Chapter 214 Lyme Disease (Borrelia burgdorferi)
Lyme disease is the most common vector-borne disease in the USA and has become an important public health problem.
Etiology
Lyme disease is caused by the spirochete Borrelia burgdorferi sensu lato (broad sense). In North America, B. burgdorferi sensu stricto (strict sense) causes virtually all cases, and in Europe, the species B. afzelii and B. garinii also cause disease. The 3 major outer-surface proteins called OspA, OspB, and OspC (which are highly charged basic proteins of molecular weights of about 31, 34, and 23 kd, respectively) and the 41 kd flagellar protein are important targets for the immune response. Differences in the molecular structure of the different species are associated with differences in the clinical manifestations of Lyme borreliosis in Europe and the USA. These differences include the greater incidence of radiculoneuritis in Europe.
Epidemiology
Lyme disease has been reported from >50 countries. In the USA approximately 20,000 cases were reported in 2006; however, because of incomplete reporting of cases, it is estimated that the actual number of cases is much higher. From 1992 through 2006, 93% of cases occurred in 10 states: Connecticut, Delaware, Maryland, Massachusetts, Minnesota, New Jersey, New York, Pennsylvania, Rhode Island, and Wisconsin (Fig. 214-1). In endemic areas, the reported annual incidence ranges from 20 to 100 cases/100,000 population, although this figure may be as high as 600 cases/100,000 population in hyperendemic areas. In Europe, most cases occur in the Scandinavian countries and in central Europe, especially Germany, Austria, and Switzerland. The reported incidence is highest among children 5-9 yr of age, with a second peak of disease activity in middle-aged adults. In the USA, Lyme disease is diagnosed in boys slightly more often than in girls, and 94% of patients are of European descent. Early Lyme disease (described later) usually occurs from spring to early fall, corresponding to deer tick activity. Late disease (chiefly arthritis) occurs year round. Among adults, outdoor occupation and leisure activities are risk factors; for children, location of residence in an endemic area is the most important risk for infection.
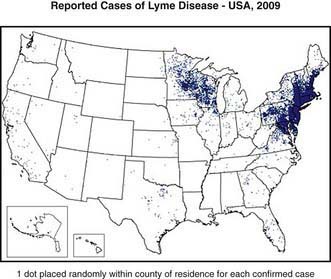
Figure 214-1 The approximate distribution of predicted risk for Lyme disease in the USA. The risk varies by the distribution of Ixodes scapularis and Ixodes pacificus, the proportion of ticks that are infected at each stage of the tick’s life cycle, and the presence of grassy or wooded locations favored by white-tailed deer.
(From the Centers for Disease Control and Prevention: Reported cases of Lyme disease—United States, 2009 [website]. http://www.cdc.gov/ncidod/dvbid/lyme/ld_Incidence.htm. Accessed November 11, 2010.)
Transmission
Lyme disease is a zoonosis caused by the transmission of B. burgdorferi to humans through the bite of an infected tick of the Ixodes genus. In the eastern and midwestern USA, the vector is Ixodes scapularis, the black-legged tick that is commonly known as the deer tick, which is responsible for most cases of Lyme disease in the USA. The vector on the Pacific Coast is Ixodes pacificus, the western black-legged tick. Ixodes ticks have a 2-yr, 3-stage life cycle. The larvae hatch in the early summer and are usually uninfected with B. burgdorferi. The tick can become infected at any stage of its life cycle by feeding on a host, usually a small mammal such as the white-footed mouse (Peromyscus leucopus), which is a natural reservoir for B. burgdorferi. The larvae overwinter and emerge the following spring in the nymphal stage, which is the stage of the tick most likely to transmit the infection. The nymphs molt to adults in the fall and then adults spend the second winter attached to white tailed deer (Odocoileus virginianus). The females lay their eggs the following spring before they die, and the 2-yr life cycle begins again.
