Chapter 220 Spotted Fever and Transitional Group Rickettsioses
Rickettsia species were classically divided into “spotted fever” and “typhus” groups based on serologic reactions. The outer membrane protein A (ompA) gene is present in spotted fever but not typhus group organisms. Complete genome sequences have further refined distinctions, and several species that possess ompA, but are genetically distinct from others in the spotted fever group, have been reassigned to a “transitional” group. Many members of the spotted fever group of rickettsiae are pathogenic for humans (Table 220-1). These include the tick-borne agents Rickettsia rickettsii, the cause of Rocky Mountain spotted fever (RMSF); R. conorii, the cause of Mediterranean spotted fever (MSF) or boutonneuse fever; R. sibirica, the cause of North Asian tick typhus; R. japonica, the cause of Oriental spotted fever; R. honei, the cause of Flinders Island spotted fever or Thai tick typhus; R. africae, the cause of African tick bite fever; and unnamed Israeli spotted fever rickettsia, and possibly others. Members of the transitional group include R. akari, the cause of mite-transmitted rickettsialpox; R. felis, the cause of cat flea–transmitted typhus; and R. australis, the cause of tick-transmitted Queensland tick typhus.
Table 220-1 SUMMARY OF RICKETTSIAL DISEASES OF HUMANS, INCLUDING RICKETTSIA, ORIENTIA, EHRLICHIA, ANAPLASMA, NEORICKETTSIA, AND COXIELLA
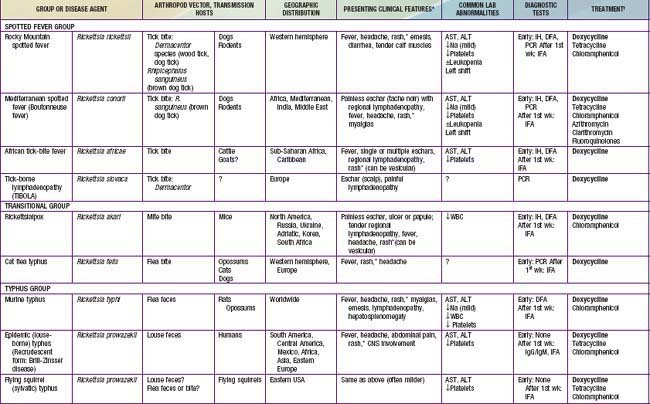
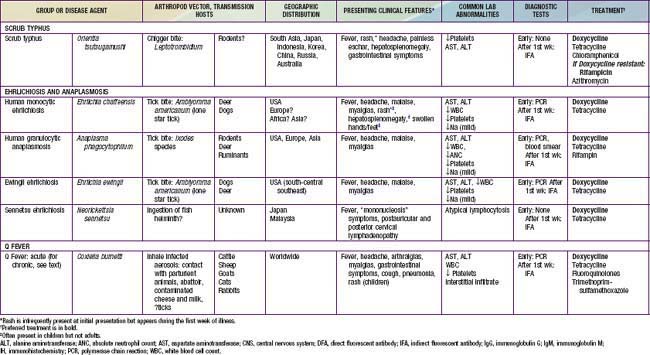
Infections with other members of the spotted fever and transitional groups are clinically similar to MSF, with fever, maculopapular rash, and eschar at the site of the tick bite. Israeli spotted fever is generally associated with a more severe course, including death in children. African tick bite fever is relatively mild, can include a vesicular rash, and often manifests with multiple eschars. New potentially pathogenic rickettsial species have been identified, including R. slovaca, the cause of TIBOLA (tick-borne lymphadenopathy), and R. parkeri, a cause of eschars in patients bitten by Amblyomma maculatum ticks in North America. R. rickettsii, R. parkeri, R. felis, and R. akari are the only members of the spotted fever and transitional groups that cause autochthonous disease in the USA.
