Pain, Temperature Regulation, Sleep, and Sensory Function
Jodi A. Allen
![]() http://evolve.elsevier.com/Rogers/pathophysiology/
http://evolve.elsevier.com/Rogers/pathophysiology/
Alterations in sensory function may involve dysfunctions of the general or the special senses. Dysfunctions of the general senses include chronic pain, abnormal temperature regulation, and tactile or proprioceptive dysfunction. Pain is a unique sensory experience that, although universally described as unpleasant, is nonetheless essential to an individual’s survival. Pain provides protection by signaling the presence of disease or injury. Like pain, variations in temperature can signal disease. Fever is a common manifestation of dysfunction and is often the first symptom observed in an infectious or inflammatory condition.
Sleep is a normal cyclic process that restores the body’s energy and maintains normal function. Sleep is so essential to physiologic and psychologic function that sleep deprivation causes a wide range of clinical manifestations. Prolonged deprivation or disruption of sleep ultimately leads to serious dysfunction.
The special senses of vision, hearing, touch, smell, and taste are the means by which individuals perceive stimuli that are essential for interacting with the environment. Special sensory receptors are connected to specific areas of the brain through the afferent pathways of the peripheral and central nervous system (CNS). Each of the special senses thus involves a connected system of organs and tissues that receives stimuli and sends sensory messages to areas of the CNS, where they are processed and guide behavior. Dysfunctions of the special senses include visual, auditory, olfactory, and gustatory (taste).
Pain
Pain is one of the body’s most important adaptive and protective mechanisms. It is a complex experience comprised of dynamic interactions among physical, cognitive, spiritual, emotional, and environmental factors and cannot be characterized as only a response to injury. McCaffery defined pain as “whatever the experiencing person says it is, existing whenever he says it does.”1 The International Association for the Study of Pain and the American Pain Society defined pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage.”2 A more recent proposal for the definition of pain is “pain is a mutually recognizable somatic experience that reflects a person’s apprehension of threat to their bodily or existential integrity.”3 Acute pain is protective and promotes withdrawal from painful stimuli, allows the injured part to heal, and teaches avoidance of painful stimuli.
Neuroanatomy of Pain
Three parts of the nervous system are responsible for the sensation, perception, and response to pain:
- 1. The afferent pathways, which begin in the peripheral nervous system (PNS), travel to the spinal gate in the dorsal horn, and then ascend to areas in the diencephalon (thalamus, epithalamus, and hypothalamus) and cortex.
- 2. The interpretive centers located in the subcortical and cortical networks, brainstem, midbrain, diencephalon, and cerebral cortex.
- 3. The efferent pathways that descend from the central nervous system (CNS) back to the dorsal horn of the spinal cord.
The processing of potentially harmful (noxious) stimuli through a normally functioning nervous system is called nociception. Nociceptors, or pain receptors, are free nerve endings in the afferent peripheral nervous system. When they are stimulated, they cause nociceptive pain. The cell bodies of nociceptors are located in the dorsal root ganglia (DRG) for the body and in the trigeminal ganglion for the face. Nociceptors have a peripheral and central axonal branch that innervates their target organ and the spinal cord, respectively. Nociceptors are unevenly distributed throughout the body, so the relative sensitivity to pain differs according to their location (Table 16.1). Nociceptors respond to different types of noxious stimuli: mechanical (pressure or mechanical distortion), thermal (extreme temperatures), or chemical (acids or chemicals of inflammation, such as bradykinin, histamine, leukotrienes, or prostaglandins). Nociception involves four phases: transduction, transmission, perception, and modulation.4
Table 16.1
Pain transduction is the process of converting a painful stimulus into an electrical signal that is transmitted to the CNS. Transduction begins when nociceptors are activated by a painful stimulus (physical, chemical, or thermal), causing ion channels (sodium, potassium, calcium) on nociceptors to open, creating electrical impulses that travel through axons of two primary types of nociceptors that are transmitted to the spinal cord, brainstem, thalamus, and cortex (see Fig. 15.15).5 The two primary types of nociceptors are A-delta (Aδ) fibers and C fibers. Aδ fibers are larger myelinated fibers that rapidly transmit sharp, well-localized “fast” pain sensations, such as intense heat or a pinprick to the skin. Activation of these fibers causes a spinal reflex withdrawal of the affected body part from the stimulus, before a pain sensation is perceived. C fibers are the most numerous, are smaller, unmyelinated, and are located in muscle, tendons, body organs, and the skin. They slowly transmit dull, aching, or burning sensations that are poorly localized and often constant.
Pain transmission is the conduction of pain impulses along the Aδ and C fibers (primary-order neurons) into the dorsal horn of the spinal cord (Fig. 16.1). Here they form synapses with excitatory or inhibitory interneurons (second-order neurons) in the substantia gelatinosa of the dorsal horn. The impulses then synapse with projection neurons (third-order neurons), cross the midline of the spinal cord, and ascend to the brain through two lateral spinothalamic tracts (Fig. 16.2). The anterior spinal thalamic tract carries fast impulses for acute sharp pain. The lateral spinothalamic tract carries slow impulses for dull or chronic pain. The fast, sharp pain is perceived first, followed by dull, throbbing pain. These tracts connect to the reticular formation, hypothalamus, thalamus (the major relay station of sensory information), and limbic system. The impulses are then projected to the somatosensory cortex for interpretation of the location and intensity of the pain (Fig. 16.3), and to other areas of the brain for an integrated response to pain.
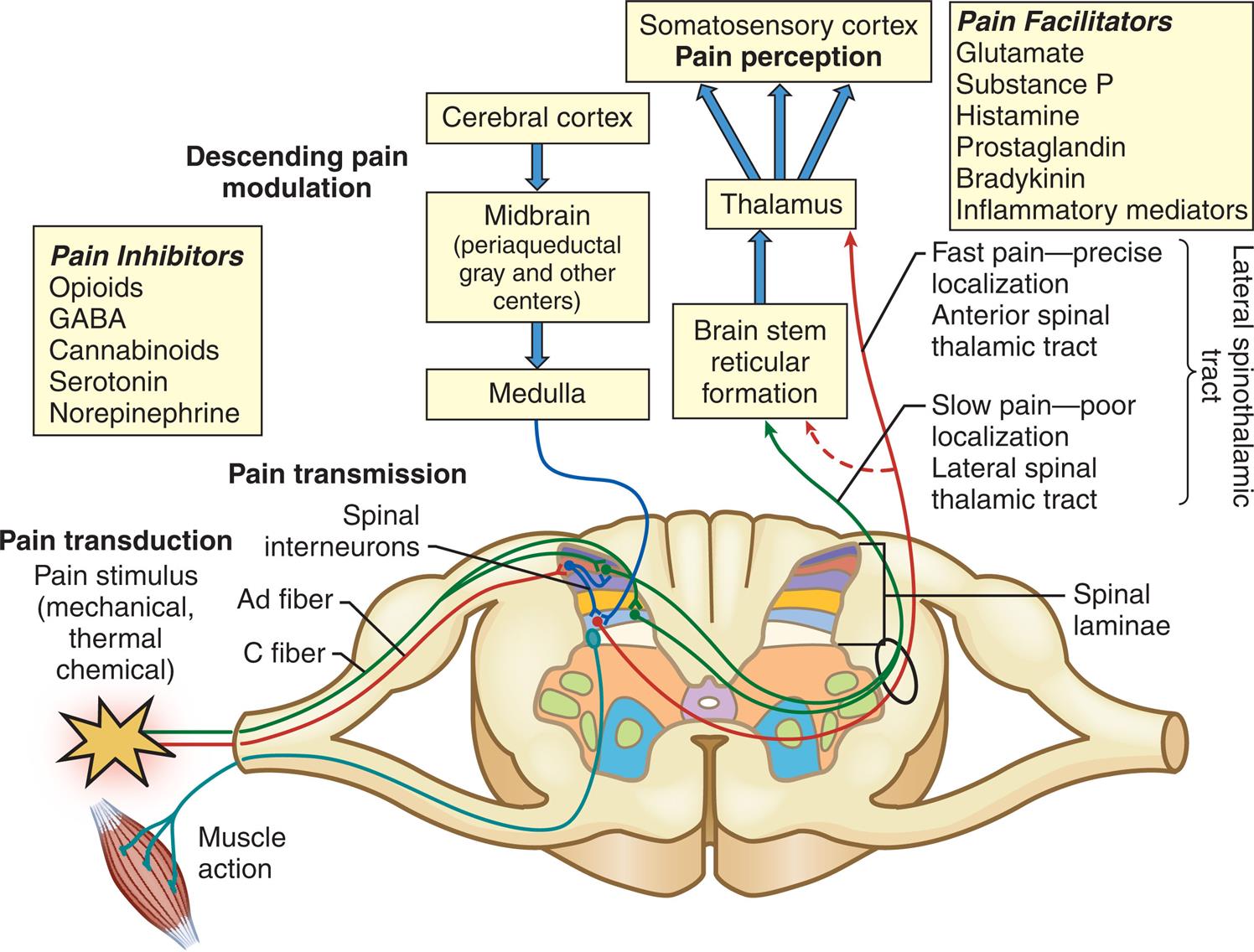
The Aδ and C fibers synapse in the laminae of the dorsal horn, cross over to the contralateral spinothalamic tract, and then ascend to synapse in the midbrain through the neospinothalamic and paleospinothalamic tracts. Impulses are then conducted to the sensory cortex. Descending pain inhibition is initiated in the cerebral cortex or from the midbrain and medulla.
An illustration accompanied by a flow chart depicts the pain pathway involved in pain transduction, pain transmission, and descending pain modulation. The illustration shows the cross-section through the spinal cord with labels as follows. Pain transduction: Pain stimulus (mechanical, thermal chemical); pain transmission: Spinal interneurons, A fiber, C fiber; spinal laminae. The pathway indicates the pain impulses along with the A fiber and C fibers into the dorsal horn by forming synapses with spinal interneurons. The flow chart represents impulse conducted to the somatosensory cortex through brain stem reticular formation and thalamus and the descending pain modulation in the cerebral cortex to form the midbrain (periaqueductal gray and other centers) and medulla. The anterior spinal thalamic tract carries fast pain through A fiber and the lateral spinal thalamic tract carries slow pain through C fiber. The pain inhibitors are opioids, G A B A, cannabinoids, serotonin, and norepinephrine. Pain facilitators are glutamate, substance P, histamine, prostaglandin, bradykinin, and inflammatory mediators.
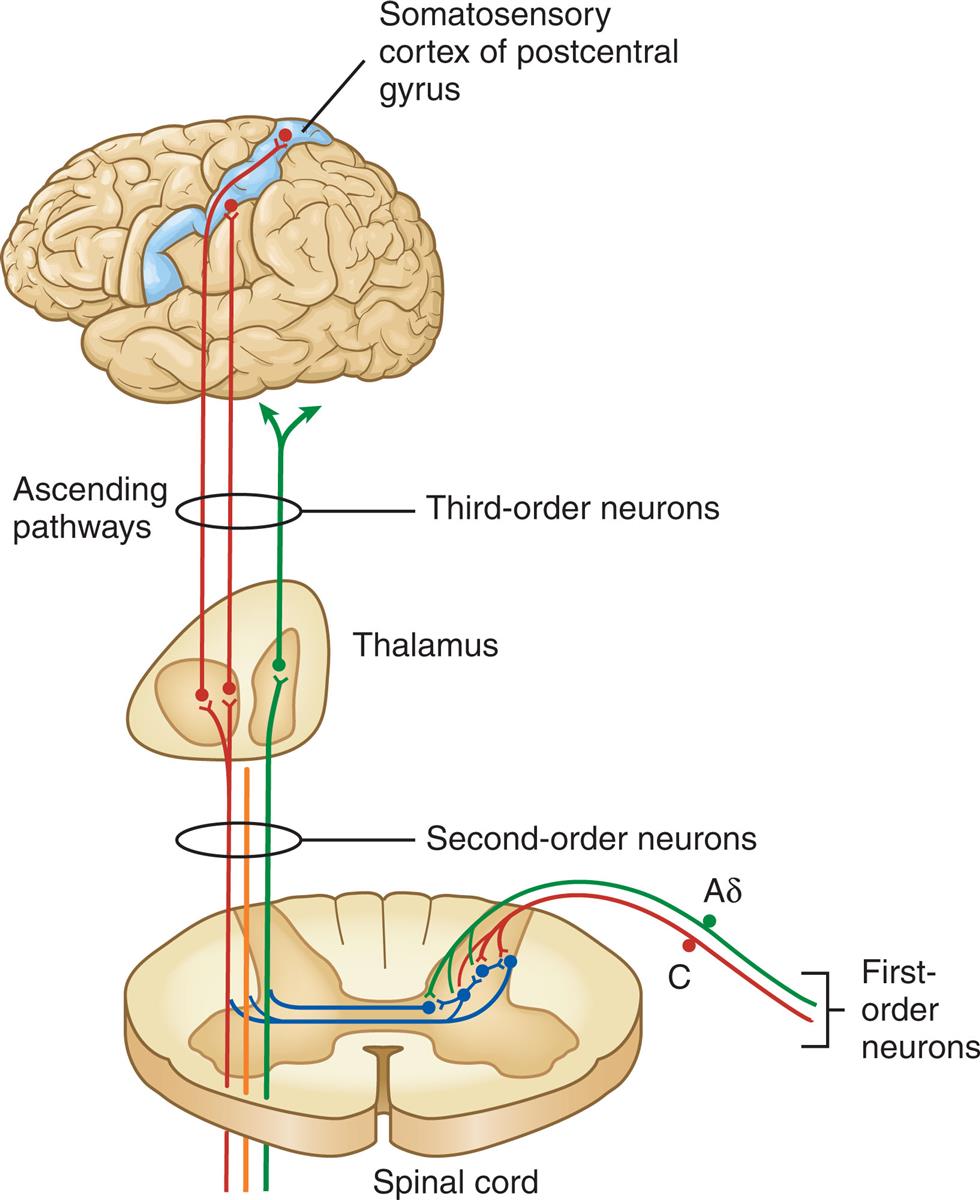
Aδ and C fibers comprise the primary, first-order sensory afferents coming into the gate at the dorsal horn of the spinal cord. Second-order neurons cross the cord (“decussate”) and ascend to the thalamus as part of the spinothalamic tract. Third-order afferents project to higher brain centers of the limbic system, the frontal cortex, and the primary sensory cortex of the postcentral gyrus of the parietal lobe.
An illustration demonstrates the pathways of nociception involving the first-order, second-order, and third-order neurons that carries action potential from the spinal cord to the somatosensory cortex of postcentral gyrus through the thalamus. The pathway indicates the impulses synapses with a projection of third-order neurons across the midline of the spinal cord, and ascend to the brain through two lateral spinothalamic tracts.
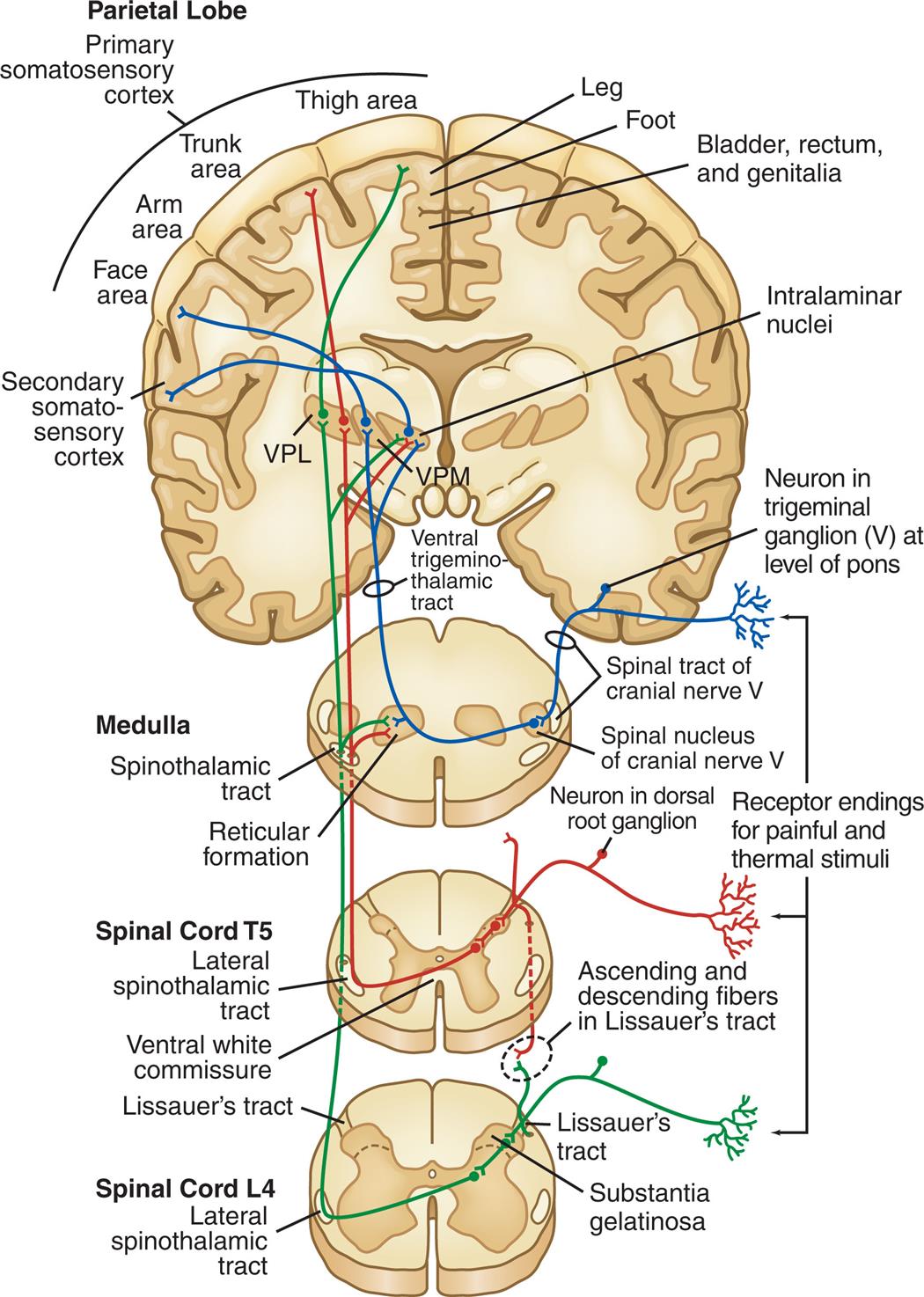
VPL, Ventral posterior lateral thalamic nuclei; VPM, ventral posterior medial thalamic nuclei.
Four series of illustrations depict the central nervous system pathways that mediate sensations of pain and temperature to the primary somatosensory cortex from the spinal cord L 4, spinal cord L 5, and medulla. The illustration of spinal cord L 4 depicts the coronal section of the spinal with labels: lateral spinothalamic tract, substantial gelatinosa, Lissauer's tract. The illustration of spinal cord T 5 depicts the coronal section of the spinal cord with labels: Lateral spinothalamic tract, ventral white commissure, Lissauer's tract, ascending and descending fibers in Lissauer's tract. The illustration of the medulla depicts the coronal section of the spinal cord with labels: Spinothalamic tract, reticular formation, a spinal tract of cranial nerve five, the spinal nucleus of cranial nerve five, a neuron in dorsal root ganglion. The illustration of the parietal lobe depicts the coronal section of the cortex with labels: Primary somatosensory cortex consisting of the face area, arm area, trunk area, thigh area, bladder, rectum and genitalia, leg, foot; secondary somatosensory cortex, ventral trigenmino-thalamic tract, a neuron in trigeminal ganglion five at the level of the pons, intralaminar nuclei. The tracts connect to the reticular formation, hypothalamus, thalamus and limbic system. The impulses are then projected to the somatosensory cortex for interpretation of the location and intensity of the pain and to other areas of the brain for an integrated response to pain.
Pain perception is the conscious awareness of pain, which occurs primarily in the reticular and limbic systems and the cerebral cortex. Interpretation of pain is influenced by many factors, including genetics, cultural preferences, sex roles, age, level of health, and past pain experiences. Three systems interact to produce the perception of pain.6
The sensory-discriminative system is mediated by the somatosensory cortex and is responsible for identifying the presence, character, location, and intensity of pain. The affective-motivational system determines an individual's conditioned avoidance behaviors and emotional responses to pain. It is mediated through the reticular formation, limbic system, and brainstem. The cognitive-evaluative system overlies the individual's learned behavior concerning the experience of pain and therefore can modulate perception of pain. It is mediated through the cerebral cortex. The integration of these three systems is referred to as the “pain matrix”7 or networks of cerebral connectivity.
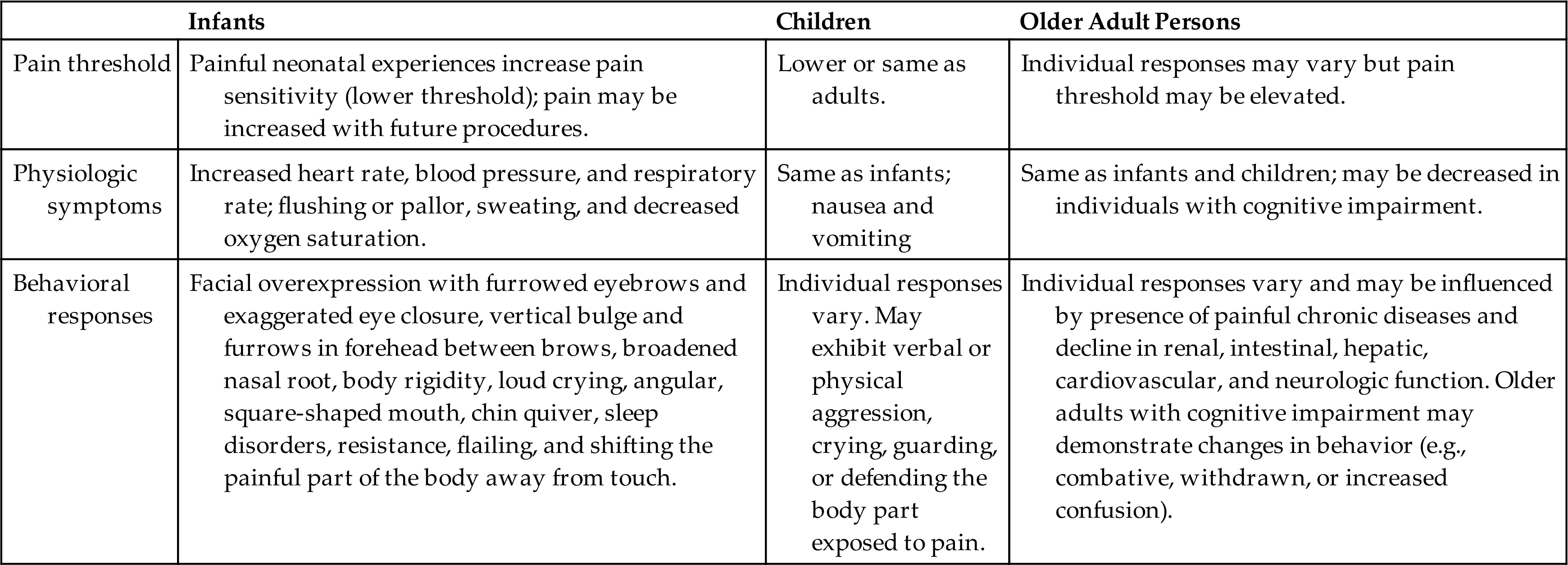
Pain threshold and tolerance are subjective phenomena that influence an individual's perception of pain. They can be influenced by genetics, sex, cultural perceptions, expectations, role socialization, physical and mental health, and age (Table 16.2A).
