Alterations of Renal and Urinary Tract Function
Julia L. Rogers
The renal system plays a major role in homeostasis by filtering nearly 200 L of blood every 24 hours. Approximately 1 L of filtered fluid is converted into urine and excreted through micturition per day. Because the kidneys filter the blood, the renal system is directly linked to every other organ system. A variety of disorders affects renal function by inhibiting the kidney’s ability to regulate plasma volume and osmolality. Disease may be limited to only the kidney and urinary tract or may include systemic diseases that cause acute kidney injury (AKI), chronic kidney disease (CKD), or difficulty eliminating urine (e.g., infection, neurologic injury, or diabetes mellitus). Infection of the kidney or urinary tract is the most common disorder affecting renal function. Stones, tumors, inflammation, or consequences of medical procedures can obstruct and/or cause injury to the upper or lower urinary tract (LUT). Renal injury, whether acute or chronic, can affect other organs and become life-threatening.
http://evolve.elsevier.com/Rogers/pathophysiology/
Urinary Tract Obstruction
Urinary tract obstruction is an anatomic (structural) or functional abnormality that causes interference with the flow of urine at any site along the urinary tract (Fig. 38.1). An obstruction impedes urine flow, increases hydrostatic pressure, dilates structures proximal to the blockage, which increases risk of infection, and compromises renal function. Anatomic changes in the urinary system caused by obstruction are referred to as an obstructive uropathy, which may be acute or chronic, partial, or complete, and unilateral or bilateral. The severity of an obstructive uropathy is determined by the:
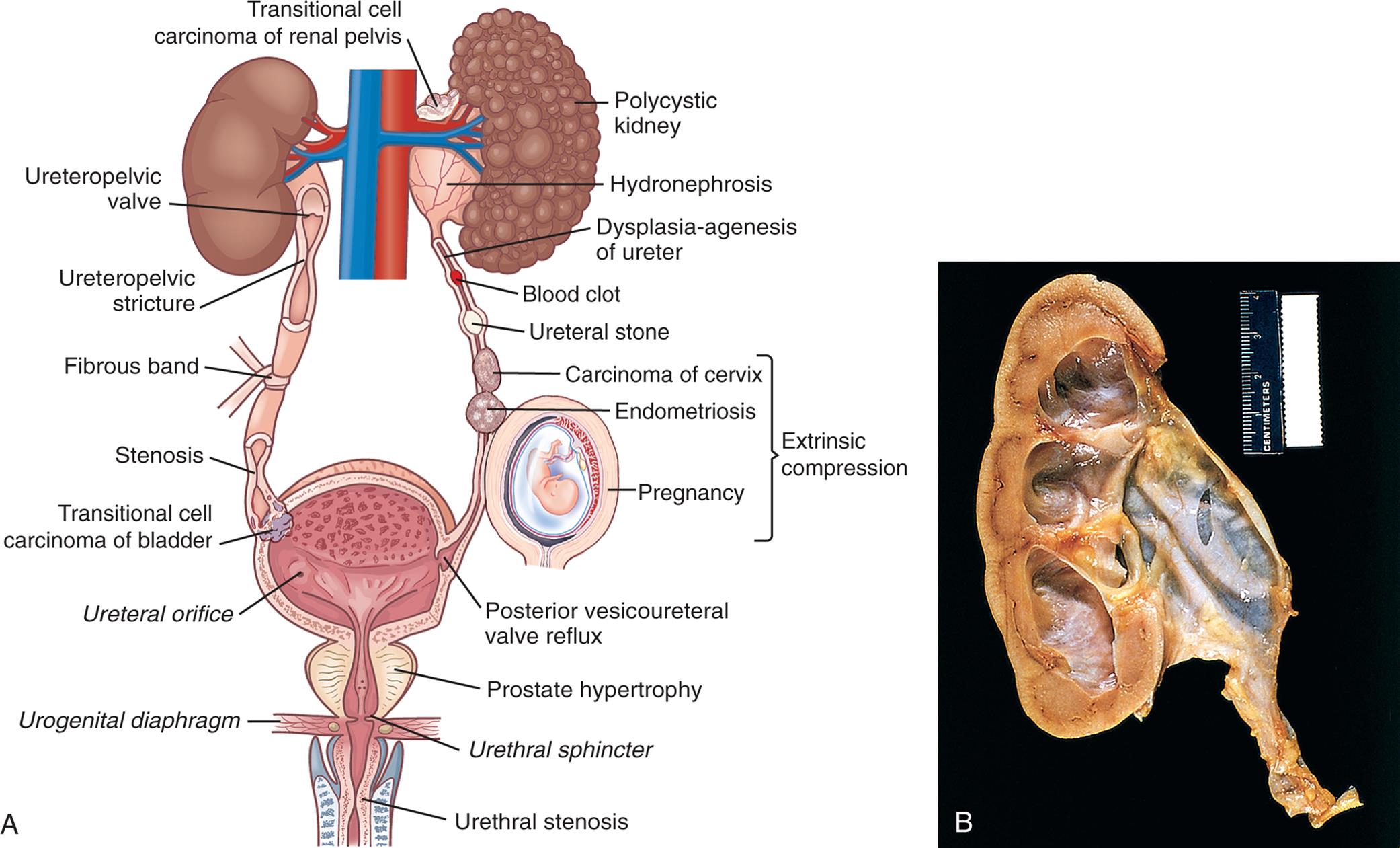
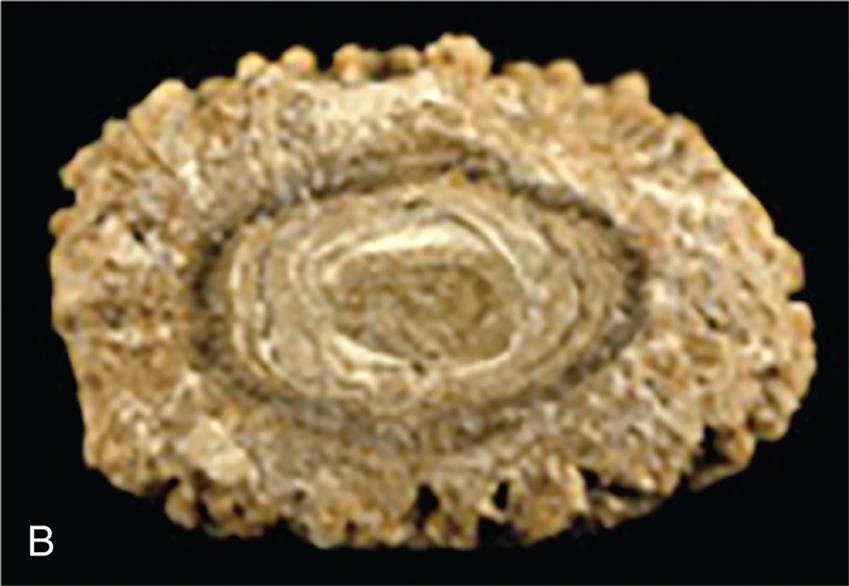
(A) Major sites of urinary tract obstruction. (B) Hydronephrosis of the kidney. There is marked dilation of the renal pelvis and calyces with thinning of the overlying cortex and medulla due to compression atrophy. (B, From Kumar V, et al. Robbins and Cotran pathologic basis of disease, 10th edition. Philadelphia: Elsevier; 2021.)
Left panel, A. The illustration shows the urinary system. The followings structures are labeled, clockwise from the top: transitional cell carcinoma of renal pelvis, polycystic kidney, hydronephrosis, dysplasia-agenesis of ureter, blood clot, ureteral stone, extrinsic compression (carcinoma of cervix, endometriosis, and pregnancy), posterior vesicoureteral valve reflux, prostate hypertrophy, urethral sphincter, urethral stenosis, urogenital diaphragm, ureteral orifice, transitional cell carcinoma of bladder, stenosis, fibrous band, ureteropelvic stricture, and ureteropelvic valve.
Obstructions may be relieved, or partially alleviated, by correction of the obstruction, although permanent impairments such as hydronephrosis occur if a complete or partial obstruction persists over a period of weeks to months or longer.
Upper Urinary Tract Obstruction
A stricture or compression of the calyx, ureteropelvic, or ureterovesical (ureter-bladder) junction is a common cause of upper urinary tract obstructions and is commonly caused by kidney stones (calculi). Ureteral compressions or blockages can be caused from an aberrant vessel, tumor, stone, or abdominal inflammation and scarring (retroperitoneal fibrosis).1 The most common cause in children is a congenital anomaly (see Chapter 39); in young adults, the common cause is renal calculi; and in older adults, renal calculi, ureteral strictures, and tumors are more common.
Obstruction of the upper urinary tract causes a “backing up” of urine and dilation of the ureter, renal pelvis, calyces, and renal parenchyma proximal to the site of urinary blockage. Dilation of the ureter is referred to as hydroureter (accumulation of urine in the ureter). Dilation of the renal pelvis and calyces proximal to a blockage is referred to as hydronephrosis or ureterohydronephrosis (dilation of both the ureter and the pelvicaliceal system) (see Fig. 38.1B). The backup of urine into the Bowman space from an obstruction opposes the hydrostatic pressure of glomerular filtration and decreases the glomerular filtration rate (GFR).1 Unless the obstruction is relieved, the dilation leads to tubulointerstitial fibrosis, which damages renal nephrons and can lead to CKD.
Tubulointerstitial fibrosis is the deposition of excessive amounts of extracellular matrix (collagen and other proteins) by activated inflammatory cells including macrophages and myofibroblasts with associated areas of tubular atrophy.2 Tubulointerstitial fibrosis occurs with kidney injury including obstructive uropathies. Although deposition of extracellular matrix is a normal process of kidney repair and maintenance, activation of inflammatory cells and production of growth factors, such as transforming growth factor-beta-1 (TGF-β1), promotes the process of tubulointerstitial fibrosis and irreversible kidney damage.3
Apoptosis is a normal process that the body uses to replace damaged or senescent cells with new ones (see Chapter 1). An imbalance in growth factors provoked by obstruction contributes to excess cellular destruction with a transition from apoptosis to necrosis and inflammation, ultimately resulting in the loss of functioning nephrons.4
The magnitude of this damage, and the kidney’s ability to recover normal homeostatic function, is affected by the severity and duration of the obstruction. With complete obstruction, damage to the renal tubules and compression of the renal vasculature occur in a matter of hours, and irreversible damage occurs within 3 to 4 weeks. Nevertheless, even in the face of a complete obstruction, the human kidney may recover at least partial homeostatic function provided the blockage is removed. Recovery can take up to 3 months.5
When there is unilateral obstruction, the body is able to partially counteract the negative consequences by a process called compensatory hypertrophy and hyperfunction. The compensatory response is guided by growth factors that cause the unobstructed kidney to increase the size and function of individual glomeruli and tubules, but not the total number of functioning nephrons. Consequently, the obstructed kidney can remain silent for a long time. Partial obstruction that is not relieved, in the absence of renal infection, leads to more subtle, but ultimately permanent impairments including loss of the kidney's ability to concentrate urine, reabsorb bicarbonate, excrete ammonia, and regulate metabolic acid-base and fluid and electrolyte balance. The process is reversible when relief of obstruction results in recovery of function by the obstructed kidney. The ability of the body to engage in compensatory hypertrophy and hyperfunction diminishes with age. Complete bilateral obstruction causes anuria because the retrograde increase in tubular hydrostatic pressure completely opposes glomerular filtration.
Relief of upper urinary tract obstruction is usually followed by a brief period of diuresis, commonly called postobstructive diuresis. Postobstructive diuresis is a physiologic response and is typically mild, representing a restoration of fluid and electrolyte imbalance caused by retention of fluid related to the obstructive uropathy. Occasionally, relief of obstruction will cause rapid excretion of large volumes of water, sodium, or other electrolytes, resulting in a urine output of 200 mL/h for two consecutive hours or more than 3 L in 24 hours. Minimal normal daily urine output is approximately 720 mL/day.6 Rapid postobstructive diuresis causes dehydration and fluid and electrolyte imbalances that must be promptly corrected. Risk factors for severe postobstructive diuresis include chronic, bilateral obstruction; impairment of one or both kidneys’ ability to concentrate urine or reabsorb sodium (nephrogenic diabetes insipidus); hypertension; edema and weight gain; congestive heart failure; and uremic encephalopathy.
Nephrolithiasis
Nephrolithiasis, also commonly known as kidney stones or renal calculi, are masses of crystals, protein, or other substances that are a common cause of urinary tract obstruction in adults. Stones can be located anywhere along the urinary tract including in the kidneys, ureters, and urinary bladder. However, the favored sites of stone formation are in the renal calyces, renal pelvis, and bladder. Stones are unilateral in about 80% of individuals. The prevalence of kidney stones in the United States is approximately 11% in males and 7% in females with an incidence of about 1% per year.7 The cumulative risk of recurrence at 5 years is approximately 53% overall, with a lower rate of 26% for those with a single stone episode.8 The risk of stone formation is influenced by a number of factors, including age, sex, race, geographic location, seasonal factors, fluid intake, diet, occupation, and genetic predisposition.9 Diseases that predispose individuals for stone formation are urinary tract infection (UTI), hypertension, atherosclerosis, metabolic syndrome, obesity, and type 2 diabetes.10 While stones are more prevalent in males before the age of 50 years, there is increasing incidence seen in females. Geographic location influences the risk of stone formation because of indirect factors. Warmer climates with high humidity and rainfall influence a person’s fluid intake and dietary patterns. Persons who regularly consume an adequate volume of water are at reduced risk when compared with persons who consume lower volumes of water.9
Stones are classified according to the primary minerals (salts) that make up the stones. The most common stone types include calcium oxalate or calcium phosphate (70% to 80%), struvite (magnesium–ammonium–phosphate) (5% to 10%), and uric acid (5% to 10%) (Table 38.1).11 Cystine stones are rare (≤2%), and so are stones that are formed from the metabolic effects of some medications (e.g., atazanavir, ceftriaxone, and N-acetyl-sulfadiazine) in individuals treated for a lengthy period of time for chronic diseases.12,13
Table 38.1

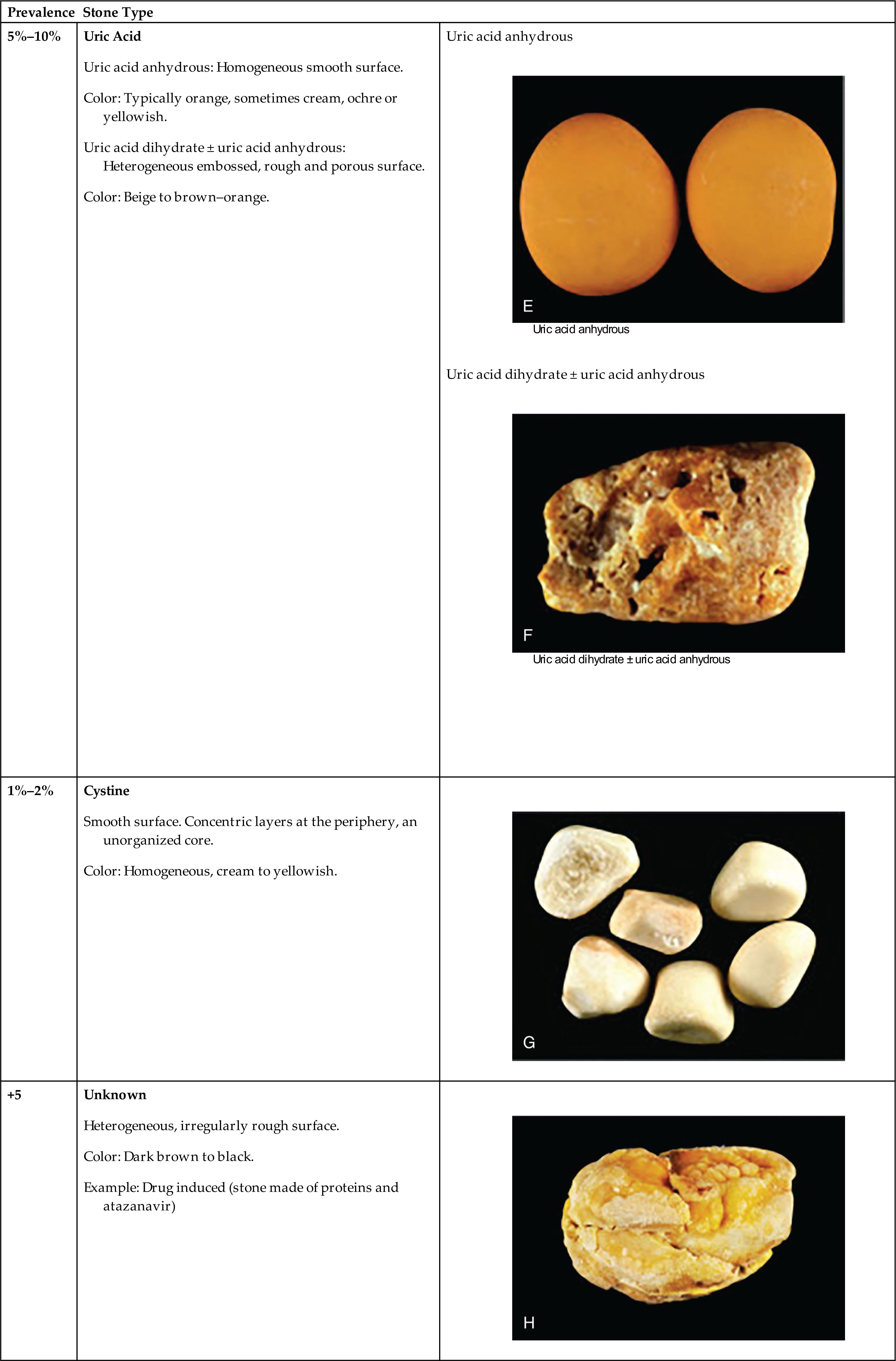
Pictures in right column from Daudon M, Dessombz A, Frochot V, et al. Comprehensive morpho-constitutional analysis of urinary stones improves etiological diagnosis and therapeutic strategy of nephrolithiasis. Comptes Rendus Chimie, 2016;19(11–12):1470–1491. https://doi.org/10.1016/j.crci.2016.05.008.
Stones are also classified according to location and size. Staghorn calculi are large and fill the minor and major calyces. Nonstaghorn calculi are of variable size but tend to be smaller and are located in the renal calyces, renal pelvis, or at various sites along the ureter.
Pathophysiology
Stone formation is complex and related to:
- 1. supersaturation of one or more salts in the urine,
- 2. precipitation of the salts from a liquid to a solid state (crystals),
- 3. growth through crystallization or agglomeration (sometimes called aggregation), and
- 4. the presence or absence of stone inhibitors (e.g., uromodulin [Tamm-Horsfall protein]).
Supersaturation is the presence of a higher concentration of a solute (salt) within a solvent (in this case, the urine) than can be dissolved. Human urine contains many ions capable of precipitating from solution and forming a variety of salts. The salts form crystals that are retained and grow into stones. Crystallization is the process by which crystals grow from a small nucleus, or nidus, to larger stones in the presence of supersaturated urine. Although supersaturation is essential for free stone formation, the urine need not remain continuously supersaturated for a stone to grow once its nucleus has precipitated from solution. Intermittent periods of supersaturation after the ingestion of a meal or during times of dehydration from limited oral intake or secondary to continued use of diuretics are sufficient for stone growth in many individuals. In addition, the renal tubules and papillae have many surfaces that may attract a crystalline nidus (known as a Randall plaque) and add biologic material (matrix), forming a stone. Matrix is an organic material (i.e., mucoprotein) in which the components of a kidney stone are embedded. Randall plaques start in the suburothelial layer and gradually grow until they break through into the renal pelvis. Once in continuous contact with urine, layers of calcium oxalate typically start to form on the calcium phosphate nidus (see Table 38.1).14 The pH of the urine influences the risk of precipitation and calculus formation. An alkaline urinary pH (pH > 7.0) significantly increases the risk of calcium phosphate stone and struvite stone formation, whereas acidic urine (pH < 5.0) increases the risk of uric acid stone formation. Cystine and xanthine also precipitate more readily in acidic urine.
Substances capable of inhibiting stone or crystal growth include potassium citrate, Tamm-Horsfall protein, pyrophosphate, and magnesium.15 These substances normally reduce the risk of calcium phosphate or calcium oxalate precipitation in the urine and prevent subsequent stone formation.
Retention of crystal particles occurs primarily at the papillary collecting ducts. Most crystals are flushed from the tract through the normal flow of urine. Urinary stasis (e.g., from benign prostatic hyperplasia, neurogenic bladder), anatomic abnormalities (strictures), or inflamed epithelium within the urinary tract may prevent prompt flushing of crystals from the system, thus increasing the risk of stone formation.
The size of a stone determines the likelihood that it will pass through the urinary tract and be excreted through micturition. Stones smaller than 5 mm have about a 50% chance of spontaneous (painful) passage, whereas stones that are larger than 1 cm have almost no chance of spontaneous passage.16
Both genetic and environmental factors may increase the susceptibility of calcium stones. Most affected individuals have idiopathic calcium oxalate urolithiasis (ICOU), a condition the exact etiology of which has not yet been determined. Stones can form freely in supersaturated urine or detach from interstitial sites of formation (e.g., from Randall plaque). Hypercalciuria, hyperoxaluria, hyperuricosuria, hypocitraturia, mild renal tubular acidosis, crystal growth inhibitor deficiencies, and alkaline urine are associated with calcium stone formation. Hypercalciuria and hyperoxaluria are usually attributable to intestinal hyperabsorption and less commonly to a defect in renal calcium reabsorption. Hyperparathyroidism and bone demineralization associated with prolonged immobilization are also known to cause hypercalciuria.17
Struvite stones primarily contain magnesium, ammonium, and phosphate as well as varying levels of a matrix. Matrix forms in an alkaline urine and during infection with a urease-producing bacterial pathogen, such as a Proteus, Klebsiella, or Pseudomonas.18 Struvite calculi may grow quite large and branch into a staghorn configuration (staghorn calculus) that approximates the pelvicaliceal collecting system. Women are at greater risk for struvite stones because they have an increased incidence of UTI.
