Chapter 232 Coccidioidomycosis (Coccidioides Species)
Etiology
Coccidioidomycosis (valley fever, San Joaquin fever, desert rheumatism, coccidioidal granuloma) is caused by Coccidioides spp., soil-dwelling dimorphic fungi. Coccidioides spp. grow in the environment as spore-bearing (arthroconidia-bearing) mycelial forms. In their parasitic form, they appear as unique, endosporulating spherules in infected tissue. The 2 recognized species, C. immitis and C. posadasii, cause similar illnesses.
Epidemiology
Coccidioides spp. inhabit soil in arid regions. C. immitis is primarily found in California’s San Joaquin Valley. C. posadasii is endemic to southern regions of Arizona, Utah, Nevada, New Mexico, western Texas, and regions of Mexico and Central and South America.
Population migrations into endemic areas and increasing numbers of immunosuppressed persons have caused coccidioidomycosis to become an important health problem. Infection rates increased from 2000 to 2007. About 150,000 newly reported infections occur annually in the USA. Coccidioidin skin test positivity in 5-7 yr old students in a highly endemic area demonstrated a decline from 10% to 2% in a 58-yr period ending in 2000. During 2002, 153 children required hospitalization for coccidioidomycosis, and infection was fatal in 9% of cases.
Infection results from inhalation of spores. Incidence increases during windy, dry periods that follow rainy seasons. Seismic events, archaeological excavations, and other activities that disturb contaminated sites have caused outbreaks. Person-to-person transmission does not occur. Rarely, infections result from spores that contaminate fomites or grow beneath casts or wound dressings of infected patients. Infection has also resulted from transplantation of organs from infected donors and from mother to fetus or newborn. Visitors to endemic areas can acquire infections, and diagnosis may be delayed when they are evaluated in nonendemic areas. Spores are highly virulent, and Coccidioides spp. are potential agents of bioterrorism (Chapter 704).
Pathogenesis
Inhaled spores reach terminal bronchioles where they transform into septated spherules that resist phagocytosis and within which many endospores develop. Released endospores transform into new spherules, and the process results in an acute focus of infection. Endospores can also disseminate lymphohematogenously. Eventually, a granulomatous reaction predominates. Both recovery and protection upon re-exposure depend on effective cellular immunity.
Clinical Manifestations
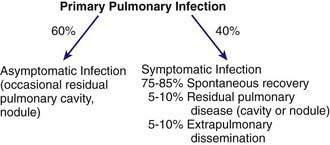
The clinical spectrum (Fig. 232-1) encompasses pulmonary and extrapulmonary disease. Pulmonary infection occurs in 95% of cases and can be divided into primary, complicated, and residual infections. About 60% of infections are asymptomatic. Symptoms in children are milder than those in adults. The incidence of extrapulmonary dissemination in children approaches that of adults.
Primary Coccidioidomycosis
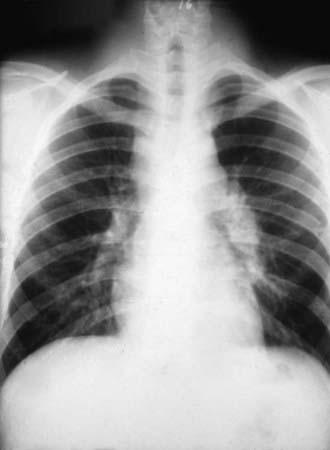
The incubation period is 1-4 wk, with an average of 10-16 days. Early symptoms include malaise, chills, fever, and night sweats. Chest discomfort occurs in 50-70% of patients and varies from mild tightness to severe pain. Headache and/or backache are sometimes reported. An evanescent, generalized, fine macular erythematous or urticarial eruption may be seen within the first few days of infection. Erythema nodosum can occur (more often in women) and is sometimes accompanied by an erythema multiforme rash, usually 3-21 days after the onset of symptoms. The clinical constellation of erythema nodosum, fever, chest pain, and arthralgias (especially knees and ankles) has been termed desert rheumatism and valley fever. The chest examination is often normal even if radiographic findings are present. Dullness to percussion, friction rub, or fine rales may be present. Pleural effusions can occur and can become large enough to compromise respiratory status. Hilar and mediastinal lymphadenopathy are common (Fig. 232-2).