Table 16.2 A
Data from Anand KJS. Defining pain in newborns: need for a uniform taxonomy? Acta Paediatr. 2017;106(9):1438–1444; Lautenbacher S, et al. Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci Biobehav Rev. 2017;75:104–113; Tracy B, Sean Morrison R. Pain management in older adults. Clin Ther. 2013;35(11):1659–1668; Walker SM. Neonatal pain. Paediatr Anaesth. 2014;24(1):39–48.
Pain threshold is defined as the lowest intensity of pain that a person can recognize.2 Intense pain at one location may increase the threshold in another location. For example, a person with severe pain in one knee is more likely to experience less intense chronic back pain (this is called perceptual dominance). Because of perceptual dominance, pain at one site may mask other painful areas. Stress, excessive physical exertion, acupuncture, sexual activity, and other factors can increase the levels of circulating neuromodulators, thereby raising the pain threshold.
Pain tolerance is defined as the greatest intensity of pain that a person can endure.2 It varies greatly among people and in the same person over time because of the body's ability to respond differently to noxious stimuli. Pain tolerance generally decreases with repeated exposure to pain, fatigue, anger, boredom, apprehension, and sleep deprivation and may increase with alcohol consumption, persistent use of opioid medications, hypnosis, distracting activities, and strong beliefs or faith.
Pediatric ConsiderationsPain Perception of Infants and Children
Infants and children have the anatomic and functional ability to perceive pain. However, in the pediatric population, pain is frequently under-recognized and inadequately treated.
Pain pathways and cortical and subcortical centers for pain perception, as well as neurochemicals associated with pain transmission and modulation, are functional in preterm and newborn infants. Alterations in biological factors (e.g., peripheral and central somatosensory function and modulation, brain structure and connectivity) and psychosocial factors (e.g., gender, coping style, mood, and parental response) that influence pain have been identified in children and young adults born very preterm or extremely preterm.8
Repetitive, painful experiences and prolonged exposure to analgesic drugs in preterm infants may permanently alter developing synaptic and neuronal pain-processing networks, causing irreversible hypersensitivity to pain with subsequent injury. Alterations occur in both the excitatory and inhibitory pathways in the spinal cord and descending inhibitory processing from the brainstem. When children are not sufficiently treated for pain, stress hormones are released into their systems, resulting in increased catabolism, immunosuppression, and hemodynamic instability.9
Facial overexpression with furrowed eyebrows and exaggerated eye closure, body rigidity, loud crying, sleep disorders, resistance, and shifting the painful part of the body away from touch are the most consistent expressions of pain in infants.9 There may be finger clenching, writhing, back arching, and head banging.
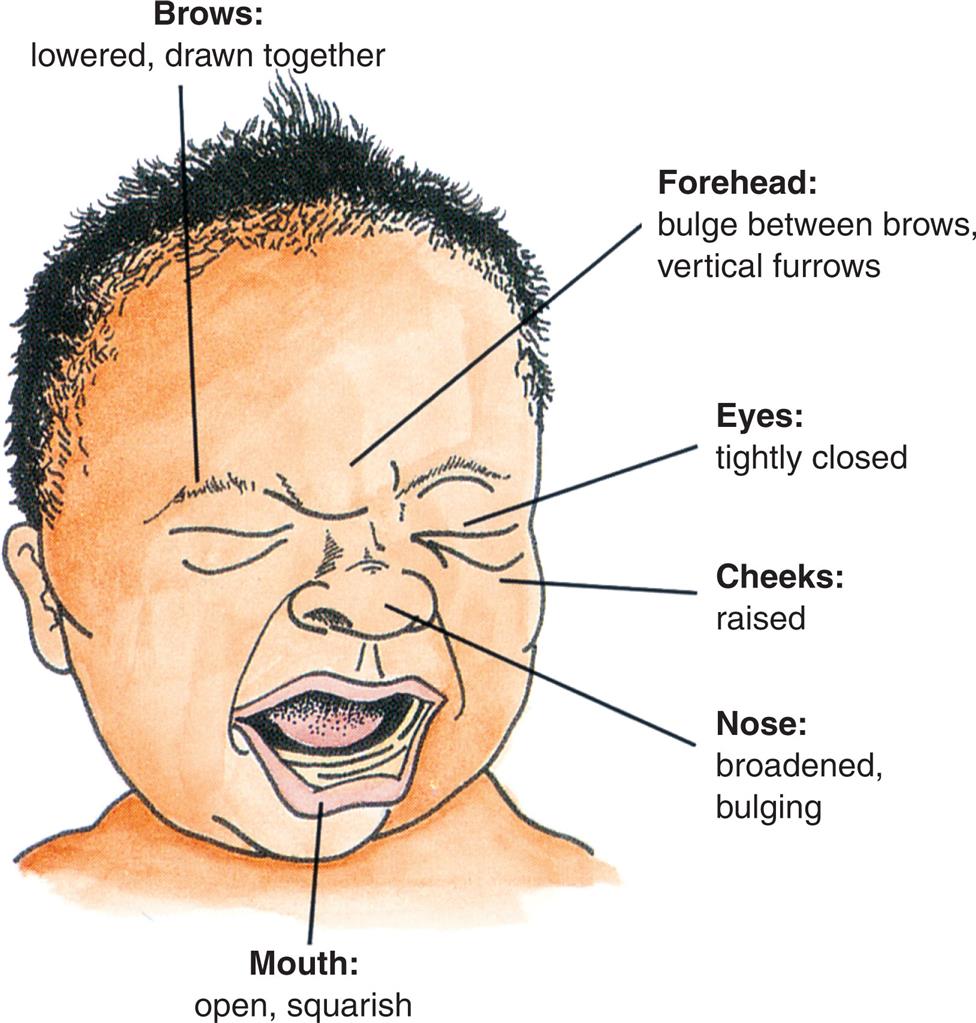
An illustration of the anterior profile of a crying infant depicts the facial expression of pain labeled as follows. Brows: Lowered, drawn together. Forehead: bulge between brows, vertical furrows; eyes: Tightly closed; cheeks: raised; Nose: broadened bulging.
Toddlers in pain may exhibit verbal or physical aggression and crying, or may guard or defend the body part exposed to pain. Children, like adults, have highly individual responses to pain. Any behavioral and physiologic indicators of pain must be carefully and accurately assessed and adequately treated for children of all ages. Physiologic responses in all age groups may include increases in heart rate, blood pressure, and respiratory rate; flushing or pallor; sweating; and decreased oxygen saturation.
Assessment of pain includes a thorough pain history received from the child and/or parent, exploring the pain quality, characteristics, location, onset, duration, aggravating and alleviating factors, and impact on function. The measurement of pain severity should be done routinely using a developmentally appropriate validated tool. Commonly used tools are listed in Table 16.2B with specified age ranges for the tool.
Table 16.2B
| Pain Assessment Tool | Age Range |
|---|---|
| Revised Premature Infant Pain Profile (PIPP-R)1 | Less than 28 weeks to greater than 36 weeks gestational age |
| Revised Face Legs Activity Cry and Consolability (r-FLACC) scale2 | 2 months to 7 years of age |
| Faces Pain Scale—Revised (FPS-R)3 | 4 years of age and older |
| Children and Infants Postoperative Pain Scale (CHIPPS)4 [Paediatr Anaesth. 2000;10(3): 303–318.] | Under 5 years of age |
| Numerical Rating Scale (NRS)5 | 8 years of age and older |
1Stevens BJ, Gibbins S, Yamada J, et al. The premature infant pain profile-revised (PIPP-R). Clin J Pain. 2014;30(3):238–243.
2Malviya S, Voepel-Lewis T, Burke C, et al. The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Pediatr Anesth. 2006;16(3):258–265.
3Hicks CL, von Baeyer CL, Spafford PA, et al. The faces pain scale-revised: toward a common metric in pediatric pain measurement. Pain. 2001;93(2):173–183.
4Büttner W, Finke W. Analysis of behavioural and physiological parameters for the assessment of postoperative analgesic demand in newborns, infants and young children: a comprehensive report on 7 consecutive studies. Paediatr Anaesth. 2000;10(3):303–318.
5Breivik H, Borchgrevink PC, Allen SM, et al. Assessment of pain. Br J Anaesth. 2008;101(1):17–24.
Data from Zieliński J, Morawska-Kochman M, Zatoński T. Pain assessment and management in children in the postoperative period: a review of the most commonly used postoperative pain assessment tools, new diagnostic methods and the latest guidelines for postoperative pain therapy in children. Adv Clin Exp Med. 2020;29(3):365–374. doi:10.17219/acem/112600. PMID: 32129952.
There are biological and psychosocial factors that influence the perception and severity of pain. Alterations in biological factors, such as peripheral and central somatosensory function, pain modulation, brain structure, and connectivity, have been identified in children and young adults born very preterm or extremely preterm and play a role in pain. There are also psychosocial factors such as gender, coping style, mood, and parental response that influence pain.8
Geriatric Considerations Aging and Pain
Pain in the older adult population is highly prevalent and is a complex issue to both identify and treat. Some older adults have an increased pain threshold while others have a decreased pain tolerance; both factors play a role in the identification and treatment of pain.10,11 It is important to assess the presence of pain in elderly persons with cognitive decline, as it is often neglected, underreported, underestimated, misdiagnosed, and not adequately treated.12 Inadequate assessment of pain or lack of proper pain management is associated with adverse outcomes including depression, anxiety, sleep disturbances, and mood changes, which often lead to significant suffering, disability, and social isolation with greater costs and burden to the health care system.10 Chronic and somatic pain have been shown to negatively affect the degree of frailty.13 The most frequent pain conditions in older adults are chronic unspecified joint pain, chronic back pain, and chronic neck pain.12 Pain must be accurately treated in relation to its effect on cognitive function, coexisting disease, drug interactions, other reactions to treatment, and an individual’s ability to express pain and maintain safety. Treatment is often compounded by the high prevalence of polypharmacy within this population. Pharmacotherapies used for pain management in older adults are usually only partially effective and are often limited by side effects.10 Older adults prescribed pharmacological treatment must be monitored carefully for any alterations in cognition, liver and renal function, physiological changes, and possible drug interactions.12
Pain Modulation
Pain modulation involves many different facilitatory and inhibitory mechanisms that increase or decrease the transmission of pain signals throughout the nervous system. Mechanisms include neurotransmitters and central and spinal pathways. Depending on the mechanism, modulation can occur before, during, or after pain is perceived.14 Analgesic drugs, anesthesia, and nonpharmacologic interventions such as transcutaneous nerve stimulation, acupuncture, hypnosis, and physical therapies are examples of strategies for enhancing pain modulation.
Neurotransmitters of Pain Modulation
A wide variety of neurotransmitters act to modulate control over transmission of pain impulses in the periphery, spinal cord, and brain. The peripheral triggering mechanisms that initiate release of excitatory neurotransmitters include tissue injury (prostaglandins, histamine, bradykinin) and chronic inflammatory lesions (lymphokines). Glutamate, aspartate, substance P, and calcitonin are common excitatory neurotransmitters in the brain and spinal cord. These substances sensitize nociceptors by reducing the activation threshold, leading to increased responsiveness of nociceptors.
Inhibitory neurotransmitters in the CNS include gamma-aminobutyric acid (GABA) and glycine. Norepinephrine and 5-hydroxytryptamine (serotonin) contribute to pain inhibition in the CNS but can excite peripheral nerves.
Endogenous opioids are a family of morphine-like neuropeptides that inhibit transmission of pain impulses in the periphery, spinal cord, and brain by binding with specific opioid receptors (mu [μ], kappa [κ], and delta [δ]) on neurons. They inhibit ion channels, preventing the release of excitatory neurotransmitters, such as substance P and glutamate, in the dorsal horn. In the midbrain they influence descending inhibitory pathways (Fig. 16.4).15 In peripheral inflamed tissue, opioids are produced and released from immune cells and activate opioid receptors on sensory nerve terminals.16 Opioid receptors are widely distributed throughout the body and are responsible for general sensations of well-being and modulation of many physiologic processes, including control of respiratory and cardiovascular functions, stress and immune responses, gastrointestinal function, reproduction, and neuroendocrine control.17,18
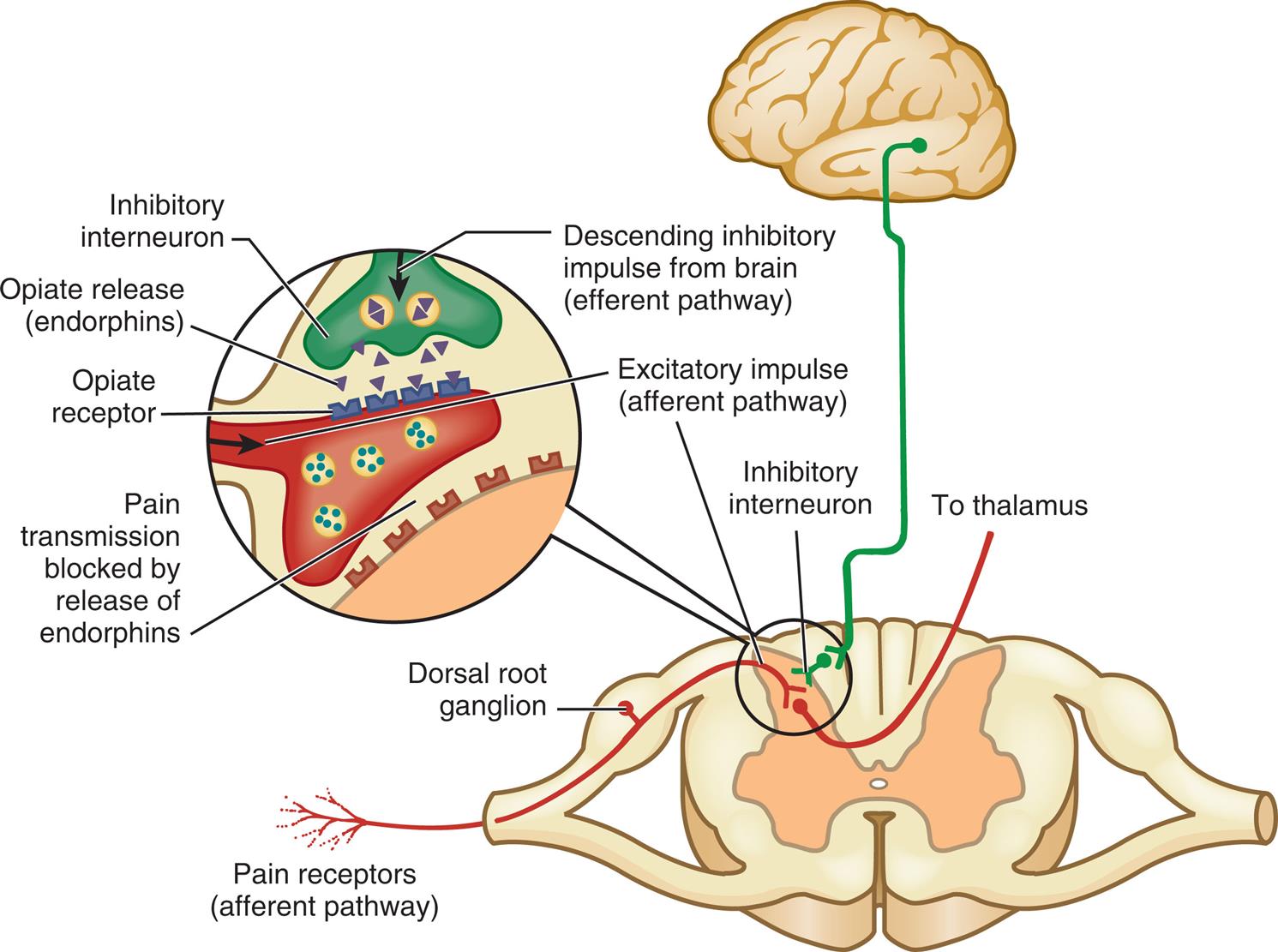
An illustration depicts the descending inhibitory impulse from the brain (efferent pathway) through the thalamus synapses at the inhibitory interneuron in the dorsal ganglion (afferent pathway) along with excitatory impulse (afferent pathway) and stimulates the opiate to release endorphins. The released endorphins activate the opioid receptor which inhibits pain transmission.
Enkephalins are the most prevalent of the natural opioids and bind to δ opioid receptors. Endorphins (endogenous morphine) are produced in the brain. The best-studied endorphin is β-endorphin, which binds to μ receptors and is purported to produce the greatest sense of exhilaration as well as substantial natural pain relief. Dynorphins are the most potent of the endogenous opioids, binding strongly with κ receptors to impede pain signals. Paradoxically, they play a role in neuropathic pain and in mood disorders and drug addiction. Endomorphins bind with μ receptors and have potent analgesic effects. Nociceptin/orphanin FQ is an opioid that induces pain or hyperalgesia but does not interact with opioid receptors. The nociceptin receptor is widely distributed throughout the PNS and CNS and is associated with numerous biological functions, including immune regulation, mood, feeding, muscle contractility, heart rate, and emotion.
Synthetic and natural opiates have pharmacologic actions similar to morphine and bind as direct agonists to the opioid receptors. Morphine has a 50 times higher affinity for μ receptors in comparison with other opioids. Naloxone is the only clinically used opioid receptor antagonist, with a higher affinity for the µ receptors than for the other receptors.
Endocannabinoids are synthesized from phospholipids and are classified as eicosanoids. They activate cannabinoid CB1 (primarily in the CNS) and CB2 receptors (primarily in immune tissue [e.g., the spleen]) to modulate pain and other functions, including memory, appetite, immune function, sleep, stress response, thermoregulation, and addiction. CB1 receptors decrease pain transmission by inhibiting release of excitatory neurotransmitters in the spinal dorsal horn, periaqueductal gray (PAG; the gray matter surrounding the cerebral aqueduct), thalamus, rostral ventromedial medulla (RVM), and amygdala. Cannabis (marijuana) produces a resin containing cannabinoids. Cannabinoids are analgesic in humans, but their use is limited by their psychoactive and addictive properties. Work is in progress to develop cannabinoid receptor agonists that do not have addictive side effects.19,20
Pathways of Modulation
Descending inhibitory and facilitatory pathways inhibit or facilitate pain. Inhibitory pathways can activate opioid receptors and inhibit release of excitatory neurotransmitters, facilitate release of inhibitory neurotransmitters, or stimulate inhibitory interneurons. Afferent stimulation of particularly the ventromedial medulla and PAG in the midbrain stimulates efferent pathways, which inhibit ascending pain signals at the dorsal horn. The RVM stimulates descending pathways that facilitate or inhibit pain in the dorsal horn.
Segmental pain inhibition occurs when A-beta (Aβ) fibers (large myelinated fibers that transmit touch and vibration sensations) are stimulated and the impulses arrive at the same spinal level or segment as impulses from Aδ or C fibers. They stimulate an inhibitory interneuron and decrease pain transmission. An example is rubbing an area that has been injured to relieve pain.
Diffuse noxious inhibitory control (DNIC) is an endogenous inhibitory pain system that involves a spinal-medullary-spinal pathway. Pain is relieved when two noxious or painful stimuli occur at the same time from different sites (pain inhibiting pain). The efficacy of DNIC is evaluated clinically by testing subjective responses to pain using conditioned pain modulation (CPM). A CPM test uses a consistent protocol to deliver a measurable test pain stimulus at one site before and during or after the application of a measurable conditioning pain stimulus at a comparable site (e.g., the arms). A subjective pain intensity rating scale (i.e., 1 to 10) is used to measure intensity of pain at both sites. The conditioning pain stimulus is expected to affect the pain experience of the test stimulus. Use of CPM assessments can be helpful in determining an individual’s endogenous pain inhibitory capacity and for managing acute or chronic pain.21,22Expectancy-related cortical activation (placebo effect [beneficial expectations] or nocebo effect [adverse expectations]) can exert control over analgesic systems to attenuate or intensify pain.23 In other words, cognitive expectations can cause real, measurable physiologic effects that share some of the same descending pain pathways as the pain modulatory systems (see Emerging Science Box: Pain Management With Pharmacogenics).
Clinical Descriptions of Pain
Pain can be described in a variety of ways. Due to the complex nature of pain, many terms overlap, and more than one description is often used. The broad categories of pain are summarized in Box 16.1. Some of the most common clinical pain presentations are summarized here.
Acute pain (nociceptive pain) is a normal protective mechanism that alerts the individual to a condition or experience that is immediately harmful to the body and mobilizes the individual to take prompt action to relieve it. Acute pain is transient, usually lasting seconds to days, sometimes up to 3 months. It begins suddenly and is relieved after the chemical mediators (usually related to inflammation) that stimulate pain receptors are removed. Stimulation of the autonomic nervous system results in physical manifestations, including increased heart rate, hypertension, diaphoresis, and dilated pupils. Anxiety related to the pain experience, including its cause, treatment, and prognosis, is common, as is the hope of recovery and expectation of limited duration.
Acute pain arises from cutaneous, deep somatic, or visceral structures and can be classified as (1) somatic, (2) visceral, or (3) referred. Somatic pain arises from the skin (i.e., from an abrasion or a laceration), joints (pain from arthritis or injured tendons), and muscles (strain from overuse or muscle injury). It is either sharp and well localized (especially fast pain carried by Aδ fibers) or dull, aching, throbbing, and poorly localized, as seen in polymodal unmyelinated C fiber transmissions. Visceral pain is transmitted by C fibers and refers to pain in internal organs and the lining of body cavities. It tends to be poorly localized with an aching, gnawing, throbbing, or intermittent cramping quality. It is carried by sympathetic fibers and is associated with nausea and vomiting, hypotension, and, in some cases, shock. Visceral pain often radiates (spreads away from the actual site of the pain) or is referred. Examples of conditions that cause visceral pain include gallstones, pancreatitis, kidney stones, bowel obstruction, appendicitis, and bladder infection. Referred pain is felt in an area removed or distant from its point of origin—the area of referred pain is supplied by the same spinal segment as the actual site of pain. Referred pain can be acute or chronic. Impulses from many cutaneous and visceral neurons converge on the same ascending neuron, and the brain cannot distinguish between the different sources of pain. Because the skin has more receptors, the painful sensation is experienced at the referred site instead of at the site of origin. Referred pain can be acute or chronic. For example, the pain of pancreatitis may be felt in the right shoulder or scapula, or pain from the heart may be referred to the left shoulder or arm. Fig. 16.5 illustrates common areas of referred pain and their associated sites of origin.
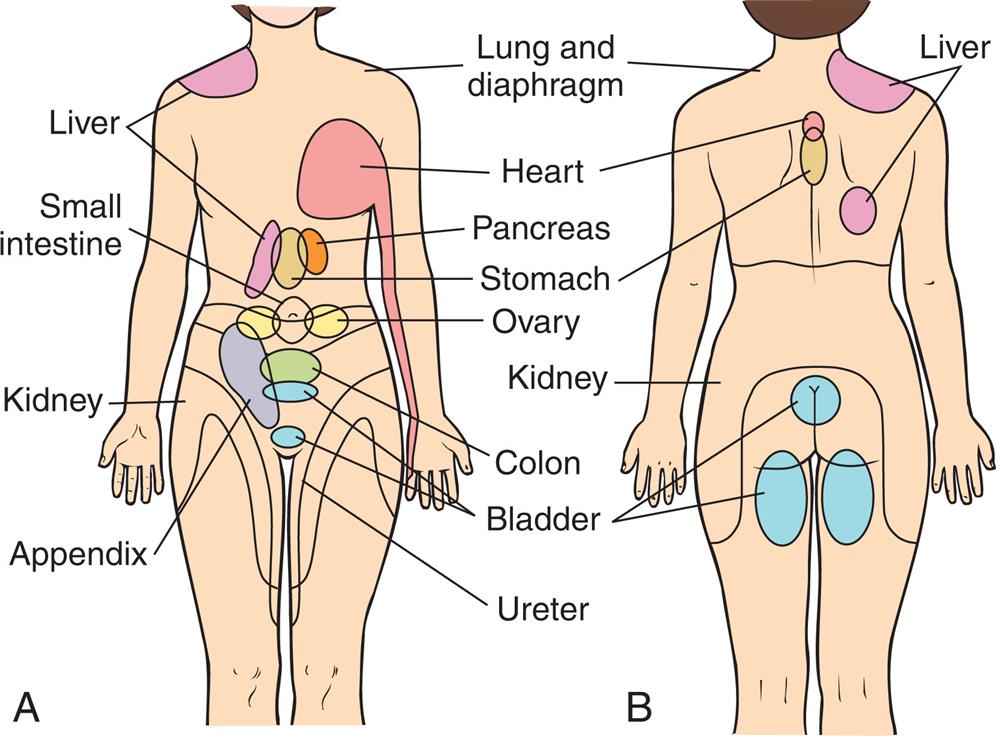
(A) Anterior view. (B) Posterior view.
