Structure and Function of the Neurologic System
Karen C. Turner
![]() http://evolve.elsevier.com/Rogers/pathophysiology/
http://evolve.elsevier.com/Rogers/pathophysiology/
The human nervous system is a remarkable structure responsible for decision-making, the body's ability to interact with the environment, and the regulation and control of activities involving our internal organs, muscles, and glands. It is a network composed of complex structures that transmit electrical and chemical signals between the brain and the body's many organs and tissues. This chapter provides a basic overview of the structure and function of the nervous system and supports the understanding of nervous system pathophysiology in the following chapters.
Overview and Organization of the Nervous System
Although the nervous system functions as a unified whole, structures and functions have been divided here to facilitate understanding. Structurally, the nervous system is divided into the central nervous system and the peripheral nervous system (Fig. 15.1). The central nervous system (CNS) consists of the brain and spinal cord, enclosed within the protective cranial vault and vertebrae, respectively. The peripheral nervous system (PNS) is composed of the cranial nerves, which project from the brain, and the spinal nerves, which project from the spinal cord. Cranial nerves can be viewed as modified spinal nerves. Cranial nerves control motor and sensory functions similarly to spinal nerves and have specialized sensory tasks, such as smell, taste, sight, and hearing (see section on Peripheral Nervous System). PNS pathways are differentiated into afferent pathways (ascending pathways), which carry sensory impulses toward the CNS, and efferent pathways (descending pathways), which innervate skeletal muscle or effector organs by transmitting motor impulses away from the CNS. Most peripheral nerves carry a combination of both afferent and efferent pathways.
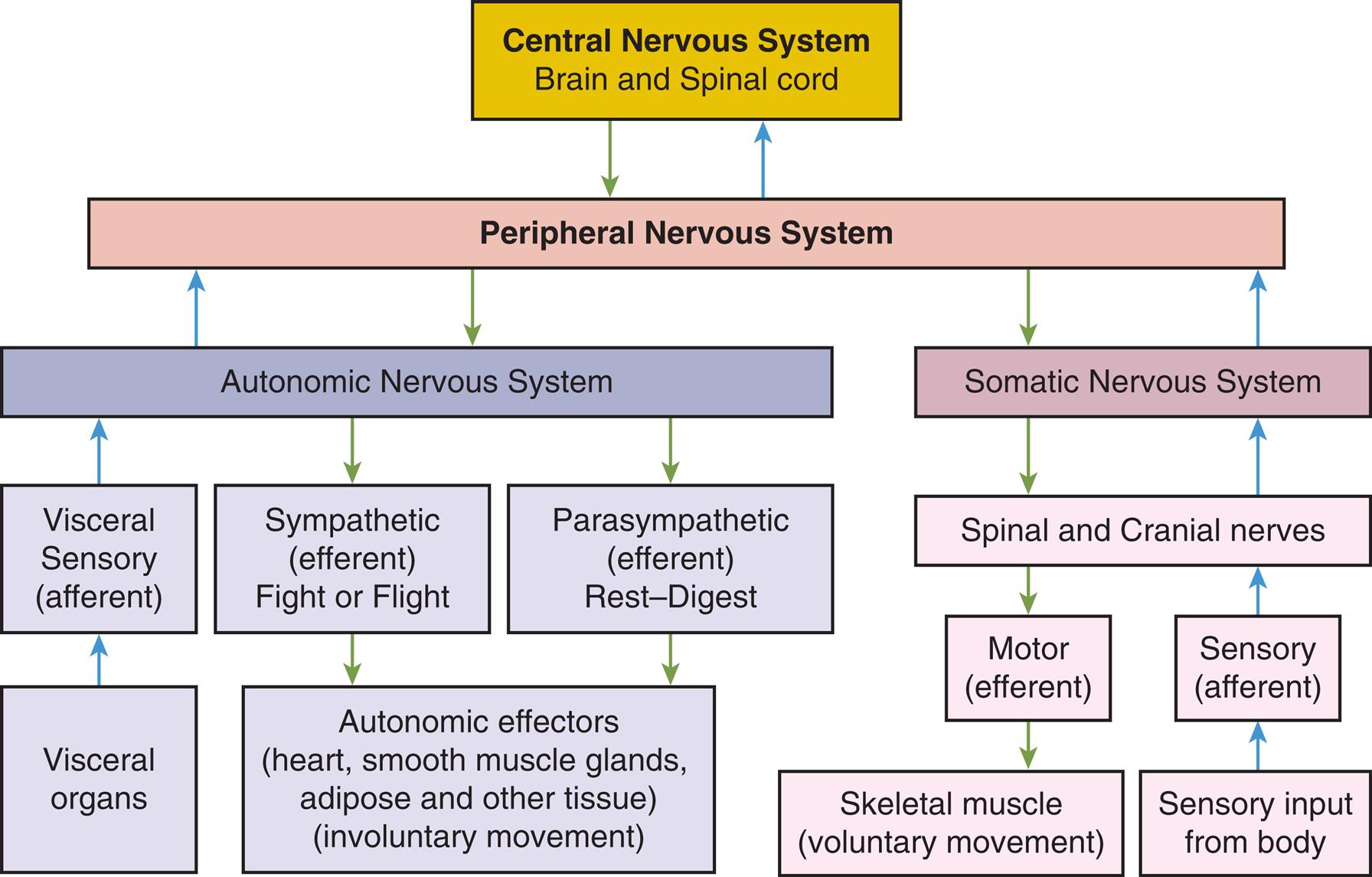
A flowchart summarizes the organization of the nervous system through afferent and efferent pathways. The afferent (ascending) pathways in the organization are as follows. • Central nervous system (brain and spinal cord) and peripheral nervous system. • Peripheral nervous system and autonomic nervous system. • Autonomic nervous system and visceral sensory (afferent). • Visceral sensory (afferent) and visceral organs. • Peripheral nervous system and somatic nervous system. • Somatic nervous system and spinal and cranial nerves. • Sensory (afferent) and spinal and cranial nerves. • Sensory input from body and sensory (afferent). The efferent (descending) pathways in the organization are as follows. • Central nervous system (brain and spinal cord) and peripheral nervous system. • Peripheral nervous system and autonomic nervous system. • Autonomic nervous system and sympathetic (efferent) fight tot flight. • Sympathetic (efferent) fight or flight and autonomic effectors (heart, smooth muscle glands, adipose and other tissue) (involuntary movement). • Parasympathetic (efferent) rest-digest and autonomic effectors (heart, smooth muscle glands, adipose and other tissue) (involuntary movement). • Peripheral nervous system and somatic nervous system. • Somatic nervous system and spinal and cranial nerves. • Spinal and cranial nerves and motor (efferent). • Motor (efferent) and skeletal muscle (voluntary movement).
Functionally, the PNS can be divided into the somatic nervous system and the autonomic nervous system. The somatic nervous system consists of pathways that regulate voluntary motor control (e.g., skeletal muscle). The autonomic nervous system (ANS) is involved in regulating the body's internal environment (viscera) through the involuntary control of organ systems. The ANS is further divided into sympathetic and parasympathetic divisions. Organs innervated by specific components of the nervous system are called effector organs.
Cells of the Nervous System
Two basic types of cells constitute nervous tissue: neurons and supporting nonneuronal neuroglial cells. The neuron is the primary cell of the nervous system. It is an electrically excitable cell, which transmits and receives information. Neuroglial cells provide structural support, protection, and nutrition for the neurons and facilitate neurotransmission. Neuroglial cells include astrocytes, microglia, and oligodendrocytes in the CNS; and Schwann (neurilemma) and satellite cells (a type of Schwann cell) in the PNS.
Neurons
Working alone or in units, neurons detect environmental changes and initiate body responses to maintain a dynamic steady state. Neuronal size and structure vary markedly, and each neuron is adapted to perform specialized functions. The cellular constituents of neurons include microtubules (transport substances within the cell), neurofibrils (very thin supportive fibers that extend throughout the neuron), microfilaments (proteins thought to be involved in the transport of cellular products), and Nissl substances (endoplasmic reticulum and ribosomes involved in protein synthesis). Although most neurons are nondividing cells, some neurons continue to divide after birth; for example, olfactory neurons in the nose continue to divide throughout life.
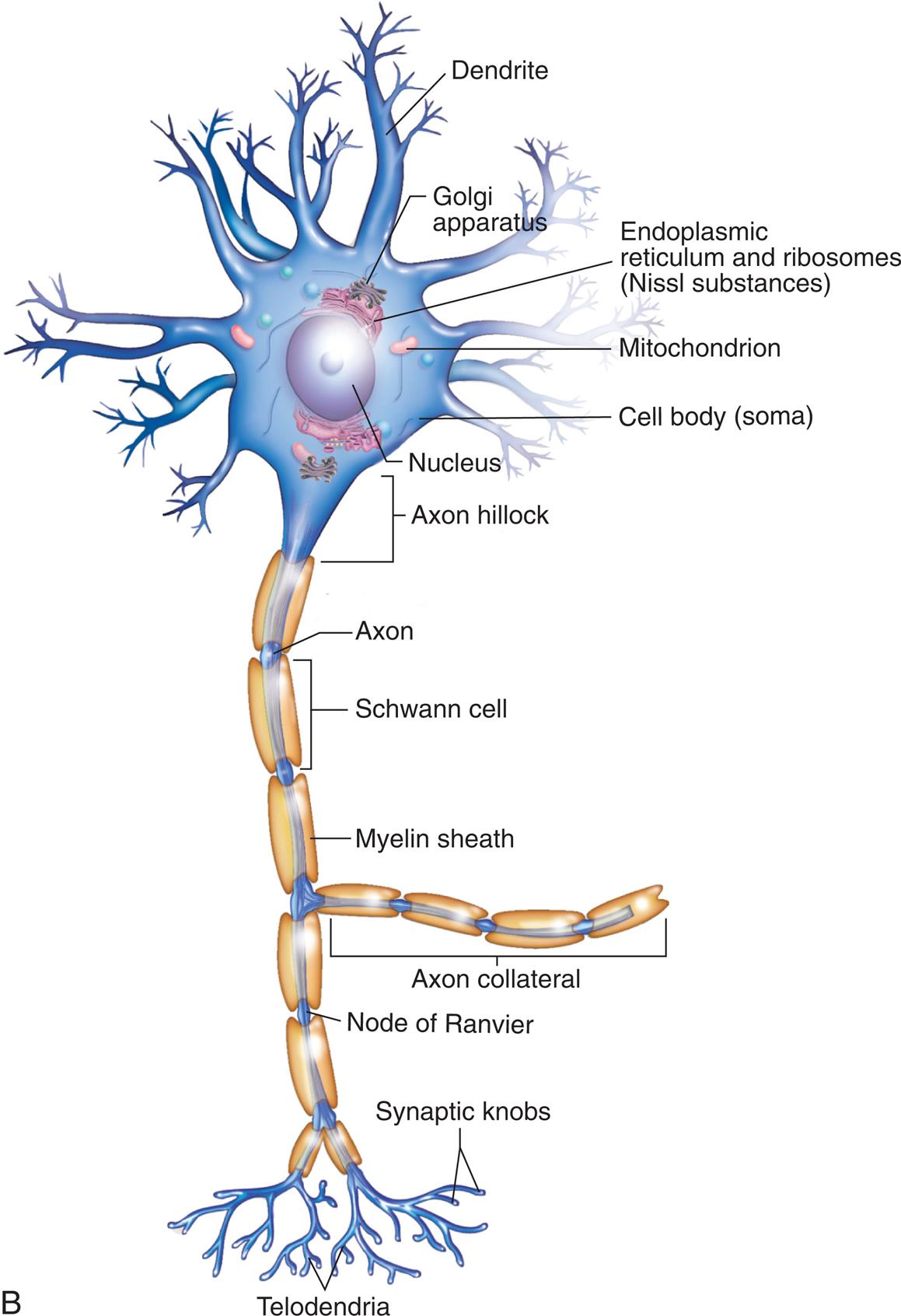
A neuron (Fig. 15.2) has three components: a cell body (soma), the dendrites (thin branching fibers of the cell), and the axons. Cell bodies for most neurons, even those extending axons into peripheral nerves, are located within the CNS. Dense collections of cell bodies in the CNS are called nuclei. Those in the PNS usually are found in groups called ganglia (or plexuses—a group of relay nerves). The dendrites are extensions that carry nerve impulses toward the cell body. The dendritic zone is the receptive portion of a neuron that receives a stimulus. Axons are long, conductive projections that carry nerve impulses away from the cell body. The axon hillock is the cone-shaped process where the axon leaves the cell body. The first part of the axon hillock has the lowest threshold for stimulation, so action potentials begin there.
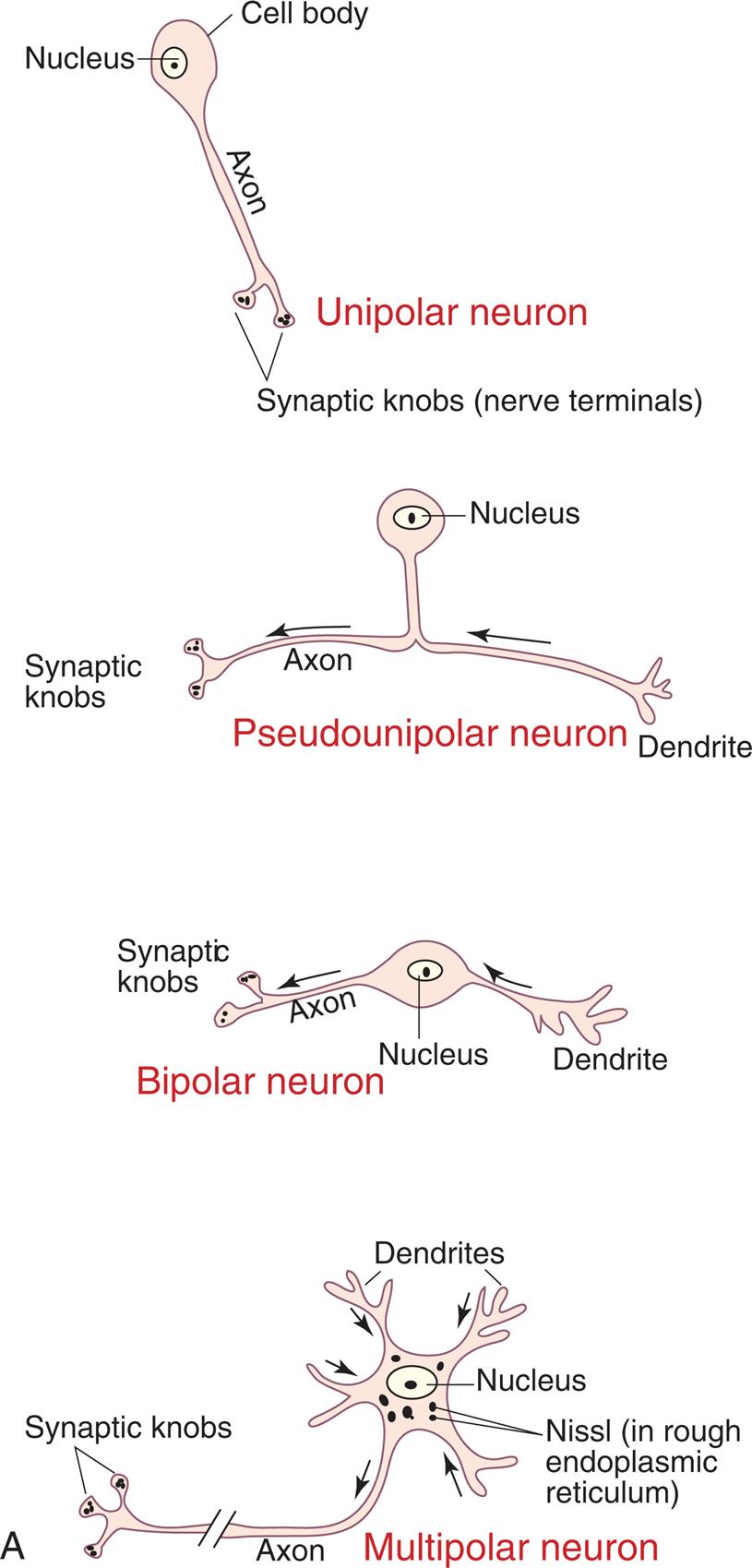
(A) Structural classifications of neurons based on the number of processes: unipolar, pseudopolar, bipolar, and multipolar. (B) Typical multipolar neuron: Peripheral nervous system neuron with multiple extensions from the cell body. (A, From Watson C, Kirkcaldie M, Paxinos G. The brain. London: Academic Press; 2011. B, Modified from Patton KT, Thibodeau GA, Douglas MM. Essentials of anatomy & physiology. St. Louis: Mosby; 2012.)
A series of illustrations show neuron structures. Illustration A. There are four types of neurons. • Unipolar neuron. The illustration shows a nucleus inside a cell body with an axon and two synaptic knobs (nerve terminals). • Pseudounipolar neuron. The illustration shows a nucleus inside a cell body with a branch that splits into a dendrite on the right and an axon with synaptic knobs on the left. • Bipolar neuron. The illustration shows a nucleus inside a cell body with a branch on either side; dendrite on the right and axon with synaptic knobs on the left. • Multipolar neuron. The illustration shows a nucleus inside a cell body. The nucleus is surrounded by Nissl (in rough endoplasmic reticulum). The cell body has multiple branches of dendrites and one axon with synaptic knobs. Illustration B shows the structure of a typical multipolar neuron. The cell body has multiple dendrites and a nucleus at the center. Other structures inside the cell body (soma) are Golgi apparatus, endoplasmic reticulum and ribosomes (Nissl substances), and mitochondrion. An axon hillock extends into the axon with Schwann cell inside a myelin sheath. The axon branches into an axon collateral and a node of Ranvier that terminates with synaptic knobs on telodendrion.
A typical neuron has only one axon, which may be wrapped with a myelin sheath—a membrane made of a lipid material called myelin. In the CNS, myelin is produced by oligodendrocytes. Regions of the brain and spinal cord with a high level of myelination constitute the white matter, whereas regions lacking significant myelination (typically primarily composed of cell bodies) are gray matter. In the PNS, myelin is produced by Schwann cells. The myelin sheaths are interrupted at regular intervals by the nodes of Ranvier. Nutrient exchange is not possible through the myelin sheath, although it can occur at the nodes of Ranvier. Axons can branch at the nodes of Ranvier, forming axon collaterals. Axons end with telodendria, branches that terminate with synaptic knobs, which are used in neurotransmission.
Where there is myelin, the velocity of nerve impulses increases. Myelin acts as an insulator that allows an action potential to leap between segments rather than flowing along the entire length of the membrane, yielding the increased velocity. This mechanism is referred to as saltatory conduction. Disorders of the myelin sheath (demyelinating diseases), such as multiple sclerosis, Guillain-Barré syndrome, and Charcot-Marie-Tooth disease, demonstrate the important role myelin plays in nerve conduction (see Chapter 17). Conduction velocities depend not only on the myelin coating but also on the diameter of the axon. Larger axons transmit impulses at a faster rate.
Neurons are structurally classified based on the number of processes (projections) extending from the cell body (see Fig. 15.2A). There are four basic types of cell configuration: (1) unipolar, (2) pseudounipolar, (3) bipolar, and (4) multipolar. Unipolar neurons have one process, an axon, which branches shortly after leaving the cell body. One example is found in the retina. Pseudounipolar neurons (unipolar) have one axon process and one dendrite process, but the dendrite and axon are fused near the cell body, and the axon then extends into the CNS. This configuration is typical of sensory neurons in both cranial and spinal nerves. Bipolar neurons have two distinct processes (one axon and one dendrite) arising directly from the cell body. This type of neuron connects the rod and cone cells of the retina. Multipolar neurons are the most common and have multiple processes capable of extensive branching. A motor neuron is typically multipolar.
Functionally, there are three types of neurons (their typical configuration is noted in parentheses): (1) sensory (mostly pseudounipolar), (2) associational (multipolar), and (3) motor (multipolar). Sensory neurons are afferent neurons, carrying impulses from peripheral sensory receptors to the CNS. Associational neurons (interneurons) transmit impulses from neuron to neuron, sensory to motor neurons. They are located solely within the CNS. Motor neurons are efferent neurons, transmitting impulses from the CNS to an effector (i.e., skeletal muscle or organs). In skeletal muscle, the end processes form a neuromuscular (myoneural) junction (see Fig. 15.14 and the Spinal Cord section later in the chapter).
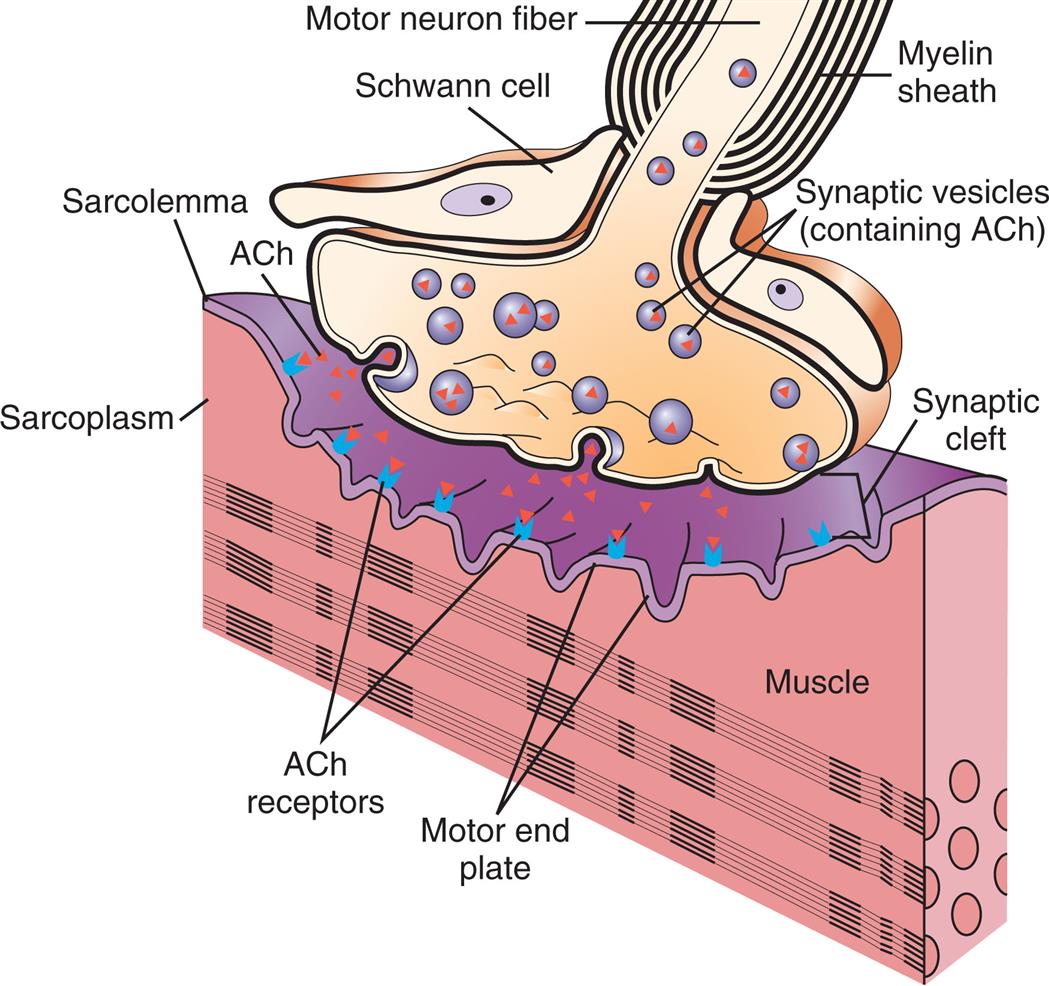
This figure shows how the distal end of a motor neuron fiber forms a synapse, or “chemical junction,” with an adjacent muscle fiber. Neurotransmitters (specifically, acetylcholine [ACh]) are released from the neuron's synaptic vesicles and diffuse across the synaptic cleft. There they stimulate receptors in the motor end-plate region of the sarcolemma. (From Damjanov I. Pathology for the health professions, 4th edition. St. Louis: Saunders; 2012.)
An illustration shows a cross-section of a normal neuromuscular junction. The following structures are labeled on the illustration: motor neuron fiber, myelin sheath, Schwann cell, synaptic vesicles (containing A C h), A C h, synaptic cleft, sarcolemma, A c h receptors, motor end plate, sarcoplasm, and muscle. Synaptic vesicles pass through the motor neuron fiber. The synaptic vesicles release A C h into the synaptic cleft which bind to the A C h receptors on the motor end plate.
Neuroglia
Neuroglia (“nerve glue”) is the general classification of nonneuronal cells that support the nervous system's neurons. They comprise approximately 50% of the total brain and spinal cord volume. Neuroglia are present in both the CNS and PNS. Astrocytes, oligodendroglia (oligodendrocytes), ependymal cells, and microglia are found in the CNS; Schwann cells, nonmyelinating Schwann cells, and satellite glial cells are found in the PNS (Fig. 15.3) (Emerging Science Box: Schwann Cells). The different types of neuroglia serve different functions (Table 15.1).
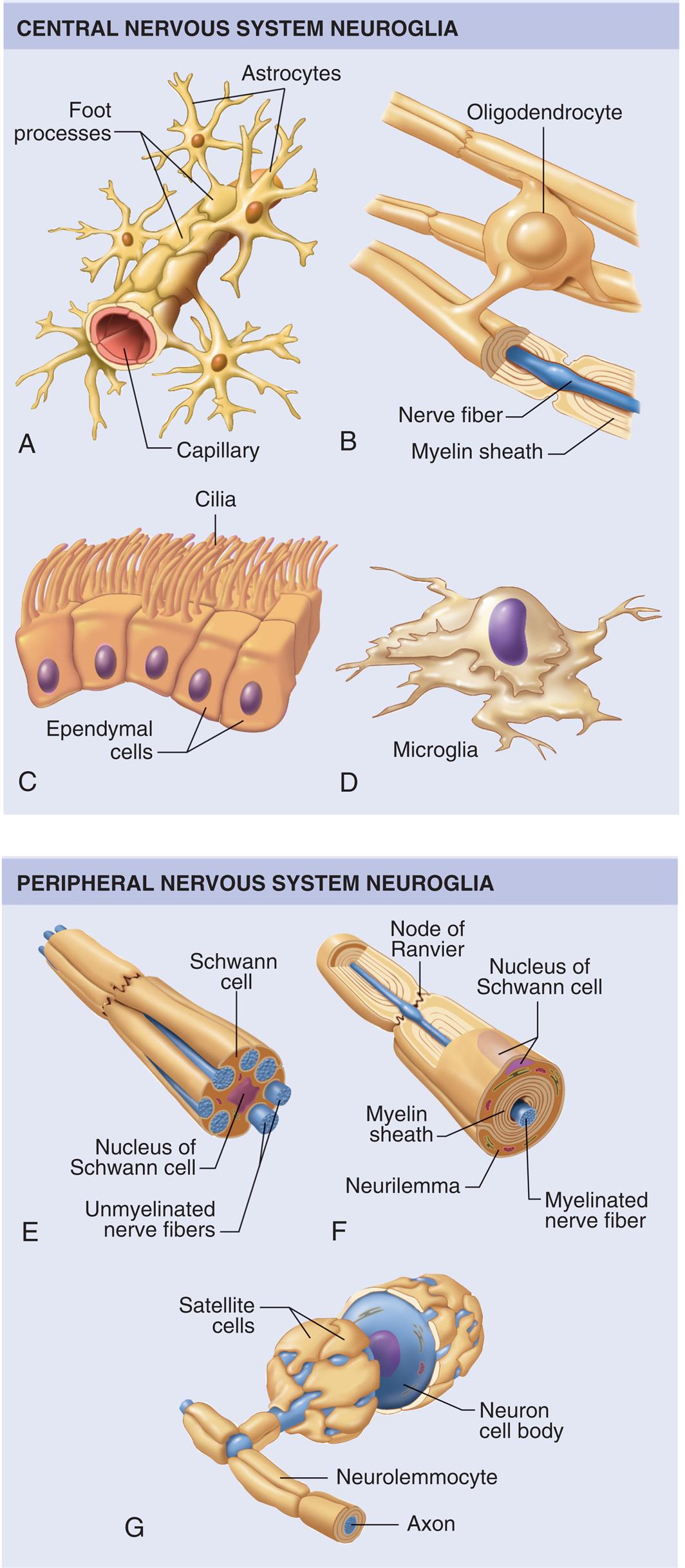
Neuroglia of the central nervous system (CNS): (A) Astrocytes attached to the outside of a capillary blood vessel in the brain. (B) An oligodendrocyte with processes that wrap around nerve fibers in the CNS to form myelin sheaths. (C) Ciliated ependymal cells forming a sheet that usually lines fluid cavities in the brain. (D) A phagocytic microglial cell. Neuroglia of the peripheral nervous system (PNS): (E) A Schwann cell supporting a bundle of nerve fibers in the PNS. (F) Another type of Schwann cell encircling a peripheral nerve fiber to form a thick myelin sheath. (G) Satellite cells, another type of Schwann cell, surround and support cell bodies of neurons in the PNS. (From Patton KT, Thibodeau GA, Douglas MM. Essentials of anatomy & physiology. St. Louis: Mosby; 2012.)
