogen to pyruvate), mitochondrial oxidative phosphorylation (conversion of pyruvate to carbon dioxide and water), or both. Dietary sources of glucose come from ingesting polysaccharides, primarily starch and disaccharides, including lactose, maltose, and sucrose. Oral intake of glucose is intermittent and unreliable. Glucose made de novo from amino acids, primarily alanine (gluconeogenesis), contributes to maintaining the euglycemic state, but this process requires time. The breakdown of hepatic glycogen provides the rapid release of glucose, which maintains a constant blood glucose concentration. Glycogen is also the primary stored energy source in muscle, providing glucose for muscle activity during exercise. Galactose and fructose are monosaccharides that provide fuel for cellular metabolism; their role is less significant than that of glucose. Galactose is derived from lactose (galactose + glucose), which is found in milk and milk products. Galactose is an important energy source in infants, but it is 1st metabolized to glucose. Galactose (exogenous or endogenously synthesized from glucose) is also an important component of certain glycolipids, glycoproteins, and glycosaminoglycans. The dietary sources of fructose are sucrose (fructose + glucose, sorbitol) and fructose itself, which is found in fruits, vegetables, and honey.
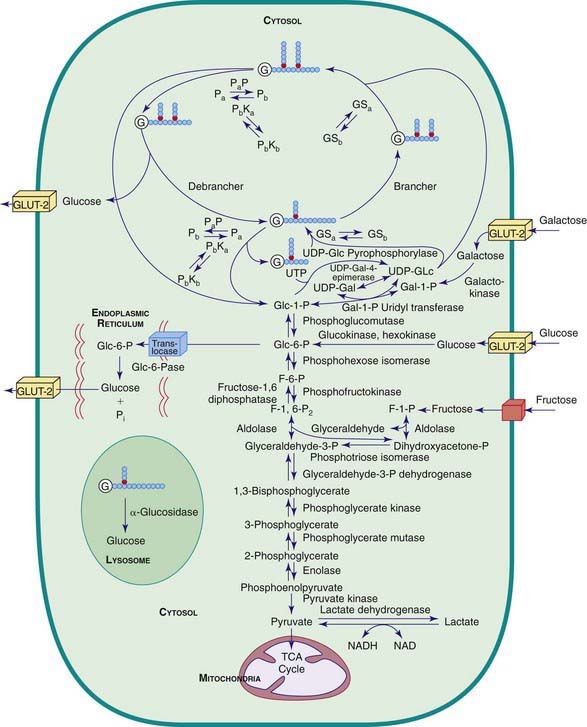
Figure 81-1 Pathway related to glycogen storage diseases and galactose and fructose disorders. GSa, active glycogen synthetase; GSb, inactive glycogen synthetase; Pa, active phosphorylase; Pb, inactive phosphorylase; PaP, phosphorylase a phosphatase; PbKa, active phosphorylase b kinase; PbKb, inactive phosphorylase b kinase; G, glycogen, the primer for glycogen synthesis; UDP, uridine diphosphate; GLUT-2, glucose transporter 2; NAD/NADH, nicotinamide-adenine dinucleotide.
(Modified from Beaudet AR: Glycogen storage disease. In Harrison TR, Isselbacher KJ, editors: Harrison’s principles of internal medicine, ed 13, New York, 1994, McGraw-Hill. Reproduced with permission of The McGraw-Hill Companies.)
Defects in glycogen metabolism typically cause an accumulation of glycogen in the tissues, hence the name glycogen storage disease (Table 81-1). Defects in gluconeogenesis or the glycolytic pathway, including galactose and fructose metabolism, do not result in an accumulation of glycogen (see Table 81-1). The defects in pyruvate metabolism in the pathway of the conversion of pyruvate to carbon dioxide and water via mitochondrial oxidative phosphorylation are more often associated with lactic acidosis and some tissue glycogen accumulation.
81.1 Glycogen Storage Diseases
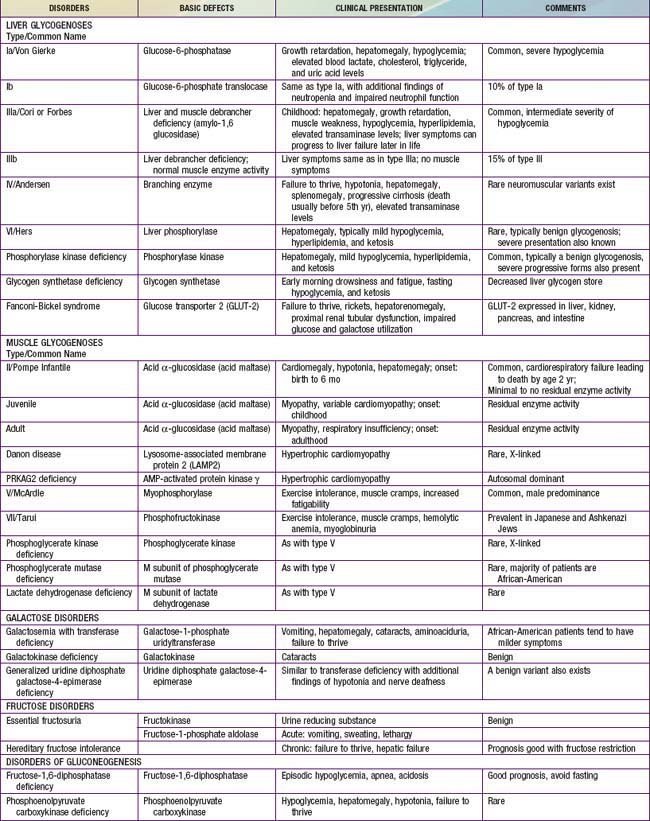
The disorders of glycogen metabolism, the glycogen storage diseases (GSDs), result from deficiencies of various enzymes or transport proteins in the pathways of glycogen metabolism (see Fig. 81-1). The glycogen found in these disorders is abnormal in quantity, quality, or both. GSDs are categorized by numeric type in accordance with the chronological order in which these enzymatic defects were identified. This numeric classification is still widely used, at least up to number VII. The GSDs can also be classified by organ involvement and clinical manifestations into liver and muscle glycogenoses (see Table 81-1).
There are more than 12 forms of glycogenoses. Glucose-6-phosphatase deficiency (type I), lysosomal acid α-glucosidase deficiency (type II), debrancher deficiency (type III), and liver phosphorylase kinase deficiency (type IX) are the most common that typically present in early childhood; myophosphorylase deficiency (type V, McArdle disease) is the most common in adolescents and adults. The frequency of all forms of GSD is ≈1/20,000 live births.
Liver Glycogenoses
The GSDs that principally affect the liver include glucose-6-phosphatase deficiency (type I), debranching enzyme deficiency (type III), branching enzyme deficiency (type IV), liver phosphorylase deficiency (type VI), phosphorylase kinase deficiency (type IX, formerly termed GSD VIa), glycogen synthetase deficiency (type 0), and glucose transporter-2 defect. Because hepatic carbohydrate metabolism is responsible for plasma glucose homeostasis, this group of disorders typically causes fasting hypoglycemia and hepatomegaly. Some (type III, type IV, type IX) can be associated with liver cirrhosis. Other organs can also be involved and may manifest as renal dysfunction in type I, myopathy (skeletal and/or cardiomyopathy) in types III and IV, as well as in some rare forms of phosphorylase kinase deficiency, and neurological involvement in types II (the brain, anterior horns cells), III (peripheral nerves), and IV (some patients can present with diffuse central and peripheral nervous system dysfunction).
Type I Glycogen Storage Disease (Glucose-6-Phosphatase or Translocase Deficiency, Von Gierke Disease)
Type I GSD is caused by the absence or deficiency of glucose-6-phosphatase activity in the liver, kidney, and intestinal mucosa. It can be divided into two subtypes: type Ia, in which the glucose-6-phosphatase enzyme is defective; and type Ib, in which a translocase that transports glucose-6-phosphate across the microsomal membrane is defective. The defects in both type Ia and type Ib lead to inadequate hepatic conversion of glucose-6-phosphate to glucose through normal glycogenolysis and gluconeogenesis and make affected individuals susceptible to fasting hypoglycemia.
Type I GSD is an autosomal recessive disorder. The structural gene for glucose-6-phosphatase is located on chromosome 17q21; the gene for translocase is on chromosome 11q23. Common mutations responsible for the disease are known. Carrier detection and prenatal diagnosis are possible with the DNA-based diagnosis.
Clinical Manifestations
Patients with type I GSD may present in the neonatal period with hypoglycemia and lactic acidosis; they more commonly present at 3-4 mo of age with hepatomegaly, hypoglycemic seizures, or both. These children often have doll-like faces with fat cheeks, relatively thin extremities, short stature, and a protuberant abdomen that is due to massive hepatomegaly; the kidneys are also enlarged, whereas the spleen and heart are normal.
The biochemical hallmarks of the disease are hypoglycemia, lactic acidosis, hyperuricemia, and hyperlipidemia. Hypoglycemia and lactic acidosis can develop after a short fast. Hyperuricemia is present in young children; gout rarely develops before puberty. Despite marked hepatomegaly, the liver transaminase levels are usually normal or only slightly elevated. Intermittent diarrhea may occur in GSD I. In patients with GSD Ib, the loss of mucosal barrier function due to inflammation, which is likely related to the disturbed neutrophil function, seems to be the main cause of diarrhea. Easy bruising and epistaxis are common and are associated with a prolonged bleeding time as a result of impaired platelet aggregation and adhesion.
The plasma may be “milky” in appearance as a result of a striking elevation of triglyceride levels. Cholesterol and phospholipids are also elevated, but less prominently. The lipid abnormality resembles type IV hyperlipidemia and is characterized by increased levels of very low density lipoprotein, low-density lipoprotein, and a unique apolipoprotein profile consisting of increased levels of apo B, C, and E, with relatively normal or reduced levels of apo A and D. The histologic appearance of the liver is characterized by a universal distention of hepatocytes by glycogen and fat. The lipid vacuoles are particularly large and prominent. There is little associated fibrosis.
All these findings apply to both type Ia and type Ib GSD, but type Ib has additional features of recurrent bacterial infections from neutropenia and impaired neutrophil function. Gut mucosa ulceration culminating in GSD enterocolitis is also common. Exceptional cases of type Ib without neutropenia and type Ia with neutropenia have been reported.
Although type I GSD affects mainly the liver, multiple organ systems are involved. Puberty is often delayed. Virtually all females have ultrasound findings consistent with polycystic ovaries; other features of polycystic ovary syndrome (acne, hirsuitism) are not seen. Nonetheless, fertility appears to be normal, as evidenced in several reports of successful pregnancy in women with GSD I. Increased bleeding during menstrual cycles, including life-threatening menorrhagia, has been noted and could be related to the impaired platelet aggregation. Symptoms of gout usually start around puberty from long-term hyperuricemia. Secondary to the lipid abnormalities, there is an increased risk of pancreatitis. The dyslipidemia, together with elevated erythrocyte aggregation, predisposes these patients to atherosclerosis. Premature atherosclerosis has not yet been clearly documented except for rare cases. Impaired platelet aggregation and increased antioxidative defense to prevent lipid peroxidation may function as a protective mechanism to help reduce the risk of atherosclerosis. Frequent fractures and radiographic evidence of osteopenia are common; bone mineral content is reduced even in prepubertal patients.
By the 2nd or 3rd decade of life, most patients with type I GSD exhibit hepatic adenomas that can hemorrhage and, in some cases, become malignant. Pulmonary hypertension has been seen in some long-term survivors of the disease.
Renal disease is another complication, and most patients with type I GSD who are >20 yr of age have proteinuria. Many also have hypertension, renal stones, nephrocalcinosis, and altered creatinine clearance. Glomerular hyperfiltration, increased renal plasma flow, and microalbuminuria are often found in the early stages of renal dysfunction and can occur before the onset of proteinuria. In younger patients, hyperfiltration and hyperperfusion may be the only signs of renal abnormalities. With the advancement of renal disease, focal segmental glomerulosclerosis and interstitial fibrosis become evident. In some patients, renal function has deteriorated and progressed to failure, requiring dialysis and transplantation. Other renal abnormalities include amyloidosis, a Fanconi-like syndrome, hypocitraturia, hypercalciuria, and a distal renal tubular acidification defect.
Diagnosis
The diagnosis of type I GSD is suspected on the basis of clinical presentation and the laboratory findings of hypoglycemia, lactic acidosis, hyperuricemia, and hyperlipidemia. Neutropenia is noted in GSD Ib patients, typically after the 1st 2-3 yr of life. Administration of glucagon or epinephrine results in little or no rise in blood glucose level, but the lactate level rises significantly. Before the glucose-6-phosphatase and glucose-6-phosphate translocase genes were cloned, a definitive diagnosis required a liver biopsy. Gene-based mutation analysis provides a noninvasive way of diagnosis for most patients with types Ia and Ib disease.
Treatment
Treatment is designed to maintain normal blood glucose levels and is achieved by continuous nasogastric infusion of glucose or oral administration of uncooked cornstarch. Nasogastric drip feeding can be introduced in early infancy from the time of diagnosis. It can consist of an elemental enteral formula or contain only glucose or a glucose polymer to provide sufficient glucose to maintain normoglycemia during the night. Frequent feedings with high-carbohydrate content are given during the day.
