Chapter 579 Gynecomastia
Gynecomastia, the proliferation of mammary glandular tissue in the male, is a common condition. True gynecomastia (the presence of glandular breast tissue) needs to be distinguished from pseudogynecomastia due to accumulation of adipose tissue in the area of the breast that is commonly seen in overweight boys. True gynecomastia is characterized by the presence of a palpable fibroglandular mass at least 0.5 cm in diameter, located concentrically beneath the nipple and areaolar region.
Physiologic Forms of Gynecomastia: Gynecomastia occurs in many newborn males as a result of normal stimulation by maternal estrogen; the effect usually disappears in a few weeks. It is then extremely rare in prepubertal boys, in whom it should always be investigated to identify the cause. It is very common in puberty.
Neonatal Gynecomastia: Transient gynecomastia occurs in 60-90% of male newborns secondary to exposure to estrogens during pregnancy. Breast development may be asymmetrical and galactorrhea is seen in approximately 5%. Most cases resolve within 4-8 wk of birth, but a few can last as long as 12 mo.
Pubertal Gynecomastia: During early to mid-puberty, up to 65% of boys develop various degrees of subareolar hyperplasia of the breasts. Incidence peaks at 14 yr of age, at Tanner stage 3-4 and at a testicular volume of 5-10 mL. Physiologic pubertal gynecomastia may involve only 1 breast; it is not unusual for both breasts to enlarge at disproportionate rates or at different times. Tenderness of the breast is common but transitory. Spontaneous regression may occur within a few months; it rarely persists longer than 2 yr. Significant psychosocial distress may be present, especially in obese boys with relatively large breasts.
The cause is thought to be an imbalance between estrogen and androgen action at the level of breast tissue. Testing usually fails to reveal any significant difference in circulating estrogen and androgen levels between affected and unaffected males, but minor degrees of imbalance in free hormone levels may still be present. Other hormones including leptin and luteinizing hormone (LH) may directly stimulate breast development and may play an important role in pubertal gynecomastia. Some cases may be due to an increased sensitivity to estrogens and/or relative androgen resistance in the affected tissue. As androgen levels continue to rise in later puberty, most cases resolve.
Pathological Gynecomastia: Monogenic forms of gynecomastia are extremely rare, but do exist. Familial gynecomastia has occurred in several kindreds as an X-linked or autosomal dominant sex-limited trait. Some of these cases were found to be due to constitutive activation of the p450 aromatase enzyme (CYP19A1 gene), leading to increased peripheral conversion of C-19 steroids to estrogens (increased aromatization). A report of this syndrome in a father and his son and daughter suggests autosomal dominant inheritance. There was gynecomastia in the 9 yr old boy and macromastia and isosexual precocity in his 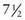 yr old sister. Excess aromatase activity was shown in skin fibroblasts and transformed lymphocytes in vitro.
yr old sister. Excess aromatase activity was shown in skin fibroblasts and transformed lymphocytes in vitro.
Exogenous sources of estrogens are an important cause of gynecomastia in prepubertal children. Very small amounts of estrogens can cause gynecomastia in male children and accidental exposure may occur by inhalation, percutaneous absorption, or ingestion. Common sources of estrogens include oral contraceptive pills, and oral and transdermal estrogen preparations. Gynecomastia has been reported in workers involved in the manufacture of estrogens and even in the children of such workers. Gynecomastia can also occur secondary to exposure to medications that decrease levels of androgens (especially free androgens), increase estradiol, or displace androgens from breast androgen receptors. Spironolactone, alkylating agents, anabolic steroids, human chorionic gonadotropin (hCG), ketoconazole, cimetidine, and androgen inhibitors such as flutamide are all associated with the occurrence of gynecomastia. Weaker associations are seen with a large number of other medications and drugs of abuse (opiates, alcohol) though the association with marijuana may not be as strong as previously thought. Lavender and certain tea oils have also been implicated as a cause of prepubertal gynecomastia.
