Chapter 266 Rabies
Rabies virus is a bullet-shaped, negative-sense, single-stranded, enveloped RNA virus from the family Rhabdoviridae, genus Lyssavirus. There currently are 7 known genotypes of Lyssavirus; more are under taxonomic consideration. The classic rabies virus (genotype 1) is distributed worldwide and naturally infects a large variety of animals. The other 6 genotypes are more geographically confined, with none found in the Americas. All 7 Lyssavirus genotypes have been associated with rabies in humans, although type 1 accounts for the great majority of cases. Within genotype 1, a number of genetic variants have been defined. Each variant is specific to a particular animal reservoir, although cross-species transmission can occur.
Epidemiology
Rabies is present on all continents except Antarctica. Rabies predominantly afflicts under-aged, poor, and geographically isolated populations. Approximately 50,000 cases of human rabies occur in Africa and Asia annually. Theoretically, rabies virus can infect any mammal (which then can transmit disease to humans), but true animal reservoirs that maintain the presence of rabies virus in the population are limited to terrestrial carnivores and bats. Worldwide, transmission from dogs accounts for >90% of human cases. In Africa and Asia, other animals serve as prominent reservoirs, such as jackals, mongooses, and raccoon dogs. In industrialized nations canine rabies has been largely controlled through the routine immunization of pets. In the USA, raccoons are the most commonly infected wild animal along the eastern seaboard. Three phylogenies of skunk rabies are endemic in the Midwest (north and south) and California, and gray foxes harbor rabies in Arizona and Texas and mongooses in Puerto Rico. Rabies occurs infrequently in livestock. Among American domestic pets, infected cats outnumber infected dogs, probably because cats frequently prowl unsupervised and are not uniformly subject to vaccine laws. Rabies is rare in small mammals, including mice, squirrels, and rabbits; to date, no animal-to-human transmission from these animals has been documented.
The epidemiology of human rabies in the USA is dominated by cryptogenic bat rabies. Bats are migratory in the spring and fall; rabid bats are identified in every state of the union except Hawaii. In one study, the largest proportion of cases of human rabies were infected with a bat variant, and in almost all cases of bat-associated human rabies there was no history of a bat bite.
In the USA, 30,000 episodes of rabies postexposure prophylaxis (PEP) occur annually. Between 1 and 3 human cases are diagnosed annually, half postmortem. There have been two outbreaks of rabies associated with solid organ and corneal transplantations.
Transmission
Rabies virus is found in large quantities in the saliva of infected animals, and transmission occurs almost exclusively through inoculation of the infected saliva through a bite or scratch from a rabid mammal. Approximately 35-50% of people bitten by a known rabies-infected animal and receiving no PEP experience rabies. The transmission rate is increased if the victim has suffered multiple bites and if the inoculation occurs in highly innervated parts of the body such as the face and the hands. Infection does not occur after exposure of intact skin to infected secretions, but virus may enter the body through intact mucous membranes. Claims that spelunkers may experience rabies after inhaling bat excreta have come under doubt, although inhalational exposure can occur during laboratory accidents.
No case of nosocomial transmission to a health care worker has been documented to date, but caregivers of a patient with rabies are advised to use full barrier precautions. The virus is rapidly inactivated in the environment, and contamination of fomites is not a mechanism of spread.
Pathogenesis
After inoculation, rabies virus replicates slowly and at low levels in muscle or skin. This slow initial step likely accounts for the disease’s long incubation period. Virus then enters the peripheral motor nerve, utilizing the nicotinic acetylcholine receptor and possibly several other receptors for entry. Once in the nerve, the virus travels by fast axonal transport, crossing synapses roughly every 12 hr. Rapid dissemination occurs throughout the brain and spinal cord before symptoms appear. Infection of the dorsal root ganglia is apparently futile but causes the characteristic radiculitis. Infection concentrates in the brainstem, accounting for autonomic dysfunction and relative sparing of cognition. Despite severe neurologic dysfunction with rabies, histopathology reveals limited damage, inflammation, or apoptosis. The pathologic hallmark of rabies, the Negri body, is composed of clumped viral nucleocapsids that create cytoplasmic inclusions on routine histology. Negri bodies can be absent in documented rabies virus infection. Rabies may be a metabolic disorder of neurotransmission; tetrahydrobiopterin (BH4) deficiency in human rabies causes severe deficiencies in dopamine, norepinephrine, and serotonin metabolism.
