Routine Urinalysis–the Microscopic Exam of Urine Sediment
After studying this chapter, the student should be able to:
- 1. Discuss the importance of standardizing the microscopic examination of urine and describe how this standardization is achieved in the clinical laboratory.
- 2. Describe microscopic and staining techniques used to enhance visualization of the formed elements in urinary sediment.
- 3. Describe the microscopic appearance and clinical significance of erythrocytes and leukocytes in urine and correlate their presence with the physical and chemical examination of urine.
- 4. Describe the microscopic characteristics and location of each type of epithelium found in the urinary tract, that is, squamous, transitional, and renal tubular epithelium (proximal, distal, and collecting duct).
- 5. Summarize briefly the clinical significance of increased sloughing of the urinary tract epithelium.
- 6. Describe the formation, composition, and clinical significance of urinary cast formation.
- 7. State the categories into which casts are classified, discuss the clinical circumstances that result in the formation of each cast type, and correlate the presence of casts with the physical and chemical examination of urine.
- 8. Describe the development of urinary crystals, including at least three factors that influence their formation.
- 9. Describe the characteristic form of each major type of urinary crystal; categorize each crystal type as being found in acid, neutral, or alkaline urine; and discuss the clinical significance of each crystal type.
- 10. Identify the following formed elements found in urine sediment, and discuss their clinical significance:
Key Terms⁎ *1
- casts
- clue cells
- collecting duct cells
- crystals
- cytocentrifugation
- crystalluria
- distal convoluted tubular cells
- iatrogenic
- KOH preparation
- lipiduria
- Maltese cross pattern
- oval fat bodies
- proximal convoluted tubular cells
- Prussian blue reaction (also called the Rous test)
- squamous epithelial cells
- Tamm-Horsfall protein
- transitional (urothelial) epithelial cells
- uromodulin
The standardized quantitative microscopic examination of urine sediment made its clinical laboratory debut in 1926. At that time, Thomas Addis developed a procedure to quantify formed elements in a 12-hour overnight urine collection. The purpose of this test, the Addis count, was to follow the progress of renal diseases, particularly acute glomerulonephritis. Increased numbers of red blood cells (RBCs), white blood cells (WBCs), or casts in the urine indicated disease progression. A disease process was indicated when one or more of the following cell changes occurred: The number of RBCs exceeded 500,000; the number of WBCs exceeded 2 million; or the number of casts exceeded 5000. Because the Addis count was time-consuming and chemical methods are currently available to monitor the progression of renal disease, it is no longer routinely performed despite its ability to accurately detect changes in the excretion of urinary formed elements. However, microscopic examination of urine sediment continues to play an important role in the initial diagnosis as well as in monitoring renal disease.
Standardization of Sediment Preparation
Ensuring the accuracy and precision of the urine microscopic examination requires standardization. This demands that established laboratory protocols for manually preparing the urine sediment, including using the same supplies, step sequences, timing intervals, and equipment, are adhered to by all personnel. Box 7.1 lists various factors that must be established and followed to obtain standardization in the microscopic examination. Note that all personnel must follow all testing aspects consistently to ensure comparable urinalysis results.
Commercial Systems
To achieve consistency, several commercial urinalysis systems are available (Table 7.1). Each system seeks to consistently (1) produce the same concentration of urine or sediment volume; (2) present the same volume of sediment for microscopic examination; and (3) control microscopic variables such as the volume of sediment viewed and the optical properties of the slides. All of these systems surpass the outdated practice of using a drop of urine on a glass slide and covering it with a coverslip. In addition, commercial slides are cost competitive, easy to adapt to, and necessary to ensure reproducible and accurate results.
Table 7.1
| Features | Count-10 System (Myers-Stevens Group) | KOVA System (Hycor Scientific) | UriSystem Features (Fisher HealthCare) |
|---|---|---|---|
| Initial volume of urine used | 12 mL | 12 mL | 12 mL |
| Final urine volume with sediment | 0.8 mL | 1.0 mL | 0.4 mL |
| Sediment concentration | 15:1 | 12:1 | 30:1 |
| Volume of sediment used | 6 µL | 6 µL | 14 µL |
| Area for viewing | 36 mm2 | 32 mm2 | 90 mm2 |
| Number of 100× fieldsa | 11 | 10 | 28 |
| Number of 400× fieldsa | 183 | 163 | 459 |
| Coverslip type | Acrylic | Acrylic | Acrylic |
| Number of specimens per slide | 10 | 4, 10 | 10 |
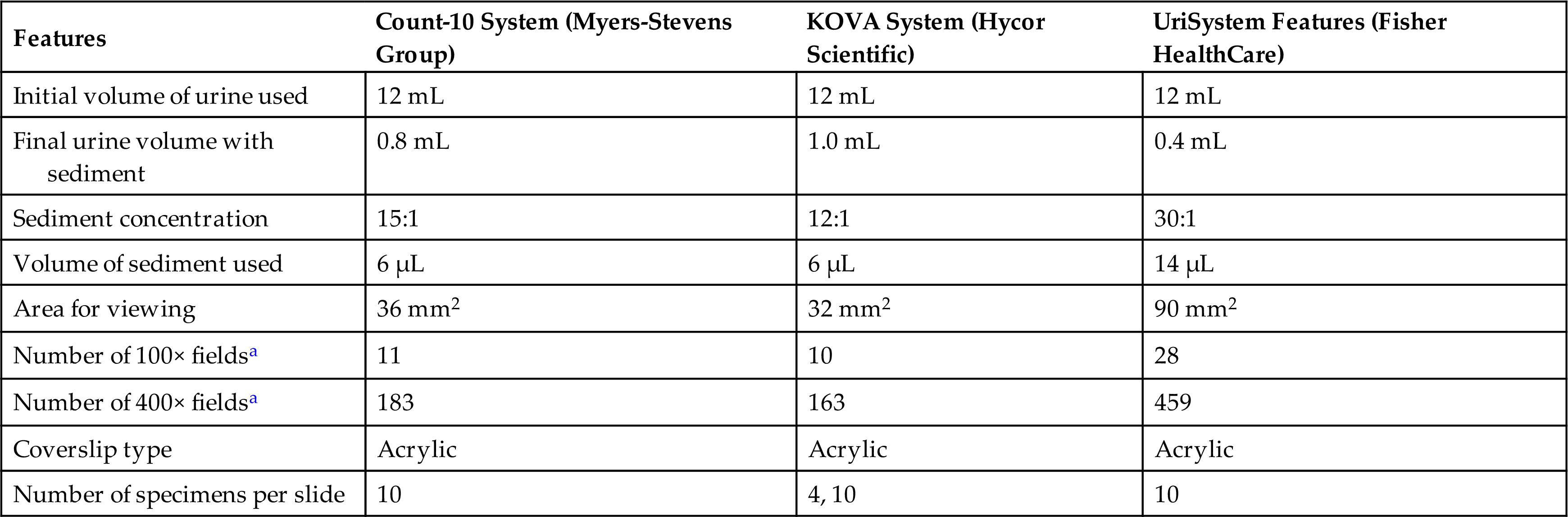
aCalculated using a “field of view” diameter for high power (×400) of 0.5 mm and for low power (×100) of 2 mm. The number of fields possible is equal to the area for viewing divided by the area per low- or high-power field. Note that the field of view diameter is determined by the lens systems of the microscope.
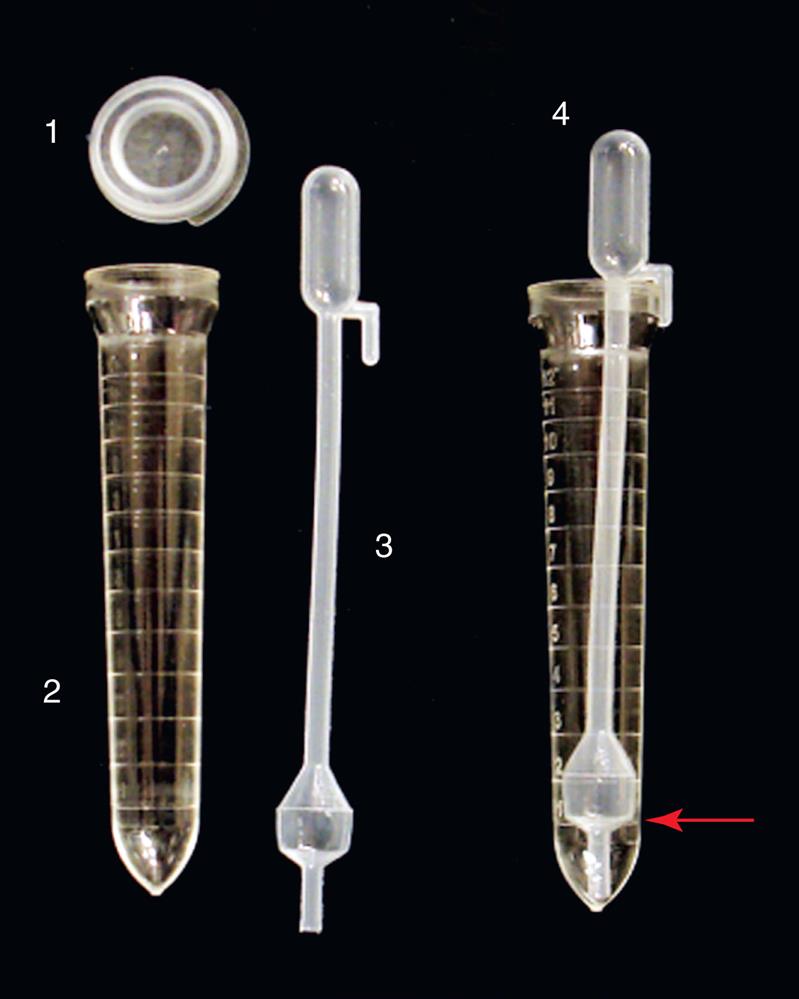
Commercial systems feature disposable plastic centrifuge tubes with gradations for consistent urine volume measurement (Fig. 7.1). The tubes are clear, allowing for assessment of urine color and clarity, and conical, which facilitates sediment concentration during centrifugation. The centrifuge tubes of each commercial system are unique. The UriSystem tube (Fisher HealthCare, Houston, TX) is designed such that after centrifugation, it can be decanted with a quick, smooth motion and consistently retains 0.4 mL of urine for sediment resuspension. The KOVA System (Hycor Biomedical, Garden Grove, CA) uses a specially designed pipette (KOVA Petter) that snuggly fits the diameter and shape of the tube to retain 1 mL of urine during decanting. The Count-10 System (V-Tech, Inc., Lake Mary, FL) offers several options to retain 0.8 mL for sediment resuspension. Each commercial system provides tight-fitting plastic caps for the tubes to prevent spillage and aerosol formation during centrifugation.
A laboratory need not purchase all aspects of a commercial system to obtain a standardized urine sediment for microscopic analysis. In fact, laboratories have considerable flexibility and can blend the systems. For example, a laboratory could choose to use KOVA System tubes to prepare the urine sediment but UriSystem slides or the RS2005 Urine Sediment Workstation (DiaSys Ltd., New York, NY), a semiautomated slide system, to view the sediment. Regardless of the system or combination of products used when preparing and performing the microscopic examination of urine sediment, the imperative is that all personnel adhere to established protocols to ensure that accurate and reproducible results are obtained.
Specimen Volume
A concentrated urine sediment is usually prepared for the microscopic examination. To ensure that a representative sampling of the formed elements in the portion is removed, the urine specimen must be well mixed. The concentrated sediment can be prepared using a variety of initial urine volumes. Frequently, the initial volume of urine is 12 mL with a 12 to 1 concentration (12:1) of sediment prepared for microscopic viewing. However, initial urine volumes ranging from 3 to 15 mL can be used.
Testing using alternate volumes when insufficient specimen is available can be achieved in two ways. One approach is to prepare the same sediment concentration by decreasing the volume of supernatant urine used to resuspend the sediment. For example, suppose the procedure details a 12:1 concentrated sediment (i.e., 12 mL initial urine with the sediment resuspended in 1 mL supernatant urine). When only 6 mL initial urine is available, the procedure is followed but the urine sediment would be resuspended in 0.50 mL—one half the volume used with 12 mL initial urine—to obtain a 12:1 concentration.
A second approach is to reduce the initial volume of urine used and multiply all numeric counts by the appropriate factor. For example, assume as previously that the procedure details a 12:1 concentration of the sediment. When only 6 mL is used, the procedure is followed and all numeric counts from the microscopic exam of the sediment are multiplied by two.
When insufficient urine is available for testing (e.g., <3 mL), well-mixed urine may be examined unconcentrated and an appropriate comment appended to the report. This comment will enable healthcare providers to evaluate the results appropriately. Note that the laboratory reference ranges for a routine microscopic exam will not apply to this unconcentrated sample.
Whenever the actual volume used to prepare the sediment for the microscopic examination is less than that routinely required, a notation should accompany the specimen report. The decision to accept specimens with volumes less than 12 mL for urinalysis, as well as the protocol used for testing, is determined by each individual laboratory.
Centrifugation
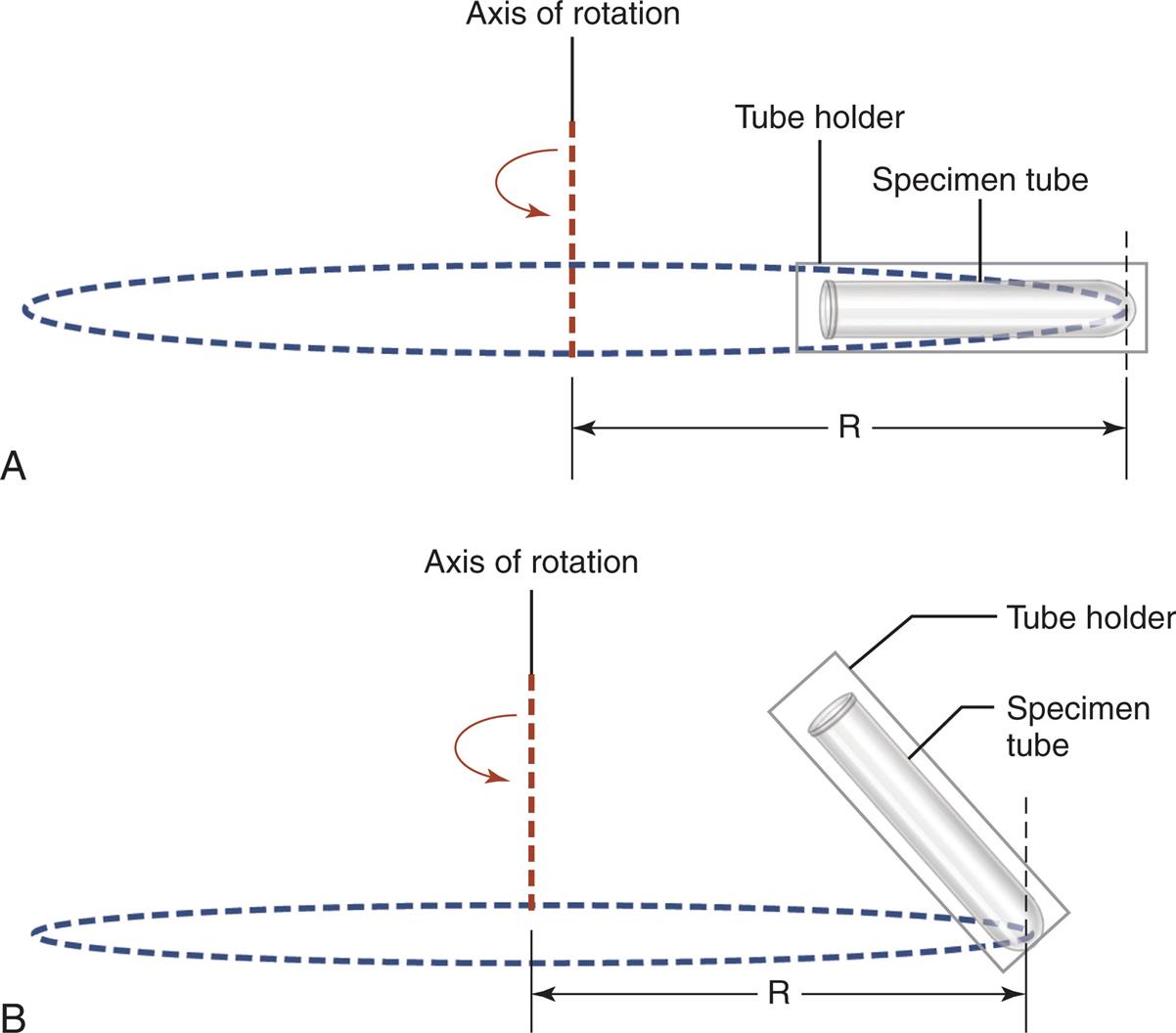
After well-mixed urine is poured into a centrifuge tube, it is covered and centrifuged at 400 to 450 g for 5 minutes. This centrifugation speed allows for optimal sediment concentration without damaging fragile formed elements such as cellular casts. All personnel must adhere to this 5-minute centrifugation time with all specimens to ensure uniformity. Note that the speed is given in relative centrifugal force (RCF, g) because this term is not dependent on the centrifuge used. In contrast, the speed in revolutions per minute (RPM) required to obtain 400 to 450 g varies with each centrifuge and is directly dependent on the rotor size. For example, one centrifuge may obtain 450 × g at 1200 RPM, whereas another may require 1500 RPM to obtain this same g-force. The RPM necessary to achieve 400 to 450 g can be determined from a nomogram or by using Equation 7.1.
 Equation 7.1
Equation 7.1
In this equation, the radius in centimeters refers to the distance from the center of the rotor to the outermost point of the cup, tube, or trunnion when the rotor is in motion (Fig. 7.2).
It is important that the centrifuge brake is not used because this will cause the sediment to resuspend, resulting in erroneously decreased numbers of formed elements in the concentrated sediment. In many laboratories, multiple personnel use centrifuges to perform numerous and varied procedures. If all centrifuge settings, including the brake, are not checked before use, the resultant urine sediments can show dramatic variations in their formed elements because of processing differences in speed, time, or braking. Using control materials for the microscopic examination or performing interlaboratory duplicate testing is valuable in its ability to detect these important changes in sediment preparation.
Sediment Concentration
After centrifugation, the covered urine specimens should be carefully removed and the sediments concentrated using the established protocol. Standardized commercial systems accomplish this task through consistent retention of a specific volume of urine. Note that different brands of centrifuge tubes and pipettes should not be intermixed. This can cause variation in the volume of urine retained, which will change the concentration of the sediment. Table 7.1 shows how commercial systems vary in the sediment concentration produced, ranging from a 12:1 to a 30:1 concentration. Manual techniques traditionally strive toward a 12:1 concentration, in which 12 mL initial urine was used; therefore supernatant urine is removed by decanting or using a pipette until 1 mL of urine is retained. Then, a pipette is used to gently resuspend the sediment. Note that too vigorous agitation of the sediment can cause fragile and brittle formed elements, such as RBC casts and waxy casts, to break into pieces.
Volume of Sediment Viewed
A standardized slide should be used for the microscopic examination of urine sediment to ensure that the same volume of sediment is presented for viewing each time. Commercial standardized slides are made of molded plastic and have a built-in coverslip or provide a glass coverslip for use (Fig. 7.3). With a disposable transfer pipette, urine sediment is presented to a chamber, which fills by capillary action. This technique facilitates uniform distribution of the formed elements throughout the viewing area of the slide.
Glass microscope slides and coverslips are not recommended because they do not yield standardized, reproducible results.1 If glass slides are used, the laboratorian should always pipette an exact amount (e.g., 15 μL) of the resuspended sediment onto the glass slide using a calibrated pipette. The volume of sediment dispensed is determined by each laboratory and depends on the size of the coverslip used. The urine sediment volume must fill the entire area beneath the coverslip without excess. Bubbles and uneven distribution of the sediment components can result when the coverslip is applied (e.g., heavier components such as casts are pushed or concentrate near the coverslip edges). If the microscopic examination reveals that the distribution of formed elements is uneven, a new suspension of the sediment should be prepared for viewing. Because all commercial systems have proved superior to the “drop on a slide” method, this technique should not be used for the microscopic examination of urine.2
Reporting Formats
In a manual microscopic examination, urine components are assessed or enumerated using at least 10 low-power (lpf) or 10 high-power fields (hpf). The quantity of some components (e.g., mucus, crystals, bacteria) is qualitatively assessed per field of view (FOV) in descriptive or numeric terms. Table 7.2 lists commonly used terms and typical descriptions. Each laboratory determines which terms are used, as well as the definition for each term. Other sediment components (RBCs, WBC, casts) are enumerated as a range of formed elements present (e.g., 0–2, 3–5, 6–10). Note that although a component may be reported using low-power magnification, high-power magnification may be needed to specifically identify or categorize it; for example, to identify the cell type in a cellular cast. In this case the cells were determined to be RBCs, and the quantity of cellular casts present is reported as the average number viewed using low power (e.g., 3–5 RBC casts/lpf).
Table 7.2
When a microscopic examination is performed, the volume of sediment viewed in each microscopic FOV is determined by two factors: the optical lenses of the microscope and the standardized slide system used. The ocular field number of the microscope and the objective lens determine the area of the FOV (see Chapter 18). The larger the FOV, the greater is the number of components that may be visible. To obtain reproducible results when manual microscopic examinations are performed, the same microscope must be used, or when multiple microscopes are available, the diameters of their FOVs (i.e., ocular field numbers) must be identical.
These viewing factors and sediment preparation protocols account for the differences observed in reference ranges for microscopic formed elements. They also prevent comparison of the microscopic results obtained in laboratories using different microscopes and commercial slides. However, if each laboratory would relate sediment elements as the “number present per volume of urine” instead of per low- or high-power field, interlaboratory result comparisons would be possible and comparisons between manual and automated microscopy systems (e.g., iQ200 [Iris Urinalysis-Beckman Coulter, Inc., Brea, CA]; UF-1000i [bioMerieux Inc., Durham, NC]) would be facilitated.
To convert the number of formed elements observed per low- or high-power field to the number present per milliliter of urine tested, a few calculations are necessary (Box 7.2). First, the area of the FOV for the low- and high-power fields must be determined. This calculation uses the diameter for the FOV, which is determined by the ocular field number of the microscope and the formula for the area of a circle (area = πr2). Because a standardized commercial microscope slide provides the same volume of sediment in a known viewing area (see Table 7.1) and the area viewed in each microscopic field is known, the “field conversion factors” remain constant. Once the field conversion factors for a particular microscope and the standardized microscope slide system used have been established, determining the number of formed elements per milliliter of urine requires a single multiplication step. Box 7.2 outlines these calculations and includes an example.
Enhancing Urine Sediment Visualization
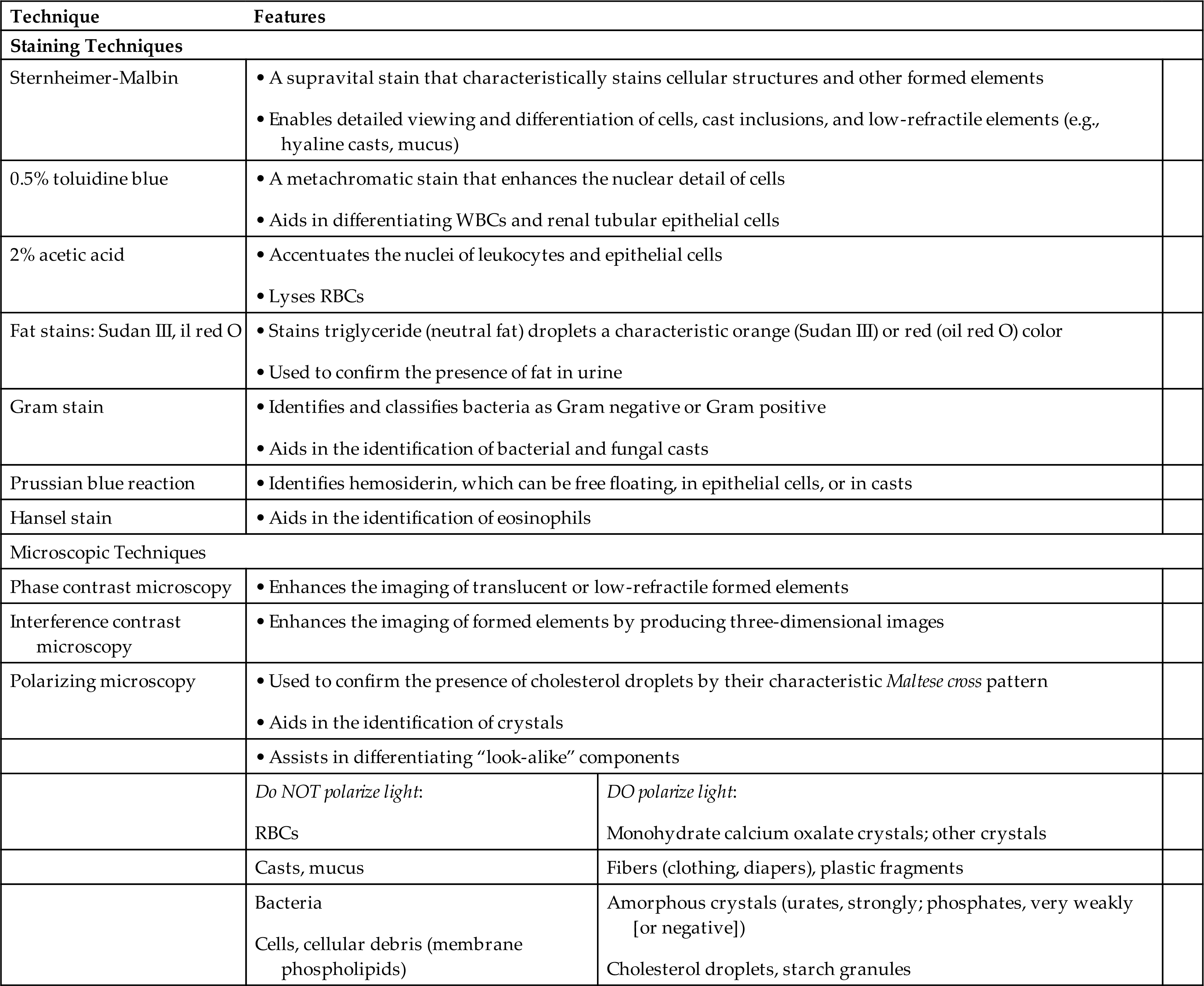
When using brightfield microscopy, it can be difficult to see urine sediment components (e.g., mucus, hyaline casts) that have a similar refractive index to that of urine. Because their refractive indexes are similar, there is insufficient contrast to enable optimal viewing. Staining changes the refractive index of formed elements and increases their visibility. Another approach is to change the type of microscopy, which can also facilitate visualization of low-refractility components or can be used to confirm the identity of suspected substances such as fat. Hyaline casts, mucus threads, and bacteria are difficult to see under brightfield microscopy; the use of stains or phase microscopy enhances their visualization. These techniques facilitate observation of the fine detail necessary for specific identification (e.g., distinguishing a WBC from a renal tubular cell). They also help to differentiate look-alike entities, such as monohydrate calcium oxalate crystals, which can resemble RBCs, and can be used to distinguish between mucus threads and hyaline casts. Table 7.3 summarizes the visualization techniques discussed in this chapter.
Table 7.3
Staining Techniques
Supravital Stains
Numerous stains have been used to enhance the visualization of urine sediment. Each laboratory should have a stain available because stains are inexpensive and can significantly assist in the identification of some urine sediment components. The most commonly used stain is a supravital stain consisting of crystal-violet and safranin, also known as the Sternheimer-Malbin stain (Fig. 7.4). This stain enhances formed element identification by enabling more detailed viewing of internal structures, particularly of WBCs, epithelial cells, and casts. Other formed elements (e.g., RBCs, mucus) stain characteristically, and their descriptions are noted on the package inserts provided with commercially prepared stains. Stabilized modifications of Sternheimer-Malbin stain are available commercially (e.g., Sedi-Stain, Becton, Dickinson and Company, Franklin Lakes, NJ), or it can be prepared by the laboratory if desired.3 One disadvantage of its use is that in strongly alkaline urines, this stain can precipitate, which obstructs the visualization of sediment components.
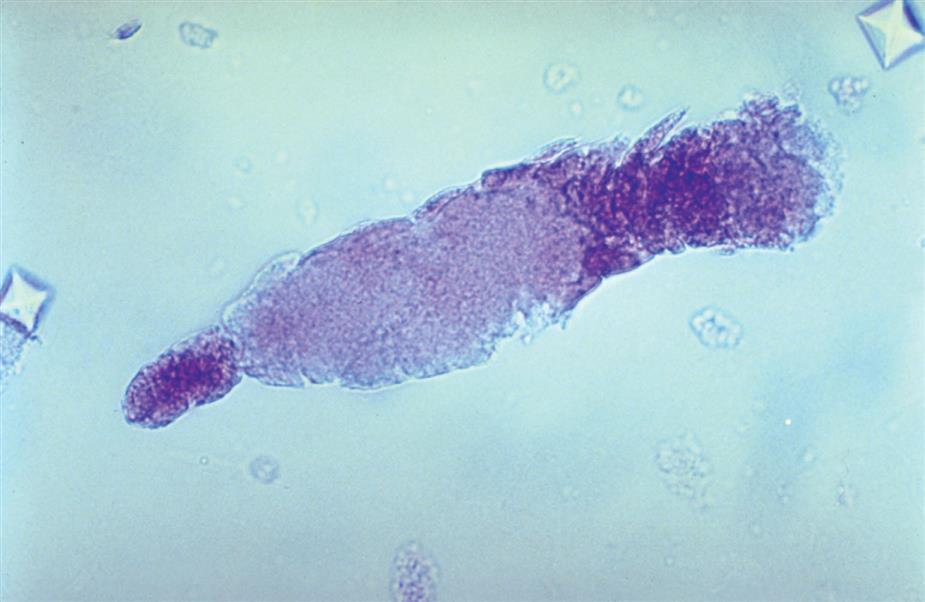
Another good supravital stain for urine sediment is a 0.5% solution of toluidine blue (Figs. 7.5 and 7.6). The stain is a metachromatic dye that stains various cell components differently; hence, the differentiation between the nucleus and the cytoplasm becomes more apparent. The toluidine blue stain enhances the specific identification of cells and aids in distinguishing cells of similar size, such as leukocytes from renal collecting duct cells.
Acetic Acid
Although acetic acid is not actually a stain, it can be helpful in identifying WBCs. WBCs can appear small, especially in hypertonic urine, with their nuclei and granulation not readily apparent. By adding 1 to 2 drops of a 2% solution of acetic acid to a few drops of urine sediment, the nuclear pattern of WBCs and epithelial cells is accentuated, whereas RBCs are lysed.
Fat or Lipid Stains
Sudan III or oil red O is often used to confirm the presence of neutral fat or triglyceride suspected during the microscopic examination (Fig. 7.7). These lipids stain orange or red and may be found (1) free floating as droplets or globules; (2) within renal cells or macrophages, aptly termed oval fat bodies; or (3) within the matrix of casts as droplets or oval fat bodies. An important note is that only neutral fats (e.g., triglycerides) stain. In contrast, cholesterol and cholesterol esters do not stain and must be confirmed by polarizing microscopy. The distinction between triglyceride and cholesterol is primarily academic because the implications for renal disease are the same regardless of the identity of the fat. In other words, changes have occurred in the glomeruli such that triglycerides and cholesterol from the bloodstream are now passing the glomerular filtration barriers with the plasma ultrafiltrate. The urinalysis laboratory can use a fat stain or polarizing microscopy to confirm the presence of fat; the confirmation method selected is usually determined by cost, personnel preference, and convenience.
Gram Stain
Although Gram stain is used primarily in the microbiology laboratory, it may at times be used in the urinalysis laboratory. Gram stain provides a means of positively identifying bacteria in the urine and differentiating them as Gram negative or Gram positive (Fig. 7.8). To perform a Gram stain, a dry preparation of the urine sediment is made on a microscope slide by smearing and air drying or by cytocentrifugation. As in the microbiology laboratory, the slide is heat fixed and then stained. Gram-negative bacteria appear pink, whereas Gram-positive bacteria appear dark purple. Because these slides can be viewed using a high-power oil immersion (×100) objective, additional characterization of the bacteria (e.g., cocci, rods) could be made, but this is rarely done by the urinalysis laboratory.

Prussian Blue Reaction
To facilitate the visualization of hemosiderin, free floating or in epithelial cells and casts, the Prussian blue reaction, also known as the Rous test, is used. First described by Rous in 1918 to identify urinary siderosis, the Prussian blue reaction stains the iron of hemosiderin granules a characteristic blue.4 See “Hemosiderin” later in this chapter for more discussion of this reaction and its use.
Hansel Stain
Hansel stain (methylene blue and eosin-Y in methanol) is used in the urinalysis laboratory specifically to identify eosinophils in the urine (Fig. 7.9). Whereas Wright’s stain or Giemsa stain also distinguishes eosinophils, Hansel stain is preferred.5 Urine eosinophils can be present in a variety of renal or urinary tract disorders, such as urinary tract infections (UTIs), acute tubular necrosis, glomerulonephritis, and acute interstitial nephritis (AIN).
Microscopy Techniques
Identification of urine sediment components is dependent on (1) the ability of the microscopist and (2) the microscope used to perform the analysis. In the United States brightfield microscopy predominates despite its inherent difficulty in detecting and identifying low-refractile entities, such as hyaline casts, ghost RBCs, and bacteria. Therefore phase contrast microscopy and the availability of supravital stains are strongly recommended in the urinalysis testing area.6 Even the most adept microscopists are restricted in their ability to identify entities when limited by inadequate equipment and supplies. A brief overview of microscopy techniques used in urinalysis testing is introduced here. See Chapter 18 for a detailed discussion of the microscope, the role of each component part, and steps for proper adjustment, as well as principles, advantages, and applications for various types of microscopy.
Phase Contrast Microscopy
Phase contrast microscopy is the preferred technique for microscopic examination of urine sediment because it enables (1) evaluation of RBC morphology and (2) detailed visualization and identification of difficult-to-view (translucent or low-refractile) formed elements such as hyaline casts, RBC ghost cells, and bacteria (Fig. 7.10). An added advantage is that microscopic examinations are generally faster to perform because of the enhanced visualization. See Chapter 18 for a detailed discussion of phase contrast microscopy and how variations in the refractive index of formed elements are converted into variations in contrast, thereby revealing low-refractile components.

