Chapter 692 Osteogenesis Imperfecta
Osteoporosis, a feature of both inherited and acquired disorders, classically demonstrates fragility of the skeletal system and a susceptibility to fractures of the long bones or vertebral compressions from mild or inconsequential trauma. Osteogenesis imperfecta (OI) (brittle bone disease), the most common genetic cause of osteoporosis, is a generalized disorder of connective tissue. The spectrum of OI is extremely broad, ranging from forms that are lethal in the perinatal period to a mild form in which the diagnosis may be equivocal in an adult.
Etiology
Structural or quantitative defects in type I collagen cause the full clinical spectrum of OI. Type I collagen is the primary component of the extracellular matrix of bone and skin. Ten percent of cases clinically indistinguishable from OI do not have a molecular defect in type I collagen. Some of these cases have biochemically normal collagen and unknown genetic defects. Other cases have overmodified collagen and severe or lethal OI-like bone dysplasia. These cases are caused by recessive null mutations in a collagen-modifying enzyme, prolyl 3-hydroxylase 1 (coded by the LEPRE1 gene on chromosome 1p34.1) or its associated protein, CRTAP.
Epidemiology
The autosomal dominant forms of OI occur equally in all racial and ethnic groups, and recessive forms occur predominantly in ethnic groups with consanguineous marriages. The West African founder mutation for type VIII OI has a carrier frequency of 1/200-300 among African Americans. The incidence of OI detectable in infancy is about 1/20,000. There is a similar incidence of the mild form OI type I.
Pathology
The collagen structural mutations cause OI bone to be globally abnormal. The bone matrix contains abnormal type I collagen fibrils and relatively increased levels of types III and V collagen. Several noncollagenous proteins of bone matrix are also reduced. The hydroxyapatite crystals deposited on this matrix are poorly aligned with the long axis of fibrils.
Pathogenesis
Type I collagen is a heterotrimer composed of two α1(I) chains and one α2(I) chain. The chains are synthesized as procollagen molecules with short globular extensions on both ends of the central helical domain. The helical domain is composed of uninterrupted repeats of the sequence Gly-X-Y, where Gly is glycine, X is often proline, and Y is often hydroxyproline. The presence of glycine at every 3rd residue is crucial to helix formation because its small side chain can be accommodated in the interior of the helix. The chains are assembled at their carboxyl ends; helix formation then proceeds linearly in a carboxyl to amino direction. Concomitant with helix assembly and formation, helical proline and lysine residues are hydroxylated by prolyl 4-hydroxylase and lysyl hydroxylase and some hydroxylysine residues are glycosylated.
Collagen structural defects are predominantly of two types: 80% are point mutations causing substitutions of helical glycine residues or crucial residues in the C-propeptide by other amino acids, and 20% are single exon splicing defects. The clinically mild OI type I has a quantitative defect, with null mutations in one α1(I) allele leading to a reduced amount of normal collagen.
The glycine substitutions in the two α chains have distinct genotype-phenotype relationships. One third of mutations in the α1 chain are lethal, and those in α2(I) are predominantly nonlethal. Two lethal regions in α1(I) align with major ligand binding regions of the collagen helix. Lethal mutations in α2(I) occur in 8 regularly spaced clusters along the chain that align with binding regions for matrix proteoglycans in the collagen fibril.
Classic OI is an autosomal dominant disorder. Some familial recurrences of OI are caused by parental mosaicism for dominant collagen mutations. Recessive OI (types VII and VIII) accounts for 5-7% of new OI in North America. These types are caused by null mutations in the genes coding for two of the components of the collagen prolyl 3-hydroxylation complex in the endoplasmic reticulum, LEPRE1, which encodes P3H1, or CRTAP. This complex is responsible for post-translational modification of a single proline residue, P986, on the α1(I) chains. It is not yet clear whether absence of the complex or the modification is the crucial feature of recessive OI.
Clinical Manifestations
OI has the triad of fragile bones, blue sclerae, and early deafness. OI was once divided into “congenita,” the forms detectable at birth, and “tarda,” the forms detectable later in childhood; this did not account for the variability of OI. The Sillence classification divides OI into four types based on clinical and radiographic criteria. Additional types have been proposed based on histologic distinctions.
Osteogenesis Imperfecta Type I (Mild)
OI type I is sufficiently mild that it is often found in large pedigrees. Many type I families have blue sclerae, recurrent fractures in childhood, and presenile hearing loss (30-60%). Both types I and IV are divided into A and B subtypes, depending on the absence (A) or presence (B) of dentinogenesis imperfecta. Other possible connective tissue abnormalities include easy bruising, joint laxity, and mild short stature compared with family members. Fractures result from mild to moderate trauma and decrease after puberty.
Osteogenesis Imperfecta Type II (Perinatal Lethal)
Infants with OI type II may be stillborn or die in the 1st yr of life. Birthweight and length are small for gestational age. There is extreme fragility of the skeleton and other connective tissues. There are multiple intrauterine fractures of long bones, which have a crumpled appearance on radiographs. There are striking micromelia and bowing of extremities; the legs are held abducted at right angles to the body in the frog-leg position. Multiple rib fractures create a beaded appearance, and the small thorax contributes to respiratory insufficiency. The skull is large for body size, with enlarged anterior and posterior fontanels. Sclerae are dark blue-gray.
