Synovial Fluid Analysis
After studying this chapter, the student should be able to:
- 1. Describe the formation and function of synovial fluid.
- 2. Summarize the four principal classifications of joint disease.
- 3. Classify synovial fluid as normal, noninflammatory, inflammatory, septic, or hemorrhagic using various laboratory results.
- 4. Discuss appropriate tubes for the collection and distribution of synovial fluid specimens; discuss the importance of timely specimen processing and testing.
- 5. State physical characteristics of normal synovial fluid and discuss how each characteristic is modified in disease states.
- 6. Correlate the cells and crystals observed during microscopic examination of synovial fluid with various joint diseases.
- 7. Compare and contrast concentrations of selected chemical constituents of synovial fluid from healthy joints with that from diseased joints.
- 8. Discuss the microbiological examination of synovial fluid and its importance in the diagnosis of infectious joint disease.
Key Terms1⁎ *
Physiology and Composition
In areas of the skeleton where friction could develop, such as the joints, bursae, and tendon sheaths, viscous synovial fluid is present. Within articulated diarthrodial joints (e.g., the knee), the ends of apposing bones are covered with articular cartilage, the joint space is lined by a synovial membrane (except in weight-bearing areas), and synovial fluid bathes and lubricates the joint (Fig. 11.1). The surface of the synovial membrane surrounding the joint consists of numerous microvilli with a layer, one to three cells deep, of synovial cells called synoviocytes (Fig. 11.2). Two types of synoviocytes are present in the synovial membrane. The most prevalent type is actively phagocytic and synthesizes degradative enzymes (e.g., collagenases). The second type of synoviocyte synthesizes hyaluronate, a mucopolysaccharide linked with approximately 2% protein. The synoviocytes are loosely organized in the synovial membrane and differ from cells in other lining membranes in that they have no basement membrane, and adjacent synovial cells are not joined with desmosomes. Beneath the synoviocytes is a thin layer of loose connective tissue containing a vast network of blood vessels, lymphatics, and nerves. Variable numbers of mononuclear cells are also found in this connective tissue layer.
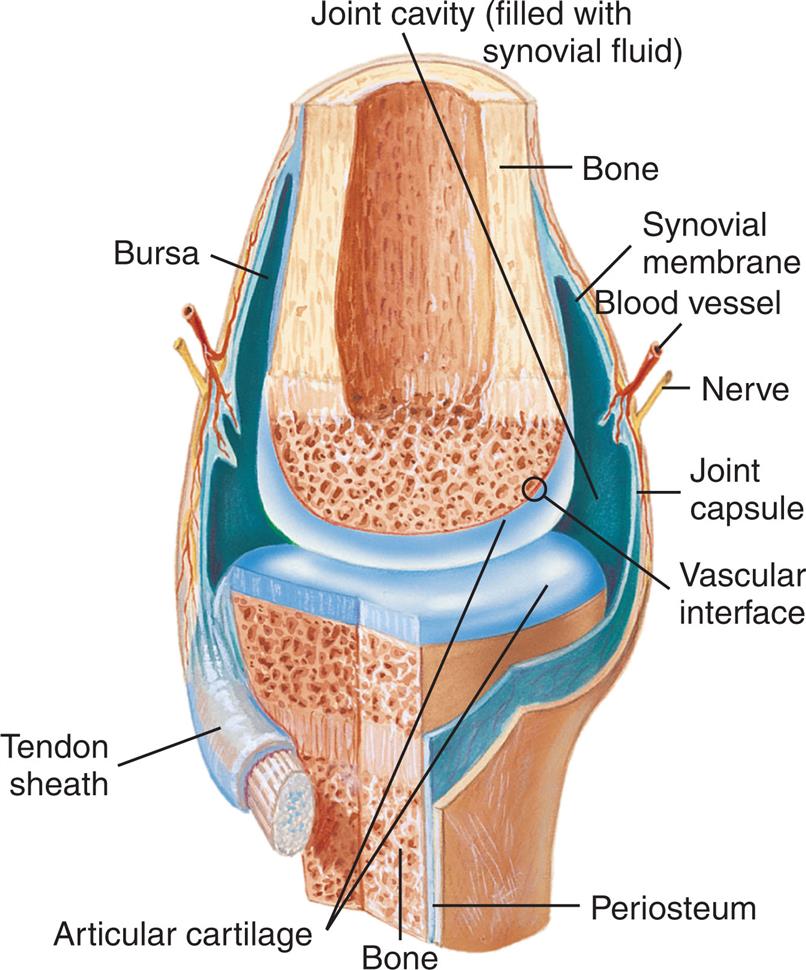
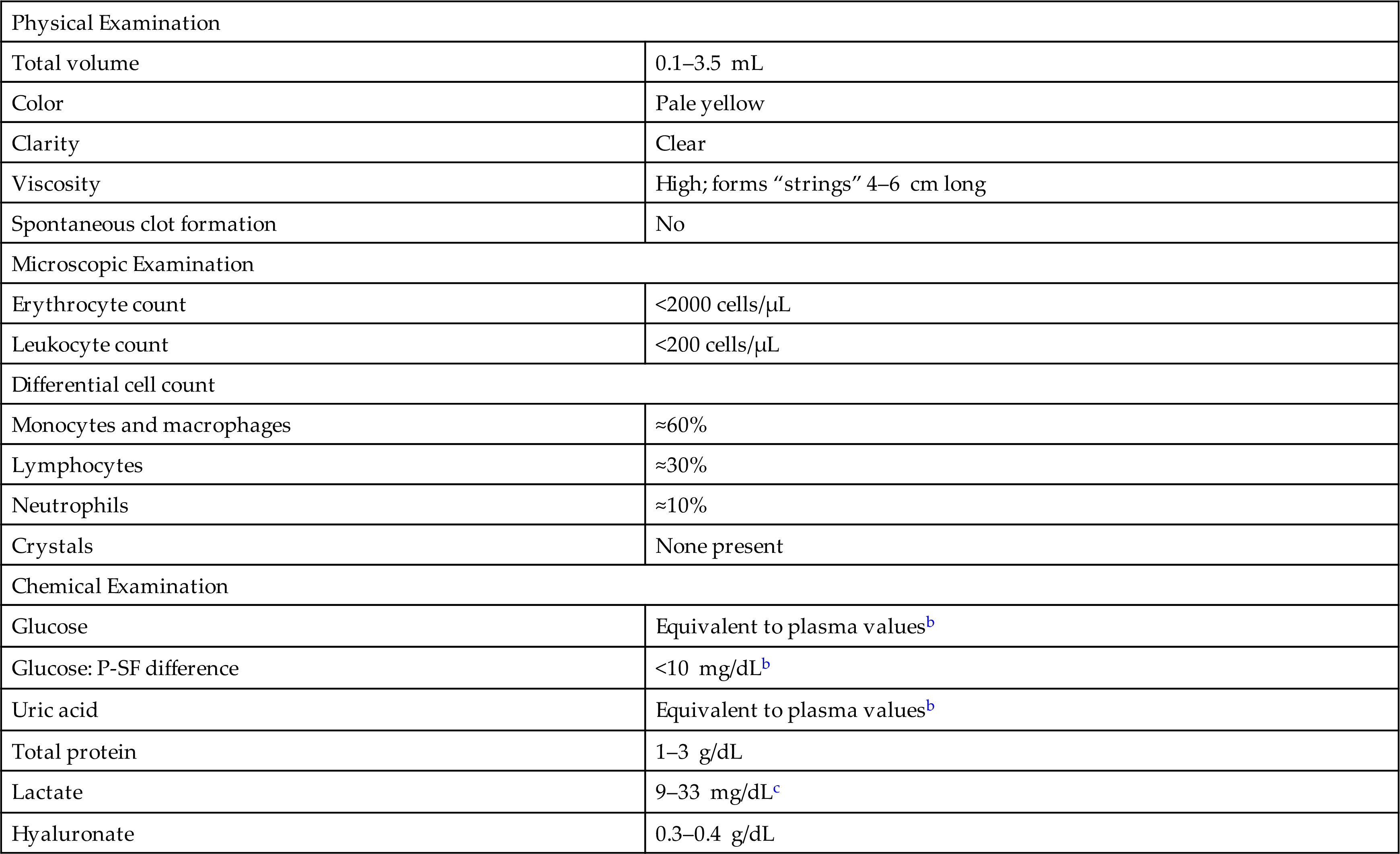
Synovial fluid is formed by the ultrafiltration of plasma across the synovial membrane and from secretions by synoviocytes. The resultant viscous fluid serves as a lubricant for the joint and is the sole nutrient source for the metabolically active articular cartilage, which lacks blood vessels, lymphatics, and nerves. The composition of synovial fluid is unique in that its glucose and uric acid concentrations are equivalent to that of the blood plasma; however its total protein and immunoglobulin concentrations vary from one-fourth to one-half that of the plasma. Table 11.1 (and Appendix C) lists reference values for various characteristics and constituents of normal synovial fluid obtained from a knee joint.
