Chapter 515 Anatomic Abnormalities Associated with Hematuria
515.1 Congenital Anomalies
Craig C. Porter and Ellis D. Avner
Gross or microscopic hematuria may be associated with many types of different malformations of the urinary tract. The sudden onset of gross hematuria after minor trauma to the flank is often associated with ureteropelvic junction obstruction or cystic kidneys (see Chapter 531).
515.2 Autosomal Recessive Polycystic Kidney Disease
Also known as infantile polycystic disease, autosomal recessive polycystic kidney disease (ARPKD) is an autosomal recessive disorder occurring with an incidence of 1 : 10,000 to 1 : 40,000. The gene for ARPKD (PKHD1) encodes fibrocystin, a large protein (>4,000 amino acids) with multiple isoforms.
Pathology
Both kidneys are markedly enlarged and grossly show innumerable cysts throughout the cortex and medulla. Microscopic studies demonstrate dilated, ectatic collecting ducts radiating from the medulla to the cortex, although transient proximal tubule cysts have been reported in the fetus. Development of progressive interstitial fibrosis and tubular atrophy during advanced stages of disease eventually leads to renal failure. Liver involvement is characterized by a basic ductal plate abnormality that leads to bile duct proliferation and ectasia, as well as hepatic fibrosis. This lesion is indistinguishable from congenital hepatic fibrosis or Caroli disease, and consequently ARPKD is increasingly referred to as ARPKD/CHF.
Pathogenesis
The function of fibrocystin in normal kidney development and the pathophysiology of its abnormal expression in ARPKD are largely unknown. Evolving information suggests that fibrocystin forms a multimeric complex with proteins of other primary genetic cystic diseases. It appears that altered intracellular signaling from these complexes, located at the apical cell surface, intercellular junction, and basolateral cell surface in association with the focal adhesion complex is a critical feature of disease pathophysiology.
Multiple mutations of PKHD1 cause disease and their average detection rate is approximately 85%. Limited available information suggests some genotype-phenotype correlation: mutations that modify fibrocystin appear to cause less-severe disease than those that truncate fibrocystin.
Clinical Manifestations
The typical child presents with bilateral flank masses during the neonatal period or early infancy. ARPKD may be associated with oligohydramnios, pulmonary hypoplasia, respiratory distress, and spontaneous pneumothorax in the neonatal period. Perinatal demise appears associated with truncating mutations. Components of the oligohydramnios complex including low-set ears, micrognathia, flattened nose, limb-positioning defects, and growth deficiency may be present. Hypertension is usually noted within the first few weeks of life and is often severe and difficult to control. Oliguria and acute renal failure are uncommonly seen, but transient hyponatremia, often in the presence of acute renal failure, often responds to diuresis. Renal function is usually impaired but may be initially normal in 20-30% of patients. Infrequently, ARPKD manifests beyond infancy, in young infants with a mixed clinical picture of renal and hepatic findings: variable degrees of portal hypertension (hepatosplenomegaly, gastroesophageal varices, prominent cutaneous periumbilical veins, reversal of portal vein flow, thrombocytopenia) and variable renal findings that range from asymptomatic abnormal renal ultrasonography to systemic hypertension and renal insufficiency.
In the newborn, clinical evidence of liver disease by radiologic or clinical laboratory assessment is present in about 45% of children. It is believed to be universal by microscopic evaluation. Patients with ARPKD are at risk for developing ascending cholangitis, varices, and hypersplenism related to portal hypertension; they are also at risk for progressive liver fibrosis, which uncommonly leads to overt liver failure and cirrhosis. A subset of older children, and even young adults with ARPKD, present with prominent hepatosplenomegaly and display mild renal disease that is discovered incidentally during imaging studies of the abdomen.
Diagnosis
The diagnosis of ARPKD is strongly suggested by bilateral palpable flank masses in an infant with pulmonary hypoplasia, oligohydramnios, and hypertension and the absence of renal cysts by sonography of the parents (Fig. 515-1). Markedly enlarged and uniformly hyperechogenic kidneys with poor corticomedullary differentiation are commonly seen on ultrasonography (Fig. 515-2). The diagnosis is supported by clinical and laboratory signs of hepatic fibrosis, pathologic findings of ductal plate abnormalities seen on liver biopsy, anatomic and pathologic proof of ARPKD in a sibling, or parental consanguinity. The differential diagnosis includes other causes of bilateral renal enlargement and/or cysts, such as multicystic dysplasia, hydronephrosis, Wilms tumor, and bilateral renal vein thrombosis (Table 515-1). Prenatal diagnostic testing using genetic linkage analysis or direct mutation analysis is available in families with ≥1 affected child.
