Structure and Function of the Reproductive Systems
Karen C. Turner
![]() http://evolve.elsevier.com/Rogers/pathophysiology/
http://evolve.elsevier.com/Rogers/pathophysiology/
The male and female reproductive systems have several anatomic and physiologic features in common. Most obvious is their major function—reproduction—through which a 23-chromosome female gamete, the ovum (pl., ova), and a 23-chromosome male gamete, the spermatozoon (sperm cell), unite to form a 46-chromosome zygote that is capable of developing into a new individual. The male reproductive system produces sperm that can be transferred to the female reproductive tract. The female reproductive system produces the ovum; if the ovum is fertilized, it is then called the embryo and developing fetus. These functions are determined not only by anatomic structures but also by complex hormonal, neurologic, and psychogenic factors.
Development of the Reproductive Systems
The structure and function of both male and female reproductive systems depend on steroid hormones called sex hormones and their precursors. Cholesterol is the precursor for steroid hormones, including sex hormones (e.g., estrogen and testosterone). Other hormones that are not steroid hormones (e.g., gonadotropins) also support reproduction. The actions of both sex and reproductive hormones are summarized in Table 24.1. Sex and reproductive hormones act on target tissues by binding with cellular receptors (see Chapter 21 hormonal regulation). Hormonal effects on the reproductive systems begin during embryonic development and continue in varying degrees throughout life.
Table 24.1
| Hormone (Source) | Action in Females | Action in Males |
|---|---|---|
| Dehydroepiandrosterone (DHEA) (adrenal gland, ovary, other tissues) | Converted to androstenedione and then to estrogens, testosterone, or both | Converted to androstenedione and then to estrogens, testosterone, or both |
| Estrogens (estrone, estradiol, estriol) (ovary and placenta, small amounts in other tissues) | Stimulates development of female sexual characteristics: maturation of breast, uterus, and vagina; promotes proliferative development of endometrium during menstrual cycle; during pregnancy promotes mammary gland development, fetal adrenal gland function, and uteroplacental blood flow (see Box 24.1) | Growth at puberty, growth plate fusion in bone, prevention of apoptosis of germ cells |
| Testosterone (adrenal glands from DHEA, testes) | Libido, learning, sleep, protein anabolism, growth of muscle and bone; growth of pubic and axillary hair; activation of sebaceous glands, accounting for some cases of acne during puberty | Stimulates spermatogenesis, stimulates development of primary and secondary sexual characteristics, promotes growth of muscle and bone (anabolic effect); growth of pubic and axillary hair; activates sebaceous glands, accounting for some cases of acne during puberty; maintains libido |
| Gonadotropin-releasing hormone (GnRH) (hypothalamus-neuroendocrine cells) | Stimulates secretion of gonadotropins (FSH and LH) from anterior pituitary | Stimulates secretion of gonadotropins (FSH and LH) from anterior pituitary |
| Follicle-stimulating hormone (FSH) (anterior pituitary, gonadotroph cells) | Gonadotropin; promotes development of ovarian follicles; stimulates estrogen secretion | Gonadotropin; promotes development and growth of testes and stimulates spermatogenesis by Sertoli cells |
| Luteinizing hormone (LH) (anterior pituitary, gonadotroph cells) | Gonadotropin; triggers ovulation; promotes development of corpus luteum | Gonadotropin; stimulates testosterone production by Leydig cells of testis |
| Inhibin (ovary and testes) | Inhibits FSH production in anterior pituitary (perhaps by limiting GnRH) | Inhibits FSH production in anterior pituitary |
| Human chorionic gonadotropin (hCG) (placenta) | Supports corpus luteum, which secretes estrogen and progesterone during first 7 weeks of pregnancy | |
| Activin (ovary) | Stimulates secretion of FSH and pituitary response to GnRH and FSH binding in dominant granulosa cells | |
| Progesterone (ovary and placenta) | Promotes secretory changes in endometrium during luteal phase of menstrual cycle; quiets uterine myometrium (muscle) activity and prevents lactogenesis during pregnancy | |
| Relaxin (corpus luteum, myometrium, and placenta) | Inhibits uterine contractions during pregnancy and softens pelvic joints and cervix to facilitate childbirth |
Sexual Differentiation and Hormone Production in Utero
Initially, in embryonic development, the reproductive structures of male and female embryos are homologous (the same) or undifferentiated. They consist of one pair of primary sex organs, or gonads, and two pairs of ducts—the wolffian ducts and the müllerian ducts (Fig. 24.1). The müllerian ducts are the precursor of the internal female sex organs (oviducts, uterus, cervix, and upper vagina). Müllerian ducts are initially formed regardless of genotypic sex and require no sex-determining region on the Y chromosome (SRY) signaling for development. SRY signaling is required in males to cause regression of the müllerian ducts, which in turn prevents the development of the female reproductive tract. The wolffian ducts are the precursor of male internal sex organs (secrete testosterone and promote the development of the male sex organs). Both pairs of ducts empty into an opening called the urogenital sinus.
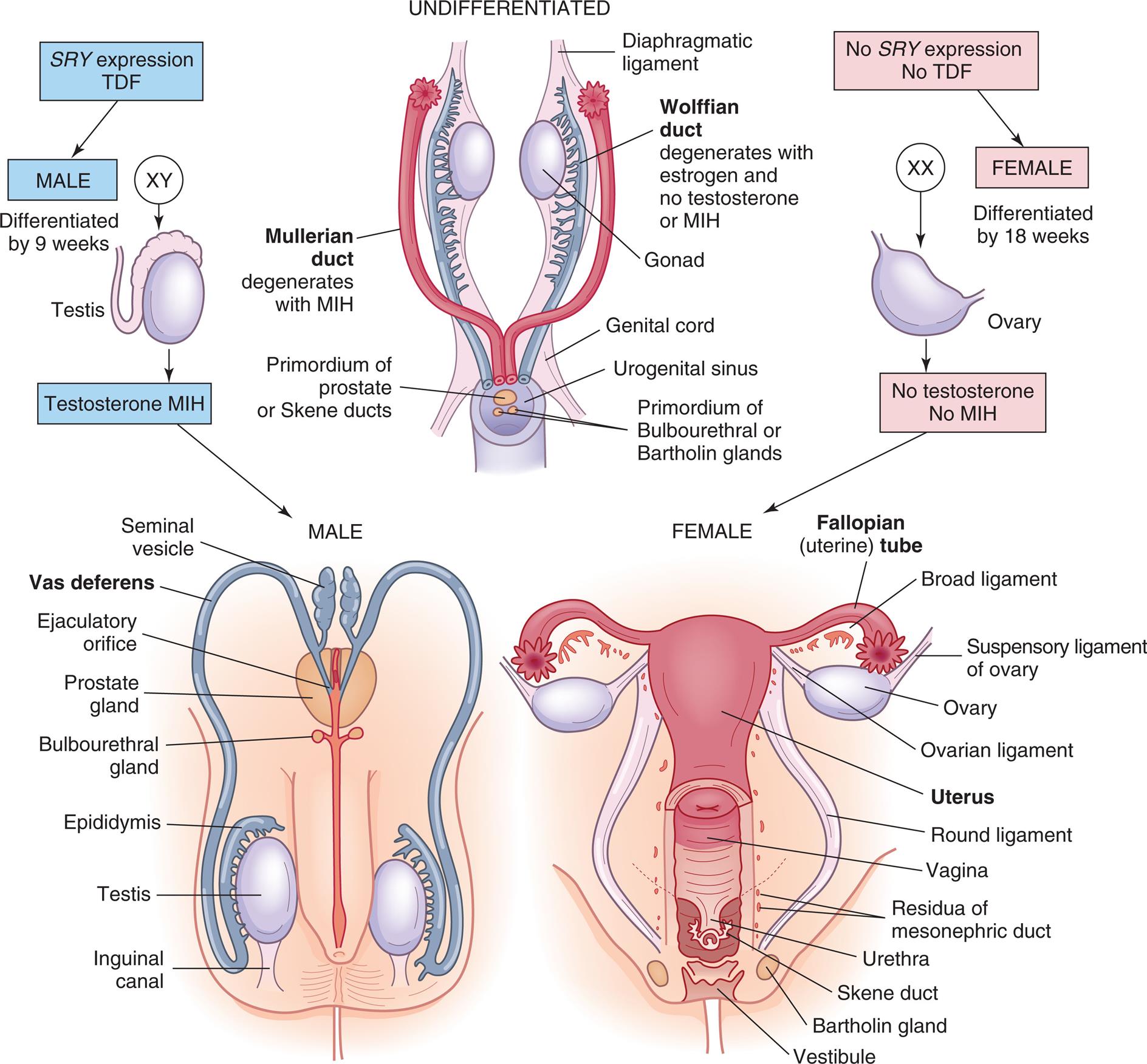
Embryonic and fetal development of the internal genitalia. MIH, Müllerian inhibitory hormone; SRY, gene that produces TDF; TDF, testosterone development factor; see text for additional details.
“A series of illustrations show and label the structures in an undifferentiated, the male, and the female sexual organs. Top panel, undifferentiated. The following parts in the structure are labeled from the top to the bottom: diaphragmatic ligament, wolffian duct (degenerates with estrogen and no testosterone or M I H), gonad, Mullerian duct (degenerates with M I H), genital cord, urogenital sinus, primordium of prostate or Skene ducts, and primordium of bulbourethral or Bartholin glands. S R Y expression and T D F result in male organs (X Y), differentiated by nine weeks, as a result of testosterone and M I H. No S R Y expression and no T D F result in female orans (X X), differentiated by eighteen weeks, as a result of no testosterone and no M I H. Bottom-left panel, male. The following structures in the illustration are labeled from the top to the bottom: seminal vesicle, vas deferens, ejaculatory orifice, prostate gland, bulbourethral gland, epididymis, testis, inguinal canal. Bottom-right panel, female. The following structures in the illustration are labeled from the top to the bottom: fallopian (uterine) tube, broad ligament, suspensory ligament of ovary, ovarian ligament, uterus, round ligament, vagina, residua of mesonephric duct, urethra, Skene duct, Bartholin gland, and vestibule.”
The first sign of development of reproductive organs (male or female) occurs during the fifth week of gestation. Between 6 and 7 weeks’ gestation, the male embryo differentiates under the influence of testes-determining factor (TDF), a protein expressed by the SRY gene. When the SRY gene is expressed, male gonadal development prevails. TDF stimulates the male gonads to develop into the two testes, and by 8 weeks’ gestation, testosterone secretion begins. Müllerian inhibitory hormone (MIH), secreted by Sertoli cells in the testes, promotes degeneration of the müllerian ducts. Without MIH, the müllerian ducts would develop, and the wolffian ducts would degenerate with loss of male sex organ development. The Leydig cells secrete testosterone and promote Wolffian duct development, which differentiates into the epididymis, vas deferens, seminal vesicles, and ejaculatory ducts. By 9 months’ gestation, the male gonads (testes) have descended into the scrotum. The testes produce sperm after puberty.
Female gonadal development occurs in the absence of SRY expression and with the expression of other genes. The presence of estrogen and the absence of testosterone and MIH cause degeneration in the wolffian ducts and maintenance of the müllerian ducts. At 6 to 8 weeks’ gestation, the two female gonads develop into ovaries, which will produce ova. By the tenth week, the wolffian ducts deteriorate, and the upper ends of the müllerian ducts become the fallopian tubules, whereas the lower ends join to become the uterus, cervix, and upper two-thirds of the vagina (see Fig. 24.1). The fallopian tubes will carry ova from the ovaries to the uterus during a female's reproductive years. Lack of testosterone and the presence of estrogen promote the development of external genitalia (lower end of vagina, labia, and clitoris).
Like the internal reproductive structures, the external structures develop from homologous embryonic tissues. During the first 7 to 8 weeks’ gestation, both male and female embryos develop an elevated structure called the genital tubercle (Fig. 24.2). Testosterone is necessary for the genital tubercle to differentiate into external male genitalia; otherwise, female genitalia develop, which may occur even in the absence of ovaries, possibly because of the presence of placental estrogens.
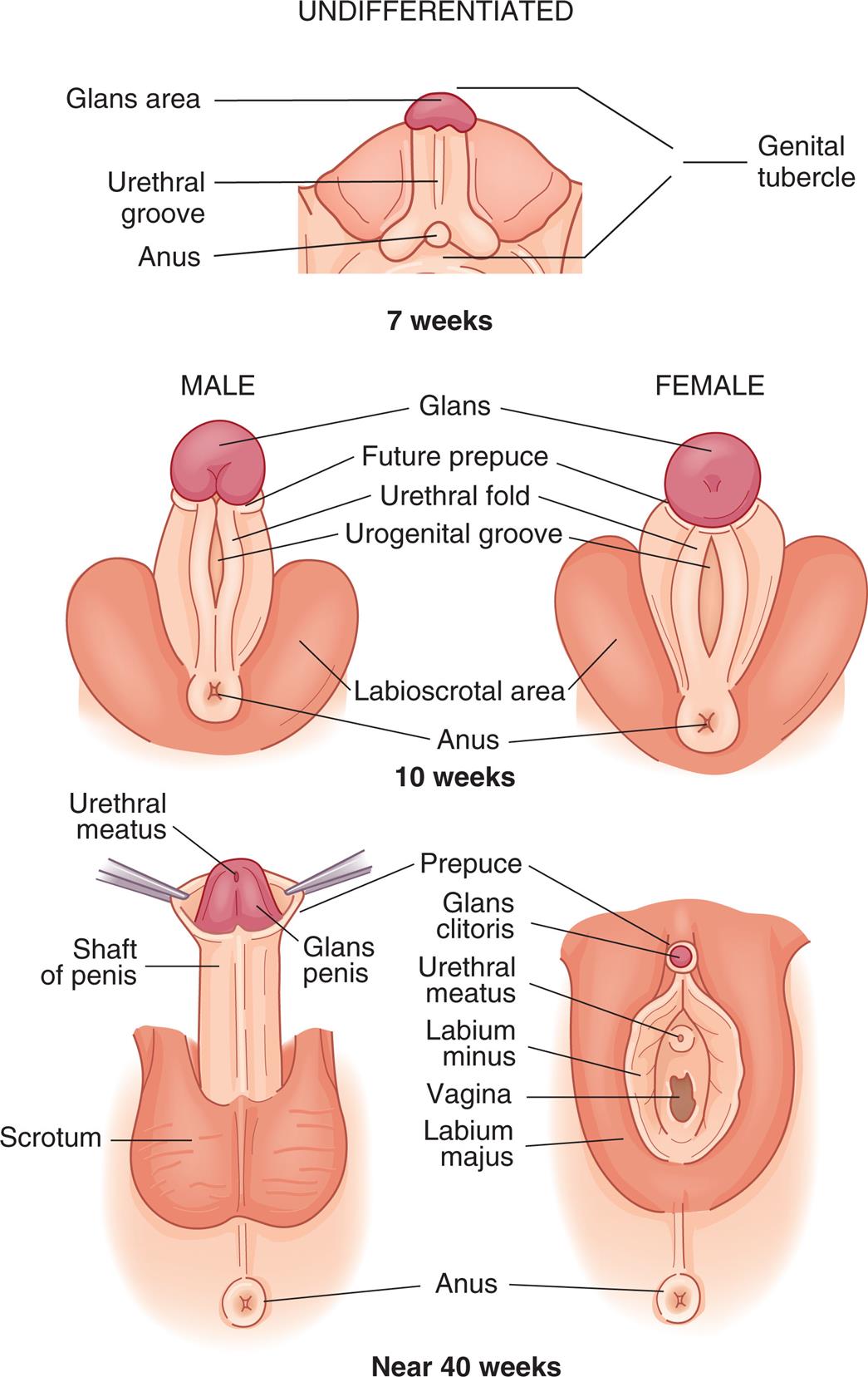
Embryonic and fetal development of the external genitalia.
“A series of illustrations show and label the structures in an undifferentiated, the male, and the female external genitalia. Top panel, undifferentiated, seven weeks. The following structures on the illustration are labeled, from the top to the bottom: genital tubercle, glans area, urethral groove, anus, and genital tubercle. Middle panel, male and female, ten weeks. The following structures on the illustration are labeled, from the top to the bottom: glans, future prepuce, urethral fold, urogenital groove, labioscrotal area, and anus. Bottom panel, male and female, forty weeks. The following structures on the male genitalia are labeled, from the top to the bottom: urethral meatus, prepuce, glans penis, shaft of penis, scrotum, and anus. The following structures on the female genitalia are labeled, from the top to the bottom: prepuce, glans clitoris, urethral meatus, labium minus, vagina, labium majus, and anus.”
Anterior pituitary development begins between the fourth and fifth weeks of fetal life, and the vascular connection between the hypothalamus and the pituitary is established by the 12th week. Gonadotropin-releasing hormone (GnRH) is produced in the hypothalamus by 10 weeks’ gestation and controls the production of two gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH), by the anterior pituitary gland. In the female fetus, high levels of FSH and LH are excreted. FSH and LH stimulate the production of estrogen and progesterone by the ovary. The production of FSH and LH increases until about 28 weeks’ gestation when the production of estrogen and progesterone by the ovaries and placenta is high enough to result in the decline of gonadotropin production. Production of primitive female gametes (ova) occurs solely during fetal life. From puberty to menopause, one female gamete matures per menstrual cycle. Production of the male gametes (sperm) begins at puberty; after that, millions are produced daily, usually for life.
By the end of pregnancy, a sensitive negative-feedback system, which includes the gonadostat, is operative in the human fetus. The gonadostat responds to high placental estrogen levels by releasing low levels of GnRH. Soon after birth, steroid hormones levels drop because of the withdrawal of maternal placental hormones. Hypothalamic pulsatile GnRH is secreted, and gonadotropins LH and FSH are released. Their levels peak at 3 to 6 months for boys and at 12 to 18 months for girls, and then fall steadily. The gonadotropins will be suppressed until the onset of puberty.
Puberty and Reproductive Maturation
Adolescence is the stage of human development between childhood and adulthood and includes social, psychological, and biologic changes. Puberty is the onset of sexual maturation and differs from adolescence. Genetics, environment, ethnicity, general health, and nutrition can influence the timing of puberty. In females, puberty begins at about age 8 to 9 years with thelarche (breast development). In males, it begins later, at about age 11 years.
Reproductive maturation involves the hypothalamic-pituitary-gonadal axis, the central nervous system, and the endocrine system (Fig. 24.3). There is a sequential series of hormonal events that promote sexual maturation as puberty approaches. Nocturnal gonadotropin secretion (i.e., LH and FSH) and an increased response in the pituitary to GnRH occur about 1 year before puberty. This, in turn, stimulates gonadal maturation (gonadarche) with estradiol secretion in females and testosterone secretion in males. Estradiol causes thelarche, maturation of the reproductive organs (vagina, uterus, ovaries), and deposition of fat in the female's hips. Estrogen and increased production of growth factors cause rapid skeletal growth in both males and females. Testosterone causes the growth of the testes, scrotum, and penis. A positive feedback loop is created with gonadotropins stimulating the gonads to produce more sex hormones. The most important hormonal effects occur in the gonads. In males, the testes begin to produce mature sperm that are capable of fertilizing an ovum. Male puberty is complete with the first ejaculation that contains mature sperm. In females, the ovaries begin to release mature ova. Female puberty is complete at the time of the first ovulatory menstrual period. Before puberty, there also is an increase in adrenal androgen in both sexes, known as adrenarche. Adrenal androgens are converted to testosterone and estradiol and contribute to the growth of axillary and pubic hair and activation of sweat and sebaceous glands during puberty. Puberty is complete when an individual is capable of reproduction.