Table 11.1
| Physical Examination | |
| Total volume | 0.1–3.5 mL |
| Color | Pale yellow |
| Clarity | Clear |
| Viscosity | High; forms “strings” 4–6 cm long |
| Spontaneous clot formation | No |
| Microscopic Examination | |
| Erythrocyte count | <2000 cells/μL |
| Leukocyte count | <200 cells/μL |
| Differential cell count | |
| Monocytes and macrophages | ≈60% |
| Lymphocytes | ≈30% |
| Neutrophils | ≈10% |
| Crystals | None present |
| Chemical Examination | |
| Glucose | Equivalent to plasma valuesb |
| Glucose: P-SF difference | <10 mg/dLb |
| Uric acid | Equivalent to plasma valuesb |
| Total protein | 1–3 g/dL |
| Lactate | 9–33 mg/dLc |
| Hyaluronate | 0.3–0.4 g/dL |
aValues for fluid obtained from a knee joint.
bSynovial fluid values are equivalent to blood plasma values if obtained from a fasting patient.
cNormal lactate values are assumed to be similar to those in blood and cerebrospinal fluid; actual reference intervals have yet to be established.
Classification of Joint Disorders
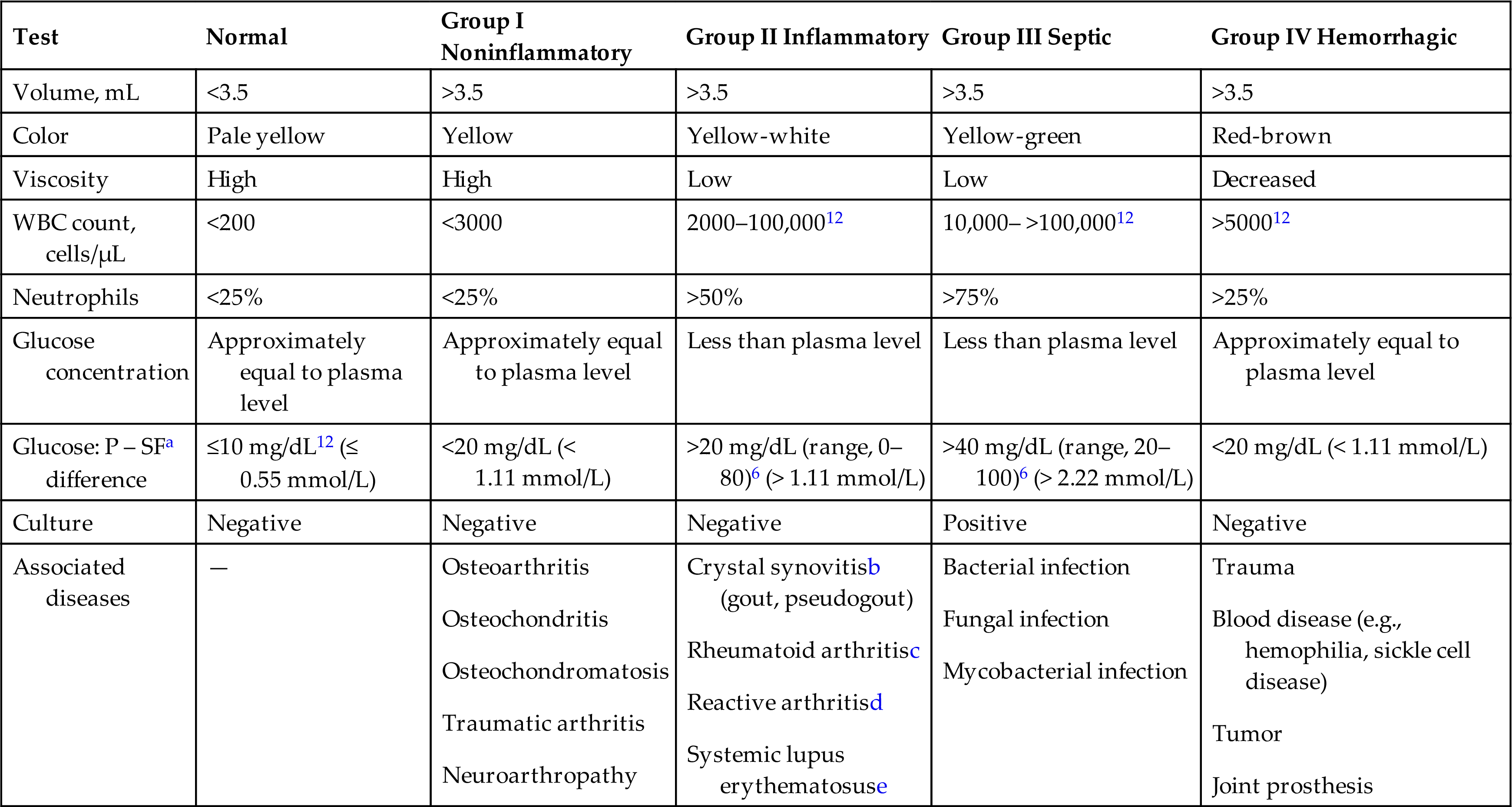
Arthritis and other joint diseases are common, and laboratory analysis of synovial fluid assists in the diagnosis and classification of these conditions. When synovial fluid is removed from a joint space, laboratory examination enables classification of the disease process into one of four principal categories: noninflammatory, inflammatory, septic, or hemorrhagic. These general classifications aid in differential diagnosis of joint disease and are summarized in Table 11.2. An important note is that (1) these categories partially overlap, (2) several conditions can occur in the joint at the same time, and (3) variations in test results can occur depending on the stage of the disease process. Consequently, these classifications are used only as a guide for the clinician in the evaluation and diagnosis of joint disease. In contrast to the tentative diagnoses possible on the basis of laboratory findings, a definitive diagnosis is possible when microorganisms are identified (septic arthritis) or crystals are present (crystal synovitis) in the synovial fluid.
Table 11.2
| Test | Normal | Group I Noninflammatory | Group II Inflammatory | Group III Septic | Group IV Hemorrhagic |
|---|---|---|---|---|---|
| Volume, mL | <3.5 | >3.5 | >3.5 | >3.5 | >3.5 |
| Color | Pale yellow | Yellow | Yellow-white | Yellow-green | Red-brown |
| Viscosity | High | High | Low | Low | Decreased |
| WBC count, cells/μL | <200 | <3000 | 2000–100,00012 | 10,000– >100,00012 | >500012 |
| Neutrophils | <25% | <25% | >50% | >75% | >25% |
| Glucose concentration | Approximately equal to plasma level | Approximately equal to plasma level | Less than plasma level | Less than plasma level | Approximately equal to plasma level |
| Glucose: P – SFa difference | ≤10 mg/dL12 (≤ 0.55 mmol/L) | <20 mg/dL (< 1.11 mmol/L) | >20 mg/dL (range, 0–80)6 (> 1.11 mmol/L) | >40 mg/dL (range, 20–100)6 (> 2.22 mmol/L) | <20 mg/dL (< 1.11 mmol/L) |
| Culture | Negative | Negative | Negative | Positive | Negative |
| Associated diseases | — |
aThe plasma–synovial fluid difference in glucose concentration when specimens are obtained simultaneously.
bWith chronic or subsiding conditions, crystal synovitis may present as group I.
cEarly stages of rheumatoid arthritis may present as group I.
dPreviously known as Reiter’s syndrome.11
eSystemic lupus erythematosus may also present as group I.
Specimen Collection
Synovial fluid is removed by arthrocentesis, which is the percutaneous aspiration of fluid from a joint using aseptic technique. Disposable, sterile needles and syringes are used most often to prevent birefringent contaminants associated with the cleaning and resterilization of reusable supplies. If possible, patients should be fasting a minimum of 4 to 6 hours to allow for the equilibration of chemical constituents between plasma and synovial fluid. A blood sample is collected at approximately the same time as the arthrocentesis procedure is performed when determination of the plasma–synovial fluid difference in glucose is requested.
The volume of synovial fluid in a joint varies with the size of the joint cavity and is normally small—about 0.1 to 3.5 mL.1 Consequently, arthrocentesis of a joint when an effusion (or fluid buildup) is not present can result in a “dry tap”—a small yield of synovial fluid. Sometimes synovial fluid is present only in the aspiration needle; this requires that the contents of the needle be expressed into an appropriate small-volume container or, at times, directly into culture media. Alternatively, the clinician may insert the needle into a sterile cork and transport the entire syringe to the laboratory for processing. However, this practice represents a significant potential biohazard and should be avoided. Note that specimens should not be rejected because of a low volume of fluid. Diagnostic crystals, cell counts with differentials, and microbial growth are possible from a few drops of synovial fluid.