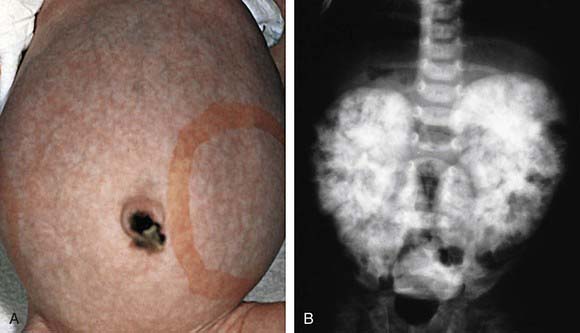
Figure 515-1 A, This infant with infantile polycystic kidney disease shows marked abdominal distention and bilaterally enlarged kidneys, as indicated by the outlined area. B, Intravenous pyelogram of the same patient shows the characteristic mottled nephrogram with brushlike medullary opacification secondary to retention of contrast material in dilated cortical and medullary collecting ducts.
(From Zitelli BJ, Davis HW, editors: Atlas of pediatric physical diagnosis, ed 4, St Louis, 2002, Mosby, p 470.)

Figure 515-2 Ultrasound examination of a neonate with autosomal recessive polycystic kidney disease demonstrating renal enlargement (9 cm) and increased diffuse echogenicity with complete loss of corticomedullary differentiation resulting from multiple small cystic interfaces.
Treatment
The treatment of ARPKD is supportive. Aggressive ventilatory support is often necessary in the neonatal period secondary to pulmonary hypoplasia, hypoventilation, and the many respiratory illnesses of prematurity (which are common). Careful management of hypertension, fluid and electrolyte abnormalities, and clinical manifestations of renal insufficiency is essential. Children with severe respiratory failure or feeding intolerance from enlarged kidneys can require unilateral or bilateral nephrectomies, prompting the need for renal replacement therapy. In families with a previous affected child, preimplantation genetic diagnosis coupled with in vitro fertilization is available in specialized centers and can lead to the birth of unaffected children in at-risk families.
Prognosis
Mortality has improved dramatically, although approximately 30% of patients die in the neonatal period of complications from pulmonary hypoplasia. Neonatal respiratory support and renal replacement therapies have increased the 10-yr survival of children surviving beyond the 1st year of life to >80%. Fifteen-year survival is estimated at 70-80%. End-stage renal disease is seen in >50% of children and usually occurs during the 1st decade of life. As a result, dialysis and renal transplantation have become standard therapies for these children. Morbidity and mortality in the older child are related to complications from chronic renal failure and liver disease.
Adeva M, El-Youssef M, Rossetti S, et al. Clinical and molecular characterization defines a broadened spectrum of autosomal recessive polycystic kidney disease (ARPKD). Medicine. 2006;85:1-21.
Bergmann C, Senderek J, Kupper F, et al. PKHD1 mutations in autosomal recessive polycystic kidney disease (ARPKD). Hum Mutat. 2004;23:453-463.
Davis ID, Ho M, Hupertz V, et al. Survival of childhood polycystic kidney disease following renal transplantation: the impact of advanced hepatobiliary disease. Pediatr Transplant. 2003;7:364-369.
Dell KM, Avner ED. Autosomal recessive polycystic kidney disease. In GeneClinics: Clinical Genetic Information Resource (database online), http://www.geneclinics.org/.
Dell KM, Sweeney WE, Avner ED. Polycystic kidney disease. In: Avner ED, Harmon WE, Niaudet P, et al, editors. Pediatric nephrology. ed 6. Heidelburg, Germany: Springer-Verlag; 2009:849-888.
Delous M, Baala L, Salomon R, et al. The ciliary gene RPGRIP1l is mutated in cerebello-oculo-renal syndrome (Joubert syndrome type B) and Meckel syndrome. Nat Genet. 2007;39:875-881.