Several factors are associated with increased risk for transmission of B. burgdorferi from ticks to humans. The proportion of infected ticks varies by geographic area and by stage of the tick’s life cycle. In endemic areas in the northeastern and midwestern USA, 15-25% of nymphal ticks and 35-50% of adult ticks are infected with B. burgdorferi. By contrast, I. pacificus often feeds on lizards, which are not a competent reservoir for B. burgdorferi, reducing the chance that these ticks will be infected. The risk for transmission of B. burgdorferi from infected Ixodes ticks is related to the duration of feeding. Experiments in animals have shown that infected nymphal ticks must feed for 36-48 hr, and infected adults must feed for 48-72 hr, before the risk for transmission of B. burgdorferi becomes substantial. If the tick is recognized and removed promptly, transmission of B. burgdorferi will not occur.
Ixodes scapularis also transmits other microorganisms, namely Anaplasma phagocytophilum and Babesia microti. Simultaneous transmission can result in co-infections with these organisms and B. burgdorferi.
Pathology and Pathogenesis
Similar to other spirochetal infections, untreated Lyme disease is characterized by asymptomatic infection, clinical disease that can occur in stages, and a propensity for cutaneous and neurologic manifestations.
The skin is the initial site of infection by B. burgdorferi. Inflammation induced by B. burgdorferi leads to the development of the characteristic rash, erythema migrans. Early disseminated Lyme disease results from the spread of spirochetes through the bloodstream to tissues throughout the body. The spirochete adheres to the surfaces of a wide variety of different types of cells, but the principal target organs are skin, central and peripheral nervous system, joints, heart, and eyes. Because the organism can persist in tissues for prolonged periods, symptoms can appear very late after initial infection.
The symptoms of early disseminated and late Lyme disease are due to inflammation mediated by interleukin 1 and other lymphokines in response to the presence of the organism. It is likely that relatively few organisms actually invade the host, but cytokines serve to amplify the inflammatory response and lead to much of the tissue damage. Lyme disease is characterized by inflammatory lesions that contain both T and B lymphocytes, macrophages, plasma cells, and mast cells. The refractory symptoms of late Lyme disease can have an immunogenetic basis. Persons with certain HLA-DR allotypes may be genetically predisposed to develop chronic Lyme arthritis. An autoinflammatory response in the synovium can result in clinical symptoms long after the bacteria have been killed.
Clinical Manifestations
The clinical manifestations of Lyme disease are divided into early and late stages (Table 214-1). Early Lyme disease is further classified as early localized or early disseminated disease. Untreated patients can progressively develop clinical symptoms of each stage of the disease, or they can present with early disseminated or with late disease without apparently having had any symptoms of the earlier stages of Lyme disease.
Table 214-1 CLINICAL STAGES OF LYME DISEASE
| DISEASE STAGE | TIMING AFTER TICK BITE | TYPICAL CLINICAL MANIFESTATIONS |
|---|---|---|
| Early localized | 3-30 days | EM (single), variable constitutional symptoms (headache, fever, myalgia, arthralgia, fatigue) |
| Early disseminated | 3-12 wk | EM (single or multiple), worse constitutional symptoms, cranial neuritis, meningitis, carditis, ocular disease |
| Late | >2 mo | Arthritis |
EM, erythema migrans.