Uncooked cornstarch acts as a slow-release form of glucose and can be introduced at a dose of 1.6 g/kg every 4 hr for infants <2 yr of age. The response of young infants is variable. As the child grows older, the cornstarch regimen can be changed to every 6 hr at a dose of 1.75-2.5 g/kg of body weight. New starch products, which are currently being developed, are thought to be longer acting, better tolerated, and more palatable. A short-term double-blind crossover pilot study comparing uncooked, physically modified cornstarch to traditional cornstarch showed that the majority of GSD patients treated with the new starch had better short-term metabolic control and longer duration of euglycemia; however, more extensive studies replicating these results are necessary at this time. Because fructose and galactose cannot be converted directly to glucose in GSD type I, these sugars are restricted in the diet. Sucrose (table sugar, cane sugar, other ingredients), fructose (fruit, juice, high fructose corn syrup), lactose (dairy foods), and sorbitol should be avoided or limited. Due to these dietary restrictions, vitamins and minerals such as calcium and vitamin D may be deficient and supplementation is required to prevent nutritional deficiencies. Dietary therapy improves hyperuricemia, hyperlipidemia, and renal function, slowing the development of renal failure. This therapy fails, however, to normalize blood uric acid and lipid levels completely in some individuals, despite good metabolic control, especially after puberty. The control of hyperuricemia can be further augmented by the use of allopurinol, a xanthine oxidase inhibitor. The hyperlipidemia can be reduced with lipid-lowering drugs such as HMG-CoA reductase inhibitors and fibrate (Chapter 80). Microalbuminuria, an early indicator of renal dysfunction in type I disease, is treated with angiotensin-converting enzyme (ACE) inhibitors. Citrate supplements can be beneficial for patients with hypocitraturia by preventing or ameliorating nephrocalcinosis and development of urinary calculi. Growth hormone should be used with extreme caution and limited to only those with a documented growth hormone deficiency. Even in those cases, there should be close monitoring of metabolic parameters and presence of adenomas.
In patients with type Ib GSD, granulocyte and granulocyte-macrophage colony–stimulating factors are successful in correcting the neutropenia, decreasing the number and severity of bacterial infections, and improving the chronic inflammatory bowel disease.
Orthotopic liver transplantation is a potential cure of type I GSD, but the inherent short- and long-term complications leave this as a treatment of last resort, usually for patients with liver malignancy, multiple liver adenomas, metabolic derangements refractory to medical management, and/or liver failure. Large adenomas (>2 cm) that are rapidly increasing in size and/or number may require partial hepatic resection. Smaller adenomas (<2 cm) can be treated with percutaneous ethanol injection or transcatheter arterial embolization. A challenge is the recurrence of liver adenomas with potential for malignant transformation in these patients, ultimately requiring a liver transplant. Bone marrow transplantation has been reported to correct the neutropenia of type Ib.
Before any surgical procedure, the bleeding status must be evaluated and good metabolic control established. Prolonged bleeding times can be normalized by the use of intensive intravenous glucose infusion for 24-48 hr before surgery. Use of 1-deamino-8-D-arginine vasopressin (DDAVP) can reduce bleeding complications. Lactated ringer solution should be avoided because it contains lactate and no glucose. Glucose levels should be maintained in the normal range throughout surgery with the use of 10% dextrose.
Prognosis
Previously, many patients with type I GSD died at a young age, and the prognosis was guarded for those who survived. The long-term complications occur mostly in adults whose disease was not adequately treated during childhood. Early diagnosis and effective treatment have improved the outcome; renal disease and formation of hepatic adenomas with potential risk for malignant transformation remain serious complications.
Type III Glycogen Storage Disease (Debrancher Deficiency, Limit Dextrinosis)
Type III GSD is caused by a deficiency of glycogen debranching enzyme activity. Debranching enzyme, together with phosphorylase, is responsible for complete degradation of glycogen. When debranching enzyme is defective, glycogen breakdown is incomplete and an abnormal glycogen with short outer branch chains and resembling limit dextrin accumulates. Deficiency of glycogen debranching enzyme causes hepatomegaly, hypoglycemia, short stature, variable skeletal myopathy, and variable cardiomyopathy. The disorder usually involves both liver and muscle and is termed type IIIa GSD. In approximately 15% of patients, the disease appears to involve only liver and is classified as type IIIb.
Type III glycogenosis is an autosomal recessive disease that has been reported in many different ethnic groups; the frequency is relatively high in Sephardic Jews from North Africa. The gene for debranching enzyme is located on chromosome 1p21. More than 30 different mutations are identified; 2 exon 3 mutations (17delAG and Q6X) are specifically associated with glycogenosis IIIb. Carrier detection and prenatal diagnosis are possible using DNA-based linkage or mutation analysis.
Clinical Manifestations
During infancy and childhood, the disease may be indistinguishable from type I GSD, because hepatomegaly, hypoglycemia, hyperlipidemia, and growth retardation are common (Fig. 81-2). Splenomegaly may be present, but the kidneys are not enlarged. Remarkably, hepatomegaly and hepatic symptoms in most patients with type III GSD improve with age and usually resolve after puberty. Progressive liver cirrhosis and failure can occur. Hepatocellular carcinoma has also been reported, more typically in patients with progressive liver cirrhosis. The frequency of adenomas in individuals with GSD III is far less, compared to GSD I. Furthermore, the relationship of hepatic adenomas and malignancy in GSD III is unclear. A single case of malignant transformation at the site of adenomas has been noted. In patients with muscular involvement (type IIIa), muscle weakness can present in childhood but can become severe after the 3rd or 4th decade of life, as evidenced by slowly progressive weakness and wasting. Because patients with GSD III can have decreased bone density, they are at an increased risk of potential fracture. Myopathy does not follow any particular pattern of involvement; both proximal and distal muscles are involved. Electromyography reveals a widespread myopathy; nerve conduction studies are often abnormal. Ventricular hypertrophy is a frequent finding, but overt cardiac dysfunction is rare. There have been some reports of life-threatening arrhythmia and need for heart transplant in some GSD III patients. Hepatic symptoms in some patients may be so mild that the diagnosis is not made until adulthood, when the patients show symptoms and signs of neuromuscular disease. The initial diagnosis has been confused with Charcot-Marie-Tooth disease. Polycystic ovaries are common; some patients can develop hirsutism, irregular menstrual cycles, and other features of polycystic ovarian syndrome. Fertility does not appear to be reduced; successful pregnancies in patients with GSD III have been reported.
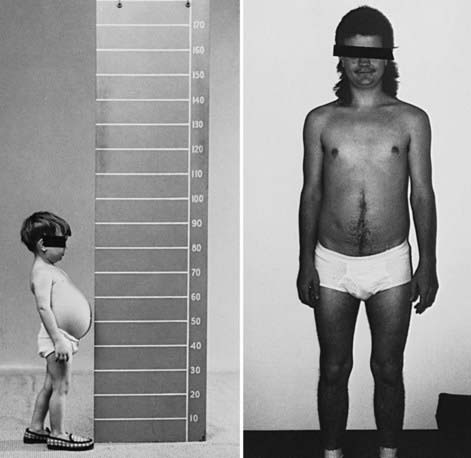
Figure 81-2 Growth and development in a patient with type IIIb glycogen storage disease. The patient has debrancher deficiency in liver but normal activity in muscle. As a child, he had hepatomegaly, hypoglycemia, and growth retardation. After puberty, he no longer had hepatomegaly or hypoglycemia, and his final adult height is normal. He had no muscle weakness or atrophy; this is in contrast to type IIIa patients, in whom a progressive myopathy is seen in adulthood.
Hypoglycemia and hyperlipidemia are common. In contrast to type I GSD, elevation of liver transaminase levels and fasting ketosis are prominent, but blood lactate and uric acid concentrations are usually normal. Serum creatine kinase levels can be useful to identify patients with muscle involvement; normal levels do not rule out muscle enzyme deficiency. The administration of glucagon 2 hr after a carbohydrate meal provokes a normal increase in blood glucose; after an overnight fast, glucagon may provoke no change in blood glucose level.
Diagnosis
The histologic appearance of the liver is characterized by a universal distention of hepatocytes by glycogen and the presence of fibrous septa. The fibrosis and the paucity of fat distinguish type III glycogenosis from type I. The fibrosis, which ranges from minimal periportal fibrosis to micronodular cirrhosis, appears in most cases to be nonprogressive. Overt cirrhosis has been seen in some patients with GSD III.
Patients with myopathy and liver symptoms have a generalized enzyme defect (type IIIa). The deficient enzyme activity can be demonstrated not only in liver and muscle, but also in other tissues such as heart, erythrocytes, and cultured fibroblasts. Patients with hepatic symptoms without clinical or laboratory evidence of myopathy have debranching enzyme deficiency only in the liver, with enzyme activity retained in the muscle (type IIIb). Definite diagnosis requires enzyme assay in liver, muscle, or both. Mutation analysis can provide a noninvasive method for diagnosis and subtype assignment in the majority of patients; however, the large size of the gene and the distribution of mutations across the entire gene present a challenge.
Treatment
Dietary management is less demanding than in type I GSD. Patients do not need to restrict dietary intake of fructose and galactose. If hypoglycemia is present, frequent meals high in carbohydrates with cornstarch supplements or nocturnal gastric drip feedings are usually effective. A high-protein diet during the daytime plus overnight protein enteral infusion can also be effective in preventing hypoglycemia and preventing endogenous protein breakdown because protein can be used as a substrate for gluconeogenesis, a pathway that is intact in type III GSD. There is no satisfactory treatment for the progressive myopathy other than recommending a high-protein diet and an exercise program. Liver transplantation has been performed in patients with end-stage cirrhosis and/or hepatic carcinoma.
Type IV Glycogen Storage Disease (Branching Enzyme Deficiency, Amylopectinosis, or Andersen Disease)
Deficiency of branching enzyme activity results in accumulation of an abnormal glycogen with poor solubility. The disease is referred to as type IV GSD or amylopectinosis because the abnormal glycogen has fewer branch points, more α 1-4 linked glucose units, and longer outer chains, resulting in a structure resembling amylopectin.
Type IV GSD is an autosomal recessive disorder. The glycogen branching enzyme gene is located on chromosome 3p21. Mutations responsible for type IV GSD have been identified, and their characterization in individual patients can be useful in predicting the clinical outcome. Some mutations are associated with a good prognosis and lack of progression of liver disease.
Clinical Manifestations
This disorder is clinically variable. The most common and classic form is characterized by progressive cirrhosis of the liver and is manifested in the 1st 18 mo of life as hepatosplenomegaly and failure to thrive. The cirrhosis progresses to portal hypertension, ascites, esophageal varices, and liver failure that usually leads to death by 5 yr of age. Rare patients survive without progression of liver disease; these patients have a milder hepatic form and do not require a liver transplant.
A neuromuscular form of the disease has been reported; there are 4 main variants recognized based on age of presentation. The perinatal form presents as a fetal akinesia deformation sequence and death in the perinatal period. The congenital form presents at birth with severe hypotonia, muscle atrophy, and neuronal involvement with death in the neonatal period; some patients have cardiomyopathy. The childhood form presents primarily with myopathy or cardiomyopathy. The adult form presents with diffuse central and peripheral nervous system dysfunction accompanied by accumulation of polyglucosan material in the nervous system (adult polyglucosan body disease). For adult polyglucosan disease, a leukocyte or nerve biopsy is needed to establish the diagnosis because the branching enzyme deficiency is limited to those tissues.
Diagnosis
Tissue deposition of amylopectin-like materials can be demonstrated in liver, heart, muscle, skin, intestine, brain, spinal cord, and peripheral nerve. The hepatic histologic findings are characterized by micronodular cirrhosis and faintly stained basophilic inclusions in the hepatocytes. The inclusions consist of coarsely clumped, stored material that is periodic acid–Schiff positive and partially resistant to diastase digestion. Electron microscopy shows, in addition to the conventional α and β glycogen particles, accumulation of the fibrillar aggregations that are typical of amylopectin. The distinct staining properties of the cytoplasmic inclusions, as well as electron microscopic findings, could be diagnostic. However, polysaccharidoses with histologic features reminiscent of type IV disease, but without enzymatic correlation, have been observed. The definitive diagnosis rests on the demonstration of the deficient branching enzyme activity in liver, muscle, cultured skin fibroblasts, or leukocytes, or on the identification of disease-causing mutations in the glycogen branching enzyme (GBE) gene. Prenatal diagnosis is possible by measuring the enzyme activity in cultured amniocytes, chorionic villi, or mutation analysis.
Treatment
There is no specific treatment for type IV GSD. Unlike patients with the other liver GSDs (I, III, VI, IX), those with GSD IV do not have hypoglycemia, which is only seen when there is overt liver cirrhosis. Liver transplantation has been performed for patients with progressive hepatic failure, but because it is a multisystem disorder involving many organ systems, the long-term success of liver transplantation is unknown. Caution should be taken in selecting type IV patients for liver transplantation because these patients have variable phenotypes, which include a nonprogressive form of the liver disease and in some cases, extrahepatic manifestations of the disease.
Type VI Glycogen Storage Disease (Liver Phosphorylase Deficiency, Hers Disease)
There are few patients with documented liver phosphorylase deficiency. Such patients usually have a benign course and present with hepatomegaly and growth retardation in early childhood; however, some cases are more severe. Hypoglycemia, hyperlipidemia, and hyperketosis are of variable severity. Lactic acid and uric acid levels are normal. The heart and skeletal muscles are not involved. The hepatomegaly and growth retardation improve with age and usually disappear around puberty; however, patients with severe hepatomegaly, recurrent severe hypoglycemia, hyperketosis, and postprandial lactic acidosis have recently been reported. Treatment is symptomatic. A high-carbohydrate diet and frequent feeding are effective in preventing hypoglycemia; most patients require no specific treatment. GSD VI is an autosomal recessive disease. Diagnosis rests on enzyme analysis of the liver biopsy. The liver phosphorylase gene (PYGL) is on chromosome 14q21-22 and has 20 exons. Many mutations are known in this gene; a splice site mutation in intron 13 has been identified in the Mennonite population.
