Chapter 253 Human Metapneumovirus
Human metapneumovirus (HMPV), a respiratory virus identified in 2001, is emerging as one of the most common causes of serious lower respiratory tract illness in children throughout the world.
Etiology
HMPV is an enveloped, single-stranded nonsegmented negative-sense RNA genome of the Paramyxoviridae family, which is divided into 2 subfamilies, Pneumovirinae and Paramyxovirinae. The Pneumovirinae subfamily includes the 2 genera Metapneumovirus and Pneumovirus, which includes respiratory syncytial virus (RSV). HMPV and the avian pneumoviruses (APVs) are highly related and are separated into the separate genus Metapneumovirus because the gene order in the nonsegmented genome is slightly altered and APVs/HMPVs lack the genes for 2 nonstructural proteins, NS1 and NS2, that are encoded at the 3′ end of RSV genomes. These proteins are thought to counteract host type I interferons. The absence of NS1/NS2 in the metapneumoviruses may contribute to decreased pathogenicity of HMPV relative to wild-type RSV strains.
Full-length sequences of a number of HMPV genomes have been determined. The genome is predicted to encode 9 proteins in the order 3′-N-P-M-F-M2-(orf1 and 2)-SH-G-L-5′. The genome also contains noncoding 3′ leader, 5′ trailer, and intergenic regions, consistent with the organization of most paramyxoviruses, with a viral promoter contained in the 3′ end of the genome. The F (fusion), G (glycosylated), and SH (short hydrophobic) proteins are integral membrane proteins on the surfaces of infected cells and virion particles. The F protein is a classic type I integral membrane viral fusion protein that contains 2 heptad repeats in the extracellular domain that facilitate membrane fusion. There is a predicted protein cleavage site near a hydrophobic fusion peptide that likely is cleaved by an extracellular protease, activating the F protein for fusion. The predicted attachment (G) protein of HMPV exhibits the basic features of a glycosylated type II mucin-like protein. The HMPV G protein differs from the RSV G protein in that it lacks a cysteine noose structure. This protein may inhibit innate immune responses. The internal proteins of the virus appear similar in function to those of other paramyxoviruses.
Epidemiology
HMPV outbreaks occur in annual epidemics during late winter and early spring in temperate climates, often overlapping with the second half of the annual RSV epidemic (Fig. 253-1). Sporadic infection does occur year round. The usual period of viral shedding is likely to be several weeks after primary infection in infants. The incubation period is 3-5 days. Humans are the only source of virus. Transmission is thought to occur by close or direct contact with contaminated secretions involving large-particle aerosols, droplets, or contaminated surfaces. Nosocomial infections have been reported, and contact isolation with excellent handwashing for health care providers is indicated in medical settings. This virus affects the elderly, immunocompromised patients, and patients with reactive airways disease more severely than otherwise healthy individuals.
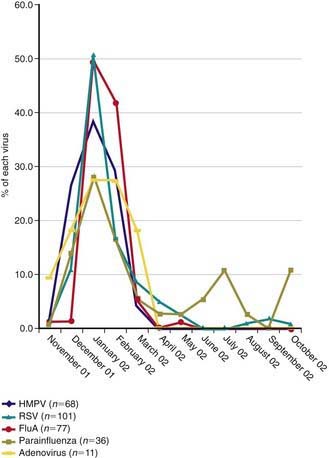
Figure 253-1 Temporal distribution of respiratory viruses among children hospitalized with lower respiratory tract infections from November 2001 through October 2002. Data are displayed as the proportion of each virus detected monthly. FluA, influenza A; HMPV, human metapneumovirus; RSV, respiratory syncytial virus.
(From Wolf DG, Greenberg D, Kalkstein D, et al: Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower reparatory tract infections in hospitalized young children, Pediatr Infect Dis J 25:320–324, 2006.)
Pathology
Infection is usually limited to the superficial layer of airway epithelial cells. Infection is associated with a local inflammatory infiltrate consisting of lymphocytes and macrophages. Immunocompromised individuals have evidence of both acute and organizing injuries during prolonged infection.
Pathogenesis
Infection occurs via inoculation of the upper respiratory tract. Infection can spread rapidly to the lower respiratory tract, but it is not clear whether the spread is mediated by cell-to-cell spread or aspiration of infected materials from the upper tract. Severe lower respiratory tract illness, especially wheezing, occurs mainly during the 1st 6 mo of life, at a time when the airways are of a small diameter and high resistance. Maternal serum neutralizing antibodies that cross the placenta may afford a relative protection against severe disease for several weeks or months after birth. Once infection is established, it is suspected that cytotoxic T cells recognize and eliminate virus-infected cells, thus terminating the infection but also causing some cytopathology. Individuals with an underlying predisposition to reactive airways disease (including adults) are susceptible to severe wheezing during re-infection later in life, suggesting that HMPV may cause smooth muscle hyperactivity, inflammation, or increased mucus production in such individuals. Infection in otherwise healthy individuals resolves without apparent long-term consequences in most cases.
Clinical Manifestations
HMPV is associated with the common cold (complicated by otitis media in about 30% of cases) and with lower respiratory tract illnesses such as bronchiolitis, pneumonia, croup, and exacerbation of reactive airways disease. The profile of signs and symptoms caused by HMPV is very similar to that caused by RSV (Table 253-1). Approximately 5-10% of outpatient lower respiratory tract illnesses in otherwise healthy young children is associated with HMPV infection, which is 2nd in incidence only to RSV. Children with RSV or HMPV infection require supplemental oxygen and medical intensive care at similar frequencies.
