Chapter 687 Disorders Involving Transmembrane Receptors
Disorders involving transmembrane receptors result from heterozygous mutations of genes encoding FGFR3 (fibroblast growth factor receptor 3) and PTHR (parathyroid hormone receptor). The mutations cause the receptors to become activated in the absence of physiologic ligands, which accentuates normal receptor function of negatively regulating bone growth. The mutations act by gain of negative function. In the FGFR3 mutation group, in which the clinical phenotypes range from severe to mild, the severity appears to correlate with the extent to which the receptor is activated. PTHR and especially FGFR3 mutations tend to recur in unrelated individuals.
Achondroplasia Group
The achondroplasia group represents a substantial percentage of patients with chondrodysplasias and contains thanatophoric dysplasia (TD), the most common lethal chondrodysplasia, with a birth prevalence of 1/35,000 births; achondroplasia, the most common nonlethal chondrodysplasia, with a birth prevalence of 1/15,000 to 1/40,000 births; and hypochondroplasia. All three have mutations in a small number of locations in the FGFR3 gene. There is a strong correlation between the mutation site and the clinical phenotype.
Thanatophoric Dysplasia
TD (OMIM 187600, 187610) manifests before or at birth. In the former situation, ultrasonographic examination in midgestation or later reveals a large head and very short limbs; the pregnancy is often accompanied by polyhydramnios and premature delivery. Very short limbs, short neck, long narrow thorax, and large head with midfacial hypoplasia dominate the clinical phenotype at birth (Fig. 687-1). The cloverleaf skull deformity known as kleeblattschädel is sometimes found. Newborns have severe respiratory distress because of their small thorax. Although this distress can be treated by intense respiratory care, the long-term prognosis is poor.
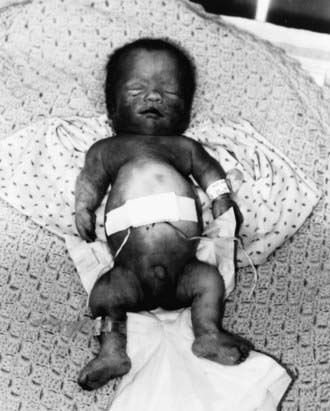
Figure 687-1 Stillborn infant with thanatophoric dysplasia. Limbs are very short, with upper limbs extending only two thirds of the way down the abdomen. The chest is narrow, exaggerating the protuberance of the abdomen. The head is relatively large.
Skeletal radiographs distinguish two slightly different forms called TD I and TD II. In the more common TD I, radiographs show large calvariae with a small cranial base, marked thinning and flattening of vertebral bodies visualized best on lateral view, very short ribs, severe hypoplasia of pelvic bones, and very short and bowed tubular bones with flared metaphyses (Fig. 687-2). The femurs are curved and shaped like a telephone receiver. TD II differs mainly in that there are longer and straighter femurs.
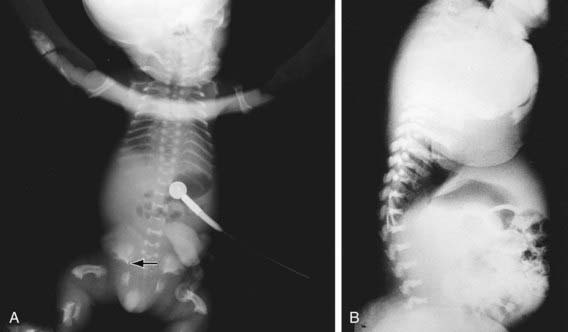
Figure 687-2 A, Neonatal radiograph of a child with thanatophoric dysplasia. Note medial acetabular spurs (arrow), hypoplastic iliac bones, bowed femora with rounded protrusion of proximal femurs, hypoplastic thorax, and wafer-thin vertebral bodies. B, Lateral radiograph of the thoracolumbar spine in thanatophoric dysplasia, showing marked vertebral flattening and short ribs. Ossification defect of the central portion of the vertebral bodies is present.
The TD II clinical phenotype is associated with mutations that map to codon 650 of FGFR3, causing the substitution of a glutamic acid for the lysine. This activates the tyrosine kinase activity of a receptor that transmits signals to intracellular pathways. Mutation of lysine 650 to methionine is associated with a clinical phenotype intermediate between TD and achondroplasia, referred to as severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN). Mutations of the TD I phenotype map mainly to two regions in the extracellular domain of the receptor, where they substitute cysteine residues for other amino acids. Free cysteine residues are thought to form disulfide bonds promoting dimerization of receptor molecules, leading to activation and signal transmission.
