Chapter 503 Clinical Evaluation of the Child with Hematuria
Hematuria is defined as the presence of at least 5 red blood cells (RBCs) per microliter of urine and occurs with a prevalence of 0.5-2.0% among school-aged children. Quantitative studies demonstrate that normal children can excrete more than 500,000 RBCs per 12-hr period; this increases with fever and/or exercise. In the clinical setting, qualitative estimates are provided by a urinary dipstick that uses a very sensitive peroxidase chemical reaction between hemoglobin (or myoglobin) and a colorimetric chemical indicator impregnated on the dipstick. Chemstrip (Boehringer Mannheim), a common commercially available dipstick, is capable of detecting 3-5 RBCs/µL of unspun urine; significant hematuria is suggested by >50 RBCs/µL. False-negative results can occur in the presence of formalin (used as a urine preservative) or high urinary concentrations of ascorbic acid (i.e., in patients with vitamin C intake >2000 mg/ day). False-positive results may be seen in a child with an alkaline urine (pH > 9), or more commonly following contamination with oxidizing agents such as hydrogen peroxide used to clean the perineum before obtaining a specimen. Microscopic analysis of 10-15 mL of freshly centrifuged urine is essential in confirming the presence of RBCs suggested by a positive dipstick.
Red urine without RBCs is seen in a number of conditions (Table 503-1). Heme-positive urine without RBCs is caused by the presence of either hemoglobin or myoglobin. Hemoglobinuria without hematuria can occur in the presence of hemolysis. Myoglobinuria without hematuria occurs in the presence of rhabdomyolysis resulting from skeletal muscle injury and is generally associated with a 5-fold increase in the plasma concentration of creatine kinase. Rhabdomyolysis can occur secondary to viral myositis, crush injury, severe electrolyte abnormalities (hypernatremia, hypophosphatemia), hypotension, disseminated intravascular coagulation, toxins (drugs, venom), metabolic disorders of muscles, and prolonged seizures. Heme-negative urine can appear red, cola colored, or burgundy, owing to ingestion of various drugs, foods (blackberries, beets), or food dyes, whereas dark brown (or black) urine can result from various urinary metabolites.
Table 503-1 OTHER CAUSES OF RED URINE
HEME POSITIVE
HEME NEGATIVE
Drugs
Dyes (Vegetable/Fruit)
Metabolites
Evaluation of the child with hematuria begins with a careful history, physical examination, and urinalysis. This information is used to determine the level of hematuria (upper vs lower urinary tract) and to determine the urgency of the evaluation based on symptomatology. Special consideration needs to be given to family history, identification of anatomic abnormalities and malformation syndromes, presence of gross hematuria, and manifestations of hypertension, edema, or heart failure.
Causes of hematuria are listed in Table 503-2. Upper urinary tract sources of hematuria originate within the nephron (glomerulus, convoluted or collecting tubules, and interstitium). Lower urinary tract sources of hematuria originate from the pelvocalyceal system, ureter, bladder, or urethra. Hematuria from within the glomerulus is often associated with brown, cola or tea-colored, or burgundy urine, proteinuria >100 mg/dL via dipstick, urinary microscopic findings of RBC casts, and deformed urinary RBCs (particularly acanthocytes). Hematuria originating within the convoluted or collecting tubules may be associated with the presence of leukocytes or renal tubular epithelial cell casts. Lower urinary tract sources of hematuria may be associated with gross hematuria that is bright red or pink, terminal hematuria (gross hematuria occurring at the end of the urine stream), blood clots, normal urinary RBC morphology, and minimal proteinuria on dipstick (<100 mg/dL).
Table 503-2 CAUSES OF HEMATURIA IN CHILDREN
UPPER URINARY TRACT DISEASE
LOWER URINARY TRACT DISEASE
GN, glomerulonephritis.
* Denotes glomerulonephritides presenting with hypocomplementemia.
† Formerly Munchausen syndrome and Munchausen syndrome by proxy.
