Chapter 284 Hookworms (Necator americanus and Ancylostoma spp.)
Etiology
Two major genera of hookworms, which are nematodes or roundworms, infect humans. Necator americanus, the only representative of its genus, is a major anthropophilic hookworm and is the most common cause of human hookworm infection. Hookworms of the genus Ancylostoma includes the major anthropophilic hookworm Ancylostoma duodenale that also causes classic hookworm infection and the less common zoonotic species A. ceylanicum, A. caninum, and A. braziliense. Human zoonotic infection with the dog hookworm A. caninum is associated with an eosinophilic enteritis syndrome. The larval stage of A. braziliense, whose definitive hosts include dogs and cats, is the principal cause of cutaneous larva migrans.
The infective larval stages of the anthropophilic hookworms live in a developmentally arrested state in warm, moist soil. Larvae infect humans either by penetrating through the skin (N. americanus and A. duodenale) or when they are ingested (A. duodenale). Larvae entering the human host by skin penetration undergo extraintestinal migration through the venous circulation and lungs before they are swallowed, whereas orally ingested larvae may undergo extraintestinal migration or remain in the gastrointestinal tract. Larvae returning to the small intestine undergo 2 molts to become adult sexually mature male and female worms ranging in length from 5 to 13 mm. The buccal capsule of the adult hookworm is armed with cutting plates (N. americanus) or teeth (A. duodenale) to facilitate attachment to the mucosa and submucosa of the small intestine. Hookworms can remain in the intestine for 1-5 yr, where they mate and produce eggs. Although approximately 2 mo is required for the larval stages of hookworms to undergo extraintestinal migration and develop into mature adults, A. duodenale larvae may remain developmentally arrested for many months before resuming development in the intestine. Mature A. duodenale female worms produce about 30,000 eggs/day; daily egg production by N. americanus is <10,000/day (Fig. 284-1). The eggs are thin shelled and ovoid, measuring approximately 40-60 µm. Eggs that are deposited on soil with adequate moisture and shade develop into 1st stage larvae and hatch. Over the ensuing several days and under appropriate conditions, the larvae molt twice to the infective stage. Infective larvae are developmentally arrested and nonfeeding. They migrate vertically in the soil until they either infect a new host or exhaust their lipid metabolic reserves and die.
Epidemiology
Hookworm infection is one of the most prevalent infectious diseases of humans, affecting an estimated 576 million individuals worldwide. Because of the requirement for adequate soil moisture, shade, and warmth, hookworm infection is usually confined to rural areas, especially where human feces are used for fertilizer or where sanitation is inadequate. Hookworm is an infection associated with economic underdevelopment and poverty throughout the tropics and subtropics. Sub-Saharan Africa, East Asia, and tropical regions of the Americas have the highest prevalence of hookworm infection. High rates of infection are often associated with cultivation of certain agricultural products such as tea in India; sweet potato, corn, cotton, and mulberry trees in China; coffee in Central and South America; and rubber in Africa. It is not uncommon to find dual N. americanus and A. duodenale infections. N. americanus predominates in Central and South America as well as in Southern China and southeast Asia, whereas A. duodenale predominates in North Africa, in northern India, in China north of the Yangtze River, and among aboriginal people in western Australia. The ability of A. duodenale to withstand somewhat harsher environmental and climatic conditions may reflect its ability to undergo arrested development in human tissues. A. ceylanicum infection occurs in India and southeast Asia.
Eosinophilic enteritis caused by A. caninum was 1st described in Queensland, Australia, with 2 reported cases in the USA. Because of its global distribution in dogs, it was initially anticipated that human A. caninum infections would be identified in many locales, but this has not been found.
Pathogenesis
The major morbidity of human hookworm infection is a direct result of intestinal blood loss. Adult hookworms adhere tenaciously to the mucosa and submucosa of the proximal small intestine by using their cutting plates or teeth and a muscular esophagus that creates negative pressure in their buccal capsules. At the attachment site, host inflammation is downregulated by the release of antiinflammatory polypeptides by the hookworm. Rupture of capillaries in the lamina propria is followed by blood extravasation, with some of the blood ingested directly by the hookworm. After ingestion, the blood is anticoagulated, the red blood cells are lysed, and the hemoglobin released and digested. Each adult A. duodenale hookworm causes loss of an estimated 0.2 mL of blood/day; blood loss is less for N. americanus. Individuals with light infections suffer from very little blood loss and, consequently, may have hookworm infection but not hookworm disease. There is a direct correlation between the number of adult hookworms in the gut and the volume of fecal blood loss. Hookworm disease results only when individuals with moderate and heavy infections experience sufficient blood loss to develop iron deficiency and anemia. Hypoalbuminemia and consequent edema and anasarca from the loss of intravascular oncotic pressure can also occur. These features depend heavily on the dietary reserves of the host.