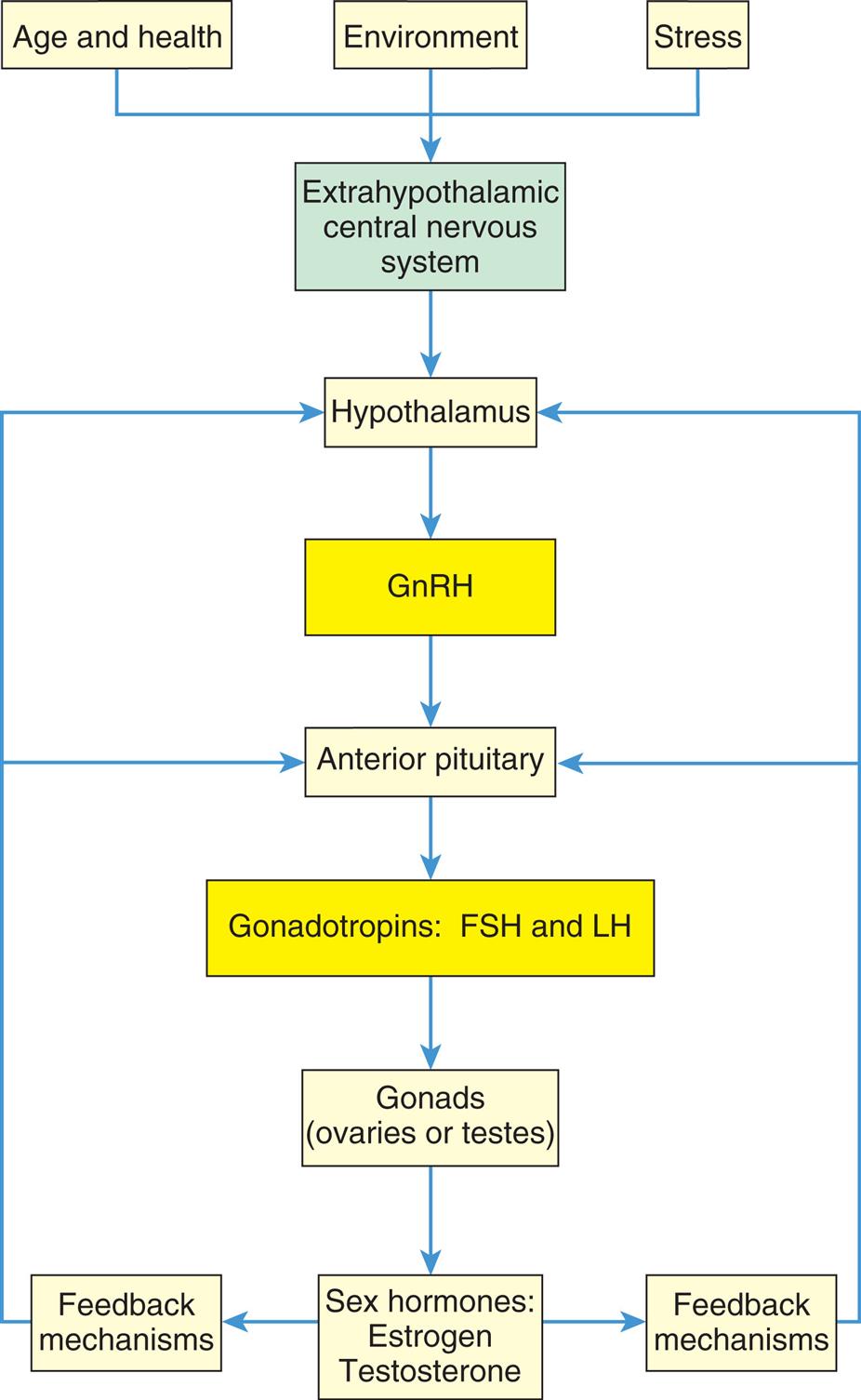
The hypothalamic-pituitary-gonadal axis. FSH, Follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
“A flowchart represents the hormonal stimulation of the gonads. 1. Age and health, environment, and stress. Impacts 2. 2. Extrahypothalamic central nervous system. Triggers 5. 3. Hypothalamus. Secretes 6. 4. G n R H. Triggers 7. 5. Anterior pituitary. Releases 8. 6. Gonadotropins: F S H and L H. Affects 9. 7. Gonads (ovaries or testes). Releases 10. 8. Sex hormones: estrogen, testosterone. Provides feedback mechanisms to 3 and 5.”
Puberty is a time during which some individuals begin to experience gender dysphoria.Gender dysphoria refers to the discomfort, distress, disharmony, and conflict caused by the discrepancy between a person’s gender identity and their personal sense of self as a man or woman, their associated primary or secondary sexual characteristics, and their expected social gender roles. Transgender is a term used to describe individuals whose gender identity or expression differs from their sex assigned at birth. Many transgender individuals choose hormonal or surgical interventions to reduce gender dysphoria (see Emerging Science Box: Gender Reassignment and Gender Affirming Hormone Therapy).
The Female Reproductive System
The function of the female reproductive system is to produce mature ova; then, if fertilization occurs, the female reproductive system provides protection and nourishment to the fetus until it is expelled at birth.
External Genitalia
The external genitalia protect body openings and play an important role in sexual functioning. Fig. 24.4 shows the external female genitalia, known collectively as the vulva or pudendum. The major structures are as follows:
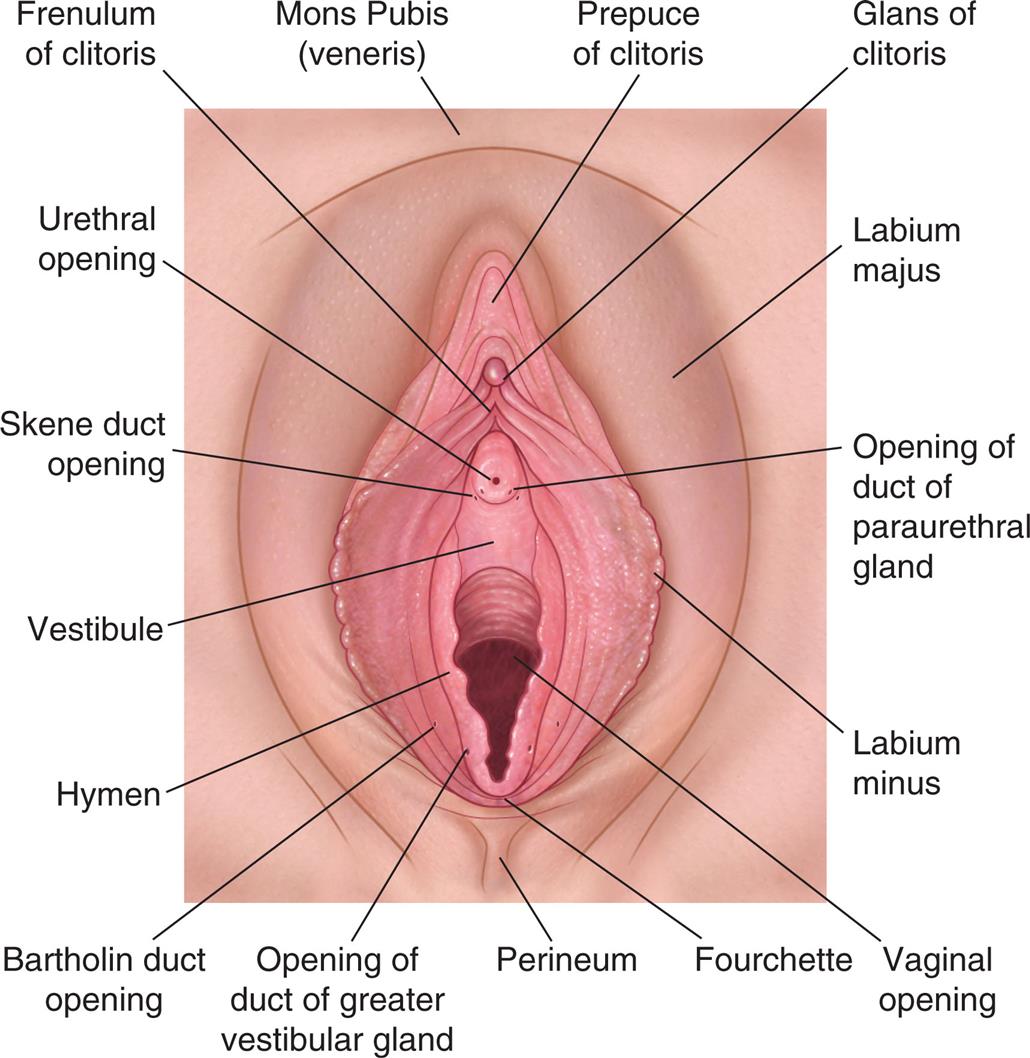
An illustration of the external female genitalia shows and labels the following structures, clockwise from the top: mons pubis (veneris), prepuce of clitoris, glans of clitoris, labium majus, opening of duct of paraurethral gland, labium minus, vaginal opening, fourchette, perineum, opening of duct of greater vestibular gland, Bartholin duct opening, hymen, vestibule, Skene duct opening, urethral opening, and frenulum of clitoris.
Mons Pubis
The mons pubis is a fatty layer of tissue over the pubic symphysis (joint formed by the union of the pubic bones) that protects the joint during sexual intercourse. During puberty, it becomes covered with pubic hair, and sebaceous and sweat glands become more active. Estrogen causes the fat to be deposited under the skin giving it a mound-like shape.
Labia Majora and Minora
The labia majora (sing., labium majus) is composed of two folds of skin arising at the mons pubis and extending back to the fourchette, forming a cleft. During puberty, the amount of fatty tissue increases, pubic hair grows on lateral surfaces, and sebaceous glands on hairless medial surfaces secrete lubricants. This structure is highly sensitive to temperature, touch, pressure, and pain and protects the inner structures of the vulva. It is homologous to the male scrotum (see Fig. 24.2).
The labia minora (sing., labium minus) is composed of two smaller, thinner, asymmetric folds of skin within the labia majora that form the clitoral hood (prepuce) and frenulum, then split to enclose the vestibule, and converge near the anus to form the fourchette. The labia minora are hairless, pink, and moist; they are well supplied by nerves, blood vessels, and sebaceous glands that secrete bactericidal fluid with a distinctive odor that lubricates and waterproofs vulvar skin. The labia swell with blood during sexual arousal.
Clitoris
The clitoris is a richly innervated erectile organ between the labia minora. It is a small, cylindric structure having a visible glans and a shaft that lies beneath the skin; the clitoris is homologous to the penis. It secretes smegma, which has a unique odor that may be sexually arousing. Like the penis, the clitoris is a major site of sexual stimulation and orgasm. With sexual arousal, erectile tissue fills with blood, causing the clitoris to enlarge slightly.
Vestibule
The vestibule is an area protected by the labia minora that contains the external opening of the vagina, called the introitus or vaginal orifice. A thin, perforated membrane, the hymen, may cover the introitus. The vestibule also contains the opening of the urethra, or urinary meatus (orifice). These structures are lubricated by two pairs of glands: Skene glands and Bartholin glands. The ducts of the Skene glands (also called the lesser vestibular or paraurethral glands) open on both sides of the urinary meatus. The ducts of the Bartholin glands (greater vestibular or vulvovaginal glands) open on either side of the introitus. In response to sexual stimulation, Bartholin glands secrete mucus that lubricates the inner labial surfaces, as well as enhances the viability and motility of sperm. Skene glands help lubricate the urinary meatus and the vestibule. In response to sexual excitement, the highly vascular tissue just beneath the vestibule also fills with blood and becomes engorged.
Perineum
The perineum is an area with less hair, skin, and subcutaneous tissue lying between the vaginal orifice and anus. Unlike the rest of the vulva, this area has little subcutaneous fat, so the skin is close to the underlying muscles. The perineum covers the muscular perineal body, a fibrous structure that consists of elastic fibers and connective tissue and serves as the common attachment for the bulbocavernosus, external anal sphincter, and levator ani muscles. The perineum varies in length from 2 to 5 cm or more and has elastic properties. The length of the perineum and the elasticity of the perineal body influence tissue resistance and injury during childbirth.
Internal Genitalia
Vagina
The vagina is an elastic, fibromuscular canal that is 9 to 10 cm long in a reproductive-age female. It extends up and back from the introitus to the lower portion of the uterus. As Fig. 24.5 shows, the vagina lies between the urethra (and part of the bladder) and the rectum. Mucosal secretions from the upper genital organs, menstrual fluids, and products of conception leave the body through the vagina. During coitus, the penis enters the vagina. During sexual arousal, the vagina lengthens and widens, and the vaginal wall becomes engorged with blood, much like the labia minora and clitoris. Engorgement pushes some fluid to the surface of the mucosa, enhancing lubrication. The vaginal wall does not contain mucus-secreting glands; rather, secretions drain into the vagina from the endocervical glands or from the Bartholin and Skene glands of the vestibule. The vagina also functions as the birth canal during childbirth. Its elasticity and relatively sparse nerve supply enhance the vagina's function in this role. During childbirth, the pelvic floor muscles and rugae of the vagina stretch to facilitate the passage of the infant.
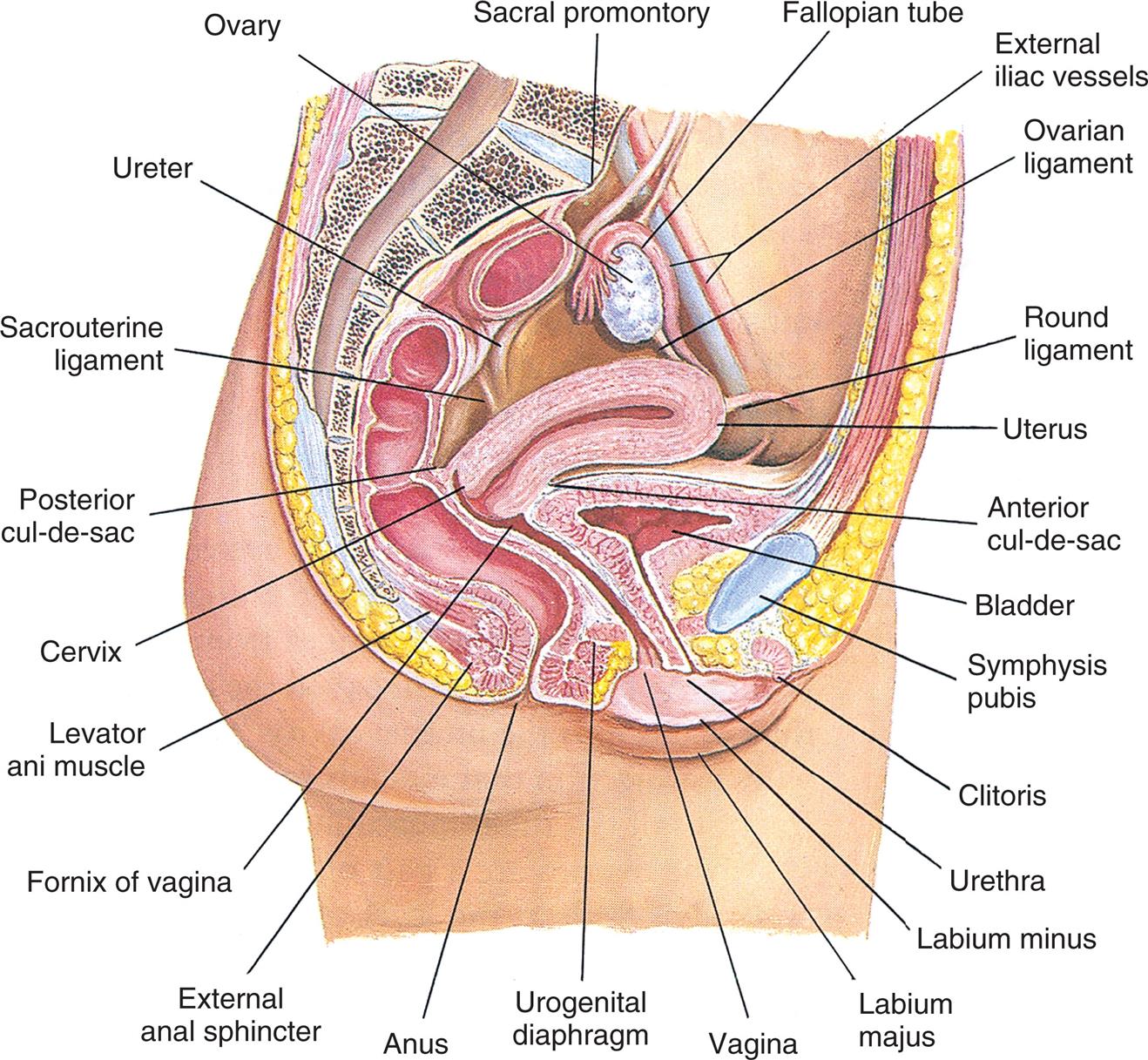
An illustration of the internal female genitalia and other pelvic organs shows and labels the following structures, clockwise from the top: sacral promontory, fallopian tube, external iliac vessels, ovarian ligament, round ligament, uterus, anterior cul-de-sac, bladder, symphysis pubis, clitoris, urethra, labium minus, labium majus, vagina, urogenital diaphragm, anus, external anus sphincter, fornix of vagina, levator ani muscle, cervix, posterior cul-de-sac, sacrouterine ligament, ureter, and ovary.
The vaginal wall is lined with a mucous membrane of squamous epithelial cells that thickens and thins in response to hormones, particularly estrogen. The squamous epithelial membrane is continuous with the membrane that covers the lower part of the uterus. In females of reproductive age, the mucosal layer is arranged in transverse wrinkles, or folds, called rugae (sing., ruga), that permit stretching during coitus and childbirth. Below the mucosal layer are three more layers: fibrous connective tissue containing numerous blood and lymphatic vessels, smooth muscle and connective tissue, and a rich network of blood vessels.
The upper part of the vagina surrounds the cervix, the lower end of the uterus (see Fig. 24.5). The recessed space around the cervix is called the fornix of the vagina. The posterior fornix is “deeper” than the anterior fornix because of the angle at which the cervix meets the vaginal canal. In most females, this angle is about 90 degrees. A pouch called the cul-de-sac separates the posterior fornix and the rectum.
Two factors help maintain the self-cleansing action of the vagina and defend it from infection, particularly during the reproductive years. They are (1) an acid-base balance that discourages the proliferation of most pathogenic bacteria and (2) the thickness of the vaginal epithelium. Before puberty, vaginal pH is about 7.0 (neutral), and the vaginal epithelium is thin. At puberty, the pH becomes more acidic (4.0 to 5.0), and the squamous epithelial lining thickens. These changes are maintained until menopause (cessation of menstruation) when the pH rises again to more alkaline levels and the epithelium thins. Therefore, protection from infection is greatest during the years when a female is most likely to be sexually active. Both defense factors are greatest when estrogen levels are high, and the vagina contains a normal population of Lactobacillus acidophilus, a harmless resident bacterium that helps maintain pH at acidic levels. Any condition that causes vaginal pH to rise—such as douching or use of vaginal sprays or deodorants, the presence of low estrogen levels, or destruction of L. acidophilus by antibiotics—lowers vaginal defenses against infection.
Uterus
The uterus is a hollow, pear-shaped organ whose lower end opens into the vagina. It anchors and protects a fertilized ovum, provides an optimal environment while the ovum develops, and pushes the fetus out at birth. In addition, the uterus plays an important role in sexual response and conception. During sexual excitement, the opening of the lower uterus (the cervix) dilates slightly. At the same time, the uterus increases in size and moves upward and backward, creating a tenting effect in the midvagina that results in the cervix “sitting” in a pool of semen. During orgasm, rhythmic contractions facilitate the movement of sperm through the cervical os while also enhancing physical pleasure.
At puberty, the uterus attains its adult size and proportions and descends from the abdomen to the lower pelvis, between the bladder and the rectum (see Fig. 24.5). The uterus of a mature, nonpregnant female is approximately 7 to 9 cm long and 6.5 cm wide, with muscular walls 3.5 cm thick, enlarging by about 1 cm in all dimensions after pregnancy.1 It is loosely held in position by ligaments, peritoneal tissue folds, and the pressure of adjacent organs, especially the urinary bladder, sigmoid colon, and rectum. In most females, the uterus is tipped forward (anteverted) so that it rests on the urinary bladder. However, it may be tipped backward (retroverted), and various degrees of forward or backward flexion are normal (Fig. 24.6).
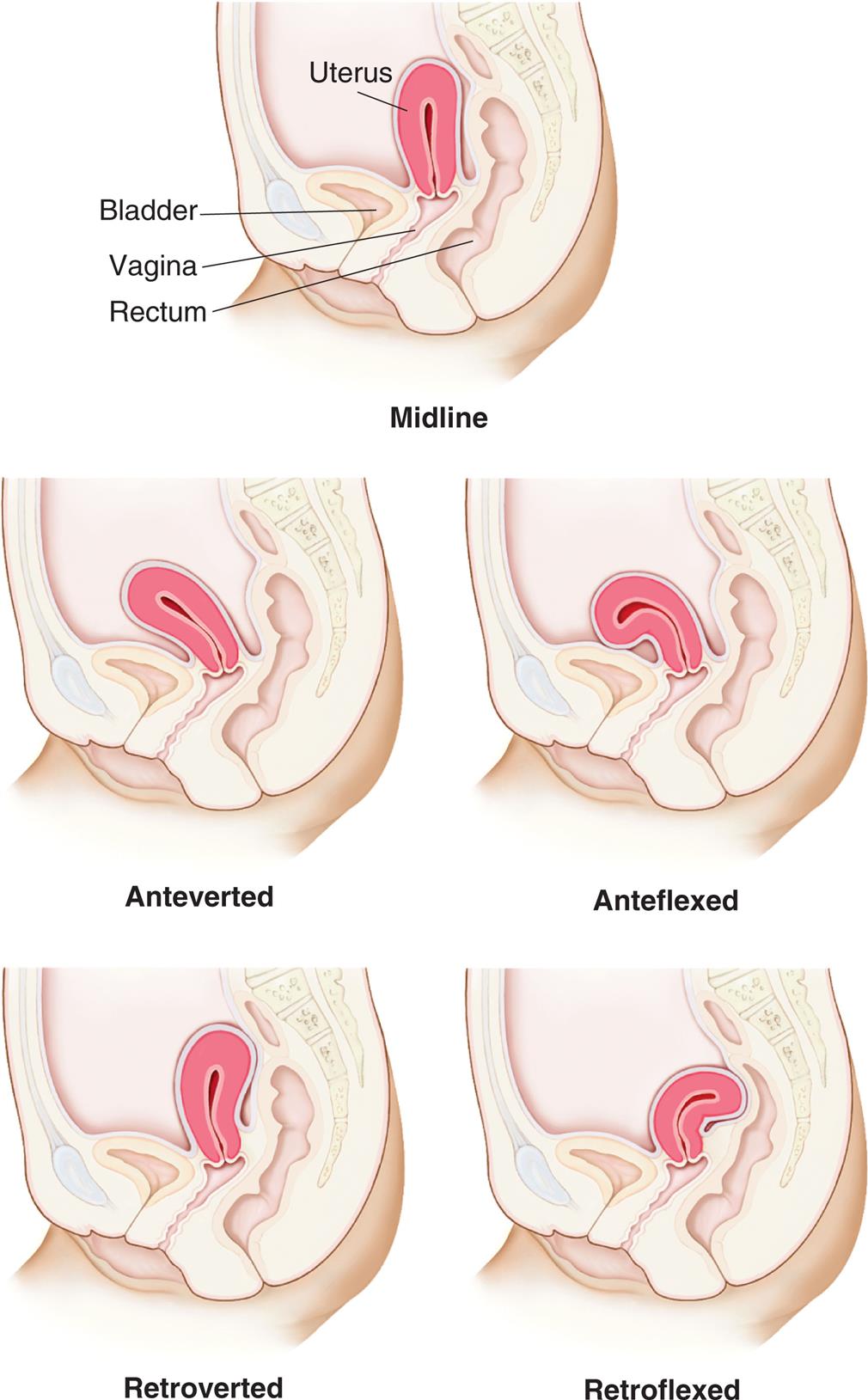
“An illustration of the lateral view of the pelvis, showing the uterus, bladder, vagina, and rectum, highlights the variations in uterine positions. Top panel, midline. The uterus is vertical. Middle-left panel, anteverted. The uterus is bent toward to the bladder. Middle-right panel, anteflexed. The uterus is curled toward the bladder. Bottom-left panel, retroverted. The uterus is bent away from the bladder. Bottom-right panel, retroflexed. The uterus is pressing at the rectal wall.”