Type IX Glycogen Storage Disease (Phosphorylase Kinase Deficiency)
This disorder represents a heterogeneous group of glycogenoses. Phosphorylase, the rate-limiting enzyme of glycogenolysis, is activated by a cascade of enzymatic reactions involving adenylate cyclase, cyclic adenosine monophosphate–dependent protein kinase (protein kinase A), and phosphorylase kinase. The latter enzyme has 4 subunits (α, β, γ, δ), each encoded by different genes on different chromosomes and differentially expressed in various tissues. This cascade of reactions is stimulated primarily by glucagon. This glycogenosis could be the result of any enzyme deficiency along this pathway; the most common is the deficiency of phosphorylase kinase. The phenotypic variability within each subtype is being uncovered with the availability of molecular testing.
The numeric classification of phosphorylase kinase deficiency is confusing, ranging from type VIa to VIII to IX. It is advisable to refrain from such a designation and to classify the various disorders according to organ involvement and mode of inheritance.
X-Linked Liver Phosphorylase Kinase Deficiency
X-linked liver phosphorylase kinase deficiency is the most common form of liver glycogenoses. In addition to liver, enzyme activity can also be deficient in erythrocytes, leukocytes, and fibroblasts; it is normal in muscle. Typically, a 1-5 yr old male presents with growth retardation, an incidental finding of hepatomegaly, and a slight delay in motor development. Cholesterol, triglycerides, and liver enzymes are mildly elevated. Ketosis may occur after fasting. Lactate and uric acid levels are normal. Hypoglycemia is typically mild, if present. The response in blood glucose to glucagon is normal. Hepatomegaly and abnormal blood chemistries gradually improve and can normalize with age. Most adults achieve a normal final height and are usually asymptomatic despite a persistent phosphorylase kinase deficiency. Liver histologic appearance shows glycogen-distended hepatocytes. The accumulated glycogen (β particles, rosette form) has a frayed or burst appearance and is less compact than the glycogen seen in type I or type III GSD. Fibrous septal formation and low-grade inflammatory changes may be present.
The structural gene for the common liver isoform of the phosphorylase kinase subunit, liver α subunit, is on the X chromosome (αL at Xp22.2). Several missense, nonsense, and splice-site mutations of this gene are known.
Autosomal Liver and Muscle Phosphorylase Kinase Deficiency
Several patients have been reported with phosphorylase kinase deficiency in liver and blood cells and an autosomal mode of inheritance. As with the X-linked form, hepatomegaly and growth retardation apparent in early childhood are the predominant symptoms. Some patients also exhibit muscle hypotonia. When measured in a few cases, reduced activity of the enzyme has been demonstrated in muscle. Mutations causing autosomally transmitted liver and muscle phosphorylase kinase deficiency are found in the autosomal β subunit gene of the PK gene (chromosome 16q12-q13). Several nonsense mutations, a single-base insertion, a splice-site mutation, and a large intragenic mutation have been identified. In addition, a missense mutation was discovered in an atypical patient with normal blood cell phosphorylase kinase activity.
Autosomal Liver Phosphorylase Kinase Deficiency
This form of phosphorylase kinase deficiency is due to mutations in the testis/liver isoform of the γ subunit of the gene (TL, PHKG2). In contrast to X-linked phosphorylase kinase deficiency, patients with mutations in the PHKG2 gene typically have more severe phenotypes with recurrent hypoglycemia and often develop progressive liver cirrhosis. PHKG2 maps to chromosome 16p12.1 and many disease-causing mutations are known for this gene.
Muscle-Specific Phosphorylase Kinase Deficiency
A few cases of phosphorylase kinase deficiency restricted to muscle are known. Patients, both male and female, present either with muscle cramps and myoglobinuria with exercise or with progressive muscle weakness and atrophy. Phosphorylase kinase activity is decreased in muscle but normal in liver and blood cells. There is no hepatomegaly or cardiomegaly. The structural gene for the muscle specific form α subunit (αM) is located at Xq12. Mutations of this gene have been found in male patients with this disorder. The gene for muscle γ subunit (γM, PHKG1) is on chromosome 7p12.29 No mutations in this gene have been reported so far.
Phosphorylase Kinase Deficiency Limited to Heart
These patients present with cardiomyopathy in infancy and rapidly progress to heart failure and death. Phosphorylase kinase deficiency is demonstrated in the heart with normal enzyme activity in skeletal muscle and liver. Studies have questioned the existence of cardiac-specific primary phosphorylase kinase deficiency because they did not find any mutations in the 8 genes encoding the phosphorylase kinase subunits.
Diagnosis
Definitive diagnosis of phosphorylase kinase deficiency requires demonstration of the enzymatic defect in affected tissues. Phosphorylase kinase can be measured in leukocytes and erythrocytes, but because the enzyme has many isozymes, the diagnosis can be missed without studies of liver, muscle, or heart in certain instances. Mutation analysis is necessary in many cases to determine the disease’s subtype.
The PHKA2 gene encoding the α subunit is most commonly involved, followed by the PHKB gene encoding the β subunit, regardless of the presence of deficiency in erythrocytes. Mutations in the PHKG2 gene underlying γ-subunit deficiency are typically associated with severe liver involvement with recurrent hypoglycemia and liver fibrosis.
Treatment
The treatment for liver phosphorylase kinase deficiency includes a high-carbohydrate diet and frequent feedings to prevent hypoglycemia; most patients require no specific treatment. Prognosis for the X-linked and certain autosomal forms is good. Patients with mutations in the γ subunit typically have a more severe clinical course with progressive liver disease. There is no treatment for the fatal form of isolated cardiac phosphorylase kinase deficiency other than heart transplantation.
Glycogen Synthetase Deficiency (GSD 0)
Deficiency of hepatic glycogen synthetase (GYS2) activity leads to a marked decrease of glycogen stored in the liver. The disease appears to be very rare in humans, and in the true sense, this is not a type of GSD because the deficiency of the enzyme leads to decreased glycogen stores.
The patients present in infancy with early-morning (before eating breakfast) drowsiness, pallor, emesis, and fatigue and sometimes convulsions associated with hypoglycemia and hyperketonemia. Blood lactate and alanine levels are low, and there is no hyperlipidemia or hepatomegaly. Prolonged hyperglycemia, glycosuria, and elevation of lactate with normal insulin levels after administration of glucose or a meal suggest a possible diagnosis of deficiency of glycogen synthetase. Definitive diagnosis requires a liver biopsy to measure the enzyme activity or identification of mutations in the liver glycogen synthetase gene, located on chromosome 12p12.2. Treatment consists of frequent meals, rich in protein, and nighttime supplementation with uncooked cornstarch. Most children with GSD 0 are cognitively and developmentally normal. Short stature and osteopenia are common features. The prognosis seems good for patients who survive to adulthood, including resolution of hypoglycemia, except during pregnancy.
Muscle Glycogen Synthase Deficiency
This GSD results from muscle glycogen synthase (glycogen synthase I, GYS1) deficiency.
It is extremely rare and was reported in 3 children of consanguineous parents of Syrian origin. Muscle biopsies showed lack of glycogen, predominantly oxidative fibers, and mitochondrial proliferation. Glucose tolerance was normal. Molecular study revealed a homozygous stop mutation (R462→ter) in the muscle glycogen synthase gene. The phenotype was variable in the 3 siblings and ranged from sudden cardiac arrest, muscle fatigability, hypertrophic cardiomyopathy, an abnormal heart rate, and hypotension while exercising, to mildly impaired cardiac function at rest.
Hepatic Glycogenosis with Renal Fanconi Syndrome (Fanconi-Bickel Syndrome)
This rare autosomal recessive disorder is caused by defects in the facilitative glucose transporter 2 (GLUT-2), which transports glucose in and out of hepatocytes, pancreatic β cells, and the basolateral membranes of intestinal and renal epithelial cells. The disease is characterized by proximal renal tubular dysfunction, impaired glucose and galactose utilization, and accumulation of glycogen in liver and kidney.
The affected child typically presents in the 1st yr of life with failure to thrive, rickets, and a protuberant abdomen from hepatomegaly and nephromegaly. The disease may be confused with type I GSD because a Fanconi-like syndrome can develop in type I disease patients.
Laboratory findings include glucosuria, phosphaturia, generalized aminoaciduria, bicarbonate wasting, hypophosphatemia, increased serum alkaline phosphatase levels, and radiologic findings of rickets. Mild fasting hypoglycemia and hyperlipidemia may be present. Liver transaminase, plasma lactate, and uric acid levels are usually normal. Oral galactose or glucose tolerance tests show intolerance, which could be explained by the functional loss of GLUT-2 preventing liver uptake of these sugars.
Tissue biopsy results show marked accumulation of glycogen in hepatocytes and proximal renal tubular cells, presumably owing to the altered glucose transport out of these organs. Diffuse glomerular mesangial expansion along with glomerular hyperfiltration and microalbuminuria similar to nephropathy in GSD Ia and diabetes have been reported.
Fanconi-Bickel syndrome is rare. Seventy percent of patients with a detectable GLUT-2 mutation have consanguineous parents. Most patients are homozygous for the disease-related mutations; some patients are compound heterozygotes. The majority of mutations detected thus far predict a premature termination of translation. The resulting loss of the C-terminal end of the GLUT-2 protein predicts a nonfunctioning glucose transporter with an inward-facing substrate-binding site.
There is no specific treatment. Growth retardation persists through adulthood. Symptomatic replacement of water, electrolytes, and vitamin D; restriction of galactose intake; and a diet similar to that used for diabetes mellitus presented in frequent and small meals with an adequate caloric intake may improve growth.
Muscle Glycogenoses
The role of glycogen in muscle is to provide substrates for the generation of ATP for muscle contraction. The muscle GSDs are broadly divided into 2 groups. The 1st group is characterized by hypertrophic cardiomyopathy, progressive skeletal muscle weakness and atrophy, or both, and includes deficiencies of acid α-glucosidase, a lysosomal glycogen degrading enzyme (type II GSD), and deficiencies of lysosomal-associated membrane protein 2 (LAMP2) and AMP-activated protein kinase γ2 (PRKAG2). The 2nd group comprises muscle energy disorders characterized by muscle pain, exercise intolerance, myoglobinuria, and susceptibility to fatigue. This group includes myophosphorylase deficiency (McArdle disease, type V) and deficiencies of phosphofructokinase (type VII), phosphoglycerate kinase, phosphoglycerate mutase, and lactate dehydrogenase. Some of these latter enzyme deficiencies can also be associated with compensated hemolysis, suggesting a more generalized defect in glucose metabolism.
Type II Glycogen Storage Disease (Lysosomal Acid α-1,4-Glucosidase Deficiency, Pompe Disease)
Pompe disease, also referred to as GSD type II or acid maltase deficiency, is caused by a deficiency of acid α-1,4-glucosidase (acid maltase), an enzyme responsible for the degradation of glycogen in lysosomes. This enzyme defect results in lysosomal glycogen accumulation in multiple tissues and cell types, with cardiac, skeletal, and smooth muscle cells being the most seriously affected. The disease is characterized by accumulation of glycogen in lysosomes, as opposed to its accumulation in cytoplasm in the other glycogenoses.
Pompe disease is an autosomal recessive disorder with an incidence of approximately 1/40,000 live births. The gene for acid α-glucosidase is on chromosome 17q25.2. Multiple pathogenic mutations have been identified that could be helpful in delineating the phenotypes. An example is a splice site mutation (IVS1-13T → G), commonly seen in late-onset patients of white racial groups.
Clinical Manifestations
The disorder encompasses a range of phenotypes, each including myopathy but differing in age at onset, organ involvement, and clinical severity. Infantile Pompe disease was uniformly lethal without enzyme replacement therapy. Affected infants present in the 1st few months of life with hypotonia, a generalized muscle weakness with a “floppy infant” appearance, neuropathic bulbar weakness, feeding difficulties, macroglossia, hepatomegaly, and a hypertrophic cardiomyopathy followed by death from cardiorespiratory failure or respiratory infection usually by 1 yr of age. Juvenile and adult-onset disease (late-onset Pompe disease) is characterized by a lack or absence of severe cardiac involvement and a less severe short-term prognosis. Symptoms can start at any age and are related to progressive dysfunction of skeletal muscles. The clinical picture is dominated by slowly progressive proximal muscle weakness with truncal involvement and greater involvement of the lower limbs than the upper limbs. The pelvic girdle, paraspinal muscles, and diaphragm are the muscle groups most seriously affected. These patients often present with proximal or limb girdle muscle weakness. With disease progression, patients become confined to wheelchairs and require artificial ventilation. The initial symptoms in some patients may be respiratory insufficiency manifested by somnolence, morning headache, orthopnea, and exertional dyspnea, which eventually lead to sleep-disordered breathing and respiratory failure. Respiratory failure is the cause of significant morbidity and mortality in this form of the disease. The age of death varies from early childhood to late adulthood, depending on the rate of disease progression and the extent of respiratory muscle involvement.
Laboratory Findings
These include elevated levels of serum creatine kinase, aspartate aminotransferase, and lactate dehydrogenase. In the infantile form a chest x-ray showing massive cardiomegaly is frequently the 1st symptom detected. Electrocardiographic findings include a high-voltage QRS complex and a shortened PR interval. Echocardiography reveals thickening of both ventricles and/or the intraventricular septum and/or left ventricular outflow tract obstruction. Muscle biopsy shows the presence of vacuoles that stain positively for glycogen; acid phosphatase is increased, presumably from a compensatory increase of lysosomal enzymes. Electron microscopy reveals glycogen accumulation within the membranous sac and in the cytoplasm. Electromyography reveals myopathic features with excessive electrical irritability of muscle fibers and pseudomyotonic discharges. Serum creatine kinase is not always elevated in adult patients. Depending on the muscle sampled or tested, the muscle histologic appearance and electromyography may not be abnormal. It is prudent to examine the affected muscle.