TD I and TD II represent new mutations to normal parents. The recurrence risk is low. Because the mutated codons in TD are mutable for unknown reasons and because of the theoretical risk of germ cell mosaicism, parents are offered prenatal diagnosis for subsequent pregnancies.
Achondroplasia
Achondroplasia (OMIM 100800) is the prototype chondrodysplasia. It typically manifests at birth with short limbs, a long narrow trunk, and a large head with midfacial hypoplasia and prominent forehead (Fig. 687-3). The limb shortening is greatest in the proximal segments, and the fingers often display a trident configuration. Most joints are hyperextensible, but extension is restricted at the elbow. A thoracolumbar gibbus is often found. Usually, birth length is slightly less than normal but occasionally plots within the low-normal range.
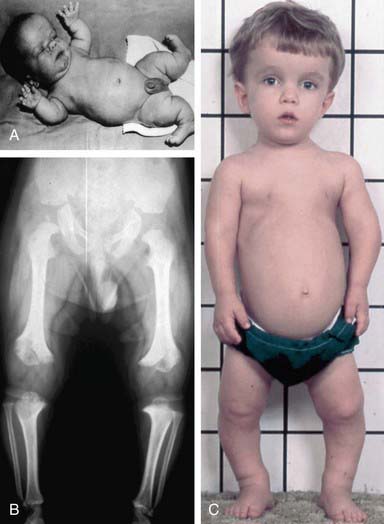
Figure 687-3 Achondroplasia phenotype at different ages. A, Infant with achondroplasia with macrocephaly, frontal bossing, midface hypoplasia, small chest, rhizomelic shortening of all the limbs, redundant skin folds, and extreme joint laxity. Note the trident hand with short fingers and abducted hips. B, Typical radiographic findings from a child with achondroplasia. All of the tubular bones are short, but the fibula is relatively long compared to the tibia. There is protrusion of the epiphysis into the metaphysis of the distal femur, creating the chevron deformity, and to a lesser extent of the proximal tibia. The iliac bones are rounded, the acetabular roof is horizontal, and the sacrosciatic notches are small. C, A 3 yr old with achondroplasia with the typical features shown in A. Note that the redundant skin folds are no longer present and that joint laxity has improved. Rhizomelic shortening of the extremities is more pronounced and accompanied by tibial bowing.
(From Horton WA, Hall JG, Hecht JT: Achondroplasia, Lancet 370:162–172, 2007.)
Diagnosis
Skeletal radiographs confirm the diagnosis (Figs. 687-3 and 687-4). The calvarial bones are large, whereas the cranial base and facial bones are small. The vertebral pedicles are short throughout the spine as noted on a lateral radiograph. The interpedicular distance, which normally increases from the 1st to the 5th lumbar vertebra, decreases in achondroplasia. The iliac bones are short and round, and the acetabular roofs are flat. The tubular bones are short with mildly irregular and flared metaphyses. The fibula is disproportionately long compared with the tibia.
Clinical Manifestations
Infants usually exhibit delayed motor milestones, often not walking alone until 18-24 mo. This is due to hypotonia and mechanical difficulty balancing the large head on a normal-sized trunk and short extremities. Intelligence is normal unless central nervous system complications develop. As the child begins to walk, the gibbus usually gives way to an exaggerated lumbar lordosis.
Infants and children with achondroplasia progressively fall below normal standards for length and height. They can be plotted against standards established for achondroplasia. Adult heights typically are 118-145 cm for men and 112-136 cm for women. Surgical limb lengthening and human growth hormone treatment have been used to increase height; both are controversial.
Virtually all infants and children with achondroplasia have large heads, although only a fraction have true hydrocephalus. Head circumference should be carefully monitored using standards developed for achondroplasia, as should neurologic function in general. The spinal canal is stenotic, and spinal cord compression can occur at the foramen magnum and in the lumbar spine. The former usually occurs in infants and small children; it may be associated with hypotonia, failure to thrive, quadriparesis, central and obstructive apnea, and sudden death. Surgical correction may be required for severe stenosis. Lumbar spinal stenosis usually does not occur until early adulthood. Symptoms include paresthesias, numbness, and claudication in the legs. Loss of bladder and bowel control may be late complications.