Polarizing Microscopy
In the urinalysis laboratory, polarizing microscopy is often used to confirm the presence of fat, specifically cholesterol. Cholesterol droplets are birefringent (i.e., they refract light in two directions) and, similar to their counterpart triglycerides, they can be found as free-floating droplets or in cells (oval fat bodies) and casts. In droplet form—within cells, free floating, or in casts—cholesterol produces a characteristic Maltese cross pattern with polarized light (Fig. 7.11A). These droplets appear as orbs against a black background divided into four quadrants forming a bright Maltese-style cross. When a first-order red compensator plate is used, the background becomes red to violet and opposing quadrants in the orbs are yellow or blue, depending on their orientation to the light (Fig. 7.11B). Note that starch granules and some drug crystals show a similar pattern, which is called a pseudo-Maltese cross because the four quadrants produced are variable in size (see Chapter 18, Table 18.1). Other neutral fats, such as fatty acids and triglycerides, cannot be identified using polarizing microscopy because they are not optically active—light passes through them unchanged. For triglyceride or neutral fat identification, see the section “Fat or Lipid Stains” earlier in this chapter.
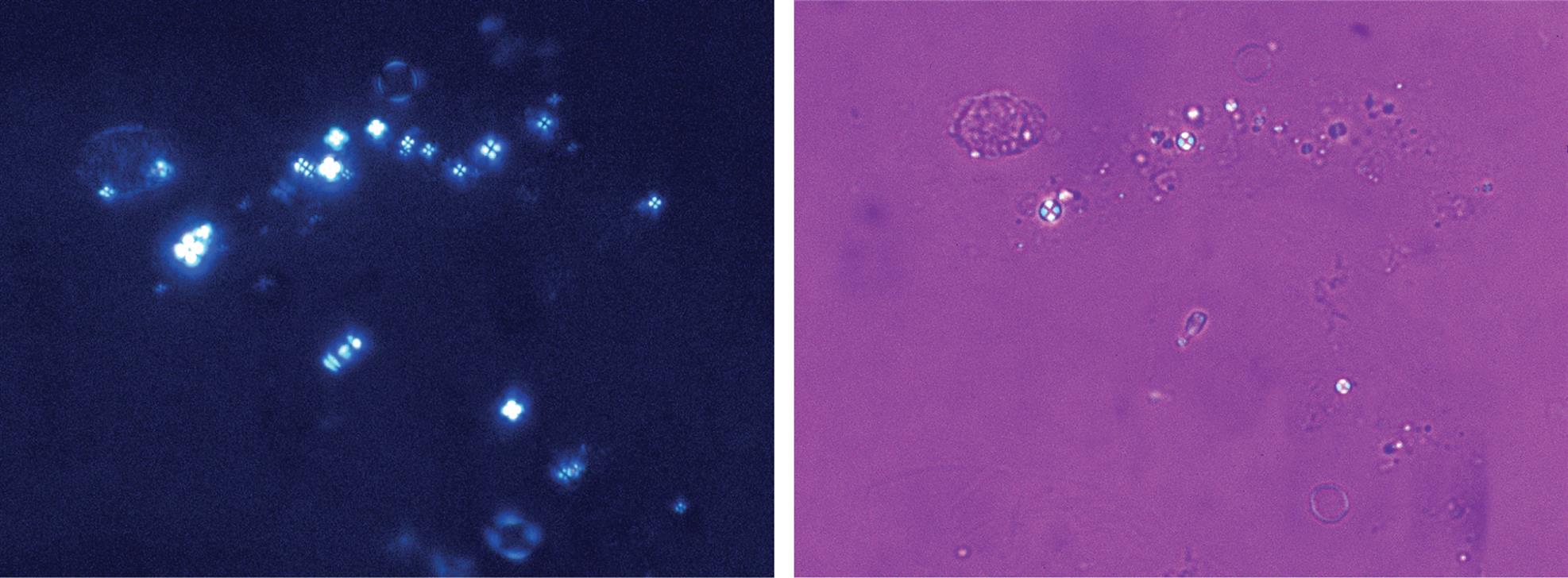
Polarizing microscopy can also assist in differentiating urine sediment components that may look alike (see Table 7.3). RBCs can be distinguished from monohydrate calcium oxalate crystals, casts or mucus from fibers, and amorphous material from coccoid bacteria. See Chapter 18 for additional information, as well as a procedure for converting a brightfield scope for polarizing microscopy (Box 18.3).
Interference Contrast Microscopy
Chapter 18 discusses two types of interference microscopy. Differential interference contrast (Nomarski) microscopy and modulation contrast (Hoffman) microscopy provide detailed three-dimensional images of high contrast and resolution (Fig. 7.12). Although their use is suited ideally for microscopic examination of the formed elements found in urine sediment, the increased cost often cannot be justified by the traditional urinalysis laboratory. With experience, however, these microscopic techniques are easy to use and less time-consuming than brightfield microscopy because of the enhanced imaging. In addition, once a brightfield microscope has been modified for modulation contrast microscopy, it can easily be used for brightfield, polarizing, and other techniques by simply removing the specialized slit aperture from the light path.
Cytocentrifugation and Cytodiagnostic Urinalysis
Cytocentrifugation
Cytocentrifugation is a technique used to produce permanent microscope slides of urine sediment and body fluids (see Chapter 17). Because a monolayer of sediment components is desired, an initial microscopic examination is required to determine the amount or volume of urine sediment to use when preparing the slide. After this step, the appropriate amount of concentrated urine sediment is added to a specially designed cartridge fitted with a microscope slide that is placed in a cytocentrifuge (e.g., Shandon Cytospin, Thermo Shandon, Pittsburgh, PA). After cytocentrifugation, a dry circular monolayer of sediment components remains on the slide. The slide is fixed permanently using an appropriate fixative and is stained. For cytologic studies, Papanicolaou’s stain is preferred; however, if Papanicolaou’s stain is not available, or if time is a factor, Wright’s stain can be used. The end result is a monolayer of the urine sediment components with their structural details greatly enhanced by staining. This enables the quantitation and differentiation of WBCs and epithelial cells in the urine sediment. If desired, these slides can also be viewed using high-power oil immersion objectives and can be retained permanently in the laboratory for later reference or review.
Cytodiagnostic Urinalysis
In 1926, Thomas Addis established the value of identifying increased numbers of urine cellular elements as evidence of disease progression. Today, the ability to perform urine differential cell counts enables identification of and discrimination between renal disease and urinary tract disorders. Although a cytodiagnostic urinalysis should not be performed on all urine specimens, it can play an important role in the early detection of renal allograft rejection and in the differential diagnosis of renal disease. Cytodiagnostic urinalysis involves making a 10:1 concentration of a first morning urine specimen, followed by cytocentrifugation of the urine sediment and Papanicolaou’s staining.7 Although cytodiagnostic urinalysis requires more time to perform, it is uniquely valuable in the identification of blood cell types, cellular fragments, epithelial cells (atypical and neoplastic), cellular inclusions (viral and nonviral), and cellular casts.
Formed Elements in Urine Sediment
A wide range of formed elements can be encountered in the microscopic examination of urine sediment. These formed components can originate from throughout the urinary tract—from the glomerulus to the urethra—or can result from contamination (e.g., menstrual blood, spermatozoa, fibers, starch granules). Many components, such as blood cells and epithelial cells, are cellular; others are chemical precipitates, such as the variety of crystalline and amorphous material that can be present in the sediment. Casts—cylindrical bodies with a glycoprotein matrix—form in the lumen of the renal tubules and are flushed out with the urine. Opportunistic microorganisms such as bacteria, yeast, and trichomonads can also be encountered in urine sediment. Not all of these formed elements indicate an abnormal or pathologic process. However, the presence of large numbers of “abnormal” components is diagnostically significant.
Identifying and enumerating the components found in urine sediment provide a means of monitoring disease progression or resolution. Determining the point at which the amount of each element present indicates a pathologic process requires familiarity with the expected normal or reference interval for each component (Table 7.4). (See Appendix C for reference intervals of all parameters in a complete urinalysis.) Normally, a few RBCs, WBCs, epithelial cells, and hyaline casts are observed in the urine sediment from normal, healthy individuals. Their actual number varies depending on the sediment preparation protocol and the standardized slide system used for the microscopic examination.8 Because changes occur in unpreserved urine, factors such as the type of urine collection and how the specimen has been stored also affect the formed elements observed during microscopic examination.
Table 7.4
| Component | Number | Magnification |
|---|---|---|
| Red blood cells | 0–3 | Per HPF |
| White blood cells | 0–8 | Per HPF |
| Casts | 0–2 hyaline (or finely granularb) | Per LPF |
| Epithelial cells: | ||
| Squamous | Few | Per LPF |
| Transitional | Few | Per HPF |
| Renal | Few (0–1) | Per HPF |
| Bacteria and yeast | Negative | Per HPF |
| Abnormal crystals | None | Per LPF |

