Chapter 259 Arboviral Encephalitis in North America
The arthropod-borne (arbovirus) viral encephalitides are a group of clinically similar severe neurologic infections caused by several different viruses. They are transmitted by mosquitoes during outdoor exposure in warmer weather in overlapping regions across most of the USA and much of southern Canada.
Etiology
The principal causes of the arthropod-borne encephalitides of North America are West Nile encephalitis (WNE), the St. Louis encephalitis (SLE), a complex of viruses included in the California encephalitis (CE) group of viruses, and, less frequently, western equine encephalitis (WEE), eastern equine encephalitis (EEE), and Colorado tick fever. The etiologic agents belong to different viral taxa: alphaviruses of the family Togaviridae (EEE and WEE), Flaviviridae (WNE, SLE), the California complex of the family Bunyaviridae (CE), and Reoviridae (Colorado tick fever virus). Alphaviruses are 69-nm, enveloped, positive-sense RNA viruses that evolved from a common Venezuelan equine encephalitis–like viral ancestor in the Western hemisphere. Flaviviruses are 40- to 50-nm, enveloped, positive-sense RNA viruses that evolved from a common ancestor. They are globally distributed and responsible for many important human viral diseases. The California serogroup, 1 of 16 Bunyavirus groups, are 75- to 115-nm enveloped viruses possessing a 3-segment, negative-sense RNA genome. Reoviruses are 60- to 80-nm double-stranded RNA viruses.
Epidemiology
Eastern Equine Encephalitis
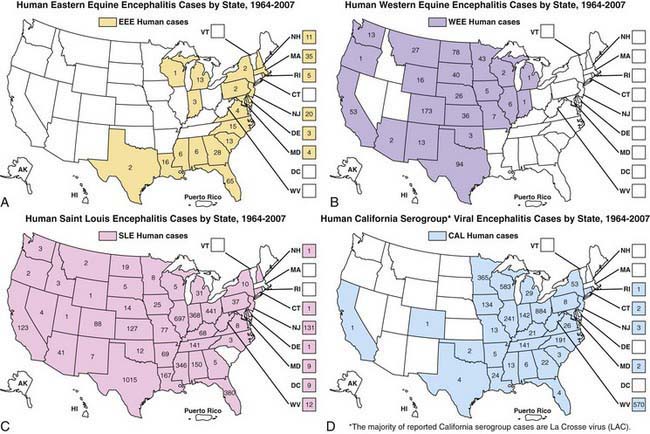
In the USA, EEE is a very low incidence disease, with a median of 8 cases occurring annually in the Atlantic and Gulf States from 1964-2007 (Fig. 259-1). Transmission occurs often in focal endemic areas of the coast of Massachusetts, the 6 southern counties of New Jersey, and northeastern Florida. In North America, the virus is maintained in freshwater swamps in a zoonotic cycle involving Culiseta melanura and birds. Various other mosquito species obtain viremic meals from birds and transmit the virus to horses and humans. Virus activity varies markedly from year to year in response to still unknown ecologic factors. Most infections in birds are silent, but infections in pheasants are often fatal, and epizootics in these species are used as sentinels for periods of increased viral activity. Cases have been recognized on Caribbean islands. The case : infection ratio is lowest in children (1 : 8) and somewhat higher in adults (1 : 29).
Western Equine Encephalitis
WEE infections occur principally in the USA and Canada west of the Mississippi River (see Fig. 259-1), mainly in rural areas where water impoundments, irrigated farmland, and naturally flooded land provide breeding sites for Culex tarsalis. The virus is transmitted in a cycle involving mosquitoes, birds, and other vertebrate hosts. Humans and horses are susceptible to encephalitis. The case : infection ratio varies by age, having been estimated at 1 : 58 in children younger than 4 yr and 1 : 1,150 in adults. Infections are most severe at the extremes of life; a third of cases occur in children younger than 1 yr. Recurrent human epidemics have been reported from the Yakima Valley in Washington State and the Central Valley of California; the largest outbreak on record resulted in 3,400 cases and occurred in Minnesota, North and South Dakota, Nebraska, and Montana as well as Alberta, Manitoba, and Saskatchewan, Canada. Epizootics in horses precede human epidemics by several weeks. For the past 20 yr, only 3 cases of WEE have been reported, presumably reflecting successful mosquito abatement.