Patients with hematuria can present with a number of symptoms suggesting specific disorders. Tea- or cola-colored urine, facial or body edema, hypertension, and oliguria are classic symptoms of acute nephritic syndrome. Diseases commonly manifesting as acute nephritic syndrome include postinfectious glomerulonephritis, immunoglobulin A (IgA) nephropathy, membranoproliferative glomerulonephritis, Henoch-Schönlein purpura (HSP) nephritis, systemic lupus erythematosus (SLE) nephritis, Wegener granulomatosis, microscopic polyarteritis nodosa, Goodpasture syndrome, and hemolytic-uremic syndrome. A history of recent upper respiratory, skin, or gastrointestinal infection suggests postinfectious glomerulonephritis, hemolytic-uremic syndrome, or HSP nephritis. Rash and joint complaints suggest HSP nephritis or SLE nephritis. Hematuria associated with glomerulonephritis is typically painless but it can be associated with flank pain. Frequency, dysuria, and unexplained fevers suggest a urinary tract infection, whereas renal colic suggests nephrolithiasis. A flank mass can signal hydronephrosis, renal cystic diseases, renal vein thrombosis, or tumor. Hematuria associated with headache, mental status changes, visual changes (diploplia), epistaxis, or heart failure suggests significant hypertension. Patients with a history of trauma require immediate evaluation (Chapter 66). Child abuse must always be suspected in the child presenting with unexplained perineal bruising and hematuria.
A careful family history is critical in the initial assessment of the child with hematuria in view of numerous genetic causes of renal disorders. Hereditary glomerular diseases include hereditary nephritis (Alport syndrome), thin glomerular basement membrane disease, SLE nephritis, and IgA nephropathy (Berger disease). Other hematuric renal disorders with a hereditary component include polycystic kidney disease (PKD) (both autosomal recessive [ARPKD] and autosomal dominant [ADPKD]), urolithiasis, and sickle cell disease/trait.
A complete physical examination is critical to assess the cause of hematuria. Hypertension, edema, or signs of heart failure suggest acute glomerulonephritis. Several malformation syndromes are associated with renal disease including VATER (vertebral body anomalies, anal atresia, tracheoesophageal fistula, and renal dysplasia) syndrome. Abdominal masses may be caused by bladder distention in posterior urethral valves, hydronephrosis in ureteropelvic junction obstruction, PKD, or Wilms tumor. Hematuria seen in patients with neurologic or cutaneous abnormalities may be the result of renal cystic disease or tumors associated with several syndromes, including tuberous sclerosis, von Hippel-Lindau syndrome, and Zellweger (cerebrohepatorenal) syndrome. Anatomic abnormalities of the external genitalia may be associated with hematuria and/or renal disease.
Patients with gross hematuria present additional challenges because of the associated parental anxiety. The most common cause of gross hematuria is bacterial urinary tract infection. Urethrorrhagia, which is urethral bleeding in the absence of urine, is associated with dysuria and blood spots on underwear after voiding. This condition, which often occurs in prepubertal boys at intervals several months apart, has a benign self-limited course. Less than 10% of patients have evidence of glomerulonephritis. Recurrent episodes of gross hematuria suggest IgA nephropathy, Alport syndrome, or thin glomerular basement membrane disease. Dysuria and abdominal or flank pain are symptoms of idiopathic hypercalciuria, or urolithiasis. Common causes of gross hematuria are listed in Table 503-3. A general approach to the laboratory and radiologic evaluation of the patient with glomerular or extraglomerular hematuria is outlined in Figure 503-1. Asymptomatic patients with isolated microscopic hematuria should not undergo extensive diagnostic evaluation because the hematuria is often transient and benign.
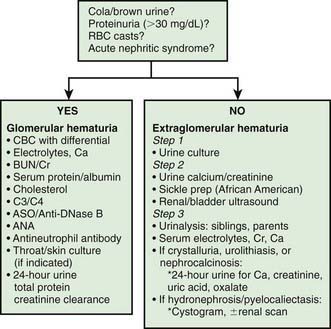
Figure 503-1 Algorithm of the general approach to the laboratory and radiologic evaluation of the patient with glomerular or extraglomerular hematuria. ANA, antinuclear antibody; ASO, antistreptolysin O; BUN, blood urea nitrogen; C3/C4, complement; CBC, complete blood cell count; Cr, creatinine; RBC, red blood cell.
