chapter 1 Surgical Anatomy of the Retroperitoneum, Adrenals, Kidneys, and Ureters
There is no greater aid to surgical expertise than an intimate knowledge of anatomy. For the urologist, the areas of greatest importance are the retroperitoneum and pelvis. In this chapter, retroperitoneal structures important to the practice of urologic surgery are described in detail and clinical correlations are provided where helpful.
Retroperitoneum
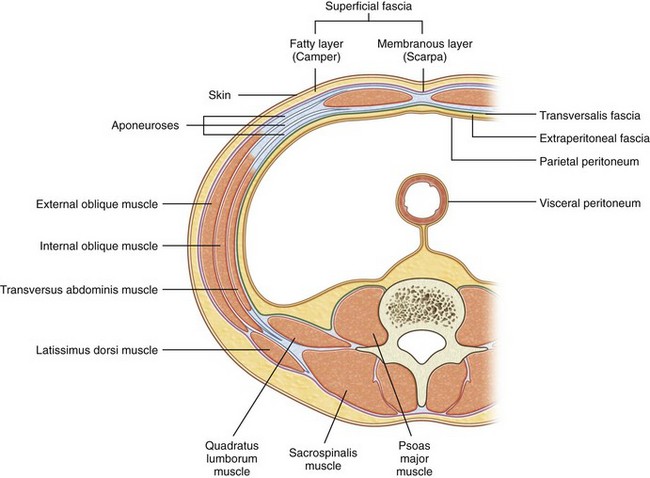
The retroperitoneum is bounded posteriorly by the abdominal wall, which consists of the lumbodorsal fascia and the enclosed sacrospinalis and quadratus lumborum muscles (Fig. 1–1). Laterally, the retroperitoneum is contiguous with the preperitoneal fat and is bounded laterally by the transversus abdominis musculature of the lateral abdominal wall. The peritoneum is the anterior limit, whereas cranially the diaphragm (Fig. 1–2) limits the retroperitoneum. Caudally the retroperitoneum is contiguous with the extraperitoneal pelvic structures.
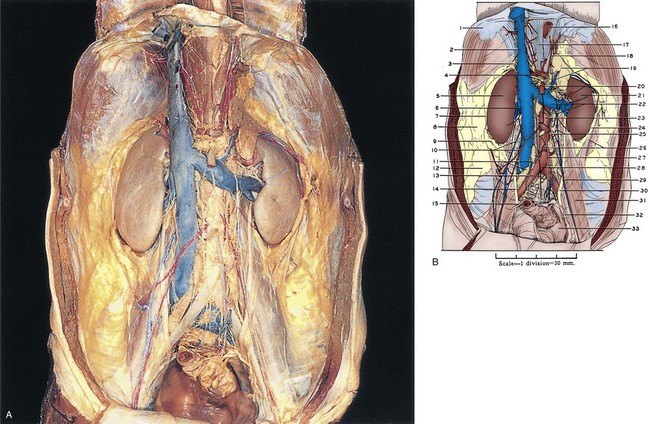
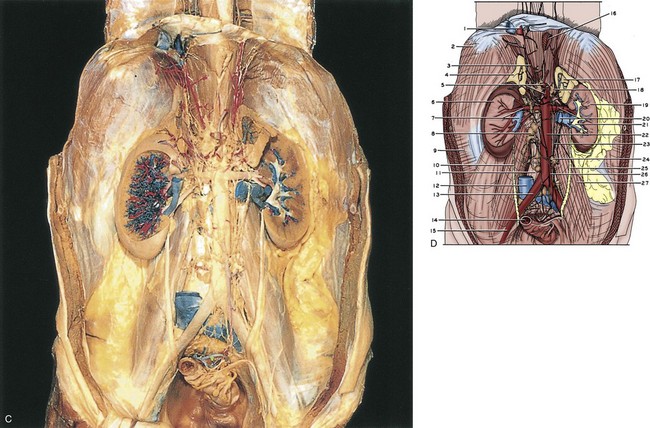
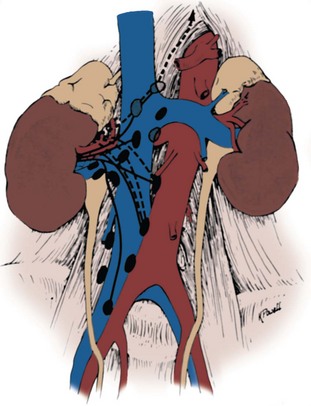
Figure 1–1 A, The retroperitoneum dissected. The anterior perirenal (Gerota) fascia has been removed. B, 1, Diaphragm. 2, Inferior vena cava. 3, Right adrenal gland. 4, Upper pointer, celiac artery; lower pointer, celiac autonomic nervous plexus. 5, Right kidney. 6, Right renal vein. 7, Gerota fascia. 8, Pararenal retroperitoneal fat. 9, Perinephric fat. 10, Upper pointer, right gonadal vein; lower pointer, right gonadal artery. 11, Lumbar lymph nodes. 12, Retroperitoneal fat. 13, Right common iliac artery. 14, Right ureter. 15, Sigmoid colon (cut). 16, Esophagus (cut). 17, Right crus of diaphragm. 18, Left inferior phrenic artery. 19, Upper pointer, left adrenal gland; lower pointer, left adrenal vein. 20, Upper pointer, superior mesenteric artery; lower pointer, left renal artery. 21, Left kidney. 22, Upper pointer, left renal vein; lower pointer, left gonadal vein. 23, Aorta. 24, Perinephric fat. 25, Aortic autonomic nervous plexus. 26, Upper pointer, Gerota fascia; lower pointer, inferior mesenteric ganglion. 27, Inferior mesenteric artery. 28, Aortic bifurcation into common iliac arteries. 29, Left gonadal artery and vein. 30, Left ureter. 31, Psoas major muscle covered by psoas sheath. 32, Cut edge of peritoneum. 33, Pelvic cavity. C, The retroperitoneum dissected. The kidneys and adrenal glands have been sectioned, and the inferior vena cava has been excised over most of its intra-abdominal course. D, 1, Inferior vena cava (cut). 2, Diaphragm. 3, Right inferior phrenic artery. 4, Right adrenal gland. 5, Upper pointer, celiac artery; lower pointer, superior mesenteric artery. 6, Right kidney. 7, Upper pointer, right renal artery; lower pointer, right renal vein (cut). 8, Lumbar lymph node. 9, Transversus abdominis muscle covered with transversalis fascia. 10, Right ureter. 11, Anterior spinous ligament. 12, Inferior vena cava (cut). 13, Right common iliac artery. 14, Sigmoid colon (cut). 15, Right external iliac artery. 16, Esophagus (cut). 17, Left adrenal gland. 18, Celiac ganglion. 19, Left kidney. 20, Upper pointer, left renal artery; lower pointer, left renal vein (cut). 21, Left renal pelvis. 22, Aorta. 23, Aortic autonomic nervous plexus. 24, Inferior mesenteric ganglion. 25, Left ureter. 26, Inferior mesenteric artery. 27, Psoas major muscle covered by psoas sheath.