220.1 Rocky Mountain Spotted Fever (Rickettsia rickettsii)
Megan E. Reller and J. Stephen Dumler
Rocky Mountain spotted fever (RMSF) is the most frequently identified and most severe rickettsial disease in the USA. It is also the most common vector-borne disease in the USA after Lyme disease. Although considered uncommon, RMSF is greatly underdiagnosed and underreported. RMSF should be considered in the differential diagnosis of fever, headache, and rash in the summer months, especially after tick exposure. Because fulminant disease and death are associated with delays in treatment, patients in whom the illness is clinically suspected should be treated promptly.
Etiology
RMSF results from systemic infection of endothelial cells by the obligate intracellular bacterium R. rickettsii.
Epidemiology
The term Rocky Mountain spotted fever is historical, because the agent was discovered in the Bitterroot Range of the Rocky Mountains of Montana. Few cases are now reported from this region. Cases have been reported throughout the continental USA (except Vermont and Maine), southwestern Canada, Mexico, Central America, and South America, but not from outside of the Western Hemisphere. In 2006, most cases were reported from North Carolina, Tennessee, Missouri, Oklahoma, Virginia, Arkansas, Alabama, Maryland, Georgia, and South Carolina. Human incursion into previously uninhabited areas has resulted in cases being described in new areas, such as the Northeast USA. The incidence of RMSF has been cyclical but has generally increased over the past decades. The mean number of cases reported each year to the Centers for Disease Control and Prevention (CDC) has steadily increased (515 during 1993-1998, 1,071 during 2001-2004, and 2,000 cases in 2006-2008). Habitats favored by ticks, including wooded areas or coastal grassland and salt marshes, are associated with disease. Foci of intense infection are found both in rural and urban areas. Clustering of cases within families likely reflects shared environmental exposures. In the USA, 90% of cases occur between April and September, months in which humans spend the most time outdoors. The highest age-specific incidence of RMSF among children is seen in those >5 yr of age, with boys outnumbering girls.
Transmission
Ticks are the natural hosts, reservoirs, and vectors of R. rickettsii. Ticks maintain the infection in nature by transovarial transmission (passage of the organism from infected ticks to their progeny). Ticks harboring rickettsiae are substantially less fecund than uninfected ticks; thus, horizontal transmission (acquisition of rickettsiae by taking a blood meal from transiently rickettsemic animal hosts such as small mammals or dogs) significantly contributes to maintenance of rickettsial infections in ticks. Ticks transmit the infectious agent to mammalian hosts (including humans) via infected saliva during feeding. The pathogen R. rickettsii in ticks becomes virulent after exposure to blood or increased temperature; thus, the longer the tick is attached, the greater the risk of transmission. The principal tick hosts of R. rickettsii are Dermacentor variabilis (the American dog tick) in the eastern USA and Canada, Dermacentor andersoni (the wood tick) in the western USA and Canada, Rhipicephalus sanguineus (the common brown dog tick) in the southwestern USA and in Mexico, and Amblyomma cajennense in Central and South America (Fig. 220-1).

Figure 220-1 Tick vectors of agents of human rickettsial diseases. An unengaged nymph (a), engorged nymph (b), and adult female (c) of Ixodes scapularis (deer tick), the vector of Anaplasma phagocytophilum, the cause of human granulocytic anaplasmosis. An adult female (d) of Amblyomma americanum (lone star tick), the vector of Ehrlichia chaffeensis and Ehrlichia ewingii, the causes of human monocytic ehrlichiosis and ewingii ehrlichiosis, respectively. An adult female (e) of Dermacentor variabilis (American dog tick), the vector of Rickettsia rickettsii, the cause of Rocky Mountain spotted fever.
Dogs can serve as reservoir hosts for R. rickettsii, can develop RMSF themselves, and can bring infected ticks into contact with humans. Serologic studies suggest that many patients with RMSF likely acquired the illness from ticks carried by the family dog.
Humans can also become infected when trying to remove an attached tick, because R. rickettsii–containing tick fluids or feces can be rubbed into the open wound at the bite site or into the conjunctivae by contaminated fingers. Finally, inhalation of aerosolized rickettsiae has caused severe infections and deaths in laboratory workers.