Early Localized Disease
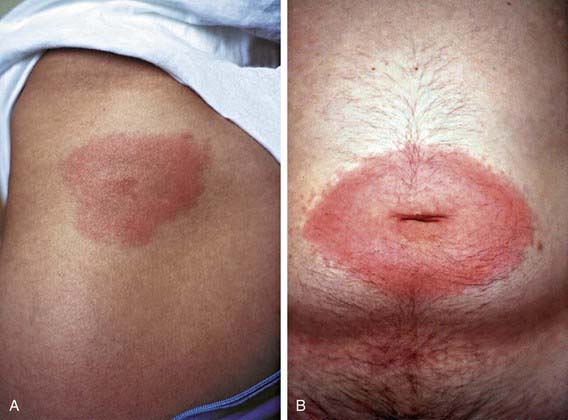
The first clinical manifestation of Lyme disease in most patients is erythema migrans (Fig. 214-2). Although it usually occurs 7-14 days after the bite, the onset of the rash has been reported from 3 to 30 days later. The initial lesion occurs at the site of the bite. The rash is generally either uniformly erythematous or a target lesion with central clearing; rarely, there are vesicular or necrotic areas in the center of the rash. Occasionally the rash is itchy or painful, though usually it is asymptomatic. The lesion can occur anywhere on the body, although the most common locations are the axilla, periumbilical area, thigh, and groin. It is not unusual for the rash to occur on the neck or face, especially in young children. Without treatment, the rash gradually expands (hence the name migrans) to an average diameter of 15 cm and typically remains present for 1-2 wk. Erythema migrans may be associated with systemic features, including fever, myalgia, headache, or malaise. Co-infection with Babesia microti or Anaplasma phagocytophilum during early infection with B. burgdorferi is associated with more severe systemic symptoms.
Early Disseminated Disease
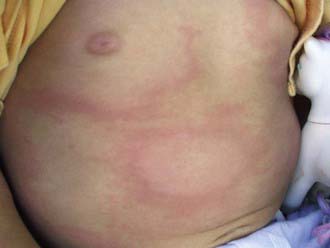
In the USA, about 20% of patients with acute B. burgdorferi infection develop secondary erythema migrans lesions, a common manifestation of early disseminated Lyme disease, caused by hematogenous spread of the organisms to multiple skin sites (Fig. 214-3). The secondary lesions, which can develop several days or weeks after the first lesion, are usually smaller than the primary lesion and are often accompanied by more severe constitutional symptoms. The most common early neurologic manifestations are peripheral facial nerve palsy and meningitis. Lyme meningitis usually has an indolent onset with days to weeks of symptoms that can include headache, neck pain and stiffness, and fatigue. Fever is variably present.
The clinical findings of papilledema, cranial neuropathy (especially cranial nerve VII), and erythema migrans, which are present individually or together in 90% of cases, help differentiate Lyme from viral meningitis, in which these findings are rarely present. Lyme aseptic meningitis can be accompanied by significant elevations of intracranial pressure, which can sometimes last weeks or even months. All of the cranial nerves except the olfactory have been reported to be involved with Lyme disease, but the most common are VI and especially VII. In endemic areas, Lyme disease is the leading cause of peripheral facial nerve palsy. It is often the initial or the only manifestation of Lyme disease and is occasionally bilateral. Laboratory findings indicating meningitis are present in more than half of the cases of peripheral facial nerve palsy. The facial paralysis usually lasts 2-8 wk and resolves completely in most cases. Radiculoneuritis and other peripheral neuropathies can occur but are more common in Europe.
Cardiac involvement occurs in 5-15% of early disseminated Lyme disease and usually takes the form of heart block, which can be 1st, 2nd, or 3rd degree, and the rhythm can fluctuate rapidly. Rarely, myocardial dysfunction can occur. Patients presenting with suspected or proven early disseminated Lyme disease should have a careful cardiac examination and might need electrocardiography. Lyme carditis is a treatable condition and is the only manifestation of Lyme disease that has been fatal.
Of the ocular conditions reported in Lyme disease, papilledema and uveitis are most common.
Late Disease
Arthritis is the usual manifestation of late Lyme disease and begins weeks to months after the initial infection. Arthritis typically involves the large joints, especially the knee, which is affected in 90% of cases; involvement is usually monoarticular. The hallmark of Lyme arthritis is joint swelling, which is due to synovial effusion and sometimes synovial hypertrophy. The swollen joint may be only mildly symptomatic or it may be painful and tender, though patients usually do not experience the severe pain and systemic toxicity that are common in pyogenic arthritis. If untreated, the arthritis can last several weeks, resolve, and then be followed by recurrent attacks in the same or other joints.