St. Louis Encephalitis
Cases of SLE are reported from nearly all states; the highest attack rates occur in the Gulf and central states (see Fig. 259-1). Epidemics frequently occur in urban and suburban areas; the largest, in 1975, involved 1,800 persons living in Houston, Chicago, Memphis, and Denver. Cases often cluster in areas where there is ground water or septic systems, which support mosquito breeding. The principal vectors are Culex pipiens and Culex quinquefasciatus in the central Gulf States, Culex nigripalpus in Florida, and Culex tarsalis in California. SLE virus is maintained in nature in a bird-mosquito cycle. Viral amplification occurs in bird species abundant in residential areas (e.g., sparrows, blue jays, and doves). Virus is transmitted in the late summer and early fall. The case : infection ratio may be as high as 1 : 300. Age-specific attack rates are lowest in children and highest in individuals older than 60 yr. The most recent small outbreaks were in Florida in 1990 and Louisiana in 2001. For the past 15 years there have been a mean of 18 cases annually.
West Nile Encephalitis
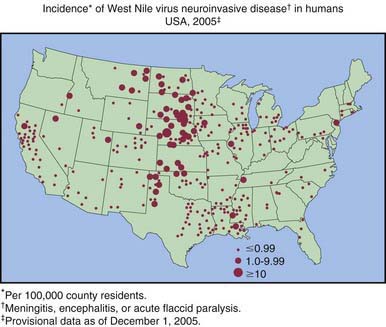
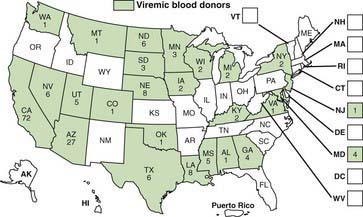
WNE virus has been implicated as the cause of sporadic summertime cases of human encephalitis and meningitis in Israel, India, Pakistan, Romania, Russia, and the USA. All American WNE viruses are genetically similar and are related to a virus recovered from a goose in Israel in 1998. WNE virus survives in a broad enzootic cycle in the USA and within 4 yr had spread to most states east of the Rocky Mountains plus California (Fig. 259-2). Every state in the continental USA plus 9 provinces in Canada have reported mosquito, bird, mammalian, or human West Nile infection. Through the end of 2008 28,813 total cases had been reported, 30-40% of which were encephalitis, with 1064 deaths. Summer/fall epidemics are common (Fig. 259-3). West Nile virus has entered the blood supply through asymptomatic viremic blood donors. Blood banks screen for West Nile virus RNA (Fig. 259-4). West Nile virus has also been transmitted to humans via the placenta, breast milk, and organ transplantation. Throughout its range, the virus is maintained in nature by transmission between mosquitoes of the Culex genus and various species of birds. In the USA, human infections are largely acquired from Culex pipiens. Horses are the non-avian vertebrates most likely to exhibit disease with WNE infection. During the 2002 transmission season, 14,000 equine cases were reported, with a mortality rate of 30%. Disease occurs predominantly in individuals >50 yr of age.
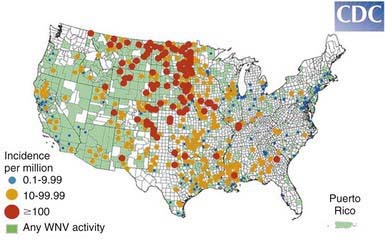
Figure 259-2 Incidence of West Nile virus neuroinvasive disease in humans—USA, 2008.
(From the Centers for Disease Control and Prevention: West Nile virus activity—United States, January 1-December 31, 2008 as reported to CDC’s ArboNET system.)