HPF, High-power field (×400); LPF, low-power field (×100).
aUsing the UriSystem. Values vary with concentration of urine sediment, microscope slide technique, and microscope optical properties. See Appendix C for reference intervals for a “complete” urinalysis.
bAfter physical exercise, cast numbers increase and include finely granular casts (1991, Haber).
This section discusses in detail the variety of formed elements possible in urine sediment and presents the origin of each component and its clinical significance, possible variations in shape and composition, and techniques used to facilitate differential identification. A wide range of additional images of urine sediment components can be found in the Urine Sediment Image Gallery at the end of this chapter.
Blood Cells
Red Blood Cells (Erythrocytes)
The name erythrocyte is derived from the Greek word erythros, meaning “red,” and the suffix -cyte, meaning “cell.” Hence these cells are more frequently called red blood cells (RBCs), and this term will be used predominantly throughout this text. RBCs were one of the first cells recognized and described after the discovery of the microscope.
Microscopic Appearance
Because of their small size—approximately 8 μm in diameter and 3 μm in depth—RBCs in urine are viewed and enumerated using high-power magnification. RBCs have no nucleus; they normally appear as smooth biconcave disks, and they are moderately refractile. When suspended in urine sediment, RBCs can be viewed from any angle. When viewed from the side, they have an hourglass shape; when viewed from above, they appear as disks with a central pallor (Fig. 7.13). The size or diameter of RBCs is affected by urine concentration (i.e., osmolality, specific gravity). In hypertonic urine, their diameter can be as small as ~ 3 μm and in hypotonic urine as large as 11.8 μm.9
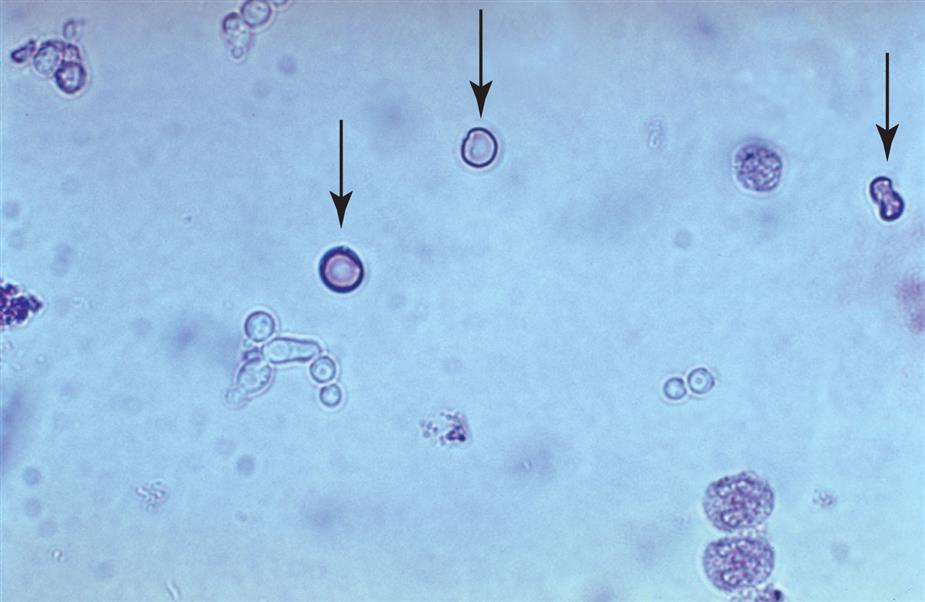
Dysmorphic or distorted forms of RBCs can also be present in urine (Fig. 7.14). At times, these forms are present with normal RBCs in the urine of healthy individuals. Some dysmorphic forms occur because of the urine’s concentration (i.e., osmolality).10 The most common dysmorphic form is crenated erythrocytes (i.e., echinocytes or burr cells). When RBCs are present in hypertonic urine (osmolality >500 mOsm/L), they become smaller as intracellular water is lost by osmosis, which causes them to become crenated. As they crenate, erythrocytes lose their biconcave disk shape and become spheres covered with evenly spaced spicules or crenations. Because of these reversible membrane changes, the surface of crenated cells appears rough or sometimes grainy, depending on the microscope adjustments, compared with normal erythrocytes. In hypotonic urine (osmolality <180 mOsm/L), erythrocytes swell and will eventually release their hemoglobin to become “ghost” cells, which are cells with intact cell membranes but no hemoglobin. These empty cells, outlined by their membranes, appear as colorless empty circles. Because their hemoglobin has been lost, ghost cells are difficult to see using brightfield microscopy; however, they are readily visible with phase contrast or interference contrast microscopy (Fig. 7.14). Note that alkaline urine promotes RBC lysis and disintegration, which results in ghost cells and erythrocyte remnants.
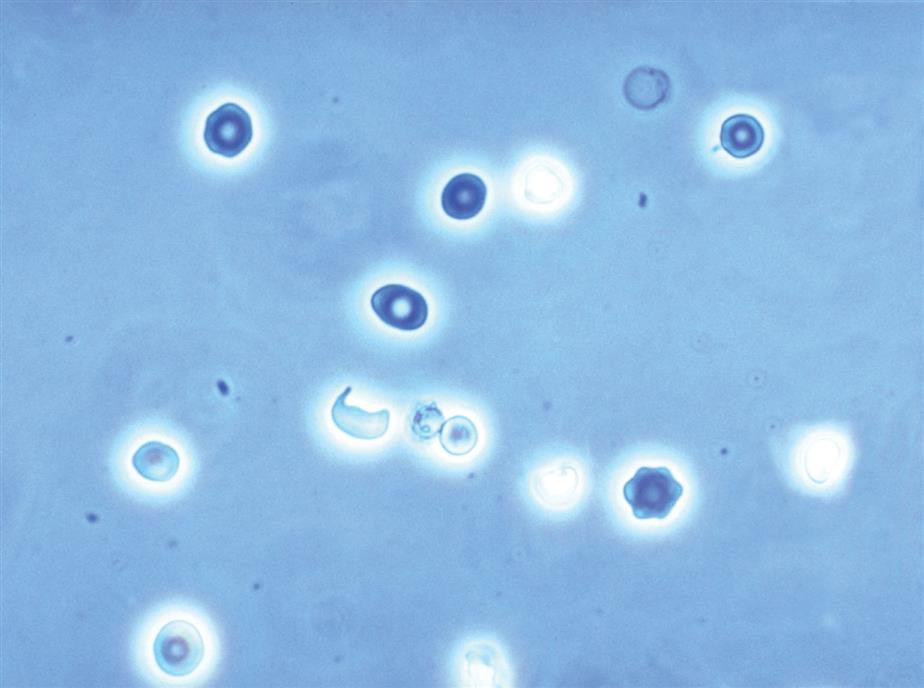
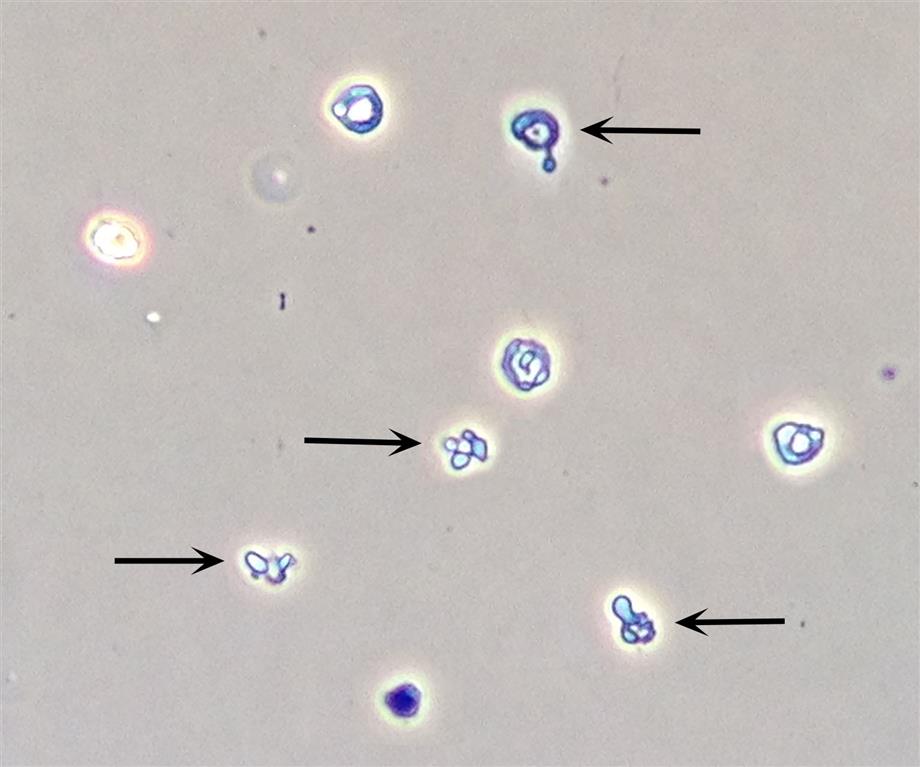
A variety of dysmorphic erythrocyte forms can be present in a single urine sediment.10 These forms include acanthocytes, schizocytes, stomatocytes, target cells, and teardrop cells (Table 7.5). Some of these forms are reversible and induced by the physical characteristics of the urine as it flows through the nephron (i.e., changes in osmolality, pH and uric acid concentration). However, the presence of acanthocytes (i.e., RBCs in a donut form with one or more protruding cytoplasmic blebs) in urine is particularly noteworthy (Fig. 7.15). The conversion of RBCs into acanthocytes is not induced by changes in osmolality or pH. Rather, the physical forces undergone by RBCs as they pass through the glomerular filtration barrier (i.e., basement membrane) disrupt and permanently alter their cell membranes. Dysmorphic RBCs tend to be smaller, and often RBC fragments and other dysmorphic forms are present with acanthocytes. Studies indicate that when 5% or more of the RBCs in urine sediment are acanthocytes, it is an indicator of hematuria due to a glomerular disorder.10–12 Rarely observed are sickle cells, which have been seen in the urine sediment of patients with sickle cell disease. Using phase contrast or interference contrast microscopy enhances the ability to evaluate RBC morphology and is recommended.
Table 7.5
| Form Name | ||||
|---|---|---|---|---|
| Category | Common Name | Bessis Nomenclaturea | Phase Microscopy Example | Form Description |
| Isomorphic | Normal cell | Discocyte | 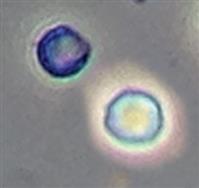 |
Biconcave disk form of normal size |
| Burr cell | Echinocyte or crenated cell |  |
Cell with evenly spaced projections or spicules over cell surface; this “reversible” shape change progresses from a “crenated” disk to a “crenated” sphere.10 | |
| Ghost cell | Ghost cell |  |
Cell with thin membrane and without hemoglobin | |
| Dysmorphic | Acanthocyte or G1 cell | Acanthocyte | 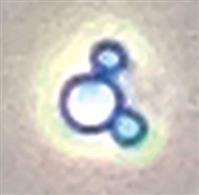 |
Cell in a ring form (donut shape) with one or more cytoplasmic blebs (i.e., vesicle-shaped protrusions or bulges) |
| Target cell | Codocyte |  |
Bull’s-eye appearance; can be bell- or cup-shaped | |
| Schistocyte | Schizocyte | 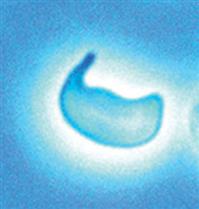 |
Cell fragment often with two or three pointed ends; size and shape vary | |
| Stomatocyte | Stomatocyte |  |
Cell with central pallor that appears slit-like; this shape change is “reversible.”10 | |
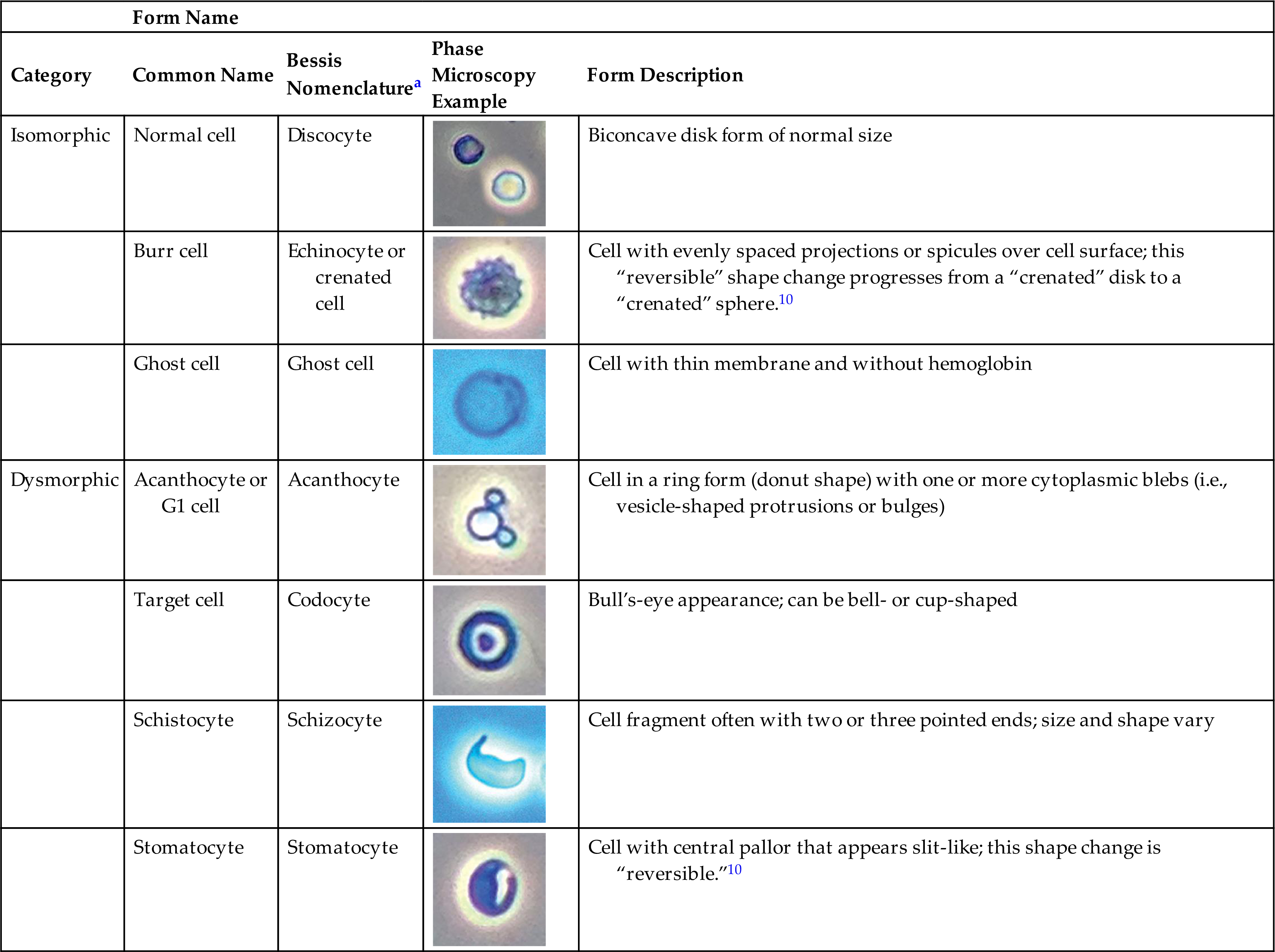
aBessis M: Red cell shapes. An illustrated classification and its rationale. Nouvelle Revue Française d’Hématologie 12:721–746, 1972.
Normally, RBCs are found in the urine of healthy individuals and do not exceed 0 to 3 per high-power field or 3 to 12 per microliter of urine sediment.13 Semiquantitation is made by observing 10 representative high-power fields and averaging the number of erythrocytes seen in each. Although RBCs are nonmotile, they are capable of passing through pores only 0.5 mm (500 nm) in diameter.14 In addition, during inflammation, RBCs can be transported out of capillaries by the same mechanism as inert, insoluble substances.12 All RBCs in urine originate from the vascular system. The integrity of the normal vascular barrier in the kidneys or the urinary tract can be damaged by injury or disease, causing leakage of RBCs into any part of the urinary tract. Increased numbers of RBCs along with red RBC casts indicate renal bleeding, either glomerular or tubular. These urines also have significant proteinuria. When an increased number of RBCs is present without casts or proteinuria, the bleed is occurring below the kidney or may be caused by contamination (e.g., menstrual, hemorrhoidal).
Correlation With Physical and Chemical Examinations
RBCs observed during microscopic examination should be correlated with physical and chemical examinations (Table 7.6). Macroscopically, the urine sediment may indicate the presence of RBCs when the sediment button is characteristically red in color. Sometimes specimens have a positive chemical test for blood, but the microscopic examination reveals no RBCs. This can be explained by the fact that RBCs readily lyse and disintegrate in hypotonic or alkaline urine; such lysis can also occur within the urinary tract before urine collection. As a result, urine specimens can be encountered that contain only hemoglobin from RBCs that are no longer intact or microscopically visible. However, it is important to note that other substances, such as myoglobin, microbial peroxidases, and strong oxidizing agents, can cause a positive blood chemical test (see Chapter 6). Note that these reactions are considered “false-positive” reactions because RBCs or blood is not present.
Table 7.6
| Microscopic features | |
| Look-alike elements | |
| Correlation with physical and chemical examinations |
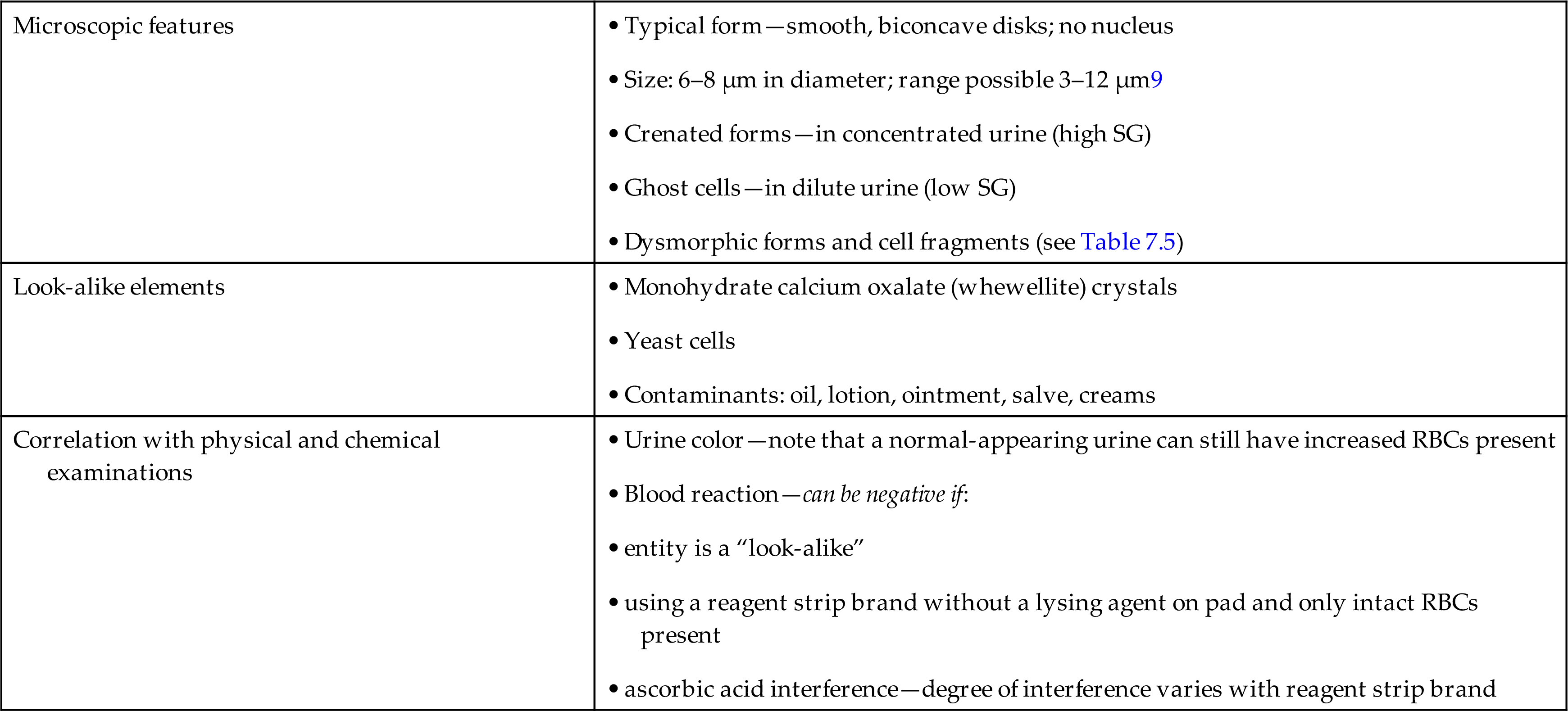
In specimens in which RBCs are present microscopically but the chemical screen for blood is negative, ascorbic acid interference should be suspected. If ascorbic acid is ruled out, it is possible that the formed elements observed are not RBCs but a “look-alike” component such as yeast or monohydrate calcium oxalate crystals. In these cases, their identity should be confirmed by an alternative technique such as staining or using polarizing microscopy.
Even though hemoglobin is a protein, in most cases of hematuria it does not contribute to the protein result obtained by the chemical reagent strip. Hemoglobin must be present in the urine in an amount exceeding 10 mg/dL before it is detected by routine protein reagent strip tests. In other words, when the chemical reagent strip test for blood reads less than large (3+), hemoglobin is not causing or contributing to the protein result; when the blood result is greater than or equal to large (3+), hemoglobin may be contributing to the protein reagent strip test result.
Look-Alikes
Other components in urine sediment such as yeast, monohydrate calcium oxalate crystals, small oil droplets, or air bubbles can resemble RBCs. Even WBCs can be difficult to distinguish from crenated RBCs in a hypertonic urine specimen. In the latter case, using acetic acid or toluidine blue stain can be advantageous because these solutions make it easier to see the nuclei of WBCs. The techniques described earlier in this chapter are useful for differentiation of these formed elements. A Sternheimer-Malbin stain characteristically colors RBCs, whereas neither yeast nor calcium oxalate crystals stain. Polarizing microscopy can identify calcium oxalate crystals or 2% acetic acid can be added, which lyses RBCs but does not eliminate yeast or calcium oxalate crystals.
Yeast varies in size, tends to be spherical or ovoid rather than biconcave, and often exhibits budding. Each of these characteristics helps to differentiate yeast from RBCs.
Small droplets or globules of oils, lotions, or ointments that were washed into the urine during collection can contaminate the urine sediment. They can be distinguished from RBCs by their variation in size, uniformity in appearance, and high refractility. Although these characteristics are usually evident to an experienced microscopist, they may not be obvious to a novice. Notably, these droplets are numerous, yet the chemical test for blood is negative.
Clinical Significance
Numerous conditions can result in hematuria. Table 6.9 categorizes them into kidney and urinary tract disorders (e.g., glomerulonephritis, pyelonephritis, cystitis, calculi, tumors); nonrenal disorders such as hypertension; appendicitis; trauma; strenuous exercise; and drugs. However, it is interesting to note that smoking, as well as normal exercise, has also been associated with hematuria.15 Anticoagulant drugs and drugs that induce a toxic reaction, such as sulfonamides, can also cause increased numbers of RBCs in the urine sediment. Therefore any condition that results in inflammation or that compromises the integrity of the vascular system throughout the urinary tract can result in hematuria. Keep in mind that specimens contaminated with blood from vaginal secretions or hemorrhoidal blood can falsely imply hematuria. Table 7.6 summarizes the microscopic features of RBCs and the expected correlation between physical and chemical examinations when RBCs are present.
White Blood Cells (Leukocytes)
Leukocyte is a collective term that refers to any type of WBC. In health, the distribution of WBCs in the urine essentially mirrors that of peripheral blood. The five types of cells that can be present are neutrophils, lymphocytes, eosinophils, basophils, and monocytes (macrophages). Because neutrophils predominate in the peripheral blood, they are the WBC most often observed in urine; however, with some renal conditions, other leukocytes may predominate in the urine. For example, with hypersensitivity to an offending drug, eosinophils may increase, whereas in renal allograft rejection, lymphocytes predominate.
Neutrophils
Microscopic Appearance
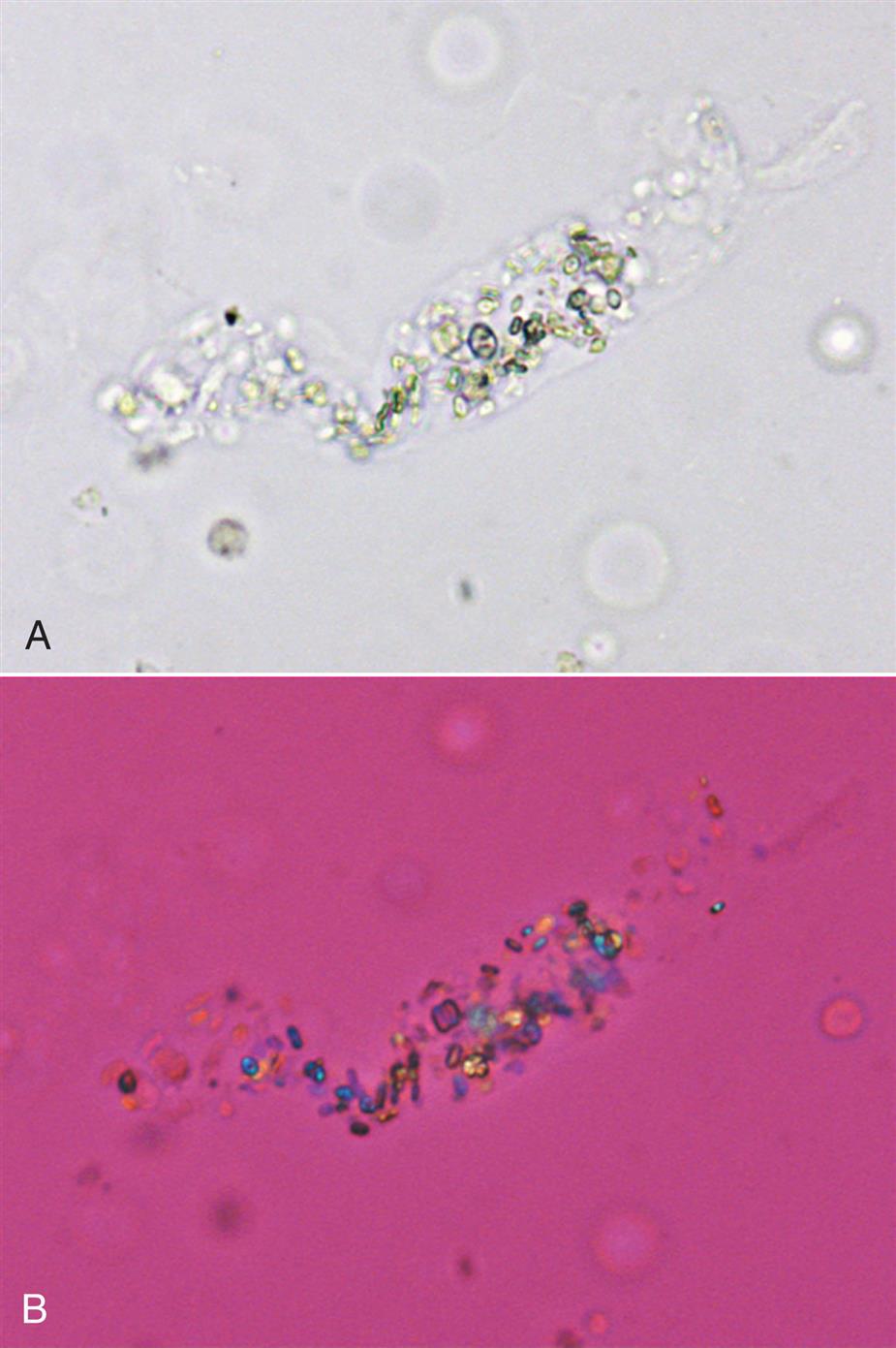
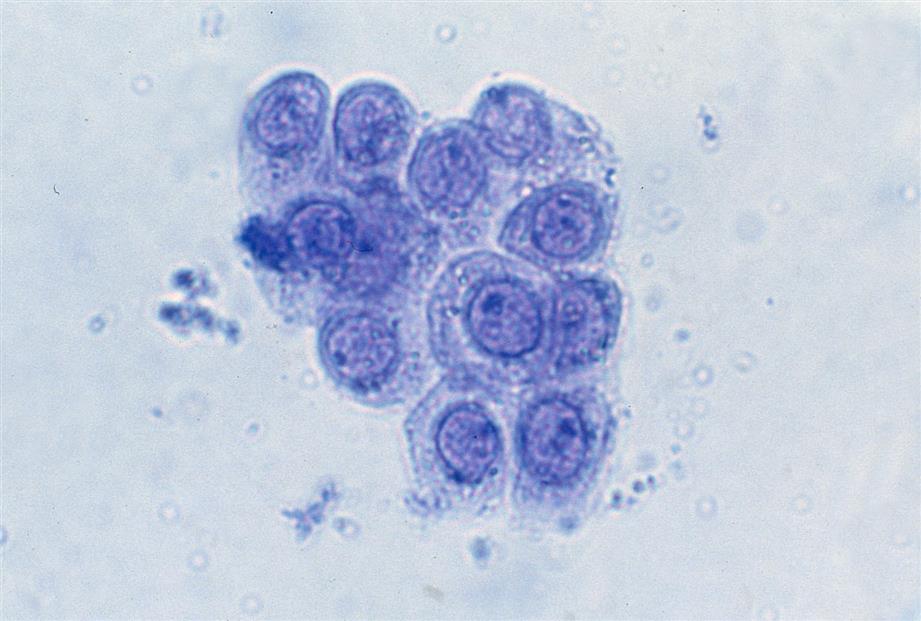
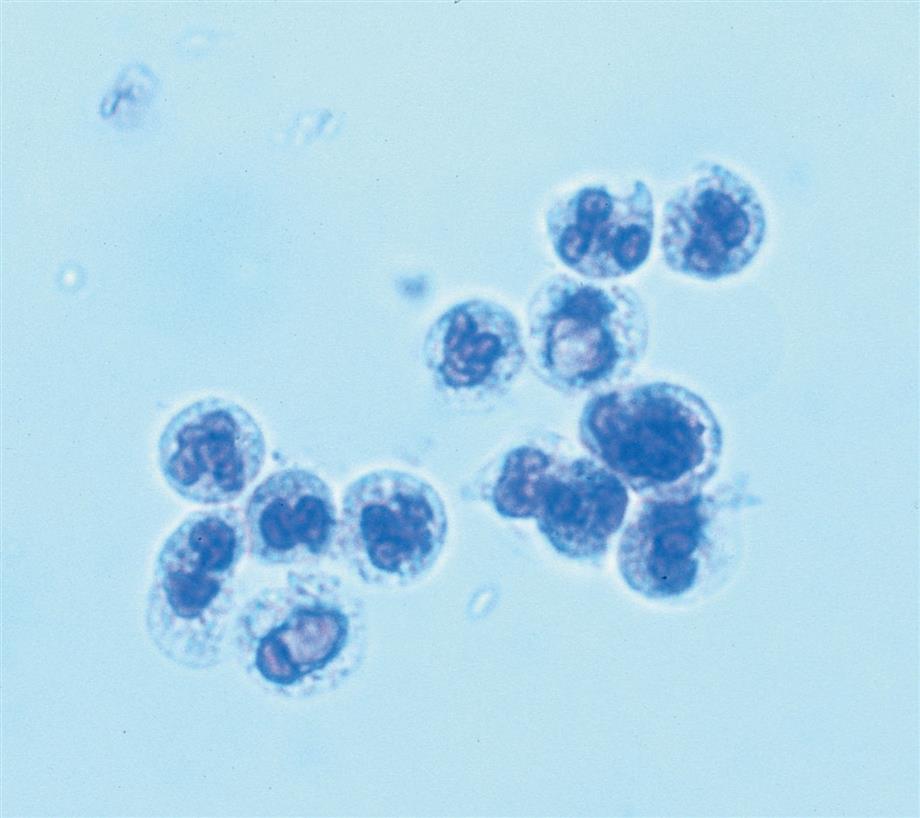
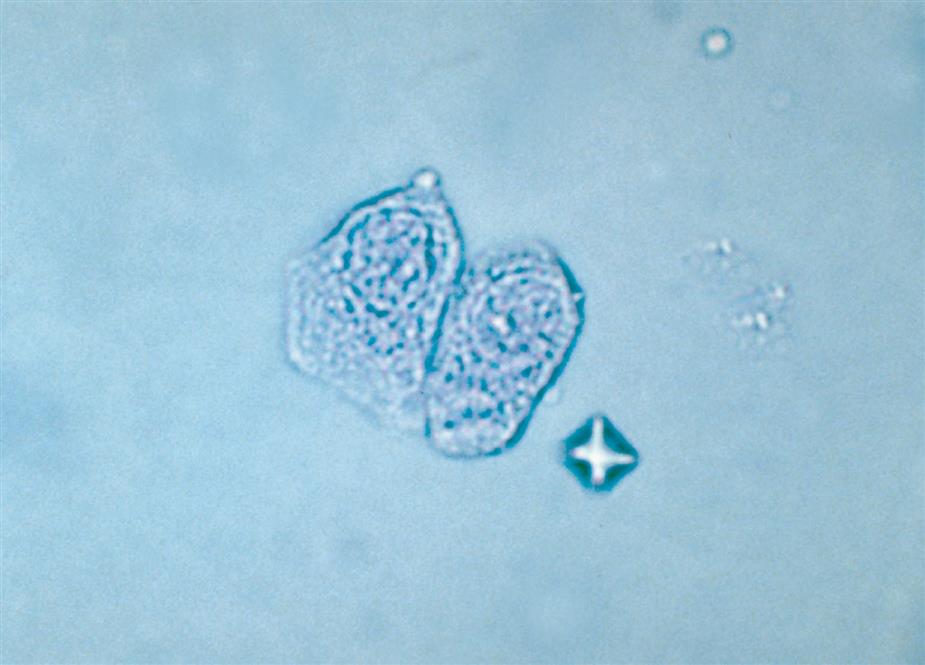
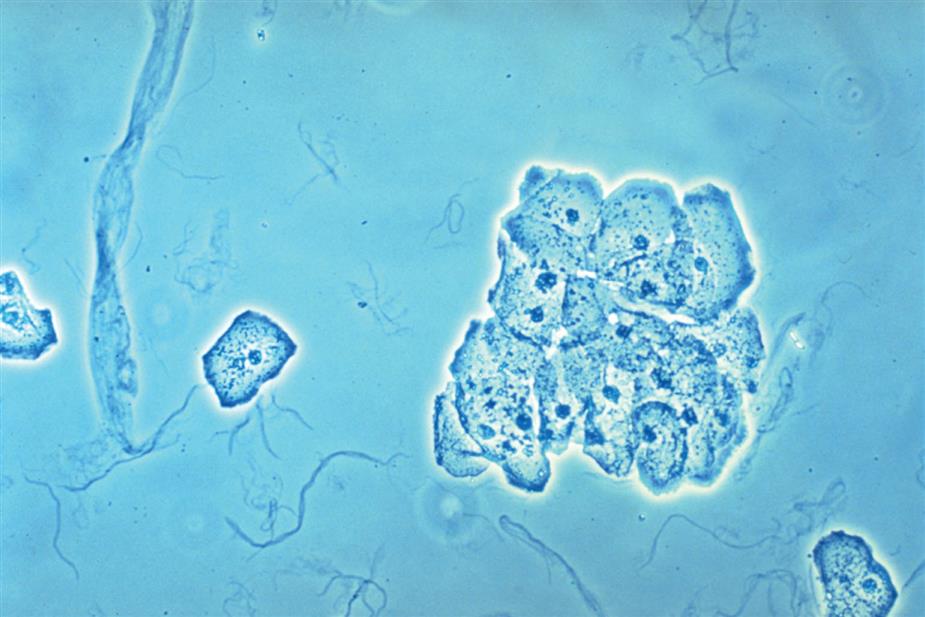
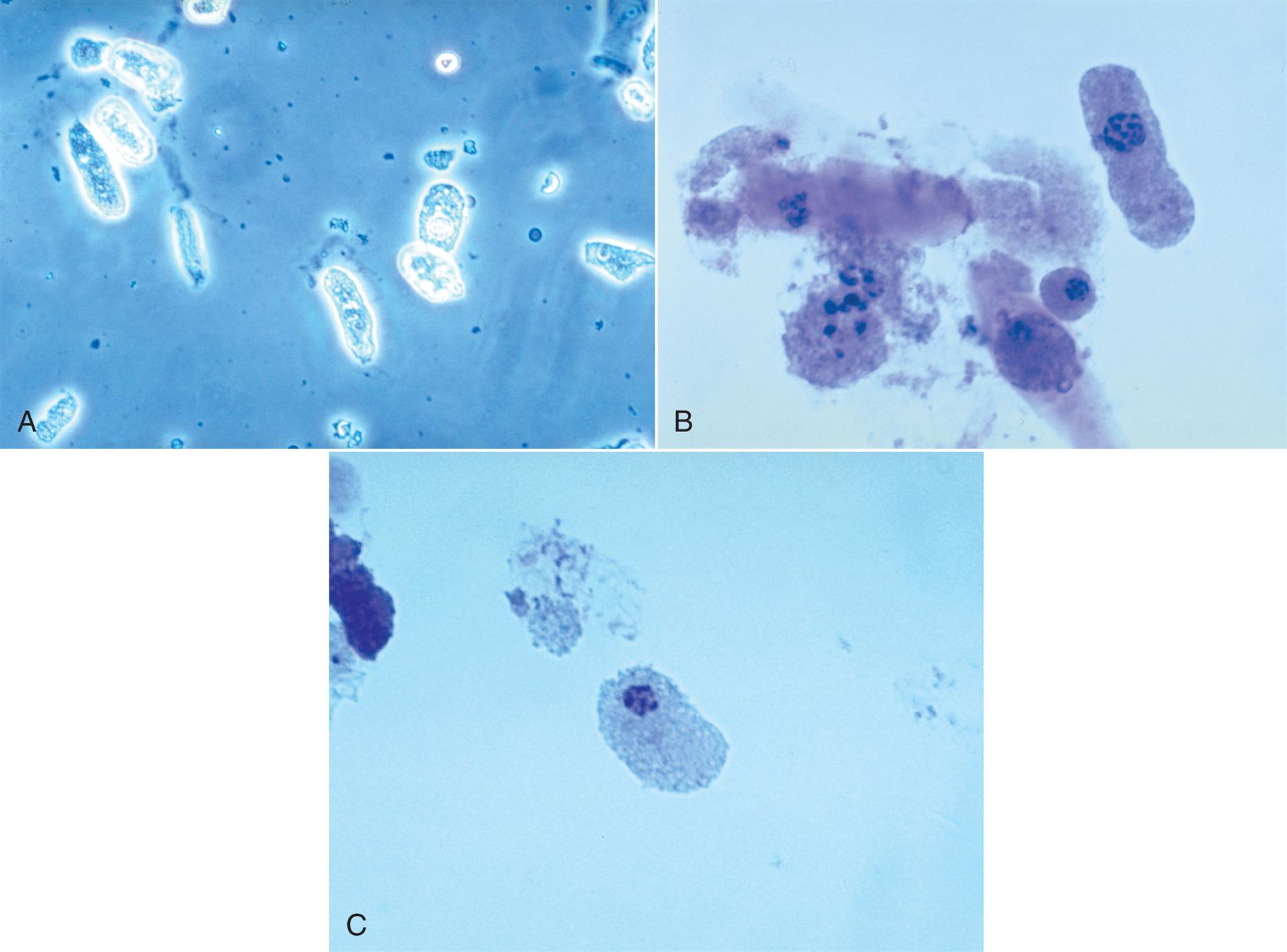
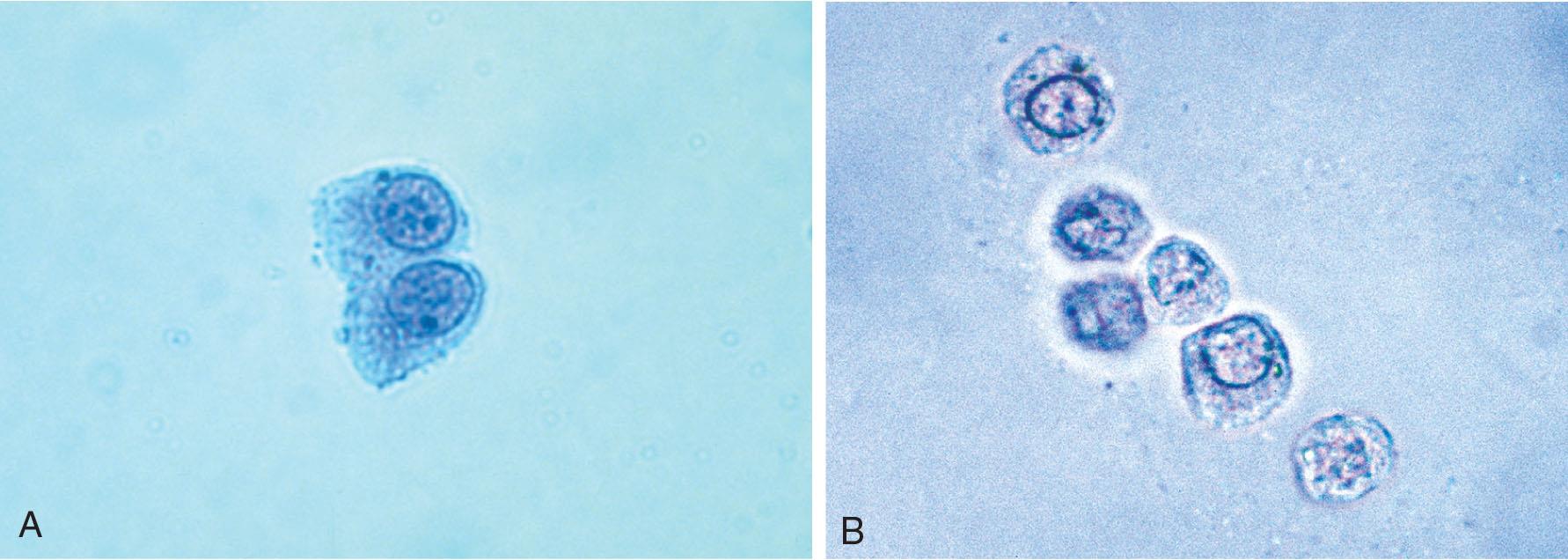
Neutrophils are the most common granulocytic leukocytes present in urine. They measure approximately 14 μm in diameter but can range from 10 to 20 μm, depending on the tonicity of the urine. They are larger than erythrocytes and can be similar in size to the small epithelial cells that line the collecting ducts of nephrons. Neutrophils are spherical cells with characteristic cytoplasmic granules and lobed or segmented nuclei (Fig. 7.16). Unstained, neutrophils have a grayish hue and appear grainy. Neutrophils may occur singly or aggregated in clumps; clumping, which often occurs in acute inflammatory conditions, makes their enumeration difficult (Fig. 7.17).
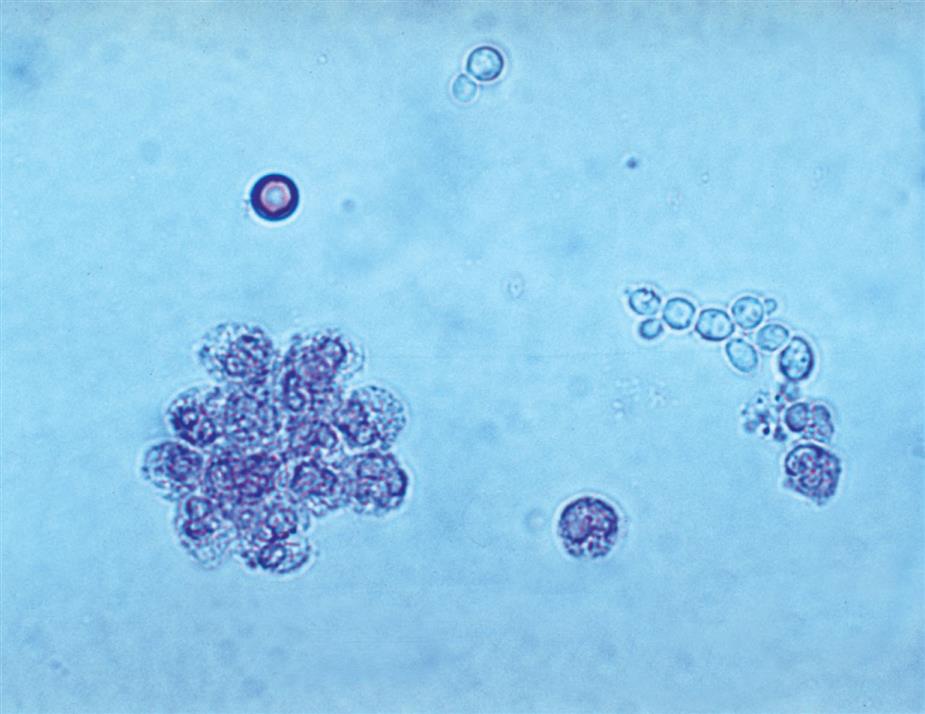
In fresh urine specimens, the characteristic features of neutrophils are often readily apparent by brightfield microscopy; however, as neutrophils age and begin to disintegrate, their lobed nuclei fuse and they can resemble a mononuclear cell. These changes can make neutrophils difficult to distinguish from renal tubular collecting duct cells. Hypotonic urine causes WBCs to swell and become spherical balls that lyse as rapidly as 50% in 2 to 3 hours at room temperature. In these large swollen cells, Brownian movement of the refractile cytoplasmic granules is often evident, giving the descriptive name “glitter cells” to these edemic leukocytes. In hypertonic urine, leukocytes become smaller as water is lost osmotically from the cells, but they do not crenate.
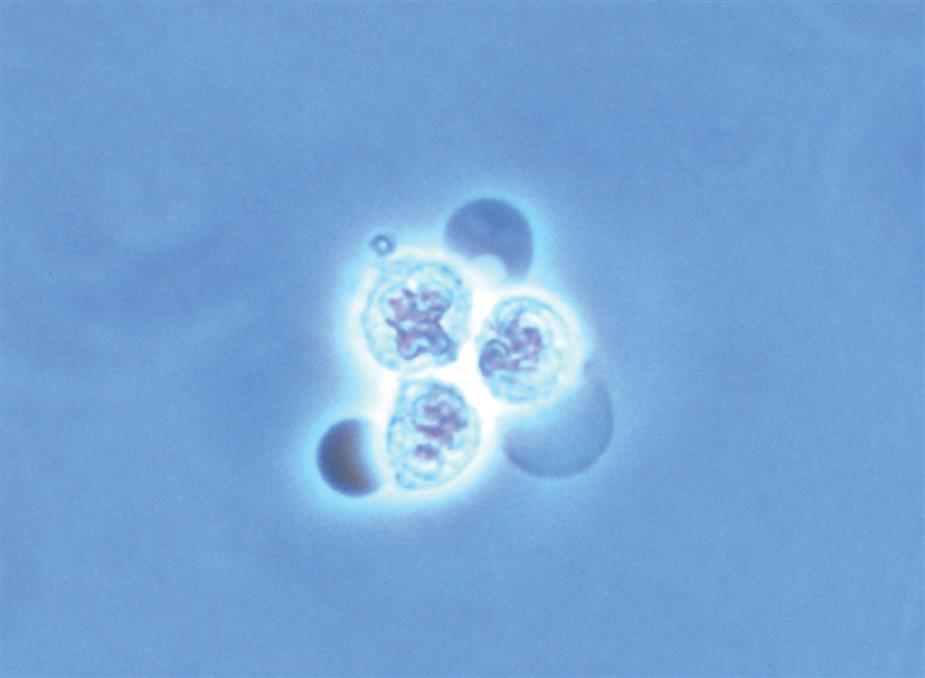
In addition to fusion of lobed nuclei (neutrophils), further evidence of cellular disintegration is seen in the formation of cytoplasmic blebs (Fig. 7.18). These blebs develop at the cell periphery on their outer membrane; they appear to be empty or may contain a few small granules. As these changes continue, the blebs can detach and become free floating in the urine or can remain within the cell, pushing the cytoplasm to one side and giving rise to large pale intracellular areas. Cytoplasmic blebs and vacuoles may also be observed with bacteriuria; the bacteria may be intracellular, extracellular, or both (Figs. 7.19 and 7.61).
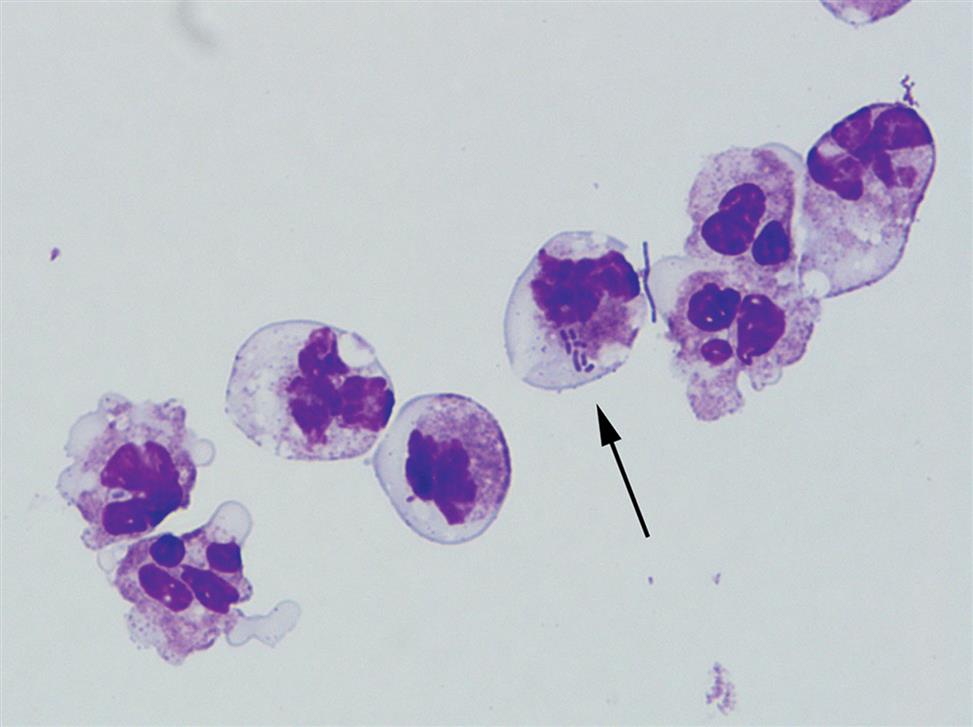
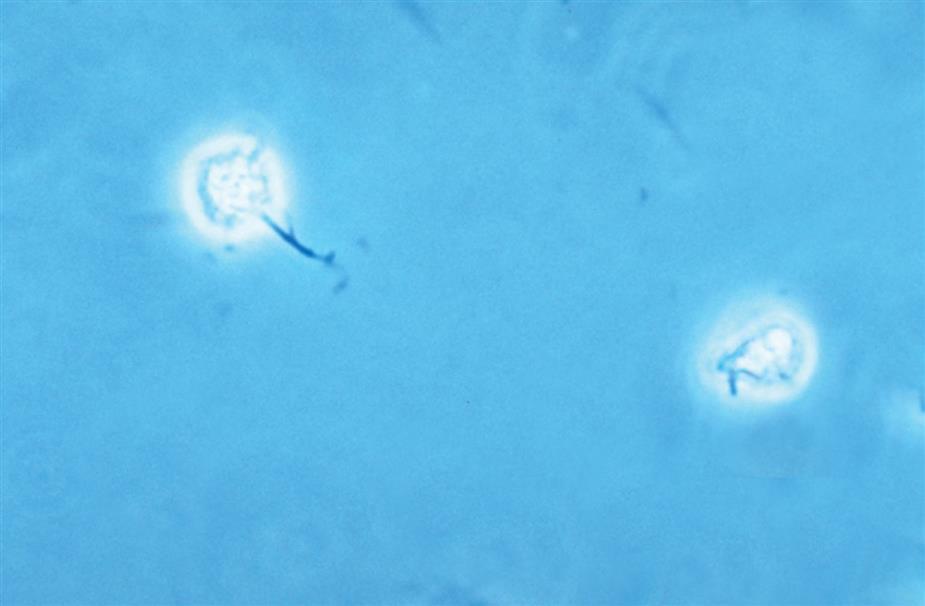
Another degenerative change is the development of numerous finger-like or worm-like projections protruding from the cell surface (Fig. 7.20). These long filaments, termed myelin forms, result from the breakdown of the cell membrane. As WBCs die, additional vacuolization, rupturing, or pseudopod formation may be observed.
Normally, leukocytes are present in the urine of healthy individuals. When manual microscopic examinations are conducted, semiquantitation is performed by observing 10 representative high-power fields and determining the average number of WBCs present in each field. Note that reference ranges depend on the protocol used. Typically in health, 0 to 8 WBCs are present per high-power field, or approximately 10 WBCs per microliter of urine sediment using a standardized microscope slide. Any clumping of WBCs evident during the microscopic examination should be included in the report because leukocyte enumeration is directly affected. The presence of WBCs in urine is not surprising because they are a normal component in secretions of the male and female genital tracts. Because WBCs are motile, they are capable of entering the urinary tract at any point. In response to an inflammatory process, WBCs are attracted to the area by chemotaxis and move ameboid-like through tissues by the formation of pseudopods. Although WBCs are generally spherical within the bloodstream and in urine, the cytoplasm and the nucleus of leukocytes can readily deform; this enables them to leave the peritubular capillaries of the kidneys and migrate through renal tissue (interstitium).
When using an automated microscopic analyzer that evaluates urine as it moves through a flowcell (e.g., flow cytometry, digital flow microscopy), the shape of WBCs may be affected–they may not appear spherical but elongated and amoeboid-like. To investigate this physical change, a cytospin slide of the urine sediment can be prepared and stained with Wright stain. One finding in a subset of samples found the presence of numerous vacuoles in WBCs, which may account for the atypical shape when the cells are moving through a flowcell system; that is, the cells are unable to maintain their typical spherical form (see Fig. 7.61B).
When microscopic examination reveals WBC casts, this finding provides diagnostic evidence of an upper UTI. Similarly, cellular casts (i.e., cell identity cannot be determined) and coarsely granular casts (which result from cell degradation) may also support a diagnosis of an upper UTI. In these cases, the protein reagent strip test should be positive. In contrast, with lower UTIs (those localized below the kidney, such as in the bladder), microscopic examination would reveal increased WBCs but without cellular casts; if protein is present, it is usually at a low level.
Correlation with Physical and Chemical Examinations
When WBCs are present in the urine in increased numbers, the urine may be cloudy. Depending on the extent of the infection, the urine may have a strong, foul odor. A macroscopic examination of the sediment button may show a large amount of gray-white material: the concentrated leukocytes. Because leukocytes readily lyse in urine, discrepancies can occur between the number of cells seen microscopically and the leukocyte esterase (LE) screening test. A positive LE test, despite few or no WBCs present microscopically, can occur due to WBC lysis and disintegration. Also, different populations of WBCs have varying quantities of cytoplasmic granules and therefore differing amounts of LE. In fact, lymphocytes have no LE. When increased numbers of WBCs are present in urine, but the LE test is negative, the microscopist must ensure that the cells are granulocytic leukocytes and that the reagent strips are functioning properly. Although the LE screening test usually detects 10 to 25 WBCs per microliter, the amount of esterase present may be insufficient to produce a positive response. Note that owing to hydration, hypotonic urine could cause the LE to be diluted such that it is below the detection limit of the LE reaction. Table 7.7 summarizes the microscopic features of WBCs and the expected correlation between physical and chemical examinations when WBCs are present.
Table 7.7
| Microscopic features | Neutrophils |
| Look-alike elements | |
| Correlation with physical and chemical examinations |
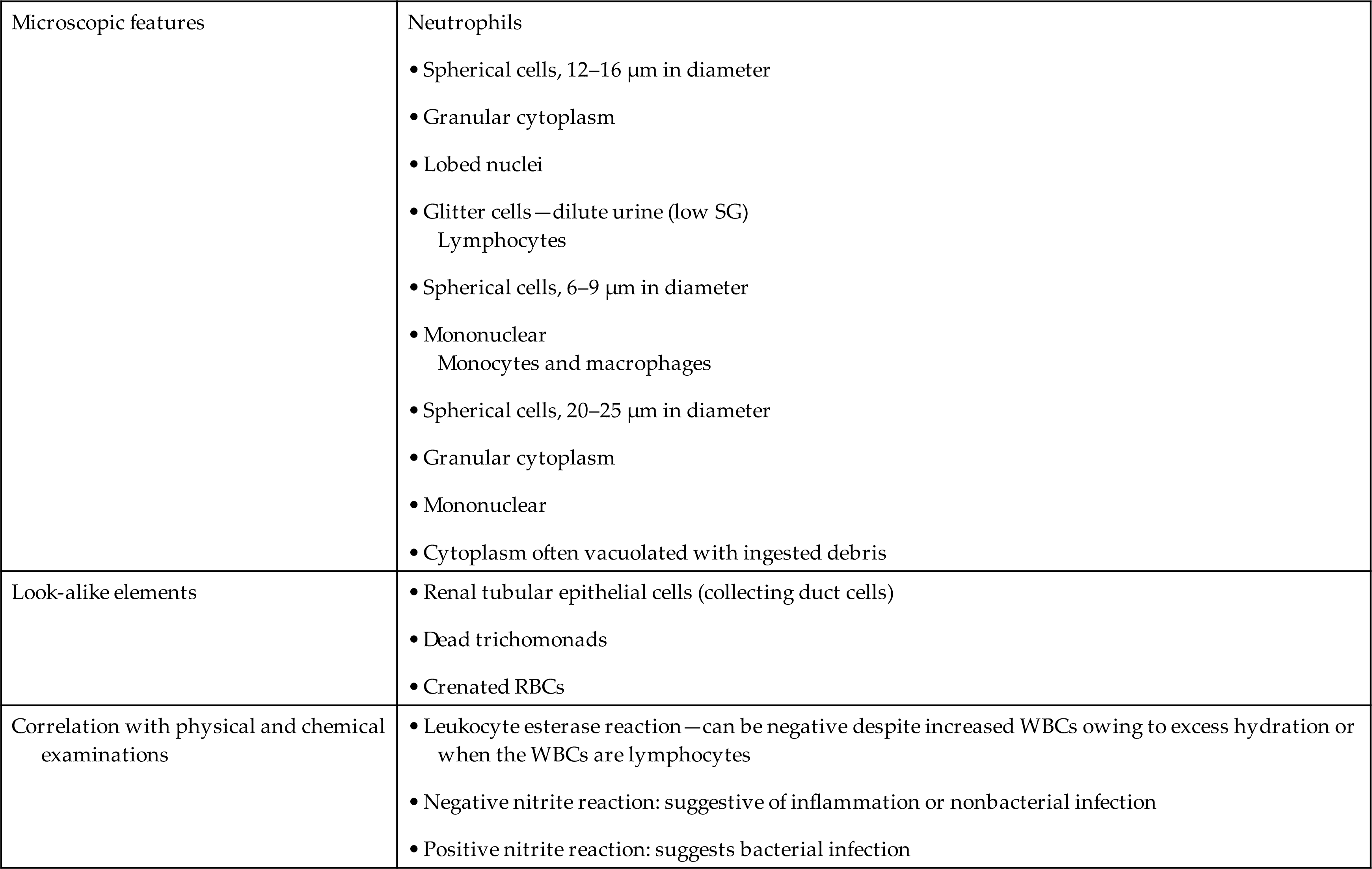
RBC, Red blood cell; SG, specific gravity; WBC, white blood cell.
Look-Alikes
As mentioned earlier, some renal tubular epithelial cells and at times even RBCs can be difficult to distinguish from leukocytes. A 2% acetic acid solution or, better yet, a 0.5% toluidine blue stain helps reveal the nuclear details of the cells present, which in turn enables proper cell identification. The large, dense nuclei of collecting duct cells and their polygonal shape (Fig. 7.21) help to distinguish them from spherical WBCs that have characteristic cytoplasmic granulation (see Figs. 7.16 and 7.17). Staining with Sternheimer-Malbin stain or toluidine blue can enhance cellular details for specific identification.
Clinical Significance
An increased number of WBCs in urine is termed leukocyturia. Inflammatory conditions of the urinary tract and almost all renal diseases show increased numbers of WBCs, particularly neutrophils, in the urine. Note that both bacterial and nonbacterial causes of inflammation can result in leukocyturia. Bacterial infections include pyelonephritis, cystitis, urethritis, and prostatitis; nonbacterial infections include nephritis, glomerulonephritis, chlamydia, mycoplasmosis, tuberculosis, trichomonads, and mycoses. The latter two organisms, trichomonads and mycoses, often appear in urine from women as contaminants from vaginal secretions. Although they can infect the urinary tract, infection is rare. In contrast, when these organisms are present in the urine from a male, a UTI is implied.
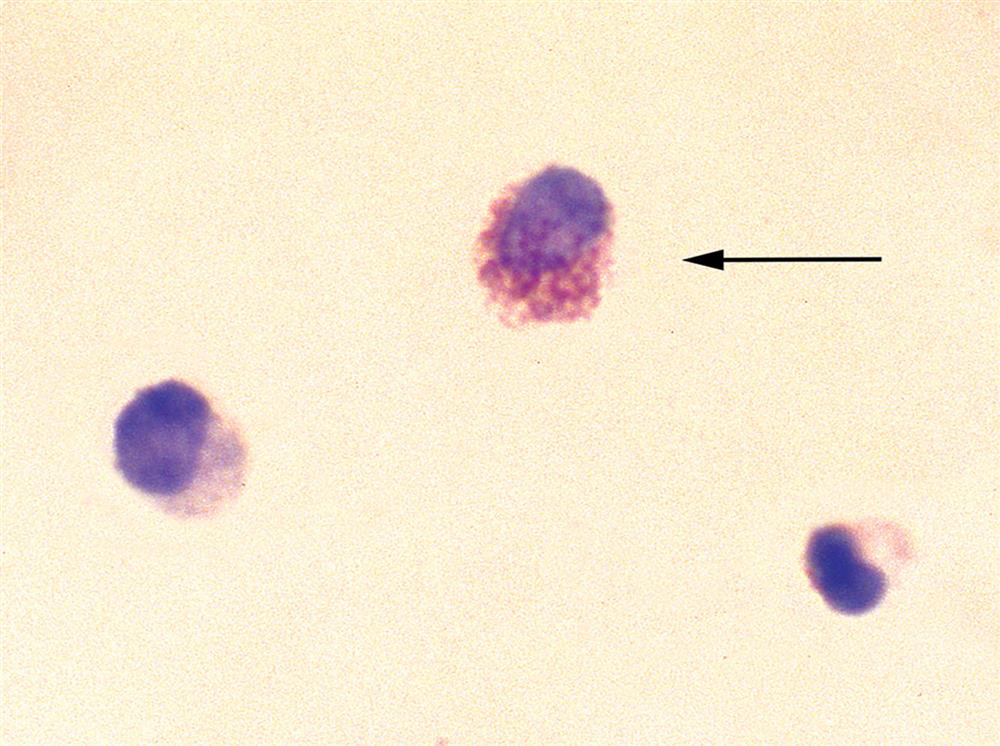
Eosinophils
In a routine microscopic examination of unstained urine sediment, the discrimination of eosinophils from neutrophils is impossible despite their bilobed nuclei and slightly larger size. When specifically requested, urine specimens for eosinophil detection should be cytocentrifuged and stained using Hansel stain. This stain is considered superior to Wright’s stain in detecting eosinophils in urine (see Fig. 7.9).
Historically, urine eosinophils were associated with AIN. However more robust studies with biopsy-proven diagnoses have shown that urine eosinophil counts are too insensitive and nonspecific to either confirm or exclude such a diagnosis.16,17 In other words, urine eosinophils should not be used as a biomarker for AIN because other causes of acute kidney injury can also present with increased urine eosinophils. These disorders include pyelonephritis, acute tubular necrosis, atheroembolic renal disease, and glomerulonephritis.
Regardless of presentation (i.e., with or without urine eosinophils), untreated AIN can lead to permanent renal damage. When AIN is suspected due to hypersensitivity to an offending drug (e.g., β-lactam antibiotics, proton pump inhibitors, nonsteroidal anti-inflammatory drugs), the offending drug should be discontinued and kidney function monitored for improvement. Currently, steroid therapy for AIN has proven beneficial in restoring kidney function and reducing the risk for progression to chronic kidney disease.
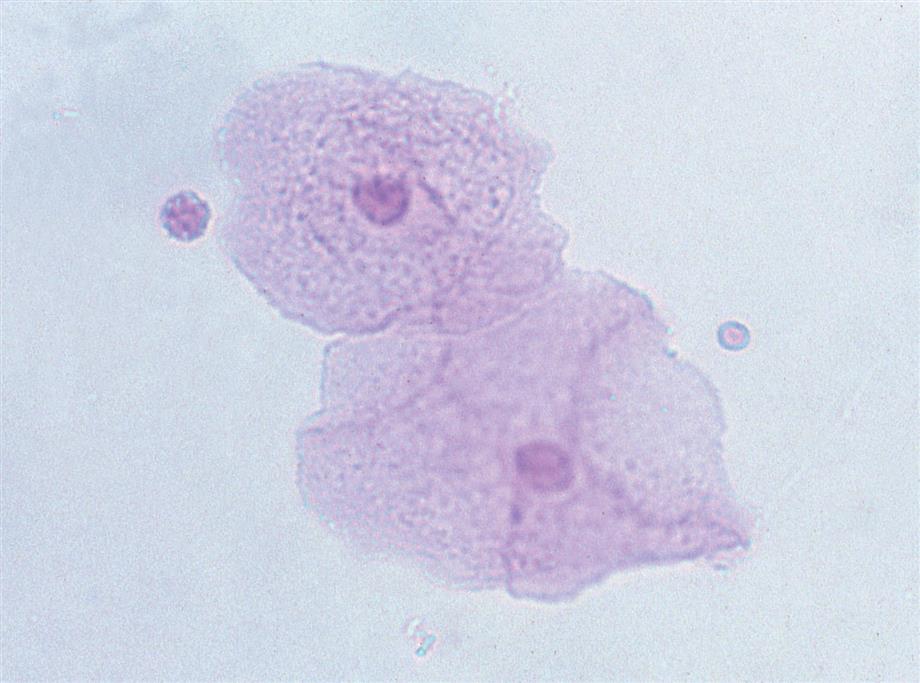
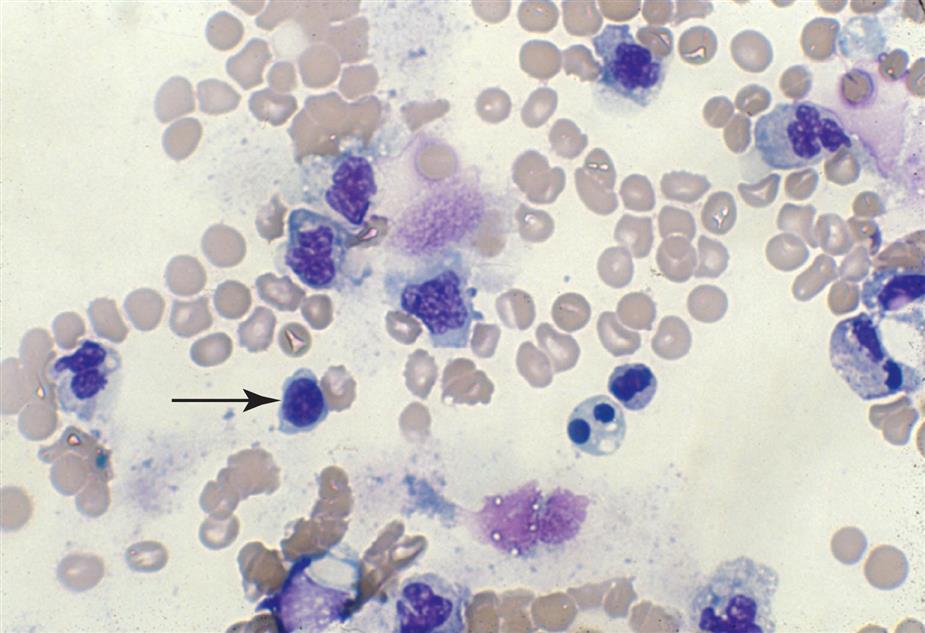
Lymphocytes
Although lymphocytes are normally present in the urine, these leukocytes are usually not recognized because of their small numbers. When supravital stains are used or a cytodiagnostic urinalysis using Wright’s or Papanicolaou’s stain is performed, lymphocytes are more readily apparent and identifiable (Fig. 7.22). Most prevalent in the urine are small lymphocytes, approximately 6 to 9 μm in diameter. They have a single, round to slightly oval nucleus and scant clear cytoplasm that usually extends out from one side of the cell. Lymphocytes are present in inflammatory conditions such as acute pyelonephritis; however, because neutrophils predominate, lymphocytes often are not recognized. In contrast, lymphocytes predominate in urine from patients experiencing renal transplant rejection. Because lymphocytes do not contain LEs, they will not produce a positive LE test regardless of the number of lymphocytes present.
Monocytes and Macrophages (Histiocytes)
Monocytes and macrophages can be observed in urine sediment. They are actively phagocytic cells that are capable of phagocytizing bacteria, viruses, antigen-antibody complexes, RBCs, and organic and inorganic substances (e.g., fat, hemosiderin). The primary functions of these cells are (1) to defend against microorganisms, (2) to remove dead or dying cells and cellular debris, and (3) to interact immunologically with lymphoid cells. Renal tubulointerstitial diseases resulting from infections or immune reactions draw monocytes and macrophages to the site of inflammation by chemotaxis, that is, their movement from the bloodstream into renal tissue occurs in response to a chemoattractant stimulus.
Monocytes range in diameter from 20 to 40 μm. They have a single large nucleus that is round to oval and often indented. The cytoplasm can be abundant and contains azurophilic granules. Because monocytes are actively phagocytic cells, large vacuoles often containing debris or organisms within them can be observed (Fig. 7.23).