Some patients with infantile Pompe disease who had peripheral nerve biopsies demonstrated glycogen accumulation in the neurons and Schwann cells, too. Infantile Pompe disease may manifest both myopathic and neuropathic clinical signs. Generally, the former predominate.
Diagnosis
The confirmatory step for a diagnosis of Pompe disease is enzyme assay demonstrating deficient acid α-glucosidase or gene sequencing showing 2 pathogenic mutations in the GAA gene. The enzyme assay is usually done in muscle, cultured skin fibroblasts, dried blood spots, leukocytes, or blood mononuclear cells using maltose, glycogen, or 4-methylumbelliferyl-α-D-glucopyranoside (4MUG) as a substrate. Deficiency is usually more severe in the infantile form than in the late onset forms. The skin fibroblast assay is usually preferred to muscle biopsy because it is a less invasive procedure with the advantage of maintaining a cell line for future use and providing information on residual enzyme activity. Blood-based assays, especially dried blood spots have the advantage of a rapid turn-around time. A muscle biopsy can yield faster results and provide additional information about glycogen content and site of glycogen storage within and outside the lysosomes of muscle cells. A major limitation of a muscle biopsy in late-onset patients is the variable pathology and glycogen accumulation in different muscles and within muscle fibers; muscle histology and glycogen content can vary depending on the site of muscle biopsy. There is also a risk from anesthesia; EKG can be helpful in making the diagnosis in suspected cases of the infantile form and should be done in patients suspected of having Pompe disease before any procedure requiring anesthesia, including muscle biopsy, is performed. Prenatal diagnosis using amniocytes or chorionic villi is available in the infantile form.
Treatment
Treatment options were once limited to supportive or palliative care. Specific enzyme replacement therapy (ERT) with recombinant human acid α-glucosidase (alglucosidase alfa, [Myozyme]) is available for treatment of Pompe disease. Recombinant acid α-glucosidase is capable of preventing deterioration or reversing abnormal cardiac and skeletal muscle functions (Fig. 81-3). ERT should be initiated as soon as possible and preferably <6 mo of age; the dose is 20 mg/kg given every 2 wk. A high-protein diet and exercise therapy may also be beneficial. Nocturnal ventilatory support, when indicated, should be used. It has been shown to improve the quality of life and is particularly beneficial during a period of respiratory decompensation.
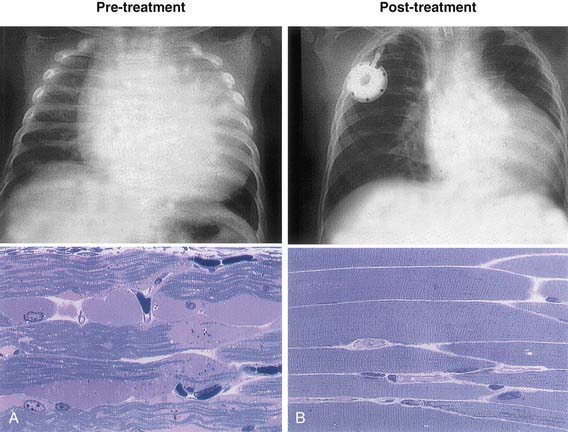
Figure 81-3 Chest x-ray and muscle histology findings of an infantile-onset Pompe disease patient before (A) and after (B) enzyme replacement therapy. Note the decrease in heart size and muscle glycogen with the therapy.
(Modified from Amalfitano A, Bengur AR, Morse RP, et al: Recombinant human acid alpha-glucosidase enzyme therapy for infantile glycogen storage disease type II: results of a phase I/II clinical trial, Genet Med 3:132–138, 2001.)
Glycogen Storage Diseases Mimicking Hypertrophic Cardiomyopathy
Deficiencies of lysosomal-associated membrane protein 2 (LAMP2, also called Danon disease) and AMP-activated protein kinase γ2 (PRKAG2) result in accumulation of glycogen in the heart and skeletal muscle. These patients present primarily as a hypertrophic cardiomyopathy, but can be distinguished from the usual causes of hypertrophic cardiomyopathy due to defects in sarcomere protein genes by their electrophysiologic abnormalities, particularly ventricular pre-excitation and conduction defects. The onset of cardiac symptoms, including chest pain, palpitation, syncope, and cardiac arrest, can occur between the ages of 8 and 15 yr for LAMP2 deficiency, younger than the average age for patients with PRKAG2 deficiency, which is 33 yr. Danon disease is transmitted as a X-linked trait and PRKAG2 mutations as a dominant trait.
The prognosis for LAMP2 deficiency is poor with progressive end-stage heart failure early in adulthood. Cardiomyopathy due to PRKAG2 mutations is compatible with long-term survival, although some patients may necessitate the implantation of a pacemaker and aggressive control of arrhythmias. A congenital form presents in early infancy with severe hypertrophic cardiomyopathy and a rapidly fatal course.
Type V Glycogen Storage Disease (Muscle Phosphorylase Deficiency, Mcardle Disease)
This is caused by the deficiency of muscle phosphorylase activity. Lack of this enzyme limits muscle ATP generation by glycogenolysis, resulting in muscle glycogen accumulation, and is the prototype of muscle energy disorders. A deficiency of myophosphorylase impairs the cleavage of glucosyl molecules from the straight chain of glycogen.
Clinical Manifestations
Symptoms usually 1st develop in late childhood or as an adult and are characterized by exercise intolerance with muscle cramps and pain. Two types of activity tend to cause symptoms: brief exercise of great intensity, such as sprinting or carrying heavy loads; and less intense but sustained activity, such as climbing stairs or walking uphill. Moderate exercise, such as walking on level ground, can be performed by most patients for long periods. Many patients experience a characteristic “second wind” phenomenon. If they slow down or pause briefly at the 1st appearance of muscle pain, they can resume exercise with more ease. Due to the underlying myopathy, these patients may be at risk for statin-induced myopathy and rhabdomyolysis. While patients typically experience episodic muscle pain and cramping due to exercise, 35% of patients with McArdle disease report permanent pain that has a serious impact on sleep and other activities.
About 50% of patients report burgundy-colored urine after exercise, which is the consequence of exercise-induced myoglobinuria secondary to rhabdomyolysis. Intense myoglobinuria after vigorous exercise may cause acute renal failure. In rare cases, electromyographic findings may suggest an inflammatory myopathy and the diagnosis can be confused with polymyositis.
The level of serum creatine kinase is usually elevated at rest and increases more after exercise. Exercise also increases the levels of blood ammonia, inosine, hypoxanthine, and uric acid. The latter abnormalities are attributed to accelerated recycling of muscle purine nucleotides owing to insufficient ATP production. Type V GSD is an autosomal recessive disorder. The gene for muscle phosphorylase (PYGM) has been mapped to chromosome 11q13.
Clinical heterogeneity is uncommon in type V GSD, but late-onset disease with no symptoms as late as the 8th decade and an early-onset, fatal form with hypotonia, generalized muscle weakness, and progressive respiratory insufficiency have been described.
Diagnosis
An ischemic exercise test offers a rapid diagnostic screening for patients with a metabolic myopathy. Lack of an increase in blood lactate levels and exaggerated blood ammonia elevations indicate muscle glycogenosis and suggest a defect in the conversion of muscle glycogen or glucose to lactate. The abnormal ischemic exercise response is not limited to type V GSD. Other muscle defects in glycogenolysis or glycolysis produce similar results (deficiencies of muscle phosphofructokinase, phosphoglycerate kinase, phosphoglycerate mutase, or lactate dehydrogenase).
Phosphorus magnetic resonance imaging (31P MRI) allows for the noninvasive evaluation of muscle metabolism. Patients with type V GSD have no decrease in intracellular pH and have excessive reduction in phosphocreatine in response to exercise. The diagnosis should be confirmed by enzymatic evaluation of muscle. A common nonsense mutation R49X in exon 1 is found in 90% of white patients, and a deletion of a single codon in exon 17 is found in 61% of Japanese patients. The R49X mutation represents 55% of alleles in Spanish patients, whereas the W797R mutation represents 14% and the G204S represents 9% of mutant alleles in the Spanish population.
Treatment
Avoidance of strenuous exercise prevents the symptoms; however, regular and moderate exercise is recommended to improve exercise capacity. Glucose or sucrose given before exercise or injection of glucagon can markedly improve tolerance in these patients. A high-protein diet may increase muscle endurance and creatine supplement has been shown to improve muscle function in some patients. Vitamin B6 supplementation reduces exercise intolerance and muscle cramps. Longevity is not generally affected.
Type VII Glycogen Storage Disease (Muscle Phosphofructokinase Deficiency, Tarui Disease)
Type VII GSD is caused by a deficiency of muscle phosphofructokinase, which catalyzes the ATP-dependent conversion of fructose-6-phosphate to fructose-1,6-diphosphate and is a key regulatory enzyme of glycolysis. Phosphofructokinase is composed of 3 isoenzyme subunits (M [muscle], L [liver], and P [platelet]) that are encoded by different genes and differentially expressed in tissues. Skeletal muscle contains only the M subunit, and red blood cells contain a hybrid of L and M forms. Type VII disease is due to a defective M isoenzyme, which causes a complete enzyme defect in muscle and a partial defect in red blood cells.
Type VII GSD is an autosomal recessive disorder and is prevalent among Japanese people and Ashkenazi Jews. The gene for muscle phosphofructokinase is located on chromosome 12q13.3. A splicing defect and a nucleotide deletion in the muscle phosphofructokinase gene account for 95% of mutant alleles in Ashkenazi Jews. Diagnosis based on molecular testing is thus possible in this population.
Clinical Manifestations
Six features of type VII are distinctive: (1) Exercise intolerance, usually evident in childhood, is more severe than in type V disease and may be associated with nausea, vomiting, and severe muscle pain; vigorous exercise causes severe muscle cramps and myoglobinuria. (2) Compensated hemolysis occurs as evidenced by an increased level of serum bilirubin and an elevated reticulocyte count. (3) Hyperuricemia is common and exaggerated by muscle exercise to a more severe degree than that observed in type V or III GSD. (4) An abnormal polysaccharide is present in muscle fibers; it is periodic acid–Schiff positive but resistant to diastase digestion. (5) Exercise intolerance is particularly acute after meals that are rich in carbohydrates because glucose cannot be utilized in muscle and because glucose inhibits lipolysis and thus deprives muscle of fatty acid and ketone substrates. In contrast, patients with type V disease can metabolize blood-borne glucose derived from either liver glycogenolysis or exogenous glucose; indeed, glucose infusion improves exercise tolerance in type V patients. (6) There is no spontaneous second-wind phenomenon because of the inability to metabolize blood glucose.
Other rare type VII variants occur. One variant presents in infancy with hypotonia and limb weakness and proceeds to a rapidly progressive myopathy that leads to death by 4 yr of age. There is a 2nd variant that occurs in infancy and results in congenital myopathy and arthrogryposis with a fatal outcome. A 3rd variant presents in infancy with hypotonia, mild developmental delay and seizures. An additional presentation is hereditary non-spherocytic hemolytic anemia. While these patients do not experience muscle symptoms, it remains unclear whether these symptoms will develop later in life. One variant presents in adults and is characterized by a slowly progressive, fixed muscle weakness rather than cramps and myoglobinuria.
Other Muscle Glycogenoses with Muscle Energy Impairment
Six additional defects in enzymes—phosphoglycerate kinase, phosphoglycerate mutase, lactate dehydrogenase, fructose-1,6- bisphosphate aldolase A, muscle pyruvate kinase, and β-enolase in the pathway of the terminal glycolysis—cause symptoms and signs of muscle energy impairment similar to those of types V and VII GSD. The failure of blood lactate to increase in response to exercise is a useful diagnostic test and can be used to differentiate muscle glycogenoses from disorders of lipid metabolism, such as carnitine palmitoyl transferase II deficiency and very long chain acyl-CoA dehydrogenase deficiency, which also cause muscle cramps and myoglobinuria. Muscle glycogen levels can be normal in the disorders affecting terminal glycolysis and assaying the muscle enzyme activity is needed to make a definite diagnosis. There is no specific treatment. Avoidance of strenuous exercise prevents acute attacks of muscle cramps and myoglobinuria. Avoidance of drugs such as statins, and malignant hyperthermia precautions for patients undergoing anesthesia should be followed.
Akman HO, Sampayo JN, Ross FA, et al. Fatal infantile cardiac glycogenosis with phosphorylase kinase deficiency and a mutation in the γ2-subunit of AMP-activated protein kinase. Pediatr Res. 2007;62:499-504.
Al-Hassnan ZN, Al Budhaim M, Al-Owain M, et al. Muscle phosphofructokinase deficiency with neonatal seizures and nonprogressive course. J Child Neurol. 2007;22:106-108.
Andersen ST, Haller RG, Vissing J. Effect of oral sucrose shortly before exercise on work capacity in McArdle disease. Arch Neurol. 2008;65:786-789.
Arad M, Maron BJ, Gorham JM, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352:362-372.
Beauchamp NJ, Dalton A, Ramaswami U, et al. Glycogen storage disease type IX: high variability in clinical phenotype. Mol Genet Metab. 2007;92:88-99.
Beauchamp NJ, Taybert J, Champion MP, et al. High frequency of missense mutations in glycogen storage disease type VI. J Inherit Metab Dis. 2007;30:722-734.
Beutler E. PGK deficiency. Br J Haematol. 2007;136:3-11.
Bhattacharya K, Orton RC, Qi X, et al. A novel starch for the treatment of glycogen storage diseases. J Inherit Metab Dis. 2007;30:350-357.
Chen LR, Chen CA, Chiu SN, et al. Reversal of cardiac dysfunction after enzyme replacement in patients with infantile-onset Pompe disease. J Pediatr. 2009;155:271-275.
Chien YH, Chiang SC, Zhang XK, et al. Early detection of Pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics. 2008;122:e39-45.
Franco LM, Krishnamurthy V, Bali D, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005;28:153-162.