After infection of the central nervous system, the virus travels anterograde through the peripheral nervous system to virtually all innervated organs. It is through this route that the virus infects the salivary glands. Many victims of rabies die from uncontrolled cardiac dysrhythmia.
Deficiency of BH4, an essential cofactor for neuronal nitric oxide synthase, is predicted to lead to spasm of the basilar arteries. Onset of vasospasm has been confirmed in a few patients within 5-8 days of first hospitalization, at about the time coma supervenes in the natural history.
Clinical Manifestations
The incubation period for rabies is 1-3 mo but is variable. In severe wounds to the head, symptoms may occur within 5 days after exposure, and occasionally the incubation period can extend to >6 mo. Rabies has 2 principal clinical forms. Encephalitic or “furious” rabies begins with nonspecific symptoms, including fever, sore throat, malaise, headache, nausea and vomiting, and weakness. These symptoms are often accompanied by paresthesias and pruritus at or near the site of the bite that then extend along the affected limb. Soon thereafter the patient begins to demonstrate typical symptoms of severe encephalitis, with agitation, depressed mentation, and occasionally seizures. Characteristically patients with rabies encephalitis initially have periods of lucidity intermittent with periods of profound encephalopathy. Hydrophobia and aerophobia are the cardinal signs of rabies; they are unique to humans and are not universal. Phobic spasms are manifested by agitation and fear created by being offered a drink or fanning of air in the face, which in turn produce choking and aspiration through spasms of the pharynx, neck, and diaphragm. The illness is relentlessly progressive. There is a dissociation of electrophysiologic or encephalographic activity with findings of brainstem coma caused by anterograde denervation. Death almost always occurs within 1-2 days of hospitalization in developing countries and by 18 days of hospitalization with intensive care.
A 2nd form of rabies known as paralytic or “dumb” rabies is seen much less frequently and is characterized principally by fevers and ascending motor weakness affecting both the limbs and the cranial nerves. Most patients with paralytic rabies also have some element of encephalopathy as the disease progresses subacutely.
Differential Diagnosis
The differential diagnosis of rabies encephalitis includes all forms of severe cerebral infections, tetanus, and some intoxications and envenomations. Rabies can be confused with psychiatric illness, drug abuse, and conversion disorders. Paralytic rabies is most frequently confused with Guillain-Barré syndrome. The diagnosis of rabies is frequently delayed in Western countries because of its rarity and the unfamiliarity of the medical staff with the infection. These considerations highlight the need to pursue a history of contact with an animal belonging to one of the known reservoirs for rabies or to establish a travel history to a rabies-endemic region.
Diagnosis
The Centers for Disease Control and Prevention (CDC) require a number of tests to confirm a clinically suspected case of rabies. The virus can be grown both in cell culture and after animal injection, but identification of rabies by these methods is prolonged. Rabies antigen is detected through immunofluorescence of saliva or biopsies of hairy skin or brain. Corneal impressions are not recommended. Rabies virus RNA has been detected in saliva, skin, and brain by the reverse transcription polymerase chain reaction (RT-PCR). RT-PCR is the most sensitive available assay for the diagnosis of rabies when done iteratively. Rabies-specific antibody can be detected in serum or cerebrospinal fluid (CSF) samples, but most patients die while seronegative. Anti-rabies antibodies are present in the sera of patients who have received an incomplete course of the rabies vaccine, precluding a meaningful interpretation in this setting. Antibody in CSF is rarely detected after vaccination and is considered diagnostic of rabies regardless of immunization status. CSF abnormalities in cell count, glucose, and protein content are minimal and are not diagnostic. MRI findings in the brain are late.
Treatment and Prognosis
Rabies is generally fatal. There are 3 survivors from rabies infection through the use of the Milwaukee Protocol (www.mcw.edu/rabies). Deep sedation is induced to avoid dysautonomia while the immune response develops. Survival is estimated at 20%; neurologic outcome can be very good. Among 6 survivors of rabies after failure of vaccine prophylaxis, only 2 had a satisfactory neurologic outcome. Neither rabies immune globulin (RIG) nor rabies vaccine alters the course of disease once symptoms have appeared. Antiviral treatments have not been effective. Ribavirin, which delays the immune response, is to be avoided during early management. In contrast, appearance of the normal antibody response at 7-10 days is associated with clearance of salivary viral load. Nimodipine is recommended as prophylaxis against cerebrovascular spasm.