A series of illustrations shows central nervous system neuroglia and peripheral nervous system neuroglia. The central nervous system neuroglia shows the following structures. • Illustration A. The structure shows the foot processes with multiple astrocytes around a single capillary. • Illustration B. The structure shows multiple nerve fibers, each covered in a myelin sheath and connected by oligodendrocyte. • Illustration C. The structure shows a layer of ependymal cells covered in cilia. • Illustration D. The structure shows microglia. The cells are distorted and shapeless. The peripheral nervous system neuroglia shows the following structures. • Illustration E. The structure shows a Schwann cell with a nucleus at the center. • Illustration F. The structure shows the node of Ranvier and labels the following structures beneath the nucleus of the Schwann cell, from the inside: myelinated nerve fiber, myelin sheath, neurilemma. • Illustration G. The structure shows a neuron cell body with satellite cells. The structure also labels the axon inside the neurolemmocyte.
Table 15.1
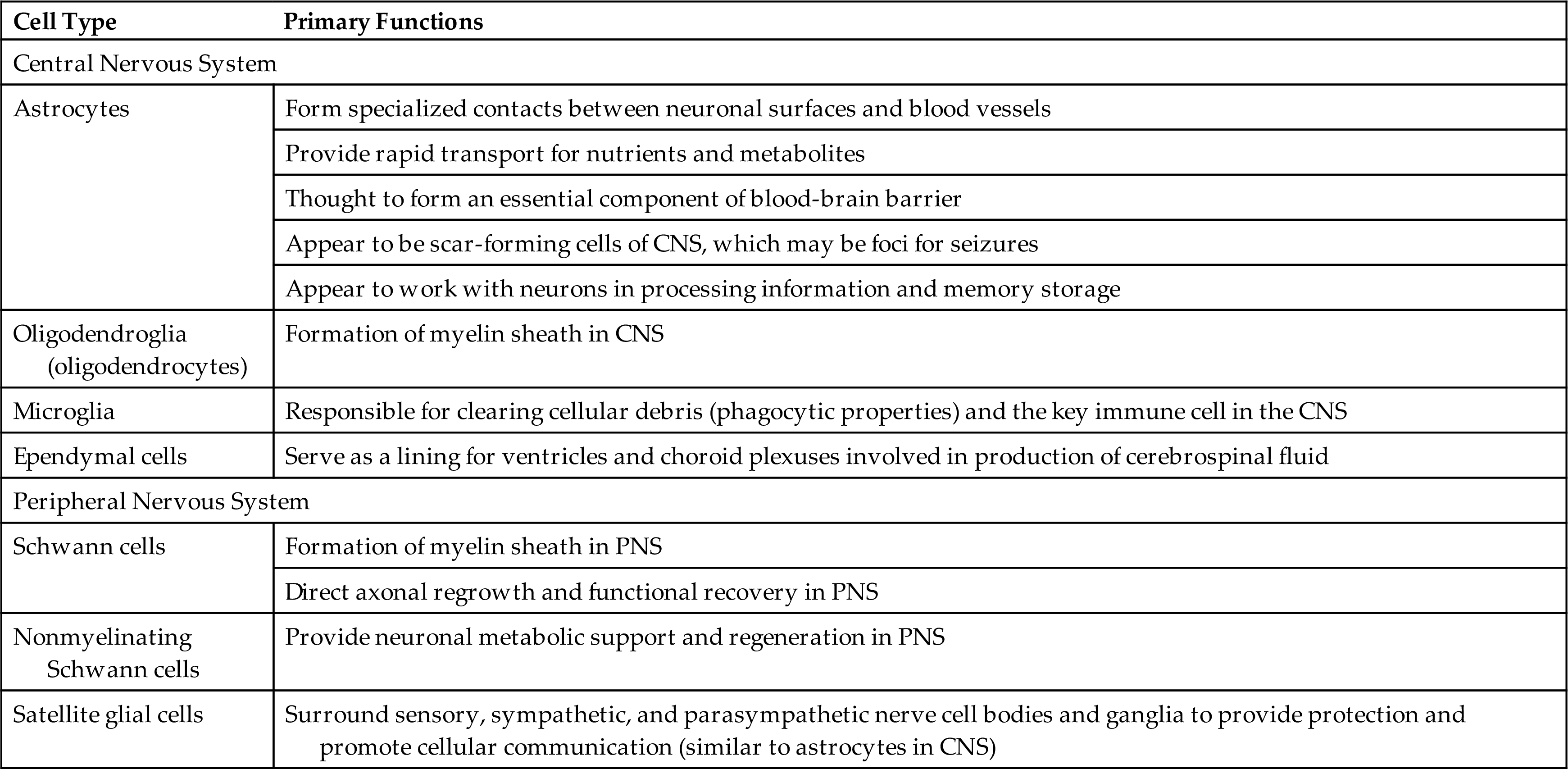
CNS, Central nervous system; PNS, peripheral nervous system.
Nerve Injury and Regeneration
Mature nerve cells do not divide; therefore, injury can cause permanent loss of function. Wallerian degeneration occurs in the distal axon when an axon is severed, the portion cut off from the cell body, within hours of the injury. The morphologic and biochemical changes that occur in the distal axon include: (1) a characteristic swelling within the portion of the axon distal to the cut; (2) neurofilaments hypertrophy; (3) shrinkage and fragmentation of the myelin sheath; and (4) axon degeneration, and disappearance. The myelin sheaths reform into Schwann cells that align in a column between the severed part of the axon and the effector organ (Fig. 15.4B).
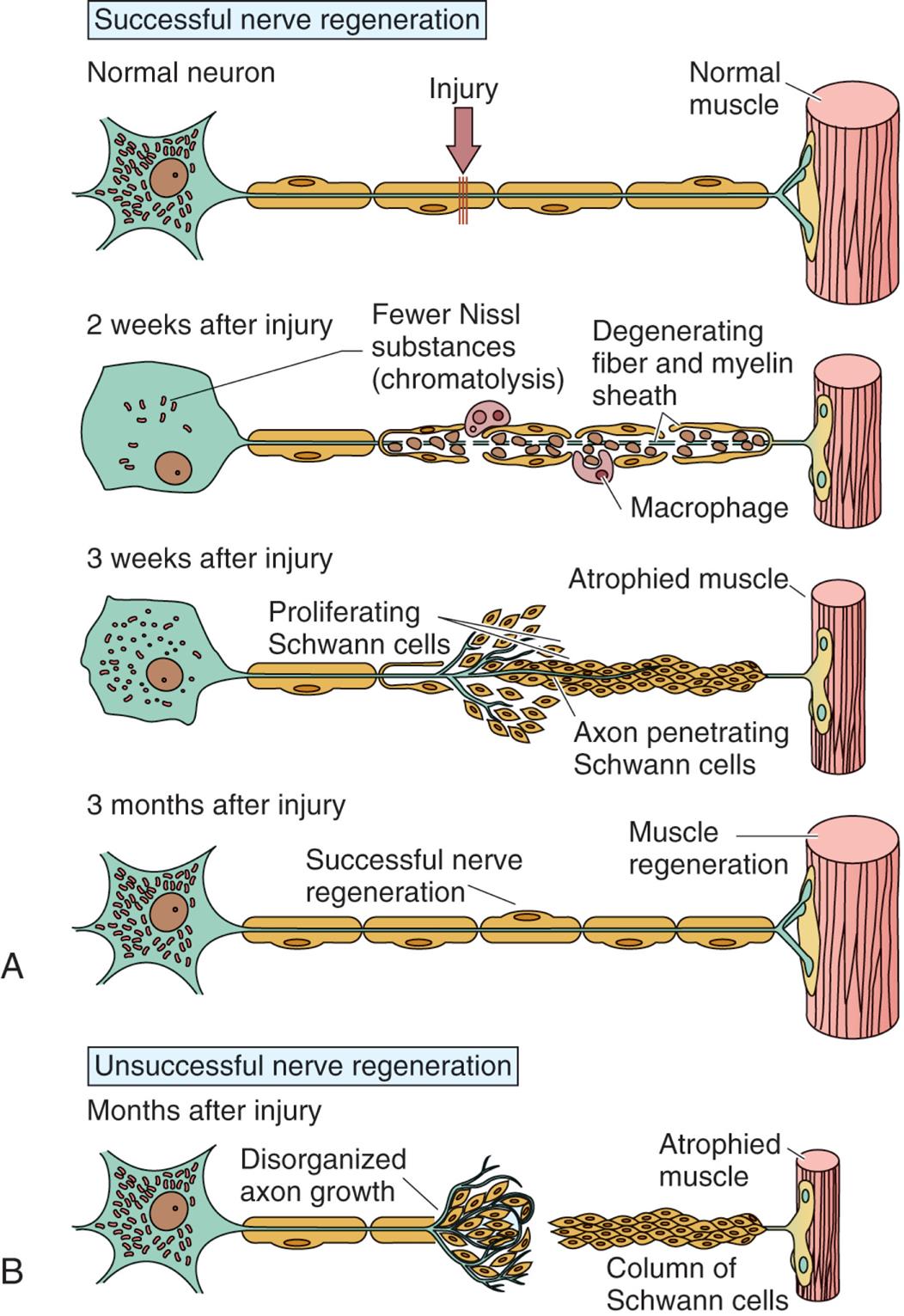
(A) In successful nerve regeneration, Schwann cells detach from the axons, proliferate, and, with recruited macrophages, help clear cellular and myelin debris. A damaged motor axon can regrow to its distal connection only if the neurilemma remains intact (to form a guiding tunnel) and if scar tissue does not block its pathway. (B) Unsuccessful nerve regeneration results in the neuron remaining cut off from the effector organ. (From Gartner LP. Textbook of histology, 4th edition. Philadelphia: Elsevier; 2017.)
A series of illustrations show the peripheral nerve regeneration following injury. Illustration A. There are four stages of successful regeneration. Stage 1. The structure shows an injury along the axon of a normal neuron connected to a normal muscle. The cell body of the neuron comprises multiple Nissl substances. Stage 2, 2 weeks after injury. The structure shows fewer Nissl substances (chromatolysis) in the cell body. There are multiple macrophages along the degenerating fiber and myelin sheath. Stage 3, 3 weeks after injury. The structure shows an increase in Nissl substances in the cell body. Schwann cells proliferate around the axon penetrating the Schwann cells, connected to the atrophied muscle. Stage 4, 3 months after injury. The structure shows normal amount of Nissl substances in the cell body. The nerve has successfully regenerated and is connected to a regenerated muscle. Illustration B. An illustration shows the structure of a neuron in an unsuccessful nerve regeneration. The structure shows a cell body with Nissl substances. The site of disorganized axon growth along the axon is identified. A column of Schwann cells is connected to the atrophied muscle, but is disconnected from the axon.
At the proximal end of the injured axon, similar changes occur but only back to the next node of Ranvier. The cell body responds to trauma by swelling and dying by chromatolysis (dispersing the Nissl substance) or apoptosis. During the repair process, the cell increases protein synthesis and mitochondrial activity. Days to weeks after injury, new terminal sprouts project from the proximal segment and may enter the remaining Schwann cell pathway. (Fig. 15.4A contains a more detailed representation of these events.)
This process is very slow (about 1 mm/day) and is limited to myelinated fibers in the PNS. The regeneration of axonal constituents in the CNS is limited by an increased incidence of glial scar formation (gliosis) and the different nature of myelin formed by the oligodendrocyte.
Nerve regeneration depends on many factors, such as the location of the injury, the type of injury, the presence of inflammatory responses, and the process of scarring. The closer the injury is to the nerve's cell body, the greater chance that the nerve cell will die and not regenerate. A crushing injury allows for fuller recovery compared with a cut injury. Crushed nerves sometimes recover fully, whereas cut nerves often form connective tissue scars that block or slow regenerating axonal branches. Peripheral nerves injured close to the spinal cord recover poorly and slowly because of the long distance between the cell body and the peripheral termination of the axon.1
Nerve Impulse
Neurons generate and conduct electrical and chemical impulses by selectively changing the electrical potential of the plasma membrane and influencing other nearby neurons by releasing chemicals (neurotransmitters). An unexcited neuron maintains a resting membrane potential. When the membrane potential is sufficiently raised, an action potential is generated, and the nerve impulse then flows to all parts of the neuron. The action potential response occurs only when the stimulus is strong enough; the membrane remains unexcited if it is too weak. This property is termed the all-or-none response (see Chapter 1 for a discussion of electrical impulse conduction).
Synapses
Neurons are not physically contiguous with one another. The region between adjacent neurons is called a synapse (Fig. 15.5). The neurons that conduct a nerve impulse are named according to whether they relay impulses toward (presynaptic neurons) or away from (postsynaptic neurons) the synapse. The synapse is composed of a bulbous end of the presynaptic neuron (synaptic knob) that is separated from the postsynaptic neuron by a gap called the synaptic cleft. Four basic types of connections occur in regions of contact between the presynaptic and postsynaptic neurons. These are between axons (axo-axonic), from axon to cell body (axo-somatic), from axon to dendrite (axo-dendritic), and from dendrite to dendrite (dendro-dendritic).
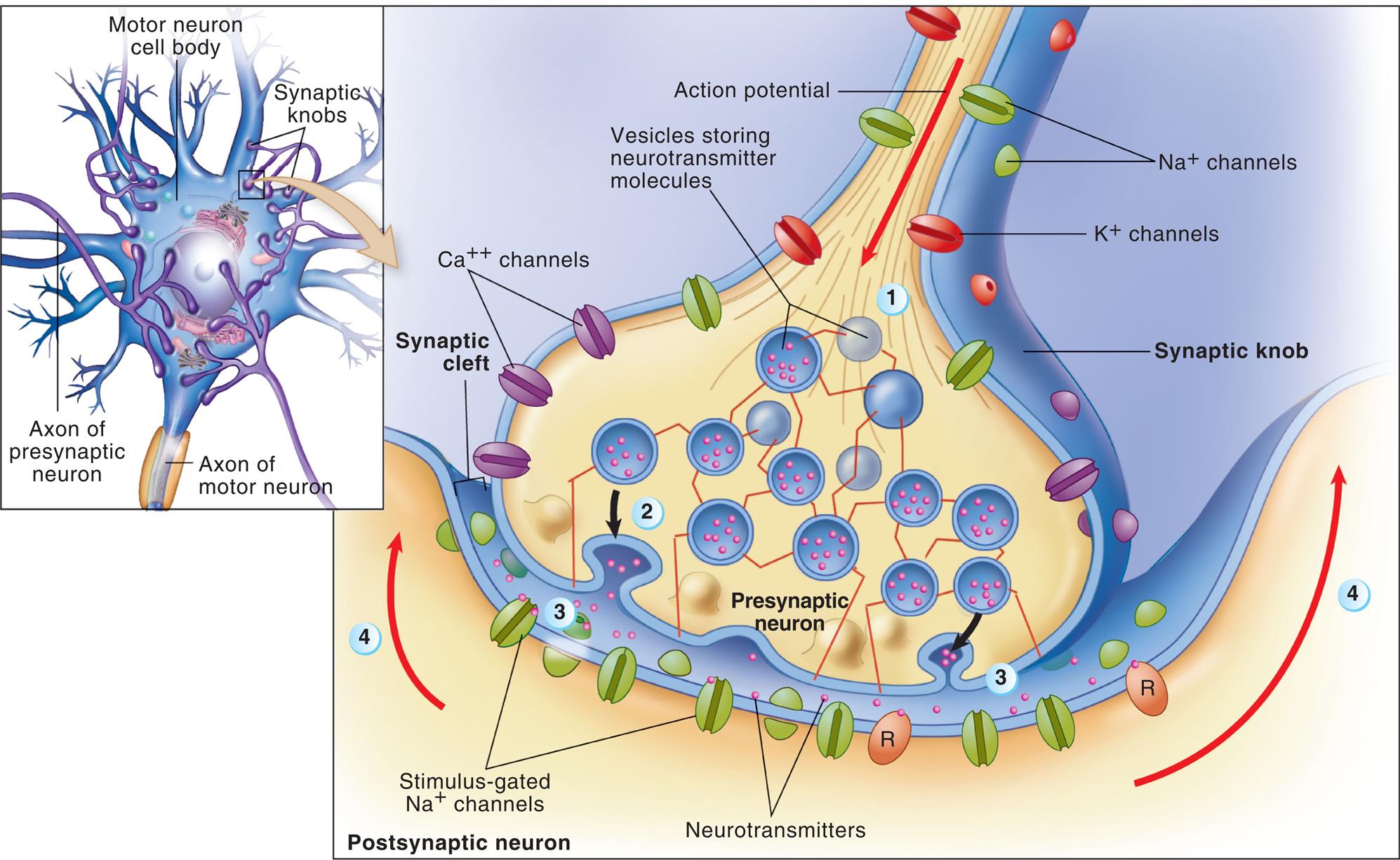
Details illustrate the synaptic bouton (knob) of a presynaptic neuron, the plasma membrane of a postsynaptic neuron, and a synaptic cleft. At step 1, the action potential arrives at the synaptic bouton. At step 2, the rapid exocytosis of neurotransmitter molecules from vesicles in the knob occurs. At step 3, neurotransmitter diffuses into the synaptic cleft and binds to receptor molecules (R) in the plasma membrane of the postsynaptic neuron. The postsynaptic receptors directly or indirectly trigger the opening of stimulus gated ion channels, initiating a local potential in the postsynaptic neuron. At step 4, the local potential may move toward the axon, where an action potential may begin. (Adapted from Patton KT. Anatomy & physiology, 10th edition. St. Louis: Mosby; 2019.)
An illustration shows neuronal transmission and synaptic cleft. The structure of a motor neuron cell body with axon of presynaptic neuron, synaptic knobs, and axon of motor neuron. An accompanying illustration shows and labels the structures in the postsynaptic neuron. The structure identifies the action potential feeding into a synaptic knob. There are sodium ion channels, potassium ion channels, and calcium ion channels on the knob. A matrix of vesicles storing neurotransmitter molecules are identified at the base of the synaptic knob. Neurotransmitters are released into the synaptic cleft that are attracted to the stimulus-gated sodium ion channels.
Impulses are transmitted across the synapse by chemical and electrical conduction (only chemical conduction is discussed here. Chapter 1 contains information on electrical conduction [see Fig.1.31].) When an impulse originates in a presynaptic neuron, the impulse reaches the vesicles, where chemicals (neurotransmitters) are stored in the synaptic bouton. Once released from the vesicles, the neurotransmitters diffuse across the synaptic cleft and bind to specific neurotransmitter (protein) receptor sites on the plasma membrane of the postsynaptic neuron, relaying the impulse (see Fig. 15.5). Brain synapses can change in strength and number throughout life, and this is known as synaptic plasticity or neuroplasticity.
Neurotransmitters
Neurotransmitters are chemicals synthesized in the neuron and localized in the presynaptic terminal (synaptic bouton). Neurotransmitters are released into the synaptic cleft in response to the arrival of an electrical impulse and bind to a receptor site (binding site) on the postsynaptic membrane of another neuron or effector, where they affect ion channels (see Fig. 15.5). Each neurotransmitter is removed by a specific mechanism from its site of action. Neurons can synthesize more than one neurotransmitter, and postsynaptic membranes can contain more than one type of transmitter-specific receptor. Many substances are neurotransmitters, including norepinephrine, acetylcholine, dopamine, histamine, and serotonin. Many of these transmitters have more than one function. Neuromodulators are chemical messengers released from a neuron in the CNS or the PNS, and this affects a group of neurons that have receptors for that messenger. They may have excitatory or inhibitory effects. Neurotransmitter and neuromodulator substances are summarized in Table 15.2.
Table 15.2
| Substance | Location | Effect | Clinical Example |
|---|---|---|---|
| Acetylcholine | Many parts of the brain, spinal cord, neuromuscular junction of skeletal muscle, and many ANS synapses | Excitatory or inhibitory | Alzheimer disease (a type of dementia) is associated with a decrease in the number of acetylcholine-secreting neurons. Muscle weakness caused by myasthenia gravis results from an autoimmune response to acetylcholine receptors on the postsynaptic terminal. |
| Monoamines | |||
| Norepinephrine | Many areas of the brain and spinal cord; also in some ANS synapses | Excitatory or inhibitory | CNS: Sleep-wake cycles and mood. Cocaine and amphetaminesa result in overstimulation of postsynaptic neurons. |
| Serotonin | Many areas of the brain and spinal cord | Generally inhibitory | Is involved with mood, anxiety, and sleep induction. Levels of serotonin are elevated in schizophrenia (delusions, hallucinations, withdrawal). |
| Dopamine | Some areas of the brain and ANS synapses | Generally excitatory | Parkinson disease (depression of voluntary motor control) results from destruction of dopamine-secreting neurons. Drugs used to increase dopamine can induce vomiting and hallucinations. |
| Histamine | Posterior hypothalamus | Excitatory (H1 and H2 receptors) and inhibitory (H3 receptors) | There is no clear indication of histamine-associated pathologic conditions. Histamine is involved with arousal and attention and links to other brain transmitter systems. |
| Amino Acids | |||
| Gamma-aminobutyric acid (GABA) | Most neurons of the CNS have GABA receptors | Majority of postsynaptic inhibition in the brain | Drugs that increase GABA function have been used to treat epilepsy by inhibiting excessive discharge of neurons. |
| Glycine | Spinal cord | Most postsynaptic inhibition in the spinal cord | Glycine receptors are inhibited by strychnine. |
| Glutamate and aspartate | Widespread in brain and spinal cord | Excitatory | Drugs that block glutamate or aspartate, such as riluzole, are used to treat amyotrophic lateral sclerosis. These drugs might prevent overexcitation from seizures and neural degeneration. |
| Neuropeptides | |||
| Endorphins and enkephalins | Widely distributed in the CNS and PNS | Generally inhibitory | Morphine and heroin bind to endorphin and enkephalin receptors on presynaptic neurons and reduce pain by blocking the release of neurotransmitters. |
| Substance P | Spinal cord, brain, and sensory neurons associated with pain, GI tract | Generally excitatory | Substance P is a neurotransmitter involved in pain transmission pathways. Blocking release of substance P by morphine reduces pain. |
| Vasoactive intestinal peptide | Gastrointestinal tract | Generally excitatory | Stimulates secretion, vasodilation, and smooth muscle relaxation (vasodilation, sphincter relaxation). |
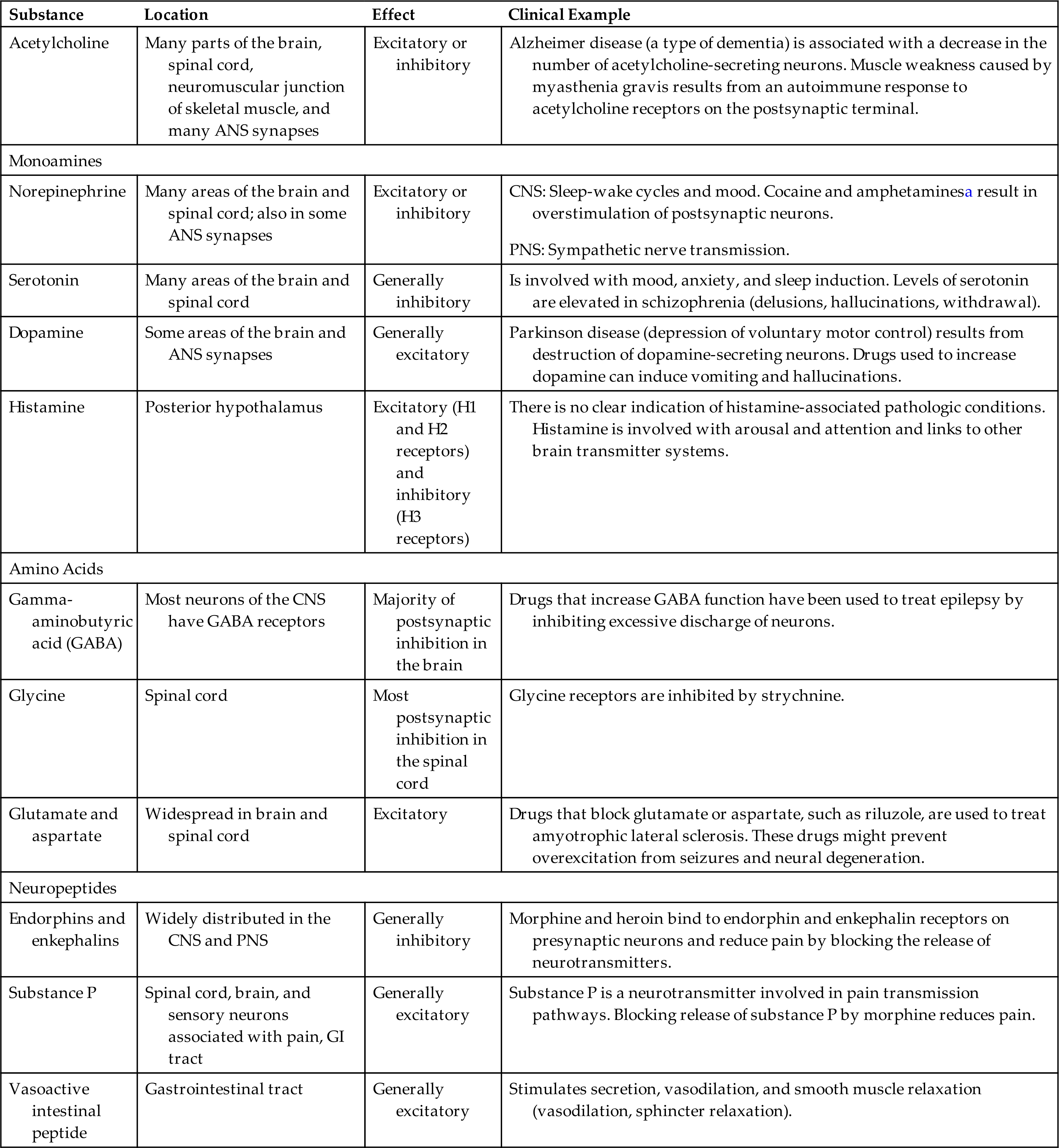
ANS, Autonomic nervous system; CNS, central nervous system; GI, gastrointestinal; PNS, peripheral nervous system.
aIncrease the release and block the reuptake of norepinephrine.