Two illustrations, A and B, depict the common areas of referred pain and their associated sites of origin labeled. Illustration A shows the anterior view of the human with the common areas of referred pain labeled as follows. Liver, small intestine, kidney, appendix, lung and diaphragm, heart, pancreas, stomach, ovary, colon, bladder, and ureter. Illustration B shows the posterior view of the human with common areas of referred pain labeled as follows. Lung and diaphragm, heart, stomach, kidney, bladder, and liver.
Chronic or persistent pain has been defined as lasting for more than 3 to 6 months in adults and is pain lasting well beyond the expected normal healing time. It varies with the type of injury, is different among age groups, and produces varietal levels of disability.24
Chronic or persistent pain serves no purpose, is poorly understood, and causes suffering. It often appears to be out of proportion to any observable tissue injury. It may be ongoing (e.g., low back pain) or intermittent (e.g., migraine headaches). Changes in the PNS and CNS that cause dysregulation of nociception and pain modulation processes (peripheral and central sensitization) are thought to lead to chronic pain (see the discussion of neuropathic pain later in this section).
Neuroimaging studies have demonstrated brain changes in individuals with chronic pain, which may lead to cognitive deficits and decreased ability to cope with pain.25,26 These negative manifestations of chronic pain are thought to be due, in part, to the stress of coping with continuous pain and may be reversible when pain is controlled. Because it is not yet possible to predict when acute pain will develop into chronic pain, early treatment of acute pain is encouraged. Comparison of acute and chronic pain is summarized in Table 16.3.
Table 16.3
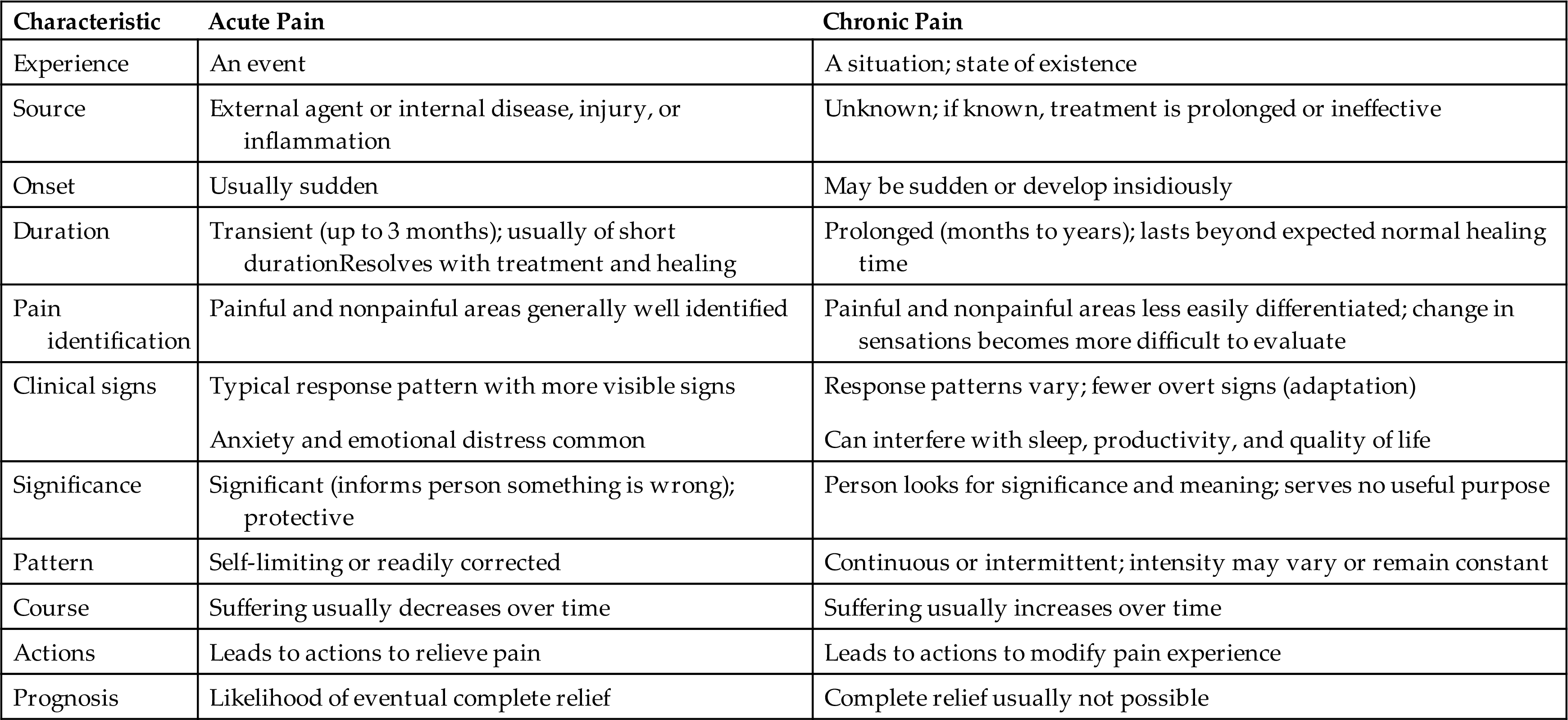
Physiologic responses to intermittent chronic pain are similar to those for acute pain, whereas persistent pain allows for physiologic adaptation, producing a normal heart rate and blood pressure. This leads many to mistakenly conclude that people with chronic pain are malingering because they do not appear to be in pain. As chronic pain progresses, certain behavioral and psychologic changes often emerge, including depression, difficulty eating and sleeping, preoccupation with the pain, and avoidance of pain-provoking stimuli.27 The desire to relieve pain and the need to hide it become conflicting drives for those with chronic pain, who fear being labeled complainers.28 Chronic pain is perceived as meaningless and is often associated with a sense of hopelessness as more time elapses; no relief seems possible. Some of the chronic pain syndromes are listed in Table 16.4. Chronic pain associated with specific organ systems is discussed in later chapters.
Table 16.4
Neuropathic pain is chronic pain initiated or caused by a primary lesion or dysfunction in the somatosensory nervous system and leads to long-term changes in pain pathway structures (neuroplasticity) and abnormal processing of sensory information. There is amplification of pain without stimulation by injury or inflammation. Neuropathic pain is often described as burning, shooting, shock-like, or tingling. It is characterized by increased sensitivity to painful or nonpainful stimuli with hyperalgesia, allodynia (the induction of pain by normally nonpainful stimuli), and the development of spontaneous pain.29 Neuropathic pain is classified as either peripheral or central and is associated with central and peripheral sensitization. Peripheral neuropathic pain is caused by peripheral nerve lesions. Peripheral sensitization is an increase in the sensitivity and excitability of primary sensory neurons and cells in the DRG. Examples include nerve entrapment, diabetic neuropathy, and chronic pancreatitis.
Central neuropathic pain is caused by a lesion or dysfunction in the brain or spinal cord. A progressive repeated stimulation of group C neurons (known as wind-up) in the dorsal horn leads to central sensitization, an increased sensitivity of central pain signaling neurons. This results in pathologic changes in the CNS that cause chronic pain. Examples include brain or spinal cord trauma, tumors, vascular lesions, multiple sclerosis, Parkinson disease, postherpetic neuralgia, and phantom limb pain.
The following mechanisms have been implicated in the cause of neuropathic pain30:
- • Changes in sensitivity of neurons—lower threshold with peripheral and central sensitization
- • Spontaneous impulses from regenerating peripheral nerves
- • Alterations in the DRG and spinothalamic tract in response to peripheral nerve injury (i.e., deafferentation pain—loss of pain-related afferent information to the brain)
- • Loss of pain inhibition and stimulation of pain facilitation by excitatory neurotransmitters in the dorsal horn (e.g., release of glutamate)
- • Loss of descending inhibitory pain modulation
- • Hyperexcitable spinal interneurons stimulated by Aβ fibers (nonpainful stimulation of pain)
- • Release of nociceptive inflammatory cytokines, chemokines, and growth factors by activated glial cells
- • Structural and functional alterations in brain processing neural networks
Because of the complexity of the causes of neuropathic pain syndromes, they are difficult to treat. Multimodal therapy is often needed, including nondrug treatment.31
An Overview of Chronic Pain Syndromes
Myofascial pain syndrome (MPS) is a regional pain syndrome associated with injury to muscle, fascia, and tendons and includes myositis, fibrositis, myofibrositis, myalgia, (see Chapter 44 for fibromyalgia), and muscle strain. MPS involves myofascial trigger points within a taut band of skeletal muscle (“muscle knots”). Compression of the trigger point causes a local twitch response (a small, quick contraction of muscle fibers) accompanied by referral of pain, motor dysfunction, and autonomic responses (e.g., flushing, diaphoresis, temperature changes). The symptoms often occur in association with poor muscle tone, inactivity, repeated muscle or tendon strain, sudden vigorous exercise, or muscle overuse. The pathophysiology is not clearly known. The pain may be the result of peripheral and central sensitization with low-threshold mechanosensitive afferents projecting to sensitized dorsal horn neurons. There may be neuroaxonal degeneration with alterations in neuromuscular transmission (e.g., extra leakage of acetylcholine at the neuromuscular junction induces persistent contraction) or muscle energy consumption that exceeds energy supply.32 During the early stages of the disorder the pain is localized, but as the disorder progresses, it becomes deep, aching, and more generalized. Chronic postoperative pain is pain that persists for at least 3 months after surgery, after ruling out any other possible causes, such as infection, tumor recurrence, or pain arising from pre-existing conditions. The types of pain can include nerve injury, complex regional pain syndrome, phantom limb pain, chronic donor site pain, post-thoracotomy pain syndrome, post-mastectomy pain syndrome, joint arthroplasty pain, and postsurgical abdominal and pelvic pain. Nerve injury and inflammation may induce plastic changes in the PNS and CNS contributing to peripheral and central nerve sensitization with allodynia and hypersensitivity. There may also be alterations in descending inhibitory pathways. Psychological factors, including anxiety and depression, also influence the occurrence of postoperative pain. Multimodal approaches to analgesia are needed for pain management including adequate management of preoperative pain and postoperative management of acute pain.33–35Cancer pain is often chronic, and the causes are multifactorial and related to site and type of cancer, extent of disease, treatment modalities, age, and access to care.36 Cancers generate and secrete mediators that sensitize and activate primary afferent nociceptors in the area of the tumor, resulting in neurochemical reorganization of the spinal cord, which contributes to spontaneous activity and enhanced pain responsiveness. Increasing pressure of a growing tumor on nerve endings, tissue destruction, inflammatory mediators, distention of visceral surfaces, obstruction of ducts and intestine, pathologic fractures, chemotherapy, radiation therapy, surgical procedures, and opioid-induced hyperalgesia also promote pain. These processes lead to both nociceptive and neuropathic pain.37 Therapeutic approaches to the management of cancer pain have advanced significantly in recent years, particularly in palliative care and hospice programs. Frequent assessment of pain, management of breakthrough pain, and implementation of individualized interdisciplinary therapeutic strategies (including pharmacotherapeutic, anesthetic, neurosurgical, psychologic, and rehabilitative techniques along with frequent evaluations) are essential to optimal cancer pain management. Research is in progress to evaluate the effects of different opioid receptors on tumor growth and suppression and will assist in selecting the optimum drugs for pain treatment.38Post-stroke pain syndromes can be acute but often occur up to 6 months after stroke and become chronic. Both nociceptive and neuropathic mechanisms of pain can be involved. Pain can include central poststroke pain or be secondary to spasticity, headache, shoulder pain, and complex region pain syndrome (see below). Central poststroke pain is pain and sensory abnormalities that manifest in the body parts that correspond to the area of the brain that have been injured by the cerebrovascular lesion. Hyperalgesia, dysesthesias, allodynia, spontaneous pain, and other sensory deficits are common.
There may be hyperexcitation in the damaged sensory pathways, damage to the central inhibitory pathways, or a combination of the two, making it difficult to differentiate from other causes of pain.39
Phantom limb pain (PLP) is pain that an individual feels in the amputated limb, usually distally (hands and feet) after the stump has completely healed (1 to 3 months after amputation). PLP is differentiated from residual limb pain, which is pain originating from the actual site of the amputated limb and can be associated with infection and neuroma formation. Both types of pain can occur at the same time. PLP can be intermittent or severe and occasional or constant, throbbing, stabbing, burning, or cramping. Both peripheral and central mechanisms of pain contribute to phantom limb pain. There is injury to peripheral nerves with increased excitability. Changes in pain processing in both the brain and spinal cord are known to occur.40 Nonpainful phantom limb sensations occur in almost all amputees, but the sensations usually fade with time. Chronic regional pain syndrome type II can also be a component of PLP.
Complex regional pain syndrome (CRPS) is chronic neuropathic pain usually associated with limb injury. Two forms are described: complex regional pain syndrome-I (CRPS-I) (previously termed reflex sympathetic dystrophy syndrome) associated with injury but no apparent nerve injury; and complex regional pain syndrome-II (CRPS-II) (previously termed causalgia) with evidence of nerve injury. The symptoms of both forms are similar. CRPS is distinguished from other chronic pain disorders by signs of autonomic and inflammatory changes in the pain region of the injured nerve. There are autonomic symptoms: changes in skin color, temperature, and sweating and alterations in hair and nail growth for the affected limb; motor symptoms: tremor or weakness may be present; and sensory symptoms: hypersensitivity, hyperalgesia, and allodynia. CRPS is further distinguished as “warm CRPS,” associated with a warm, red, and edematous extremity; and “cold CRPS,” associated with a cold, dusky, and sweaty extremity. Peripheral and central sensitization contribute to the pain syndrome, but the mechanisms are unknown. A combination of injury and the presence of inflammatory cytokines and neuropeptides may lead to peripheral nociceptive sensitization and physiologic change in pain transmission and in autonomic and motor systems.41
Temperature Regulation
Human thermoregulation is achieved through precise balancing of heat production, heat conservation, and heat loss The normal range of body temperature is considered to be 36.2°C to 37.7°C (96.2°F to 99.4°F) overall, but a person's individual body parts will vary in temperature. Body temperature rarely exceeds 41°C (105.8°F). The extremities are generally cooler than the trunk, and the temperature at the core of the body (as measured by rectal temperature) is generally 0.5°C higher than the surface temperature (as measured by oral temperature). Internal temperature varies in response to activity, environmental temperature, and daily fluctuation (circadian rhythm). Oral temperatures fluctuate within 0.2°C to 0.5°C during a 24-hour period. Women tend to have wider fluctuations that follow the menstrual cycle, with a sharp rise in temperature just before ovulation. The daily fluctuating temperature in both sexes peaks around 6 p.m. and is at its lowest during sleep. Maintenance of body temperature within the normal range is necessary for life.
Control of Body Temperature
Temperature regulation (thermoregulation) is mediated primarily by the hypothalamus and endocrine system. Peripheral thermoreceptors in the skin, liver, and skeletal muscle (unmyelinated C fibers and thinly myelinatewd Aδ fibers) and central thermoreceptors in the hypothalamus, spinal cord, viscera, and great veins provide the hypothalamus with information about body temperatures. If these temperatures are low or high, the hypothalamus triggers heat production and heat conservation or heat loss mechanisms.42
Body heat is produced by the chemical reactions of metabolism and skeletal muscle tone and contraction. The heat-producing mechanism (chemical or non-shivering thermogenesis) begins with hypothalamic thyrotropin-releasing hormone (TRH); it stimulates the anterior pituitary to release thyroid-stimulating hormone (TSH), which acts on the thyroid gland and stimulates the release of thyroxine. Thyroxine then acts on the adrenal medulla, causing the release of epinephrine into the bloodstream. Epinephrine causes cutaneous vasoconstriction, stimulates glycolysis, and increases metabolic rate, thus increasing body heat. Norepinephrine and thyroxine activate brown fat thermogenesis where energy is released as heat (non-shivering thermogenesis) instead of as adenosine triphosphate (ATP). Heat is distributed by the circulatory system.
The hypothalamus also triggers heat conservation by stimulating the sympathetic nervous system and results in increased skeletal muscle tone, initiating the shivering response and producing vasoconstriction. Sympathetic stimulation also constricts peripheral blood vessels and redistributes blood flow. Centrally warmed blood is shunted away from the periphery to the core of the body, where heat can be retained. This involuntary mechanism takes advantage of the insulating layers of the skin and subcutaneous fat to protect the core temperature. The hypothalamus relays information to the cerebral cortex about cold, and voluntary responses result. Individuals typically bundle up, keep moving, or curl up in a ball. These types of voluntary physical activities provide insulation, increase skeletal muscle activity, and decrease the amount of skin surface available for heat loss through radiation, convection, and conduction.
The hypothalamus responds to warmer core and peripheral temperatures by reversing the same mechanisms resulting in heat loss. Heat loss is achieved through (1) radiation, (2) conduction, (3) convection, (4) vasodilation, (5) evaporation (sweating), (6) decreased muscle tone, (7) increased respiration, (8) voluntary measures, and (9) adaptation to warmer climates (i.e., increasing or decreasing the volume of sweat). Table 16.5 summarizes further information about heat production and loss.
Table 16.5
Temperature Regulation in Infants and Older Adult Persons
Infants (particularly low-birth-weight infants) and older adult persons require special attention to maintenance of body temperature. Term infants produce sufficient body heat, primarily through metabolism of brown fat, but cannot conserve heat produced because of their small body size, greater ratio of body surface to body weight, and inability to shiver. Infants also have little subcutaneous fat and thus are not as well insulated as adults. Children also have a greater ratio of body surface to body weight, lower sweating rate, higher peripheral blood flow in the heat, and a greater extent of vasoconstriction in the cold than adults. They can acclimatize to changes in environmental temperatures but do so at a lower rate than adults.
Older adult persons respond poorly to environmental temperature extremes because of their slowed blood circulation, structural and functional skin changes, overall decreased heat-producing activities, and the presence of disease (i.e., congestive heart failure, chronic lung disease, diabetes mellitus, or peripheral vascular disease). Cold stress in older adults also decreases coronary perfusion.43 In addition, older adult persons have a decreased shivering response (delayed onset and decreased effectiveness), slowed metabolic rate, decreased vasoconstrictor response, diminished or absent ability to sweat, decreased peripheral sensation, desynchronized circadian rhythm, decreased perception of heat and cold, decreased thirst, decreased nutritional reserves, decreased brown adipose tissue, and decreased shivering response.44,45
Pathogenesis of Fever
Fever (febrile response) is a temporary resetting of the hypothalamic thermostat to a higher level in response to exogenous or endogenous pyrogens. Exogenous pyrogens (endotoxins produced by pathogens; see Chapter 10) stimulate the release of inflammatory endogenous pyrogens from phagocytic cells (primarily macrophages), including Prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), interleukin-6 (IL-6), and interferon γ (IFNγ). These pyrogenic cytokines raise the thermal set point by acting on the hypothalamus to induce an integrated endocrine, autonomic nervous system, and behavioral response and also initiate the acute phase response (described below). The hypothalamic set point for temperature regulation is elevated, signaling an increase in heat production and conservation. Peripheral vasoconstriction shunts blood from the skin to the body core. Epinephrine release increases metabolic rate and muscle tone. Decreased release of vasopressin (anti-diuretic hormone) reduces the volume of body fluid to be heated. The individual feels colder, dresses more warmly, decreases body surface area by curling up, and may go to bed in an effort to get warm. Symptoms of lassitude and anorexia are common and may be related to energy conservation to support the increased metabolic demands of fever. Body temperature is maintained at the new level until the fever “breaks,” when the set point begins to return to normal with decreased heat production and increased heat reduction mechanisms. The individual feels very warm, dons cooler clothes, throws off the covers, and stretches out. Once the body has returned to a normal temperature, the individual feels more comfortable and the hypothalamus adjusts thermoregulatory mechanisms to maintain the new temperature (Fig. 16.6).
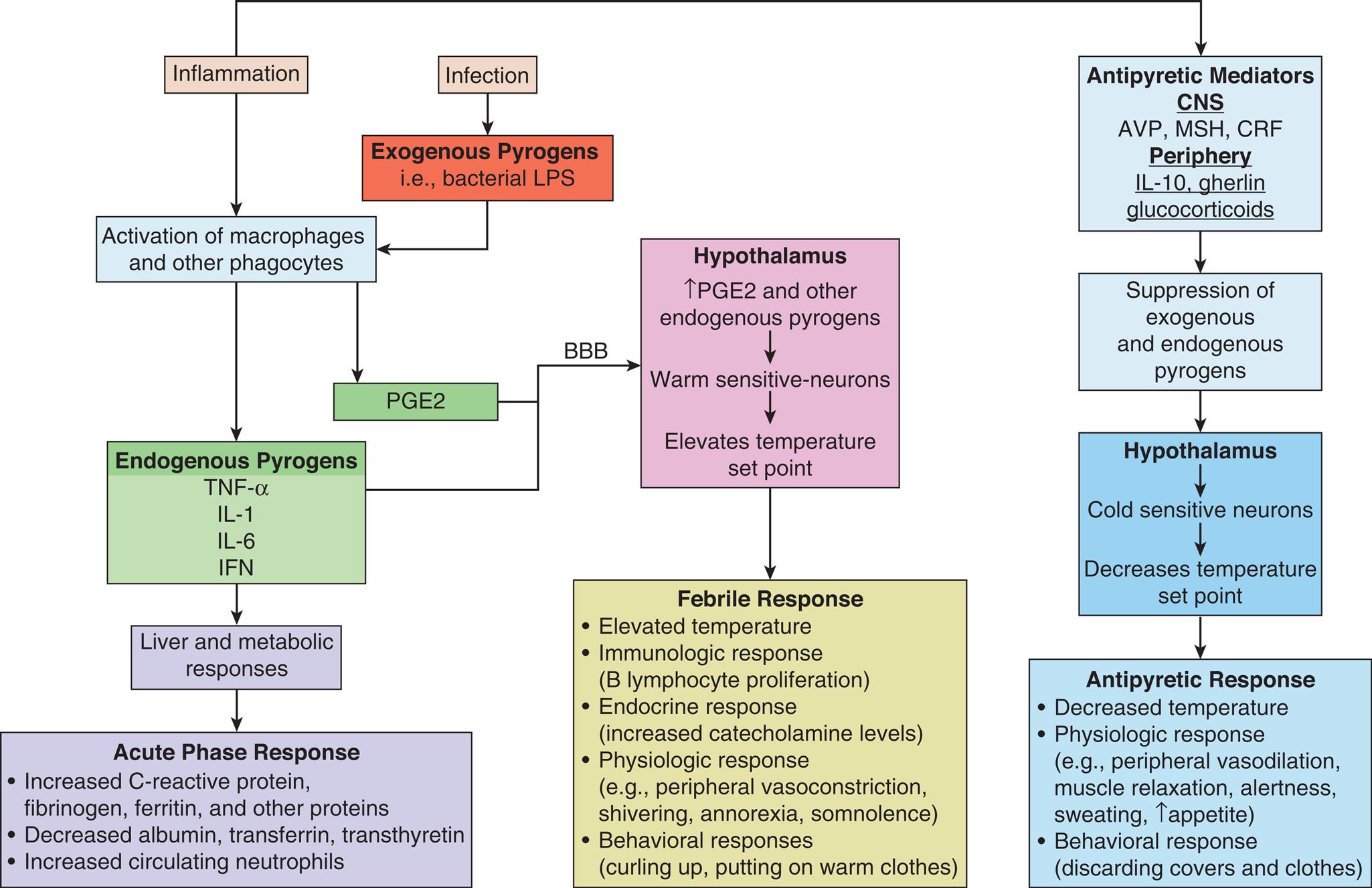
Infection and inflammation initiate the release of exogenous pyrogens from pathogens and endogenous pyrogens from macrophages and other immune cells. Endogenous pyrogens and PGE2 act on the hypothalamus to elevate the temperature set point and initiate fever and the acute phase response. Fever is modulated by antipyretic mediators (cryogens) from both the CNS and periphery which suppress the febrile response and prevent damage from excessively high temperatures. BBB, Blood brain barrier; AVP, arginine vasopressin; CRP, C-reactive protein; IFN, interferon; IL, interleukin-1, interleukin-6; MCH, melanocortin; PGE2, prostaglandin E2; TNF-α, tumor necrosis factor-alpha.