Macrophages are derived from monocytes; when they reside in interstitial tissues, they are often called histiocytes. Although macrophages average 30 to 40 μm in diameter, they can be as small as 10 μm or as large as 100 μm in diameter. When they are small, their oval nuclei and azurophilic granules make them difficult to distinguish from neutrophils. Because macrophages are transformed from monocytes, they usually have irregular, kidney-shaped nuclei and abundant cytoplasm. They are actively phagocytic, so their cytoplasm is often vacuolated. Because of their variable size and appearance, macrophages can be difficult to identify in an unstained urine sediment.
Monocytes and macrophages are identified more easily by using supravital stains on the urine sediment or by making a cytocentrifuged preparation followed by Wright’s or Papanicolaou’s stain. In addition, because monocytes and macrophages contain azurophilic granules, they can be detected by the chemical screening test for LE if they are present in sufficient numbers.
During microscopic examination of an unstained urine sediment, monocytes can be misidentified as renal tubular cells. They are of similar size, and both are mononucleated. However, monocytes or macrophages are spherical in urine, whereas renal tubular epithelial cells have dense nuclei and tend to be polygonal with one or more flat edges.
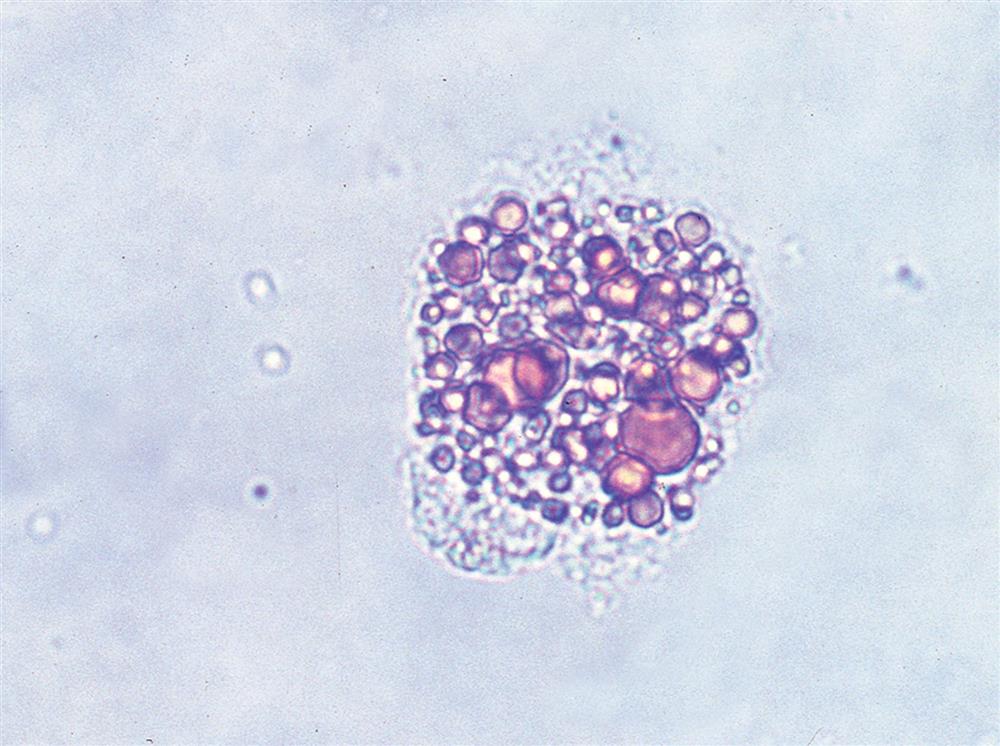
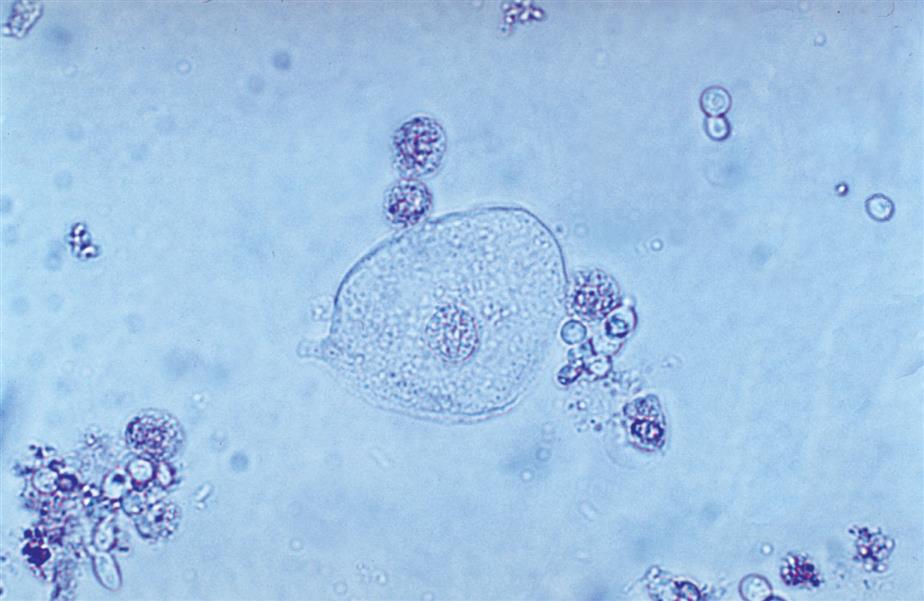
When monocytes or macrophages have ingested lipoproteins and fat, these globular inclusions are distinctly refractile (Fig. 7.24). Called oval fat bodies, these cells are impossible to distinguish from renal tubular cells that can also absorb fat. The microscopist can use polarizing microscopy or fat stains to confirm the identity of the lipid inclusions.
Epithelial Cells
Various types of epithelial cells are seen in urine sediment. Some epithelial cells result from normal cell turnover of aging cells, whereas others represent epithelial damage and sloughing caused by inflammatory processes or renal disease. Familiarity with the type of epithelium present in each portion of a nephron and in the urinary tract (e.g., urethra, bladder, ureters) facilitates identification of cells in urine sediment. In addition, the presence of large numbers of some cell types can indicate an improperly collected specimen, whereas increased numbers of others indicate a severe pathologic process. Whenever epithelial cells with abnormal characteristics are observed, such as unusual size, shape, inclusions, or nuclear chromatin pattern, additional cytologic studies are necessary. These cells may indicate neoplasia in the genitourinary tract or can result from treatments, such as chemotherapy or radiation.
Basically three types of epithelial cells are observed in urine sediment: squamous, transitional (urothelial), and renal tubular epithelial cells (Table 7.8). By far the most common epithelial cells encountered are squamous epithelial cells. Renal epithelial cells are those from the nephrons of the kidney. They consist of several distinctively different cell types, with each originating from a specific part of the nephron (i.e., collecting duct cells, proximal convoluted tubular cells, distal convoluted tubular cells). The type of cell encountered depends on the location of the disease process that is causing the epithelium to be injured and sloughed. Although identification of some epithelial cells can be difficult in wet preparations, techniques are available to facilitate proper cell identification. Each laboratory should have a policy that addresses urine sediments with unusual or abnormal cellularity, such as atypical cells or cellular fragments. This policy may simply involve forwarding the specimen to the cytology department for analysis or performing a cytodiagnostic urinalysis. Because both the presence of certain types of epithelial cells and the number of epithelial cells present can be clinically significant, it is important that the microscopist use any techniques available to ensure the proper identification and reporting of epithelial cells.
Table 7.8
| Cell Type | Site | Relative Size and Diameter | Morphology | Clinical Significance |
|---|---|---|---|---|
| Squamous | 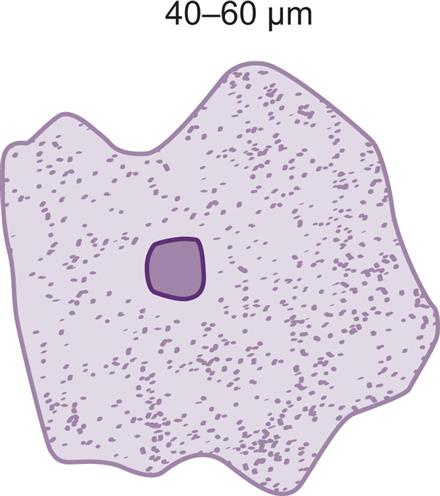 | • Shape: thin, irregularly angled (or flagstone-shaped) with distinct cell borders • Abundant cytoplasm; cytoplasmic granulation increases as cell ages • Nucleus: ≈8–14 μm,a centrally located; can be anucleated or multinucleated |
||
| Transitional | 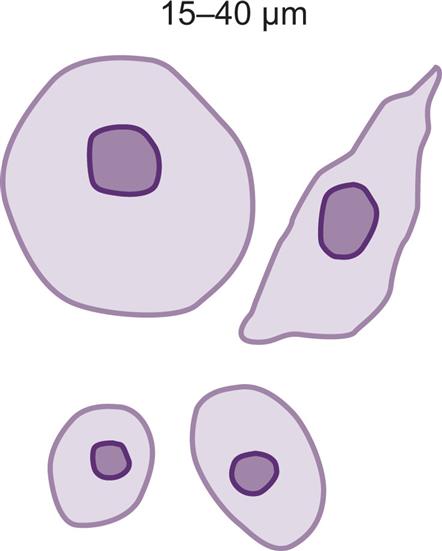 |
Superficial cells: round or pear-shaped Intermediate layer: club-like,caudate (with tail), elongated Basal layer: small, rectangular (or columnar-like) • Moderate amount of cytoplasm • Distinct cell borders that appear “firm” • Nucleus: ≈8–14 μm,a round or oval, centrally located in oval/round cells; off-center in elongated cells |
||
| Renal | Collecting duct cells | 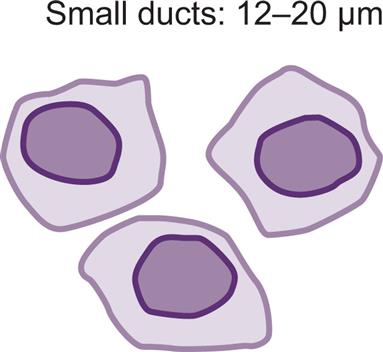 |
Small duct cells • Shape: polygonal or cuboidal (Hint: Look for a flat edgea) |
ShockAnoxiaSepsis |
 |
Large duct cells | |||
| Convoluted tubular cells | 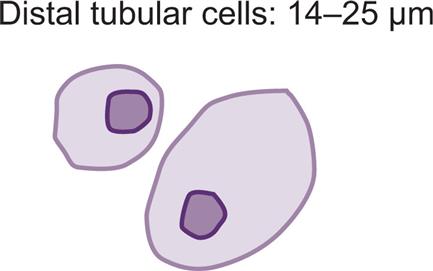 |
Distal tubular cells | Heavy metalsHemoglobinuria, myoglobinuria | |
 |
Proximal tubular cells |
bOver time, cells in urine absorb water to become swollen, and the flat edge may not be as noticeable.
aApproximately the size of a red blood cell or a white blood cell.
During the microscopic examination, squamous epithelial cells are easily observed using low-power magnification because of their large size. In contrast, transitional and renal epithelial cells are better assessed using high-power magnification. After epithelial cells are observed in 10 representative FOVs at the appropriate magnification, the report should indicate each type of epithelial cell encountered. The report format may use descriptive terms such as few, moderate, or many per FOV or may be numeric such as 5 to 10 cells per FOV.
Squamous Epithelial Cells
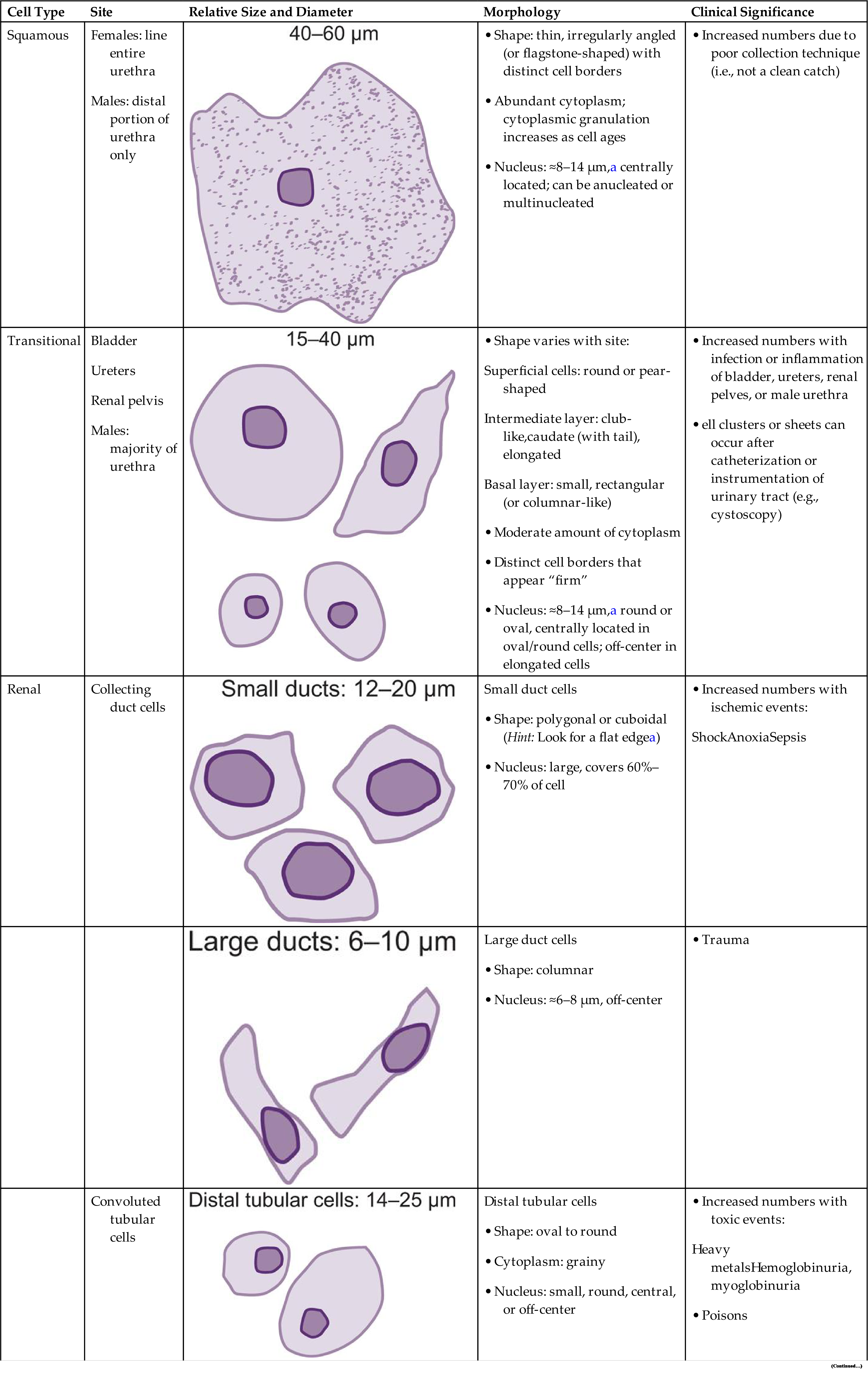
Squamous epithelial cells are the most common and the largest epithelial cells found in the urine (Figs. 7.25 and 7.26). These cells line the entire urethra in the female but only the distal portion of the urethra in the male. Routinely, the superficial layers of the squamous epithelium are desquamated and replaced by new, underlying epithelium. In women, large numbers of squamous epithelial cells in the urine sediment often indicate vaginal or perineal contamination; similarly in uncircumcised men, large numbers suggest specimen contamination. Squamous epithelial cells are large (40–60 μm), thin, flagstone-shaped (i.e., irregularly angled) cells with distinct edges that may be present in clumps. They have a small, condensed, centrally located nucleus about 8 to 14 μm (i.e., the size of an RBC or WBC) or they can be anucleated. Their large amount of cytoplasm is often stippled with fine granulation (keratohyalin granules), which increases as the cells degenerate. Squamous epithelial cells can be observed in unusual conformations because their edges can fold over or curl while they are suspended in urine, making a full or partial tubular form (Fig. 7.27).
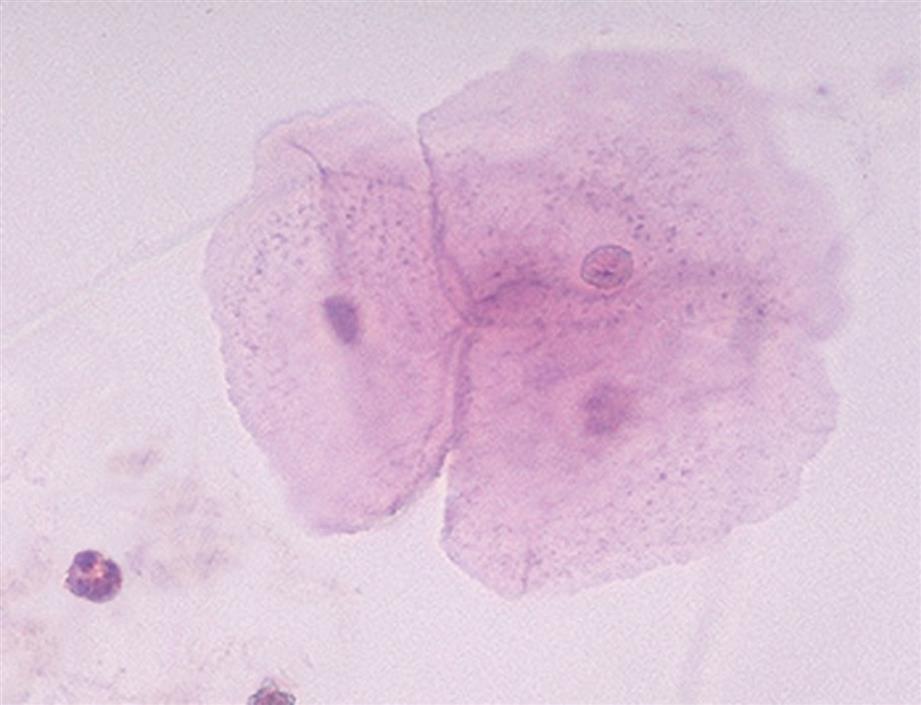
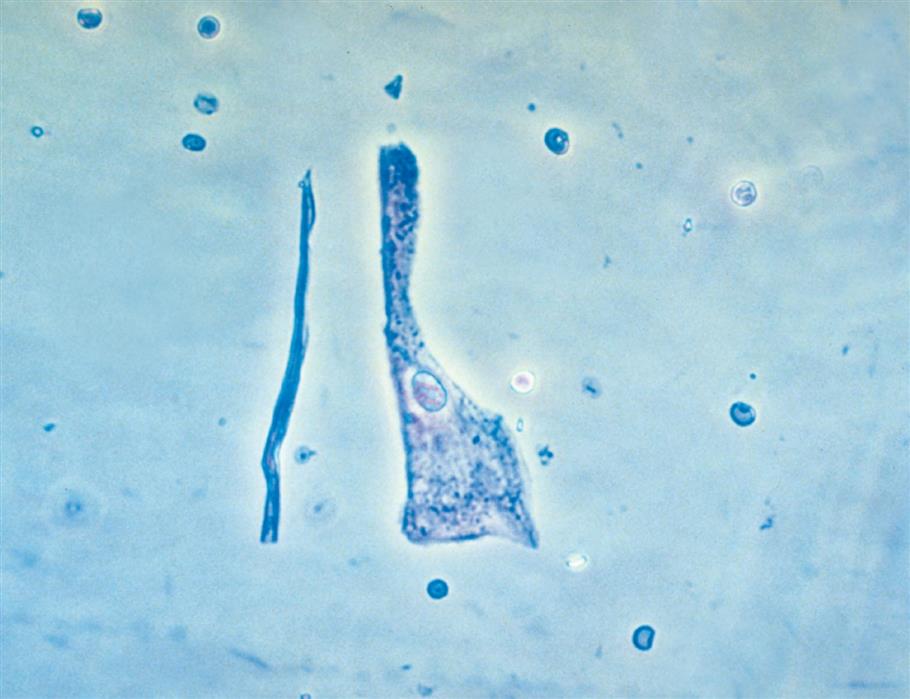
Squamous cells, which are easily identified using low-power magnification, are the only epithelial cells evaluated using this magnification. Squamous epithelial cells in urine specimens rarely have diagnostic significance and usually indicate that the specimen was not a midstream clean catch.
Transitional (Urothelial) Epithelial Cells
The renal calyces, renal pelvis, ureters, and bladder are lined with several layers of transitional epithelium. In the male, this type of epithelium also lines the urethra except for the distal portion, whereas in the female, transitional epithelium ceases at the base of the bladder. Transitional (urothelial) epithelial cells vary considerably in size and shape (Figs. 7.28 and 7.29). This variation relates primarily to the layers of transitional epithelium in the bladder. The cells in the uppermost or superficial layer are large (30–40 μm) and usually round or pear-shaped. Cells from the intermediate layers or from the trigone region of the bladder are elongated, caudate (i.e., with a cytoplasmic tail), or club-like, and their nucleus is usually off-center (see Fig. 7.28B, and 7.29A). Those from the deep basal layer are smaller (15–30 μm) and tend to be rectangular.
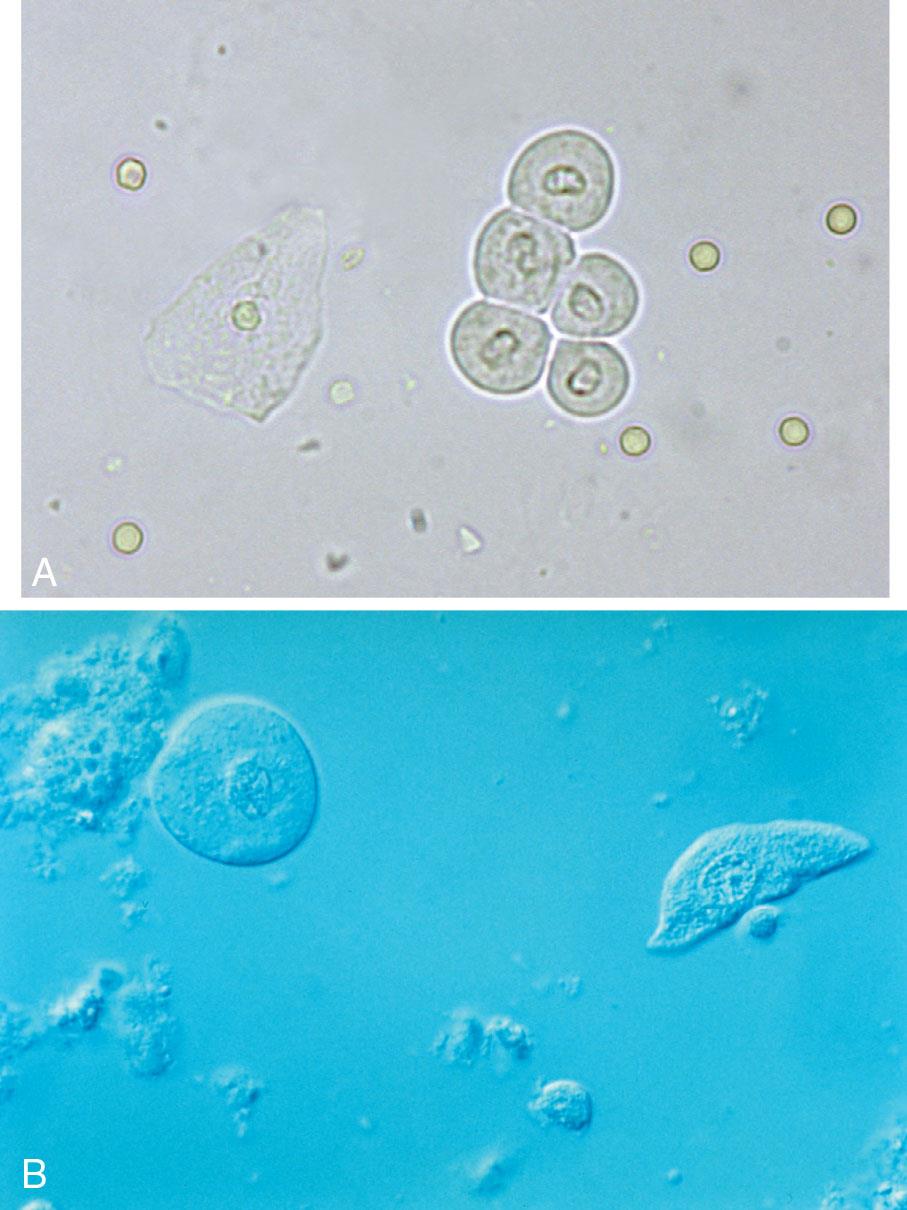
A few transitional epithelial cells can be present in the urine sediment from normal, healthy individuals and represent routine sloughing of old epithelium. The most prevalent form of transitional cells is the superficial type: round or pear-shaped, with a dense oval to round nucleus and abundant cytoplasm (see Fig. 7.28A). The nucleus is about the size of a RBC or WBC, and the peripheral borders of the nucleus and cell membrane are distinctly outlined.
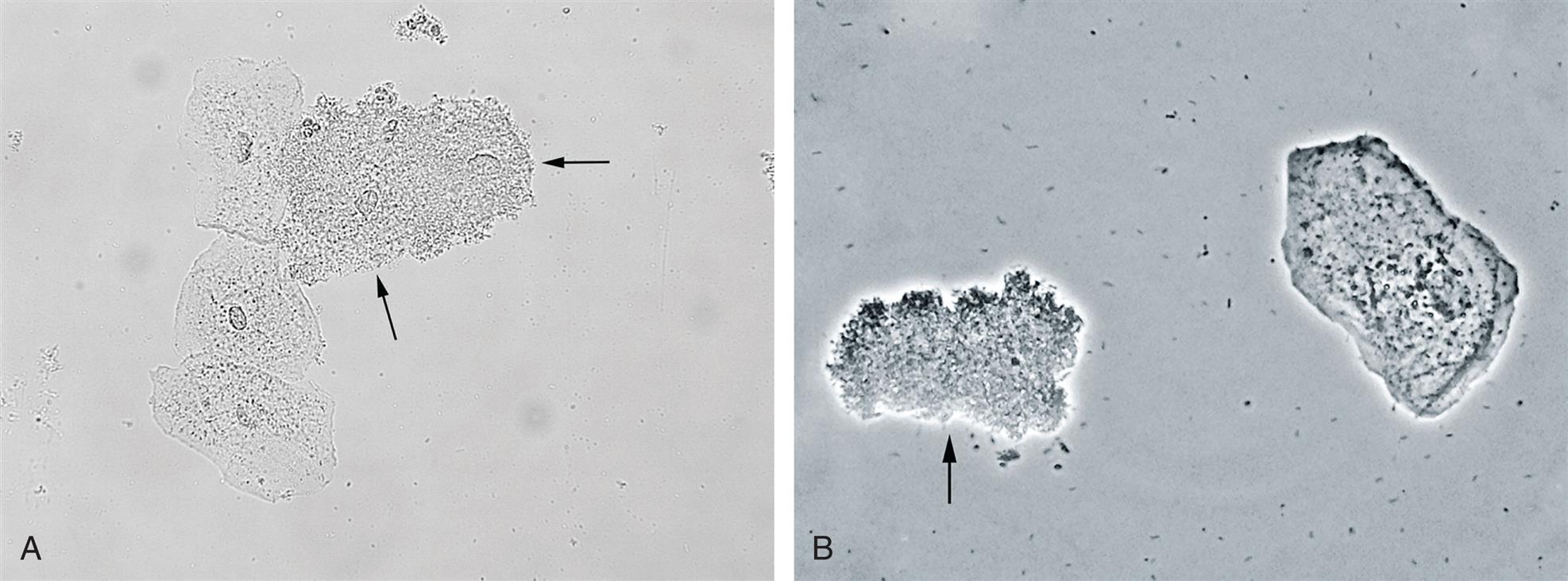
With UTI or inflammation, the epithelium can be irritated and increased numbers of transitional epithelial cells sloughed in the urine. At times, fragments or sheets of transitional epithelium are observed after bladder instrumentation, such as catheterization or cystoscopy (see Fig. 7.29B). However, when clusters of cells appear without these procedures or the cell nuclei are large with nuclear irregularities, they could indicate a pathologic process that requires further investigation, such as transitional cell carcinoma (i.e., bladder cancer).
Decoy Cells
Decoy cells are transitional or renal tubular epithelial cells that are infected with polyomavirus of the BK virus (BKV) strain. The name decoy originated because of the resemblance and potential misidentification of these cells in urine as malignant cells. These infected epithelial cells have enlarged nuclei with large homogeneous, intranuclear basophilic inclusions (i.e., hyperchromatic nuclei) (Fig. 7.30). In other types of decoy cells, an intranuclear halo resembling cytomegalovirus (CMV) infection can be present or the cells can be multinucleated. Common nuclear features of BKV-infected cells include: (1) nuclear enlargement (basophilic, homogeneous, ground glass-like intranuclear inclusions) with displacement of the nucleus to the cell periphery—making it appear as if the nucleus was “trying to escape from” the cell (i.e., “comet-like” cells); (2) chromatin clumping along the nuclear membrane (i.e., margination); (3) abnormal chromatin patterns—coarse granules of variable size, shape, and irregular arrangement; and (4) the presence of cytoplasmic vesicles.9 Note that laboratories should have a protocol for actions to be taken when unusual or abnormal epithelial cells are encountered in urine sediment. Differentiation of epithelial cells as atypical, malignant, or decoy cells requires an experienced microscopist or cytologist.18 To assist in identification, additional procedures are performed such as preparing cytospins of the urine sediment followed by Papanicolaou staining.