Guay-Woodford LM, Desmond RA. Autosomal recessive polycystic kidney disease: the clinical experience in North America. Pediatrics. 2003;111:1072-1080.
Guay-Woodford M, Parisi MA, Diherty D, et al. MKS3-related celiopathy with features of autosomal recessive polycystic kidney disease, nephronophthisis, and Joubert syndrome. J Pediatr. 2009;155:386-392.
Plaisier E, Gribouval O, Alamowitch S, et al. COL4A1 mutations and hereditary angiopathy nephropathy, aneurysms, and muscle cramps. N Engl J Med. 2007;357:2687-2695.
Rosetti S, Harris PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18:1374-1380.
Sweeney WEJr, Avner ED. Molecular and cellular pathophysiology of autosomal recessive polycystic kidney disease (ARPKD). Cell Tissue Res. 2006;326:671-685.
515.3 Autosomal Dominant Polycystic Kidney Disease
Autosomal dominant polycystic kidney disease (ADPKD) is the most common hereditary human kidney disease, with an incidence of 1/500 to 1/1,000.
Pathology
Both kidneys are enlarged and show cortical and medullary cysts originating from all regions of the nephron.
Pathogenesis
Approximately 85% of patients with ADPKD have mutations that map to the PKD1 gene on the short arm of chromosome 16, which encodes polycystin, a transmembrane glycoprotein. Another 10-15% of ADPKD mutations map to the PKD2 gene on the long arm of chromosome 4, which encodes polycystin 2, a proposed nonselective cation channel. The majority of mutations appear to be unique to a given family. At present, a mutation can be found in 90% of patients with well-characterized disease. Mutations of PKD1 are associated with more severe renal disease than mutations of PKD2. The pathophysiology of the disease appears to be related to disruption of normal multimeric cystoprotein complexes, with consequent abnormal intracellular signaling resulting in abnormal proliferation, tubular secretion, and cyst formation. Abnormal growth factor expression, coupled with low intracellular calcium and elevated cyclic adenosine monophosphate (cAMP), appear to be important features leading to formation of cysts and progressive enlargement.
Clinical Presentation
The severity of renal disease and the clinical manifestations of ADPKD are highly variable. Although symptomatic ADPKD commonly occurs in the 4th or 5th decade of life, symptoms, including gross or microscopic hematuria, bilateral flank pain, abdominal masses, hypertension, and urinary tract infection, may be seen in children and neonates. Renal ultrasonography usually demonstrates multiple bilateral macrocysts in enlarged kidneys (Fig. 515-3), although normal kidney size and unilateral disease may be seen in the early phase of the disease.
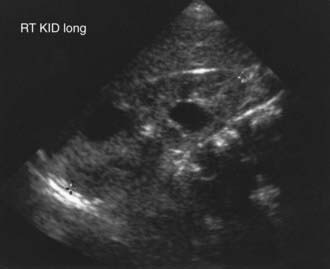
Figure 515-3 Ultrasound examination of an 18 mo old boy with autosomal dominant polycystic kidney disease demonstrating renal enlargement (10 cm) and two large cysts.
ADPKD is a systemic disorder affecting many organ systems. Cysts may be present within the liver, pancreas, spleen, and ovaries and when present help confirm the diagnosis. Intracranial aneurysms, which appear to cluster within certain families, have an overall prevalence of 5% and are an important cause of mortality in adults but are rarely reported in children. Mitral valve prolapse is seen in approximately 12% of children. Hernias and intestinal diverticula can also occur in these children. Renal cell carcinoma has been reported in association with ADPKD.
Diagnosis
ADPKD is confirmed by the presence of enlarged kidneys with bilateral macrocysts in a patient with an affected first-degree relative. De novo mutations occur in 5-10% of patients with newly diagnosed disease. The diagnosis might be made in children before their affected parent, making parental renal sonography an important diagnostic test in families with no apparent family history. Among patients with genetically defined ADPKD, screening renal ultrasonography results may be normal in ≤20% by 20 yr of age and <5% by 30 yr of age.
Prenatal diagnosis is suggested from the presence of enlarged kidneys with or without cysts on ultrasonography in families with known ADPKD. Prenatal DNA testing is available in families with affected members whose disease is caused by identified mutations in the PKD1 or PKD2 genes.