(A to D, Reproduced from the Bassett anatomic collection, with permission granted by Dr. Robert A. Chase.)
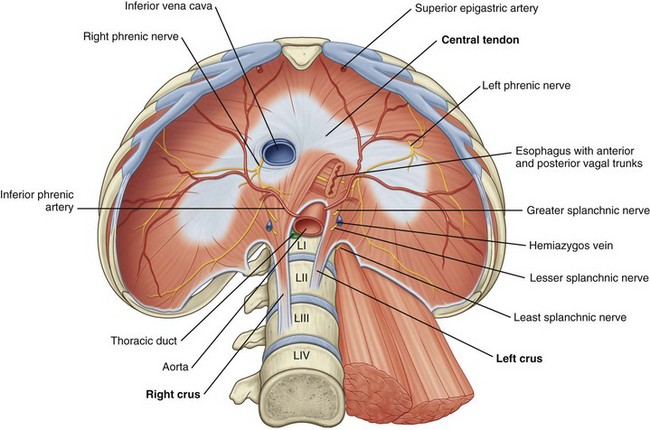
Figure 1–2 The diaphragm: abdominal surface.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 317.)
Posterior Abdominal Wall
Posterior Musculature and Lumbodorsal Fascia
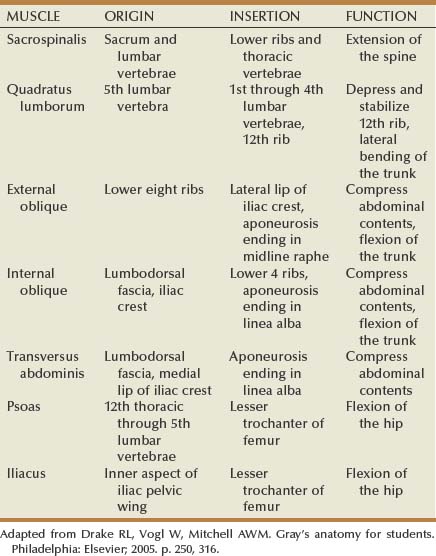
See Figures 1-3 to 1-6 and Table 1–1. The lumbodorsal fascia surrounds the sacrospinalis and quadratus lumborum, which together comprise the posterior abdominal wall. The lumbodorsal fascia originates from the spinous processes of the lumbar vertebrae and extends anteriorly and cranially. As it progresses upward, it separates into three layers: posterior, middle, and anterior.
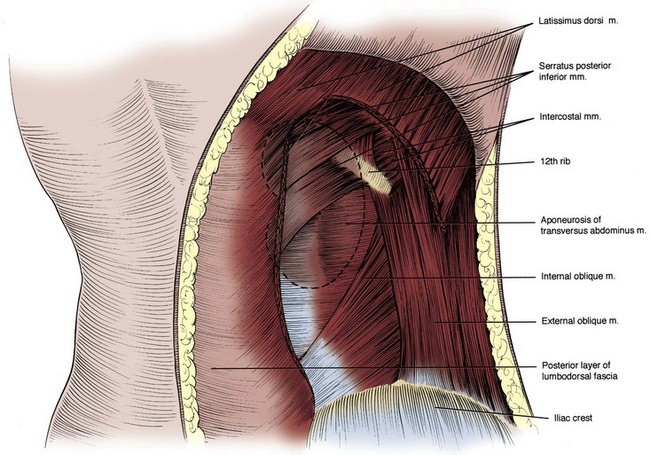
Figure 1–3 Posterior abdominal wall musculature, superficial dissection. A section of the latissimus dorsi muscle has been removed. The location of the right kidney within the retroperitoneum is shown by dashed outline.
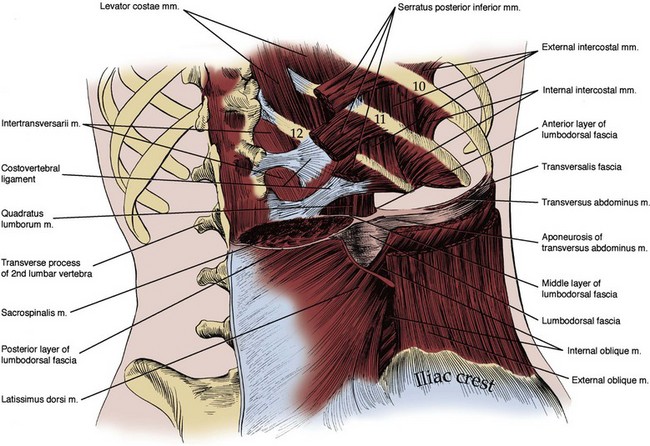
Figure 1–4 Posterior abdominal wall musculature, intermediate dissection. The sacrospinalis muscle and three anterolateral flank muscle layers are seen in cut section, and the three layers of the lumbodorsal fascia posteriorly can be appreciated.
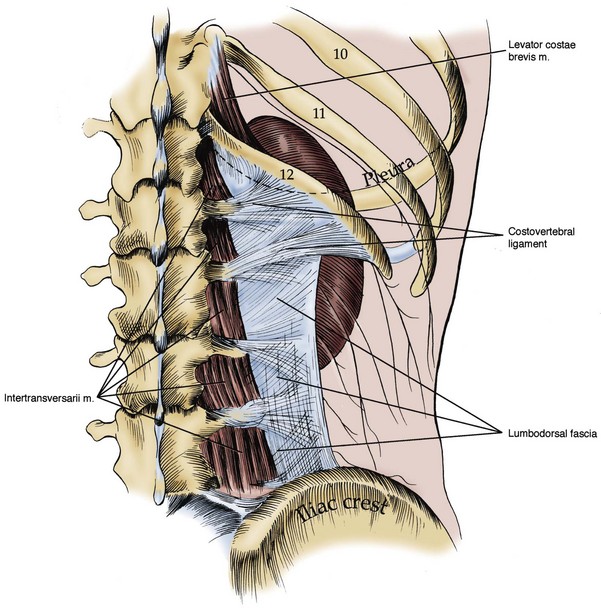
Figure 1–5 Posterior abdominal wall musculature, deep dissection. The lumbodorsal fascia and costovertebral ligament are visualized, arising from the transverse processes of the lumbar vertebrae. The relation of the kidney and pleura is also shown.