Kishnani PS, Corzo D, Leslie ND, et al. Early treatment with alglucosidase alfa prolongs long-term survival of infants with Pompe disease. Pediatr Res. 2009;66:329-335.
Koeberl DD, Kishnani PS, Bali D, et al. Emerging therapies for glycogen storage disease type I. Trends Endocrinol Metab. 2009;20:252-258.
Koeberl DD, Kishnani PS, Chen YT. Glycogen storage disease types I and II: treatment updates. J Inherit Metab Dis. 2007;30:159-164.
Kollberg G, Tulinius M, Gilljam T, et al. Cardiomyopathy and exercise intolerance in muscle glycogen storage disease 0. N Engl J Med. 2007;357:1507-1514.
Llerena JCJr, Horovitz DM, Marie SKN, et al. The Brazilian consensus on the management of Pompe disease. J Pediatr. 2009;155:S47-S56.
Lucchiari S, Pagliarani S, Salani S, et al. Hepatic and neuromuscular forms of glycogenosis type III: nine mutations in AGL. Hum Mutat. 2006;27:600-601.
Nicolino M, Byrne B, Wraith JE, et al. Clinical outcomes after long-term treatment with alglucosidase alfa in infants and children with advanced Pompe disease. Genet Med. 2009;11:210-219.
Pierre G, Chakupurakal G, McKiernan P, et al. Bone marrow transplantation in glycogen storage disease type 1b. J Pediatr. 2008;152:286-288.
Rommel O, Kley RA, Dekomien G, et al. Muscle pain in myophosphorylase deficiency (McArdle’s disease): the role of gender, genotype, and pain-related coping. Pain. 2006;124:295-304.
Spiegel R, Mahamid J, Orho-Melander M, et al. The variable clinical phenotype of liver glycogen synthase deficiency. J Pediatr Endocrinol Metab. 2007;20:1339-1342.
van der Ploeg AT, Clemens PR, Corzo D, et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N Engl J Med. 2010;362(15):1396-1406.
81.2 Defects in Galactose Metabolism
Milk and dairy products contain lactose, the major dietary source of galactose. The metabolism of galactose produces fuel for cellular metabolism through its conversion to glucose-1-phosphate (see Table 81-1). Galactose also plays an important role in the formation of galactosides, which include glycoproteins, glycolipids, and glycosaminoglycans. Galactosemia denotes the elevated level of galactose in the blood and is found in 3 distinct inborn errors of galactose metabolism defective in 1 of the following enzymes: galactose-1-phosphate uridyl transferase, galactokinase, and uridine diphosphate galactose-4-epimerase. The term galactosemia, although adequate for the deficiencies in any of these disorders, generally designates the transferase deficiency.
Galactose-1-Phosphate Uridyl Transferase Deficiency Galactosemia
Two forms of the deficiency exist: Infants with complete or near complete deficiency of the enzyme (classic galactosemia) and those with partial transferase deficiency. Classic galactosemia is a serious disease with onset of symptoms typically by the 2nd half of the 1st wk of life. The incidence is 1/60,000. The newborn infant receives high amounts of lactose (up to 40% in breast milk and certain formulas), which consists of equal parts of glucose and galactose. Without the transferase enzyme, the infant is unable to metabolize galactose-1-phosphate, the accumulation of which results in injury to kidney, liver, and brain. This injury may begin prenatally in the affected fetus by transplacental galactose derived from the diet of the heterozygous mother or by endogenous production of galactose in the fetus.
Clinical Manifestations
The diagnosis of uridyl transferase deficiency should be considered in newborn or young infants with any of the following features: jaundice, hepatomegaly, vomiting, hypoglycemia, seizures, lethargy, irritability, feeding difficulties, poor weight gain or failure to regain birth weight, aminoaciduria, nuclear cataracts, vitreous hemorrhage, hepatic failure, liver cirrhosis, ascites, splenomegaly, or mental retardation. Symptoms are milder and improve when milk is temporarily withdrawn and replaced by intravenous or lactose-free nutrition. Patients with galactosemia are at increased risk for Escherichia coli neonatal sepsis; the onset of sepsis often precedes the diagnosis of galactosemia. Pseudotumor cerebri can occur and cause a bulging fontanel. Death from liver and kidney failure and sepsis may follow within days. When the diagnosis is not made at birth, damage to the liver (cirrhosis) and brain (mental retardation) becomes increasingly severe and irreversible.
Partial transferase deficiency is generally asymptomatic. It is more frequent than classic galactosemia and is diagnosed in newborn screening because of moderately elevated blood galactose and/or low transferase activity. Galactosemia should be considered for the newborn or young infant who is not thriving or who has any of the preceding findings. Light and electron microscopy of hepatic tissue reveals fatty infiltration, the formation of pseudoacini, and eventual macronodular cirrhosis. These changes are consistent with a metabolic disease but do not indicate the precise enzymatic defect.
Diagnosis
The preliminary diagnosis of galactosemia is made by demonstrating a reducing substance in several urine specimens collected while the patient is receiving human milk, cow’s milk, or any other formula containing lactose. The reducing substance found in urine by Clinitest (glucose, galactose, others) can be identified by chromatography or by an enzymatic test specific for galactose. Galactosuria is present, provided the last milk feed does not date back more than a few hours and the child is not vomiting excessively. Clinistix urine test results are usually negative because the test materials rely on the action of glucose oxidase, which is specific for glucose and is nonreactive with galactose. Owing to a proximal renal tubular syndrome, the acutely ill baby may also excrete glucose together with amino acids. Because galactose is injurious to persons with galactosemia, diagnostic challenge tests dependent on administering galactose orally or intravenously should not be used. Direct enzyme assay using erythrocytes establishes the diagnosis. One needs to confirm that the patient did not receive a blood transfusion before the collection of the blood sample, as a diagnosis could be missed. Deficient activity of galactose-1-phosphate uridyl transferase is demonstrable in hemolysates of erythrocytes, which also exhibit increased concentrations of galactose-1-phosphate.
Genetics
Transferase deficiency galactosemia is an autosomal recessive disorder. There are several enzymatic variants of galactosemia. The Duarte variant, a single amino acid substitution (N314D), has diminished red cell enzyme activity (50% of normal), but usually no clinical significance. This variant is the most common, with a carrier frequency of 12% in the general population. Some African-American patients have milder symptoms despite the absence of measurable transferase activity in erythrocytes; these patients retain 10% enzyme activity in liver and intestinal mucosa, whereas most white patients have no detectable activity in any of these tissues. In African-Americans, 62% of alleles are represented by the S135L mutation, a mutation that is responsible for a milder disease course. In the white population, 70% of alleles are represented by the Q188R and K285N missense mutations and are associated with severe disease. Carrier testing and prenatal diagnosis can be performed by direct enzyme analysis of amniocytes or chorionic villi; testing can also be DNA based.
Treatment And Prognosis
Because of newborn screening for galactosemia, patients are being identified and treated early. Various non–lactose containing milk substitutes are available (casein hydrolysates, soybean-based formula). Elimination of galactose from the diet reverses growth failure and renal and hepatic dysfunction. Cataracts regress, and most patients have no impairment of vision. Early diagnosis and treatment have improved the prognosis of galactosemia; however, on long-term follow-up, patients still manifest ovarian failure with primary or secondary amenorrhea, decreased bone mineral density, developmental delay, and learning disabilities that increase in severity with age. Hypergonadotrophic hypogonadism is reported in 80% to over 90% of female patients with classic galactosemia. Although most women with classic galactosemia are infertile when they reach childbearing age, a small number have given birth. Most patients manifest speech disorders, whereas a smaller number demonstrate poor growth and impaired motor function and balance (with or without overt ataxia). The relative control of galactose-1-phosphate levels does not always correlate with long-term outcome, leading to the belief that other factors, such as elevated galactitol, decreased uridine diphosphate galactose (UDP-galactose, a donor for galactolipids and proteins), and endogenous galactose production may be responsible.
Galactokinase Deficiency
The deficient enzyme is galactokinase, which normally catalyzes the phosphorylation of galactose. The principal metabolites accumulated are galactose and galactitol. Two genes have been reported to encode galactokinase: GK1 on chromosome 17q24 and GK2 on chromosome 15. Cataracts are usually the sole manifestation of galactokinase deficiency; pseudotumor cerebri is a rare complication. The affected infant is otherwise asymptomatic. Heterozygote carriers may be at risk for presenile cataracts. Affected patients have an increased concentration of blood galactose levels, provided they have been fed a lactose-containing formula. The diagnosis is made by demonstrating an absence of galactokinase activity in erythrocytes or fibroblasts. Transferase activity is normal. Treatment is dietary restriction of galactose.
Uridine Diphosphate Galactose-4-Epimerase Deficiency
The abnormally accumulated metabolites are similar to those in transferase deficiency; however, there is also an increase in cellular UDP-galactose. There are 2 distinct forms of epimerase deficiency. The 1st is a benign form discovered incidentally through neonatal screening programs. Affected persons are healthy and without problems; the enzyme deficiency is limited to leukocytes and erythrocytes. No treatment is required. The 2nd form of epimerase deficiency is severe, and clinical manifestations resemble transferase deficiency, with the additional symptoms of hypotonia and nerve deafness. The enzyme deficiency is generalized, and clinical symptoms respond to restriction of dietary galactose. Although this form of galactosemia is rare, it must be considered in a symptomatic patient with measurable galactose-1-phosphate who has normal transferase activity. Diagnosis is confirmed by the assay of epimerase in erythrocytes.
Patients with the severe form of epimerase deficiency cannot synthesize galactose from glucose and are galactose dependent. Because galactose is an essential component of many nervous system structural proteins, patients are placed on a galactose-restricted diet rather than a galactose-free diet.
Infants with the mild form of epimerase deficiency have not required treatment. It is advisable to follow urine specimens for reducing substances and exclude aminoaciduria within a few weeks of diagnosis while the infant is still on lactose-containing formula.
The gene for UDP-galactose-4-epimerase is located on chromosome 1 at 1p36. Carrier detection is possible by measurement of epimerase activity in the erythrocytes. Prenatal diagnosis for the severe form of epimerase deficiency, using an enzyme assay of cultured amniotic fluid cells, is possible.
Barbouth DS, Velazquez DL, Konopka S, et al. Screening newborns for galactosemia using total body galactose oxidation to CO2 in expired air. Pediatr Res. 2007;62:720-724.
Bosch AM. Classical galactosaemia revisited. J Inherit Metab Dis. 2006;29:516-525.
Coman DJ, Murray DW, Byrne JC, et al. Galactosemia, a single gene disorder with epigenetic consequences. Pediatr Res. 2010;67(3):286-292.
Holton JB, Walter JH, Tyfield IA, et al. Galactosemia. In: Scriver CM, Beaudet AL, Sly WS, et al, editors. The metabolic and molecular bases of inherited disease. ed 8. New York: McGraw-Hill; 2008:1553-1587.
Hughes J, Ryan S, Lambert D, et al. Outcomes of siblings with classical galactosemia. J Pediatr. 2009;154:721-726.
Noelmans L, Jacquemyn Y, De Naeyer S, et al. Pregnancy and galactosaemia. J Obstet Gynaecol. 2006;26:812-814.
Rubio-Gozalbo ME, Panis B, Zimmermann LJ, et al. The endocrine system in treated patients with classical galactosemia. Mol Genet Metab. 2006;89:316-322.
81.3 Defects in Fructose Metabolism
Two inborn errors are known in the specialized pathway of fructose metabolism: benign or essential fructosuria and hereditary fructose intolerance (HFI). Fructose-1,6-bisphosphatase deficiency, although strictly speaking not a defect of the specialized fructose pathway, is discussed in Chapter 81.4.
Deficiency of Fructokinase (Essential or Benign Fructosuria)
Deficiency of fructokinase is not associated with any clinical manifestations. It is an accidental finding usually made because the asymptomatic patient’s urine contains a reducing substance. No treatment is necessary and the prognosis is excellent. Inheritance is autosomal recessive with an incidence of 1/120,000. The gene encoding fructokinase is located on chromosome 2p23.3.
Fructokinase catalyzes the 1st step of metabolism of dietary fructose: conversion of fructose to fructose-1-phosphate (see Fig. 81-1). Without this enzyme, ingested fructose is not metabolized. Its level is increased in the blood, and it is excreted in urine because there is practically no renal threshold for fructose. Clinitest results reveal the urinary-reducing substance, which can be identified as fructose by chromatography.
Deficiency of Fructose-1,6-Bisphosphate Aldolase (Aldolase B, Hereditary Fructose Intolerance)
Deficiency of fructose-1,6-bisphosphate aldolase is a severe condition of infants that appears with the ingestion of fructose-containing food and is caused by a deficiency of fructose aldolase B activity in the liver, kidney, and intestine. The enzyme catalyzes the hydrolysis of fructose-1,6-bisphosphate into triose phosphate and glyceraldehyde phosphate. The same enzyme also hydrolyzes fructose-1-phosphate. Deficiency of this enzyme activity causes a rapid accumulation of fructose-1-phosphate and initiates severe toxic symptoms when exposed to fructose.
Epidemiology and Genetics
The true incidence of HFI is unknown but may be as high as 1/26,000. The gene for aldolase B is on chromosome 9q22.3. Several mutations causing HFI are known. A single missense mutation, a G→C transversion in exon 5 resulting in the normal alanine at position 149 being replaced by a proline, is the most common mutation identified in northern Europeans. This mutation, plus 2 other point mutations (A174D and N334K), account for 80-85% of HFI in Europe and the USA. Diagnosis of HFI can be made by direct DNA analysis.