The uterus has two major parts: the corpus (body of the uterus) and the cervix (Fig. 24.7). The top of the corpus, above the insertion of the fallopian tubes, is called the fundus. The diameter of the uterine cavity is widest at the fundus and narrowest at the isthmus, just above the cervix (see Fig. 24.5). The cervix, or “neck of the uterus,” extends from the isthmus to the vagina. The passageway between the upper opening (the internal os) and the lower opening (the external os) of the cervix is called the endocervical canal (see Fig. 24.7). The entire uterus, like the upper vagina, is innervated exclusively by motor and sensory fibers of the autonomic nervous system.
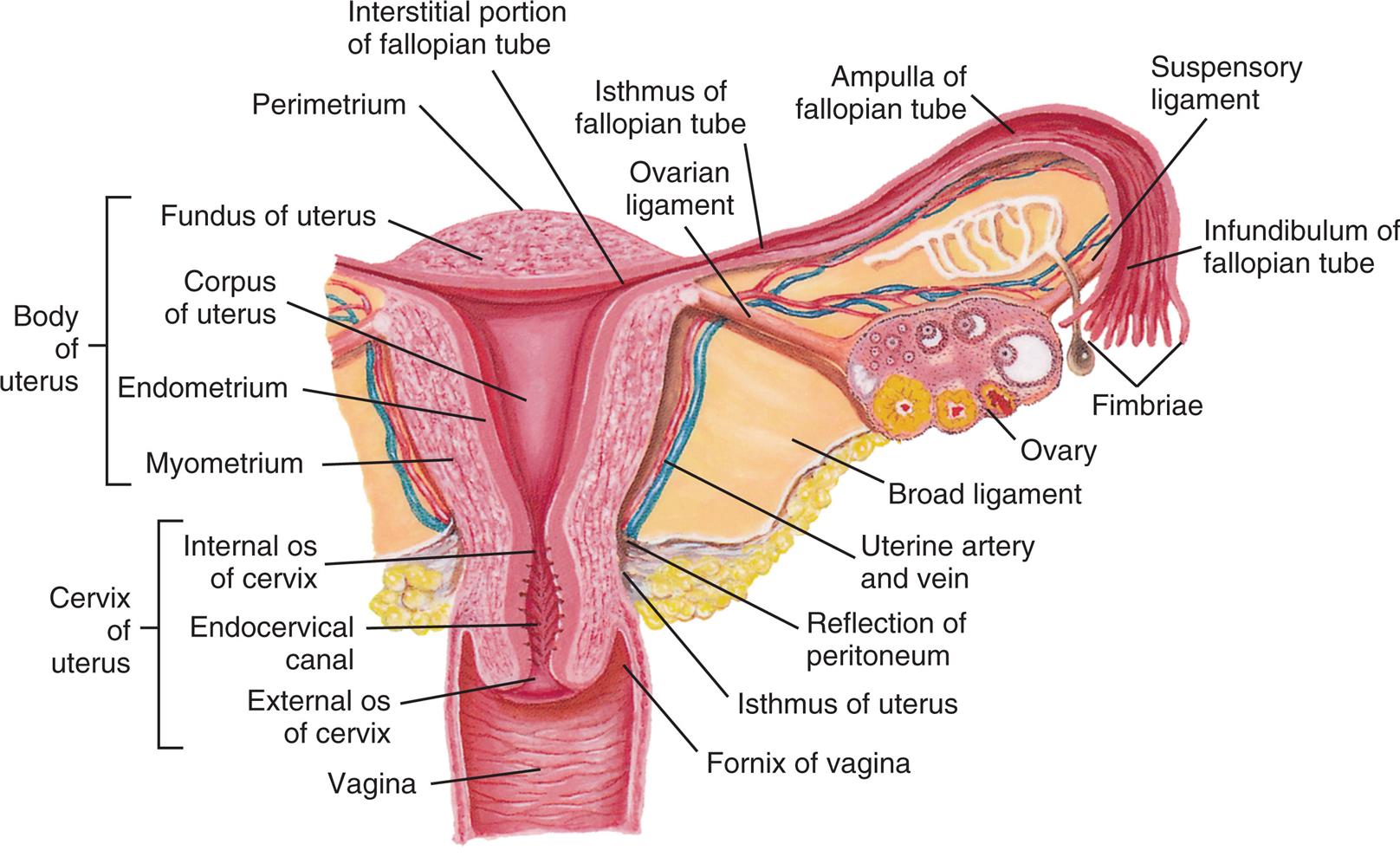
An illustration of the cross-section of the uterus, fallopian tube, and ovary, shows and labels the following structures, clockwise from the top: perimetrium, interstitial portion of fallopian tube, ovarian ligament, isthmus of fallopian tube, ampulla of fallopian tube, suspensory ligament, infundibulum of fallopian tube, fimbriae, ovary, broad ligament, uterine artery and vein, reflection of peritoneum, isthmus of uterus, fornix of vagina, vagina, cervix of uterus, and body of uterus. The cervix of uterus includes the following parts, from the top: internal o s of cervix, endocervical canal, and external o s of cervix. The body of uterus includes the following parts, from the top: fundus of uterus, corpus of uterus, endometrium, and myometrium.
The uterine wall is composed of three layers (see Fig. 24.7). The perimetrium (parietal peritoneum) is the outer serous membrane that covers the uterus. The myometrium is the thick, muscular middle layer. It is thickest at the fundus, apparently to facilitate birth. The endometrium, or uterine lining, is composed of a functional layer (superficial compact layer and spongy middle layer) and a basal layer. The functional layer of the endometrium responds to the sex hormones estrogen and progesterone. Between puberty and menopause, this layer proliferates and is shed monthly. The basal layer, which is attached to the myometrium, regenerates the functional layer after shedding (menstruation).
The endocervical canal does not have an endometrial layer but is lined with columnar epithelial cells. It is continuous with the lining of the outer cervix and vagina, which are lined with squamous epithelial cells. The point where the two types of cells meet is called the transformation zone, or squamous-columnar junction (see Fig. 25.20). The transformation zone is vulnerable to the human papillomavirus (HPV), especially HPV types 16 and 18, which can lead to cervical dysplasia or carcinoma in situ. Cells of the transformation zone are removed for examination during a Papanicolaou (Pap test) smear.
The cervix acts as a mechanical barrier, protecting the uterus from infectious microorganisms from the vagina. The external cervical os is a very small opening that contains thick, sticky mucus (the mucous “plug”) during the luteal phase of the menstrual cycle and throughout pregnancy. During ovulation, the mucus changes under the influence of estrogen and forms watery strands, or spinnbarkeit mucus, to facilitate the transport of sperm into the uterus. In addition, the downward flow of cervical secretions moves microorganisms away from the cervix and uterus. In females of reproductive age, the pH of these secretions is inhospitable to many bacteria. Further, mucosal secretions contain enzymes and antibodies (mostly immunoglobulin A [IgA]) of the secretory immune system. Uterine pathophysiologic disorders include infection, displacement of the uterus within the pelvis, benign growths (fibroids) of the uterine wall, hyperplasia of the endometrium, endometriosis, and cancer (see Chapter 25).
Fallopian Tubes
The two fallopian tubes (oviducts, uterine tubes) enter the uterus bilaterally just beneath the fundus (see Fig. 24.7). They direct the ova from the spaces around the ovaries to the uterus. From the uterus, the fallopian tubes curve up and over the two ovaries. Each tube is 8 to 12 cm long and about 1 cm in diameter, except at its ovarian end, which resembles the bell of a trumpet and is fringed or fimbriated (infundibulum). The fimbriae (fringes) move, creating a current that draws the ovum into the infundibulum. Once the ovum enters the fallopian tube, cilia (hairlike structures) and peristalsis (muscle contractions) keep it moving toward the uterus.
The ampulla, or distal third, of the fallopian tube is the usual site of fertilization (see Fig. 24.7). Sperm released into the vagina travel upward through the endocervical canal and uterine cavity and enter the fallopian tubes. If an ovum is present in either tube, fertilization can occur. Whether or not the ovum encounters sperm, it continues to travel through the fallopian tube to the uterus. If fertilized, the ovum (then called a blastocyst) implants itself in the endometrial layer of the uterine wall. If not fertilized, the ovum fragments and leaves the uterus with menstrual fluids. Disorders that affect the fallopian tubes (e.g., congenital malformations, infection, and inflammation) can block the path of both sperm and the ovum and may cause infertility or ectopic (tubal) pregnancy.
Ovaries
The ovaries, the female gonads, are the primary female reproductive organs (Fig. 24.8). Their two main functions are secretion of female sex hormones and development and release of female gametes, or ova.
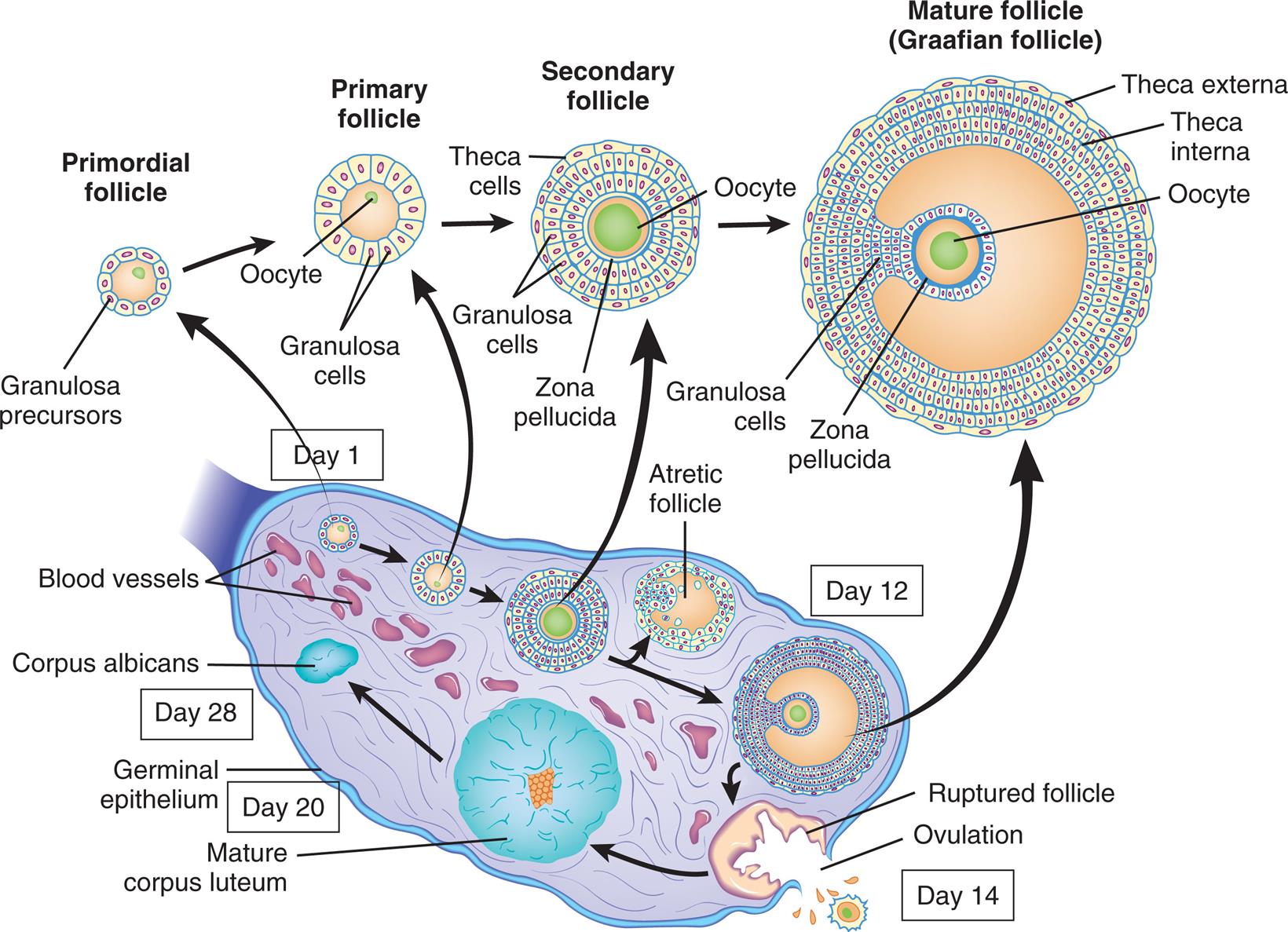
Schematic representation (not to scale) of the structure of the ovary, showing the various stages in the development of the follicle and its successor structure, the corpus luteum. (Adapted from Berne RM, Levy MN, eds. Physiology, 5th edition. St. Louis: Mosby; 2003.)
“A series of illustration of the cross-section of the ovary tracks the development of ovarian follicle. Day 1. The primordial follicle is an oocyte surrounded by a layer of granulosa precursors. The follicle undergoes the following changes until Day 12. • The primary follicle has a larger oocyte surrounded by a layer of granulosa cells. • The secondary follicle is filled with oocyte and is surrounded by a zona pellucida, multiple layers of granulosa cells, and an outer layer of theca cells. • The atretic follicle develops. Day 12. The mature follicle (Graafian follicle) shows an oocyte surrounded by a thicker zona pellucida, proliferated granulosa cells, theca interna, and theca externa. Day 14. The follicle ruptures and results in ovulation. A mature corpus luteum develops. Day 20. Geminal epithelium. Mature corpus luteum develops. Day 28. Corpus albicans.”
The almond-shaped ovaries are located on both sides of the uterus and are suspended and supported by a portion of the broad ligament (the mesovarium component), ovarian ligaments, and suspensory ligaments (see Fig. 24.7). The ovaries are smaller than their male homologs, the testes. In females of reproductive age, each ovary is about 3 to 5 cm long, 2.5 cm wide, and 2 cm thick, weighing 4 to 8 g. Size and weight vary slightly during each phase of the menstrual cycle (see the Menstrual (Ovarian) Cycle section).
The central part of the ovary, or medulla, is composed of connective tissue and contains many small arteries, veins, and lymphatics that enter at the hilum. Surrounding the medulla is the cortex. At birth, the cortex of each ovary contains approximately 1 to 2 million ova within primordial (immature) ovarian follicles. By puberty, the number ranges between 300,000 and 500,000, and some of the follicles and the ova within them begin to mature. Follicles grow and undergo atresia continuously and irrevocably throughout a woman's life. Between puberty and menopause, the ovarian cortex always contains follicles and ova in various stages of development (primary and secondary follicles). Once every menstrual cycle (about every 28 days), one of the follicles reaches maturation and discharges its ovum through the ovary's outer covering, the germinal epithelium. During the reproductive years, 400 to 500 ovarian follicles mature completely and release an ovum (ovulation). The remaining follicles either fail to develop at all or degenerate without maturing completely and are known as atretic follicles (see Fig. 24.8).
After the release of the mature ovum (ovulation), the follicle develops into another structure, the corpus luteum (see Fig. 24.8). If fertilization occurs, the corpus luteum enlarges and begins to secrete hormones that maintain and support pregnancy. If fertilization does not occur, the corpus luteum secretes these hormones for approximately 14 days and then degenerates, which triggers the maturation of another follicle. The ovarian cycle—the process of follicular maturation, ovulation, corpus luteum development, and corpus luteum degeneration—is continuous from puberty to menopause, except during pregnancy or hormonal contraceptive use. At menopause, this process ceases, and the ovaries atrophy to the point that they cannot be felt during a pelvic examination.
Sex hormones are secreted by cells present within the ovarian cortex, including two types of cells in the ovarian follicle—theca cells (produce androgens that migrate to granulosa cells) and granulosa cells (convert androgens to estradiol)—and cells of the corpus luteum (secrete primarily progesterone, estrogen, and inhibin) (see Fig. 24.8). These cells all contain receptors for gonadotropins (LH, FSH) or for sex hormones, which are discussed in the next section.
Female Sex Hormones
The sex hormones are all steroid hormones and are synthesized from cholesterol (see Chapter 21). The dominant female sex hormones, estrogen and progesterone, are produced primarily by the ovaries (see Table 24.1). During fetal development, infancy, and childhood, sex hormone production is low. At puberty, hormone production surges, triggering sexual maturation and the development of secondary sex characteristics. From puberty to menopause, the sex hormones are produced cyclically; production surges and diminishes monthly, creating the ovarian and uterine changes associated with the menstrual cycle. These hormones also are produced in higher levels during pregnancy by the placenta, inhibiting ovulation. Individual effects of sex hormones depend on the amount and concentration in the blood.
Both male and female sex hormones are present in all adults. However, the female body contains low levels of testosterone and other androgens, and the male body contains low levels of estrogen.
Estrogens and Androgens
Estrogen is a generic term for any of three similar hormones derived from cholesterol: estradiol, estrone, and estriol. Estradiol (E2) is the most potent and plentiful of the three and is principally produced (95%) by the ovaries (ovarian follicle and corpus luteum). Limited amounts are secreted by the cortices of the adrenal glands and the placenta during pregnancy. Androgens are converted to estrone in ovarian and adipose tissue; estriol is the peripheral metabolite of estrone and estradiol.
Estrogen has numerous biologic effects, many of which involve interactions with other hormones. It is needed for the maturation of reproductive organs, development of secondary sex characteristics, growth, and maintenance of pregnancy. It also is needed for many nonreproductive effects, including closure of long bones after the pubertal growth spurt (in both males and females), maintenance of bone and skin, and systemic organ function (see Table 24.1 and Box 24.1). After menopause, the ovaries dramatically reduce the production of estradiol, and secretion of estrone is markedly diminished (see the Aging and the Female Reproductive System section). At this time, the majority of estradiol is derived from intracellular synthesis in peripheral tissues. Estradiol acts locally to meet physiologic needs according to cell type and is then inactivated without systemic effects.2
Like other steroid hormones, estrogens are derived from cholesterol in a complex, enzyme-mediated series of reactions. The hypothalamus secretes GnRH in a pulsatile manner that stimulates gonadotropin (LH and FSH) release from the anterior pituitary. Gonadotropins trigger the ovarian production of estrogen. The primary function of LH is to stimulate theca cells of the ovarian follicle to produce androgens, mainly androstenedione. (Androgens are discussed further under the section titled Male Sex and Reproductive Hormones.) Some of these androgens are converted to estrogen by the theca cells themselves, and others diffuse into the granulosa cells. Within the granulosa layer, FSH induces conversion (aromatization) of androgens to estrogens. Estrogens are then released into the bloodstream.
Although androgens are primarily male sex hormones produced in the testes, small amounts are produced in the adrenal cortex in both males and females and in the ovaries in females. Some androgens (dehydroepiandrosterone and its metabolite androstenedione) are precursors of estrogens (estrone, estradiol) (see Table 24.1). At puberty, androgens contribute to the skeletal growth spurt and cause the growth of pubic and axillary hair. Androgens also activate sebaceous glands, accounting for some cases of acne during puberty, and play a role in libido.