Uric acid stones occur in persons who excrete excessive uric acid in the urine, such as those with gouty arthritis. Uric acid is primarily a product of biosynthesis of endogenous purines and is secondarily affected by consumption of purines (e.g., meat and beer) in the diet. A consistently acidic urine greatly increases this risk, including defective excretion. Cystinuria and xanthinuria are genetic disorders of amino acid metabolism, and their excess in urine can cause cystine or xanthine stone formation in the presence of acidic urine.19
Clinical Manifestations
Renal colic is pain related to dilation and spasms of smooth muscle related to ureteral obstruction. Moderate to severe pain often originates in the flank and radiates to the groin, and usually indicates obstruction of the renal pelvis or proximal ureter. Colic that radiates to the lateral flank or lower abdomen typically indicates obstruction in the midureter. Bothersome LUT symptoms (urinary urgency, frequency, incontinence) indicate obstruction of the lower ureter or ureterovesical junction. The pain can be incapacitating and may be accompanied by nausea and vomiting. Gross or microscopic hematuria may be present.
Evaluation and Treatment
The evaluation and diagnosis of nephrolithiasis is based on presenting symptoms and history combined with a focused physical assessment. Imaging studies determine the location of the stone, severity of obstruction, and associated obstructive uropathy. Imaging of kidney stones can include plain abdominal radiography, ultrasound, intravenous pyelography, computed tomography (CT), and magnetic resonance imaging (MRI). The history queries dietary habits, age of the first stone episode, stone analysis, and presence of complicating factors, including hyperparathyroidism or recent gastrointestinal or genitourinary surgery. Urinalysis (including pH) is obtained, and a 24-hour urine is completed to identify calcium oxalate, calcium citrate, and other significant constituents. In addition, every effort is made to retrieve and analyze stones that are passed spontaneously or retrieved through aggressive intervention. To diagnose and manage underlying metabolic disorders, additional tests are completed for those with suspected hyperparathyroidism (elevated serum calcium levels), cystine calculi, or uric acid (high purine diet) stones.
The goals of treatment are to manage acute pain, promote stone passage, reduce the size of stones already formed, and prevent new stone formation.20 The components of treatment include:
- 1. managing pain (can require narcotic medication),
- 2. reducing the concentration of stone-forming substances by increasing urine flow rate with high fluid intake,
- 3. adjusting the pH of the urine (e.g., make it more alkaline with potassium citrate administration or more acid with potassium acid phosphate),
- 4. decreasing the amount of stone-forming substances in the urine by decreasing dietary intake or endogenous production or by altering urine pH, and
- 5. removing stones using percutaneous nephrolithotomy, ureteroscopy, or ultrasonic or laser lithotripsy to fragment stones for excretion in the urine.21
Obstructing kidney stones with a suspected proximal UTI are urologic emergencies requiring emergent decompression, stone removal, and antibiotics.22 Prevention of recurrent stones includes increasing fluid intake to generate 2.5 L of urine per day, avoiding intake of colas and other soft drinks acidified with phosphoric acid, avoiding dietary oxalate (e.g., chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, wheat bran), eating less animal protein, and limiting sodium intake. Maintaining a dietary calcium intake of 1000 to 1200 mg/day is helpful for calcium stone prevention. Potassium citrate may be used to prevent calcium stone aggregation and to raise urinary pH.23
Lower Urinary Tract Obstruction
Obstructions of the LUT are inherently caused by a structural or anatomic disorder or an alteration in neurologic function (neurogenic bladder). These disorders are related to alterations of urine storage in the bladder or emptying of urine through the bladder outlet. Incontinence is a common symptom associated with LUT obstructions. The types of incontinence are summarized in Table 38.2.
Table 38.2

Data from: Wyndaele M, Hashim H. Pathophysiology of urinary incontinence. Surgery (Oxford), 2020;38(4): 185–190.
Anatomic Obstructions to Urine Flow
Anatomic causes of resistance to urine flow include urethral stricture, prostatic enlargement in men, pelvic prolapse (bladder and uterus) in women, and tumor compression. A urethral stricture is a narrowing of its lumen and occurs when infection, injury, or surgical manipulation produces a scar that reduces the caliber of the urethra. The severity of obstruction is influenced by its location within the urethra, its length, and the severity of the stricture. Strictures that are longer than 1 centimeter and in the proximal urethra cause more severe obstruction. They are more common in men because of a longer urethra (see Chapter 26).24 Urethral stricture is treated with urethral dilation accomplished by using a steel instrument shaped like a catheter (urethral sound) or a series of incrementally increasing catheter-like tubes (filiforms and followers). Long, dense strictures typically require surgical repair (urethroplasty) to prevent recurrence. Prostate enlargement is caused by acute inflammation, benign prostatic hyperplasia, or prostate cancer (see Chapter 26). Severe pelvic organ prolapse (see Chapter 25) in a woman causes bladder outlet obstruction when a cystocele (the downward protrusion/herniation of the bladder into the vagina) or the uterus descends into the vagina below the level of the urethral outlet. In men, the bladder may rarely herniate into the scrotum, causing a similar type of obstruction. Each of these disorders can cause compression of the urethra with obstruction to urine flow.
Partial obstruction of the bladder outlet or urethra initially causes an increase in the force of detrusor contraction. If the obstruction persists, afferent nerves within the bladder wall are adversely affected, leading to urinary urgency and, in some cases, overactive detrusor contractions (a myogenic cause of overactive bladder). When obstruction persists, there is an increased deposition of collagen within the smooth muscle bundles of the detrusor muscle (trabeculation). Ultimately, the bladder wall loses its ability to stretch and accommodate urine, a condition called low bladder wall compliance (loss of elasticity), and the detrusor loses its ability to contract efficiently, resulting in urine retention. This underactive bladder (UAB) syndrome can also occur as a consequence of bladder radiation treatment. Low bladder wall compliance chronically elevates intravesicular pressure, increasing the likelihood of hydroureter, hydronephrosis, impaired renal function, incontinence, and UTI.
Symptoms of obstruction include:
- 1. frequent daytime voiding (micturition more than every 2 hours while awake);
- 2. nocturia (awakening more than once each night to urinate for adults younger than 65 years of age or more than twice for older adults);
- 3. poor force of stream;
- 4. intermittency of urinary stream;
- 5. bothersome urinary urgency, often combined with hesitancy; and
- 6. feelings of incomplete bladder emptying despite micturition.
Overactive and Underactive Bladder Syndrome
Overactive bladder syndrome (OAB) is a dysfunction of urine storage with nonspecific symptoms characterized by urinary urgency, frequency, and nocturia with or without incontinence in the absence of UTI or other known pathology (e.g., neurologic disorders). OAB affects a significant number of adults (approximately 16%).25 The specific cause is not clearly known and several mechanisms could be involved including myogenic or neurogenic alterations in sensory and motor function.26 The symptoms are usually associated with involuntary contractions of the detrusor muscle during the bladder-filling phase, often resulting in urge incontinence and nocturia. Risk factors in women include vaginal birth with episiotomy or use of forceps, surgery for pelvic organ prolapse, and decreased estrogen associated with menopause or hysterectomy. Loss of estrogen results in thinning and loss of urethral muscle strength. Risk factors in men include enlarged prostate with urinary obstruction and surgical treatment for prostate cancer. Risk factors include the use of diuretics, antidepressants, alpha-agonists, beta-antagonists, sedatives, anticholinergics, and analgesics.
Both behavioral and pharmacologic therapy are first- and second-line treatments for OAB. Behavioral therapy includes pelvic floor (Kegel) exercises (detrusor contraction can be inhibited by pelvic floor muscle contraction providing time to get to the toilet), bladder training with timed voiding, management of fluid intake and use of caffeine and alcohol, managing constipation, and biofeedback techniques. Drug therapy to manage incontinence includes topical vaginal estrogen in women and drugs that increase urethral sphincter contraction or relax the bladder wall.
Because the parasympathetic nervous system controls detrusor muscle contraction with cholinergic (muscarinic) signals and the bladder neck consists of circular smooth muscle with α-adrenergic innervation, OAB may be managed by anticholinergic therapy (antimuscarinic) and adrenergic medications. Anticholinergics increase urethral pressure, and β3-adrenergic agonists relax the bladder wall, increasing bladder capacity. These medications must be monitored closely for adverse side effects. When these therapies are not successful, neuromodulation therapy is considered, including intradetrusor injection of onabotulinumtoxinA (Botox) (inhibits release of acetylcholine), peripheral tibial nerve stimulation, and sacral neuromodulation. Low bladder wall compliance (loss of elasticity) may be managed by antimuscarinic drugs, intradetrusor onabotulinumtoxin A injections, and intermittent catheterization.27 OAB syndrome should be discussed during health assessments; however, many individuals are reluctant to discuss OAB syndrome with their health care provider. Untreated OAB is an economic burden, impairs health and quality of life, and causes symptoms such as skin breakdown because of leakage, sleep disturbance, fall-related injuries, depression, prolonged hospital stays, and admission to long-term care facilities.
UAB syndrome is a voiding dysfunction characterized by the International Continence Society Working Group as bladder contraction of reduced strength and/or duration, resulting in prolonged bladder emptying or a failure to achieve complete bladder emptying, or both, within a normal time span. Symptoms include a slow urinary stream, hesitancy, and straining to void, with or without a feeling of incomplete bladder emptying and dribbling.28 Disruption of bladder innervation can occur with spinal cord injury, stroke, multiple sclerosis, Parkinson disease, and diabetic neuropathy. Aging can be a contributing factor. The symptoms may be indistinguishable from symptoms of LUT obstruction, including weak stream, intermittency, hesitancy, and straining to void. In some cases, UAB and OAB may occur together with detrusor overactivity during storage but poor detrusor contraction in the voiding phase. Urodynamic studies are required for evaluation. Treatment depends on the cause of the disorder and may include sacral neuromodulation, drugs that increase bladder contractility, and/or drugs that induce urethral relaxation.29
Evaluation and Treatment
Diagnosis of LUT obstructions requires a detailed history; physical examination, including neurologic and pelvic examinations; urinalysis; and determining if pathologic causes of urgency and frequency, such as prostatic enlargement, pelvic organ prolapse, urethral strictures, and neurologic disorders or systemic disease, are present. Diaries and questionnaires are helpful to determine the pattern and severity of incontinence. However, no symptom or cluster of symptoms has been identified that accurately differentiates the various causes of these disorders. For example, symptoms such as urgency, urge incontinence, frequent urination, and nocturia may develop because of overactive bladder or either increased or decreased bladder outlet resistance. Reduced resistance is associated with the symptom of stress incontinence (incontinence with coughing or sneezing), and symptoms of increased resistance are similar to bladder outlet obstruction, including poor force of urinary stream, hesitancy, and feelings of incomplete bladder emptying. Various urodynamic tests (Box 38.1) assist with the evaluation of how efficient the bladder, sphincters, and urethra are in storing and releasing urine. An evaluation of renal function, including functional imaging studies and measurement of serum creatinine (SCr) level, is completed particularly when the obstruction is severe and associated with elevated residual urine or UTI.
Neurogenic Bladder
Neurogenic bladder is a general term for bladder dysfunction caused by neurologic disorders (Table 38.3).30 The types of dysfunctions are related to the sites in the nervous system that control sensory and motor bladder function (Fig. 38.2). Lesions in the upper motor neurons of the brain and spinal cord result in detrusor hyperreflexia (overactive bladder) and bladder dyssynergia (loss of coordinated neuromuscular contraction). Lesions in the sacral area of the spinal cord or peripheral nerves result in underactive, hypotonic, or atonic (flaccid) bladder function, often with loss of bladder sensation. (See Chapter 15 for upper and lower motor neuron function.)
Table 38.3

An illustration shows the sites of neurologic injury associated with neurogenic bladder. The illustration shows and labels the following structures, from the top to the bottom: the detrusor motor area, lesions above C 2, the pontine micturition center, lesions above S 1 and below C 2, lesions below S 1, and bladder. Reflex urinary incontinence in the detrusor motor area is detrusor hyperreflexia. The diseases (upper motor neuron lesion) include stroke, traumatic brain injury, multiple sclerosis, hydrocephalus, cerebral palsy, Alzheimer disease, and brain tumors. Reflex urinary incontinence in the pontine micturition center is detrusor hyperreflexia with vesicosphincter dyssynergia. The diseases (upper motor neuron lesions) include spinal cord injury (C 2 to T 12), multiple sclerosis, transverse myelitis, Guillain-Barre syndrome, and disk problems. Reflex urinary incontinence in the bladder is detrusor areflexia, with or without urethral sphincter incompetence. The diseases (lower motor neuron lesions) include myelodysplasia, peripheral polyneuropathies, multiple sclerosis, tabes dorsalis, spinal injury (T 12 to S), cauda equina syndrome, and herpes simplex or zoster.
Neurologic disorders that develop above the pontine micturition center (located near the posterior pons) result in detrusor (bladder muscle) hyperreflexia (overactivity), also known as an uninhibited or reflex bladder or neurogenic overactive bladder. This is an upper motor neuron disorder in which the bladder empties automatically (without voluntary control) when it becomes full and the urethral sphincter functions normally. Because the pontine micturition center remains intact, there is coordination between detrusor muscle contraction and relaxation of the urethral sphincter. Stroke, traumatic brain injury, dementia, and brain tumors are examples of disorders that result in detrusor hyperreflexia. Symptoms include urine leakage and incontinence.
Neurologic lesions that occur below the pontine micturition center but above the sacral micturition center (between C2 and S1) are also upper motor neuron lesions and result in detrusor sphincter dyssynergia (detrusor hyperreflexia with vesicosphincter dyssynergia) (loss of coordinated function between the bladder and sphincter). There is loss of pontine coordination of detrusor muscle contraction and external sphincter relaxation, so both the bladder and the sphincter are contracting at the same time (dyssynergia), causing a functional obstruction of the bladder outlet. Spinal cord injury, multiple sclerosis, Guillain-Barré syndrome, and vertebral disk problems are causes of this disorder. There is diminished bladder relaxation during storage with small urine volumes and high intravesicular (inside the bladder) pressures. The result is an OAB with symptoms of frequency, urgency, urge incontinence, and increased risk for UTI. Diagnosis includes a medical history, physical examination, urinalysis, and urodynamic testing. Detrusor sphincter dyssynergia may be managed by intermittent catheterization in combination with higher dose antimuscarinic drugs to prevent overactive detrusor contractions and associated dyssynergia, while ensuring regular, complete bladder evacuation by catheterization. Transurethral botulinum toxin A injection has shown temporary efficacy in reducing bladder outlet obstruction. Transurethral sphincterotomy can be beneficial.31
Neurologic lesions involving the sacral micturition center (below S1, also termed cauda equina syndrome) or peripheral nerve lesions result in detrusor areflexia (acontractile detrusor, atonic bladder, or UAB), a lower motor neuron disorder. The atonic bladder causes retention of urine and distention with stress and overflow incontinence. There is prolonged urination time with or without a sensation of incomplete bladder emptying, usually with hesitancy, reduced sensation on filling, and a slow stream. If the sensory innervation of the bladder is intact, the full bladder will be sensed but the detrusor may not contract. Myelodysplasia, multiple sclerosis, tabes dorsalis (deterioration of the posterior columns of the spinal cord associated with untreated syphilis), spinal cord injury, and peripheral polyneuropathies (i.e., diabetic neuropathy) are associated with this disorder.
Diagnosis includes disease history, clinical examination, urinalysis, and urodynamic studies (see Box 38.1). Bethanechol chloride (Urecholine) is a cholinergic agent (muscarinic agonist) that stimulates the bladder to empty and can be helpful in some cases. Intermittent catheterization or indwelling catheters are commonly required.
Tumors
Kidney (Renal) Tumors
Kidney (Renal) tumors were estimated at 76,080 (4%) of new cancer cases and 13,780 deaths for 2021.32 Renal cell carcinoma (RCC) (also known as renal cell adenocarcinoma) usually occurs in men (about three times more often than in women) between 50 and 60 years of age. Risk factors include cigarette smoking, obesity, and uncontrolled hypertension. With surgical resection, 5-year survival is about 93% for stage I (encapsulated) cancer.
Pathophysiology
There are several different types of RCCs. They are classified according to subtypes and extent of metastasis. Clear cell RCC is the most common renal neoplasm (80% of all renal neoplasms) and represents about 2% of cancer deaths.32,33 It occurs primarily in the proximal tubule of the renal cortex. Other types include papillary (small fingerlike growths) and chromophobe RCC (larger cells), and both occur in the distal tubules of the kidneys.34 Confinement within the renal capsule, together with treatment, is associated with a better survival rate. The tumors usually occur unilaterally (Fig. 38.3). Renal transitional cell carcinoma (RTCC) is rare and primarily arises in the renal parenchyma and renal pelvis near the ureteral orifice. Renal adenomas (benign tumors) are uncommon but are increasing in number. The tumors are encapsulated and are usually located near the cortex of the kidney. Some tumors are unclassified because they have multiple cell types. Because the tumors can become malignant, they are usually surgically removed.

Renal cell carcinomas usually are spheroidal masses composed of yellow tissue mottled with hemorrhage, necrosis, and fibrosis. (From Damjanov I, Linder J, eds. Anderson’s pathology, 10th edition. St. Louis, MA: Mosby; 1996.)
Clinical Manifestations
The classic clinical manifestations of renal tumors are hematuria, dull and aching flank pain, palpable flank mass, and weight loss, but all these symptoms occur in fewer than 10% of cases. Further, they represent an advanced stage of disease, whereas earlier stages are often silent (painless hematuria). About 25% to 30% of individuals with RCC present with metastasis.35 The most common sites of distant metastasis are the lung, lymph nodes, liver, bone, thyroid gland, and central nervous system.
Evaluation and Treatment
Diagnosis is based on the clinical symptoms and imaging procedures. The tumor, node, metastasis (TNM) classification is used to stage RCC. Treatment for localized disease is surgical removal of the affected kidney (radical nephrectomy) or partial nephrectomy for smaller tumors, with combined use of chemotherapeutic agents. Radiofrequency ablation also may be used for early-stage tumors when surgery is not an option. Metastatic disease is treated with immunotherapy and targeted molecular therapies.36 Survival is related to tumor grade, tumor cell type, and extent of metastasis.
Bladder Tumors
Bladder tumors represent about 4.5% of all malignant tumors with 64,280 new cases each year and 12,260 deaths.32 The development of bladder cancer is most common in men older than 60 years. Risk factors include smoking, exposure to occupational chemicals, heavy consumption of phenacetin, uroepithelial schistosomiasis infection, or a genetic predisposition. Transitional cell (urothelial) carcinoma is the most common bladder malignancy, and tumors are usually superficial. More advanced tumors are muscle invasive. Less common forms are squamous cell and adenocarcinoma (cells that produce mucus).
Pathophysiology
Tumors present as flat or papillary and progress from in situ to invasive into the muscle and bladder wall (Fig. 38.4). Metastasis is usually to lymph nodes, liver, bones, or lungs. The TNM classification is used for staging bladder carcinoma. Secondary bladder cancer develops by invasion of cancer from bordering organs, such as cervical carcinoma in women or prostatic carcinoma in men.

(A) Papillary transitional cell carcinoma arising in the dome of the bladder as a cauliflower-like lesion (black arrow); location and frequency of bladder tumor types noted. (B) Bladder cancer with morphologic patterns of most common tumors. (A, From Stevens A, Lowe J, Scott I, eds. Core pathology, 3rd edition. London: Mosby; 2009; 2009. B, Adapted from Kissane JM, ed. Anderson’s pathology, 9th edition. St. Louis: Mosby; 1990.)
Top panel, A. A closeup of a cross-section of the dome of the bladder. The locations of tumors are as follow: 10 percent in the dome; 70 percent in the posterior and lateral wall; and 20 percent in trigone and bladder neck. Tumor types are 80 percent papillary and 3 percent carcinoma in situ. Bottom panel, B. The illustration on the top-left shows a flat tumor on the surface. The illustration on the top-right shows a flat invasive tumor, penetrating the layers. The illustration on the bottom-left shows a papilloma on the surface. The illustration on the bottom-right shows a papillary and invasive tumor, penetrating the layers.
Clinical Manifestations
Gross painless hematuria is the archetypal clinical manifestation of bladder cancer. Episodes of hematuria tend to recur, and they are often accompanied by bothersome LUT symptoms including daytime voiding frequency, nocturia, urgency, and urge urinary incontinence, particularly for carcinoma in situ. Flank pain may occur if tumor growth obstructs one or both ureterovesical junctions.