Complicated Pulmonary Infection
Complicated infections include severe and persistent pneumonia, progressive primary coccidioidomycosis, progressive fibrocavitary disease, transient cavities that develop in areas of pulmonary consolidation, and empyema that follows rupture of a cavity into the pleural space. Some cavities persist, are thin-walled, peripheral, and cause no symptoms; occasionally there is mild hemoptysis, and rarely there is serious hemorrhage. Rarely, acute respiratory insufficiency occurs following intense exposure; this is associated with high mortality.
Residual Pulmonary Coccidioidomycosis
Residual pulmonary coccidioidomycosis includes fibrosis as well as persisting pulmonary nodules. Nodules are present in 5-7% of infections and sometimes require differentiation from malignancy.
Dissseminated (Extrapulmonary) Infection
Clinically apparent dissemination occurs in 0.5% of patients. Its incidence is increased in infants; men; persons of Filipino, African, and Latin American ancestry; and in other Asians. Primary or acquired disorders of cellular immunity (Table 232-1) markedly increase the risk of dissemination.
Table 232-1 RISK FACTORS FOR POOR OUTCOME IN PATIENTS WITH ACTIVE COCCIDIOIDOMYCOSIS
| PRIMARY INFECTIONS |
| Severe, prolonged (≥6 wk), or progressive infection |
| RISK FACTORS FOR EXTRAPULMONARY DISSEMINATION |
| Primary or acquired cellular immune dysfunction (including patients receiving tumor necrosis factor inhibitors) |
| Neonates, infants, the elderly |
| Male sex (adult) |
| Filipino, African, Native American, or Latin American ethnicity |
| Late-stage pregnancy and early postpartum period |
| Standardized complement fixation antibody titer >1:16 or increasing titer with persisting symptoms |
| Blood group B |
| HLA class II allele-DRBI*1301 |
Symptoms usually occur within 6 mo of primary infection and can follow directly. Prolonged fever, toxicity, skin lesions, subcutaneous and/or osseous cold abscesses, and laryngeal lesions can herald onset. Organism-specific skin lesions have a predilection for the nasolabial area and appear initially as papules, which evolve to form pustules, plaques, abscesses, and verrucous plaques. Biopsy of these lesions demonstrates spherules. Basilar meningitis is the most common manifestation and may be accompanied by ventriculitis, ependymitis, cerebral vasculitis, abscess, and syringomyelia. Headache, vomiting, meningismus, and cranial nerve dysfunction are often present. Untreated meningitis is almost invariably fatal. Bone infections account for 20-50% of extrapulmonary manifestations, are often multifocal, and can affect adjacent structures. Miliary dissemination and peritonitis can mimic tuberculosis.
Diagnosis
Nonspecific tests have limited usefulness. The complete blood count might show an elevated eosinophil count, and marked eosinophilia can accompany dissemination.
Culture, Histopathologic Findings, and Antigen Detection
Although diagnostic, culture is positive in only 8.3% of respiratory tract specimens and in only 3.2% of all other sites. Coccidioides is isolated from clinical specimens as the spore-bearing mold form, and thus the laboratory should be informed and use special precautions when the diagnosis is suspected. The observation of endosporulating spherules in histopathologic specimens is also diagnostic.
A quantitative enzyme immunoassay (EIA) (MiraVista Diagnostics) that detects coccidioidal galactomannan in urine has excellent specificity and is positive in 70% of patients with severe infections. Although the EIA can cross-react with other endemic mycoses, interpretation is often straightforward because there is negligible geographic overlap with areas endemic for other mycoses.
Cerebrospinal fluid (CSF) analysis should be performed in patients with suspected dissemination. The findings in meningitis are similar to those seen with tuberculous meningitis (Chapter 207). Eosinophilic pleocytosis may be present. Fungal stains and culture are usually negative. Volumes of 10 mL in adults have improved the yield of culture.