Late manifestations of Lyme disease involving the central nervous system, sometimes termed late neuroborreliosis, are rarely reported in children. In adults, chronic encephalitis and polyneuritis have been attributed to Lyme disease. The term Lyme encephalopathy has been used to describe chronic encephalitis (demonstrable by objective measures), but other literature has also used this term in reference to memory loss and other cognitive sequelae after Lyme disease has been treated. At times, the vague term chronic Lyme disease has been used to describe symptomatology in persons who might have never had well-documented infection with B. burgdorferi at all, have serologic evidence of prior infection but current symptoms not consistent with Lyme disease, or have persistent symptoms after having received appropriate antibiotic therapy. Post–Lyme disease syndrome is now the preferred term for this last group.
Congenital Lyme Disease
In endemic areas, infection can occur during pregnancy, although congenital infection appears to be a rare event. B. burgdorferi has been identified from several abortuses and from a few liveborn children with congenital anomalies; however, the tissues in which the spirochete has been identified usually have not shown histologic evidence of inflammation. Severe skin and cardiac manifestations have been described in a few cases, but no consistent pattern of fetal damage has been identified to suggest a clinical syndrome of congenital infection. Furthermore, studies conducted in endemic areas have indicated that there is no difference in the prevalence of congenital malformations among the offspring of women with serum antibodies against B. burgdorferi and the offspring of those without such antibodies.
Laboratory Findings
Standard laboratory tests rarely are helpful in diagnosing Lyme disease because any associated laboratory abnormalities usually are nonspecific. The peripheral white blood cell count may be either normal or elevated. The erythrocyte sedimentation rate may be elevated. Liver transaminases are occasionally mildly elevated. In Lyme arthritis, the white blood cell count in joint fluid can range from 25,000 to 100,000/mL, often with a preponderance of polymorphonuclear cells. When meningitis is present, there usually is a low-grade pleocytosis with a lymphocytic and monocytic predominance. The CSF protein level may be elevated, but the glucose concentration usually is normal. Gram stain and routine bacterial cultures are negative. Imaging of the CNS (e.g., magnetic resonance imaging and single photon emission computed tomography [SPECT]) occasionally reveals abnormalities, but there is no definitive pattern in Lyme disease. The main role of imaging is to exclude other diagnoses.
Diagnosis
In the appropriate epidemiologic setting, typical erythema migrans is virtually pathognomonic. The diagnosis of erythema migrans may be difficult because the rash initially can be confused with nummular eczema, tinea corporis, granuloma annulare, an insect bite, or cellulitis. The relatively rapid expansion of erythema migrans helps distinguish it from these other skin lesions. The other clinical manifestations of Lyme disease are less specific and may be confused with other conditions; the monoarticular or pauciarticular arthritis sometimes is confused with a septic joint or other causes of arthritis in children, such as juvenile rheumatoid arthritis or rheumatic fever; the facial nerve palsy due to Lyme disease is clinically indistinguishable from idiopathic Bell palsy, although bilateral involvement is much more common with Lyme disease; Lyme meningitis generally occurs in the warmer months, the same period that enteroviral disease is prevalent. Therefore, for all disease manifestations other than erythema migrans, it is recommended to have laboratory confirmation of infection with B. burgdorferi.
Although B. burgdorferi has been isolated from blood, skin, CSF, myocardium, and the synovium of patients with Lyme disease, the organism is difficult to isolate in culture (cultivation is largely relegated to research laboratories). Infection is usually identified by the detection of antibody in serum. Although some laboratories offer polymerase chain reaction (PCR) as a diagnostic test for Lyme disease, its sensitivity may be poor because of the low concentrations of bacteria in many sites, especially cerebrospinal fluid (CSF). Other antigen-based tests, including a test for B. burgdorferi antigens in urine, have been unreliable. Clinicians should be aware that some laboratories use alternative diagnostic tests and/or alternative interpretive criteria that are not evidence based, leading to a false diagnosis of Lyme disease.