Pathology and Pathogenesis
Systemic infection is most obvious on the skin (rash), but nearly all organs and tissues are affected. Following inoculation of tick saliva into the dermis, the rickettsiae attach to the vascular endothelium via protein ligands and initiate rickettsia-mediated injury to host cell membranes. Damage to the membranes induces endocytosis, and the internalized rickettsiae then gain access to the cytosol by continued lysis of vacuolar membranes. Members of the spotted fever group actively initiate intracellular actin polymerization to achieve directional movement, and rickettsiae can easily invade neighboring cells despite minimal initial damage to host cells. The rickettsiae proliferate and damage the host cells by peroxidative membrane alterations, protease activation, or continued phospholipase activity.
The histologic correlate of the initial macular or maculopapular rash is perivascular infiltrates of lymphoid and histiocytic cells with edema but without significant endothelial damage. Proliferation of rickettsiae within the cytoplasm of infected endothelial cells leads to lymphohistiocytic or leukocytoclastic vasculitis of small venules and capillaries, which manifests as a petechial rash. This process ultimately results in microvascular leakage, tissue hypoperfusion, and possibly end-organ ischemic injury. Rickettsiae are contained within endothelial cells of inflamed vessels that may be eccentrically involved. Infrequently, inflammation leads to nonocclusive thrombi. Very rarely, small and large vessels become completely obliterated by thrombosis, which leads to tissue infarction or hemorrhagic necrosis. Interstitial pneumonitis and vascular leakage in the lungs can lead to noncardiogenic pulmonary edema, and meningoencephalitis can cause significant cerebral edema.
The presence of the infectious agent initiates an inflammatory cascade, including release of cytokines such as tumor necrosis factor-α (TNF-α), interleukin 1β, and interferon-γ (IFN-γ). Infection of endothelial cells by R. rickettsii induces surface E-selectin expression and procoagulant activity. Release of chemokines and expression of vascular selectin results in infiltration of the damaged endothelial cells by lymphocytes, macrophages, and occasionally neutrophils. Local inflammatory and immune responses have been suspected to contribute to the vascular injury characteristic of rickettsioses; however, the benefits of effective inflammation and immunity are greater. Blockade of TNF-α and IFN-γ action in animal models diminishes survival and increases the morbidity of spotted fever group infections, probably by abrogating upregulation of nitric oxide synthase and arginine-dependent intracellular killing. Direct contact of infected endothelial cells with perforin-producing CD8 T lymphocytes and IFN-γ–producing natural killer cells helps control the infection. Infection of endothelial cells with Rickettsia leads to upregulated expression of procoagulant molecules and consumption of coagulation factors, platelet adhesion, dissolution of endothelium junctional proteins, and emigration of leukocytes and can lead to disseminated intravascular coagulation (DIC).
Clinical Manifestations
The incubation period of RMSF in children varies from 2 to 14 days, with a median of 7 days. In 60% of cases, patients or their parents report a history of removing an attached tick, although the site of the tick bite is usually unapparent. Epidemiologic clues include living in or visiting an endemic area, playing or hiking in the woods, typical season, similar illness in family members, and close contact with a dog (especially one that is sick). Inapparent or mild illness probably occurs infrequently, but the illness might not be reported. In patients presenting for care, the illness is initially nonspecific, with symptoms including headache, anorexia, myalgias, and restlessness. Pain and tenderness of calf muscles are particularly common in children. Gastrointestinal symptoms including nausea, vomiting, diarrhea, and abdominal pain occur commonly (39-63%) early in the disease.
The typical clinical triad of fever, headache, and rash is observed in 44% of patients overall but is present in only 3% at presentation. Fever and headache persist if the illness is untreated. Fever can exceed 40°C and may be persistently elevated or can fluctuate dramatically. Headache is severe, unremitting, and unresponsive to analgesics.