Table 253-1 CLINICAL MANIFESTATIONS OF HUMAN METAPNEUMOVIRUS IN CHILDREN
COMMON (>50%)
LESS COMMON
RARE
About 50% of the cases of lower respiratory tract illness in children occur in the 1st 6 mo of life, suggesting that young age is a major risk factor for severe disease. Both young adults and the elderly can have HMPV infection that requires medical care including hospitalization, but severe disease occurs at much lower frequencies in adults than in young children. Severe disease in older subjects is most common in immunocompromised patients and can be fatal. A significant number of both adult and pediatric patients with asthma exacerbations have HMPV infection; it is not clear whether the virus causes long-term wheezing. RSV and HMPV co-infections have been reported; coinfections may be more severe, resulting in pediatric intensive care unit admissions. It is difficult to define true co-infections because the viral genome can be detected by reverse transcriptase polymerase chain reaction (RT-PCR) in respiratory secretions for at least several weeks after illness, even when virus shedding has terminated.
Laboratory Findings
The virus can be seen only with electron microscopy. The virus grows in primary monkey kidney cells or LLC-MK2 cell or Vero cell line monolayer cultures, but its efficient isolation requires an experienced laboratory technician. Conventional bright-field microscopy of infected cell monolayer cultures often reveals cytopathic effect only after multiple passages in cell culture. The characteristics of the cytopathic effect are not sufficiently distinct to allow identification of the virus on this basis alone, even by a trained observer. Direct antigen tests for identification of HMPV antigens in nasopharyngeal secretions are available. Some laboratories have success with the use of immunofluorescence staining with monoclonal or polyclonal antibodies to detect HMPV in nasopharyngeal secretions and shell vial cultures or in monolayer cultures in which virus has been cultivated. The most sensitive test for identification of HMPV in clinical samples is RT-PCR, usually performed with primers directed to internal genes such as the nucleoprotein gene. Detection by this modality is also available in some multiplex PCR tests for panels of respiratory viruses. Real-time RT-PCR tests offer enhanced sensitivity and specificity, including assays designed to detect viruses from the 4 known genetic lineages.
Diagnosis and Differential Diagnosis
In temperate areas, the diagnosis should be suspected during the late winter in infants or young children with wheezing or pneumonia and a negative RSV diagnostic test result. The diseases caused by RSV and HMPV cannot be distinguished clinically. Many other common respiratory viruses, such as parainfluenza viruses, influenza viruses, adenoviruses, rhinoviruses, and coronaviruses, can cause similar disease in young children. Some of these viruses can be identified by PCR genetic testing or conventional cell culture means.
Complications
Co-infection with bacteria is not common in HMPV infection, except for the local complication of otitis media.
Treatment
There is no specific treatment at this time for HMPV infection. Management consists of supportive care. The rate of bacterial lung infection or bacteremia associated with HMPV infection is not fully defined but is suspected to be very low. Antibiotics are usually not indicated in treatment of infants hospitalized for HMPV bronchiolitis or pneumonia.
Supportive Care
Treatment is supportive and includes careful attention to hydration, monitoring of respiratory status by physical examination and measurement of oxygen saturation, use of supplemental oxygen, and, if necessary, mechanical ventilation.
Prognosis
Most infants and children recover from acute HMPV infection without apparent long-term consequences. Many experts believe an association exists between severe HMPV infections in infancy and risk for recurrent wheezing or the development of asthma; however, it is not clear whether the virus causes these conditions or precipitates their 1st manifestations.
Prevention
The only method of prevention of HMPV infection is reduction of exposure. Contact precautions are recommended for the duration of HMPV-associated illness among hospitalized infants and young children. Patients known to have HMPV infection should be housed in single rooms or with a cohort of HMPV-infected patients. It may be wise to care for patients with RSV infection in a separate cohort from HMPV-infected patients to prevent co-infection. Preventive measures include limiting, where feasible, exposure to contagious settings during annual epidemics (daycare centers) and emphasis on hand hygiene in all settings, including the home, especially during periods when the contacts of high-risk children have respiratory infections. However, providers should keep in mind that infection is universal in the first several years of life. Therefore, reduction of exposure makes most sense during the 1st 6 mo of life, when infants are at highest risk for severe disease.
Arnold JC, Singh KK, Milder E, et al. Human metapneumovirus associated with central nervous system infection in children. Pediatr Infect Dis J. 2009;28:1057-1060.
Bao X, Liu T, Shan Y, et al. Human meta-pneumovirus glycoprotein G inhibits innate immune responses. PLoS Pathog. 2008;4:e1000077.
Crowe JEJr. Human metapneumovirus as a major cause of human respiratory tract disease. Pediatr Infect Dis J. 2004;23(Suppl):S215-S221.
Foulongne V, Buyon G, Rodiere M, et al. Human metapneumovirus infection in young children hospitalized with respiratory tract disease. Pediatr Infect Dis J. 2006;25:34-39.
Klein MI, Coviello S, Bauer G, et al. The impact of infection with human metapneumovirus and other respiratory viruses in young infants and children at high risk for severe pulmonary disease. J Infect Dis. 2006;193:1544-1551.
Semple MG, Cowell A, Dove W, et al. Dual infection of infants by human metapneumovirus and human respiratory syncytial virus is strongly associated with severe bronchiolitis. J Infect Dis. 2005;191:382-386.
Williams JV, Crowe JEJr, Enriquez R, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis. 2005;192:1149-1153.
Williams JV, Tollefson SJ, Heymann PW, et al. Human metapneumovirus infection in children hospitalized for wheezing. J Allergy Clin Immunol. 2005;115:1311-1312.
Williams JV, Wang CK, Yang CF, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387-395.
Wolf DG, Greenberg D, Kalkstein D, et al. Comparison of human metapneumovirus, respiratory syncytial virus and influenza A virus lower reparatory tract infections in hospitalized young children. Pediatr Infect Dis J. 2006;25:320-324.