Serology
Serologic tests provide valuable diagnostic information but may be falsely negative early in self-limited infections and in immunocompromised patients. Three major methods are used, including EIA, complement fixation (CF), and immunodiffusion (ID). EIA and CF tests are best done in experienced reference laboratories.
Immunoglobulin M (IgM) specific antibody becomes measurable in 50% of infected patients 1 wk after onset and in 90% of infected patients by 3 wk. EIA is sensitive and can detect IgM and IgG antibody; it is less specific than other methods, and confirmation with ID or CF may be needed. IgG antibodies measured by CF appear between the 2nd and 3rd wk but can take several months; follow-up testing is needed if tests are negative and clinical suspicion persists. In the presence of CF titers of 1:2 or 1:4, a positive IDF test can help corroborate significance. IgG-specific antibody can persist for months, with titers elevated in proportion to the severity of illness. CF titers >1:16 are suggestive of dissemination. Direct comparison of the results of CF (IgG) antibody tests measured by different methodologies should be interpreted with caution. IgG antibody titers used to monitor disease activity should be tested concurrently with serum samples taken earlier in the illness using the same methodology.
C. immitis antibody is present in CSF in 95% of patients with meningitis and is usually diagnostic. Rarely, “spillover” in patients without meningitis but with high IgG titers in serum can be present in CSF. Isolation of Coccidioides from CSF culture of patients with meningitis is uncommon, although culture of large volumes of CSF may improve sensitivity.
Imaging Procedures
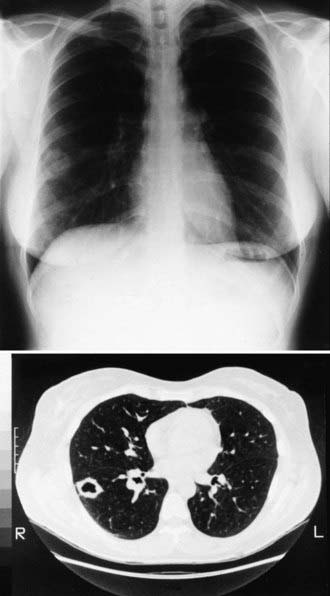
During primary infection, chest radiography may be normal or demonstrate consolidation, single or multiple circumscribed lesions, or soft pulmonary densities. Hilar and subcarinal lymphadenopathy is often present (see Fig. 232-2). Cavities tend to be thin-walled (Fig. 232-3). Pleural effusions vary in size. The presence of miliary or reticulonodular lesions is prognostically unfavorable. Isolated or multiple osseous lesions are usually lytic and often affect cancellous bone. Lesions can affect adjacent structures, and vertebral lesions can impact the spinal cord.
Treatment
Based on the few rigorous clinical trials performed in adults and the opinions of experts in the management of coccidioidomycosis, consensus treatment guidelines have been developed (Table 232-2). Consultation with experts in an area of endemicity should be considered when formulating a plan of management.
Table 232-2 INDICATIONS FOR TREATMENT OF COCCIDIOIDOMYCOSIS IN ADULTS
| INDICATION | TREATMENT |
|---|---|
| Acute pneumonia, mild | Observe without antifungal treatment at 1-3 mo intervals for ≥1 yr; some experts recommend antifungal treatment |
| Weight loss >10%; sweats >3 wk; infiltrates at least half of one lung or parts of both lungs; prominent or persistent hilar lymphadenopathy; CF titers >1:16; inability to work, symptoms >2 mo | Treat with an azole daily for 3-6 mo, with follow up at 1-3 mo intervals for ≥1 yr |
| Uncomplicated acute pneumonia, special circumstances: immunosuppression, late pregnancy, Filipino or African ancestry, age >55 yr, other chronic diseases (diabetes, cardiopulmonary disease), symptoms >2 mo | Treat with an azole daily for 3-6 mo, with follow up at 1-3 mo intervals for ≥1 yr Treat with amphotericin B if in late pregnancy |
| Diffuse pneumonia: reticulonodular or miliary infiltrates suggest underlying immunodeficiency and possible fungemia, pain | Treat initially with amphotericin B if significant hypoxia or rapid deterioration, followed by an azole for ≥1 yr In mild cases, an azole for ≥1 yr |
| Chronic pneumonia | Treat with an azole for ≥1 yr |
| Disseminated disease, nonmeningeal | Treat with an azole for ≥1 yr except in severe or rapidly worsening cases for which amphotericin B is recommended |
| Disseminated disease, meningeal | Treat with fluconazole (some add intrathecal amphotericin B) and treat indefinitely |
CF, complement fixation.
