Chapter 273 Amebiasis
Entamoeba histolytica infects up to 10% of the world’s population; endemic foci are particularly common in the tropics, especially in areas with low socioeconomic and sanitary standards. In most infected individuals, E. histolytica parasitizes the lumen of the gastrointestinal tract and causes few symptoms or sequelae. The 2 most common forms of disease caused by E. histolytica are amebic colitis with parasitic invasion of the intestinal mucosa and amebic liver abscess with dissemination of the parasite to the liver.
Etiology
Two morphologically identical but genetically distinct species of Entamoeba commonly infect humans. Entamoeba dispar, the more prevalent species, does not cause symptomatic disease. E. histolytica, the pathogenic species, causes a spectrum of disease and can become invasive. Patients previously described as asymptomatic carriers of E. histolytica based on microscopy findings were likely harboring E. dispar. Five other species of nonpathogenic Entamoeba can colonize the human gastrointestinal tract: E. coli, E. hartmanni, E. gingivalis, E. moshkovskii, and E. polecki.
Infection is acquired through the ingestion of parasite cysts, which measure 10-18 mm in diameter and contain 4 nuclei. Cysts are resistant to harsh environmental conditions including the concentrations of chlorine commonly used in water purification but can be killed by heating to 55°C. After ingestion, cysts are resistant to gastric acidity and digestive enzymes and germinate in the small intestine to form trophozoites. These large, actively motile organisms colonize the lumen of the large intestine and may invade the mucosal lining. Infection is not transmitted by trophozoites, as these rapidly degenerate outside the body and are unable to survive the low pH of the stomach if swallowed.
Epidemiology
Prevalence of infection with E. histolytica varies greatly depending on region and socioeconomic status. Most prevalence studies have not distinguished between E. histolytica and E. dispar, and thus the true prevalence of E. histolytica infection is not known. It is estimated that infection with E. histolytica leads to 50 million cases of symptomatic disease and 40,000-110,000 deaths annually. Amebiasis is the 3rd leading parasitic cause of death worldwide. Prospective studies have shown that 4-10% of individuals infected with E. histolytica develop amebic colitis and that <1% of infected individuals develop disseminated disease, including amebic liver abscess. These numbers vary by region; for example, in South Africa and Vietnam, liver abscesses form a disproportionately large number of the cases of invasive disease due to E. histolytica. Amebic liver abscesses are rare in children and occur equally in male and female children; in adults, amebic liver abscesses occur predominantly in men.
Amebiasis is endemic to Africa, Latin America, India, and Southeast Asia. In the USA, amebiasis is seen most frequently in immigrants from and in travelers to developing countries. Residents of mental health institutions and men who have sex with men are also at increased risk for invasive amebiasis. Food or drink contaminated with Entamoeba cysts and direct fecal-oral contact are the most common means of infection. Untreated water and night soil (human feces used as fertilizer) are important sources of infection. Food handlers shedding amebic cysts play a role in spreading infection. Direct contact with infected feces also results in person-to-person transmission.
Pathogenesis
Trophozoites are responsible for tissue invasion and destruction. These attach to colonic epithelial cells by a galactose and N-acetyl-D-galactosamine (Gal/GalNac)-specific lectin. This lectin is also thought to be responsible for resistance to complement-mediated lysis. Once attached to the colonic mucosa, amebae release proteinases that allow for penetration through the epithelial layer. Host cells are destroyed by cytolysis and apoptosis. Cytolysis is mediated by trophozoite release of amoebapores (pore-forming proteins), phospholipases, and hemolysins. Amoebapores may also be partially responsible for the induction of apoptosis that occurs in mice with amebic liver disease and colitis. Early invasive amebiasis produces significant inflammation, due in part to parasite-mediated activation of nuclear factor-κB (NF-κB). Once E. histolytica trophozoites invade the intestinal mucosa, the organisms multiply and spread laterally underneath the intestinal epithelium to produce the characteristic flask-shaped ulcers. Amebae produce similar lytic lesions if they reach the liver. These lesions are commonly called abscesses, although they contain no granulocytes. Well-established ulcers and amebic liver abscesses demonstrate little local inflammatory response.
