Chapter 188 Moraxella catarrhalis
Moraxella catarrhalis, an unencapsulated gram-negative diplococcus, is a human-specific pathogen that colonizes the respiratory tract beginning in infancy. Colonization and infection with M. catarrhalis are increasing in countries in which pneumococcal conjugate vaccines are used widely. The most important clinical manifestation of M. catarrhalis infection in children is otitis media.
Etiology
M. catarrhalis has long been considered to be an upper respiratory tract commensal. Initially named Micrococcus catarrhalis, its name was changed to Neisseria catarrhalis in 1970 because of its phenotypic similarities and similar ecological niche with commensal Neisseria species. On the basis of more modern analyses of genetic relatedness, Moraxella catarrhalis is now the accepted name.
Substantial genetic heterogeneity exists among strains of M. catarrhalis. Several outer membrane proteins demonstrate sequence differences among strains, particularly in regions of the proteins that are exposed on the bacterial surface. M. catarrhalis endotoxin lacks repeating polysaccharide side chains and is thus a lipo-oligosaccharide. In contrast to other gram-negative respiratory pathogens, such as Haemophilus influenzae and Neisseria meningitidis, the lipo-oligosaccharide of M. catarrhalis is relatively conserved among strains; only 3 serotypes (A, B, and C) that are based on oligosaccharide structure have been identified. Genetic and antigenic differences among strains account for the observation that resolving an infection by one strain does not induce protective immunity to other strains. M. catarrhalis causes recurrent infections, which generally represent re-infection by new strains.
Epidemiology
The ecological niche of M. catarrhalis is the human respiratory tract. The bacterium has not been recovered from animals or environmental sources. Age is the most important determinant of the prevalence of upper respiratory tract colonization. Common throughout infancy, nasopharyngeal colonization is a dynamic process with active turnover due to acquisition and clearance of strains of M. catarrhalis. Some geographic variation in rates of colonization is observed. On the basis of monthly or bimonthly cultures, colonization during the 1st year of life may range from 33% to 100%. Several factors likely account for this variability among studies, including living conditions, daycare attendance, hygiene, environmental factors (e.g., household smoking), and genetics of the population. The prevalence of colonization steadily decreases with age. Understanding nasopharyngeal colonization patterns is important, because the pathogenesis of otitis media involves migration of the bacterium from the nasopharynx to the middle ear via the eustachian tube.
The widespread use of pneumococcal polysaccharide vaccines in some countries has resulted in alteration of patterns of nasopharyngeal colonization in the population. A relative increase in colonization by non-vaccine pneumococcal serotypes, nontypable H. influenzae, and M. catarrhalis has occurred. These changes in colonization patterns may account for the increased rates of otitis media due to nontypable H. influenzae and M. catarrhalis. Similar shifts in etiology are being observed in children with sinusitis as well.
Pathogenesis of Infection
Strains of M. catarrhalis differ in their virulence properties. The species is composed of complement-resistant and complement-sensitive genetic lineages, the complement-resistant strains being more strongly associated with virulence. Strains that cause infection in children differ in several phenotypic characteristics from strains that cause infection in adults, in whom the most common clinical manifestation is lower respiratory tract infection in the setting of chronic obstructive pulmonary disease.
The presence of several adhesin molecules with differing specificities for various host cell receptors reflects the importance of adherence to the human respiratory epithelial surface in the pathogenesis of infection. M. catarrhalis has long been viewed as an exclusively extracellular pathogen. However, the bacterium is now known to invade multiple cell types, including bronchial epithelial cells, small airway cells, and type 2 alveolar cells. In addition, M. catarrhalis resides intracellularly in lymphoid tissue, providing a potential reservoir for persistence in the human respiratory tract.
M. catarrhalis forms biofilms in vitro and in the middle ears of children with chronic and recurrent otitis media. Biofilms are communities of bacteria encased in a matrix attached to a surface. Bacteria in biofilms are more resistant to antibiotics and to host immune responses than bacteria growing individually in planktonic form.
Clinical Manifestations
M. catarrhalis causes predominantly mucosal infections in children. The mechanism of infection is migration of the infecting strains from the nasopharynx to the middle ear in the case of otitis media or to the sinuses in the case of sinusitis. The inciting event for both otitis media and sinusitis is often a preceding viral infection.
Acute Otitis Media
Approximately 80% of children have one or more episodes of otitis media by age 3 yr. Otitis media is the most common reason for which children receive antibiotics. On the basis of culture of middle ear fluid obtained by tympanocentesis, the predominant causes of acute otitis media are S. pneumoniae, H. influenzae, and M. catarrhalis (Fig. 188-1). Overall, M. catarrhalis causes 15-20% of cases of otitis media. The distribution of the causative agents of otitis media is changing as a result of widespread administration of pneumococcal conjugate vaccines, with a relative increase in H. influenzae and M. catarrhalis.
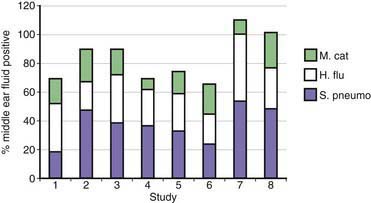
Figure 188-1 Bacterial causes of otitis media based on results of culture of middle ear fluid obtained by tympanocentesis from 8 studies published from 1992 to 2006. Bars represent % positive results of cultures of middle ear fluids. Some cultures had more than one species, accounting for totals greater that 100%. S. pneumo, Streptococcus pneumoniae; H. flu, nontypable Haemophilus influenzae; M. cat, Moraxella catarrhalis.
(From Murphy TF, Paramswaran GI: Moraxella catarrhalis, a human respiratory tract pathogen, Clin Infect Dis 49:124–131, 2009.)