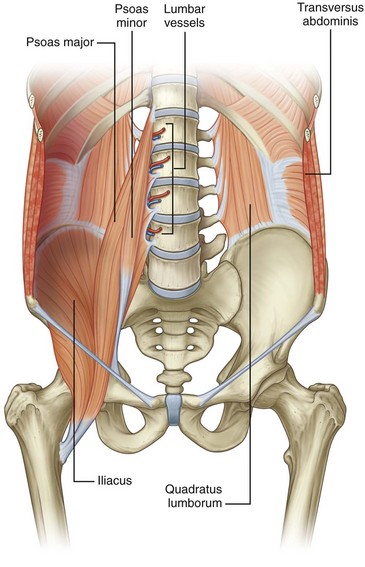
Figure 1–6 Muscles of the posterior abdominal wall.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 316.)
Key Points
Retroperitoneum
The posterior layer provides the posterior covering for the sacrospinalis muscle and is the origin of the latissimus dorsi muscle. The middle layer forms the fascial layer separating the anterior aspect of the sacrospinalis muscle from the posterior aspect of the quadratus lumborum. The anterior layer of the lumbodorsal fascia provides the anterior covering to the quadratus lumborum muscle and forms the posterior margin of the retroperitoneum. As one moves laterally away from the sacrospinalis and quadratus lumborum muscles, the lumbodorsal fascial layers fuse together and then connect with the transversus abdominis muscle.
The quadratus lumborum and sacrospinalis muscles (see Figs. 1-6 and 1-7) form the muscular portion of the posterior abdominal wall, filling the space among the 12th rib, spine, and iliac crest. The quadratus lumborum serves a number of functions. It supports the 12th rib, thus improving diaphragmatic contraction and inspiration, as well as aiding intercostal muscle function during forced expiration. Finally, it controls lateral bending of the trunk. The sacrospinalis also controls movement of the trunk by promoting extension of the spine. These muscular and fascial relationships become important clinically when performing a dorsal lumbotomy incision. As seen in Figure 1–7, this is a vertical incision lateral to the border of the sacrospinalis and quadratus lumborum. This approach allows entrance to the retroperitoneum without violation of the musculature.
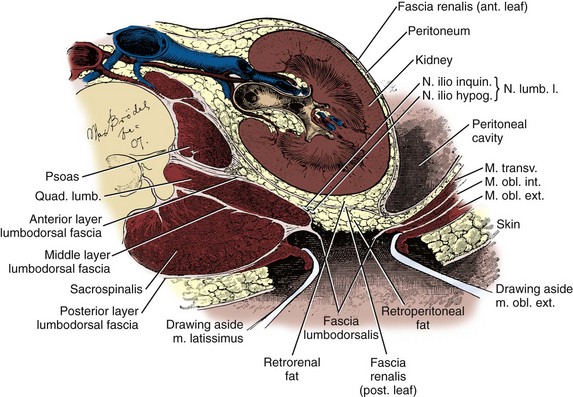
Figure 1–7 Transverse section through the kidney and posterior abdominal wall showing the lumbodorsal fascia incised. Note that through such a lumbodorsal incision, the kidney can be reached without incising muscle.
(After Kelly and Burnam, from McVay C. Anson & McVay surgical anatomy. 6th ed. Philadelphia: WB Saunders; 1984.)
Lateral Flank Musculature
See Figure 1–8 and Table 1–1. Three muscular layers comprise the lateral flank musculature. From superficial to internal, these are the external oblique, internal oblique, and transversus abdominis muscles. The most superficial structure is the external oblique muscle. This muscle arises from the lower ribs and moves from lateral to medial as it progresses caudally. Final attachment is to the iliac crest caudally and the rectus sheath anteriorly. The posterior border remains free as it terminates before reaching the lumbodorsal fascia. Next is the internal oblique muscle. Again, this muscle arises from the lower rib cage, but the orientation of the fibers is from medial to lateral as they move caudally. Final attachment is to the iliac crest and lumbodorsal fascia. The final structures are the transversus abdominis muscle and transversalis fascia. The transversus abdominis muscle arises from the lumbodorsal fascia with fibers running directly transversely until it attaches anteriorly and medially onto the rectus sheath. Immediately deep to the transversus abdominis muscle is the transversalis fascia and then the retroperitoneal space. The function of the lateral flank musculature is to compress and stabilize the abdomen and trunk. This provides controlled movement and protection for the abdominal organs.
Psoas and Iliacus Muscles
The psoas major muscle originates on the 12th thoracic through the 5th lumbar vertebrae (see Fig. 1–6). A smaller psoas minor is identifiable in about one half of the population and resides medial to the psoas major. The psoas muscle(s) is covered by the psoas fascia. In close proximity to the psoas muscle is the iliacus muscle, which attaches to the inner aspect of the iliac pelvic wing. As the iliacus progresses caudally it joins with the psoas muscle to form the iliopsoas muscle. This combined muscle then joins to the lesser trochanter of the femur and controls flexion of the hip.
Lower Rib Cage
See Figure 1–9. In addition to the protection provided by the muscular layers of the posterior and lateral abdominal wall, the 10th, 11th, and 12th ribs safeguard the upper retroperitoneal space and are intimately related to the adrenal glands and kidneys. Given the close proximity, injury to these ribs can be associated with significant retroperitoneal injury. While providing protection, the lower ribs and the accompanying pleura and lung limit surgical exposure to the upper retroperitoneum. The limits of the pleura are the 8th rib anteriorly, the 10th rib in the midaxillary line, and the 12th rib posteriorly. Given this location of the pleura, flank incisions at or above the 11th or 12th ribs risk pleural violation.
Great Vessels
The abdominal aorta and inferior vena cava are the great vessels of the abdomen, providing vascular supply to the abdominal organs and lower extremities (Figs. 1-10 and 1-11).
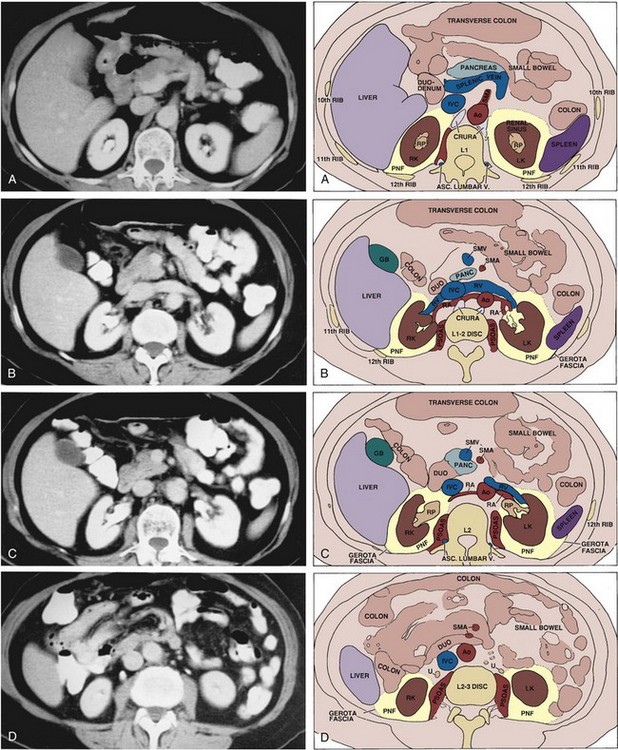
Figure 1–11 Cross-sectional anatomy of the upper abdomen at the level of the kidneys demonstrated with transverse sections obtained by computed tomography. Sections are arranged from most cephalic to caudal. A, Section through the upper poles of the kidneys, superior to the renal vascular pedicles. B, Section through the level of the renal arteries and veins. C, Slightly more inferior section showing the renal pelves and relation of the duodenum to the right renal hilum. D, Section through the lower poles of the kidneys showing the upper ureters. Ao, aorta; DUO, duodenum; GB, gallbladder; IVC, inferior vena cava; LK, left kidney; PANC, pancreas; PNF, perinephric fat; RA, renal artery; RK, right kidney; RP, renal pelvis; RV, renal vein; SMA, superior mesenteric artery; SMV, superior mesenteric vein; U, ureter.