Bowing of the legs is common and might need to be corrected surgically. Other common problems include dental crowding, articulation difficulties, obesity, and frequent episodes of otitis media, which can contribute to hearing loss.
Genetics
All patients with typical achondroplasia have mutations at FGFR3 codon 380. The mutation maps to the transmembrane domain of the receptor and is thought to stabilize receptor dimers that enhance receptor signals, the consequences of which inhibit linear bone growth. Achondroplasia behaves as an autosomal dominant trait; most cases arise from a new mutation to normal parents.
Because of the high frequency of achondroplasia among dwarfing conditions, it is relatively common for adults with achondroplasia to marry. Such couples have a 50% risk of transmitting their condition, heterozygous achondroplasia, to each offspring, as well as a 25% risk of homozygous achondroplasia. The latter condition exhibits intermediate severity between thanatophoric dysplasia and heterozygous achondroplasia and is usually lethal in the newborn period. Prenatal diagnosis is available and has been used to diagnose homozygous achondroplasia.
Hypochondroplasia
Hypochondroplasia (OMIM 146000) resembles achondroplasia but is milder. Usually, it is not apparent until childhood, when mild short stature affecting the limbs becomes evident. Children have a stocky build and slight frontal bossing of the head. Learning disabilities may be more common in this condition. Radiographic changes are mild and consistent with the mild achondroplastic phenotype. Complications are rare; in some patients the condition is never diagnosed. Adult heights range from 116 to 146 cm. An FGFR3 mutation at codon 540 has been found in many patients with hypochondroplasia. Genetic heterogeneity exists in hypochondroplasia, and other genetic loci are expected to be identified.
Jansen Metaphyseal Dysplasia
Jansen metaphyseal chondrodysplasia (OMIM 156400) is a rare, dominantly inherited chondrodysplasia characterized by severe shortening of limbs associated with an unusual facial appearance. Sometimes it is accompanied by clubfoot and hypercalcemia. At birth, a diagnosis can be made from these clinical findings and radiographs that show short tubular bones with characteristic metaphyseal abnormalities that include flaring, irregular mineralization, fragmentation, and widening of the physeal space. The epiphyses are normal.
The joints become enlarged and limited in mobility with age. Flexion contractures develop at the knees and hips, producing a bent-over posture. Intelligence is normal, although there may be hearing loss.
Jansen metaphyseal chondrodysplasia is caused by activating mutations of PTHR1. This G protein–coupled transmembrane receptor serves as a receptor for both PTH and PTHrP. Signaling through this receptor serves as a brake on the terminal differentiation of cartilage cells at a critical step in bone growth. Because the mutations activate the receptor, they enhance the braking effect and thereby slow bone growth. Loss of function mutations of PTHR1 are observed in Blomstrand chondrodysplasia, whose clinical features are the mirror image of Jansen metaphyseal chondrodysplasia.
American Academy of Pediatrics Committee on Genetics. Health supervision for children with achondroplasia. Pediatrics. 1995;95:443-451.
Collins WO, Choi SS. Otolaryngologic manifestations of achondroplasia. Arch Otolaryngol Head Neck Surg. 2007;133:237-244.
Ednick M, Tinkle BT, Phromchairak J, et al. Sleep-related respiratory abnormalities and arousal pattern in achomdroplasia during early infancy. J Pediatr. 2009;155:510-515.
Ho NC, Guarnieri M, Brant LJ, et al. Living with achondroplasia: quality of life evaluation following cervico-medullary decompression. Am J Med Genet A. 2004;131:163-167.
Hoover-Fong JE, McGready J, Schulze KJ, et al. Weight for age charts for children with achondroplasia. Am J Med Genet A. 2007;143:2227-2235.
Horton WA, Hall JG, Hecht JT. Achondroplasia. Lancet. 2007;370:162-172.
Schipani E, Langman CB, Parfitt AM, et al. Constitutively activated receptors for parathyroid hormone and parathyroid hormone–related peptide in Jansen’s metaphyseal chondrodysplasia. N Engl J Med. 1996;335:708-714.
Sciubba DM, Noggle JC, Marupudi NI, et al. Spinal stenosis surgery in pediatric patients with achondroplasia. J Neurosurg. 2007;106:372-378.
