Chapter 331 Intestinal Transplantation in Children with Intestinal Failure
The introduction of tacrolimus and the development of the abdominal multiorgan procurement techniques allowed the tailoring of various types of intestine grafts, which can contain other intra-abdominal organs such as the liver, pancreas, and stomach; this tailoring has been critical to the application of this type of organ transplant, given the wide scope of diseases for which replacement of the intestine may be necessary. Also, the understanding that the liver protects the intestine against rejection had been suggested by previous combinations of liver plus other organs such as the kidney. The first survivors of intestine transplantation would also go on to demonstrate the interaction (host-versus-graft and graft-versus-host) between recipient and donor immunocytes (brought with the allograft), which under the cover of immunosuppression allows varying degrees of graft acceptance and eventual minimization of drug therapy.
Indications for Intestinal Transplant
Intestinal failure (IF) describes a patient who has lost the ability to maintain nutritional support with his or her intestine and is permanently dependent on total parenteral nutrition (TPN). The majority of these patients have short bowels as a result of a congenital deficiency or acquired condition (Chapter 330.7). In others, the cause of IF is a functional disorder of motility or absorption (Table 331-1). Rarely do patients receive intestinal transplants for benign neoplasms. IF is a syndrome that includes “satellite” complications such as loss of venous access, life-threatening infections, and TPN-induced cholestatic liver disease. Patients who develop these complications have a ∼70% 1 yr mortality and thus require organ replacement therapy with intestinal transplantation.
Paucity of Venous Access
Administration of TPN requires the insertion of a centrally placed venous catheter, and there are only 6 readily accessible sites (bilateral internal jugulars, subclavians, and iliac veins). The loss of venous access generally occurs in the setting of recurrent catheter sepsis and thrombosis; loss of 50% of these venous access sites places the patient at risk of not being able to be treated with TPN.
Life-Threatening Infections
Life-threatening infections are usually catheter-related; the absence of significant lengths of intestine may be associated with abnormal motility of the residual bowel (producing both delayed or rapid emptying), with varying degrees of bacterial overgrowth and possible bacterial translocation as a consequence of loss of intestinal barrier function and/or loss of gut immunity. This situation can produce cholestatic liver disease, multisystem organ failure, and metastatic infectious foci in lungs, kidneys, liver, and the brain.
Liver Disease
The development of cholestatic liver disease may be a consequence of the toxic drug effects of TPN on hepatocytes, a disruption of bile flow and bile acid metabolism, and the frequent occurrence of bacterial translocation and sepsis with endotoxin release into the portal circulation. This complication varies in frequency depending on age and the etiology of the IF; it is most common in neonates with extremely short gut. The effects on the liver include fatty transformation, steatohepatitis and necrosis, fibrosis, and then cholestasis. Development of clinical jaundice (total bilirubin >3 mg/dL) and thrombocytopenia are significant risk factors for poor outcome, because these changes lead to the development of portal hypertensive gastroenteropathy, hypersplenism, coagulopathy, and uncontrollable bleeding.
Transplant Operation
Donor Selection
Intestinal grafts are usually procured from hemodynamically stable, ABO-identical brain-dead donors who have minimal clinical or laboratory evidence suggesting intra-abdominal ischemia. Human leukocyte antigen (HLA) has been random, and cytomegalovirus-positive donors have been avoided in recipients of isolated intestine grafts. Exclusion criteria include a history of malignancy and intra-abdominal evidence of infection; systemic viral or bacterial infections are not excluded. Donor preparation has been limited to the administration of systemic and enteral antibiotics. Prophylaxis for graft versus host disease (GVHD) with graft pretreatment using irradiation or a monoclonal antilymphocyte antibody has varied over time and among the centers performing this procedure. Grafts have been preserved with the University of Wisconsin solution.
Types of Intestinal Grafts
Intestinal allografts are used in various forms, either alone (as an isolated intestine graft) or as a composite graft, which can include the liver, duodenum, and pancreas (liver-intestine graft); when this composite graft includes the stomach, and the recipient operation requires the replacement of all of the patient’s gastrointestinal tract (as with intestinal pseudo-obstruction) and liver, then this replacement graft is known as a multivisceral graft.
