Chapter 399 Inherited Disorders of Surfactant Metabolism
Pulmonary surfactant is a mixture of phospholipids and proteins synthesized, packaged, and secreted by type II pneumocytes that line the distal air spaces. This mixture forms a monolayer at the air-liquid interface that lowers surface tension at end-expiration of the respiratory cycle and thereby prevents atelectasis and ventilation-perfusion mismatch. Four surfactant-associated proteins have been described: surfactant proteins A and D (SP-A, SP-D) participate in host defense in the lung, whereas surfactant proteins B and C (SP-B, SP-C) contribute to the surface tension–lowering activity of the pulmonary surfactant. The ATP (adenosine triphosphate) binding cassette protein member A3, ABCA3, is a transporter located on the limiting membrane of lamellar bodies, the storage organelle for surfactant within alveolar type II cells, and has an essential role in surfactant phospholipids metabolism. Two genes for SP-A [SFTPA1, SFTPA2] and one gene for SP-D [SFTPD] are located on human chromosome 10, whereas single genes encode SP-B [SFTPB] and SP-C [SFTPC] and ABCA3 [ABCA3], which are located on human chromosomes 2, 8, and 16, respectively. Genetically engineered mice deficient in SP-A or SP-D are susceptible to viral and bacterial infections, and lineages deficient in SP-D accumulate lipids and foamy macrophages in their lungs and demonstrate emphysema as they age. Although inherited deficiencies of SP-D have not been identified in humans, mutations in the gene encoding SP-A2 may result in pulmonary fibrosis and lung cancer, although the disease did not manifest until adulthood in these cases. Inherited disorders of SP-B, SP-C, and ABCA3 have been identified in humans (see Table 399-1 on the Nelson Textbook of Pediatrics website at www.expertconsult.com).
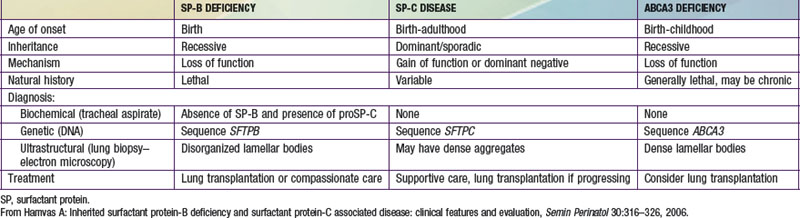
Deficiency of Surfactant Protein B (Surfactant Metabolism Dysfunction, Pulmonary, 1; SMDP1; OMIM #265120)
Genetics
More than 30 loss-of-function mutations in SFTPB have been identified. The most common is a net 2 base-pair insertion in codon 121 (termed 121ins2) that results in a frameshift, an unstable SP-B transcript, and absence of SP-B protein production. This mutation has accounted for 60-70% of the alleles found to date in patients identified with SP-B deficiency. Most other mutations have been family specific.
Pathology
Although SP-B deficiency was first described in a patient with newborn-onset alveolar proteinosis, this histology is neither specific for SP-B deficiency nor universally present in lungs of affected infants. The histologic characteristics seen in infants with SP-B deficiency are also present in children with mutations in other surfactant-associated genes and include alveolar type II cell hyperplasia, alveolar macrophage accumulation, interstitial thickening and inflammation, and alveolar proteinosis. This unique constellation of histologic features is specific for these disorders and is now classified as representing a disorder of surfactant metabolism, or surfactant dysfunction. Ultrastructural findings in SP-B deficiency include a lack of tubular myelin, disorganized lamellar bodies, and an accumulation of abnormal-appearing multivesicular bodies, suggesting abnormal lipid packaging and secretion.
Clinical Manifestations
Infants with an inherited deficiency of SP-B present in the immediate neonatal period with respiratory failure. This autosomal recessive disorder is clinically and radiographically similar to the respiratory distress syndrome (RDS) of premature infants (Chapter 95.3) but typically affects full-term infants. The initial degree of respiratory distress is variable, but the disease is progressive and is refractory to mechanical ventilation, surfactant replacement therapy, glucocorticoid administration, and extracorporeal membrane oxygenation. SP-B deficiency has been recognized in diverse racial and ethnic groups. Almost all affected patients have died without lung transplantation, but prolonged survival is possible in cases of partial deficiency of SP-B. Although murine lineages heterozygous for SP-B deficiency are susceptible to oxidant injury and pulmonary infection, humans heterozygous for loss-of-function mutations in SFTPB are clinically normal as adults and have normal pulmonary function.