Abdominal Aorta
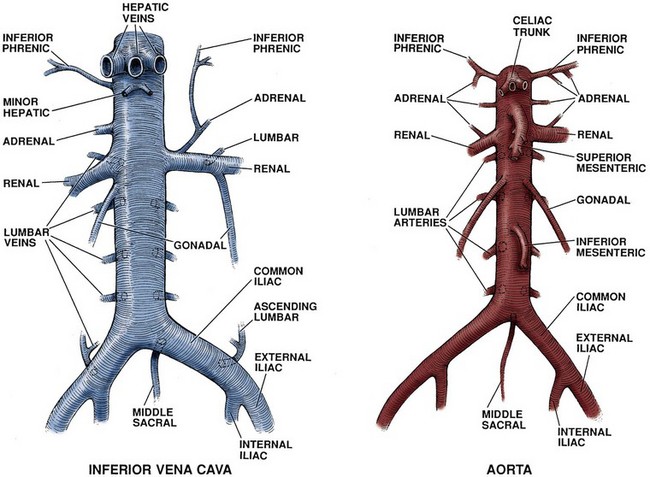
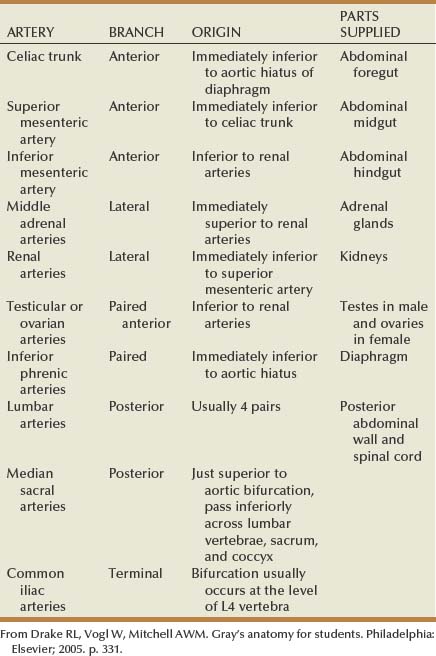
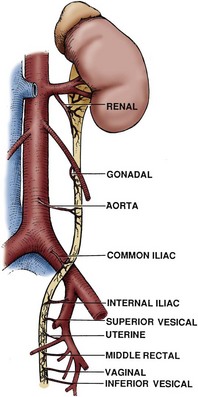
The aorta enters the abdomen via the aortic hiatus found between the diaphragmatic crura in the posterior diaphragm at the level of the 12th thoracic vertebrae (see Fig. 1–2). It continues caudally to the 4th lumbar vertebrae, where it bifurcates into the common iliac arteries. During its course through the abdomen the aorta gives off a number of large branches (Table 1–2). The paired inferior phrenic arteries are first. They supply the inferior diaphragm and the superior portion of the adrenal gland (see Fig. 1–2). Next is the celiac trunk, which is the origin for the common hepatic, left gastric, and splenic arteries that supply the liver, stomach, and spleen, respectively. The paired adrenal arteries follow with an artery going to each adrenal gland. The superior mesenteric artery leaves the aorta on the anterior side and supplies the entire small intestine and majority of the large intestine. Also of note, this artery communicates with the celiac trunk vasculature via the pancreaticoduodenal artery. Overlying the 2nd lumbar vertebrae, the paired renal arteries are the next branching point of the aorta. To the urologist, renal artery anatomy is obviously of great importance and is discussed in detail in the kidney section.
Moving distally on the aorta, the paired gonadal arteries are encountered. In the male this artery is also called the testicular artery and in the female it is the ovarian artery. The initial course in both the male and female is similar, with the artery moving caudally and laterally from the aorta, with the right gonadal artery crossing anterior to the inferior vena cava. In men, the gonadal artery then crosses over the ureter and exits the retroperitoneum at the internal inguinal ring. In women the course is different: Instead of exiting the pelvis, the artery crosses medially back over the external iliac vessels and enters the pelvis. It then proceeds via the suspensory ligament to the ovary. The destination of the gonadal artery (the testis in the male and the ovary in the female) has significant collateral sources of arterial blood, from the deferential and cremasteric arteries in the male and the uterine artery in the female. Thus the gonadal artery can generally be ligated during retroperitoneal surgery without detrimental effect.
After the gonadal arteries, the inferior mesenteric artery is found on the anterior side of the aorta before its bifurcation into the common iliac vessels. This vessel provides vascular supply to the left third of the transverse colon, descending colon, sigmoid colon, and rectum. In patients without significant vascular disease, this artery can be sacrificed without ill effect because there is collateral circulation to these bowel segments from the superior mesenteric, middle hemorrhoidal, and inferior hemorrhoidal arteries.
In addition to the listed arteries that exit the aorta from its anterior or lateral aspect, there are a number of small branches from the posterior side of the aorta. Lumbar arterial branches are found at regular intervals along the length of the aorta, with generally four pairs located within the retroperitoneum. These branches supply the posterior body wall and spine. Again these arteries can generally be ligated without detrimental effects, although spinal ischemia and paralysis has occurred after ligation at multiple levels. The final posterior branch from the aorta is the middle sacral artery, which exits the aorta immediately before the branching of the common iliac arteries and then sends branches to the rectum and anterior sacrum. The common iliac arteries then proceed into the pelvis, thus completing the arterial course through the retroperitoneum.
