Chapter 215 Mycoplasma pneumoniae
Among the 5 Mycoplasma species isolated from the human respiratory tract, Mycoplasma pneumoniae is the only recognized human pathogen and is a major cause of respiratory infections in school-aged children and young adults.
Etiology
Mycoplasmas are the smallest self-replicating biologic system and are dependent on attachment to host cells for obtaining essential precursors such as nucleotides, fatty acids, sterols, and amino acids. They are distinguished by the complete absence of a cell wall, double-stranded DNA, and small genomes ranging from 577 to 1,380 kb. M. pneumoniae is fastidious, and growth in commercially available culture systems is too slow to be of practical clinical use.
Epidemiology
M. pneumoniae infections occur worldwide and throughout the year. In contrast to the acute, short-lived epidemics of some respiratory viral agents, M. pneumoniae infection is endemic in larger communities, with epidemic outbreaks occurring every 4-7 yr. In smaller communities, infections are sporadic, with long-lasting and smoldering outbreaks occurring at irregular intervals. Community outbreaks can spread largely through school contacts, but up to 40% of household members of an infected student also develop mycoplasma infection.
The occurrence of mycoplasmal illness is related, in part, to age and pre-exposure immunity. Overt illness is unusual before 3 yr of age. Children <5 yr of age appear to have mild illness associated with upper respiratory tract involvement, vomiting, and diarrhea. The peak incidence of lower respiratory illness occurs in school-aged children. M. pneumoniae accounts for 7-40% of all community-acquired pneumonias among children 3-15 yr of age. Recurrent infections occur infrequently but are well documented to occur in adults at intervals of 4-7 yr.
Transmission
Infection occurs through the respiratory route by large droplet spread. The incubation period is 1-3 wk. High transmission rates have been documented within families, with a high percentage of secondary cases developing lower respiratory tract infections. Outbreaks can occur in closed settings (military recruits, institutions, summer camps for children) or can occur as community-wide epidemics.
Pathology and Pathogenesis
Cells of the ciliated respiratory epithelium are the target cells of M. pneumoniae infection. The organism is an elongated snakelike structure with an attachment tip characterized by an electron-dense core and a trilaminar outer membrane. Attachment to the ciliary membrane is mediated by a complex network of interactive adhesion and adherence-accessory proteins localized to this specialized attachment tip (P1/B/C, P30, P65, P24, and P41). These proteins cooperate structurally and functionally to mobilize and concentrate adhesion proteins at the tip and permit mycoplasmal colonization of mucous membranes. Avirulent phenotypes that arise through spontaneous mutations at high frequency cannot synthesize specific cytoadherence-related proteins or are unable to stabilize them at the tip organelle.
Virulent organisms attach to ciliated respiratory epithelial cell surfaces through sialated glycoprotein or sulfated glycolipid receptors and burrow down between cells, resulting in ciliostasis and eventual sloughing of the cells. Mechanisms of cytopathology have not been determined completely; one possibility is the transmission to cells of various cytotoxins such as hydrogen peroxide. M. pneumoniae can also produce a protein similar to the S1 subunit of pertussis toxin. Intracellular organisms have not been found in vivo, and M. pneumoniae rarely invades beyond the respiratory tract basement membrane. However, M. pneumoniae can invade certain cell lines in vitro and survive in the cytoplasm or perinuclear regions for prolonged periods. M. pneumoniae has been detected by polymerase chain reaction (PCR) in many nonrespiratory sites. These observations suggest that M. pneumoniae causes more extrapulmonary infections and chronic disease than appreciated.
A possible mechanism of M. pneumoniae disease is the release of various proinflammatory and anti-inflammatory cytokines. M. pneumoniae infection may induce numerous interleukins, interferons, tumor necrosis factor-α, and other cytokines. The disease produced by M. pneumoniae is complex; the immunologic response of the host may be responsible for the manifestations of disease itself as well as for protection against infection, depending on the qualitative and quantitative balance of humoral and cellular immunity. Although it is well documented that specific cell-mediated immunity and antibody titers against M. pneumoniae increase with age (and therefore probably follow repeated infections), the immune mechanisms that protect against or clear infection are not defined. Patients with immunodeficiencies such as hypogammaglobulinemia and sickle cell disease can have more-severe mycoplasmal pneumonia than do immunocompetent hosts. M. pneumoniae can persist for years in the respiratory tract of patients with hypogammaglobulinemia despite multiple courses of antibiotics. M. pneumoniae is a common infectious cause of acute chest syndrome in sickle cell disease, but it is not prevalent in patients with AIDS.