Progesterone
Luteinizing hormone from the anterior pituitary stimulates the corpus luteum to secrete progesterone, the second major female sex hormone. With estrogen, progesterone controls the ovarian menstrual cycle. LH surge occurs when there is a peak level of estrogen, about 24 to 36 hours before ovulation. LH promotes luteinization of the granulosa in the dominant follicle, resulting in progesterone production and the development of blood vessels and connective tissue. During the follicular phase, the ovary and adrenal glands each contribute approximately 50% of progesterone production. Conversely, large amounts are cyclically secreted from the ovary while the corpus luteum is active for about 9 to 13 days after ovulation. (The complementary and opposing effects of progesterone and estrogen are listed in Table 24.2.) Progesterone secreted by the corpus luteum stimulates the thickened endometrium to become more complex in preparation for implantation of a blastocyte. If conception and implantation do occur, the corpus luteum persists and secretes progesterone (and estrogen) until the placenta is well established at approximately 8 to 10 weeks’ gestation and undertakes progesterone production.
Table 24.2
Progesterone is sometimes called the hormone of pregnancy. Progesterone's effects in pregnancy include:
- • maintaining the thickened endometrium;
- • relaxing the smooth muscle in the myometrium, which prevents premature contractions and helps the uterus to expand;
- • thickening (hypertrophy) the myometrium, which prepares it for the muscular work of labor;
- • promoting the growth of lobules and alveoli in the breast in preparation for lactation, but preventing lactation until the fetus is born and then promoting lactation in collaboration with prolactin after birth;
- • preventing additional maturation of ova by suppressing FSH and LH, thereby stopping the menstrual cycle;
- • providing immune modulation, allowing tolerance against fetal antigens (the mother's immune system does not attack the fetus); and
- • preventing preterm birth.
Menstrual (Ovarian) Cycle
In addition to pregnancy, the obvious manifestation of female reproductive functioning is menstrual bleeding (the menses), which starts with menarche (first menstruation) and ends with menopause (cessation of menstrual flow for 1 year). In the United States, the age of first menstruation is about 12 years.3
The onset of menarche appears to be related to body weight, especially a high percentage of body fat (a high ratio of fat to lean tissue), which many trigger a change in the metabolic rate and lead to hormonal changes associated with early menarche. The hormone leptin increases before the onset of menarche. Leptin (a regulatory hormone of appetite and energy metabolism) promotes the secretion of kisspeptin from the hypothalamus and leads to the release of GnRH, which in turn enhances the release of FSH and LH and estradiol, triggering ovulation and the onset of puberty. A high percentage of body fat is associated with higher levels of leptin. Childhood obesity is associated with an increase in leptin and with early menarche (age 11 years or younger).4
Cycles are not ovulatory at first and may vary in length from 10 to 60 days or more. As adolescence proceeds, regular patterns of menstruation and ovulation are established at intervals ranging between 21 and 45 days.5 Menstruation continues to recur in a recognizable and characteristic pattern during adulthood, with the length of the menstrual cycle varying considerably among individuals. The commonly accepted cycle average is 28 (25 to 30) days, with rhythmic intervals of 21 to 35 days considered normal (Fig. 24.9). Approximately 4 to 10 years before menopause, cycles begin to lengthen again with variation related to changing hormone levels.6
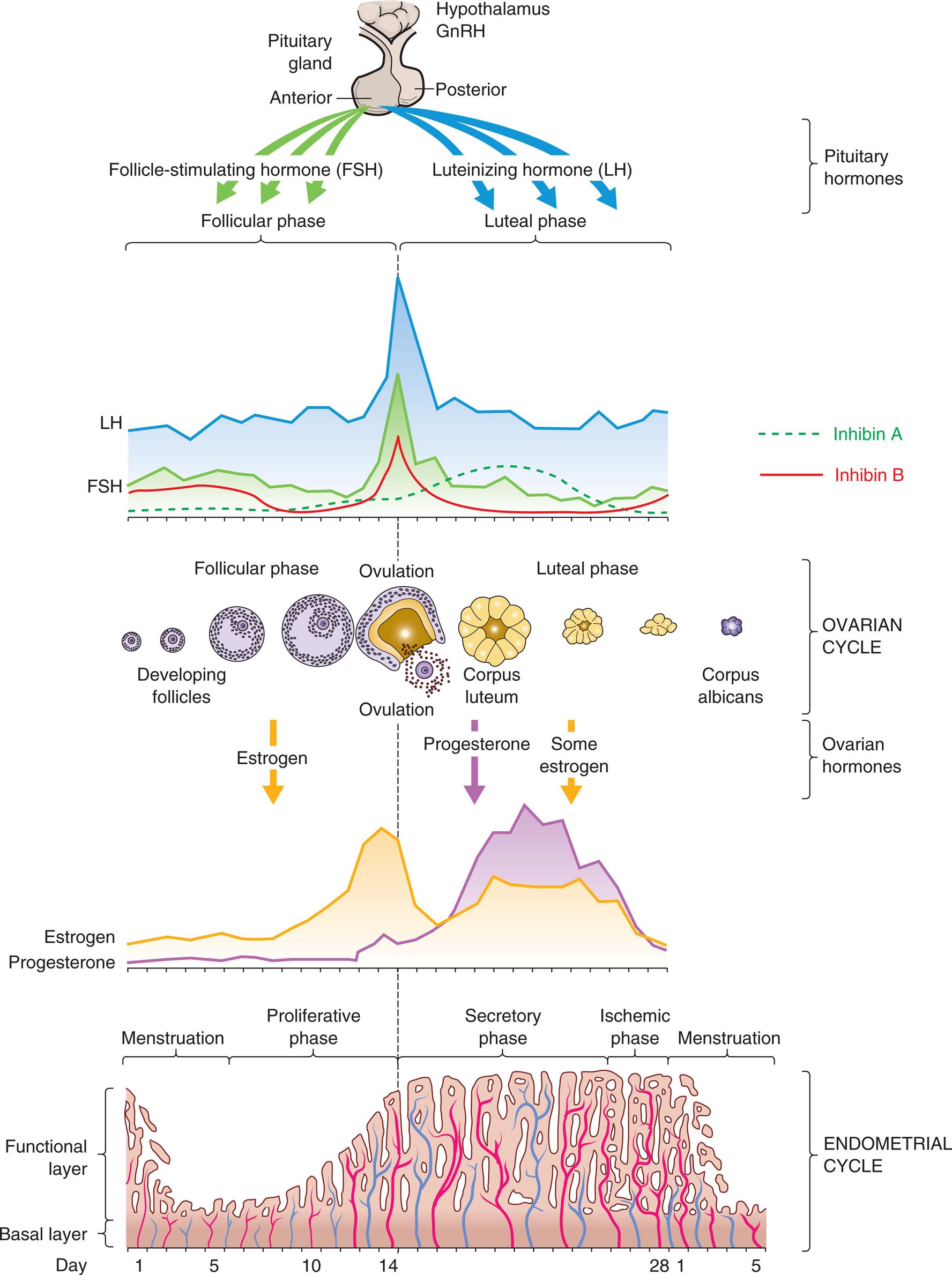
GnRH, Gonadotropin-releasing hormone. (Adapted from Lowdermilk DL, et al. Maternity and women’s health care, 10th edition. St. Louis: Mosby; 2012.)
Four illustrations show the four phases of the menstrual cycle. The cycle consists of follicular or proliferative phases, luteal or secretory phases, follicular or luteal phases, and proliferative, secretory, or ischemic phases.
Phases of the Menstrual Cycle
The menstrual (ovarian) cycle (see Fig. 24.9) is the process of menstruation (menses) in the uterine endometrium and ovulation in the ovary. The cycle consists of the follicular/proliferative phase (postmenstrual) followed by the luteal/secretory phase (premenstrual) and then the ischemic/menstrual phase if conception does not occur. These are named for processes that occur in the ovary (follicular and luteal phases) and the uterine endometrium (proliferative, secretory, and ischemic phases) during the menstrual cycle.
During menstruation (menses), the functional layer of the endometrium disintegrates and is discharged through the vagina. Menstruation is followed by the follicular/proliferative phase. This phase is named for two simultaneous processes: maturation of an ovarian follicle and proliferation of the uterine endometrium (see Fig. 24.9). During this phase, GnRH and a balance between activin and inhibin levels from the granulosa cells contribute to the increase of FSH level, which stimulates a number of follicles. The result is a rescue of a dominant ovarian follicle from normal dissolution by days 5 to 7 of the cycle. Together, estrogen and FSH make the granulosa cells of the primary follicle more sensitive to FSH and promote LH stimulation, which causes a more rapid secretion of follicular estrogen. A surge in the levels of both LH and FSH is then required for final follicular growth and ovulation. Estrogen levels increase, and inhibin B inhibits the secretion of FSH level by the granulosa cells in the dominant follicle. This drop in FSH concentration decreases the growth of less developed follicles (see Fig. 24.8). Estrogen causes cells of the endometrium to proliferate.
Ovulation is the release of an ovum from a mature follicle and marks the beginning of the luteal/secretory phase of the menstrual cycle. The ovarian follicle begins its transformation into a corpus luteum (see Fig. 24.8), hence the name luteal phase. Pulsatile secretion of LH from the anterior pituitary stimulates the corpus luteum to secrete progesterone, estrogen, and inhibin A (suppresses FSH secretion), which in turn initiates the secretory phase of endometrial development. Estrogen maintains the thickness of the endometrium, and progesterone stimulates the growth of glands and blood vessels in the endometrium. The glands begin to secrete a thin, glycogen-containing fluid, hence the name secretory phase. At this point, one of the following two paths occurs:
- • If conception and implantation do not occur, the corpus luteum degenerates and ceases its production of progesterone and estrogen. Without progesterone or estrogen to maintain it, the endometrium enters the ischemic (“blood-starved”) phase and disintegrates, hence the name ischemic/menstrual phase. Menstruation then occurs, marking the beginning of another cycle.
- • If conception occurs, the nutrient-laden endometrium is ready for implantation. Human chorionic gonadotropin (hCG) is secreted 3 days after fertilization by the blastocytes and maintains the corpus luteum once implantation occurs at about day 6 or 7. Levels of hCG can be detected in maternal blood and urine 8 to 10 days after ovulation. The production of estrogen and progesterone will continue until the placenta can adequately maintain hormonal production.
Ovulatory cycles appear to have a minimum length of 24 to 26.5 days: the ovarian follicle requires 10 to 12.5 days to develop, and the luteal phase appears fixed at 14 days (±3 days). Menstrual blood flow usually lasts 3 to 7 days but may be between 2 and 8 days and still be considered within normal limits. Bleeding is consistently scant to heavy and varies from 30 to 80 ml, with most blood loss occurring during the first 3 days of menses. Menstrual discharge consists of blood, mucus, and desquamated endometrial tissue and does not clot under normal circumstances. It is usually dark and produces a characteristic musty odor on oxidation. Environmental factors such as severe emotional stress, illness, malnutrition, obesity, extreme exercise, and seasonal variation may affect the length of the menstrual cycle.7,8
Cervical mucus also undergoes cyclic changes during the menstrual cycle. During the proliferative phase, the cervical mucus is thin and watery. Peak estrogen levels occur just before ovulation and maximally stimulate the cervical glands to produce mucus. Cervical mucus becomes abundant and more elastic (spinnbarkeit). In the presence of estrogen, tiny channels develop in the mucus, which allows sperm access to the interior of the uterus. Changes in the consistency of cervical mucus can be used to identify fertile intervals. After ovulation, the ovary begins to secrete progesterone under the influence of the corpus luteum. The amount of cervical mucus is reduced, becomes thicker and stickier, and blocks sperm migration.
The vaginal epithelium also responds to the cyclic hormonal changes of the menstrual cycle. Under the influence of estrogen, cells of the vaginal epithelium become thicker during the follicular/proliferative phase. After ovulation, layers of keratinized cells overgrow the basal epithelium (cells become larger and flatter), a process known as cornification. Near the end of the luteal phase, leukocytes invade the vaginal epithelium, removing the outer layers in a process termed decornification with thinning of the epithelium.
Basal body temperature (BBT) undergoes characteristic biphasic changes during menstrual cycles in which ovulation occurs. During the follicular phase, the BBT fluctuates around 98°F (37°C). During the luteal phase, the average temperature increases by 0.4°F to 1.0°F (0.2°C to 0.5°C). At the end of the luteal phase, 1 to 3 days before the onset of menstruation, BBT declines to follicular-phase levels. The shift in temperature is related to ovulation, corpus luteum formation, and increased serum progesterone levels. Progesterone acts on the thermoregulatory center of the hypothalamus to increase body temperature. Changes in BBT are used to estimate ovulatory cycles but when used alone may not be the best method to support fertility awareness.9
Hormonal Control
Hormonal control of the menstrual cycle depends on complex interactions among the hypothalamus, the anterior pituitary, and the ovaries (or hypothalamic-pituitary-ovarian [HPO] axis) (Table 24.3). Hormonal control is dependent on negative and positive ovarian feedback mechanisms. In the hypothalamus, kisspeptin activates the release of GnRH to stimulate the gonadotropin production of FSH and LH. The constant and pulsatile release of GnRH is critical to the timing of the menstrual cycle. GnRH is secreted into the hypophyseal portal system and travels to the anterior pituitary, where it stimulates the secretion of LH and FSH. FSH and LH are released from the anterior pituitary in pulses that correspond to the pulsatile secretion of GnRH.
Table 24.3
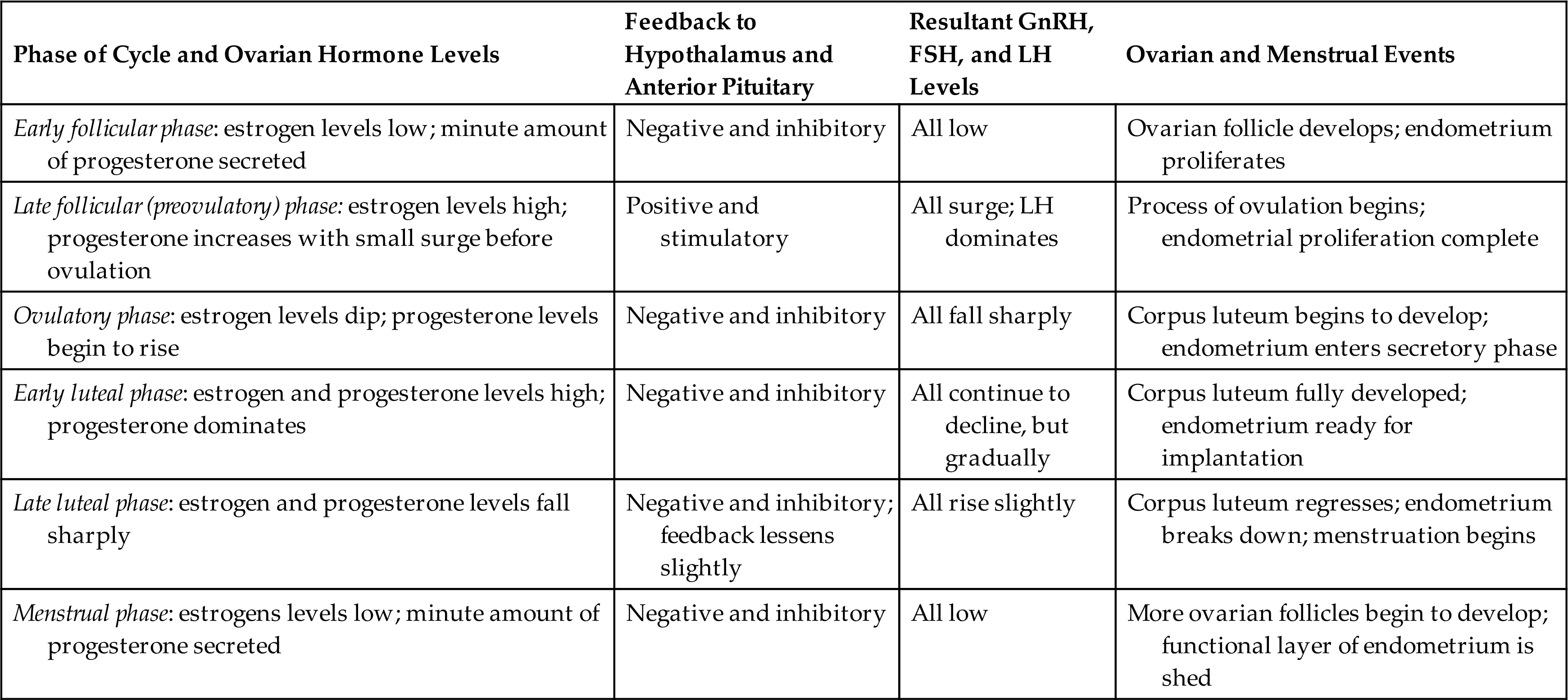
FSH, Follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone.
During the early follicular phase, estrogen levels rise steadily and, through negative feedback, suppress FSH and positively increase the production of LH. During the late follicular phase, the preovulatory rise in progesterone concentration facilitates the positive feedback of estrogen; estrogen levels begin to increase, stimulating a surge of LH secretion from the anterior pituitary. The midcycle surge of LH and FSH induces ovulation. A nonsteroidal ovarian factor, gonadotropin surge–attenuating factor (GnSAF), may antagonize the effect of estrogen on the pituitary and regulate the surge of LH at midcycle.10 Rising estrogen and progesterone levels during the luteal phase may inhibit the anterior pituitary and thus reduce LH and FSH secretion. Just before the onset of menstruation, FSH and LH levels begin to increase slightly, probably because of declining estrogen and progesterone levels (see Fig. 24.9).
A variety of growth factors and autocrine/paracrine peptides influence hormonal control and follicular response. During the early follicular stage, FSH stimulates FSH receptors and LH receptors and the release of insulin-like growth factor one, as well as the production of inhibin and activin in the ovary. Activin from granulosa cells stimulates the secretion of FSH and increases the pituitary response to GnRH, and increases FSH binding in the granulosa cells in the dominant follicle. FSH stimulates inhibin secretion from granulosa cells, and it, in turn, suppresses FSH synthesis. Inhibin B is primarily secreted in the follicular phase of the cycle but sharply spikes when ovulation occurs. Inhibin A is secreted in the luteal phase and further suppresses FSH. Inhibin also restrains prolactin and growth hormone release, interferes with GnRH receptors, and promotes the breakdown of intracellular gonadotropins. In summary, the balance between activin and inhibin regulates FSH secretion. Follistatin inhibits activin and boosts inhibin activity. Inhibin and activin also regulate LH stimulation of androgen synthesis (required for ovarian estrogen biosynthesis) in theca cells.11Fig. 24.9 depicts fluctuating estrogen, progesterone, gonadotropin, and inhibin levels. Research continues to advance understanding of the function and structural complexity of these polypeptides and their interaction with GnRH, gonadotropins, and sex hormones.12
Structure and Function of the Breast
The breasts are modified sebaceous glands that lie on the ventral surface of the thorax, within the superficial fascia of the chest wall. They extend vertically from the second rib to the sixth or seventh intercostal space and laterally from the side of the sternum to the midaxillary line. Breast tissue also may extend into the axilla; this tissue is known as the tail of Spence.