Patients should be followed closely because late relapse can occur, especially in patients who are immunosuppressed or have severe manifestations. Treatment is recommended for all HIV-infected patients with active coccidioidomycosis and CD4 counts <250/µL. Following successful treatment, antifungals may be stopped if the CD4 count exceeds 250/µL. Treatment should be continued if the CD4 count remains less than 250/µL and should be given indefinitely in all HIV-infected patients with coccidioidal meningitis.
First-line agents include oral and intravenous preparations of fluconazole (6-12 mg/kg/day IV or PO) and itraconazole (5-10 mg/kg/day). Serum levels of itraconazole should be monitored.
Amphotericin B is preferred for initial treatment of severe infections. Amphotericin B deoxycholate is less costly than lipid formulations and is often well tolerated in children. Once a daily dose of amphotericin B deoxycholate of 1-1.5 mg/kg/day is achieved, the frequency of administration can be reduced to 3 times weekly. The recommended total dosage ranges from 15 mg/kg to 45 mg/kg and is determined by the clinical response. Lipid formulations of amphotericin are recommended for patients with impaired renal function, patients receiving other nephrotoxic agents, or if amphotericin B deoxycholate is not tolerated. Some experts prefer liposomal amphotericin to treat CNS infections because it achieves higher levels in brain parenchyma. Amphotericin B preparations do not cross the blood-brain barrier to effectively treat Coccidioides spp., but they can mask the signs of meningitis. Infections during pregnancy should be treated with amphotericin B, because the azoles are potentially teratogenic. Voriconazole and posaconazole have been used successfully as salvage therapy in infections failing the standard agents.
Primary Pulmonary Infection
Primary pulmonary coccidioidomycosis resolves in 95% of patients without risk factors for dissemination; antifungal therapy does not lessen the frequency of dissemination or pulmonary residua. When it is elected to defer antifungal therapy, visits are recommended as needed and at 3-6 mo intervals for 2 yr and as needed.
Patients with significant or prolonged symptoms are more likely to incur benefit from antifungal agents, but there are no established criteria upon which to base the decision. Commonly used indicators in adults are summarized in Table 232-2. A treatment trial in adults with primary respiratory infections examined outcomes of antifungal therapy prescribed on the basis of severity and compared them to an untreated group with less severe symptoms; complications occurred only in patients in the treatment group and only in those in whom treatment was stopped. If treatment is elected, a 3-6 mo course of fluconazole (6-12 mg/kg/day) or itraconazole (5-10 mg/kg/day) is recommended.
Diffuse Pneumonia
Diffuse reticulonodular densities or miliary infiltrates, sometimes accompanied by severe illness, can occur in dissemination or follow exposure to a large fungal inoculum. In this setting, amphotericin B is recommended for initial treatment followed thereafter by extended treatment with high-dose fluconazole (Table 232-2).
Disseminated Infection (Extrapulmonary)
For nonmeningeal infection (see Table 232-2), oral fluconazole and itraconazole are effective for treating disseminated coccidioidomycosis that is not extensive, is not progressing rapidly, and has not affected the CNS. Some experts recommend higher doses for adults than were used in clinical trials. A subgroup analysis showed a tendency for improved response of skeletal infections that were treated with itraconazole. Amphotericin B deoxycholate is used as an alternative, especially if there is rapid worsening and lesions are in critical locations. Voriconazole has been used successfully as salvage therapy. The optimal duration of therapy with the azoles has not been clearly defined. Late relapses have occurred after lengthy treatment and favorable clinical response.