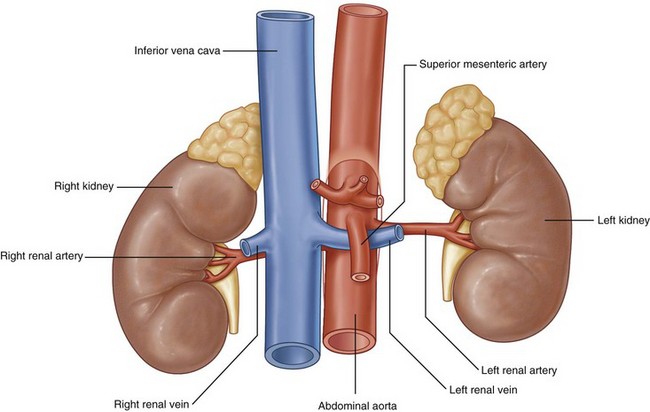
Inferior Vena Cava
The inferior vena cava (IVC) arises from the confluence of the common iliac veins at the level of the fifth lumbar vertebra (see Fig. 1–10). Because the common iliac veins lie medial and posterior to the iliac arteries, the confluence of the iliac veins is posterior and to the right of the aortic bifurcation. As the IVC progresses cranially through the abdomen, tributaries include the gonadal, renal, adrenal, and hepatic veins. In addition, the middle sacral vein enters the inferior vena cava posteriorly and the lumbar veins enter throughout the length of the abdominal vena cava.
The first tributary encountered along the IVC is the middle sacral vein, which enters at the junction of the common iliac veins. Also entering along the posterior aspect of the IVC throughout its course are lumbar veins. These veins course anterior to the spinal transverse processes and generally parallel the lumber arteries. In addition to providing vascular drainage, the lumbar veins connect the IVC to the azygous venous system on the right side and hemiazygos venous system on the left side of the thorax. This provides alternate routes of venous drainage within the retroperitoneum (Fig. 1–12).
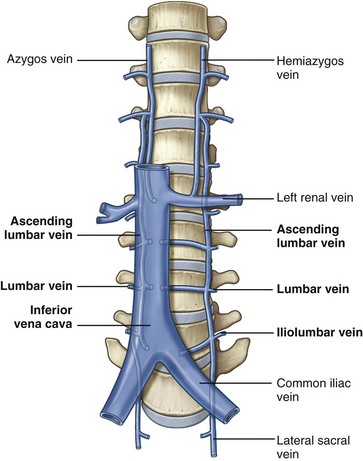
Figure 1–12 Lumbar, azygos, and hemiazygos veins.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 332.)
The next tributaries to the IVC are the gonadal veins, whose course is analogous to the gonadal arteries until approaching the IVC. During the cranial portion of their course these veins are more lateral and closer to the ipsilateral ureter. Of surgical importance is their terminal drainage because the right gonadal vein drains directly into the IVC and the left empties into the inferior aspect of the left renal vein (see Fig. 1–10).
After the gonadal veins, the renal veins are encountered. The renal veins are generally directly anterior to the accompanying renal artery, but it is not unusual for them to be separated by 1 to 2 cm in the craniocaudal direction. The right renal vein typically is short and without branches, but in a small minority of patients the right gonadal vein can enter the right renal vein as opposed to the IVC. In a second anatomic variation, a lumbar vein will enter on the posterior aspect of the right renal vein as opposed to entering the IVC directly. The left renal vein is significantly longer than the right and receives additional branches before entering the IVC. Typically after exiting the renal hilum, the left renal vein receives a lumbar vein posteriorly, the left gonadal vein inferiorly, and the adrenal vein superiorly. Next, the left renal vein crosses anterior to the aorta and under the caudal edge of the superior mesenteric artery before draining into the IVC. Rarely the left renal vein crosses the aorta to the IVC in a retroaortic or circumaortic path.
Proceeding cranially, the posterior aspect of the IVC receives the right adrenal vein. This short vein is located posteriorly on the IVC, making it challenging to expose during right adrenal or renal surgery. As noted already, the left adrenal vein drains into the left renal vein as opposed to the IVC. The inferior phrenic vein on the right side enters along the posterior or posterior lateral aspect of the IVC, with the left inferior phrenic vein typically entering the left renal vein. The final tributaries to the IVC before it leaves the retroperitoneum are the short hepatic veins draining the liver. Inferiorly these veins are small, but superiorly three large hepatic trunks are encountered.
Lymphatics
Lymphatic drainage of the lower extremities, external genitalia, testes, kidneys, and intestines is located in the retroperitoneum (Fig. 1–13). Knowledge of these lymphatic channels is useful not only for urologic oncology (e.g., testis cancer) but also for prevention of complications such as lymphocele. Drainage of the lower extremities, perineum, and external genitalia progresses through the retroperitoneum via common iliac lymph vessels and then forms ascending vertical lumbar lymphatic chains. There is flow not only cranially but also laterally, predominantly from the right to the left. Gastrointestinal lymphatic drainage also follows the vascular supply, with the majority of the lymphatics paralleling the inferior mesenteric, superior mesenteric, and celiac arteries. Eventually these lymphatics join posterior to the aorta at the level of the first or second lumbar vertebrae to form the thoracic duct. This coalescence is classically marked by a local dilation called the cisterna chyli, which tends to lie within the thorax just to the right of the aorta in a retrocrural position.
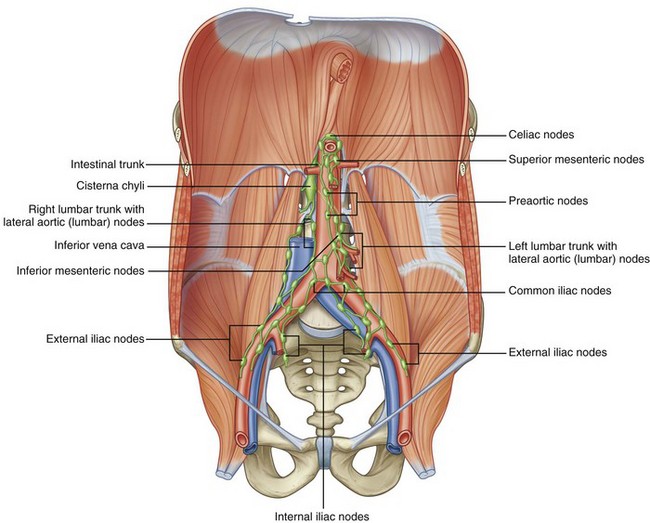
Figure 1–13 Retroperitoneal lymphatics.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 335.)
For the urologist, the lumbar lymphatics are important as the primary lymphatic drainage from two urologic organs: the kidneys and testes. Given the kidney’s retroperitoneal location, the lumbar path of its lymphatic drainage is not surprising and is discussed in more depth later in this chapter. Embryologically, the testes develop within the retroperitoneum and maintain both blood flow (testicular arteries) and lymphatic drainage through this area even after they descend into the scrotum. To better describe the lymphatic drainage within the retroperitoneum, a practical system has been developed. This system defines three major nodal areas: right paracaval, interaortocaval, and left para-aortic. The right paracaval nodal region extends from the midline of the IVC to the right ureter. The interaortocaval region extends from the midline of the IVC to the midline of the aorta, and the left para-aortic region extends from the midline of the aorta to the left ureter.
