Chapter 325 Ileus, Adhesions, Intussusception, and Closed-Loop Obstructions
325.1 Ileus
Ileus is the failure of intestinal peristalsis caused by loss of coordinated gut motility without evidence of mechanical obstruction. In children, it is most often associated with abdominal surgery or infection (pneumonia, gastroenteritis, peritonitis). Ileus also accompanies metabolic abnormalities (e.g. uremia, hypokalemia, hypercalcemia, hypermagnesemia, acidosis) or administration of certain drugs, such as opiates, vincristine, and antimotility agents such as loperamide when used during gastroenteritis.
Ileus manifests as increasing abdominal distention, emesis, and pain that worsens with distention. Bowel sounds are minimal or absent, in contrast to early mechanical obstruction, when they are hyperactive. Plain abdominal radiographs demonstrate multiple air-fluid levels throughout the abdomen. Serial radiographs usually do not show progressive distention as they do in mechanical obstruction. Contrast radiographs, if performed, demonstrate slow movement of barium through a patent lumen.
Treatment of ileus involves correcting the underlying abnormality. Nasogastric decompression is used to relieve recurrent vomiting or abdominal distention associated with pain. Ileus after abdominal surgery generally resolves in 24-72 hr. Prokinetic agents such as metoclopramide or erythromycin have been thought to hasten the return of normal bowel motility, but clinical data are inconclusive. The development of selective peripheral opioid antagonists such as methylnaltrexone holds promise in decreasing postoperative ileus, but pediatric data are lacking.
325.2 Adhesions
Adhesions are fibrous tissue bands that result from peritoneal injury. They can constrict hollow organs and are a major cause of postoperative small bowel obstruction. Most remain asymptomatic, but problems can arise anytime after the 2nd postoperative wk to years after surgery, regardless of surgical extent. In 1 study, the 5-year readmission risk due to adhesions varied by operative region (2.1% for colon to 9.2% for ileum) and procedure (0.3% for appendectomy to 25% for ileostomy formation/closure). The overall risk was 5.3% excluding appendectomy and 1.1% when they were included.
The diagnosis is suspected in patients with abdominal pain, constipation, emesis, and a history of intraperitoneal surgery. Nausea and vomiting quickly follow onset of pain. Initially, bowel sounds are hyperactive, and the abdomen is flat. Subsequently, bowel sounds disappear, and bowel dilation can cause abdominal distention. Fever and leukocytosis suggest bowel necrosis and peritonitis. Plain radiographs demonstrate obstructive features, and a CT scan or contrast studies may be needed to define the etiology.
Management includes nasogastric decompression, intravenous fluid resuscitation, and broad-spectrum antibiotics in preparation for surgery. Nonoperative intervention is contraindicated unless a patient is stable with obvious clinical improvement. In children with repeated obstruction, fibrin-glued plication of adjacent small bowel loops can reduce the risk of recurrent problems. Long-term complications include female infertility, failure to thrive, and chronic abdominal and/or pelvic pain.
325.3 Intussusception
Intussusception occurs when a portion of the alimentary tract is telescoped into an adjacent segment. It is the most common cause of intestinal obstruction between 3 mo and 6 yr of age and the most common abdominal emergency in children <2 yr. Sixty percent of patients are <1 yr of age, and 80% of the cases occur before age 24 mo; it is rare in neonates. The incidence varies from 1 to 4/1,000 live births. The male : female ratio is 3 : 1. A few intussusceptions reduce spontaneously, but if left untreated, most lead to intestinal infarction, perforation, peritonitis, and death.
Etiology and Epidemiology
Approximately 90% of cases of intussusception in children are idiopathic. The seasonal incidence has peaks in spring and autumn. Correlation with prior or concurrent respiratory adenovirus (type C) infection has been noted, and the condition can complicate otitis media, gastroenteritis, Henoch-Schönlein purpura, or upper respiratory tract infections. The risk of intussusception was increased in infants ≤1 yr of age after receiving a tetravalent rhesus-human reassortant rotavirus vaccine within 2 wk of immunization. The Advisory Committee on Immunization Practices no longer recommends this vaccine, and it is no longer available. Although rotavirus produces an enterotoxin, there is no association between wild-type human rotavirus and intussusception. The currently approved rotavirus vaccines have not been associated with an increased risk of intussusception.