A flow chart represents the pathogenesis of fever and the acute phase response. • The inflammation initiates the activation of macrophages and other phagocytes from endogenous pyrogens such as T N F alpha, I L 1, I L 8, I F N and antipyretic mediators such as A V P, M S H, C R F in C NS and I L 10, ghrelin, glucocorticoids in the periphery leads to suppression of exogenous and endogenous pyrogens that acts on hypothalamus involves in cold-sensitive neurons and decreases temperature set point leading to an antipyretic response. • The inflammation that initiates endogenous pyrogens acts on liver and metabolic responses leads to acute phase response. • The infection initiates the exogenous pyrogens that are bacterial L P S from the activation of macrophages and other phagocytes and P G E 2 which acts on the hypothalamus and increases the P G E G and other endogenous pyrogens, warm-sensitive neurons and elevate temperature set point leading to a febrile response. • The acute phase response initiates an increase in C reactive protein, fibrinogen, ferritin, and other proteins; decreases albumin, transferrin, transthyretin; increases circulating neutrophils. • Febrile response initiates elevated temperature; immunologic response (B lymphocyte proliferation); endocrine response (increased catecholamine levels); physiologic response for example peripheral vasoconstriction, shivering, anorexia, somnolence; behavioral responses (curling up, putting on warm clothes). • Antipyretic responses initiate decreased temperature; physiologic responses for example peripheral vasodilation, muscle relaxation, alertness, sweating, and increase appetite; behavioral response (discarding covers and clothes).
An acute phase response (see Chapter 7) is a defensive reaction to control pathogens that occurs when pyrogenic and other cytokines are released in response to infection and inflammation. Protective proteins such as C-reactive protein, fibrinogen, and ferritin are produced. Other proteins such as albumin, transferrin, and transthyretin are reduced. Neutrophils numbers are increased. In addition to fever, other symptoms occur, including anorexia, fatigue, malaise, somnolence, and loss of concentration. At the cellular level, inflammatory pyrogenic cytokines promote muscle catabolism and hyperglycemia (gluconeogenesis, glycogenolysis, and insulin resistance) by stimulating release of adrenocorticotropic hormone (ACTH) and glucocorticoids to support glucose-consuming cells.46
During inflammation and fever, cryogens modulate the duration and intensity of the febrile response. Antipyretic cytokines such as arginine vasopressin (AVP), melanocyte-stimulating hormone (MSH), and corticotropin-releasing factor (CRF) are released from the brain, and systemic anti-inflammatory mediators (e.g., IL-10, ghrelin, glucocorticoids) are released from the periphery. These mediators act as endogenous cryogens or antipyretics to decrease the thermal set point in the hypothalamus (see Fig. 16.6).47 This antipyretic effect constitutes a negative-feedback loop for temperature control and prevents lethal effects of uncontrolled temperature elevation. When this antipyretic effect is ineffective or the physiologic response to fever is dangerous to organ system function, antipyretic medications (e.g., aspirin, acetaminophen, or ibuprofen) may be given to suppress PGE2 and fever.
Febrile seizures may occur in children with temperatures greater than 38°C (100.4°F) without CNS infection, hypoglycemia, or electrolyte disorders. The seizures are caused by systemic infection with the release of inflammatory cytokines that cross the blood-brain barrier and stimulate neuronal hyperexcitability, triggering the seizure.48 Febrile seizures are more predominant in boys before age 5 years, and genetic factors contribute to susceptibility. Simple febrile seizures are generally brief and self-limiting, lasting less than 5 minutes and without recurrence. Complex febrile seizures last more than 15 minutes with focal neurologic signs (e.g., one side of body involved, stiff neck) and recur within 24 hours. Although in most instances there appear to be no long-term effects on the child, a small percentage of children may develop epilepsy.49 Prolonged febrile seizures are associated with the development of temporal lobe epilepsy in children and are probably associated with functional changes in neurons and neural networks. Treatment includes antipyretic and/or antiepileptic drugs.50
Fever of unknown origin (FUO) is a body temperature of greater than 38.3°C (101°F) for longer than 3 weeks’ duration that remains undiagnosed after 3 days of hospital investigation, three outpatient visits, or 1 week of ambulatory investigation. The clinical categories of FUO include infectious, rheumatic/inflammatory, neoplastic, HIV-associated, and miscellaneous and undiagnosed disorders.51
Benefits of Fever
Moderate fever helps the body respond to infectious processes through several mechanisms.52,53
- 1. Raising of body temperature kills many pathogens and adversely affects their growth and replication.
- 2. Higher body temperatures decrease serum levels of iron, zinc, and copper—minerals needed for bacterial replication.
- 3. Increased temperature causes lysosomal breakdown and autodestruction of cells, preventing viral replication in infected cells.
- 4. Heat increases lymphocytic transformation and motility of polymorphonuclear neutrophils, facilitating the immune response.
- 5. Phagocytosis is enhanced, and production of antiviral interferon is augmented.
Suppression of fever with antipyrogenic medications can be effective but should be used with caution.54
Infection and fever responses in older adult persons and children may vary from those in normal adults. Box 16.2 lists the principal features associated with fever at these extremes of age.45,55
Disorders of Temperature Regulation
Hyperthermia
Hyperthermia is elevation of the body temperature without an increase in the hypothalamic set point. Hyperthermia can produce nerve damage, coagulation of cell proteins, and death. At 41°C (105.8°F), nerve damage produces convulsions in the adult. Death results at 43°C (109.4°F). Hyperthermia may be therapeutic, accidental, or associated with stroke or head trauma. Prevention of hyperthermia in stroke and head trauma assists in limiting brain injury.56
Therapeutic hyperthermia is a form of local, regional, or whole-body hyperthermia used to destroy pathologic microorganisms or tumor cells by facilitating the host's natural immune process or tumor blood flow or activate drugs.57,58
The forms of accidental hyperthermia are summarized as follows.59
- 1. Heat cramps—severe, spasmodic cramps in the abdomen and extremities that follow prolonged sweating and associated sodium loss. Usually occur in those not accustomed to heat or those performing strenuous work in very warm climates. Fever, rapid pulse rate, and increased blood pressure accompany the cramps. Treatment includes administration of oral dilute salt solutions.
- 2. Heat exhaustion—results from prolonged high core or environmental temperatures that cause profound vasodilation and profuse sweating, leading to dehydration, decreased plasma volumes, hypotension, decreased cardiac output, and tachycardia. Symptoms include weakness, dizziness, confusion, nausea, and fainting. Treatment includes administration of oral or parenteral dilute salt solution.
- 3. Heat stroke—a potentially lethal condition associated with multiorgan failure. Heat stroke can be caused by exertion, by overexposure to environmental heat, or from impaired physiologic mechanisms for heat loss. With very high core temperatures (>40°C [104°F]), there is cell injury and loss of body heat regulation. Symptoms include high core temperature, absence of sweating, rapid pulse rate, confusion, agitation, and coma. The increase in skin blood flow requirements decreases intestinal blood flow and increases intestinal membrane permeability. Increased circulating endotoxin, release of inflammatory mediators, and hypovolemia promote coagulation with microvascular thrombosis leading to multiorgan system and neuronal dysfunction. Complications include cerebral edema, degeneration of the CNS, swollen dendrites, renal tubular necrosis, and hepatic failure with delirium, coma, and eventually death if treatment is not undertaken. There is controversy about whether there is failure of the hypothalamic thermoregulatory center during the acute phase of recovery.60,61
- 4. Malignant hyperthermia—a potentially lethal hypermetabolic complication of a rare autosomal dominant inherited muscle disorder that may be triggered by inhaled halogenated anesthetics and depolarizing muscle relaxants.62 The syndrome involves uncontrolled release of calcium from muscle cells with hypermetabolism, uncoordinated muscle contractions, increased muscle work, increased oxygen consumption, hypercarbia, and a raised level of lactic acid production. Acidosis develops, and body temperature rises, with resulting tachypnea, tachycardia, cardiac dysrhythmias, hypotension, decreased cardiac output, and cardiac arrest. Signs resemble those of coma—unconsciousness, absent reflexes, fixed pupils, apnea, and occasionally a flat electroencephalogram. Oliguria and anuria are common. It is most common in children and adolescents. Treatment includes withdrawal of the provoking agent, oxygen therapy, body cooling therapy, administration of drugs that inhibit calcium release from muscle (dantrolene), treatment of arrhythmias, and maintenance of urine output.
Hypothermia
Hypothermia (core body temperature less than 35°C [95°F]) produces depression of the CNS and respiratory system, vasoconstriction, alterations in microcirculation and coagulation, and ischemic tissue damage. Hypothermia may be accidental or therapeutic (Box 16.3). Most tissues can tolerate low temperatures in controlled situations, such as surgery. However, in severe hypothermia, ice crystals form on the inside of the cell, causing cells to rupture and die. Tissue hypothermia slows cell metabolism, increases the blood viscosity, slows microcirculatory blood flow, facilitates blood coagulation, and stimulates profound vasoconstriction (also see Frostbite, Chapter 46).
Trauma and Temperature
Major body trauma can affect temperature regulation through various mechanisms. Damage to the CNS, release of inflammatory mediators, increased intracranial pressure, or intracranial bleeding typically produces a body temperature of greater than 39°C (102.2°F), generally higher than infectious fever. This sustained noninfectious fever, often referred to as a central fever or neurogenic fever, appears with or without bradycardia. A central fever does not induce sweating and is very resistant to antipyretic therapy.63 Other traumatic mechanisms that produce temperature alterations include accidental injuries, hemorrhagic shock, major surgery, and thermal burns. The severity and type of alteration (hyperthermia or hypothermia) vary with the severity of the cause and the body system affected.
Accidental Hypothermia Accidental hypothermia is generally the result of sudden immersion in cold water or prolonged exposure to cold environments. At particular risk for accidental hypothermia are infants and older adults, because thermoregulatory mechanisms are immature or altered in these two groups. Also at risk are individuals with conditions that diminish the ability to generate heat. Such conditions include hypothyroidism, hypopituitarism, decreased liver function, malnutrition, Parkinson disease, and rheumatoid arthritis. Other risk factors include chronic increased vasodilation and decreased thermoregulatory control caused by cerebral injuries, ketoacidosis, uremia, and drug overdoses. In acute hypothermia, peripheral vasoconstriction shunts blood away from the cooler skin to the core in an effort to decrease heat loss, which produces peripheral tissue ischemia. Intermittent reperfusion of the extremities (the Lewis phenomenon) helps preserve peripheral oxygenation. Intermittent peripheral perfusion continues until core temperatures drop dramatically.The hypothalamic center stimulates shivering in an effort to increase heat production. Severe shivering occurs at core temperatures of 35°C (95°F) and continues until core temperature (measure by esophageal probe) drops to about 30° to 32°C (86° to 89.6°F). Prolonged shivering can lead to exhaustion of liver glycogen stores. Thinking becomes sluggish and coordination is decreased at 34°C (93.2°F). As hypothermia deepens, paradoxical undressing may occur as hypothalamic control of vasoconstriction is lost and vasodilation occurs with loss of core heat to the periphery. The hypothermic individual therefore feels suddenly warm and begins to remove clothing.At 30°C (86°F), the individual becomes stuporous, heart rate and respiratory rate decline, and cardiac output is diminished. Cerebral blood flow is decreased. Metabolic rate declines, further decreasing core temperature. Sinus node depression occurs with slowing of conduction through the atrioventricular node. In severe hypothermia (core temperature of 26° to 28°C [78.8° to 82.4°F]), pulse and respirations may be undetectable and require resuscitation. Acidosis is moderate to severe. Coagulopathy, ventricular fibrillation, and asystole are common.117 Surface cooling may cause frostbite and fat necrosis.If hypothermia is mild, passive rewarming may be sufficient. If core temperature is greater than 30°C (86°F), active rewarming also may be required. Active rewarming uses warm-water baths, warm blankets, heating pads, and warm oral fluids when the individual is fully alert. Core rewarming may be accomplished through administration of warm intravenous (IV) solutions, warm gastric lavage, warm peritoneal lavage, inhalation of warmed gases, and, in extreme cases, exchange transfusions, warming blood in a pump oxygenator circuit, and mediastinal lavage.Rewarming generally should proceed no faster than a few degrees per hour. Short-term complications of rewarming include acidosis, rewarming shock, and dysrhythmias. Long-term complications include congestive heart failure, hepatic and renal failure, abnormal erythropoiesis, myocardial infarction, pancreatitis, and neurologic dysfunctions.
Therapeutic Hypothermia Therapeutic hypothermia is used to slow metabolism and preserve ischemic tissue after brain trauma or during brain surgery, after cardiac arrest, and in neonatal hypoxic encephalopathy. Hypothermia protects the brain by reduction in metabolic rate, ATP consumption, oxidative stress, and the critical threshold for oxygen delivery; modulation of excitotoxic neurotransmitters and calcium antagonism; preservation of protein synthesis and the blood-brain barrier; decreased edema formation; and modulation of the inflammatory response.
Sleep
Sleep is an active multiphase process that provides restorative functions and promotes memory consolidation. Complex neural circuits, interacting hormones, and neurotransmitters involving the hypothalamus, thalamus, brainstem, and cortex control the timing of the sleep-wake cycle and coordinate this cycle with circadian rhythms (24-hour rhythm cycles).64 Normal sleep has two primary phases that can be documented by electroencephalogram (EEG), a test that detects electrical activity in your brain: rapid eye movement (REM) sleep (20% to 25% of sleep time) and slow-wave (non-REM) sleep. Non-REM sleep is further divided into three stages (N1, N2, N3) from light to deep sleep. REM cycles do not typically start to occur until about 90 minutes into sleep. Four to six cycles of REM and non-REM sleep occur each night in an adult. Sleep duration and sleep architecture do not mature in children until after adolescence.65 The hypothalamus is a major sleep center, and the hypocretins (orexins), acetylcholine, and glutamate are neuropeptides secreted by the hypothalamus that promote wakefulness. Prostaglandin D2, adenosine, melatonin, serotonin, L-tryptophan, GABA, and growth factors promote sleep. The pontine reticular formation is primarily responsible for generating REM sleep, and projections from the thalamocortical network produce non-REM sleep.66
Rapid eye movement (REM) sleep is initiated by REM-on and REM-off neurons in the pons and mesencephalon. REM sleep occurs about every 90 minutes beginning 1 to 2 hours after non-REM sleep begins. This sleep is known as paradoxical sleep because the EEG pattern is similar to that of the normal awake pattern and the brain is very active with dreaming. REM and non-REM sleep alternate throughout the night, with lengthening intervals of REM sleep and fewer intervals of deeper stages of non-REM sleep toward morning. The changes associated with REM sleep include increased parasympathetic activity and variable sympathetic activity associated with rapid eye movement; muscle relaxation; loss of temperature regulation; altered heart rate, blood pressure, and respiration; penile erection in men and clitoral engorgement in women; release of steroids; and many memorable and often bizarre dreams. Respiratory control appears largely independent of metabolic requirements and oxygen variation. Loss of normal voluntary muscle control in the tongue and upper pharynx may produce respiratory obstruction which, in turn, can precipitate apneic events. Cerebral blood flow increases.
Non-REM sleep accounts for 75% to 80% of sleep time in adults and is initiated when inhibitory signals are released from the hypothalamus. Sympathetic tone is decreased and parasympathetic activity is increased during non-REM sleep, creating a state of reduced activity. The basal metabolic rate falls by 10% to 15%; temperature decreases 0.5°C to 1°C (0.9°F to 1.8°F); heart rate, respiration, blood pressure, and muscle tone decrease; and knee jerk reflexes are absent. Pupils are constricted. During the various stages, cerebral blood flow to the brain decreases and growth hormone is released, with corticosteroid and catecholamine levels depressed. Non-REM sleep is associated with memory consolidation during slow wave sleep.67Box 16.4 summarizes sleep characteristics in infants, children, and older adult persons.
Sleep Disorders
Because classification of sleep disorders is complex, a system has been established by the American Academy of Sleep Medicine and includes seven major categories: (1) insomnia, (2) sleep-related breathing disorders, (3) central disorders of hypersomnolence, (4) circadian rhythm sleep-wake disorders, (5) parasomnias, (6) sleep-related movement disorders, and (7) other sleep disorders.68 The most common disorders are summarized here.
Common Dyssomnias
Insomnia is the inability to fall or stay asleep; it is accompanied by fatigue, malaise, and difficulty with performance during wakefulness and may be mild, moderate, or severe. It may be transient, lasting a few days or months (primary insomnia), and related to travel across time zones or caused by acute stress, or very commonly inadequate “sleep hygiene.” Sleep hygiene simply refers to behavioral and environmental practices that are intended to promote better-quality sleep (e.g., avoiding all-nighters and caffeine late in the evening). Chronic insomnia lasts at least 3 months and can be idiopathic, start at an early age, and be associated with drug or alcohol abuse, chronic pain disorders, chronic depression, the use of certain drugs, obesity, aging, genetics, and environmental factors that result in hyperarousal.69
Obstructive sleep apnea syndrome (OSAS) is the most commonly diagnosed sleep disorder and occurs in all age groups. However, the incidence of OSAS increases with age beyond 60 years. Major risk factors include obesity, male sex, older age, and postmenopausal status (not on hormone therapy) in women, craniofacial anomalies, and increased size of tonsillar and adenoid tissue.70 OSAS results from partial or total upper airway obstruction to airflow recurring during sleep with continuous respiratory efforts made against a closed airway. It is often accompanied by excessive loud snoring, gasping, and multiple apneic episodes that last 10 seconds or longer. Central sleep apnea is the temporary absence or diminution of ventilatory effort during sleep with decreased sensitivity to carbon dioxide and oxygen tensions, and decreased airway dilator muscle activation. It may be associated with heart failure, neurologic disease, high altitude, or narcotic medications. Obesity hypoventilation syndrome is a combination of obesity (body mass index ≥ 30 kg·m-2), daytime hypercapnia (arterial carbon dioxide tension ≥ 45 mmHg), and sleep disordered breathing not caused by other disorders of hypoventilation. It may be related to leptin resistance because leptin also is a strong respiratory stimulant. The periodic breathing eventually produces arousal, which interrupts the sleep cycle, reducing total sleep time and producing sleep and REM deprivation. Sleep apnea produces hypercapnia and low oxygen saturation and if left untreated, eventually leads to polycythemia, pulmonary hypertension, systemic hypertension, stroke, right-sided congestive heart failure, dysrhythmias, liver congestion, cyanosis, and peripheral edema.
Hypersomnia (excessive daytime sleepiness) is associated with OSAS. Individuals may fall asleep while driving a car, working, or even while conversing, with significant safety concerns.71 Sleep deprivation also can result in impaired mood and cognitive function characterized by impairments of attention, episodic memory, working memory, and executive functions (i.e., decision-making ability).
Polysomnography and home sleep testing are used to diagnose OSAS, in addition to the history and physical examination. Treatments include use of continuous positive airway pressure (CPAP) and dental devices, surgery of the upper airway, hypoglossal nerve stimulation in selected individuals, and management of obesity.72 Adenotonsillar hypertrophy is the major cause of obstructive sleep apnea in children, and obesity increases the risk. Tonsillectomy with or without adenoidectomy is the treatment of choice.73
Narcolepsy is a primary hypersomnia with disruption in REM sleep-wake cycles characterized by hallucinations, sleep paralysis, excessive daytime sleepiness, and, rarely, cataplexy (brief spells of muscle weakness). Narcolepsy is usually sporadic or can occur in families. Type I narcolepsy (narcolepsy with cataplexy) is associated with immune-mediated T-cell destruction of hypocretin (orexin)-secreting cells in the hypothalamus. Orexins stimulate wakefulness. Type II narcolepsy (narcolepsy without cataplexy) is less severe and associated with normal levels of orexins (hypocretins). The cause is unknown.74
Circadian rhythm sleep disorders are common disorders of the 24-hour sleep-wake schedule with disruption in the timing of sleep. They involve difficulty falling asleep, waking up during the sleep cycle, or waking up too early and being unable to fall back to sleep. These disorders can result from extrinsic causes, such as rapid time zone changes (or jet-lag syndrome), alternating the sleep schedule (rotating work shifts) involving 3 hours or more in sleep time, or changing the total sleep time from day to day. Common types of these disorders include advanced sleep phase disorder (early evening sleeping, e.g., 6:00 pm and early morning waking, e.g., 3:00 to 5:00 am), or delayed sleep phase disorder (late night sleeping, e.g., at 2:00 am and late morning or afternoon waking). A circadian rhythm sleep disorder known as shift work sleep disorder affects many shift workers who rotate or swing long shifts (such as nurses), particularly between the hours of 2200 (10 PM) and 0600 (6 AM). Jet lag disorder is a disturbance in the circadian rhythm from crossing time zones more rapidly than the circadian system can keep pace. Eastward travel is more difficult than westward travel because it is easier to delay sleep than to advance sleep.
The disruption of circadian rhythms may cause problems in the short term, such as cognitive deficits, poor vigilance, difficulty concentrating, and inadequate performance of psychomotor tasks. However, long-term health consequences of shift work sleep disorder may be quite serious and include depression/anxiety, increased risk for cardiovascular disease, and increased all-cause mortality. Sleep cycle phenotype also has a genetic basis and influences the timing and cycles of sleep and can affect advances or delays in sleep-wake times (see Emerging Science Box: Shift Work Disorder).75
Common Parasomnias
Parasomnias are unusual behaviors occurring during non-REM stage 3 (slow-wave) sleep (disorders of arousal) and REM-related sleep behavior disorder.76 Non-REM sleep behaviors are associated with an inability to maintain deep sleep and an increased number of arousals. Behaviors include sleepwalking, having night terrors, rearranging furniture, eating food, exhibiting sleep sex or violent behavior, and having restless leg syndrome. REM sleep behavior disorder (RBD) is manifested by loss of REM paralysis, leading to potentially injurious dream enactment. Nonmotor symptoms are nonspecific and include olfactory dysfunction, abnormal color vision, autonomic dysfunction, excessive daytime sleepiness, depression, and cognitive impairment. RBD is a common prodromal non-motor manifestation of Parkinson disease.77
Two dysfunctions of sleep (somnambulism and night terrors) are common in children and may be related to CNS immaturity. Somnambulism (sleepwalking) is a non-REM parasomnia disorder primarily of childhood and appears to resolve within a few years. Sleepwalking is therefore not associated with dreaming, and the child has no memory of the event on awakening. Sleepwalking in adults is often associated with sleep-disordered breathing. Night terrors are characterized by sudden apparent arousals in which the child expresses intense fear or emotion. However, the child is not awake and can be difficult to arouse. Once awakened, the child has no memory of the night terror event. Night terrors are not associated with dreams. Although this problem occurs most often in children, adults also may experience it with corresponding daytime anxiety.