La Crosse/California Encephalitis
La Crosse viral infections are endemic in the USA, occurring annually from July to September, principally in the north-central and central states (See Fig. 259-1). Infections occur in peridomestic environments as the result of bites from Aedes triseriatus mosquitoes, which often breed in tree holes. The virus is maintained vertically in nature by transovarial transmission and can be spread between mosquitoes by copulation and amplified in mosquito populations by viremic infections in various vertebrate hosts. Amplifying hosts include chipmunks, squirrels, foxes, and woodchucks. A case : infection ratio of 1 : 22-300 has been surmised. La Crosse encephalitis is principally a disease of children, who may account for to 75% of cases. A mean of 100 cases have been reported annually for the past 10 years.
Colorado Tick Fever
Colorado tick fever virus is transmitted by the wood tick Dermacentor andersoni, which inhabits high-elevation areas of states extending from the central plains to the Pacific coast. The tick is infected with the virus at the larval stage and remains infected for life. Squirrels and chipmunks serve as primary reservoirs. Human infections typically occur in hikers and campers in indigenous areas during the spring and early summer.
Clinical Manifestations
The arboviruses produce symptoms of encephalitis, except West Nile virus and Colorado tick fever virus, which most commonly manifest as flulike illnesses and only occasionally cause encephalitis.
Eastern Equine Encephalitis
EEE virus Infections result in fulminant encephalitis with a rapid progression to coma and death in one third of cases. In infants and children, abrupt onset of fever, irritability, and headache are followed by lethargy, confusion, seizures, and coma. High temperature, bulging fontanel, stiff neck, and generalized flaccid or spastic paralysis are observed. There may be a brief prodrome of fever, headache, and dizziness. Unlike most other viral encephalitides, the peripheral white blood cell count usually demonstrates a marked leukocytosis, and the cerebrospinal fluid (CSF) may show marked pleocytosis. Pathologic changes are found in the cortical and gray matter, with viral antigens localized to neurons. There is necrosis of neurons, neutrophilic infiltration, and perivascular cuffing by lymphocytes.
Western Equine Encephalitis
In WEE, there may be a prodrome with symptoms of an upper respiratory tract infection. The onset is usually sudden with chills, fever, dizziness, drowsiness, increasing headache, malaise, nausea and vomiting, stiff neck, and disorientation. Infants typically present with the sudden cessation of feeding, fussiness, fever, and protracted vomiting. Convulsions and lethargy develop rapidly. On physical examination, patients are somnolent, exhibit meningeal signs, and have generalized motor weakness and reduced deep tendon reflexes. In infants, a bulging fontanel, spastic paralysis, and generalized convulsions may be observed. On pathologic examination, disseminated small focal abscesses, small focal hemorrhages, and patchy areas of demyelination are distinctive.
St. Louis Encephalitis
Clinical manifestations of SLE vary from a mild flulike illness to fatal encephalitis. There may be a prodrome of nonspecific symptoms with subtle changes in coordination or mentation of several days to 1wk in duration. Early signs and symptoms include fever, photophobia, headache, malaise, nausea, vomiting, and neck stiffness. About half of patients exhibit abrupt onset of weakness, incoordination, disturbed sensorium, restlessness, confusion, lethargy, and delirium or coma. The peripheral white blood cell count is modestly elevated, with 100-200 cells/mm3 found in the CSF. On autopsy, the brain shows scattered foci of neuronal damage and perivascular inflammation.
West Nile Encephalitis
WNE may be asymptomatic, but when clinical features appear, they include an abrupt onset of high fever, headache, myalgias, and nonspecific signs of emesis, rash, abdominal pain, or diarrhea. Most infections manifest as a flulike febrile illness, whereas a minority of patients demonstrate meningitis or encephalitis or both. Rarely there may be cardiac dysrhythmias, myocarditis, rhabdomyolysis, optic neuritis, uveitis, retinitis, orchitis, pancreatitis, or hepatitis. WNE disease in the USA has been accompanied by prolonged lymphopenia and an acute asymmetric polio-like paralytic illness with CSF pleocytosis involving the anterior horn cells of the spinal cord. A striking but uncommon feature has been Parkinsonism and movement disorders (with tremor and myoclonus).