It is postulated that gastrointestinal infection or the introduction of new food proteins results in swollen Peyer patches in the terminal ileum. Lymphoid nodular hyperplasia is another related risk factor. Prominent mounds of lymph tissue lead to mucosal prolapse of the ileum into the colon, thus causing an intussusception. In 2-8% of patients, recognizable lead points for the intussusception are found, such as a Meckel diverticulum, intestinal polyp, neurofibroma, intestinal duplication cysts, hemangioma, or malignant conditions such as lymphoma. Lead points are more common in children >2 yr of age; the older the child, the higher the risk of a lead point. Intussusception can complicate mucosal hemorrhage, as in Henoch-Schönlein purpura or hemophilia. Cystic fibrosis is another risk factor. Postoperative intussusception is ileoileal and usually occurs within several days of an abdominal operation. Intrauterine intussusception may be associated with the development of intestinal atresia. Intussusception in premature infants is rare.
Pathology
Intussusceptions are most often ileocolic, less commonly cecocolic, and rarely exclusively ileal. Very rarely, the appendix forms the apex of an intussusception. The upper portion of bowel, the intussusceptum, invaginates into the lower, the intussuscipiens, pulling its mesentery along with it into the enveloping loop. Constriction of the mesentery obstructs venous return; engorgement of the intussusceptum follows, with edema, and bleeding from the mucosa leads to a bloody stool, sometimes containing mucus. The apex of the intussusception can extend into the transverse, descending, or sigmoid colon, even to and through the anus in neglected cases. This presentation must be distinguished from rectal prolapse. Most intussusceptions do not strangulate the bowel within the 1st 24 hr but can eventuate in intestinal gangrene and shock.
Clinical Manifestations
In typical cases, there is sudden onset, in a previously well child, of severe paroxysmal colicky pain that recurs at frequent intervals and is accompanied by straining efforts with legs and knees flexed and loud cries. The infant may initially be comfortable and play normally between the paroxysms of pain; but if the intussusception is not reduced, the infant becomes progressively weaker and lethargic. At times, the lethargy is out of proportion to the abdominal signs. Eventually, a shocklike state, with fever, can develop. The pulse becomes weak and thready, the respirations become shallow and grunting, and the pain may be manifested only by moaning sounds. Vomiting occurs in most cases and is usually more frequent in the early phase. In the later phase, the vomitus becomes bile stained. Stools of normal appearance may be evacuated in the 1st few hours of symptoms. After this time, fecal excretions are small or more often do not occur, and little or no flatus is passed. Blood is generally passed in the 1st 12 hr, but at times not for 1-2 days, and infrequently not at all; 60% of infants pass a stool containing red blood and mucus, the currant jelly stool. Some patients have only irritability and alternating or progressive lethargy. The classic triad of pain, a palpable sausage-shaped abdominal mass, and bloody or currant jelly stool is seen in <15% of patients with intussuscpetion.
Palpation of the abdomen usually reveals a slightly tender sausage-shaped mass, sometimes ill defined, which might increase in size and firmness during a paroxysm of pain and is most often in the right upper abdomen, with its long axis cephalocaudal. If it is felt in the epigastrium, the long axis is transverse. About 30% of patients do not have a palpable mass. The presence of bloody mucus on rectal examination supports the diagnosis of intussusception. Abdominal distention and tenderness develop as intestinal obstruction becomes more acute. On rare occasions, the advancing intestine prolapses through the anus. This prolapse can be distinguished from prolapse of the rectum by the separation between the protruding intestine and the rectal wall, which does not exist in prolapse of the rectum.
Ileoileal intussusception can have a less-typical clinical picture, the symptoms and signs being chiefly those of small intestinal obstruction. Recurrent intussusception is noted in 5-8% and is more common after hydrostatic than surgical reduction. Chronic intussusception, in which the symptoms exist in milder form at recurrent intervals, is more likely to occur with or after acute enteritis and can arise in older children as well as in infants.