Diagnosis
A rapid, definitive diagnosis can be established with sequence analysis of SFTPB, which is available through clinical laboratories (www.genetest.org). In families in which a mutation has been previously identified, antenatal diagnosis can be established by molecular assays of DNA from chorionic villus biopsy or amniocytes, which permits advanced planning of a therapeutic regimen. Other laboratory tests are investigational; they include analysis of tracheal effluent by enzyme-linked immunosorbent assay or Western blotting for the presence or absence of SP-B protein and for aberrantly processed precursor proSP-C peptides that have been found in SP-B–deficient human infants and animals. Immunostaining of lung biopsy tissue for the surfactant proteins can also support the diagnosis, although immunohistochemical assays for SP-B and SP-C are generally available only on a research basis. Staining for SP-B is usually absent, but robust extracellular staining for proSP-C due to aberrantly processed proSP-C peptides is observed and is diagnostic for SP-B deficiency. Such studies require a lung biopsy in a critically ill child but may be performed on lung blocks acquired at the time of autopsy, allowing for retrospective diagnosis.
Treatment
Virtually all patients with SP-B deficiency die within the first year of life. Conventional neonatal intensive care interventions can maintain extrapulmonary organ function for a limited time (weeks to months). Replacement therapy with commercially available surfactants is ineffective. Lung transplantation has been successful, but the pre-transplant, transplant, and post-transplant medical and surgical care is highly specialized and available only at pediatric pulmonary transplant centers; prompt recognition is critical if patients are to be considered for lung transplantation. Genetic counseling of the parents is also important to convey the risks for future pregnancies and the availability of antenatal diagnosis and therapeutic options. Palliative care consultation is helpful.
Surfactant Protein C Gene Abnormalities (Surfactant Metabolism Dysfunction, Pulmonary, 2; SMDP2; OMIM #610913)
Surfactant protein C (SP-C) is a very low molecular weight, extremely hydrophobic protein that, along with SP-B, enhances the surface tension–lowering properties of surfactant phospholipids. It is derived from proteolytic processing of a larger precursor protein (proSP-C).
Genetics
More than 35 dominantly expressed mutations in SFTPC have been identified in association with acute and chronic lung disease in patients ranging in age from newborn to adult. Approximately 55% of these mutations arise spontaneously, resulting in sporadic disease, but the remainder is inherited. A threonine substitution for isoleucine in codon 73 (termed I73T) has accounted for 25-35% of the cases identified to date. Mutations in SFTPC are thought to result in production of misfolded proSP-C that accumulates within the alveolar type II cell and causes cellular injury. The frequency of mutations or disease due to mutations in SFTPC is unknown but is probably low. Mutations have been identified in diverse racial and ethnic groups.
Pathology
The histopathology of lung tissue from patients with mutations in SFTPC falls into the category of disorders of surfactant metabolism, with alveolar type II cell hyperplasia and interstitial thickening and inflammation. Immunostaining may demonstrate proSP-C aggregates but is available only on a research basis.
Clinical Manifestations
The clinical presentation of patients with SFTPC mutations is quite variable. Some patients present at birth with symptoms, signs, and radiographic findings typical of RDS. Others present later in life, ranging from early infancy until well into adulthood, with gradual onset of respiratory insufficiency, hypoxemia, failure to thrive, and chest radiograph demonstration of interstitial lung disease. The age and severity of disease vary even within families with the same mutation. The natural history is also quite variable, with some patients improving either spontaneously or due to therapy, some with persistent respiratory insufficiency, and some progressing to the point of requiring lung transplantation. This variability in severity and course of the disease does not appear to correlate with the specific mutation and also hinders accurate assessment of prognosis.
Diagnosis
Sequencing of SFTPC, the only definitive diagnostic test, is available in clinical laboratories. The relatively small size of the gene facilitates such analyses, which are quite sensitive, but since most SFTPC mutations are missense mutations, distinguishing true disease causing mutations from rare yet benign sequence variants may be difficult.
Treatment
No specific treatment is available for patients with lung disease due to mutations in SFTPC. Therapeutic approaches used for interstitial lung diseases, such as the use of quinolones or corticosteroids, have been tried but not systematically evaluated. The variable natural history makes predictions of prognosis difficult. Lung transplantation is reserved for patients with progressive and refractory respiratory failure who would otherwise qualify for transplantation irrespective of their diagnosis. Genetic counseling is important to define the risks for future pregnancies.