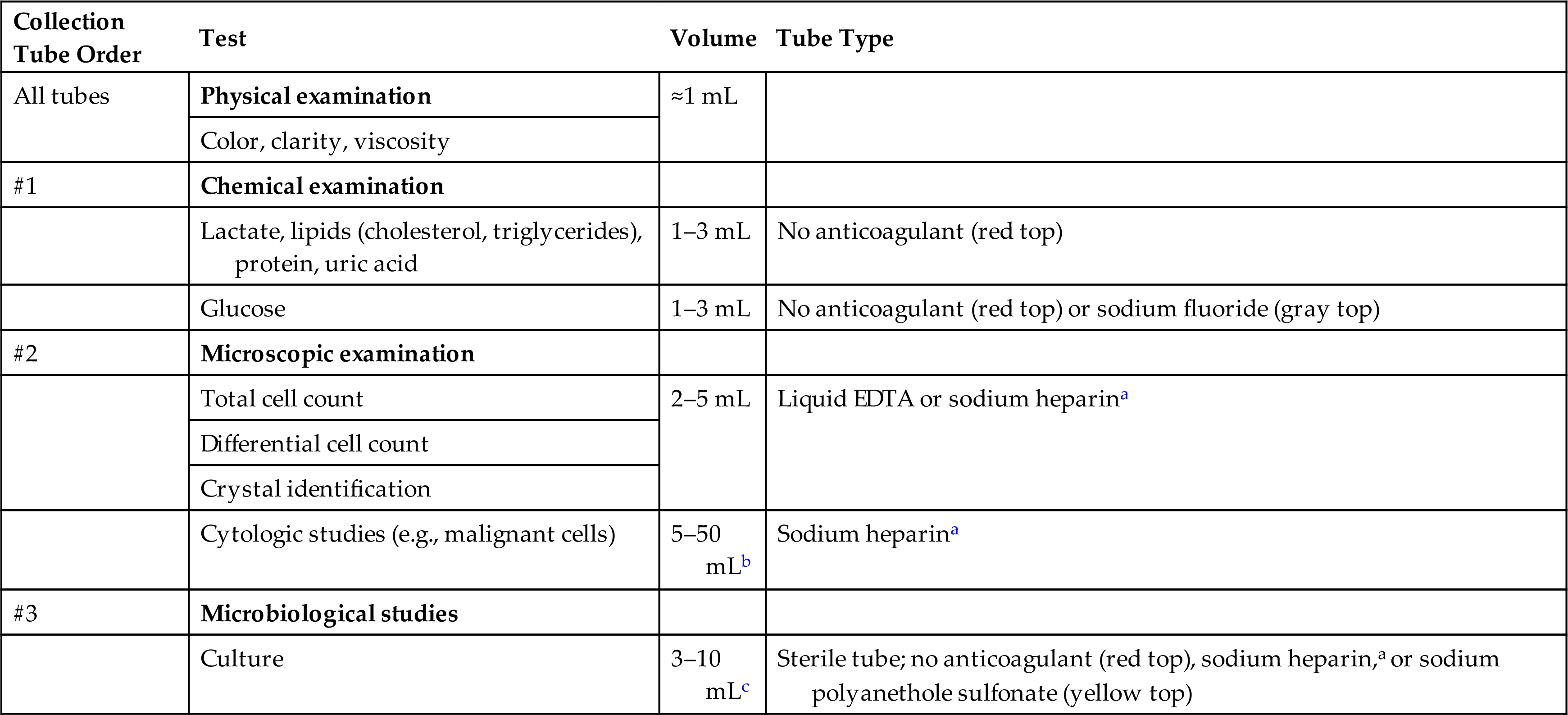
Synovial fluid handling and volume requirements are summarized in Table 11.3. It is recommended by the Clinical Laboratory Standards Institute (CLSI) that the first portion of fluid obtained (tube #1) be placed into a plain red-top tube (no anticoagulant) for chemical and immunologic evaluation. The next portion (tube #2) is collected in an anticoagulant tube for microscopic examinations, and the last portion (tube #3) is placed in a sterile anticoagulant tube for microbiological studies. Because the volume of synovial fluid present can vary significantly, the amount collected and distributed into each collection tube will also vary.
Table 11.3
| Collection Tube Order | Test | Volume | Tube Type |
|---|---|---|---|
| All tubes | Physical examination | ≈1 mL | |
| Color, clarity, viscosity | |||
| #1 | Chemical examination | ||
| Lactate, lipids (cholesterol, triglycerides), protein, uric acid | 1–3 mL | No anticoagulant (red top) | |
| Glucose | 1–3 mL | No anticoagulant (red top) or sodium fluoride (gray top) | |
| #2 | Microscopic examination | ||
| Total cell count | 2–5 mL | Liquid EDTA or sodium heparina | |
| Differential cell count | |||
| Crystal identification | |||
| Cytologic studies (e.g., malignant cells) | 5–50 mLb | Sodium heparina | |
| #3 | Microbiological studies | ||
| Culture | 3–10 mLc | Sterile tube; no anticoagulant (red top), sodium heparin,a or sodium polyanethole sulfonate (yellow top) |
EDTA, Ethylenediaminetetraacetic acid.
aSodium heparin concentration at 25 U/mL synovial fluid.
bNo upper limit to the amount of fluid that can be submitted; large volumes of fluid increase the recovery of cellular elements.
cLarge fluid volumes may increase the recovery of viable microbial organisms.
Note that using larger volumes of synovial fluid for cultures can enhance the recovery of microbial organisms; similarly, greater volumes of fluid will increase the numbers of cells recovered for cytologic evaluation. For cell counts and crystal identification, the best anticoagulant for synovial fluid is “liquid” ethylenediaminetetraacetic acid (EDTA) or sodium heparin at approximately 25 units (U) per milliliter of synovial fluid. These anticoagulants prevent clotting when fibrinogen is present but do not form crystals. Oxalate, lithium heparin, and powdered EDTA must be avoided because they can produce crystalline structures that resemble monosodium urate crystals, thereby interfering with the microscopic examination for in vivo crystals.
For microbial studies, synovial fluid placed in a sterile tube without anticoagulant or with sodium polyanetholsulfonate (SPS, yellow-top tube) is acceptable.2 Note that the concentration of SPS must not exceed 0.025% (wt/vol) because some Neisseria spp. and certain anaerobes are inhibited by higher concentrations.3 For anaerobic culture, fluid can be placed into an anaerobic transport vial or tube. Because synovial fluid specimens are often sent to different laboratories for testing, the total volume of fluid removed should be recorded in the patient’s chart and on specimen test request forms at the time of fluid collection.
Synovial fluid should be transported and analyzed at room temperature. As with other body fluids, it should be processed and tested as soon as possible after collection. If processing is delayed, several adverse changes can occur: (1) Cells in the synovial fluid can alter the chemical composition, (2) detection of microbial organisms can be jeopardized, and (3) blood cells (white blood cells [WBCs], red blood cells [RBCs]) can lyse. Note that refrigeration adversely affects the viability of microorganisms and could erroneously induce in vitro crystalline precipitation.
Physical Examination
Color
The hematology laboratory often performs the physical and microscopic examinations of synovial fluid. Physical examination includes visual assessment for color, clarity, and viscosity. Normally, synovial fluid appears pale yellow or colorless and is clear. Color variations of red and brown are associated with trauma during the arthrocentesis and with disorders that disrupt the synovial membrane, allowing blood to enter the joint cavity, such as joint fracture, tumor, and traumatic arthritis. A traumatic procedure is indicated when the amount of blood in the fluid decreases as collection continues, or when a streak of blood is noticed in the fluid. With some joint disorders, particularly infections, synovial fluid can appear greenish or purulent; with other conditions (e.g., tuberculous arthritis, systemic lupus erythematosus), synovial fluid can appear milky.