BKV primarily colonizes the superficial transitional epithelium of the lower urinary tract—bladder, ureters, and renal pelves—where it is asymptomatic, does not last long, and does not affect kidney function. However in kidney transplant patients receiving immunosuppressive medications, BKV can reactivate. If the infection spreads into the collecting ducts of the nephrons, it can cause a condition known as BKV nephropathy (BKVN). The infected renal tubular cells become damaged and lyse, causing inflammation and the release of viral particles into the interstitium of the kidney and the bloodstream.
In kidney transplant patients, decoy cells are associated with transplant rejection. However, their negative predictive value (>99%) for kidney transplant rejection is even stronger, such that when decoy cells are absent, it is unlikely that the patient is rejecting the transplanted organ.
Renal Tubular Epithelial Cells
As described in Chapter 3, each portion of a nephron or renal tubule is lined with a single layer of a characteristic epithelium. A few renal tubular cells can appear in urine from normal, healthy individuals and represent routine replacement of aging or old epithelium. However, the presence of more than 15 renal tubular cells in 10 high-power fields suggests intrinsic renal disease.19 Newborn infants have more renal tubular cells in their urine than do older children or adults.
In the microscopic examination of urine, two categories of renal epithelial cells can be present: convoluted tubular cells and collecting duct cells. Often, these are not distinguished but are enumerated and reported collectively as “renal epithelial cells.”
Convoluted Renal Tubular Cells
Because the cytoplasm of convoluted tubular cells is coarsely granular, their nuclei are not readily visible when phase contrast microscopy is used, and these cells can resemble granular casts. Using brightfield microscopy and staining the urine sediment greatly enhance visualization of the nuclei and correct identification of these cells. Cytocentrifugation followed by Papanicolaou’s staining of the urine sediment can be used to specifically identify these cells.
Differentiating between proximal convoluted tubular cells and distal convoluted tubular cells is difficult and is based primarily on size and shape. Usually differentiation between proximal and distal convoluted tubular cells is not necessary, and these cells are collectively reported as “convoluted” renal tubular cells.
Proximal Convoluted Tubular Cells
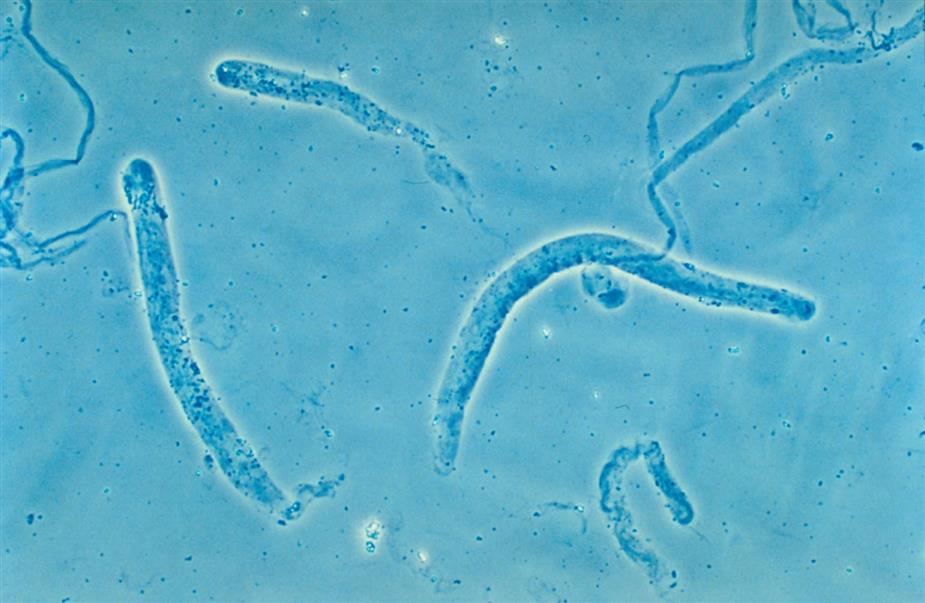
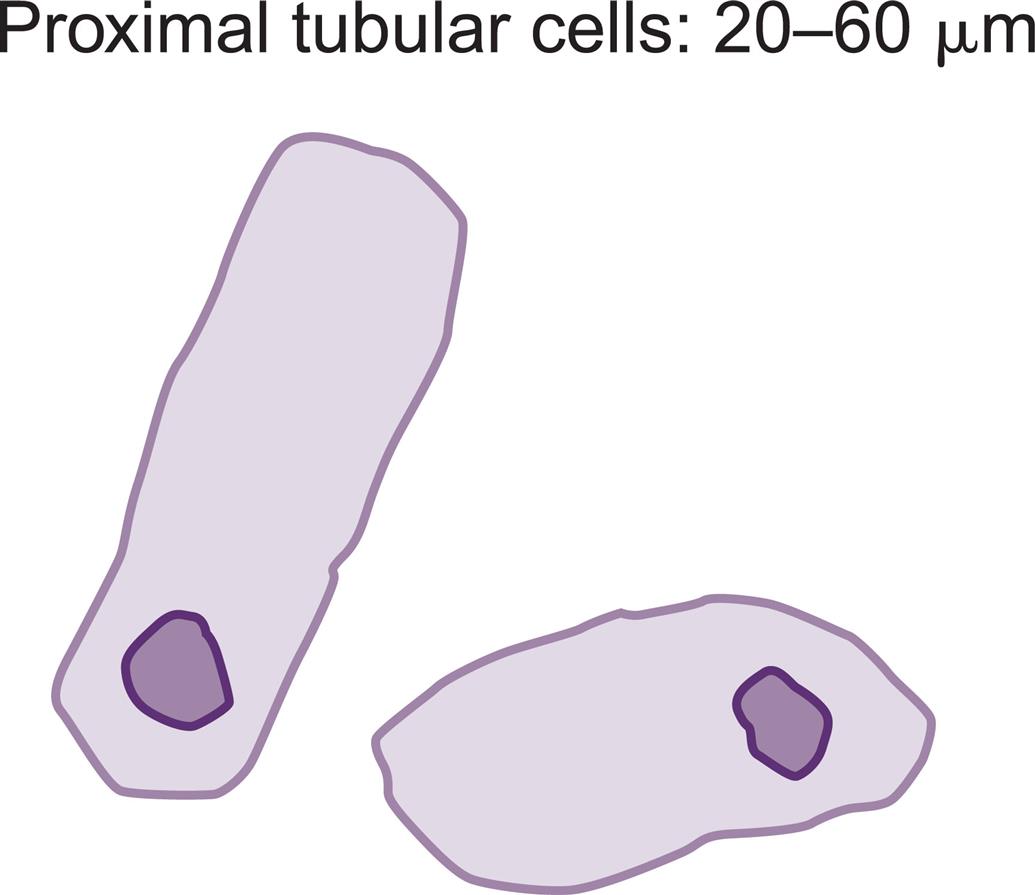
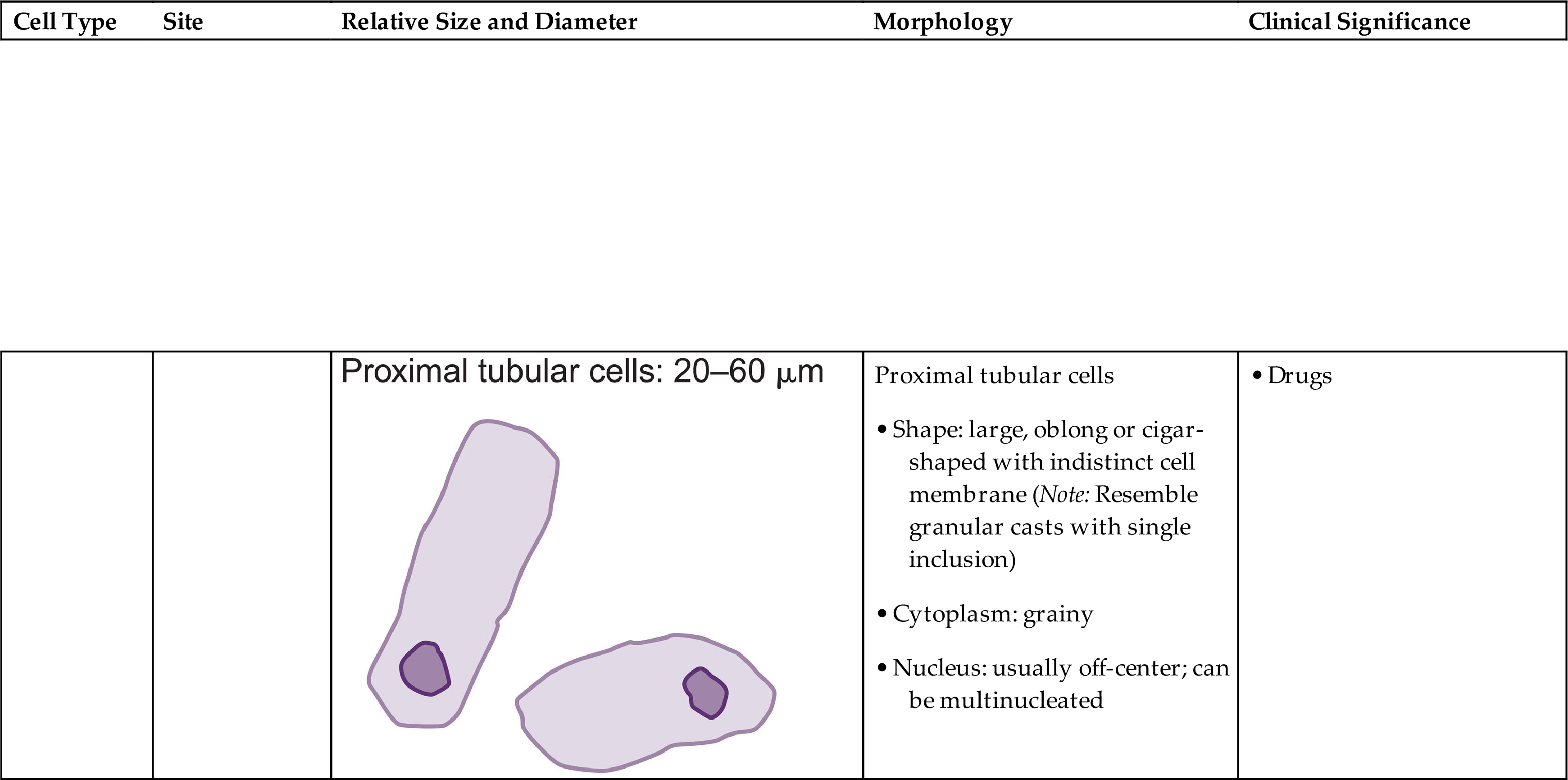
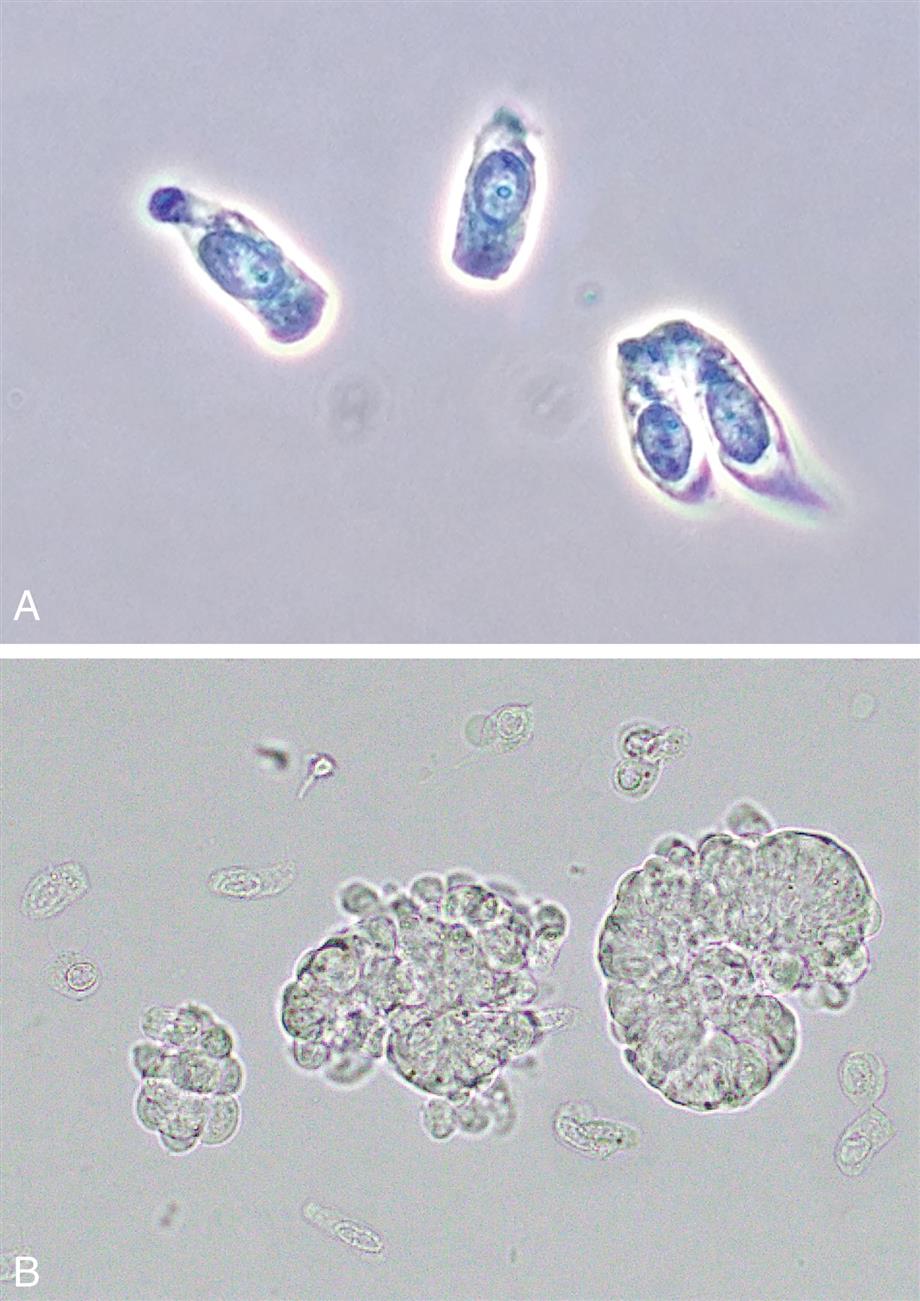
These are large cells (20–60 μm in diameter or length) with granular cytoplasm. They are oblong or cigar-shaped (Fig. 7.31)—a characteristic that makes them resemble granular casts. They have a nucleus with a dense chromatin pattern that is usually eccentric, and they can be multinucleated.
Distal Convoluted Tubular Cells
These round to oval cells measuring approximately 14 to 25 μm in diameter are smaller than cells of the proximal tubule (Fig. 7.31B). They have a small, dense nucleus that is usually eccentric and they have a granular cytoplasm, much like that of proximal tubular cells.
Proximal and distal convoluted tubular cells are found in the urine as a result of acute ischemic or toxic renal tubular disease (e.g., acute tubular necrosis) from heavy metals or drug (aminoglycosides) toxicity (see Chapter 8, section “Acute Tubular Necrosis”).
Collecting Duct Cells
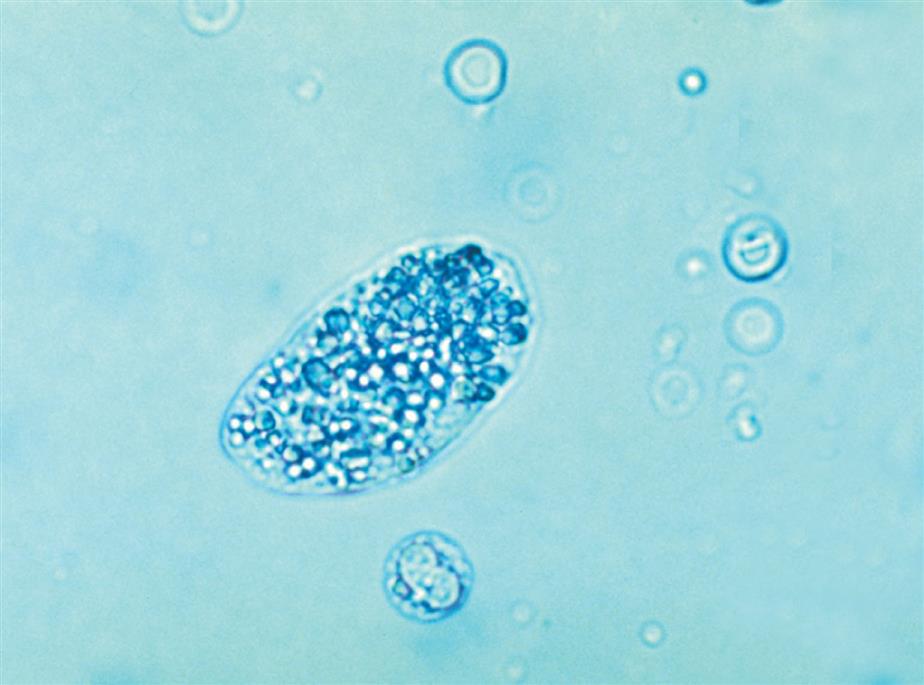
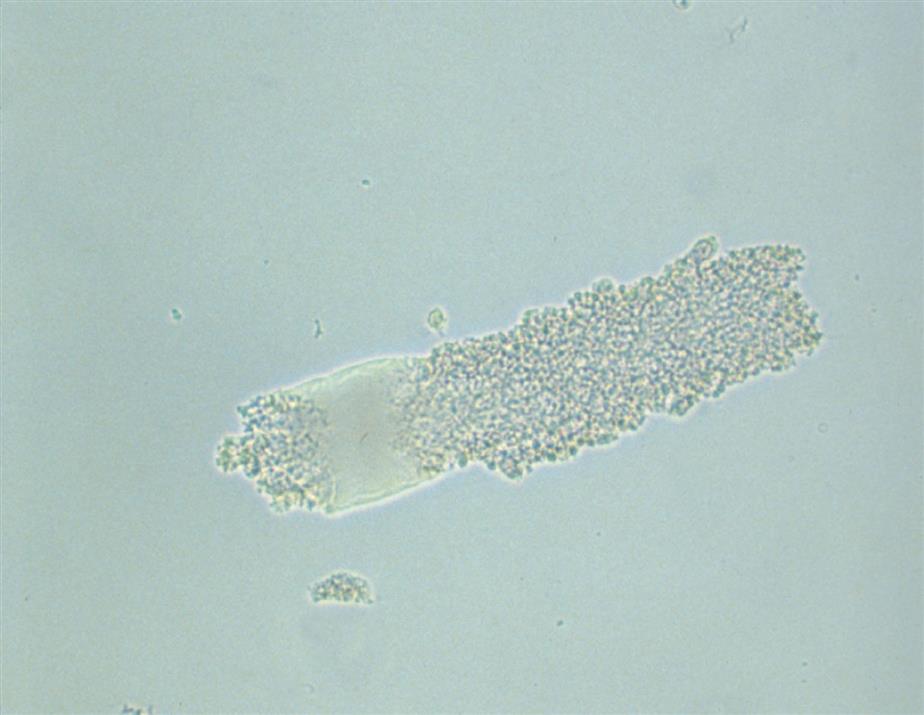
Collecting duct cells range from 12 to 20 μm in diameter and are cuboidal, polygonal, or columnar (Fig. 7.32). They are rarely round or spherical. Therefore always look for a corner or a flat edge on the cell by which to identify it. Macrophages or monocytes are round or spherical and may be misidentified as collecting duct cells. Collecting duct cells have a single large, moderately dense nucleus that takes up approximately two-thirds of its relatively smooth cytoplasm. The collecting ducts become wider as they approach the renal calyces, and their epithelial cells become larger and more columnar (Fig. 7.33 and Urine Sediment Image Gallery, Fig. 73). Increased numbers of collecting duct cells accompany all types of renal diseases, including nephritis, acute tubular necrosis, kidney transplant rejection, and salicylate poisoning.
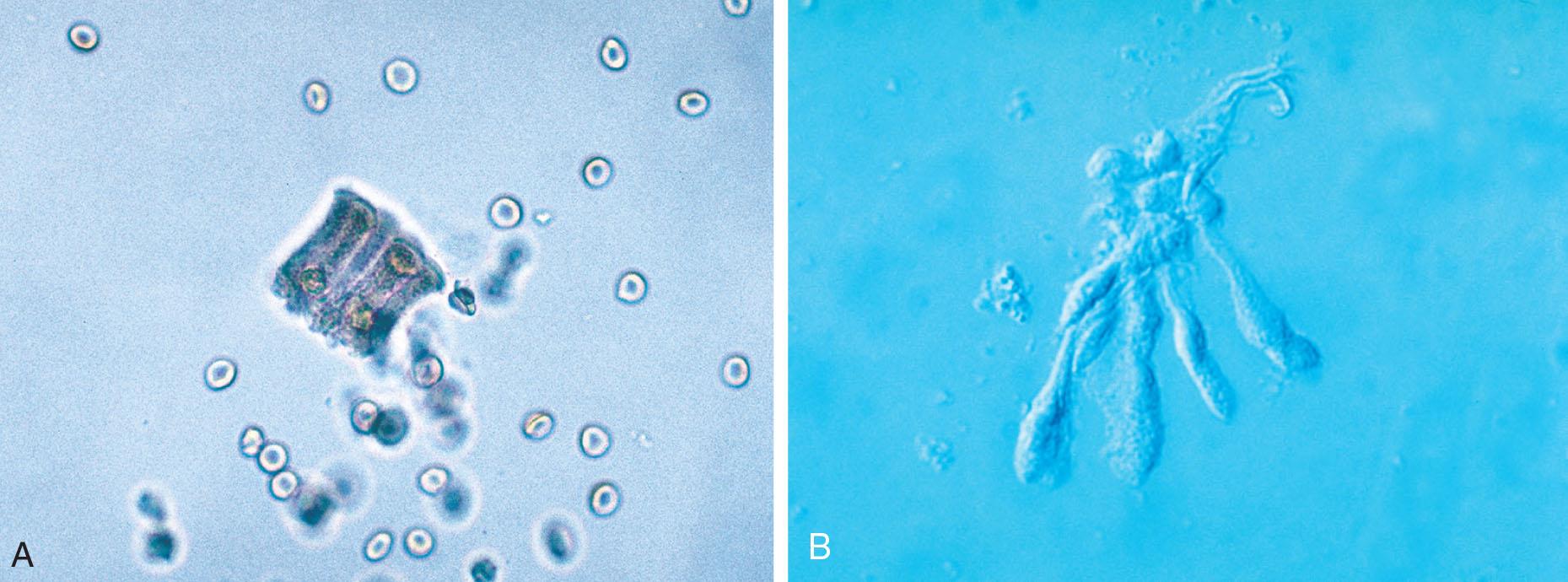
In contrast to proximal and distal convoluted tubular cells, collecting duct cells can be observed as fragments of undisrupted tubular epithelium (Fig. 7.33; see Fig. 7.5). To be identified as a fragment, at least three cells must be sloughed together with a bordering edge intact. Their presence reveals severe tubular injury and damage to the epithelial basement membrane. Collecting duct fragments are found after trauma, shock, or sepsis and indicate ischemic necrosis of the tubular epithelium. In addition to these renal cell fragments, pathologic casts (e.g., granular, waxy, renal tubular cell) and increased numbers of blood cells are usually present.
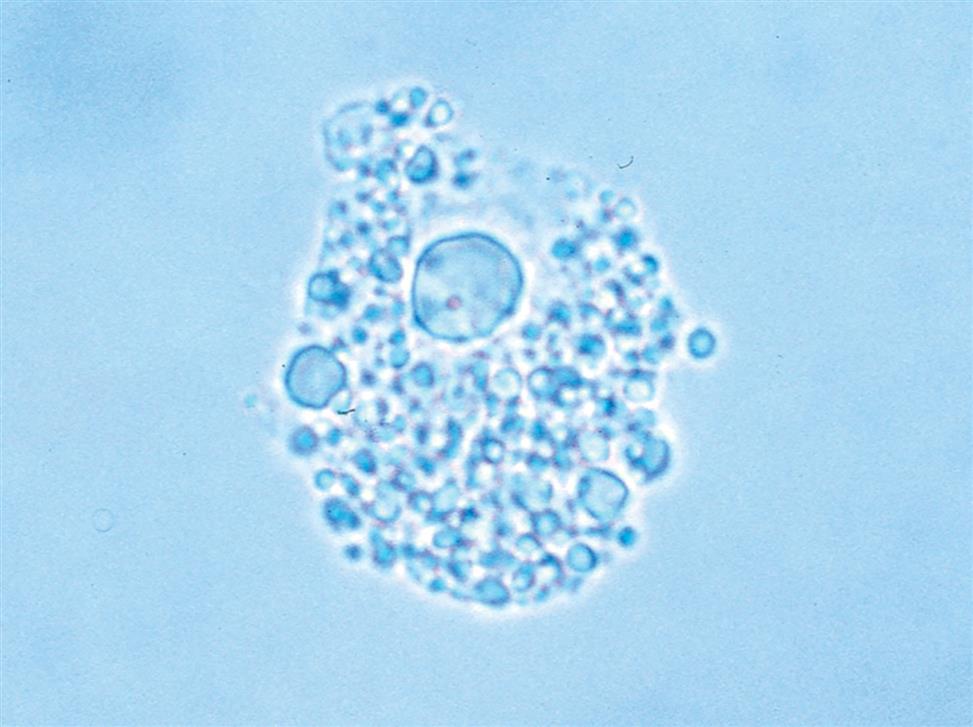
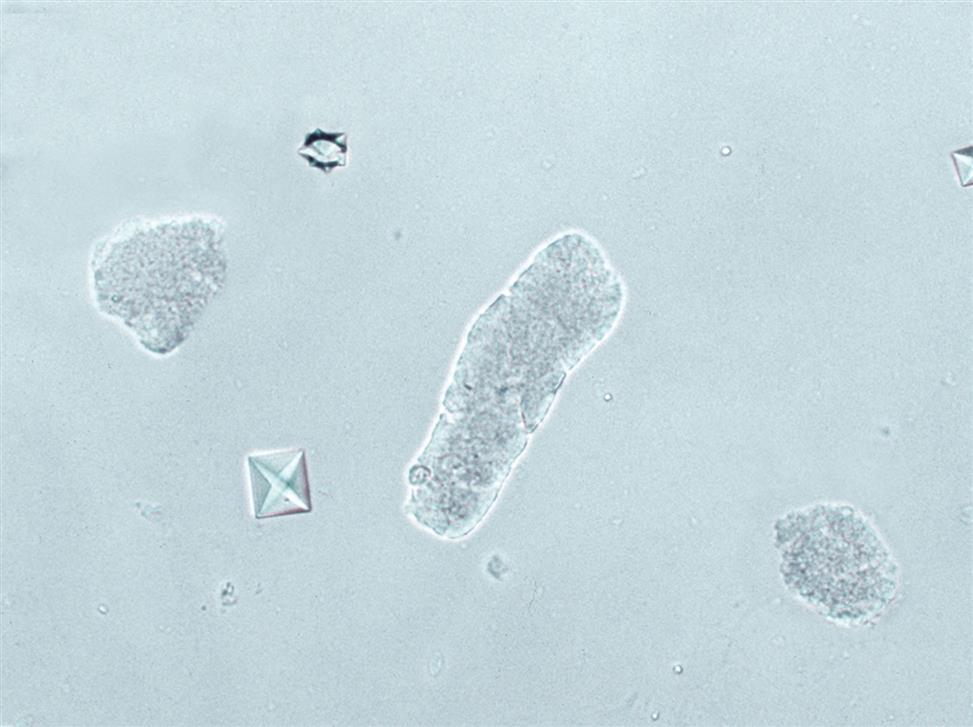
Renal Tubular Cells with Absorbed Fat
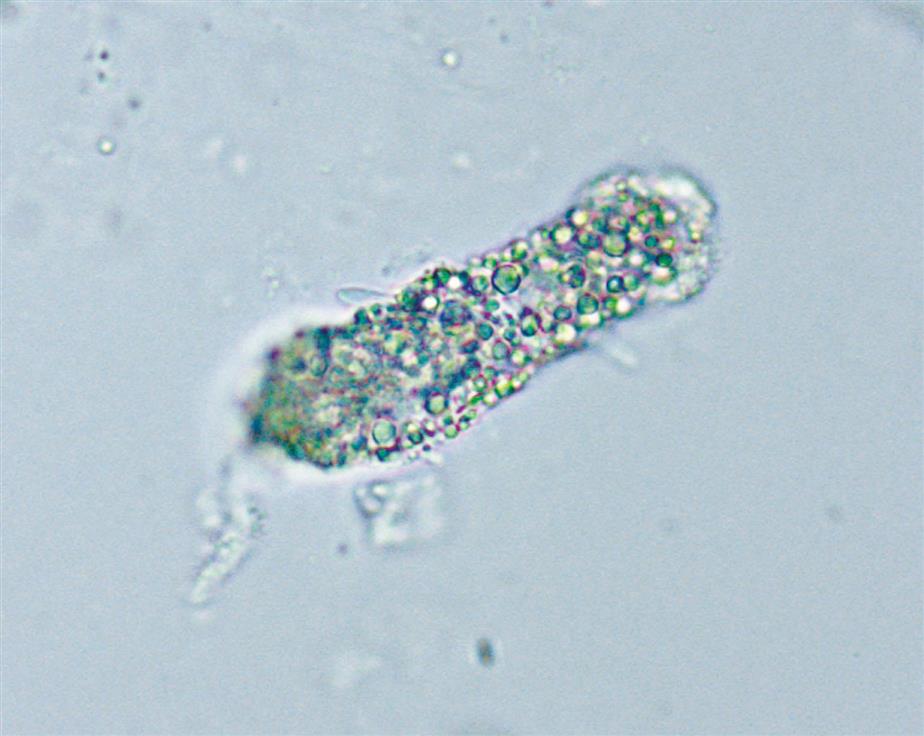
Renal tubular cells that are engorged with absorbed fat from the tubular lumen are called oval fat bodies (Fig. 7.34). These cells may have many large, highly refractile droplets, or they can have only a small number of apparently glistening granules. Because oval fat bodies often indicate glomerular dysfunction and renal tubular cell death, they are always accompanied by an increased amount of urine protein and cast formation. Oval fat bodies are positively identified using polarizing microscopy or fat stains such as Sudan III or oil red O (see Fig. 7.7). (For continued discussion on fat identification in urine, see the section “Fat” later in this chapter.)
Other Epithelial Cells
Bladder Diversion
In some individuals, whether due to congenital anomalies or disease, the bladder must be removed (cystectomy), bypassed, or replaced. There are basically three surgical options for bladder diversion and urine elimination. An ileal conduit (urostomy) procedure uses a piece of small intestine or colon to create a channel that carries urine to an opening on the abdomen (stoma), where it is collected in an external drainable pouch. When a continent urinary diversion is performed, an internal pouch is made from intestine and it is connected to the abdominal surface at an opening called a stoma. Urine is drained from the internal pouch by passing a catheter through the stoma about every 3 to 4 hours. Last, in an orthotopic neobladder procedure a new bladder is made using intestine, and it is placed in the same location as the original bladder. The ureters and urethra are attached to this new bladder. Urine drains from the ureters into the neobladder and is eliminated via the urethra.
Note that these three procedures use small intestine or colon to reconstruct a channel or pouch for urine conveyance or storage. Consequently, the urine excreted will now contain cellular and other elements that originate from the intestinal mucosa. Typically the urine contains increased amounts of mucus, WBCs, and epithelium from the intestinal lining, including goblet cells, as well as increased amounts of degenerated cells and debris. Bacteria are often present in the urine, as the presence of intestinal epithelium appears to promote asymptomatic bacterial colonization of this tissue area. Therefore in individuals with a bladder diversion, a diagnosis of an infection of the urinary tract is more complicated. Healthcare providers rely on patient symptoms and other parameters instead of a urinalysis test or urine culture.
Casts
Formation and General Characteristics
Unique to the kidney, urinary casts are formed in the distal and collecting tubules with a core matrix of uromodulin (formerly known as Tamm-Horsfall protein). This glycoprotein is secreted by the renal tubular cells of the thick ascending limb of the loop of Henle (i.e., the straight portion of the distal tubules) and by the distal convoluted tubules.20,21 As the contents of the tubular lumen become concentrated, uromodulin forms fibrils that attach it to the lumen cells, holding it temporarily in place while it enmeshes into its matrix many substances that are present. Any urinary component, whether chemical or a formed element, can be found incorporated into a cast. Eventually, the formed cast detaches from the tubular epithelial cells and is flushed through the remaining portions of the nephron with the lumen fluid.
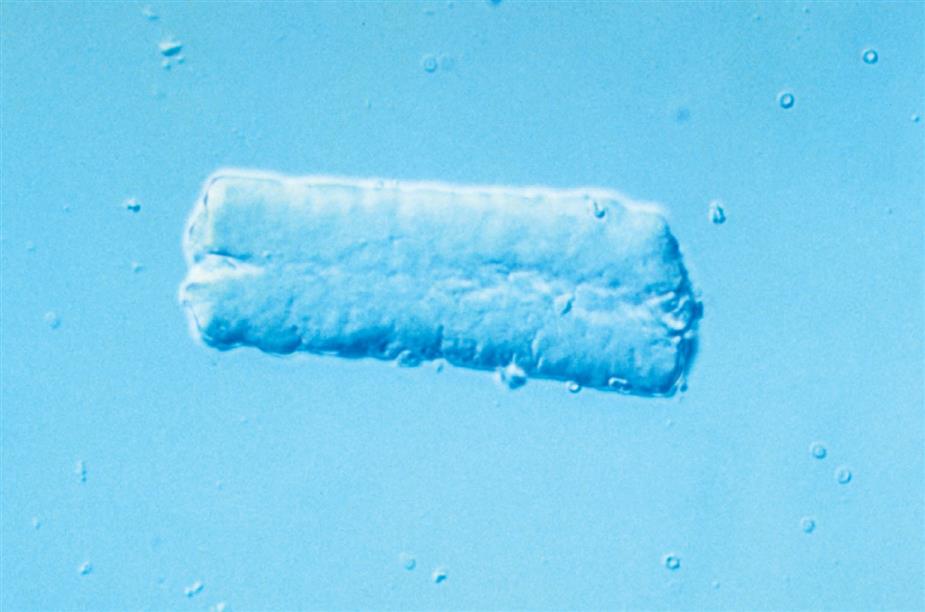
Because casts are formed within the tubules, they are cylindrical and microscopically always appear thicker in the middle than along their edges (Fig. 7.35). They have essentially parallel sides with ends that can be rounded or straight (abrupt). The shape and size of urinary casts can vary greatly depending on the diameter and shape of the tubule in which they form. The narrower the tubular lumen, the narrower is the resulting cast. Sometimes casts are well formed at one end but are tapered or have a tail at the other end (Fig. 7.36). It is postulated that these casts, sometimes referred to as cylindroids, result because of insufficient time for complete cast formation. They can be hyaline or have inclusions of granules, cells, or fat. Because they are casts and have the same clinical significance, cylindroids should be enumerated in the same categories as fully formed casts. When wide or broad casts are observed, they indicate cast formation in extremely dilated tubules or in a wide collecting duct (Fig. 7.37). Because a single collecting duct serves numerous nephrons, cast formation within them indicates pronounced urine stasis and renal disease.
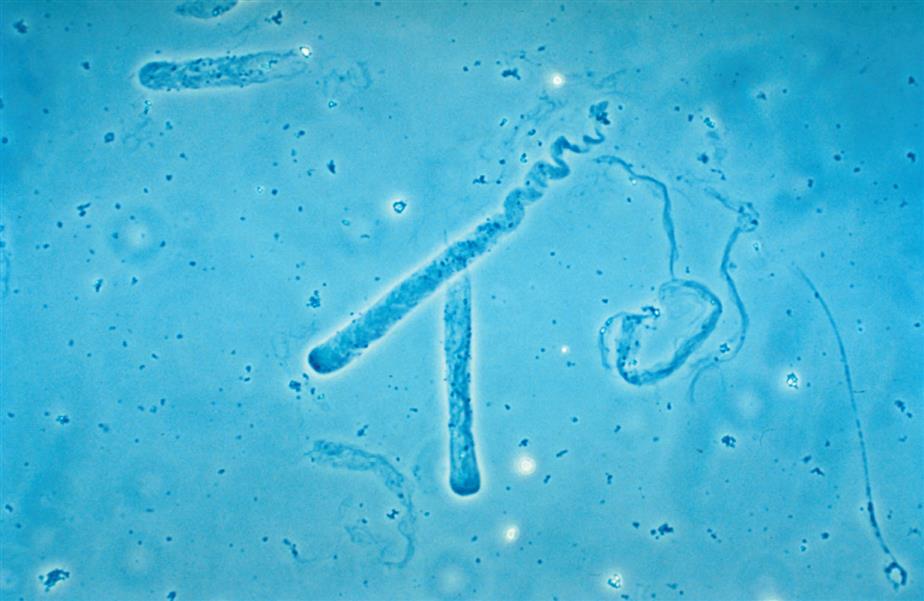

