Chapter 261 Dengue Fever and Dengue Hemorrhagic Fever
Dengue fever, a benign syndrome caused by several arthropod-borne viruses, is characterized by biphasic fever, myalgia or arthralgia, rash, leukopenia, and lymphadenopathy. Dengue hemorrhagic fever (Philippine, Thai, or Singapore hemorrhagic fever; hemorrhagic dengue; acute infectious thrombocytopenic purpura) is a severe, often fatal, febrile disease caused by dengue viruses. It is characterized by capillary permeability, abnormalities of hemostasis, and, in severe cases, a protein-losing shock syndrome (dengue shock syndrome), which is thought to have an immunopathologic basis.
Etiology
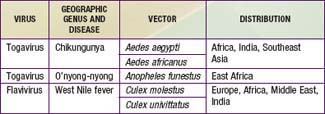
There are at least 4 distinct antigenic types of dengue virus, which is a member of the family Flaviviridae. In addition, 3 other arthropod-borne viruses (arboviruses) cause similar or identical febrile diseases with rash (Table 261-1).
Epidemiology
Dengue viruses are transmitted by mosquitoes of the Stegomyia family. Aedes aegypti, a daytime biting mosquito, is the principal vector, and all 4 virus types have been recovered from it. In most tropical areas, A. aegypti is highly urbanized, breeding in water stored for drinking or bathing and in rainwater collected in any container. Dengue viruses have also been recovered from Aedes albopictus, as in the 2001 Hawaiian epidemic, whereas outbreaks in the Pacific area have been attributed to several other Aedes species. These species breed in water trapped in vegetation. In Southeast Asia and West Africa, dengue virus may be maintained in a cycle involving canopy-feeding jungle monkeys and Aedes species, which feed on monkeys.
Epidemics were common in temperate areas of the Americas, Europe, Australia, and Asia until early in the 20th century. Dengue fever and dengue-like disease are now endemic in tropical Asia, the South Pacific Islands, northern Australia, tropical Africa, the Caribbean, and Central and South America. Dengue fever occurs frequently among travelers to these areas. Locally acquired disease has been reported in Florida and Texas, and imported cases in the USA occur in travelers to endemic areas.
Dengue outbreaks in urban areas infested with A. aegypti may be explosive; up to 70-80% of the population may be involved. Most disease occurs in older children and adults. Because A. aegypti has a limited flight range, spread of an epidemic occurs mainly through viremic human beings and follows the main lines of transportation. Sentinel cases may infect household mosquitoes; a large number of nearly simultaneous secondary infections give the appearance of a contagious disease. Where dengue is endemic, children and susceptible foreigners may be the only persons to acquire overt disease, adults having become immune.
Dengue-Like Diseases
Dengue-like diseases may also occur in epidemics. Epidemiologic features depend on the vectors and their geographic distribution (see Table 261-1). Chikungunya virus is widespread in the most populous areas of the world. In Asia, A. aegypti is the principal vector; in Africa, other Stegomyia species may be important vectors. In Southeast Asia, dengue and chikungunya outbreaks occur concurrently. Outbreaks of o’nyong-nyong and West Nile fever usually involve villages or small towns, in contrast to the urban outbreaks of dengue and chikungunya.
Dengue Hemorrhagic Fever
Dengue hemorrhagic fever occurs where multiple types of dengue virus are simultaneously or sequentially transmitted. It is endemic in all of tropical America and Asia, where warm temperatures and the practices of water storage in homes plus outdoor breeding sites result in large, permanent populations of A. aegypti. Under these conditions, infections with dengue viruses of all types are common, and 2nd infections with heterologous types are frequent.
Second dengue infections are relatively mild in the majority of instances, ranging from an inapparent infection through an undifferentiated upper respiratory tract or dengue-like disease, but may also progress to dengue hemorrhagic fever. Nonimmune foreigners, both adults and children, who are exposed to dengue virus during outbreaks of hemorrhagic fever have classic dengue fever or even milder disease. The differences in clinical manifestations of dengue infections between natives and foreigners in Southeast Asia are related more to immunologic status than to racial susceptibility. Dengue hemorrhagic fever can occur during primary dengue infections, most frequently in infants whose mothers are immune to dengue.
Dengue 3 virus strains circulating in mainland Southeast Asia since 1983 are associated with a particularly severe clinical syndrome, characterized by encephalopathy, hypoglycemia, markedly elevated liver enzyme values, and, occasionally, jaundice.
Pathogenesis
Fatalities with chikungunya and West Nile fever infections have been ascribed to hemorrhage or viral encephalitis.