Prevention
Primary prevention of rabies infection includes vaccination of domestic animals and education to avoid wild animals, stray animals, and animals with unusual behavior.
Immunization and Fertility Control of Animal Reservoirs
The introduction of routine rabies immunization for domestic pets in the USA and Europe during the middle of the 20th century virtually eliminated infection in dogs, which prior to that time had been the principal transmitter of rabies to humans in developed as well as nonindustrialized countries. Since the 1990s, efforts in Europe and North America have shifted to immunization of wildlife reservoirs of rabies, where rabies is newly emerging. These programs have employed bait laced with either an attenuated rabies vaccine or a recombinant rabies surface glycoprotein inserted into vaccinia, distributed by air or hand into areas inhabited by rabid animals. Human contact with vaccine-laden bait has occurred infrequently. Adverse events after such contact have been rare, but the vaccinia vector poses a threat to the same population at risk for vaccinia itself, namely, pregnant women, immunocompromised persons, and people with chronic dermatologic conditions. Mass culling of endemic reservoirs has never worked; vaccination and fertility control abort outbreaks. Bats are ubiquitous and very important for insect control. Less than 1% of free-flying bats but >8% of downed bats and bats found in dwellings are rabid.
Postexposure Prophylaxis
The relevance of rabies for most pediatricians centers on evaluating whether an animal exposure warrants PEP (Table 266-1). No case of rabies has been documented in a person receiving the fully recommended schedule of PEP since introduction of modern cellular vaccines in the 1970s.
Table 266-1 RABIES POSTEXPOSURE PROPHYLAXIS GUIDE
| ANIMAL TYPE | EVALUATION AND DISPOSITION OF ANIMAL | POSTEXPOSURE PROPHYLAXIS RECOMMENDATIONS |
|---|---|---|
| Dogs, cats, and ferrets | Healthy and available for 10 days of observation | Prophylaxis only if animal shows signs of rabies* |
| Rabid or suspected of being rabid† | Immediate immunization and RIG | |
| Unknown (escaped) | Consult public health officials for advice | |
| Bats, skunks, raccoons, foxes, and most other carnivores; woodchucks | Regarded as rabid unless geographic area is known to be free of rabies or until animal proven negative by laboratory tests† | Immediate immunization and RIG |
| Livestock, rodents, and lagomorphs (rabbits, hares, and pikas) | Consider individually | Consult public health officials. Bites of squirrels, hamsters, guinea pigs, gerbils, chipmunks, rats, mice and other rodents, rabbits, hares, and pikas almost never require antirabies treatment. |
RIG, rabies immune globulin.
* During the 10-day observation period, at the first sign of rabies in the biting dog, cat, or ferret, treatment of the exposed person with RIG (human) and vaccine should be initiated. The animal should be euthanized immediately and tested.
† The animal should be euthanized and tested as soon as possible. Holding for observation is not recommended. Immunization is discontinued if immunofluorescent test result for the animal is negative.
From American Academy of Pediatrics: Red book 2009: report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL, 2009, American Academy of Pediatrics.
Given the incubation period for rabies, PEP is a medical urgency, not emergency. Algorithms have been devised to aid practitioners in deciding when to initiate rabies PEP (Fig. 266-1). The decision to proceed ultimately depends on the local epidemiology of animal rabies as determined by active surveillance programs, information that can be obtained from local and state health departments. In general, bats, raccoons, skunks, coyotes, and foxes should be considered rabid unless proven otherwise through euthanasia and testing of brain tissue, whereas bites from small herbivorous animals (squirrels, hamsters, gerbils, chipmunks, rats, mice, and rabbits) can be discounted. The response to bites from a pet, particularly a dog, cat, or ferret, depends on local surveillance statistics and on whether the animal is available for observation.