Serology
Following the transmission of B. burgdorferi from a tick bite, specific immunoglobulin M (IgM) antibodies appear first, usually at 3-4 wk, peak at 6-8 wk, and subsequently decline. Sometimes a prolonged elevation of IgM antibodies occurs despite effective antimicrobial treatment. (For that reason, the results of tests for specific IgM antibodies alone should not be used as a reliable indicator of either active or recent infection.) Specific IgG antibodies usually appear at 4-8 wk, peak after 4-6 mo, and can remain elevated for years, particularly in patients with arthritis. The antibody response to B. burgdorferi may be blunted in patients with early Lyme disease who are treated promptly with an effective antimicrobial agent.
By far the most common method used to detect IgG and IgM antibodies is the enzyme-linked immunosorbent assay (ELISA). This method is sensitive but not optimally specific. The ELISA sometimes produces false-positive results because of antibodies that cross-react with other spirochetal infections (e.g., syphilis, leptospirosis, or relapsing fever), or certain viral infections (e.g., Epstein-Barr virus or parvovirus B19) or that occur in certain autoimmune diseases (e.g., systemic lupus erythematosus). The positive predictive value of the ELISA result depends primarily on the plausibility that the patient has Lyme disease based on the clinical and epidemiologic history and the physical examination (the pretest probability). For patients who have been in endemic areas with opportunities for Ixodes tick exposure and who have typical clinical manifestations of Lyme disease, the pretest probability is high and positive ELISA results are usually true positives. For patients who are from nonendemic areas and/or who have little risk for Ixodes tick exposures and/or have nonspecific symptoms (low pretest probability), rates of false-positive results are high.
Western immunoblotting is well standardized, and there are accepted criteria for interpretation. Five of 10 IgG bands and 2 of 3 IgM bands is considered positive. The Western blot is not as sensitive as ELISA, especially in early infection, but it is highly specific. Any positive or equivocal ELISA should be confirmed with Western blotting. This two-tier testing is the recommended laboratory evaluation of most cases of Lyme disease and is associated with a high degree of sensitivity and specificity when used appropriately.
Clinicians should be aware that Lyme disease might not be the cause of a patient’s symptoms despite the presence of antibodies to B. burgdorferi. The test result may be falsely positive (as described for ELISA), or the patient might have been infected previously. Antibodies to B. burgdorferi that develop with infection can persist for many years despite adequate treatment and clinical cure of the disease. In addition, because some people who become infected with B. burgdorferi are asymptomatic, the background rate of seropositivity among patients who have never had clinically apparent Lyme disease may be substantial in endemic areas. Finally, because antibodies against B. burgdorferi persist after successful treatment, there is no reason to obtain follow-up serologic tests.
Treatment
Treatment recommendations are given in Table 214-2. These have been developed by the Infectious Diseases Society of America (IDSA) and are based on the best available evidence. There have been more clinical trials involving adults than children, so some of the pediatric recommendations derive from results of studies in adults.
Table 214-2 RECOMMENDED TREATMENT OF LYME DISEASE
| DRUG | PEDIATRIC DOSING |
|---|---|
| Amoxicillin | 50 mg/kg/day in 3 divided doses (max 1,500 mg/day) |
| Doxycycline | 4 mg/kg/day in 2 divided doses (max 200 mg/day) (see text regarding doxycycline use in children) |
| Cefuroxime axetil | 30 mg/kg/day in 2 divided doses (max 1,000 mg/day) |
| Ceftriaxone (IV)*† | 50-75 mg/kg/day once daily (max 2,000 mg/day) |
| RECOMMENDED THERAPY BASED ON CLINICAL MANIFESTATION | |
| Erythema migrans | Oral regimen, 14-21 days |
| Meningitis | Ceftriaxone, 10-28 days |
| Cranial nerve palsy | Oral regimen, 14-21 days (see text regarding possible need for lumbar puncture) |
| Cardiac disease | Oral regimen or ceftriaxone, 14-21 days (see text for specifics) |
| Arthritis‡ | Oral regimen, 28 days |
| Late neurologic disease | Ceftriaxone, 14-28 days |
* Cefotaxime and penicillin G are alternative parenteral agents
† Doses of 100 mg/kg/day can be used for meningitis
‡ Persistent arthritis can be treated with a second oral regimen or ceftriaxone.