Osteogenesis Imperfecta Type III (Progressive Deforming)
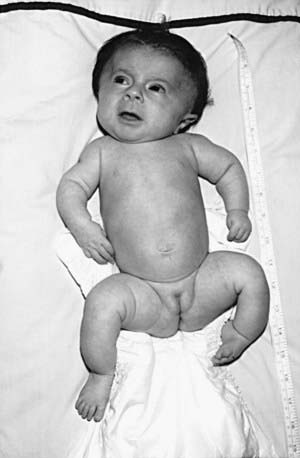
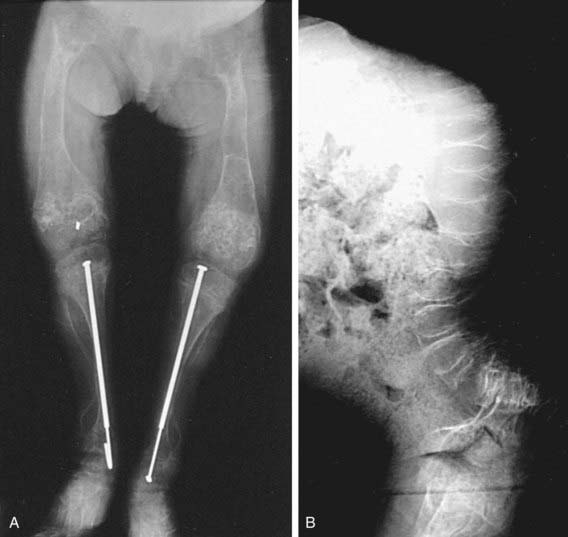
OI type III is the most severe nonlethal form of OI and results in significant physical disability. Birthweight and length are often low normal. Fractures usually occur in utero. There is relative macrocephaly and triangular facies (Fig. 692-1). Postnatally, fractures occur from inconsequential trauma and heal with deformity. Disorganization of the bone matrix results in a “popcorn” appearance at the metaphyses (Fig. 692-2). The rib cage has flaring at the base, and pectal deformity is frequent. Virtually all type III patients have scoliosis and vertebral compression. Growth falls below the curve by the 1st year; all type III patients have extreme short stature. Scleral hue ranges from white to blue.
Osteogenesis Imperfecta Type IV (Moderately Severe)
Patients with OI type IV can present at birth with in utero fractures or bowing of lower long bones. They can also present with recurrent fractures after ambulation. Most children have moderate bowing even with infrequent fractures. Children with OI type IV require orthopedic and rehabilitation intervention, but they are usually able to attain community ambulation skills. Fracture rates decrease after puberty. Radiographically, they are osteoporotic and have metaphyseal flaring and vertebral compressions. Patients with type IV have moderate short stature. Scleral hue may be blue or white.
Osteogenesis Imperfecta Type V (Hyperplastic Callus) and Type VI (Mineralization Defect)
Types V and VI OI patients clinically have OI type IV, but they have distinct findings on bone histology. Type V patients also have hyperplastic callus, calcification of the interosseous membrane of the forearm, and a radiodense metaphyseal band. The absence of a collagen defect supports the distinct grouping; the genetic etiology is unknown. They constitute <5% of OI populations.
Osteogenesis Imperfecta Types VII and VIII (Recessive Form)
Types VII and VIII patients have null mutations in P3H1 and CRTAP, respectively. Clinically they overlap types II and III OI but have distinct features including white sclerae, rhizomelia, and small to normal head circumference. Surviving children have severe osteochondrodysplasia with extreme short stature and dual energy x-ray absorptiometry (DEXA) L1-L2 z-score in the −6 to −7 range.
Laboratory Findings
The diagnosis of types I-IV and VII/VIII is confirmed by collagen biochemical studies using cultured dermal fibroblasts. Both dominant and recessive types have a positive biochemical test (over modified collagen on gel electrophoresis), although dominant cases caused by collagen defects in the amino third of the chains have a false negative biochemical test. In OI type I, the reduced amount of type I collagen results in an increase in the ratio of type III to type I collagen on gel electrophoresis. DNA sequencing to identify mutations in COL1A1, COL1A2, LEPRE1, or CRTAP is useful to distinguish types and to facilitate family screening and prenatal diagnosis.
Severe OI can be detected prenatally by level II ultrasonography as early as 16 wk of gestation. OI and thanatophoric dysplasia may be confused. Fetal ultrasonography might not detect OI type IV and rarely detects OI type I. For recurrent cases, chorionic villus biopsy can be used for biochemical or molecular studies. Amniocytes produce false-positive biochemical studies but can be used for molecular studies in appropriate cases.
In the neonatal period, the normal to elevated alkaline phosphatase levels present in OI distinguish it from hypophosphatasia.
Complications
The morbidity and mortality of OI are cardiopulmonary. Recurrent pneumonias and declining pulmonary function occur in childhood, and cor pulmonale is seen in adults.