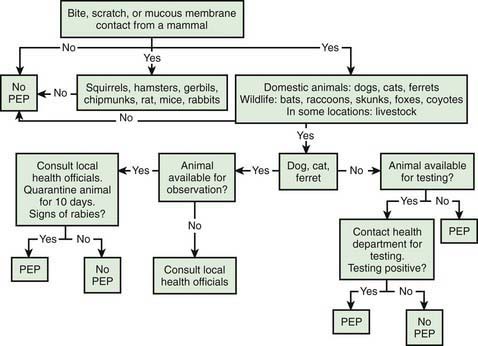
Figure 266-1 Algorithm for evaluating a child for rabies postexposure prophylaxis (PEP). This and any other algorithm should be used in concert with local epidemiologic information regarding the incidence of animal rabies in any given location.
The approach to nonbite bat exposures is controversial. In response to the observation that most cases of rabies in the USA have been caused by bat variants and that the majority of affected patients had no recollection of a bat bite, the CDC has recommended that rabies PEP be considered after any physical contact with bats and when a bat is found in the same room as persons who may not be able to accurately report a bite, assuming that the animal is unavailable for testing. Such people include young children, the mentally disabled, and intoxicated individuals. Other nonbite contacts (e.g., handling a carcass, exposure to an animal playing with a carcass, or coming into contact with blood or excreta from a potentially rabid animal) usually do not require PEP.
In all instances of a legitimate exposure, effort should be made to recover the animal for quarantine and observation or brain examination after euthanasia. Testing obviates the need for PEP over half the time. In most instances PEP can be deferred until the results of observation or brain histology are known. In dogs, cats, and ferrets, symptoms of rabies always occur within several days of viral shedding; therefore, in these animals a 10-day observation period is sufficient to eliminate the possibility of rabies.
No duration of time between exposure and onset of symptoms should preclude rabies prophylaxis. Rabies PEP is most effective when applied expeditiously. Nevertheless, the series should be begun in the asymptomatic person as soon as possible, regardless of the length of time since the bite. The vaccine and RIG are contraindicated once symptoms develop.
The 1st step in rabies PEP is to cleanse the wound thoroughly. Soapy water is sufficient for an enveloped virus, and its effectiveness is supported by broad experience. Other commonly used disinfectants, such as iodine-containing preparations, are virucidal and should be used in addition to soap when available. Probably the most important aspect of this component is that the wound is cleansed with copious volumes of disinfectant and primary closure is avoided. Antibiotics and tetanus prophylaxis (Chapter 203) should be applied with the use of usual wound care criteria.
The 2nd component of rabies PEP consists of passive immunization with RIG. Most failures of PEP are attributed to not using RIG. Human RIG, the formulation used in industrialized countries, is administered at a dose of 20 IU/kg. As much of the dose is infused around the wound as possible, and the remainder is injected intramuscularly in a limb distant from the one injected with the killed vaccine. Like other immune globulin preparations, RIG interferes with the take of live viral vaccines for at least 4 mo after administration of the RIG dose. Human RIG is not available in many parts of the developing world. Equine RIG serves as a substitute for the human immune globulin preparation in some areas. Modern preparations of equine RIG are associated with fewer side effects than prior products composed of crude horse serum. Regrettably, for a large segment of the world’s population, no passive immunization product is available at all. Monoclonal antibody products are in clinical trials and may alleviate this deficiency.
The 3rd component of rabies PEP is immunization with inactivated vaccine. In the developed world, cell-based vaccines have replaced previous preparations. Two formulations currently are available in the USA, namely, RabAvert (Chiron Behring Vaccines, Maharashtra, India), a purified chick-embryo cell cultivated vaccine, and Imovax Rabies (Aventis Pasteur, Bridgewater, NJ), cultivated in human diploid cell cultures. In both children and adults, both vaccines are administered intramuscularly in a 1-mL volume in the deltoid or anterolateral thigh on days 0, 3, 7, and 14 after presentation. Injection into the gluteal area has been associated with a blunted antibody response, so this area should not be used. The rabies vaccines can be safely administered during pregnancy. In most persons the vaccine is well tolerated; most adverse effects are related to booster doses. Pain and erythema at the injection site occur commonly, and local adenopathy, headache, and myalgias occur in 10-20% of patients. Approximately 5% of patients who receive the human diploid cell vaccine experience an immune complex–mediated allergic reaction, including rash, edema, and arthralgias, several days after a booster dose. The World Health Organization (WHO) has approved schedules using smaller amounts of vaccine, administered intradermally, that are immunogenic and protective (www.who.int/rabies/human/postexp/en/), but none is approved for use in the USA. Other cell culture–derived rabies virus vaccines are available in the developing world. A few countries still produce nerve tissue–derived vaccines; these preparations are poorly immunogenic, and cross reactivity with human nervous tissue may occur with their use, producing severe neurologic symptoms even in the absence of rabies infection.