Immunity to infection is associated with a mucosal secretory IgA response against the Gal/GalNac lectin. Neutrophils appear to be important in initial host defense, but E. histolytica–induced epithelial cell damage releases neutrophil chemoattractants, and E. histolytica is able to kill neutrophils, which then release mediators that further damage epithelial cells. The disparity between the extent of tissue destruction by amebae and the absence of a local host inflammatory response in the presence of systemic humoral (antibody) and cell-mediated responses may reflect both parasite-mediated apoptosis and the ability of the trophozoite to kill not only epithelial cells but neutrophils, monocytes, and macrophages.
The sequencing of the E. histolytica genome has led to further insights into the pathogenesis of E. histolytica disease. The genome is functionally tetraploid, and there is evidence of lateral gene transfer from bacteria. It has been demonstrated that the amoebapore-A (Ap-A) gene along with other important genes can be epigenetically silenced using plasmids with specifically engineered sequences or short hairpin RNAs. Transcriptional profiling using proteomics and microarrays have likewise identified several candidate virulence factors. Several classes of proteases that may be associated pathogenesis have been identified, including a novel E. histolytica rhomboid protease 1 (EhROM1), which may be involved in immune evasion.
Clinical Manifestations
Clinical presentations range from asymptomatic cyst passage to amebic colitis, amebic dysentery, ameboma, and extraintestinal disease. E. histolytica infection is asymptomatic in about 90% of persons but has the potential to become invasive and should be treated. Severe disease is more common in young children, pregnant women, malnourished individuals, and persons taking corticosteroids. Extraintestinal disease usually involves the liver, but less common extraintestinal manifestations include amebic brain abscess, pleuropulmonary disease, ulcerative skin, and genitourinary lesions.
Amebic Colitis
Amebic colitis may occur within 2 wk of infection or may be delayed for months. The onset is usually gradual, with colicky abdominal pains and frequent bowel movements (6-8/day). Diarrhea is frequently associated with tenesmus. Almost all stool is heme-positive, but most patients do not present with grossly bloody stools. Generalized constitutional symptoms and signs are characteristically absent, with fever documented in only one third of patients. Amebic colitis affects all age groups, but its incidence is strikingly high in children 1-5 yr of age. Severe amebic colitis in infants and young children tends to be rapidly progressive with more frequent extraintestinal involvement and high mortality rates, particularly in tropical countries. Amebic dysentery can result in dehydration and electrolyte disturbances.
Amebic Liver Abscess
Amebic liver abscess, a serious manifestation of disseminated infection, is uncommon in children. Although diffuse liver enlargement has been associated with intestinal amebiasis, liver abscesses occur in <1% of infected individuals and may appear in patients with no clear history of intestinal disease. Amebic liver abscess may occur months to years after exposure, so obtaining a careful travel history is critical. In children, fever is the hallmark of amebic liver abscess and is frequently associated with abdominal pain, distention, and enlargement and tenderness of the liver. Changes at the base of the right lung, such as elevation of the diaphragm and atelectasis or effusion, may also occur.
HIV Co-Infection
Though some studies have shown higher rates of amebic colonization in HIV-positive men in East Asia compared to HIV-uninfected men, this association was not found in other areas of the world. While cellular immune dysfunction associated with HIV infection may, in turn affect humoral immunity, the higher prevalence of amebiasis in this group of patients is more likely due to higher intestinal colonization rates rather than a true association with immunodeficiency.
Laboratory Findings
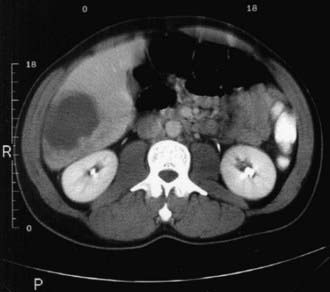
Laboratory examination findings are often unremarkable in uncomplicated amebic colitis, although mild anemia may be seen. Laboratory findings in amebic liver abscess are a slight leukocytosis, moderate anemia, high erythrocyte sedimentation rate, and elevations of hepatic enzyme (particularly alkaline phosphatase) levels. Stool examination for amebae is negative in more than half of patients with documented amebic liver abscess. Ultrasonography, CT, or MRI can localize and delineate the size of the abscess cavity (Fig. 273-1). The most common finding is a single abscess in the right hepatic lobe in about a half of these cases. Higher resolution ultrasound and CT studies have shown that left lobe abscess and multiple abscesses occur more often than previously recognized.