The procurement of these various types of grafts focuses on the preservation of the arterial vessels of celiac and/or superior mesenteric arteries, as well as appropriate venous outflow, which would include the superior mesenteric vein or the hepatic veins in the composite grafts. The various etiologies precipitating intestinal failure have prompted the development of these various combinations of intestinal allografts, where the component organs can be removed or retained according to the clinical needs of the individual patient. The larger composite grafts inherently retain the celiac and superior mesenteric arteries; this includes multivisceral grafts, liver plus small bowel grafts, and modified multivisceral grafts in which the liver is excluded but the entire gastrointestinal tract is replaced, including stomach. The isolated intestine graft retains the superior mesenteric artery and vein; this graft can be accomplished with preservation of the vessels going to the pancreas, when that organ has been allocated to another recipient. The graft that is to be used in a particular recipient is dissected out in situ and then removed after cardiac arrest of the donor, with core cooling of the organs, using an infusion of preservation solution (Fig. 331-1).
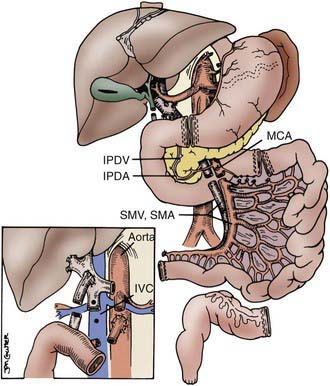
Figure 331-1 The various abdominal organs can be dissected in situ, providing isolated or composite grafts to fit the individual patient’s needs. Separation of intestine and pancreas is feasible, with preservation of the inferior pancreaticoduodenal artery (IPDA) and vein (IPDV). The use of vascular grafts from the donor allow connections to the superior mesenteric pedicle (SMA and SMV) to aorta and inferior vena cava (IVC) or portal vein (inset).
(Reproduced with permission from Abu-Elmagd K, Fung J, Bueno J, et al: Logistics and technique for procurement of intestinal, pancreatic and hepatic grafts from the same donor. Ann Surg 232:680–697, 2000).
Various modifications in these grafts have included the preservation of visceral ganglia at the base of the arteries, the inclusion of donor duodenum and pancreas for the liver and intestine graft, the inclusion of colon, the reduction of the liver graft (into left or right side) and variable reduction of the intestine graft, and the development of living donor intestine grafts.
The Recipient Operation
Because many children have had multiple previous abdominal operations, intestinal transplantation can be a formidable technical challenge; most children require replacement of the liver because of TPN-induced disease and often present with advanced liver failure. Transplantation of an isolated intestinal allograft involves exposure of the lower abdomen, infrarenal aorta, and inferior vena cava. Placement of vascular homografts using donor iliac artery and vein to these vessels allows arterialization and venous drainage of the intestinal graft. In patients who have retained their intestine and then undergo an enterectomy at the time of transplantation, use of the native superior mesenteric vessels is feasible.
Transplantation of a larger composite graft (liver with intestine or multivisceral grafts) requires the removal and replacement of native organs. In a similar fashion, the infrarenal aorta is exposed for placement of an arterial conduit graft (donor thoracic aorta) for arterialization of the graft. When the operation involves removal of the native liver, the venous drainage is achieved through the retained hepatic veins, which are fashioned to a single conduit for anastomosis to the allograft liver.
The intestinal anastomosis to native proximal and distal bowel is performed, leaving an enterostomy of distal allograft ileum; this will be used for routine post-transplant surveillance endoscopy and biopsy. This ostomy is closed 3 to 6 mo after transplant (Fig. 331-2).
