Chapter 469 Hemostasis
Hemostasis is the active process that clots blood in areas of blood vessel injury yet simultaneously limits the clot size only to the areas of injury. Over time, the clot is lysed by the fibrinolytic system, and normal blood flow is restored. If clotting is impaired, hemorrhage occurs. If clotting is excessive, thrombotic complications ensue. The hemostatic response needs to be rapid and regulated such that trauma does not trigger a systemic reaction but must initiate a rapid, localized response. Key to the speed and coordination of response is that when a platelet adheres to a site of vascular injury, the platelet surface provides a reaction surface where clotting factors bind. The active enzyme is brought together with its substrate and a catalytic cofactor on a reaction surface, accelerating reaction rates and providing activated products for reaction with clotting factors further down the coagulation cascade. Active clotting is controlled by negative feedback loops that inhibit the clotting process when the procoagulant process comes in contact with intact endothelium. The main components of the hemostatic process are the vessel wall, platelets, coagulation proteins, anticoagulant proteins, and fibrinolytic system. Most components of hemostasis are multifunctional; fibrinogen serves as the ligand between platelets during platelet aggregation and also serves as the substrate for thrombin that forms the fibrin clot. Platelets provide the reaction surface on which clotting reactions occur, form the plug at the site of vessel injury, and contract to constrict and limit clot size.
The Process
The intact vascular endothelium is the primary barrier against hemorrhage. The endothelial cells that line the vessel wall normally inhibit coagulation and provide a smooth surface that permits rapid blood flow.
After vascular injury, vasoconstriction occurs and flowing blood comes in contact with the subendothelial matrix (Fig. 469-1). In flowing blood, when exposed to subendothelial matrix proteins, von Willebrand factor (VWF) changes conformation and provides the glue to which the platelet VWF receptor, the glycoprotein Ib complex, binds, tethering platelets to sites of injury. When the VWF receptor binds its ligand, complex signaling occurs from the outside membrane receptor to intracellular pathways, activating the platelets and triggering secretion of storage granules containing adenosine diphosphate (ADP), serotonin, and stored plasma and platelet membrane proteins. Upon activation, the platelet receptor for fibrinogen, α2bβ3, is switched on (“inside out” signaling) to bind fibrinogen and triggers the aggregation and recruitment of other platelets to form the platelet plug. Multiple physiologic agonists can trigger platelet activation and aggregation, including ADP, collagen, thrombin, and arachidonic acid. Aggregation involves the interaction of specific receptors on the platelet surface with plasma hemostatic proteins, primarily fibrinogen.
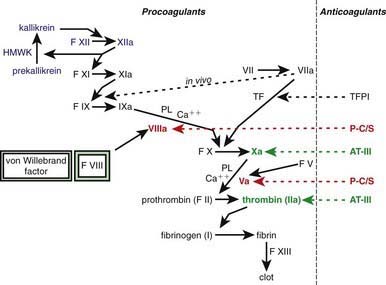
Figure 469-1 The clotting cascade, with sequential activation and amplification of clot formation. Many of the factors (F) are activated by the clotting factors shown above them in the cascade. The activated factors are designated by the addition of an a. On the right side, the major anticoagulants and the sites that they regulate are shown: Tissue factor pathway inhibitor (TFPI) regulates tissue factor (TF]); factor VIIa, protein C, and protein S (P-C/S) regulate factors VIII and V; and antithrombin III (AT-III) regulates factor Xa and thrombin (factor IIa). The dotted line shows that, in vivo, TF and factor VIIa activate both factors IX and X but that, in vitro, only the activation of factor X is measured. Unactivated factor VIII, when bound to its carrier protein, von Willebrand factor, is protected from protein C inactivation. When thrombin, or factor Xa activates factor VIII, it becomes unbound from von Willebrand factor, whereupon it can participate with factor IXa in the activation of factor X in the presence of phospholipid (PL) and Ca2+ (the “tenase” complex). Factor XIIIa cross links the fibrin clot and thereby makes it more stable. Prekallikrein, high molecular weight kininogen (HMWK), and factor XII are shown in blue because they do not have a physiologic role in coagulation, although they contribute to the clotting time in partial thromboplastin time (PTT).