From Wormser GP, Dattwyler RJ, Shapiro ED, et al: The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America, Clin Infect Dis 43:1089–1134, 2006.
Most patients can be treated with an oral regimen of antibiotic therapy. Young children are generally treated with amoxicillin. Doxycycline has the advantages of good CNS penetration and is active against Anaplasma phagocytophilum, which may be transmitted at the same time as B. burgdorferi in certain geographic areas. In general, children <8 yr of age should not be treated with doxycycline because of the risk of permanent staining of the teeth (although courses of ≤2 wk are usually safe in this regard). Patients who are treated with doxycycline should be alerted to the risk for developing photosensitivity in sun-exposed areas while taking the medication; long sleeves, long pants, and hat are recommended for activities in direct sunlight. The only oral cephalosporin proved to be effective for the treatment of Lyme disease is cefuroxime axetil, which is an alternative for persons who cannot take doxycycline and who are allergic to penicillin (it is also more expensive). Results with macrolide antibiotics, including azithromycin, have been disappointing.
Parenteral therapy is recommended for patients with central nervous system infection and higher degrees of heart block. Patients with arthritis that fails to resolve after an initial course of oral therapy can be re-treated with an oral regimen or can receive intravenous antibiotic therapy. Ceftriaxone is usually favored because of its excellent anti-borrelial activity, tolerability, and once-daily dosing regimen, which can usually be done on an outpatient basis.
Peripheral facial nerve palsy can be treated using an oral antibiotic. However, many of these patients have concomitant meningitis. Patients with meningitis should receive a parenteral antibiotic. Experts are divided on whether every patient with Lyme-associated facial palsy should have a CSF analysis, but clinicians should consider lumbar puncture for patients with significant headache, neck pain or stiffness, or papilledema.
Patients with symptomatic cardiac disease, second- or third-degree heart block, or significantly prolonged PR interval should be hospitalized and monitored closely. These patients should receive a parenteral antibiotic. Patients with mild first-degree heart block can be treated with an oral antibiotic.
Some patients develop a Jarisch-Herxheimer reaction soon after treatment is initiated; this results from lysis of the borrelia. The manifestations of this reaction are fever, diaphoresis, and myalgia. These symptoms resolve spontaneously within 24-48 hr, although administration of nonsteroidal anti-inflammatory drugs (NSAIDs) often is beneficial. NSAIDs also may be useful in treating symptoms of early Lyme disease and of Lyme arthritis. Co-infections with other pathogens transmitted by Ixodes ticks should be treated according to standard recommendations.
Criteria for the post–Lyme disease syndrome have been proposed by the IDSA. There is no clear evidence that this condition is related to persistence of the organism. Studies in adults have shown little benefit associated with prolonged or repeated treatment with oral or parenteral antibiotics.
Prognosis
There is a widespread misconception that Lyme disease is difficult to cure and that chronic symptoms and clinical recurrences are common. The most likely reason for apparent treatment failure is an incorrect diagnosis of Lyme disease.
The prognosis for children treated for Lyme disease is excellent. Children treated for erythema migrans rarely progress to late Lyme disease. The long-term prognosis for patients who are treated beginning in the late phase of Lyme disease also is excellent. Although chronic and recurrent arthritis does occur rarely, especially among patients with certain HLA allotypes (an autoimmune process), most children who are treated for Lyme arthritis are cured and have no sequelae. Although there are rare reports of adults who have developed late neuroborreliosis, usually among persons with Lyme disease in whom treatment was delayed for months or years, similar cases in children are rare.