From Mtui E, Gruener G, Dockery P. Fitzgerald’s clinical neuroanatomy and neuroscience, 7th edition. Philadelphia: Elsevier; 2016.
Because the neurotransmitter is normally stored on one side of the synaptic cleft, and the receptor sites are on the other side, chemical synapses operate in one direction. Therefore, action potentials are transmitted along a multineuronal pathway in one direction. The binding of the neurotransmitter at the receptor site changes the permeability of the postsynaptic neuron and, consequently, its membrane potential. Two possible scenarios can occur:
- 1. The postsynaptic neuron may be excited (depolarized), called excitatory postsynaptic potentials (EPSPs). If the EPSP reaches the threshold potential, an action potential is initiated.
- 2. The postsynaptic neuron's plasma membrane may be inhibited (hyperpolarized), called inhibitory postsynaptic potentials (IPSPs). This makes the membrane less likely to reach the threshold potential, meaning the action potential is inhibited.
Chapter 1 reviews electrical impulses and membrane potentials.
Usually, a single EPSP cannot induce a neuron's action potential and the nerve impulse propagation. Whether this occurs, depends on the number and frequency of potentials the postsynaptic neuron receives—a concept known as summation. Temporal summation (time relationship) refers to the effects of successive, rapid impulses received from a single neuron at the same synapse. Spatial summation (spacing effect) is the combined effects of impulses from several neurons onto a single neuron simultaneously. Facilitation refers to the effect of EPSP on the plasma membrane potential. The plasma membrane is facilitated when summation brings the membrane closer to the threshold potential and decreases the stimulus required to induce an action potential. The effect that a chemical neurotransmitter has on the plasma membrane potential depends on the balance of these effects. The mechanisms of convergence (many neurons firing and converging on one neuron), divergence (one neuron firing and diverging on many neurons), summation, and facilitation allow for the integrative processes of the nervous system.
Central Nervous System
Brain
The brain is a functionally integrated circuit of millions of neurons with different genomes, structures, molecular composition, networks, and connections. It weighs approximately 3 pounds and receives 15% to 20% of the total cardiac output. The brain enables a person to reason, function intellectually, express personality and mood and perceive and interact with the environment.
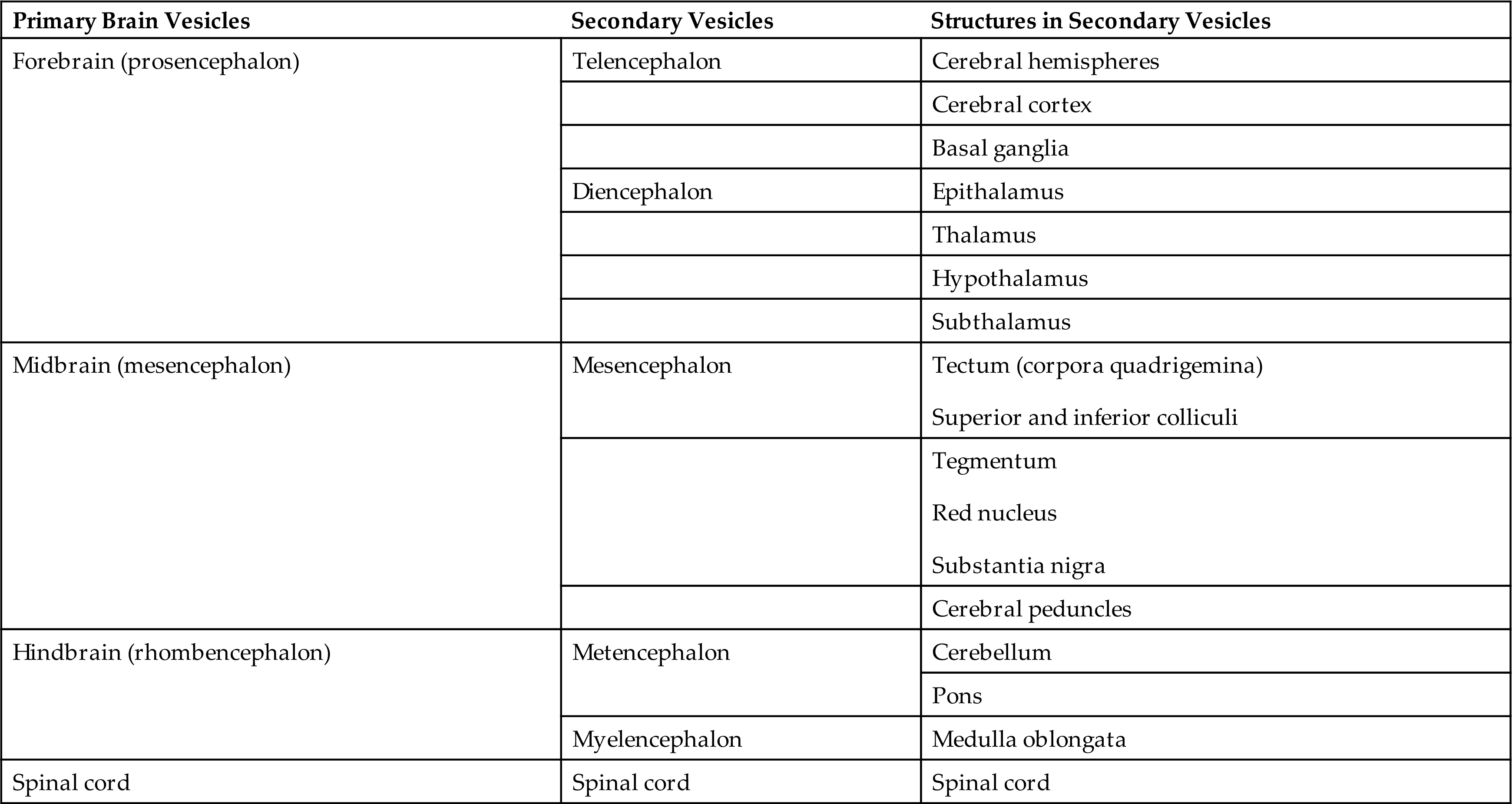
The cerebrum is the largest part of the brain and contains both gray matter and white matter. The three primary embryonic vesicles (structural divisions) of the brain are (1) the forebrain (prosencephalon), (2) the midbrain (mesencephalon), and (3) the hindbrain (rhombencephalon). These three vesicles then develop further into the secondary vesicles and their structures, which are summarized in Table 15.3 and Fig. 15.6. The midbrain, medulla, and pons comprise the brainstem, which connects the brain's hemispheres, cerebellum, and spinal cord. A collection of nerve cell bodies (nuclei) within the brainstem makes up the reticular formation. The reticular formation is a large network of diffuse nuclei that connect the brainstem to the cortex and control vital reflexes, such as cardiovascular function and respiration. It is essential for maintaining wakefulness and attention and is referred to as the reticular activating system (Fig. 15.7). Some nuclei within the reticular formation support specific motor movements, such as balance and posture.
Table 15.3
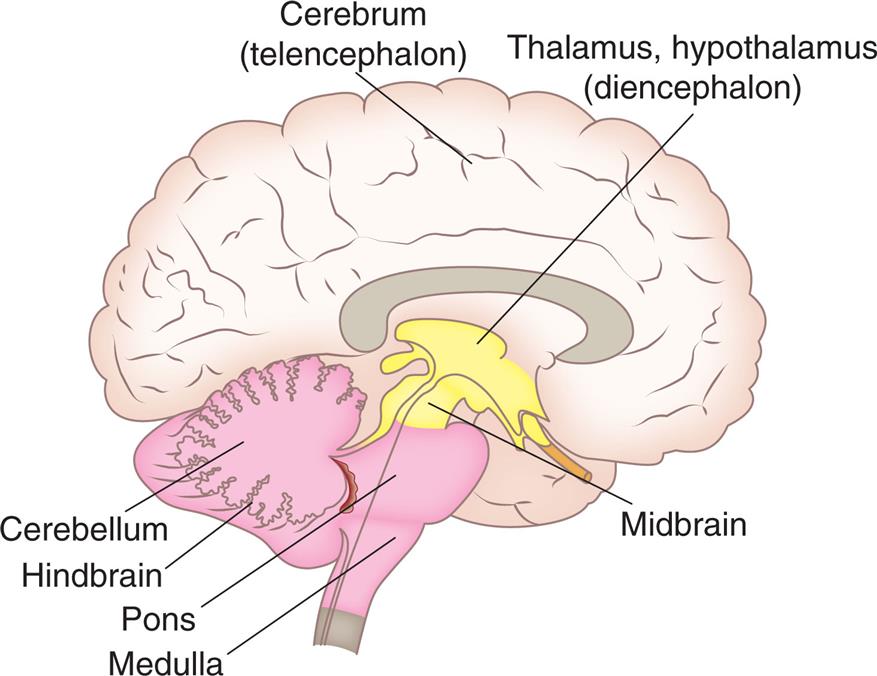
An illustration shows the right lateral view of the brain and identifies the following structures, from the forebrain to the hindbrain: cerebrum (telencephalon), thalamus, hypothalamus (diencephalon), midbrain, cerebellum, hindbrain, pons, and medulla.
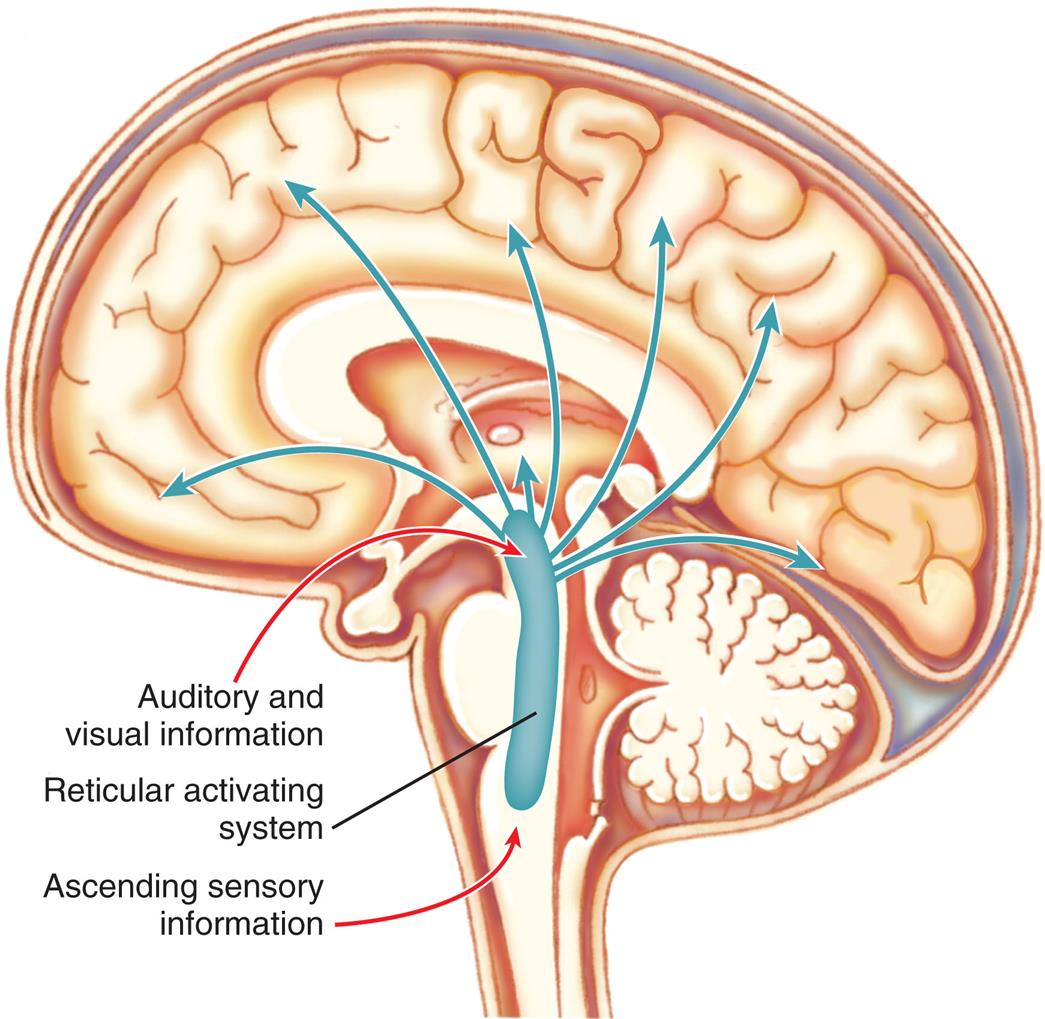
The reticular activating system consists of nuclei in the brainstem reticular formation plus fibers that conduct sensory information to the nuclei and fibers that conduct from the nuclei to widespread areas of the cerebral cortex. Functioning of the reticular activating system is essential for consciousness.
An illustration shows the left lateral view of the human brain and traces the paths through reticular activating system. Auditory and visual information and ascending sensory information pass through the reticular activating system and spread inside the brain.
Divisions of the brain are associated with different functions but attributing specific functions to definite brain regions is not entirely accurate. However, functional specificity is very useful for localizing pathologic conditions in various nervous system regions for clinical considerations. Brodmann areas are used to correlate functional activities to many regions of the cerebral cortex.2Fig. 15.8C illustrates these regions and describes some of the areas. The mapping of brain networks is also helpful in discovering how varying parts of the brain are interconnected when performing a specific function (see Emerging Science: Brain Networks).
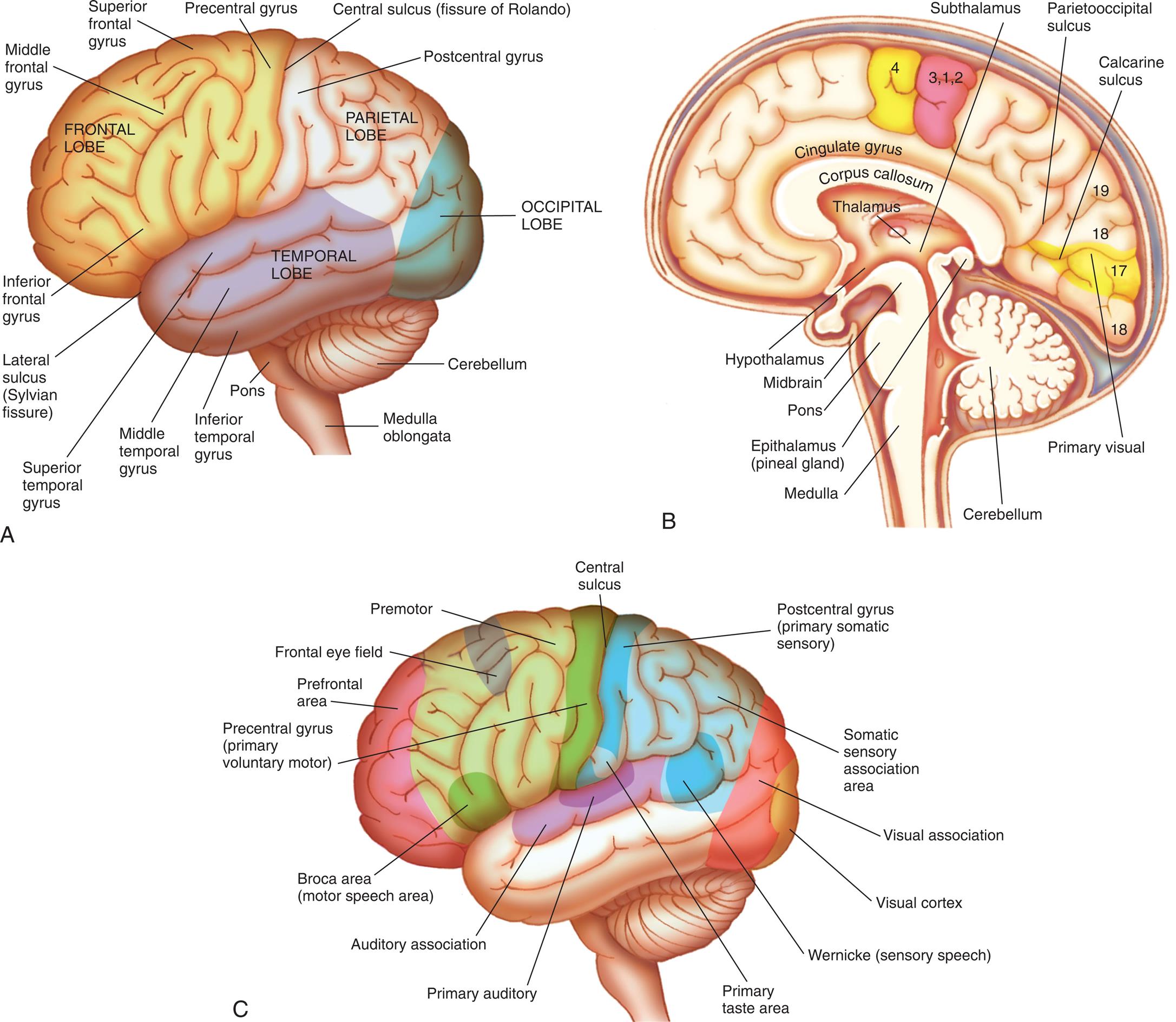
(A) Left hemisphere of cerebrum, lateral view. (B) Functional areas of the cerebral cortex, midsagittal view. (C) Functional areas of the cerebral cortex, lateral view.
Three illustrations of the left lateral and midsagittal views of the human brain label the cerebral hemispheres. Illustration A. The following structures are identified in the frontal lobe: inferior frontal gyrus, middle frontal gyrus, superior frontal gyrus, and precentral gyrus. The central sulcus (fissure of Ronaldo) separates the frontal lobe from the parietal lobe. The postcentral gyrus in the parietal lobe is identified. The lateral sulcus (Sylvian fissure) separates the frontal lobe from the temporal lobe. The following structures are identified in the temporal lobe: inferior temporal gyrus, middle temporal gyrus, and superior temporal gyrus. The occipital lobe is identified at the back of the brain. The cerebellum is labeled at the base of the brain, followed by the pons and the medulla oblongata. Illustration B. The following structures are labeled from above the midbrain to the hindbrain: thalamus, hypothalamus, subthalamus, corpus callosum, cingulate gyrus, parietooccipital sulcus, calcarine sulcus, and primary visual. The following structures are identified below the thalamus: epithalamus (pineal gland), midbrain, pons, cerebellum, and medulla. Illustration C. The following structures of the brain are labeled, clockwise from the frontal lobe: bronco area (motor speech area), prefrontal area, frontal eye field, premotor, precentral gyrus (primary voluntary motor), central sulcus, postcentral gyrus (primary somatic sensory), somatic sensory association area, visual association, visual cortex, Wernicke (sensory speech), primary taste area, primary auditory, auditory association, and Broca area (motor speech area).
Forebrain
Telencephalon
The telencephalon (cerebral hemispheres) consists of the cerebral cortex (the largest portion of the brain) and the basal ganglia (composed of several nuclei). The surface of the cerebral cortex is covered with convolutions called gyri (see Fig. 15.8A), which greatly increase the cortical surface area and the number of neurons. Grooves between adjacent gyrus are termed sulci; deeper grooves are fissures. The cerebral cortex contains an outer layer of cell bodies of neurons called gray matter, which is organized into columns perpendicular to the surface that receive, integrate, store, and transmit information. The cerebral cortex is located in the frontal, parietal, temporal, and occipital lobes. White matter lies beneath the cerebral cortex and is composed of myelinated nerve fibers (axons).
Lobes
The two cerebral hemispheres are separated by a deep groove known as the longitudinal fissure (see Fig. 15.10B). The surface of each hemisphere is divided into lobes named after the region of the skull under which each lobe lies: frontal, parietal, occipital, and temporal lobes.
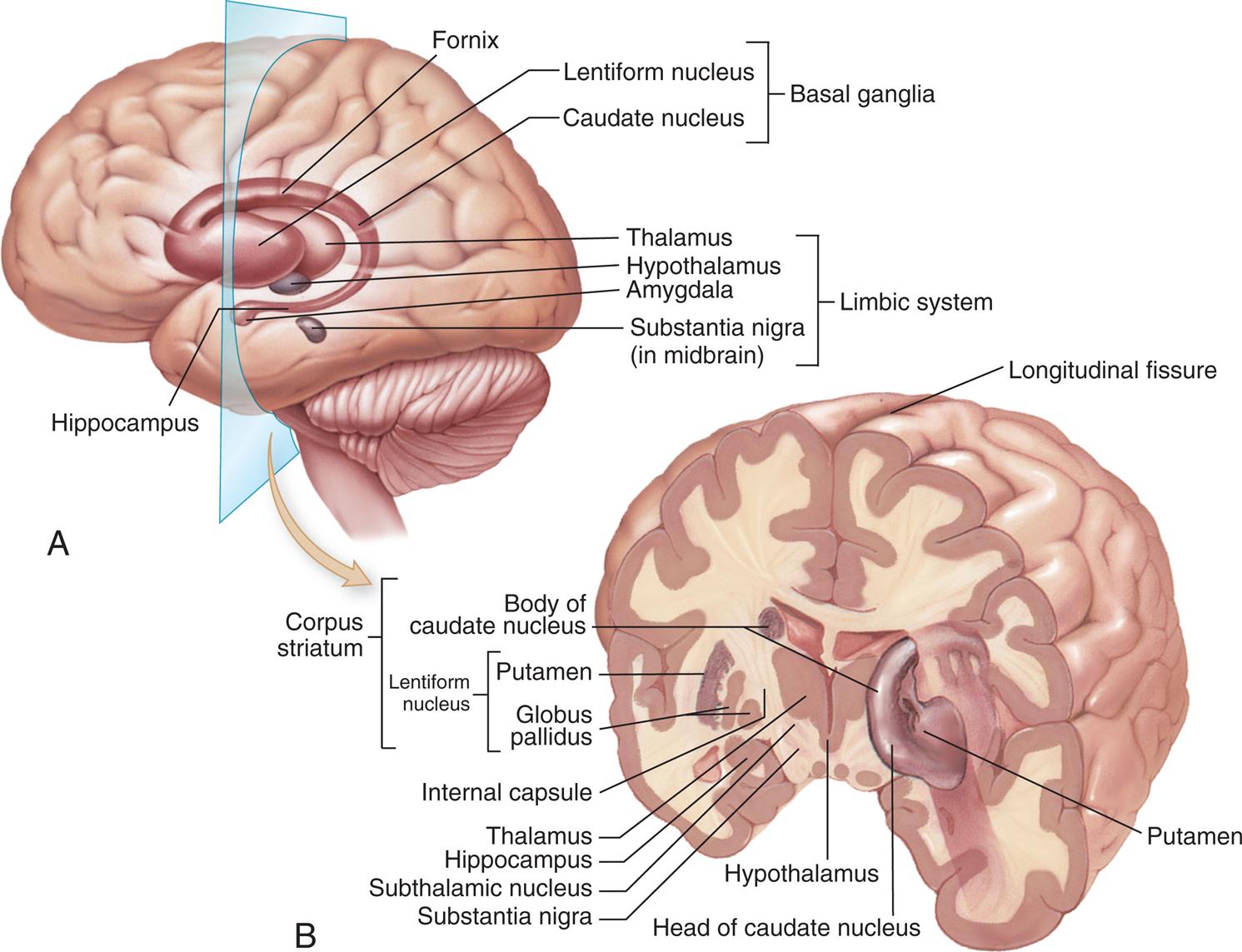
(A) The basal ganglia seen through the cortex of the left cerebral hemisphere. (B) The basal ganglia seen in a frontal (coronal) section of the brain. The nucleus accumbens is not visible in this figure; it lies between the caudate nucleus and putamen. (From Patton KT, Thibodeau GA. Anatomy & Physiology. 9th edition. St. Louis: Mosby; 2016.)
Illustration A shows the left cerebral hemisphere and labels the following structures: hippocampus, fornix, basal ganglia (lentiform nucleus, caudate nucleus), limbic system (thalamus, hypothalamus, amygdala, and substantia nigra in midbrain). Illustration B shows a cross-sectional view of the coronal section of the brain shows and labels the following structures: longitudinal fissure, putamen, head of caudate nucleus, hypothalamus, substantia nigra, subthalamic nucleus, hippocampus, thalamus, internal capsule, and corpus striatum. Corpus striatum is made of body of caudate nucleus and lentiform nucleus. The lentiform nucleus is made of putamen and globus pallidus.
The posterior margin of the frontal lobe is on the central sulcus (fissure of Rolando), and it borders inferiorly on the lateral sulcus (Sylvian fissure, lateral fissure) (see Fig. 15.8A). The prefrontal area is responsible for goal-oriented behavior (e.g., ability to concentrate), short-term or recall memory, the elaboration of thought, and inhibition of the limbic areas of the CNS. The premotor area (Brodmann area 6) (see Fig. 15.8C) is involved in programming motor movements. This area contains the cell bodies that form part of the basal ganglia system. The frontal eye fields (the lower portion of Brodmann area 8), which control eye movements, are located on the middle frontal gyrus.
The primary motor area (Brodmann area 4) is located along the precentral gyrus forming the primary voluntary motor area. It has a specific correspondence between a body region and an area in the brain (somatotopic organization) that is often referred to as a homunculus (little man) (Fig. 15.9). Electrical stimulation of specific areas of this cortex causes specific muscles of the body to move. For example, stimulation of Brodmann area 4 in the medial longitudinal fissure affects the lower limb and foot, whereas stimulation of the superior lateral surface of the precentral gyrus affects the torso and arm, the middle third of the hand, and the lower third of the face and mouth/throat. The axons traveling from the cell bodies in and on either side of this gyrus project fibers (axons) that form the pyramidal system. This system includes the corticobulbar tract that synapses in the brainstem and provides voluntary control of muscles in the head and neck and the corticospinal tracts that descend into the spinal cord and provide voluntary control of muscles throughout the body. Cerebral impulses control function on the opposite side of the body, a phenomenon called contralateral control (see Fig. 15.15 later in the chapter). The Broca speech area is on the inferior frontal gyrus (Brodmann areas 44, 45). It is usually on the left hemisphere and is responsible for the motor aspects of speech. Damage to this area, commonly as a result of a cerebrovascular accident (stroke), results in the inability to form words or at least some difficulty in forming words (expressive aphasia) (see Chapter 17).