The pathogenesis of dengue hemorrhagic fever is incompletely understood, but epidemiologic studies suggest that it is usually associated with 2nd infections with dengue types 1-4. Retrospective studies of sera from human mothers whose infants acquired dengue hemorrhagic fever and prospective studies in children acquiring sequential dengue infections have shown that the circulation of infection-enhancing antibodies at the time of infection is the strongest risk factor for development of severe disease. Absence of cross-reactive neutralizing antibodies and presence of enhancing antibodies from passive transfer or active production are the best correlates of risk for dengue hemorrhagic fever. Monkeys that are infected sequentially or are receiving small quantities of enhancing antibodies have enhanced viremias. In humans studied early during the course of secondary dengue infections, viremia levels directly predicted disease severity. When dengue virus immune complexes attach to macrophage Fc receptors, a signal is sent that suppresses innate immunity, resulting in enhanced viral production. In the Americas, dengue hemorrhagic fever and dengue shock syndrome have been associated with dengue types 1-4 strains of recent Southeast Asian origin. Recent occurrences of sizable dengue hemorrhagic fever outbreaks in India, Pakistan, and Bangladesh also appear to be related to imported dengue strains.
Early in the acute stage of secondary dengue infections, there is rapid activation of the complement system. Shortly before or during shock, blood levels of soluble tumor necrosis factor receptor, interferon-γ, and interleukin-2 are elevated. C1q, C3, C4, C5-C8, and C3 proactivators are depressed, and C3 catabolic rates are elevated. These factors or virus itself may interact with endothelial cells to produce increased vascular permeability through the nitric oxide final pathway. The blood clotting and fibrinolytic systems are activated, and levels of factor XII (Hageman factor) are depressed. The mechanism of bleeding in dengue hemorrhagic fever is not known, but a mild degree of disseminated intravascular coagulopathy, liver damage, and thrombocytopenia may operate synergistically. Capillary damage allows fluid, electrolytes, small proteins, and, in some instances, red blood cells to leak into extravascular spaces. This internal redistribution of fluid, together with deficits caused by fasting, thirsting, and vomiting, results in hemoconcentration, hypovolemia, increased cardiac work, tissue hypoxia, metabolic acidosis, and hyponatremia.
Usually no pathologic lesions are found to account for death. In rare instances, death may be due to gastrointestinal or intracranial hemorrhages. Minimal to moderate hemorrhages are seen in the upper gastrointestinal tract, and petechial hemorrhages are common in the interventricular septum of the heart, on the pericardium, and on the subserosal surfaces of major viscera. Focal hemorrhages are occasionally seen in the lungs, liver, adrenals, and subarachnoid space. The liver is usually enlarged, often with fatty changes. Yellow, watery, and at times blood-tinged effusions are present in serous cavities in about 75% of patients.
Dengue virus is frequently absent in tissues at the time of death; viral antigens or RNA have been localized to macrophages in liver, spleen, lung, and lymphatic tissues.
Clinical Manifestations
The incubation period is 1-7 days. The clinical manifestations are variable and are influenced by the age of the patient. In infants and young children, the disease may be undifferentiated or characterized by fever for 1-5 days, pharyngeal inflammation, rhinitis, and mild cough. A majority of infected older children and adults experience sudden onset of fever, with temperature rapidly increasing to 39.4-41.1°C (103-106°F), usually accompanied by frontal or retro-orbital pain, particularly when pressure is applied to the eyes. Occasionally, severe back pain precedes the fever (back-break fever). A transient, macular, generalized rash that blanches under pressure may be seen during the 1st 24-48 hr of fever. The pulse rate may be slow relative to the degree of fever. Myalgia and arthralgia occur soon after the onset and increase in severity. Joint symptoms may be particularly severe in patients with chikungunya or o’nyong-nyong infection. From the 2nd to 6th day of fever, nausea and vomiting are apt to occur, and generalized lymphadenopathy, cutaneous hyperesthesia or hyperalgesia, taste aberrations, and pronounced anorexia may develop.
About 1-2 days after defervescence, a generalized, morbilliform, maculopapular rash appears that spares the palms and soles. It disappears in 1-5 days; desquamation may occur. Rarely there is edema of the palms and soles. About the time this 2nd rash appears, the body temperature, which has previously decreased to normal, may become slightly elevated and demonstrate the characteristic biphasic temperature pattern.