Prevention
The best way to avoid Lyme disease is to avoid tick-infested areas. Children should be examined for deer ticks after known or potential exposure. If a deer tick attachment is noted, the tick should be grasped at the mouthparts with a forceps or tweezers; if these are not available, the tick should be covered with a tissue. The recommended method of tick removal is to pull directly outward without twisting; infection is usually preventable if the tick is removed before 48 hours of attachment. The overall risk for acquiring Lyme disease after a tick bite is low (1-3%) in most endemic areas. Patients and families can be advised to watch the area for development of erythema migrans and to seek medical attention if the rash or constitutional symptoms occur. If infection develops, early treatment of the infection is highly effective. A study of prophylaxis after a tick bite found that a single dose of doxycycline in adults (200 mg PO) was 87% effective in preventing Lyme disease; data in children using this strategy are lacking. Most people are not able to identify the species or the stage of the tick (in some instances reported “tick” bites prove not to be from ticks), and therefore administration of antimicrobial prophylaxis is not recommended routinely. The routine testing of ticks that have been removed from humans for evidence of B. burgdorferi is not recommended, because the value of a positive test result for predicting infection in the human host is unknown.
Personal protective measures that may be effective in reducing the chance of tick bites include wearing protective clothing (long pants tucked into socks, long-sleeved shirts) when entering tick-infested areas, checking for and promptly removing ticks, and using tick repellents such as DEET. This chemical can safely be used on pants, socks and shoes; care must be used with heavy or repeated application on skin, particularly in infants, because of the risk of systemic absorption and toxicity.
Avery RA, Frank G, Glutting JJ, et al. Prediction of Lyme meningitis in children from a Lyme disease endemic region: a logistic regression model using history, physical, and laboratory findings. Pediatrics. 2006;117:e1-e7.
Costello JM, Alexander ME, Greco KM, et al. Lyme carditis in children: presentation, predictive factors, and clinical course. Pediatrics. 2009;123:e835-841.
Feder HM, Johnson BJB, O’Connell S, et al. A critical appraisal of chronic Lyme disease. N Engl J Med. 2007;357:1422-1430.
Garro AC, Rutman M, Simonsen K, et al. Prospective validation of a clinical prediction model for lyme meningitis in children. Pediatrics. 2009;123:e829-e834.
Hayes EB, Piesman J. How can we prevent Lyme disease? N Engl J Med. 2003;348:2424-2430.
Klempner MS, Hu LT, Evans J, et al. Two controlled trials of antibiotic treatment in patients with persistent symptoms and a history of Lyme disease. N Engl J Med. 2001;345:85-92.
Kowalski TJ, Tata S, Berth W, et al. Antibiotic treatment duration and long-term outcomes of patients with early Lyme disease from a Lyme disease–hyperendemic area. CID. 2010;50:512-520.
2010 The Medical Letter: Treatment of Lyme disease. Med Lett. 2010;52(1342):53-54.
Nigrovic LE, Thompson AD, Fine AM, et al. Clinical predictors of Lyme disease among children with a peripheral facial palsy at an emergency department in a Lyme disease–endemic area. Pediatrics. 2008;122:e1080-e1085.
Shah SS, Zaoutis TE, Turnquist J, et al. Early differentiation of Lyme from enteroviral meningitis. Pediatr Infect Dis J. 2005;24:542-545.
Thompson A, Mannix R, Bachur R. Acute pediatric monoarticular arthritis: distinguishing Lyme arthritis from other etiologies. Pediatrics. 2009;123:959-965.
Tuerlinckx D, Bodart E, Jamart J, et al. Prediction of Lyme meningitis based on a logistic regression model using clinical and cerebrospinal fluid analysis. Pediatr Infect Dis J. 2009;28:394-396.
Vázquez M, Muehlenbein C, Cartter M, et al. Effectiveness of personal protective measures to prevent Lyme disease. Emerg Infect Dis. 2008;14:210-216.
Wormser GP. Early Lyme disease. N Engl J Med. 2006;354:2794-2801.
Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Disease Society of America. CID. 2006;43:1089-1134.