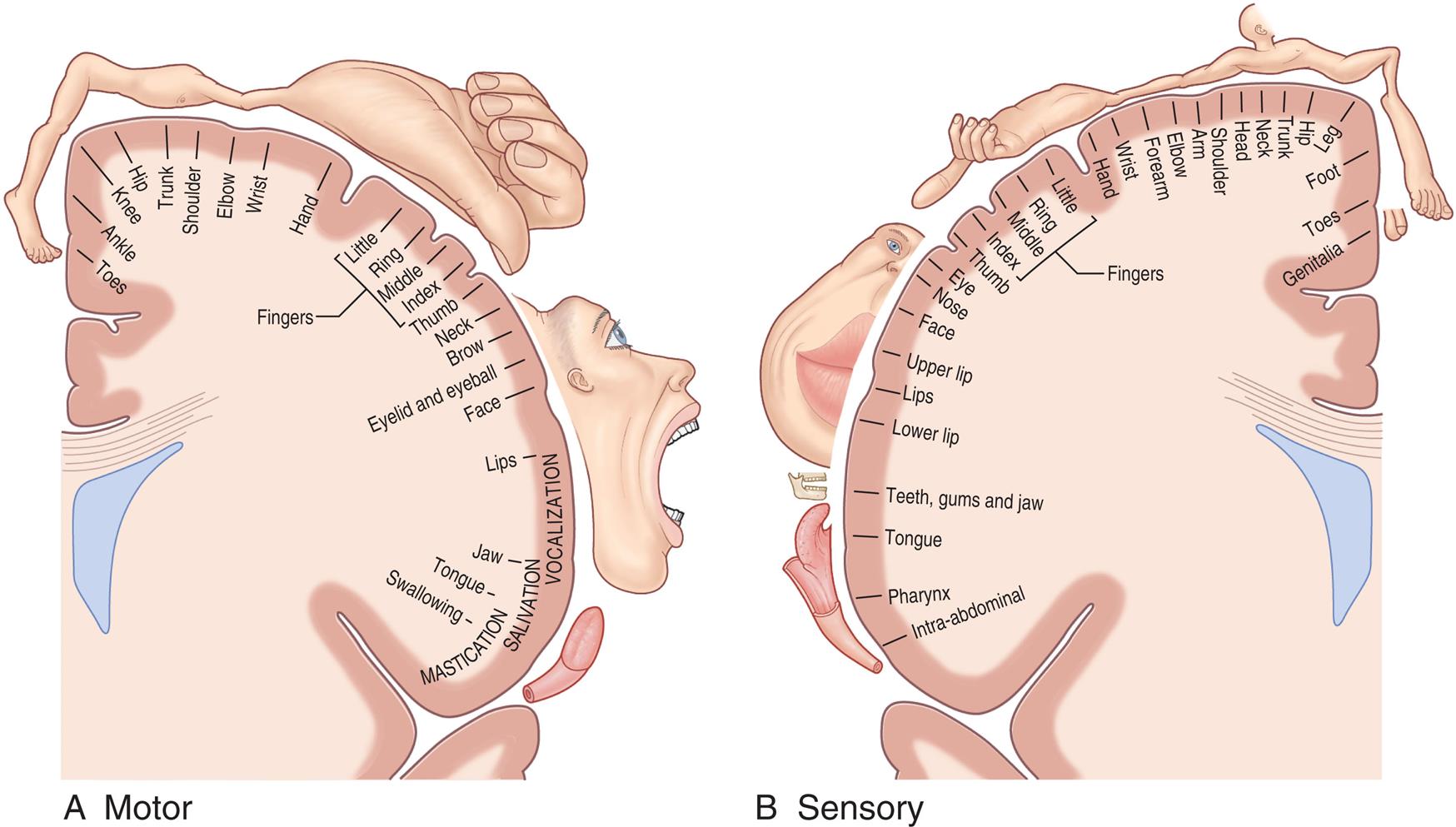
(A) The motor homunculus shows proportional somatotopic representation in the main motor area. (B) The sensory homunculus shows proportional somatotopic representation in the somaesthetic cortex. (From Standring S. Gray's Anatomy. 40th edition. Edinburgh: Churchill Livingstone; 2008.)
Illustration A shows the representations in the main motor area, clockwise from the top: toes, ankle, knee, hip, trunk, shoulder, elbow, wrist, hand, fingers (little, ring, middle, index, thumb), neck, brow, eyelid and eyeball, face, lips (vocalization), jaw (salivation), tongue, and swallowing (mastication). Illustration B shows the representation in the somaesthetic cortex, counterclockwise from the top: genitalia, toes, foot, leg, hip, trunk, neck, head, shoulder, arm, elbow, forearm, hand, fingers (little, ring, middle, index, thumb), eye, nose, face, upper lip, lips, lower lip, teeth, gums, and jaw, tongue, pharynx, and intra-abdominal.
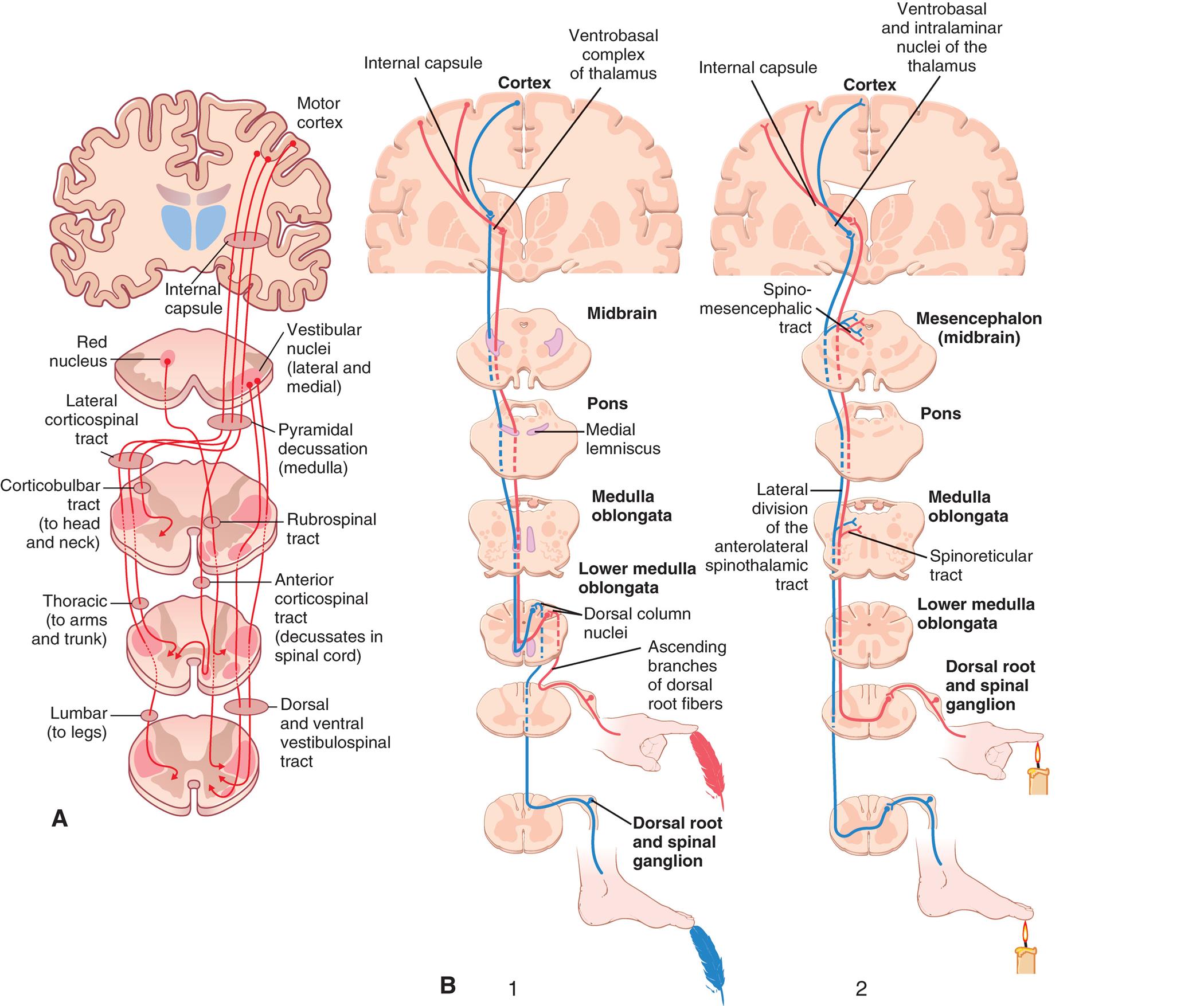
(A) Motor tracts. The pyramidal pathway through the lateral corticospinal tract and the extrapyramidal pathways through the rubrospinal, reticulospinal, and vestibulospinal tracts. Note that the pathways from the motor cortex cross over to the opposite side of the body, demonstrating contralateral control. (B) Sensory tracts. 1, The dorsal column-medial lemniscal pathway for transmitting critical types of tactile signals: touch/proprioception. Note the lateral corticospinal tract decussation; the point where it crosses to the other side is in the lower medulla. 2, Anterior and lateral divisions of the anterolateral spinothalamic sensory tract: pain/temperature. Note the decussation is in the spinal cord. (A, From Compston A, Lassmann H, McDonald I. McAlpine’s multiple sclerosis, 4th edition. London: Churchill Livingstone; 2006. B, From Hall JE. Guyton and Hall textbook of medical physiology, 13th edition. Philadelphia: Saunders; 2016.)
Illustration A traces the somatic motor pathways from the motor cortex. Three pathways traced on the illustration are as follows • Motor cortex through internal capsule, vestibular nuclei (lateral and medial), pyramidal decussation (medulla), lateral corticospinal tract, corticobulbar tract (to head and neck), and thoracic (to arms and trunk), to lumbar (to legs). • Vestibular nuclei (lateral and medial) through rubrospinal tract and anterior corticospinal tract (decussates in spinal cord), to dorsal and ventral vestibulospinal tract. • Red nucleus through rubrospinal tract and anterior corticospinal tract (decussates in spinal cord), to dorsal and ventral vestibulospinal tract. Illustration B traces two sensory pathways from the cortex. 1. The tip of the index finger touches a feather, activating the ascending branches of dorsal root fibers. The tip of the big toe touches a feather, activating the dorsal root and spinal ganglion. The pathways are then traced through the following structures: ascending branches of dorsal root fibers, dorsal column nuclei in lower medulla oblongata, medulla oblongata, pons (medial lemniscus), midbrain, spino-mesencephalic tract in mesencephalon (midbrain), and internal capsule (pathway from foot) or ventrobasal complex of thalamus (pathway from hand). 2. The tip of the index finger and the big toe touch a candle flame each, activating the dorsal root and spinal ganglion. The pathways are then traced through the following structures: dorsal root and spinal ganglion, lower medulla oblongata, spinoreticular tract, medulla oblongata, lateral division of the anterolateral spinothalamic tract, pons, and internal capsule (pathway from hand) or ventrobasal and intralaminar nuclei of the thalamus (pathway from foot).
The parietal lobe lies within the borders of the central, parietooccipital, and lateral sulci. This lobe contains the major area for somatic sensory input, located primarily along the postcentral gyrus (Brodmann areas 3, 1, 2) (see Fig. 15.8), which is adjacent to the primary motor area precentral gyrus. Communication between the motor and sensory areas (and among other regions in the cortex) is provided by association fibers. Much of this region is involved in sensory association (storage, analysis, and interpretation of stimuli). Fig. 15.9 shows the distribution of functions associated with both the primary motor area and the primary sensory area of the cerebral cortex.
The occipital lobe lies caudal to the parietooccipital sulcus and is superior to the cerebellum. The primary visual cortex (Brodmann area 17) is located in this region and receives input from the retinas. Much of the remainder of this lobe is involved in visual association (Brodmann areas 18, 19). The temporal lobe lies inferior to the lateral fissure and is composed of the superior, middle, and inferior temporal gyri. The primary auditory cortex (Brodmann area 41) and related association area (Brodmann area 42) lie deep within the lateral sulcus on the superior temporal gyrus. The Wernicke area (posterior portion of Brodmann area 22) and adjacent portions of the parietal lobe constitute a sensory speech area. This area is responsible for reception and interpretation of speech, and dysfunction may result in receptive aphasia or dysphasia. The temporal lobe also is involved in memory consolidation and smell.
Another lobe, the insula (insular lobe), lies hidden from view in the lateral sulci between each hemisphere's temporal and frontal lobes. The insula processes sensory and emotional information and routes the information to other areas of the brain. Lying directly beneath the longitudinal fissure is a mass of white matter pathways called the corpus callosum (transverse or commissural fibers). This structure connects the two cerebral hemispheres through sensory and motor contralateral projection of axons and is essential in coordinating activities between hemispheres (see Fig. 15.8B).
Basal ganglia
Inside the cerebrum are numerous tracts (white matter) and nuclei (gray matter). The major subcortical nuclei are called the basal ganglia (basal nuclei) system. The basal ganglia system is a group of nuclei that includes the caudate nucleus, putamen, and globus pallidus (Fig. 15.10). The putamen and the globus pallidus together are called the lentiform nucleus (because they are shaped like a lentil). The caudate nucleus, putamen, and nucleus accumbens together are called the striatum. Functionally, the substantia nigra is a component of the basal ganglia. It synthesizes dopamine, a neurotransmitter and precursor of norepinephrine. The basal ganglia nuclei are important for voluntary movement and cognitive and emotional functions (i.e., the nucleus accumbens has pleasure and reward functions).
The internal capsule is a thick layer of white matter in which axons of afferent (sensory) and efferent (motor) pathways pass to and from the cerebral cortex through the center of the cerebral hemispheres and between the caudate and lentiform nuclei (see Fig. 15.10B). The basal ganglia, plus their direct and indirect interconnections with the thalamus, premotor cortex, red nucleus, reticular formation, and spinal cord, have been considered part of the extrapyramidal system. The extrapyramidal system is part of the motor control system that causes involuntary reflexes and coordinated movement and stabilizes motor control. Parkinson disease and Huntington disease are characterized by disruption of the extrapyramidal system and various involuntary or exaggerated motor movements (see Chapter 17).
Limbic system
The limbic system is a group of interconnected structures between the telencephalon and diencephalon and surrounding the corpus callosum. It comprises the amygdala, hippocampus, fornix, hypothalamus, and related autonomic nuclei (see Fig. 15.10A). It is an extension or modification of the olfactory system and influences the autonomic and endocrine systems. Its principal effects are involved in primitive behavioral responses, visceral reaction to emotion, motivation, mood, feeding behaviors, biologic rhythms, and the sense of smell. The limbic system mediates emotion and long-term memory through connections in the prefrontal cortex (limbic cortex).
Diencephalon
The diencephalon (interbrain) is surrounded by the cerebrum and sits on top of the brainstem. It controls vital functions and visceral activities and is closely associated with those of the limbic system. The diencephalon has four divisions: epithalamus, thalamus, hypothalamus, and subthalamus (see Table 15.3 and Fig. 15.8). The epithalamus forms the roof of the third ventricle (a brain cavity) and composes the most superior portion of the diencephalon.
The thalamus is the largest component of the diencephalon, and it borders and surrounds the third ventricle. It is a major integrating center for afferent impulses to the cerebral cortex. Various sensations are perceived at this level, but cortical processing is required for interpretation. The thalamus also serves as a relay center for information from the basal ganglia and cerebellum to the appropriate motor area.
The hypothalamus forms the base of the diencephalon. The hypothalamus functions to (1) maintain a constant internal environment and (2) implement behavioral patterns. Integrative centers control ANS function, regulate body temperature and endocrine function, and adjust emotional expression. The hypothalamus exerts its influence through the endocrine system and neural pathways (Box 15.1). The subthalamus flanks the hypothalamus laterally. It serves as an important basal ganglia center for motor activities.
Midbrain
Mesencephalon
The midbrain (mesencephalon) is com-posed of the tectum (corpora quadrigemina [forms roof of the midbrain]), the tegmentum, and the cerebral peduncles. The tectum includes two pairs of superior colliculi and two pairs of inferior colliculi. The superior colliculi are involved with voluntary and involuntary visual-motor movements (e.g., the ability of the eyes to track moving objects in the visual field). The inferior colliculi accomplish similar motor activities but involve movements affecting the auditory system (e.g., positioning the head to improve hearing). The tegmentum (the floor of the midbrain) is composed of the red nucleus and substantia nigra. The red nucleus receives ascending sensory information from the cerebellum and projects a minor motor pathway, the rubrospinal tract, to the cervical spinal cord. The substantia nigra synthesizes dopamine. The cerebral peduncles of the anterior midbrain are made up of efferent fibers of the corticospinal, corticobulbar, and corticopontocerebellar tracts (tracts that link the cortex to the brainstem).
Other notable structures of this region are the nuclei of the third and fourth cranial nerves. The cerebral aqueduct (aqueduct of Sylvius), which carries cerebrospinal fluid (CSF), also traverses this structure. Obstruction of this aqueduct is often the cause of hydrocephalus.
Hindbrain
Metencephalon
The major structures of the metencephalon are the cerebellum and the pons. The cerebellum (seeFig. 15.8) is composed of two lobes of gray matter and white matter, and its cortical surface is convoluted, similar to the surface of the cerebrum. It also is divided by a central fissure into the right and left lobes connected by the vermis.
The cerebellum is responsible for reflexive, involuntary fine-tuning of motor control and maintaining balance and posture through extensive neural connections with the medulla (through the inferior cerebellar peduncle) and the midbrain (through the superior cerebellar peduncle). The two hemispheres are connected to the pons by the middle cerebellar peduncles. These connections allow extensive sampling of visual, vestibular, and proprioceptive data from other regions of the CNS and periphery. The cerebellum has ipsilateral control (same side) of the body, in contrast to the cerebral cortex, which has contralateral control of the body.
The pons (bridge) is easily recognized by its bulging appearance below the midbrain and above the medulla. Primarily it transmits information from the cerebellum to the brainstem and between the two cerebellar hemispheres. The nuclei of the fifth through eighth cranial nerves are located in this structure.
Myelencephalon
The myelencephalon usually is called the medulla oblongata and forms the lowest portion of the brainstem. Reflex activities, such as heart rate, respiration, blood pressure, coughing, sneezing, swallowing, and vomiting, are controlled in this area. The nuclei of cranial nerves IX through XII are located in this region.
A major portion of the descending motor pathways (i.e., corticospinal tracts) cross to the other side, or decussate, at the medulla (see Fig. 15.15 later in the chapter). These pathways, together with other areas of decussation in the CNS, are the basis for the phenomenon of contralateral control when cerebral impulses control function on the opposite side of the body. Sleep–wake rhythms also are processed by neural influences from lower brain centers and are associated with a complex group of diffuse structures and functions (see Chapter 16), including the reticular activating system (cells that receive collateral signals from the afferent sensory pathways and project the signals to the higher brain centers, thus controlling CNS activity) (see Fig. 15.7).
Spinal Cord
The spinal cord is the portion of the CNS that lies within the vertebral canal and is surrounded and protected by the vertebral column. The spinal cord has many functions, including connecting the brain and the body through a long nerve cable, somatic and autonomic reflexes, motor pattern control, and sensory and motor modulation. The spinal cord originates in the medulla oblongata and ends at the first or second lumbar vertebra level in adults (Fig. 15.11A). The end of the spinal cord, the conus medullaris, is cone-shaped. Spinal nerves continue from the end of the spinal cord and form a nerve bundle called the cauda equine. The filament anchor from the conus medullaris to the coccyx is the filum terminale. The coverings of the spinal cord are illustrated in Fig. 15.11C.
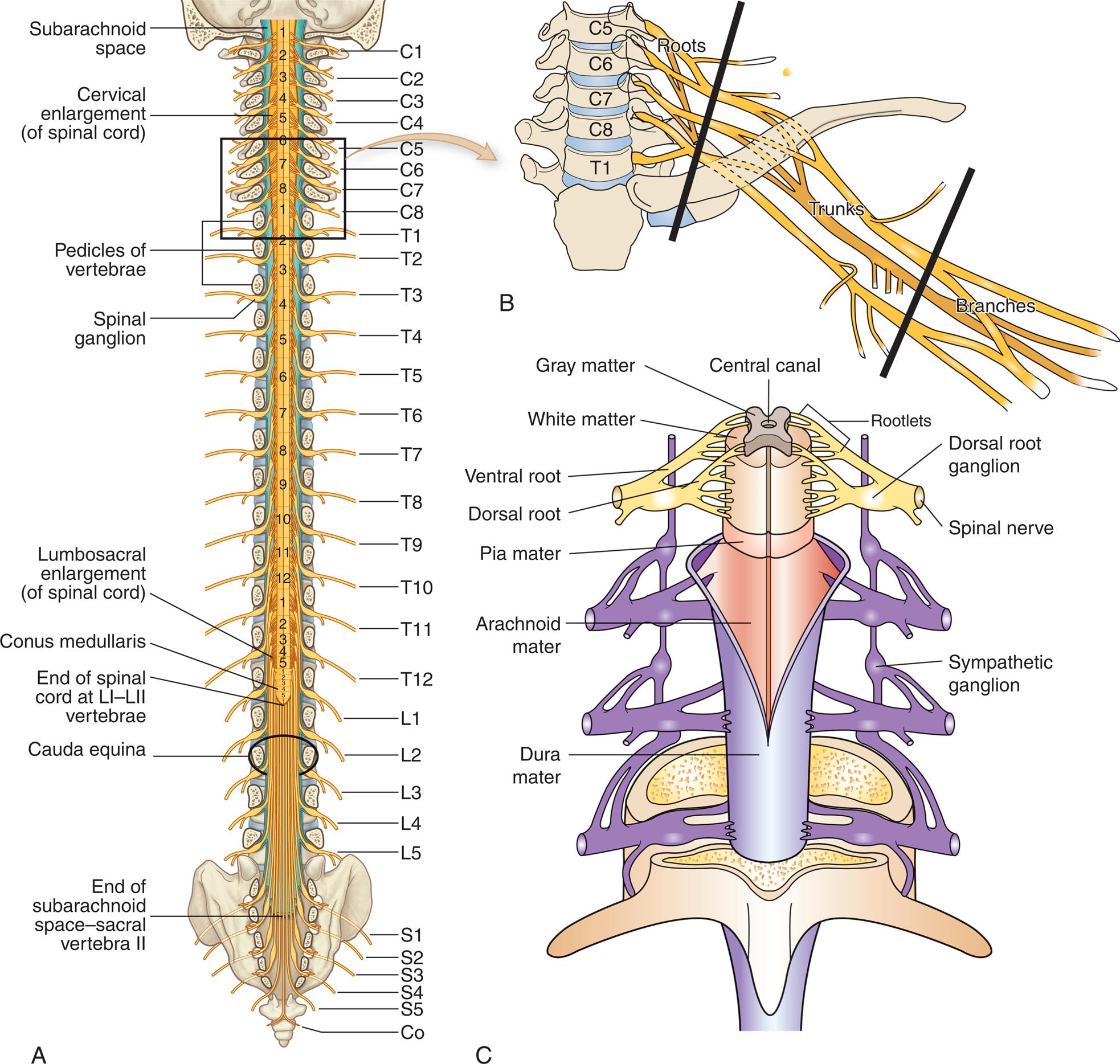
Illustration A shows the spinal cord and labels its different sections. From the top, the sections are as follows: subarachnoid space, cervical enlargement (of spinal cord), pedicles of vertebrae, spinal ganglion, lumbosacral enlargement (of spinal cord), conus medullaris, end of spinal cord at L 1 and L 2 vertebrae, cauda equina, and end of subarachnoid space-sacral vertebra 2. The vertebrae are labeled from the top as follows: C 1 to C 8, T 1 to T 12, L 1 to L 5, S 1 to S 5, and C o. Illustration B shows a section of spinal cord, C 5 to T 1. The spinal nerves connected to the spinal cord comprise branches, trunks, and roots. Illustration C shows the coverings of the spinal cord. From the inside to the outside, the structures are as follows: central canal, gray matter, white matter, rootlets, ventral root, dorsal root, dorsal root ganglion, spinal nerve, pia mater, arachnoid mater, dura mater, and sympathetic ganglion.
Grossly, the spinal cord is divided into vertebral sections (8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal) that correspond to paired nerves (see Fig. 15.11A). A cross section of the spinal cord (Fig. 15.12) is characterized by a butterfly-shaped inner core of gray matter (containing nerve cell bodies). The central canal is filled with CSF, lies in the center of this region, and extends through the spinal cord from its origin in the fourth ventricle. The gray matter of the spinal cord is divided into three regions and displays specific functional characteristics. These regions include the posterior horn, the lateral horn, and the anterior horn. The posterior horn, or dorsal horn, is composed primarily of interneurons and axons from sensory neurons whose cell bodies lie in the dorsal root ganglion. At the tip of the posterior horn is the substantia gelatinosa, a structure involved in pain transmission (see Chapter 16). The lateral horn contains cell bodies involved with the ANS. The anterior horn, or ventral horn, contains the nerve cell bodies for efferent pathways that leave the spinal cord by way of spinal nerves.