Pre-Exposure Prophylaxis
The killed rabies vaccine can be given to prevent rabies in persons at high risk for exposure to wild-type virus, including laboratory personnel working with rabies virus, veterinarians, and others likely to be exposed to rabid animals as part of their occupation. Pre-exposure prophylaxis should be considered for persons traveling to a rabies-endemic region where there is a credible risk for a bite or scratch from a rabies-infected animal, particularly if there is likely to be a shortage of RIG or cell culture–based vaccine (Chapter 168). Rabies vaccine as part of the routine vaccine series is under investigation in some countries. The schedule for pre-exposure prophylaxis consists of 3 intramuscular injections on days 0, 7, and 21 or 28. PEP in the patient who has received pre-exposure prophylaxis or a prior full schedule of PEP consists of 2 doses of vaccine (1 each on days 0 and 3) and does not require RIG. Immunity from pre-exposure prophylaxis wanes after several years and requires boosting if the potential for exposure to rabid animals recurs.
Bakker AB, Python C, Kissling CJ, et al. First administration to humans of a monoclonal antibody cocktail against rabies virus: safety, tolerability, and neutralizing activity. Vaccine. 2008;26:5922-5927.
Centers for Disease Control and Prevention. Public health response to a rabid dog in an animal shelter—North Dakota and Minnesota, 2010. MMWR. 2011;59(51):1678-1680.
Centers for Disease Control and Prevention. Use of reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies. MMWR Morb Mortal Wkly Rep. 2010;59:1-9.
Centers for Disease Control and Prevention. Presumptive abortive human rabies—Texas, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:185-190.
Centers for Disease Control and Prevention. Human vaccinia infection after contact with a raccoon rabies vaccine bait—Pennsylvania, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1204-1207.
Centers for Disease Control and Prevention. Human exposures to a rabid bat—Montana, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:557-560.
De Serres G, Skowronski DM, Mimault P, et al. Bats in the bedroom, bats in the belfry: reanalysis of the rationale for rabies postexposure prophylaxis. Clin Infect Dis. 2009;48:1493-1499.
Hampson K, Dobson A, Kaare M, et al. Rabies exposures, post-exposure prophylaxis and deaths in a region of endemic canine rabies. PLoS Negl Trop Dis. 2008;2:e339.
Hu WT, Willoughby REJr, Dhonau H, et al. Long-term follow-up after treatment of rabies by induction of coma. N Engl J Med. 2007;357:945-946.
Manning SE, Rupprecht CE, Fishbein D, et al. Human rabies prevention—United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2008;57(RR-3):1-28.
Mattner F, Henke-Gendo C, Martens A, et al. Risk of rabies infection and adverse effects of postexposure prophylaxis in healthcare workers and other patient contacts exposed to a rabies virus-infected lung transplant recipient. Infect Control Hosp Epidemiol. 2007;28:513-518.
Pengsaa K, Limkittikul K, Sabchareon A, et al. A three-year clinical study on immunogenicity, safety, and booster response of purified chick embryo cell rabies vaccine administered intramuscularly or intradermally to 12- to 18-month-old Thai children, concomitantly with Japanese encephalitis vaccine. Pediatr Infect Dis J. 2009;28:335-337.
Velasco-Villa A, Reeder SA, Orciari LA, et al. Enzootic rabies elimination from dogs and reemergence in wild terrestrial carnivores, United States. Emerg Infect Dis. 2008;14:1849-1854.
Willoughby REJr, Opladen T, Maier T, et al. Tetrahydrobiopterin deficiency in human rabies. J Inherit Metab Dis. 2008;32:65-68.
Willoughby RE, Roy-Burman A, Martin KW, et al. Generalized cranial artery spasm in human rabies. Dev Biol (Basel). 2008;131:367-375.