Clinical Manifestations
Tracheobronchitis and bronchopneumonia are the most commonly recognized clinical syndromes associated with M. pneumoniae infection. Although the onset of illness may be abrupt, it is usually characterized by gradual onset of headache, malaise, fever, and sore throat, followed by progression of lower respiratory symptoms, including hoarseness and cough. Coryza is unusual with M. pneumoniae pneumonia and usually suggests a viral etiology. Although the clinical course in untreated patients is variable, coughing usually worsens during the 1st wk of illness, with all symptoms usually resolving within 2 wk. The cough is initially nonproductive, but older children and adolescents might produce frothy, white sputum. The symptoms are usually more severe than the physical signs, which appear later in the disease. Crackles are fine and are the most prominent signs. With progression of the disease, the fever intensifies, the cough becomes more troublesome, and the patient can become dyspneic.
Radiographic findings are not specific. Pneumonia is usually described as interstitial or bronchopneumonic, and involvement is most common in the lower lobes, with unilateral, centrally dense infiltrates present in 75% of cases. Lobar pneumonia is seen infrequently. Hilar lymphadenopathy occurs in up to one third of patients. Significant amounts of pleural fluid are unusual, but patients with large pleural effusions due to M. pneumoniae have been described as having severe disease associated with lobar infiltrates and necrotizing pneumonia. The white blood cell and differential counts are usually normal, whereas the erythrocyte sedimentation rate is often elevated.
Additional respiratory illnesses caused occasionally by M. pneumoniae include undifferentiated upper respiratory tract infections, pharyngitis, sinusitis, croup, and bronchiolitis. M. pneumoniae may be a common trigger of wheezing in asthmatic children or can cause chronic colonization and resulting lung dysfunction in adolescent and adult asthma patients. Otitis media and bullous myringitis have been described but are rarely seen without associated lower respiratory tract infection. Encephalitis and postinfectious demyelination occur but are less common than respiratory tract infections.
Diagnosis
No specific clinical, epidemiologic, or laboratory observations permit a definite diagnosis of mycoplasmal infection early in the clinical course. However, certain observations are suggestive and can be helpful. Pneumonia in school-aged children and young adults always suggests M. pneumoniae disease, especially if cough is a prominent finding. Cultures on special media of the throat or sputum might demonstrate M. pneumoniae, but growth generally requires incubation for >1 wk, and few commercial laboratories maintain the capability of culturing M. pneumoniae. Positive M. pneumoniae immunoglobulin M (IgM) antibody identified by indirect fluorescence or enzyme-linked immune assay (EIA) more specifically supports the diagnosis. IgM antibodies may be positive for 6-12 mo after infection. A 4-fold increase in IgG M. pneumoniae antibody titer by complement fixation or EIA between acute and convalescent sera obtained 10 days to 3 wk after the onset of illness is diagnostic. PCR of a nasopharyngeal or throat swab (doing both can increase sensitivity) for M. pneumoniae DNA is very specific (>97%) in many studies but has a sensitivity of only 50-70% when compared to 4-fold rise in antibody titer. Combined use of PCR and IgM antibody may be the most reliable approach to diagnose the acute illness. When M. pneumoniae is confirmed in the community in a few patients, the probability of the existence of more widespread mycoplasmal illness is greatly increased.