Restless Leg Syndrome
Restless legs syndrome (RLS)/Willis Ekbom disease is a common sensorimotor disorder associated with unpleasant sensations (prickling, tingling, crawling) and nonvolitional periodic leg movements that occurs at rest and is worse in the evening or at night. There is a compelling urge to move the legs for relief, with a significant effect on sleep and quality of life. The disorder is more common in women, during pregnancy, in older adults, and in individuals with iron deficiency. RLS has a familial tendency, although no monogenetic cause has been found. RLS is associated with a circadian fluctuation of dopamine in the substantia nigra. Iron is a cofactor in dopamine production, and some individuals respond to iron administration as well as low-dose dopamine agonists. Diagnostic and treatment guidelines have been established to assist with disease management.78
The Special Senses
Vision
The eyes are complex sense organs responsible for vision. Within a protective casing, each eye has receptors, a lens system for focusing light on the receptors, and a system of nerves for conducting impulses from the receptors to the brain. Visual dysfunction may be caused by abnormal ocular movements or alterations in visual acuity, refraction, color vision, or accommodation. Visual dysfunction also may be the secondary effect of another neurologic disorder.
The Eye
The wall of the eye consists of three layers: (1) sclera, (2) choroid, and (3) retina (Fig. 16.7). The sclera is the thick, white, outermost layer. It becomes transparent at the cornea—the portion of the sclera in the central anterior region that allows light to enter the eye. The choroid is the deeply pigmented middle layer that prevents light from scattering inside the eye. The iris, part of the choroid, has a round opening, the pupil, through which light passes. Smooth muscle fibers control the size of the pupil so that it adjusts to bright light or dim light and to close or distant vision.
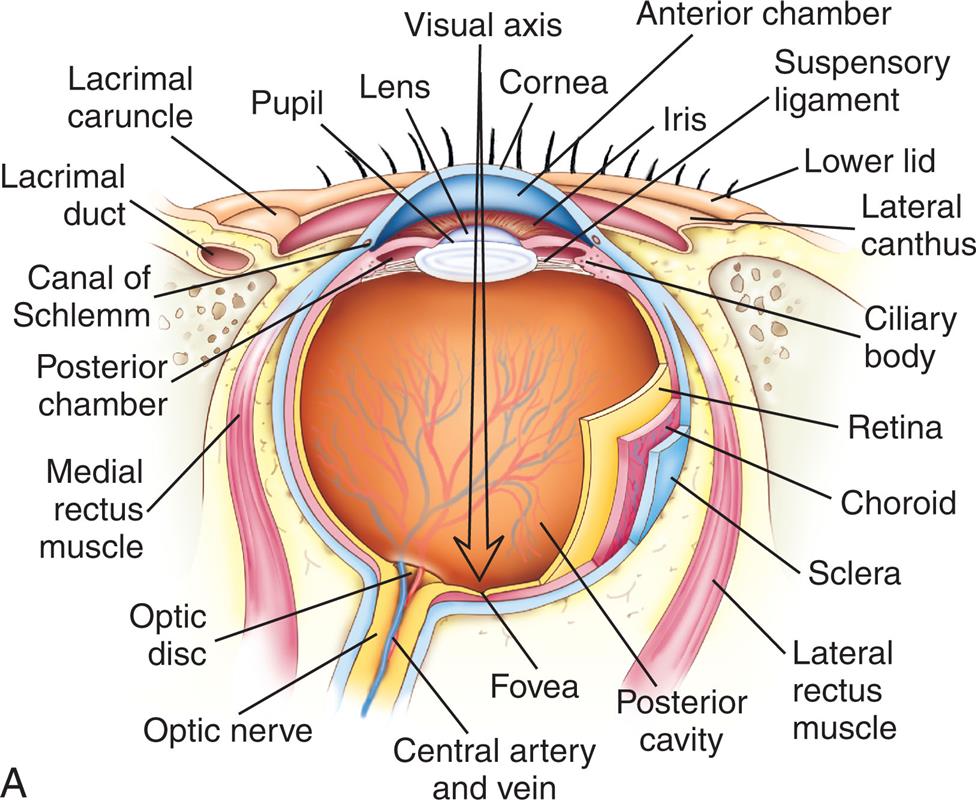
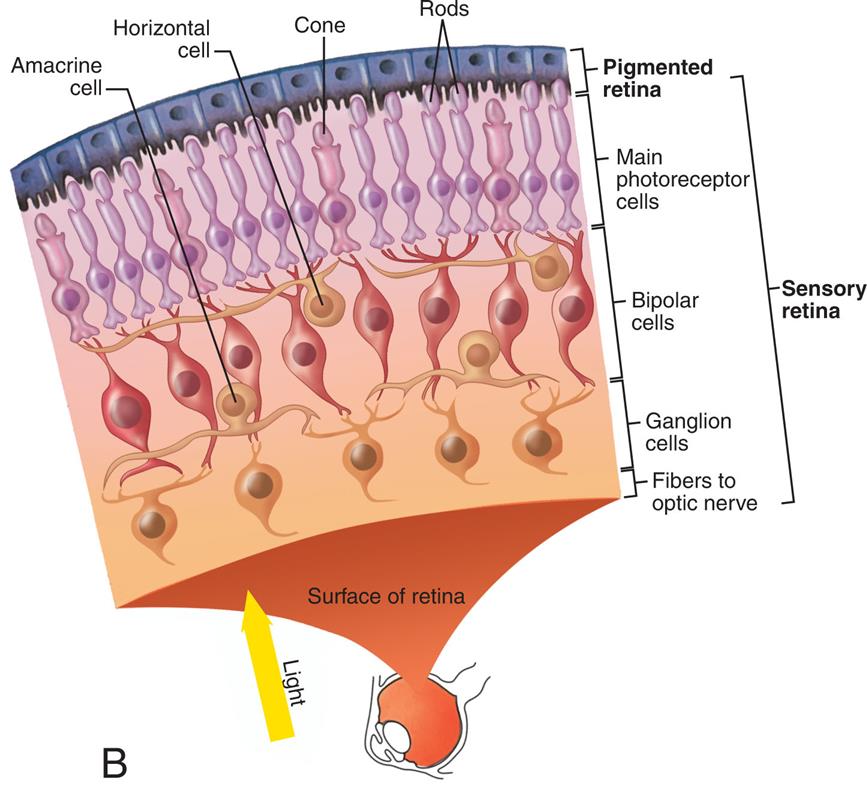
Illustration A depicts the cross-section of the eye with its internal structure labeled clockwise from the left: Visual axis, cornea, anterior chamber, suspensory ligament, lower lid, lateral canthus, ciliary body, retina, choroid, sclera, lateral rectus muscle, posterior cavity, fovea, central artery and vein, optic nerve, optic disc, medial rectus muscle, posterior chamber, the canal of Schlemm, lacrimal duct, lacrimal caruncle, pupil, and lens. Illustration B depicts the enlarged view of the retina with its structure labeled: Pigmented retina consisting of rods and cone; sensory retina consisting of main photoreceptor cells, bipolar cells, ganglion cells, fibers to the optic nerve, amacrine cell, and horizontal cell main.
The retina is the innermost layer of the eye and contains millions of rods and cones—special photoreceptors that convert light energy into nerve impulses (see Fig. 16.7B). Rods mediate peripheral and dim light vision, do not mediate color, and are densest at the periphery. Cones are color and detail receptors, and densest in the center of the retina. There are no photoreceptors where the optic nerve leaves the eyeball; this creates the optic disc, or blind spot. Lateral to each optic disc is the macula lutea, the area of most distinct vision, and in the center is the fovea centralis, a tiny area that contains only cones and provides the greatest visual acuity (see Fig. 16.7).
Nerve impulses pass through the optic nerves (second cranial nerve) to the optic chiasm (Fig. 16.8). The nerves from the inner (nasal) halves of the retinas cross to the opposite side and join fibers from the outer (temporal) halves of the retinas to form the optic tracts (see Fig. 16.8). The fibers of the optic tracts synapse in the dorsal lateral geniculate nucleus and pass by way of the optic radiation (or geniculocalcarine tract) to the primary visual cortex in the occipital lobe of the brain. Some fibers terminate in the suprachiasmatic nucleus (SCN) of the hypothalamus (located above the optic chiasm) and are involved in circadian regulation of the sleep-wake cycle. Light entering the eye is focused on the retina by the lens—a flexible, biconvex, crystal-like structure. The flexibility of the lens allows a change in curvature with contraction of the ciliary muscles, called accommodation, and allows the eye to focus on objects at different distances. The eye has two segments divided by the lens: the anterior and posterior segments. The anterior segment has two chambers. The anterior chamber lies between the cornea and the iris; the posterior chamber lies between the iris and the lens. Aqueous humor fills the anterior segment and helps maintain pressure inside the eye, as well as provide nutrients to the lens and cornea. Aqueous humor is secreted by the ciliary processes in the posterior chamber, flows through the pupil into the anterior chamber and drains though the trabecular meshwork, and is absorbed by endothelial cells in the canal of Schlemm. It then passes into the venous circulation. If drainage is blocked, intraocular pressure increases, causing glaucoma. The posterior segment extends from the back of the lens to the retinae and is filled with a gel-like substance called vitreous humor that cannot regenerate. Vitreous humor maintains intraocular pressure and prevents the eyeball from collapsing inward.
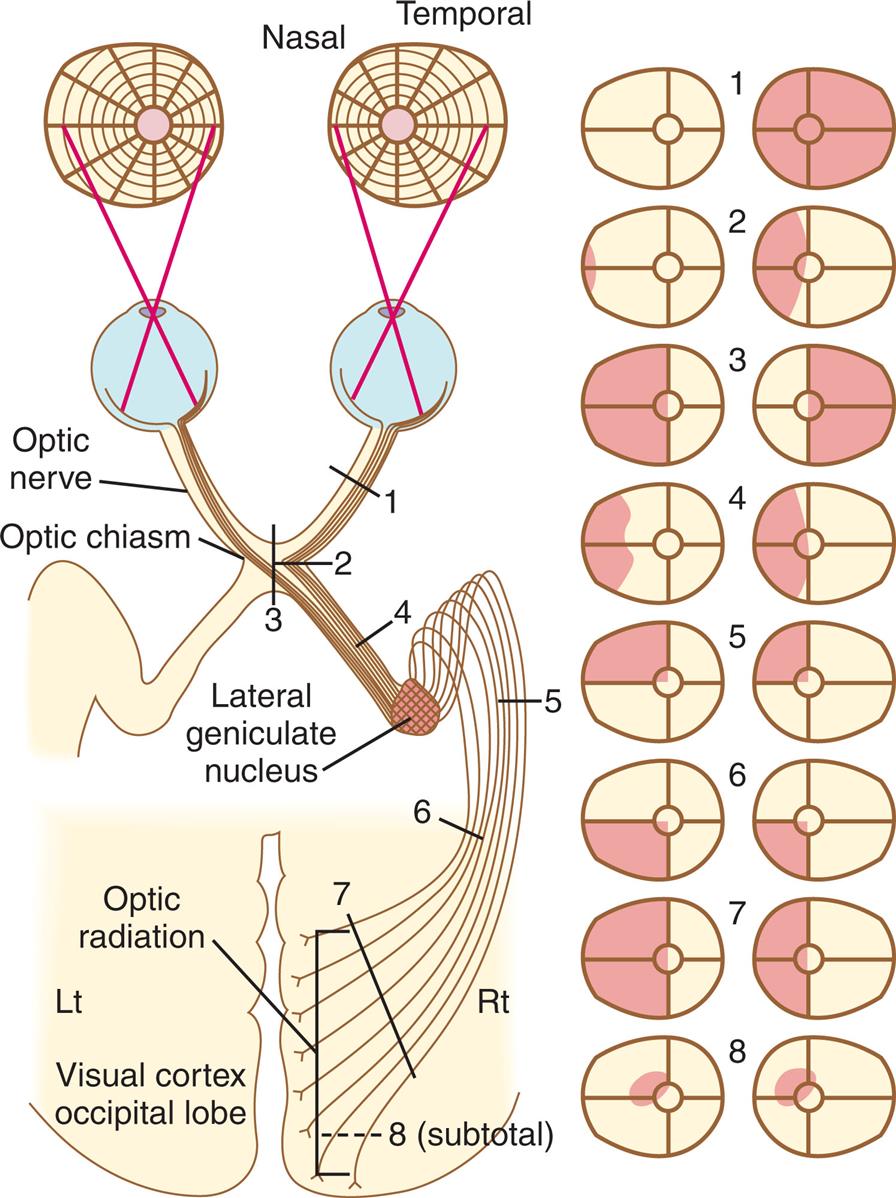
1, Optic nerve: blindness. 2, Lateral optic chiasm: grossly incongruous, incomplete (contralateral) homonymous hemianopsia. 3, Central optic chiasm: bitemporal hemianopsia. 4, Optic tract: incongruous, incomplete homonymous hemianopsia. 5, Temporal loop of the optic radiation: congruous partial or complete (contralateral) homonymous superior quadrantanopia. 6, Parietal (superior) projection of the optic radiation: congruous partial or complete homonymous inferior quadrantanopia. 7, Complete parieto-occipital interruption of the optic radiation: complete congruous homonymous hemianopsia with psychophysical shift of the foveal point, often sparing central vision and resulting in “macular sparing.” 8, Incomplete (subtotal) damage to the visual cortex: congruous homonymous scotomas, usually encroaching at least acutely on central vision. (From Goldman C, ed. Goldman’s Cecil Medicine. 24th ed. Vol 2. Philadelphia: Saunders; 2012.)
Two illustrations depict the visual field deficits produced by lesions at various points along the visual pathway. The illustration on the left depicts the visual pathway and the lesions at various points numbered from 1 through 8. The labels on the visual pathways are optic nerve, optic chiasm, lateral geniculate nucleus, optic radiation, and visual cortex occipital lobe. The illustration on the right depicts the eight visual pathway lesions.
Blood supply to the eye is provided by branches of the ophthalmic artery. The ciliary artery and its branches provide blood to the anterior eye and the layers of the eye wall. The central retinal artery provides blood to the inner retinal surface, and the choroid supplies nutrients to the outer surface of the retina. Six extrinsic eye muscles allow gross eye movements and permit eyes to follow a moving object (Fig. 16.9).
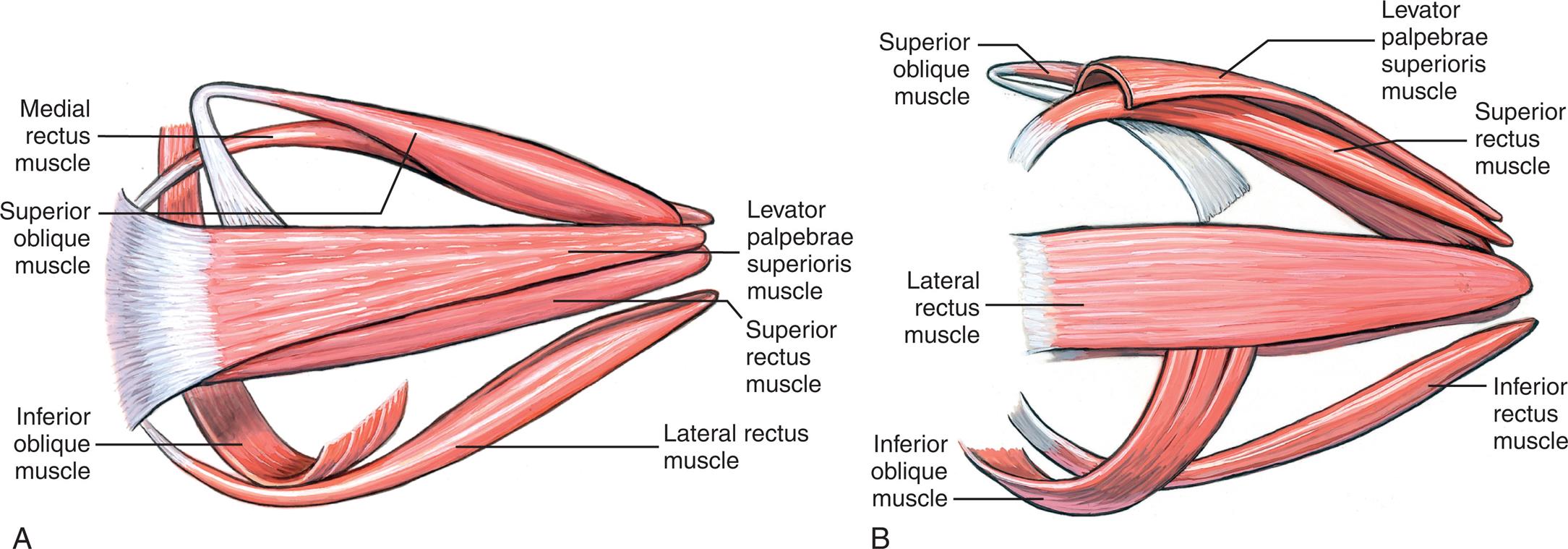
(A) Superior view. (B) Lateral view. (From Dutton JJ. Atlas of Clinical and Surgical Orbital Anatomy. 2nd ed. Philadelphia: Saunders; 2011.)
Illustration A depicts the superior view of the extrinsic muscle of the right eye with labels as follows. Medial rectus muscle, superior oblique muscle, inferior oblique muscle, levator palpebrae muscle, superior rectus muscle, and the lateral rectus muscle. Illustration B depicts the lateral view of the extrinsic muscle of the right eye with labels as follows. Superior oblique muscle, lateral rectus muscle, inferior oblique muscle, levator palpebrae superioris muscle, superior rectus muscle, and inferior rectus muscle.
Visual Dysfunction
Alterations in ocular movements
Abnormal ocular movements result from oculomotor, trochlear, or abducens cranial nerve dysfunction (see Table 15.6). The three types of eye movement disorders are (1) strabismus, (2) nystagmus, and (3) paralysis of individual extraocular muscles.
In strabismus, one eye deviates from the other when the person is looking at an object. This is caused by a weak or hypertonic muscle in one eye. The deviation may be upward, downward, inward (esotropia), or outward (exotropia). Strabismus in children requires early intervention to prevent amblyopia (reduced vision in the affected eye caused by cerebral blockage of the visual stimuli). The primary symptom of strabismus is diplopia (double vision). Causes include neuromuscular disorders of the eye muscle, diseases involving the cerebral hemispheres, or thyroid disease.
Nystagmus is an involuntary unilateral or bilateral rapid, rhythmic oscillatory movement of the eyes. It may be present at rest or when the eye moves. Pendular nystagmus is characterized by a regular back and forth movement of the eyes with only slow phases. In jerk nystagmus, one phase of the eye movement is faster than the other. Nystagmus may be caused by imbalanced reflex activity of the inner ear, vestibular nuclei, cerebellum, medial longitudinal fascicle, or nuclei of the oculomotor, trochlear, and abducens cranial nerves (see Table 15.6 and Fig. 15.25). Drugs, retinal disease, diseases involving the cervical cord, stroke syndromes, brain tumors, and brain trauma also may produce nystagmus.79
Paralysis or loss of neuromuscular coordination of specific extraocular muscles may cause limited abduction, abnormal closure of the eyelid, ptosis (drooping of the eyelid), or diplopia (double vision) as a result of unopposed muscle activity. Trauma or pressure in the area of the cranial nerves or diseases such as diabetes mellitus and myasthenia gravis also paralyze specific extraocular muscles.
Alterations in visual acuity
Visual acuity is the ability to see objects in sharp detail. With advancing age, the lens of the eye becomes less flexible and adjusts slowly, and there is altered refraction of light by the cornea and lens. Thus visual acuity declines with age. Table 16.6 contains a summary of changes in the eye caused by aging. Specific causes of visual acuity changes are (1) amblyopia, (2) scotoma (blind spot in visual field), (3) cataracts, (4) papilledema, (5) dark adaptation, (6) glaucoma, (7) retinal detachment, and (8) macular degeneration (Table 16.7).
Table 16.6
| Structure | Change | Consequence |
|---|---|---|
| Cornea | ||
| Anterior chamber | Decrease in size and volume caused by thickening of lens | Occasionally exerts pressure on Schlemm canal and may lead to increased intraocular pressure and glaucoma |
| Lens | Increase in opacity | Decrease in refraction with increased light scattering (blurring) and decreased color vision (green and blue); can lead to cataracts |
| Ciliary muscles | Reduction in pupil diameter, atrophy of radial dilation muscles | Persistent constriction (senile miosis); decrease in critical flicker frequencya |
| Retina | Reduction in number of rods at periphery, loss of rods and associated nerve cells | Increase in minimum amount of light necessary to see an object |
| Macula | Atrophy (age-related macular degeneration) | Loss of vision |
| Vitreous | Liquefaction of vitreous and decrease in gel volume | Posterior vitreous detachment causing “floaters;” risk for retinal detachment |
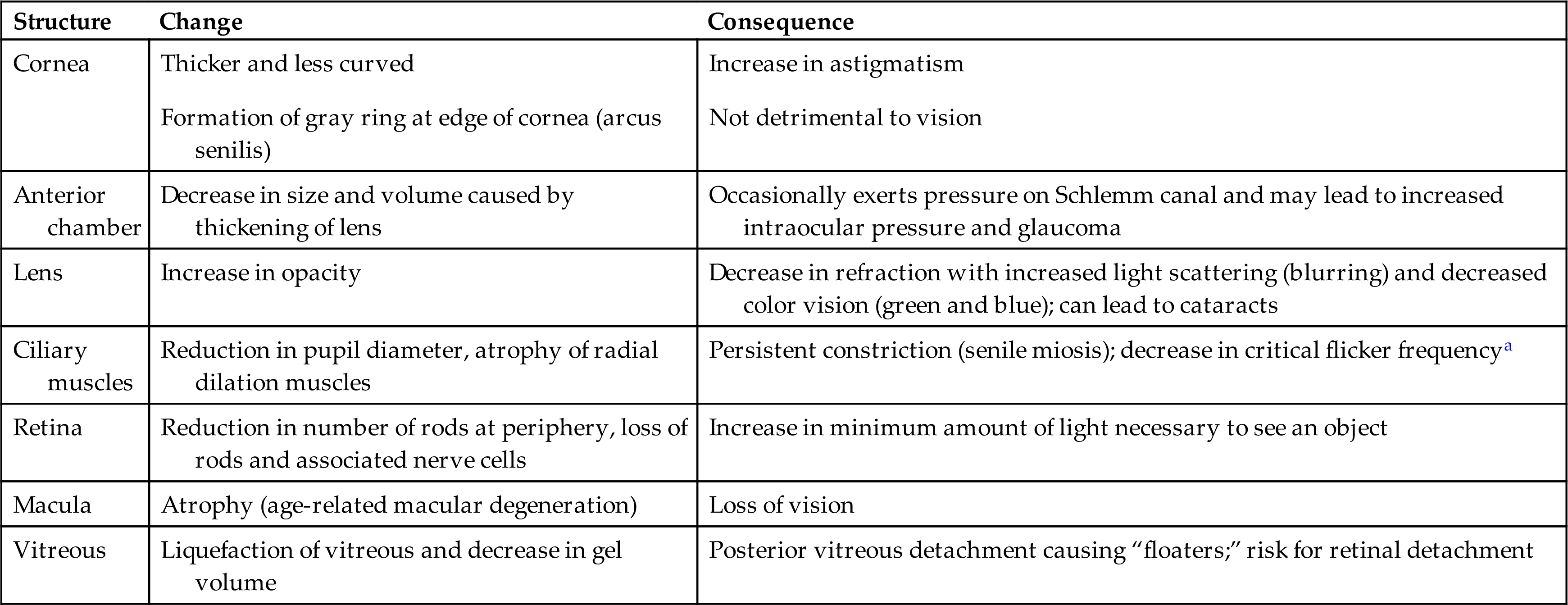
aThe rate at which consecutive visual stimuli can be presented and still be perceived as separate.