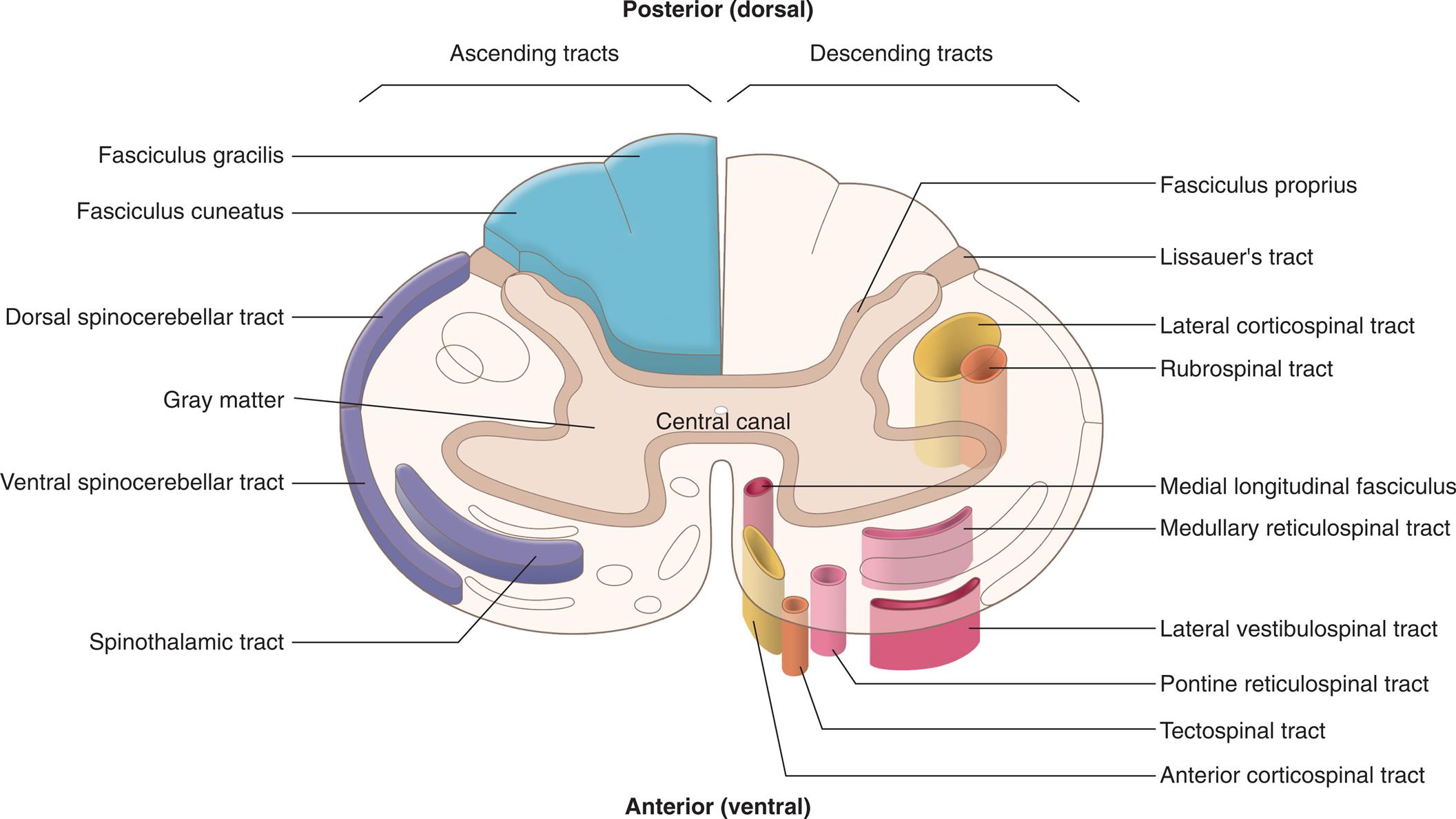
An illustration of the central canal with the posterior (dorsal) side at the top and the anterior (ventral) side at the bottom identifies the ascending descending tracts in the spinal cord. The ascending tracts, from the top to the bottom, are as follows: fasciculus gracilis, fasciculus cuneatus, dorsal spinocerebellar tract, gray matter, ventral spinocerebellar tract, and spinothalamic tract. The descending tracts, from the top to the bottom, are as follows: fasciculus proprius, Lissauer’s tract, lateral corticospinal tract, rubrospinal tract, medial longitudinal fasciculus, medullary reticulospinal tract, lateral vestibulospinal tract, pontine reticulospinal tract, tectospinal tract, and anterior corticospinal tract.
Surrounding the gray matter is white matter, which forms ascending and descending pathways called spinal tracts. Spinal tracts are named to denote their beginning and ending points. For example, the spinothalamic tract (see Fig. 15.12) carries sensory nerve impulses from the spinal cord to the thalamus in the diencephalon. Numerous spinal tracts are grouped into columns according to their location within the white matter. These include the anterior columns, lateral columns, and posterior columns (see Fig. 15.12).
Neural circuits in the spinal cord, when activated, display specific sets of motor responses. Reflex arcs form basic units that respond to stimuli and provide protective circuitry for motor output. Structures needed for a reflex arc are a receptor, an afferent (sensory) neuron, an efferent (motor) neuron, and an effector muscle or gland. A simple reflex arc (e.g., knee-jerk reflex) may contain only two neurons (Fig. 15.13). Interneurons are usually present and provide a link between sensory and motor neurons. The motor effects of reflex arcs generally occur before the event is perceived in the brain's higher centers. Much internal environmental regulation is mediated by the ANS's reflex activity (e.g., cardiac muscle and smooth muscle contraction/relaxation and glandular responses).
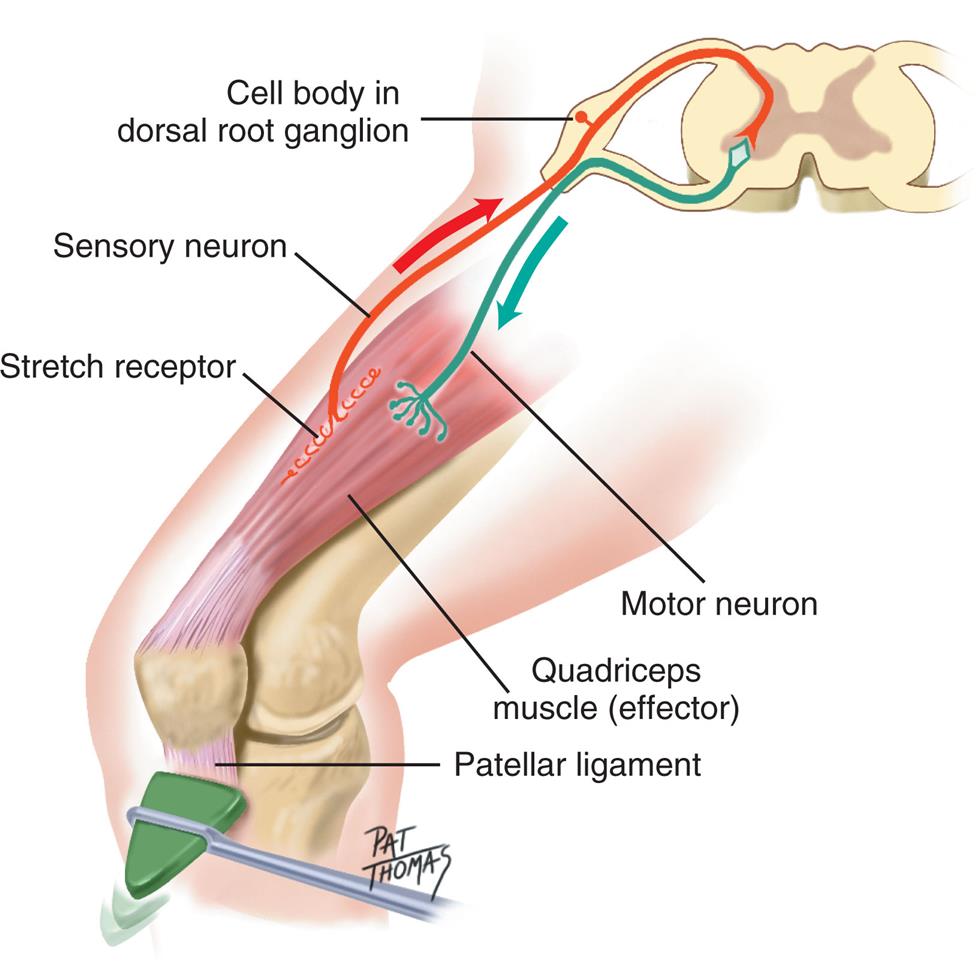
An illustration shows the cross-section of the spinal cord. The following structures are labeled along structure: cell body in dorsal root ganglion, sensory neuron, stretch receptor, motor neuron, quadriceps muscle (effector), and patellar ligament.
Afferent pathways transmit sensory information from peripheral receptors toward the cerebrum. The pathways terminate in the cerebral or cerebellar cortex or both. Efferent pathways primarily relay information away from the cerebrum to the brainstem or spinal cord. Upper motor neurons (i.e., corticospinal and corticobulbar tracts) are completely contained within the CNS. Their primary roles are controlling fine motor movement and influencing/modifying spinal reflex arcs and circuits. Generally, upper motor neurons form synapses with interneurons, forming synapses with lower motor neurons that project into the periphery. Lower motor neurons directly influence muscles. Their cell bodies lie in the brainstem and spinal cord's gray matter, but their processes extend out of the CNS and into the PNS. Destruction of upper motor neurons usually results in initial paralysis followed within days or weeks by partial recovery, whereas destruction of the lower motor neuronsleads to paralysis unless peripheral nerve damage is followed by nerve regeneration and recovery (see Fig. 15.4). Differences in injury to upper and lower motor neurons are presented in Chapter 17.
Nerve impulses regulate muscle activity (i.e., stimulation and contraction). Motor neurons innervate one or more muscle cells, forming motor units, consisting of a neuron and the skeletal muscles it stimulates. The junction between the axon of the motor neuron and the plasma membrane of the muscle cell is called the neuromuscular (myoneural) junction (Fig. 15.14). (Injury to motor neurons is discussed in Chapter 17.)
Motor Pathways
Clinically relevant motor pathways are the lateral corticospinal (connects motor cortex with anterior horn cells in the spinal cord) and pyramidal tracts (connects the motor cortex with the medullary pyramids and descend to synapse with lower motor neurons of several cranial nerves in the brain stem or spinal cord); and the extrapyramidal reticulospinal, vestibulospinal, and rubrospinal tracts. The corticospinal and corticobulbar pathways are essentially the same tract and consist of a two-neuron chain (Fig. 15.15). The cell bodies (upper motor neurons) originate in and around the precentral gyrus; pass through the corona radiata (connects motor and sensory pathways) of the cerebrum, the internal capsule, middle three-fifths of the cerebral pedunculus, pons, and pyramid; and decussate (cross contralaterally) in the medulla oblongata and form the lateral corticospinal tract of the spinal cord (see Figs. 15.12 and 15.15A) and thus control the opposite side of the body. The corticobulbar tract axons synapse on motor cranial nuclei within the brainstem that control the face, head, and neck muscles. The lateral corticospinal tract axons leave the tract to go to specific interneurons or motor neurons in the anterior horn. The lateral corticospinal tract has the same somatotopic organization as the body (see Figs. 15.9 and 15.15A). These lower motor neurons project through nerves to specific muscles.
The extrapyramidal tracts are involved in precise motor movements. The reticulospinal tract arises in the reticular formation of the medulla or pons (see Fig. 15.12) and modulates motor movement by inhibiting and exciting spinal activity. The vestibulospinal tract arises from a vestibular nucleus in the pons and causes the extensor muscles of the body to rapidly contract, most dramatically witnessed when a person starts to fall backward. The rubrospinal tract originates in the red nucleus, decussates, and terminates in the cervical spinal cord. It is important for muscle movement and fine muscle control in the upper extremities.
Sensory Pathways
The three clinically important spinal afferent pathways are the posterior column, anterior spinothalamic tract, and lateral spinothalamic tract (see Figs. 15.12 and 15.15B). The posterior (dorsal) column (fasciculus gracilis and fasciculus cuneatus) carries fine-touch sensation, two-point discrimination, and proprioceptive information (i.e., epicritic information). The posterior column is formed by a three-neuron chain. The first neuron of the chain is the primary afferent neuron. It also is the sensory neuron of the reflex arc. After entering the spinal cord, it sends its axon ipsilaterally (on the same side) up the spinal cord to a specific part of the posterior column and synapses in the three posterior column nuclei in the medulla oblongata. For example, a basketball player who is above 6 feet tall has primary afferent neurons that could be 6 feet long, running from the great toe up to the medulla oblongata. The axon of the second-order neuron crosses contralaterally in the medial lemniscus and ascends in the medulla and pons to synapse with a specific nucleus of the thalamus. The third-order neuron, originating in the thalamus, continues the tract into the internal capsule, corona radiata, and postcentral gyrus (Brodmann areas 3, 1, 2) (see Fig. 15.8, and 15.15B).
The anterior and lateral spinothalamic tracts are responsible for vague touch sensation and pain and temperature perception, respectively (see Figs. 15.12 and 15.15; see Chapter 16). These modalities are referred to as protopathic. These tracts also form a three-neuron chain. However, their primary afferent neurons synapse in the posterior horn of the spinal cord is not just at the level they enter the intervertebral foramen but in several spinal segments above and below their point of entry. This is an example of divergence. The axons of the second-order neurons in the posterior horn cross to the contralateral side in the spinal cord in the lateral column, ascend to the same thalamic nucleus as the posterior column pathway, and continue with the posterior column pathway to the postcentral gyrus.
Protective Structures
Cranium
The cranial vault encloses and protects the brain and its associated structures. The bony cranium comprises eight bones (frontal, two parietal, two temporal, ethmoid, sphenoid, and occipital). The galea aponeurotica, a thick, fibrous band of tissue overlying the cranium between the frontal and occipital muscles, affords added protection to the skull. The subgaleal space has venous connections with the dural sinuses. If there is increased intracranial pressure, blood can be shunted to the space, thus reducing pressure in the intracranial cavity. The subgaleal space is also a common site for wound drains after intracranial surgery.
The floor of the cranial vault is irregular and contains many foramina (openings) for cranial nerves, blood vessels, and the spinal cord to exit. The cranial floor is divided into three fossae (depressions). The frontal lobes lie in the anterior fossa, the temporal lobes and base of the diencephalon lie in the middle fossa (temporal fossa), and the cerebellum lies in the posterior fossa. These terms are commonly used anatomic landmarks to describe the location of intracranial lesions.
Meninges
Surrounding the brain and spinal cord are three protective membranes: the dura mater, the arachnoid, and the pia mater. Collectively they are called the meninges (Fig. 15.16C). The dura mater (meaning literally “hard mother”) is composed of two layers, with the venous sinuses formed between them. The outermost layer forms the periosteum (endosteal layer) of the skull. The inner dura (meningeal layer) is responsible for forming rigid membranes that support and separate various brain structures. One of these membranes, the falx cerebri, dips between the two cerebral hemispheres along the longitudinal fissure. The falx cerebri is anchored anteriorly to the base of the brain at the crista galli of the ethmoid bone. The tentorium cerebelli, a common landmark, is a membrane that separates the cerebellum below from the cerebral structures above. Internal to the dura mater is the location of the arachnoid, a spongy, web-like structure that loosely follows the contours of the cerebral structures.
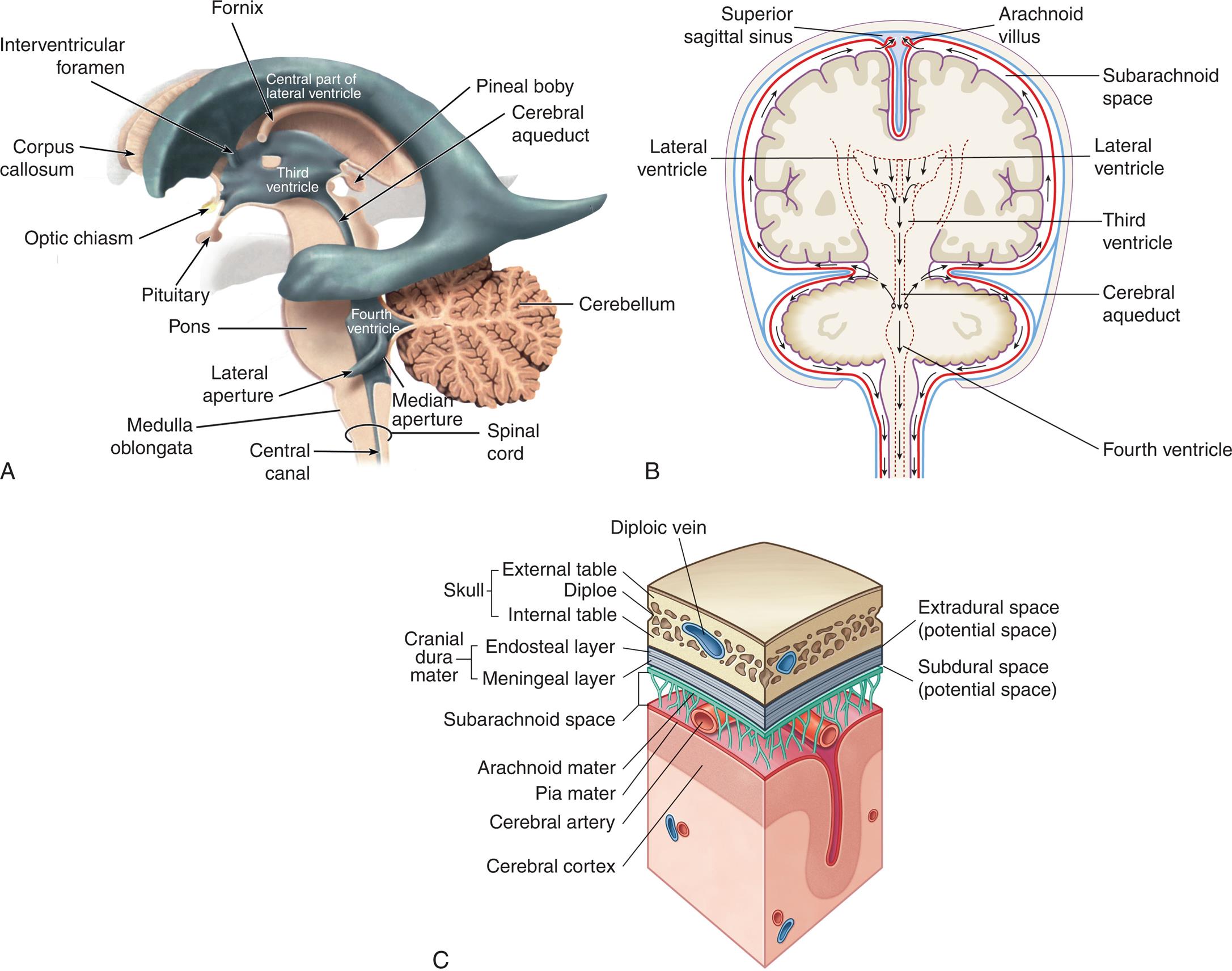
(A) Ventricles highlighted in blue within a translucent brain in a left lateral view. (B) Flow of cerebral spinal fluid. The fluid produced by filtration of blood by the choroid plexus of each ventricle flows inferiorly through the lateral ventricles, interventricular foramen, third ventricle, cerebral aqueduct, fourth ventricle, and subarachnoid space to the blood. (C) Meninges of the brain in relation to cerebrospinal fluid and venous blood flow. (A and B, From Waugh A, Grant A. Ross and Wilson anatomy and physiology in health and illness, 12th edition. London: Churchill Livingstone; 2012. C, From Drake RL, Gray H, Vogl W, et al. Gray’s anatomy for students, 3rd edition. London: Churchill Livingstone; 2015.)
Three illustrations, A, B, and C, show the flow of cerebrospinal fluid and meninges of the brain. Illustration A identifies the following structures with reference to the different ventricles. • Central part of lateral ventricle. Corpus callosum, interventricular foramen, and fornix. • Third ventricle. Pituitary, optic chiasm, pineal boby, cerebral aqueduct. • Fourth ventricle. Pons, lateral aperture, medulla oblongata, cerebellum, median aperture, central canal, and spinal cord. Illustration B identifies the following structures on the posterior view of the brain, clockwise from the top: arachnoid villus, subarachnoid space, lateral ventricle, cerebral aqueduct, fourth ventricle, lateral ventricle, and superior sagittal sinus. Illustration shows a cross-sectional view of meninges of the brain. The following structures on the labeled on the illustration, from the top to the bottom: skull (external table, dipole, and internal table), diploic vein, extradural space (potential space), cranial dura mater (endosteal layer and meningeal layer, subdural space (potential space), subarachnoid space, arachnoid mater, cerebral artery, pia mater, and cerebral cortex.
The subdural space lies between the dura and arachnoid. Many small bridging veins that have little structural support traverse the subdural space. Their disruption results in a subdural hematoma (see Chapter 18). The subarachnoid space lies between the arachnoid and the pia mater and contains CSF (see Fig. 15.16C). Unlike the dura mater and the arachnoid, the delicate pia mater adheres to the brain and spinal cord contours. It provides support for blood vessels serving brain tissue. The choroid plexuses, which are structures that produce CSF, arise from the pia membrane.
The spinal cord is anchored to the vertebrae by extension of the meninges (see Fig. 15.11C). The meninges continue beyond the end of the spinal cord (at vertebrae levels L1 and L2) to the lower portion of the sacrum. CSF within the subarachnoid space also circulates down to the large lumbar cistern, extending from the second lumbar vertebra to the second sacral vertebra. Cisterns are expanded areas of the subarachnoid space. The cerebellomedullary cistern (cisterna magna) and the pontine cistern are two other important cisterns.
The meninges form potential and real spaces important to understanding functional and pathologic mechanisms. For example, between the dura mater and skull lies a potential space termed the extradural space (also called the epidural space) (see Fig. 15.16C). The arterial supply to the meninges consists of blood vessels that lie within grooves in the skull. A skull fracture can sever one of these vessels and produce an epidural hematoma.
Cerebrospinal Fluid and the Ventricular System
Cerebrospinal fluid (CSF) is a clear, colorless fluid similar to blood plasma and interstitial fluid. The intracranial and spinal cord structures float in CSF and are thereby partially protected from jolts and blows. The buoyant properties of the CSF also prevent the brain from tugging on meninges, nerve roots, and blood vessels. (Constituents of CSF are listed in Table 15.4.) Between 125 and 150 mL of CSF is circulating within the ventricles (small cavities) and subarachnoid space at any given time. Approximately 600 mL of CSF is produced daily.
Table 15.4
Ependymal cells in the choroid plexuses of the lateral, third, and fourth ventricles produce the major portion of CSF. (Ventricles are illustrated in Fig. 15.16A.) These plexuses are characterized by a rich network of blood vessels supplied by the pia mater and lie close to the ependymal cells of the ventricles. The tight junctions of the choroid blood vessel provide a limiting barrier between CSF and blood, which functions similarly to the blood-brain barrier (see the Blood-Brain Barrier section).
CSF exerts pressure within the brain and the spinal cord. When a person is supine, CSF pressure is about 80 to 180 mm of water pressure or approximately 5 to 14 mm of mercury pressure, but doubles when the person moves to the upright position. CSF flow results from the pressure gradient between the arterial system and the CSF-filled cavities. Beginning in the lateral ventricles, the CSF flows through the interventricular foramen (foramen of Monro) into the third ventricle and then passes through the cerebral aqueduct (aqueduct of Sylvius) into the fourth ventricle (see Fig. 15.16B). The CSF may pass through the fourth ventricle, through the paired lateral apertures (foramen of Luschka), or the median aperture (foramen of Magendie) before communicating with the subarachnoid spaces of the brain and spinal cord. CSF is produced continually but does not accumulate. Instead, it is reabsorbed into the venous circulation through the arachnoid villi. The arachnoid villi protrude from the arachnoid space through the dura mater and lie within the blood flow of the venous sinuses (see Fig. 15.16B). CSF is reabsorbed through a pressure gradient between the arachnoid villi and the cerebral venous sinuses. The villi function as one-way valves directing CSF outflow into the blood but preventing blood flow into the subarachnoid space. Thus, CSF is formed from blood, and after circulating throughout the CNS, it returns to blood.
Vertebral Column
The vertebral column (Fig. 15.17) is composed of 33 vertebrae: 7 cervical, 12 thoracic, 5 lumbar, 5 fused sacral, and 4 fused coccygeal. Between each vertebra (except for the fused sacral and coccygeal vertebrae) is an intervertebral disk (Fig. 15.18). At the center of the intervertebral disk is the nucleus pulposus, a pulpy mass of elastic fibers. The intervertebral disk absorbs shocks, preventing damage to the vertebrae. The intervertebral disk is also a common source of back problems. If too much stress is applied to the vertebral column, the disk contents may rupture and protrude into the spinal canal, causing spinal cord compression or nerve roots. The disks can also degenerate.
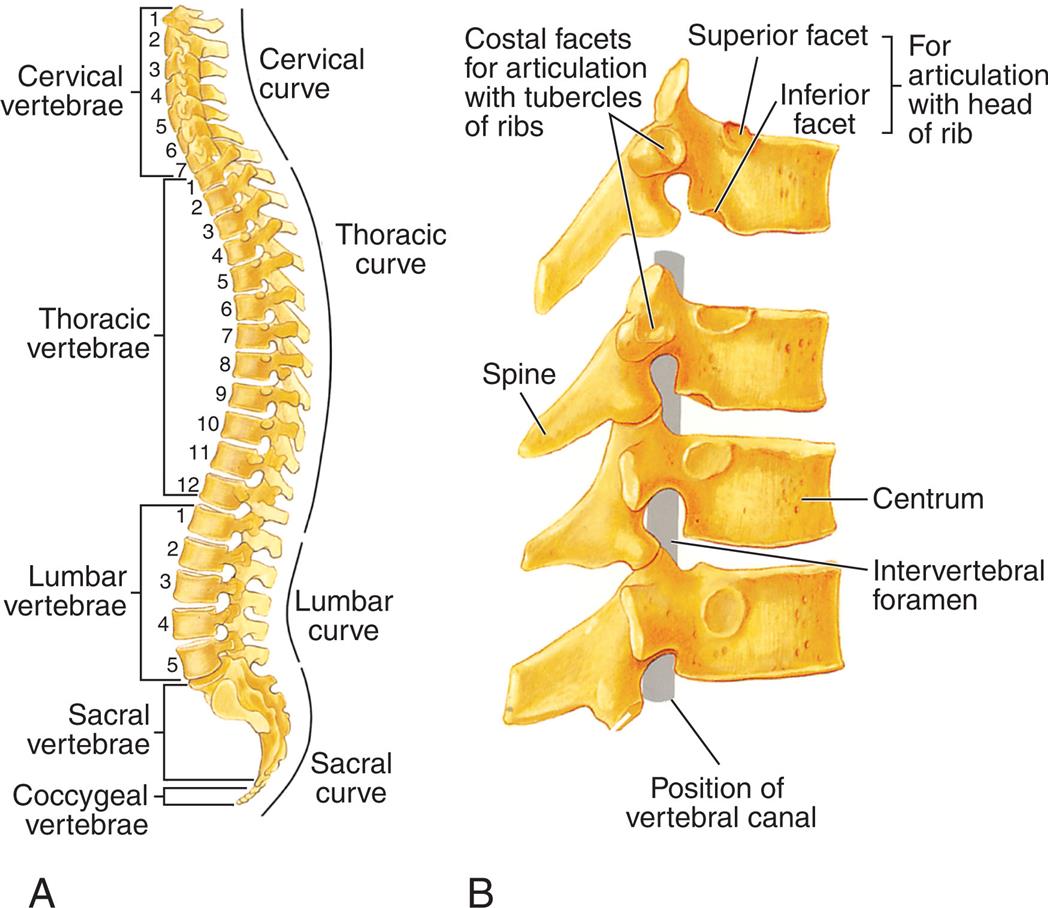
(A) The normal curves and regions of the vertebral column. The vertebrae in each region are numbered. (B) Lateral view of several vertebrae showing how they articulate. (From Solomon E. Introduction to human anatomy and physiology, 4th edition. St. Louis: Saunders; 2016.)
Illustration A is the right lateral view of the spinal cord, identifying the curves by location of the vertebrae. Cervical vertebrae (C 1 to C 7), cervical curve. Thoracic vertebrae (T 1 to T 12), thoracic curve. Lumbar vertebrae (L 1 to L 5), lumbar curve. Sacral vertebrae and coccygeal vertebrae, sacral curve. Illustration B shows a lateral view of the spine, identifying the following structures from the inside: position of vertebral canal, intervertebral foramen, centrum, and spine. An accompanying illustration shows the costal facets for articulation with tubercles of ribs and for articulation with head of rib (superior facet and inferior facet for articulation with head of rib).