Evaluation and Treatment
Urine cytologic study (pathologic analysis of sloughed cells within the urine) is used for screening. Cystoscopy with tissue resection and biopsy is the first stage of treatment and confirms the diagnosis of bladder cancer. Use of biologic markers for bladder cancer diagnosis and treatment prognosis are available.37 Transurethral resection or laser ablation, combined with intravesical chemotherapy or immunotherapy, is effective for superficial tumors. Radical cystectomy (removal of the prostate and seminal vesicles in men and removal of the uterus, ovaries, and part of the vagina in women) with urinary diversion and adjuvant chemotherapy is required for locally invasive tumors.38
Urinary Tract Infection
Causes of Urinary Tract Infection
A UTI is an inflammation of the urinary epithelium (mucosa) usually caused by bacteria from gut flora. UTI can occur anywhere along the urinary tract, including the urethra, prostate, bladder, ureter, or kidney. At risk are premature newborns; prepubertal children; sexually active and pregnant women; women treated with antibiotics that disrupt vaginal flora; spermicide users; estrogen-deficient postmenopausal women; individuals with indwelling catheters; and individuals with diabetes mellitus, neurogenic bladder, or urinary tract obstruction. Cystitis is more common in women because of the shorter urethra and the closeness of the urethra to the anus (increasing the possibility of bacterial contamination). Adult women have a 50% to 60% lifetime incidence of UTI.39
Several factors normally combine to protect against UTIs. Most bacteria are washed out of the urethra during micturition. The low pH and high osmolality of urea, the presence of Tamm-Horsfall protein or uromodulin (secreted by renal tubular cells in the distal loop of Henle), and secretions from the uroepithelium provide a bactericidal effect. The ureterovesical junction closes during bladder contraction, preventing reflux of urine to the ureters and kidneys. Both the longer urethra and the presence of prostatic secretions decrease the risk of infection in men. UTI occurs when a pathogen circumvents or overwhelms the body’s natural defense mechanisms and rapidly reproduces. Uncomplicated UTIs are mild with a self-limiting course and occur in individuals without any functional or anatomical anomalies in the urinary tract. A complicated UTI develops when there is an abnormality in the urinary system or a secondary disease, syndrome, or illness that compromises an individual’s defenses, such as diabetes mellitus, neurogenic bladder, urinary tract obstruction, renal transplant, or spinal cord injury.40 UTI may occur alone or in association with pyelonephritis, prostatitis, or nephrolithiasis. Up to 30% of cases of septic shock are caused by urosepsis (a systemic response to an infection in the urogenital tract that can include symptoms of shock).41 The mechanisms associated with UTI including bacterial and human factors are summarized in Fig. 38.5. Recurrent UTI is commonly defined as three or more UTIs within 12 months or two or more occurrences within 6 months. Recurrence is more common in women as compared to men. UTI may occur as a relapse when there is a second infection within the urinary tract caused by the same pathogen within 2 weeks of the original treatment or a reinfection that occurs more than 2 weeks after completion of treatment for the same or different pathogen.40 Guidelines are available for clinical management.42
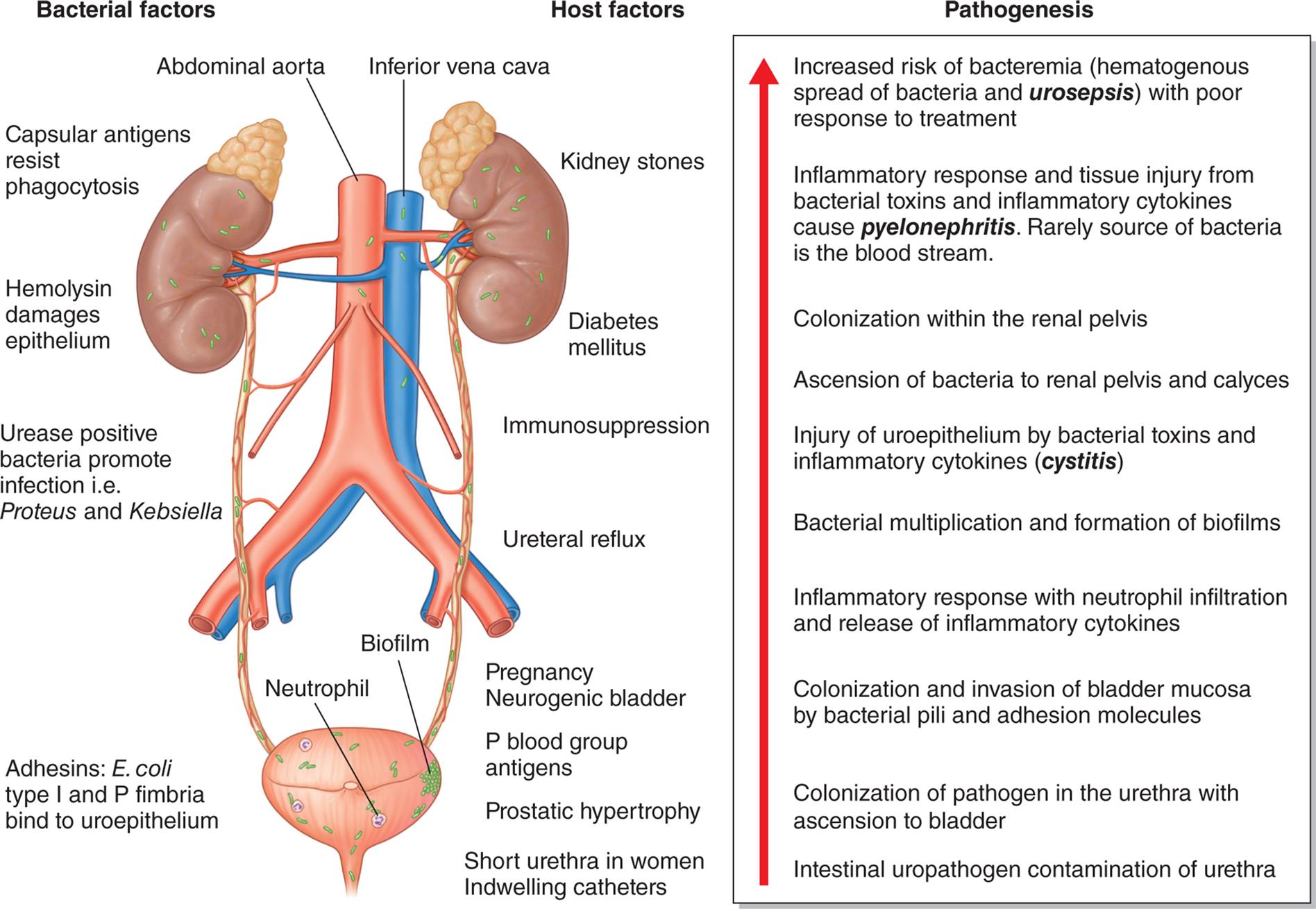
An illustration shows the mechanisms of urinary tract infection. The illustration shows and labels the following structures on the urinary system: abdominal aorta, inferior vena cava, biofilm, and neutrophil. The bacterial factors include capsular antigens resist phagocytosis; hemolysin damages epithelium; urease positive bacteria promote infection, that is proteus and kebsiella; and adhesins: E coli type 1 and P fimbria bind to uroepithelium. The host factors include kidney stones; diabetes mellitus; immunosuppression; ureteral reflux; pregnancy neurogenic bladder; P blood group antigens; prostatic hypertrophy; and short urethra in women indwelling catheters. The pathogenesis, from the bottom to the top, is as follows. • Intestinal uropathogen contamination of urethra. • Colonization of urethra with ascension to bladder. • Colonization and invasion of bladder mucosa by bacterial pili and adhesion molecules. • Inflammatory response with neutrophil infiltration and release of inflammatory cytokines. • Bacterial multiplication and formation of biofilms. • Injury of uroepithelium by bacterial toxins and inflammatory cytokines (cystitis). • Ascension of bacteria to renal pelvis and calyces. • Colonization of renal pelvis. • Inflammatory response and tissue injury from bacterial toxins and inflammatory cytokines cause pyelonephritis. Rarely source of bacteria is the blood stream. • Increased risk of bacteremia (hematogenous spread of bacteria and urosepsis) with poor response to treatment.
Types of Urinary Tract Infection
Cystitis
Cystitis is an inflammation of the bladder, which is the most common site of UTI. The appearance of the bladder through a cystoscope describes the different types of cystitis:
- 1. Mild cystitis shows a hyperemic (red) mucosa.
- 2. Hemorrhagic cystitis shows diffuse mucosal hemorrhages and occurs with more advanced inflammation.
- 3. Suppurative cystitis shows mucosal pus formation or suppurative exudates.
- 4. Ulcerative cystitis results from prolonged infection with ulcers that may lead to sloughing of the mucosa.
- 5. Gangrenous cystitis is necrosis of the bladder wall and occurs with the most severe infections.
Pathophysiology
Two factors account for the development of UTI: the virulence of the pathogen and the efficiency of host defense mechanisms. The most common infecting microorganisms are uropathic strains of Escherichia coli, and the second most common is Staphylococcus saprophyticus. Less common microorganisms include Klebsiella, Proteus, Pseudomonas, fungi, viruses, parasites, or tubercular bacilli. Schistosomiasis is the most common parasitic invasion of the urinary tract on a global basis, particularly Africa and areas of the Middle East, and has a strong association with bladder cancer.43 Bacterial contamination of the normally sterile urine usually occurs by retrograde (backward) movement of gastrointestinal gram-negative bacilli into the urethra and bladder from the opening of the urethra. The microorganisms overcome normal defense mechanisms and can then move into the ureter and kidney. Uropathic strains of E. coli have type-1 fimbriae (also termed pili or fingerlike projections) that bind to receptors on the uroepithelium. Consequently, they resist flushing during normal micturition. They also can bind to latex catheters used for urinary drainage. Some women may be genetically susceptible to certain strains of E. coli attachment. In these cases, women have P blood group antigen (a glycolipid) that binds to P. fimbriae (pyelonephritis-associated fimbriae) of E. coli on the uroepithelium, allowing the pathogen to ascend the urinary tract. Infection initiates an inflammatory response and the symptoms of cystitis. The inflammatory edema in the bladder wall stimulates activation of stretch receptors. The activated stretch receptors initiate symptoms of bladder fullness with small volumes of urine, producing the urgency and frequency of urination associated with cystitis.
Clinical Manifestations
Clinical manifestations of cystitis are related to the inflammatory response and usually include polyuria, urinary frequency, urgency, dysuria (painful urination), and suprapubic and low back pain. Hematuria, cloudy or malodorous urine, flank pain, and mental status changes are more serious symptoms. Many individuals with bacteriuria are asymptomatic. Individuals with a complicated UTI may present with systemic symptoms such as fever, chills, mental status changes, tachycardia, hypotension, nausea, vomiting, pain, and incontinence. The elderly have the highest risk and may present with only confusion or vague abdominal discomfort.
Evaluation and Treatment
Cystitis in symptomatic individuals is diagnosed by urine culture of specific microorganisms with counts of 100,000/mL or more from freshly voided urine.44 The standard diagnostic test for UTI is urinalysis, which is both cost-effective and noninvasive. The most accurate way to obtain a urine specimen is a midstream clean catch. Positive nitrates and leukocyte esterase on the dipstick analysis are accurate indicators of UTI. Urinalysis screening of asymptomatic bacteriuria is only recommended in women during pregnancy and for individuals prior to undergoing invasive urologic procedures.45 Women reporting typical symptoms of uncomplicated lower UTI do not require any laboratory or diagnostic testing.46 Risk factors, such as urinary tract obstruction, should be identified and treated.
Evidence of bacteria from urine culture and antibiotic sensitivity warrants treatment with a microorganism-specific antibiotic. Optimal therapy depends on the severity and local bacterial resistance patterns. Acute uncomplicated cystitis in nonpregnant women can be diagnosed without an office visit or urine culture. If urine culture and sensitivity are ordered, the urine specimen must be obtained before the initiation of any antibiotic therapy; 3 to 7 days of treatment is most common.42
Complicated UTI requires 7 to 14 days of treatment. Relapsing infection within 7 to 10 days requires prolonged antibiotic treatment. Clinical symptoms are frequently relieved, but bacteriuria may still be present. Repeat cultures are not necessary as a test for cure post-treatment. For chronic infection with a continuation of symptoms, cultures should be obtained every 3 to 4 months until 1 year after treatment for evaluation and treatment of recurrent infection. Guidelines are available for the treatment of community-acquired UTIs, uncomplicated cystitis and pyelonephritis in women, complicated cystitis, and for the prevention of catheter-associated cystitis.47
Painful Bladder Syndrome/Interstitial Cystitis
Interstitial cystitis/bladder pain syndrome (IC/BPS) is defined as an unpleasant sensation (pain, pressure, discomfort) perceived to be related to the urinary bladder associated with LUT symptoms of more than 6 weeks’ duration in the absence of infection or other identifiable causes. It is most commonly diagnosed in women and in the fourth decade of life or after.
Pathophysiology. The cause of IC/BPS is unknown. IC/BPS can be conceptualized as a bladder pain disorder that is often associated with voiding and other systemic chronic disorders such as fibromyalgia, irritable bowel disease, chronic fatigue syndrome, Sjogren’s syndrome, chronic headaches, and vulvodynia.48 An autoimmune reaction may be responsible for the inflammatory response, which includes mast cell activation, altered uroepithelial permeability, and increased sensory nerve sensitivity. The inflammation is associated with a derangement of the glycosaminoglycan layer of the bladder mucosa that makes it more susceptible to penetration by bacteria and noxious urinary solutes.Clinical Manifestations. Inflammation and fibrosis of the bladder wall (uroepithelium) are accompanied by pain. Bladder volume may decrease as a result of fibrosis. IC/BPS is also categorized by the presence or absence of hemorrhagic ulcers (Hunner ulcers or lesions). Absence of Hunner ulcers is a non-inflammatory phenotype with little evidence of bladder etiology and presents with somatic and/or psychological symptoms that commonly result in central nervous sensitization.49
Evaluation and Treatment. Diagnosis of IC/PBS requires a thorough history, physical examination and urinalysis, analysis of cystoscopy findings, the presence or absence of Hunner ulcers, and the exclusion of other diagnoses. The hallmark symptom of IC/PBS is pain, including sensations of pressure and discomfort located in the suprapubic area, urethra, vulva, vagina, rectum, lower abdomen, or back lasting longer than 6 weeks. Other characteristic symptoms of IC/PBS include bladder fullness, urinary urgency, frequency (including nocturia) with small urine volume, and chronic pelvic pain. No single treatment is effective. Treatment should focus on improving quality of life through self-care practices and behavioral modifications. Oral and intravesical therapies, sacral nerve stimulation, and intradetrusor botulinum toxin A are used for symptom relief. Fulguration with laser or electrocautery and/or injection with triamcinolone should be performed if Hunner ulcers are present. Surgery is used in refractory cases.50 Guidelines are available for the treatment of IC/BPS.51
Acute Pyelonephritis
Pyelonephritis is an infection of one or both upper urinary tracts (ureter, renal pelvis, and kidney interstitium). Common causes are summarized in Table 38.4. Urinary obstruction and reflux of urine from the bladder (vesicoureteral reflux) are the most common underlying risk factors, with most cases occurring in women. One or both kidneys may be involved.
Table 38.4
Pathophysiology
Microorganisms usually associated with acute pyelonephritis include E. coli (most common), Proteus, or Pseudomonas. The latter two microorganisms are more commonly associated with infections after urethral instrumentation or urinary tract surgery. These microorganisms also split urea into ammonia, making an alkaline urine that increases the risk of stone formation. The infection is likely spread by ascending uropathic microorganisms along the ureters. Dissemination also may occur by way of the bloodstream. The inflammatory process primarily affects the pelvis, calyces, and medulla of the kidney. The infection causes infiltration of leukocytes with renal inflammation, renal edema, and purulent urine. In severe infections, localized abscesses may form in the medulla and extend to the cortex. The tubules are primarily affected, while the glomeruli are usually spared. Necrosis of renal papillae can develop. After the acute phase, healing occurs with fibrosis and atrophy of affected tubules. The number of bacteria decreases until the urine again becomes sterile. Acute pyelonephritis rarely causes renal failure.
Clinical Manifestations
The onset of symptoms is usually acute, with fever, chills, tachycardia, nausea, vomiting, and flank or groin pain. Symptoms characteristic of UTI, including frequency, dysuria, incontinence, and costovertebral tenderness, may precede systemic signs and symptoms.46 Older adults may have early nonspecific symptoms, such as low-grade fever, confusion, and malaise.
Evaluation and Treatment
Differentiating symptoms of cystitis from those of pyelonephritis by clinical assessment alone is difficult. The specific diagnosis is established by urine culture, urinalysis, and clinical manifestations. White blood cell casts formed in the renal tubules and flushed into the urine indicate pyelonephritis. However casts are not always present in the urine. A urine culture assay establishes a definitive diagnosis through identification of the uropathogen. A positive culture is characterized by bacteriuria of at least 105 CFU/mL.44 Individuals with complicated pyelonephritis may require blood cultures and diagnostic imaging if there is no response to antibiotic treatment or if there is a suspected obstruction. Optimal therapy for acute uncomplicated pyelonephritis depends on severity and local resistance patterns.52 Current guidelines recommend empiric therapy with a broad-spectrum antibiotic (e.g., fluoroquinolone) for patients with pyelonephritis, not requiring hospitalization. A urine specimen for culture and sensitivity must be collected prior to initiation of antibiotics.46 A post-treatment test of cure urinalysis or urine culture in asymptomatic individuals is not performed.42 However, follow-up urine cultures are obtained at 1 and 4 weeks after treatment if symptoms recur. Antibiotic-resistant microorganisms or reinfection may occur in cases of urinary tract obstruction or reflux. Intravenous pyelography and voiding cystourethrography are used to identify surgically correctable lesions.
Chronic Pyelonephritis
Chronic pyelonephritis is a persistent or recurrent infection of one or both of the kidneys leading to scarring. The specific cause of chronic pyelonephritis may be unknown (idiopathic) or associated with chronic UTIs, vesicoureteral reflux, or kidney stone obstructive uropathy. Other causes include drug toxicity from analgesics, such as nonsteroidal anti-inflammatory drugs (NSAIDs), ischemia, irradiation, and immune-complex diseases.
Pathophysiology
Chronic urinary tract obstruction prevents elimination of bacteria and starts a process of progressive inflammation. Alterations occur within the renal pelvis and calyces from the obstruction and inflammation (Fig. 38.6). There is destruction of the tubules and diffuse scarring, which impairs urine-concentrating ability. The lesions of chronic pyelonephritis are sometimes termed chronic interstitial nephritis because the inflammation and fibrosis are located in the interstitial spaces between the tubules.

(Right) Small, shrunken, irregularly scarred kidney of an individual with chronic pyelonephritis. (Left) Kidney is of normal size but also shows scarring on the upper pole. From Damjanov I. Pathology for the health professions, 4th edition. Philadelphia: Saunders; 2012.
Clinical Manifestations
The early symptoms of chronic pyelonephritis are often minimal and may include urinary frequency, dysuria, flank pain, and hypertension. Progression can lead to kidney failure, particularly in the presence of other risk factors (e.g., obstructive uropathy or diabetes mellitus). There is an inability to conserve sodium with loss of tubular function and development of hyperkalemia and metabolic acidosis. Risk for dehydration must be considered if there is loss of the ability to concentrate the urine.
Evaluation and Treatment
Urinalysis, intravenous pyelography, and ultrasound are used as diagnostic tests to evaluate chronic pyelonephritis. Treatment is related to the underlying cause. Obstruction must be relieved. Antibiotics may be given, with prolonged antibiotic therapy for recurrent infection.
Glomerular Disorders
Glomerulonephritis
Acute glomerulonephritis is an inflammation isolated to the kidney glomerulus caused by primary glomerular injury, including infection, immunologic responses, ischemia, free radicals, drugs, toxins, and vascular disorders. Secondary glomerular injury is a glomerular injury that occurs as a consequence of systemic diseases, including diabetes mellitus, hypertension, bacterial toxins, systemic lupus erythematosus, congestive heart failure, and HIV-related kidney disease.
Pathophysiology
Immune mechanisms are a major component of both primary and secondary glomerular injury. (Fig. 38.7). The injury damages the glomerular capillary filtration membrane. The most common type of immune injury is related to the presence of antigen-antibody complexes within the glomerulus (Table 38.5). Nonimmune glomerular injury is related to injury or ischemia from metabolic disorders, toxin exposure, drugs, vascular disorders, and infection. Different causes of injury may result in more than one type of glomerular lesion; thus, lesions are not necessarily disease specific (Table 38.6).
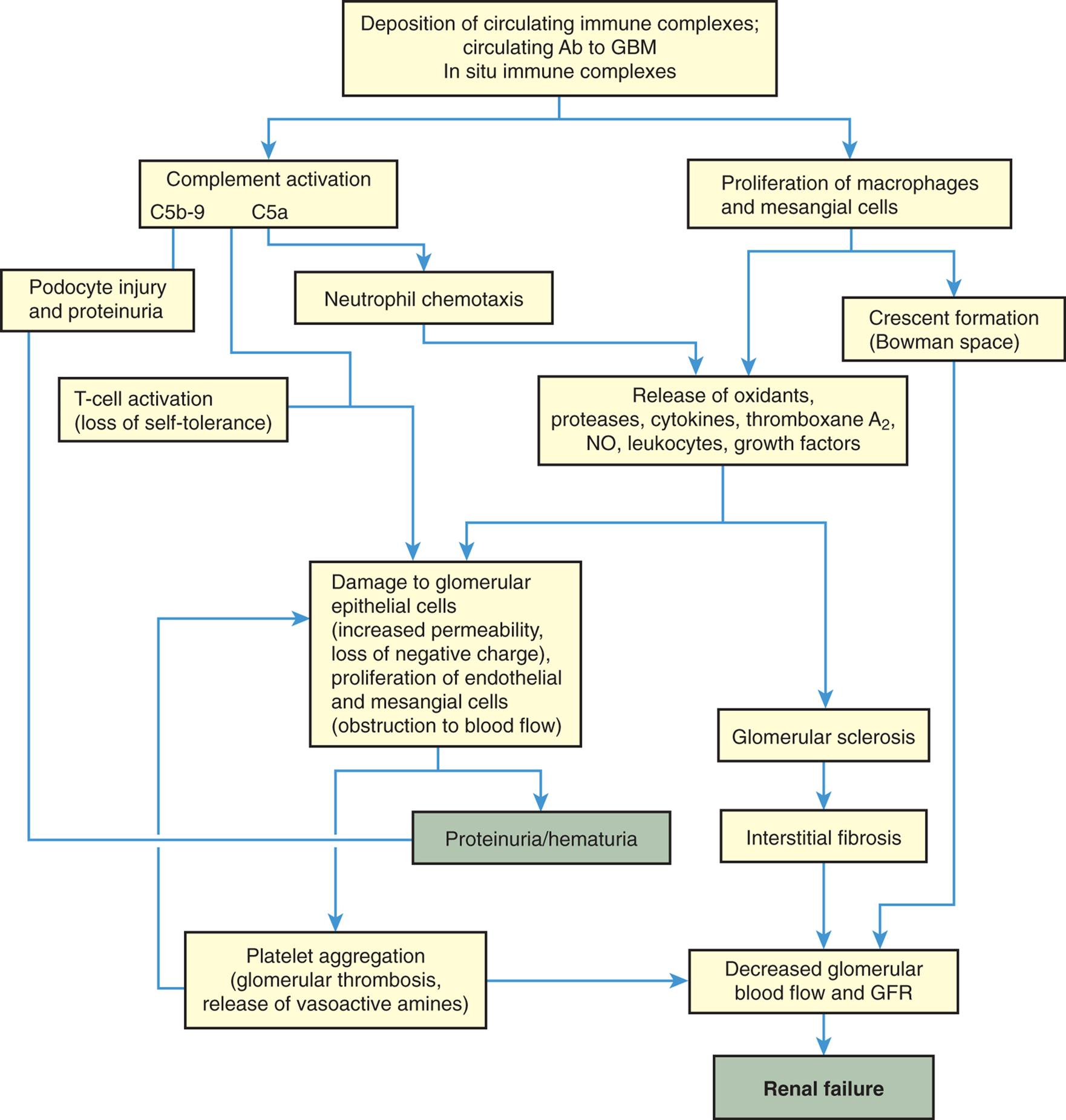
Ab, Antibody; GBM, glomerular basement membrane; GFR, glomerular filtration rate; NO, nitric oxide.