Clinical Manifestations
Patients with HFI are asymptomatic until fructose or sucrose (table sugar) is ingested (usually from fruit, fruit juice, or sweetened cereal). Symptoms may occur early in life, soon after birth if foods or formulas containing these sugars are introduced into the diet. Certain patients are very sensitive to fructose, whereas others can tolerate moderate intakes (up to 250 mg/kg/day). The average intake of fructose in Western societies is 1-2 g/kg/day. Early clinical manifestations resemble galactosemia and include jaundice, hepatomegaly, vomiting, lethargy, irritability, and convulsions. There may also be a higher incidence of celiac disease in HFI patients (>10%) than in the general population (1-3%). Laboratory findings include a prolonged clotting time, hypoalbuminemia, elevation of bilirubin and transaminase levels, and proximal tubular dysfunction. Acute fructose ingestion produces symptomatic hypoglycemia; the higher the intake, the more severe is the clinical picture. Chronic ingestion results in failure to thrive and hepatic disease. If the intake of the fructose persists, hypoglycemic episodes recur, and liver and kidney failure progress, eventually leading to death.
Diagnosis
Suspicion of the enzyme deficiency is fostered by the presence of a reducing substance in the urine during an episode.
The fructose challenge although an effective method of diagnosis, causes a rapid fall, first of serum phosphate and then of blood glucose, and a subsequent increase in uric acid and magnesium. Due to high risks to the patient who can become acutely ill after the oral tolerance test, it should not be performed. Definitive diagnosis is made by assay of fructaldolase B activity in the liver. Gene-based diagnosis is available for most patients with this disease; a common mutation (substitution of Pro for Ala at position 149) accounts for 53% of HFI alleles worldwide.
Treatment
Treatment consists of the complete elimination of all sources of sucrose, fructose, and sorbitol from the diet. It may be difficult because these sugars are widely used additives, found even in most medicinal preparations. With treatment, liver and kidney dysfunction improves, and catch-up in growth is common. Intellectual development is usually unimpaired. As the patient matures, symptoms become milder even after fructose ingestion; the long-term prognosis is good. Because of voluntary dietary avoidance of sucrose, affected patients have few dental caries.
81.4 Defects in Intermediary Carbohydrate Metabolism Associated with Lactic Acidosis
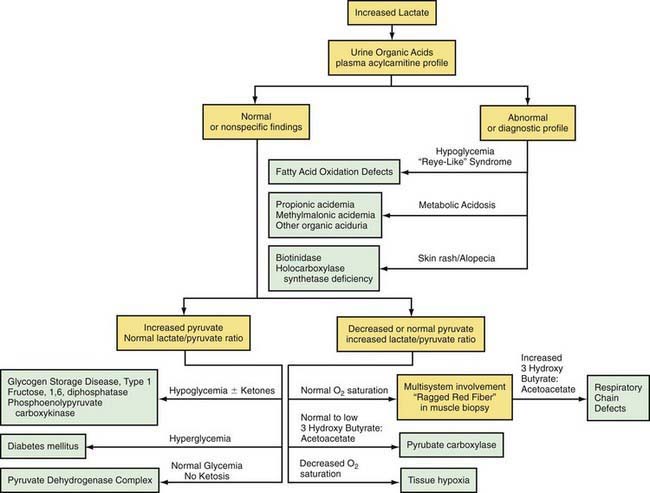
Lactic acidosis occurs with defects of carbohydrate metabolism that interfere with the conversion of pyruvate to glucose via the pathway of gluconeogenesis or to carbon dioxide and water via the mitochondrial enzymes of the Krebs cycle. Figure 81-4 depicts the relevant metabolic pathways. Type I GSD, fructose-1,6-diphosphatase deficiency, and phosphoenolpyruvate carboxylase deficiency are disorders of gluconeogenesis associated with lactic acidosis. Pyruvate dehydrogenase complex deficiency, respiratory chain defects, and pyruvate carboxylase deficiency are disorders in the pathway of pyruvate metabolism causing lactic acidosis. Lactic acidosis can also occur in defects of fatty acid oxidation, organic acidurias (Chapters 79.6, 79.10, and 80.1), or biotin utilization diseases. These disorders are easily distinguishable by the presence of abnormal acylcarnitine profiles, amino acids in the blood, and unusual organic acids in the urine. Blood lactate, pyruvate, and acylcarnitine profiles and the presence of these unusual urine organic acids should be determined in infants and children with unexplained acidosis, especially if there is an increase of anion gap.
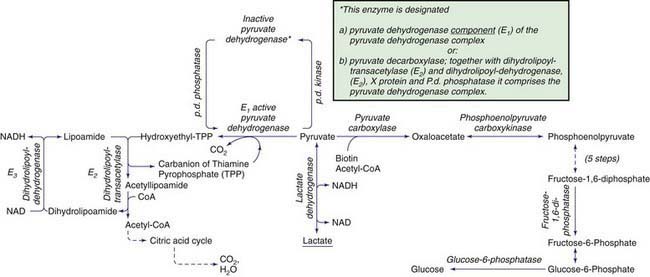
Figure 81-4 Enzymatic reactions of carbohydrate metabolism, deficiencies of which can give rise to lactic acidosis, pyruvate elevations, or hypoglycemia. The pyruvate dehydrogenase complex comprises, in addition to E1, E2, and E3, an extra lipoate-containing protein (not shown), called protein X, and pyruvate dehydrogenase phosphatase.
Lactic acidosis unrelated to an enzymatic defect occurs in hypoxemia. In this case, as well as in defects in the respiratory chain, the serum pyruvate concentration may remain normal (<1.0 mg/dL with an increased lactate : pyruvate ratio), whereas pyruvate is usually increased when lactic acidosis results from an enzymatic defect in gluconeogenesis or pyruvate dehydrogenase complex (both lactate and pyruvate are increased and the ratio is normal). Lactate and pyruvate should be measured in the same blood specimen and on multiple blood specimens obtained when the patient is symptomatic because lactic acidosis can be intermittent. An algorithm for the differential diagnosis of lactic acidosis is shown in Figure 81-5.
Disorders of Gluconeogenesis
Deficiency of Glucose-6-Phosphatase (Type I Glycogen Storage Disease)
Type I GSD is the only glycogenosis associated with significant lactic acidosis. The chronic metabolic acidosis predisposes these patients to osteopenia; after prolonged fasting, the acidosis associated with hypoglycemia is a life-threatening condition (Chapter 81.1).
Fructose-1,6-Diphosphatase Deficiency
Fructose-1,6-diphosphatase deficiency impairs the formation of glucose from all gluconeogenic precursors, including dietary fructose. Hypoglycemia occurs when glycogen reserves are limited or exhausted. The clinical manifestations are characterized by life-threatening episodes of acidosis, hypoglycemia, hyperventilation, convulsions, and coma. In about half of the cases, the deficiency presents in the 1st wk of life. In infants and small children, episodes are triggered by febrile infections and gastroenteritis if oral food intake decreases. The frequency of the attacks decreases with age. Laboratory findings include low blood glucose, high lactate and uric acid levels, and metabolic acidosis. In contrast to HFI, there is usually no aversion to sweets; renal tubular and liver functions are normal.
The diagnosis is established by demonstrating an enzyme deficiency in either liver or intestinal biopsy. The enzyme defect can also be demonstrated in leukocytes in some cases. The gene coding for fructose-1,6-diphosphatase is located on chromosome 9q22; mutations are characterized, making carrier detection and prenatal diagnosis possible. Treatment of acute attacks consists of correction of hypoglycemia and acidosis by intravenous glucose infusion; the response is usually rapid. Avoidance of fasting, aggressive management of infections and restriction of fructose and sucrose from the diet can prevent further episodes. For long-term prevention of hypoglycemia, a slowly released carbohydrate such as cornstarch is useful. Patients who survive childhood develop normally.
Phosphoenolpyruvate Carboxykinase (PEPCK) Deficiency
PEPCK is a key enzyme in gluconeogenesis. It catalyzes the conversion of oxaloacetate to phosphoenolpyruvate (see Fig. 81-4). PEPCK deficiency is both a mitochondrial enzyme deficiency and a cytosolic enzyme deficiency, encoded by 2 distinct genes.
The disease has been reported in only a few cases. The clinical features are heterogeneous, with hypoglycemia, lactic acidemia, hepatomegaly, hypotonia, developmental delay, and failure to thrive as the major manifestations. There may be multisystem involvement, with neuromuscular deficits, hepatocellular damage, renal dysfunction, and cardiomyopathy. The diagnosis is based on the reduced activity of PEPCK in liver, fibroblasts, or lymphocytes. Fibroblasts and lymphocytes are not suitable for diagnosing the cytosolic form of PEPCK deficiency because these tissues possess only mitochondrial PEPCK. To avoid hypoglycemia, patients should be treated with slow-release carbohydrates such as cornstarch, and fasting should be avoided.
Disorders of Pyruvate Metabolism
Pyruvate is formed from glucose and other monosaccharides, from lactate, and from alanine. It is metabolized through 4 main enzyme systems: lactate dehydrogenase, alanine aminotransferase, pyruvate carboxylase, and pyruvate dehydrogenase complex. Deficiency of the M subunit of lactate dehydrogenase causes exercise intolerance and myoglobinuria (Chapter 81.1). Genetic deficiency of alanine aminotransferase has not been reported in humans.
Pyruvate Dehydrogenase Complex Deficiency
After entering the mitochondria, pyruvate is converted into acetyl coenzyme A (acetyl CoA) by the pyruvate dehydrogenase complex (PDHC), which catalyzes the oxidation of pyruvate to acetyl CoA, which then enters the tricarboxylic acid cycle for ATP production. The complex comprises 5 components: E1, an α-keto acid decarboxylase; E2, a dihydrolipoyl transacylase; E3, a dihydrolipoyl dehydrogenase; protein X, an extra lipoate-containing protein; and pyruvate dehydrogenase phosphatase. The most common is a defect in the E1 (see Fig. 81-4).
Deficiency of the PDHC is the most common of the disorders leading to lactic acidemia and central nervous system dysfunction. The central nervous system dysfunction is because the brain obtains its energy primarily from oxidation of glucose. Brain acetyl CoA is synthesized nearly exclusively from pyruvate.
The E1 defects are caused by mutations in the gene coding for E1 α subunit, which is X-linked. Although X-linked, its deficiency is a problem in both males and females even though only 1 E1 α allele in females carries a mutation.
Clinical Manifestations
The disease has a wide spectrum of presentations from the most severe neonatal presentation to a mild late-onset form. The neonatal onset is associated with lethal lactic acidosis, white matter cystic lesions, agenesis of the corpus callosum, and the most severe enzyme deficiency. Infantile onset can be lethal or associated with psychomotor retardation and chronic lactic acidosis, cystic lesions in the brainstem and basal ganglia, and pathologic features resembling Leigh disease (see later). Older children, usually boys, may have less acidosis, have greater enzyme activity, and manifest ataxia with high-carbohydrate diets. Intelligence may be normal. Patients of all ages may have facial dysmorphology, features similar to those of fetal alcohol syndrome.
The E2 and protein X-lipoate defects are rare and result in severe psychomotor retardation. The E3 lipoamide dehydrogenase defect leads to deficient activity not only in the PDHC, but also in the α-ketoglutarate and branched-chain keto acid dehydrogenase complexes. Pyruvate dehydrogenase phosphatase deficiency has also been reported. These other PDHC defects have clinical manifestations within the variable spectrum associated with PDHC deficiency due to E1 deficiency.
Treatment
The general prognosis is poor except in rare cases in which mutation is associated with altered affinity for thiamine pyrophosphate, which may respond to thiamine supplementation. Because carbohydrates can aggravate lactic acidosis, a ketogenic diet is recommended. The diet has been found to lower the blood lactate level; the long-term benefit to patient outcome is unclear. A potential treatment strategy is to maintain any residual PDHC in its active form by dichloroacetate, an inhibitor of E1 kinase. Beneficial effects in controlling postprandial lactic acidosis in some patients have been shown. Young children with congenital acidosis generally tolerate oral dichloroacetate well, but continued exposure is associated with peripheral neuropathy, a condition that could be attributable to the drug or the disease.
Deficiency of Pyruvate Carboxylase
Pyruvate carboxylase is a mitochondrial, biotin-containing enzyme essential in the process of gluconeogenesis; it catalyzes the conversion of pyruvate to oxaloacetate. The enzyme is also essential for Krebs cycle function as a provider of oxaloacetate and is involved in lipogenesis and formation of nonessential amino acids. Clinical manifestations of this deficiency have varied from neonatal severe lactic acidosis accompanied by hyperammonemia, citrullinemia, and hyperlysinemia (type B) to late-onset mild to moderate lactic acidosis and developmental delay (type A). In both types, patients who survived usually had severe psychomotor retardation with seizures, spasticity, and microcephaly. Some patients have pathologic changes in the brainstem and basal ganglia that resemble Leigh disease (see later). The clinical severity appears to correlate with the level of the residual enzyme activity. A “benign” form of PC deficiency characterized by recurrent attacks of lactic acidosis and mild neurologic deficits has also been described. Laboratory findings are characterized by elevated levels of blood lactate, pyruvate, alanine, and ketonuria. In the case of type B, blood ammonia, citrulline, and lysine levels are also elevated, which might suggest a primary defect of the urea cycle. The mechanism is likely caused by depletion of oxaloacetate, which leads to reduced levels of aspartate, a substrate for argininosuccinate synthetase in the urea cycle (Chapter 79.12).
Treatment consists of avoidance of fasting, and eating a carbohydrate meal before bedtime. During acute episodes of lactic acidosis, patients should receive continuous intravenous glucose. Aspartate and citrate supplements restore the metabolic abnormalities; whether this treatment can prevent the neurologic deficits is not known. Liver transplantation has been attempted; its benefit remains unknown. Diagnosis of pyruvate carboxylase deficiency is made by the measurement of enzyme activity in liver or cultured skin fibroblasts and must be differentiated from holocarboxylase synthetase or biotinidase deficiency.