Dengue Hemorrhagic Fever
Differentiation between dengue fever and dengue hemorrhagic fever is difficult early in the course of illness. A relatively mild 1st phase with abrupt onset of fever, malaise, vomiting, headache, anorexia, and cough is followed after 2-5 days by rapid clinical deterioration and collapse. In this 2nd phase, the patient usually has cold, clammy extremities, a warm trunk, flushed face, diaphoresis, restlessness, irritability, mid-epigastric pain, and decreased urinary output. Frequently, there are scattered petechiae on the forehead and extremities; spontaneous ecchymoses may appear, and easy bruising and bleeding at sites of venipuncture are common. A macular or maculopapular rash may appear, and there may be circumoral and peripheral cyanosis. Respirations are rapid and often labored. The pulse is weak, rapid, and thready, and the heart sounds are faint. The liver may enlarge to 4-6 cm below the costal margin and is usually firm and somewhat tender. Approximately 20-30% of cases of dengue hemorrhagic fever are complicated by shock (dengue shock syndrome). Fewer than 10% of patients have gross ecchymosis or gastrointestinal bleeding, usually after a period of uncorrected shock. After a 24- to 36-hr period of crisis, convalescence is fairly rapid in the children who recover. The temperature may return to normal before or during the stage of shock. Bradycardia and ventricular extrasystoles are common during convalescence.
Diagnosis
A clinical diagnosis of dengue fever derives from a high index of suspicion and knowledge of the geographic distribution and environmental cycles of causal viruses. Because clinical findings vary and there are many possible causative agents, the term dengue-like disease should be used until a specific diagnosis is established. A case is confirmed by isolation of the virus, virus antigen, or genome by polymerase chain reaction (PCR) analysis as well as demonstration of a fourfold or greater increase in antibody titers. A probable case is a typical acute febrile illness with supportive serology and occurrence at a location where there are confirmed cases.
The World Health Organization criteria for dengue hemorrhagic fever are fever (2-7 days in duration or biphasic), minor or major hemorrhagic manifestations, thrombocytopenia (≤100,000/mm3), and objective evidence of increased capillary permeability (hematocrit increased by ≥20%), pleural effusion or ascites (by chest radiography or ultrasonography), or hypoalbuminemia. Dengue shock syndrome criteria include those for dengue hemorrhagic fever as well as hypotension, tachycardia, narrow pulse pressure (≤20 mm Hg), and signs of poor perfusion (cold extremities).
Virologic diagnosis can be established by serologic tests, by detection of viral proteins or viral RNA or the isolation of the virus from blood leukocytes or acute phase serum. Following primary and secondary dengue infections, there is a relatively transient appearance of anti-dengue immunoglobulin (Ig) M antibodies. These disappear after 6-12 wk, a feature that can be used to time a dengue infection. In 2nd primary dengue infections, most antibody is of the IgG class. Serologic diagnosis depends on a fourfold or greater increase in IgG antibody titer in paired sera by hemagglutination inhibition, complement fixation, enzyme immunoassay, or neutralization test. Carefully standardized IgM and IgG capture enzyme immunoassays are now widely used to identify acute-phase antibodies from patients with primary or secondary dengue infections in single-serum samples. Usually such samples should be collected not earlier than 5 days nor later than 6 wk after onset. It may not be possible to distinguish the infecting virus by serologic methods alone, particularly when there has been prior infection with another member of the same arbovirus group. Virus can be recovered from acute-phase serum after inoculating tissue culture or living mosquitoes. Viral RNA can be detected in blood or tissues by specific complementary RNA probes or amplified first by = PCR or by real-time PCR. A viral nonstructural protein, NS1, is released by infected cells into the circulation and can be detected using monoclonal or polyclonal antibodies. The detection of NS1 is the basis of commercial tests, including rapid lateral flow tests. These tests offer reliable point of care diagnosis of acute dengue infection.
Differential Diagnosis
The differential diagnosis of dengue fever includes dengue-like diseases, viral respiratory and influenza-like diseases, the early stages of malaria, mild yellow fever, scrub typhus, viral hepatitis, and leptospirosis.
Four arboviral diseases have dengue-like courses but without rash: Colorado tick fever, sandfly fever, Rift Valley fever, and Ross River fever. Colorado tick fever occurs sporadically among campers and hunters in the western USA; sandfly fever in the Mediterranean region, the Middle East, southern Russia, and parts of the Indian subcontinent; and Rift Valley fever in North, East, Central, and South Africa. Ross River fever is endemic in much of eastern Australia, with epidemic extension to Fiji. In adults, Ross River fever often produces protracted and crippling arthralgia involving weight-bearing joints.
Because meningococcemia, yellow fever (Chapter 262), other viral hemorrhagic fevers (Chapter 263), many rickettsial diseases, and other severe illnesses caused by a variety of agents may produce a clinical picture similar to dengue hemorrhagic fever, the etiologic diagnosis should be made only when epidemiologic or serologic evidence suggests the possibility of a dengue infection.