Table 16.7
A cataract is a cloudy or opaque area in the ocular lens and leads to visual loss when located on the visual axis (see Fig. 16.7). It is the leading cause of blindness in the world. The incidence of cataracts increases with age as lens proteins break down, leading to opacification. Cataracts develop because of alterations of metabolism and transport of nutrients within the lens. Although the most common form of cataract is degenerative, cataracts also may occur congenitally or as a result of infection, radiation, trauma, drugs, or diabetes mellitus. Cataracts cause decreased visual acuity, blurred vision, glare, and decreased color perception. Cataracts are treated by removal of the entire lens and replacement with an intraocular artificial lens.
Glaucomas are the second leading cause of blindness and are characterized by intraocular pressures greater than 12 to 20 mm Hg with death of retinal ganglion cells and their axons and irreversible loss of central and sometimes peripheral vision. There is a strong genetic tendency with higher prevalence among Black Americans. Chronic use of corticosteroids or drugs with anticholinergic properties can predispose to glaucoma. The two primary types of age-related glaucoma are differentiated by the configuration of the anterior chamber drainage angle and the location of where aqueous humor is obstructed from exiting the eye (Fig. 16.10).80
- 1. Open angle. This type of glaucoma is the most common and is characterized by outflow obstruction of aqueous humor at the trabecular meshwork or canal of Schlemm, even though there is adequate space for drainage. Often this is an inherited disease and is a leading cause of blindness with few preliminary symptoms. Obstruction results in high intraocular pressures with compression of the optic nerve head, which can cause blindness within hours or days. With normal tension glaucoma there may be neurodegeneration of the optic nerve head that leads to loss of vision without elevated intraocular pressures.
- 2. Angle closure or narrow angle. In this type of glaucoma there is displacement of the iris toward the cornea with obstruction of the trabecular meshwork and obstruction of outflow of aqueous humor from the anterior chamber; it may occur acutely with a sudden rise in intraocular pressure, causing pain and visual disturbances, and will need immediate treatment.
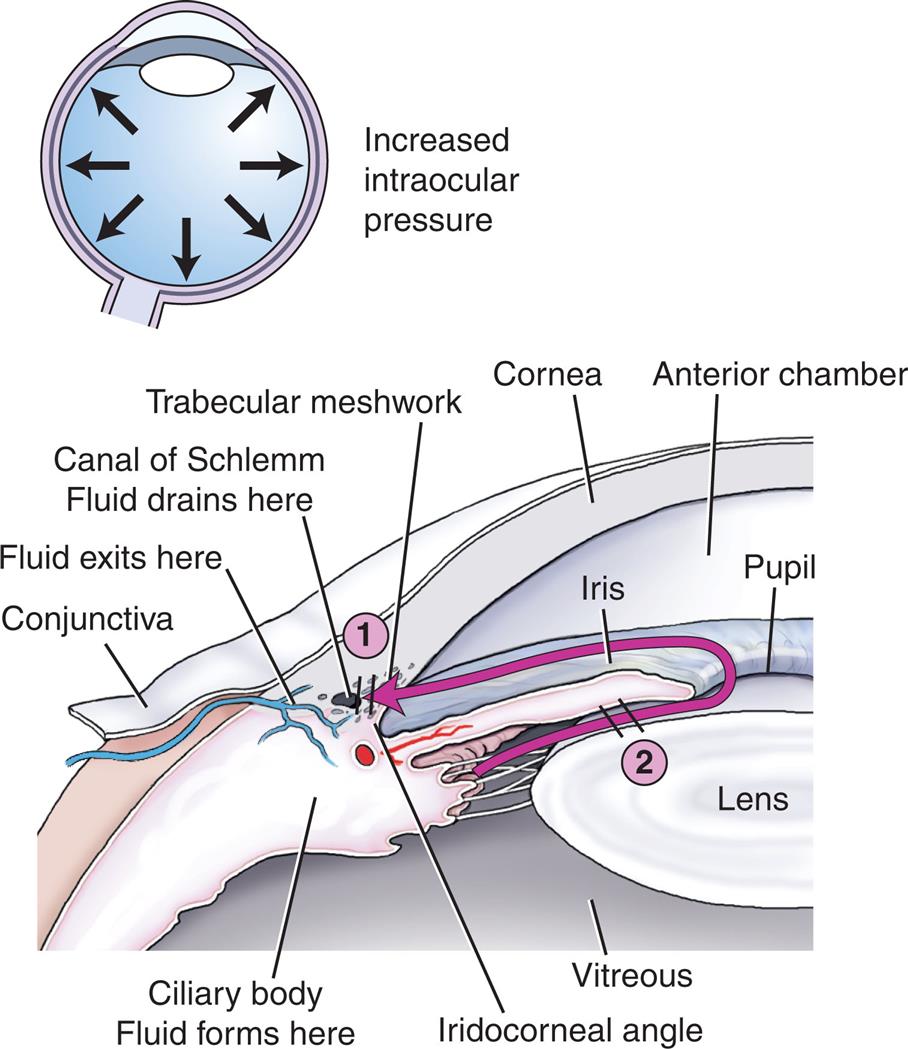
1, Open-angle glaucoma. The obstruction to aqueous humor flow lies in the trabecular meshwork near canal of Schlemm, 2, Closed-angle glaucoma. The iris presses against the lens, blocking aqueous flow into the anterior chamber.
The illustration on the top depicts the increased intraocular pressure. Illustration on the bottom depicts the section of the outer eye with the structures labeled: conjunctiva where fluid exists here, the canal of Schlemm where fluid drains here, trabecular meshwork, cornea, anterior chamber, ciliary body where fluid forms, vitreous, iridocorneal angle, iris, pupils, and lens. An arrow mark at trabecular meshwork indicates open-angle glaucoma and at the iris indicates closed-angle glaucoma.
Congenital closure glaucoma is a rare disease associated with congenital malformations and other genetic anomalies. There is a defect in the development of the drainage angle formed by the cornea and iris. Aqueous humor cannot flow out normally. The intraocular pressure increases and leads to optic nerve damage. The disease is usually diagnosed by one year of age.
Glaucoma is often treated with pharmaceutical eyedrops to reduce secretion or increase absorption of aqueous humor. Angle closure glaucoma is a medical emergency. Surgical therapies are available to open the spaces of the trabeculae to control intraocular pressure.
Age-related macular degeneration (AMD) is a severe and irreversible loss of vision and a major cause of blindness in older individuals. Hypertension, cigarette smoking, diabetes mellitus, and a family history of AMD are risk factors. The degeneration usually occurs after the age of 60 years. The cause of AMD is unknown and complex because of the many components of eye structure, genetic diversity, and variable environmental factors. There are two forms: dry or non-neovascular (atrophic) and wet or exudative (neovascular). The dry form is more common and is slowly progressive with accumulation of drusen (waste products from photoreceptors) in the macula, choriocapillary loss, accumulation of lipofuscin (a lysosomal pigmented residue) in the retinae, and atrophy, leading to permanent central vision loss. Early symptoms include limited night vision and difficulty reading. The wet form includes abnormal choroidal blood vessel growth within the macula (neovascularization), leakage of blood or serum, retinal tears or detachment, fibrovascular scarring, loss of photoreceptors, and more severe and rapid loss of central vision.81 There are no approved treatments for dry AMD. Daily high doses of vitamins C and E, beta-carotene, and the minerals zinc and copper—called the AREDS formulation—can help slow the progression of dry AMD.82 Treatment of wet AMD includes anti–vascular endothelial growth factor (anti-VEGF) intraocular injection to provide an anti-angiogenesis effect. New treatments are being evaluated including anti-VEGF antibodies, stem cell, and gene therapy.81
Retinal detachment is a common cause of visual impairment and blindness. Risk factors include retinal holes and vitreoretinal traction. Fluid (exudate, hemorrhage, or liquid vitreous) separates the retinal pigment epithelium from the photoreceptors in the neuroepithelium (see Fig. 16.7). The separation deprives the outer retina of oxygen and nutrients because the diffusion distance is increased. Communication is also disrupted between the pigment epithelium and photoreceptors. There is retinal degeneration and fibrosis with loss of vision. Rhegmatogenous retinal detachment (full-thickness retinal breaks caused by vitreoretinal traction) is the most common form of retinal detachment. Causes include intracapsular cataract extraction, severe myopia, age-related lattice degeneration, vitreoretinal traction, and trauma. Contraction of fibrous membranes can cause tractional separation of the retinal layers as occurs in proliferative diabetic retinopathy. Symptoms include dark floaters or squiggly lines in the field of vision; flashes of light; or a dark curtain or shadow on the sides or center of the visual field. Retinal detachment is a medical emergency. Treatment involves immediate surgical retinal reattachment.83
Alterations in accommodation
Accommodation refers to changes in the shape of the lens and allows for a change of focus from distant to near images. Accommodation is mediated through the oculomotor nerve. Pressure, inflammation, age, and disease of the oculomotor nerve may alter accommodation, causing diplopia, blurred vision, and headache.
Loss of accommodation with advancing age is termed presbyopia, a condition in which the ocular lens becomes larger, firmer, and less elastic. The major symptom is reduced near vision, causing the individual to hold reading material at arm's length. Treatment includes corrective forward, contact, and intraocular lenses, corneal inlays, or laser refractive surgery for monovision.84
Alterations in refraction
Alterations in refraction are the most common visual problem. Causes include irregularities of the corneal curvature, the focusing power of the lens, and the length of the eye. The major symptoms of refraction alterations are blurred vision and headache. Three types of refraction alterations are as follows (Fig. 16.11):
- Myopia—nearsightedness: The eyeball is lengthened, and light rays are focused in front of the retina when the person is looking at a distant object causing blurry vision. A concave lens is needed for correction. There is increased risk for retinal detachment, cataract formation, and glaucoma.
- Hyperopia—farsightedness: the axis of the eyeball is too short and light rays are focused behind the retina when a person is looking at a near object and vision is blurry. A convex lens is needed for correction.
- Astigmatism—unequal curvature of the cornea: Light rays enter the eye at two points and do not come to a single focus on the retina and cause blurry vision. Astigmatism may coexist with myopia, hyperopia, or presbyopia. Corrective lenses or laser surgery provide correction.
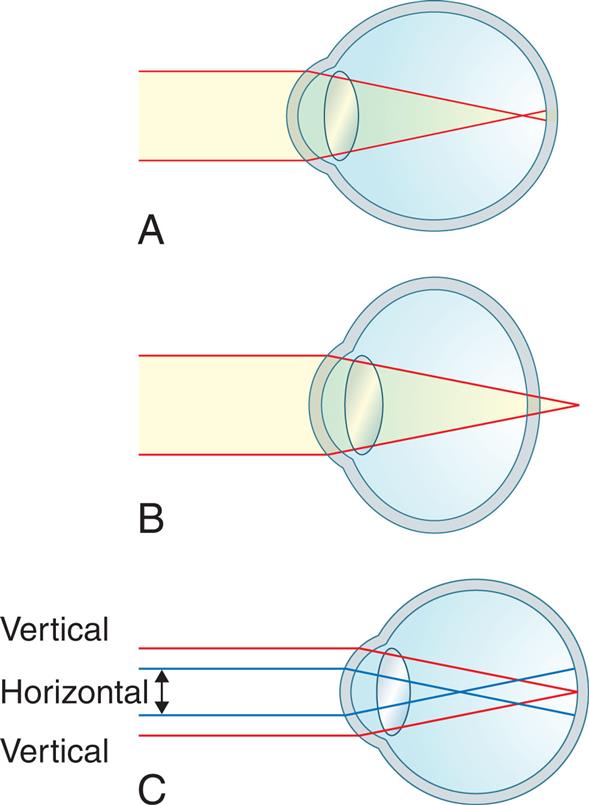
(A) Myopic eye. Parallel rays of light are brought to a focus in front of the retina. (B) Hyperopic eye. Parallel rays of light come to a focus behind the retina in the unaccommodative eye. (C) Simple myopic astigmatism. The vertical bundle of rays is focused on the retina; the horizontal rays are focused in front of the retina. (From Stein HA, et al. The Ophthalmic Assistant: A Text for Allied and Associated Ophthalmic Personnel. 9th ed. Philadelphia: Saunders; 2013.)
Three illustrations A through C depict the three types of refraction alterations. Illustration A shows parallel rays of light from a distant object focused in front of the retina. Illustration B shows the parallel rays of light from a distant object focused behind the retina. Illustration C shows the horizontal and vertical rays focused in front and on the retina respectively.
Alterations in color vision
Normal sensitivity to color diminishes with age because of the progressive yellowing of the lens that occurs with aging. All colors become less intense, although color discrimination for blue and green is greatly affected. Color vision deteriorates more rapidly for individuals with diabetes mellitus than for the general population because diabetic neuropathy can also affect photoreceptors.
Abnormal color vision also may be caused by color blindness and is an X-linked genetic trait.85 Congenital color blindness affects 6% to 8% of the male population and about 0.5% of the female population. Mutations in the genes coding for wavelength sensitivity of retinal cone photopigments leads to color blindness. Although many forms of color blindness exist, most commonly the affected individual cannot distinguish red from green rather than yellow from blue. In the most severe form (achromatopsia) individuals see only shades of gray, black, and white. Acquired color vision deficiency occurs with ocular, neurologic, or systemic disease.
Neurologic disorders causing visual dysfunction
Various neurologic disorders may cause visual dysfunction including stroke, traumatic brain injury, tumors, and neurodegenerative diseases. Vision may be disrupted at many points along the visual pathway, causing a variety of defects in fields of vision.86 Visual changes do not always cause defects or blindness in the entire visual field; hemianopia is the term that describes defective vision in half of a visual field. Fig. 16.8 illustrates areas along the visual pathway that may be damaged and the associated visual changes. Because of the anatomy of the optic nerves, injury to the optic nerve causes ipsilateral (same side) blindness but a normal contralateral (opposite side) visual field. Injury to the optic chiasm (the X-shaped crossing of the optic nerves), often caused by atherosclerotic ischemia or external compression from trauma or aneurysm, can cause a variety of defects, depending on the location of injury. These defects vary because at the optic chiasm, nerve fibers from the medial half of each retina separate from the lateral half and enter the opposite optic tract.
Because of the normal structure of the visual pathways, destruction of one optic tract causes homonymous hemianopsia (complete loss of vision in the inner half of one eye and the outer half of the other). Thus, if an injury to the left optic tract occurs, the individual is blind in the right eye’s medial (inner) field and the left eye’s lateral (outer) field. If the compression of the optic tract is asymmetric, an incongruous (or uneven) homonymous defect results. Injury to one optic radiation (an ocular pathway in the internal capsule, temporal lobe, or occipital lobe) also causes a homonymous (same field) defect. A major injury in the optic radiation causes homonymous hemianopsia. A lesser injury may cause an upper quadrant homonymous defect. Generally the defects are the same size in both eyes. When the homonymous hemianopsia is caused by an occipital lobe lesion, the area of hemianopsia is split. Although visual acuity may remain unimpaired, reading is difficult because of the inability to group words.
Papilledema is edema of the optic nerve at its point of entrance into the eyeball. Papilledema is caused by increased intracranial pressure (e.g., brain tumors, intracranial hemorrhage, hydrocephalus, or cerebral edema). The subarachnoid space of the brain is continuous with the optic nerve sheath. As cerebrospinal fluid (CSF) pressure increases, the pressure is transmitted to the optic nerve and the optic nerve sheath compresses the nerve and impedes axoplasmic transport. This leads to accumulation of axoplasmic substances at the level of the lamina cribrosa (a mesh-like structure in the sclera where the retinal nerves exit the eye and form the optic nerve), resulting in the characteristic swelling of the optic disc. Obliteration of the physiologic cup (a bright area normally located in the center of the optic disc) follows. Later the optic disc becomes raised above the level of the surrounding retina, and the margins become blurred and indistinct. With severe swelling, hemorrhage and patches of white exudate (caused by nerve infarcts) surround the disc margins. The edematous nerves compress the small retinal veins, causing venous stasis and engorgement. Headache is common and there may be no visual changes, blurred vision, or constriction of visual fields.87
External Eye Structure and Disorders
Protective external eye structures include the eyelids (palpebrae), conjunctivae, and lacrimal apparatus. The eyelids control the amount of light reaching the eyes, and the conjunctiva lines the eyelids. Tears released from the lacrimal apparatus bathe the surface of the eye and prevent friction, maintain hydration, and wash out foreign bodies and other irritants (Fig. 16.12).
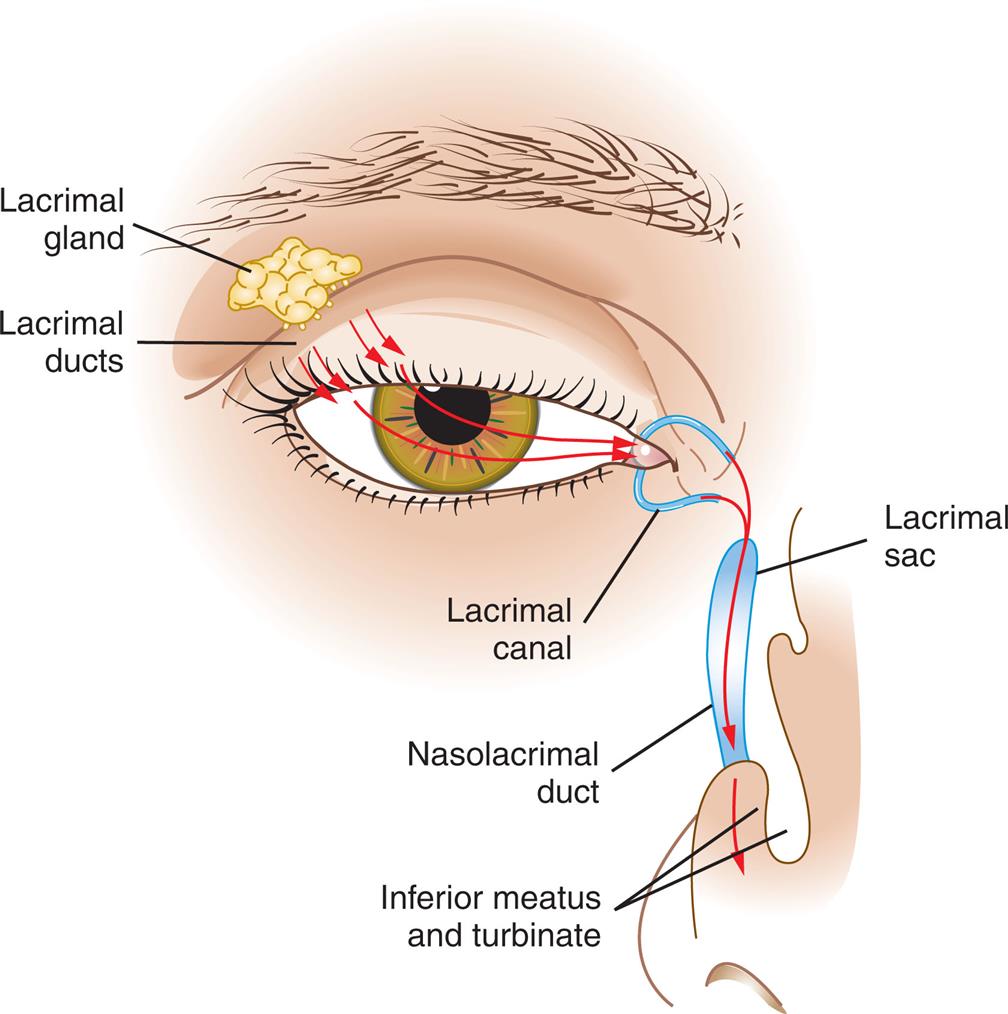
Fluid produced by lacrimal glands (tears) streams across the eye surface, enters the canals, and then passes through the nasolacrimal duct to enter the nose. (From Applegate E. The Anatomy and Physiology Learning System. 4th ed. St Louis: Saunders; 2011.)
An illustration of the anterior view of an eye depicts the structures labeled within the lacrimal apparatus. The labels are as follows. Lacrimal gland, lacrimal ducts, lacrimal canal, lacrimal sac, nasolacrimal duct, inferior meatus, and turbinate. The arrow marks indicate the pathway of tears from streaming across the eye surface to the nose through the lacrimal canal and nasolacrimal duct.
Infection and inflammatory responses are the most common conditions affecting the supporting structures of the eyes. Blepharitis is an inflammation of the eyelids caused by Staphylococcus or seborrheic dermatitis. A hordeolum (stye) is an infection (usually staphylococcal) of the sebaceous glands of the eyelids, usually centered near an eyelash. A chalazion is a noninfectious lipogranuloma of the Meibomian (oil-secreting) gland that often occurs in association with a hordeolum and appears as a deep nodule within the eyelid. These conditions present with redness, swelling, and tenderness and are treated symptomatically. Entropion is a common eyelid malposition in which the lid margin turns inward against the eyeball. In ectropion, the eyelid turns outward away from the eye. Trichiasis is abnormally positioned eyelashes that grow back toward the eye. There are both surgical and nonsurgical treatments to reposition the lid margin. There are both surgical and nonsurgical treatments to reposition the lid margin.
Conjunctivitis is an inflammation of the conjunctiva (mucous membrane covering the front part of the eyeball) caused by viruses (most common), bacteria, allergies, or chemical irritants. Conjunctivitis can be acute, recurrent, or chronic and can be associated with systemic disease.88
Acute bacterial conjunctivitis (pinkeye) is highly contagious and often caused by Staphylococcus, Haemophilus, Streptococcus pneumoniae, and Moraxella catarrhalis, although other bacteria may be involved (Fig. 16.13). In children younger than 6 years, Haemophilus infection often leads to otitis media (conjunctivitis-otitis syndrome). Bilateral matting of the eyelids, gluing of eyelashes on awakening, lack of itching, and no previous history of conjunctivitis are predictors of bacterial conjunctivitis and differentiate it from viral conjunctivitis. Preventing the spread of the microorganism with meticulous hand washing and use of separate towels is important. The disease is also treated with topical antibiotics.
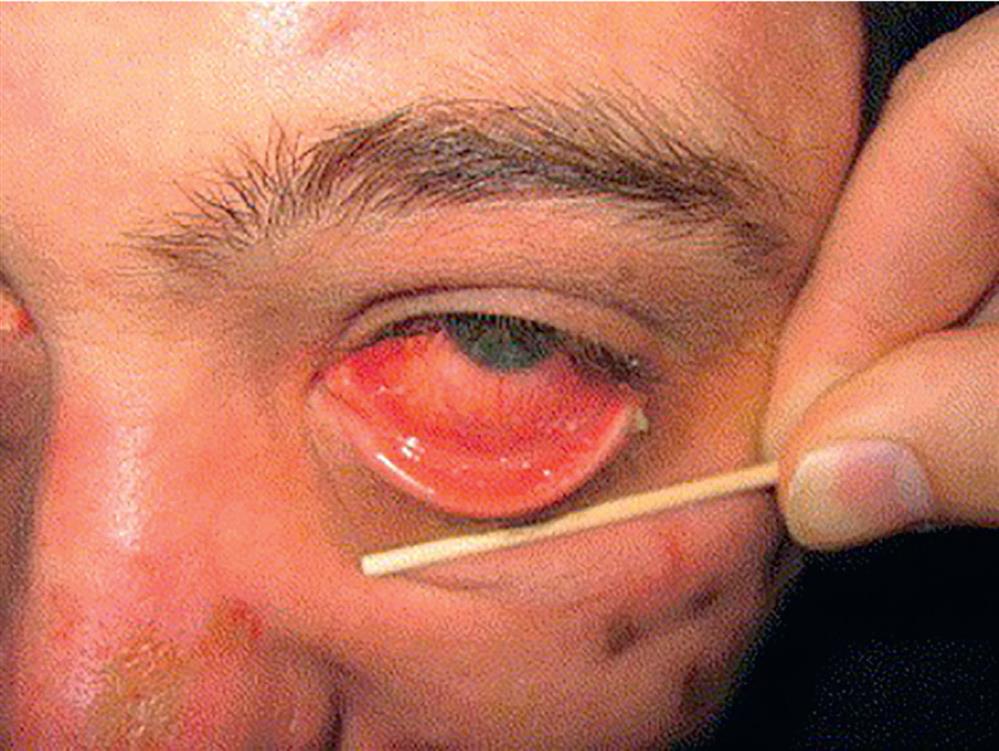
Staphylococcal conjunctivitis of the left eye with mild erythema and inflammatory edema of the eyelids. Purulent exudate can be seen at the lateral canthus. (From Durkin SR, et al. Recurrent staphylococcal conjunctivitis associated with facial impetigo contagiosa. Am J Ophthalmol. 2006;141(1):189–190. https://doi.org/10.1016/j.ajo.2005.07.079.)