A flowchart shows the mechanisms of glomerular injury. 1. Deposition of circulating immune complexes; circulating Ab to G B M; and In situ immune complexes. Leads to 2 and 3. 2. Complement activation (C 5 b-9 and C 5 a). Leads to 4, 5, 7, and 9. 3. Proliferation of macrophages and mesangial cells. Leads to 6 and 8. 4. Podocyte injury and proteinuria. Leads to 11. 5. Neutrophil chemotaxis. Leads to 8. 6. Crescent formation (Bowman space). Leads to 14. 7. T-cell activation (loss of self-tolerance). Leads to 9. 8. Release of oxidants, proteases, cytokines, thromboxane A sub 2, N O, leukocytes, growth factors. Leads to 9 and 10. 9. Damage to glomerular epithelial cells (increased permeability, loss of negative charge), proliferation of endothelial and mesangial cells (obstruction to blood flow). Leads to 11 and 13. 10. Glomerular sclerosis. Leads to 12. 11. Proteinuria/hematuria. 12. Interstitial fibrosis. Leads to 14. 13. Platelet aggregation (glomerular thrombosis, release of vasoactive amines). Leads to 9 and 14. 14. Decreased glomerular blood flow and G F R. Leads to 15. 15. Renal failure.
Table 38.5
Data from Foster MH. Basement membranes and autoimmune diseases. Matrix Biology, 2017;57–58:149–168; Nester CM, Smith RJ. Complement inhibition in C3 glomerulopathy. Seminars in Immunology, 2016;28(3):241–249; Rodríguez-Iturbe B, Batsford S. Pathogenesis of poststreptococcal glomerulonephritis a century after Clemens von Pirquet. Kidney International, 2007;71(11):1094–1104.
Table 38.6
| Lesion | Distribution When Single Glomeruli Considered |
|---|---|
| Global | A lesion involving the entire glomerulus |
| Segmental-local | Changes in one part of the glomerulus with other parts unaffected |
Immune injury is caused by activation of the inflammatory response (i.e., complement activation, leukocyte recruitment, and release of cytokines from leukocytes). Injury begins after the antigen-antibody complexes have deposited or formed in the glomerular capillary wall or mesangium. Complement is deposited with the antibodies and complement activation can cause cell injury or serve as a chemotactic stimulus for attraction of leukocytes (neutrophils, monocytes, and T lymphocytes). These phagocytes, along with activated platelets, further the inflammatory reaction by releasing mediators that injure the glomerular filtration membrane including epithelial cells, glomerular basement membrane, and endothelial cells (podocytes and filtration slits).53 The injury increases glomerular filtration membrane permeability and reduces glomerular membrane surface area.
There may be hypertrophy and proliferation of mesangial cells and expansion of the extracellular matrix in the Bowman space. The deposition of these substances and cell proliferation forms a crescent shape within the Bowman space that can be seen under a microscope and can assist with diagnosis if a biopsy is performed. The result of these processes is compression of glomerular capillaries, decreased glomerular blood flow, hypoxic injury, decreased driving glomerular hydrostatic pressure, alteration in the filtration membrane, and decreased GFR. Crescent formation is associated with rapidly progressive glomerulonephritis.54
Loss of the normal negative electrical charge across the glomerular filtration membrane and increase in filtration pore size enhance movement of proteins into the urine. Proteins are normally repelled because they also have a negative charge and thus are not filtered into the urine. Red blood cells also escape if pore size is large enough. Consequently, proteinuria and/or hematuria develop. The severity of glomerular damage and decline in glomerular function is related to the size, number, and location (i.e., focal [affecting some glomeruli] or diffuse [affecting glomeruli throughout the kidney]) of cells injured; duration of exposure; and type of antigen-antibody complexes formed.
Clinical Manifestations
The onset of glomerulonephritis may be sudden or insidious. A significant loss of nephron function can occur before symptoms develop. Acute glomerulonephritis may be silent, mild, moderate, or severe in symptom presentation. Severe or progressive glomerular disease causes oliguria (urine output of 30 mL/h or less), hypertension, and renal failure. Focal lesions tend to produce less severe clinical symptoms. Salt and water are reabsorbed, contributing to fluid volume expansion, edema, and hypertension.
Two distinct symptoms of more severe or rapidly progressive glomerulonephritis are (1) hematuria with red blood cell casts (the red blood cells accumulate in the kidney tubules and are washed into the urine in the form of a cast of the tubule) and (2) proteinuria exceeding 3 to 5 g/day with albumin (macroalbuminuria). Glomerular bleeding provides prolonged contact with the acidic urine and transforms hemoglobin to methemoglobin, which has a brownish color and no blood clots.
Evaluation and Treatment
The diagnosis of glomerular disease is confirmed by the progressive development of clinical manifestations and abnormal laboratory findings. Common urinalysis findings associated with glomerular disease include proteinuria, red blood cells, white blood cells, and casts. Reduced GFR during glomerulonephritis is evidenced by elevated plasma urea, cystatin C, and creatinine concentrations, or by reduced renal creatinine clearance (see Chapter 37). Microscopic evaluation from renal biopsy can provide a specific determination of renal injury and the type of pathologic lesion (i.e., the formation of glomerular crescents as previously described and location and character of glomerular lesions). Patterns of antigen-antibody complex deposition within the glomerular capillary filtration membrane have been established using light, electron, and immunofluorescent microscopy. Electron microscopy differentiates morphologic changes within the glomerular capillary wall (e.g., subendothelial and mesangial electron-dense deposits, increased mesangial matrix, mesangialization of capillary loops, and foot process fusion). Staining with fluorescein identifies complement and different antibodies (i.e., immunoglobulin G [IgG] or immunoglobulin A [IgA]) and associated configurations when viewed under ultraviolet light with light microscopy. Findings with microscopy provide information about the distribution and lesions of immune response injury and guide therapy.55
Reduced GFR during glomerulonephritis is evidenced by elevated plasma urea, cystatin C, and creatinine concentrations, or by reduced creatinine clearance (see Tests of Renal Function in Chapter 37). Edema, caused by excessive sodium and water retention and or loss of plasma proteins (see Chapter 3 for the pathophysiology of edema), may require the use of diuretics or dialysis.
Management principles for treating glomerulonephritis are related to treating the primary cause, preventing or minimizing immune responses, and correcting accompanying problems. Accompanying problems include edema, hypertension, hypoalbuminemia, and hyperlipidemia. Specific treatment regimens are necessary for particular types of glomerulonephritis. Antibiotic therapy is essential for the management of underlying infections that may be contributing to ongoing antigen-antibody responses. Corticosteroids decrease antibody synthesis and suppress inflammatory responses. Cytotoxic agents (e.g., cyclophosphamide) may be used to suppress the immune response in corticosteroid-resistant cases. Anticoagulants may be useful for controlling fibrin crescent formation in rapidly progressive glomerulonephritis.
Types of Glomerulonephritis
The types of glomerulonephritis can be described according to cause, pathologic lesions determined by biopsy (Table 38.7), disease progression (acute, rapidly progressive, chronic), or clinical presentation (nephrotic syndrome, nephritic syndrome, acute or chronic renal failure). In nearly all types of glomerulonephritis, the epithelial or podocyte layer of the glomerular capillary membrane is disturbed with loss of negative charges and changes in membrane permeability. Plasma proteins (albumin) and red blood cells can escape into the urine and can cause proteinuria and/or hematuria. The mesangial matrix may be expanded, or the basement membrane thickened decreasing blood flow through the glomerular capillaries and decreasing GFR. Many types of glomerular injury occur most often in children or young adults, including acute postinfectious glomerulonephritis and minimal change nephropathy (lipoid nephrosis). Details of these diseases are presented in Chapter 39.
Table 38.7
Complications of systemic diseases, such as diabetic nephropathy and systemic lupus erythematosus, can affect the entire nephron with significant glomerular injury. Different patterns of injury develop over the course of these diseases, and there is usually chronic progression. They are described in the next section.
Chronic Glomerulonephritis
Chronic glomerulonephritis encompasses several glomerular diseases with a progressive course leading to chronic kidney failure. There may be no history of kidney disease before the diagnosis. Hypercholesterolemia and proteinuria have been associated with progressive glomerular and tubular injury (Fig. 38.8). The proposed mechanism is related to those observed in glomerulosclerosis and interstitial injury, such as inflammatory processes and glomerular hyperfiltration. The primary cause may be difficult to establish because advanced pathologic changes may obscure specific disease characteristics. Diabetes nephropathy and lupus nephritis are examples of secondary causes of chronic glomerular injury. Renal insufficiency usually begins to develop after 10 to 20 years of disease, followed by nephrotic syndrome (see next section) and an accelerated progression to end-stage renal failure (ESRF). Symptom patterns vary depending on the underlying cause and the areas of the kidney that are damaged. The specific pathologic changes are identified by renal biopsy, which is best performed in the early stages of CKD to identify specific treatment options. Management of the underlying disease and use of steroids, immunosuppressive agents, dipeptidyl peptidase-4 (DPP-4) inhibitors, glucagon-like peptide-1 (GLP-1) receptor agonists, sodium-glucose co-transporter-2 (SGLT2) inhibitors, renin-angiotensin-aldosterone (RAAS) inhibitors, and angiotensin-receptor blockers (ARBs) can prolong remissions and preserve renal function (see Emerging Science Box: Renin–Angiotensin–Aldosterone System Inhibitors in Patients With COVID-19). Dialysis or kidney transplantation ultimately may be needed.
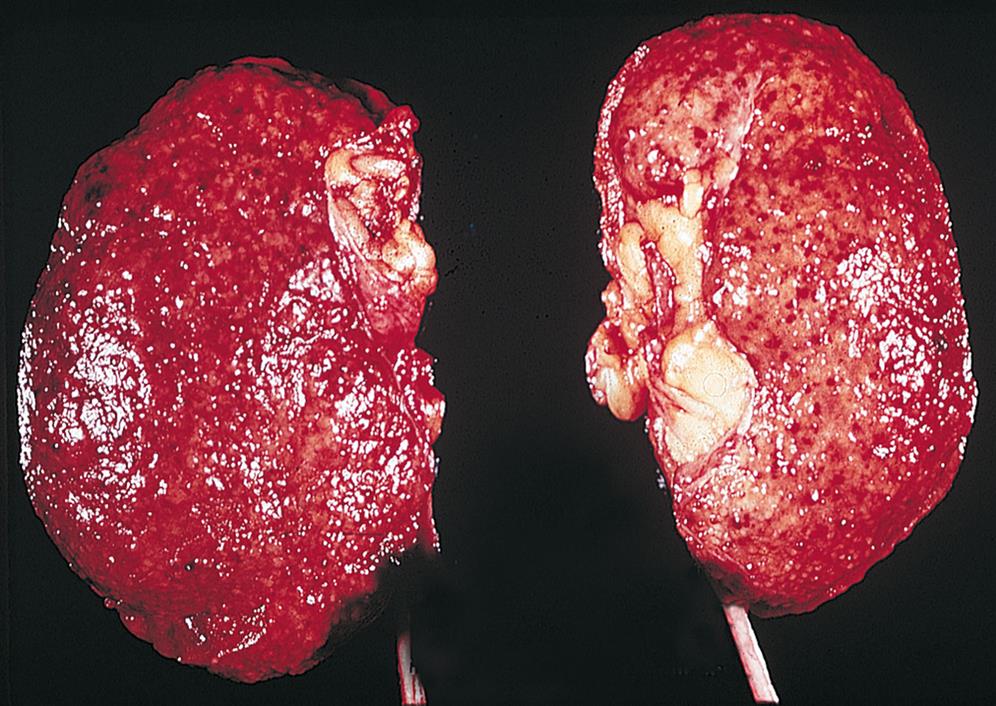
The kidneys appear small, are uniformly shrunken, and have a finely granular external surface. (From Damjanov I. Pathology for the health professions, 4th edition. Philadelphia: Saunders, 2012.)
Diabetic nephropathy develops from metabolic (accumulation of advanced glycosylated end products), inflammatory (transforming growth factor-beta and protein kinase C), and macrovascular and microvascular complications related to chronic hyperglycemia (see Chapter 22). Changes in the glomerulus are characterized by podocyte injury, progressive thickening and fibrosis of the glomerular basement membrane, expansion of the mesangial matrix (diffuse diabetic glomerulosclerosis), and nodular glomerulosclerosis (Kimmelstiel-Wilson nodules; see Fig. 38.9) with albuminuria, loss of tubular cells, and progression to chronic kidney disease. Although albuminuria is the classic phenotype of progressive diabetic renal disease, two new phenotypes have emerged (i.e., “nonalbuminuric renal impairment” and “progressive renal decline”), suggesting there can be progressive failure of renal function (i.e., declining GFR) without albuminuria. These “new” phenotypes may be the consequence of improved treatment. Work is in progress to determine if diagnostic and treatment guidelines should be modified for the management of these different phenotypes.56 Diabetic nephropathy is the most common cause of CKD and end-stage renal disease (ESRD) for both type 1 and type 2 diabetes.57 ESRD requires treatment with dialysis or renal transplantation.58
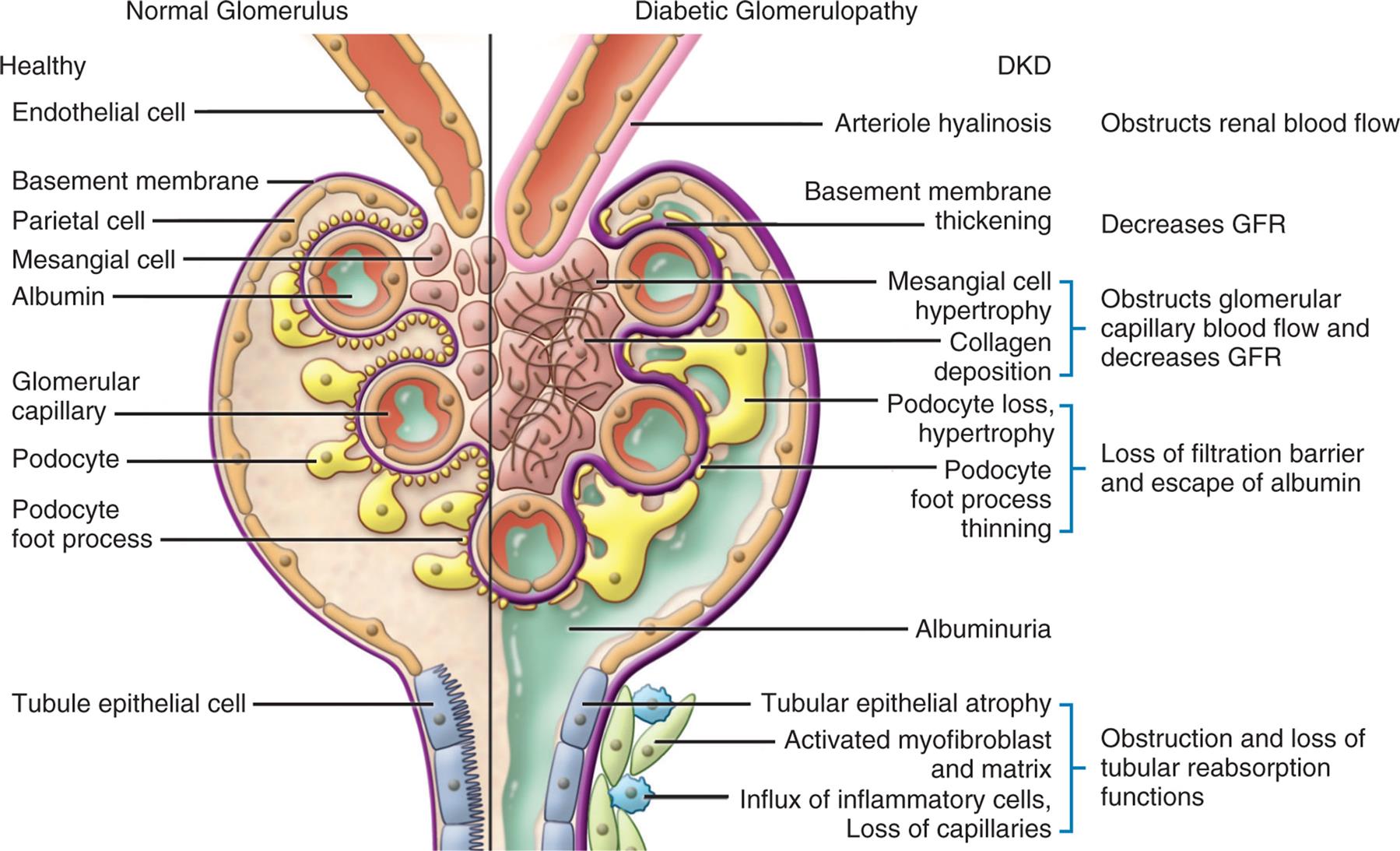
An illustration of the cross-section of the glomerulus compares the structures of normal glomerulus and diabetic glomerulopathy. Normal glomerulus: healthy endothelial cell, basement membrane, parietal cell, mesangial cell, albumin, glomerular capillary, podocyte foot process, and tubule epithelial cell. Diabetic glomerulopathy: D K D, obstructs renal blood flow (arteriole hyalinosis), decreases G F R (basement membrane thickening), obstructs glomerular capillary blood flow and decreases G F R (mesangial cell hypertrophy, collagen deposition), loss of filtration barrier and escape of albumin (podocyte loss, hypertrophy, podocyte foot process thinning), albuminuria, and obstruction and loss of tubular reabsorption functions (tubular epithelial atrophy, activated myofibroblast and matrix, and influx of inflammatory cells, loss of capillaries).
Lupus nephritis is an inflammatory complication of the chronic autoimmune syndrome systemic lupus erythematosus (see Chapter 9). The renal component of the disease may be caused by the formation of autoantibodies against double-stranded DNA and nucleosomes with glomerular deposition of the immune complexes. Immune complexes also may be formed in situ by binding to planted antigens of circulating autoantibodies. There is complement activation and a cascade of inflammatory events resulting in damage to the glomerular membrane with mesangial expansion (see Chapter 8).59 Various glomerular lesion patterns are identifiable on biopsy, including membranous, mesangial, membranoproliferative, and diffuse proliferative glomerulonephritis; tubular fibrosis can also be present (Table 38.8). Symptom presentation is variable depending on lesion involvement and can include proteinuria, microscopic hematuria, edema, and other signs of nephrotic syndrome. Disease progression may be silent or may progress to ESRD over a period of years. Treatment includes the use of immunosuppressive agents and efforts to protect the kidney from secondary nonimmune consequences of acute injury.60
Table 38.8
| Type and Cause | Histopathophysiology |
|---|---|
| Associated with Nephritic Syndrome | |
| Acute postinfectious glomerulonephritis (PIGN) (e.g., group A beta-hemolytic streptococci [more common in children]; staphylococcus [more common in older adults]) | Subepithelial deposits of IgG and complement complexes; infiltration of neutrophils and monocytes; proliferation of mesangial and epithelial cells with occlusion of glomerular capillary blood flow and decreased glomerular filtration; usually diffuse lesions |
| Rapidly progressive or crescentic glomerulonephritis (a clinical syndrome): Type I: Formation of IgG antibodies against pulmonary capillary and glomerular basement membrane (Goodpasture syndrome); activation of complement and neutrophils; more common in young men; causes pulmonary hemorrhage and renal failure Type II: Mesangial immune-complex deposition (PIGN, SLE, IgA nephropathy) Type III: Pauci-immune, lack of antiglomerular basement membrane antibodies antibodies or immune complexes; presence of serum antineutrophil cytoplasmic (ANC) antibodies associated with systemic vasculitides (usually idiopathic); nonspecific response to glomerular injury; can occur in any severe glomerular disease | Accumulation of fibrin, macrophages, and epithelial cell proliferation into the Bowman space forms crescents and occludes glomerular capillary blood flow, decreasing glomerular filtration; antiglomerular basement membrane antibodies lead to necrotizing, proliferative glomerulonephritis, and renal failure; diffuse lesions |
| Deposits of immune complexes in the mesangium with mesangial cell proliferation; results in decreased glomerular blood flow and glomerular filtration; leads to hematuria/proteinuria and nephrotic syndrome | |
| Associated with Nephrotic Syndrome | |
Minimal change nephropathy (lipoid nephrosis) Glomerular basement membrane appears normal Most common cause of nephrotic syndrome in children (see Chapter 39) |
Glomeruli look normal under light microscopy; electron microscopy reveals uniform diffuse effacement of epithelial (podocyte) foot processes; loss of negative charge in basement membrane and increased permeability lead to severe proteinuria and nephrotic syndrome |
| Focal proliferation of endothelial and mesangial cells and glomerulosclerosis from hyaline deposits in segmental parts of the glomerular membrane; there is effacement (thinning or deletion) of epithelial podocytes, with a significant increase in pore size resulting in proteinuria and nephrotic syndrome; can progress to involve entire glomerulus and development of tubulointerstitial fibrosis | |
| Diffuse thickening of glomerular basement membrane and capillary wall from deposits of antibody, complement, and release of inflammatory cytokines; increased permeability with proteinuria and leading cause of nephrotic syndrome in white adults | |
| Membranoproliferative glomerulonephritis (MPGN) | Mesangial cell proliferation; thickening of basement membrane; subendothelial deposits of immune-complex occlude glomerular capillary blood flow and decrease glomerular filtration; diffuse lesions |
| Usually idiopathic; associated with hypocomplementemia Type I: Activation of classical complement pathway with nephrotic syndrome (hepatitides B and C, SLE) Type II: Activation of alternate complement pathway with hematuria (idiopathic); no circulating immune complexes Type III: Activation of alternative complement pathway with nephrotic syndrome; can be familial | |
IgA Nephropathy (Berger Disease) Usually idiopathic (can be associated with cirrhosis and minimal change disease); elevated IgA plasma levels (also see Henoch-Schönlein purpura nephritis in Chapter 39) |
Mesangial proliferation with deposition of IgA; release of inflammatory mediators with cellular proliferation; crescent formation, glomerulosclerosis, interstitial fibrosis, decreased GFR and hematuria; usually focal, some diffuse lesions |

GBM, Glomerular basement membrane; GFR, glomerular filtration rate; HIV, human immunodeficiency virus; IgA, immunoglobulin A; IgG, immunoglobulin G; SLE, systemic lupus erythematosus.
Nephrotic and Nephritic Syndromes
Nephrotic and nephritic syndromes are consequences of glomerular injury and present with a pattern of clinical manifestations. Nephrotic syndrome is the excretion of 3.5 g or more of protein in the urine per day. It occurs when glomerular filtration of plasma proteins, particularly albumin, exceeds tubular reabsorption. Primary causes of nephrotic syndrome include particular types of glomerular injury including minimal change nephropathy (lipoid nephrosis) (see Chapter 39), membranous glomerulonephritis, and focal segmental glomerulosclerosis (see Table 38.8. Secondary forms of nephrotic syndrome occur in systemic diseases, including diabetes mellitus (see Chapter 22), amyloidosis, systemic lupus erythematosus (see Chapter 9), and IgA vasculitis (Henoch–Schönlein purpura) (see Chapter 39). Nephrotic syndrome is also associated with certain medications (e.g., NSAIDs), infections, malignancies, and vascular disorders. Familial or inherited forms of nephrotic syndrome result from genetic defects that affect the function and composition of the glomerular capillary wall (i.e., alterations in basement membrane type IV collagen [Alport syndrome] and podocyte dysfunction resulting in steroid resistance).61 It often signifies a more serious prognosis when present as a secondary complication. Nephrotic syndrome is more common in children (see Chapter 39) than in adults and is more commonly idiopathic in adults.