Female Breast
The adult female breast is composed of 15 to 20 pyramid-shaped lobes that are separated and supported by suspensory (Cooper) ligaments (Fig. 24.10). Each lobe contains 20 to 40 lobules, which subdivide further into many functional units called acini (sing., acinus). Each acinus is lined with a layer of epithelial cells capable of secreting milk during lactation and a layer of subepithelial cells capable of contracting to squeeze milk from the acinus. The acini empty into a network of lobular collecting ducts, which empty into interlobular collecting and ejecting ducts. Ductal elongation and organized branching are achieved with collagen fiber alignment. The ducts reach the skin through openings (pores) in the nipple. The lobes and lobules are surrounded and separated by muscle strands and fatty connective tissue. The amount of fatty connective tissue varies among individuals depending on weight, genetic, and endocrine factors; this contributes to the diversity of breast size and shape and the function of the mammary epithelium. Fat increases in the breast after menopause and is a local source of estrogen and other steroid hormones.13
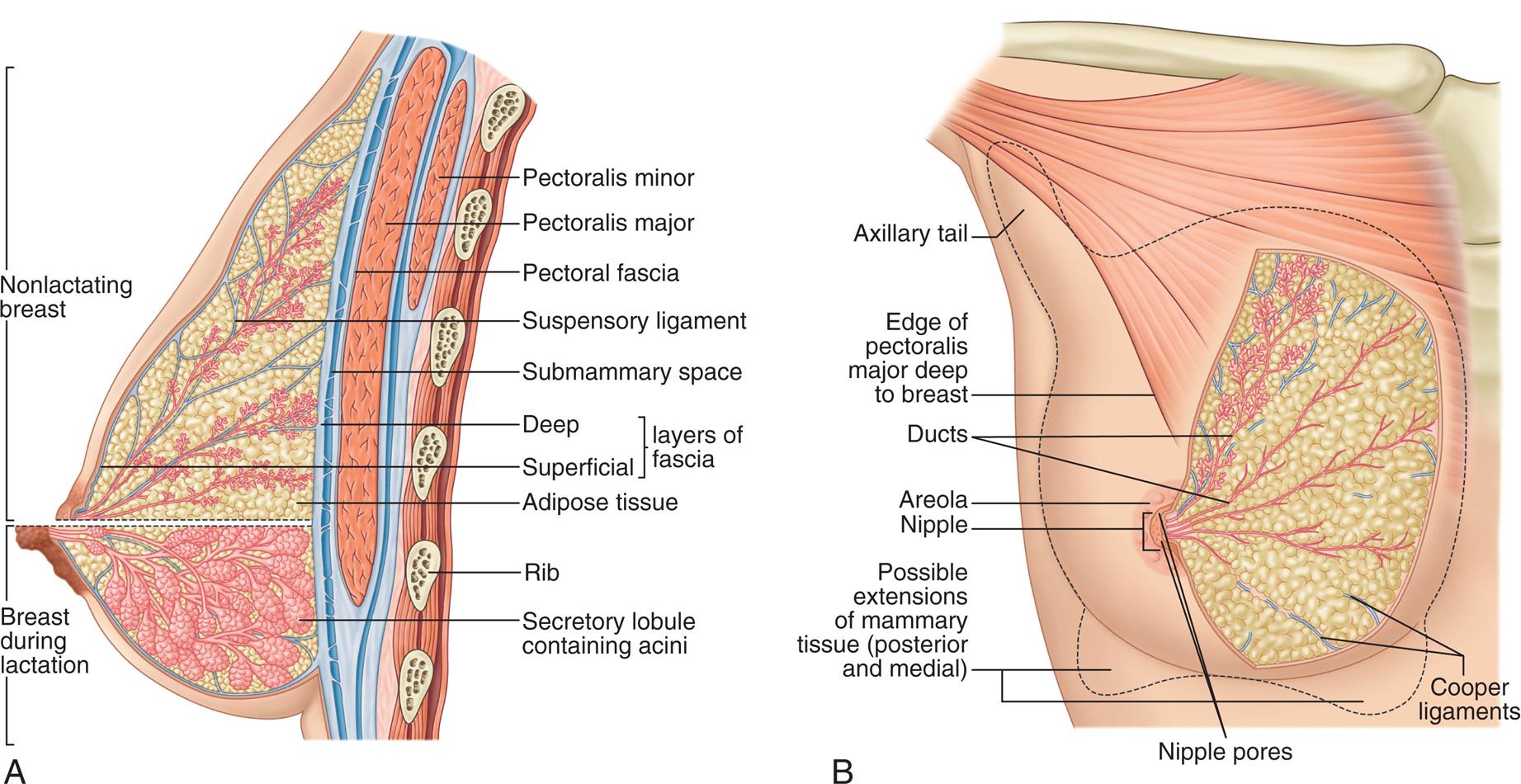
(A) Lactating breast. (B) Structure of the breast. (From Standring S. Gray’s anatomy, 42nd edition. London: Elsevier; 2021.)
“Illustration A shows a lateral, cross-sectional view of a breast. The following structures of the breast are labeled, from the top to the bottom: pectoralis minor, pectoralis major, pectoral fascia, suspensory ligament, submammary space, layers of fascia (deep, superficial), adipose tissue, rib, and secretory lobule. The upper part of the breast, above the nipple, represents a nonlactating breast, and shows tiny secretory lobules. The lower part of the breast, below the nipple, represents a lactating breast, and shows larger secretory lobules containing acini. Illustration B is an anterior view of a breast. The following structures of the breast are labeled, from the top to the bottom: axillary tail, edge of pectoralis major deep to breast, ducts, areola, nipple, cooper ligaments, and possible extensions of mammary tissue (posterior and medial).”
An extensive capillary network surrounds the acini and is supplied by branches of the internal mammary, thoracoacromial, internal and lateral thoracic, and intercostal arteries. Venous return follows arterial supply, with relatively rapid emptying into the superior vena cava. The breasts receive sensory innervation from branches of the second through sixth intercostal nerves and the cervical plexus. This accounts for the fact that breast pain may be referred to the chest, back, scapula, medial arm, and neck. Lymphatic drainage of the breast occurs largely through axillary nodes, but there may be a predominance of superficial mammary routes with resultant asymmetry between a person's breasts. Lymphatic drainage from one breast may drain to the opposite side and is a factor in cancer metastasis (Fig. 24.11).
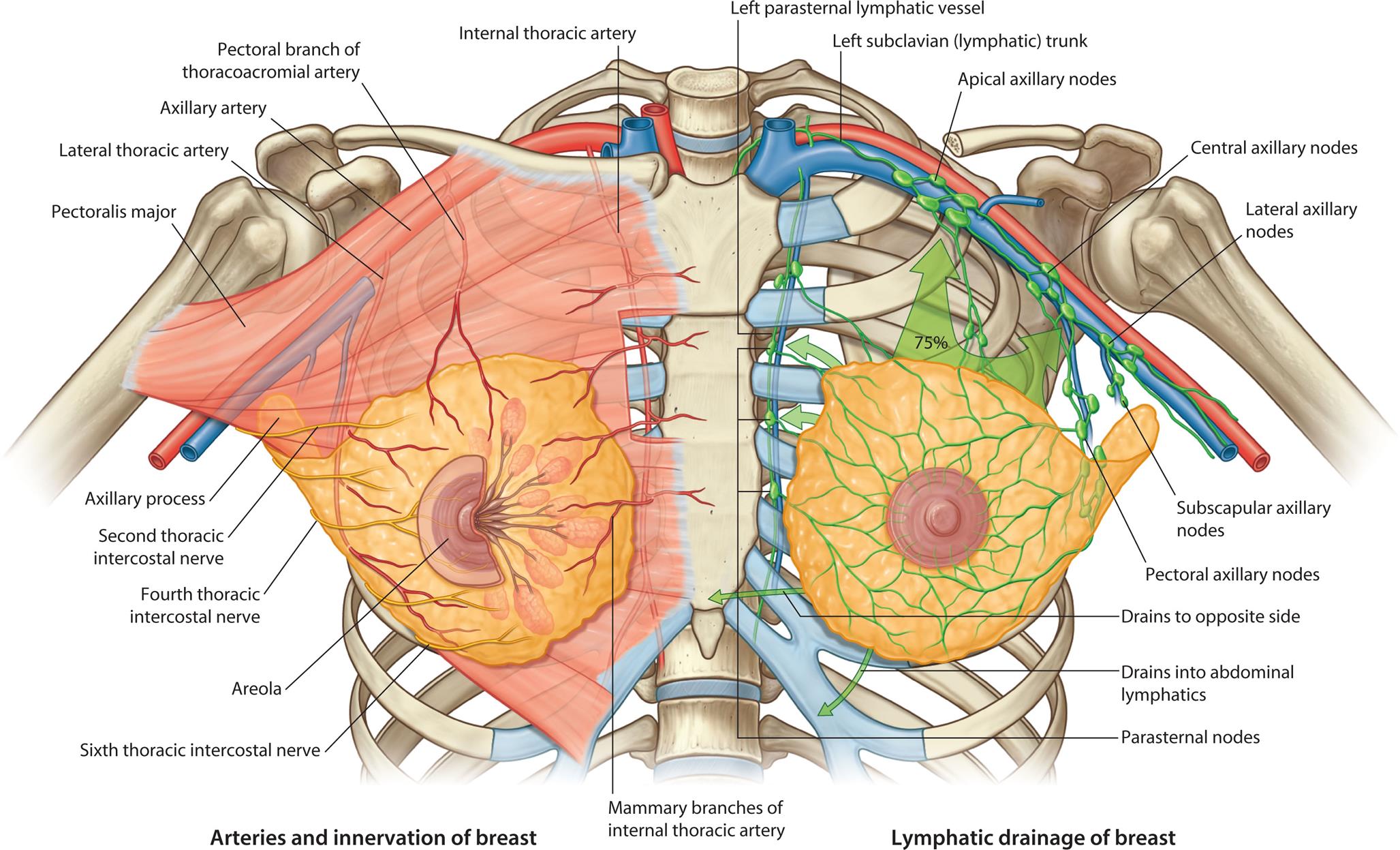
“An illustration shows the vasculature, innervation, and lymphatic drainage of the female breast. The left side of the illustration shows the arteries and innervation of breast, labeling the following structures from the top to the bottom: internal thoracic artery, pectoral branch of thoracoacromial artery, axillary artery, pectoralis major, axillary process, second thoracic intercostal nerve, fourth thoracic intercostal nerve, mammary branches of internal thoracic artery, areola, and sixth thoracic intercostal nerve. The right side of the illustration shows the lymphatic draining of breast, labeling the following structures from the top to the bottom: left parasternal lymphatic vessel, left subclavian (lymphatic) trunk, apical axillary nodes, central axillary nodes, lateral axillary nodes, subscapular axillary nodes, pectoral axillary nodes, drains to opposite side, drains into abdominal lymphatics, and parasternal nodes.”
The nipple is a pigmented cylindric structure usually located in the fourth or fifth intercostal space. It measures 0.5 to 1.3 cm in diameter and is approximately 10 to 12 mm in height when erect. On its surface lie multiple pores, one from each lobe. The areola is the pigmented circular area around the nipple. It may be 15 to 60 mm in diameter. A number of sebaceous glands, the glands of Montgomery, are located within the areola and aid in the lubrication of the nipple during lactation. The nipple and areola contain smooth muscles, which receive motor innervation from the sympathetic nervous system. Breast-feeding, sexual stimulation, and exposure to cold cause the nipple to become erect.
The fetal and early postnatal development of breast tissue does not depend on hormones, although fetal breast tissue does become progressively responsive to hormonal stimulation. The neonatal breasts are rudimentary, containing 10 to 12 branching ducts. During childhood, breast growth is latent, and growth of the nipple and areola keeps pace with body surface growth. (Male breast development normally does not progress any further.) At the onset of puberty in the female, growth hormone, insulin-like growth factor 1 (IGF1), and estrogen stimulate mammary growth. Thelarche is usually the first sign of puberty in the female. Full differentiation and development of breast tissue occur over approximately four years and are mediated by the levels of several hormones, including estrogen, progesterone, prolactin, growth hormone, thyroid and parathyroid hormones, insulin, and cortisol. Estrogen promotes the increase in the size of the breast by the formation of a mass of tissue under the areola, increases the size and pigmentation of the areola, and promotes the development of the lobular ducts. The breast cells of parous females (those who have given birth) are different than those of females who never become pregnant, as the expansion of acini only occurs with pregnancy when the mammary gland prepares for lactation. During menopause, the lobules of the parous breast involute to prepregnancy composition and become identical to the nulliparous breast.14
During the reproductive years, the breast undergoes cyclic changes in response to changes in the levels of estrogen and progesterone associated with the menstrual cycle. Estrogen promotes the development of the lobular ducts; progesterone stimulates the development of cells lining the acini. During the follicular/proliferative phase of the menstrual cycle, high estradiol levels increase the vascularity of breast tissue and stimulate the proliferation of ductal and acinar tissue. This effect is sustained into the luteal/secretory phase of the cycle. During this phase, progesterone levels increase and contribute to the breast changes induced by estradiol. Specific effects of progesterone include dilation of the ducts and conversion of the acinar cells into secretory cells. Most females experience some degree of premenstrual breast fullness, tenderness, and increased breast nodularity. Breast volume may increase as much as 10 to 30 ml. Because the length of the menstrual cycle does not allow for complete regression of new cell growth, breast growth continues at a slow rate until approximately 35 years of age. Because of the cyclic changes that occur in breast tissue, breast examination should be conducted at the conclusion of or a few days after the menstrual cycle, when hormonal effects are minimal and breasts are at their smallest and least tender.
The function of the female breast is primarily to provide a source of nourishment for the newborn. During pregnancy, the breast remodels into a milk-secreting organ and reaches its ultimate mature developmental stage. With increased levels of estrogen, the lobules further differentiate. Progesterone stimulates the development of cells lining the alveoli to produce milk. Lactation (milk production) occurs after childbirth in response to increased levels of prolactin. Prolactin secretion, in turn, increases by continued breastfeeding. Oxytocin, another hormone released during and after delivery, controls milk ejection from alveolar cells. Milk is continuously secreted into the alveolar lumen and is stored there until suckling by the infant stimulates oxytocin, which triggers the let-down reflex. The alveoli empty into a network of lactiferous ducts. These ducts reach the skin through 9 or 10 pores in the nipple.
Physiologically, breast milk is the most appropriate nourishment for newborns. Colostrum, produced in low quantities in the first few days postpartum, is rich in immunologic components, including secretory IgA, lactoferrin, leukocytes, and developmental factors, such as epidermal growth factor. The nutrient composition changes over time to meet the changing digestive capabilities and nutritional requirements of the infant. Secretory IgA and nonspecific antimicrobial factors, such as lysosomes and lactoferrin, protect the infant against infection. During lactation, high prolactin levels interfere with hypothalamic-pituitary hormones that stimulate ovulation. This mechanism suppresses the menstrual cycle and can prevent ovulation.
Male Breast
Until puberty, the development of the male breast is similar to that of the female breast. In the absence of sufficiently high levels of estrogen and progesterone, and with antagonistic effects of androgens, the male breast does not develop any further. The normal male breast consists mostly of fat with a small, underdeveloped nipple and a few ductlike structures in the subareolar area. The male breast may appear enlarged in obese males because of the accumulation of fatty tissue. During puberty, some males experience benign gynecomastia (benign proliferation of male breast glandular tissue), a condition in which the breasts enlarge temporarily as a result of hormonal fluctuations and which should be differentiated from any underlying systemic disorders.
The Male Reproductive System
The external genitalia in males perform the major functions of reproduction. Sperm are produced in the male gonads (testes) and delivered by the penis to the female vagina. The internal male genitalia consist of conducting tubes and fluid-producing glands, all of which aid in the transport of sperm from the testes to the urethral opening of the penis. The male reproductive and urinary structures are shown in Fig. 24.12.
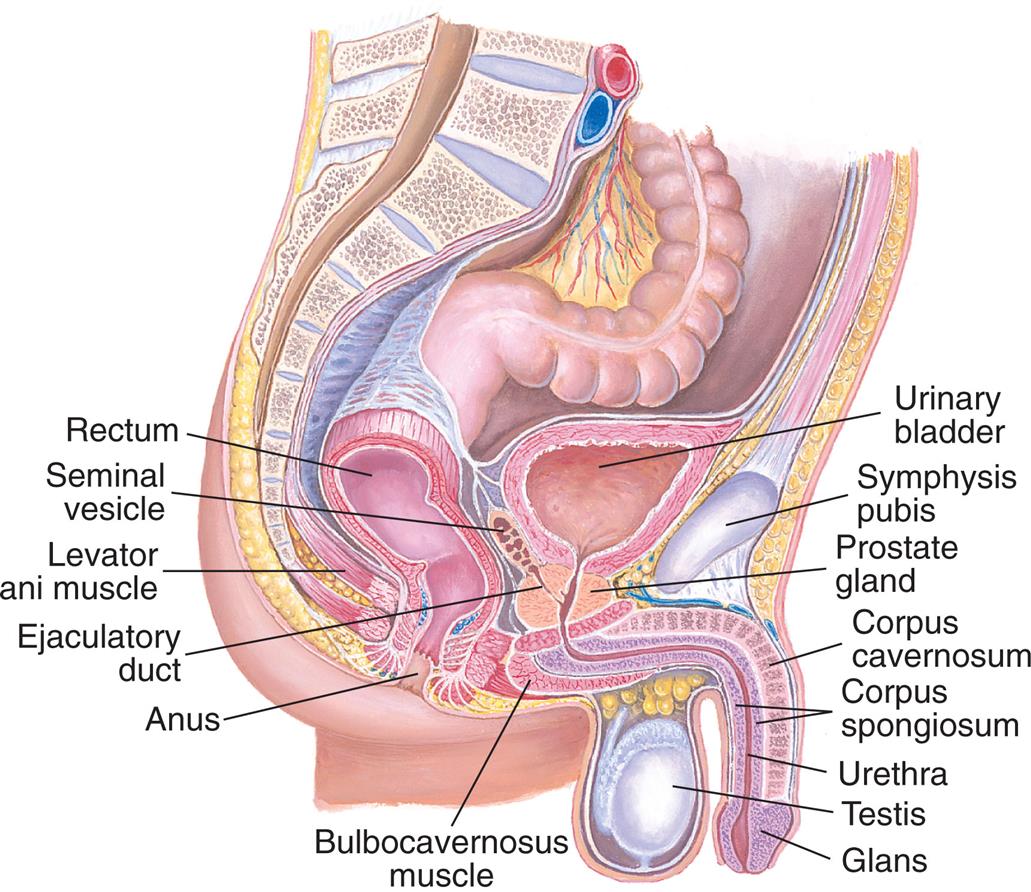
An illustration of the right lateral view of the male reproductive organs shows and labels the followings structures, clockwise from the top: urinary bladder, symphysis pubis, prostate gland, corpus cavernosum, corpus spongiosum, urethra, testis, glans, bulbocavernosus muscle, anus, ejaculatory duct, levator ani muscle, seminal vesicle, and rectum.
External Genitalia
Testes
The testes (sing., testis) are the essential organs of male reproduction. Like the ovaries, the testes have two functions: (1) production of gametes (i.e., sperm) and (2) production of sex hormones (i.e., androgens and testosterone).
During embryonic and fetal life, the testes develop within the abdomen (see Fig. 24.1). About 3 months before birth, the testes start to descend toward the developing scrotum (Fig. 24.13). About 1 month before birth, they enter twin passageways called inguinal canals. Vaginal processes created by outpouchings of the peritoneum (lining of the abdominal cavity) also descend through the inguinal canals. When descent is complete, the abdominal end of each vaginal process closes, and the scrotal end of each process becomes the outer covering of the testis, the tunica vaginalis. (See Fig. 24.16, A for the inguinal canal in a mature adult.) Failure of the testes to descend through the inguinal canal is known as cryptorchidism.
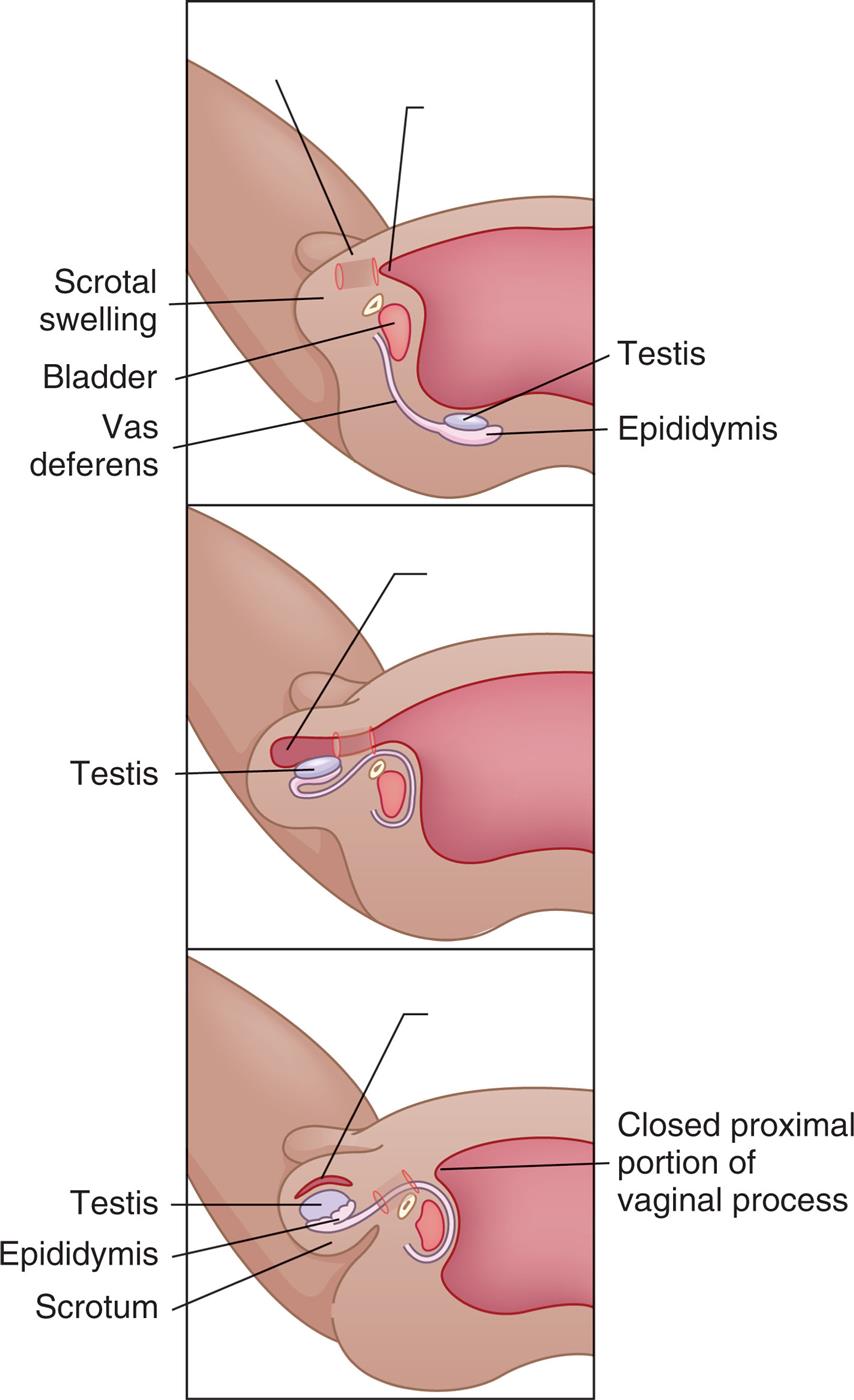
The testes descend from the abdominal cavity to the scrotum during the last 3 months of fetal development.