Viral conjunctivitis is caused by an adenovirus and is very contagious. Symptoms include edema, watering, redness, petechial hemorrhages of the conjunctiva, and photophobia. Treatment is usually symptomatic and can include antihistamines and cold compresses.
Allergic conjunctivitis is associated with a variety of antigens, including pollens. Chronic conjunctivitis results from any persistent conjunctivitis lasting more than 4 weeks. Trachoma (chlamydial conjunctivitis) is caused by Chlamydia trachomatis and often is associated with poor hygiene and leads to corneal scarring. It is the leading cause of preventable blindness in the world and is treated with azithromycin.89Keratitis is an infection of the cornea caused by bacteria, viruses, fungus, or parasites. Bacterial infections can cause corneal ulceration, and type 1 herpes simplex virus can involve both the cornea and the conjunctiva. Acanthamoeba keratitis can occur from contact lens wear because of poor hygiene. Fungal infections are most common in tropical and subtropical climates. Severe ulcerations with residual scarring require corneal transplantation.90
Hearing
The Normal Ear
The ear is divided into three areas: (1) the external ear, involved only with hearing; (2) the middle ear, involved only with hearing; and (3) the inner ear, involved with both hearing and equilibrium.
The external ear is composed of the pinna (auricle), which is the visible portion of the ear, and the external auditory canal, a tube that leads to the middle ear (Fig. 16.14). The external auditory canal is surrounded by the bones of the cranium. The opening (meatus) of the canal is just above the mastoid process. The air-filled sinuses, called mastoid air cells, of the mastoid process promote conductivity of sound between the external and the middle ear. The tympanic membrane separates the external ear from the middle ear. Sound waves entering the external auditory canal hit the tympanic membrane (eardrum) and cause it to vibrate.
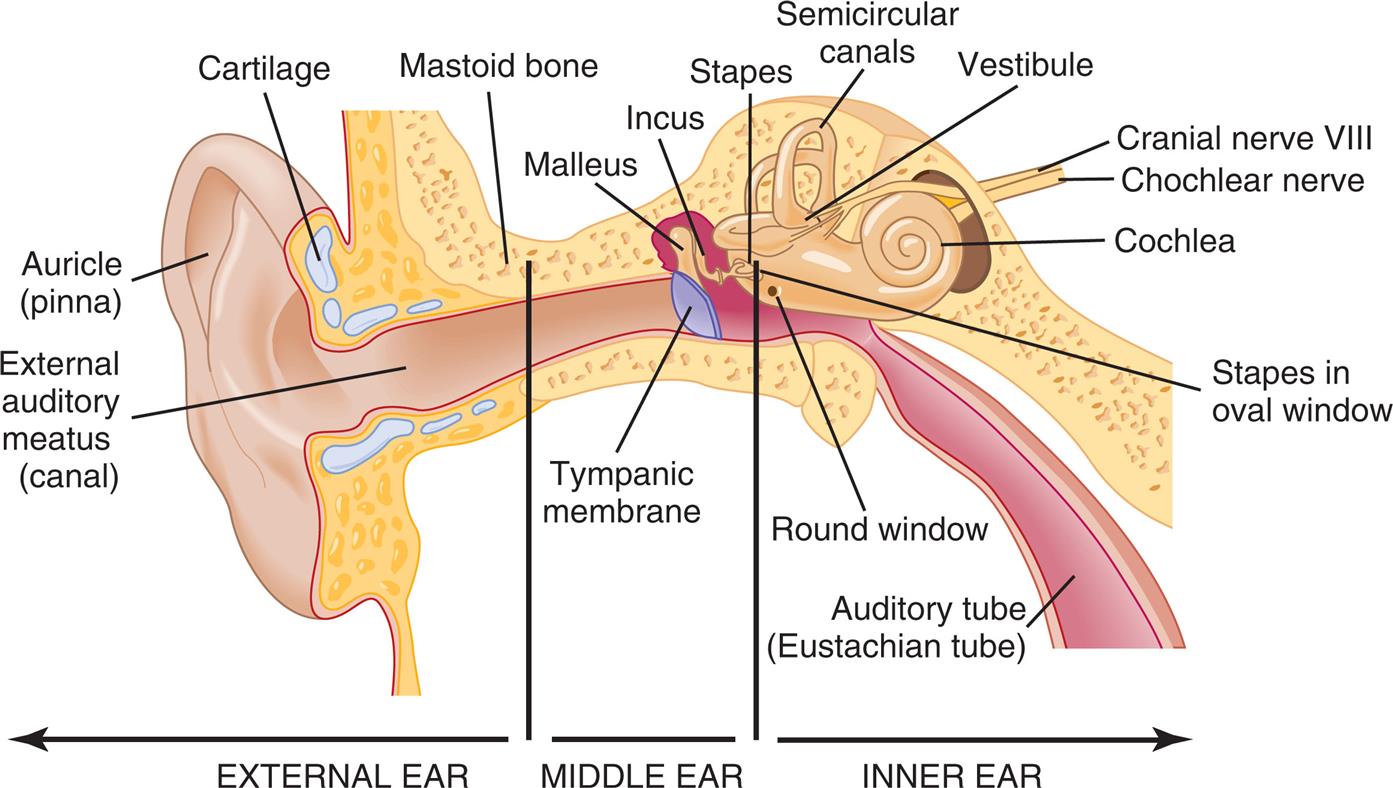
(Anatomic structures are not drawn to scale.) (From Applegate E. The Anatomy and Physiology Learning System. 4th ed. St Louis: Saunders; 2011.)
An illustration depicts the cross-section of an ear with its parts labeled as follows. External ear: Mastoid bone, cartilage, auricle or pinna, and external auditory meatus or canal. Middle ear: Malleus, incus, stapes, and tympanic membrane; inner ear: Semicircular canals, vestibule, cranial nerve eight, cochlear nerve, cochlea, stapes in the oval window, round window, and auditory tube or eustachian tube.
The middle ear is composed of the tympanic cavity, a small chamber in the temporal bone. Three ossicles (small bones known as the malleus [hammer], incus [anvil], and stapes [stirrup]) transmit the vibration of the tympanic membrane to the inner ear. When the tympanic membrane moves, the malleus moves with it and transfers the vibration to the incus, which passes it on to the stapes. The stapes presses against the oval window, a small membrane of the inner ear. The movement of the oval window promotes movement of the round window and sets the fluids of the inner ear in motion (Fig. 16.15).
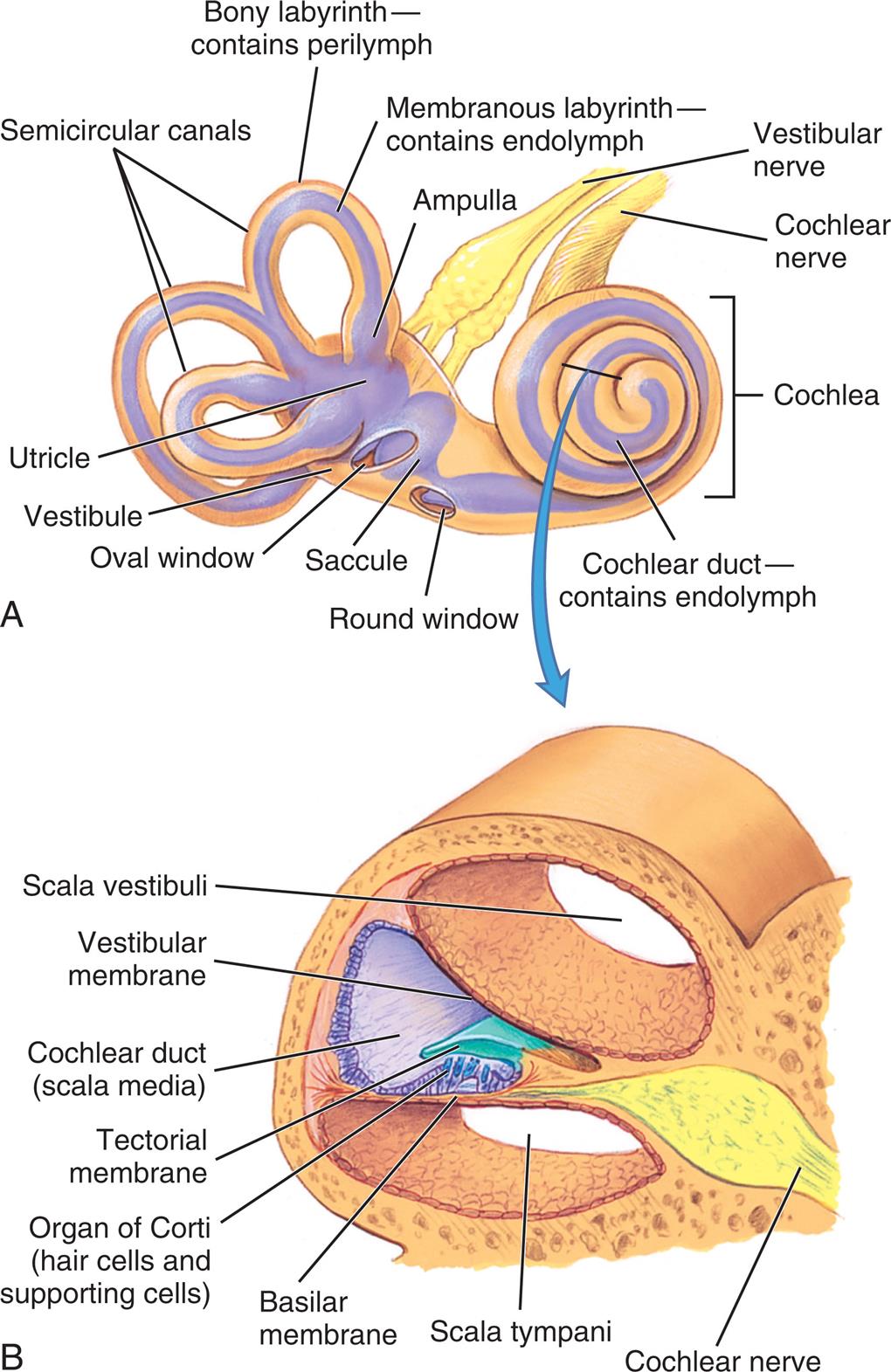
(A) The bony labyrinth (tan) is the hard outer wall of the entire inner ear and includes the semicircular canals, vestibule, and cochlea. Within the bony labyrinth is the membranous labyrinth (purple), which is surrounded by perilymph and filled with endolymph. Each ampulla in the vestibule contains a crista ampullaris that detects changes in head position and sends sensory impulses through the vestibular nerve to the brain. (B) Section of the membranous cochlea. Hair cells in the organ of Corti detect sound and send the information through the cochlear nerve. The vestibular and cochlear nerves join to form the eighth cranial nerve. (From Applegate E. The Anatomy and Physiology Learning System. 4th ed. St Louis: Saunders.)
Illustration A depicts the inner ear with its major structures labeled clockwise from the left: Bony labyrinth contains perilymph, membranous labyrinth containing endolymph, ampulla, vestibular nerve, cochlea, cochlear duct containing endolymph, round window, saccule, oval window, vestibule, utricle, and semicircular canals. Illustration B depicts the enlarged view of a section of the cochlea with its structure labeled: Scala vestibuli, vestibular membrane, cochlear duct or Scala media, tectorial membrane, organ of Corti or hairs cells and supporting cells, basilar membrane, Scala tympani, and cochlear nerve.
The eustachian (pharyngotympanic) tube connects the middle ear with the thorax. Normally flat and closed, the eustachian tube opens briefly when a person swallows or yawns, and it equalizes the pressure in the middle ear with atmospheric pressure. Equalized pressure permits the tympanic membrane to vibrate freely. Through the eustachian tube the mucosa of the middle ear is continuous with the mucosal lining of the throat.
The inner ear is a system of osseous labyrinths (bony, maze-like chambers) filled with a fluid, the perilymph. The bony labyrinth is divided into the cochlea, the vestibule, and the semicircular canals (see Fig. 16.15). Suspended in the perilymph is the endolymph-filled membranous labyrinth that basically follows the shape of the bony labyrinth.
Within the cochlea is the organ of Corti, which contains hair cells (hearing receptors). Sound waves that reach the cochlea through vibrations of the tympanic membrane, ossicles, and oval window set the cochlear fluids into motion. Receptor cells on the basilar membrane are stimulated when their hairs are bent or pulled by fluid movement. Once stimulated, hair cells transmit impulses along the cochlear nerve (a division of the vestibulocochlear nerve) to the auditory cortex of the temporal lobe in the brain (see Fig. 16.15 and view an animation at www.youtube.com/watch?v=46aNGGNPm7s). This is where interpretation of the sound occurs.
The semicircular canals and vestibule of the inner ear contain equilibrium receptors. In the semicircular canals the dynamic equilibrium receptors respond to changes in direction of movement. Within each semicircular canal is the crista ampullaris, a receptor region composed of a tuft of hair cells covered by a gelatinous cupula. When the head is rotated, the endolymph in the canal lags behind and moves in the direction opposite to the head’s movement. The hair cells are stimulated, and impulses are transmitted through the vestibular nerve (a division of the vestibulocochlear nerve) to the cerebellum.
The vestibule in the inner ear contains maculae—receptors essential to the body's sense of static equilibrium. As the head moves, otoliths (small pieces of calcium salts) move in a gel-like material in response to changes in the pull of gravity. The otoliths pull on the gel, which in turn pulls on the hair cells in the maculae. Nerve impulses in the hair cells are triggered and transmitted to the brain (see Fig. 16.15). Thus the ear not only permits the hearing of a large range of sounds but also assists with maintaining balance through the sensitive equilibrium receptors.
Auditory Dysfunction
Between 5% and 10% of the general population have impaired hearing, and it is the most common sensory defect. The major categories of auditory dysfunction are conductive hearing loss, sensorineural hearing loss, mixed hearing loss, and functional hearing loss. Hearing loss may range from mild to profound. Auditory changes caused by aging are common and incremental (see the box Geriatric Considerations: Aging and Changes in Hearing).
Conductive hearing loss
A conductive hearing loss occurs when physical anomalies in the outer or middle ear impair conduction of sound from the outer to the inner ear. Conditions that commonly cause a conductive hearing loss include impacted cerumen, foreign bodies lodged in the ear canal, benign tumors of the middle ear, carcinoma of the external auditory canal or middle ear, eustachian tube dysfunction, otitis media, acute viral otitis media, chronic suppurative otitis media, cholesteatoma (accumulation of keratinized epithelium), and otosclerosis.
Symptoms of conductive hearing loss include diminished hearing and a soft speaking voice. A soft speaking voice is often used because the individual hears his or her voice, conducted by bone, as loud.
Sensorineural hearing loss
A sensorineural hearing loss is caused by impairment of the organ of Corti or its central connections to the auditory cortex. The loss may occur gradually or suddenly. Conditions causing sensorineural loss include congenital and hereditary factors, noise exposure, aging, Ménière disease, ototoxicity, systemic disease (syphilis, Paget disease, collagen diseases, diabetes mellitus), neoplasms, and autoimmune processes. Congenital and neonatal sensorineural hearing loss may be caused by maternal rubella, ototoxic drugs, prematurity, traumatic delivery, erythroblastosis fetalis, bacterial meningitis, and congenital hereditary malfunction. Diagnosis is often made when delayed speech development is noted. Sudden-onset bilateral sensorineural hearing loss can be associated with stroke or trauma and is a medical emergency.91
Presbycusis is the most common form of sensorineural hearing loss in older adults and is usually bilateral and symmetric. Its cause may be atrophy of the basal end of the organ of Corti, loss of auditory receptors, changes in vascularity, or stiffening of the basilar membranes. Drug ototoxicities (drugs that cause destruction of auditory function) have been observed after exposure to various chemicals; for example, antibiotics such as aminoglycosides (streptomycin, neomycin, gentamicin), and vancomycin; diuretics such as ethacrynic acid and furosemide; and chemicals such as salicylate, quinine, carbon monoxide, nitrogen mustard, arsenic, mercury, gold, tobacco, and alcohol. In most instances, the drugs and chemicals listed initially cause tinnitus (ringing in the ear) followed by a progressive high-tone sensorineural hearing loss that is permanent. With presbycusis there is loss of sound clarity related to cochlear impairment and hearing loss at high frequencies.92
Mixed and functional hearing loss
A mixed hearing loss is caused by a combination of conductive and sensorineural losses. With functional hearing loss, which is rare, the individual does not respond to voice and appears not to hear. It is thought to be caused by emotional or psychologic factors.
Ménière disease
Ménière disease (endolymphatic hydrops) is an episodic chronic disorder of the middle ear with an unknown etiology that can be unilateral or bilateral and with variable presentations. The most likely cause is excessive endolymph and pressure in the membranous labyrinth that disrupts both vestibular and hearing functions. There are four symptoms: recurring episodes of vertigo (often accompanied by severe nausea and vomiting), hearing loss, ringing in the ears (tinnitus), and a feeling of fullness in the ear. Treatment is symptomatic with medical management or surgical management when medications fail.93
Ear Infections
Otitis Externa
Otitis externa is the most common inflammation of the outer ear and may be acute or chronic, infectious or noninfectious. Risk factors include swimming with ear plugs, chronic dermatitis, and irritants such as hair spray or hair dyes. The most common origins of acute infections are bacterial microorganisms including Pseudomonas aeruginosa, Staphylococcus aureus, and, less commonly, Escherichia coli. Fungal infections are less common. Infection usually follows prolonged exposure to moisture (swimmer’s ear). The earliest symptoms are inflammation with pruritus, swelling, and clear drainage, progressing to purulent drainage with obstruction of the canal. Tenderness and pain with earlobe retraction accompany inflammation. Acidifying solutions are used for early treatment and prevention. Topical antimicrobials and steroids usually provide effective treatment for later stages of disease. Chronic infections are more often related to allergy or skin disorders. Malignant otitis externa is a very rare serious complication of the spread of otitis externa into the mastoid and/or temporal bone causing osteomyelitis. It presents with headache and otalgia. Diagnosis requires advanced imaging, and treatment requires intravenous antibiotics.94
Otitis media
Otitis media (OM) is a common infection of infants and children. Most children have one episode by 3 years of age. The most common pathogens are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Respiratory viruses have also been associated with OM.95 Predisposing factors include allergy, sinusitis, submucosal cleft palate, adenoidal hypertrophy, eustachian tube dysfunction, and immune deficiency. Breast-feeding is a protective factor. Recurrent acute otitis media may be genetically determined.96
Acute otitis media (AOM) is associated with ear pain, fever, irritability, inflamed tympanic membrane, fluid in the middle ear, and holding or tugging at the ear. The appearance of the tympanic membrane progresses from erythema to opaqueness with bulging as fluid accumulates. There is an increasing prevalence of AOM caused by penicillin-resistant microorganisms. Otitis media with effusion (OME) is the presence of fluid in the middle ear without symptoms of acute infection (Fig. 16.16). Treatment includes symptom management, particularly of pain, with watchful waiting, antimicrobial therapy for severe illness, and placement of tympanostomy tubes when there is persistent bilateral effusion and significant hearing loss (chronic suppurative otitis media). Complications include mastoiditis, brain abscess, meningitis, and chronic otitis media with hearing loss. Persistent middle ear effusions may affect speech, language, and cognitive abilities. Multivalent vaccines for influenza result in modest prevention of acute otitis media.97,98
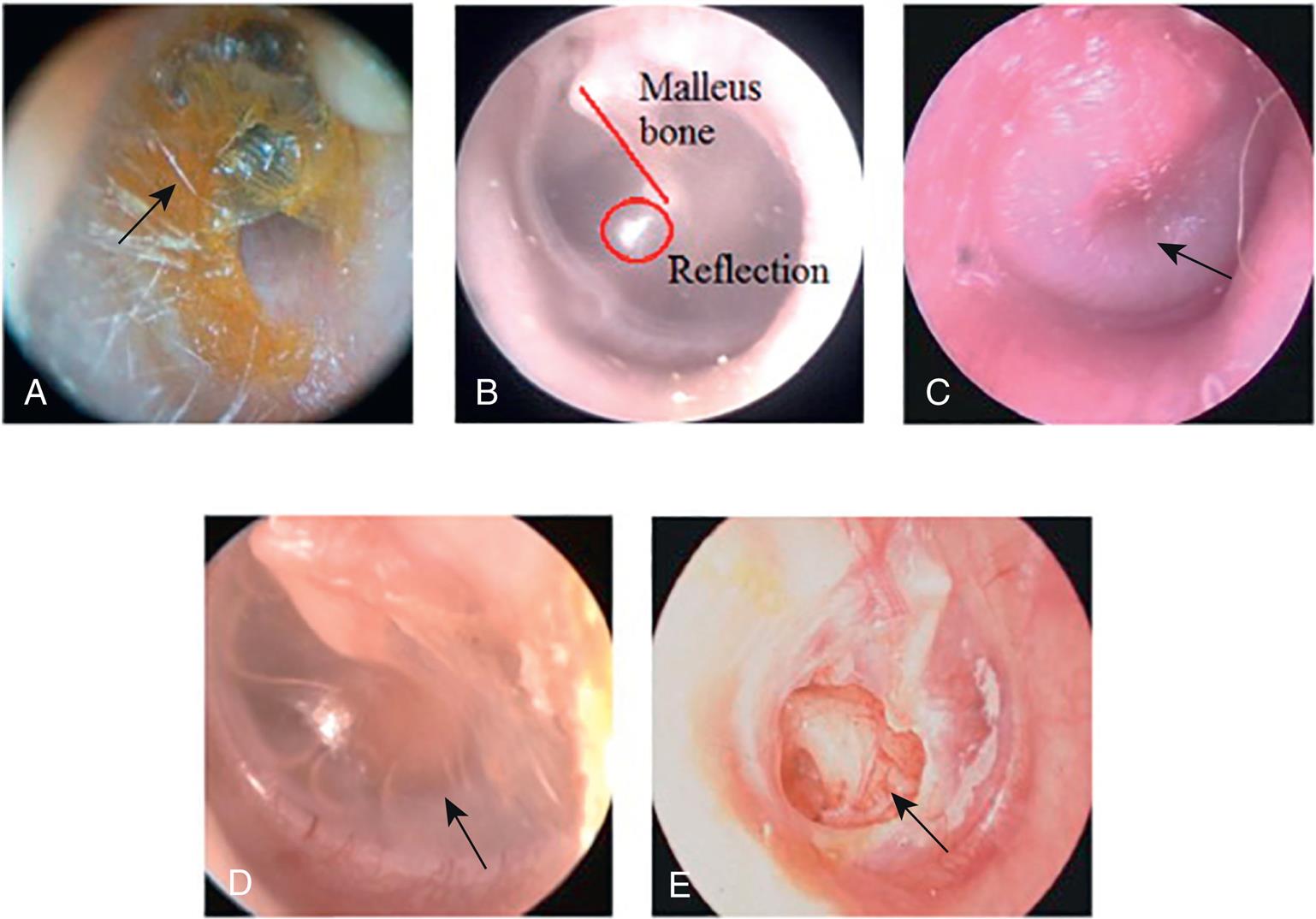
Photomicrography A shows the external ear canal and an arrow indicates the presence of obstructing wax. Photomicrography B shows the external ear canal with a light reflection indicated by a circle and a line indicating the malleus bone. Photomicrography C shows the external ear canal and an arrow mark indicates the bulged tympanic membrane. Photomicrography D shows the external ear canal and an arrow mark indicates the presence of fluid in the middle ear with a retracted tympanic membrane. Photomicrography E shows the external ear canal and an arrow mark indicates the perforated tympanic membrane.