The child with asymptomatic isolated microscopic hematuria that persists on at least 3 urinalyses observed over a minimum of a 2-wk period poses a dilemma in regard to the degree of further diagnostic testing that should be performed. Significant disease of the urinary tract is uncommon with this clinical presentation. The initial evaluation of these children should include a urine culture followed by a spot urine for hypercalciuria with a calcium:creatinine ratio in culture-negative patients. In African-American patients, a sickle cell screen should be included. If these studies are normal, urinalysis of all first-degree relatives is indicated. Renal and bladder ultrasonography should be considered to rule out structural lesions such as tumor, cystic disease, hydronephrosis, or urolithiasis. Ultrasonography of the urinary tract is most informative in patients presenting with gross hematuria, abdominal pain, flank pain, or trauma. If these initial studies are normal, assessment of serum creatinine and electrolytes is recommended.
The finding of certain hematologic abnormalities can narrow the differential diagnosis. Anemia in this setting may be caused by intravascular dilution secondary to hypervolemia associated with acute renal failure; decreased RBC production in chronic renal failure; hemolysis from hemolytic-uremic syndrome or SLE; or blood loss from pulmonary hemorrhage, as seen in Goodpasture syndrome, or melena in patients with Henoch-Schönlein purpura or hemolytic-uremic syndrome. Inspection of the peripheral blood smear might reveal a microangiopathic process consistent with the hemolytic-uremic syndrome. The presence of autoantibodies in SLE can result in a positive Coombs test, the presence of antinuclear antibody, leukopenia, and multisystem disease. Thrombocytopenia can result from decreased platelet production (malignancies) or increased platelet consumption (SLE, idiopathic thrombocytopenic purpura, hemolytic-uremic syndrome, renal vein thrombosis). Although urinary RBC morphology may be normal with lower tract bleeding and dysmorphic from glomerular bleeding, cell morphology is not reliable to unequivocally delineate the site of hematuria. A bleeding diathesis is an unusual cause of hematuria. Coagulation studies are not routinely obtained unless personal or family history suggests a bleeding tendency.
A voiding cystourethrogram is only required in patients with a urinary tract infection, renal scarring, hydroureter, or pyelocaliectasis. Cystoscopy is an unnecessary and costly procedure in most pediatric patients with hematuria, and carries the associated risks of anesthesia. The diagnosis of “possible urethral stenosis” as an indication for cystoscopy should be viewed with a high degree of suspicion, because true urethral stenosis is quite rare. This procedure should be reserved for evaluating the rare child with a bladder mass noted on ultrasound, urethral abnormalities caused by trauma, posterior urethral valves, or tumor. The finding of unilateral gross hematuria localized by cystoscopy is rare, but it can indicate a vascular malformation or another anatomic abnormality.
Children with persistent asymptomatic isolated hematuria and a normal evaluation should have their blood pressure and urine checked every 3 months until the hematuria resolves. Referral to a pediatric nephrologist should be considered for patients with persistent asymptomatic hematuria greater than 1 yr’s duration and is recommended for patients with nephritis (glomerulonephritis, tubulointerstitial nephritis), hypertension, renal insufficiency, urolithiasis or nephrocalcinosis, or a family history of renal disease such as PKD or hereditary nephritis. Renal biopsy is indicated for some children with persistent microscopic hematuria and most children with recurrent gross hematuria associated with decreased renal function, proteinuria, or hypertension.
Bergstein J, Leiser J, Andreoli S. The clinical significance of asymptomatic gross and microscopic hematuria in children. Arch Pediatr Adolesc Med. 2005;159:353-355.
Cohen RA, Brown RS. Microscopic hematuria. N Engl J Med. 2003;348:2330-2338.
Higashihara E, Nishiyama T, Horie S, et al. Hematuria: definition and screening test methods. Int J Urol. 2008;15:281-284.
Meyers KE. Evaluation of hematuria in children. Urol Clin North Am. 2004;3:559-573.
Omoloja AA, Patel H, Ey E, et al. Common renal problems in pediatric medicine. Curr Prob Pediatr Adolesc Health Care. 2007;37:145-200.
Pan CG. Evaluation of gross hematuria. Pediatr Clin North Am. 2007;53:401-412.
Quigley R. Evaluation of hematuria and proteinuria: how should a pediatrician proceed. Curr Opin Pediatr. 2008;20:140-144.