Diagnosis
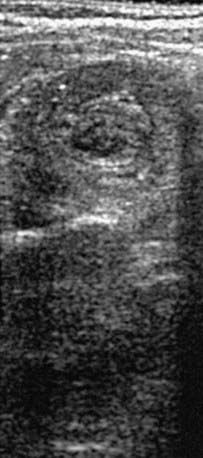
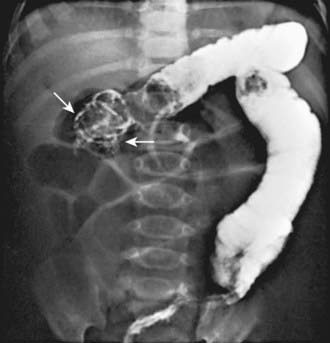
When the clinical history and physical findings suggest intussusception, an ultrasound is typically performed. A plain abdominal radiograph might show a density in the area of the intussusception. Screening ultrasounds for suspected intussusception increases the yield of diagnostic or therapeutic enemas and reduces unnecessary radiation exposure in children with negative ultrasound examinations. The diagnostic findings of intussusception on ultrasound include a tubular mass in longitudinal views and a doughnut or target appearance in transverse images (Fig. 325-1). Ultrasound has a sensitivity of approximately 98-100% and a sensitivity of about 88% in diagnosing intussusception. Air, hydrostatic (saline), and, less often, water-soluble contrast enemas have replaced barium examinations. Contrast enemas demonstrate a filling defect or cupping in the head of the contrast media where its advance is obstructed by the intussusceptum (Fig. 325-2). A central linear column of contrast media may be visible in the compressed lumen of the intussusceptum, and a thin rim of contrast may be seen trapped around the invaginating intestine in the folds of mucosa within the intussuscipiens (coiled-spring sign), especially after evacuation. Retrogression of the intussusceptum under pressure and visualized on x-ray or ultrasound documents successful reduction. Air reduction is associated with fewer complications and lower radiation exposure than traditional contrast hydrostatic techniques.
Differential Diagnosis
It may be particularly difficult to diagnose intussusception in a child who already has gastroenteritis; a change in the pattern of illness, in the character of pain, or in the nature of vomiting or the onset of rectal bleeding should alert the physician. The bloody stools and abdominal cramps that accompany enterocolitis can usually be differentiated from intussusception because in enterocolitis the pain is less severe and less regular, there is diarrhea, and the infant is recognizably ill between pains. Bleeding from a Meckel diverticulum is usually painless. Joint symptoms, purpura, or hematuria usually but not invariably accompany the intestinal hemorrhage of Henoch-Schönlein purpura. Because intussusception can be a complication of this disorder, ultrasonography may be needed to distinguish the conditions.
Treatment
Reduction of an acute intussusception is an emergency procedure and should be performed immediately after diagnosis in preparation for possible surgery. In patients with prolonged intussusception and signs of shock, peritoneal irritation, intestinal perforation, or pneumatosis intestinalis, hydrostatic reduction should not be attempted.
The success rate of radiologic hydrostatic reduction under fluoroscopic or ultrasonic guidance is approximately 80-95% in patients with ileocolic intussusception. Spontaneous reduction of intussusception occurs in about 4-10% of patients. Bowel perforations occur in 0.5-2.5% of attempted barium and hydrostatic (saline) reductions. The perforation rate with air reduction is 0.1-0.2%.
An ileoileal intussusception is best demonstrated by abdominal ultrasonography. Reduction by instillation of contrast agents, saline, or air might not be possible. Such intussusceptions can develop insidiously after bowel surgery and require reoperation if they do not spontaneously reduce. If manual operative reduction is impossible or the bowel is not viable, resection of the intussusception is necessary, with end-to-end anastomosis.
Prognosis
Untreated intussusception in infants is usually fatal; the chances of recovery are directly related to the duration of intussusception before reduction. Most infants recover if the intussusception is reduced in the 1st 24 hr, but the mortality rate rises rapidly after this time, especially after the 2nd day. Spontaneous reduction during preparation for operation is not uncommon.