Nephritic syndrome is characterized by hematuria and red blood cell casts in the urine. Hypertension, edema, and oliguria also are components of the syndrome. Proteinuria is present but is usually less severe than in nephrotic syndrome. It occurs primarily with infection-related glomerulonephritis (e.g., hepatitis B and C and acute poststreptococcal glomerulonephritis), rapidly progressive crescentic glomerulonephritis, antiphospholipid syndrome (production of antiphospholipid antibodies that cause thrombotic microangiopathy), and lupus nephritis.59
Pathophysiology
In nephrotic syndrome, injury to the glomerular filtration membrane leads to increased permeability and loss of an electrical negative charge. Normally, plasma proteins, which carry a negative charge, are repelled by the negative charge at the glomerular filtration membrane and thus remain in the plasma. Movement of plasma proteins, particularly albumin and some immunoglobulins, occurs across the injured membrane. The plasma proteins are then lost into the urine, resulting in decreased plasma oncotic pressure and edema (Fig. 38.10). Hypoalbuminemia results from urinary loss of albumin combined with a diminished synthesis of replacement albumin by the liver. Albumin is lost in the greatest quantity because of its high plasma concentration and low molecular weight. Decreased dietary intake of protein from anorexia, malnutrition, or accompanying liver disease may also contribute to lower levels of plasma albumin. Loss of albumin stimulates lipoprotein synthesis by the liver, causing hyperlipidemia, which can promote the progression of glomerular disease. Loss of immunoglobulins may increase susceptibility to infections. Sodium retention is common, further contributing to edema and hypertension.
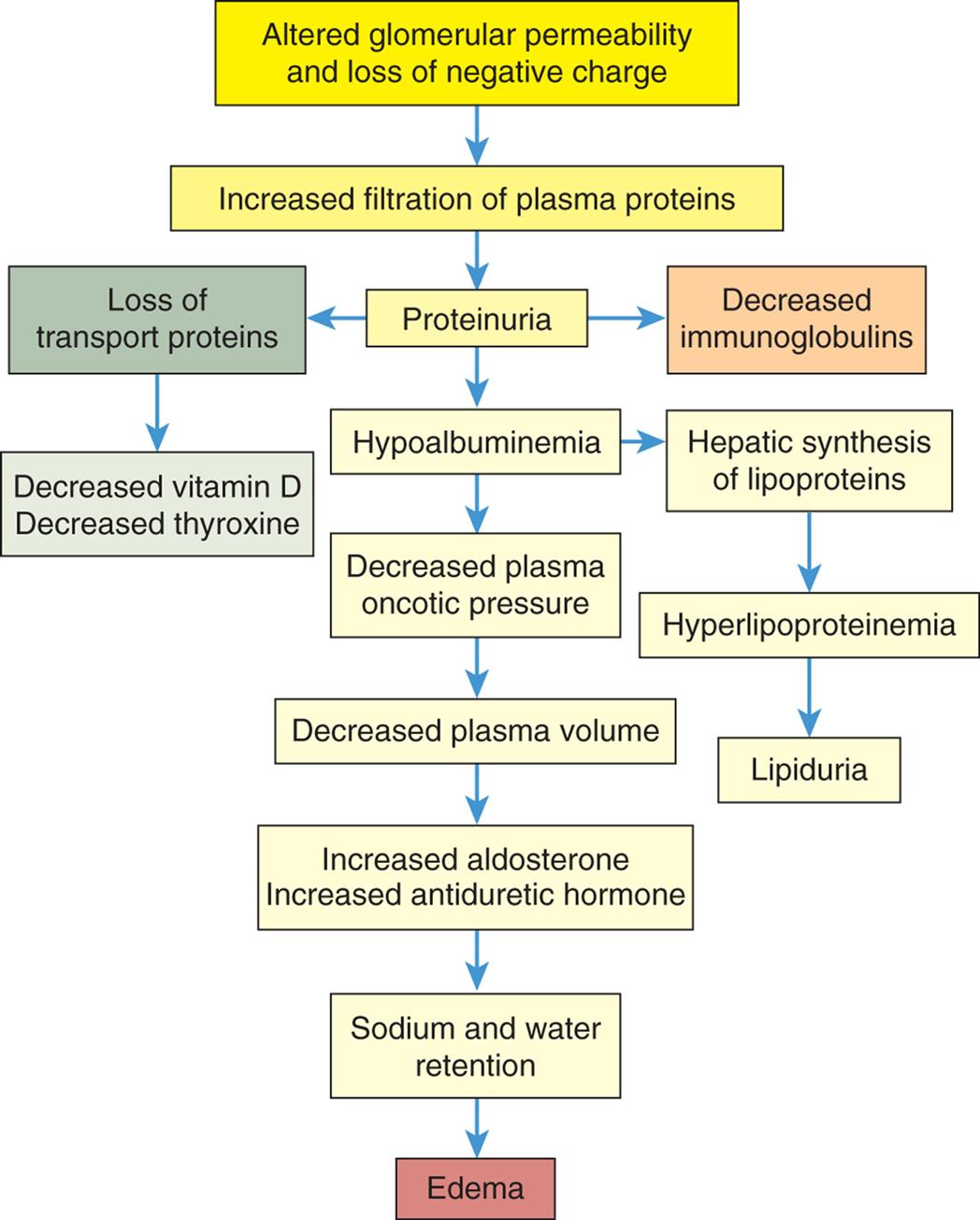
A flowchart shows the pathophysiology of nephrotic syndrome. 1. Altered glomerular permeability and loss of negative charge. Leads to 2. 2. Increased filtration of plasma proteins. Leads to 3. 3. Proteinuria. Leads to 4, 10, and 12. 4. Hypoalbuminemia. Leads to 5 and 13. 5. Decreased plasma oncotic pressure. Leads to 6. 6. Decreased plasma volume. Leads to 7. 7. Increased aldosterone Increased antidiuretic hormone. Leads to 8. 8. Sodium and water retention. Leads to 9. 9. Edema. 10. Loss of transport proteins. Leads to 11. 11. Decreased vitamin D; decreased thyroxine. 12. Decreased immunoglobulins. 13. Hepatic synthesis of lipoproteins. Leads to 14. 14. Hyperlipoproteinemia. Leads to 15. 15. Lipiduria.
Nephritic syndrome is caused by increased permeability of the glomerular filtration membrane with pore sizes large enough to allow the passage of red blood cells and protein. The pathophysiology is related to immune injury of the glomerulus, as previously described. Hypertension and uremia (accumulation of urea and other nitrogen-based metabolic products) occur in advanced stages of disease.
Clinical Manifestations
Many clinical manifestations of nephrotic and nephritic syndrome are related to loss of serum proteins and associated sodium retention (Table 38.9). The manifestations of both nephrotic and nephritic syndrome include edema (periorbital and pedal), hypoproteinemia, proteinuria, hyperlipidemia, lipiduria, vitamin D deficiency, and hypothyroidism. In addition, voided urine that appears foamy from protein loss is associated with nephrotic syndrome, and hematuria, hypertension, and oliguria are associated with nephritic syndrome. Vitamin D deficiency is related to loss of serum transport proteins and decreased vitamin D activation by the kidney. Hypothyroidism can result from urinary loss of thyroid-binding protein and thyroxine. Renal loss of anti-clotting factors and increased liver synthesis of clotting factors can cause hypercoagulability and may lead to thromboembolic events.62
Table 38.9
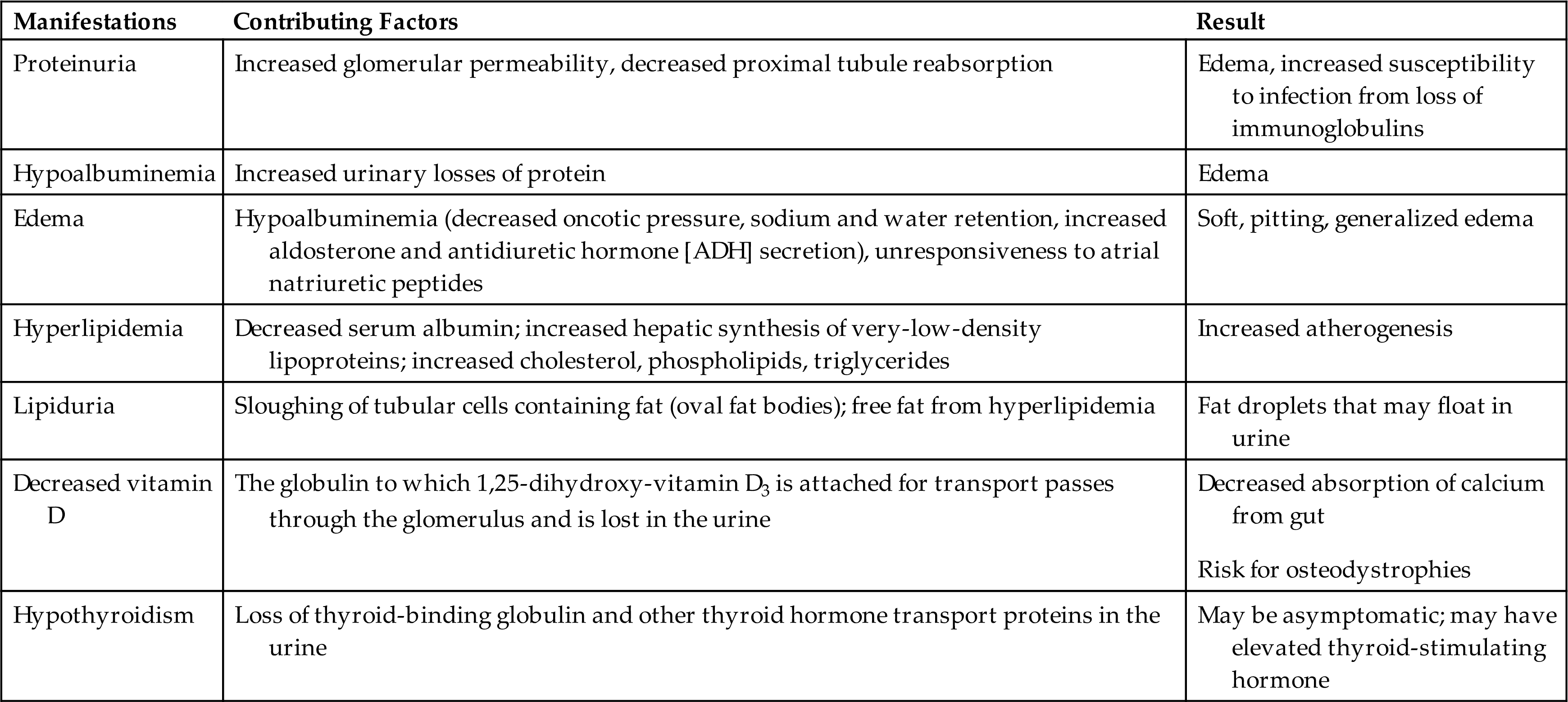
Evaluation and Treatment
Nephrotic syndrome is diagnosed when the protein level in a 24-hour urine collection is greater than 3.5 g. Serum albumin level decreases (to <3 g/dL), and concentrations of serum cholesterol, phospholipids, and triglycerides increase. Fat bodies may be present and float in the urine. The presence of lipiduria (lipids within tubular cells or casts, or as free glubules) suggests a glomerular disorder.
Nephrotic syndrome is commonly treated by consuming a moderate protein restriction (i.e., 0.8 g/kg body weight/day), low-fat, salt-restricted diet, and by prescribing corticosteroids and diuretics. Glucocorticoids are used to control immune-mediated disease and may be combined with immunosuppressive drugs (e.g., rituximab). Targeted immune therapy is considered for steroid-resistant nephrotic syndrome.63 Diuretics are used to control hypertension and eliminate fluid. Care must be taken to observe for hypovolemia and hypokalemia or potassium toxicity in the presence of renal insufficiency. Spironolactone may be combined with loop diuretics to suppress aldosterone activity to conserve potassium. Anticoagulants are used for prophylactic anticoagulation. Angiotensin-converting enzyme (ACE) inhibitors or ARBs lower urine protein excretion and control blood pressure.63
The evaluation and treatment of nephritic syndrome are similar to those described for nephrotic syndrome. Red blood cells and red blood cell casts will be found in the urine. The course of glomerulonephritis is usually more severe with nephritic syndrome. High-dose corticosteroids and cyclophosphamide represent the standard therapy for rapidly progressive crescentic glomerulonephritis. Treatment also may include supportive care with antihypertensives, diuretics, and antibiotics. The addition of plasma exchange (plasmapheresis) and dialysis may be required.
Acute Kidney Injury
Classification of Kidney Dysfunction
Kidney injury may be acute and rapidly progressive (within hours) or may become chronic, progressing to ESRD over several months or years. The terms renal insufficiency, renal failure, uremia, and azotemia are associated with decreasing kidney function but are not specific in relation to the cause of kidney disease. Generally, renal insufficiency refers to a decline in renal function to about 25% of normal or a GFR of 25 to 30 mL/min. Levels of SCr and urea are mildly elevated. AKI captures the diverse nature of this syndrome, ranging from minimal or subtle changes in kidney function to complete kidney failure requiring renal replacement therapy. Renal failure refers to a significant loss of function. When less than 10% of kidney function remains, this is termed ESRD. Specific criteria for AKI are discussed in the next section. Uremia (uremic syndrome) is a syndrome of renal failure and includes elevated blood urea nitrogen (BUN) and creatinine levels accompanied by fatigue, anorexia, nausea, vomiting, pruritus, and neurologic changes. Uremia represents numerous consequences related to kidney failure, including retention of toxic wastes, deficiency states, electrolyte disorders, and immune activation promoting a proinflammatory state. Azotemia is characterized by increased BUN levels (normal is 8 to 20 mg/dL) and frequently increased SCr levels (normal is 0.7 to 1.4 mg/dL). Renal insufficiency or kidney failure causes azotemia. Both azotemia and uremia indicate an accumulation of nitrogenous waste products in the blood, a common characteristic that explains the overlap in definitions of terms.
Acute Kidney Injury
AKI is a sudden decline in kidney function with a decrease in glomerular filtration caused by a defect in the excretion of water, salts, and nitrogenous waste products, which accumulate in the blood as demonstrated by an elevation in SCr level and decrease in urine volume.64 Classification criteria have been developed to guide the diagnosis of AKI (Table 38.10). The Kidney Disease: Improving Global Outcomes (KDIGO) developed clinical practice guidelines for AKI and is based on the Risk, Injury, Failure, Loss, End-Stage Renal Disease (RIFLE) and AKI Network (AKIN) criteria.65 The KDIGO guideline is the commonly accepted definition and classification system for AKI clinical trials and clinical practice.64 AKI is a complex syndrome that is classified according semi-anatomical categories (i.e., pre-renal, intrinsic, and post-renal). However, in recent years, AKI has been further differentiated into specific syndromes (i.e., hepatorenal, cardiorenal, nephrotoxic, and sepsis-associated), each having a unique pathophysiologic process and treatment course.64
Table 38.10

AKI, Acute kidney injury; ESRD, end-stage renal disease; GFR, glomerular filtration rate; SCr, serum creatinine; UOP, urine output.
Data from Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. Kidney Inter Suppl. 2012;2:1–138. http://www.kdigo.org/clinical_practice_guidelines/AKI.php; Bellomo R, Kellum JA, Mehta R, et al. Acute dialysis quality initiative II: The Vicenza conference. Current Opinion in Critical Care, 2002;8(6):505–508; Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Current Opinion in Critical Care, 2004;8(4):R204–R212; Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: A critical and comprehensive review. Clinical Kidney Journal, 2013;6(1):8–14. doi:10.1093/ckj/sfs160.
Pathophysiology
AKI results from ischemic injury related to extracellular volume depletion, renal hypoperfusion, exposure from chemicals, drugs, or endogenous toxins, sepsis, intra-abdominal hypertension, progressive glomerulonephritis, acute interstitial nephritis, infection, or an obstructive process. Injured, hypoxic, and ischemic tissues initiate a complex inflammatory immunopathophysiological response that results in microcirculatory and macrocirculatory disturbances in the kidney, functional impairment, and ultimately cell death.66 The simultaneous activation of components of innate immunity, including leukocytes, coagulation factors, and complement proteins, drives kidney inflammation, glomerular and tubular damage, and breakdown of the blood-urine barrier.66 This profound immune response is an integral part of multi-organ dysfunction associated with AKI. Alterations in kidney function may be minimal or severe.67 AKI is an evolving clinical syndrome with multiple, often simultaneous, and overlapping causes. AKI is currently broadly classified according to the underlying pathophysiologic process as prerenal (renal hypoperfusion), intrarenal (intrinsic disorders involving renal parenchymal or interstitial tissue), or postrenal (urinary tract obstructive disorders) (Table 38.11).68
Table 38.11


ACEi, Angiotensin-converting enzyme inhibitor; (a)HUS, (atypical) Hemolytic uremic syndrome; AIN, acute interstitial nephritis; AKI, acute kidney injury; APS, antiphospholipid syndrome; ARB, angiotensin-receptor blocker; ATN, acute tubular necrosis; DIC, disseminated intravascular coagulation; FSGS, focal segmental glomerulosclerosis; HELLP, hemolysis, elevated liver enzymes, low platelet count) syndrome; HIV, human immunodeficiency virus; NSAID, nonsteroidal anti-inflammatory drug; TTP, thrombotic thrombocytopenic purpura.
Data from Moore PK, Hsu RK, Liu KD. Management of acute kidney injury: Core curriculum 2018. American Journal of Kidney Disease, 2018;72(1):136–148. doi:10.1053/j. ajkd.2017.11.021; Gameiro J, Fonseca JA, Outerelo C, et al. Acute kidney injury: From diagnosis to prevention and treatment strategies. Journal of Clinical Medicine, 2020;9(6):1704. doi:10.3390/jcm9061704.
Prerenal AKI or inadequate kidney perfusion is the most common reason for AKI. Poor perfusion can result from hypovolemia, reduced cardiac output, renal vasomodulation/shunting, and systemic vasodilation (see Table 38.11).69 During the early phases of hypoperfusion, protective autoregulatory mechanisms maintain GFR at a relatively constant level through afferent arteriolar dilation and efferent arteriolar vasoconstriction (mediated by angiotensin II). Tubuloglomerular feedback mechanisms also maintain GFR and distal tubular nephron flow (see Chapter 37). The GFR declines because of the decrease in glomerular filtration pressure. Failure to restore blood volume or blood pressure and oxygen delivery can cause ischemic cell injury and acute tubular necrosis (ATN) or acute interstitial necrosis, a more severe form of AKI. Reperfusion (reoxygen) injury with cell death also can occur (see Fig. 2.10 also see Chapters 32 and 48). AKI can occur with CKD if a sudden stress is imposed on already poorly functioning kidneys, hastening the progression to ESRD.
Intrarenal (intrinsic) AKI can result from vascular, microvascular, glomerular, and tubulointerstitium causes (see Table 38.11). The most commonly seen cause of intrarenal AKI is ATN. Ischemic ATN most often occurs after surgery but also is associated with prerenal causes such as sepsis, obstetric complications, and severe hemorrhagic trauma or severe burns. Whereas nephrotoxic ATN is usually caused by exposure to radiocontrast media or nephrotoxic medications (e.g., aminoglycosides, NSAIDs, ACEi, ARBs, and antibiotics).
Hypotension associated with hypovolemia produces ischemia and the inflammatory response, generating toxic oxygen free radicals that cause cellular swelling, injury, and necrosis. Intrarenal microcirculatory vasoconstriction occurs in response to injury and inflammation and decreases blood flow. Ischemic necrosis results and tends to be patchy and may be distributed along any part of the nephron. Nephrotoxic ATN is associated with radiocontrast media and numerous antibiotics, particularly the aminoglycosides (neomycin, gentamicin, tobramycin) because these drugs accumulate in the renal cortex.69 Other substances, such as excessive myoglobin (oxygen-transporting substance from muscles; released with crush injuries), carbon tetrachloride, heavy metals (mercury, arsenic), or methoxyflurane anesthetic, and bacterial toxins may promote kidney injury. Dehydration, advanced age, concurrent renal insufficiency, and diabetes mellitus tend to enhance nephrotoxicity. Necrosis caused by nephrotoxins is usually uniform and limited to the proximal tubules. This is due to the high surface area of the brush border (microvilli) of the proximal tubular cells and the reabsorption properties of epithelial cells, which make the proximal tubules more vulnerable to toxic injury.
Postrenal AKI is caused by an obstruction within the urinary tract that affects the kidneys bilaterally (e.g., bladder outlet obstruction, ureteral obstruction, or renal pelvis obstruction) (see Table 38.11). A pattern of several hours of anuria with flank pain followed by polyuria is a characteristic finding. The obstruction causes an increase in intraluminal hydrostatic pressure upstream from the site of obstruction with a gradual decrease in GFR.
Oliguria, or a urine output of <400 mL/24 h, occurs in AKI and can be differentiated from prerenal, intrarenal, and ATN (Table 38.12). Three mechanisms have been proposed to account for the decrease in urine volume in AKI. All three mechanisms contribute to oliguria in varying combinations and degrees throughout the course of the disease (Fig. 38.11). These mechanisms are as follows70:
- 1. Alterations in renal blood flow. Efferent arteriolar vasoconstriction may be produced by intrarenal release of angiotensin II or there may be a redistribution of blood flow from the cortex to the medulla. Autoregulation of blood flow may be impaired, resulting in decreased GFR. Microthrombi may obstruct blood flow. Changes in glomerular permeability and decreased GFR also may result from ischemia.
- 2. Tubular obstruction. Necrosis of the tubules causes sloughing of cells, cast formation, and obstruction to urine flow. There can be ischemic edema that results in tubular obstruction, which causes a retrograde increase in hydrostatic pressure, and opposes the hydrostatic pressure of glomerular filtration, thus reducing the GFR. Kidney failure can occur within 24 hours.
- 3. Tubular backleak. Tubular reabsorption of filtrate is accelerated as a result of increased permeability caused by ischemia and increased tubular pressure from obstruction. The increased reabsorption of filtrate contributes to oliguria.