Study of lymphatic metastases from testicular tumors have shown that testicular lymphatic drainage is consistent and follows the general scheme of vertical drainage with lateral flow from right to left. Lymphatic metastases from the right testis drain primarily into the interaortocaval nodes with significant drainage to the right paracaval nodes. In addition there is a small amount of drainage to the left para-aortic nodes. On the other hand, the left testis drains primarily to the left para-aortic nodes with significant drainage to the interaortocaval nodes. There is essentially no drainage to the right paracaval nodes from left-sided tumors.
Nervous System Structures
The nervous structures within the retroperitoneum are part of the peripheral nervous system and can be divided into two categories: autonomic and somatic nerves. The autonomic nerves provide afferent and efferent innervation to organs, blood vessels, glands, and smooth muscles. They are further characterized by the presence of peripheral synapses. Thus there are at least two peripheral nerves between the central nervous system and the viscera. The somatic nerves supply afferent and efferent innervation to the skin, skeletal muscles, and joints. Although these two nerve types leave the spinal column within shared spinal nerves, their course and functions quickly diverge.
Autonomic System
The autonomic system is further divided into sympathetic and parasympathetic fibers. The origin of these two nerve types is quite different, with the sympathetic preganglionic fibers originating from the thoracic and lumbar portions of the spinal column and the parasympathetic preganglionic fibers beginning in the cranial and sacral spinal column segments. Preganglionic sympathetic fibers enter the retroperitoneum through both the paired sympathetic chains and input from the lumbar spinal nerves (Fig. 1–14). The lumbar portion of this sympathetic chain then sends preganglionic fibers to autonomic plexuses associated with the major branches of the abdominal aorta. Within these aortic plexuses the preganglionic fibers synapse and postganglionic fibers are then distributed to the various abdominal viscera and organs. Parasympathetic input from the vagus nerve also supplies these ganglia.
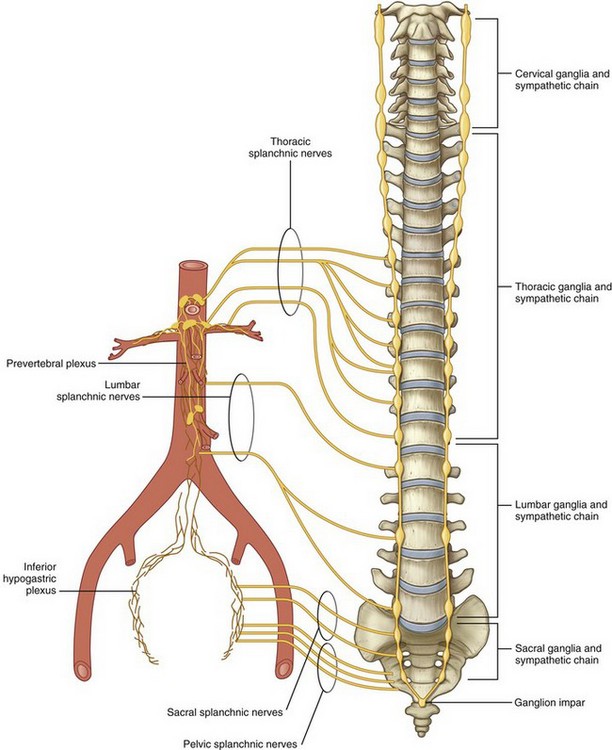
Figure 1–14 Sympathetic chain and splanchnic nerves.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 309.)
In more detail, the thoracic and lumbar portions of the sympathetic chain originate from preganglionic sympathetic fibers arising from the first thoracic through the third lumbar spinal nerves (see Fig. 1–14). This chain then courses vertically along the anterolateral aspect of the spine just medial to the psoas muscle. Within the retroperitoneum, lumbar arteries and veins are closely associated with the lumbar sympathetic chain, in some instances even splitting the fibers as they cross the chain perpendicularly. From this sympathetic chain preganglionic fibers follow one of three courses. First, preganglionic fibers are sent to the various autonomic plexuses (splanchnic nerves). Once in the plexus, the preganglionic fibers synapse within a ganglion to postganglionic fibers, which in turn proceed to the abdominal viscera. Second, preganglionic fibers can synapse within the sympathetic chain ganglia and send postganglionic fibers to the body wall and lower extremities. Finally, preganglionic sympathetic fibers can proceed directly to the adrenal gland without synapsing. Within the adrenal medulla, these preganglionic fibers control release of catecholamines.
The major autonomic nerve plexuses are associated with the primary branches of the aorta. These plexuses include the celiac, superior hypogastric, and inferior hypogastric plexuses (Fig. 1–15). These plexuses receive sympathetic input from the sympathetic chains via the greater, lesser, and least thoracic splanchnic nerves originating from the 5th through 12th thoracic spinal nerves. They also receive input from the lumbar portion of the sympathetic chain via the lumbar splanchnic nerves, as well as parasympathetic input via the vagus nerve.
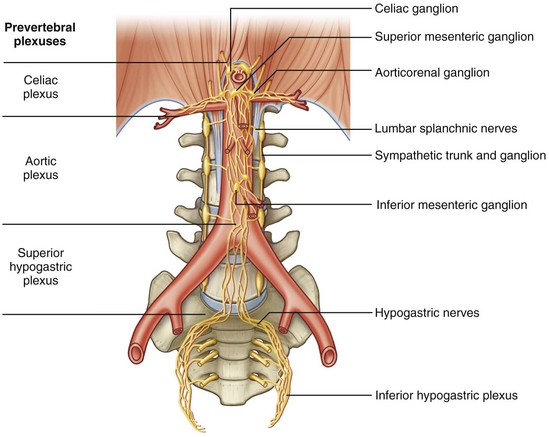
Figure 1–15 Autonomic plexuses associated with branches of the aorta.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 337.)
The largest is the celiac plexus and is located on either side of the celiac arterial trunk as a paired structure. It is through this plexus that much or all of the autonomic input to the kidney, adrenal, renal pelvis, and ureter passes. In addition, some of the sympathetic innervation to the testes passes through this ganglion before continuing in parallel with the testicular artery to the testis. A separate aorticorenal ganglion usually exists as an inferior extension of the celiac ganglion, forming part of the renal autonomic plexus. The latter plexus surrounds the renal artery and its branches and is contiguous with the celiac plexus. At the lower extent of the abdominal aorta, much of the autonomic input to the pelvic urinary organs and genital tract travels through the superior hypogastric plexus. This plexus lies on the aorta anterior to its bifurcation and extends inferiorly on the anterior surface of the fifth lumbar vertebra. This plexus is contiguous bilaterally with inferior hypogastric plexuses, which extend into the pelvis. Disruption of the sympathetic nerve fibers that travel through these plexuses during retroperitoneal dissection can cause loss of seminal vesicle emission and/or failure of bladder neck closure, resulting in retrograde ejaculation.