Clinical Manifestations
Chronically infected children with moderate and heavy hookworm infections suffer from intestinal blood loss that results in iron deficiency and can lead to anemia as well as protein malnutrition. Prolonged iron deficiency associated with hookworms in childhood can lead to physical growth retardation and cognitive and intellectual deficits.
Anthropophilic hookworm larvae elicit dermatitis sometimes referred to as ground itch when they penetrate human skin. The vesiculation and edema of ground itch are exacerbated by repeated infection. Infection with a zoonotic hookworm, especially A. braziliense, can result in lateral migration of the larvae to cause the characteristic cutaneous tracts of cutaneous larva migrans (Chapter 284.1). Cough subsequently occurs in A. duodenale and N. americanus hookworm infection when larvae migrate through the lungs to cause laryngotracheobronchitis, usually about 1 wk after exposure. Pharyngitis also can occur.
Intestinal hookworm infection may occur without specific gastrointestinal complaints, although pain, anorexia, and diarrhea have been attributed to the presence of hookworms. Eosinophilia is often 1st noticed in early gastrointestinal infection. The major clinical manifestations are related to intestinal blood loss. Heavily infected children exhibit all of the signs and symptoms of iron deficiency anemia and protein malnutrition. In some cases, children with chronic hookworm disease acquire a yellow-green pallor known as chlorosis.
An infantile form of ancylostomiasis resulting from heavy A. duodenale infection has been described. Affected infants experience diarrhea, melena, failure to thrive, and profound anemia. Infantile ancylostomiasis has significant mortality.
Eosinophilic enteritis caused by A. caninum is associated with colicky abdominal pain that begins in the epigastrium and radiates outward and is usually exacerbated by food. Extreme cases may mimic acute appendicitis.
Diagnosis
Children with hookworm release eggs that can be detected by direct fecal examination (Fig. 284-2). Quantitative methods are available to determine whether a child has a heavy worm burden that can cause hookworm disease. The eggs of N. americanus and A. duodenale are morphologically indistinguishable. Species identification typically requires egg hatching and differentiation of 3rd stage infective larvae; newer methods using polymerase chain reaction methods are under development.
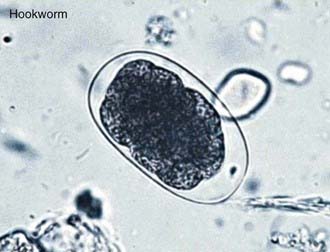
Figure 284-2 Soil-transmitted helminth eggs.
(From Bethony J, Brooker S, Albonico M, et al: Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm, Lancet 367:1521–1532, 2006.)
In contrast, eggs are generally not present in the feces of patients with eosinophilic enteritis caused by A. caninum. Eosinophilic enteritis is often diagnosed by demonstrating ileal and colonic ulcerations by colonoscopy in the presence of significant blood eosinophilia. An adult canine hookworm may occasionally be recovered during colonoscopic biopsy. Patients with this syndrome develop IgG and IgE serologic responses.
Treatment
The goal of deworming is removal of the adult hookworms with an anthelmintic drug. The benzimidazole anthelmintics, mebendazole and albendazole, are effective at eliminating hookworms from the intestine, although multiple doses are sometimes required. Albendazole (400 mg PO once, for all ages) usually achieves high cure rates, although N. americanus adult hookworms are sometimes more refractory and require additional doses. Mebendazole (100 mg bid PO for 3 days, for all ages) is also effective. In many developing countries, mebendazole is administered as a single dose of 500 mg; however, the cure rates with this regimen can be as low as 10% or less. According to the World Health Organization, children should be encouraged to chew tablets of albendazole or mebendazole because forcing very young children to swallow large tablets may cause choking or asphyxiation. Mebendazole is recommended for A. caninum–associated eosinophilic enteritis, although recurrences are common. Because the benzimidazoles have been reported to be embryotoxic and teratogenic in laboratory animals, their safety during pregnancy and in young children is a potential concern and the risks vs benefits must be carefully considered. The World Health Organization currently support the use of benzimidazoles in infected children ≥1 yr of age but at a reduced dose (200 mg for albendazole) in the youngest age group (1-2 yr old). Pyrantel pamoate (11 mg/kg PO once daily for 3 days, maximum dose 1 g) is available in liquid form and is an effective alternative to the benzimidazoles. Replacement therapy with oral iron is not usually required to correct hookworm-associated iron deficiency in children.