Clarity
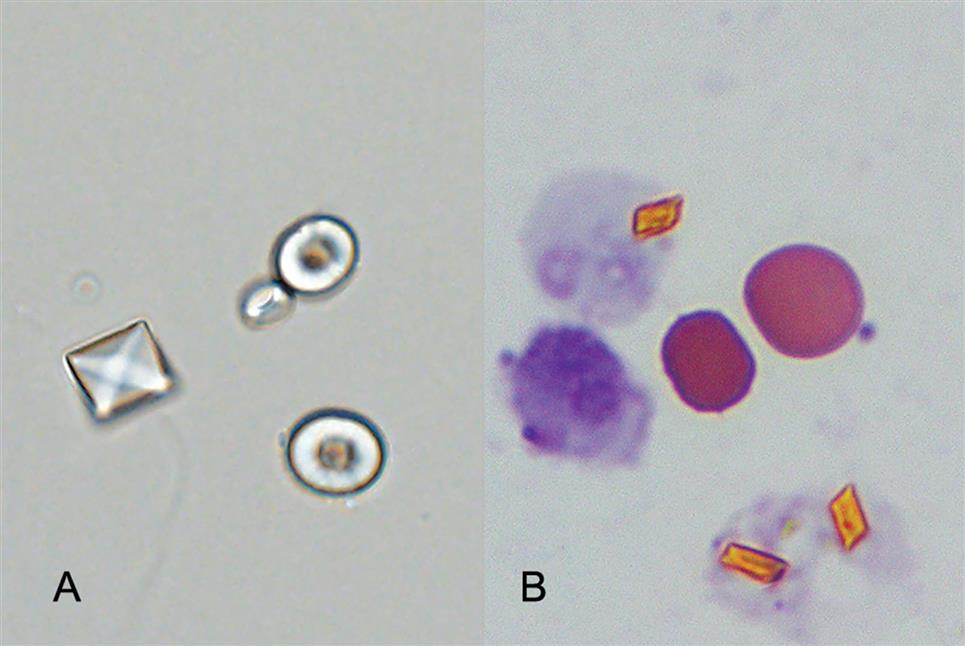
Numerous substances can modify the clarity of synovial fluid; these substances include WBCs, RBCs, synoviocytes, crystals, fat droplets, fibrin, cellular debris, rice bodies, and ochronotic shards. The specific entity or entities causing the observed turbidity are usually identified by microscopic examination. Some substances are evident upon gross visual examination of synovial fluid. Rice bodies are white, free-floating particles made up of collagen covered by fibrinous tissue.4 They resemble polished, shiny grains of rice and can vary greatly in size. Although they may be seen in many arthritic conditions, they are observed most commonly with rheumatoid arthritis. Dark, pepper-like particles called ochronotic shards can be present in synovial fluid from individuals with ochronotic arthropathy, a consequence of alkaptonuria and ochronosis.5 These pepper-like particles are pieces of pigmented cartilage that has eroded and broken loose into the fluid.
Viscosity
Synovial fluid has high viscosity compared to water because of its high concentration of the mucoprotein hyaluronate. This high-molecular-weight polymer of repeating disaccharide units is secreted by synoviocytes and serves as a joint lubricant. During inflammatory conditions, hyaluronate can be depolymerized by the action of the enzyme hyaluronidase, which is present in neutrophils, as well as by some bacteria (e.g., Staphylococcus aureus, Streptococcus pyogenes, Clostridium perfringens). In addition, some disease processes inhibit the production and secretion of hyaluronate by synoviocytes.
Synovial fluid viscosity can be assessed at the bedside by observing the fluid as it is expelled from the collection syringe. A drop of normal synovial fluid forms a string at least 4 cm long before breaking. The viscosity of the fluid is considered abnormally low when the string breaks earlier or forms discrete water-like droplets. Because viscosity measurements have little diagnostic or clinical value and are not reliable, they are rarely performed.
Clot Formation
Spontaneous clot formation in synovial fluid indicates the abnormal presence of fibrinogen. Because of its high molecular weight (340,000), fibrinogen cannot pass through a normal or healthy synovial membrane. Pathologic processes that damage the synovial membrane or a traumatic arthrocentesis with blood contamination can cause fibrinogen to be present in synovial fluid, which will result in clot formation. Therefore a portion of a synovial fluid collection should always be anticoagulated using liquid EDTA or sodium heparin to prevent potential fibrin clots that interfere with the microscopic examination.
Microscopic Examination
To eliminate the potential for clot formation and reduce the viscosity of synovial fluid, before analysis a well-mixed portion is transferred to another tube and a few grains of hyaluronidase are added. This enzyme will eliminate hyaluronate, thereby preventing clot formation, reducing viscosity, and enhancing an even distribution of cells in the counting chambers of the hemacytometer. Alternatively when the fluid requires dilution because it is cloudy or turbid, a hyaluronidase buffer solution can be used as the diluent.6 Note that if the synovial fluid has not been “pretreated” with hyaluronidase, an acetic acid diluent cannot be used because it will cause hyaluronate to form a mucin clot and cells to clump, which interfere with the microscopic examination.
A manual microscopic examination is performed using a hemacytometer and well-mixed synovial fluid, either undiluted or diluted. See Chapter 17 for procedural details for performing manual cell counts and Appendix D for optional diluents when needed. Analysis should occur as soon as possible to avoid potential changes in cell counts (lysis) and crystal formation.
Total Cell Count
Normally, the number of RBCs in synovial fluid is fewer than 2000 RBCs/μL. Some RBCs originate from the procedure itself; those resulting from hemorrhagic effusions are usually obvious from their large numbers and the initial red-brown appearance of the fluid. When the number of RBCs present is excessive and they must be eliminated to allow performance of an accurate WBC and differential count, hypotonic saline (0.3%) is used as the diluent because it will selectively lyse the RBCs while retaining the WBCs.
WBCs are normally present in synovial fluid at cell counts lower than 200 WBCs/μL. Although WBC counts greater than 2000 cells/μL are typically associated with bacterial arthritis, leukocytosis can occur with other conditions, such as acute gouty arthritis and rheumatoid arthritis. Although a total WBC count has limited value in identifying a specific disease process, when abnormal, it is providing clinically valuable information.
Differential Cell Count
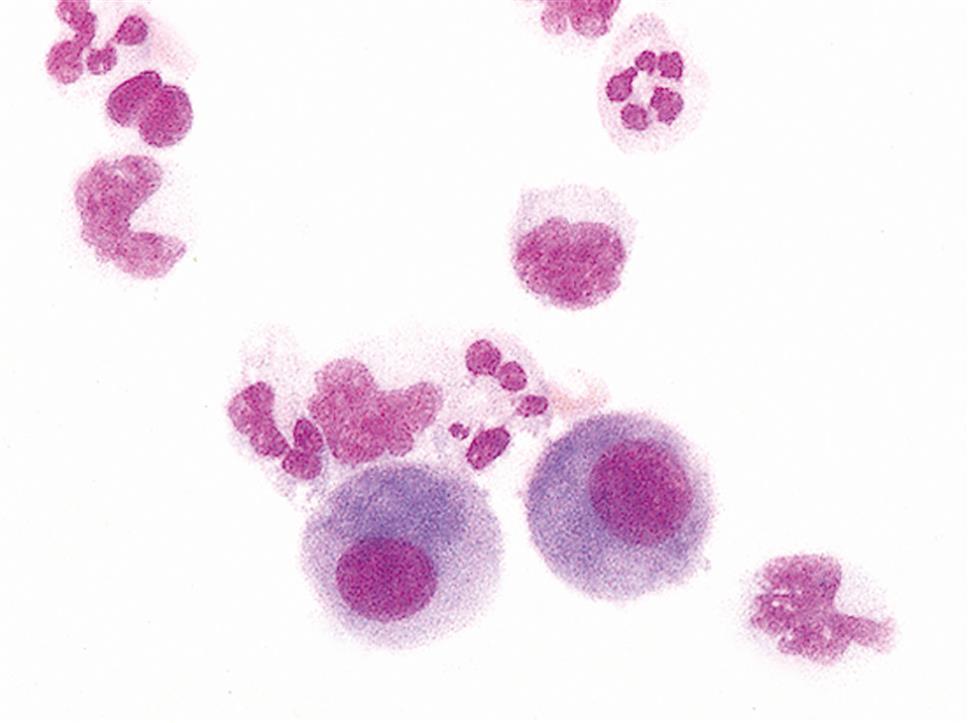
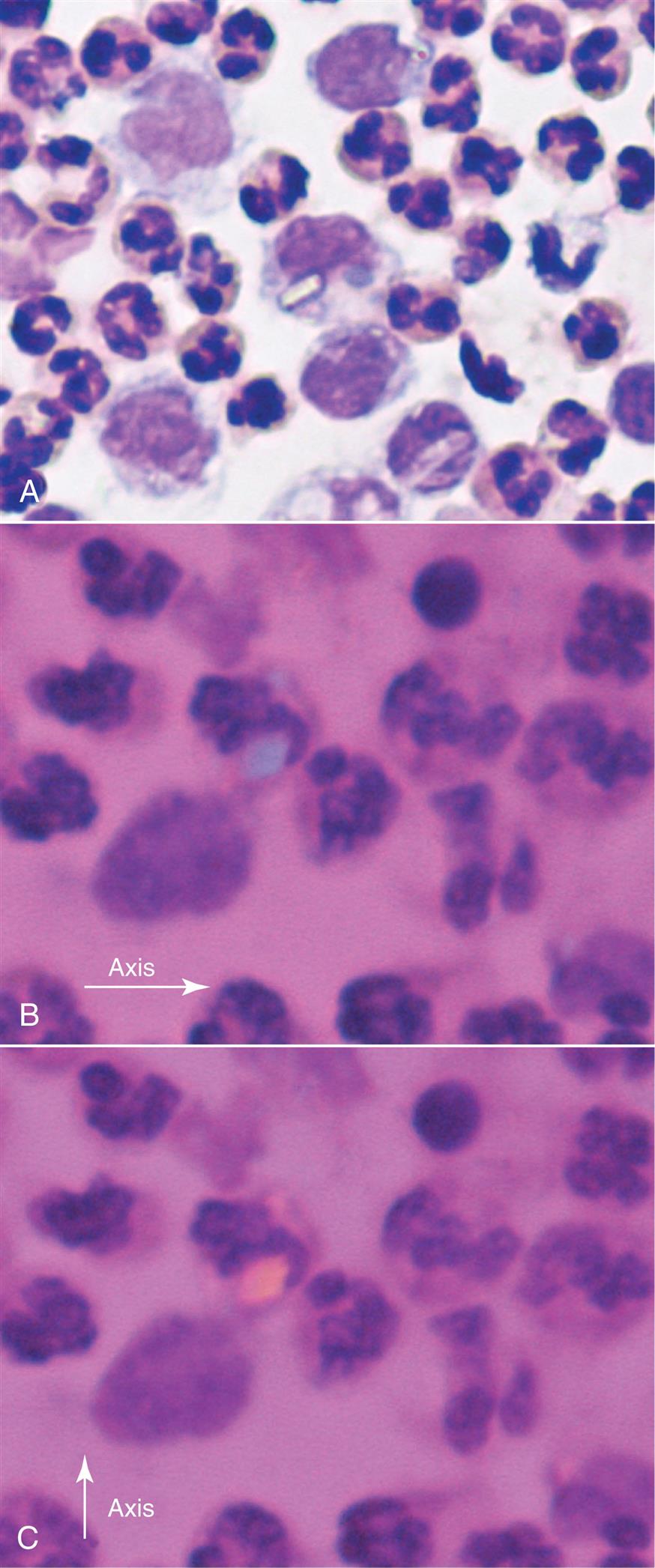
Synovial fluid can be concentrated by several techniques; however, cytocentrifugation preserves cellular morphology better than routine centrifugation procedures.7 Normally, about 60% of synovial fluid’s nucleated cells are monocytes or macrophages, approximately 30% are lymphocytes, and approximately 10% are neutrophils.8 Nucleated cell differentials have limited clinical value because they can vary in a particular disease as well as with the stage of the disease.
A nucleated cell differential with more than 80% neutrophils is associated with bacterial arthritis and urate gout, regardless of the total cell count. An increase in the lymphocyte percentage often occurs in the early stages of rheumatoid arthritis, whereas neutrophils predominate in later stages. An increased eosinophil count (greater than 2%) has been associated with a variety of disorders, including rheumatic fever, parasitic infestations, and metastatic carcinoma, and often follows procedures such as arthrography and radiation therapy.