One of the subendothelial matrix proteins that are exposed after vascular injury is tissue factor. Just as exposed subendothelial matrix proteins bind VWF, exposed tissue factor binds to factor VII and activates the clotting cascade, as shown in Figure 469-2. The activated clotting factor then initiates the activation of the next sequential clotting factor in a systematic manner. Our understanding of the sequence of steps in the cascade followed assignment of the numerals for the clotting factors for the participant proteins, and thus the sequence seems “out of numerical order.” During the process of platelet activation, internalized platelet phospholipids (primarily phosphatidylserine) become externalized and interact at 2 specific, rate-limiting steps in the clotting process—those involving the cofactors factor VIII (X-ase complex) and factor V (prothrombinase complex). Both of these reactions are localized to the platelet surface and bring together the active enzyme, an activated cofactor, and the zymogen that will form the next active enzyme in the cascade. This sequence results in amplification of the process, which supplies a burst of clotting where it is physiologically needed. In vivo, autocatalysis of factor VII generates small amounts of VIIa continuously, so the system is always poised to act. Near the bottom of the cascade, the multipotent enzyme thrombin is formed. Thrombin converts fibrinogen into fibrin, activates factors V, VIII, and XI, and aggregates platelets. Activation of factor XI by thrombin amplifies further thrombin generation and contributes to inhibition of fibrinolysis. Thrombin also activates factor XIII. The stable fibrin-platelet plug is ultimately formed through clot retraction and cross linking of the fibrin clot by factor XIIIa.
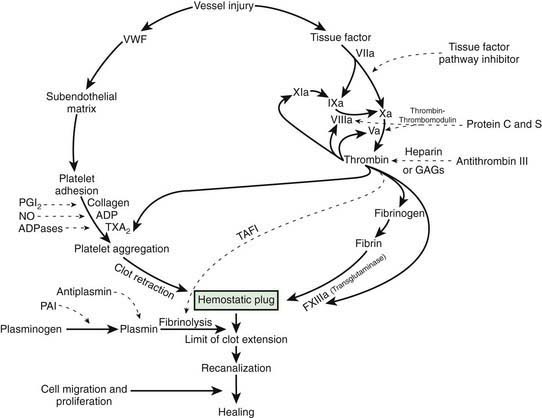
Figure 469-2 The hemostatic mechanism. ADP, adenosine diphosphate; GAGs, glycosaminoglycans; NO, nitric oxide; PGI2, prostacyclin; PAI, plasminogen activator inhibitor; TAFI, thrombin-activated fibrinolytic inhibitor; TXA2, thromboxane A2; VWF, von Willebrand factor.
Virtually all procoagulant proteins are balanced by an anticoagulant protein that regulates or inhibits procoagulant function. Four clinically important, naturally occurring anticoagulants regulate the extension of the clotting process. They are antithrombin III (AT-III), protein C, protein S, and tissue factor pathway inhibitor (TFPI). AT-III is a serine protease inhibitor that regulates factor Xa and thrombin primarily and factors IXa, XIa, and XIIa to a lesser extent. When thrombin in flowing blood encounters intact endothelium, thrombin binds to thrombomodulin, its endothelial receptor. The thrombin-thrombomodulin complex then converts protein C into activated protein C. In the presence of the cofactor protein S, activated protein C proteolyses and inactivates factor Va and factor VIIIa. Inactivated factor Va is, in fact, a functional anticoagulant that inhibits clotting. TFPI limits activation of factor X by factor VIIa and tissue factor and shifts the activation site of tissue factor and factor VIIa to that of factor IX (see Figs. 469-1 and 469-2).