Laboratory Findings
In dengue fever, pancytopenia may occur after the 3-4 days of illness. Neutropenia may persist or reappear during the latter stage of the disease and may continue into convalescence with white blood cell counts <2,000/mm3. Platelet counts rarely fall below 100,000/mm3. Venous clotting, bleeding and prothrombin times, and plasma fibrinogen values are within normal ranges. The tourniquet test result may be positive. Mild acidosis, hemoconcentration, increased transaminase values, and hypoproteinemia may occur during some primary dengue virus infections. The electrocardiogram may show sinus bradycardia, ectopic ventricular foci, flattened T waves, and prolongation of the P-R interval.
The most common hematologic abnormalities during dengue hemorrhagic fever and dengue shock syndrome are hemoconcentration with an increase of >20% in hematocrit, thrombocytopenia, prolonged bleeding time, and moderately decreased prothrombin level that is seldom <40% of control. Fibrinogen levels may be subnormal, and fibrin split product values are elevated. Other abnormalities include moderate elevations of serum transaminase levels, consumption of complement, mild metabolic acidosis with hyponatremia, occasionally hypochloremia, slight elevation of serum urea nitrogen, and hypoalbuminemia. Roentgenograms of the chest reveal pleural effusions (right > left) in nearly all patients with dengue shock syndrome.
Treatment
Treatment of uncomplicated dengue fever is supportive. Bed rest is advised during the febrile period. Antipyretics should be used to keep body temperature <40°C (104°F). Analgesics or mild sedation may be required to control pain. Aspirin is contraindicated and should not be used because of its effects on hemostasis. Fluid and electrolyte replacement is required for deficits caused by sweating, fasting, thirsting, vomiting, and diarrhea.
Dengue Hemorrhagic Fever and Dengue Shock Syndrome
Management of dengue hemorrhagic fever and dengue shock syndrome includes immediate evaluation of vital signs and degrees of hemoconcentration, dehydration, and electrolyte imbalance. Close monitoring is essential for at least 48 hr, because shock may occur or recur precipitously early in the disease. Patients who are cyanotic or have labored breathing should be given oxygen. Rapid intravenous replacement of fluids and electrolytes can frequently sustain patients until spontaneous recovery occurs. Normal saline is more effective in treating shock than the more expensive Ringer lactated saline. When pulse pressure is ≤10 mm Hg or when elevation of the hematocrit persists after replacement of fluids, plasma or colloid preparations are indicated.
Care must be taken to avoid overhydration, which may contribute to cardiac failure. Transfusions of fresh blood or platelets suspended in plasma may be required to control bleeding; they should not be given during hemoconcentration, but only after evaluation of hemoglobin or hematocrit values. Salicylates are contraindicated because of their effect on blood clotting.
Sedation may be required for children who are markedly agitated. Use of vasopressors has not resulted in a significant reduction of mortality over that observed with simple supportive therapy. Disseminated intravascular coagulation may require treatment (Chapter 477). Corticosteroids do not shorten the duration of disease or improve prognosis in children receiving careful supportive therapy.
Hypervolemia during the fluid reabsorptive phase may be life threatening and is heralded by a decrease in hematocrit with wide pulse pressure. Diuretics and digitalization may be necessary.
Complications
Primary infections with dengue fever and dengue-like diseases are usually self-limited and benign. Fluid and electrolyte losses, hyperpyrexia, and febrile convulsions are the most frequent complications in infants and young children. Epistaxis, petechiae, and purpuric lesions are uncommon but may occur at any stage. Swallowed blood from epistaxis, vomited or passed by rectum, may be erroneously interpreted as gastrointestinal bleeding. In adults and possibly in children, underlying conditions may lead to clinically significant bleeding. Convulsions may occur during high temperature, especially with chikungunya fever. Infrequently, after the febrile stage, prolonged asthenia, mental depression, bradycardia, and ventricular extrasystoles may occur in children.
In endemic areas, dengue hemorrhagic fever should be suspected in children with a febrile illness suggestive of dengue fever who experience hemoconcentration and thrombocytopenia.
Prognosis
The prognosis of dengue fever may be adversely affected by passively acquired antibody or by prior infection with a closely related virus that predisposes to development of dengue hemorrhagic fever.
Dengue Hemorrhagic Fever
Death has occurred in 40-50% of patients with shock, but with adequate intensive care deaths should occur in <1% of cases. Survival is directly related to early and intense supportive treatment. Infrequently, there is residual brain damage due to prolonged shock or occasionally to intracranial hemorrhage.