“Three illustrations demonstrate the descent of a testis. Top panel. The illustration shows the scrotal swelling, the bladder, vas deferens, testis, and epididymis. The testis and the epididymis are away from the scrotal swelling and bladder. Middle panel. The testis has descended from the back and into the scrotal swelling. Top panel. The illustration shows the testis and the epididymis in the scrotum. The closed proximal portion of vaginal process is highlighted.”
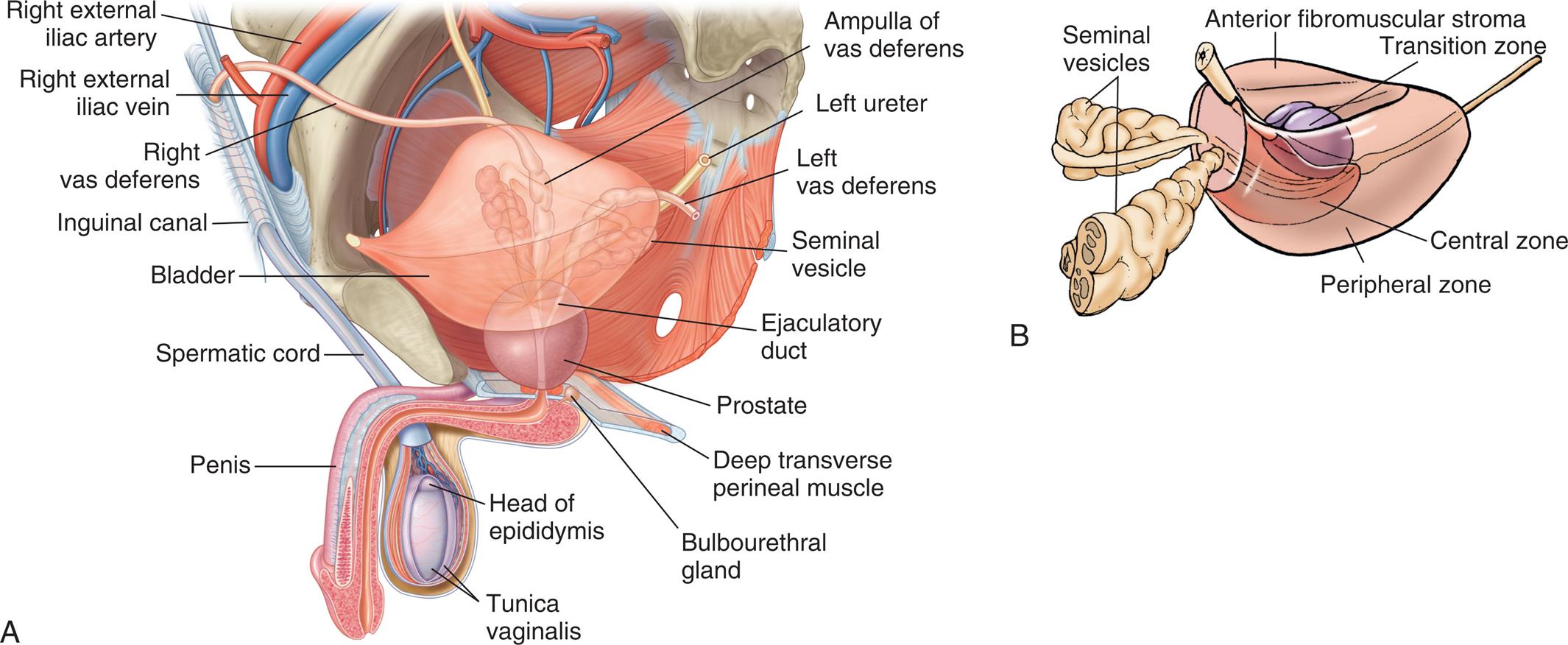
The peripheral zone, accounting for 70% of the prostate gland, is the site of origin of ≤70% of prostate cancers; the central zone, approximately 25% of the prostate gland, gives rise to only 1% to 5% of prostate cancers; and the transition zone, approximately 5% to 10% of the prostate gland, gives rise to 20% of prostate cancers and is the site of origin of benign prostatic hyperplasia (BPH) (B), Glands and ducts within the male reproductive system. (A, From Drake R, et al. Gray’s atlas of anatomy, 3rd edition. Philadelphia: Elsevier; 2020. B, Copyright Baylor College of Medicine, Houston, TX.)
“Illustration A shows and labels the following structures of the male reproductive organs, clockwise from the top: ampulla of vas deferens, left ureter, left vas deferens, seminal vesicle, ejaculatory duct, prostate, deep transverse perineal muscle, bulbourethral gland, head of epididymis, tunica vaginalis, penis, spermatic cord, bladder, inguinal canal, right vas deferens, right external iliac vein, and right external iliac artery. Illustration B shows and labels the following structures of the male reproductive organs: anterior fibromuscular stroma, transition zone, central zone, peripheral zone, and seminal vesicles.”
Fig. 24.14 shows a sagittal section of a mature testis. The adult testis is oval and varies considerably in length (3 to 6 cm), width (2 to 3.5 cm), depth (3 to 4 cm), and weight (10 to 40 g). The testis is almost entirely surrounded by an outer covering called the tunica vaginalis, which separates the testis from the scrotal wall, and an inner covering called the tunica albuginea. Inward extensions of the tunica albuginea form septa that separate the testis into about 250 compartments, or lobules, each of which contains several tortuously coiled ducts called seminiferous tubules. The seminiferous tubules constitute the bulk (80%) of testicular volume and are the site of sperm production. (Sperm production is described in the Spermatogenesis section.) The tissue surrounding these ducts contains blood and lymphatic vessels, fibroblastic support cells, macrophages, mast cells, andLeydig cells, which occur in clusters and produce androgens, chiefly testosterone.
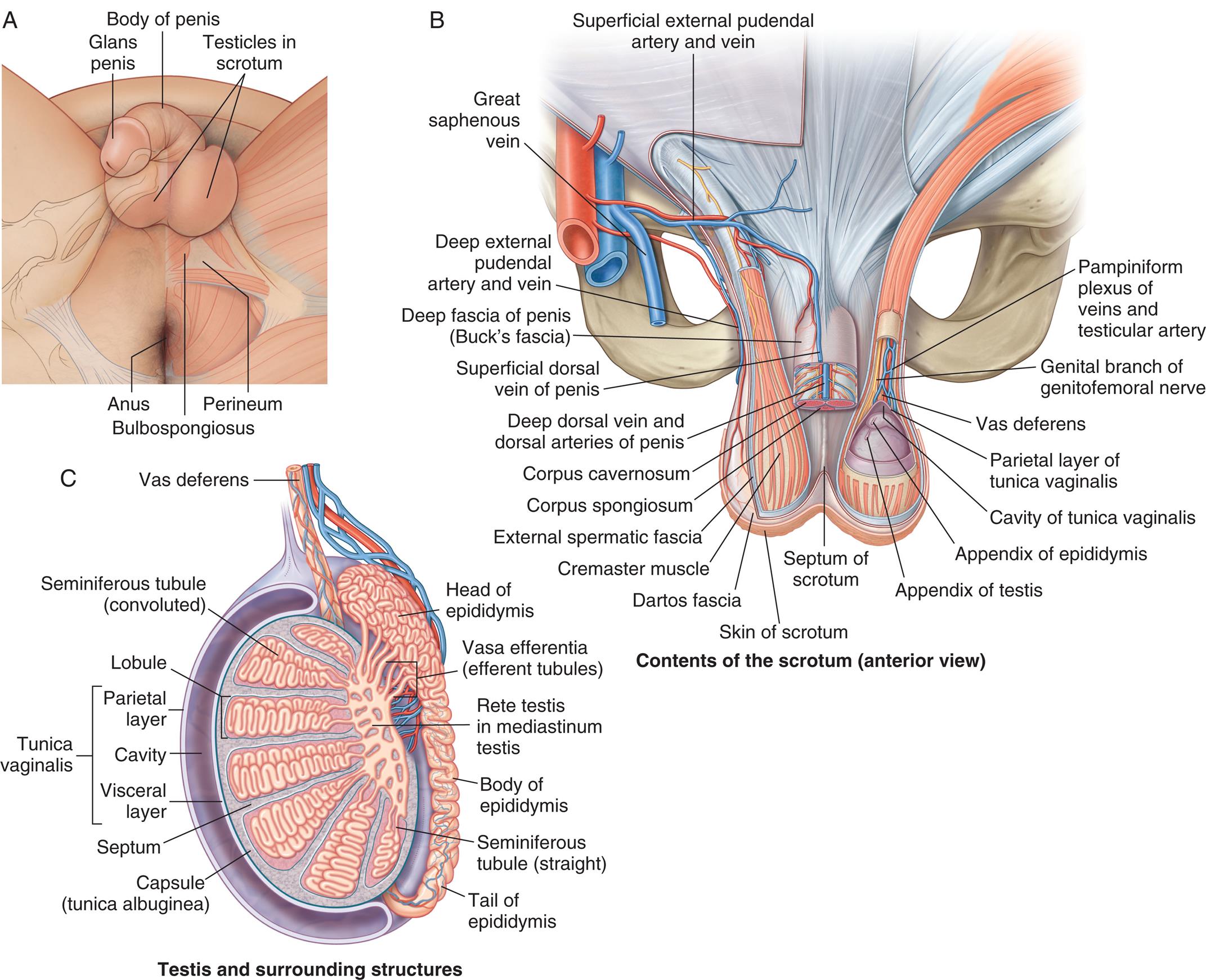
“Illustration A is an inferior view of the external male genitalia. The following structures on the genitalia are labeled from the top to the bottom: glans penis, body of penis, testicles in scrotum, perineum, bulbospongiosus, and the anus. Illustration B is an anterior view of the male genitalia, with the scrotum. The following structures are labeled, clockwise from the right: pampiniform plexus of veins and testicular artery, genital branch of genitofemoral nerve, vas deferens, parietal layer of tunica vaginalis, cavity of tunica vaginalis, appendix of epididymis, appendix of testis, septum of scrotum, skin of scrotum, dartos fascia, cremaster muscle, external spermatic fascia, corpus spongiosum, corpus cavernosum, deep dorsal vein and dorsal arteries of penis, superficial dorsal vein of penis, deep fascia of penis (buck's fascia), deep external pudendal artery and vein, great saphenous vein, and superficial external pudendal artery and vein. Illustration C is the sagittal view of the testis and surrounding structures. The following structures on the genitalia are labeled, clockwise from the top: vas deferens, head of epididymis, vasa efferentia (efferent tubules), rete testis in mediastinum testis, body of epididymis, seminiferous tubule (straight), tail of epididymis, capsule (tunica albuginea), septum, tunica vaginalis (parietal layer, cavity, visceral layer), lobule, and seminiferous tubule (convoluted).”
The two ends of each seminiferous tubule join and leave the lobule through the tubulus rectus, which leads to the central portion of the testis, the rete testis. The sperm then move through the efferent tubules, or vasa efferentia, to the epididymis, where they mature.
The testes are innervated by adrenergic fibers whose sole function is to regulate blood flow to the Leydig cells. Arterial blood from the internal spermatic and differential arteries flows over the surface of the testes before entering the parenchyma (functional tissues). Surface flow cools the blood to temperatures to approximately 35°C to 36°C, which promotes spermatogenesis.15 Additionally, the testes are suspended outside the pelvic cavity to facilitate cooling.
Epididymis
The epididymis (pl., epididymides) is a comma-shaped structure that curves over the posterior portion of each testis (see Fig. 24.14). It consists of a single, densely packed, and markedly coiled duct measuring 5 to 7 cm in length (but about 6 meters in length when uncoiled). The epididymis has structural and physiologic functions. Its structural function is to conduct sperm from the efferent tubules to the vas deferens, whereas physiologic functions include sperm maturation, mobility, and fertility. When sperm enter the head of the epididymis, they are not fully mature or motile, nor can they fertilize an ovum. During the 12 or more days sperm take to travel the length of the epididymis, they receive nutrients and testosterone, and their capacity for fertilization is enhanced.16 After traveling the length of the epididymis, sperm are stored in the epididymal tail and vas deferens. The vas deferens is a duct with muscular layers capable of powerful peristalsis that transports sperm toward the urethra (see Fig. 24.14). The vas deferens enters the pelvic cavity through the spermatic cord.
Scrotum
The testes, epididymides, and spermatic cord are enclosed and protected by the scrotum, a skin-covered, fibromuscular sac homologous to the female labia majora (see Fig. 24.2). The skin of the scrotum is thin and has rugae (wrinkles or folds), which enable it to enlarge or relax away from the body. At puberty, the scrotal skin darkens, develops active sebaceous glands, and becomes sparsely covered with hair. Just under the skin lies a layer of connective tissue (fascia) and smooth muscle, the tunica dartos (see Fig. 24.14). The tunica dartos also forms a septum that separates the two testes. Exposure to cold temperatures causes the tunica dartos to contract, pulling the testes close to the warm body. In warm temperatures, the tunica dartos relaxes, suspending the testes away from body heat. These mechanisms promote optimal temperatures for spermatogenesis. In addition, scrotal sensitivity to touch, pressure, temperature, and pain protects the testes from potential harm. During sexual excitement, the scrotal skin and tunica thicken, the scrotum tightens and lifts, and the spermatic cords shorten, partially elevating the testes toward the body. As excitement plateaus, the engorged testes increase 50% in size, rotate anteriorly, and flatten against the body, signaling impending ejaculation.
Penis
The penis has two main functions: delivery of sperm to the female vagina and elimination of urine. Embryonically, the penis is homologous to the female clitoris (see Fig. 24.2).
Fig. 24.12 shows a sagittal section of the adult penis and its anatomic relation to other urogenital structures, and Fig. 24.15 shows a cross section of the shaft of the penis. Internally, the penis consists of the urethra and three compartments or sinusoids: two corpora cavernosa (sing., corpus cavernosum) and the corpus spongiosum separated by Buck fascia. Like the testes, these compartments are enclosed by the fibrous tunica albuginea. The urethra passes through the corpus spongiosum and ends at a sagittal slit in the glans.
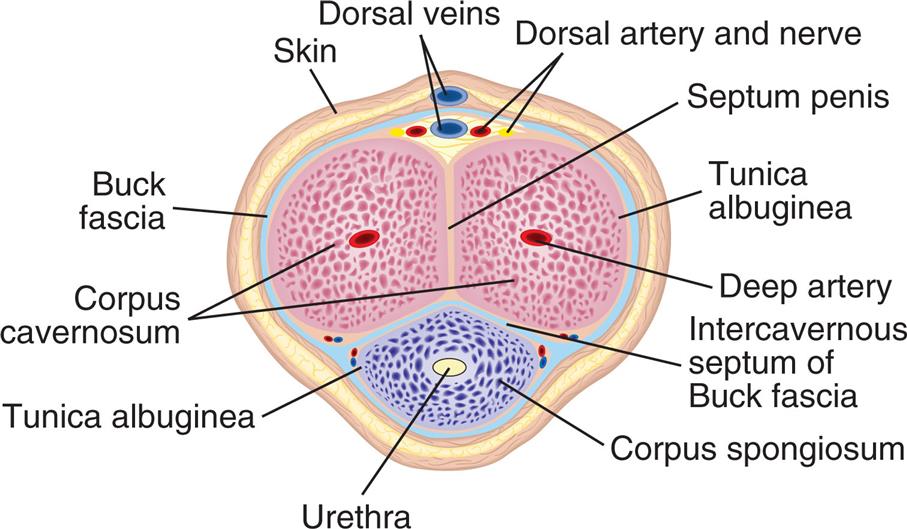
The Buck fascia is the blue layer separating the corpora cavernosa from the corpus spongiosum. (From Thompson JM, et al., eds. Mosby’s clinical nursing, 5th edition. St. Louis: Mosby; 2002.)
An illustration of the cross-section of the penis shows and labels the following structures, clockwise from the top: dorsal veins, dorsal artery and nerve, septum penis, tunica albuginea, deep artery, intercavernous septum of Buck fascia, corpus spongiosum, urethra, tunica albuginea, corpus cavernosum, buck fascia, and skin.
Externally, the penis consists of a shaft with a tip (the glans) that contains the opening of the urethra (see Fig. 24.14). The skin of the glans folds over the tip of the penis, forming the prepuce, or foreskin. At birth, the foreskin is adhered to the glans. Penile erections, which commonly occur, cause the adhesions to break so that by age 3 years, the foreskin becomes completely retractable. The skin of the penis is continuous with that of the groin, scrotum, and inner thighs. It is hairless, movable, and darker than the surrounding skin.
Penetration of the female vagina is made possible by the erectile reflex, a process in which erectile tissues within the corpora cavernosa and corpus spongiosum become engorged with blood, generally 20 to 50 mL. The erectile tissues consist of vascular spaces, or chambers, supplied with blood by arterioles (small arteries). Usually, the arterioles are constricted so that not much blood flows through the erectile tissues. Sexual stimulation, however, causes the arterioles to dilate and fill with blood, expanding the erectile tissues and causing an erection. The corpora cavernosa increases in length and width and becomes rigid. Erection apparently is maintained by compression or constriction of veins that drain the corpora cavernosa and corpus spongiosum. When sexual stimulation ceases, or orgasm and ejaculation occur, these veins open, blood flows out of the arterioles, and the penis becomes flaccid (soft and pendulous). Erection is under the control of the autonomic nervous system but can be stimulated or inhibited by CNS input.
Stimulation of the glans, which is endowed with copious sensitive nerve endings, provides maximum erotic sensation. With sexual arousal, skin color deepens, the glans doubles in size, and the urethral meatus dilates. Ejaculation occurs with frequent, strong contractions of the vas deferens, epididymis, seminal vesicles, prostate, urethra, and penis. Erection and ejaculation can occur independently of each other, but it is not common.17
Erections begin in utero and continue throughout life, but ejaculation does not occur until sperm production begins at puberty. Growth of the penis and scrotal contents continues well past puberty, however, and may not be complete until the late teens or early twenties. Penis size, when flaccid, varies considerably; with an erection, the difference in penis size diminishes.
Internal Genitalia
Fig. 24.12 shows the anatomy of the internal genitalia and their relation to other pelvic organs. The internal genitalia consist of ducts and glands.
- • Ducts consist of two vasa deferentia, the ejaculatory duct and the urethra. They conduct sperm and glandular secretions from the testes to the urethral opening of the penis.
- • Glands consist of the prostate gland, two seminal vesicles, and two Cowper (bulbourethral) glands. They secrete fluids that serve as a vehicle for sperm transport and create a nutritious alkaline medium that promotes sperm motility and survival. Together the sperm and the glandular fluids compose semen.
Sperm leaves the epididymides and travels rapidly through the internal ducts (emission). Emission occurs just seconds before ejaculation, at the moment when sexual arousal peaks. It always leads to ejaculation.
Emission occurs as smooth muscle in the walls of the epididymides and vasa deferentia begins to contract rhythmically, pushing sperm and epididymal secretions through the vasa deferentia. Each vas deferens is a firm, elastic, fibromuscular tube that begins at the tail of the epididymis, enters the pelvic cavity within the spermatic cord, loops up and over the bladder, and ends in the prostate gland (Fig. 24.16). Sperm are conducted by peristaltic contractions of smooth muscle in the walls of the vas deferens.
The seminal vesicles are glands about 4 to 6 cm long that lie behind the urinary bladder and in front of the rectum. As sperm leave the ampulla (wide portion) of the vas deferens, the seminal vesicles secrete a nutritive, glucose-rich fluid into the ejaculate (semen). The seminal vesicles provide fructose as a source of energy for ejaculated sperm and secrete prostaglandins that promote smooth muscle contraction, assisting with sperm transport. The ducts of the seminal vesicles join the ampulla of the vas deferens to become the ejaculatory duct, which contracts rhythmically during emission and ejaculation. As seen in Figs. 24.12 and 24.16B, the ejaculatory duct joins the urethra, where both pass through the prostate gland. During emission and ejaculation, a sphincter (muscle surrounding a duct) closes, preventing urine from entering the prostatic urethra.
The prostate gland is about the size of a walnut, surrounds the urethra, and is composed of glandular alveoli and ducts embedded in fibromuscular tissue. The prostate gland has three zones (see Fig. 24.16A), which are significant in the study of prostate cancers. Prostate growth, development, and function are regulated by androgens and the androgen receptor. Nerves required for penile erection travel along the posterolateral surface of the prostate.