Once a stable fibrin-platelet plug is formed, the fibrinolytic system limits its extension and also lyses the clot (fibrinolysis) to reestablish vascular integrity. Plasmin, generated from plasminogen by either urokinase-like or tissue-type plasminogen activator, degrades the fibrin clot. In the process of dissolving the fibrin clot, fibrin degradation products (FDPs) are produced. The fibrinolytic pathway is regulated by plasminogen activator inhibitors and α2-antiplasmin as well as by the thrombin-activatable fibrinolysis inhibitor (TAFI). Finally, the flow of blood in and around the clot is crucial, because flowing blood returns to the liver, where activated clotting factor complexes are removed and new procoagulant and anticoagulant proteins are synthesized to maintain homeostasis of the hemostatic system.
Pathology
Congenital deficiency of an individual procoagulant protein leads to a bleeding disorder, whereas deficiency of an anticoagulant (clotting factor inhibitor) predisposes the patient to excessive thrombosis. In acquired hemostatic disorders, there are frequently multiple problems with homeostasis that perturb and dysregulate hemostasis. A primary illness (sepsis) and its secondary effects (shock and acidosis) activate coagulation and fibrinolysis and impair the host’s ability to restore normal hemostatic function. When sepsis triggers disseminated intravascular coagulation (DIC), platelets, procoagulant clotting factors, and anticoagulant proteins are consumed, leaving the hemostatic system unbalanced and prone to bleeding or clotting. Similarly, newborn infants and patients with severe liver disease have synthetic deficiencies of both procoagulant and anticoagulant proteins. Such dysregulation causes the patient to be predisposed to both hemorrhage and thrombosis with mild or moderate triggers that result in major alterations in the hemostatic process.
In the laboratory evaluation of hemostasis, parameters are manipulated to allow assessment of isolated aspects of hemostasis and limit the multifunctionality of some of its components. The coagulation process is studied in plasma anticoagulated with citrate to bind calcium, with added phospholipid to mimic the reaction surface normally provided by the platelet membrane and with a stimulus to trigger clotting. Calcium is added to restart the clotting process. This results in anomalies such that the in vivo physiologic pathway of clotting in which factor VIIa activates factor IX is bypassed; instead, in prothrombin time (PT), factor VIIa activates factor X. If this were truly the physiologic situation, then there would be an in vivo bypass mechanism that would ameliorate severe factor VIII and factor IX deficiencies, the 2 most common severe bleeding disorders.
469.1 Clinical and Laboratory Evaluation of Hemostasis
History
For most hemostatic disorders, the clinical history provides the most useful information. To evaluate for a bleeding disorder, the history should determine the site or sites of bleeding, the severity and duration of hemorrhage, and the age at onset. Was the bleeding spontaneous, or did it occur after trauma? Was there a previous personal or family history of similar problems? Did the symptoms correlate with the degree of injury or trauma? Does bruising occur spontaneously? Are there lumps with bruises for which there is minimal trauma? If the patient had previous surgery or significant dental procedures, was there any increased bleeding? If a child or adolescent has had surgery that affects the mucosal surfaces, such as a tonsillectomy or major dental extractions, the absence of bleeding usually rules out a hereditary bleeding disorder. Delayed or slow healing of superficial injuries may suggest a hereditary bleeding disorder. In postpubertal females, it is important to take a careful menstrual history. Because some common bleeding disorders, such as von Willebrand disease (VWD), have a fairly high prevalence, mothers and family members may have the same mild bleeding disorder and may not be cognizant that the child’s menstrual history is abnormal. Women with mild VWD who have a moderate history of bruising frequently have a reduction of that bruising during pregnancy or after administration of oral contraceptives. Some medications, such as aspirin and other nonsteroidal anti-inflammatory drugs, inhibit platelet function and increase bleeding symptoms in patients with a low platelet count or abnormal hemostasis. Standardized bleeding scores have been developed and are undergoing investigation for their sensitivity and specificity in children.