Disease Due to Mutations in ABCA3 (Surfactant Metabolism Dysfunction, Pulmonary, 3; SMDP3; OMIM #610920)
Genetics
Recessive mutations in ABCA3 were first described in infants who presented with lethal respiratory distress in the newborn period, but now have been identified in older infants and children with interstitial lung disease. There is considerable allelic heterogeneity: More than 150 mutations scattered throughout the gene have been identified, most of which are family specific. A missense mutation that results in a valine substitution for glutamine in codon 292 (E292V) is present in approximately 0.4% of the general population and has been identified, in association with another ABCA3 mutation, in children with severe neonatal respiratory failure and in older children with interstitial lung disease. The frequency of mutations and disease is unknown, but ABCA3 mutations may contribute to a substantial proportion of unexplained fatal lung disease in term infants and of interstitial lung disease in older children. ABCA3 mutations have been identified in diverse racial and ethnic groups.
Pathology
The lung pathology of infants with ABCA3 mutations is classified as a disorder of surfactant metabolism, and is similar to that of infants with mutations in SFTPB and SFTPC. On ultrastructural examination, the lamellar bodies appear small and contain electron-dense inclusions, a finding that may be characteristic for mutations in ABCA3 and one that indicates that ABCA3 function is necessary for lamellar body biogenesis.
Clinical Manifestations
Disease due to mutations in ABCA3 presents in two forms: a severe, lethal form that manifests in the immediate newborn period much like SP-B deficiency, and a chronic form that appears most typically in the first year of life with interstitial lung disease similar to SP-C–associated disease. Currently no clear genotype-phenotype correlations permitting prediction of severity or course of disease based on the location or predicted function of individual mutations have been recognized.
Diagnosis
Sequence analysis of ABCA3 is available in clinical laboratories and is the most definitive approach for diagnosis. Considerable variation in ABCA3 necessitates careful interpretation regarding the functionality of an individual variant and its contribution to the clinical presentation. In these situations, lung biopsy is a useful adjunct to the diagnostic approach because the presence of the dense lamellar body inclusions on electron microscopy supports the diagnosis. There are no biochemical markers to establish the diagnosis.
Treatment
Specific treatment for patients with ABCA3 mutations is not available. Quinolones and/or corticosteroids have been used but have not been systematically evaluated. Infants with progressive respiratory failure may be candidates for lung transplantation. Genetic counseling is important to define the risks for future pregnancies.
Brasch F, Schimanski S, Muhlfeld C, et al. Alteration of the pulmonary surfactant system in full-term infants with hereditary ABCA3 deficiency. Am J Respir Crit Care Med. 2006;174:571-580.
Bullard JE, Wert SE, Whitsett JA, et al. ABCA3 mutations associated with pediatric interstitial lung disease. Am J Respir Crit Care Med. 2005;172:1026-1031.
Cameron HS, Somaschini M, Carrera P, et al. A common mutation in the surfactant protein C gene associated with lung disease. J Pediatr. 2005;146:370-375.
Deutsch GH, Young LR, Deterding RR, et al. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120-1128.
Dunbar AE, Wert SE, Hamvas A, et al. Prolonged survival in hereditary surfactant protein B (SP-B) deficiency associated with a novel splicing mutation. Pediatr Res. 2000;48:275-282.
Garmany TH, Wambach JA, Heins HB, et al. Population and disease-based prevalence of the common mutations associated with surfactant deficiency. Pediatr Res. 2008;63:645-649.
Hamvas A, Nogee LM, Wegner DJ, et al. Inherited surfactant deficiency caused by uniparental disomy of rare mutations in the surfactant protein-B and ATP binding cassette, subfamily A, member 3 genes. J Pediatr. 2009;155:854-859.
Matsumura Y, Ban N, Inagaki N. Aberrant catalytic cycle and impaired lipid transport into intracellular vesicles in ABCA3 mutants associated with nonfatal pediatric interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2008;295:698-707.
Nogee LM, Dunbar AEIII, Wert SE, et al. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573-579.
Nogee LM, Wert SE, Proffit SA, et al. Allelic heterogeneity in hereditary surfactant protein B (SP-B) deficiency. Am J Respir Crit Care Med. 2000;161:973-981.
Palomar LM, Nogee LM, Sweet SC, et al. Long-term outcomes after infant lung transplantation for surfactant protein B deficiency related to other causes of respiratory failure. J Pediatr. 2006;149:548-553.
Shulenin S, Nogee LM, Annilo T, et al. ABCA3 gene mutations in newborns with fatal surfactant deficiency. N Engl J Med. 2004;350:1296-1303.
Wang Y, Kuan PJ, Xing C, et al. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52-59.