The nucleated cell types that can be present in synovial fluid include granulocytes (i.e., neutrophils, eosinophils, basophils/mast cells), lymphocytes, plasma cells, mononuclear phagocytes (i.e., monocytes, macrophages), synoviocytes (lining cells of synovial membrane), and, although rare, malignant cells. Note that all nucleated cells are counted in the differential, including synoviocytes, the lining cells of the synovial membrane.
Synoviocytes can be difficult to differentiate from monocytes and they closely resemble mesothelial cells that are found in pleural and peritoneal fluids (compare Fig. 11.2 with Fig 10.6). As with mesothelial cells in serous fluid, the presence of synoviocytes in synovial fluid is expected and does not have any diagnostic value. Many institutions group synoviocytes, monocytes, and macrophages in a single category without differentiating between them.
Some nucleated cells are associated with a specific disease process, such as LE cells with lupus erythematosus, cells with hemosiderin inclusions following a hemorrhagic process, multinucleated cartilaginous cells in patients with osteoarthritis, and malignant cells in patients with metastatic tumors.
Crystal Identification
One of the most important laboratory tests routinely performed on synovial fluid is microscopic examination for crystals. Identification of some crystals is pathognomonic of a specific joint disease, thereby enabling a rapid definitive diagnosis (Table 11.4). Because temperature and pH changes affect crystal formation and solubility, synovial fluid specimens should be maintained at room temperature and examined as soon as possible after collection. Time delays before microscopic examination can result in a decrease in WBCs (lysis) and in phagocytosis of crystals by WBCs during storage. Note that pretreatment with hyaluronidase has no effect on crystals if present.
Table 11.4
| Crystal | Microscopic Characteristicsa | Clinical Conditions |
|---|---|---|
| Monosodium urate monohydrate | Fine, needle-like, with pointed ends; strong negative birefringence | Urate arthritis (gouty arthritis) |
| Calcium pyrophosphate dihydrate | Rod-like or rhombic; weak positive birefringence | Pseudogout (i.e., chondrocalcinosis) |
| Cholesterol | Flat, parallelogram-shaped plates, with notched corners; negative birefringence; intensity varies with crystal thickness | Chronic arthritic conditions (e.g., rheumatoid arthritis), monoarthritis |
| Hydroxyapatite | Requires electron microscopy for visualization; not birefringent | Apatite-associated arthropathies |
| Corticosteroid | Irregular with jagged or serrated edges; broken pieces; varies with corticosteroid used | Indicates previous intraarticular injection |
| Calcium oxalate | Calcium oxalate dihydrate (Weddellite) and monohydrate (Whewellite) | Oxalate gout in long-term renal dialysis patients or those with primary hyperoxaluria (rare, inherited disorder) |
| Hematin | Yellow-brown (golden) rhomboid crystals under brightfield microscopy | Indicates a previous hemorrhage in the joint |
aCharacteristics of typical crystalline forms; however, other crystalline forms can also be present.
When crystals are present in synovial fluid, they are typically of a single type. However, although uncommon, both monosodium urate and calcium pyrophosphate dihydrate (CPPD) crystals have been found. Note that clinically significant crystals will not have irregular, jagged edges or appear uneven or broken. In these cases, artifacts or corticosteroid crystals should be suspected.
Microscope Slide Preparations
Synovial fluid is viewed microscopically using a cytocentrifuged (or cytospin) slide preparation or a wet preparation. Several advantages are associated with the use of cytospin slides. First, cytocentrifugation concentrates the fluid components in a small area on a slide; this increases the sensitivity of the recovery and the detection of small numbers of crystals. Second, they are permanent slides that can be retained and used for review, training, and competency assessment. Lastly, cytospin slides can be viewed stained or unstained using polarizing microscopy because the appearance and birefringence of crystals are identical to those observed in wet preparations. The only disadvantage is the initial cost of a cytocentrifuge (see discussion of cytocentrifugation in Chapter 17). Be aware when staining prepared slides using a technique that involves a “methanol fixative” step, such as with Wright-Giemsa procedures, as monosodium urate and cholesterol crystals can dissolve during processing.9 Therefore, synovial fluid should be viewed using both a wet mount and a stained smear to determine the presence of crystals.
When a manual “wet” preparation is made, a drop of synovial fluid is placed onto an alcohol-cleaned microscope slide and a coverslip is applied. The specimen should just fill the area beneath the coverslip; too much specimen will cause the coverslip to float, increasing the number of focal planes and possibly hampering the viewing of important components.
Thorough examination of synovial fluid preparations by a skilled microscopist is imperative to ensure visualization and proper identification of synovial fluid crystals. Such examination is necessary because (1) the number of crystals present can vary significantly with disease (i.e., only a few small crystals may be present); (2) the differentiation of crystals is difficult because different types of crystals closely resemble each other; (3) free crystals can become enmeshed in fibrin or debris and can easily be overlooked; and (4) numerous artifacts are birefringent and must be appropriately identified. In addition, synovial fluid findings in infectious arthritis and crystal synovitis can be similar, which makes microscopic examination for crystals an important tool in differentiating these conditions.