Outside the neonatal period, thrombotic disorders are relatively rare until adulthood. In the neonate, physiologic deficiencies of procoagulants and anticoagulants cause the hemostatic mechanism to be dysregulated, and clinical events can lead to either hemorrhage or thrombosis. If a child or teenager presents with deep venous thrombosis or pulmonary emboli, a detailed family history must be obtained to evaluate for deep venous thrombosis, pulmonary emboli, myocardial infarction, or stroke in other family members. Even in the absence of a family history, the presence of thrombosis in the child or teenager should trigger consideration whether the individual should be evaluated for a hereditary or acquired predisposition to thrombosis.
Physical Examination
The physical examination should focus on whether bleeding symptoms are associated primarily with the mucous membranes or skin (mucocutaneous bleeding) or with the muscles and joints (deep bleeding). The examination should determine the presence of petechiae, ecchymoses, hematomas, hemarthroses, or mucous membrane bleeding. Patients with defects in platelet-blood vessel wall interaction (VWD or platelet function defects) usually have mucocutaneous bleeding, which may include epistaxis, menorrhagia, petechiae, ecchymoses, occasional hematomas, and less commonly, hematuria and gastrointestinal bleeding. Individuals with a clotting factor deficiency of factor VIII or IX (hemophilia A or B) have symptoms of deep bleeding into muscles and joints, with much more extensive ecchymoses and hematoma formation. Patients with mild VWD or other mild bleeding disorders may have no abnormal findings on physical examination. Individuals with disorders of the collagen matrix and vessel wall may have loose joints and lax skin associated with easy bruising (Ehlers-Danlos syndrome).
Patients undergoing evaluation for thrombotic disorders should be asked about swollen, warm, tender extremities or internal organs (venous thrombosis), unexplained dyspnea or persistent “pneumonia,” especially in the absence of fever (pulmonary emboli), and varicosities and postphlebitic changes. Arterial thrombi usually cause an acute, dramatic impairment of organ function, such as stroke, myocardial infarction, or a painful, white, cold extremity.
Laboratory Tests
In patients who have a positive bleeding history or who are actively hemorrhaging, a platelet count, PT, and partial thromboplastin time (PTT) should be performed. If the results are normal, a thrombin time to evaluate fibrinogen function and VWF testing should be considered. In individuals with abnormal screening test results, further specific factor work-up should be undertaken. In a patient with an abnormal bleeding history and a positive family history, normal screening tests should not preclude further laboratory evaluation.
There are no useful routine screening tests for hereditary thrombotic disorders. If the family history is positive or clinical thrombosis is unexplained, specific anticoagulant assays should be undertaken. Thrombosis is rare in children, and when it is present, the possibility of a hereditary predisposition should be considered.
Platelet Count
Platelet count is essential in the evaluation of the child with a positive bleeding history because thrombocytopenia is the most common acquired cause of a bleeding diathesis in children. Patients with a platelet count of > 50,000/mm3 rarely have significant clinical bleeding. Thrombocytosis in children is usually reactive and is not associated with bleeding or thrombotic complications. Persistent, severe thrombocytosis in the absence of an underlying illness may require evaluation for the very rare pediatric presentation of essential thrombocythemia or polycythemia vera.
Prothrombin Time and Activated Partial Thromboplastin Time
Because clotting factors were named in the order of discovery, they do not necessarily reflect the sequential order of activation (Table 469-1). In fact, factors III, IV, and VI were not subsequently found to be independent proteins; thus, these terms are no longer used. The dual mechanisms of activating clotting have been termed the intrinsic (surface activation) and extrinsic (tissue factor–mediated) pathways. Study of the hemostatic mechanism is further complicated in that the interactions in vivo may use different pathways from those studied in clinical laboratory testing. PT measures the activation of clotting by tissue factor (thromboplastin) in the presence of calcium. Addition of tissue factor causes a burst of factor VIIa generation. The tissue factor–factor VIIa complex activates factor X. Whether factor X is activated by the intrinsic or the extrinsic pathway, factor Xa on the platelet phospholipid surface complexes with factor V and calcium (the “prothrombinase” complex) to activate prothrombin to thrombin (also referred to as factor IIa). Once thrombin is generated, fibrinogen is converted to a fibrin clot, the end-point of the reaction (see Fig. 469-2). PT is not prolonged with deficiencies of factors VIII, IX, XI, and XII. In most laboratories, the normal PT value is 10-13 sec. PT has been standardized using the International Normalized Ratio (INR) so that values can be compared from 1 laboratory or instrument to another. This ratio is used to determine similar degrees of anticoagulation with warfarin (Coumadin)–like medications.