Meningitis
Therapy with oral fluconazole is currently preferred for coccidioidal meningitis. In adults, a dosage >400 mg/day is recommended by some experts. Itraconazole at a dosage of 400-600 mg/day in adults has been reported to have a comparable effect. Some experts use intrathecal, intraventricular, or intracisternally administered amphotericin B in addition to an azole, believing that the clinical response may be faster. Patients who respond to the azole should continue treatment indefinitely. Hydrocephalus is a common occurrence and is not necessarily a marker of treatment failure. In the event of treatment failure with azoles, intrathecal therapy with amphotericn B deoxycholate is indicated, with or without the azole treatment. Cerebral vasculitis can occur and can predispose to cerebral ischemia, infarction, or hemorrhage. The efficacy of steroids in high dosage is unresolved. Salvage therapy with voriconazole has been found to be effective.
Surgical Management
If a cavity is located peripherally or there is recurrent bleeding or pleural extension, excision may be needed. Infrequently, bronchopleural fistula or recurrent cavitation occur as surgical complications; rarely, dissemination can result. Perioperative intravenous therapy with amphotericin B may be considered. Drainage of cold abscesses, synovectomy, and curettage or excision of osseous lesions are sometimes needed. Local and systemic administration of amphotericin B can be used to treat coccidioidal articular disease.
Prevention
Prevention relies on education about ways to reduce exposure. Physicians practicing in nonendemic regions should incorporate careful travel histories when evaluating patients with symptoms compatible with coccidioidomycosis.
Ampel NM. The complex immunology of human coccidioidomycosis. Ann NY Acad Sci. 2007;1111:245-258.
Ampel NM, Giblin A, Mourani JP, et al. Factors and outcomes associated with the decision to treat primary pulmonary coccidioidomycosis. Clin Inf Dis. 2009;48:172-178.
Blair J. Approach to the solid organ transplant patient with latent infection and disease caused by Coccidioides sp. Curr Opin Infect Dis. 2008;21:415-420.
Blair JE. State-of-the-art treatment of coccidioidomycosis skeletal infections. Ann N Y Acad Sci. 2007;1111:422-433.
Centers for Disease Control and Prevention. Increase in coccidioidomycosis—California, 2000-2007. MMWR Morb Mortal Wkly Rep. 2009;58:105-108.
DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis. Arch Dermatol. 2006;142:744-746.
Durkin M, Connolly P, Kuberski T, et al. Diagnosis of coccidioidomycosis with use of the Coccidioides antigen enzyme immunoassay. Clin Infect Dis. 2008;47:e69.
Fisher BT, Chiller TM, Prasad PA, et al. Hospitalizations for coccidioidomycosis at forty-one children’s hospitals in the United States. Pediatr Infect Dis J. 2010;29:243-247.
Freifeld A, Proia L, Andes D, et al. Voriconazole use for endemic fungal infections. Antimicrob Agents Chemother. 2009;53:1648-1651.
Homans JD, Spencer L. Itraconazole treatment of nonmeningeal coccidioidomycosis in children. Pediatr Infect Dis J. 2010;29:65-67.
Laniado-Laborin R. Expanding understanding of epidemiology of coccidioidomycosis in the western hemisphere. Ann N Y Acad Sci. 2007;1111:19-34.
Mathisen G, Shelub A, Truong J, et al. Coccidioidal meningitis. Medicine. 2010;89(5):251-284.
Saubolle MA. Laboratory aspects in the diagnosis of coccidioidomycosis. Ann N Y Acad Sci. 2007;1111:301-314.
Spinello IM, Munoz A, Johnson RH. Pulmonary coccidioidomycosis. Semin Respir Crit Care Med. 2008;29:166-173.
Stevens DA, Rendon A, Gaona-Flores V, et al. Posaconazole therapy for chronic refractory coccidioidomycosis. Chest. 2007;132:952-958.
Williams PL. Coccidioidal meningitis. Ann N Y Acad Sci. 2007;1111:377-384.