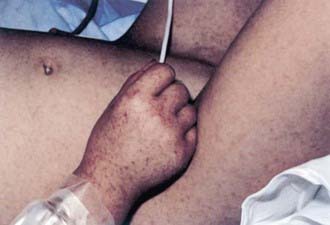
Rash usually appears only after 2-4 days of illness, and approximately 5% of children and up to 20% of adults never develop a rash that is recognized. Rash occurs more reliably in children than in adults. Initially, discrete, pale, rose-red blanching macules or maculopapules appear, characteristically on the extremities, including the ankles, wrists, or lower legs (Fig. 220-2). The rash then spreads rapidly to involve the entire body, including the soles and palms. After several days, the rash becomes more petechial or hemorrhagic, sometimes with palpable purpura. In severe disease, the petechiae can enlarge into ecchymosis, which can become necrotic. Severe vascular obstruction secondary to the rickettsial vasculitis and thrombosis is uncommon but can result in gangrene of the digits, earlobes, scrotum, nose, or an entire limb.
Central nervous system infection usually manifests as meningismus and changes in sensorium. In addition, patients can manifest ataxia, seizures, coma, or auditory deficits. CSF parameters are usually normal, but one third have mononuclear pleocytosis (<10-300 cells/µL) and 20% have elevated protein (<200 mg/dL). Neuroimaging studies generally reveal only subtle abnormalities that do not alter treatment. Cerebral edema, meningeal enhancement, and prominent perivascular spaces have been observed in patients with severe disease.
Other pulmonary disease occurs more commonly in adults than in children and can manifest clinically as rales, infiltrates, and noncardiogenic pulmonary edema. Other findings can include conjunctival suffusion, periorbital edema, dorsal hand and foot edema, and hepatosplenomegaly. Severe disease can include myocarditis, acute renal failure, and vascular collapse.
Persons with glucose-6-phosphate dehydrogenase (G6PD) deficiency are at increased risk for fulminant RMSF, defined as death from R. rickettsii infection within 5 days. The clinical course of fulminant RMSF is characterized by profound coagulopathy and extensive thrombosis leading to kidney, liver, and respiratory failure. Clinical features associated with a fatal outcome include stupor, respiratory distress, acute renal failure, hepatomegaly, jaundice, and a DIC-like syndrome.
Occasionally, infection of vessels with rickettsiae appear to be a local process, such as appendicitis or cholecystitis. Thorough evaluation usually reveals evidence of a systemic process, and unnecessary surgical interventions are avoided.
Laboratory Findings
Laboratory abnormalities are common but nonspecific. Often, the total white blood cell count is initially normal or low, but leukocytosis can develop as the illness progresses. Other characteristic abnormalities include a left-shifted leukocyte differential, anemia (33%), thrombocytopenia (<150,000 platelets/µL in 33%), hyponatremia (<130 mEq/mL in 20%), and elevated serum aminotransferase levels (50%).
Diagnosis
Delays in diagnosis and treatment are associated with severe disease and death. Because no reliable diagnostic test is available to confirm RMSF acutely, the decision to treat must be based on compatible epidemiologic, clinical, and laboratory features. RMSF should be considered in patients presenting spring through fall with an acute febrile illness accompanied by headache and myalgia (particularly if they report exposure to ticks or contact with a dog or have been in forested or tick-infested rural areas). A history of tick exposure, a rash (especially if on the palms or soles), a normal or low leukocyte count with a marked left shift, a relatively low or decreasing platelet count, and a low serum sodium concentration are all clues that can support a diagnosis of RMSF. In patients without a rash or in dark-skinned patients in whom a rash can be difficult to appreciate, the diagnosis can be exceptionally elusive and delayed. One half of pediatric deaths occur within 9 days of onset of symptoms. Thus, treatment should not be withheld pending definitive laboratory results for a patient with clinically suspected illness. Further, prompt response to early treatment is diagnostically helpful.
If a rash is present, a vasculotropic rickettsial infection can be diagnosed as early as day 3 of illness with biopsy of a petechial lesion and immunohistochemical or immunofluorescent demonstration of specific rickettsial antigen in the endothelium. Although very specific, the sensitivity of this method is probably 70% at most. Further, it can be adversely influenced by prior antimicrobial therapy, suboptimal selection of skin lesions for biopsy, and examination of insufficient tissue because the infection is very focal. Tissue or blood can also be evaluated for R. rickettsii nucleic acids by polymerase chain reaction (PCR) at the CDC and selected public health or reference laboratories; however, PCR on blood is less sensitive than PCR on tissue and of similar sensitivity to tissue immunohistology, probably because the level of rickettsemia is generally very low (<6 rickettsiae/mL).