The recurrence rate after reduction of intussusceptions is about 10%, and after surgical reduction it is 2-5%; none has recurred after surgical resection. Corticosteroids can reduce the frequency of recurrent intussusception. Recurrent intussusception can usually be reduced radiologically. It is unlikely that an intussusception caused by a lesion such as lymphosarcoma, polyp, or Meckel diverticulum will be successfully reduced by radiologic intervention. With adequate surgical management, operative reduction carries a very low mortality rate in early cases.
Bajaj L, Roback MG. Postreduction management of intussusception in a children’s hospital emergency department. Pediatrics. 2003;112:1302-1307.
Bines JE, Kohl KS, Forster J, et al. Acute intussusception in infants and children as an adverse event following immunizations: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22:569-574.
Bines JE, Liem NT, Justice FA, et al. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatr. 2006;149:452-460.
Bonnard A, Demarche M, Dimitriu C, et al. Indications for laparoscopy in the management of intussusception. A multicenter retrospective study conducted by the French study group for pediatric laparoscopy (GECI). J Pediatr Surg. 2008;43:1249-1253.
Buettcher M, Baer G, Bonhoeffer J, et al. Three-year surveillance of intussusception in children in Switzerland. Pediatrics. 2007;120:473-480.
Burke MS, Ragi JM, Karamanoukian HL, et al. New strategies in nonoperative management of meconium ileus. J Pediatr Surg. 2002;37:760-764.
Centers for Disease Control and Prevention. Postmarketing monitoring of intussusception after Rota Teg vaccination—United States, February 1, 2006-February 15, 2007. MMWR. 2007;56:218-222.
Crystal P, Hertzanu Y, Farber B, et al. Sonographically guided hydrostatic reduction of intussusception in children. J Clin Ultrasound. 2002;30:343-348.
Fischer TK, Bihrmann K, Perch M, et al. Intussusception in early childhood: a cohort study of 1.7 million children. Pediatrics. 2004;114:782-785.
Greenberg D, Givon-Lavi N, Newman N, et al. Intussusception in children in southern Israel: disparity between 2 populations. Pediatr Infect Dis J. 2008;27:236-240.
Henrikson S, Blane CE, Koujok K, et al. The effect of screening sonography on the positive rate of enemas for intussusception. Pediatr Radiol. 2003;33:190-193.
Henry MCW, Brever CK, Tashjian DB, et al. The appendix sign: a radiographic marker for irreducible intussusception. J Pediatr Surg. 2006;41:487-489.
Koumanidou C, Vakaki M, Pitsoulakis G, et al. Sonographic detection of lymph nodes in the intussusception of infants and young children: clinical evaluation and hydrostatic reduction. AJR Am J Roentgenol. 2002;178:445-450.
Kubota A, Imura K, Yagi M, et al. Functional ileus in neonates: Hirschsprung’s disease–allied disorders versus meconium-related ileus. Eur J Pediatr Surg. 1999;9:392-395.
O’Ryan M, Lucero Y, Pena A, et al. Two year review of intestinal intussusception in six large public hospitals of Santiago, Chile. Pediatr Infect Dis J. 2003;22:717-721.
Shteyer E, Koplewitz BZ, Gross E, et al. Medical treatment of recurrent intussusception associated with intestinal lymphoid hyperplasia. Pediatrics. 2003;111:682-685.
Sorantin E, Lindbichler F. Management of intussusception. Eur Radiol. 2004;14:146-154.
Williams H. Imaging and intussusception. Arch Dis Child Educ Pract Ed. 2008;93:30-36.
325.4 Closed-Loop Obstructions
Closed-loop obstructions (i.e., internal hernia) are caused by small bowel loops that enter windows created by mesenteric defects or adhesions and become trapped. Vascular engorgement of the strangulated bowel results in intestinal ischemia and necrosis unless promptly relieved. Symptoms include abdominal pain, distention, and bilious emesis. Peritoneal signs suggest ischemic bowel. Plain radiographs demonstrate signs of small bowel obstruction or free air if the bowel has perforated. Supportive management includes intravenous fluids, antibiotics, and nasogastric decompression. Prompt surgical relief of the obstruction is indicated to prevent bowel necrosis. Symptoms occasionally are intermittent if the herniated bowel slides in and out of the defect, spontaneously relieving and regenerating the obstruction.