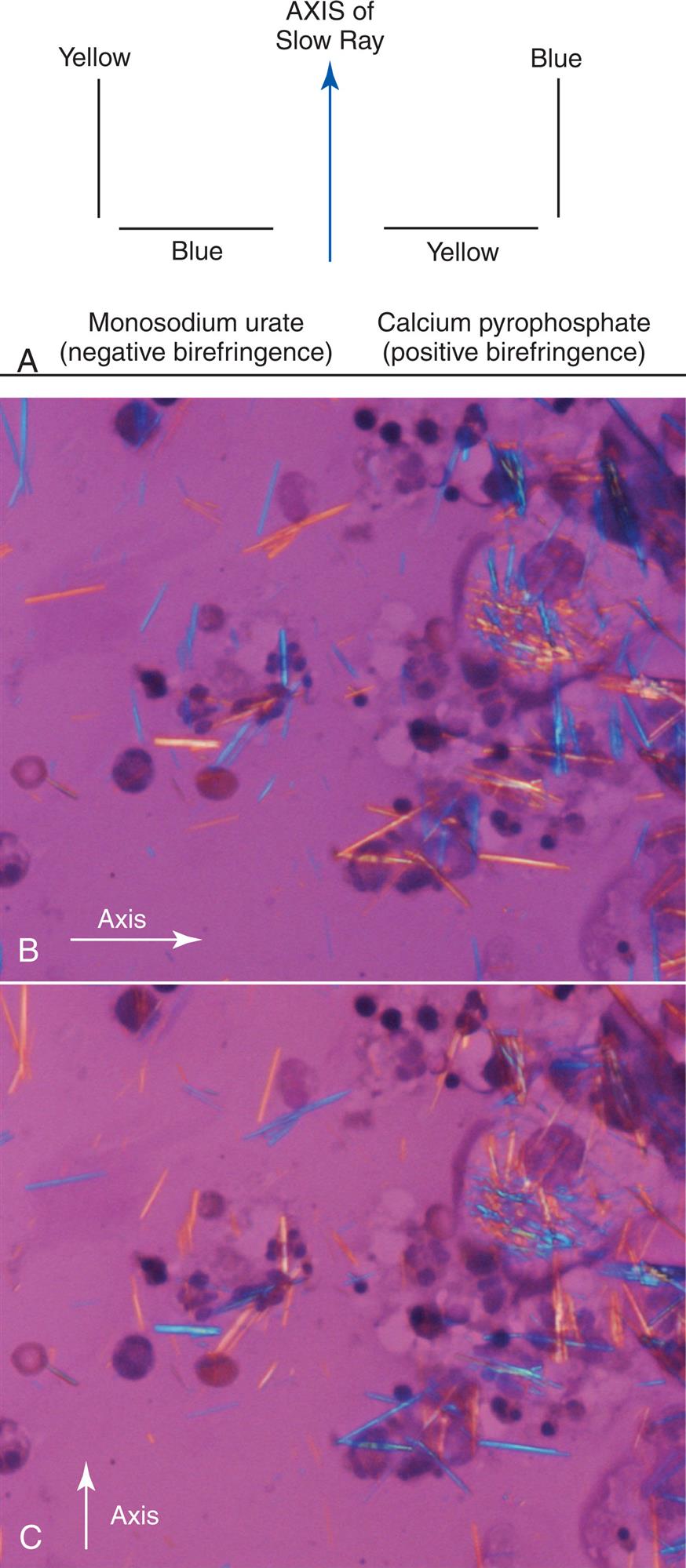
Slide preparations for crystal identification are viewed microscopically using direct and compensated polarizing microscopy. Under polarizing microscopy, birefringent substances appear as bright objects against a black background, and the intensity of their birefringence varies with the substance. For example, monosodium urate crystals are bright and easy to visualize compared to CPPD crystals. Compensated polarizing microscopy (using a red compensator plate) enables the identification and differentiation of positive and negative birefringent substances based on the color produced when the crystals are oriented parallel and perpendicular to the axis of the red compensator (Fig. 11.3A). See Chapter 18 for additional discussion of polarizing microscopy and for examples of different types of birefringence and intensities, see Tables 18.1 and 18.2.
Monosodium Urate Crystals
Monosodium urate (MSU) crystals in synovial fluid indicate gouty arthritis. Present intracellularly in leukocytes during acute stages of the disease, these needle-like crystals with pointed ends can distend the cytoplasm of WBCs. Free-floating crystals enmeshed in fibrin can also be present. Under polarizing microscopy, monosodium urate crystals are strongly birefringent, appearing bright against a black background. When a red compensator or full-wave plate is used, these negatively birefringent crystals appear yellow when their longitudinal axes are parallel to the axis of the red compensator plate (Fig. 11.3B) and blue when their longitudinal axes are perpendicular to it (Fig. 11.3C ). This characteristic enables the differentiation of MSU crystals from other similarly appearing crystals (e.g., EDTA crystals, betamethasone acetate crystals).
Calcium Pyrophosphate Dihydrate Crystals
A group of diseases is associated with CPPD crystals in synovial fluid. These conditions, often referred to as pseudogout or chondrocalcinosis, are associated with the calcification of articular cartilages and include degenerative arthritis and arthritides accompanying metabolic diseases (e.g., hypothyroidism, hyperparathyroidism, diabetes mellitus). Several characteristics enable the differentiation of CPPD crystals from MSU crystals. Calcium pyrophosphate dihydrate crystals are smaller and blunter, rod-like, or rhomboid (Fig. 11.4A). With compensated polarizing microscopy, CPPD crystals display weak positive birefringence with their color opposite to that observed for MSU crystals. CPPD crystals are blue when their longitudinal axes are parallel to the red compensator plate and yellow when their longitudinal axes are perpendicular (Fig. 11.4B and C).
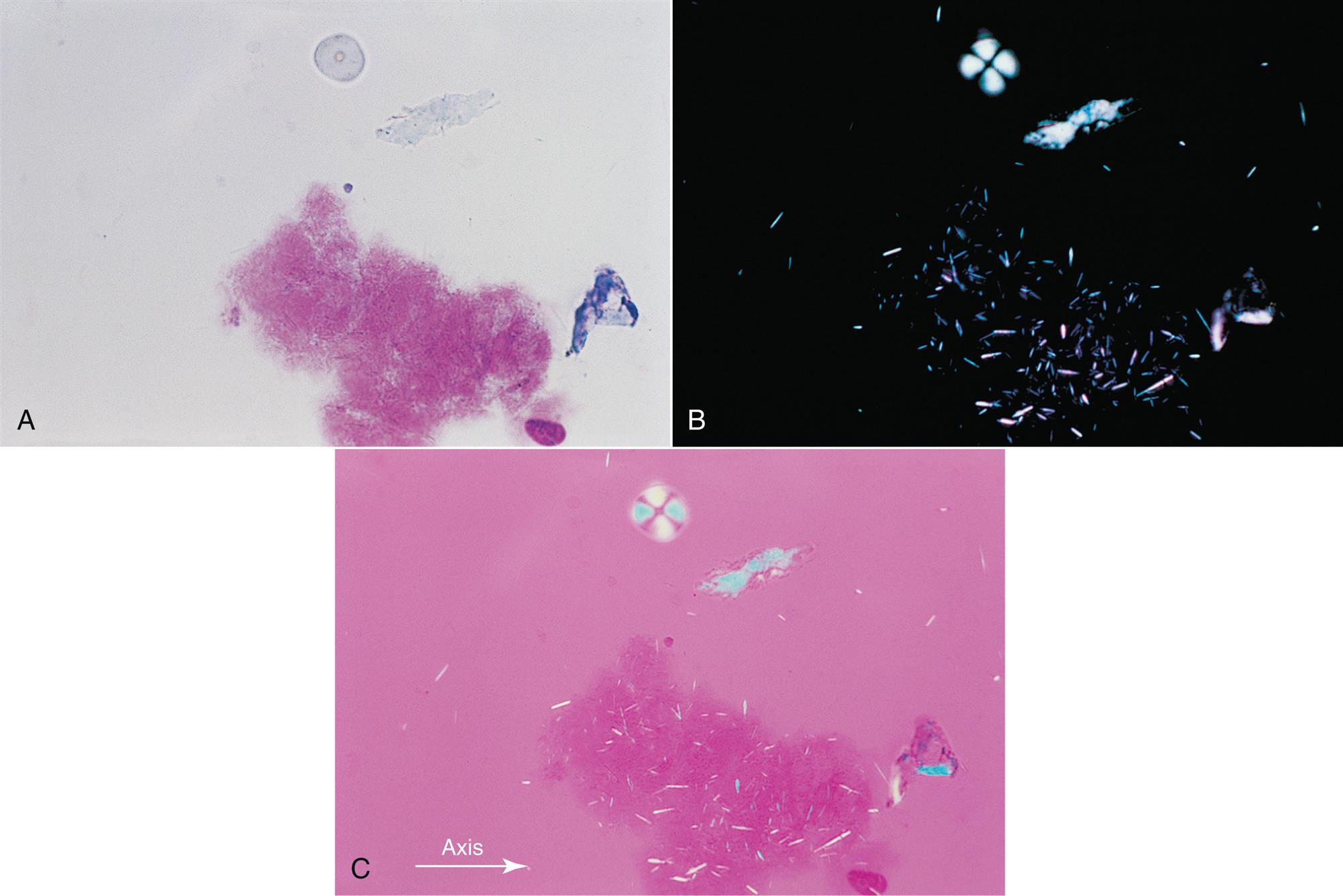
Cholesterol Crystals
Note that cholesterol crystals are best identified using a wet prep or an “unstained” cytospin slide because Wright-Giemsa staining can cause cholesterol crystals to dissolve. Cholesterol crystals usually appear as flat, rectangular plates with notched corners (Fig. 11.5). However, rhomboid or needle-like forms that resemble MSU and CPPD crystals have also been observed in synovial fluid. Under polarizing microscopy, the birefringence of cholesterol crystals varies with the thickness of the crystal. Associated with chronic inflammatory conditions (e.g., rheumatoid arthritis), cholesterol crystals are considered nonspecific and frequently occur in chronic effusions found in other body cavities.