Definitive diagnosis is most often accomplished by serology, which is retrospective, because a rise in titer is not seen until after the 1st week of illness. The gold standard for the diagnosis of RMSF is a 4-fold increase in IgG antibody titer by indirect fluorescent antibody assay (IFA) between acute and convalescent (at 2-4 wk) sera or demonstration of seroconversion. A single titer is neither sensitive (patients can die before seroconversion) nor specific (an elevated titer can represent prior infection). With current serologic methods, RMSF cannot be reliably distinguished from other spotted fever group rickettsiae infections. Cross reactions with typhus group rickettsiae also occur, but titers may be lower for the typhus group. Cross reactions are not seen with Ehrlichia or Anaplasma infections. Weil-Felix antibody testing should not be performed, because it lacks both sensitivity and specificity. RMSF is a reportable disease in the USA.
Differential Diagnosis
Other rickettsial infections are easily confused with RMSF, especially all forms of human ehrlichiosis and murine typhus. RMSF can also mimic a variety of other pathogens, such as meningococcemia and enteroviruses. Negative blood cultures can exclude meningococcemia. PCR can differentiate enterovirus from R. rickettsii in patients with aseptic meningitis and a lymphocytic pleocytosis in the CSF. Other diseases in the differential diagnosis are typhoid fever, secondary syphilis, Lyme disease, leptospirosis, rat-bite fever, scarlet fever, toxic shock syndrome, rheumatic fever, rubella, parvovirus infection, Kawasaki disease, idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, Henoch-Schönlein purpura, hemolytic uremic syndrome, aseptic meningitis, acute gastrointestinal illness, acute abdomen, hepatitis, infectious mononucleosis, hemophagocytic syndromes, dengue fever, and drug reactions.
Treatment
The time-proven effective therapies for RMSF are tetracyclines and chloramphenicol. The treatment of choice for suspected RMSF in patients of all ages, including for young children, is doxycycline (4 mg/kg/day divided every 12 hr PO or IV, maximum 200 mg/day). Tetracycline (25-50 mg/kg/day divided every 6 hr PO, maximum 2 g/day) is an alternative. Chloramphenicol (50-100 mg/kg/day divided every 6 hr IV, maximum 4 g/day) should be reserved for patients with doxycycline allergy and for pregnant women, because chloramphenicol has been shown to be an independent risk factor for increased mortality vs. tetracycline. If used, chloramphenicol should be monitored to maintain serum concentrations of 10-30 µg/mL. Chloramphenicol is preferred for pregnant women because of doxycycline’s potential adverse effects on the fetus’s teeth and bone and the mother’s liver. Although tetracycline and doxycycline may be associated with tooth discoloration in children <8 yr of age, RMSF is a life-threatening illness for which prompt therapy is imperative. Tooth discoloration is dose dependent and it is unlikely that children with RMSF will require multiple courses. Chloramphenicol has rarely been associated with aplastic anemia and is no longer available as an oral preparation in the USA. An additional benefit of doxycycline over chloramphenicol is its effectiveness against potential concomitant ehrlichial infection. Sulfonamides should not be used, because they are associated with greater morbidity and mortality with RMSF. Other antibiotics, including penicillins, cephalosporins, and aminoglycosides, are not effective. The use of alternative antimicrobial agents, such as fluoroquinolones and the macrolides (azithromycin and clarithromycin), has not been evaluated.
Therapy should be continued for a minimum of 5-7 days and until the patient has been afebrile for at least 3 days to avoid relapse, especially in patients treated early. Treated patients usually defervesce within 48 hr, so the duration of therapy is usually <10 days.
Supportive Care
Most infections resolve rapidly with appropriate antimicrobial therapy and do not require hospitalization or other supportive care. On occasion, severe infections require intensive care. Particular attention to hemodynamic status is required in severely ill children, because iatrogenic pulmonary or cerebral edema is easy to precipitate owing to diffuse microvascular injury of the lungs, meninges, and brain. Judicious use of corticosteroids for meningoencephalitis has been advocated by some, but no controlled trials have been conducted.