The differential diagnosis includes renal cysts associated with glomerulocystic kidney disease, tuberous sclerosis, and von Hippel-Lindau disease, which may be inherited in an autosomal dominant pattern (see Table 515-1). The neonatal manifestations of ADPKD and ARPKD may be indistinguishable.
Treatment and Prognosis
Treatment of ADPKD is primarily supportive. Control of blood pressure is critical because the rate of disease progression in ADPKD correlates with the presence of hypertension. Angiotensin-converting enzyme inhibitors and/or angiotensin II receptor antagonists are agents of choice. Obesity, caffeine ingestion, smoking, multiple pregnancies, male sex, and possibly the use of calcium channel blockers appear to accelerate disease progression. Older patients with a family history of intracranial aneurysm rupture may be considered candidates for screening for aneurysms.
Although neonatal ADPKD may be fatal, long-term survival of the patient and the kidneys is possible for children surviving the neonatal period. ADPKD that occurs initially in older children has a favorable prognosis, with normal renal function during childhood seen in >80% of children.
An important resource for patients and their families worldwide is the Polycystic Kidney Disease Foundation (www.pkdcure.org).
Bisceglia M, Galliani CA, Senger C, et al. Renal cystic diseases: a review. Adv Anat Pathol. 2006;13:26-56.
Dell KM, Sweeney WE, Avner ED. Polycystic kidney disease. In: Avner ED, Harmon W, Niaudet P, et al, editors. Pediatric nephrology. ed 6. Heidelburg, Germany: Springer-Verlag; 2009:849-888.
Fick-Brosnahan GM, Tran ZV, Johnson AM, et al. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int. 2001;59:1979-1980.
Grantham JJ. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359:1477-1484.
Harris PC. 2008 Homer W: Smith Award: insights into the pathogenesis of polycystic kidney disease from gene discovery. J Am Soc Nephrol. 2009;20:1188-1198.
Hildebrandt F. Genetic kidney diseases. Lancet. 2010;375:1287-1294.
Lau EC, Janson MM, Roesler MR, et al. Birth of a healthy infant following preimplantation PKHD1 haplotyping for autosomal recessive polycystic kidney disease using multiple displacement amplification. J Assist Reprod Genet. 2010;27(2):397-407.
Mekahli D, Woolf AS, Bockenhauer D. Similar renal outcomes in children with ADPKD diagnosed by screening or presenting with symptoms. Pediatr Nephrol. 2010;25:2275-2282.
Ong AC. Screening for intracranial aneurysms in ADPKD. BMJ. 2009;339:706-707.
Ong AC, Harris PC. Molecular pathogenesis of ADPKD: the polycystin complex gets complex. Kidney Int. 2005;67:1234-1247.
Takiar V, Caplan MJ. Telling kidneys to cease and decyst. Nat Med. 2010;16(7):751-752.
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet. 2007;369:1287-1301.
Verdeguer F, Le Corre S, Fischer E, et al. A mitotic transcriptional switch in polycystic kidney disease. Nat Med. 2010;16(1):106-110.
515.4 Trauma
Infants and children are more susceptible to renal injury following blunt or penetrating injury to the back or abdomen owing to their decreased muscle mass “protecting” the kidney. Gross or microscopic hematuria, flank pain, and abdominal rigidity can occur; associated injuries may be present (Chapter 66). In the absence of hemodynamic instability, most renal trauma can be managed nonoperatively. Urethral trauma can result from crush injury, often associated with a fractured pelvis or from direct injury. Such injury is suspected when gross blood appears at the external urethral meatus. Rhabdomyolysis and consequent renal failure is another complication of crush injury that can be ameliorated by vigorous fluid resuscitation.
Dreitlein DA, Suner S, Basler J. Genitourinary trauma. Emerg Med Clin North Am. 2001;19:569-590.
Gunal AI, Celiker H, Dogukan A, et al. Early and vigorous fluid resuscitation prevents acute renal failure in the crush victims of catastrophic earthquakes. J Am Soc Nephrol. 2004;15:1862-1867.
Henderson CG, Sedberry-Ross S, Pickard R, et al. Management of high grade renal trauma: 20-year experience at a pediatric level 1 trauma center. J Urol. 2007;178:246-250.