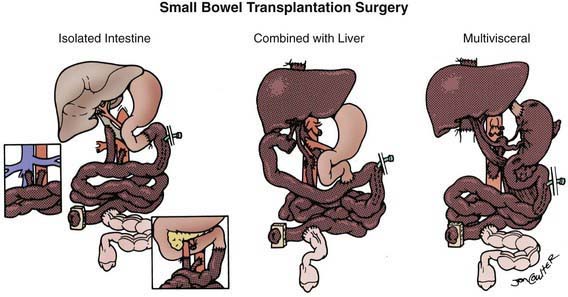
Figure 331-2 The 3 basic intestinal transplant procedures (the graft is shaded). With the isolated intestine, the venous outflow may be to the recipient portal vein (main figure), inferior vena cava (inset left), or superior mesenteric vein (inset right). With the composite grafts, which include the liver, the arterialization is from the aorta with venous drainage out from the liver graft to the recipient inferior vena cava.
Postoperative Management
Immunosuppression
Immunosuppression for intestinal transplantation was based on tacrolimus and corticosteroid induction. This required high levels of tacrolimus (in the nephrotoxic range), and although initial success rates were high, they were followed by rejection rates of >80%, infection, and late drug toxicities, resulting in a gradual loss of patients and grafts. The next generation of protocols incorporated the addition of other agents such as azathioprine, cyclophosphamide, mycophenolate mofetil, rapamycin, and induction with an interleukin-2 (IL-2) antibody antagonist. This modification resulted in a decreased incidence in the severity of initial rejection; the ability to decrease immunosuppression later did not allow for stabilization of long-term survival. The introduction of recipient pretreatment using antilymphocyte antibodies and the elimination of recipient therapy with steroids have resulted in improved transplant survival, with a significant decrease in incidence of rejection and infection, permitting the gradual decrease of immunosuppressive drug therapy within 3 mo and a decline in drug toxicity events.
Allograft Assessment
There are no simple laboratory tools that allow assessment of the intestinal allograft. The gold standard for diagnosis of intestinal allograft rejection has been serial endoscopic surveillance and biopsies through the allograft ileostomy. Clinical signs and symptoms of rejection or infection of the allograft can overlap and mimic each other, producing either rapid diarrhea or complete ileus with pseudo-obstruction syndromes, or gastrointestinal bleeding. Any changes in clinical status should warrant thorough evaluation for rejection with endoscopic biopsies and an evaluation for opportunistic infection, malabsorption, and other enteral infections.
The diagnosis of acute rejection is based on seeing destruction of crypt epithelial cells from apoptosis, in association with a mixed lymphocytic infiltrate. These histologic findings may or may not correlate with endoscopic evidence of injury, which varies from diffuse erythema and friability to ulcers and, in cases of severe rejection, exfoliation of the intestinal mucosa. Chronic rejection of the allograft can be diagnosed only through full thickness sampling of the intestine, which shows the typical vasculopathy that can result in progressive ischemia of the allograft.
Rejection and Graft Versus Host Disease
Acute rejection rates for the intestinal allograft are significantly higher than with any other organ, in the range of 80-90%, and severe rejection requiring the use of antilymphocyte antibody preparations may be as high as 30%. Triple-drug regimens and the use of IL-2 antibody inhibitors have resulted in significant decreases in rejection rates; nonetheless, the amount of immunosuppression was incompatible with improvements in long-term patient and graft survival. Rejection rates of 40% are achievable with the use of antilymphocyte globulin. These protocols induce varying degrees of “tolerance” and eventually minimize immunosuppression, thus reducing the risk of drug toxicity and infection. Vascular rejection has been an uncommon occurrence, and chronic rejection has been seen in about 15% of cases. Graft versus host disease has been seen in about 8% of recipients, with varying degrees of circulating donor cells.
Infections
The most common infections after intestinal transplantation occur as a result of the continuing need for venous catheter placement for as long as 1 yr after transplant. Infections as a consequence of immunosuppressive drug management are due to cytomegalovirus (CMV) infection (22% incidence), Epstein-Barr virus (EBV)-induced infections (21% incidence), and adenovirus enteritis (40% incidence). Successful management of these viral infections is achieved through early detection and preemptive therapy, for both CMV and EBV, before the development of a serious life-threatening infection. This approach has improved outcomes for CMV, eliminating the mortality in the pediatric patient population (Chapters 171, 246, and 247).