Table 38.12
| Urine Volume | Urine Specific Gravity | Urine Osmolality | Urine Sodium Concentration | Blood Urea Nitrogen (BUN)/Plasma Creatinine Ratio | FENaa | |
|---|---|---|---|---|---|---|
| Normal values | 800–2000 mL | 1.010–1.030 | 500–800 mOsm | 20 mEq/L | 10:1–20:1 | 1% |
| Prerenal failure | <400 mL | 1.016–1.020 | >500 mOsm | <10 mEq/L | ||
| Intrarenal failure (i.e., acute tubular necrosis) | <400 mL | 1.010–1.012 | <400 mOsm | >30 mEq/L |
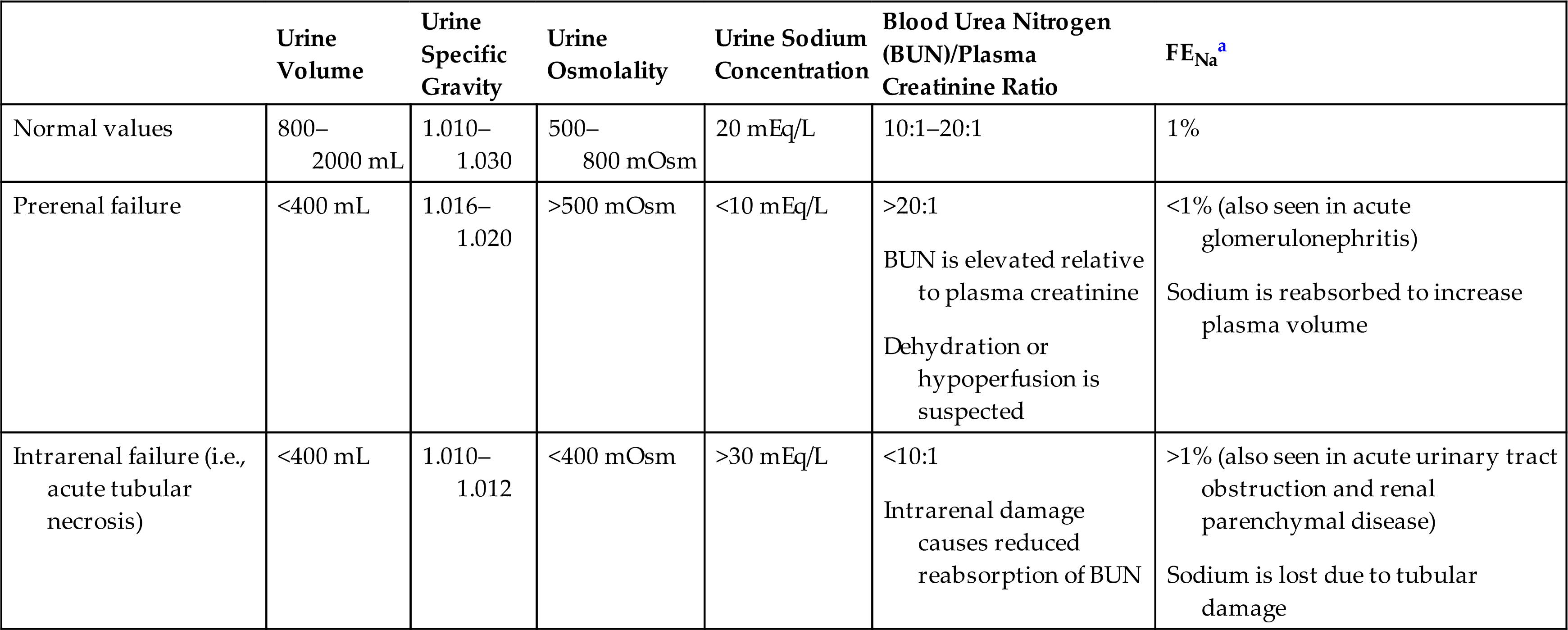
a.
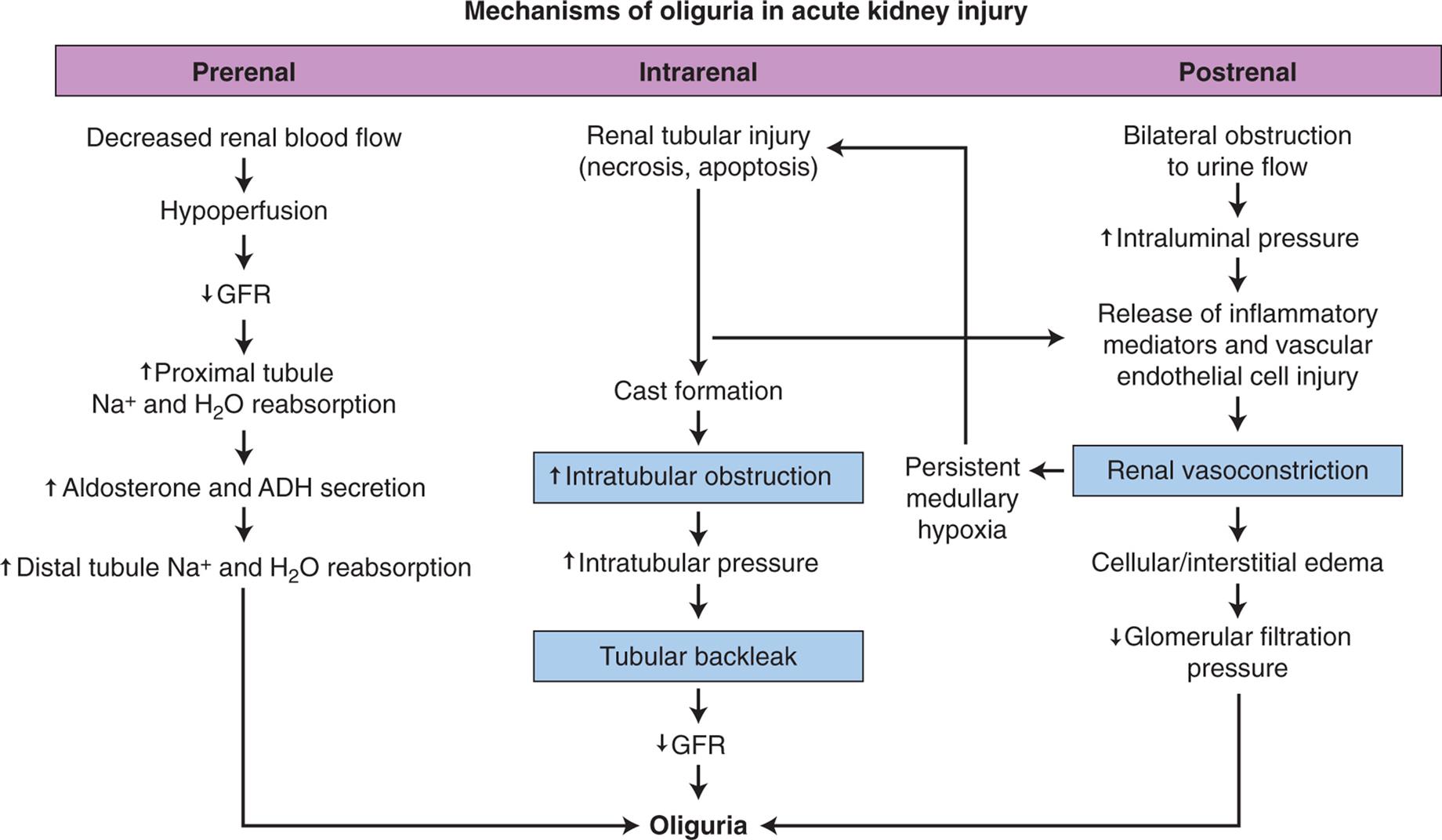
ADH, Antidiuretic hormone; GFR, glomerular filtration rate.
A flowchart shows prerenal, intrarenal, and postrenal mechanisms of oliguria in acute kidney injury. The mechanism for prerenal oliguria is as follows. 1. Decreased renal blood flow. Leads to 2. 2. Hypoperfusion. Leads to 3. 3. Decreased G F R. Leads to 4. 4. Increased proximal tubule; sodium ion and water reabsorption. Leads to 5. 5. Increased aldosterone and A D H secretion. Leads to 6. 6. Increased distal tubule sodium ions and water reabsorption. The mechanism for intrarenal oliguria is as follows. 1. Renal tubular injury (necrosis, apoptosis). Leads to 2 or 3 of postrenal oliguria. 2. Cast formation. Leads to 3. 3. Increased intratubular obstruction. Leads to 4. 4. Increased intratubular pressure. Leads to 5. 5. Tubular back leak. Leads to 6. 6. Increased G F R. The mechanism for postrenal oliguria is as follows. 1. Bilateral obstruction to urine flow. Leads to 2. 2. Increased intraluminal pressure. Leads to 3. 3. Release of inflammatory mediators and vascular endothelial cell injury. Leads to 4. 4. Cellular or interstitial edema. Leads to 5 or persistent medullary hypoxia leading to 1 of intrarenal oliguria. 5. Decreased glomerular filtration pressure.
Oliguria begins within 1 day after a hypotensive event and lasts 1 to 3 weeks, but may regress in several hours or extend for several weeks depending on the duration of ischemia or the severity of injury or obstruction.
AKI also can present with nonoliguric kidney failure (high output kidney failure) and represents less severe injury, particularly with intrinsic kidney injury associated with nephrotoxins. The renal tubules have impaired reabsorption and concentration and dilution function. The urine output may be normal or high in volume, but the BUN and plasma creatinine concentrations increase. Anuria (urine output <50 mL/day) can occur in AKI but is uncommon in ATN. It involves both kidneys and suggests bilateral renal artery occlusion, obstructive uropathy, or acute cortical necrosis.
Clinical Manifestations
The clinical progression of AKI due to ATN, the most common cause of AKI, occurs in four overlapping phases: initiation, extension, maintenance, and recovery.71 Each phase is described as follows:
- 1. The initiation phase occurs with the onset of renal hypoperfusion or toxicity resulting in severe cellular ATP depletion leading to acute cell injury and dysfunction (renal tubular epithelial cell injury is a key feature). Prevention of injury is possible during this phase and the next phase with therapeutic interventions, but there is a short window of opportunity.
- 2. The extension phase is initiated by continued hypoxia following the initial ischemic event and an inflammatory response. Cells continue to undergo injury and death with necrosis and apoptosis present in the outer medulla. However, in the outer cortex, where blood flow has returned to near normal levels, the proximal tubule cells undergo cellular repair during this phase.
- 3. The maintenance or oliguric phase is the period of established kidney injury and dysfunction after the initiating event has been resolved. Cells undergo repair, migration, apoptosis, and proliferation, which may last from weeks to months with urine output lowest during this phase. During this phase, SCr, BUN, and serum potassium levels increase; metabolic acidosis develops; there is salt and water overload; and urine output is decreased with oliguria.
- 4. The recovery or polyuria phase is the interval when glomerular function returns but the regenerating tubules cannot yet concentrate the filtrate. Diuresis is common during this phase, with a decline in SCr and urea concentrations and an increase in creatinine clearance. Cellular differentiation continues, epithelial polarity is reestablished, and homeostasis is established. As renal function improves, the increase in urine volume (diuresis) is progressive. The tubules are still damaged early in the recovery phase and have not recovered secretion and reabsorption functions. Polyuria can result in excessive loss of sodium, potassium, and water. Fluid and electrolyte balance must be carefully monitored, and excessive losses replaced. Serial measurements of SCr concentration provide an index of renal function during the recovery phase. Return to normal status may take 3 to 12 months; some individuals do not gain full recovery of a normal GFR or tubular function, and plasma creatinine concentration will remain higher than normal.
The hallmark features of AKI are increased SCr, reduced GFR, and decreased urine output. In the presence of fever, rash, joint pains, pulmonary infiltrates, abnormal urine analysis, thrombocytopenia, and hemolytic anemia, less common causes (e.g., glomerulopathy, vasculitis, and hemolytic uremic syndrome) of AKI should be considered.67 Other manifestations of altered urine excretion include hyperkalemia, hyperphosphatemia, and metabolic acidosis. Edema and congestive heart failure can be associated with fluid retention.
A diagnostic challenge is to differentiate prerenal AKI from ATN. Urine composition may provide helpful diagnostic clues to changes in tubular function (Table 38.13). The ratios of BUN to plasma creatinine concentration and fractional excretion of sodium (the ratio of filtered sodium to excreted sodium) are helpful diagnostic indicators because the tests reflect renal tubular reabsorption ability. In prerenal AKI, tubular function is maintained, and salt, water, and urea are reabsorbed. With ATN, reabsorption and urinary concentration abilities are compromised. Other causes of renal failure also may exhibit similar clinical findings. Cystatin C, a serum protein constantly produced by nucleated cells, is freely filtered by the glomerulus, and its concentration can serve as a measure of GFR and may be useful for detecting early changes in GFR. Serial measurements of plasma creatinine concentration provide an index of renal function during the recovery phase. However, changes in SCr level occur only if more than 50% of glomerular filtration is lost. The SCr level may not reflect this decrease in glomerular filtration for 24 hours or more. Such diagnostic delays make the implementation of early therapy very difficult, contributing to disease progression and mortality.
Table 38.13
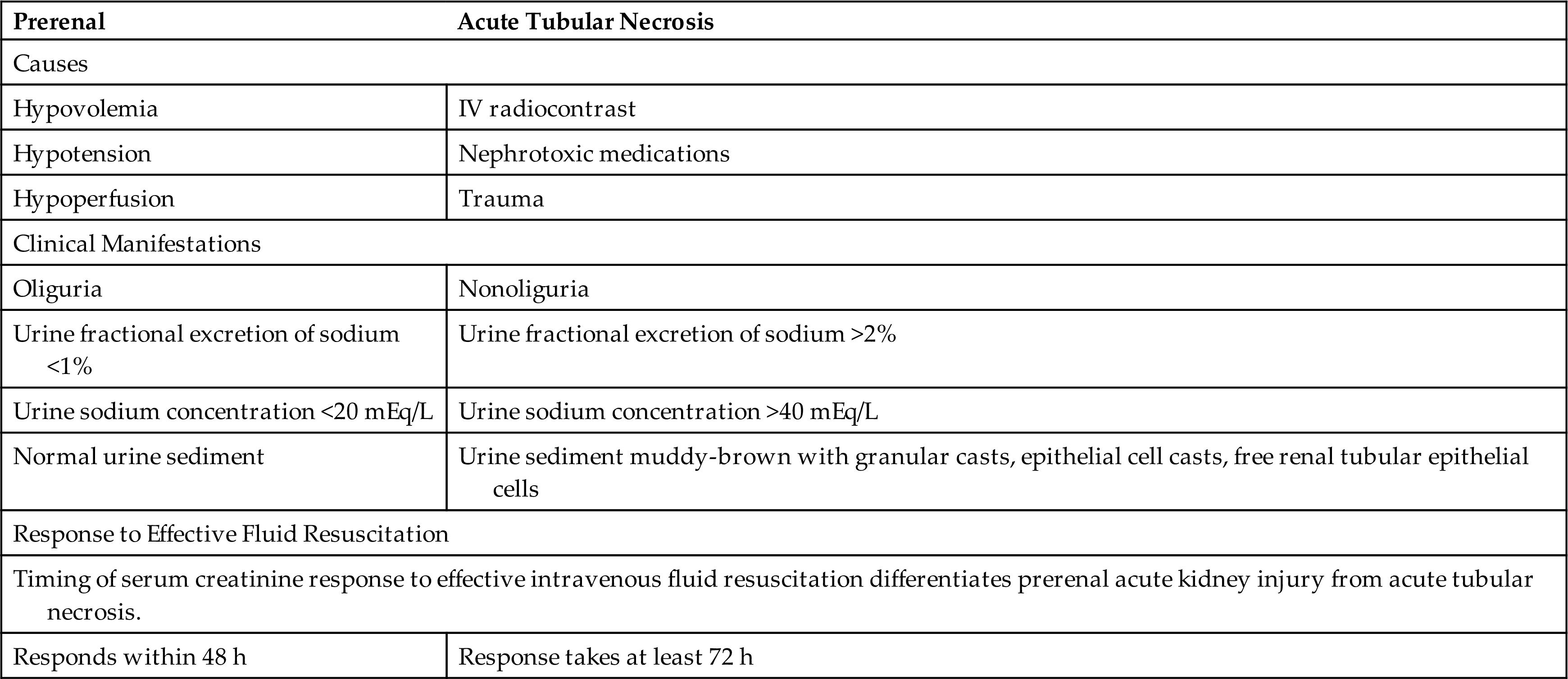
Evaluation and Treatment
Prevention of AKI is the most important therapeutic approach and involves avoidance of hypotension, hypovolemia, and nephrotoxicity. However, once AKI has occurred, determination of the etiology of AKI is essential for appropriate management. There are recent developments in ways to assess, monitor, and evaluate AKI progression before the SCr increases. One advancement is the emergence of biomarker testing to aid in the risk assessment for moderate or severe AKI (see Emerging Science Box: Biomarkers for AKI Risk Stratification).72 Other newer developments related to monitoring and evaluating risk progression include e-alert systems, machine-learning algorithms and artificial intelligence for AKI recognition and monitoring, as well as models based upon the renal angina index, and furosemide stress test (FST).73
The diagnosis of AKI is related to the cause of the disease. Therefore, a complete history must be obtained and include medications (prescribed and over the counter); recent exposure to nephrotoxic agents; recent surgery, trauma, or infection; and past medical history of cardiovascular disorders, obstructive uropathies (e.g., an enlarged prostate or kidney stones), or AKI (see Table 38.13).
Laboratory evaluation should include SCr, serum urea, electrolytes, complete blood count, liver function tests, glucose level, bone profile, urine analysis, and urine microscopic examination.67 Other laboratory testing can provide information related to the cause such as an assessment of antineutrophil cytoplasmic antibodies (ANCA), antiglomerular basement membrane antibodies (anti-GBM), antinuclear antibodies (ANA), anti-double-stranded DNA (anti-dsDNA) antibodies, complement factors, rheumatoid factor, antistreptolysin O titer (ASOT), cryoglobulins, serum electrophoresis, immunoglobulins, serum free light chains, hepatitis, and HIV serology.67 Diagnostic imaging should include a renal ultrasound, to exclude obstruction, and chest x-ray to determine a potential cause, such as pneumonia or vasculitis, and to evaluate volume status.67
Management principles directly related to physiologic alterations generally include the following69,73:
- 1. ensuring adequate hydration and volume status;
- 2. correcting electrolyte disturbances, particularly hyperkalemia;
- 3. managing hemodynamics, including blood pressure;
- 4. preventing and treating infections;
- 5. avoiding nephrotoxic agents and drugs;
- 6. maintaining adequate nutrition; and
- 7. glucose control.
Fluid and electrolyte replacement must be carefully calculated with consideration of urine losses, insensible losses (up to 1000 mL/day), and production of endogenous water by oxidation (450 mL/day). Overhydration of individuals dilutes plasma sodium concentration and can precipitate pulmonary, cerebral, myocardial, and liver edema. A positive fluid balance is independently associated with increased mortality in individuals with AKI and contributes to worse outcomes in critically ill persons.67 Metabolic acidosis is treated when serum bicarbonate concentration is less than 22 mEq/L.
Hyperkalemia can be managed by restricting dietary sources of potassium, using loop diuretics, and using cation exchange resins, which may be administered orally or rectally. These resins exchange potassium for another cation, such as sodium in the bowel, and the potassium is then excreted attached to the resin.69 With severe hyperkalemia (more than 6.5 mEq/L), dialysis may be required, or potassium can be temporarily driven back into the cells by administering insulin (followed by glucose to prevent hypoglycemia), or by infusing sodium bicarbonate or administering albuterol. Insulin administered with glucose facilitates the uptake of glucose into the cell, which results in potassium shifting from the extracellular environment to the intracellular fluid. (Glucose metabolism is discussed in (see Chapters 1 and 22). Using sodium bicarbonate to cause alkalemia also shifts potassium into cells in exchange for hydrogen ions but requires consideration of hypervolemia. Careful monitoring of the electrocardiogram for peaking T waves is essential for individuals with hyperkalemia. Intravenous infusion of calcium is the most rapid method of treating cardiac effects of hyperkalemia. Calcium decreases the threshold potential and reduces the membrane excitability caused by hyperkalemia (see Chapter 3). Calcium should be used only in emergencies, however, because hypercalcemia also may cause cardiac arrest.
Azotemia is generally controlled and nutrition maintained with a low-protein, high-carbohydrate diet. Essential amino acid replacement can be given orally or parenterally. Adequate carbohydrate intake slows protein catabolism and helps prevent release of potassium from cellular breakdown. Because sepsis is a common serious and potentially fatal complication of renal failure, observation for signs of infection and early treatment with antibiotics are necessary. Drug dosage levels may require adjustment if they are metabolized or excreted by the kidneys. Recovery may take up to 1 year.
Continuous renal replacement therapy (CRRT) (mechanical removal of water, electrolytes, and toxins from the blood) is indicated for uncontrollable hyperkalemia, acidosis, or severe fluid overload. CRRT is particularly promising in critically ill individuals with multiple organ dysfunction or sepsis. According to the KDIGO guideline, CRRT is a complementary therapy in the treatment of AKI.69 The timing and optimal dose-response relationships for CRRT are individually determined with consideration to hemodynamic status, degree of volume overload, bleeding risk, and the treating facility’s availability/experience.74
Chronic Kidney Disease
CKD is the progressive and irreversible loss of renal function indicated by a decline in GFR to below 60 mL/min/1.73 m2 for 3 months or more with implications for health. CKD is associated with systemic diseases, such as diabetes mellitus (most significant risk factor), hypertension, and systemic lupus erythematosus. CKD also is associated with intrinsic kidney diseases, such as AKI, chronic glomerulonephritis, chronic pyelonephritis, obstructive uropathies, or vascular disorders (Table 38.14).