Cholesterol globules or droplets can also be observed in synovial fluid. When using polarizing microscopy, cholesterol droplets will show positive birefringence, in contrast to negative birefringence seen with MSU spherules. Note that starch granules also demonstrate positive birefringence (Fig. 11.6C), but they can be distinguished from cholesterol by their morphology when observed using brightfield microscopy.
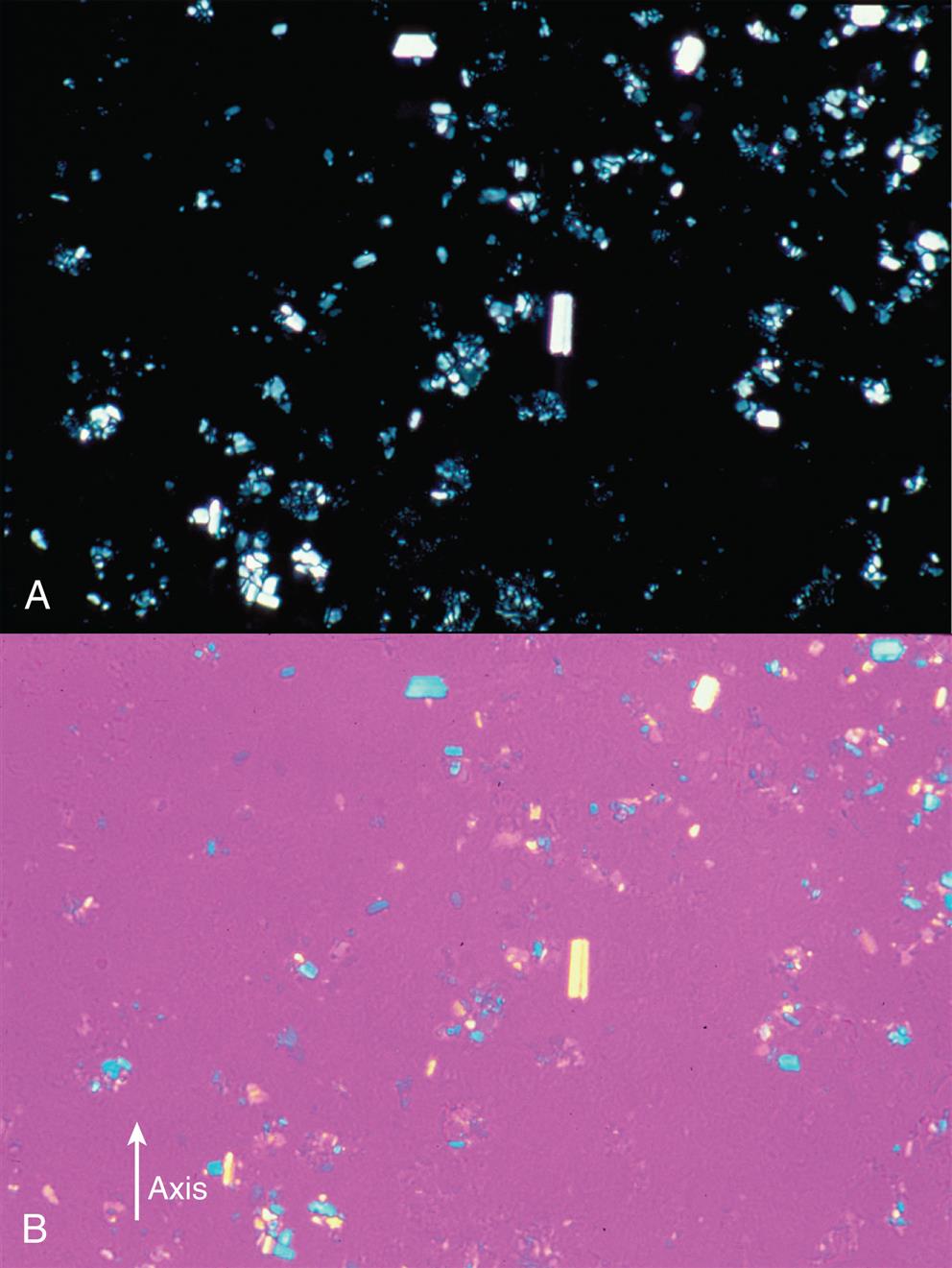
Hydroxyapatite Crystals
Hydroxyapatite crystals are present intracellularly in WBCs and require electron microscopy for visualization. They are tiny, needle-like crystals and are not birefringent. Hydroxyapatite crystals are associated with conditions characterized by calcific deposition and are collectively termed apatite-associated arthropathies. Apatite is the principal component of bone and is also present in cartilage. In crystalline form, hydroxyapatite crystals can induce an acute inflammatory reaction similar to that caused by monosodium urate and CPPD crystals.10
Corticosteroid Crystals
Because corticosteroid crystals can be found in synovial fluid for months after intraarticular injection, the laboratory must be informed of any injections when requested to do a synovial fluid microscopic examination. The crystals are often irregular, having jagged or serrated edges or appearing as broken pieces of variable sizes. Depending on the drug preparation used, corticosteroid crystals can closely resemble monosodium urate or CPPD crystals but yield conflicting results based on their birefringence (Fig. 11.7). In fact, laboratories can use betamethasone acetate (Celestone, Soluspan) as a microscopic control material because its crystals closely resemble MSU morphologically and exhibit similar negative birefringence. Corticosteroid crystals have no clinical significance other than indicating previous injection of the drug into the joint.
Calcium Oxalate Crystals
Calcium oxalate dihydrate (Weddellite) and calcium oxalate monohydrate (Whewellite) crystals can be present in synovial fluid and are associated with two conditions: (1) patients that have been undergoing renal dialysis for a long time and (2) patients with primary hyperoxaluria—a rare, inherited liver disorder that results in increased production of oxalate in the body.
Calcium oxalate dihydrate (Weddellite) crystals have a characteristic eight-faced bipyramidal structure whereas calcium oxalate monohydrate (Whewellite) crystals are ovoid or dumbbell-shaped (hourglass) (Fig. 11.8A).
Hematin Crystals
Hematin crystals are readily apparent using brightfield microscopy and are distinctively golden yellow-brown, rhomboid-shaped crystals. Their presence indicates that a hemorrhagic event (i.e., a bleed) previously occurred in the joint. When significant blood is released into synovial fluid, the RBCs are taken up by monocyte/macrophages and their hemoglobin is catabolized. This process of hemoglobin degradation and eventual formation of hematin crystals takes about 2 weeks.
Because of hematin’s rhomboid shape, these crystals look similar to CPPD crystals. Note, however, that the distinct yellow color of hematin crystals under brightfield microscopy should readily differentiate them (Fig. 11.8B).
Artifacts
Numerous artifacts in synovial fluid show birefringence using polarizing microscopy and differentiating artifacts from crystals is necessary (see Fig. 11.6). Birefringent artifacts include anticoagulant crystals, starch granules from examination gloves (see Table 18.1), cartilage and prosthesis fragments, collagen fibers, fibrin, and dust particles. An experienced microscopist is able to differentiate artifacts based on their irregular or indistinct morphologic appearance compared to crystals. Note that anticoagulant-associated crystals (e.g., calcium oxalate, powdered EDTA) can also be phagocytosed by WBCs after collection and storage. Therefore only sodium heparin or liquid EDTA, which do not form crystals, should be used as an anticoagulant for synovial fluid specimens.
Chemical Examination
Although numerous chemical constituents in synovial fluid can be analyzed, few analytes provide diagnostically useful information. Regardless of joint disease, some analytes such as uric acid maintain the same concentration in synovial fluid as in blood plasma, and they are often monitored using blood plasma measurements. In contrast, joint diseases can cause other analytes (e.g., glucose) to differ in synovial fluid concentration compared to blood plasma. In these conditions, determination of the plasma–synovial fluid difference can aid in identification and differentiation of the joint disease. Currently, lipid (cholesterol, triglycerides) and enzyme analysis of synovial fluid has limited clinical value in rare cases of joint disorders, thus these chemical tests are rarely performed on synovial fluid.