Complications
Complications of RMSF include noncardiogenic pulmonary edema from pulmonary microvascular leakage, cerebral edema from meningoencephalitis, and multiorgan damage (hepatitis, pancreatitis, cholecystitis, epidermal necrosis, and gangrene) mediated by rickettsial vasculitis and/or the accumulated effects of hypoperfusion and ischemia (acute renal failure). Long-term neurologic sequelae are more likely to occur in patients who have been hospitalized for ≥2 wk and include paraparesis; hearing loss; peripheral neuropathy; bladder and bowel incontinence; cerebellar, vestibular, and motor dysfunction; and language disorders. Learning disabilities and behavioral problems are the most common neurologic sequelae among children who have survived severe disease.
Prognosis
Delays in diagnosis and therapy are significant factors associated with death or severe illness. Before the advent of effective antimicrobial therapy for RMSF, the case fatality rate was 10% for children and 30% for adults. A case fatality rate of 8.5% was documented in Texas from 1986 through 1996. The overall mortality rate is now 2-7%. Diagnosis based on serology alone underestimates the true mortality of RMSF, because patients often die before developing a serologic response. Deaths occur despite the availability of effective therapeutic agents, indicating the need for clinical vigilance and a low threshold for early empiric therapy. Even with administration of appropriate antimicrobials, delayed therapy can lead to irreversible vascular or end-organ damage and long-term sequelae or death. Early therapy in uncomplicated cases usually leads to rapid defervescence within 1-3 days and recovery within 7-10 days. A slower response may be seen if therapy is delayed. In those who survive despite no treatment, fever subsides in 2-3 wks.
Prevention
No vaccines are available. Prevention of RMSF is best accomplished by preventing or treating tick-infestation in dogs, avoiding wooded or grassy areas where ticks reside, using insect repellents containing DEET, wearing protective clothing, and carefully inspecting children who have been playing in the woods or fields for ticks. Recovery from infection yields lifelong immunity.
Prompt and complete removal of attached ticks helps reduce the risk for transmission because rickettsiae in the ticks need to be reactivated to become virulent, and this requires at least several hours to days of exposure to body heat or blood. Contrary to popular belief, the application of petroleum jelly, 70% isopropyl alcohol, fingernail polish, or a hot match are not effective in removing ticks. A tick can be safely removed by grasping the mouth parts with a pair of forceps at the site of attachment to the skin and applying gentle and steady pressure to achieve retraction without twisting, thereby removing the entire tick and its mouth parts. The site of attachment should then be disinfected. Ticks should not be squeezed or crushed, because their fluids may be infectious. The removed tick should be soaked in alcohol or flushed down the toilet, and hands should be washed to avoid accidental inoculation into conjunctivae, mucous membranes, or breaks in skin. Prophylactic antimicrobial therapy should not be administered, because tetracyclines and chloramphenicol are only rickettsiastatic, simply delaying the onset of illness and confusing the clinical picture by prolonging the incubation period.
220.2 Mediterranean Spotted Fever or Boutonneuse Fever (Rickettsia conorii)
Megan E. Reller and J. Stephen Dumler
Boutonneuse fever is caused by R. conorii and its related subspecies and was first described in Tunisia in 1909. It is also called Mediterranean spotted fever (MSF), Kenya tick typhus, Indian tick typhus, Israeli spotted fever, and Astrakhan fever. It is a moderately severe vasculotropic rickettsiosis that is often initially associated with an eschar at the site of the tick bite. Minor differences in clinical presentation could be associated with genetic diversity of the rickettsial subspecies.
Etiology
MSF is caused by systemic endothelial cell infection by the obligate intracellular bacterium R. conorii. Similar species are distributed globally, such as R. sibirica in Russia, China, Mongolia, and Pakistan; R. australis and R. honei in Australia; R. japonica in Japan; and R. africae in South Africa (see Table 220-1). Analysis of antigens and related DNA sequences show that all are closely related to R. rickettsii, the cause of Rocky Mountain Spotted Fever (RMSF).