Table 469-1 COAGULATION FACTORS
| CLOTTING FACTOR | SYNONYM | DISORDER |
|---|---|---|
| I | Fibrinogen | Congenital deficiency (afibrinogenemia) or dysfunction (dysfibrinogenemia) |
| II | Prothrombin | Congenital deficiency or dysfunction |
| V | Labile factor, proaccelerin | Congenital deficiency (parahemophilia) |
| VII | Stable factor or proconvertin | Congenital deficiency |
| VIII | Antihemophilic factor | Congenital deficiency is hemophilia A (classic hemophilia) |
| IX | Christmas factor | Congenital deficiency is hemophilia B |
| X | Stuart-Prower factor | Congenital deficiency |
| XI | Plasma thromboplastin antecedent | Congenital deficiency, sometimes referred to as hemophilia C |
| XII | Hageman factor | Congenital deficiency is not associated with clinical symptoms |
| XIII | Fibrin-stabilizing factor | Congenital deficiency |
Partial Thromboplastin Time
The intrinsic pathway involves the initial activation of factor XII, which is accelerated by 2 other plasma proteins, prekallikrein and high molecular weight kininogen. In the clinical laboratory, factor XII is activated using a surface (silica or glass) or a contact activator, such as ellagic acid. Factor XIIa, in turn, activates factor XI to factor XIa, which then catalyzes factor IX to factor IXa. On the platelet phospholipid surface, factor IXa complexes with factor VIII and calcium to activate factor X (“tenase” complex).
This process is accelerated by interaction with phospholipid and calcium at the steps involving factors V and VIII. An isolated deficiency of a single clotting factor may result in isolated prolongation of PT, PTT, or both, depending on the location of the factor in the clotting cascade. This approach is useful in determining hereditary clotting factor deficiencies; however, in acquired hemostatic disorders encountered in clinical practice, > 1 clotting factor is frequently deficient, so the relative prolongation of PT and PTT must be assessed.
Measurement of PTT as performed in the clinical laboratory is actually “activated” PTT; however, most refer to it as PTT. This test measures the initiation of clotting at the level of factor XII through sequential steps to the final clot end-point. It does not measure factor VII, factor XIII, or anticoagulants. PTT uses a contact activator (silica, kaolin, or ellagic acid) in the presence of calcium and phospholipid. Because of differences in reagents and laboratory instruments, the normal range for PTT varies among hospital laboratories. Normal ranges for PTT are much more variable from laboratory to laboratory than those for PT.
Thus, the mechanisms studied by PT and PTT allow the evaluation of clotting factor deficiencies, even though these pathways may not be the same as those occurring physiologically In vivo, factor VIIa activates factors IX and X, but as routinely studied in the clinical laboratory, the pathway through which factor VIIa activates factor IX is not evaluated. If the tissue factor–factor VIIa complex activated only factor X, it would be difficult to explain why the most severe bleeding disorders are deficiencies of factor VIII (hemophilia A) and factor IX (hemophilia B). In vivo, thrombin is generated and feeds back to activate factor XI and accelerate the clotting process. Clotting in PTT can be prolonged by deficiencies of factor XII, prekallikrein, and high molecular weight kininogen, yet these deficiencies are asymptomatic conditions.
Thrombin Time
Thrombin time measures the final step in the clotting cascade, in which fibrinogen is converted to fibrin. The normal thrombin time varies between laboratories but is usually 11-15 sec. Prolongation of thrombin time occurs with reduced fibrinogen levels (hypofibrinogenemia or afibrinogenemia), with dysfunctional fibrinogen (dysfibrinogenemia), or in the presence of substances that interfere with fibrin polymerization, such as heparin and fibrin split products. If heparin contamination is a potential cause of prolonged thrombin time, a reptilase time is usually ordered.