Treatment
M. pneumoniae illness is usually mild, and hospitalization is generally unnecessary. M. pneumoniae is typically sensitive to erythromycin, clarithromycin, azithromycin, and the tetracyclines in vitro, although macrolide resistant strains have been reported from Asia, Europe, and the USA. Macrolides are effective in shortening the course of mycoplasmal illnesses, although they do not have bactericidal activity. Hence, there may be delay in eradicating the organism from the respiratory tract. Two multicenter studies of pediatric community-acquired pneumonia demonstrated equal efficacy between erythromycin and clarithromycin or azithromycin. These newer macrolides were better tolerated and more effective at eradication of M. pneumoniae from the respiratory tract. The recommended treatment is clarithromycin (15 mg/kg/day divided bid PO for 10 days) or azithromycin (10 mg/kg once PO on day 1 and 5 mg/kg once daily PO on days 2-5), which eradicate M. pneumoniae in 100% of patients studied. Prophylaxis with azithromycin has been shown to substantially reduce the secondary attack rate in institutional outbreaks.
Complications
Complications are unusual. Despite the reportedly rare isolation of M. pneumoniae from nonrespiratory sites such as joints, pleural fluid, and cerebrospinal fluid (CSF), the availability of PCR to detect specific segments of M. pneumoniae DNA has led to increasing identification of M. pneumoniae in nonrespiratory sites, particularly the central nervous system (CNS). Nonrespiratory illness can therefore involve direct invasion with M. pneumoniae or can involve autoimmune mechanisms, which is reflected by the frequency with which human antigens cross react with M. pneumoniae. Patients with or without respiratory symptoms can manifest illness involving the skin, CNS, blood, heart, gastrointestinal tract, and joints. Skin lesions include a variety of exanthemas, most notably maculopapular rashes, erythema multiforme, and Stevens-Johnson syndrome (SJS). M. pneumoniae is the most common infectious agent identified as a cause of SJS, which usually develops 3-21 days after initial respiratory symptoms, lasts <14 days, and is rarely associated with severe complications (Figs. 215-1 and 215-2). M. pneumoniae has been linked to an atypical SJS with oral mucositis but absence of rash.
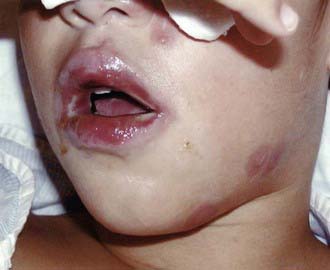
Figure 215-1 Lip changes found in Stevens-Johnson syndrome associated with Mycoplasma pneumoniae infection.
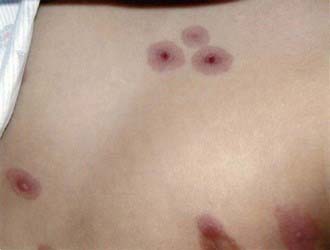
Figure 215-2 Classic erythema multiforme skin lesions found in Stevens-Johnson syndrome associated with Mycoplasma pneumoniae infection.
Neurologic complications include meningoencephalitis, transverse myelitis, aseptic meningitis, cerebellar ataxia, Bell palsy, deafness, brainstem syndrome, acute demyelinating encephalitis, and Guillain-Barré syndrome. Neurologic complications occur 3-28 days (mean, 10 days) after respiratory illness but may not be preceded by respiratory illness in 20% of cases. Encephalitis occurring within 5 days of the onset of prodromal symptoms may be caused by direct M. pneumoniae infection of the CNS, although CSF PCR is positive in <5% of cases. Encephalitis occurring >7 days after onset of prodromal symptoms is more likely to be due to an autoimmune response. M. pneumoniae accounts for 5-15% of all forms of childhood encephalitis and most commonly manifests as fever, lethargy, and impaired consciousness. Seizures, focal motor deficit, ataxia, or meningeal signs are less common. Concomitant infection with viral agents such as herpes simplex virus, human herpesvirus 6, enteroviruses, or respiratory viruses is found in about one third of patients. Involvement of the brainstem can result in severe dystonia and movement disorders. The CSF may be normal or have a mild mononuclear pleocytosis. Diagnosis is confirmed with positive CSF PCR, positive PCR from a throat swab, or the presence of definitive serum antibody titers. Findings on MRI include focal ischemic changes, ventriculomegaly, diffuse edema, or multifocal white matter inflammatory lesions consistent with postinfectious demyelinating encephalomyelitis.