Included in prostate epithelial secretions are prostate-specific antigen (PSA), cytokeratins, prostate-specific membrane antigen (PSMA), and prostate-specific acid phosphatase. Prostate secretions contribute to the ejaculate. While semen moves through the prostatic portion of the urethra, the prostate gland contracts rhythmically and secretes prostatic fluid (a thin, milky substance with an alkaline pH that helps sperm survive in the acidic environment of the female reproductive tract) into the mixture. In addition, clotting enzymes and fibrinolysin in prostatic fluids help to mobilize sperm after ejaculation.
Bulbourethral glands (Cowper glands) are the last pair of glands to add fluid to the ejaculate; their ducts secrete mucus into the urethra near the base of the penis. Ejaculation occurs as semen reaches the base of the penis, where muscles rhythmically contract and expel semen. Normally a male ejaculates between 2 and 6 ml of semen, containing 75 million to 400 million sperm. About 98% of the ejaculate consists of glandular fluids, 60% to 70% of the volume originates from the seminal vesicles, and 20% from the prostate. The ejaculate of a man who has undergone a vasectomy (a surgical procedure that prevents sperm from entering the vas deferens) is reduced by about 2%.
Spermatogenesis
Spermatogenesis (the production of sperm) begins at puberty and continues for life. In this respect, spermatogenesis differs markedly from oogenesis (production of primordial ova), which occurs during fetal life only. Spermatogenesis takes place within the seminiferous tubules of the testes (Fig. 24.17). The basement membrane of each seminiferous tubule is lined with diploid (46-chromosome) germ cells called spermatogonia (sing., spermatogonium). These cells undergo continuous mitotic division. Some spermatogonia move away from the basement membrane and mature, becoming primary spermatocytes. These undergo meiosis, cell division that results in two haploid (23-chromosome) cells called secondary spermatocytes. The secondary spermatocytes also undergo meiosis, resulting in four spermatids. Spermatids then differentiate into spermatozoa, or sperm, each of which contains 23 chromosomes (Fig. 24.18).
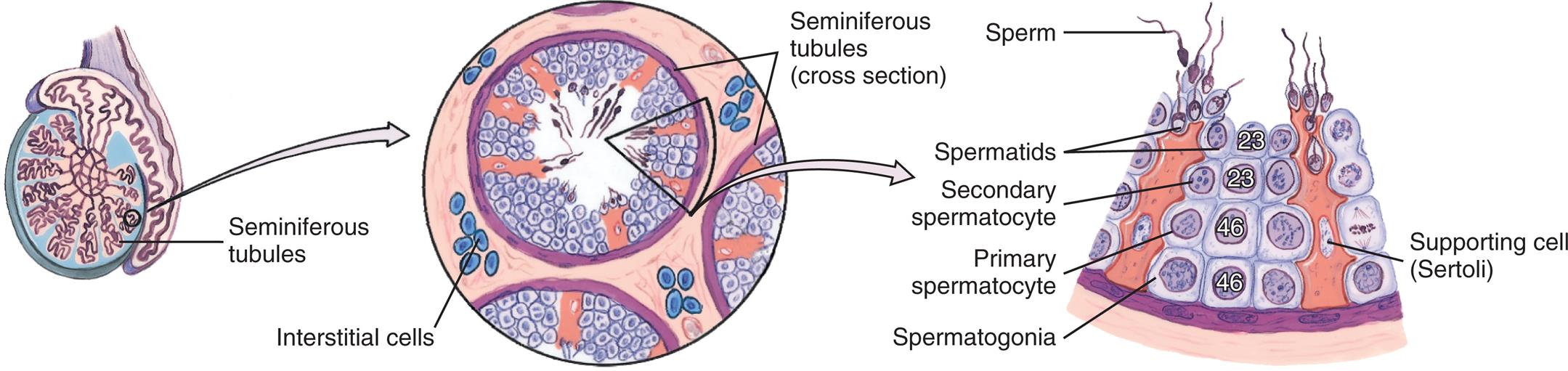
Cross section of a seminiferous tubule showing the different cell types. Interstitial cells that produce testosterone are between the seminiferous tubules. Spermatids in the lumen become sperm by a process called spermiogenesis. The numbers in white represent the number of chromosomes. (From Applegate E. The anatomy and physiology learning system, 4th edition. St. Louis: Saunders; 2011.)
“A series of illustration demonstrates spermatogenesis. Left panel. The illustration shows a cross-section of testis with seminiferous tubules. Middle panel. The illustration shows an enlarged view of cross-section of seminiferous tubules with interstitial cells in between the tubules. Bottom panel. The illustration shows an enlarged cross-sectional view of portion of seminiferous tubule and labels the following structures from the top: sperm, spermatids, secondary spermatocyte, primary spermatocyte, supporting cell (Sertoli), and spermatogonia.”
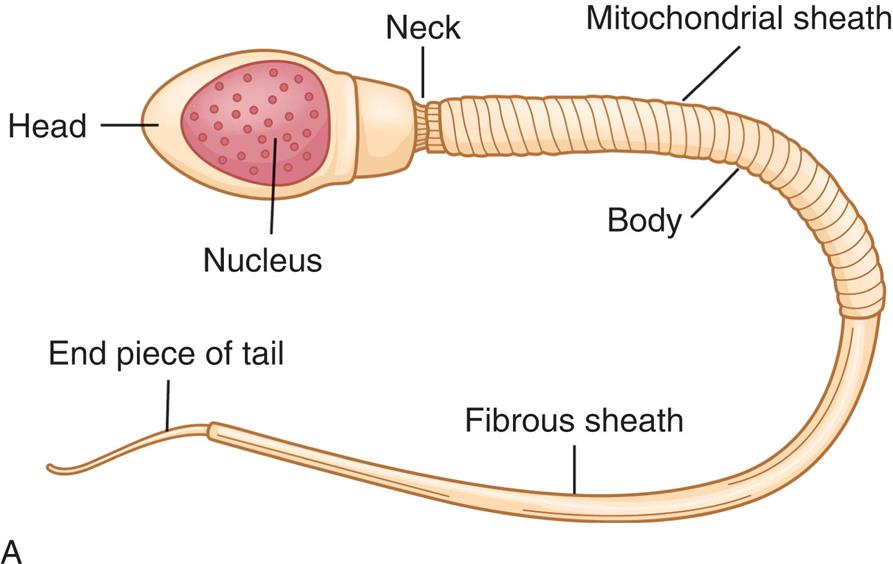
(A) Anatomy of mature sperm cell. (B) Human sperm with nuclear material glowing with a fluorescent dye. (B From Lennart Nilsson.)
“Illustration A shows and labels the following structures of a mature sperm: head, nucleus, neck, mitochondrial sheath, body, fibrous sheath, and end piece of tail. Photomicrograph B shows a cluster of sperms swimming.”
The development of spermatids into sperm depends on the presence of Sertoli cells (nondividing support cells) within the seminiferous tubules. Spermatids attach themselves to the Sertoli cells (see Fig. 24.17), where they receive nutrients and hormonal signals (e.g., testosterone) necessary to develop into sperm.
The process of spermatogenesis, from the mitotic division of a spermatogonium to the maturation of the spermatids, takes about 70 to 80 days. Mature sperm migrate from the seminiferous tubules to the epididymides, where their capacity for fertilization continues to develop. Although they are completely mature by the time they are ejaculated, the sperm do not become motile (capable of movement) until they are activated by biochemicals in the epididymis and in the female reproductive tract (known as sperm capacitation).
Male Sex and Reproductive Hormones
The male sex hormones are androgens. Testosterone, the primary male sex hormone, and other androgens are produced mainly by Leydig cells of the testes, but they are also produced by the adrenal glands in both males and females and by the ovary in females (see Table 24.1). In males, sex hormone production is relatively constant and does not occur in a cyclic pattern, as it does in females.
The physiologic actions of androgen are related to the growth and development of male tissues and organs. Androgens are responsible for the fetal differentiation and development of the male urogenital system and have some effects on the fetal brain. After birth, the Leydig cells become dormant until activated by the gonadotropins during puberty. Then androgens cause the sex organs to grow and secondary sex characteristics to develop.
Testosterone affects nervous and skeletal tissues, bone marrow, skin and hair, and sex organs. It has an anabolic effect on skeletal muscle tissue, thereby contributing to the difference in body weight and composition between males and females. Testosterone also stimulates the growth of the musculature and cartilage of the larynx, causing a permanent deepening of the voice. Testosterone directly stimulates the bone marrow and indirectly stimulates renal erythropoietin production to achieve increased hemoglobin and hematocrit levels. Because sebaceous gland activity is stimulated by testosterone, acne may develop. Hair becomes coarser in texture, and facial, axillary, and pubic hair grows in male patterns. Testosterone is required for spermatogenesis and for the secretion of fluid by the prostate gland, seminal vesicles, and bulbourethral glands. Testosterone is also associated with libido (sex drive). Other, less-understood effects of testosterone include regulatory proteins involved in glycolysis, glycogen synthesis, insulin action, and lipid and cholesterol metabolism.
The regulation of androgen production and spermatogenesis is achieved by a complex feedback system involving the extrahypothalamic CNS, the hypothalamus, the anterior pituitary, the testes, and the androgen-sensitive end organs. These make up the hypothalamic-pituitary-testicular (HPT) axis (Fig. 24.19). These relationships are essentially the same in females (see Fig. 24.3). Extrahypothalamic influences include such variables as physiologic and psychologic stress, which may inhibit or augment hypothalamic activity. In the hypothalamus, neurotransmitters regulate GnRH synthesis and pulsatile release (about every 3 hours) into the hypophyseal portal veins. Norepinephrine stimulates GnRH secretion, and serotonin and dopamine inhibit GnRH secretion. GnRH is transported by portal flow to the median eminence of the pituitary gland, where it binds to receptors and stimulates the synthesis and secretion of the gonadotropins LH and FSH. These gonadotropins are named for their effects in the female reproductive system but have important effects on the male system as well. LH acts on the Leydig cells to regulate testosterone secretion. FSH acts on the seminiferous tubule Sertoli cells to promote spermatogenesis. FSH secretion is inhibited by inhibin secreted by the Sertoli cells. Similar to their action in the female gonad, inhibin functions as an autocrine/paracrine regulator in the male gonad. Inhibin inhibits the proliferation of spermatogonia by regulating pituitary FSH levels. In addition, inhibin facilitates LH stimulation of androgen biosynthesis in Leydig cells.18
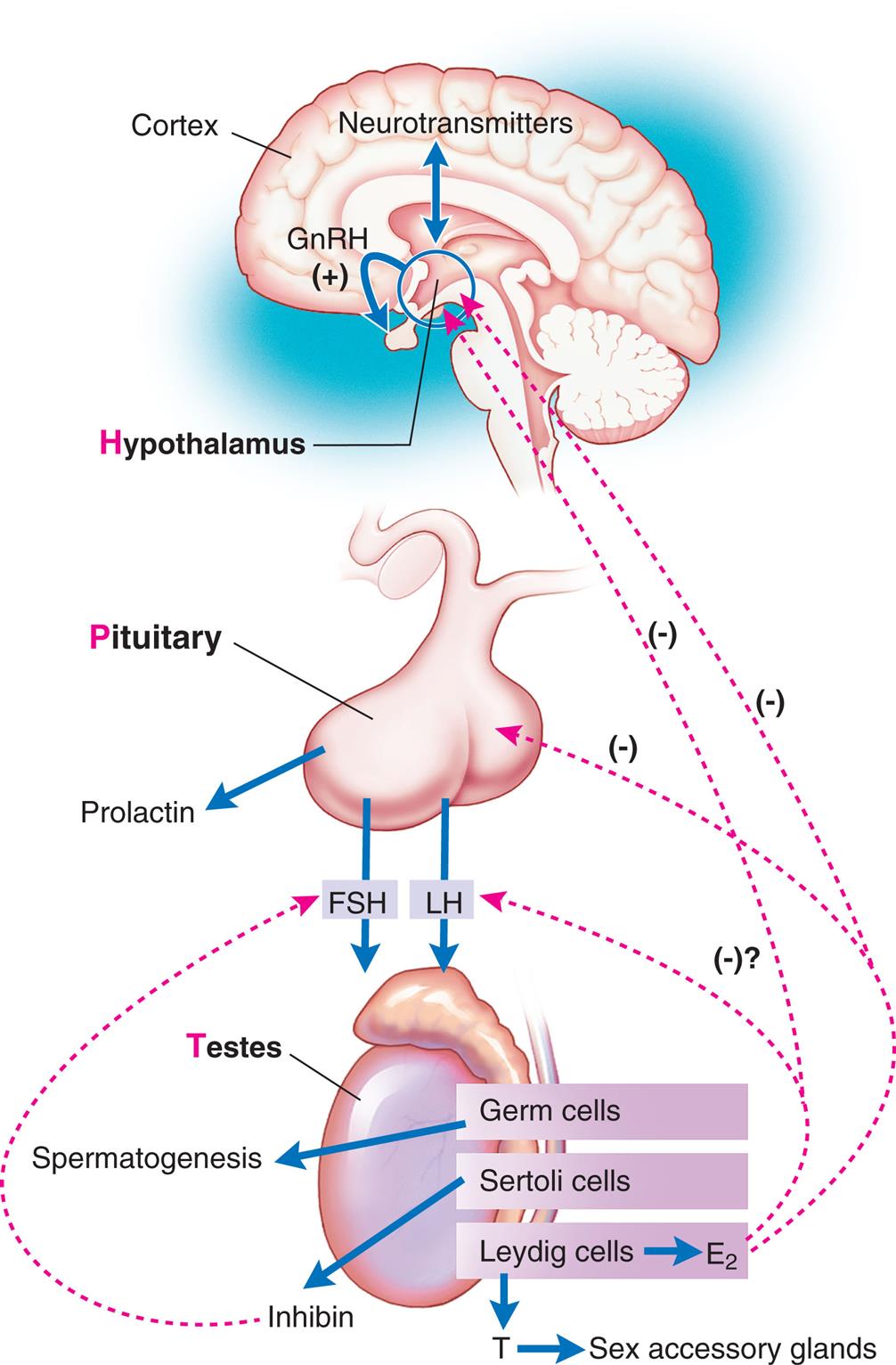
E2, Estrogen; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; T, testosterone.
“An illustration represents the activity along the hypothalamus-pituitary-testicular axis. The illustration shows a brain. There are neurotransmitters in the cortex. The hypothalamus releases G n R H. G n R H stimulates pituitary. The pituitary releases prolactin, F S H, and L H. The F S H and L H impact the testes. Germ cells trigger spermatogenesis. Sertoli cells releases inhibin that provide positive feedback to the F S H. Leydig cells release E 2 which provides negative feedback to L H, the pituitary, and the hypothalamus. Leydig cells also release testosterone which acts on sex accessory glands.”
Ninety-eight percent of testosterone, the major steroid hormone produced by the testes, binds to either sex hormone-binding globulin (SHBG) (50%) or albumin (48%). The remaining 2% remains unbound in the plasma and is free to enter cells and wield its metabolic effects. Changes in the amount of available SHBG affect the amount of testosterone within tissues. The testes secrete only 25% of circulating estrogen (estradiol). The majority is produced by the peripheral conversion of testosterone and androstenedione. Estrogens help regulate GnRH and LH secretion. Peripheral conversion of testosterone also produces dihydrotestosterone (DHT), another potent androgen. DHT is necessary for external virilization during embryogenesis and androgen activity beginning at puberty and continuing throughout adulthood. Prolactin, a polypeptide synthesized and secreted from the pituitary, helps maintain the biosynthesis of testosterone. However, elevated prolactin levels are associated with low levels of testosterone.19
In summary, hormones secreted at each level of the HPT axis control and coordinate testicular function (see Fig. 24.19). This control is exerted through positive and negative feedback signals by (1) sex steroids that inhibit hypothalamic GnRH secretion and pituitary LH responsiveness to GnRH; and (2) testicular inhibin that inhibits pituitary FSH and, possibly, circulating estrogens (E2). Any disruption along the HPT axis may lead to hypogonadism or infertility.
Tests of Reproductive Function
Diagnostic tests of the male and female reproductive systems may be performed to detect the presence of endometriosis or cancerous lesions or identify the presence of sexually transmitted infections. Most commonly, however, tests of reproductive function are performed when infertility exists. In cases of infertility, both partners are examined, and several diagnostic evaluations may be completed. The types of tests available to assess reproductive function and fertility are summarized in Table 24.4. A list of serum hormone tests and normal values is found in Table 24.5. The male is evaluated for number, amount, structure, and motility of sperm and obstruction along the reproductive tract. Tests for females determine whether (1) the reproductive tract (cervix, uterus, fallopian tubes) is adequately patent to allow for the passage of ovum and sperm, (2) ovulation occurs normally, (3) the endometrium is responding normally to hormones, and (4) reproductive tissues are free of tumors or infections. Hormonal assays evaluate the adequacy of pituitary function and target organ response. The position and size of organs or the presence of tumors can be detected by direct observation procedures using a laparoscope or by ultrasound, radiographic, and other imaging studies.20
Table 24.4
FSH, Follicle-stimulating hormone; TSH, thyroid-stimulating hormone; WBC, white blood cell.
Table 24.5
Aging and Reproductive Function
Aging and the Female Reproductive System
Menopause is the cessation of ovulation and menses caused by ovarian failure. It is a normal developmental and transitional event that is universally experienced by the average age of 51 years with a range of 40 to 60 years. Premature menopause is the cessation of ovulation before 40 years of age. A number of factors are thought to influence the age of menopause, including genetics, socioeconomic status, race, parity, oral contraceptive use, early menarche, and lifestyles such as smoking or weight.21 The term “climacteric” refers to gradual changes of ovarian function that start before menopause and result in the symptoms associated with loss of ovarian function.22 For clarity, the term menopause will be used here. Reproductive changes are caused primarily by declining ovarian function and a resulting decrease in ovarian hormone secretion.
Perimenopause is the transitional period between reproductive and nonreproductive years and can last 1 to 8 years. About 5 to 10 years before menopause, approximately 90% of females note mild to extreme variability in frequency and quality of menstrual flow. Changes in hormones occur during this time, including erratically higher estradiol levels, decreased progesterone levels (in normal ovulatory, short luteal phase, or anovulatory cycles), and a disturbed ovarian-pituitary-hypothalamic feedback relationship with higher LH levels. A decrease in the sensitivity of the target tissue receptors and the development of perimenopausal symptoms are commonly experienced. Symptoms usually begin with a lengthening of the menstrual cycle, which correlates with anovulatory cycles. Unpredictable or irregular ovulation uniformly precedes menopause. The perimenopause experience varies among females and from cycle to cycle in the same person.
Menopause is defined by the point that marks 12 consecutive months of amenorrhea. This means that it is determined retrospectively after a female has not had a menstrual period for 1 year. It is characterized by loss of ovarian function, low estrogen and progesterone levels, and high FSH and LH levels (Fig. 24.20). Early menopause is the 5 years after menopause onset. Late menopause follows and continues until death.
Mean circulating hormone levels. FSH, Follicle-stimulating hormone; LH, luteinizing hormone; MP, menopause.
“A graph plots the mean circulating hormone levels. The vertical axis represents picomoles per liter (0 through 1000, in increments of 200) and micrograms per liter (0 to 25, in increments of 5). The horizontal axis represents M P (negative 8 to 0, in increments of negative 2) and years (0 to 10, in increments of 2). The graph plots curves for F S H, L H, estrone, and estradiol. A vertical dashed lined is plotted at 0. F S H. The line rises from (negative 6.5 M P, 100 picomoles per liter or 2.5 micrograms per liter) to (10 years, 700 picomoles per liter or 18 micrograms per liter), passing through (0, 500 picomoles per liter or 12.5 micrograms per liter). L H. The line rises from (negative 6.5 M P, 120 picomoles per liter or 3 micrograms per liter) to (10 years, 300 picomoles per liter or 7.5 micrograms per liter), passing through (0, 350 picomoles per liter or 8 micrograms per liter). Estrone. The lines falls from (negative 6.5 M P, 350 picomoles per liter or 7.5 micrograms per liter) to (10 years, 200 picomoles per liter or 5 micrograms per liter), passing through (0, 300 picomoles per liter or 7.5 micrograms per liter). Estradiol. The line falls from (negative 6.5 M P, 480 picomoles per liter or 12 micrograms per liter) to (10 years, 100 picomoles per liter or 2.5 micrograms per liter), passing through (0, 200 picomoles per liter or 5 micrograms per liter).”
The primary changes of perimenopause and menopause are as follows:
- • Ovarian changes: Beginning in utero, the number of follicles steadily decreases through activation, maturation, and atresia. Around 37 to 38 years of age, females experience accelerated follicular loss, which ends when the supply of follicles is depleted at menopause. This accelerated loss is correlated with increased FSH stimulation, declining inhibin production, slightly elevated estradiol levels, and decreasing amounts of anti-müllerian hormone (normally decreases FSH effects) (see Fig. 24.20). Attenuated LH surges are associated with impaired hypothalamic responses to estradiol positive feedback. The ovarian response to high FSH level recruits increasing numbers of follicles (Box 24.2); these follicles only partially develop, with a net effect of irregular ovulation, lower progesterone levels, depleted follicle reserve, and infertility. The ovaries begin to decrease in size around age 30; this decrease accelerates after age 60. Of the 500,000 follicles present at the onset of puberty, the number dwindles to between 100 and 1000 with menopause.31Table 24.6 summarizes endocrine events occurring during perimenopause. Table 24.7 provides a template to visualize the complex physiology of perimenopause and the dynamic changes that occur during this time.