Table 38.14
| Stage | Description | Signs/Symptoms |
|---|---|---|
| I | ||
| II | Mild kidney damage, mild reduction in GFR (60–89 mL/min) | |
| III | ||
| IV | ||
| V |
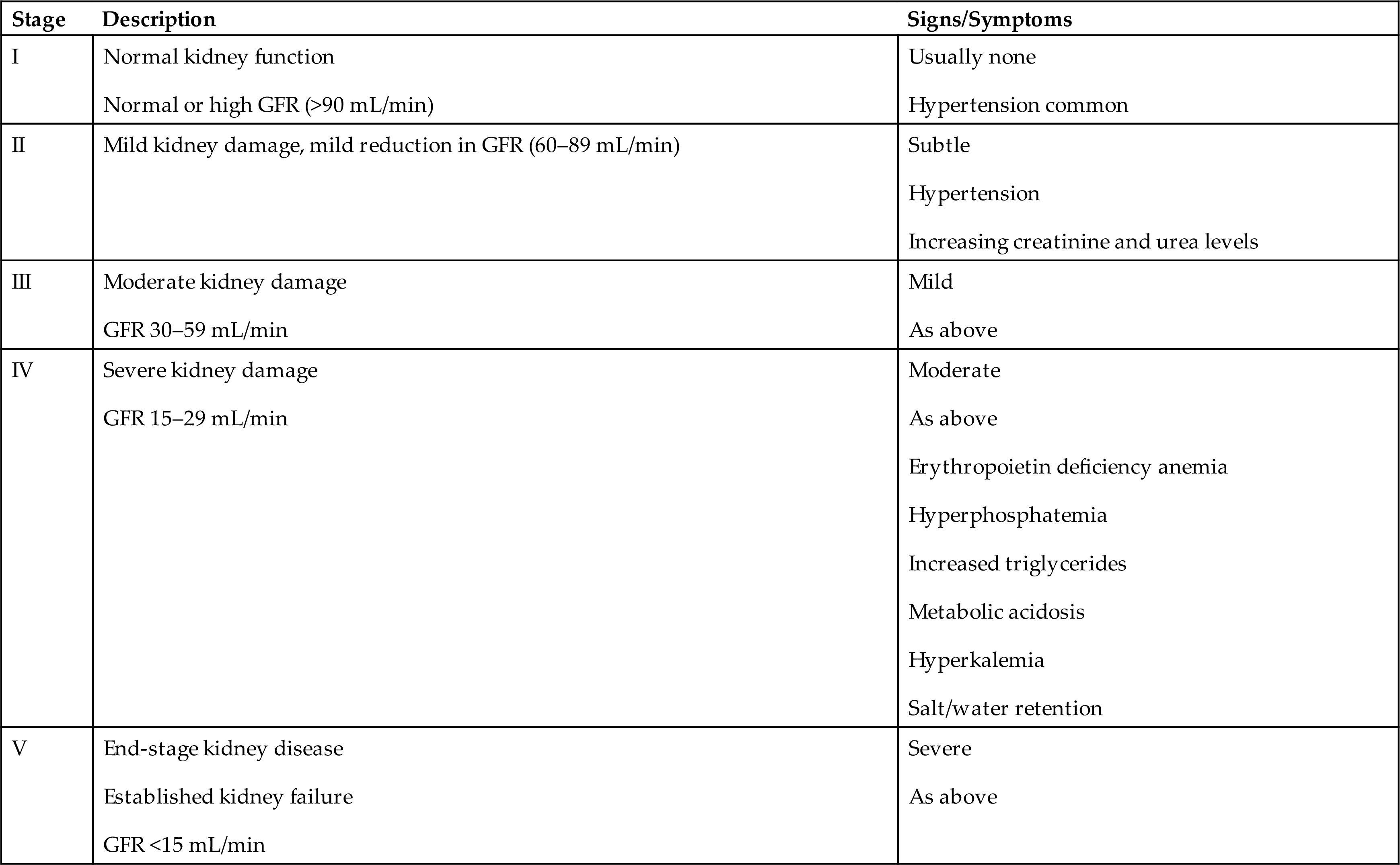
Pathophysiology
The progression phase of the disease is characterized by a persistent state of inflammation and hypoxia and oxidative stress that contribute to the development of renal fibrosis.75 The kidneys have a remarkable ability to adapt to the loss of nephron mass. Symptomatic changes result from increased plasma levels of creatinine, urea, and potassium. Alterations in salt and water balance usually do not become apparent until renal function declines to less than 25% of normal when adaptive renal reserves have been exhausted.76
Different theories have been proposed to account for the adaptation to the loss of renal function. The intact nephron hypothesis proposes that loss of nephron mass with progressive kidney damage causes the surviving nephrons to increase their capacity to maintain solute and water regulation. These nephrons are capable of a compensatory hypertrophy and expansion or hyperfunction in their rates of filtration, reabsorption, and secretion. Although the urine of an individual with CKD may contain abnormal amounts of protein and red and white blood cells or casts, the major end products of excretion are similar to those of normally functioning kidneys until the advanced stages of renal failure when there is a significant reduction of functioning nephrons.
The particular location of kidney damage influences loss of kidney function. For example, tubular interstitial diseases damage primarily the tubular or medullary parts of the nephron, producing problems such as renal tubular acidosis, salt wasting, and difficulty diluting or concentrating the urine. Proteinuria, hematuria, and nephrotic syndrome are more prominent when the damage is primarily vascular or glomerular. With severe or repeated injury, interstitial capillary loss, and fibroblast proliferation result in progressive glomerulosclerosis and tubulointerstitial fibrosis. These conditions contribute to CKD and ESRD. A summary of factors involved in the progression of CKD is outlined in Table 38.15 and Fig. 38.12.
Table 38.15
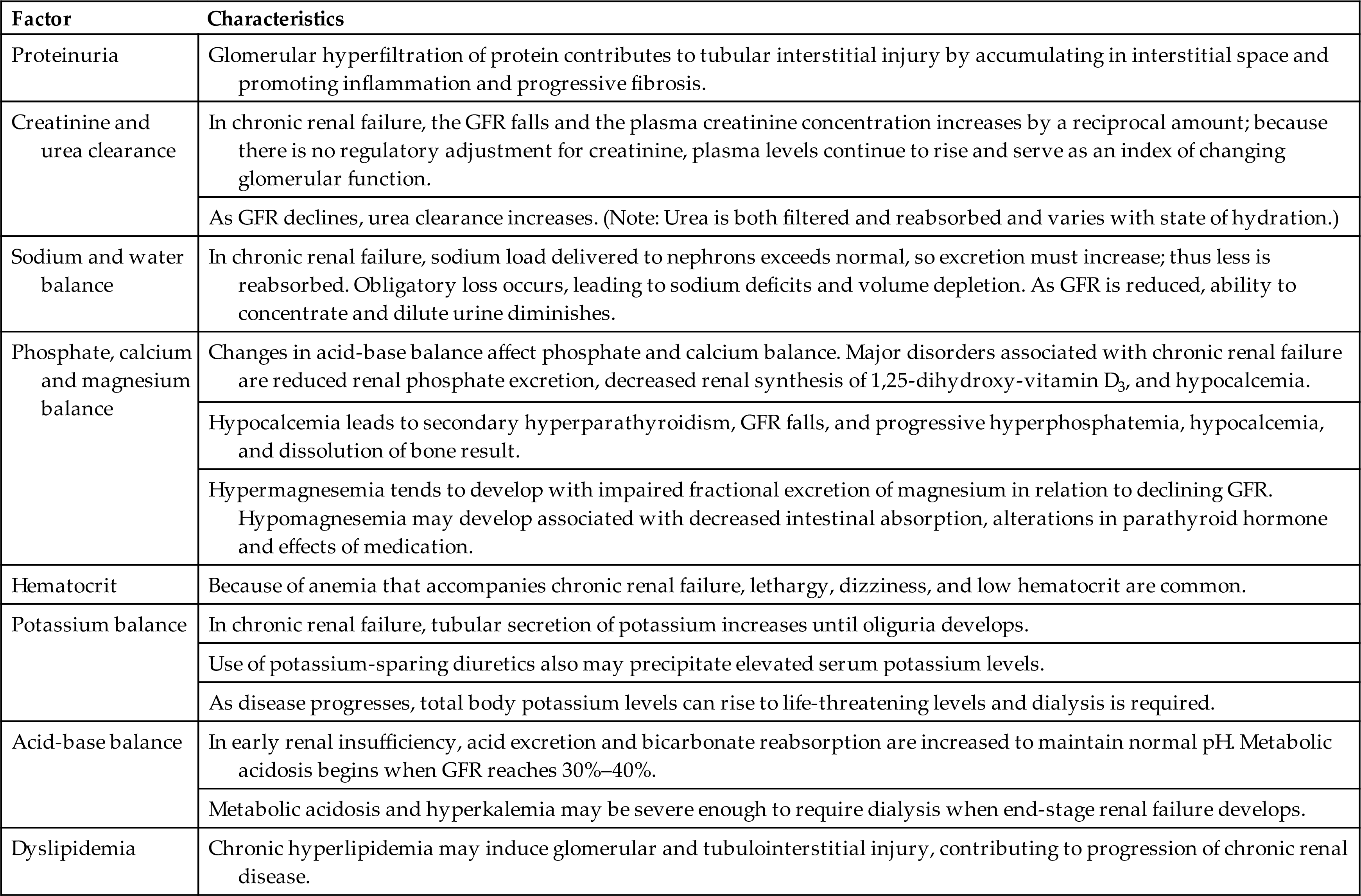

A flowchart shows the mechanisms related to the progression of chronic kidney disease. 1. Renal injury; ischemia. Leads to 2. 2. Loss of nephrons. Leads to 3 and 4. 3. Increased angiotensin 2. Leads to 3 and 8. 4. Glomerular capillary hypertension. Leads to 5. 5. Increased glomerular permeability and filtration. Leads to 6. 6. Proteinuria. Leads to 7. 7. Increased tubular protein reabsorption. Leads to 8. 8. Tubulointerstitial inflammation and fibrosis. Leads to 9. 9. Renal scarring. Leads to 2 and 10. 10. Systemic hypertension.
The factors that contribute to the pathogenesis of CKD are complex and involve the interaction of many cells, cytokines, and structural alterations. Two factors that have consistently been recognized to advance renal disease are proteinuria and angiotensin II activity. Glomerular hyperfiltration, increased glomerular capillary permeability, and loss of negative charge may lead to proteinuria. Proteinuria contributes to tubulointerstitial injury by accumulating in the interstitial space of the nephron tubules. There is activation of complement proteins and other mediators and cells, such as macrophages, that promote inflammation and progressive fibrosis. Angiotensin II (from activation of the renin-angiotensin-aldosterone system [RAAS]) causes efferent arteriolar vasoconstriction that promotes glomerular hypertension, systemic hypertension, and hyperfiltration. Hyperfiltration is also associated with hyperglycemia and diabetic nephropathy. The chronically high intraglomerular pressure increases glomerular capillary permeability, contributing to proteinuria. Angiotensin II promotes the activity of inflammatory cells and growth factors that participate in tubulointerstitial fibrosis and scarring.3
The progression of AKI to CKD is related to incomplete or maladaptive tissue repair, setting in motion processes promoting the development of interstitial fibrosis.77 CKD and progressive renal dysfunction are also characterized by an amplification of oxidative stress.78 Oxidative stress accompanied by inflammation (in which the cells of the innate immune response system are mainly involved) has a significant role in the pathogenesis of CKD.78 The effect of reactive oxidative stress (ROS) disrupts the excretory function of each section of the nephron, preventing the maintenance of intra-systemic homeostasis and leading to the accumulation of metabolic products.78 Renal regulatory mechanisms, such as tubular glomerular feedback and the RAAS, are also affected, making it impossible for the kidney to compensate for water–electrolyte and acid-base disturbances. Without appropriate compensatory mechanisms, there is further intensification of oxidative stress resulting in the progression of CKD and a spectrum of complications such as malnutrition, calcium phosphate abnormalities, atherosclerosis, and anemia.78 Hypertension can be used as an example. Oxidative stress in the kidney and vascular tissue causes hypertension, and hypertension promotes oxidative stress. Now add in the inflammation component. Chronic inflammation is a significant contributor to the promotion of oxidative stress.78 Therefore, oxidative stress, along with inflammation, is a critical component of CKD-related pathologies that can adversely affect the human body (e.g., diabetes mellitus, hypertension).78
Clinical Manifestations
The clinical manifestations of CKD are often described using the terms azotemia and uremia. Azotemia is manifested by increased levels of serum urea, SCr, and other nitrogenous compounds related to decreasing kidney function. Uremia is the accumulation of urea and other nitrogenous compounds and toxins. Sources of toxins include the accumulation of end products of protein metabolism, alterations in fluid and electrolytes, metabolic acidosis, intestinal absorption of toxins produced by gut bacteria, and results of altered renal hormone synthesis (e.g., anemia, hyperphosphatemia, and hypocalcemia). The accumulation of toxins has systemic effects known as uremic syndrome.79 The manifestations involve almost every organ system and include hypertension; anorexia; nausea; vomiting; diarrhea or constipation; malnutrition and weight loss; pruritus; edema; anemia; clotting disorders; neurologic, cardiovascular, and endocrine disease; and skin and skeletal changes. The manifestations are summarized in Table 38.16 and Fig. 38.13. Details of the systemic manifestations associated with CKD are discussed in the following sections.
Table 38.16
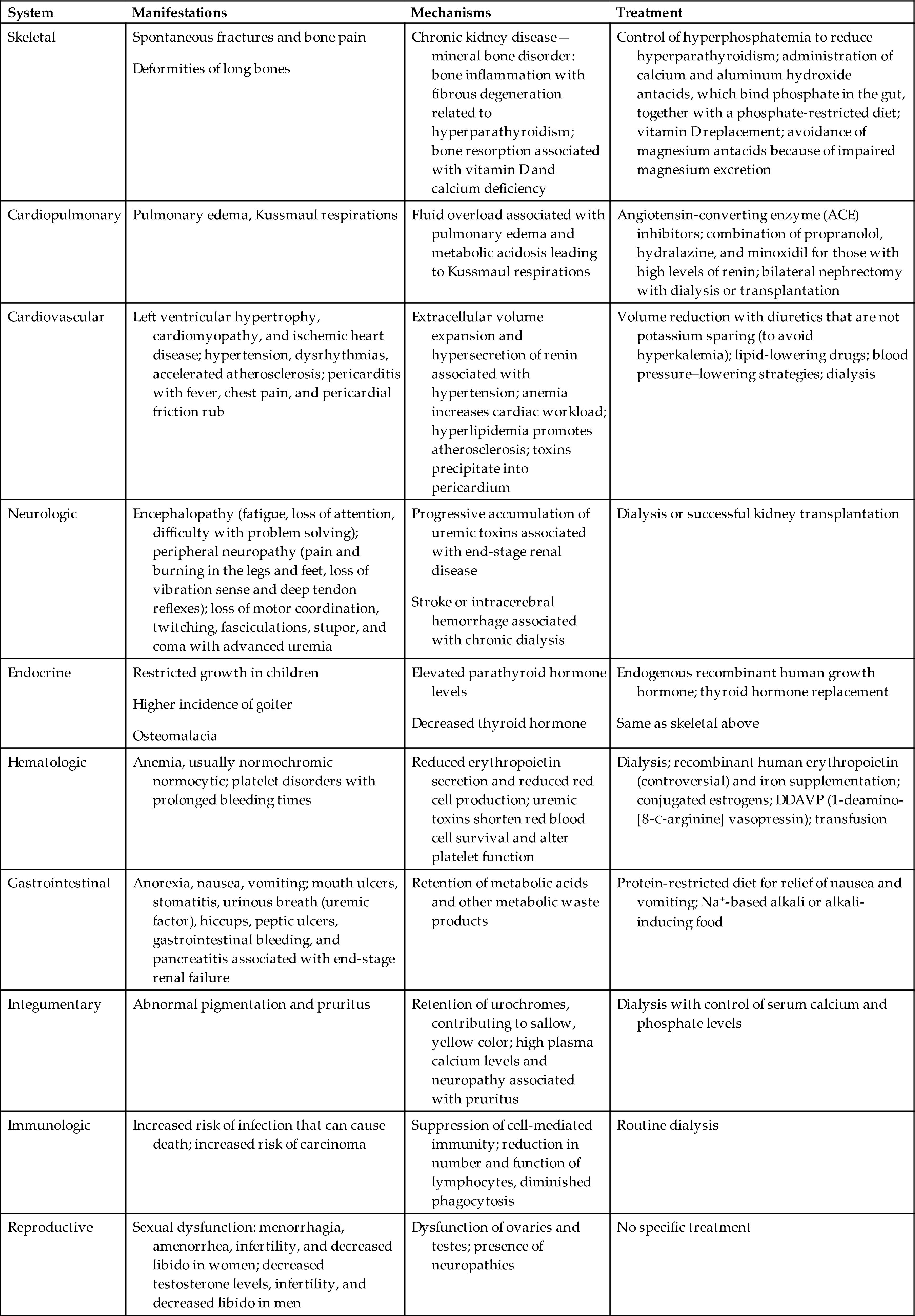
Data from Almeras C, Argilés A. The general picture of uremia. Seminars in Dialysis, 2009;22(4):329–333; Keane WF. The role of lipids in renal disease: Future challenges. Kidney International Supplement, 75:S27–S31, 2000; Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Primary Care, 2008;35(2):329–344.
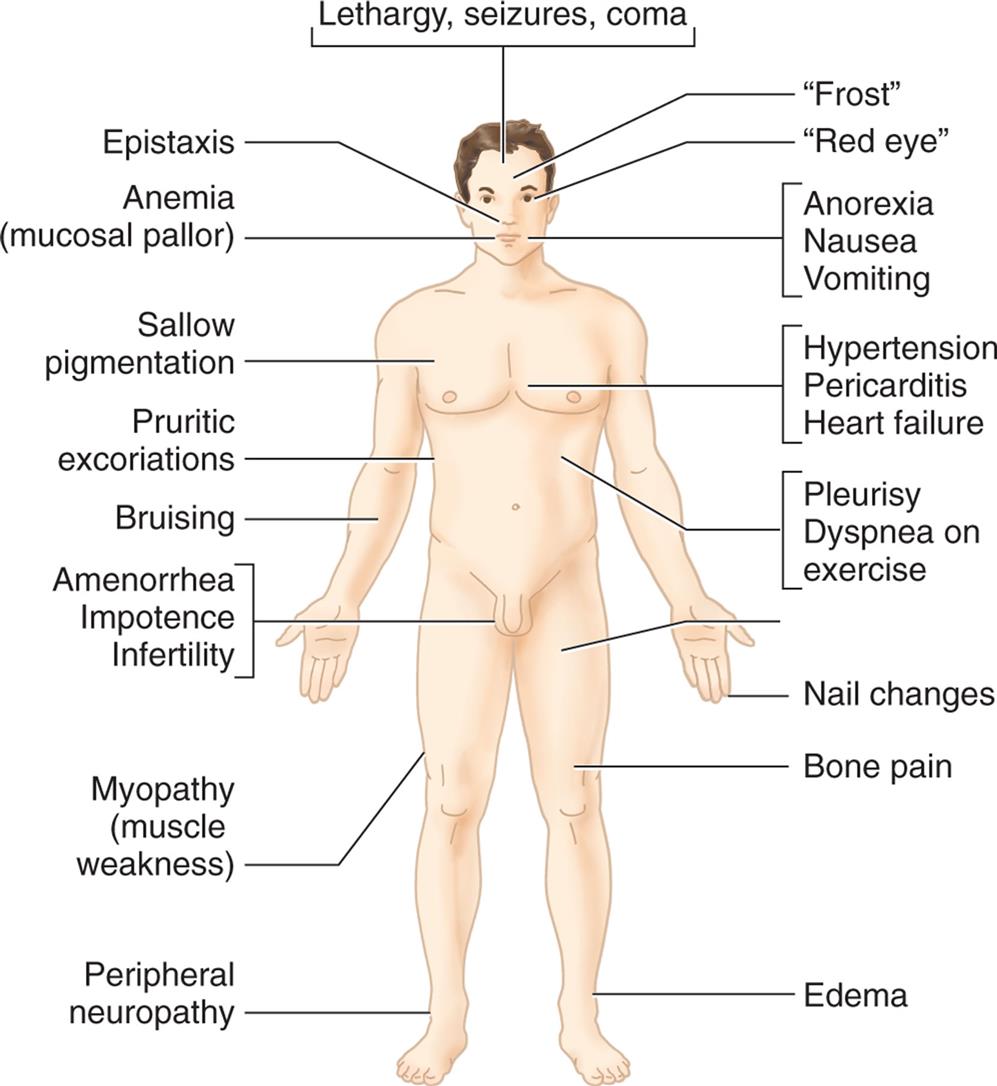
An illustration of the anterior view of the human body identifies the common signs and symptoms of kidney dysfunction, clockwise from the top: lethargy, seizures, coma; frost; red eye; anorexia, nausea, vomiting; hypertension, pericarditis, heart failure; pleurisy, dyspnea on exercise; nail changes; bone pain; edema; peripheral neuropathy; myopathy (muscle weakness); amenorrhea, impotence, infertility; bruising; pruritic excoriations; sallow pigmentation; anemia (mucosal pallor); and epistaxis.
Creatinine, Cystatin C, and Urea Clearance
Creatinine is constantly released from muscle and excreted primarily by glomerular filtration. In CKD, as GFR declines, the SCr level increases by a reciprocal amount to maintain a constant rate of excretion (Fig. 38.14). With continuing decline in GFR, the plasma creatinine concentration increases. Measures of SCr can serve as an index of changing glomerular function. However, SCr as an estimate of GFR is limited when there is reduced muscle mass or fluid overload. Equations including cystatin C or combined with SCr provide a better index. The clearance of urea follows a similar pattern, but urea is both filtered and reabsorbed. The measured urea level varies with the state of hydration; therefore, urea concentration is not a good index of GFR. However, as the GFR decreases, plasma urea concentration increase.