Prevention
In 2001, the World Health Assembly urged its member states to implement programs of periodic deworming in order to control the morbidity of hookworm and other soil-transmitted helminth infections. Although anthelmintic drugs are effective at eliminating hookworms from the intestine, the high rates of reinfection among children suggest that drug chemotherapy alone is not effective for controlling hookworm in highly endemic areas. Moreover data suggest that the efficacy of mebendazole decreases with frequent, periodic use, leading to concerns about the possible emergence of anthelmintic drug resistance. In order to reduce the reliance exclusively on anthelmintic drugs, a recombinant human hookworm vaccine has been developed and is undergoing clinical testing. Economic development and associated improvements in sanitation, health education, and avoidance of human feces as fertilizer remain critical for reducing hookworm transmission and endemicity.
284.1 Cutaneous Larva Migrans
Etiology
Cutaneous larva migrans (creeping eruption) is caused by the larvae of several nematodes, primarily hookworms, which are not usually parasitic for humans (Table 284-1). A. braziliense, a hookworm of dogs and cats, is the most common cause, but other animal hookworms may also produce the disease.
Table 284-1 ETIOLOGIES OF THE CUTANEOUS LARVA MIGRANS SYNDROME ACCORDING TO CUTANEOUS PRESENTATION
| CAUSATIVE AGENT | CUTANEOUS TRACK | OTHER CUTANEOUS SIGNS |
|---|---|---|
| Animal hookworm | 1-10 burrows, on the feet or buttocks, about 3 mm wide and up to 15-20 cm long, slow-moving (2-5 cm/day), chronic (weeks to months) | Highly pruritic, vesiculobullous lesions, impetiginization, hookworm folliculitis |
| Pelodera strongyloides | 10-100 burrows, on abdomen or buttocks, 1-2 cm long, 2-3 mm wide, may persist for months | Pruritus, follicular papules and pustules |
| Strongyloides stercoralis | Usually 1 burrow, on the abdomen or buttocks; lasts for hours only, may recur, fast-moving (larva currens) | Pruritus, urticaria |
| Gnathostoma spp. (G. hispidum, etc.) | Usually 1 burrow located anywhere, lasts for days, medium-fast-moving | Cutaneous migratory edema (eosinophilic panniculitis), cellulitis, papules, and nodules |
From Caumes E, Danis M: From creeping eruption to hookworm-related cutaneous larva migrans, Lancet Infect Dis 4:659–660, 2004.
Epidemiology
Cutaneous larva migrans is usually caused by A. braziliense, which is endemic to the southeastern USA and Puerto Rico.
Clinical Manifestations
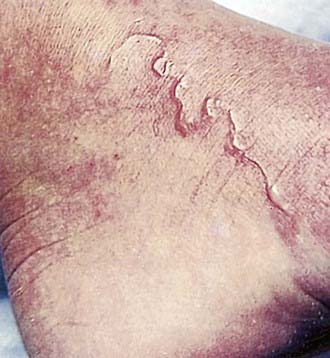
After penetrating the skin, larvae localize at the epidermal-dermal junction and migrate in this plane, moving at a rate of 1-2 cm/day. The response to the parasite is characterized by raised, erythematous, serpiginous tracks, which occasionally form bullae (Fig. 284-3). These lesions may be single or numerous and are usually localized to an extremity, although any area of the body may be affected. As the organism migrates, new areas of involvement may appear every few days. Intense localized pruritus, without any systemic symptoms, may be associated with the lesions. Bacterial superinfection can occur.
Diagnosis
Cutaneous larva migrans is diagnosed by clinical examination of the skin. Patients are often able to recall the exact time and location of exposure, because the larvae produce intense itching at the site of penetration. Eosinophilia may occur but is uncommon.
Treatment
If left untreated, the larvae die, and the syndrome resolves within a few weeks to several months. Treatment with ivermectin (200 µg/kg daily PO for 1-2 days), albendazole (400 mg daily PO for 3 days, for all ages), or topical thiabendazole hastens resolution, if symptoms warrant treatment. Nausea and vomiting frequently preclude repeated administration of oral thiabendazole. The safety of ivermectin in young children (<15 kg) and pregnant women remains to be established. Ivermectin should be taken on an empty stomach with water.
Budhatohoki S, Shah D, Bhurtyal KK, et al. Hookworm causing melaena and severe anaemia in early infancy. Ann Trop Paediatr. 2008;28:293-296.
Centers for Disease Control and Prevention. Outbreak of cutaneous larva migrans at a children’s camp—Miami, Florida, 2006. MMWR Morb Mortal Wkly Rep. 2007;56:1285-1287.
Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
Hotez PJ, Brindley PJ, Bethony JM, et al. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311-1321.
Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937-1948.