- • Uterine changes: The increase in anovulatory cycles allows for proliferative growth of the endometrium. With this longer exposure to unopposed estrogen and greater thickness of the endometrium, 50% of perimenopausal females will experience dysfunctional uterine bleeding that is heavy and unpredictable. Increased endometrial bleeding is correlated with a change from ovulatory to anovulatory cycles and is associated with unopposed high estrogen levels the week before menses. Estrogen causes endometrial tissue to thicken. However, without corresponding stromal support from progesterone, estrogen production leads to heavier periods, menometrorrhagia (excessive bleeding often caused by submucosal myomas and endometrial polyps), or metrorrhagia (midcycle bleeding) (Table 24.8). In the past, this has put females at high risk for hysterectomy or endometrial ablation. Medical and hormonal management is the first line of therapy if the uterus is normal.23
- • Breast tissue changes: Breast tissue becomes involuted, fat deposits and connective tissue increase, and breasts are reduced in size and firmness. There can be an increase in white adipose tissue inflammation with elevated aromatase levels (increases circulating estrogen), particularly in obese women.24
- • Genitourinary tract changes: The ovaries shrink; the uterus atrophies; and the vagina shortens, narrows, and loses some elasticity. Lubrication of the vagina diminishes, and vaginal pH increases, creating a higher incidence of vaginitis. The cervix atrophies; the cervical os shrinks; vaginal epithelium atrophies; labia majora and minora become less prominent; some pubic hair is lost; urethral tone declines along with muscle tone throughout the pelvic area; urinary frequency or urgency, urinary tract infections, and incontinence may occur. Regular sexual activity and orgasm may diminish some of these changes. Sexually active females have less vaginal atrophy.
- • Skeletal changes: Bone mass is reduced, leading to increased brittleness and porosity, which increases the risk of osteoporosis and fracture, particularly in the lumbar spine and femoral neck.25
- • Cardiovascular changes: The risk of cardiovascular disease increases significantly and is the leading cause of death in postmenopausal females. Blood pressure and total LDL cholesterol increase, and HDL cholesterol decreases. There is an increased risk for metabolic syndrome.26
- • Systemic changes: Vasomotor flushes are characterized by a rise in skin temperature, dilation of peripheral blood vessels, increased blood flow in the hands, increased skin conductance, and a transient increase in heart rate followed by a temperature drop and profuse perspiration over the area of flush distribution. This usually occurs in the face and neck and may radiate into the chest and other parts of the body. Night sweats, dizziness, nausea, headaches, or palpitations may accompany the flush. These flushes can vary in frequency, intensity, and duration and are experienced by up to 85% of perimenopausal to postmenopausal females from 1 to 15 years. The physiology of vasomotor flushes is poorly understood. Interestingly, emerging evidence shows that the intensity of vasomotor flushes may be associated with an increased risk for cardiovascular disease.27
- • Other changes: Emotional stress with unpredictable mood swings, weight gain, migraine headaches, insomnia, and depression often accompany the change in estrogen levels. Lower estrogen levels decrease skin thickness and diminish skin elasticity, thereby causing increased skin dryness, wrinkling, and poor wound healing. Alopecia and unwanted facial hair are common.28 Hormone replacement therapy can relieve the symptoms of menopause, but risks and benefits must be evaluated for each person.
Table 24.6
FP, Follicular phase; FSH, follicle-stimulating hormone; LH, luteinizing hormone; LP, luteal phase.
Table 24.7
| Phase | Menstrual Physiology | Hormonal Changes | Symptomatology |
|---|---|---|---|
| A | |||
| B | |||
| C | |||
| D | |||
| E |
E2, Estradiol; FP, follicular phase; FSH, follicle-stimulating hormone; LP, luteal phase.
Table 24.8
| Associated Physiologic Change | Signs/Symptoms |
|---|---|
| Short follicular phase (FP) | Short cycles |
| Long FP | Long cycles |
| Thickened endometrium | Heavy, long, or unpredictable flow (including clotting and flooding)a |
| Increase in glandular cells without stromal support produced by progesterone → unstable endometrium | |
| Possible increased production of prostaglandins within endometrial tissue |
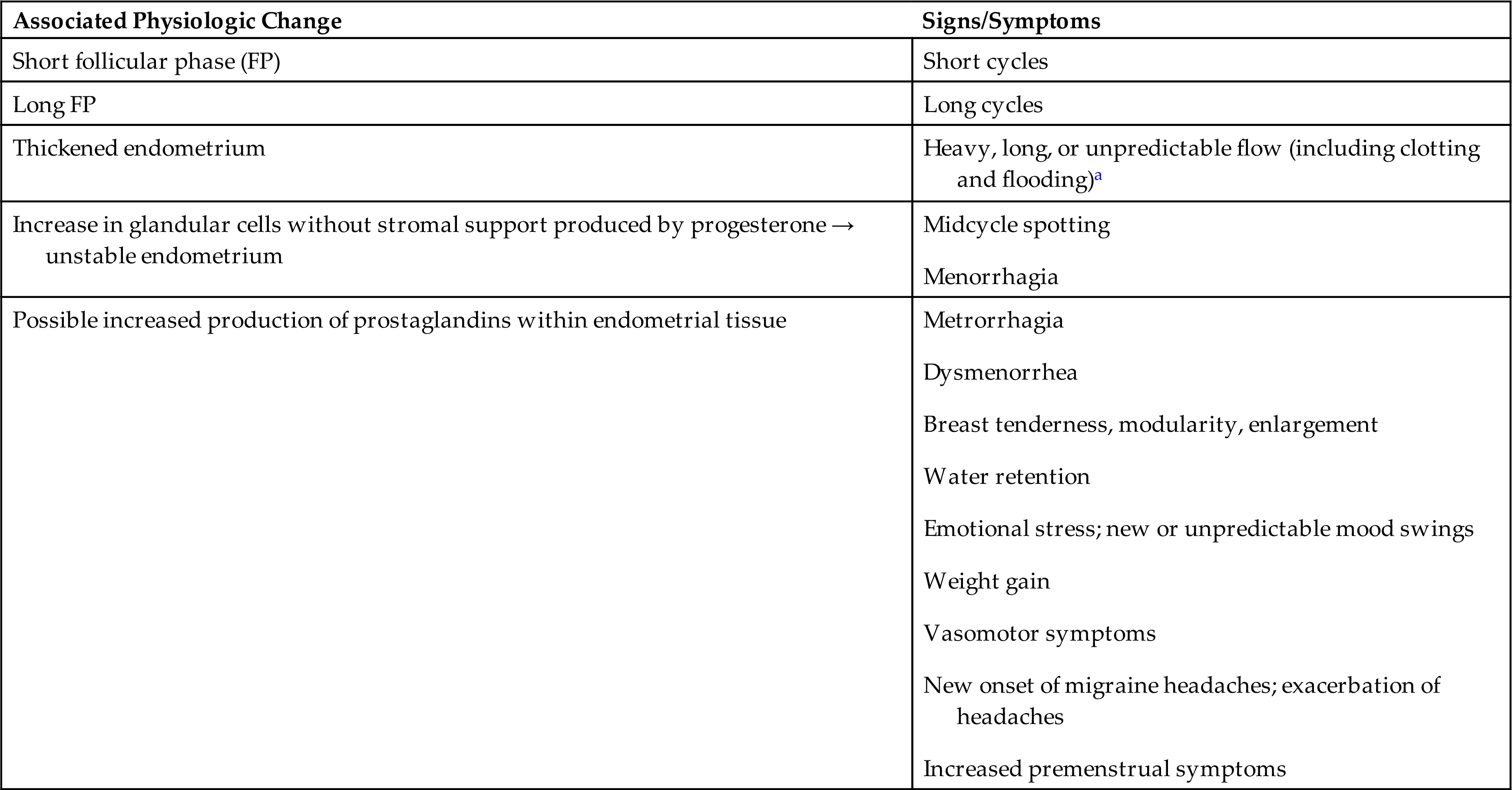
aSymptoms aggravated by anovulatory cycles; leads to dysfunctional uterine bleeding.
Menopause increases the risk of ovarian, breast, and uterine cancers. The risk is greater in females who began menstruating before age 12 or experience menopause after age 55. Females who menstruate longer than normal during a lifetime are exposed to more estrogen and have more ovulations. A longer exposure to estrogen increases the risk of uterine and breast cancers and having more ovulations than normal increases the risk of ovarian cancer.
Aging and the Male Reproductive System
Males maintain reproductive capacity longer than females. There is no known discrete event comparable to menopause that characterizes aging of the male reproductive system, although the term andropause is sometimes used to describe the changes associated with male aging and lower levels of testosterone. Gradual changes do occur, and aging in the male reproductive system is characterized by hypogonadism, testosterone deficiency, erectile dysfunction, and proliferative disorders of the prostate gland (see Chapter 26). Aging changes are also influenced by chronic diseases and the use of medications.29
Components of male sexual behavior include both sexual drive and erectile and ejaculatory capacity. Libido, or sexual drive, is a complex phenomenon that is influenced significantly by health status and environmental, social, and psychological factors. In males older than 40 years, organic factors and chronic disease (e.g., vascular, endocrine, and neurologic disorders) are involved in more than half of the cases of male sexual dysfunction. Aging causes specific physical changes that may influence erectile and ejaculatory capabilities. Alterations in sexual response include the need for longer stimulation to achieve a full erection; slower and less forceful ejaculation, with less pelvic muscle involvement; decreased vasocongestive response; and longer refractory period (time during which erection and ejaculation are not possible), up to 24 hours in some men.
The testes undergo several age-related structural changes, including decreased weight, atrophy, and softening. Degenerative changes in the seminiferous tubules may include thickening of the basement membrane; increase in lumen size; germ cell (spermatogonium) arrest and a decrease in spermatogenic activity; and collapse of tubules, followed by complete obstruction caused by sclerosis and fibrosis. Areas of mild to severe degenerative change may be interspersed with areas having intact tubules. These morphologic changes may result from atherosclerosis (arterial clogging) in the testicular vascular bed. Alterations of the seminiferous tubules do not appear to diminish sperm counts (20 million sperm per milliliter of semen is estimated as the minimum concentration for fertility), but they do reduce fertility because a greater percentage of the sperm lack motility or have structural abnormalities.
About 20% of healthy males more than 60 years of age and 30% to 50% older than 80 years of age have testosterone levels below the reference range, and about 2% have hypogonadism. Aging can cause changes in the production of male sex hormones, levels of SHBG, and responsiveness of target tissue receptors. Hormone synthesis by the testes and testicular responsiveness to the gonadotropins (FSH and LH) are diminished, and pituitary secretion of these gonadotropins is elevated. The reduced levels of testosterone may be related to alterations in the Leydig cells, the testosterone producers of the testes. The number of Leydig cells and their function decrease as age increases, perhaps because of decreased arterial perfusion of the testes, and varicoceles and decreased LH or responsiveness to LH. Decreases in testosterone with advancing age and symptoms of libido loss, erectile dysfunction, loss of muscle mass, increased body fat, anemia, osteoporosis, depressed mood, decreased vitality, sweating, and hot flushes are described as late-onset hypogonadism.30
Even if testosterone levels are not decreased, older males may have less unbound testosterone in their blood, decreasing the amount of unbound hormone available to stimulate target tissues. Decreased testosterone levels have several effects, including functional deterioration of the accessory sex organs (the prostate gland, seminal vesicles, epididymis, and ductus deferens); loss of muscle mass, strength, and endurance; increased visceral fat, osteopenia, and cognitive decline; and, in many men, decrease in libido. This last effect also may be caused by alterations in other variables that affect libido. Modifiable risk factors for low testosterone levels and symptoms of androgen deficiency include health status and waist circumference.
Summary Review
Development of the Reproductive Systems
- 1. Differentiation of female and male genitalia begins around 7 to 8 weeks of embryonic development when the gonads of genetically male embryos begin to secrete male sex hormones, primarily testosterone, under the influence of SRY gene expression and testosterone-determining factor (TDF). Female gonadal development occurs in the absence of SRY gene expression. Until that time, the primitive reproductive organs of males and females are homologous (the same).
- 2. Production of primitive female gametes (ova) occurs solely during fetal life. From puberty to menopause, one female gamete matures per menstrual cycle. Production of male gametes (sperm) begins at puberty; after that, millions are produced daily, usually for life.
- 3. Puberty is the onset of sexual maturation. Adolescence is a stage of human development between childhood and adulthood and includes social, psychological, and biologic changes.
- 4. The structure and function of both male and female reproductive systems depend on interactions among the central nervous system (hypothalamus), the endocrine system (anterior pituitary), the gonads (ovaries, testes), and the hypothalamic-pituitary-gonadal axis. A set of complex neurologic and hormonal interactions accelerate at puberty and lead to sexual maturation and reproductive capability.
- 5. One year before puberty, secretion of gonadotropin-releasing hormone (GnRH), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) stimulate the gonads (ovaries and testes) to secrete female (estrogen and progesterone) or male sex hormones (testosterone). These stimulate the maturation of the gonads, reproductive organs, and breasts (in females). Puberty is complete in females with the first ovulatory menstrual period and is complete in males with the first ejaculation that contains mature sperm.
The Female Reproductive System
- 1. The function of the female reproductive system is to produce mature ova and, when they are fertilized, to protect and nourish them through embryonic and fetal life and expel them at birth.
- 2. The external female genitalia are the mons pubis, labia majora, labia minora, clitoris, vestibule (urinary and vaginal openings), Bartholin and Skene glands, and perineum. They protect body openings and may play a role in sexual functioning.
- 3. The internal female genitalia are the vagina, uterus, fallopian tubes, and ovaries. Although all these organs are needed for reproduction, the ovaries are the most essential because they produce the female gametes and female sex hormones.
- 4. The vagina is a fibromuscular canal that receives the penis during sexual intercourse and is the exit route for menstrual fluids and products of conception. The vagina leads from the introitus (its external opening) to the cervical portion of the uterus.
- 5. The uterus is the hollow, muscular organ in which a fertilized ovum develops until birth. The uterine walls have three layers: the endometrium (lining), myometrium (muscular layer), and perimetrium (outer covering, which is continuous with the pelvic peritoneum). The endometrium proliferates (thickens) and is shed in response to cyclic changes in levels of female sex hormones. The cervix is the narrow, lower portion of the uterus that opens into the vagina.
- 6. The two fallopian tubes extend from the uterus to the ovaries. Their function is to direct ova from the spaces around the ovaries to the uterus. Fertilization normally occurs in the distal third of the fallopian tubes.
- 7. From puberty to menopause, the ovaries are the site of (1) ovum maturation and release and (2) production of female sex hormones (estrogen, progesterone) and androgens. The female sex hormones are involved in sexual differentiation and development, the menstrual cycle, pregnancy, and lactation. Although they are primarily male sex hormones, androgens in females are precursors of female sex hormones and contribute to the prepubertal growth spurt, pubic and axillary hair growth, and activation of sebaceous glands.
- 8. Estrogen (primarily estradiol) is produced by cells in the developing ovarian follicle (the structure that encloses the ovum). Progesterone is produced by cells of the corpus luteum, the structure that develops from the ruptured ovarian follicle after ovulation (ovum release). Androgens are produced within the ovarian follicle, adrenal glands, and adipose tissue.
- 9. The average menstrual cycle lasts 25 to 30 days and consists of three phases, which are named for ovarian and endometrial changes: the follicular/proliferative phase, the luteal/secretory phase, and the ischemic menstrual phase.
- 10. The follicular/proliferative phase is the maturation of an ovarian follicle, the proliferation of the uterine endometrium, and the release of the ovum. FSH stimulates follicle and ovum maturation, then a surge of LH causes ovulation. Estrogen causes proliferation of the endometrium.
- 11. During the luteal/secretory phase, the ovum transforms into the corpus luteum. LH stimulates the corpus luteum to secrete progesterone and estrogen. Progesterone stimulates blood vessel and glandular growth, and estrogen maintains the thickened endometrium. Glands in the endometrium begin to secrete a thin, glycogen-containing fluid, hence the name secretory phase.
- 12. During the ischemic/menstrual phase, the corpus luteum degenerates, production of progesterone and estrogen drops sharply, and the “starved” endometrium degenerates and is shed, causing menstruation.
- 13. Cyclic changes in hormone levels also cause thinning and thickening of the vaginal epithelium, thinning and thickening of cervical secretions, and changes in basal body temperature.
Structure and Function of the Breast
- 1. The basic functional unit of the female breast is the lobe, a system of ducts that branches from the nipple to milk-producing units called lobules. Each breast contains 15 to 20 lobes, which are separated and supported by Cooper ligaments. The lobules contain acini cells, which are convoluted spaces lined with epithelial cells. Contraction of the subepithelial cells of each acinus moves milk into the system of ducts that leads to the nipple.
- 2. Until puberty, the female and male breasts are similar, consisting of a small, underdeveloped nipple and some fatty and fibrous tissue. At puberty, however, a variety of hormones (estrogen, progesterone, prolactin, growth hormone, insulin, cortisol) cause the female breast to develop into a system of glands and ducts that is capable of producing and ejecting milk.
- 3. During the reproductive years, breast tissue undergoes cyclic changes in response to hormonal changes of the menstrual cycle.
- 4. Milk production occurs in response to prolactin, a hormone that is secreted in larger amounts after childbirth. Milk ejection is under the control of oxytocin, another hormone of pregnancy and lactation.
- 5. The male breast does not develop because of the absence of sufficiently high levels of estrogen and progesterone and antagonistic effects of androgens.
The Male Reproductive System
- 1. The function of the male reproductive system is to produce male gametes (sperm) and deliver them to the female reproductive tract.
- 2. The external male genitalia are the testes, epididymides, scrotum, and penis. The internal genitalia are the vas deferens, ejaculatory duct, prostatic and membranous sections of the urethra, seminal vesicles, prostate gland, and bulbourethral glands.
- 3. The testes (male gonads) are paired glands suspended within the scrotum. The testes have two functions: spermatogenesis (sperm production) and the production of male sex hormones (androgens, chiefly testosterone).
- 4. The epididymis is a long, coiled tube arranged in a comma-shaped compartment that curves over the top and rear of the testis. The epididymis receives sperm from the testis and stores them while they develop further. Sperm travel the length of the epididymis and then are ejaculated into the vas deferens, which transports sperm to the urethra.
- 5. The scrotum is a skin-covered, fibromuscular sac that encloses the testes and epididymides, which are suspended within the scrotum by the spermatic cord. The scrotum keeps these organs at optimal temperatures for sperm survival (about 1°C to 2°C lower than body temperature) by contracting in cold environments and relaxing in warm environments.
- 6. The penis has two functions: delivery of sperm and elimination of urine.
- 7. The penis is a cylindric organ consisting of three longitudinal compartments (two corpora cavernosa and one corpus spongiosum) and the urethra. The urethra runs through the corpus spongiosum. The corpora cavernosa and corpus spongiosum consist of erectile tissue. Externally the penis consists of a shaft and a tip, which is called the glans.
- 8. Sexual intercourse is made possible by the erectile reflex, in which tactile or psychogenic stimulation of the parasympathetic nerves causes arterioles in the corpora cavernosa and corpus spongiosum to dilate and fill with blood, causing the penis to enlarge and become firm.
- 9. Emission, which occurs at the peak of sexual arousal, is the movement of semen from the epididymides to the penis. Ejaculation, which is a continuation of emission, is the pulsatile ejection of semen from the penis.
- 10. Spermatogenesis is a continuous process because spermatogonia, the primitive male gametes, undergo continuous mitosis within the seminiferous tubules of the testes. Some spermatogonia develop into primary spermatocytes, which divide meiotically into secondary spermatocytes and then spermatids. The spermatids develop into sperm with the help of nutrients and hormonal signals from Sertoli cells.
- 11. Production of the male sex hormones (androgens) is controlled by interactions among the hypothalamus, anterior pituitary, and gonads. The male hormones are produced steadily rather than cyclically, however.
Tests of Reproductive Function
Aging and Reproductive Function
- 1. Perimenopause is the transitional period between reproductive and nonreproductive years in females. During this transition, the ovaries produce erratic and high levels of estrogen that contribute to such symptoms as hot flashes, breast tenderness and nodularity, and migraine headaches. Menstrual cycles shorten and then become irregular as anovulation occurs.
- 2. Menopause, the point that marks 12 consecutive months of amenorrhea, includes atrophic changes in the ovaries, vagina, and breast.
- 3. Males maintain reproductive capacity into their later years. In some males, there are gradual changes with testosterone deficiency, hypogonadism, proliferative disorders of the prostate, erectile dysfunction, and some loss of muscle mass and strength. Andropause is an androgen deficiency in the aging male.