Chapter 287 Strongyloidiasis (Strongyloides stercoralis)
Etiology
Strongyloidiasis is caused by the nematode, or roundworm, Strongyloides stercoralis. Only adult female worms inhabit the small intestine. The nematode reproduces in the human host by parthenogenesis and releases eggs containing mature larvae into the intestinal lumen. Rhabditiform larvae immediately emerge from the ova and are passed in feces, where they can be visualized by stool examination. Rhabditiform larvae either differentiate into free-living adult male and female worms or metamorphose into the infectious filariform larvae. Sexual reproduction occurs only in the free-living stage. Humans are usually infected through skin contact with soil contaminated with infectious larvae. Larvae penetrate the skin, enter the venous circulation and then pass to the lungs, break into alveolar spaces, and migrate up the bronchial tree. They are then swallowed and pass through the stomach, and adult female worms develop in the small intestine. Egg deposition begins about 28 days after initial infection.
The hyperinfection syndrome occurs when large numbers of larvae transform into infective organisms during their passage in feces and then reinfect (autoinfect) the host by way of the lower gastrointestinal tract or perianal region. This cycle may be accelerated in immunocompromised persons, particularly those with depressed T-cell function.
Epidemiology
S. stercoralis infection is prevalent in tropical and subtropical regions of the world and endemic in several areas of Europe, the southern USA, and Puerto Rico. Transmission requires appropriate environmental conditions, particularly warm, moist soil. Poor sanitation and crowded living conditions are conducive to high levels of transmission. Dogs and cats can act as reservoirs. The highest prevalence of infection in the USA (4% of the general population) is in impoverished rural areas of Kentucky and Tennessee. Infection may be especially common among residents of mental institutions, veterans who were prisoners of war in areas of high endemicity, and refugees and immigrants. Because of internal autoinfection, individuals may remain infected for decades. Individuals with hematologic malignancies, autoimmune diseases, malnutrition, and drug-induced immunosuppression (especially corticosteroids) are at high risk for the hyperinfection syndrome. Patients with AIDS may experience a rapid course of disseminated strongyloidiasis with a fatal outcome.
Pathogenesis
The initial host immune response to infection is production of immunoglobulin E (IgE) and eosinophilia in blood and tissues, which presumably prevents dissemination and hyperinfection in the immunocompetent host. Adult female worms in otherwise healthy and asymptomatic individuals may persist in the gastrointestinal tract for years. If infected persons become immunocompromised, the reduction in cellular and humoral immunity may lead to an abrupt and dramatic increase in parasite load with systemic dissemination.
Clinical Manifestations
Approximately 30% of infected individuals are asymptomatic. The remaining patients have symptoms that correlate with the 3 stages of infection: invasion of the skin, migration of larvae through the lungs, and parasitism of the small intestine by adult worms. Larva currens is the manifestation of an allergic reaction to filariform larvae that migrate through the skin, where they leave pruritic, tortuous, urticarial tracks. The lesions may recur and are typically found over the lower abdominal wall, buttocks, or thighs, resulting from larval migration from defecated stool. Pulmonary disease secondary to larval migration through the lung rarely occurs and may resemble Loeffler syndrome (cough, wheezing, shortness of breath, transient pulmonary infiltrates accompanied by eosinophilia). Gastrointestinal strongyloidiasis is characterized by indigestion, crampy abdominal pain, vomiting, diarrhea, steatorrhea, protein-losing enteropathy, protein-caloric malnutrition, and weight loss. Edema of the duodenum with irregular mucosal folds, ulcerations, and strictures can be seen radiographically. Infection may be chronic in nature and is associated with eosinophilia.
Strongyloidiasis is potentially lethal because of the ability of the parasite to cause overwhelming hyperinfection in immunocompromised persons. The hyperinfection syndrome is characterized by an exaggeration of the clinical features that develop in symptomatic immunocompetent individuals. The onset is usually sudden, with generalized abdominal pain, distention, and fever. Multiple organs can be affected as massive numbers of larvae disseminate throughout the body and introduce bowel flora. The latter may result in bacteremia and septicemia. Cutaneous manifestations may include petechiae and purpura. Cough, wheezing, and hemoptysis are indicative of pulmonary involvement. Whereas eosinophilia is a prominent feature of strongyloidiasis in immunocompetent persons, this sign may be absent in immunocompromised persons. Because of the low incidence of strongyloidiasis in industrialized countries, it is often misdiagnosed, resulting in a significant delay in treatment.
Diagnosis
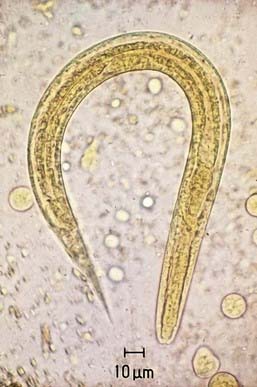
Intestinal strongyloidiasis is diagnosed by examining feces or duodenal fluid for the characteristic larvae (Fig. 287-1). Several stool samples should be examined either by direct smear, Koga agar plate method, or the Baermann test. Alternatively, duodenal fluid can be sampled by the enteric string test (Entero-Test) or aspiration via endoscopy. In children with the hyperinfection syndrome, larvae may be found in sputum, gastric aspirates, and rarely in small intestinal biopsy specimens. An enzyme-linked immunosorbent assay for IgG antibody to Strongyloides may be more sensitive than parasitologic methods for diagnosing intestinal infection in the immunocompetent host. The utility of the assay in diagnosing infection in immunocompromised subjects with the hyperinfection syndrome has not been determined. Eosinophilia is common.
Treatment
Treatment is directed at eradication of infection. Ivermectin (200 µg/kg/day once daily PO for 1-2 days) is the drug of choice for uncomplicated strongyloidiasis. It is equally effective and associated with fewer adverse effects than thiabendazole (25 mg/kg/dose bid PO for 2 days, maximum 3 g/day), which is the traditional treatment. Patients with the hyperinfection syndrome should be treated with ivermectin for 7-10 days and may require repeated courses. Reducing the dose of immunosuppressive therapy and treatment of concomitant bacterial infections are essential in the management of the hyperinfection syndrome. Close follow-up with repeated stool examination is necessary to ensure complete elimination of the parasite. Strongyloides antibodies decrease within 6 mo after successful treatment.
Prevention
Sanitary practices designed to prevent soil and person-to-person transmission are the most effective control measures. Wearing shoes is a main preventive strategy. Reduction in transmission in institutional settings can be achieved by decreasing fecal contamination of the environment such as by the use of clean bedding. Because infection is uncommon in most settings, case detection and treatment are advisable. Individuals who will be given prolonged high dose corticosteroids, immunosuppressive drugs before organ transplantation, or cancer chemotherapy should have a screening examination for S. stercoralis. If infected, they should be treated before immunosuppression is initiated.
Biggs B, Caruana S, Mihrshahi S. Management of chronic strongyloidiasis in immigrants and refugees: is serologic testing useful? Am J Trop Med Hyg. 2009;80:788-791.
Keiser PB, Nutman TB. Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev. 2004;17:208-217.
Knopp S, Mgeni AF, Khamis IS, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:e331.
Marcos LA, Terashima A, DuPont HL. Strongyloides hyperinfection syndrome: an emerging global infectious disease. Trans R Soc Trop Med Hyg. 2008;102:314-318.
Segarra-Newnham M. Manifestations, diagnosis, and treatment of Strongyloides stercoralis infection. Ann Pharmacother. 2007;41:1992-2001.