Common hematologic complications include mild degrees of hemolysis with a positive Coombs test and minor reticulocytosis 2-3 wk after the onset of illness. Severe hemolysis is associated with high titers of cold hemagglutinins (≥1 : 512) and occurs rarely. Thrombocytopenia and coagulation defects occur occasionally. Mild hepatitis, pancreatitis, and protein-losing hypertrophic gastropathy are rarely reported gastrointestinal complications. Myocarditis, pericarditis, and a rheumatic fever–like syndrome are uncommon manifestations, but arrhythmias, ST- and T-wave changes, and cardiac dilation with heart failure can accompany M. pneumoniae infection, particularly in adults. Transient monoarticular arthritis has been reported in up to 1% of patients.
It is unclear from existing literature whether antibiotic treatment of M. pneumoniae infection decreases the risk for complications. There is also no specific established therapy for most of the complications. Corticosteroids have been the most commonly used agents in the management of severe M. pneumoniae complications, particularly neurologic complications.
Prognosis
Fatal M. pneumoniae infections are rare. Anatomic abnormalities such as altered lung perfusion, mild bronchiectasis, and bronchial wall thickening have been detected by high-resolution CT in approximately one third of children 1-2 yr following M. pneumoniae pneumonia. Abnormalities in pulmonary gas diffusion have been reported in nearly half of children 6 mo after recovery from M. pneumoniae. Patients generally recover without complications, although sequelae of encephalitis may be severe and permanent.
Atkinson TP, Balish MF, Waites KB. Epidemiology, clinical manifestations, pathogenesis and laboratory detection of Mycoplasma pneumoniae infections. FEMS Microbiol Rev. 2008;32:956-973.
Chig-Yung C, Chiang L-M, Chen T-P. Mycoplasma pneumoniae infection complicated by necrotizing pneumonitis with massive pleural effusion. Eur J Pediatr. 2006;165:275-277.
Christie LJ, Honarmand S, Talkington DF, et al. Pediatric encephalitis: what is the role of Mycoplasma pneumoniae? Pediatrics. 2007;120:305-313.
Defilippi A, Silvestri M, Tacchella A. Epidemiology and clinical features of Mycoplasma pneumoniae infection in children. Resp Med. 2008;102:1762-1768.
Korppi M, Heiskanen-Kosma T, Kleemola M. Incidence of community-acquired pneumonia in children caused by Mycoplasma pneumoniae: serological results of a prospective, population-based study in primary health care. Respirology. 2004;9:109-114.
Krause DC, Balish MF. Cellular engineering in a minimal microbe: structure and assembly of the terminal organelle of Mycoplasma pneumoniae. Mol Microbiol. 2004;51:917-924.
Li X, Atkinson P, Hagood J, et al. Emerging macrolide resistance in Mycoplasma pneumonia in children. Pediatr Infect Dis J. 2009;28:693-696.
Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701-707.
Ravin KA, Rappaport LD, Zucherbraun NS, et al. Mycoplasma pneumoniae and atypical Stevens-Johnson syndrome: a case series. Pediatrics. 2007;119:e1002-e1005.
Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697-728.
Walter ND, Grant GB, Bandy U, et al. Community outbreak of Mycoplasma pneumoniae infection: school-based cluster of neurologic disease associated with household transmission of respiratory illness. J Infect Dis. 2008;98:1365-1374.
Wang ND, Grant GB, Bandy U, et al. Community outbreak of Mycoplasma pneumoniae infection: school-based cluster of neurologic disease associated with household transmission of respiratory illness. J Infect Dis. 2008;198:1365-1374.
Wang RS, Wang SY, Hsieh KS, et al. Necrotizing pneumonitis caused by Mycoplasma pneumoniae in pediatric patients: report of five cases and review of literature. Pediatr Infect Dis J. 2004;23:564-567.
Yang J, Hooper WC, Phillips DJ, et al. Cytokines in Mycoplasma pneumoniae infections. Cytokine Growth Factor Rev. 2004;15:157-168.