Reptilase Time
Reptilase time uses snake venom to clot fibrinogen. Unlike thrombin time, reptilase time is not sensitive to heparin and is prolonged only by reduced or dysfunctional fibrinogen and fibrin split products. Therefore, if thrombin time is prolonged but reptilase time is normal, the prolonged thrombin time is due to heparin and does not indicate the presence of fibrin split products or reduced concentration or function of fibrinogen.
Mixing Studies
If there is unexplained prolongation of PT, PTT, or thrombin time, a mixing study is usually performed. Normal plasma is added to the patient’s plasma, and the PT or PTT is repeated. Correction of PT or PTT by 1 : 1 mixing with normal plasma suggests deficiency of a clotting factor, because a 50% level of individual clotting proteins is sufficient to produce normal PT or PTT. If the clotting time is not corrected or only partially corrected, an inhibitor is usually present. An inhibitor of clotting may be either a chemical similar to heparin that delays coagulation or an antibody directed against a specific clotting factor or the phospholipid used in clotting tests. In the inpatient setting, the most common cause of this finding is heparin contamination of the sample. The presence of heparin in the sample can be ruled in or out with the use of thrombin time and reptilase time, as noted earlier. If the mixing study is not corrected or if its result becomes more prolonged and the patient has clinical bleeding, an inhibitor of a specific clotting factor (antibody directed against the factor), most commonly factor VIII, factor IX, or factor XI, may be present. If the patient has no bleeding symptoms and both PTT and the mixing study are prolonged, a lupus-like anticoagulant (Chapter 476) is often present. Patients with these findings usually have a long PTT, do not bleed, and may have a clinical predisposition to excessive clotting.
Bleeding Time
Bleeding time assesses the function of platelets and their interaction with the vascular wall. Disposable standardized devices have been developed that control the length and depth of the skin incision. A blood pressure cuff is applied to the upper arm and inflated to 40 mm Hg for children and adults. In term newborns and younger children, a modified device has been developed that is used with a lower blood pressure cuff pressure. Bleeding time is a difficult laboratory test to standardize, and there is much interlaboratory and interindividual variation. Although platelet counts of < 100,000/mm3 are associated with prolonged bleeding time, disproportionate prolongation of bleeding time may suggest a qualitative platelet defect or VWD. After an incision is made with the bleeding time device, blood is blotted from the margin of the incision at 30-sec intervals until bleeding ceases. Although each laboratory must establish its own normal range, bleeding usually stops within 4-8 min. Accurate evaluation of bleeding time requires the cooperation of the patient (often a challenge in the young child) and a skilled technician. Use of the bleeding time is declining in many centers.
Platelet Function Analyzer
In an attempt to evaluate the early stages of hemostasis (platelet function and VWF interaction under high shear), several in vitro platelet analyzers have been developed. The greatest experience has been with the platelet function analyzer (PFA-100, Siemens Healthcare Diagnostics, Inc., Deerfield, IL). The PFA-100 measures platelet adhesion-aggregation in whole blood at high shear when exposed to either collagen-epinephrine or collagen-ADP. Results are reported as the closure time measured in sec. The PFA-100 appears to be sensitive to severe forms of VWD and platelet dysfunction. The PFA-100 has variable sensitivity, particularly in the detection of mild VWD and some platelet function defects. Its use as a preoperative screening tool has been disappointing in some studies.
D-Dimer
D-dimer is formed by plasmin degradation of cross-linked fibrin, produced when fibrinogen is clotted by thrombin and cross-linked by factor XIIIa, and is more specific for fibrinolysis than FDPs. D-dimer is elevated in patients with DIC or acute deep vein thrombosis but is relatively nonspecific, in that other ill hospitalized patients often have elevated levels of D-dimer. Adult studies show that the D-dimer can be useful to help exclude venous thrombosis and pulmonary embolus because of its high negative predictive value; for example, a patient with a normal D-dimer value is unlikely to have an acute thrombosis.