Epidemiology
R. conorii is distributed over a large geographic region, including India, Pakistan, Russia, Ukraine, Georgia, Israel, Morocco, southern Europe, Ethiopia, Kenya, and South Africa. Reported cases of MSF in southern Europe have steadily increased since 1980, and the seroprevalence is 11-26% in some areas. The peak in reported cases occurs during July and August in the Mediterranean basin; in other regions it occurs during warm months when ticks are active.
Transmission
Transmission occurs after the bite of the brown dog tick, R. sanguineus, or other tick species such as Dermacentor, Haemaphysalis, Amblyomma, Hyalomma, and Ixodes. Clustering of human cases of boutonneuse fever, infected ticks, and infected dogs implicate the household dog as a potential vehicle for transmission.
Pathology and Pathogenesis
The underlying pathology seen with MSF is nearly identical to that of RMSF, except that eschars are often present at the site of tick bite where inoculation of rickettsiae occurs. The histopathology of the resultant lesion includes necrosis of dermal and epidermal tissues with a superficial crust; a dermis densely infiltrated by lymphocytes, histiocytes, and scattered neutrophils; and damaged capillaries and venules in the dermis. Immunohistochemical stains confirm that the lesions contain rickettsia-infected endothelial cells, but the vasculature structure might not be apparent owing to extensive inflammation and necrosis. The necrosis results from both direct rickettsia-mediated vasculitis and resultant extensive local inflammation. Rickettsiae thus have ready access to lymphatics and venous blood and disseminate to cause systemic disease.
Clinical Manifestations and Laboratory Findings
Typical findings include fever, headache, myalgias, and a maculopapular rash that appears 3-5 days after onset of fever. In about 70% of patients, a painless eschar or tache noire appears at the site of the tick bite with accompanying regional lymphadenopathy. Although previously considered self-limited, this infection can be severe, mimicking RMSF. Findings can include purpuric skin lesions, neurologic deficits, respiratory and/or acute renal failure, severe thrombocytopenia, and death in 1.4-5.6% of cases. As with RMSF, a particularly severe form occurs in patients with G6PD deficiency and in patients with underlying conditions such as alcoholic liver disease or diabetes mellitus. Fortunately, disease is generally milder in children.
Diagnosis
Laboratory diagnosis of MSF and related spotted fever group rickettsioses is the same as that for RMSF. Cases may be confirmed by immunohistologic or immunofluorescent demonstration of rickettsiae in skin biopsies, in vitro cultivation via centrifugation-assisted shell vial tissue culture, or demonstration of seroconversion or a 4-fold rise in serum antibody titer to spotted fever group rickettsiae between acute and convalescent sera. Antibodies to spotted fever group antigens cross react, so RMSF in the USA or MSF in Europe, Africa, and Asia cannot be distinguished by these methods.
Differential Diagnosis
The differential diagnosis includes conditions also associated with single eschars, such as anthrax, bacterial ecthyma, brown recluse spider bite, rat-bite fever (caused by Spirillum minus), and other rickettsioses (such as rickettsialpox, African tick-bite fever, and scrub typhus). The spotted fever group rickettsia R. africae causes African tick typhus, a milder illness than MSF that is often associated with multiple eschars and occasionally a vesicular rash. African tick typhus can be contracted in North Africa, where MSF also occurs and is a common infection of travelers to sub-Saharan Africa who encounter bush or high grasslands on safari.
Treatment and Supportive Care
MSF is effectively treated with tetracycline, doxycycline, chloramphenicol, ciprofloxacin, ofloxacin, levofloxacin, azithromycin, or clarithromycin. The treatment of choice is doxycycline (4 mg/kg/day divided every 12 hr PO or IV, maximum 200 mg/day). Tetracycline and chloramphenicol are alternatives, as for RMSF. Azithromycin (10 mg/kg/day once daily PO for 3 days) and clarithromycin (15 mg/kg/day divided twice daily PO for 7 days) are also used. Specific fluoroquinolone regimens effective for children have not been established. Intensive care may be required.