Casts can be short and stubby, long and thin, or any combination. They may be straight, curved, or convoluted. A cast becomes convoluted when after formation and release from a tubule, it encounters a tubular obstruction, such as another cast being formed. The first (narrower) cast becomes compressed to form a cast that appears convoluted (Fig. 7.38). Because casts can be retained in the tubule for varying lengths of time, the substances enmeshed in their matrices can disintegrate. In addition, the cast matrix itself can undergo changes that become apparent microscopically, for example, transition from a granular to a waxy cast (see Figs. 7.37 and 7.39). Some casts are fragile and are easily broken into chunks if the urine sediment is mixed too vigorously during resuspension (Fig. 7.40). Also note that hypotonic and alkaline urine promotes the disintegration of casts in the urine sediment.
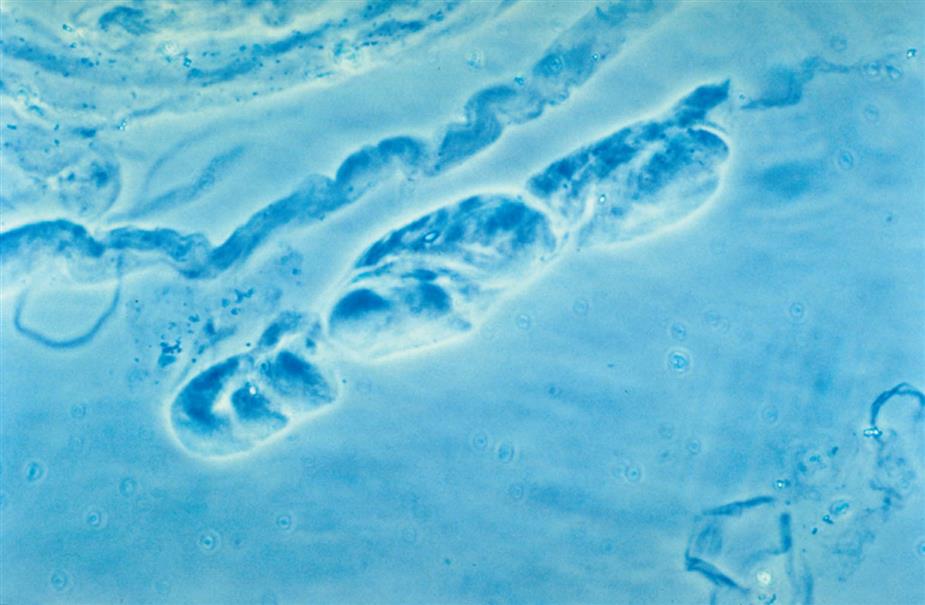
Numerous factors, such as an acid pH, increased solute concentration, urine stasis, and increased plasma proteins (particularly albumin), enhance cast formation.20 In an acidic environment, gelation of protein and the precipitation of solutes are enhanced. Because acidification and concentration of urine occur in the distal and collecting tubules, these tubules are the sites of most cast formation. Urinary stasis can occur because of obstruction from disease processes or congenital abnormalities. This stasis promotes the accumulation and concentration of ultrafiltrate components, hence cast formation. In conditions that cause increased quantities of plasma proteins (e.g., albumin, globulins, hemoglobin, myoglobin) in the lumen ultrafiltrate, cast formation is enhanced greatly. These proteins become incorporated into the uromodulin protein matrix, along with any cells and cellular or granular debris that happen to be present.
Clinical Significance
A few hyaline or finely granular casts may be present in the urine sediment from normal, healthy individuals. Casts reflect the status of the renal tubules; therefore, with renal disease, increased numbers of casts are found in urine sediment (Fig. 7.41). The number of casts reflects the extent of tubular involvement and the severity of disease. Both the types of casts and their numbers provide valuable information to healthcare providers. Two exceptions are notable. After strenuous exercise such as marathon running, increased numbers of casts can be found in the urine of normal individuals (athletic pseudonephritis); their presence does not indicate renal disease. These casts are linked to the increased albuminuria resulting from exercise-induced glomerular permeability changes. The urine sediment may show as many as 30 to 50 hyaline or finely granular casts per low-power field but returns to normal (showing no proteinuria or casts) within 24 to 48 hours. Increased numbers of casts have also been associated with some diuretic therapies.20

The importance of a patient history including the diagnosis and a list of medications cannot be overemphasized. A patient history provides information that can support or account for the numbers and types of formed elements observed. Note that physical exercise and emotional stress can affect the number of formed elements observed in the urine sediment. Increased excretion of casts is thought to be caused at least in part by increased secretion of uromodulin by renal tubular cells. A careful patient history can prevent misdiagnosis or overdiagnosis of renal dysfunction.
Classification of Casts
Casts are classified microscopically on the basis of the composition of their matrix and the types of substances or cells enmeshed within them (Box 7.3). Because any substance can be incorporated into a cast, Box 7.3 could be expanded to include all possible inclusions; those that are listed represent the most commonly encountered casts. One should keep in mind that casts can contain more than one formed element or can be of two matrix types. Mixed cellular casts often are reported as such with a description of the entities involved—for example, cellular cast: leukocytes and renal tubular cells. When a cast of two matrix types (half granular and half waxy) is encountered, the cast is identified using the term that has the greatest clinical significance. In this example, the cast should be enumerated and reported as a waxy cast. Table 7.9 lists the characteristic features, chemical examination, and other correlations that are associated with each cast type.
Table 7.9
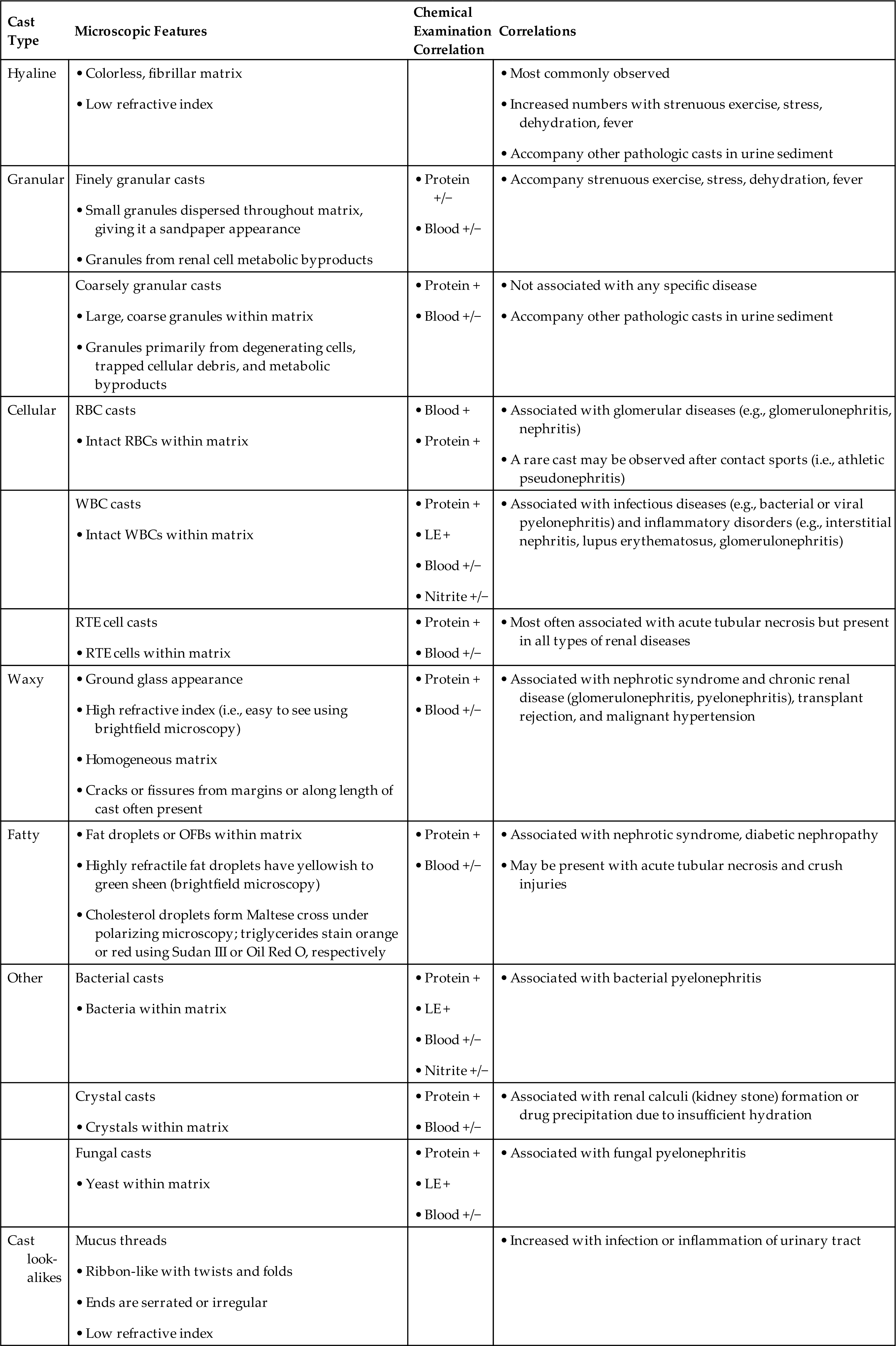
LE, Leukocyte esterase; OFB, oval fat body; RBC, red blood cell; RTE, renal tubular epithelial; WBC, white blood cell.
Homogeneous Matrix Composition
Hyaline Casts
Hyaline casts, composed primarily of a homogeneous uromodulin protein matrix, are the most commonly observed casts in the urine sediment (Fig. 7.42). This protein matrix gives hyaline casts a low refractive index that is similar to that of urine and makes them difficult to see using brightfield microscopy. These casts appear colorless in unstained urine sediment, with rounded ends and in various shapes and sizes. When phase contrast or interference contrast microscopy is used, their fibrillar protein matrix is more apparent and often includes some fine granulation (Fig. 7.43).
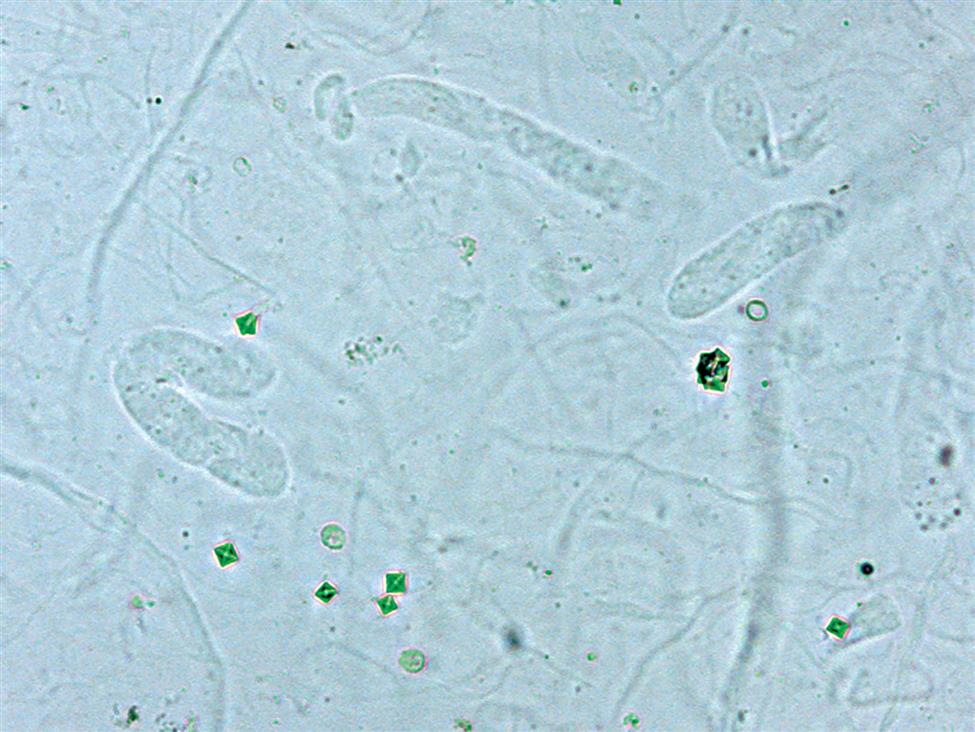
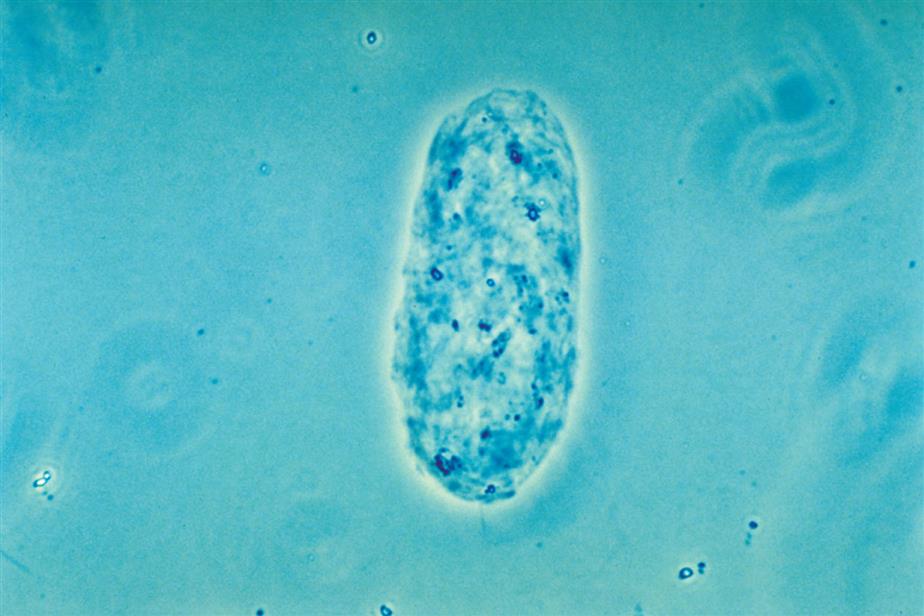
In healthy individuals, two or fewer hyaline casts per low-power field is considered normal. Increased numbers of hyaline casts can be found after extreme physiologic conditions such as strenuous exercise, dehydration, fever, or emotional stress. They also accompany pathologic casts in renal disease and in cases of congestive heart failure.
If brightfield microscopy is used, staining the sediment greatly enhances the visualization of hyaline casts and helps differentiate them from mucus threads that may also be present. Hyaline casts become pink with Sternheimer-Malbin stain, and their edges are more clearly defined. With phase contrast or interference contrast microscopy, hyaline casts are readily identified by the homogeneity of their matrix and by their characteristic shape. Occasionally, a hyaline cast may have a single epithelial or blood cell within its matrix. These casts, as well as cylindroids, are enumerated as hyaline casts and have no diagnostic significance.
Waxy Casts
Named as such because of their waxy appearance, these casts have a high refractive index and are readily visible using brightfield microscopy. Waxy casts appear homogeneous, with their edges well defined, and often have sharp, blunt, or uneven ends. Cracks or fissures from their lateral margins or along their axes are often present and are characteristic of these casts (Fig. 7.44). In unstained urine sediment they are colorless, gray, or yellow; with Sternheimer-Malbin stain they become darker pink than hyaline casts and have a diffuse, ground glass appearance.
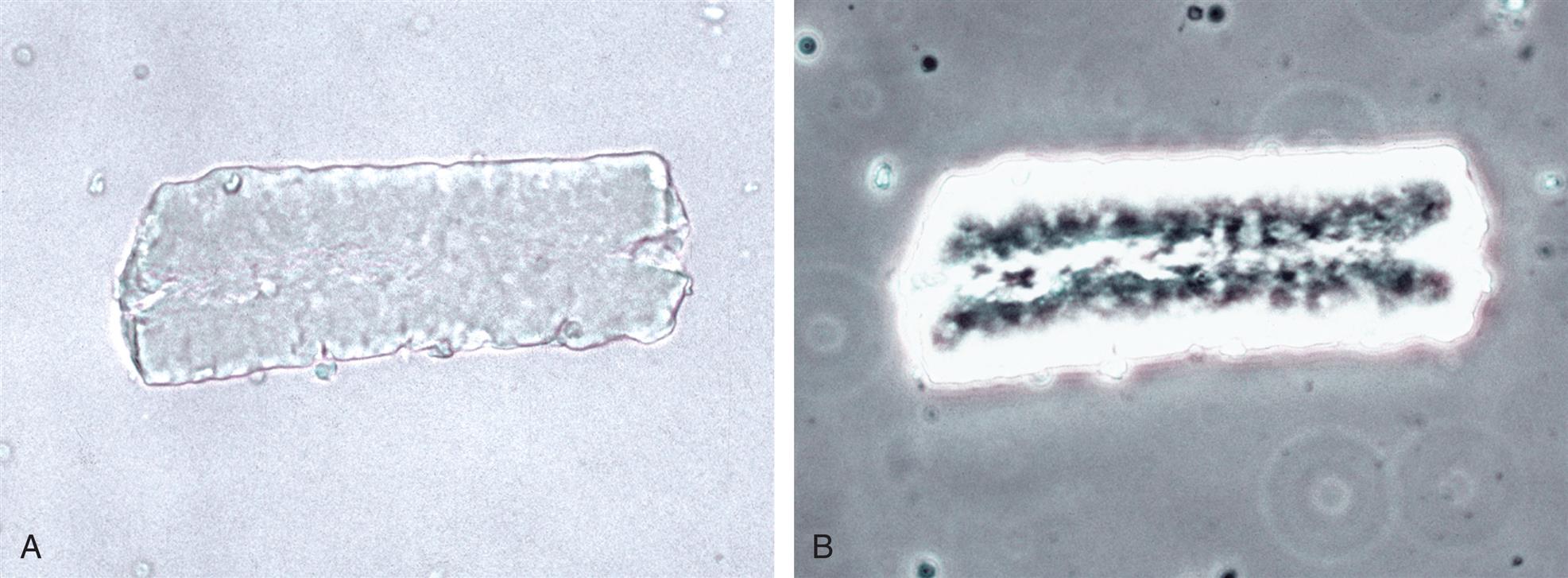
Waxy casts indicate prolonged stasis and tubular obstruction. They are believed to represent an advanced stage of other casts (e.g., hyaline, granular, cellular) that are transformed during urinary stasis, taking as long as 48 hours or more to form (Fig. 7.45). They are often broad, indicating their formation in dilated tubules or collecting ducts. Waxy casts are found most frequently in patients with chronic renal failure; hence they are frequently referred to as renal failure casts. They are also encountered in patients with acute renal disease (e.g., acute glomerulonephritis, nephrotic syndrome) or malignant hypertension and during renal allograft rejection.
Cellular Inclusion Casts
Red Blood Cell Casts
The microscopic appearance of RBC casts varies. Some casts are packed with RBCs; others may present principally as a hyaline cast with several clearly defined RBCs embedded within its matrix (Figs. 7.46 and 7.47). In either case, the RBCs must be unmistakably identified in at least a portion of the cast before it can be called an RBC cast. In unstained urine sediments, erythrocytes within the cast matrix cause them to be characteristically yellow or red-brown. The latter color indicates degeneration of the erythrocytes with hemoglobin oxidation. In Sternheimer-Malbin–stained sediments, intact RBCs may appear colorless or lavender in a pink homogeneous matrix.
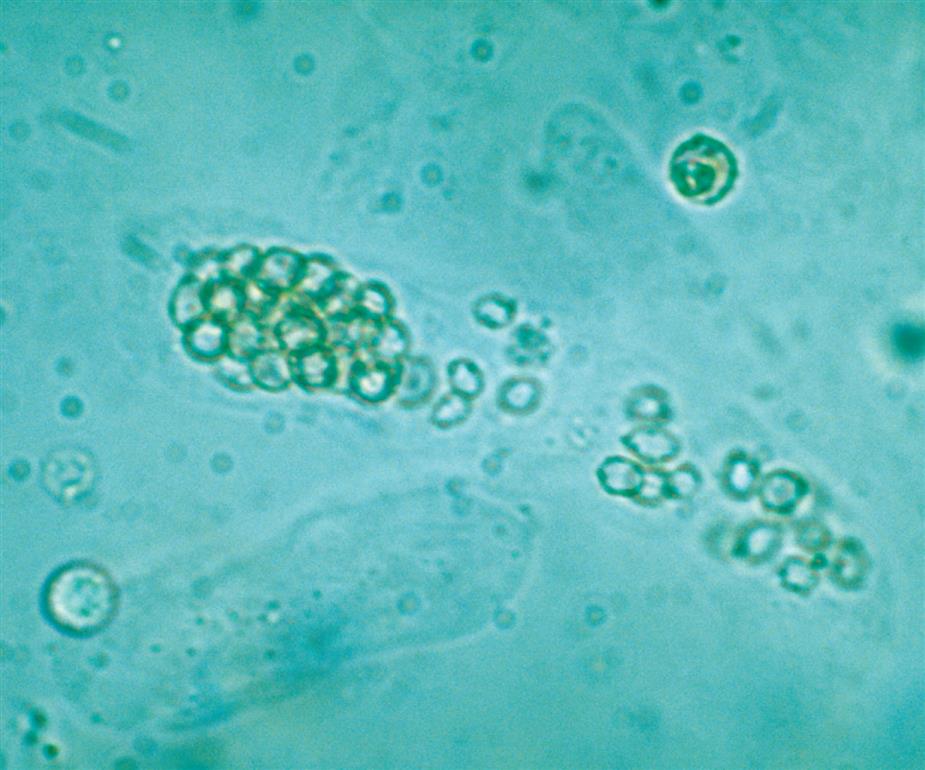
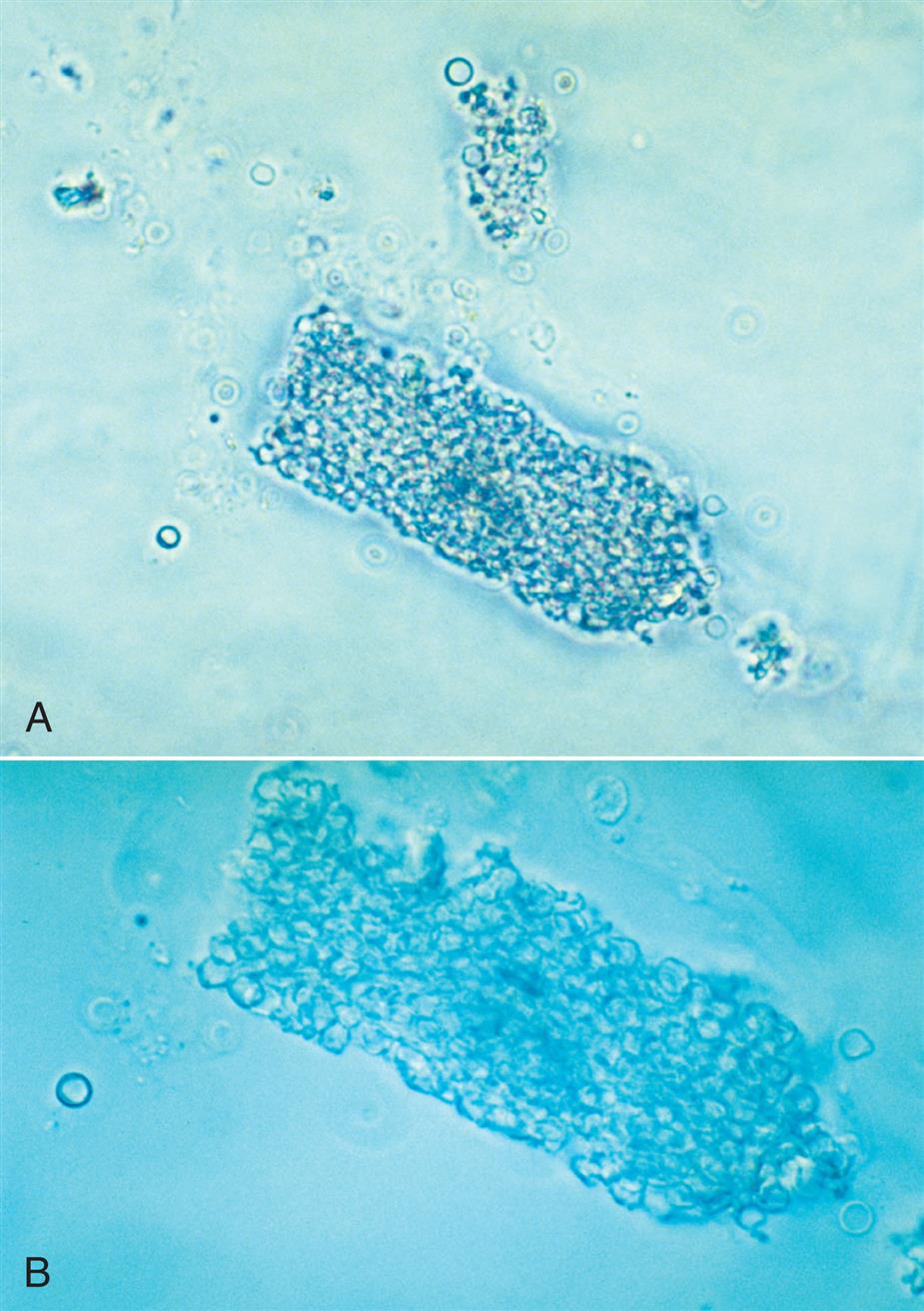
If urine stasis is sufficient, erythrocyte casts degenerate into pigmented, granular casts called blood casts or muddy brown casts (Fig. 7.48). These red to golden-brown granular casts contain no distinct RBCs in their matrix because the cells have lysed and undergone degeneration. This process can also occur in vitro when the urine specimen is old and improperly stored. RBC casts are fragile, and overly vigorous resuspension of the urine sediment can result in breakage of the casts into pieces. Microscopically, chunks of casts would be present and may be difficult to identify.
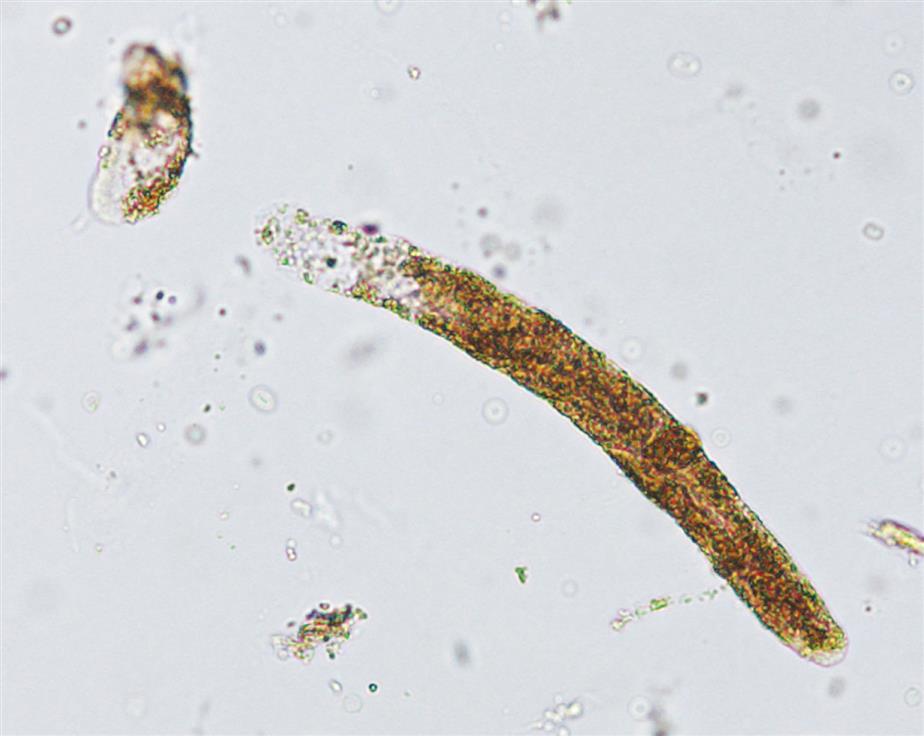
Phase contrast and interference contrast microscopy aid in identification of RBC casts by enhancing the detail of cells trapped within the cast matrix. Because free-floating RBCs are also present, the optical sectioning ability of these techniques enables better visualization to ensure that cells are actually within the cast matrix and are not simply superimposed on its surface.
RBC casts are diagnostic of intrinsic renal disease. The RBCs are most often of glomerular origin (i.e., passage across glomerular filtration barriers as in glomerulonephritis) but can result from tubular damage (i.e., blood leakage into the tubules, as with AIN). When RBCs are able to pass into the tubular ultrafiltrate, so are plasma proteins; therefore varying degrees of proteinuria are present (see Table 7.9). The detection and monitoring of RBC casts in urine sediment provide a means of evaluating a patient’s response to treatment. Occasionally, an RBC cast may be observed in the urine of a healthy individual. This finding usually is noted after strenuous exercise (i.e., athletic pseudonephritis), particularly after participation in contact sports such as football, basketball, or boxing. As with other urine findings associated with this condition, the urine sediment returns to normal within 24 to 48 hours.
White Blood Cell Casts
WBC casts consist of leukocytes embedded in a hyaline cast matrix (Fig. 7.49). Because of the refractility of the cells within them, leukocyte casts are readily apparent and identifiable with brightfield microscopy. When the characteristic multilobed nuclei and granular cytoplasm of these cells are readily apparent, these casts are easy to identify. However, when these characteristics are not evident because of cellular degeneration, the use of supravital stains or contrast microscopy is necessary to differentiate them from renal epithelial cells. The presence of increased numbers of WBCs, free floating or in clumps, would suggest strongly that the cells within these casts are leukocytes.