Deficiency of Pyruvate Carboxylase Secondary to Deficiency of Holocarboxylase Synthetase or Biotinidase
Deficiency of either holocarboxylase synthetase (HCS) or biotinidase, which are enzymes of biotin metabolism, result in multiple carboxylase deficiency (pyruvate carboxylase and other biotin-requiring carboxylases and metabolic reactions) and in clinical manifestations associated with the respective deficiencies, as well as rash, lactic acidosis, and alopecia (Chapter 79.6). The course of HCS or biotinidase deficiency can be protracted, with intermittent exacerbation of chronic lactic acidosis, failure to thrive, seizures, and hypotonia leading to spasticity, lethargy, coma, and death. Auditory and optic nerve dysfunction can lead to deafness and blindness, respectively. Late-onset milder forms have also been reported. Laboratory findings include metabolic acidosis and abnormal organic acids in the urine. In HCS deficiency, biotin concentrations in plasma and urine are normal. Diagnosis can be made in skin fibroblasts or lymphocytes by assay for HCS activity, and in the case of biotinidase, in the serum by a screening blood spot.
Treatment consists of biotin supplementation, 5-20 mg/day, and is generally effective if treatment is started before the development of brain damage. Patients identified through newborn screening and treated with biotin have remained asymptomatic.
Both enzyme deficiencies are autosomal recessive traits. HCS and biotinidase are located on chromosome 21q22 and 3p25, respectively. Ethnic-specific mutations in the HCS gene have been identified. Two common mutations (del7/ins3 and R538C) in the biotinidase gene account for 52% of all mutant alleles in symptomatic patients with biotinidase deficiency.
Mitochondrial Respiratory Chain Defects (Oxidative Phosphorylation Disease)
The mitochondrial respiratory chain catalyzes the oxidation of fuel molecules and transfers the electrons to molecular oxygen with concomitant energy transduction into ATP (oxidative phosphorylation). The respiratory chain produces ATP from nicotinamide adenine dinucleotide (NADH) or FADH2 and includes 5 specific complexes (I: NADH–coenzyme Q reductase; II: succinate–coenzyme Q reductase; III: coenzyme QH2 cytochrome C reductase; IV: cytochrome C oxidase; V: ATP synthase). Each complex is composed of 4-35 individual proteins and, with the exception of complex II (which is encoded solely by nuclear genes), is encoded by nuclear or mitochondrial DNA (inherited only from the mother by mitochondrial inheritance). Defects in any of these complexes or assembly systems produce chronic lactic acidosis presumably due to a change of redox state with increased concentrations of NADH. In contrast to PDHC or pyruvate carboxylase deficiency, skeletal muscle and heart are usually involved in the respiratory chain disorders, and in muscle biopsy, “ragged red fibers” (indicating mitochondrial proliferation) are very suggestive when present (see Fig. 81-5). Because of the ubiquitous nature of oxidative phosphorylation, a defect of the mitochondrial respiratory chain accounts for a vast array of clinical manifestations and should be considered in patients in all age groups presenting with multisystem involvement. Some deficiencies resemble Leigh disease (see later), whereas others cause infantile myopathies such as MELAS (mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes), MERRF (myoclonus epilepsy, with ragged red fibers), and Kearns-Sayre syndrome (external ophthalmoplegia, acidosis, retinal degeneration, heart block, myopathy, and high cerebrospinal fluid protein) (Table 81-2; Chapters 591.2 and 603.4). There is a higher incidence of psychiatric disorders in adults with a primary oxidative phosphorylation disease than in the general population. Diagnosis requires demonstration of abnormalities of oxidative phosphorylation enzyme complex activities in tissues or of mitochondrial DNA (mtDNA) or a nuclear gene coding form mitochondrial functions, or both (Fig. 81-6). Analysis of oxidative phosphorylation complexes I-IV from intact mitochondria isolated from fresh skeletal muscle is the most sensitive assay for mitochondrial disorders. Specific criteria may assist in making a diagnosis (Table 81-3). Clues to the diagnosis of mitochondrial diseases are noted in Table 81-4.
Table 81-2 CLINICAL AND GENETIC HETEROGENEITY OF DISORDERS RELATED TO MUTATIONS IN MITOCHONDRIAL DNA (mtDNA)*
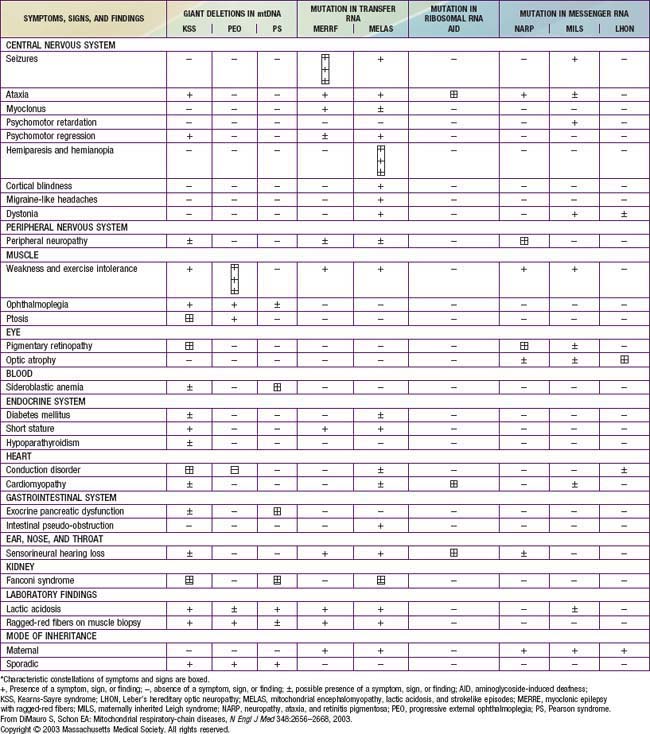
Figure 81-6 Mutations in the human mitochondrial genome that are known to cause disease. Disorders that are frequently or prominently associated with mutations in a particular gene are shown in bold. Diseases due to mutations that impair mitochondrial protein synthesis are shown in blue. Diseases due to mutations in protein-coding genes are shown in red. ECM, encephalomyopathy; FBSN, familial bilateral striatal necrosis; LHON, Leber hereditary optic neuropathy; LS, Leigh syndrome; MELAS, mitochondrial encephalomyopathy, lactic acidosis, and strokelike episodes; MERRF, myoclonic epilepsy with ragged-red fibers; MILS, maternally inherited Leigh syndrome; NARP, neuropathy, ataxia, and retinitis pigmentosa; PEO, progressive external ophthalmoplegia; PPK, palmoplantar keratoderma; and SIDS, sudden infant death syndrome.
(From DiMauro S, Schon EA: Mitochondrial respiratory-chain diseases, N Engl J Med 348:2656–2668, 2003.
Table 81-3 MODIFIED WALKER CRITERIA APPLIED TO CHILDREN REFERRED FOR EVALUATION OF MITOCHONDRIAL DISEASE
| MAJOR CRITERIA | MINOR CRITERIA | |
|---|---|---|
| Clinical | Clinically complete RC encephalomyopathy* or a mitochondrial cytopathy defined as fulfilling 3 criteria† | Symptoms compatible with an RC defect‡ |
| Histology | >2% ragged red fibers (RRF) in skeletal muscle | Smaller numbers of RRF, SSAM, or widespread electron microscopy abnormalities of mitochondria |
| Enzymology | Cytochrome c oxidase–negative fibers or residual activity of an RC complex <20% in a tissue; <30% in a cell line, or <30% in 2 or more tissues | Antibody-based demonstration of an RC defect or residual activity of an RC complex 20-30% in a tissue, 30-40% in a cell line, or 30-40% in 2 or more tissues |
| Functional | Fibroblast ATP synthesis rates >3 SD below mean | Fibroblast ATP synthesis rates 2-3 SD below mean, or fibroblasts unable to grow in galactose media |
| Molecular | Nuclear or mtDNA mutation of undisputed pathogenicity | Nuclear or mtDNA mutation of probable pathogenicity |
| Metabolic | One or more metabolic indicators of impaired metabolic function |
ATP, adenosine triphosphate; RC, respiratory chain; SSAM, subsarcolemmal accumulation of mitochondria.
* Leigh disease, Alpers disease, LIMD, Pearson syndrome, Kearns-Sayre syndrome, MELAS, MERRF, NARP, MNGIE, and LHON.
† (1) Unexplained combination of multisystemic symptoms that is essentially pathognomonic for an RC disorder, (2) a progressive clinical course with episodes of exacerbation or a family history strongly indicative of an mtDNA mutation, and (3) other possible metabolic or nonmetabolic disorders have been excluded by appropriate testing.
‡ Added pediatric features: stillbirth associated with a paucity of intrauterine movement, neonatal death or collapse, movement disorder, severe failure to thrive, neonatal hypotonia, and neonatal hypertonia as minor clinical criteria.
From Scaglia F, Towbin JA, Craigen WJ, et al: Clinical spectrum, morbidity and mortality in 113 pediatric patients with mitochondrial disease, Pediatrics 114:925–931, 2004.
Table 81-4 CLUES TO THE DIAGNOSIS OF MITOCHONDRIAL DISEASE
NEUROLOGIC
CARDIOVASCULAR
OPHTHALMOLOGIC
GASTROENTEROLOGIC
OTHER
From Haas RH, Parikh S, Falk MJ, et al: Mitochondrial disease: a practical approach for primary care physicians, Pediatrics 120:1326–1333, 2007, Table 1, p 1327.
Treatment remains largely symptomatic and does not significantly alter the outcome of disease. Some patients appear to respond to cofactor supplements, typically coenzyme Q10 plus L-carnitine at pharmacologic doses. The addition of creatine monohydrate and alpha-lipoic acid supplementation may add a significant benefit.
Leigh Disease (Subacute Necrotizing Encephalomyelopathy)
Leigh disease is a heterogenous neurologic disease that remains a neuropathologic description characterized by demyelination, gliosis, necrosis, relative neuronal sparing, and capillary proliferation in specific brain regions. In decreasing order of severity, the affected areas are the basal ganglia, brainstem cerebellum, and cerebral cortex (Chapter 591). The classic presentation is of an infant who presents with central hypotonia, developmental regression or arrest, and signs of brainstem or basal ganglia involvement. The clinical presentation is highly variable. Diagnosis is usually confirmed by radiologic or pathologic evidence of symmetric lesions affecting the basal ganglia, brainstem, and subthalamic nuclei. Patients with Leigh disease have defects in several enzyme complexes. Dysfunction in cytochrome C oxidase (complex IV) is the most commonly reported defect, followed by NADH-coenzyme Q reductase (complex I), PDHC, and pyruvate carboxylase. Mutations in the nuclear SURF1 gene, which encodes a factor involved in the biogenesis of cytochrome C oxidase and mitochondrial DNA mutations in the ATPase 6 coding region, are common molecular findings in patients with Leigh disease. Patients with Leigh disease frequently present with developmental delay, seizures, altered consciousness, failure to thrive, pericardial effusion, and dilated cardiomyopathy. The prognosis for Leigh syndrome is poor. In a study of 14 cases, there were 7 fatalities before the age of 1.5 yr.
Copeland WC. Inherited mitochondrial diseases of DNA replication. Annu Rev Med. 2008;59:131-146.
Debray FG, Lambert M, Chevalier I, et al. Long-term outcome and clinical spectrum of 73 pediatric patients with mitochondrial diseases. Pediatrics. 2007;119:722-733.
DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91-123.
Koene S, Kozicz TL, Rodenburg RJ, et al. Major depression in adolescent children consecutively diagnosed with mitochondrial disorder. J Affect Disord. 2009;114:327-332.
Garcoa-Cazorla A, De Lonlay P, Nassogne MC, et al. Long-term follow-up of neonatal mitochondrial cytopathies: a study of 57 patients. Pediatrics. 2005;116:1170-1177.
Genc GA, Sivri-Kalkanoglu HS, Dursun A, et al. Audiologic findings in children with biotinidase deficiency in Turkey. Int J Pediatr Otorhinolaryngol. 2007;71:333-339.
Haas RH, Parikh S, Falk MJ, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120:1326-1333.
Huntsman RJ, Sinclair DB, Bhargava R, et al. Atypical presentations of Leigh syndrome: a case series and review. Pediatr Neurol. 2005;32:334-340.
Lee HF, Tsai CR, Chi CS, et al. Leigh syndrome: clinical and neuroimaging follow up. Pediatr Neurol. 2009;40:88-93.
Lee NC, Dimmock D, Hwu WL, et al. Simultaneous detection of mitochondrial DNA depletion and single-exon deletion in the deoxyguanosine gene using array-based comparative genomic hybridization. Arch Dis Child. 2009;94:55-58.
Montini G, Malaventura C, Saliati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med. 2008;358:2849-2850.
Pithukpakorn M. Disorders of pyruvate metabolism and the tricarboxylic acid cycle. Mol Genet Metab. 2005;85:243-246.
Sarzi E, Bourdon A, Chretien D, et al. Mitochondrial DNA depletion is a prevalent cause of multiple respiratory chain deficiency in childhood. J Pediatr. 2007;105:531-534.
Scheers I, Bachy V, Stephenne X, et al. Risk of hepatocellular carcinoma in liver mitochondrial respiratory chain disorders. J Pediatr. 2005;146:414-417.
Schon EA, DiMauro S. Mitochondrial mutations: genotype to phenotype. Novartis Found Symp. 2007;287:214-225.
Shanske S, Coku J, Lu J, et al. The G13513A mutation in the ND5 gene of mitochondrial DNA as a common cause of MELAS or Leigh syndrome: evidence from 12 cases. Arch Neurol. 2008;65:368-372.