Prevention
Several types of dengue type 1-4 vaccines are under development, and a killed vaccine for chikungunya is efficacious but not licensed. Prophylaxis consists of avoiding mosquito bites through the use of insecticides, repellents, body covering with clothing, screening of houses, and destruction of A. aegypti breeding sites. If water storage is mandatory, a tight-fitting lid or a thin layer of oil may prevent egg laying or hatching. A larvicide, such as Abate [O,O′-(thiodi-p-phenylene) O,O,O,O′-tetramethyl phosphorothioate], available as a 1% sand-granule formation and effective at a concentration of 1 ppm, may be added safely to drinking water. Ultra-low-volume spray equipment effectively dispenses the adulticide malathion from truck or airplane for rapid intervention during an epidemic. Only personal anti-mosquito measures are effective against mosquitoes in the field, forest, or jungle.
The possibility exists that incomplete dengue vaccination may sensitize a recipient so that ensuing dengue infection could result in hemorrhagic fever. Vaccination with yellow fever 17D strain has no effect on the severity of dengue illness, although seroconversion rates to a dengue type 2 vaccine was enhanced in persons immune to yellow fever.
Bethell DB, Gamble J, Pham PL, et al. Noninvasive measurement of microvascular leakage in patients with dengue hemorrhagic fever. Clin Infect Dis. 2001;32:243-253.
Blaney JEJr, Matro JM, Murphy BR, et al. Recombinant, live-attenuated tetravalent dengue vaccine formulations induce a balanced, broad and protective neutralizing antibody response against each of the four serotypes in rhesus monkeys. J Virol. 2005;79:5516-5528.
Capeding RZ, Brion JD, Caponpon MM, et al. The incidence, characteristics, and presentation of dengue virus infections during infancy. Am J Trop Med Hyg. 2010;82:330-336.
Centers for Disease Control and Prevention. Health advisory—increased potential for dengue infection in travelers returning from international and selected domestic areas. Health Alert Network. July 25, 2010. CDCHAN-00315-2010-07-25-ADV-N
Centers for Disease Control and Prevention. Locally acquired dengue—Key West, Florida, 2009–2010. MMWR Morb Mortal Wkly Rep. 2010;59:577-581.
Centers for Disease Control and Prevention. Travel associated dengue surveillance—United States, 2006–2008. MMWR Morb Mortal Wkly Rep. 2010;59:715-720.
Centers for Disease Control and Prevention. Dengue fever among US travelers returning from the Dominican Republic—Minnesota and Iowa, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:654-656.
Chau TN, Quyen NT, Thuy TT, et al. Dengue in Vietnamese infants—results of infection-enhancement assays correlate with age-related disease epidemiology and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198:518-524.
Dung NM, Day NP, Tam DT, et al. Fluid replacement in dengue shock syndrome: A randomized, double-blind comparison of four intravenous-fluid regimens. Clin Infect Dis. 1999;29:787-794.
Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011;377:849-860.
Guirakhoo F, Pugachev K, Zhang Z, et al. Safety and efficacy of chimeric yellow fever—Dengue virus tetravalent vaccine formulations in nonhuman primates. J Virol. 2004;78:461-475.
Guzman MG, Kouri G. Dengue haemorrhagic fever integral hypothesis: confirming observations, 1987–2007. Trans R Soc Trop Med. 2008;102:522-523.
Halstead SB. Seminar: Dengue. The Lancet. 2007;370:1644-1652.
Kliks SC, Nisalak A, Brandt WE, et al. Evidence that maternal dengue antibodies are important in the development of dengue hemorrhagic fever in infants. Am J Trop Med Hyg. 1988;38:411-419.
Monath TP, McCarthy K, Bedford P, et al. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20:1004-1018.
Růžek D, Yakimenko VV, Karan L, et al. Omsk haemorrhagic fever. Lancet. 2010;376:2104-2112.
Simasthien S, Thomas SJ, Watanaveeradej V, et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus naïve children. Am J Trop Med Hyg. 2008;78:426-433.
Teixeria MG, Barreto ML. Diagnosis and management of dengue. BMJ. 2009;339:1189-1193.
Ubol S, Masrinoul P, Chaijaruwanich J, et al. Differences in global gene expression in peripheral blood mononuclear cells indicate a significant role of the innate responses in progression of dengue fever but not dengue hemorrhagic fever. J Infect Dis. 2008;197:1459-1467.
Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2-9.
Wilder-Smith A, Schwartz E. Dengue in travelers. N Engl J Med. 2005;353:924-933.
Wills BA, Dung NM, Loon HT, et al. Comparison of three fluid solutions for resuscitation in dengue shock syndrome. N Engl J Med. 2005;353:877-889.