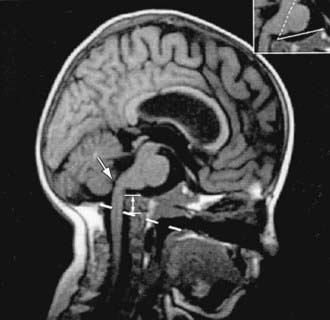
Neurologic complications include basilar invagination, brainstem compression, hydrocephalus, and syringohydromyelia. Most children with OI types III and IV have basilar invagination, but brainstem compression is uncommon. Basilar invagination is best detected with spiral CT of the craniocervical junction (Fig. 692-3).
Treatment
There is no cure for OI. For severe nonlethal OI, active physical rehabilitation in the early years allows children to attain a higher functional level than orthopedic management alone. Children with OI type I and some with type IV are spontaneous ambulators. Children with types III and IV OI benefit from gait aids and a program of swimming and conditioning. Severely affected patients require a wheelchair for community mobility but can acquire transfer and self-care skills. Teens with OI can require psychologic support with body image issues. Growth hormone improves bone histology in growth-responsive children (usually types I and IV).
Orthopedic management of OI is aimed at fracture management and correction of deformity to enable function. Fractures should be promptly splinted or cast; OI fractures heal well, and cast removal should be aimed at minimizing immobilization osteoporosis. Correction of long bone deformity requires an osteotomy procedure and placement of an intramedullary rod.
A several-year course of treatment of children with OI with bisphosphonates (IV pamidronate or oral olpadronate) confers some benefits. Bisphosphonates decrease bone resorption by osteoclasts; OI patients have increased bone volume that still contains the defective collagen. Bisphosphonates are more beneficial for vertebrae (trabecular bone) than long bones (cortical bone). Treatment for 1-2 yr results in increased L1-4 DEXA and, more importantly, improved vertebral compressions and area, which can prevent or delay the scoliosis of OI. Relative risk of long bone fractures is modestly decreased. However, the material properties of long bones are weakened by prolonged treatment and nonunion after osteotomy is increased. There is no effect of bisphosphonates on mobility scores, muscle strength, or bone pain. Limiting treatment duration to 2-3 yr in mid-childhood can maximize benefits and minimize detriment to cortical material properties. Benefits appear to persist several years after the treatment interval. Side effects include abnormal long bone remodeling, increased incidence of fracture non-union, and osteopetrotic-like brittleness to bone.
Prognosis
OI is a chronic condition that limits both life span and functional level. Infants with OI type II usually die within months to a year of life. An occasional child with radiographic type II and extreme growth deficiency survives to the teen years. Persons with OI type III have a reduced life span with clusters of mortality from pulmonary causes in early childhood, the teen years, and the 40s. OI types I and IV are compatible with a full life span.
Individuals with OI type III are usually wheelchair dependent. With aggressive rehabilitation, they can attain transfer skills and household ambulation. OI type IV children usually attain community ambulation skills either independently or with gait aids.
Genetic Counseling
For autosomal dominant OI, the risk of an affected individual passing the gene to his or her offspring is 50%. An affected child usually has about the same severity of OI as the parent; however, there is variability of expression, and the child’s condition can be either more or less severe than that of the parent. The empirical recurrence risk to an apparently unaffected couple of having a second child with OI is 5-7%; this is the statistical chance that one parent has germ line mosaicism. The collagen mutation in the mosaic parent is present in some germ cells and may be present in somatic tissues. If a parent is a mosaic carrier, the risk of recurrence may be as high as 50%. For recessive OI, the recurrence risk is 25% per pregnancy. No known individual with severe nonlethal recessive OI has had a child.
Antoniazzi F, Bertoldo F, Mottes M, et al. Growth hormone treatment in osteogenesis imperfecta with quantitative defect of type I collagen synthesis. J Pediatr. 1996;129:432-439.
Åström E, Jorulf H, Söderhäll S. Intravenous pamidronate treatment of infants with severe osteogenesis imperfecta. Arch Dis Child. 2007;92:332-338.
Barnes AM, Chang W, Morello R, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med. 2006;355:2757-2764.
Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nat Genet. 2007;39:359-365.
Glorieux FH, Rauch F, Plotkin H, et al. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000;15:1650-1658.
Glorieux FH, Rauch F, Ward LM, et al. Alendronate in the treatment of pediatric osteogenesis imperfecta. J Bone Miner Res. 2004;19(Suppl 1):S12.
Glorieux FH, Ward LM, Rauch F, et al. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res. 2002;17:30-38.
Letocha A, Cintas HL, Troendle JF, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res. 2005;20:977-986.
Marini JC. Should children with osteogenesis imperfecta be treated with bisphosphonates? Nat Clin Prac Endocrinol Metab. 2006;2:14-15.
Marini JC, Hopkins E, Glorieux FH, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta. J Bone Miner Res. 2003;18:237-243.
Plotkin H, Rauch F, Bishop NJ. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab. 2000;85:1846-1850.
Sakkers R, Kok D, Engelbert R, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomized placebo-controlled study. Lancet. 2004;363:1427-1431.