Clotting Factor Assays
Each of the clotting factors can be measured in the clinical laboratory using individual factor–deficient plasmas. For most clotting factors, activity is measured against pooled normal plasma or against a standard, by which 100% activity is expressed as 100 IU/dL. By definition, 1 international unit (IU) of each factor is defined as that amount in 1 mL of normal plasma referenced against a standard established by the World Health Organization (WHO). For most clotting factors, the normal range is 50-150 IU/dL (50-150%) (Table 469-2).
In patients with hemophilia A or hemophilia B, inhibitors of factor VIII or factor IX may develop after exposure to replacement therapy. To quantitate the amount of inhibitor present, the standardized clinical assay of these clotting inhibitors is the Bethesda assay. One Bethesda unit is defined as the amount that will inhibit 50% of the clotting factor in normal plasma.
Platelet Aggregation
When a qualitative platelet function defect is suspected, platelet aggregation testing is usually ordered. Platelet-rich plasma from the patient is activated with 1 of a series of agonists (ADP, epinephrine, collagen, thrombin or thrombin-receptor peptide, and ristocetin). Some platelet aggregometers measure specific ATP release from the platelets, as reflected in generating luminescence (lumiaggregometer), and are more sensitive in detecting abnormalities of the platelet release reaction from storage granules. Repeat testing or testing of other symptomatic family members can help to determine the hereditary nature of the defect. Many medications, especially aspirin, other nonsteroidal anti-inflammatory drugs, and valproic acid, alter platelet function testing.
Testing for Thrombotic Predisposition
Hereditary predisposition to thrombosis is associated with a reduction of anticoagulant function (protein C, protein S, AT-III); the presence of a factor V molecule that is resistant to inactivation by protein C (factor V Leiden); elevated levels of procoagulants (a mutation of the prothrombin gene); or a deficiency of fibrinolysis (plasminogen deficiency). When patients are being screened for prothrombotic tendencies, specific tests of the natural anticoagulants are warranted. Although both immunologic and functional tests are usually available, functional assays of protein C, protein S, and AT-III are clinically more useful.
Factor V Leiden is a common mutation in factor V that is associated with an increased risk of thrombosis. A point mutation in the factor V molecule prevents the inactivation of factor Va by activated protein C and, thereby, the persistence of factor Va. This defect, also known as activated protein C resistance, is easily diagnosed with DNA testing.
The prothrombin gene mutation (G20210A) is a mutation in the noncoding portion of the prothrombin gene, with a glycine (G) at position 20210 being replaced by an alanine (A). This mutation increases the amount of prothrombin messenger RNA, is associated with elevations of prothrombin, and causes a predisposition to thrombosis. This abnormality is easily identified with molecular diagnostic (DNA) testing.
Elevated Homocysteine
Levels of homocysteine may be increased as a result of genetic mutations, causing homocystinuria. Patients with homocysteine elevation are predisposed to arterial and venous thrombosis as well as to an increase in arteriosclerosis.
Tests of the Fibrinolytic System
Euglobulin clot lysis time is a screening test used in some laboratories to assess fibrinolysis. More specific tests are available in most laboratories to determine the levels of plasminogen, plasminogen activator, and inhibitors of fibrinolysis. An increase in fibrinolysis may be associated with hemorrhagic symptoms, and a delay in fibrinolysis is associated with thrombosis.