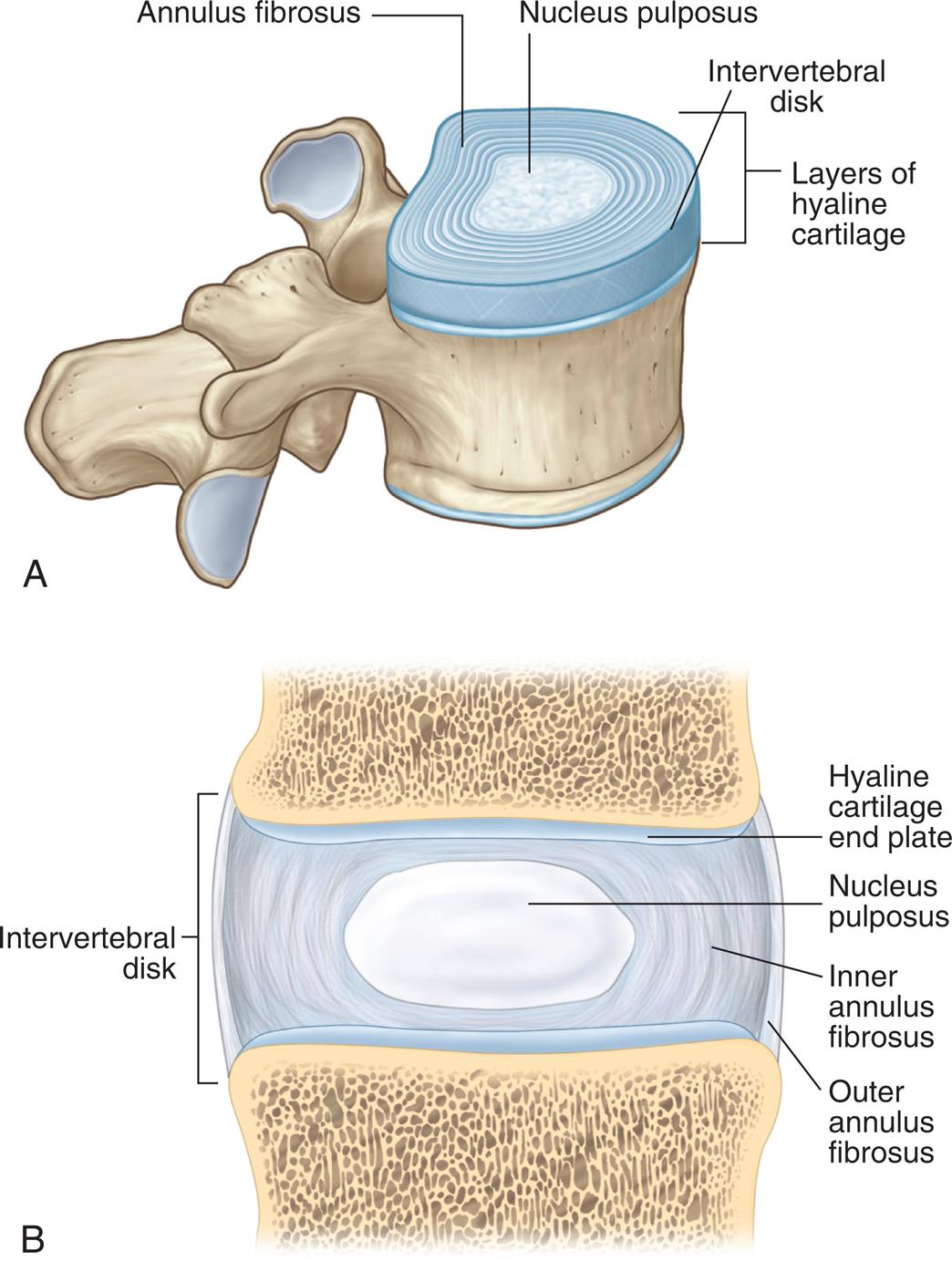
(A) Sagittal view of vertebra and intervertebral disk. (B) Lateral view of several vertebrae showing how they articulate. (A, From Drake R, Vogl AW, Mitchell AWM. Gray’s anatomy for students, 3rd edition. London: Churchill Livingstone; 2015. B, From Lawry GV, Kreder HJ, Hawker G, Jerome, D. Fam’s musculoskeletal examination and joint injection techniques, 2nd edition. Philadelphia: Mosby; 2010.)
Illustration A shows a sagittal view of a vertebra and identifies the following structures from the inside: nucleus pulposus, annulus fibrosus, and intervertebral disk followed by layers of hyaline cartilage. Illustration B shows a lateral view of a vertebra and identifies the following structures on the intervertebral disk, from the top to the bottom: hyaline cartilage end plate, nucleus pulposus, inner annulus fibrosus, and outer annulus fibrosus.
Blood Supply
Blood Supply to the Brain
The brain receives approximately 20% of the cardiac output or 800 to 1000 mL of blood flow per minute. Cerebral blood flow is autoregulated to maintain a stable flow during fluctuating perfusion pressures. Carbon dioxide is a primary regulator of blood flow within the CNS. It is a potent vasodilator, and its effects ensure an adequate blood supply.
The brain derives its arterial supply from the internal carotid arteries and the vertebral arteries (Fig. 15.19). The internal carotid arteries supply a proportionately greater amount of blood flow. They originate at the common carotid arteries, enter the cranium through the base of the skull, and pass through the cavernous sinus. After forming some small branches, these arteries divide into the anterior and middle cerebral arteries (Fig. 15.20). The vertebral arteries originate at the subclavian arteries and pass through the transverse foramina of the cervical vertebrae, entering the cranium through the foramen magnum. They join at the junction of the pons and medulla to form the basilar artery (see Fig. 15.20). The basilar artery divides at the level of the midbrain to form paired posterior cerebral arteries.
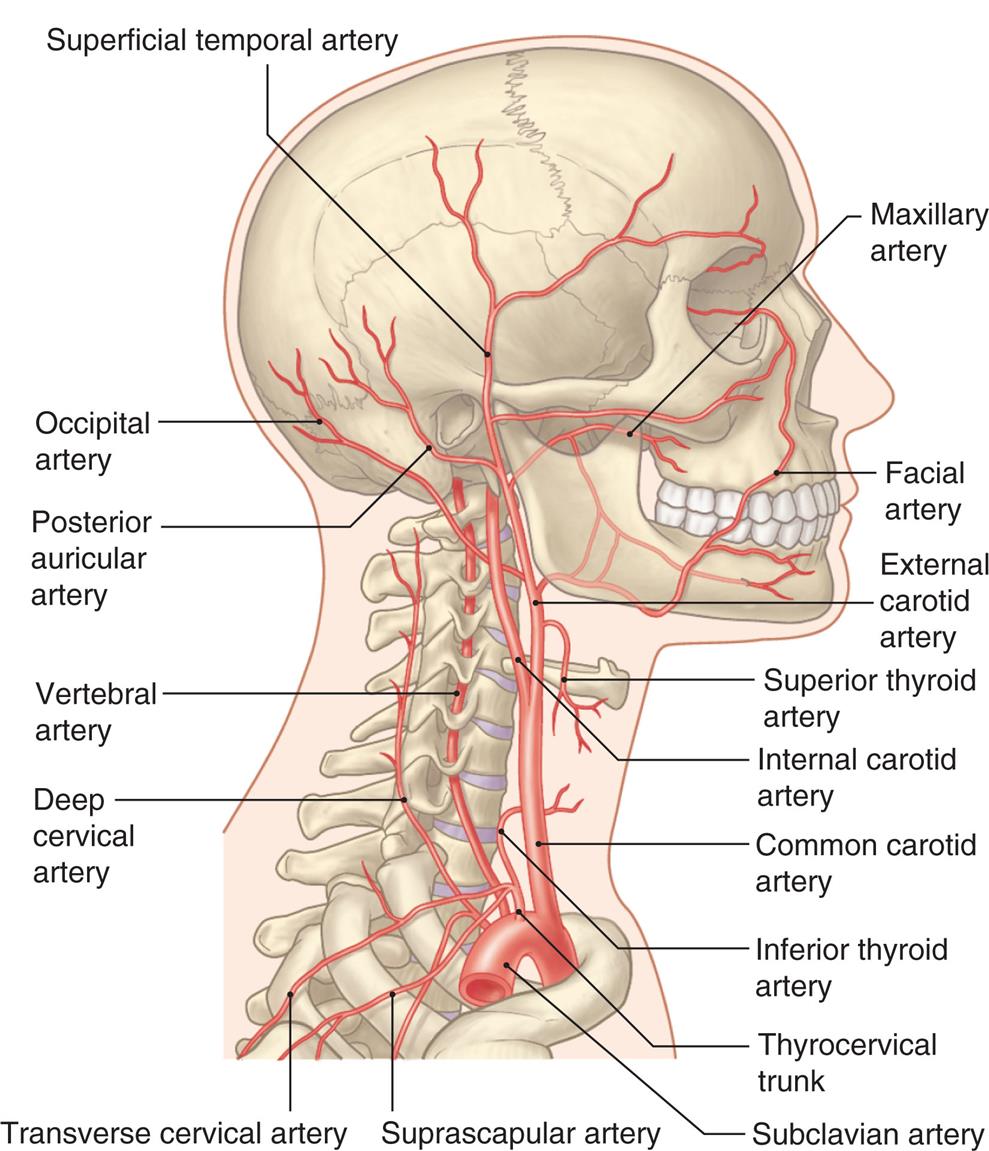
An illustration shows the right lateral view of the human skull and neck, highlighting the major arteries within the structure. The following arteries are identified from the top to the bottom along the anterior side: maxillary artery, facial artery, external carotid artery, superior thyroid artery, internal carotid artery, common carotid artery, inferior thyroid artery, thyrocervical trunk, and subclavian artery. The following arteries are identified from the top to the bottom along the posterior side: superficial temporal artery, occipital artery, posterior auricular artery, vertebral artery, deep cervical artery, transverse cervical artery, and suprascapular artery.
![Illustration A shows the inferior view of the human brain and identifies the following structures from the top to the bottom: olfactory bulb, anterior cerebral artery, optic nerve [3] (cut), middle cerebral artery, posterior cerebral artery, internal carotid artery, vertebral artery, brainstem, and cerebellum. Illustration B shows and labels the following arteries at the base of the brain, from the top to the bottom: circle of Willis, anterior communicating artery, anterior cerebral artery, middle cerebral artery, posterior communicating artery, posterior cerebral artery, basilar artery, and vertebral artery.](../images/F00015Xf15-20-9780323789875.jpg)
(A) View of the arteries at the base of the brain. (B) Circle of Willis. The arteries that compose the circle of Willis are the two anterior cerebral arteries, joined to each other by the anterior communicating artery and two short segments of the internal carotids, off of which the posterior communicating arteries connect to the posterior cerebral arteries. (A, From Moses KP. Atlas of clinical gross anatomy, 2nd edition. Philadelphia: Saunders; 2013. B, From Hagen-Ansert S. Textbook of diagnostic sonography, 7th edition. St. Louis: Mosby; 2012.)
Illustration A shows the inferior view of the human brain and identifies the following structures from the top to the bottom: olfactory bulb, anterior cerebral artery, optic nerve [3] (cut), middle cerebral artery, posterior cerebral artery, internal carotid artery, vertebral artery, brainstem, and cerebellum. Illustration B shows and labels the following arteries at the base of the brain, from the top to the bottom: circle of Willis, anterior communicating artery, anterior cerebral artery, middle cerebral artery, posterior communicating artery, posterior cerebral artery, basilar artery, and vertebral artery.
Three major paired arteries perfuse the cerebellum and brainstem: the posterior inferior cerebellar artery, the anterior inferior cerebellar artery, and the superior cerebellar arteries. They originate from the basilar artery. The basilar artery also gives rise to small pontine arteries. The large arteries on the surface of the brain and their branches are called superficial arteries (conducting arteries). Small branches that project into the brain are termed projecting arteries (nutrient arteries).
The circle of Willis (see Fig. 15.20B) provides an alternative route for blood flow when one of the contributing arteries is obstructed (collateral blood flow). The circle of Willis is formed by the posterior cerebral arteries, posterior communicating arteries, internal carotid arteries, anterior cerebral arteries, and anterior communicating arteries. The anterior cerebral, middle cerebral, and posterior cerebral arteries leave the circle of Willis and extend to various brain structures, serving their associated brain territories. The border zone is the area between the major arterial territories. (Table 15.5 and Fig. 15.21 describe the structures served, the functional relationships, and the pathologic considerations related to occlusion of cerebral arteries.)
Table 15.5
| Arterial Origin | Structures Served | Conditions Caused by Occlusion |
|---|---|---|
| Anterior cerebral artery | Basal ganglia; corpus callosum; medial surface of cerebral hemispheres; superior surface of frontal and parietal lobes | Hemiplegia on contralateral side of body, greater in lower than in upper extremities |
| Middle cerebral artery | Frontal lobe; parietal lobe; temporal lobe (primarily cortical surfaces) | Aphasia in dominant hemisphere and contralateral hemiplegia (see Chapter 17) |
| Posterior cerebral artery | Part of diencephalon (thalamus, hypothalamus) and temporal lobe; occipital lobe | Visual loss; sensory loss; contralateral hemiplegia if cerebral peduncle affected |
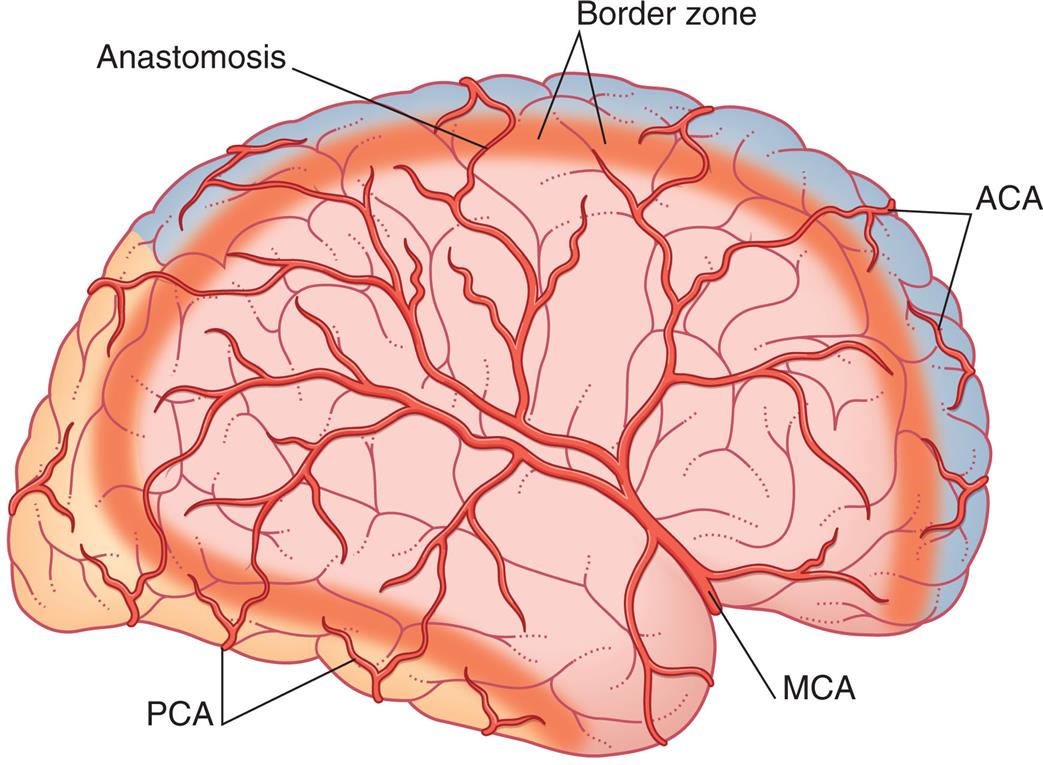
ACA, Gray area affected by occlusion of branches of anterior cerebral artery; MCA, pink area affected by occlusion of branches of middle cerebral artery; PCA, orange area affected by occlusion of branches of posterior cerebral artery. Occlusions can occur in the cortical or deep areas of the border zone. (From Mtui E, Gruener G, Fitzgerald MJT. Clinical neuroanatomy and neuroscience, 6th edition. Philadelphia: Saunders; 2012.)
Cerebral venous drainage does not parallel its arterial supply, whereas the venous drainage of the brainstem and cerebellum does parallel the arterial supply of these structures. The cerebral veins are classified as superficial and deep veins. The veins drain into venous plexuses and dural sinuses (formed between the dural layers) and eventually join the internal jugular veins at the skull base (Fig. 15.22). Adequacy of venous outflow can significantly affect intracranial pressure. For example, when individuals with a head injury turn or let their heads fall to the side, the action partially occludes venous return, and intracranial pressure can increase because of decreased flow through the jugular veins.
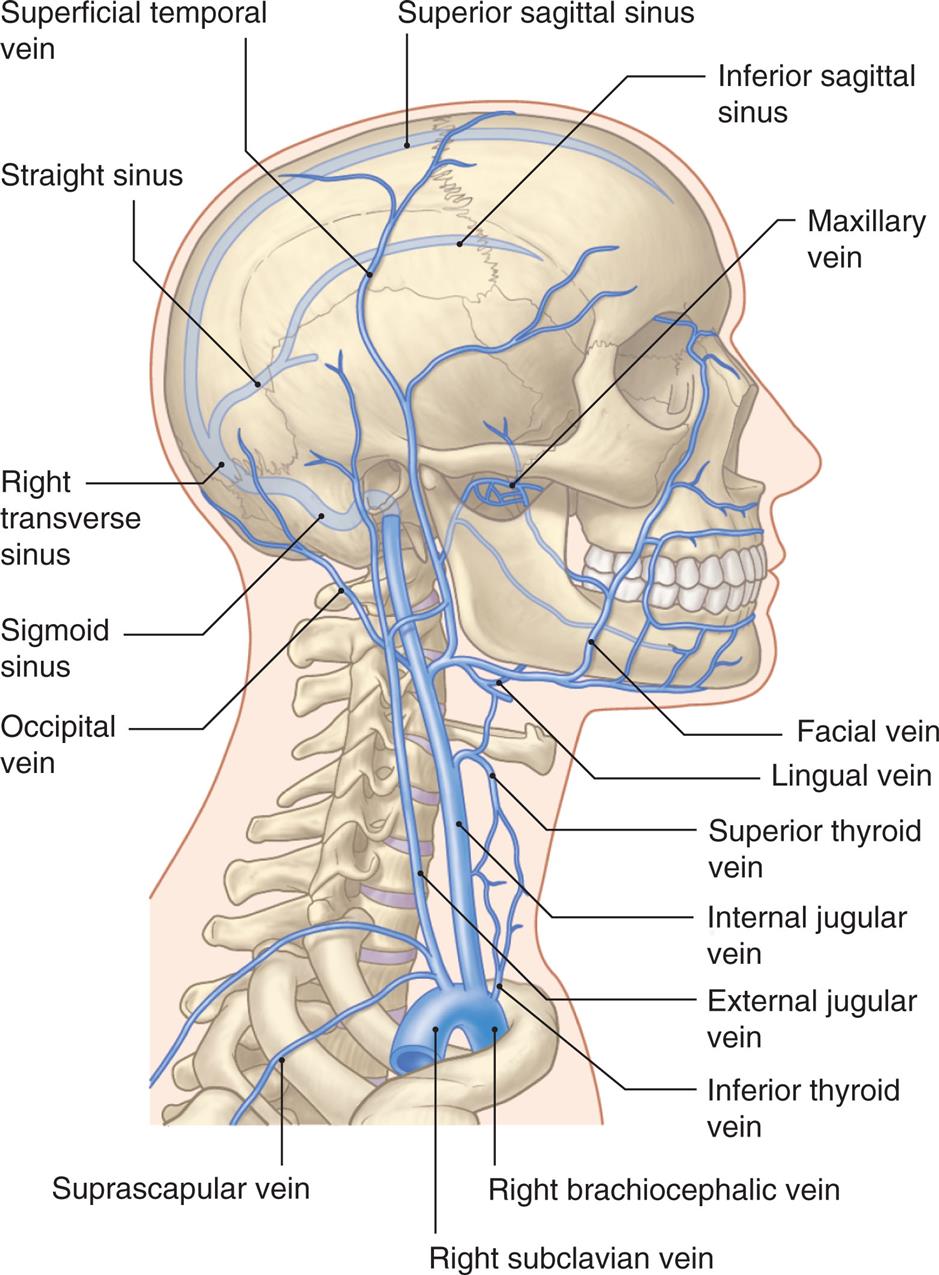
Deep veins and dural sinuses are projected on the skull. Note two superficial veins in the face are tributaries that send blood through emissary veins in the skull foramen into deep veins inside the skull, terminating in the internal jugular vein. (From Moses KP. Atlas of clinical gross anatomy, 2nd edition. Philadelphia: Saunders; 2013.)
An illustration shows the right lateral view of the human skull and neck, highlighting the major veins within the structure. The following veins are identified from the top to the bottom along the anterior side: maxillary vein, facial vein, lingual vein, superior thyroid vein, internal jugular vein, external jugular vein, inferior thyroid vein, right brachiocephalic vein, and right subclavian vein. The following veins are identified from the top to the bottom along the posterior side: superior sagittal sinus, superficial temporal vein, straight sinus, right transverse sinus, sigmoid sinus, occipital vein, and suprascapular vein.
Blood-Brain Barrier
The blood-brain barrier (BBB) describes cellular structures that selectively inhibit certain potentially harmful substances in the blood from entering the interstitial spaces of the brain or CSF, thus allowing neurons to function normally. Endothelial cells in brain capillaries with their intracellular tight junctions are the site of the BBB (Fig. 15.23). Supporting cells include astrocytes, pericytes, and microglia.3 Some substances, including glucose, lipid-soluble molecules, electrolytes, and chemicals, can cross into and out of the brain facilitated by transport molecules. This has substantial implications for drug therapy because certain antibiotics and chemotherapeutic drugs show a greater propensity than others to cross this barrier. Breakdown of the BBB can contribute to brain invasion by toxic molecules or pathogens promoting neuroinflammation and neurodegeneration. The epithelium of the choroid plexus and the arachnoid membrane also provide barrier functions.4
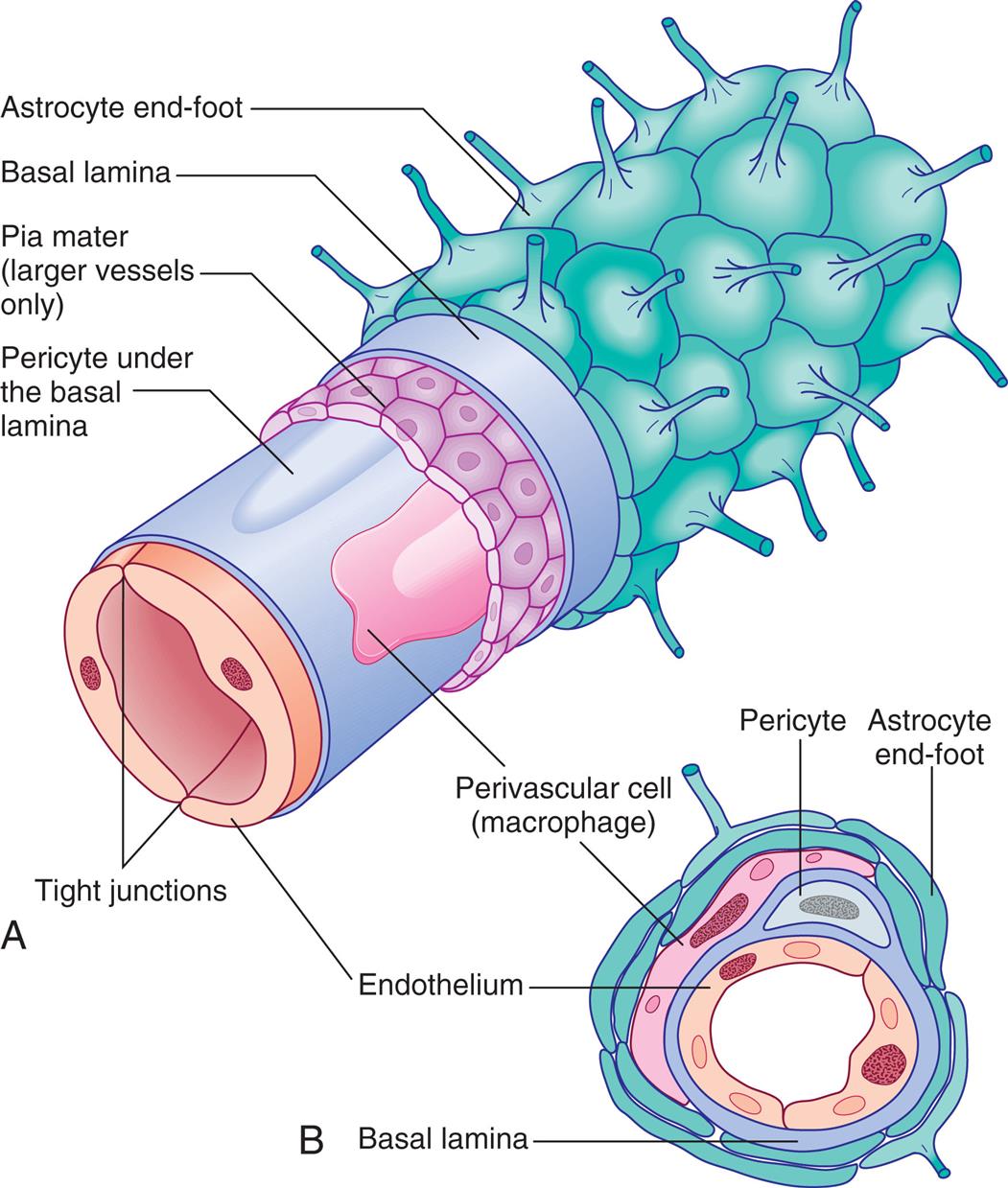
(A) Cellular structure of brain capillary. Endothelial cell membranes with tight junctions create a physical barrier between capillary blood and the brain, restricting movement of bacteria or neurotoxic substances. The pia mater is present only in larger vessels. (B) Cross-section. (From Standring S. Gray’s anatomy, 41st edition. London: Elsevier; 2016.)
Illustration A is a lateral view of a brain capillary. The following structures are labeled, from the inside: tight junctions, endothelium, pericyte under the basal lamina, perivascular cell (macrophage), pia mater (larger vessels only), basal lamina, and astrocyte end-foot. Illustration B is a cross-sectional view of a brain capillary. The following structures are labeled, from the inside: endothelium, basal lamina, pericyte, perivascular cell (macrophage), and astrocyte end-foot.
Blood Supply to the Spinal Cord
The spinal cord derives its blood supply from branches of the vertebral arteries and from branches from various regions of the aorta. The anterior spinal artery and the paired posterior spinal arteries branch from the vertebral artery at the base of the cranium and descend alongside the spinal cord. Arterial branches from vessels exterior to the spinal cord follow the spinal nerve through the intervertebral foramina, pass through the dura, and divide into the anterior and posterior radicular arteries (Fig. 15.24).