Olfaction and Taste
Olfaction (smell) is a function of cranial nerve I and part of cranial nerve V. Taste (gustation) is a function of multiple nerves in the tongue, soft palate, uvula, pharynx, and upper esophagus innervated by cranial nerves VII and IX. Both cranial nerves are influenced by hormones within the sensory cells. Dysfunctions of smell and taste may occur separately or jointly. The strong relationship between smell and taste creates the sensation of flavor. If either sensation is impaired, the perception of flavor is altered. Olfactory structures are illustrated in Fig. 16.17.
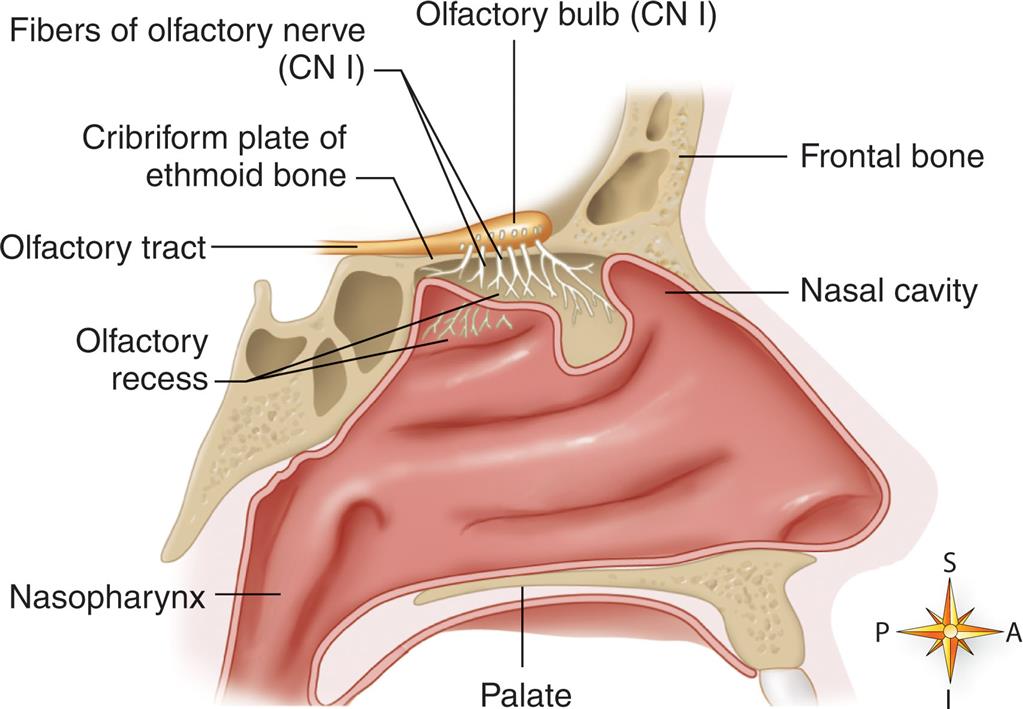
Midsagittal section of the nasal area shows the location of major olfactory sensory structures. (From Patton KT, Thibodeau GA, Douglas MM. Essentials of Anatomy & Physiology. St Louis: Mosby; 2012.)
An illustration depicts the midsagittal section of the nasal area with the major olfactory sensory structures labeled clockwise from the left: Olfactory bulb (CN 1), fibers of the olfactory nerve (C N 1), cribriform plate of the ethmoid bone, olfactory tract, olfactory recess, nasopharynx, palate, nasal cavity, and frontal bone.
Olfactory cells, located in the olfactory epithelium, are the receptor cells for smell. Seven different primary classes of olfactory stimulants have been identified: (1) camphoraceous, (2) musky, (3) floral, (4) peppermint, (5) ethereal, (6) pungent, and (7) putrid. Olfaction is important for detection of hazards in the environment, generating feelings of pleasure, promoting adequate nutrition, influencing sexuality, and maintenance of mood.99
Olfactory dysfunctions include hyposmia, anosmia, hallucinations, and parosmia. Hyposmia is the impaired sense of smell, and anosmia is the complete loss of smell. Both conditions are associated with aging, neurodegenerative and nasal/sinus disorders, and head trauma. When hyposmia or anosmia occurs bilaterally, it is usually the result of rhinitis (inflammation of nasal mucosa), sinusitis, nasal polyps, or excessive smoking. Unilateral hyposmia or anosmia may indicate compression of one olfactory bulb (a bulb-like portion of the olfactory nerves) or nerve tract (olfactory nerve pathway), possibly by tumor or head trauma. Olfactory hallucinations arise from hyperactivity in cortical neurons and involve smelling odors that are not really present. They are associated with temporal lobe seizures and rarely with schizophrenia. Parosmia, an abnormal or perverted sense of smell, may occur with severe depression and in Parkinson and Alzheimer disease.100 Sensitivity to odors declines steadily with aging. (See the box Geriatric Considerations: Aging and Changes in Olfaction and Taste for a summary of changes in olfaction and taste with aging.)
The primary sensations of taste are (1) sour, (2) salty, (3) sweet, (4) bitter, and (5) umami (savory taste of glutamate). Taste buds (fungiform, foliate, and circumvallate) sensitive to each of the primary sensations are located in specific areas of the tongue and are continuously renewing: sweet near the tip, salty on frontal sides, sour on the posterior sides, bitter on the very back, and umami overall surface of tongue. Taste receptors also are found on airway smooth muscle, bladder, breast cancer cells, ovarian cancer cells, and neuroblastoma (bitter) and in the gastrointestinal tract (bitter and sweet). Their function is not for taste. In the lung they stimulate bronchodilation and in the gastrointestinal tract they may participate in metabolic and digestive regulation. These receptors are being evaluated as targets for diagnosis and treatment.101,102
Olfactory and Taste Dysfunctions
Olfactory dysfunctions include the following:
The sense of taste can be impaired by injury, medications, oral infections, or aging. A change in taste may be attributed to impaired smell associated with injury near the hippocampus.
Hypogeusia is a decrease in taste sensation, whereas ageusia is an absence of the sense of taste. These disorders result from cranial nerve injuries and can be specific to the area of the tongue innervated. Dysgeusia is a perversion of taste in which substances possess an unpleasant flavor (i.e., metallic). Ageusia affecting the entire tongue may follow head injury. Hypogeusia and ageusia occur with viral respiratory and oral infections. Autoimmune disease (e.g., systemic lupus erythematosus) and cancer chemotherapy alter taste sensitivity. Damage to the glossopharyngeal nerve (cranial nerve IX, which innervates the posterior one-third of the tongue) causes the loss of the ability to detect bitterness. This loss occurs because the receptors for bitter are located on the base of the tongue. Damage to the facial nerve (cranial nerve VII, which innervates the anterior two-thirds of the tongue) causes loss of the ability to detect sour, sweet, and salty tastes. Only bitter tastes can be detected. These losses occur because sour, sweet, and salt receptors are located on the anterior portion of the tongue.103 Alterations in taste may compromise adequate nutrition or cause anorexia. Variations in taste and smell are important early manifestations of SARS-CoV-2 viral infection (see Emerging Science Box: Loss of Taste and Smell in SARS-CoV-2-Positive Persons).104
Somatosensory Function
Touch
The sensation of touch involves four afferent fiber types that mediate tactile sensation and there may be an additional sensory nerve that transmits pleasurable touch.105 Receptors sensitive to touch are present in the skin with high densities in the fingers and lips. Meissner and Pacinian corpuscles sense movement across the skin and vibration, respectively. Merkel disks sense sustained light touch, and Ruffini endings respond to deep sustained pressure, stretch, and joint position. Specific sensory input is carried to the higher levels of the CNS by the dorsal column of the spinal cord and the anterior spinothalamic tract.
The cutaneous senses develop before birth, but structural growth continues into early adulthood. Then a gradual decline occurs, with loss in tactile discrimination with advancing age. Abnormal tactile perception may be caused by alterations at any level of the nervous system, from the receptor to the cerebral cortex. Factors that interrupt or impair reception, transmission, perception, or interpretation of touch—including trauma, tumor, infection, metabolic changes, vascular changes, and degenerative diseases—may cause tactile dysfunction. In addition, most tactile sensations evoke affective responses that determine whether the sensation is unpleasant, pleasant, or neutral. With aging, touch sensitivity declines and gentle touch becomes more pleasant. There is a decline in skin elasticity and tactile receptors and changes in central somatotropic organization.106
Proprioception
Proprioception is the awareness of the position of the body and its parts. It depends on impulses from the inner ear and from receptors in joints and ligaments. Sensory data are transmitted to higher centers, primarily through the dorsal columns and the spinocerebellar tracts, with some data passing through the medial lemnisci and thalamic radiations to the cortex. These stimuli are necessary for the coordination of movements, the grading of muscular contraction, and the maintenance of equilibrium.
As with tactile dysfunction, any factor that interrupts or impairs the reception, transmission, perception, or interpretation of proprioceptive stimuli also alters proprioception and increases the risk for falls and injury. A progressive loss of proprioception has been reported in older adult persons with an increased risk for falls and injury.107 Two common causes are vestibular dysfunction and neuropathy.
Specific vestibular dysfunctions are vestibular nystagmus and vertigo. Vestibular nystagmus is the constant, involuntary movement of the eyeball and develops when the semicircular canal system is overstimulated. Vertigo is the sensation of spinning that occurs with inflammation of the semicircular canals in the ear. The individual may feel either that he or she is moving in space or that the world is revolving. Vertigo often causes loss of balance, and nystagmus may occur. Ménière disease can cause loss of proprioception during an acute attack, so that standing or walking is impossible.
Peripheral neuropathies also can cause proprioceptive dysfunction. They may be caused by several conditions and commonly are associated with renal disease and diabetes mellitus. Although the exact sequence of events is unknown, neuropathies cause a diminished or absent sense of body position or position of body parts. Gait changes often occur.
Summary Review
Pain
- 1. Pain (nociception) is a complex, sensory experience involving emotion, cognition, and motivation. Acute pain is protective, promoting withdrawal from painful stimuli.
- 2. Three portions of the nervous system are responsible for the sensation, perception, and response to pain: (1) the afferent pathways, (2) the interpretive centers in the central nervous system, and (3) the efferent pathways.
- 3. Nociception involves four phases: transduction, transmission, perception, and modulation.
- 4. Pain transduction begins when nociceptors (pain receptors) are activated by noxious stimulants. There are two types of nociceptors: myelinated Aδ fibers transmit sharp, “fast” pain; smaller, unmyelinated C fibers more slowly transmit dull, less localized pain.
- 5. Pain transmission is the conduction of pain impulses along the nociceptors into the spinal cord and eventually to the brain.
- 6. Pain perception is the conscious awareness of pain. It occurs with the integration of three systems. The sensory-discriminative system (mediated by the somatosensory cortex) identifies the location and intensity of pain. The affective-motivational system (mediated by the reticular formation, limbic system, and brain stem) controls emotional and affective responses to pain. The cognitive-evaluative system (mediated by the cortex) coordinates the meaning and experience of pain.
- 7. Pain threshold is the lowest intensity of pain that a person can recognize. Pain tolerance is the greatest level of pain that an individual is prepared to tolerate. Both are subjective and influenced by many factors.
- 8. Pediatric pain considerations
- 9. Geriatric pain considerations
- 10. Pain modulation facilitates or inhibits the transmission of pain signals throughout the nervous system. Neuromodulators of pain include substances that (1) stimulate pain nociceptors (e.g., prostaglandins, bradykinins, lymphokines, substance P, glutamate) and (2) suppress pain (e.g., GABA, endogenous opioids, endocannabinoids). Some substances excite peripheral nerves but inhibit central nerves (e.g., serotonin, norepinephrine).
- 11. Endogenous opioids inhibit pain transmission and include enkephalins, endorphins, dynorphins, and endomorphins. They are produced in the central nervous system and by immune cells.
- 12. Descending inhibitory and facilitatory pathways and nuclei inhibit or facilitate pain. Efferent pathways from the ventromedial medulla and PAG inhibit pain impulses at the dorsal horn. The RVM stimulates efferent pathways that facilitate or inhibit pain in the dorsal horn.
- 13. Segmental pain inhibition occurs when impulses from Aβ fibers (touch and vibration sensations) arrive at the same spinal level as impulses from Aδ or C fibers.
- 14. DNIC occurs when pain signals from two different sites are transmitted simultaneously and inhibit pain through a spinal-medullary-spinal pathway. Conditioned pain modulation uses a test pain stimulus and a conditioning pain stimulus with subjective measures of pain intensity to evaluate the efficacy of DNIC responses.
- 15. Because of the complex nature of pain, classifications of pain often overlap, and more than one description is often used.
- 16. Acute pain is a signal to the person of a harmful stimulus and may be (1) somatic (skin, joints, muscles), (2) visceral (inner organs, body cavities), or (3) referred (present in an area distant from its origin). The area of referred pain is supplied by the same spinal segment as the actual site of pain.
- 17. Chronic pain is pain lasting well beyond the expected normal healing time and may be ongoing (e.g., low back pain) or intermittent (e.g., migraine headaches). Psychologic, behavioral, and physiologic responses to chronic pain include depression, sleep disorders, preoccupation with pain, lifestyle changes, and physiologic adaptation.
- 18. Neuropathic pain is chronic pain with increased sensitivity to painful or nonpainful stimuli and results from abnormal processing of pain information in the peripheral or central nervous system.
- 19. Chronic pain syndromes include myofascial pain, chronic postoperative pain, cancer pain, post-stroke pain, phantom limb pain, and complex regional pain syndrome. These syndromes are complex, often involve both nociceptive and neuropathic pain mechanisms, and are difficult to treat.
Temperature Regulation
- 1. Temperature regulation (thermoregulation) is achieved through precise balancing of heat production, heat conservation, and heat loss. Body temperature is maintained in a range around 37°C (98.6°F).
- 2. Temperature regulation is mediated by the hypothalamus and endocrine system through peripheral thermoreceptors in the skin, liver, and skeletal muscle and central thermoreceptors in the hypothalamus, spinal cord, viscera, and great veins.
- 3. Heat is produced through chemical reactions of metabolism and skeletal muscle contraction. Heat is distributed by the circulatory system.
- 4. Heat is lost through radiation, conduction, convection, vasodilation, decreased muscle tone, evaporation of sweat, increased respiration, voluntary mechanisms, and adaptation to warmer climates.
- 5. Heat conservation is accomplished through vasoconstriction and voluntary mechanisms.
- 6. Infants do not conserve heat well because of their greater body surface/mass ratio and decreased amounts of subcutaneous fat. Older adult persons have poor responses to environmental temperature extremes as a result of slowed blood circulation, structural and functional changes in the skin, and overall decrease in heat-producing activities.
- 7. Fever involves the temporary “resetting of the hypothalamic thermostat” to a higher level. When the fever breaks, the set point returns to normal. Fever is triggered by the release of exogenous pyrogens from bacteria or the release of endogenous pyrogens (cytokines) from phagocytic cells.
- 8. Fever kills many pathogens and decreases serum levels of iron, zinc, and copper that are needed for bacterial replication.
- 9. The acute phase response is a defensive reaction to infection or injury that includes an inflammatory response, fever, and the production of immune cytokines and proteins that support the immune system in eliminating pathogens.
- 10. Endogenous cryogens are antipyretic substances that diminish fever and control the febrile response.
- 11. Febrile seizures occur in children with temperatures greater than 38°C (100.4°F) without CNS infection, hypoglycemia, or electrolyte disorders. They are usually brief and without recurrence.
- 12. Fever of unknown origin is a body temperature greater than 38.3°C (101°F) for longer than 3 weeks that remains undiagnosed after 3 days of investigation.
- 13. Fever production aids responses to infectious processes. Higher temperatures kill many microorganisms, promote immune responses, and decrease serum levels of iron, zinc, and copper, which are needed for bacterial replication.
- 14. Hyperthermia (marked warming of core temperature) can produce nerve damage, coagulation of cell proteins, and death. Therapeutic hyperthermia may be used to promote natural immune processes or promote tumor blood flow. Forms of accidental hyperthermia include heat cramps, heat exhaustion, heat stroke, and malignant hyperthermia. Heat stroke and malignant hyperthermia are potentially lethal.
- 15. Hypothermia (marked cooling of core temperature) slows the rate of cell metabolism, increases the viscosity of the blood, slows blood flow through the microcirculation, facilitates blood coagulation, and stimulates profound vasoconstriction. Hypothermia may be accidental or therapeutic.
- 16. Major body trauma can affect temperature regulation by damaging the CNS or causing inflammation, increased intracranial pressure, or intracranial bleeding. It results in a sustained, noninfectious fever called central fever.
Sleep
- 1. Sleep is an active process that provides restorative functions and promotes memory consolidation. Sleep is divided into REM and non-REM stages, each of which has its own series of stages. While asleep, an individual progresses through REM and non-REM (slow wave) sleep multiple times in a predictable cycle.
- 2. REM sleep is controlled by mechanisms in the pons and mesencephalon. It is known as paradoxical sleep because the EEG pattern is similar to that of an awake person. The brain is very active with dreaming.
- 3. Non-REM sleep is controlled by release of inhibitory signals from the hypothalamus and accounts for 75% to 80% of sleep time. The body is in a state of reduced activity.
- 4. The sleep patterns of the newborn and young child vary from those of the adult in total sleep time, cycle length, and percentage of time spent in each sleep cycle. Older adult persons experience a total decrease in sleep time.
- 5. The restorative, reparative, and growth processes occur during slow-wave (non-REM) sleep. Sleep deprivation can cause profound changes in personality and functioning.
- 6. Sleep disorders include (1) dyssomnias, which are disorders of initiating or maintaining sleep (i.e., insomnia, obstructive sleep apnea syndrome, hypersomnia, or disorders of the sleep-wake schedule) and (2) parasomnias, which are unusual behaviors during sleep (i.e., sleepwalking or night terrors and restless leg syndrome).
The Special Senses
- 1. The special senses include vision, hearing, olfaction, and taste.
- 2. The eyes are responsible for vision. The wall of the eye has three layers: sclera, choroid, and retina. The retina contains millions of baroreceptors known as rods and cones that receive light through the lens and then convey signals to the optic nerve and subsequently to the visual cortex of the brain.
- 3. The eye is filled with vitreous and aqueous humor, which prevent it from collapsing.
- 4. The major alterations in ocular movement include strabismus, nystagmus, and paralysis of the extraocular muscles.
- 5. Alterations in visual acuity (the ability to see objects in sharp detail) can be caused by amblyopia, scotoma, cataracts, papilledema, dark adaptation, glaucoma, retinal detachment, and macular degeneration. Visual acuity decreases with age due to structural eye changes.
- 6. A cataract is a cloudy or opaque area in the ocular lens and leads to visual loss when located on the visual axis. Cataracts are the leading cause of blindness in the world.
- 7. Glaucoma is characterized by intraocular pressure with death of retinal ganglion cells and their axons. Open angle, angle closure or narrow angle, and congenital closure are the various forms, with angle closure glaucoma being a medical emergency.
- 8. Age-related macular degeneration is irreversible loss of vision with atrophic (dry) or neovascular (wet) forms.
- 9. Retinal detachment is a separation of the retinal pigment epithelium from the photoreceptors in the neuroepithelium with loss of vision.
- 10. Alterations in accommodation (changes in lens shape that changes focus from distant to near images) develop with increased intraocular pressure, inflammation, age, and disease of the oculomotor nerve. Presbyopia is loss of accommodation caused by loss of elasticity of the lens with aging.
- 11. Alterations in refraction, including myopia, hyperopia, and astigmatism, are the most common visual disorders.
- 12. Alterations in color vision can be related to yellowing of the lens with aging and color blindness, an inherited trait.
- 13. Trauma or disease of the optic nerve pathways can cause defects or blindness in the entire visual field or in half of the visual field (hemianopia).
- 14. The eyelids, conjunctivae, and lacrimal apparatus protect the eye externally. Infections are the most common disorders; they include blepharitis, conjunctivitis, chalazion, and hordeolum.
- 15. Blepharitis is an inflammation of the eyelid; a hordeolum (stye) is an infection of the eyelid’s sebaceous gland; and a chalazion is an infection of the eyelid’s Meibomian gland.
- 16. Conjunctivitis is an inflammation of the conjunctiva, and can be acute or chronic, bacterial, viral, or allergic. Redness, edema, pain, and lacrimation are common symptoms. Trachoma (chlamydial conjunctivitis) is the leading cause of preventable blindness in the world and is associated with poor hygiene.
- 17. Keratitis is a bacterial or viral infection of the cornea that can lead to corneal ulceration.
- 18. The ears are responsible for hearing. The ear is composed of external, middle, and inner structures.
- 19. The external ear structures are the pinna, auditory canal, and tympanic membrane. The external ear is only involved in hearing.
- 20. The middle ear is composed of the tympanic cavity (containing three bones: the malleus, the incus, and the stapes), oval window, eustachian tube, and fluid. These transmit sound vibrations to the inner ear. The middle ear is only involved in hearing.
- 21. The inner ear is involved in both hearing and equilibrium. It includes the bony and membranous labyrinths that transmit sound waves through the cochlea and to the cochlear nerve and ultimately to the brain. The semicircular canals and vestibule help maintain balance through the equilibrium receptors.
- 22. Impaired hearing is the most common sensory defect, occurring in 5% to 10% of the general population.
- 23. Hearing loss can be classified as conductive, sensorineural, mixed, or functional.
- 24. Conductive hearing loss occurs when sound waves cannot be conducted through the middle ear.
- 25. Sensorineural hearing loss develops with impairment of the organ of Corti or its central connections. Presbycusis is the most common form of sensorineural hearing loss in older adults.
- 26. A combination of conductive and sensorineural loss is mixed hearing loss. Loss of hearing with no known organic cause is functional hearing loss.
- 27. Ménière disease is a disorder of the middle ear that affects hearing and balance.
- 28. Otitis externa is an infection of the outer ear associated with prolonged exposure to moisture.
- 29. Otitis media is an infection of the middle ear that is common in children. Accumulation of fluid (effusion) behind the tympanic membrane is a common finding.
- 30. Olfaction (smell) is a function of cranial nerve I and part of cranial nerve V. Taste (gustation) is a function of multiple nerves in the tongue, soft palate, uvula, pharynx, and upper esophagus innervated by cranial nerves VII and IX.
- 31. The perception of flavor is altered if olfaction or taste dysfunctions occur. Sensitivity to odor and taste decreases with aging.
- 32. Hyposmia is an impaired sense of smell, and anosmia is the complete loss of the sense of smell.
- 33. Hypogeusia is a decrease in taste sensation, and ageusia is the absence of the sense of taste.
Somatosensory Function
- 1. The sensation of touch is a function of receptors present in the skin, and the sensory response is conducted to the brain through the dorsal column and anterior spinothalamic tract.
- 2. Alterations in touch can result from alterations at any level of the nervous system.
- 3. Proprioception is the awareness of the position and location of the body and its parts. Proprioceptors are located in the inner ear, joints, and ligaments. Proprioceptive stimuli are necessary for balance, coordinated movement, and grading of muscular contraction.
- 4. Disorders of proprioception can occur at any level of the nervous system and result in impaired balance and lack of coordinated movement. Vestibular nystagmus is the constant, involuntary movement of the eyeball and develops when the semicircular canal system is overstimulated. Vertigo is the sensation of spinning that occurs with inflammation of the semicircular canals in the ear.