220.3 Rickettsialpox (Rickettsia akari)
Megan E. Reller and J. Stephen Dumler
Rickettsialpox is caused by Rickettsia akari, a transitional group Rickettsia species that is transmitted by the mouse mite, Allodermanyssus sanguineus. The mouse host for this mite is widely distributed in cities in the USA, Europe, and Asia. Seroepidemiologic studies suggest a high prevalence of this infection in urban settings. The disease is uncommon and is usually mild. Unlike the situation with most forms of rickettsiosis, the macrophage is an important target cell for R. akari.
Rickettsialpox is best known because of its association with a varicelliform rash. In fact, this rash is a modified form of an antecedent typical macular or maculopapular rash like those seen in other vasculotropic rickettsioses. At presentation, most patients have fever, headache, and chills. In up to 90% of cases, there is a painless papular or ulcerative lesion or eschar at the initial site of inoculation, which may be associated with regional lymphadenopathy that is often tender. In some patients, the maculopapular rash becomes vesicular, involving the trunk, head, and extremities. The infection generally resolves spontaneously and does not require therapy. However, a short course of doxycycline hastens resolution and is sometimes used in patients >8 yr of age and in young children with relatively severe illness. Complications and fatalities are rare.
Billings AN, Rawlings JA, Walker DH. Tick-borne disease in Texas: a 10-year retrospective examination of cases. Tex Med. 1998;94:66-76.
Buckingham SC. Rocky Mountain spotted fever: a review for the pediatrician. Pediatr Ann. 2002;31:163-168.
Buckingham SC, Marshall GS, Schutze GE, et al. Clinical and laboratory features, hospital course, and outcome of Rocky Mountain spotted fever in children. J Pediatr. 2007;150:180-184.
Cascio A, Colomba C, Antinori S, et al. Clarithromycin versus azithromycin in the treatment of Mediterranean spotted fever in children: a randomized controlled trial. Clin Infect Dis. 2002;34:154-158.
Centers for Disease Control and Prevention. Consequences of delayed diagnosis of Rocky Mountain spotted fever in children—West Virginia, Michigan, Tennessee, and Oklahoma, May-July 2000. MMWR Morb Mortal Wkly Rep. 2000;49:885-888.
Centers for Disease Control and Prevention. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever, ehrlichioses, and anaplasmosis—United States. MMWR Recomm Rep. 2006;55:1-29.
Centers for Disease Control and Prevention. Fatal cases of Rocky Mountain spotted fever in family clusters—three states, 2003. MMWR Morb Mortal Wkly Rep. 2004;53:407-410.
Comer JA, Tzianabos T, Flynn C, et al. Serologic evidence of rickettsialpox (Rickettsia akari) infection among intravenous drug users in inner-city Baltimore, Maryland. Am J Trop Med Hyg. 1999;60:894-898.
Demma LJ, Traeger MS, Nicholson WL, et al. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N Engl J Med. 2005;353:587-594.
Dumler JS, Walker DH. Rocky Mountain spotted fever—changing ecology and persisting virulence. N Engl J Med. 2005;353:551-553.
Holman RC, Paddock CD, Curns AT, et al. Analysis of risk factors for fatal Rocky Mountain spotted fever: evidence for superiority of tetracyclines for therapy. J Infect Dis. 2001;184:1437-1444.
Marshall GS, Stout GG, Jacobs RF, et al. Antibodies reactive to Rickettsia rickettsii among children living in the southeast and south central regions of the United States. Arch Pediatr Adolesc Med. 2003;157:443-448.
Sexton DJ, Kaye KS. Rocky Mountain spotted fever. Med Clin North Am. 2002;86:351-360.
Thorner AR, Walker DH, Petri WA. Rocky Mountain spotted fever. Clin Infect Dis. 1998;27:1353-1359.
Treadwell TA, Holman RC, Clarke MJ, et al. Rocky Mountain spotted fever in the United States, 1993–1996. Am J Trop Med Hyg. 2000;63:21-26.
Walker DH. Tick-transmitted infectious diseases in the United States. Annu Rev Public Health. 1998;19:237-269.