Chapter 290 Toxocariasis (Visceral and Ocular Larva Migrans)
Etiology
Most cases of human toxocariasis are caused by the dog roundworm, Toxocara canis. Adult female T. canis worms live in the intestinal tracts of young puppies and their lactating mothers. Large numbers of eggs are passed in the feces of dogs and embryonate under optimal soil conditions. Toxocara eggs can survive relatively harsh environmental conditions and are resistant to freezing and extremes of moisture and pH. Humans ingest embryonated eggs contaminating soil, hands, or fomites. The larvae hatch and penetrate the intestinal wall and travel via the circulation to the liver, lung, and other tissues. Humans do not excrete T. canis eggs because the larvae are unable to complete their maturation to adult worms in the intestine. The cat roundworm, Toxocara cati, is responsible for far fewer cases of visceral larva migrans (VLM) than T. canis. Ingestion of infective larvae of the raccoon ascarid Baylisascaris procyonis rarely leads to VLM, but can cause neural larva migrans resulting in fatal eosinophilic meningitis. Ingestion of larvae from the opossum ascarid Lagochilascaris minor leads to VLM rarely.
Epidemiology
Human T. canis infections have been reported in nearly all parts of the world, primarily in temperate and tropical areas where dogs are popular household pets. Young children are at highest risk because of their unsanitary play habits and tendency to place fingers in the mouth. Other behavioral risk factors include pica, contact with puppy litters, and institutionalization. In North America, the highest prevalences of infection are in the southeastern USA and Puerto Rico, particularly among socially disadvantaged African-American and Hispanic children. In the USA, serosurveys show that 4.6-7.3% of children are infected. Assuming an unrestrained and untreated dog population, toxocariasis is prevalent in settings where other geohelminth infections such as ascariasis, trichuriasis, and hookworm infections are common.
Pathogenesis
T. canis larvae secrete large amounts of immunogenic glycosylated proteins. These antigens induce immune responses that lead to eosinophilia and polyclonal and antigen-specific immunoglobulin E (IgE) production. The characteristic histopathologic lesions are granulomas containing eosinophils, multinucleated giant cells (histiocytes), and collagen. Granulomas are typically found in the liver but may also occur in the lungs, central nervous system (CNS), and ocular tissues. Clinical manifestations reflect the intensity and chronicity of infection, anatomic localization of larvae, and host granulomatous responses.
Clinical Manifestations
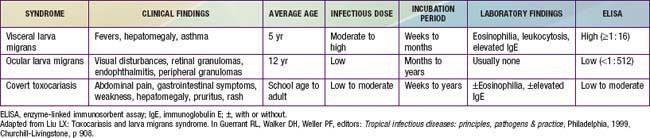
There are 3 major clinical syndromes associated with human toxocariasis: VLM, ocular larva migrans (OLM), and covert toxocariasis (Table 290-1). The classic presentation of VLM includes eosinophilia, fever, and hepatomegaly, and occurs most commonly in toddlers with a history of pica and exposure to puppies. The findings include fever, cough, wheezing, bronchopneumonia, anemia, hepatomegaly, leukocytosis, eosinophilia, and positive Toxocara serology. Cutaneous manifestations such as pruritis, eczema, and urticaria can be present. OLM tends to occur in older children without signs or symptoms of VLM. Presenting symptoms include unilateral visual loss, eye pain, white pupil, or strabismus that develops over a period of weeks. Granulomas occur on the posterior pole of the retina and may be mistaken for retinoblastoma. Serologic testing for Toxocara has allowed the identification of individuals with less obvious or covert symptoms of infection. These children may have nonspecific complaints that do not constitute a recognizable syndrome. Common findings include hepatomegaly, abdominal pain, cough, sleep disturbance, failure to thrive, and headache with elevated Toxocara antibody titers. Eosinophilia may be present in only 50-75% of cases. The prevalence of positive Toxocara serology in the general population supports the notion that most children with T. canis infection are asymptomatic and will not develop overt clinical sequelae over time. A correlation between positive Toxocara serology and allergic asthma has also been described.
Diagnosis
A presumptive diagnosis can be established in a young child with eosinophilia (>20%), leukocytosis, hepatomegaly, fevers, wheezing, and a history of geophagia and exposure to puppies or unrestrained dogs. Supportive laboratory findings include hypergammaglobulinemia and elevated isohemagglutinin titers to A and B blood group antigens. Most patients with VLM have an absolute eosinophil count of >500/µL. Eosinophilia is less common in subjects with OLM. Biopsy confirms the diagnosis. When biopsies cannot be obtained, an enzyme-linked immunosorbent assay using excretory-secretory proteins harvested from T. canis larvae maintained in vitro is the standard serologic test used to confirm toxocariasis. The sensitivity is approximately 78% and specificity 92% at a titer of 1 : 42. The sensitivity for OLM is significantly less. The diagnosis of OLM can be established in patients with typical clinical findings of a retinal or peripheral pole granuloma or endophthalmitis with elevated antibody titers. Vitreous and aqueous humor fluid anti-Toxocara titers are usually greater than serum titers. The diagnosis of covert toxocariasis should be considered in individuals with chronic weakness, abdominal pain, or allergic signs with eosinophilia and increased IgE. In temperate regions of the world, nonparasitic causes of eosinophilia that should be considered in the differential diagnosis include allergies, drug hypersensitivity, lymphoma, vasculitis, and the idiopathic hypereosinophilic syndrome (Chapter 123).
Treatment
Most cases do not require treatment because signs and symptoms are mild and subside over a period of weeks to months. Several anthelmintic drugs have been used for symptomatic cases, often with adjunctive corticosteroids to limit inflammatory responses that presumably result from release of Toxocara antigens by dying parasites. Albendazole (400 mg bid PO for 5 days for all ages) has demonstrated efficacy in both children and adults. Mebendazole (100-200 mg bid PO for 5 days for all ages) is also useful. Anthelmintic treatment of CNS and ocular disease should be extended (3-4 wk). Even though there are no clinical trials regarding therapy of OLM, a course of oral corticosteroids such as prednisone (1 mg/kg/day PO for 2-4 wk) has been recommended to suppress local inflammation while treatment with anthelmintic agents is initiated.
Prevention
Transmission can be minimized by public health measures that prevent dog feces from contaminating the environment. These include keeping dogs on leashes and excluding pets from playgrounds and sandboxes that toddlers use. Children should be discouraged from putting dirty fingers in their mouth and eating dirt. Vinyl covering of sandboxes reduces the viability of T. canis eggs. Widespread veterinary use of broad-spectrum anthelmintics effective against Toxocara may lead to a decline in parasite transmission to humans.
Gavignet B, Piarroux R, Aubin F, et al. Cutaneous manifestations of human toxocariasis. J Am Acad Dermatol. 2008;59:1031-1042.
Graeff-Teixeira CM, da Silva ACA, Yoshimura K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev. 2009;22:322-348.
Hotez PJ, Wlikins PP. Toxocariasis: America’s most common neglected infection of poverty and a helminthiasis of global importance? PLoS Negl Trop Dis. 2009;3:e400.
Litwin CM. Pet-transmitted infections: diagnosis by microbiologic and immunologic methods. Pediatr Infect Dis J. 2003;22:768-777.
Sabrosa NA, Zajdenweber M. Nematode infections of the eye: toxocariasis, onchocerciasis, diffuse unilateral subacute neuroretinitis, and cysticercosis. Ophthalmol Clin North Am. 2002;15:351-356.
Schantz PM, Glickman LT. Toxocaral visceral larva migrans. N Engl J Med. 1978;298:436-439.