Somatic
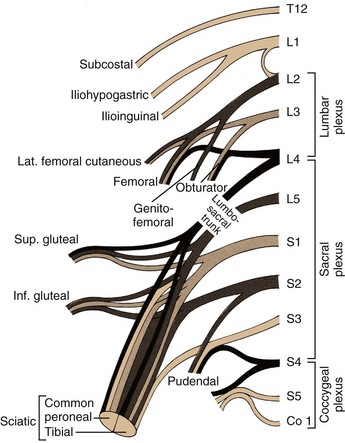
The somatic sensory and motor innervation to the abdomen and lower extremities arises in the retroperitoneum and is called the lumbosacral plexus. The lumbosacral plexus is formed from branches of all lumbar and sacral spinal nerves, with some contribution from the 12th thoracic spinal nerve as well (Fig. 1–16). Superiorly, nerves of this plexus form within the body of the psoas muscle and pierce this muscle, with more inferior branches passing medial to the psoas as the pelvis is entered (Fig. 1–17). The origins and functions of these lumbosacral somatic nerves are summarized in Table 1–3.
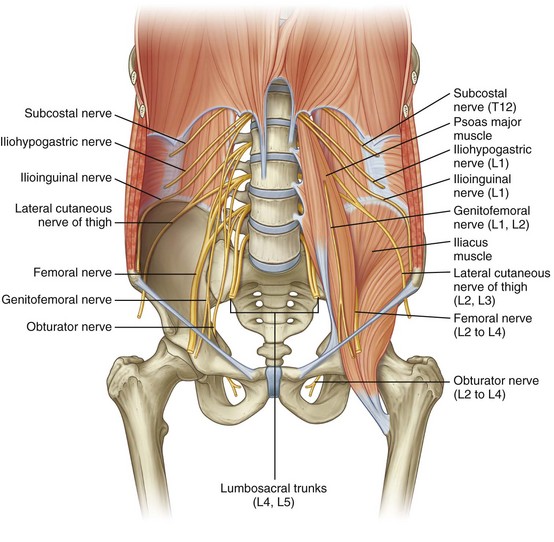
Figure 1–17 Lumbar plexus in the posterior abdominal region.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 341.)
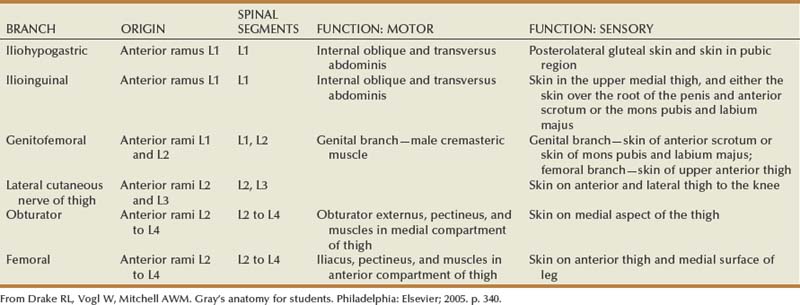
The subcostal nerve is the anterior extension of the 12th thoracic nerve and extends laterally beneath the 12th rib. As one proceeds inferiorly, the iliohypogastric nerve and the ilioinguinal nerve originate together as an extension from the first lumbar spinal nerve. These three nerves cross the anterior or inner surface of the quadratus lumborum muscle before piercing the transversus abdominis muscle and continuing their course between this and the internal oblique muscle. Together they provide multiple motor branches to the muscles of the abdominal wall, as well as sensory innervation to the skin of the lower abdomen and genitalia. The lateral femoral cutaneous nerve and the genitofemoral nerve arise from the 1st through 3rd lumbar nerves and are primarily sensory nerves to the skin of the upper thigh and genitalia; however, the genital branch of the genitofemoral nerve also supplies the cremaster and dartos muscles in the scrotum. The genitofemoral nerve lies directly atop and parallels the psoas muscle throughout most of its retroperitoneal course and is easily identified in this position.
The femoral nerve is a larger structure arising from the second through fourth lumbar spinal nerves and is largely hidden by the body of the psoas muscle before exiting the abdomen just lateral to the femoral artery. This important nervous structure supplies the psoas and iliacus muscles, as well as the large muscle groups of the anterior thigh. It also provides sensory innervation of the anteromedial portions of the lower extremity. Intraoperatively, it may be compressed by retractor blades placed inferolaterally against the inguinal ligament in lower abdominal incisions, producing a significant motor palsy that prevents active extension of the knee.
The final lumbosacral plexus nerves include the obturator and sciatic nerves. The obturator nerve, an important pelvic landmark, arises behind the psoas muscle in the retroperitoneum from the third and fourth lumbar spinal nerves. It then courses inferiorly, where its major function is to supply the adductor muscles of the thigh. The sciatic nerve receives input from the fourth lumbar through third sacral spinal nerves, taking final form in the deep posterior pelvis as the body’s single largest nerve, supplying the bulk of both sensory and motor innervation to the lower extremity.
Duodenum, Pancreas, Colon
See Figure 1–18 on the Expert Consult website![]() . The duodenum is divided into four anatomic components. The first (ascending) portion is short (5 cm) and intimately related to the gallbladder. The second (descending portion) is of most importance to the urologist because it lies immediately anterior to the right renal hilum and pelvis. This portion of the duodenum is frequently mobilized (referred to as a Kocher maneuver) to expose the right kidney, right renal pelvis, and additional upper abdominal structures. The second portion of the duodenum also receives the common bile duct and surrounds the head of the pancreas. The third (horizontal) and fourth (ascending) portions of the duodenum cross from right to left over the IVC and aorta before transitioning into the jejunum.
. The duodenum is divided into four anatomic components. The first (ascending) portion is short (5 cm) and intimately related to the gallbladder. The second (descending portion) is of most importance to the urologist because it lies immediately anterior to the right renal hilum and pelvis. This portion of the duodenum is frequently mobilized (referred to as a Kocher maneuver) to expose the right kidney, right renal pelvis, and additional upper abdominal structures. The second portion of the duodenum also receives the common bile duct and surrounds the head of the pancreas. The third (horizontal) and fourth (ascending) portions of the duodenum cross from right to left over the IVC and aorta before transitioning into the jejunum.
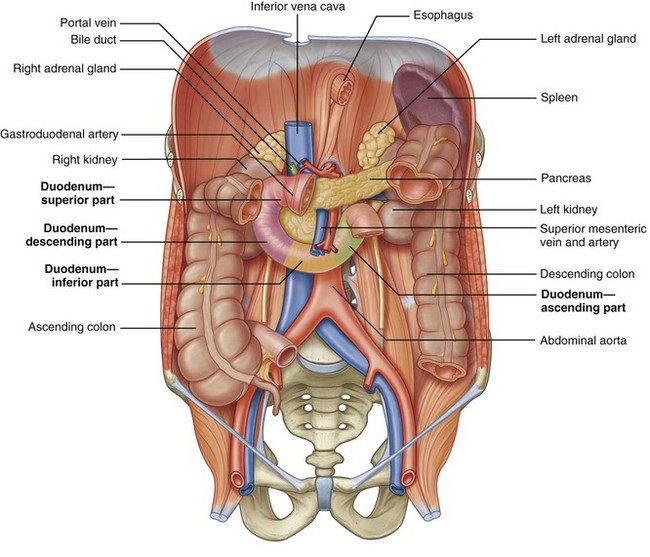
Figure 1–18 Colon, duodenum, and pancreas within the retroperitoneum.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 274.)