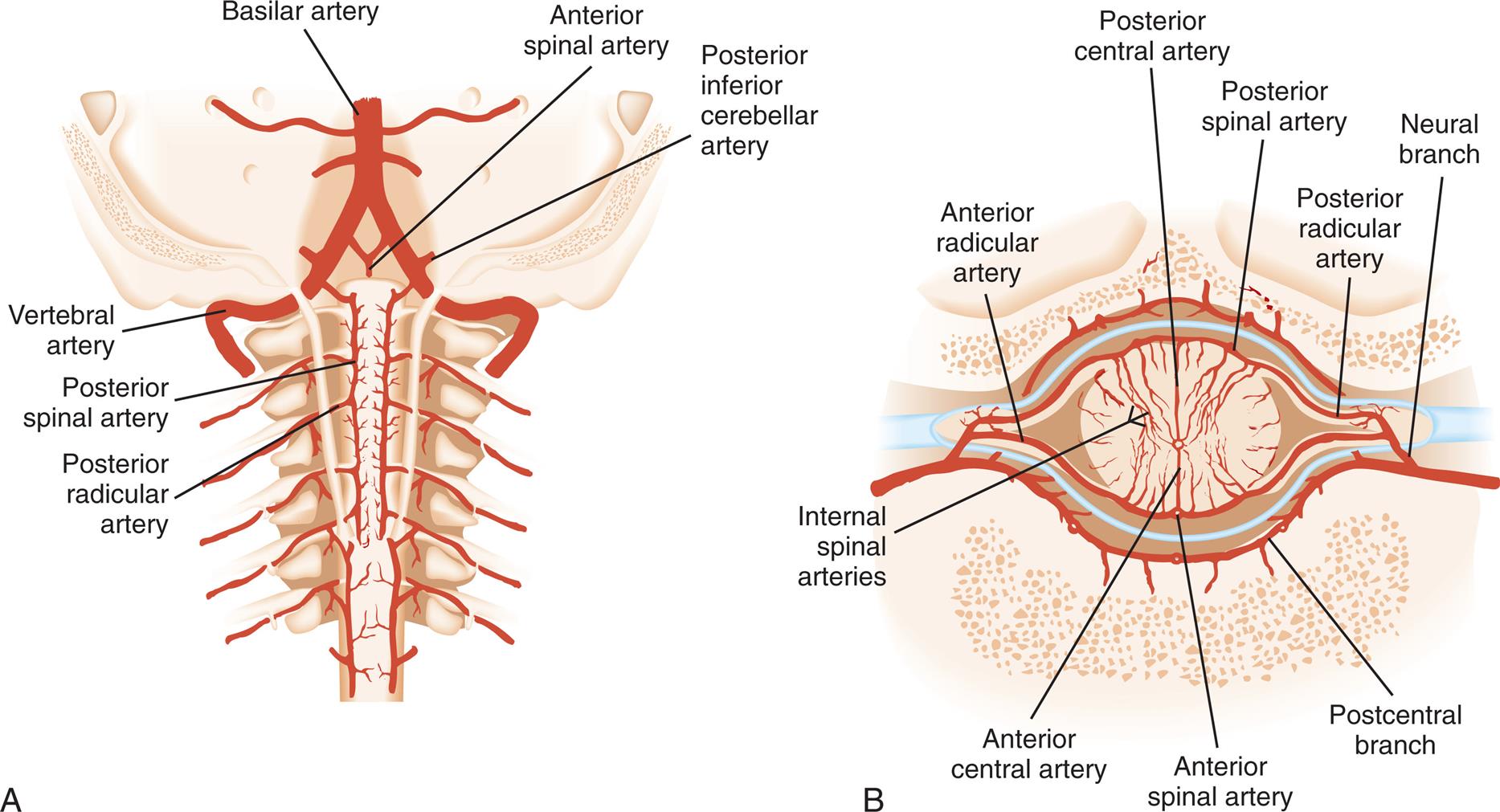
(A) Arteries of cervical cord exposed from the rear. (B) Arteries of spinal cord diagrammatically shown in horizontal section. (Redrawn from Rudy EB, eds. Advanced neurological and neurosurgical nursing. St. Louis: Mosby; 1984.)
Illustration A is a posterior view of the cervical cord, identifying the following arteries from the top to the bottom: basilar artery, anterior spinal artery, posterior inferior cerebellar artery, vertebral artery, posterior spinal artery, and posterior radicular artery. Illustration B is a cross-section view of the cervical cord, identifying the following arteries from the inside: anterior central artery, internal spinal arteries, posterior central artery, anterior spinal artery, posterior spinal artery, anterior radicular artery, posterior radicular artery, postcentral branch, and neural branch.
The radicular arteries eventually connect to the spinal arteries. Branches from the radicular and spinal arteries form plexuses whose branches penetrate the spinal cord, supplying the deeper tissues. Venous drainage parallels the arterial supply closely and drains into venous sinuses located between the dura and periosteum of the vertebrae.
Peripheral Nervous System
The peripheral nervous system (PNS) includes the nerves outside the CNS (see Fig. 15.1). The somatic nervous system is the part of the PNS that controls voluntary muscle movement (efferent nerves) and sensory information (afferent nerves). The cranial and spinal nerves, including their branches and ganglia, constitute the PNS. The spinal nerves originate in the spinal cord. The cranial nerves originate in the brain and pass out of the skull. A peripheral nerve is composed of individual axons/dendrites, with most wrapped in a myelin sheath. These individual fibers are arranged in bundles called fascicles (Fig. 15.25B). The coverings provide structural support, a blood supply, and interstitial compartments necessary to deliver essential electrolytes to support nerve impulse conduction.
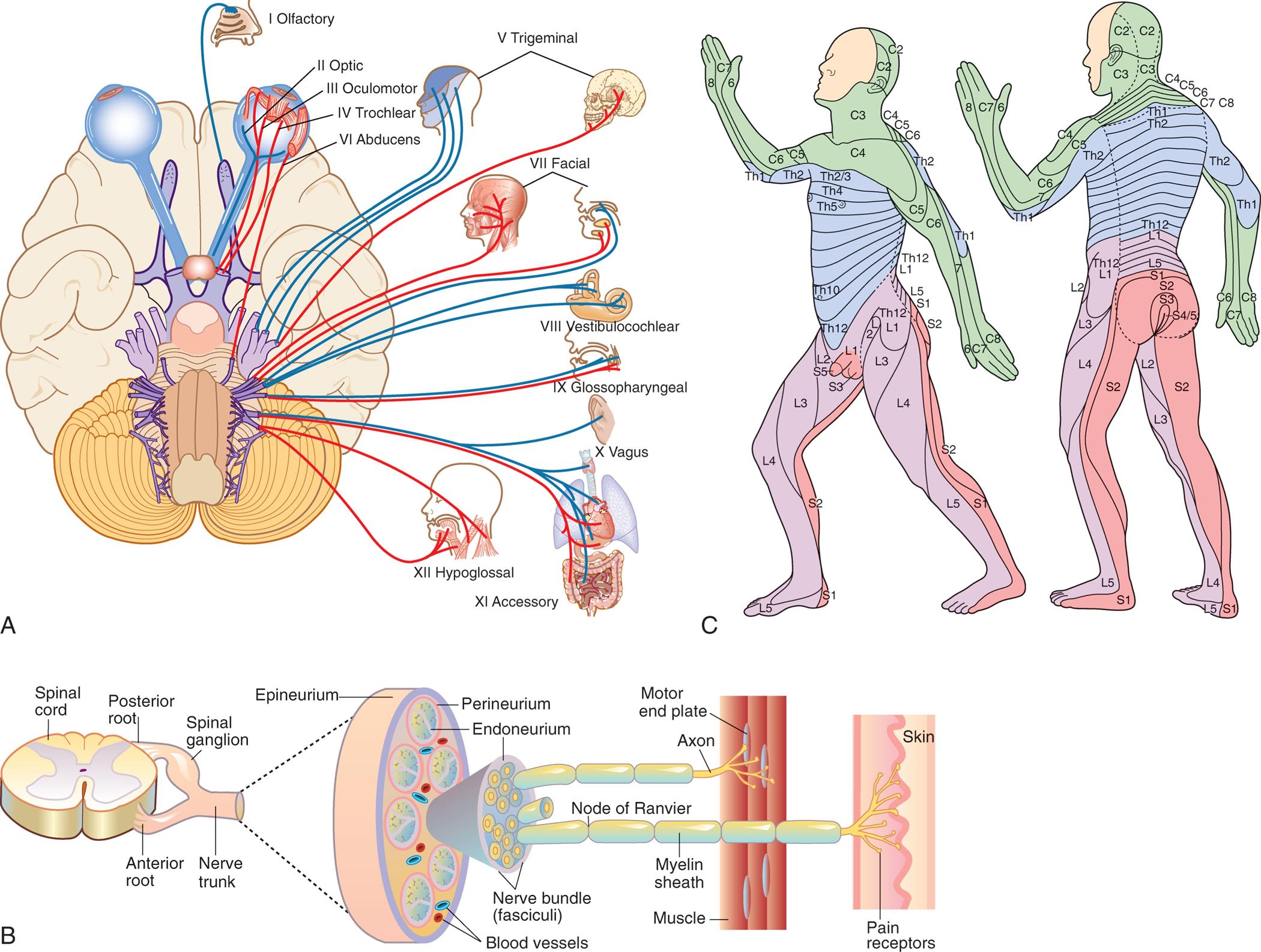
(A) Ventral surface of the brain showing attachment of the cranial nerves. The red lines indicate motor function, and the blue lines indicate sensory function. (B) Peripheral nerve trunk and coverings. (C) Dermatome map, anterolateral view (left), and posterolateral view (right). (A, From Applegate E. The anatomy and physiology learning system, 4th edition. St. Louis: Saunders; 2011. C, From Salvo SG. Mosby’s pathology for massage therapists, 3rd edition. St. Louis: Mosby; 2014.)
Illustration A shows the ventral surface of the brain, identifying the motor and sensory functions linked to the cranial nerves. The motor functions are: 3 oculomotor, 4 trochlear, 6 abducens, 7 facial, 9 glossopharyngeal, and 12 hypoglossal. The sensory functions are: 1 olfactory, 2 optic, 5 trigeminal, 8 vestibulocochlear, 10 vagus, and 11 accessory. Here, facial 7, trigeminal 5, glossopharyngeal 9, and vagus 10 nerves are both sensory and motor. Illustration B shows a cross-sectional view of the spinal cord with posterior root, anterior root, spinal ganglion, and nerve trunk. An accompanying illustration shows the lateral view of the nerve trunk, identifying the following structures from the outside: epineurium, perineurium, endoneurium, blood vessels, nerve bundle (fasciculi), node of Ranvier, myelin sheath, axon, muscle, motor end plate, pain receptors, skin. Illustration C shows the lateral and posterior view of a human figure and color-codes the different parts of the body by vertebrae connections. • C 1 to C 8: back of the head, neck, shoulders, front of the arms, and hands. • T h 1 to T h 12: back of the arms, chest, and upper back. • L 1 to L 5: front of the legs. • S 1 to S 5: back of the legs.
The 31 pairs of spinal nerves derive their names from the vertebral level from which they exit: 8 cervical, 12 thoracic, 5 lumbar, 5 sacral, and 1 coccygeal. The first cervical nerve exits above the first cervical vertebra, and the rest of the spinal nerves exit below their corresponding vertebrae. From the thoracic region (and inferiorly), nerves correspond to the vertebral level above their exit (see Fig. 15.11).
Spinal nerves contain both sensory and motor neurons and are called mixed nerves. Their structure can be compared to that of a tree with roots, trunk, and branches (see Fig. 15.11B). They arise as rootlets that combine into roots lateral to anterior and posterior horns of the spinal cord. These two spinal nerve roots converge in the region of the intervertebral foramen to form the spinal nerve trunk. Shortly after converging, the spinal nerve divides into anterior and posterior rami (branches). The anterior rami (except the thoracic) initially form plexuses (networks of nerve fibers), branching into the peripheral nerves. Instead of forming plexuses, the thoracic nerves pass through the thorax's intercostal spaces and innervate regions.
The main spinal nerve plexuses innervate the skin and the underlying muscles of the limbs. The brachial plexus, for example, is formed by the last four cervical nerves (C5 to C8) and the first thoracic nerve (T1) (see Fig. 15.11B). The brachial plexus innervates the nerves of the arm, wrist, and hand.
The posterior rami of each spinal nerve, with their many processes, are distributed to a specific area in the body. Sensory signals thus arise from specific sites associated with a specific spinal cord segment. Specific areas of cutaneous (skin) innervation at these spinal cord segments are called dermatomes (Fig. 15.25C). The dermatomes of various spinal nerves are distributed in a fairly regular pattern, although adjacent regions between dermatomes can be innervated by more than one spinal nerve.
Like spinal nerves, cranial nerves are categorized as peripheral nerves. Most of these are mixed nerves (like the spinal nerves), although some are purely sensory or purely motor. Cranial nerves connect to nuclei in the brain and brainstem (see Fig. 15.25A). Table 15.6 describes the cranial nerves and how to test them.
Table 15.6
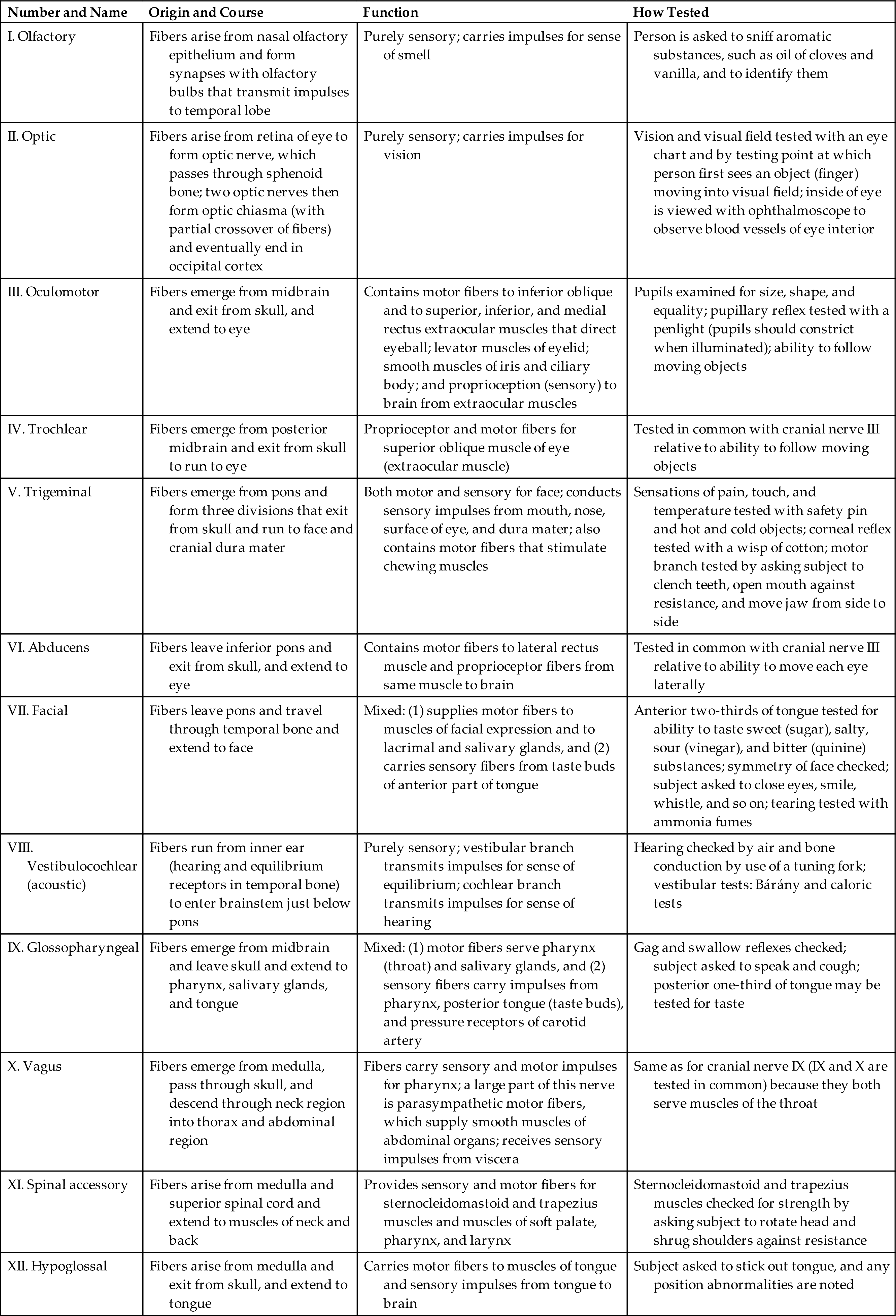
Autonomic Nervous System
The autonomic nervous system (ANS) coordinates and maintains a steady state among body organs and regulates cardiac muscle, smooth muscle, and the body's glands.5 This system is considered an involuntary system because one generally cannot will these functions to happen. Components of the ANS are located in both the CNS and the PNS, but the ANS is considered to be part of the efferent division of the PNS. The ANS is separated both structurally and functionally into two divisions: (1) the sympathetic nervous system and (2) the parasympathetic nervous system (Fig. 15.26). The effects of these two divisions are usually antagonistic, which means one stimulates an effector and one inhibits an effector. In this way, an effector, such as the heart or the blood vessels, can be precisely controlled. Many neurons of the ANS travel in the spinal nerves and certain cranial nerves. The widespread activity of this system indicates that its components are distributed all over the body. The peripheral autonomic nerves carry mainly efferent fibers, and that will be the emphasis here. The visceral or enteric autonomic nervous system controls the gastrointestinal tract's sensory and motor function and operates independently of the brain and spinal cord.
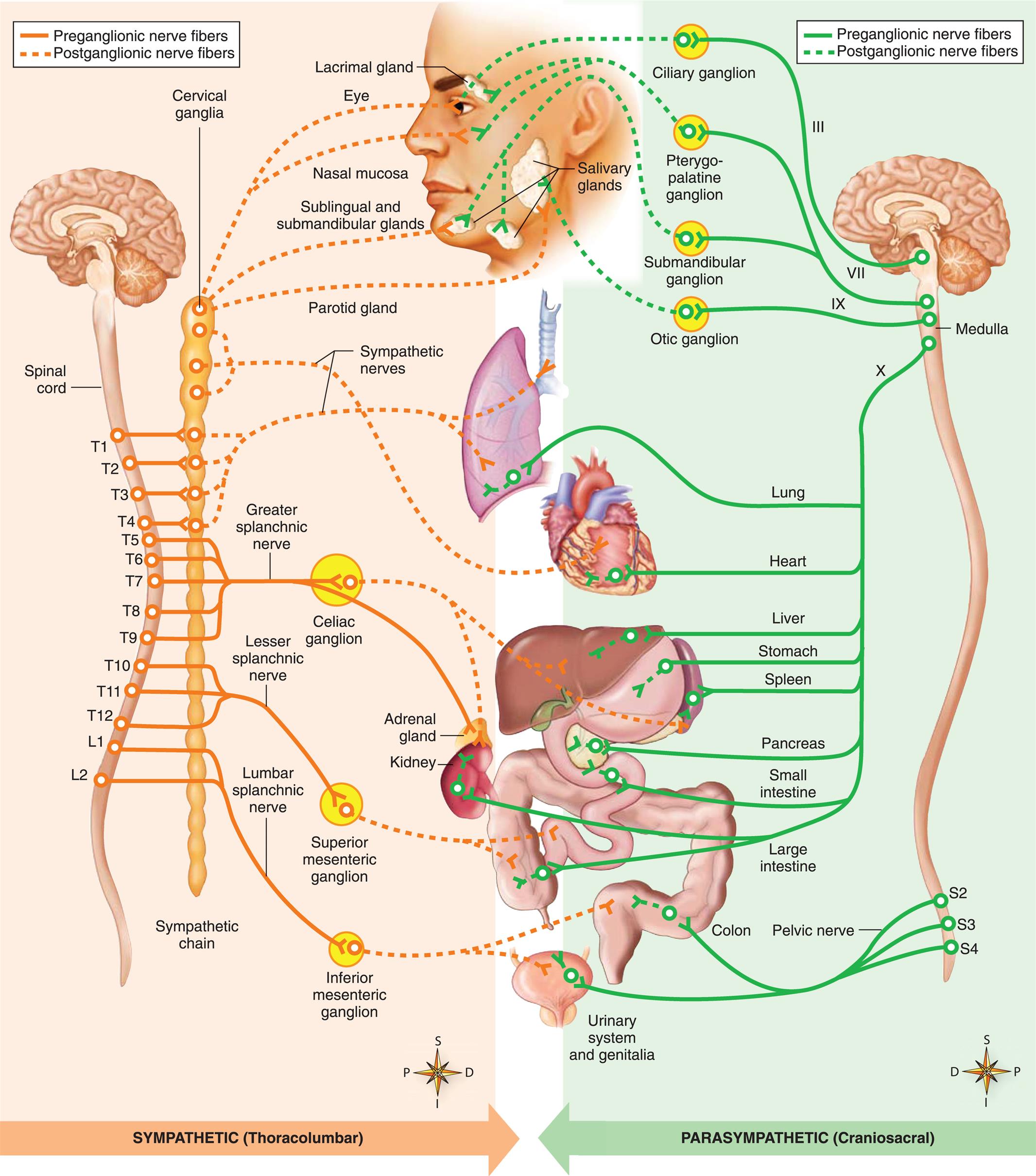
Preganglionic neuron cell bodies are located in the brainstem and sacral cord segments (parasympathetic or “craniosacral” division) and thoracic and upper lumbar cord segments (sympathetic or “thoracolumbar” division). The axons of these neurons synapse with postganglionic neurons, which innervate smooth muscle, cardiac muscle, and glands of the body. The postganglionic neuron cell bodies may be located in distinct autonomic ganglia (represented by circles) or very near the wall of the innervated visceral organ. Note that sympathetic fibers provide the only innervation to peripheral effectors (sweat glands, arrector pili muscles, adipose tissue, and blood vessels). (From Patton KA. Anatomy & physiology, 10th edition. St. Louis: Elsevier; 2019.)
An illustration maps the sympathetic and parasympathetic divisions of the autonomic nervous system. The sympathetic (thoracolumbar) divisions show the following preganglionic nerve fibers. • T 1 to T 4 from the spinal cord to the sympathetic nerves. • T 5 to T 9 from the spinal cord to the greater splanchnic nerve. • T 10 to T 12 from the spinal cord to the lesser splanchnic nerve. • L 1 and L 2 from the spinal cord to the lumbar splanchnic nerve. The sympathetic (thoracolumbar) divisions show the following postganglionic nerve fibers. • Cervical ganglia to the eye and nasal mucosa. • Cervical ganglia to the sublingual and submandibular glands. • Cervical ganglia to the parotid gland. • Cervical ganglia to the sympathetic nerves. • Greater splanchnic nerve to the liver, spleen, and adrenal gland. • Lumbar splanchnic nerve to the colon and urinary system and genitalia. The parasympathetic (craniosacral) divisions show the following preganglionic nerve fibers. • 3 to ciliary ganglion. • 6 to pterygo-palatine ganglion and submandibular ganglion. • 9 to otic ganglion. • 10 to lung, heart, liver, stomach, spleen, pancreas, small intestine, and large intestine. • S 2, S 3, and S 4 to pelvic nerve, colon, and urinary system and genitalia. The parasympathetic (craniosacral) divisions show the following postganglionic nerve fibers. • Ciliary ganglion to lacrimal gland. • Pterygo-palatine ganglion to eye and nasal mucosa. • Submandibular ganglion to sublingual and submandibular glands, including the salivary glands. • Otic ganglion to salivary glands.
The CNS has cardiovascular and respiratory centers in the reticular formation and the sympathetic and parasympathetic areas in the hypothalamus. CNS pathways interconnect all these areas and project to autonomic areas in the brain stem and spinal cord. Both divisions of the ANS are composed of autonomic nerves, ganglia, and plexuses. These structures, in turn, are made up of efferent (motor) autonomic neurons that conduct impulses away from the brainstem or spinal cord and down to the autonomic effectors. The efferent component of the ANS is a two-neuron system. Preganglionic neurons (myelinated) conduct impulses from the brainstem or spinal cord to an autonomic ganglion, where they synapse with a postganglionic neuron. Postganglionic neurons (unmyelinated) conduct impulses away from the ganglion to the effector (Fig. 15.27). This arrangement contrasts with the efferent somatic nervous system, where a single motor neuron travels from the CNS to the innervated structure without passing through the ganglia (see Fig. 15.27D).
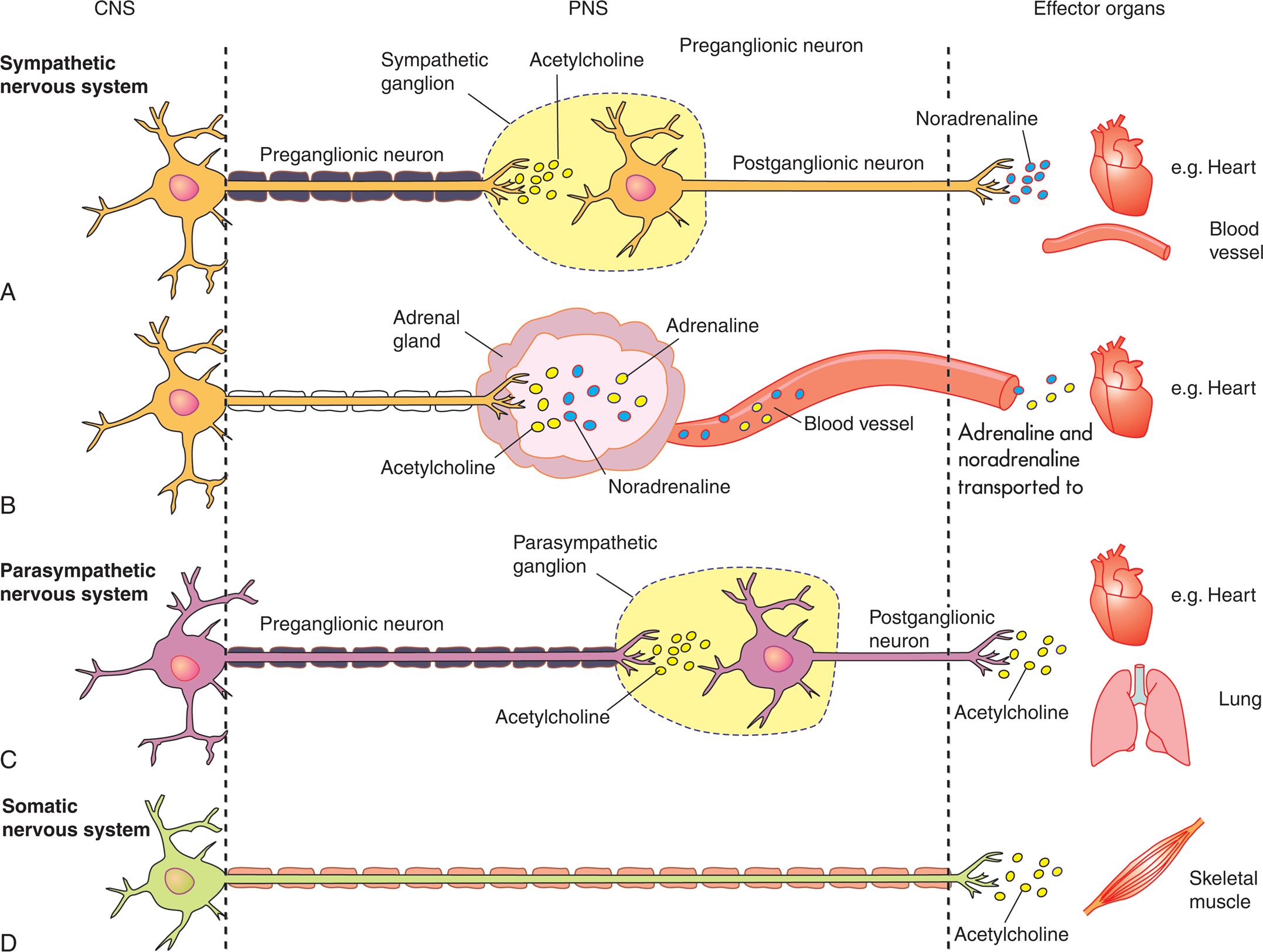
Although not part of the autonomic nervous system, the somatic nervous system is included because of its similarity for comparison. In each case, the cell body of the first neuron is located in the central nervous system. (From Craft J, Gordon C, Huether SE, et al. Understanding pathophysiology, Australian adaptation, 2nd edition. Marrickville, Australia: Elsevier Australia; 2015.)