Acute otitis media due to M. catarrhalis is clinically milder than otitis media due to H. influenzae or S. pneumoniae, with less fever and lower prevalence of a red, bulging tympanic membrane. However, substantial overlap in symptoms is seen so that it is not possible to predict etiology in an individual child on the basis of clinical features. Tympanocentesis is required to make an etiologic diagnosis but is not performed routinely, and thus, treatment of otitis media is generally empirical.
Recurrent Otitis Media and Otitis Media with Effusion
Otitis media with effusion refers to the presence of fluid in the middle ear in the absence of signs and symptoms of acute infection. Children who experience four or more episodes of acute otitis media in a year or who have at least 8 mo of middle ear effusion in a year are defined as otitis prone. These children suffer conductive hearing loss, which may lead to delays in speech and language development. Analysis of middle ear fluid from children with otitis media with effusion using sensitive molecular techniques such as polymerase chain reaction (PCR) indicates that bacterial DNA is present in up to 80% of samples from such children. Indeed, M. catarrhalis DNA is present in a larger proportion of cases of otitis media with effusion than of acute otitis media. Biofilms may account for these observations, although definitive evidence for this conclusion is lacking.
Sinusitis
A small proportion of viral upper respiratory tract infections are complicated by bacterial sinusitis. According to findings of studies that use sinus puncture, M. catarrhalis accounts for approximately 20% of cases of acute bacterial sinusitis in children and in a smaller proportion in adults. Sinusitis caused by M. catarrhalis is clinically indistinguishable from that caused by S. pneumoniae or H. influenzae.
Bacteremia
M. catarrhalis rarely causes bacteremia or invasive infections in children. When bacteremia occurs, the usual source is the respiratory tract. Some children have underlying immunocompromising conditions, but no particular immunodeficiency has been associated with invasive M. catarrhalis infections.
Diagnosis
The clinical diagnosis of otitis media is made by demonstration of fluid in the middle ear by pneumatic otoscopy. A tympanocentesis is required to establish an etiologic diagnosis, but this procedure is not performed routinely. Thus, the choice of antibiotic for otitis media is empirical and generally based on guidelines. Management of bacterial sinusitis is also empirical, because determining the etiology of sinusitis requires a sinus puncture, also a procedure that is not performed routinely.
The key to making a microbiologic diagnosis is distinguishing M. catarrhalis from commensal Neisseria that are part of the normal upper respiratory tract flora. Indeed, the difficulty in distinguishing colonies of M. catarrhalis from Neisseria species explains in part why M. catarrhalis has been overlooked in the past as a respiratory tract pathogen. M. catarrhalis produces round, opaque colonies that can be slid across the agar surface without disruption, the “hockey puck sign.” In addition, after 48 hr, M. catarrhalis colonies tend to be larger than Neisseria and take on a pink color. A variety of biochemical tests distinguish M. catarrhalis from Neisseria species, and commercially available kits based on these tests are available.
Sensitive tests that employ PCR to detect respiratory tract bacterial pathogens in human respiratory tract secretions are in development. The application of such assays when they become available is likely to contribute new information about the epidemiology and disease patterns of M. catarrhalis.
Treatment
A proportion of cases of M. catarrhalis otitis media resolve spontaneously. Treatment of otitis media is empirical, and clinicians are advised to follow guidelines of the American Academy of Pediatrics (Chapter 632).
Strains of M. catarrhalis rapidly acquired β-lactamase worldwide in the 1970s and 1980s, rendering essentially all strains resistant to amoxicillin. Antimicrobial susceptibility patterns have remained relatively stable since then. Most strains of M. catarrhalis are susceptible to amoxicillin/clavulanic acid, extended-spectrum cephalosporins, macrolides (azithromycin, clarithromycin), trimethoprim/sulfamethoxazole, and fluoroquinolones.
Prevention
Vaccines to prevent otitis media and other infections caused by M. catarrhalis are under development, but none is available yet.
Deshpande LM, Sader HS, Fritsche TR, Jones RN. Contemporary prevalence of BRO beta-lactamases in Moraxella catarrhalis: report from the SENTRY antimicrobial surveillance program (North America, 1997 to 2004). J Clin Microbiol. 2006;44:3775-3777.
Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;296:202-211.
Heiniger N, Spaniol V, Troller R, et al. A reservoir of Moraxella catarrhalis in human pharyngeal lymphoid tissue. J Infect Dis. 2007;196:1080-1087.
Murphy TF, Paramswaran GI. Moraxella catarrhalis, a human respiratory tract pathogen. Clin Infect Dis. 2009;49:124-131.
Revai K, McCormick DP, Patel J, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal bacterial colonization during acute otitis media. Pediatrics. 2006;117:1823-1829.
Ruckdeschel EA, Kirkham C, Lesse AJ, et al. Mining the Moraxella catarrhalis genome: identification of potential vaccine antigens expressed during human infection. Infect Immun. 2008;76:1599-1607.
Slevogt H, Seybold J, Tiwari KN, et al. Moraxella catarrhalis is internalized in respiratory epithelial cells by a trigger-like mechanism and initiates a TLR2- and partly NOD1-dependent inflammatory immune response. Cell Microbiol. 2007;9:694-707.
Tan TT, Riesbeck K. Current progress of adhesins as vaccine candidates for Moraxella catarrhalis. Expert Rev Vaccines. 2007;6:949-956.
Verhaegh SJ, Streefland A, Dewnarain JK, et al. Age-related genotypic and phenotypic differences in Moraxella catarrhalis isolates from children and adults presenting with respiratory disease in 2001–2002. Microbiology. 2008;154:1178-1184.
Wirth T, Morelli G, Kusecek B, et al. The rise and spread of a new pathogen: seroresistant Moraxella catarrhalis. Genome Res. 2007;17:1647-1656.