Lacrosse/California Encephalitis
The clinical spectrum includes a mild febrile illness, aseptic meningitis, and fatal encephalitis. Children typically present with a prodrome of 2-3 days with fever, headache, malaise, and vomiting. The disease evolves with clouding of the sensorium, lethargy, and, in severe cases, focal or generalized seizures. On physical examination, children are lethargic but not disoriented. Focal neurologic signs, including weakness, aphasia, and focal or generalized seizures, have been reported in 16-25% of cases. CSF shows low to moderate leukocyte counts. On autopsy, the brain shows focal areas of neuronal degeneration, inflammation, and perivascular cuffing.
Colorado Tick Fever
Colorado tick fever begins with the abrupt onset of a flulike illness, including high temperature, malaise, arthralgias and myalgia, vomiting, headache, and decreased sensorium. Rash is uncommon. The symptoms rapidly disappear after 3 days of illness. However, in approximately half of patients, a 2nd identical episode recurs 24-72 hr after the 1st, producing the typical “saddleback” temperature curve of Colorado tick fever. Complications, including encephalitis, meningoencephalitis, and a bleeding diathesis, develop in 3-7% of infected persons and may be more common in children younger than 12 yr.
Diagnosis
The etiologic diagnosis of a specific arboviral infection is established by testing an acute-phase serum ≥5 days after onset of illness for the presence of virus-specific immunoglobulin (Ig) M antibodies using an indirect immunofluorescence test or an enzyme-linked immunosorbent assay (ELISA) IgM capture test. Alternatively, acute and convalescent sera can be tested for a fourfold or greater increase in ELISA, hemagglutination inhibition, or neutralizing IgG antibody titers. Commercial serologic diagnostic kits are marketed, especially for West Nile viral infections. Serum and CSF should be tested for West Nile virus–specific IgM. However, IgM may reflect past infection, because it may be present up to 12 mo after infection. The diagnosis may also be established by isolation in cell cultures of virus in brain tissue, obtained by brain biopsy or at autopsy, or by identification of viral RNA reverse transcriptase polymerase chain reactions.
The diagnosis of encephalitis may be aided by CT or MRI and by electroencephalography. Focal seizures or focal findings on CT or MRI or electroencephalography should suggest the possibility of herpes simplex encephalitis, which should be treated with acyclovir (Chapter 244).
Treatment
There is no specific treatment for arboviral encephalitides, although oral ribavirin may have been of benefit in a case of La Crosse encephalitis. The treatment of acute arboviral encephalitis is intensive supportive care (Chapter 62), including control of seizures (Chapter 586).
Prognosis
Fatalities occur with all arboviral encephalitides. With the exception of EEE, most resolve without residua.
Eastern Equine Encephalitis
The prognosis in EEE is better for patients with a prolonged prodrome; the occurrence of convulsions conveys a poor prognosis. Patient fatality rates are 33-75% and are highest in the elderly. Residual neurologic defects are common, especially in children.
Western Equine Encephalitis
Patient fatality rates in WEE are 3-9% and highest in the elderly. Major neurologic sequelae have been reported in up to 13% of cases and may be as high as 30% in infants. Parkinsonian syndrome has been reported as a residual in adult survivors.
St. Louis Encephalitis
The principal risk factor for fatal outcome of SLE is advanced age, with patient fatality rates being as high as 80% in early outbreaks. In children, mortality rates are 2-5%. In adults, underlying hypertensive cardiovascular disease has been a risk factor for fatal outcome. Recovery from SLE is usually complete, but the rate of serious neurologic sequelae has been reported to be as high as 10% in children.
West Nile Encephalitis
Cases of and deaths due to WNE occur mainly in the elderly, although many serologic surveys show that persons of all ages are infected. During 2002-2004, there were 648 deaths among 16,557 cases, a 3.8% mortality rate. Paralysis may result in permanent weakness.
Prevention
Killed EEE, WEE, and WNE vaccines are available for horses, and an experimental killed vaccine is administered to human laboratory workers who handle EEE virus. Flocks of sentinel chickens or pheasants have been stationed at various locations along the Atlantic coast during the late summer or early fall to obtain early warning of increased transmission of EEE virus.