Klinefelter syndrome and other causes of hypergonadotropic hypogonadism are strongly associated with gynecomastia. It is also seen in other conditions characterized by male undervirilization, including androgen insensitivity syndromes and 17-ketosteroid reductase deficiency. Gynecomastia has been observed in children with congenital virilizing adrenal hyperplasia (11β-hydroxylase deficiency) and may be associated with Leydig cell tumors of the testis or with feminizing tumors of the adrenal gland. Several boys with Peutz-Jeghers syndrome and gynecomastia had sex cord tumors of the testes. The testes may not be enlarged in these cases and the tumor is usually multifocal and bilateral. Excessive aromatase production accounts for the gynecomastia. When gynecomastia is associated with galactorrhea, a prolactinoma should be considered. Hyperthyroidism alters the androgen to estrogen ratio by increasing bound androgen and decreasing the free testosterone and may result in gynecomastia in up to 40% of cases. Gynecomastia is also seen in malnourished patients after restoration of normal nutrition (refeeding syndrome), in whom it may be due to hepatic dysfunction or abnormal activation of the gonadotropin axis.
Evaluation of Gynecomastia: In pubertal cases a detailed history and physical examination is all that is needed to exclude rare pathologic causes. Historical evaluation should include family history of male relatives with gynecomastia, history of liver or renal disease, use of medications or drugs of abuse, and exposure to herbal and cosmetic products that may contain phytoestrogens. Physical examination should include special attention to the breasts (looking for overlying skin changes, fixation, local lymphadenopathy, and nipple discharge) as well as a testicular exam. No laboratory evaluation is indicated in routine cases. All prepubertal cases, as well as pubertal cases with suspicious features should be investigated; initial laboratory evaluation should include thyroid function tests (to rule out hyperthyroidism), testosterone, estradiol, hCG and LH levels. If there is any evidence of galactorrhea, then prolactin level should also be obtained. Due to circadian variation, these levels should ideally be obtained in the morning. Other tests that may be indicated in selected cases include a karyotype, DHEAS, and liver and renal function tests.
Treatment in case of benign pubertal gynecomastia usually consists of reassuring the boy and his family of the physiologic and transient nature of the phenomenon. When the enlargement is striking and persistent and causes serious emotional disturbance to the patient, specific treatment may be justified. Unfortunately, medical treatment is generally ineffective in long standing cases. Early cases respond better to medical treatment but it is harder to justify treatment since most cases will resolve spontaneously. Agents that may be used for such treatment include androgens, aromatase inhibitors, and estrogen antagonists. The effectiveness of synthetic androgens is variable and side effects are a concern, so these are rarely used in pediatrics. Aromatase inhibitors make physiologic sense, but placebo-controlled trials have been disappointing. Estrogen antagonists like tamoxifen and raloxifene are more effective, with raloxifene being the superior agent in at least 1 well designed trial. Therefore, if medical treatment is attempted, it should be in early cases (less than 12 mo standing) using raloxifene (in a dose of 60 mg/day) or tamoxifen (10-20 mg/day) for 3-9 mo.
In those cases where breast development is excessive (Tanner stages 3-5), causes significant psychologic distress or fails to regress in 18-24 mo, surgical removal of the enlarged breast tissue is indicated. Various surgical approaches, including ultrasound assisted liposuction, are available.
Felner EI, White PC. Prepubertal gynecomastia: indirect exposure to estrogen cream. Pediatrics. 2000;105:1-3.
Henley DV, Lipson N, Korach KS, et al. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007;356:479-485.
Lawrence SE, Faught KA, Vethamuthu J, et al. Beneficial effects of raloxifene and tamoxifen in the treatment of pubertal gynecomastia. J Pediatr. 2004;145:71-76.
Ma NS, Geffner ME. Gynecomastia in prepubertal and pubertal men. Curr Opin Pediatr. 2008;20:465-470.
Narula HS, Carlson HE. Gynecomastia. Endocrinol Metab Clin North Am. 2007;36:497-519.
Nordt CA, DiVasta AD. Gynecomastia in adolescents. Curr Opin Pediatr. 2008;20:375-382.
Pacynski A, Budzynska A, Przylecki S, et al. Hyperestrogenism in pharmaceutical factory workers and their children as an occupational disease. Endokrynol Pol. 1971;22:149-154.
Plourde PV, Reiter EO, Jou H, et al. Safety and efficacy of anastrozole for the treatment of pubertal gynecomastia: a randomized, double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89:4428-4433.
Stratakis CA, Vottero A, Brodies A, et al. The aromatase excess syndrome is associated with feminization of both sexes and autosomal dominant transmission of aberrant P450 aromatase gene transcription. J Clin Endocrinol Metab. 1998;83:1348-1357.