Four illustrations, A, B, C, and D, compare neurotransmitters and receptors between C N S, P N A, and the effector organs. Illustration A, sympathetic nervous system. The illustration shows a preganglionic neuron releasing acetylcholine in the sympathetic ganglion. The postganglionic neuron releases noradrenaline to heart or blood vessels. Illustration B, sympathetic nervous system. The illustration shows a neuron releasing acetylcholine into the adrenal gland. Adrenaline and noradrenaline pass through blood vessel to the heart. Illustration C, parasympathetic nervous system. The illustration shows a preganglionic neuron releasing acetylcholine into the parasympathetic ganglion. The postganglionic neuron releases acetylcholine to the heart or the lungs. Illustration D, somatic nervous system. The illustration shows a neuron releasing acetylcholine to the skeletal muscle.
Anatomy of the Sympathetic Nervous System
The sympathetic nervous system mobilizes energy stores in times of need (e.g., in the “fight or flight” or stress response) (see Chapter 11 and Fig. 11.2). The sympathetic division is innervated by cell bodies located from the first thoracic (T1) through the second lumbar (L2) regions of the spinal cord and therefore is called the thoracolumbar division (see Fig. 15.26). The preganglionic axons of the sympathetic division form synapses shortly after leaving the spinal cord in the sympathetic (paravertebral) ganglia (see Fig. 15.27A). These preganglionic axons travel in several different ways: (1) directly synapsing with postganglionic neurons in the sympathetic chain ganglion at their level; (2) by traveling up the sympathetic chain ganglia; (3) by traveling down the sympathetic chain ganglion before forming synapses with a higher or lower postganglionic neuron; or (4) by traveling through the sympathetic chain ganglion to synapse with collateral ganglia (i.e., the splanchnic nerves, which lead to collateral ganglia on the front of the aorta). The collateral ganglia are named according to the branches of the aorta nearest them, namely the celiac, superior mesenteric, and inferior mesenteric ganglia (see Fig. 15.26). Postganglionic neurons leave the collateral ganglia and innervate the viscera below the diaphragm.
Preganglionic sympathetic neurons that innervate the adrenal medulla also travel in the splanchnic nerves and do not synapse before reaching the gland (see Figs. 15.26 and 15.27B). The secretory cells in the adrenal medulla are considered modified postganglionic neurons. Because preganglionic sympathetic fibers are all myelinated, travel to the adrenal medulla is quick, and innervation causes the rapid release of epinephrine and norepinephrine, which are mediators of the fight-or-flight response (see Chapter 11).
Anatomy of the Parasympathetic Nervous System
The parasympathetic nervous system conserves and restores energy when a person is at rest. The nerve cell bodies of this division are located in the cranial nerve nuclei and the sacral region of the spinal cord and therefore constitute the craniosacral division. Unlike the sympathetic branch, the preganglionic fibers in the parasympathetic division are longer and travel close to the organs they innervate before forming synapses with the relatively short postganglionic neurons (see Fig. 15.26). Parasympathetic nerves arising from nuclei in the brainstem travel to the viscera of the head, thorax, and abdomen within cranial nerves—including the oculomotor (III), facial (VII), glossopharyngeal (IX), and vagus (X) nerves.
Preganglionic parasympathetic nerves that originate from the sacral region of the spinal cord run either separately or together with some spinal nerves. The preganglionic axons unite to form the pelvic splanchnic nerve, which innervates the viscera of the pelvic cavity. These preganglionic axons synapse with postganglionic neurons in terminal ganglia located close to the organs they innervate.
Similar to the spinal nerves, the autonomic nerves also form plexuses. These are networks of nerves that have both parasympathetic and sympathetic neurons. They are usually located near the organs they innervate. Examples include the celiac (solar) plexus (contains the celiac ganglia and innervates many abdominal and pelvic organs), the cardiac plexus (innervates the heart), and Meissner and Auerbach plexuses (innervate the gastrointestinal tract).
Neurotransmitters and Neuroreceptors
Sympathetic preganglionic fibers and parasympathetic preganglionic and postganglionic fibers release acetylcholine—the same neurotransmitter released by somatic efferent neurons (see Fig. 15.27). These fibers are characterized by cholinergic transmission. Most postganglionic sympathetic fibers release norepinephrine (noradrenaline) and thus are considered to function by adrenergic transmission. A few postganglionic sympathetic fibers, such as those that innervate the sweat glands, release acetylcholine.
The action of catecholamines (adrenalin, noradrenaline, dopamine) varies with the type of neuroreceptor stimulated. Catecholamines are also released by the adrenal medulla gland, which physiologically and biochemically resembles the sympathetic nervous system. Two types of adrenergic receptors exist, α and β. Cells of the effector organs may have one or both types of adrenergic receptors. α-Adrenergic receptors have been further subdivided according to the action produced. α1-Adrenergic activity is associated mostly with excitation or stimulation; α2-adrenergic activity is associated with relaxation or inhibition. Most of the α-adrenergic receptors on effector organs belong to the α1 class. β-Adrenergic receptors are classified as β1-adrenergic receptors (which facilitate increased heart rate and contractility and cause the release of renin from the kidney) and β2-adrenergic receptors (which facilitate all remaining effects attributed to β receptors).6 Norepinephrine stimulates all α1 and β1 receptors and only certain β2 receptors. The primary response from norepinephrine, however, is stimulation of α1-adrenergic receptors that cause vasoconstriction. Epinephrine strongly stimulates all four types of receptors and induces general vasodilation because of the predominance of β receptors in muscle vasculatures. (Table 15.7 summarizes the effects of neuroreceptors on their effector organs.)
Table 15.7
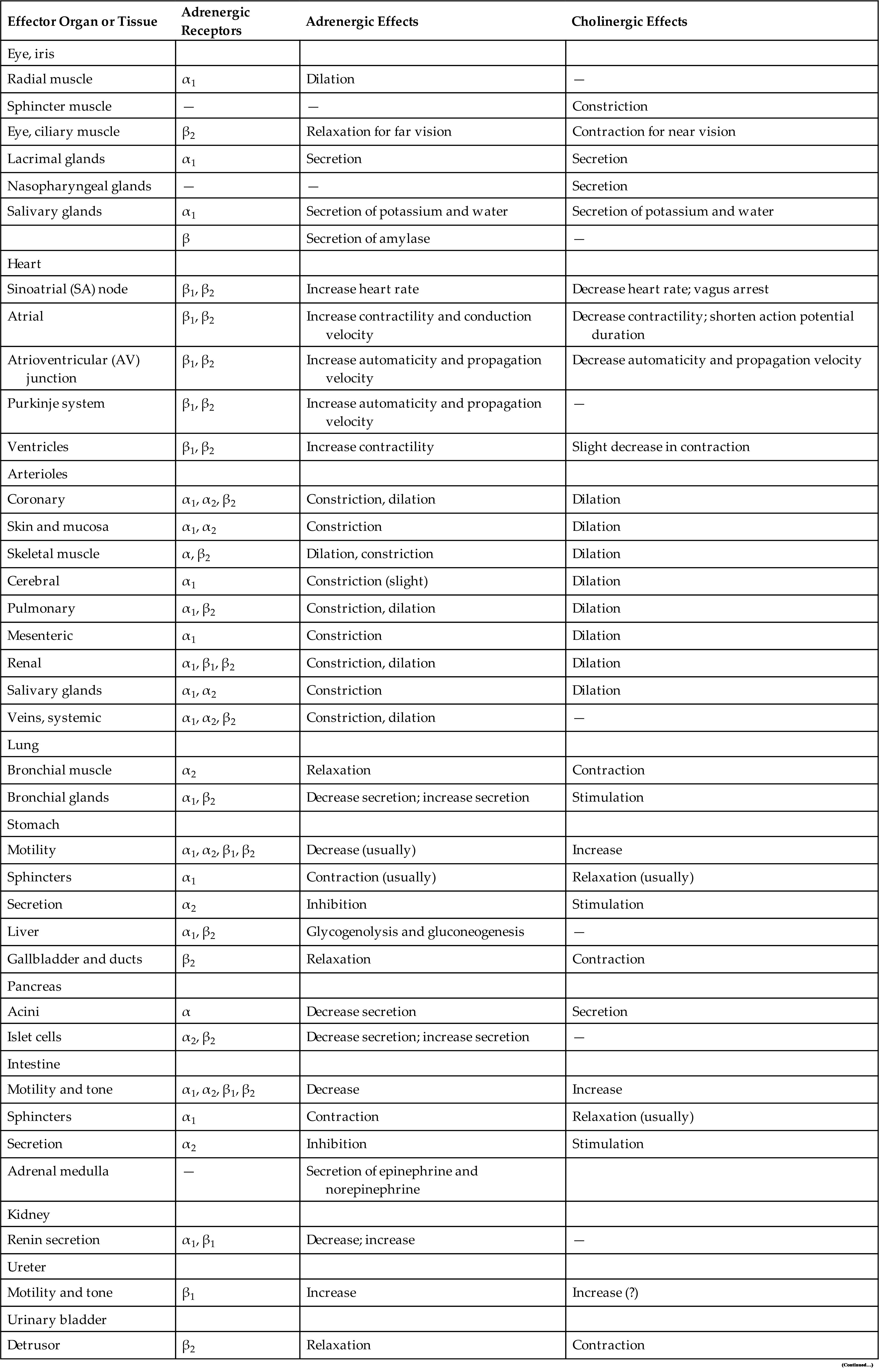
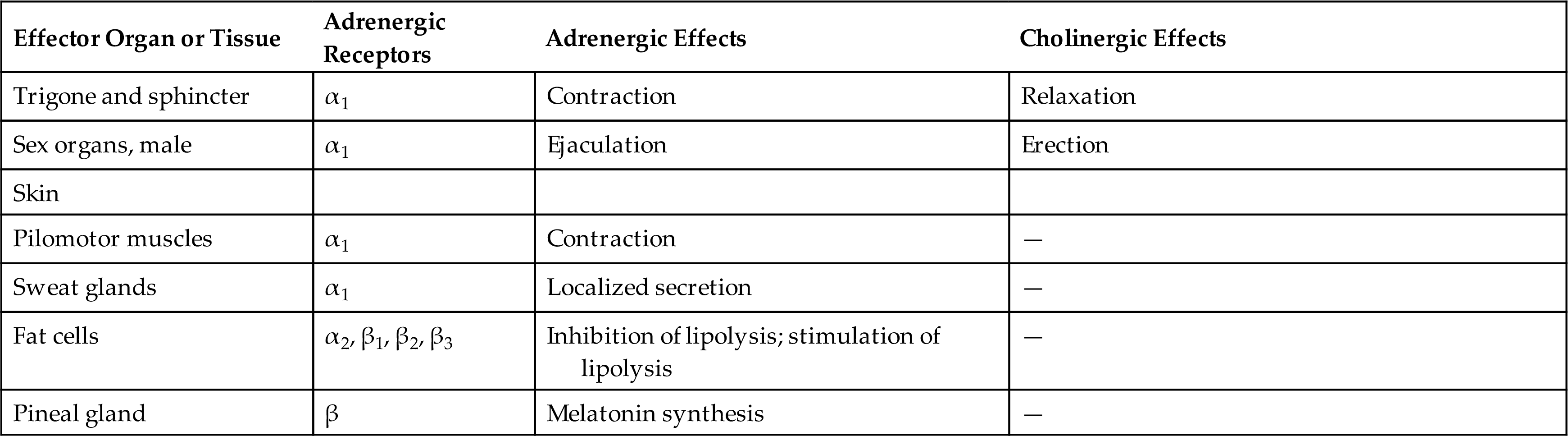
Modified from Brunton LL, Chabner B, Knollmann BC, eds. Goodman & Gilman’s pharmacological basis of therapeutics, 12th edition. New York: McGraw-Hill; 2010; Dowd FJ, Yagiela JA, Johnson B, et al. Pharmacology and therapeutics for dentistry, 6th edition. St. Louis: Mosby; 2011.
Functions of the Autonomic Nervous System
Many body organs are innervated by both the sympathetic and parasympathetic nervous systems. The two divisions often cause opposite responses; for example, sympathetic stimulation of the stomach causes decreased peristalsis, whereas parasympathetic stimulation of the intestine increases peristalsis. In general, sympathetic stimulation promotes responses for the protection of the individual. For example, sympathetic activity increases blood glucose levels and temperature and increases heart rate and blood pressure. In emergency situations, a generalized and widespread discharge of the sympathetic system occurs and is known as the “fight or flight” reflex or acute stress response (see Chapter 11). This is accomplished by an increased firing frequency of sympathetic fibers and by activation of sympathetic fibers normally silent and at rest (fibers to the sweat glands, pilomotor muscles, and the adrenal medulla, as well as vasodilator fibers to muscle). Regulation of vasomotor tone is considered the single most important function of the sympathetic nervous system. (Fig. 15.28 illustrates some of the most important functions of the sympathetic nervous system.)
(A) Regulation of vasomotor tone. (B) Regulation of strenuous muscular exercise (“fight-or-flight” or stress response). (See also Chapter 11 and Fig. 11.2 for more detail on the stress response.)
Flowchart A shows the functions of the sympathetic activation (vasomotor tone). • Adrenal medulla activation; release of epinephrine and norepinephrine; and increased strength of contraction of heart. • Contraction of arteriolar smooth muscles; vasoconstriction; increased peripheral resistance; and increased blood pressure. Flowchart B shows the functions of sympathetic activation (strenuous muscular exercise). • Peripheral vasoconstriction; and increased venous return leading to increased cardiac output or shifting of cardiac output to muscles. • Stimulation of beta-receptors of muscle vasculature; vasodilation; and increased blood flow to muscles. • Stimulation of beta-receptors of bronchiole vasculature; and increased bronchodilation; increased oxygenation. • Metabolic effects; and increased release of epinephrine. Increased release of epinephrine leads to the following three responses. • Glycogenolysis in the liver; and increased blood sugar. • Glycolysis in muscle; and increased lactic acid. • Breakdown of adipose tissue; and release of free fatty acids.
Increased parasympathetic activity promotes rest and tranquility and is characterized by reduced heart rate and enhanced visceral functions concerned with digestion. Stimulation of the vagus nerve (cranial nerve X) in the gastrointestinal tract increases peristalsis and secretion, as well as the relaxation of sphincters. Activation of parasympathetic fibers in the head, provided by cranial nerves III, VII, and IX, causes pupil constriction, tear secretion, and increased salivary secretion. Stimulation of the sacral division of the parasympathetic system contracts the urinary bladder and facilitates the process of penile erection.
The parasympathetic system lacks the generalized and widespread response of the sympathetic system. Specific parasympathetic fibers are activated to regulate particular functions. Although the actions of the parasympathetic and sympathetic systems are usually antagonistic, there are exceptions. Peripheral vascular resistance, for example, is increased dramatically by sympathetic activation but is not altered appreciably by activity of the parasympathetic system. Most blood vessels involved in the control of blood pressure are innervated by sympathetic nerves. To decrease blood pressure, therefore, it is more important to block or paralyze the continuous (tonic) discharge of the sympathetic system than to promote parasympathetic activity.
The CNS mechanisms involved in the aging process are extremely complex, and many questions have yet to be answered. Geriatric Considerations: Aging & the Nervous System summarizes the structural, cellular, cerebrovascular, and functional changes that occur in the nervous system with aging.
Tests of Nervous System Structure and Function
Skull and Spine Roentgenograms
Roentgenograms (x-ray films) of the skull or spine from multiple angles (views) are used primarily to localize bony defects, bone density, erosion, or calcified structures. The pineal gland in older people becomes calcified and is useful as an internal brain landmark. Probably the most commonly used radiologic studies are x-ray films.
Computed Tomography
Computed tomography (CT) creates two-dimensional reconstructions from multiple radiologic images (x-rays) using computer-assisted analysis. It can demonstrate fine distinctions in shape, size, and densities of a variety of tissues based on differential absorption of x-rays. CT imaging is a noninvasive procedure used in evaluating cranial and spinal structures and hemorrhages, tumors, and distortions in the brain caused by pressure differences. A variety of contrast media are also commonly used in conjunction with this procedure to enhance the delineation of selected structures. Spiral or helical CT uses a multidetector scanner mounted on a rotating gantry to provide several axial images of a large continuous anatomic area in a matter of seconds. Helical CT angiography uses contrast media for the detection of aneurysms or ruptured aneurysms.
Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) uses a static magnetic field, instead of x-rays, to orient physiologic atomic particles. Disruption of this orientation by excitation of the particles using serial radiofrequency pulsations provides the image data. The specific tissue reaction is computer-analyzed to give an image with much better spatial resolution than that provided by CT. The MRI also reconstructs images in three angles at right angles (i.e., axial, sagittal, coronal). MRI is reported to have none of the adverse effects associated with radiation examinations. Magnetic resonance spectroscopy (MRS) can be completed simultaneously as a standard MRI by analyzing the chemical composition of proton (hydrogen) or phosphorous-based molecules and is useful for differentiating the chemical composition and function of various regions of the brain. Functional MRI (fMRI) detects changes in blood oxygenation and flow and can produce maps showing the parts of the brain that are functioning during a mental process. Diffusion tensor imaging (DTI) is an MRI technique that quantifies the size and direction of white matter tracts by measuring asymmetries in the magnitude and direction of the diffusion of water molecules. Color coding and fiber tracking (tractography) add detail.
Magnetic Resonance Angiography
Magnetic resonance angiography (MRA) uses special imaging techniques to visualize blood vessels in great detail. MRA can be used in conjunction with cerebral angiography to detect and localize pathologic lesions of the circulatory system of the brain, particularly the arterial system. In some cases, contrast agents are used.
Positron-Emission Tomography Scan
The positron-emission tomography (PET) scan uses CT imaging to detect the emission of positive electrons from trace amounts of radioactive substances injected into the bloodstream or administered as inhaled gases. With radioactive decay, radioactive substances emit a positron. As they are distributed in tissues, they display characteristic patterns that indicate physiologic and metabolic processes, for example, glucose and oxygen uptake, cerebral blood flow, neural and neurotransmitter function, and the effects of drugs. As a research tool, PET is being used to visualize the specific brain sites involved in the processing of information in the brain.
Single Photon Emission Computed Tomography
Single photon emission computed tomography (SPECT) uses radiotracers with longer half-lives than those used in PET scans. CT creates images after radionuclide substances (technetium [99mTc]) have been introduced into the bloodstream. For example, visualization of tissue uptake of the radioactive agent indicates blood-brain barrier integrity (increased uptake of the agent indicates disruption). This scanning technique also can identify abnormalities in blood-flow dynamics and cellular metabolic function. The brain scan is particularly helpful in detecting abnormal vascularity resulting from neoplasms, abscesses, and vascular lesions.
Cerebral Angiography
Cerebral angiography is a radiologic technique that demonstrates cerebrovascular blood flow. The introduction of a small catheter commonly performs this technique into the femoral artery. The catheter is then passed to the level of the cerebral circulation and through the aorta, and a contrast dye is injected. Serial x-ray films are then taken. These films demonstrate the flow of the dye through the cerebral vasculature and provide information on the patency, location, size, and flow pattern of the vessels. Another technique used in cerebral angiography is the retrograde (reverse flow) injection of the dye through catheterization of a brachial, axillary, subclavian, or femoral vein.
Echoencephalography (Ultrasound)
Echoencephalography, or ultrasound, is a safe, noninvasive procedure using sound waves deflected at differing rates, depending on the density of the tissue. Information is processed and displayed on an oscilloscope screen. It is useful primarily in detecting structural characteristics of intracranial space-occupying mass lesions and the determination of ventricular dimensions, especially in newborns where open fontanelles provide good acoustic windows.
Electroencephalography
The electroencephalograph (EEG) is a recording of electrical impulses arising from the brain's cortical surface that are detected by scalp electrodes. The recording of brain wave patterns is analyzed for alterations or localization (or both) of specific electrical activity. This test is especially useful in detecting and localizing foci that initiate seizure activity. It is also an important technique in the assessment of encephalopathy (altered mental status). Complete absence of electrical activity is seen in individuals who are legally “brain dead.”
Magnetoencephalography
Magnetoencephalography (MEG) is a specialized imaging technique used to measure magnetic fields induced by electrical activity in the brain. Electrical activity in the brain, such as that detected by EEG, induces an orthogonally oriented magnetic field. MEG uses multiple sensors that allow vectoring and ultimately localization of the origin of the electrical activity that generated the field. The use of MEG has the advantage over EEG in that the induced magnetic field is not significantly dispersed by passing through the skull and soft tissues of the scalp. Co-registration of MEG and MRI data allow precise localization of the electrical focus for seizure onset.
Evoked Potentials
Evoked potentials (EPs) are a method of detecting electrical brain activity that results from a stimulus—primarily auditory, visual, or peripheral sensory. Electrical activity is computer-formatted to display changes in trends. The primary uses of EPs include perioperative detection of sensory pathway integrity and disease- or drug-related sensory dysfunction.
Cerebrospinal Fluid Analysis
CSF generally is obtained from the lumbar or cisternal subarachnoid space using a hollow needle that allows passive flow. The lumbar puncture is performed most often at the L3–L4 interspace (below the level of the spinal cord at L1–L2). Cisternal puncture is performed by inserting a needle into the cerebellomedullary cistern using an approach from the back of the neck in the region of the foramen magnum. CSF pressure is commonly measured during these procedures. The CSF can also be analyzed for gross characteristics and constituents (color, blood cells, electrolytes, and protein) and cultured for microorganisms.
Summary Review
Overview and Organization of the Nervous System
- 1. The nervous system divisions have been categorized as either structural (central nervous system [CNS] and peripheral nervous system [PNS]) or functional (somatic nervous system and autonomic nervous system [ANS]).
- 2. The CNS is contained within the brain and spinal cord.
- 3. The PNS is composed of cranial and spinal nerves, which carry impulses toward the CNS (afferent—sensory) and away from the CNS (efferent—motor) to and from target organs or skeletal muscle.
- 4. The somatic nervous system consists of pathways regulating voluntary motor control.
The ANS is involved in the involuntary control of organ systems. The ANS is divided into sympathetic and parasympathetic divisions.
Cells of the Nervous System
- 1. The neuron and neuroglial cells (nonnerve cells) make up nervous tissue. The neuron is specialized to transmit and receive electrical and chemical impulses, whereas the neuroglial cell provides supportive and maintenance functions.
- 2. The neuron is composed of a cell body, dendrite(s), and an axon. A myelin sheath around selected axons forms insulation that allows faster nerve impulse conduction, referred to as saltatory conduction.
- 3. The neuron is further divided into unipolar, pseudounipolar, bipolar, and multipolar categories, according to its structure and particular mechanics of impulse transmission. Functionally, the neuron can be sensory, associational, and motor.
- 4. Neuroglial cells (“nerve glue”) support the CNS and comprise approximately half of the total brain and spinal cord volume.
- 5. Nerve injury usually leads to the degeneration of the portion cut off from the cell body, called Wallerian degeneration. The part of the axon connected to the cell body can undergo slow regeneration depending on the type and location of the injury.
The Nerve Impulse
- 1. The region between adjacent neurons is the synapse, and the region between the neuron and muscle is the neuromuscular junction.
- 2. Neurotransmitters are responsible for chemical conduction across the synapse, and the nerve impulse is regulated predominantly by a balance of inhibitory postsynaptic potentials (IPSPs) and excitatory postsynaptic potentials (EPSPs), temporal and spatial summation, and convergence and divergence.
The Central Nervous System
- 1. The cerebrum is the largest part of the brain and contains gray matter and white matter. The cerebral cortex is the brain's outer layer and contains only gray matter arranged in folds, composed mostly of cell bodies.
- 2. The brain is contained within the cranial vault and is divided into three distinct regions: (1) forebrain, (2) hindbrain, and (3) midbrain.
- 3. The forebrain includes the telencephalon (the two cerebral hemispheres) and allows conscious perception of internal and external stimuli, thought and memory processes, and voluntary control of skeletal muscles. The deep portion of the forebrain is termed the diencephalon and processes incoming sensory data. The center for the voluntary control of skeletal muscle movements is located along the precentral gyrus in the frontal lobe, whereas the center for perception is along the postcentral gyrus in the parietal lobe. The Broca area (inferior frontal gyrus—motor function) and the Wernicke area (superior temporal gyrus—sensory function) are major speech centers.
- 4. The midbrain is primarily a relay center for motor and sensory tracts and a center for auditory and visual reflexes.
- 5. The hindbrain allows sampling and comparison of sensory data received from the periphery and motor impulses of the cerebral hemispheres for coordination and refinement of skeletal muscle movement.
- 6. The spinal cord contains most of the nerve fibers that connect the brain with the periphery. The corticospinal tracts are descending pyramidal (motor) pathways from the motor cortex. The rubrospinal and reticulospinal tracts are descending extrapyramidal tracts that coordinate movement. The anterior, posterior, and lateral spinothalamic tracts carry sensory information to the brainstem and thalamus, where information is relayed to the sensory cortex. Reflex arcs are sensory and motor circuits completed in the spinal cord and influenced by the higher centers in the brain.
- 7. The CNS is protected by the bony cranium, meninges (dura mater, arachnoid, membrane, and pia mater), cerebrospinal fluid (CSF), and vertebral column. CSF is formed from blood components in the choroid plexuses of the ventricles and is reabsorbed in the arachnoid villi (located in the dural venous sinuses) after circulating through the brain and subarachnoid space.
- 8. The paired carotid and vertebral arteries supply blood to the brain and connect to form the circle of Willis. The major branches projecting from the circle of Willis are the anterior, middle, and posterior cerebral arteries. Drainage of blood from the brain is accomplished through the venous sinuses and jugular veins.
- 9. The blood-brain barrier is provided by tight junctions between the cells of brain capillary endothelial cells and surrounding supporting cells.
- 10. Blood supply to the spinal cord originates from the vertebral arteries and branches arising from the aorta.
The Peripheral Nervous System
The Autonomic Nervous System
- 1. The ANS is responsible for maintaining a steady state in the internal environment. Two opposing systems make up the ANS: (1) the sympathetic nervous system (thoracolumbar division) responds to stress by mobilizing energy stores and prepares the body to defend itself, and (2) the parasympathetic nervous system (craniosacral division) conserves energy and the body's resources. Both systems function, more or less, at the same time. The enteric autonomic nervous system controls the function of the gastrointestinal tract.