No human vaccine is licensed for arboviral encephalitides, although WNE vaccines are being developed. Killed WNE vaccines are licensed for veterinary use. Extensive water management and mosquito abatement programs in California have reduced transmission of WEE and the incidence of human infections. Urban WNE and SLE outbreaks in the eastern USA, Texas, and the Midwest have been controlled by the application of ultra-low-volume adulticide chemicals applied from trucks or low-flying aircraft.
Because infections in children may occur as a result of summer daytime mosquito biting in residential areas, sealing mosquito breeding sites, using insect repellents, and instructing children to play in open, sunny areas away from forest fringe may help prevent disease.
Armstrong PM, Andreadis TG, Anderson JF, et al. Tracking eastern equine encephalitis virus perpetuation in the northeastern United States by phylogenetic analysis. Am J Trop Med Hyg. 2008;79:291-296.
Balfour HHJr, Siem RA, Bauer H, et al. California arbovirus (La Crosse) infections I: clinical and laboratory findings in 66 children with meningoencephalitis. Pediatrics. 1973;52:680-691.
Blivich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008;9:71-86.
Centers for Disease Control and Prevention. West Nile virus activity—United States, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:769-772.
Centers for Disease Control and Prevention. Detection of West Nile virus in blood donations—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:769-772.
Civen R, Villacorte F, Robles DT, et al. West Nile infection in the pediatric population. Pediatr Infect Dis J. 2006;25:75-78.
Cunha BA, Minnaganti V, Johnson DH, et al. Profound and prolonged lymphocytopenia with West Nile encephalitis. Clin Infect Dis. 2000;31:1116-1117.
Custer B, Kamel H, Kiely NE, et al. Associations between West Nile infections and symptoms reported by blood donors identified by nucleic acid screening. Transfusion. 2009;49:278-288.
Davis LE, Beckham JD, Tyler KL. North American encephalitic arboviruses. Neurol Clin. 2008;26:727-757.
Earnest MP, Goolishian HA, Calverley JR, et al. Neurologic, intellectual, and psychologic sequelae following western encephalitis: a follow-up study of 35 cases. Neurology. 1971;21:969-974.
Forrester NL, Kenney JL, Deardorff E, et al. Western equine encephalitis submergence: lack of evidence for a decline in virus virulence. Virology. 2008;380:170-172.
Haddow AD, Haddow AD. The use of oral ribavirin in the management of La Crosse viral infections. Med Hypotheses. 2009;72:190-192.
Hayes EB, Komar N, Nasci RS, et al. Epidemiology and transmission dynamics of West Nile Virus disease. Emerg Infect Dis. 2005;11:1167-1173.
Hayes EB, O’Leary DR. West Nile virus infection: a pediatric perspective. Pediatrics. 2004;113:1375-1381.
Hinckley AF, O’Leary DR, Hayes EB. Transmission of West Nile virus through human breast milk seems to be rare. Pediatrics. 2007;119:e666-e671.
Komar N, Spielman A. Emergence of eastern encephalitis in Massachusetts. Ann N Y Acad Sci. 1995;740:157-168.
Marfin AA, Bleed DM, Lofgren JP, et al. Epidemiological aspects of a St. Louis encephalitis epidemic in Jefferson County, Arkansas. Am J Trop Med Hyg. 1993;49:30-37.
Minke JM, Audonnet JC, Fischer L. Equine viral vaccines: the past, present and future. Vet Res. 2004;35:425-443.
Reimann CA, Hayes EB, DiGuiseppi C, et al. Epidemiology of neuroinvasive arboviral diseases in the United States, 1999–2007. Am J Trop Med Hyg. 2008;79:974-979.
Sejvar JJ, Hadded MB, Tierney BC, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511-515.
Solomon T. Flavivirus encephalitis. N Engl J Med. 2004;351:370-378.
Spruance SL, Bailey A. Colorado tick fever: a review of 115 laboratory confirmed cases. Arch Intern Med. 1973;131:288-293.
Stramer SL, Fang CT, Foster GA, et al. West Nile virus among blood donors in the United States, 2003 and 2004. N Engl J Med. 2005;353:451-459.