Outcomes
Intestinal transplantation is the standard of care for children with intestinal failure who have significant complications of total parenteral nutrition. Data from the International Transplant Registry, the OPTN/SRTR Annual Report 2008, and center-specific data reports have documented significant improvements with short- and long-term survivals for transplantations occurring principally in the last 10 years; intestinal transplantation survival rates are now 97% and 89% for 1 and 3 yr, respectively. It is hoped that with the minimization strategies currently used, the long-term survival will plateau, as occurs with other organ transplants; also, rehabilitation and quality-of-life studies have shown that >80% of survivors reach total independence from TPN and have meaningful life activities. Consequently, there has been a shift in efforts to improve long-term outcomes and quality of life.
As a result of the success of intestinal transplantation, multidisciplinary intestinal centers have focused on intestinal rehabilitation and improving the devastating effects of TPN on the liver. Medicare recognizes intestinal transplantation as a clinical standard of care for patients with IF who have complications of TPN management.
Abu-Elmagd K, Fung J, Bueno J, et al. Logistics and technique for procurement of intestinal, pancreatic and hepatic grafts from the same donor. Ann Surg. 2000;232:680-697.
Berg CL, Steffick DE, Edwards EB, et al. Liver and intestine transplantation in the United States 1998–2007. Am J Transplant. 2009;9(Part 2):907-931.
Bond G, Reyes J, Mazariegos G, et al. The impact of positive T-cell lymphocytotoxic crossmatch on intestinal allograft rejection and survival. Transplant Proc. 2000;32:1197-1198.
Fishbein TW, Matsumoto CS. Intestinal replacement therapy: timing and indications for referral of patients to an intestinal rehabilitation and transplant program. Gastroenterology. 2006;130:S147-S151.
Grant D, Abu-Elmagd K, Reyes J, et al. 2003 report of the Intestine Transplant Registry: a new era has dawned. Ann Surg. 2005;241:607-613.
Green M, Reyes J, Webber S, et al. The role of antiviral and immunoglobulin therapy in the prevention of Epstein-Barr virus infection and post-transplant lymphoproliferative disease following solid organ transplantation, Transplant. Infect Dis. 2001;3:97-103.
Kauffman SS, Atkinson JB, Bianchi A, et al. Indications for pediatric intestinal transplantation. Pediatr Transplant. 2001;5:80-87.
Kelly DA. Intestinal failure associated liver disease—what do we know today? Gastroenterology. 2006;130(2 Suppl):S70-S77.
Organ Procurement and Transplantation Network. Data (website), http://optn.transplant.hrsa.gov/data/. Accessed May 7, 2010
Puntis J, Jenkins HR. Intestinal failure. Arch Dis Child. 2009;94:919-920.
Reyes J, Mazariegos GV, Abu-Elmagd K, et al. Intestinal transplantation under tacrolimus monotherapy after perioperative lymphoid depletion with rabbit anti-thymocyte globulin (thymoglobulin). Am J Transplant. 2005;5:1430-1436.
Reyes J, Mazariegos GV, Bond GM, et al. Pediatric intestinal transplantation: historical notes, principles and controversies. Pediatr Transplant. 2002;6:193-207.
Rogers J, Bueno J, Shapiro R, et al. Results of simultaneous and sequential pediatric liver and kidney transplantation. Transplantation. 2001;72:1666-1670.
Salvia G, Guarino A, Terrin G, et al. Neonatal onset intestinal failure: an Italian multicenter study. J Pediatr. 2008;153:674-676.
Sherman PM, Mitchell DJ, Cutz E. Neonatal enteropathies: defining the causes of protracted diarrhea of infancy. J Pediatr Gastroeinerol Nutr. 2004;38:16-26.
Starzl TE, Demtris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127-1156.
Testa G, Holterman M, John E, et al. Combined living donor liver/small bowel transplantation. Transplantation. 2005;27:1401-1404.