The presence of WBC casts indicates renal inflammation or infection and requires further clinical investigation. The origin of the WBCs, glomerular or tubular, can be difficult to determine. If glomerular (e.g., glomerulonephritis), RBC casts will also be present and in greater numbers than WBC casts. With tubular diseases (e.g., pyelonephritis), leukocytes migrating into the tubular lumen from the interstitium are enmeshed in the cast matrix. In these cases, bacteriuria and varying degrees of proteinuria and hematuria usually accompany the WBC casts (see Table 7.9). Renal infections from agents other than bacteria (e.g., CMV) are possible and must be considered when bacteriuria is not present and negative bacterial cultures are obtained.
Renal Tubular Cell Casts
Renal tubular cells can become enmeshed in the uromodulin matrix of casts; these casts are nonspecific markers of tubular injury. They have a high refractive index and are readily visible on brightfield microscopy. When the characteristic large central nuclei and shape of these cells are apparent, these casts are easily identified (Figs. 7.50 and 7.58). However, as renal tubular cells become damaged, they undergo degenerative changes that can make specific identification of these casts difficult. Individual renal tubular cells may be found randomly arranged within a cast, or they may appear aligned as fragments of the tubular lining removed intact from the tubule. These latter casts indicate that a portion of a nephron has been severely damaged, with the tubular basement membrane stripped of its epithelium.

Because the size of some renal tubular cells is similar to that of leukocytes, degenerating renal tubular cells in casts may need enhanced visualization to be differentiated and specifically identified. Supravital stains or microscopy techniques such as phase contrast or interference contrast microscopy can be used. The presence of degenerating tubular cell casts in urine sediment indicates intrinsic renal tubular disease. Proteinuria and often granular casts accompany renal tubular cell casts. See Urine Sediment Image Gallery for additional images of cellular casts.
Mixed Cell Casts
It is common to find casts that have incorporated within their matrix multiple cell types, such as renal epithelial cells and leukocytes or erythrocytes and leukocytes. Any combination is possible. These casts are often enumerated and reported as cellular casts, with their composition provided in the report.
Bacterial Casts
Because visualizing these small organisms within the cast matrix is difficult, bacterial casts are rarely identified as such. Bacterial casts are diagnostic of pyelonephritis. Because these casts usually include leukocytes, they are often reported as leukocyte casts. They are actually mixed casts. With the use of brightfield microscopy and a stain (supravital or Gram), careful scrutiny of the cast matrix between leukocytes can often reveal embedded bacteria. Contrast interference microscopy allows even better visualization of bacteria within casts because of its optical sectioning ability. Occasionally, casts that consist of bacteria without leukocytes incorporated in the protein matrix have been observed.
Casts with Inclusions
Granular Casts
Granular casts come in a variety of granular textures. They range from small, fine granules dispersed throughout the cast matrix to large, coarse granules (Figs. 7.51 and 7.52). They are composed primarily of uromodulin protein, and cast granulation is not clinically significant. Easily viewed with brightfield microscopy because of their high refractive index, granular casts often appear colorless to shades of yellow. Granular casts can appear in all shapes and sizes, and broad granular casts are considered to be an indicator of poor prognosis.
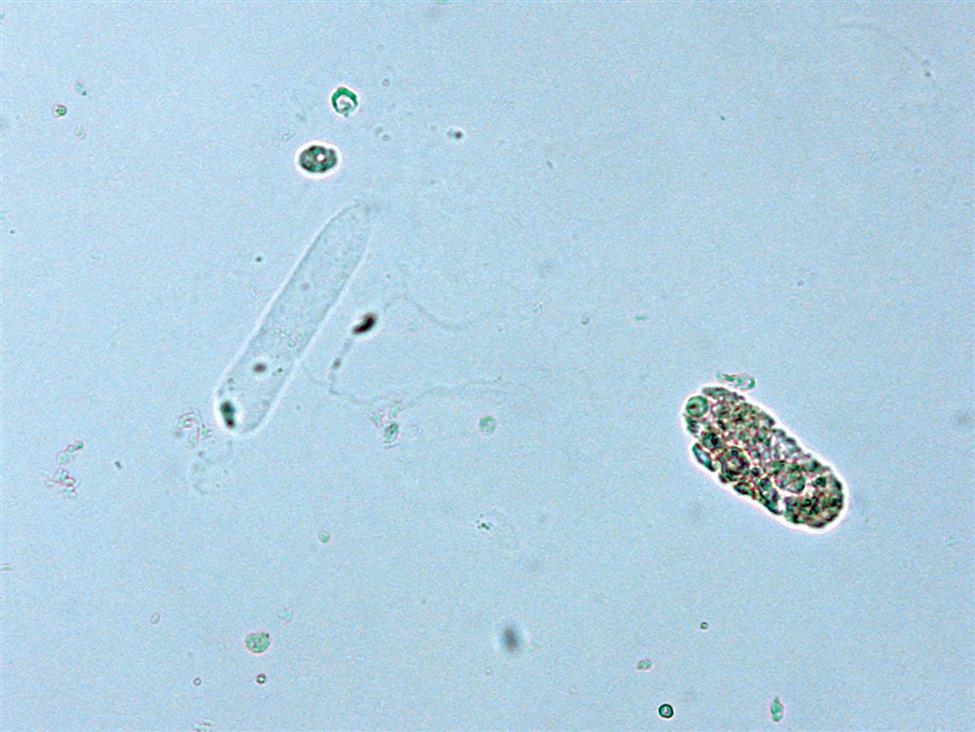
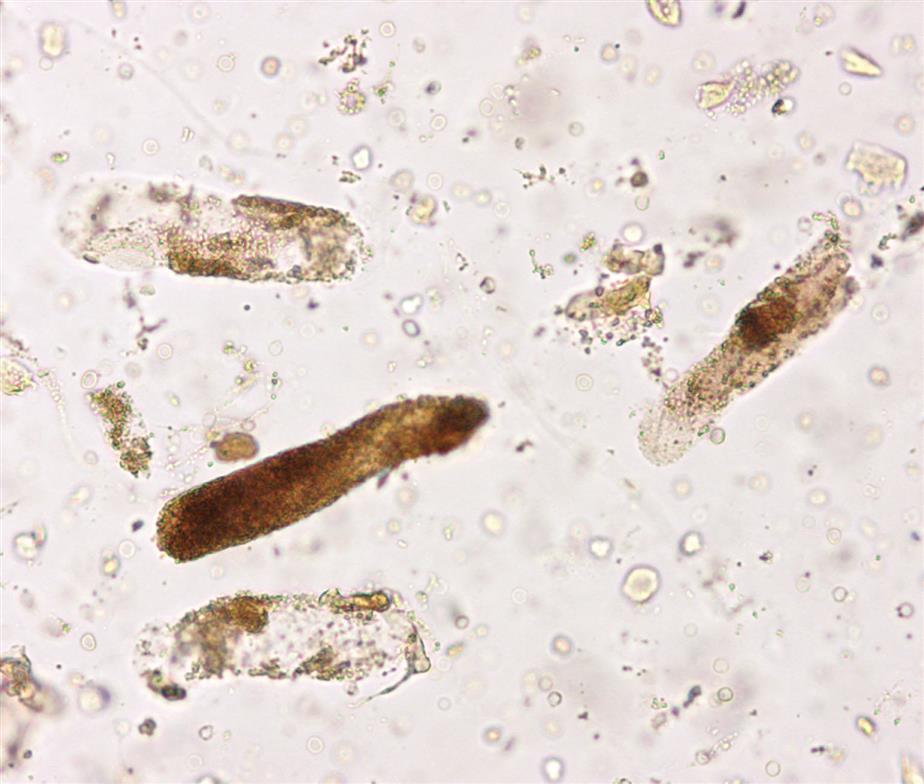
Several mechanisms account for the granular casts observed in the urine sediment. The granules in finely granular casts have been identified as byproducts of protein metabolism, in part lysosomal, that are excreted by renal tubular epithelial cells22—this accounts for the appearance of granular casts in the urine of normal, healthy individuals. A variation of this mechanism is believed to account for the finding of some casts with large, coarse granulation, particularly when no accompanying cellular casts are present. In these cases, as tubular cells degenerate, their intracellular components are released into the tubular lumen and become enmeshed in a cast. Other coarsely granular casts result from the degeneration of cellular casts. These casts often contain identifiable cellular remnants. In patients with intrinsic renal disease, these coarsely granular casts are usually accompanied by cellular casts. Further degeneration of granular casts into waxy casts can occur during urine stasis (see Figs. 7.39 and 7.45).
Urine sediment from normal, healthy individuals may have an occasional finely granular cast. These casts are not as common as hyaline casts, but their numbers can increase after exercise. Patients with various types of renal diseases can have varying quantities of coarse and finely granular casts.
Fatty Casts
Fatty casts contain free fat droplets, oval fat bodies, or both, and their matrix can be hyaline or granular. Within the cast, fat droplets can vary in size and are highly refractile (Figs. 7.53 and 7.54). Oval fat bodies in casts are identified by their intact cellular membranes. Because oval fat bodies often indicate renal tubular cell death, the presence of oval fat bodies in fatty casts indicates a significant renal pathologic condition. Cells other than oval fat bodies may also be present within the fatty cast matrix.
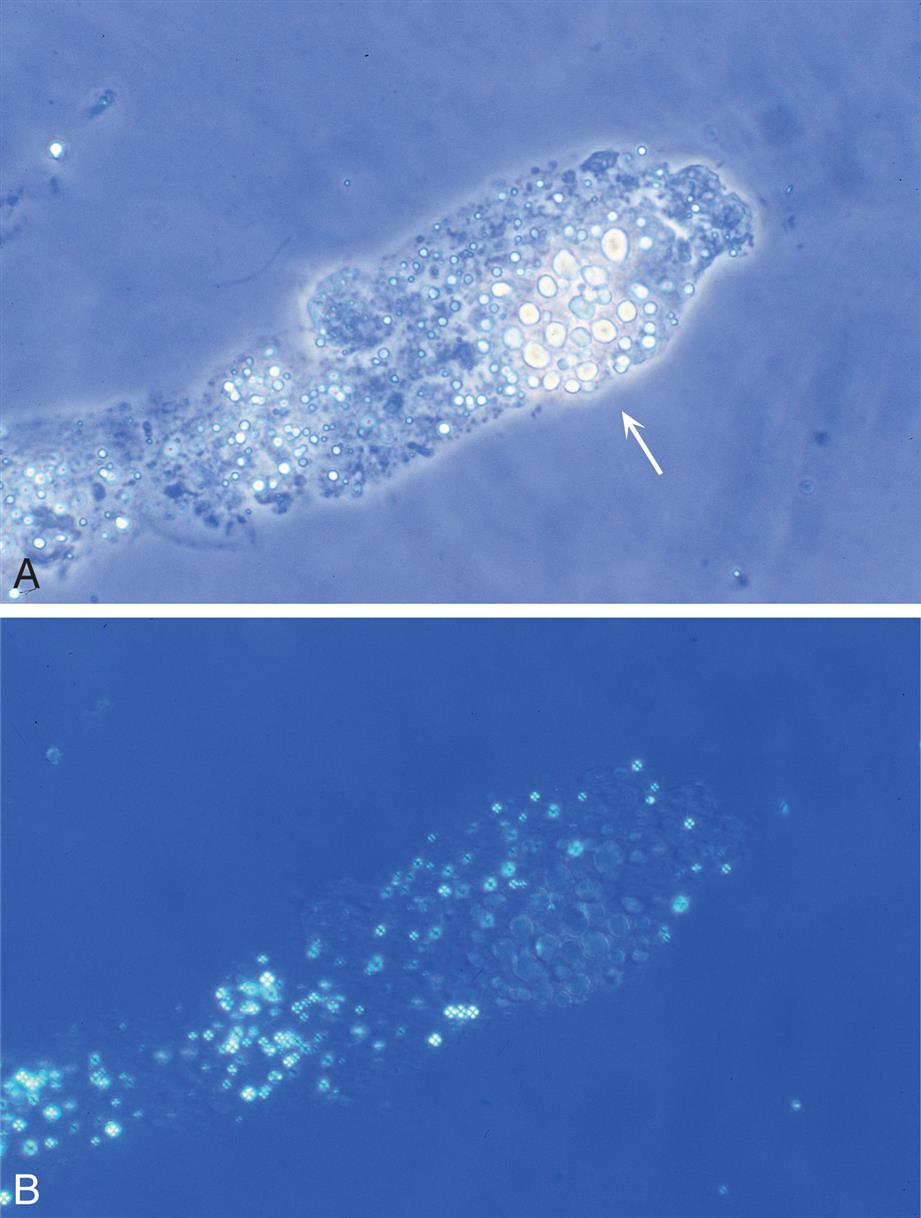
In unstained urine sediment examined by brightfield microscopy, lipid droplets may appear light yellow or darker, depending on microscope adjustments. If fat stains such as Sudan III or oil red O are used, triglyceride (neutral fat) droplets within casts stain characteristically orange or red (see Fig. 7.7 and Urine Sediment Image Gallery, Fig. 26), whereas cholesterol and cholesterol esters do not. In contrast, polarized microscopy can identify cholesterol and cholesterol esters by their characteristic birefringence; these droplets form a Maltese cross pattern (see Figs. 7.11 and 7.54B,). Note that lipids do not take up Sternheimer-Malbin stain, although the protein matrix of the cast does.
Fatty casts are accompanied by significant proteinuria and may be found in numerous renal diseases, particularly nephrotic syndrome (see Table 7.9). In addition, a severe crush injury with disruption of body fat can result in the presence of fatty casts in the urine sediment.
Other Inclusion Casts
Because during cast formation any substance present in the tubular lumen can be incorporated into the uromodulin matrix, hemosiderin granules and crystals have been found in casts. As crystals can aggregate along mucus threads to simulate a cast, it is important that the hyaline matrix is observed and that it actually encases the crystals. Crystal casts are not common; those encountered are usually composed of calcium oxalate or sulfonamide crystals (Figs. 7.55 and 7.56). The presence of crystal casts indicates crystal precipitation within the tubules, which can damage tubular epithelium as well as cause tubular obstruction. As a result, varying amounts of hematuria usually accompany crystalline casts in the urine sediment.
Pigmented Casts
Pigmented casts, usually of a hyaline matrix with distinct coloration, are characterized by incorporation of the pigment within the casts (see Figs. 7.52 and 7.57). Hemoglobin, myoglobin, or bilirubin (bile) casts can be encountered in the urine sediment. Hemoglobin casts appear yellow to brown and are accompanied by hematuria. Because myoglobin casts are similar in appearance to hemoglobin casts, differentiation requires a patient history with a possible diagnosis of rhabdomyolysis or confirmation that myoglobin is present. Bilirubin characteristically colors all urine sediment constituents, including casts, yellow- or golden-brown (Fig. 7.58). In contrast, urobilin, a pigment that can impart an orange-brown color to urine, does not color the formed elements of the sediment. Highly pigmented drugs, such as phenazopyridine, can also characteristically color sediment elements.

Size
Broad casts indicate cast formation in dilated tubules or in the large collecting ducts (Fig. 7.59). Because several nephrons empty into a single collecting duct, cast formation here indicates significant urinary stasis due to obstruction or disease. The presence of many broad casts in urine sediment indicates a poor prognosis. Broad casts may be of any type; however, when a significant amount of urinary stasis is involved, they principally present as granular or waxy casts (see Fig. 7.37). In chronic renal diseases in which nephrons have sustained previous damage, broad hyaline casts may be encountered. These casts form as a result of continued proteinuria and other factors that enhance their formation.
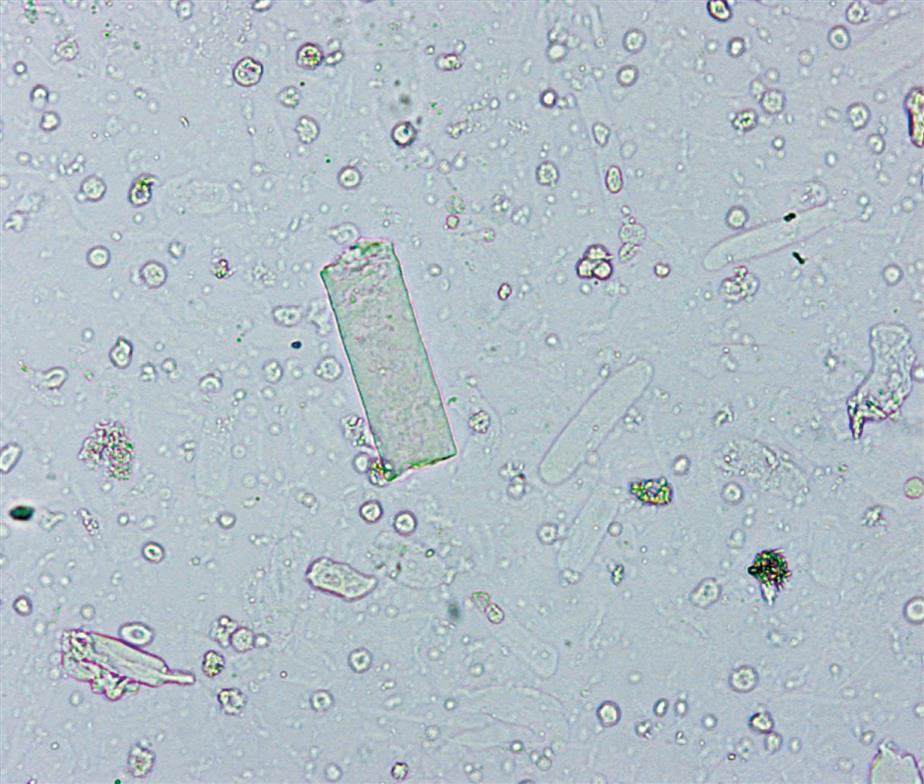
Correlation With Physical and Chemical Examinations
When significant numbers of pathologic casts (i.e., those associated with disease) are identified in urine sediment, correlation with the physical and chemical examinations must be made (see Table 7.9). Increased numbers of abnormal casts should be accompanied by proteinuria, although the degree of proteinuria can vary. In contrast, proteinuria can occur without cast formation. If RBC casts are identified, the chemical test for blood should be positive, or its negativity accounted for before these casts are reported. Leukocyte casts may or may not be associated with a positive LE test, depending on the types and numbers of leukocytes present. Leukocyte casts often are accompanied by bacteriuria, the most common causative agent of UTI. In these cases, the nitrite test may also be positive. Bile-pigmented casts should be accompanied by a positive chemical test for bilirubin; similarly, hemoglobin- or myoglobin-pigmented casts should be accompanied by a positive chemical test for blood.
Look-Alikes
For the novice microscopist, several formed elements in urine sediment can be confused with casts. Mucus threads can be misidentified as hyaline casts (see Fig. 7.35). Although mucus threads have a similar low refractive index, they are ribbon-like and their ends are not rounded but are serrated. They are irregular, whereas hyaline casts are more formed.
Various fibers, such as cotton threads or absorbent fibers from diapers and other hygiene products, can resemble waxy casts (Fig. 7.60). Several distinguishing characteristics allow for their differentiation. Fibers tend to be flatter in the middle and thicker at their margins, whereas casts are cylindrical and thicker in the center. In addition, fibers are more refractile than casts. Under polarizing microscopy, fibers polarize light, whereas casts do not (see Fig. 7.56B, noting the appearance of the “cast matrix”). Finally, fibers can contaminate the urine at any time, whereas casts, particularly waxy casts, must be accompanied by proteinuria. Other entities, such as squamous epithelial cells folded into a tubular shape or scratches on the coverslip surface, may be misidentified as casts. With practice, proper identification of these components is not difficult.

Crystals such as amorphous urates and phosphates can aggregate together or along a mucus thread to simulate a cast. With polarizing microscopy, their birefringence identifies them as crystalline entities, and the lack of a distinct matrix differentiates them from a true cast.
Microorganisms in Urine Sediment
In health, the urinary tract is sterile; no microorganisms are present. Consequently, the presence of bacteria, yeast, trichomonads, or parasites in urine indicates an infection or that contamination occurred during the collection process. Table 7.10 lists characteristic microscopic features and urinalysis findings associated with commonly observed microorganisms as well as some less common.
Table 7.10
| Organism | Characteristic Features | UA Correlations |
|---|---|---|
| Bacteria | ||
| Yeast | ||
| Trichomonads | ||
| Other parasites | None; fecal contaminant | |
| None; fecal contaminant | ||
Football-shapeda or ovoid eggs with a spike at one end |
LE, Leukocyte esterase; WBC, white blood cell; RBC, red blood cell.
aShape of the American football.
Bacteria
Observing bacteria in the urine sediment requires high-power magnification (Fig. 7.61A), and they are easier to observe using phase contrast microscopy compared with brightfield microscopy. The most commonly encountered bacteria in urine are rod-shaped (bacilli), but coccoid forms can also be present. These microorganisms can vary in size from long, thin rods to short, plump rods. They may appear singly or in chains, depending on the species present. In wet preparations, their motility often distinguishes bacteria from amorphous substances that may be present. Because the skin, vagina, and gastrointestinal tract normally contain bacteria, the presence of bacteria in urine often reflects contamination from these sources.
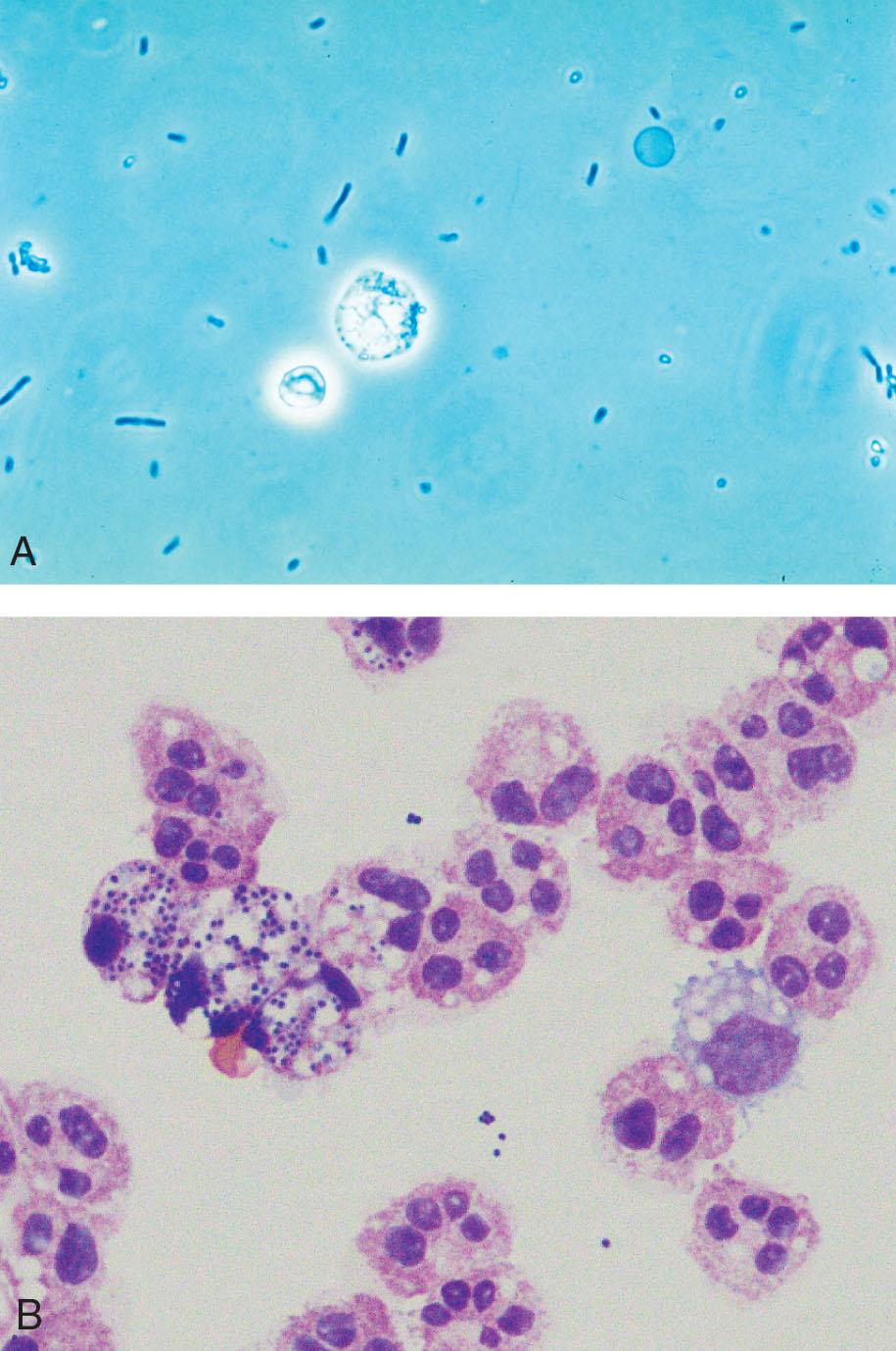
Bacteria are reported as few, moderate, or many per high-power field. Because urine from normal, healthy individuals is sterile, the presence of bacteria in the urine sediment implies a UTI or urine contamination. Bacteria most often ascend the urethra to cause a UTI. They can also be present because of a fistula—a narrow pathway—between the urinary tract and the bowel. It is important to note that contaminating bacteria rapidly multiply in improperly stored urine. Therefore the presence of bacteria has clinical significance only if the urine specimen has been properly collected and stored.
For urine sediment in which identification of bacteria is difficult, a cytospin preparation followed by Gram staining could be performed (Fig. 7.61B), although time-consuming. During UTI, bacteriuria is usually accompanied by leukocytes in the urine sediment. When significant bacteriuria is present without leukocytes, the specimen collection and handling should be investigated.
Yeast
Yeasts are colorless ovoid cells that can closely resemble RBCs (Fig. 7.62). More refractile than erythrocytes, yeasts often have characteristic budding forms and pseudohyphae (Fig. 7.63). Yeasts can vary in size, and some species are very large (10–12 μm). Yeasts do not dissolve in acid and usually do not stain with supravital stains; these two characteristics can aid in differentiating them from erythrocytes. In addition, yeast are resistant to KOH—place a drop of urine sediment on a microscope slide, add a drop of KOH and a coverslip, and review microscopically for yeast elements.
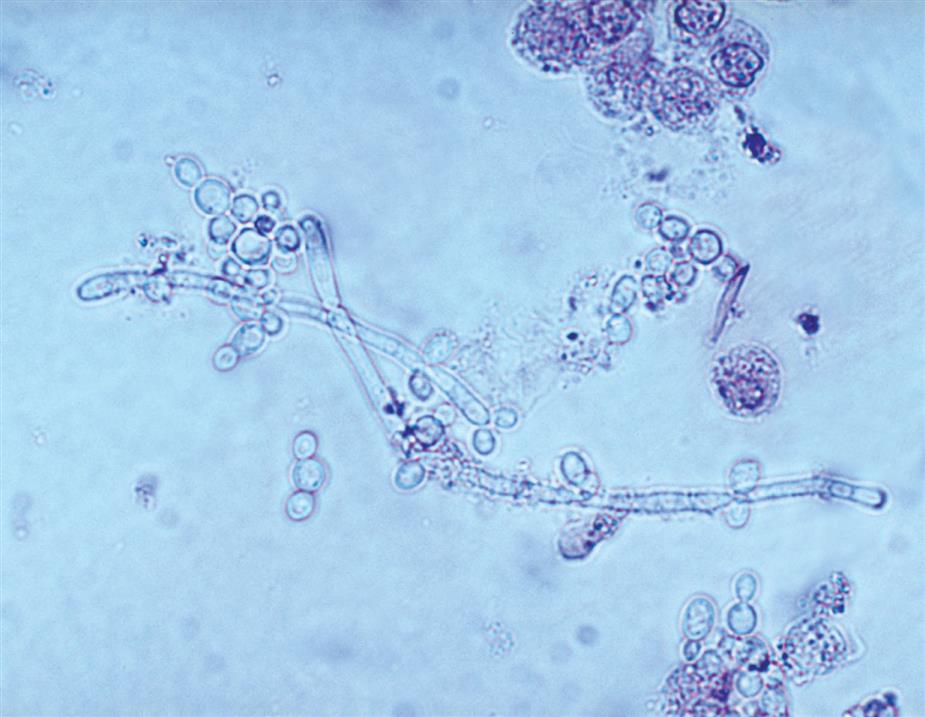
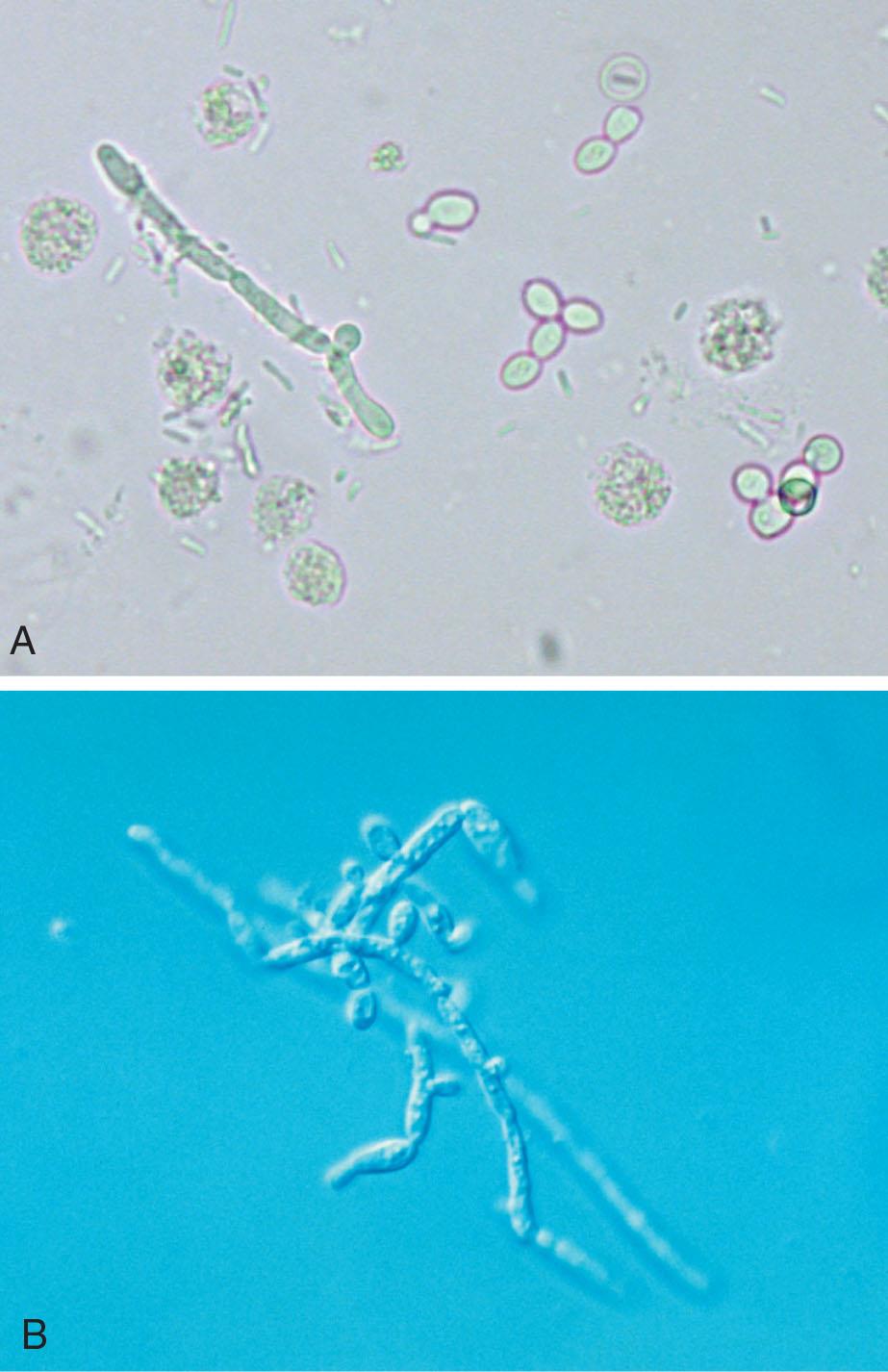
In women, yeast in the urine sediment most often indicates contamination of the urine with vaginal secretions. However, because yeasts are ubiquitous—present in the air and on skin—their presence could indicate contamination from these sources. Although infrequent, primary UTIs resulting from yeasts are possible; hence healthcare providers must correlate the finding of yeast with the patient’s clinical picture to determine whether an actual infection, vaginal or urethral, is present. Certain situations, such as pregnancy, use of oral contraceptives, and diabetes mellitus, promote the development of vaginal yeast infection.
The most commonly encountered yeast in urine sediment is Candida albicans. The characteristic budding and the development of pseudohyphae make C. albicans readily identifiable as yeast. Another species found less frequently is C. glabrata, formerly called Torulopsis glabrata. This species does not form pseudohyphae, and these yeast cells may be found phagocytized within WBCs (Fig. 7.64). In immunosuppressed patients, systemic Candida infections are common, and yeasts have a predilection for the kidneys. Note that during the microscopic examination, only the presence of yeast can be determined; identification of the species present requires fungal culture.