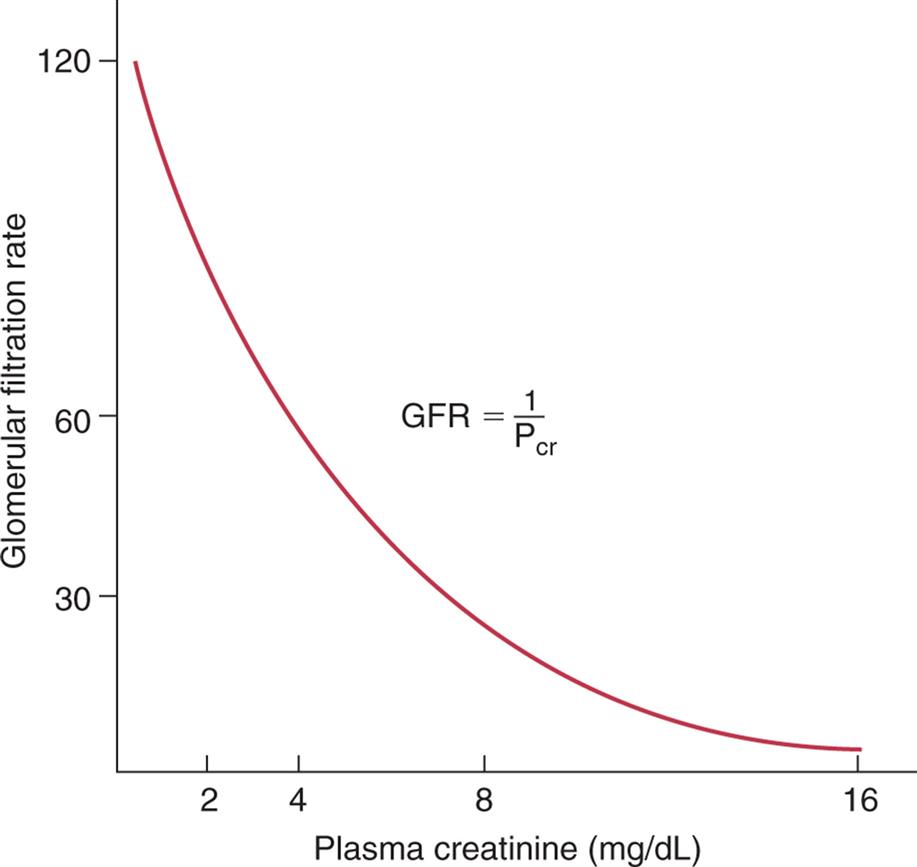
A graph plots glomerular filtration rate against plasma creatinine (in milligrams per deciliter). The horizontal axis ranges from 0 to 16, and the vertical axis ranges from 0 to 120. A concave-up downward sloping curve represents the equation G F R equals 1 over P sub c r.
Fluid and Electrolyte Balance
Fluid and electrolyte and acid-base balances are significantly disturbed with CKD.80 A summary of electrolyte and acid-base balance alterations is presented in Table 38.17.
Table 38.17
Levels of sodium must be regulated within narrow limits because sodium is the major extracellular solute. In CKD, sodium and water balance is maintained very close to normal until the development of stage V ESRD (see Table 38.14). This occurs because of the increased fractional excretion of sodium, particularly in the distal nephron, in relation to decreasing GFR. Hormones including aldosterone, prostaglandins, and natriuretic peptides also modulate sodium excretion, and their levels are elevated with progressive kidney disease. Individual variation in the underlying pathology of CKD must be considered in the management of sodium intake or restriction. Sodium wasting may be present with tubulointerstitial causes of CKD, and there may also be extra renal losses of sodium from vomiting, diarrhea, or fever. Sodium retention is more likely in ESRD particularly in the presence of nephrotic syndrome or heart failure. Sodium retention contributes to hypertension, edema, heart failure, and mortality. Interdialytic water intake can cause volume overload and dilutional hyponatremia prior to dialysis. Management of salt and water balance requires individual assessment, and both hyponatremia and hypernatremia require management.81,82
The regulation of water balance and osmolality is normally achieved by urinary concentration mediated by antidiuretic hormone (ADH). As GFR is reduced, the ability to concentrate and dilute the urine diminishes. In earlier stages of renal disease, this may be caused by osmotic diuresis produced by increased fractional excretion of solutes by the remaining functioning nephrons or by a decreased tubular response to ADH. Individual nephrons can maintain water balance until severe renal failure occurs and GFR declines to 15% to 20% of normal with extensive loss of nephron and tubular function. At this stage, the urinary concentration becomes fixed and approaches that of the plasma at 285 mOsm/L with a specific gravity of about 1.010.
Urinary excretion of potassium is related primarily to distal tubular secretion mediated by aldosterone and sodium-potassium adenosine triphosphatase (see Chapter 3). In renal failure, there is increased tubular secretion that provides effective regulation until the onset of oliguria. With hyperkalemia, larger amounts of potassium can be eliminated through the bowel.83 Although nonoliguric patients can maintain potassium excretion with normal dietary intake, they are more prone to develop hyperkalemia with increased loading (i.e., use of salt substitutes). Use of potassium-sparing diuretics, such as spironolactone (aldactone), volume depletion, acute infection, severe acidosis, or marked hyperglycemia also may precipitate elevated levels of serum potassium. With progression of disease to ESRD, total body potassium can increase to life-threatening levels and must be controlled by dietary restriction, loop diuretics, cation exchange resins, and dialysis. Sodium polystyrene sulfonate (a nonspecific sodium-cation exchange resin in the colon), patiromer sorbitex calcium (exchanges calcium for potassium in the colon), and sodium zirconium cyclosilicate (traps potassium in the colon) are effective in managing hyperkalemia.84 Hyperkalemia also can be a side effect of ACE inhibitors or angiotensin receptor blockers (ARB) often used to prevent diabetic nephropathy. Severe acute hyperkalemia is treated with intravenous 10% calcium gluconate or 10% calcium chloride, intravenous dextrose, and insulin, and nebulized or intravenous salbutamol (sympathetic beta2 agonist, promotes Na+-K+-ATPase pump and intracellular movement of potassium). Renal replacement therapy may be required to manage hyperkalemia (support of renal function using hemodialysis or peritoneal dialysis).
The intake of a normal diet produces 50 to 100 mEq of hydrogen per day. These ions are secreted from the renal tubules and excreted in the urine combined with phosphate and ammonia buffers (buffering is described in Chapter 3). Metabolic acidosis (Chapter 3) develops when the GFR decreases to less than 20% to 25% of normal. The causes of acidosis are primarily related to decreased hydrogen ion elimination and decreased bicarbonate reabsorption. With ESRD, metabolic acidosis may be severe enough to require alkali therapy and dialysis.85
Calcium, Phosphate, Magnesium, and Bone
Bone and skeletal changes develop with alterations in calcium, phosphate, magnesium, and vitamin D metabolism (Table 38.18).86 These changes begin when the GFR decreases to 25% or less. Hypocalcemia is accelerated by impaired renal synthesis of 1,25-dihydroxy-vitamin D3 (calcitriol) with decreased intestinal absorption of calcium. Renal phosphate excretion also decreases, and the increased serum phosphate binds calcium, further contributing to hypocalcemia. Acidosis also contributes to a negative calcium balance. Decreased serum calcium level stimulates parathyroid hormone secretion with mobilization of calcium from bone. The combined effect of secondary hyperparathyroidism and vitamin D deficiency can result in renal osteodystrophies (i.e., osteomalacia and osteitis fibrosa with increased risk for fractures) (see Chapter 44) and vascular calcification, including coronary artery calcification. Fractional excretion of magnesium increases as compensation for the decline in GFR with the progression of CKD. With ESRD, fractional excretion of magnesium is inadequate and serum magnesium levels must be monitored considering dietary intake, intestinal absorption, alterations in parathyroid hormone, and effects of different medications. Generally, there is a tendency to develop hypermagnesemia. However, hypomagnesemia can develop in some cases and requires modification of the dialysate magnesium concentration.87 Hypomagnesemia has been associated with osteoporosis in postmenopausal women,88 but the effects on bone in ESRD are not clearly known.
Table 38.18
CaHPO4, Calcium hydrogen phosphate; PTH, parathyroid hormone.
Protein, Carbohydrate, and Fat Metabolism
Protein, carbohydrate, and fat metabolism are altered in CKD. Proteinuria, metabolic acidosis, inflammation, and a catabolic state contribute to a negative nitrogen balance. Levels of serum proteins diminish, including albumin, complement, and transferrin, and there is loss of muscle mass. The amount of proteinuria is also related to the extent of renal injury and predicts disease progression. Proteinuria may independently cause renal damage by promoting tubular inflammation and fibrosis from particular protein-bound fatty acids.89 Monitoring of proteinuria using the urine albumin-to-creatinine ratio can assist in staging CKD.90
Insulin resistance and glucose intolerance are common and may be related to proinflammatory cytokines and alterations in adipokines (high leptin and low adiponectin levels) that interfere with insulin action. In both nondiabetic and diabetic persons with CKD, oxidative stress can contribute to renal tubular and vascular injury as antioxidant systems become inadequate.91
Dyslipidemia is common among individuals with CKD. There is a high ratio of low-density lipoprotein (LDL) to high-density lipoprotein (HDL), a high level of triglycerides, and an accumulation of LDL particles with accelerated atherosclerosis and vascular calcification. Uremia causes a deficiency in lipoprotein lipase and a decreased level of hepatic triglyceride lipase. Decreased lipolytic activity results in a reduction and protection effects of the HDL level. Apolipoprotein concentration also is elevated, thereby accelerating atherogenesis.92
Hematologic System
Hematologic alterations include normochromic-normocytic anemia, impaired platelet function, and hypercoagulability. Inadequate production of erythropoietin decreases red blood cell production and causes anemia. Chronic inflammation, iron deficiency, and decreased half-life of erythrocytes are also contributing factors. Anemia contributes to decreased tissue oxygenation and to progression of kidney disease. Low serum levels of hemoglobin and symptoms of anemia, such as lethargy, weakness, and dizziness, are common findings. Treatment of anemia includes infusion of erythropoiesis stimulating agents (ESAs) (i.e., recombinant human erythropoietin), and intravenous iron. Hypoxia-inducible factor-prolyl hydroxylase inhibitors, oral agents that stimulate endogenous erythropoietin production and enhance iron availability, are in clinical trials and may replace ESAs.93
Disorders of hemostasis in CKD are primarily related to defective platelet aggregation, impaired adhesion of platelets to the vascular endothelium, and alterations in coagulation factors and in the fibrinolytic pathway. The consequence is either (1) an increased bleeding tendency (more common with later stages of CKD) manifested by bruising, epistaxis and other mucosal bleeding, gastrointestinal bleeding, and cerebrovascular hemorrhage or (2) excessive formation of thrombi (e.g., deep vein thrombosis, pulmonary embolism, and cardiovascular events), which is more common in earlier stages of CKD.94
Cardiovascular System
Cardiovascular disease is a major cause of morbidity and mortality in CKD. Proinflammatory cytokines, oxidative stress, metabolic derangements, dyslipidemia, uremic toxins, and anemia are significant contributors.
Hypertension is the result of excess sodium and fluid volume and arteriosclerosis. Endothelial cell dysfunction and calcium deposits lead to a loss of vessel elasticity and vascular calcification. Elevated renin concentration also stimulates the secretion of aldosterone, increasing sodium and water reabsorption.
Dyslipidemia promotes atheromatous plaque formation (see section on Protein, Carbohydrate and Fat Metabolism). The resulting vascular disease increases the risk for ischemic heart disease, left ventricular hypertrophy, congestive heart failure, stroke, and peripheral vascular disease in individuals with uremia.95
Pericarditis can develop from pericardial inflammation caused by the presence of uremic toxins. Accumulation of fluid in the pericardial space can compromise ventricular filling and cardiac output.96
Congestive heart failure can develop from fluid overload, hypertension, cardiac remodeling with hypertrophy and fibrosis (cardiorenal syndrome).97
Anemia increases demands for cardiac output and adds to the cardiac workload.
Pulmonary System
Pulmonary complications are associated with fluid overload causing pulmonary edema, congestive heart failure, and dyspnea. Pulmonary edema leads to respiratory acidosis causing Kussmaul respirations. Pulmonary hypertension is associated with left ventricular dysfunction or uremic-related pulmonary vascular changes.98
Immune System
Immune system dysregulation develops with the uremia of CKD.Chemotaxis, phagocytosis, antibody production, and cell-mediated immune responses are suppressed. Malnutrition, metabolic acidosis, and hyperglycemia may amplify immunosuppression. Release of inflammatory cytokines results in systemic inflammation. Failure of antioxidant systems also promotes inflammation. There are deficient responses to vaccination, increased risk for infection, and virus-associated cancers (e.g., human papillomavirus, hepatitis B and C viruses, Epstein–Barr virus).99
Neurologic System
Neurologic symptoms are common and progressive with CKD. Symptoms are related to dysfunction of lower motor and sensory neurons associated with uremic toxicity, chronic hyperkalemic depolarization, and anemia. The symptoms may include headache, pain, drowsiness, sleep disorders, impaired concentration, memory loss, and impaired judgment (known as uremic encephalopathy). In advanced stages of kidney failure, symptoms may progress to seizures and coma. Neuromuscular irritation can cause hiccups, muscle cramps, and muscle twitching. Peripheral neuropathies associated with uremic toxins also can develop with impaired sensations, particularly in the lower limbs causing decreased tendon reflexes, muscle weakness, and muscle atrophy. Symptoms improve with renal replacement therapy (i.e., hemodialysis or peritoneal dialysis) or kidney transplant.
Gastrointestinal System
Gastrointestinal complications are common in individuals with CKD. Uremic gastroenteritis can cause bleeding ulcer and significant blood loss. Nonspecific symptoms include anorexia, nausea, vomiting, constipation, or diarrhea. Uremic fetor is a form of bad breath caused by the breakdown of urea by salivary enzymes. Malnutrition is common.
Endocrine and Reproductive Systems
Endocrine and reproductive alterations develop with progression of CKD. Both males and females have a decrease in levels of circulating sex steroids. Males often experience a reduction in testosterone levels and may be impotent. Oligospermia and germinal cell dysplasia can result in infertility. Females have reduced estrogen levels, amenorrhea, anovulation, difficulty maintaining a pregnancy to term, and early menopause.100–102
Insulin resistance is common in uremia, and as CKD progresses, the ability of the kidney to degrade insulin is reduced and the half-life of insulin is prolonged. Individuals with diabetes mellitus and CKD need to carefully manage their insulin dosages.
CKD also causes alterations in thyroid hormone metabolism, particularly hypothyroidism, known as nonthyroidal illness syndrome. Uremia delays the response of thyroid-stimulating hormone receptors, and triiodothyronine (T3) levels are often low.
Integumentary System
Skin changes are associated with other complications that develop with CKD. Anemia can cause pallor and bleeding into the skin and results in hematomas and ecchymosis. Retained urochromes manifest as a sallow skin color. Hyperparathyroidism and uremic skin residues (known as uremic frost) are associated with inflammation, irritation, and pruritus with scratching, excoriation, and increased risk for infection. Half-and-half nails (half white and half red or brown) are common.
Evaluation and Treatment
Early screening and evaluation of CKD is based on risk factors, health history, presenting signs and symptoms, and diagnostic testing. Decreased GFR (< 80) with elevated SCr and BUN concentrations are consistent with CKD. Markers of kidney damage include measurement of urine protein level, particularly albumin, and examination of urine sediment. Imaging will show small kidney size, and renal biopsy confirms the diagnosis.
Management involves promotion of adequate caloric intake with dietary restriction of protein, sodium, potassium, and phosphate. Supplementation with vitamin D or vitamin D receptor activators aids in the management of hyperphosphatemia. Maintenance of sodium and fluid balance may require fluid restriction. Management of dyslipidemias includes both pharmacological (statins and fibrates) and nonpharmacological measures (lifestyle and behavioral modifications). Erythropoietin can be used as needed to increase red blood cells. ACE inhibitors or ARBs are often used to control systemic hypertension, reduce proteinuria, provide renoprotection, and prevent progressive renal damage. CKD related to diabetic nephropathy can be significantly reduced with glycemic control. SGLT2 inhibitors are nephroprotective and provide blood pressure and hemodynamic regulation, protection from lipotoxicity, uric acid control, anti-inflammatory actions, and renal gluconeogenesis inhibition.103 ESRD is treated with conservative care, CRRT, supportive therapy, and renal transplantation.
Summary Review
Urinary Tract Obstruction
- 1. Obstruction can occur anywhere in the urinary tract. It may be anatomic (structural) or functional. Obstruction impedes flow and dilates structures proximal to the blockage, increases risk for infection, and compromises renal function.
- 2. Upper urinary tract obstructions are caused by kidney stones or tumors within the kidney; or compression from a tumor, stone, or fibrosis along the ureter or at the ureter-bladder junction,
- 3. Complications of upper urinary tract obstruction include hydronephrosis, hydroureter, ureterohydronephrosis, and tubulointerstitial fibrosis.
- 4. Hypertrophy and hyperfunction of the unobstructed kidney compensate for loss of function of the kidney with obstructive disease.
- 5. Relief of obstruction is usually followed by postobstructive diuresis and may cause fluid and electrolyte imbalance.
- 6. Kidney stones are caused by supersaturation of the urine with precipitation of stone-forming substances and changes in urine pH.
- 7. Stones can be located in the kidneys, ureters, and urinary bladder. The most common kidney stone is formed from calcium oxalate.
- 8. LUT obstructions include both structural or anatomic disorders and alterations in neurologic function (neurogenic bladder), or both. They are related to urine storage and emptying.
- 9. Anatomic causes of resistance to urine flow include urethral stricture, prostatic enlargement in men, pelvic prolapse in women, and tumor compression.
- 10. Partial obstruction of the bladder can result in an increase in the force of detrusor contraction. If obstruction persists, there is deposition of collagen in the bladder wall over time, resulting in decreased bladder wall compliance and ineffective detrusor muscle contraction.
- 11. OAB is a dysfunction of urine storage with uncontrollable or premature contraction of the bladder that results in urgency with or without incontinence, frequency, and nocturia.
- 12. UAB syndrome is a voiding dysfunction resulting in prolonged bladder emptying or a failure to achieve complete bladder emptying, or both, within a normal time span.
- 13. A neurogenic bladder is caused by a neural lesion that interrupts innervation of the bladder.
- 14. Upper motor neuron lesions above the pontine micturition center result in overactive (hyperreflexive) bladder function and bladder dyssynergia (loss of coordinated neuromuscular contraction).
- 15. Lesions in the sacral area of the spinal cord or peripheral nerves result in underactive, hypotonic, or atonic bladder function.
- 16. Detrusor sphincter dyssynergia is failure of the urethrovesical junction smooth muscle to release urine during bladder contraction and causes a functional obstruction.
- 17. Kidney (renal) tumors include RCC (the most common), RTCC s and renal adenomas. RCC can metastasize to the lung, lymph nodes, liver, bone, thyroid gland, and central nervous system.
- 18. Bladder tumors are commonly composed of transitional cells with a papillary appearance and a high rate of recurrence
Urinary Tract Infection
- 1. UTIs are commonly caused by the retrograde movement of bacteria into the urethra and bladder and can ascend to the kidney. UTIs are uncomplicated when the urinary system is normal or complicated when there is an abnormality. Types of UTI include cystitis, interstitial cystitis, and acute or chronic pyelonephritis.
- 2. Host defenses that protect against UTI include high osmolality and acidic pH of urine, mucus, uromodulin, and other antimicrobial proteins that activate the immune response, sphincters that prevent reflux, and urine flow that washes out bacteria.
- 3. Virulent uropathogens have pili or fimbriae, or both, that promote binding to the uroepithelium and retrograde movement in the urinary tract. Formation of biofilms enhances colonization and resists host defenses and antimicrobial therapy.
- 4. Cystitis is an inflammation of the bladder commonly caused by bacteria and may be mild, hemorrhagic, suppurative, ulcerative, or gangrenous.
- 5. Interstitial cystitis/painful bladder syndrome is a bladder pain disorder associated with LUT symptoms of more than 6 weeks’ duration in the absence of infection or other identifiable causes. It is likely related to an inflammatory autoimmune reaction with a derangement of the glycosaminoglycan layer of the bladder mucosa that makes it more susceptible to penetration by bacteria and noxious urinary solutes.
- 6. Acute pyelonephritis is an inflammation of the upper urinary tracts (ureter, renal pelvis, and kidney interstitium). Infection due to urinary obstruction and reflux of urine from the bladder are the most common underlying risk factors. Severe infections may cause localized abscess formation.
- 7. Chronic pyelonephritis is an acute or chronic inflammation of the renal pelvis often related to ascending infection and obstructive uropathies leading to scarring of one or both kidneys and alteration in renal function. Untreated, chronic pyelonephritis can lead to kidney failure.
Glomerular Disorders
- 1. Glomerular disorders are a group of related diseases of the glomerulus that can be primary and caused by immune injury, infection, ischemia, toxins or drugs, or vascular disorders or, secondarily, caused by systemic diseases.
- 2. Acute glomerulonephritis commonly results from inflammatory damage to the glomerulus as a consequence of immune reactions including deposition of circulating immune complexes, antibodies reacting in situ to planted antigens, antibodies directed against the glomerular basement membrane, and complement activation.
- 3. Chronic glomerulonephritis is related to a variety of diseases that cause deterioration of the glomerulus and a progressive course leading to chronic kidney failure.
- 4. Diabetic nephropathy is the most common cause of glomerular injury progressing to CKD and is related to chronic hyperglycemia, inflammatory mediators, and microvascular and macrovascular complications.
- 5. Lupus nephritis is caused by the formation of autoantibodies against double-stranded DNA and nucleosomes with glomerular deposition of the immune complexes or formation of immune complexes against planted antigens in the glomerulus.
- 6. Nephrotic syndrome is the excretion of at least 3.5 g of protein (primarily albumin) in the urine per day primarily because of glomerular injury with increased capillary permeability and loss of membrane negative charge.
- 7. Nephritic syndrome is characterized by hematuria and red blood cell casts with less severe proteinuria.
- 8. The manifestations of both nephrotic and nephritic syndrome include edema, hypoproteinemia, proteinuria, hyperlipidemia, lipiduria, vitamin D deficiency, and hypothyroidism.
Acute Kidney Injury
- 1. AKI is a sudden decline in kidney function with a decrease in GFR and urine output and with an elevation in plasma creatinine and BUN levels.
- 2. Prerenal AKI is caused by decreased renal perfusion with ischemia, decreased GFR, and tubular necrosis.
- 3. Intrarenal AKI is associated with several systemic diseases but is commonly related to acute tubular necrosis.
- 4. Postrenal kidney injury is associated with diseases that obstruct the flow of urine from the kidneys.
- 5. Oliguria is urine output that is less than 400 mL/24 h and can be caused by alterations in renal blood flow, tubular obstruction, or tubular fluid backleak, or by a combination of these events.
Chronic Kidney Disease
- 1. CKD is the progressive loss of renal function for 3 months or more, with implications for health. Plasma creatinine levels gradually become elevated as GFR declines; sodium is lost in the urine; potassium is retained; acidosis develops; calcium, phosphate, magnesium, and vitamin D metabolism are altered; and erythropoietin production is diminished. All organ systems are affected by CKD.
- 2. Symptomatic changes usually do not become evident until renal function declines to less than 25%.
- 3. Glomerular hypertension, hyperfiltration, and chronic inflammation, oxidative stress, and fibrosis contribute to the progression of CKD. Proteinuria and angiotensin II promote the pathologic changes of chronic renal injury.
- 4. Uremic syndrome is a proinflammatory state with the accumulation of urea and other nitrogenous compounds as well as toxins and alterations in fluid, electrolyte, and acid-base balance that result from chronic kidney failure.
- 5. All organ systems are affected and contribute to disease symptoms.