Diagnosis and Differential Diagnosis
A diagnosis of amebic colitis is made in the presence of compatible symptoms with detection of E. histolytica antigens in stool. This approach has a greater than 95% sensitivity and specificity and coupled with a positive serology test is the most accurate means of diagnosis in developed countries. The E. histolytica II stool antigen detection test (TechLab, Blacksburg, VA) is able to distinguish E. histolytica from E. dispar infection. Microscopic examination of stool samples has a sensitivity of 60%. Sensitivity can be increased to 85-95% by examining 3 stools, since excretion of cysts can be intermittent. However, microscopy cannot differentiate between E. histolytica and E. dispar unless phagocytosed erythrocytes (specific for E. histolytica) are seen. In highly endemic areas, trophozoites without phagocytosed erythrocytes may reflect co-infection with E. dispar in a patient with another cause of colitis, such as shigellosis. Endoscopy and biopsies of suspicious areas should be performed when stool sample results are negative and suspicion for amebiasis remains high.
Various serum antiamebic antibody tests are available. Serologic results are positive in 70-80% of patients with invasive disease (colitis or liver abscess) at presentation and in >90% of patients after 7 days of disease symptoms. The most sensitive serologic test, indirect hemagglutination, yields a positive result even years after invasive infection. Therefore, many uninfected adults and children in highly endemic areas demonstrate antibodies to E. histolytica. Polymerase chain reaction (PCR) detection in stool of E. histolytica is also able to distinguish E. histolytica from E. dispar but is less sensitive (72%) than the stool antigen test. Rapid antigen and antibody tests for bedside diagnosis in the developing world have been developed and are currently being tested. In addition, a loop-mediated isothermal amplification assay (LAMP) that can be optimized for field use is under development.
The differential diagnosis for amebic colitis includes colitis due to bacterial (Shigella, Salmonella, enteropathogenic Escherichia coli, Campylobacter, Yersinia, Clostridium difficile), mycobacterial (tuberculosis and atypical mycobacteria), and viral (cytomegalovirus) pathogens, as well as noninfectious causes such as inflammatory bowel disease (IBD). Pyogenic liver abscess due to bacterial infection, hepatoma, and echinococcal cysts are in the differential for amebic liver abscess. However, echinococcal cysts are rarely associated with systemic symptoms such as fever unless there is cyst rupture or leakage.
Complications
Complications of amebic colitis include acute necrotizing colitis, ameboma, toxic megacolon, extraintestinal extension, or local perforation and peritonitis. Less commonly, a chronic form of amebic colitis develops, often recurring over several years. Amebomas are nodular foci of proliferative inflammation that sometimes develops in the wall of the colon. Chronic amebiasis should be excluded before initiating corticosteroid treatment for IBD, as corticosteroid therapy given during active amebic colitis is associated with high mortality rates.
An amebic liver abscess may rupture into the peritoneum, pleural cavity, skin, and pericardium. Cases of amebic abscesses in extrahepatic sites, including the lung and brain, have been reported.
Treatment
Invasive amebiasis is treated with a nitroimidazole such as metronidazole or tinidazole and then a luminal amebicide (Table 273-1). Tinidazole has similar efficacy to metronidazole with shorter and simpler dosing and less frequent adverse effects. These adverse effects include nausea, abdominal discomfort, and a metallic taste that disappears after completion of therapy. Therapy with a nitroimidazole should be followed by treatment with a luminal agent, such as paromomycin (which is preferred) or iodoquinol. Diloxanide furoate can also be used in children >2 yr of age, but it is no longer available in the USA. Paromomycin should not be given concurrently with metronidazole or tinidazole, because diarrhea is a common side effect of paromomycin and may confuse the clinical picture. Asymptomatic intestinal infection with E. histolytica should be treated preferably with paromomycin or alternatively with either iodoquinol or diloxanide furoate. For fulminant cases of amebic colitis, some experts suggest adding dehydroemetine (1 mg/kg/day subcutaneously or IM, never IV), available only through the Centers for Disease Control and Prevention. Patients should be hospitalized for monitoring if dehydroemetine is administered. Dehydroemetine should be discontinued if tachycardia, T-wave depression, arrhythmia, or proteinuria develops.