Glucose
To evaluate synovial fluid glucose concentrations, a blood sample must be drawn at the same time that the arthrocentesis is performed. In a healthy fasting patient, the glucose concentrations in the blood and in synovial fluid are equivalent. In other words, the plasma–synovial fluid glucose difference is equal to or less than 10 mg/dL (0.55 mmol/L) (see Table 11.2). In contrast, because of the time required in vivo for this dynamic equilibrium to occur, the plasma–synovial fluid glucose difference observed in a nonfasting patient can be greater than 10 mg/dL (greater than 0.55 mmol/L). Another guideline for a nonfasting patient is that the synovial fluid glucose is considered significantly low when the glucose concentration is less than half that of the plasma glucose value.
With various joint diseases, the concentration of glucose in synovial fluid is decreased, which causes an increased plasma–synovial fluid glucose difference. Typically, in noninflammatory and hemorrhagic joint disorders, the plasma–synovial fluid difference is less than 20 mg/dL (<1.11 mmol/L). When the difference is greater than 20 mg/dL (>1.11 mmol/L) or greater than 40 mg/dL (>2.22 mmol/L), it is suggestive of inflammatory or septic conditions, respectively (see Table 11.2).
Synovial fluid specimens for glucose quantitation should be assayed within 1 hour of collection,2 or, when quantitation will be delayed, a portion of the specimen should be placed into a sodium fluoride anticoagulant tube (gray top). These precautions will eliminate falsely low glucose values due to the glycolytic activity of WBCs that may be present in the fluid.
Total Protein
Normally, the total protein concentration of synovial fluid is approximately one-third the protein concentration of blood plasma. An increased amount of protein in the synovial fluid results from changes in the permeability of the synovial membrane or from increased synthesis within the joint. A variety of joint diseases (e.g., rheumatoid arthritis, crystal synovitis, septic arthritis) routinely have increased protein levels. However, determination of the synovial fluid protein content does not assist in the differential diagnosis of joint disease or in its treatment. Rather, increased total protein in synovial fluid indicates only the presence of an inflammatory process in the joint. Consequently, synovial fluid protein determinations are not performed routinely.
Uric Acid
The uric acid concentration in synovial fluid is equivalent to that in blood plasma; hence an increased plasma uric acid level, along with patient symptoms, usually enables the clinician to establish a diagnosis of gout. Gout is frequently diagnosed by the presence of MSU crystals in the synovial fluid. In cases in which MSU crystals are not observed microscopically, plasma or synovial fluid uric acid levels can be particularly valuable. It is worth noting that some individuals with gout do not have an increased plasma uric acid level. Therefore uric acid concentration alone cannot be used to diagnose gout.
Lactate
Increased synovial fluid lactate concentrations are believed to result from anaerobic glycolysis in the synovium. With severe inflammatory conditions comes an increased demand for energy, and tissue hypoxia can occur. Although the lactate level in synovial fluid can be determined easily, its clinical use remains uncertain. Some conditions of the joint, particularly septic arthritis, have been associated with significantly increased synovial fluid lactate levels. In contrast, gonococcal arthritis presents with normal or low lactate levels. Despite numerous studies, the clinical value of routine lactate quantitation in synovial fluid has yet to be established.
Microbiological Examination
Gram Stain
To aid in the differential diagnosis of joint disease, the routine examination of synovial fluid includes a Gram stain, preferably a cytocentrifuged smear, and routine aerobic and anaerobic cultures. When Gram stains are positive, they provide immediately useful clinical and diagnostic information. Most infectious agents in synovial fluid are bacteria that originate from the bloodstream (bacteremia); other infective agents in synovial fluid include fungi, viruses, and mycobacteria. The sensitivity of the Gram stain depends on the organism involved; approximately 75% of patients with staphylococcal infection (Gram-positive cocci), 50% of patients with Gram-negative organisms, and 40% of patients with gonococcal infection (Gram-negative cocci) are identified as positive by Gram stain.
Staphylococcus aureus is the most frequent causative organism of septic arthritis, with the incidence of methicillin-resistant S. aureus increasing. The second most common organisms are Streptococcus spp.—Streptococcus pyogenes, Streptococcus pneumoniae, and Streptococcus agalactiae (in infants < 2 months).13 In more than 20% of septic arthritis cases, Gram-negative cocci are involved, with Neisseria gonorrheae and Neisseria meningitidis predominating.13 Other bacteria involved in infectious arthritis include Gram-negative bacilli such as Haemophilus influenzae (pediatric population), Escherichia coli, Proteus mirabilis, Klebsiella, and Enterobacter.
Culture and Molecular Methods
Whether or not the Gram stain is positive, all synovial fluid specimens should be cultured. In most cases of bacterial or septic arthritis, the synovial fluid culture is positive. Recovery of microbial organisms requires careful and rapid processing of a fresh synovial fluid specimen. Specimens should be maintained at room temperature and cultures set up as soon as possible, within 24 hours of collection. Special culture media considerations are required if fungal, mycobacterial, and anaerobic organisms are suspected, hence consultation between the clinician and the microbiology laboratory is important.
Molecular methods using the polymerase chain reaction (PCR) are currently used to identify difficult-to-detect microorganisms, such as Borrelia burgdorferi, which causes Lyme arthritis, and Mycobacterium tuberculosis, which can cause osteoarticular tuberculosis.
Study Questions
- 2. Which of the following statements is a characteristic of normal synovial fluid?
- 3. Which of the following components is not normally present in synovial fluid?
- 4. Which of the following substances will not increase the turbidity of synovial fluid?
- 5. Abnormally decreased viscosity in synovial fluid results from
- 6. A synovial fluid specimen is received in the laboratory 2 hours after collection. Which of the following changes to the fluid will most likely have taken place?
- 7. Which of the following anticoagulants does not have the potential to precipitate out in crystalline form when used for synovial fluid specimens?
- 8. A synovial fluid specimen has a high cell count and requires dilution to be counted. Which of the following diluents should be used?
- 9. Which of the following results from synovial fluid analysis indicates a joint disease process?
- 10. Differentiation of synovial fluid crystals, based on their birefringence, is achieved using
- 11. The microscopic examination of synovial fluid for crystals can be difficult because
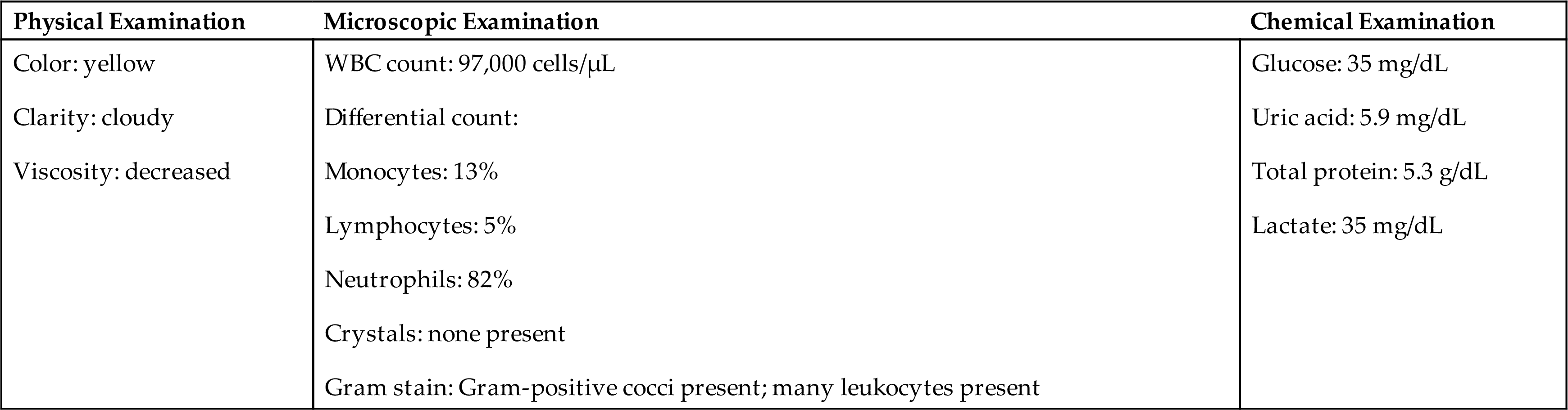
- 16. Analysis of a synovial fluid specimen reveals the following:
- • Cloudy, yellow-green fluid of low viscosity
- • Total leukocyte count of 98,000 cells/μL
- • Plasma–synovial fluid glucose difference of 47 mg/dL
Based on the information provided and Table 11.2, this specimen most likely would be classified as
Based on the information provided and Table 11.2, this specimen would most likely be classified as