Shoffner JM. Oxidative phosphorylation diseases. In: Scriver CR, Beaudet AL, Sly WS, et al, editors. The online metabolic and molecular bases of inherited disease. New York: McGraw-Hill, 2008.
Stacpoole PW, Gilbert LR, Neiberger RE, et al. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics. 2008;121:e1223-1228.
Stacpoole PW, Kerr DS, Barnes C, et al. Controlled clinical trial of dichloro-acetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519-1531.
Rodriquez MC, MacDonald JR, Mahoney DJ, et al. Beneficial effects of creatine, CoQ10, and lipoic acid in mitochondrial disorders. Muscle Nerve. 2007;35:235-242.
Wani AA, Ahanger SH, Bapat SA, et al. Analysis of mitochondrial DNA sequences in childhood encephalomyopathies reveals new disease-associated variants. PLoS One. 2007;2:e942.
81.5 Defects in Pentose Metabolism
About 90% of glucose metabolism in the body is via the glycolytic pathway, with the remaining 10% via the hexose monophosphate pathway. The hexose monophosphate shunt leads to formation of pentoses, as well as providing NADH. One of the metabolites is ribose-5-phosphate, which is used in the biosynthesis of ribonucleotides and deoxyribonucleotides. Through the transketolase and transaldolase reactions, the pentose phosphates can be converted back to fructose-6-phosphate and glucose-6-phosphate.
Essential Pentosuria
Essential pentosuria is a benign disorder encountered principally in Ashkenazi Jews and is an autosomal recessive trait. The urine contains L-xylulose, which is excreted in increased amounts because of a block in the conversion of L-xylulose to xylitol due to xylitol dehydrogenase deficiency. The condition is usually discovered accidentally in a urine test for reducing substances; no treatment is required.
Transaldolase Deficiency
Few patients have reported symptoms that include liver cirrhosis, hepatosplenomegaly, severe neonatal hepatopathy, and cardiomyopathy. Biochemical abnormalities revealed elevated levels of arabitol, ribitol, and erythritol in the urine. Enzyme assay in the lymphoblasts and fibroblasts demonstrated low transaldolase activity, which was confirmed by mutations in the transaldolase gene. Transaldolase deficiency can be diagnosed by assessing urinary concentrations of polyols.
Ribose-5-Phosphate Isomerase Deficiency
Only 1 case of this disorder has been reported. The affected male had psychomotor retardation from early in life and developed epilepsy at 4 yr of age. Thereafter, a slow neurologic regression developed, with prominent cerebellar ataxia, some spasticity, optic atrophy, and a mild sensorimotor neuropathy. MRI of the brain at ages 11 yr and 14 yr showed extensive abnormalities of the cerebral white matter. Proton magnetic resonance spectroscopy (MRS) of the brain revealed elevated levels of ribitol and D-arabitol. These pentitols were also increased in urine and plasma similar to the patient found in transaldolase deficiency. Enzyme assays in cultured fibroblasts showed deficient ribose-5-phosphate isomerase activity, which was confirmed by a molecular study.
Wamelink MM, Smith DE, Jakobs C, et al. Analysis of polyols in urine by liquid chromatography-tandem mass spectrometry: a useful tool for recognition of inborn errors affecting polyol metabolism. J Inherit Metab Dis. 2005;28:951-963.
Wamelink MM, Struys EA, Jakobs C. The biochemistry, metabolism and inherited defects of the pentose phosphate pathway: a review. J Inherit Metab Dis. 2008;31:703-717.
Verhoeven NM, Wallot M, Huck JH, et al. A newborn with severe liver failure, cardiomyopathy and transalodase deficiency. J Inherit Metab Dis. 2005;28:169-179.
81.6 Disorders of Glycoprotein Degradation and Structure
The disorders of glycoprotein degradation and structure include several lysosomal storage diseases that result from defects in glycoprotein degradation, and the congenital disorders of glycosylation (CDGs), which are pathophysiologically unrelated. Glycoproteins are macromolecules that are composed of oligosaccharide chains linked to a peptide backbone. They are synthesized by 2 pathways: the glycosyltransferase pathway, which synthesizes oligosaccharides linked O-glycosidically to serine or threonine residues; and the dolichol, lipid-linked pathway, which synthesizes oligosaccharides linked N-glycosidically to asparagine.
The glycoprotein lysosomal storage diseases result from the deficiency of the enzymes that normally participate in the degradation of oligosaccharides and include sialidosis, galactosialidosis, aspartylglucosaminuria, and α-mannosidosis. In some instances, the underlying abnormality that leads to glycoprotein accumulation also results in abnormal degradation of other classes of macromolecules that contain similar oligosaccharide linkages, such as certain glycolipids and proteoglycans. In these instances, the underlying enzymatic deficiency results in the accumulation of both glycoproteins and glycolipids. The classification of these types of disorders as lipidoses or glycoproteinoses is dependent on the nature of the predominantly stored substance. In general, the glycoprotein disorders are characterized by autosomal recessive inheritance and a progressive disease course with clinical features that resemble those seen in the mucopolysaccharidoses.
Sialidosis and Galactosialidosis
Sialidosis is an autosomal recessive disorder that results from the primary deficiency of neuraminidase due to mutations in the gene that encodes this protein, which is located on chromosome 10. In contrast, galactosialidosis is due to the deficiency of 2 lysosomal enzymes, neuraminidase and β-galactosidase. The loss of these enzymatic activities results from mutations in a gene located on chromosome 20 that encodes protective protein/cathepsin A (PPCA), which functions to stabilize these enzymatic activities. Neuraminidase normally cleaves terminal sialyl linkages of several oligosaccharides and glycoproteins. Its deficiency results in the accumulation of oligosaccharides, and the urinary excretion of sialic acid terminal oligosaccharides and sialylglycopeptides. Examination of tissues from affected individuals reveals pathologic storage of substrate in many tissues including liver, bone marrow, and brain.
The clinical phenotype associated with neuraminidase deficiency is variable and includes type I sialidosis which usually presents in the 2nd decade of life with myoclonus and the presence of a cherry red spot. These patients typically come to attention secondary to gait disturbances, myoclonus, or visual complaints. In contrast, type II sialidosis occurs as congenital, infantile, and juvenile forms. The congenital and infantile forms result from isolated neuraminidase deficiency, whereas the juvenile form results from both neuraminidase and β-galactosidase deficiency. The congenital type II disease is characterized by hydrops fetalis, neonatal ascites, hepatosplenomegaly, stippling of the epiphyses, periosteal cloaking, and stillbirth or death in infancy. The type II infantile form presents in the 1st yr of life with dysostosis multiplex, moderate mental retardation, visceromegaly, corneal clouding, cherry red spot, and seizures. The juvenile type II form of sialidosis, which is sometimes designated galactosialidosis, has a variable age of onset ranging from infancy to adulthood. In infancy, the phenotype is similar to that of GM1 gangliosidosis, with edema, ascites, skeletal dysplasia, and cherry red spot. Patients with later-onset disease have dysostosis multiplex, visceromegaly, mental retardation, dysmorphism, corneal clouding, progressive neurologic deterioration, and bilateral cherry red spots. No specific therapy exists for any form of the disease, although studies in animal models have demonstrated improvement in the phenotype after bone marrow transplantation. The diagnosis of sialidosis and galactosialidosis is achieved by the demonstration of the specific enzymatic deficiency. Prenatal diagnosis using cultured amniotic cells is also possible.
Aspartylglucosaminuria (AGU)
This is a rare autosomal recessive lysosomal storage disorder, except in Finland, where the carrier frequency is estimated at 1/36. The disorder results from the deficient activity of aspartylglucosaminidase and the subsequent accumulation of aspartylglucosamine, particularly in the liver, spleen, and thyroid. The gene for the enzyme has been localized to the long arm of chromosome 4 and the cDNA has been cloned and sequenced. In the Finnish population, a single mutation in the gene (C163S) accounts for most mutant alleles, whereas outside of Finland, a large number of private mutations have been described. Affected individuals with AGU typically present in the 1st yr of life with recurrent infections, diarrhea, and hernias. Coarsening of the facies and short stature usually develop later. Other features include joint laxity, macroglossia, hoarse voice, crystal-like lens opacities, hypotonia, and spasticity. Psychomotor development is usually near normal until the age of 5 yr when a decline is noted. Behavioral abnormalities are typical and IQ values in affected adults are usually <40. Survival to adulthood is common, with most early deaths attributable to pneumonia or other pulmonary causes. Definitive diagnosis requires measurement of the enzyme in peripheral blood leukocytes. Molecular diagnosis by analysis of DNA for the C163S mutation is possible for Finnish patients. Several patients have undergone allogeneic bone marrow transplants with some reports of stabilization of the neurologic phenotype, but this approach has not been proven effective and no specific treatment is available. Prenatal diagnosis by the determination of the level of aspartylglucosaminidase in cultured amniocytes or chorionic villi has been reported.
α-Mannosidosis
This autosomal recessive disorder results from the deficient activity of α-mannosidase and the accumulation of mannose-rich compounds. The gene encoding the enzyme has been localized to chromosome 19p13.2-q12, although the cDNA has not been cloned. Affected patients with this disorder display clinical heterogeneity. There is a severe infantile form, or type I disease, and a milder juvenile variant, type II disease. All patients have psychomotor retardation, facial coarsening, and dysostosis multiplex. The infantile form of the disorder, however, is characterized by more rapid mental deterioration, with death occurring between the ages of 3 and 10 yr. Patients with the infantile form also have more severe skeletal involvement and hepatosplenomegaly. The juvenile disorder is characterized by onset of symptoms in early childhood or adolescence with milder somatic features and survival to adulthood. Hearing loss, destructive synovitis, pancytopenia, and spastic paraplegia have been reported in type II patients. No specific therapy exists for the disorder. The diagnosis is made by the demonstration of the deficiency of α-mannosidase activity in white blood cells or cultured fibroblasts, and prenatal diagnosis has also been achieved.
Congenital Disorders of Glycosylation (CDGs)
These are a heterogenous group of autosomal recessive disorders that result from defective protein and lipid glycosylation. The protein glycosylation disorders result from defects of N-glycosylation, combined N- and O-glycosylation, the dolichol pathway and the conserved oligomeric Golgi complex (COG). The more recently discovered lipid-glycosylation disorders include defects of ganglioside synthesis (GM3 synthase deficiency) and the GPI anchor system. In addition, there are patients with glycosylation defects for which the molecular and biochemical bases are not yet known.
To date, more than 30 CDG subtypes have been identified (Table 81-5). In general most CDG disorders are multisystemic and present with variable involvement of the central nervous system (most often hypotonia and ataxia), abnormal fat distribution, ocular movement defects, coagulation abnormalities, gastrointestinal symptoms including protein-losing enteropathy, retinitis pigmentosa, hormonal abnormalities, and in some cases dysmorphic features. CDG Type Ia, which results from mutations in the gene that encodes phosphomannomutase, is the most common form. The most consistent clinical features of this disorder include variable degrees of psychomotor retardation, subcutaneous fat pads and inverted nipples. Frequent neurologic findings in infancy include cerebellar atrophy (Fig. 81-7), hypotonia, weakness, hyperreflexia, and strokelike episodes.
Figure 81-7 Sagittal T2-weighted MR image shows severe spinal cord compression with myelopathy (white arrow), together with cerebellar atrophy (black arrow), and cortical atrophy of the parietal lobe (red arrow).
(From Schade van Westrum SM, Nederkoorn PJ, Schuurman PR, et al: Skeletal dysplasia and myelopathy in congenital disorder of glycosylation type 1A, J Pediatr 148:115–117, 2006.)
In childhood, ataxia, muscle atrophy, decreased deep tendon reflexes, toe walking, and continued strokelike episodes are observed. The latter events may be related to coagulopathies characterized by reduced antithrombin III, protein C and S in conjunction with abnormal levels of factors VIII, IX, XI, and XIII, which together increase risk for bleeding and thrombosis. Growth failure, liver dysfunction, retinal degeneration, and skeletal abnormalities have also been described. The skeletal features can include contractures, kyphoscoliosis, and pectus carinatum, all of which may be secondary to the neurologic effects of the disorder. Pericardial effusion in older patients and hypertrophic obstructive cardiomyopathy in the infant also may occur.
Among the other types, few have distinctive phenotypes. These include CDG Ib, which is characterized by protein-losing enteropathy and normal neurologic function; CDG If, which includes ichthyosis and growth retardation; and CDG IIf, in which macrothrombocytopenia is present.
Diagnostic testing for CDG types that result from defects of N-glycosylation begins with isoelectric focusing of N-glycosylated serum transferring. For some types further confirmation of the diagnosis by enzymatic analysis in fibroblasts or leukocytes and/or mutation analysis of the relevant gene is available. Although prenatal diagnosis by analysis of transferrin has been attempted, it has not proven reliable. Treatment of these disorders is symptomatic, except for CDGIb, which responds to oral mannose (100-150 mg/kg/day every 4-6 hr).
Collins AE, Ferriero DM. The expanding spectrum of congenital disorders of glycosylation. J Pediatr. 2005;147:728-730.
Denecke J, Kranz C, Von Kleist-Retzow JC, et al. Congenital disorder of glycosylation type 1d: clinical phenotype, molecular analysis, prenatal diagnosis, and glycosylation of fetal proteins. Pediatr Res. 2005;58:248-253.
Dinopoulos A, Mohaned I, Jones B, et al. Radiologic and neurophysiologic aspects of stroke-like episodes in children with congenital disorder of glycosylation type Ia. Pediatrics. 2007;119:e768-e772.
Leroy JG. Congenital disorders of N-glycosylation including diseases associated with O- as well as N-glycosylation defects. Pediatr Res. 2006;60:643-656.
Vodopiutz J, Bodamer OA. Congenital disorders of glycosylation—a challenging group of IEMs. J Inherit Metab Dis. 2008;31:267-269.