Table 273-1 DRUG TREATMENT FOR AMEBIASIS
| MEDICATION | ADULT DOSAGE (ORAL) | PEDIATRIC DOSAGE (ORAL)* |
|---|---|---|
| INVASIVE DISEASE | ||
| Metronidazole | Colitis or liver abscess: 750 mg tid for 7-10 days | Colitis or liver abscess: 35-50 mg/kg/day in 3 divided doses for 7-10 days |
| or | ||
| Tinidazole | Colitis: 2 g once daily for 3 days | Colitis: 50 mg/kg/day once daily for 3 days |
| Liver abscess: 2 g once daily for 3-5 days | Liver abscess: 50 mg/kg/day once daily for 3-5 days | |
| Followed by: | ||
| Paromomycin (preferred) | 500 mg tid for 7 days | 25-35 mg/kg/day in 3 divided doses for 7 days |
| or | ||
| Diloxanide furoate† or | 500 mg tid for 10 days | 20 mg/kg/day in 3 divided doses for 7 days |
| Iodoquinol | 650 mg tid for 20 days | 30-40 mg/kg/day in 3 divided doses for 20 days |
| ASYMPTOMATIC INTESTINAL COLONIZATION | ||
| Paromomycin (preferred) | As for invasive disease | As for invasive disease |
| or | ||
| Diloxanide furoate† | ||
| or | ||
| Iodoquinol | ||
* All pediatric dosages are up to a maximum of the adult dose.
Broad-spectrum antibiotic therapy may be indicated in fulminant colitis to cover possible spillage of intestinal bacteria into the peritoneum and translocation into the bloodstream. Intestinal perforation and toxic megacolon are indications for surgery. In amebic liver abscess, image-guided aspiration of large lesions or left lobe abscesses may be necessary if rupture is imminent or if the patient shows a poor clinical response 4-6 days after administration of amebicidal drugs. A Cochrane meta-analysis comparing metronidazole and metronidazole plus aspiration in uncomplicated amebic liver abscess showed that there is insufficient evidence to make any recommendation for or against this approach. Chloroquine, which concentrates in the liver, may also be a useful adjunct to nitroimidazoles in the treatment of amebic liver abscess. To confirm cure, stool examination should be repeated every 2 wk following completion of therapy until clear.
Prognosis
Most infections evolve to either an asymptomatic carrier state or eradication. Extraintestinal infection carries about a 5% mortality rate.
Prevention
Control of amebiasis can be achieved by exercising proper sanitation and avoiding fecal-oral transmission. Regular examination of food handlers and thorough investigation of diarrheal episodes may help identify the source of infection No prophylactic drug or vaccine is currently available for amebiasis. Immunization with a combination of Gal/GalNAc lectin and CpG oligodeoxynucleotides has been shown to be protective to amebic trophozoite challenge in animals.
Baxt LA, Singh U. New insights into Entamoeba histolytica pathogenesis. Curr Opin Infect Dis. 2008;21:489-494.
Bercu TE, Petri WA, Behm JW. Amebic colitis: new insights into pathogenesis and treatment. Curr Gastroenterol Rep. 2007;9:429-433.
Carrero JC, Cervantes-Rebolledo C, Aguilar-Díaz H, et al. The role of the secretory immune response in the infection by Entamoeba histolytica. Parasite Immunol. 2007;29:331-338.
Chavez-Tapia NC, Hernandez-Calleros J, Tellez-Avila FI, et al: Image-guided percutaneous procedure plus metronidazole versus metronidazole alone for uncomplicated amoebic liver abscess, Cochrane Database Syst Rev 1:CD004886, 2009.
Fotedar R, Stark D, Beebe N, et al. Laboratory diagnostic techniques for Entamoeba species. Clin Microbiol Rev. 2007;20:511-532.
Karp CL, Auwaerter PG. Coinfection with HIV and tropical infectious diseases. I. Protozoal pathogens. Clin Infect Dis. 2007;45:1208-1213.
Liang SY, Chan YH, Hsia KT, et al. Development of loop-mediated isothermal amplification assay for diagnosis of Entamoeba histolytica. J Clin Microbiol. 2009;47:1892-1895.
Santi-Rocca J, Rigothier MC, Guillén N. Host-microbe interactions and defense mechanisms in the development of amoebic liver abscesses. Clin Microbiol Rev. 2009;22:65-75.