A KOH preparation is often used to detect yeast, hyphae, and other fungal cells in vaginal secretions. See Chapter 13.
Trichomonas vaginalis
Trichomonads, protozoan flagellates, can be observed in the urine sediment. Trichomonads appear as turnip-shaped flagellates whose unicellular bodies average 15 μm in length, although organisms as small as 5 μm and as large as 30 μm are possible. They have four anterior flagella, a single posterior axostyle, and an undulating membrane that extends halfway down the body of the organism (Fig. 7.65). The beating flagella propel the organism while the undulating membrane rotates it. The result is a characteristic flitting or jerky motility in wet preparations. Because of their similarity in size to both leukocytes and renal tubular cells, this motility is critical for their identification (Fig. 7.66 and Urine Sediment Image Gallery Figs. 83–85).
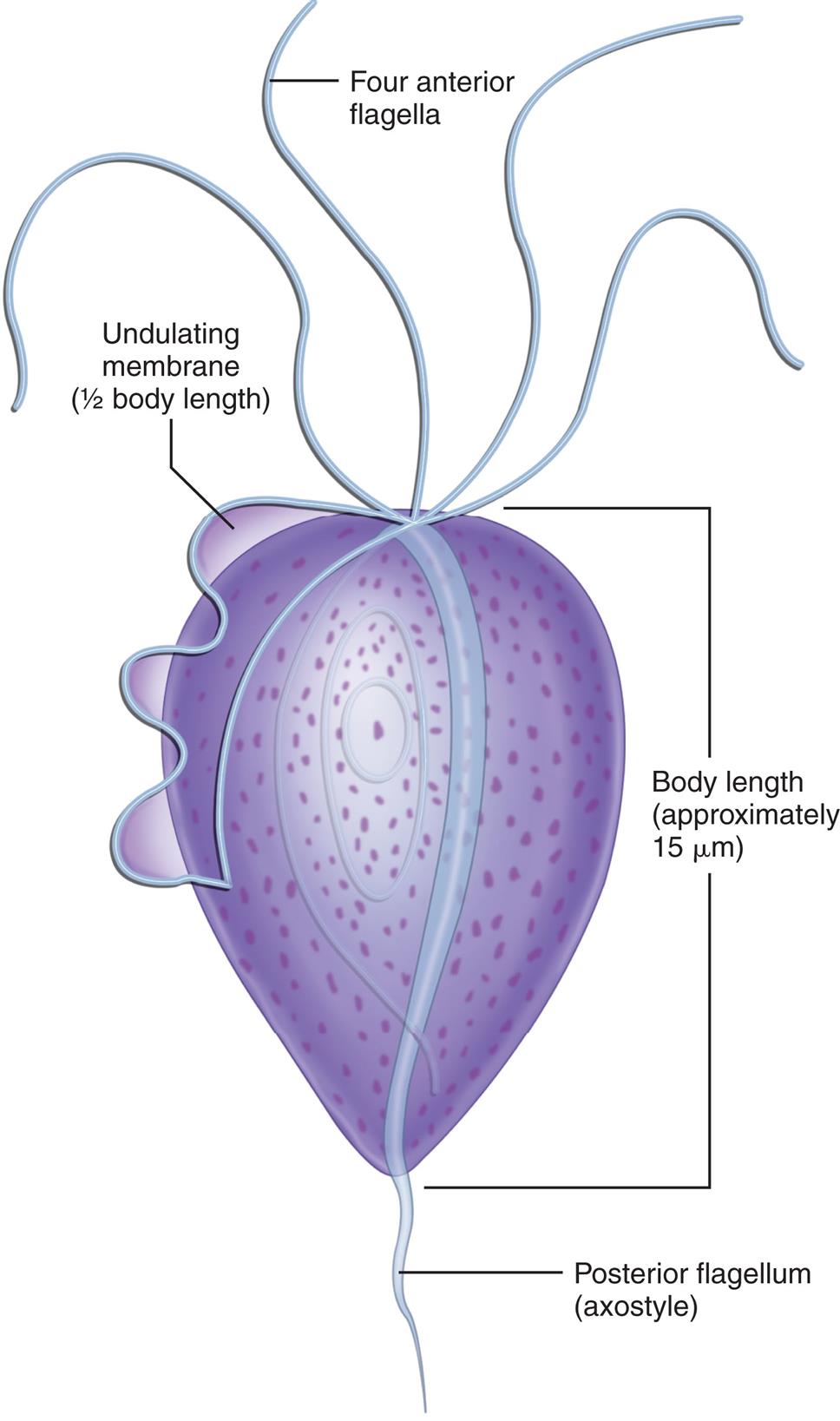

Trichomonas vaginalis is the most common cause of parasitic gynecologic infection in female patients (see Chapter 13). Transmitted sexually, trichomonads most frequently represent an infection of the vagina and/or urethra, and their presence in the urine often indicates contamination with vaginal secretions. In male patients, Trichomonas infections of the urethra are usually asymptomatic. In either case, when observed in urine sediment, trichomonads are not quantitated but are simply reported as present.
Urine is not an optimal medium for trichomonads. Because their characteristic motility provides the best means of positively identifying them, a fresh urine specimen is needed. Once in urine, trichomonads proceed to die. First, they lose their motility; later, their undulating membrane ceases, and eventually they ball up to resemble WBCs or renal tubular epithelial cells. With the loss of motility or movement of the undulating membrane, differentiation of trichomonads from other cells in the sediment can be impossible. Supravital stains do not enhance trichomonad identification whether the organisms are dead or alive; in fact, unless the stain concentration is tightly controlled it will kill them. Phase contrast microscopy and interference contrast microscopy permit enhanced imaging and visualization of the flagella and undulating membranes of trichomonads; however, their characteristic movement is still required to specifically identify them.
Clue Cells and Gardnerella vaginalis
Squamous epithelial cells from the vaginal mucosa with large numbers of bacteria adhering to them are called clue cells; they can be present in urine specimens contaminated with vaginal secretions. These characteristic cells are indicative of bacterial vaginosis (BV), a synergistic infection most often involving Gardnerella vaginalis and an anaerobe, usually Mobiluncus spp. (e.g., Mobiluncus curtisii). A Gram stain can clearly reveal the bacterial flora adhering to these epithelial cells (Fig. 7.67).
When there is a disruption in the normal vaginal flora (lactobacilli) with subsequent proliferation of the usually minor, endogenous bacterial species, a foul-smelling vaginal discharge and the sloughing of clue cells will occur. Clue cells appear soft and finely granular with indistinct cell borders caused by the numerous bacteria adhering to them; hence they are often described as bearded or as having shaggy edges (Fig. 7.68). In these bacteria-laden cells, the nucleus may not be visible. To be considered a clue cell, the bacteria should cover at least 75% of the cell surface and the bacterial organisms must extend beyond the cell’s cytoplasmic borders.23,24 Be aware that with an inexperienced microscopist, normal intracellular keratohyalin granulation, which is more evident using phase contrast microscopy, could be misidentified as bacteria adhering to squamous epithelial cells. However, keratohyalin granules are variable in size and are usually larger and more refractile than bacteria (Fig. 7.68B). When a healthcare provider suspects BV, a pelvic examination is performed and a vaginal secretions specimen is collected for evaluation (see Chapter 13).
Parasites
Several parasites, in addition to trichomonads and yeast, can be observed in the urine sediment. The eggs, or ova, of Enterobius vermicularis (pinworm) can be found in urine from school-aged children; however, individuals of any age can be infected. The adult female pinworm lays eggs in the area around the rectum; this causes itching. Consequently, the eggs can be present in urine sediment if the specimen is contaminated during collection. Pinworm eggs are characteristically American football–shaped, with one side appearing flatter. They are large, transparent cells (50–60 μm long; 20–30 μm wide), and the developing larvae can be seen inside (Fig. 7.69).
Cysts of Giardia lamblia (also known as Giardia intestinalis or Giardia duodenalis) may be observed in urine sediment as the result of fecal contamination from infected individuals. Giardiasis is most often acquired by drinking contaminated water—from inadequate sanitation of city water supplies or from contaminated freshwater lakes and streams. Giardia organisms have also contaminated recreational water sources such as swimming pools, hot tubs, and water parks. The cysts are small ovoid cells usually 11 to 14 μm in length, but can range from 8 to 19 μm; they have smooth, well-defined cell walls (Fig. 7.70). When viewing using brightfield microscopy and high-power (×400) magnification, the cytoplasm appears to be filled with nuclear material, but distinct nuclei (up to four) usually are not apparent without specific staining.
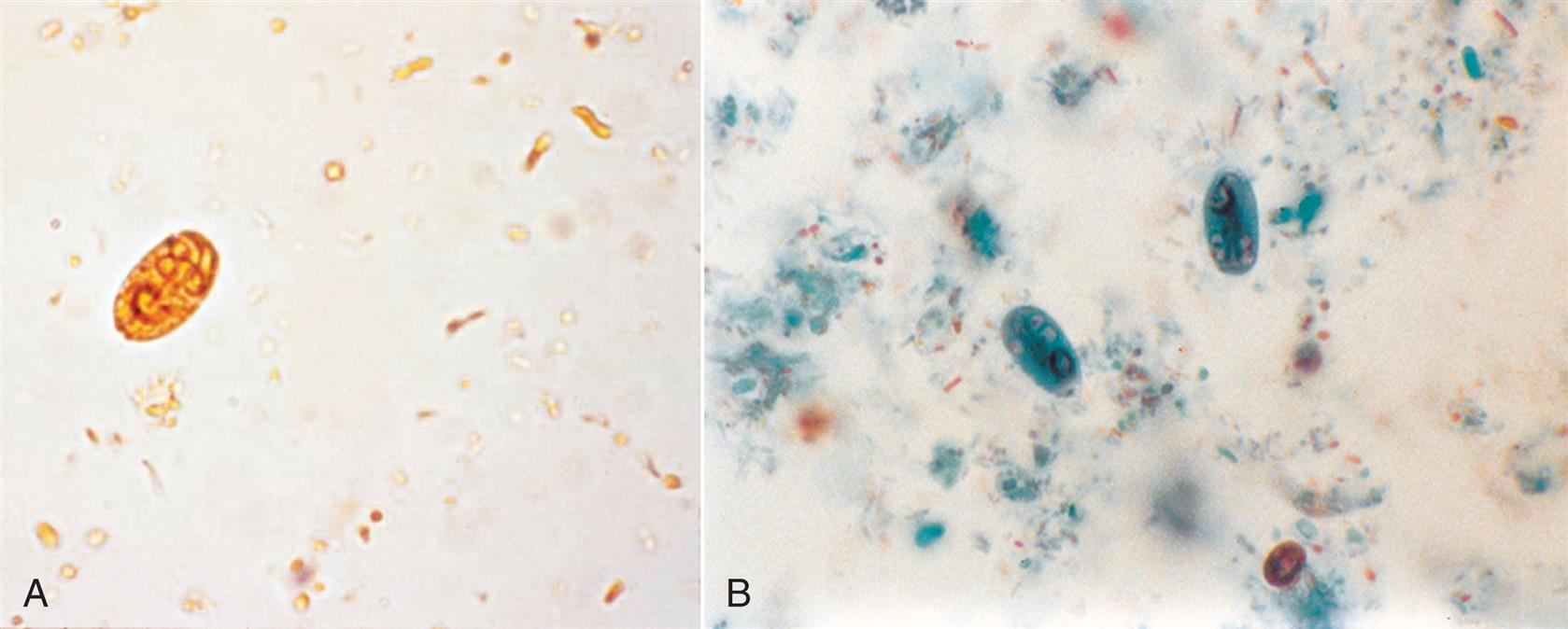
Finally, the eggs of the blood fluke Schistosoma haematobium can be present in urine sediment. Schistosomiasis is endemic in Africa and the Middle East, and is acquired upon exposure to water where infected snails live (e.g., by fishermen, swimmers, workers in irrigation canals). Infections are most often diagnosed when the eggs are found in urine sediment or in biopsies of the bladder, rectum, or vaginal wall. Schistosoma eggs are distinctively large (100–170 μm long and 40–70 μm wide) and shaped like an American football with a spike or spine at one end (Fig. 7.71). Be aware that the terminal spine can be very small and is not always evident; therefore, numerous ova should be carefully viewed. Their cell wall is thick but transparent, revealing the larva that fills the interior. Hematuria is often present as well.

Miscellaneous Formed Elements
Mucus
Mucus, a fibrillar protein, commonly appears in urine sediment and has no clinical significance. In unstained urine sediment with brightfield microscopy, mucus can be difficult to observe because of its low refractive index. However, when phase contrast or interference contrast microscopy is used, mucus threads are readily identifiable by their delicate, ribbon-like strands and irregular or serrated ends. Mucus strands appear wavy and can take various forms as they surround other sediment elements. They can be present as distinct strands or as a clumped mass (Fig. 7.72).

Because some mucus has been shown immunohistochemically to contain uromodulin (formerly Tamm-Horsfall protein) and because this protein is produced solely by the renal tubular epithelium, some mucus found in urine is derived at least partially from the renal tubules.22 The genitourinary tract, particularly the vaginal epithelium, is also a source of the mucus frequently observed in urine sediments from women.
Mucus threads can be misidentified as hyaline casts because of their similar low refractive index and fibrillar protein structure. The cylindrical form of casts and their rounded ends aid in their differentiation from mucus.
Fat
Fats or lipids are found in urine sediment in three forms: as free-floating fat droplets or globules, within oval fat bodies (cells with fat droplets), or within a cast matrix as fat droplets or entrapped oval fat bodies. During the microscopic examination, a distinguishing feature of lipids is their high refractility. When using brightfield microscopy, these highly refractive droplets, whether triglyceride or cholesterol, are spherical, vary in size, and, depending on microscope adjustment, can appear colorless to yellow-green or even brownish (see Figs. 7.24 and 7.34).
The type of fat present can vary; often both triglyceride and cholesterol can be demonstrated (Fig. 7.73). Triglyceride, a neutral fat, is composed of a glycerol backbone with three fatty acids esterified to it. Adding a Sudan III or an oil red O stain to the urine sediment causes triglyceride droplets to become characteristically orange or red (Fig. 7.74). In contrast, cholesterol and cholesterol esters do not stain but are identified by their characteristic birefringence with polarizing microscopy. Cholesterol droplets produce a distinctive Maltese cross pattern—that is, an orb that appears divided into four quadrants by a bright Maltese-style cross (Fig. 7.75). The quadrants can be symmetric or equal, which is usually observed with free floating droplets. Asymmetric or unequal quadrants are typically seen when multiple fat droplets are clustered together.In urine specimens that contain significant amounts of fat, cholesterol crystals may form as the urine cools or with storage at refrigerator temperature. See the section titled “Crystals” later in this chapter for more discussion of the unique features of cholesterol crystals.


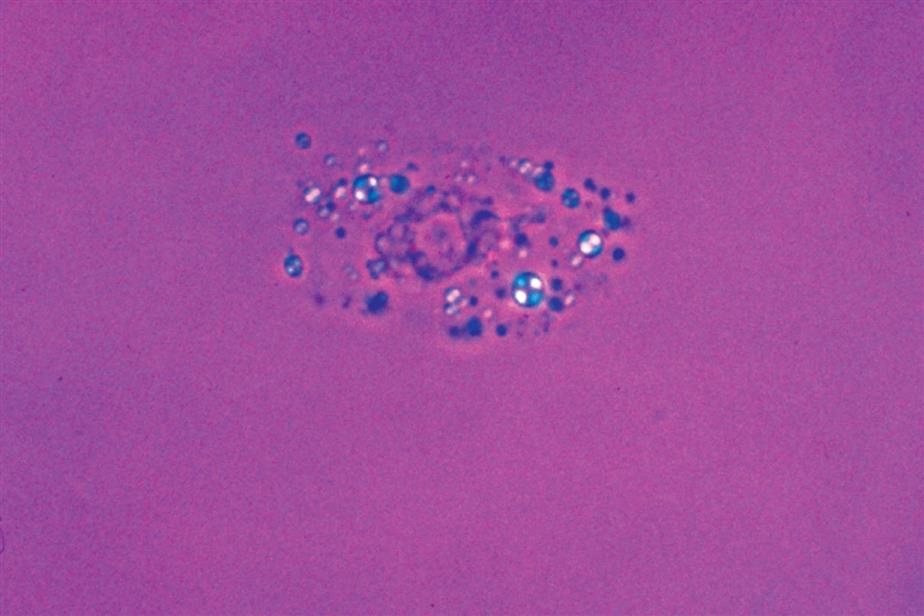
Lipiduria is always clinically significant, although its presence does not pinpoint a specific diagnosis. Lipiduria is present with a variety of renal diseases and may occur after severe crush injuries. It is a characteristic feature of the nephrotic syndrome, along with severe proteinuria, hypoproteinemia, hyperlipidemia, and edema. Because the nephrotic syndrome can occur with other kidney diseases, as well as with metabolic diseases such as diabetes mellitus, lipids are often encountered in the urine sediment from these patients. In preeclampsia, fat is often present and can persist for several weeks after delivery. Extreme physical exercise (e.g., marathon racing) can also cause fat to appear in the urine sediment.20 Identifying the presence of lipids in urine sediment and monitoring the level of lipiduria aid health care providers in determining whether a disease process is progressing or resolving.
Lipids most often enter the urine because of adverse changes in the glomerular filtration barriers, which allow the plasma lipids to pass. If large lipid molecules are able to cross into Bowman’s space, so can plasma proteins, most notably albumin. Therefore, lipiduria is always accompanied by some degree of proteinuria. Note, however, that the level of proteinuria in a random urine specimen can be disguised by hydration. In other words, a low reagent strip protein test can be caused by the large amount of water excreted (dilute urine). Because fat is uncommon and easy to overlook, a good laboratory practice is to specifically screen for fat when protein excretion is high (e.g., 300–500 mg/dL or greater). When entities are present in the urine sediment that resemble free fat droplets, oval fat bodies, or fatty casts, their identity can be verified by using either polarizing microscopy (for cholesterol) or fat stains (for neutral fats). Polarizing microscopy is preferred because it is less labor intensive and more economical. Note that detection of either type of fat (cholesterol or neutral fat) is sufficient for confirmation. The amount of fat in the sediment is graded qualitatively (see Table 7.2) for each fat-laden entity present—free-floating droplets, oval fat bodies, and fatty casts.
It is also important to note that urine from men with prostatitis could be contaminated with prostatic fluid. During prostatitis, prostatic fluid contains many WBCs and macrophages, as well as oval fat bodies (fat-laden macrophages) and free-floating fat droplets.25 Note that urine contaminated with this type of prostatic fluid could resemble the nephrotic syndrome—lipiduria and proteinuria—and may require investigation. Contamination of urine with normal prostatic fluid may be suspected when numerous fine secretory granules are present, giving the urine a grainy appearance microscopically. These secretory granules vary in size, from fine granules to about half the size of an RBC. In elderly men, prostatic fluid may contain free-floating fat droplets. Therefore, when the finding of urinary fat is unexpected in the urine from a male, it could be the result of prostatic fluid contamination.
Because other entities in urine sediment can resemble fat, it is important to distinguish these look-alike substances. Starch granules form a pseudo-Maltese pattern with polarizing microscopy; however, to an experienced microscopist, they are easily distinguished from fat droplets using brightfield microscopy. Starch granules are highly refractile, tend to have a central dimple, and are not spherical (see Fig. 7.114). The variation in size demonstrated by fat droplets aids in differentiating them from RBCs. In addition, RBCs do not stain with fat dyes and are not birefringent. When a modified Sternheimer-Malbin stain is used, lipids retain their high refractility and yellow-green to gold color, whether free floating, held intracellularly (oval fat bodies), or enmeshed within a cast matrix.
Oils and fats from lubricants, ointments, creams, and lotions can also contaminate urine. They may be introduced during specimen collection from vaginal creams, topical ointments, or catheter lubricants. In the laboratory, immersion oil left on an objective lens can contaminate urine sediment during the microscopic examination. Regardless of the source, these contaminating lipids are often indicated by the lack of associated abnormalities (proteinuria, fatty casts, oval fat bodies) and are identified by (1) their presence only as free-floating droplets, (2) homogeneity, (3) lack of structure, and (4) size (often droplets coalesce to become unusually large).
Hemosiderin
Hemosiderin is a form of iron that results from ferritin denaturation. These insoluble granules can become large enough to be observed microscopically in urine sediment, especially after they have been stained to a Prussian blue color. Unstained hemosiderin granules appear as coarse yellow-brown granules and are difficult to distinguish from amorphous crystalline material in sediment (Fig. 7.76A).
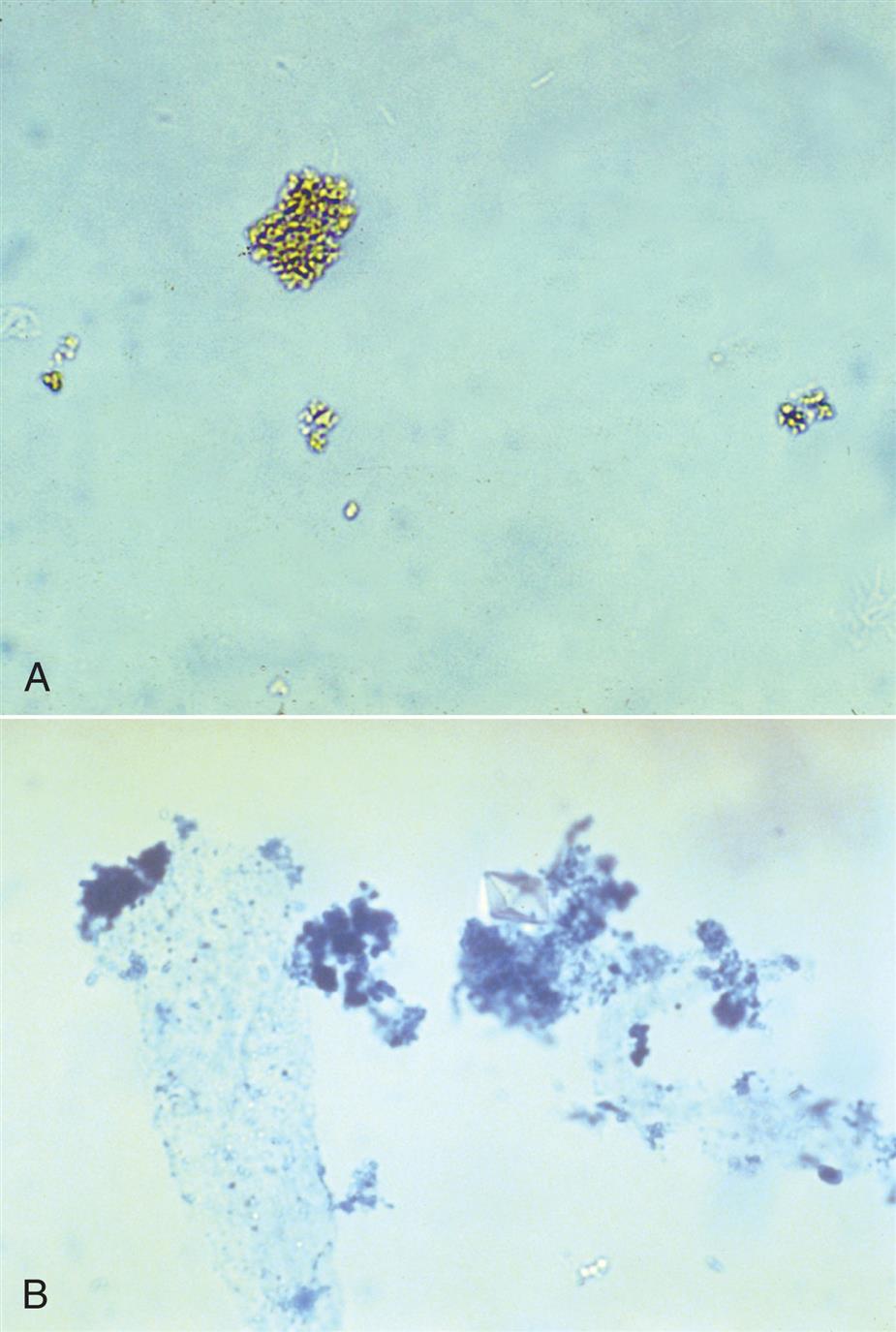
Hemosiderin granules can be found in urine sediment 2 to 4 days after a severe hemolytic episode (e.g., transfusion reaction, paroxysmal nocturnal hemoglobinuria). In these cases, plasma haptoglobin is saturated with hemoglobin, and any remaining free hemoglobin is able to pass through the glomerular filtration barrier and is absorbed by the renal tubular epithelium. The tubular cells metabolize the hemoglobin to ferritin and subsequently denature it to form hemosiderin. Note that hemoglobin is toxic to cells, and as these cells degenerate, hemosiderin granules appear in the urine. Hemosiderin granules can be present free floating or within macrophages, casts, or tubular epithelial cells.
The Prussian blue reaction is used to identify hemosiderin in urine sediment and in tissues. Initially, a concentrated urine sediment is examined for the presence of coarse yellow-brown hemosiderin granules, free floating or within casts or tubular epithelial cells. The urine sediment is treated at room temperature with potassium ferricyanide–HCl. After treatment, the sediment is reexamined for the presence of coarse Prussian blue–colored granules. The blue color results from the staining of iron in the hemosiderin granules (Fig. 7.76B). For procedural details and performance of the Rous test, see Appendix E, “Manual and Historic Methods of Interest.”
Sperm
Sperm cells or spermatozoa may be present in urine sediment from males and females. They have oval heads approximately 3 to 5 μm long and thin, thread-like tails about 40 to 60 μm long (Fig. 7.77). A variety of forms may be encountered, and at times sperm may be found in clumps of mucus in the sediment. See Chapter 12 for additional discussion of sperm morphology.
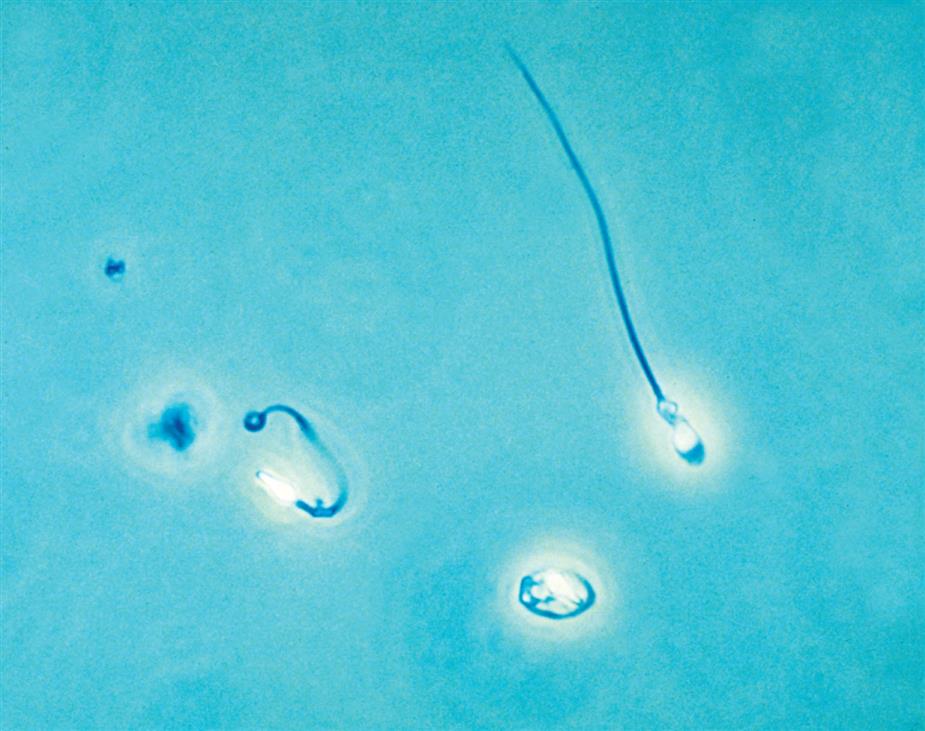
Because urine is not a viable medium for sperm, the presence of motile sperm indicates ejaculation or recent intercourse. In urine from women, sperm are usually considered a vaginal contaminant. It is important to report the presence of sperm in urine from females because it could potentially identify sexual abuse in underage vulnerable individuals and those with intellectual or developmental disabilities. This information enables the healthcare provider to appropriately intervene if necessary.
In urine from men, sperm can be present owing to nocturnal emissions, from normal or retrograde ejaculation. Sperm in urine sediment have no clinical significance and are simply reported as present.
Crystals
Crystals result from the precipitation of urine solutes out of solution. They are not normally present in freshly voided urine but form as urine cools to room or refrigerator temperature (depending on storage). When crystals are present in freshly voided urine, they indicate formation in vivo and are always clinically significant. Regardless of the crystal type, crystal formation within the nephrons can cause significant tubular damage. However, most crystals form in the urine after it has been excreted and are not clinically significant. When they are present in large numbers, they can be distracting and can make visualization of important formed elements difficult during the microscopic examination. Some crystals are significant and may indicate a metabolic or pathologic process; therefore it is important that they are correctly identified and reported. Crystals are identified on the basis of their microscopic appearance and the pH at which they are present. The urine pH provides key information to positively identify several look-alike crystals (e.g., amorphous urates from phosphates, ammonium biurate from sulfonamethoxazole).
When the identity of crystals cannot be determined by visual characteristics (e.g., shape, pH, color, birefringence), solubility, and patient history, a sample can be sent to a laboratory capable of performing Fourier transform infrared (FTIR) spectroscopy. This sophisticated technique is able to conclusively determine the chemical composition of crystals.
Contributing Factors
Several factors influence crystal formation, including (1) the concentration of the solute in the urine, (2) the urine pH, and (3) the flow of urine through the tubules. As the glomerular ultrafiltrate passes through the tubules, solutes within the lumen fluid are concentrated. If an increased amount of a solute is present because of dehydration, dietary excess, or medications, the ultrafiltrate can become supersaturated. This can result in precipitation of the solute into its characteristic crystalline form. Because solutes differ in their solubility, this characteristic provides a means of identifying and differentiating them. For example, inorganic salts such as oxalate, phosphate, calcium, ammonium, and magnesium are less soluble in neutral or alkaline urine (pH >6). As a result, when the urine pH becomes neutral or alkaline, these solutes can precipitate out in their crystalline form. In contrast, organic solutes such as uric acid, bilirubin, and cystine are less soluble in acidic conditions and can form crystals in acidic urine (pH <6). Most clinically significant crystals (e.g., cystine, tyrosine, leucine) are found in acidic urine, including many drugs associated with crystalluria.
Crystal formation, similar to cast formation, is enhanced by slow urine flow through the renal tubules as well as by pH. Flow reduction allows time for maximum concentration of solutes in the ultrafiltrate, while small pH changes can cause the solute to crystallize. For example, at a pH of 5.0, uric acid will crystallize at a concentration of about 2 mmol/L, whereas at a pH of 5.9 to 6.0 a concentration of ≥4 mmol/L is required for crystallization.26 In other words, as the specific gravity of the ultrafiltrate increases, the possibility of crystal formation also increases. In summary, when the pH and solute concentration become optimal, crystals can form.
Although these factors account for crystal formation within the renal tubules, they are also involved in the development of crystals during urine storage. The solute concentration, the pH, the time allowed for formation, as well as temperature play a role in crystal formation. When these conditions are optimized, the chemicals in urine can exceed their solubility and precipitate in their uniquely characteristic crystalline or amorphous forms.
The following section discusses normal and abnormal crystals and loosely categorizes them according to the pH at which they typically form. Crystals are routinely reported as few, moderate, or many under high-power magnification. The characteristics and clinical significance of crystals formed from normal urine solutes are provided in Table 7.11, those from abnormal solutes in Table 7.12, and those from drugs in Table 7.13. Because the feature most notable when viewing crystals microscopically is shape, Table 7.16 located at the end of this chapter arranges crystals into categories based on shape to enhance rapid identification.
Table 7.11
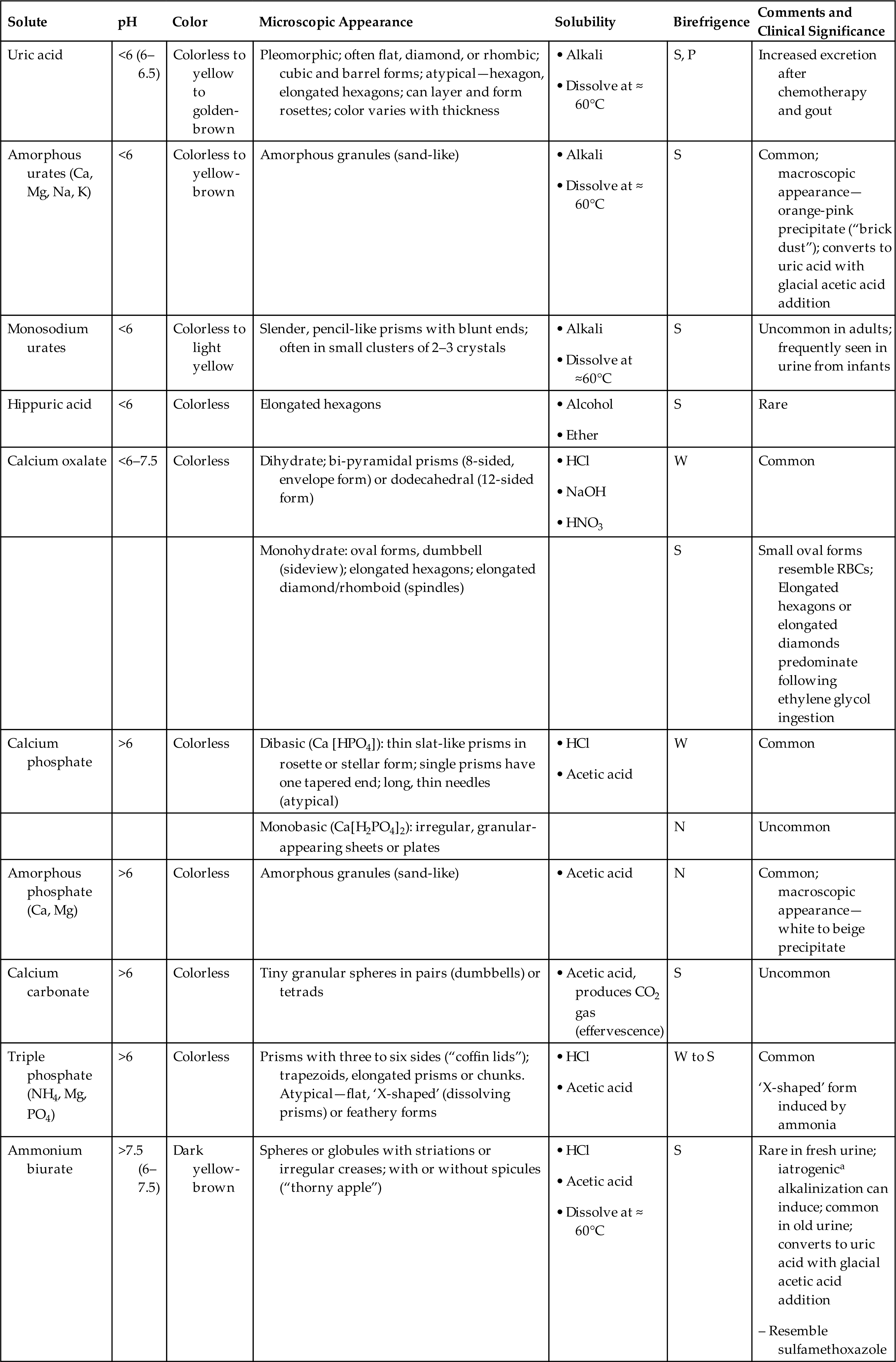
M, Moderate; P, polychromatic; pMC, forms pseudo-Maltese cross pattern; S, strong; SG, specific gravity; W, weak.
Table 7.12
| Solute | pH | Color | Microscopic Appearance | Solubility | Birefringence | Comments and Clinical Significance |
|---|---|---|---|---|---|---|
| Bilirubin | <6 | Yellow-brown; highly pigmented | Small balls of fine golden needles or granules that form clusters | N to W | Rare; crystals induced by storage at 2–8°C; liver disease or obstruction | |
| Leucine | <6 | Dark yellow to brown | Spheres with radial striations and concentric circles | S, pMC | Rare; crystals induced by storage at 2–8°C; liver disease; confirm by amino acid analysis | |
| Tyrosine | <6 | Colorless to yellow | Fine, delicate needles; singly or in clusters, bundles, or sheaves | S | Rare; crystals induced by storage at 2–8°C; liver disease; confirm by amino acid analysis | |
| Cholesterol | <6 (6–7.5) | Colorless | Flat plates (parallelograms) with notched corners; may layer |