Developmental Hemostasis
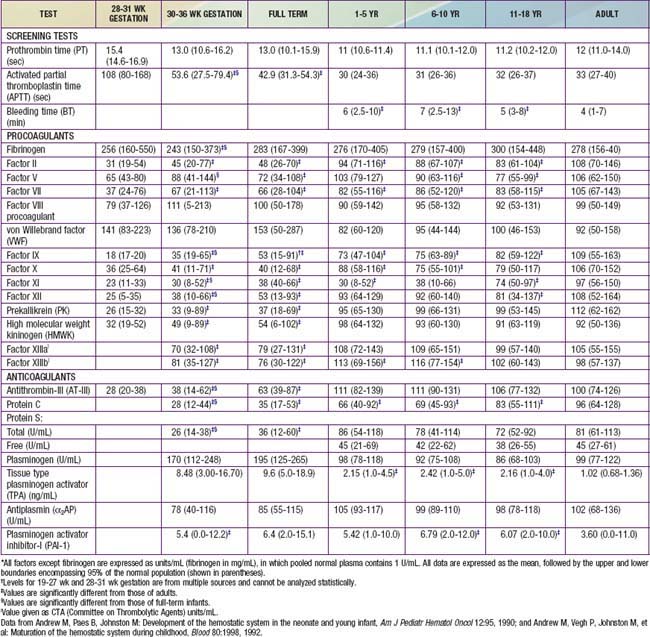
The normal newborn infant has reduced levels of most procoagulants and anticoagulants (see Table 469-1). In general, there is a more marked abnormality in the preterm infant. Although major differences exist in the normal ranges for newborn and preterm infants, these ranges vary greatly among laboratories. During gestation, there is progressive maturation and increase of the clotting factors synthesized by the liver. The extremely premature infant has prolonged PT and PTT values as well as a marked reduction in anticoagulant protein levels (protein C, protein S, and AT-III). Levels of fibrinogen, factors V and VIII, VWF, and platelets are near-normal throughout the later stages of gestation (Chapter 97.4). Because protein C and protein S are physiologically reduced, the normal factors V and VIII are not balanced with their regulatory proteins. In contrast, the physiologic deficiency of vitamin K–dependent procoagulant proteins (factors II, VII, IX, and X) is partially balanced by the physiologic reduction of AT-III. The net effect is that newborns (especially premature infants) are at increased risk for complications of bleeding, clotting, or both.
Cantor AB. Developmental hemostasis: relevance to newborns and infants. In: Orkin SH, Nathan DG, Ginsberg D, et al, editors. Nathan and Oski’s hematology of infancy and childhood. ed 7. Philadelphia: Saunders Elsevier; 2009:147-191.
Colman RW, Clowes AW, George JN, et al. Overview of hemostasis. In: Colman RW, Marder VJ, Clowes AW, et al, editors. Hemostasis and thrombosis: basic principles and clinical practice. ed 5. Lippincott: Williams and Wilkins; 2006:3-16.
Harrison P. The role of PFA-100 testing in the investigation and management of haemostatic defects in children and adults. Br J Haematol. 2005;130:3-10.
Lippi G, Franchini M, Montagnana M, et al. Coagulation testing in pediatric patients: the young are not just miniature adults. Semin Thromb Hemost. 2007;33:816-820.
Parmar N, Albisetti M, Berry LR, Chan AK. The fibrinolytic system in newborns and children. Clin Lab. 2006;52:115-124. erratum in Clin Lab 2006;52:324
Rajpurkar M, Lusher J. Clinical and laboratory approach to the patient with bleeding. In: Orkin SH, Nathan DG, Ginsberg D, et al, editors. Nathan and Oski’s hematology of infancy and childhood. ed 7. Philadelphia: Saunders Elsevier; 2009:1449-1462.
Saxonhouse MA, Manco-Johnson MJ. The evaluation and management of neonatal coagulation disorders. Semin Perinatol. 2009;33:52-65.
Sugar NF, Taylor JA, Feldman KW. Bruises in infants and toddlers: those who don’t cruise rarely bruise. Arch Pediatr Adolesc Med. 1999;153:399-403.
Mann KG, Brummel-Ziedens K. Blood coagulation. In: Orkin SH, Nathan DG, Ginsberg D, et al, editors. Nathan and Oski’s hematology of infancy and childhood. ed 7. Philadelphia: Saunders Elsevier; 2009:1399-1424.
Newman PK, Newman DK. Platelets and the vessel wall. In: Orkin SH, Nathan DG, Ginsberg D, et al, editors. Nathan and Oski’s hematology of infancy and childhood. ed 7. Philadelphia: Saunders Elsevier; 2009:1379-1399.