As noted earlier, the head of the pancreas is on the medial border of the descending duodenum. The body and tail of the pancreas continue across the IVC and aorta to the left side of the abdomen, where the pancreas is closely related to the left adrenal gland and the upper pole of the left kidney. The splenic artery and vein travel laterally along the posterior aspect of the pancreas, with the artery just superior to the vein. In this position these vascular structures are also closely related to the upper pole of the left kidney.
The final retroperitoneal gastrointestinal structure is the colon, with the ascending and descending portions being retroperitoneal. Both the ascending colon at the hepatic flexure and descending colon at the splenic flexure overlie the ipsilateral kidney. In addition, the hepatocolic ligament and splenocolic ligament tether the liver and spleen to the respective portions of the colon. Given the close anatomic relationship to the kidneys, mobilization of the colon and its mesentery is important for transperitoneal exposure of the kidneys and ureters.
Adrenal Glands
See Figure 1–19 on the Expert Consult website.![]()
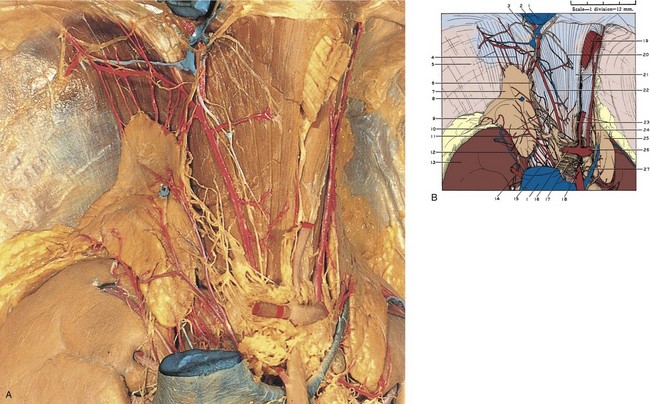
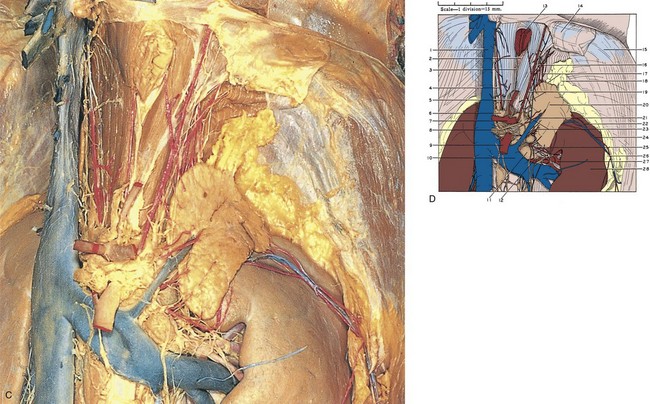
Figure 1–19 A, Right adrenal gland dissected. The inferior vena cava has been excised to fully expose the gland. The celiac arterial trunk, its branches, and associated autonomic nervous plexus are also well demonstrated. B, 1, Inferior vena cava (cut). 2, Right inferior phrenic vein. 3, Right phrenic nerve. 4, Superior adrenal arteries (branching from right inferior phrenic artery). 5, Diaphragm. 6, Inferior phrenic ganglion. 7, Right adrenal gland. 8, Right adrenal vein (cut). 9, Pararenal retroperitoneal fat. 10, Autonomic nerves to adrenal gland. 11, Middle adrenal artery (from aorta). 12, Inferior adrenal artery (from renal artery). 13, Right kidney. 14, Branch of right renal artery. 15, Celiac ganglion. 16, Common hepatic artery. 17, Celiac autonomic nervous plexus. 18, Superior mesenteric artery. 19, Esophagus (cut). 20, Branch of phrenic nerve. 21, Upper pointer, right crus of diaphragm; lower pointer, vagus nerve. 22, Right inferior phrenic artery. 23, Upper pointer, left gastric artery; lower pointer, superior extension of celiac autonomic nervous plexus. 24, Left inferior phrenic artery. 25, Left adrenal gland. 26, Splenic artery. 27, Left adrenal vein. C, Left adrenal gland dissected. D, 1, Inferior vena cava. 2, Esophageal hiatus. 3, Vagus nerve. 4, Right inferior phrenic artery. 5, Left gastric artery. 6, Right celiac ganglion. 7, Celiac artery. 8, Left celiac ganglion. 9, Superior mesenteric artery. 10, Left renal vein. 11, Renal hilar lymph node. 12, Renal autonomic nervous plexus. 13, Esophagus (cut). 14, Peritoneum (cut). 15, Diaphragm. 16, Phrenic autonomic nervous plexus. 17, Upper pointer, superior adrenal arteries (from inferior phrenic artery); lower pointer, superior margin of left adrenal gland. 18, Perinephric fat. 19, Upper pointer, left inferior phrenic artery; lower pointer, medial margin of left adrenal gland. 20, Left adrenal gland. 21, Left adrenal vein. 22, Inferior adrenal artery (in this case branching from perinephric/capsular artery of kidney). 23, Middle adrenal arteries (from aorta). 24, Perinephric blood vessels within Gerota fascia. 25, Inferior adrenal artery (from renal artery). 26, Perinephric fat. 27, Branch of left renal artery. 28, Left kidney.
(A to D, Reproduced from the Bassett anatomic collection, with permission granted by Dr. Robert A. Chase.)
Key Points
Adrenal Glands
Anatomic Relationships
The adult adrenal glands are 3 to 5 cm in greatest transverse dimension and weigh approximately 5 g. Grossly, they are yellow-orange and noticeably more orange than the surrounding adipose tissue. The position of this bilateral gland varies from right to left, but both glands are enclosed within the perirenal (Gerota) fascia and are separated from the upper pole of the kidneys by a layer of connective tissue.
The right gland is more superiorly located in the retroperitoneum and is pyramidal. It is almost directly cranial to the upper pole of the right kidney. Surrounding structures include the liver anterolaterally, the duodenum anteromedially, and the inferior vena cava medially. It is also important to note that there is often a retrocaval extension of one wing. The left gland is more crescenteric and medial to the upper pole of the left kidney. The upper and anterior aspects are related to the stomach, tail of the pancreas, and splenic vessels.
Composition
Embryologically, the adrenal is distinct from the kidney. Thus in cases of renal ectopia, the adrenal gland is not affected. Histologically, the adrenal is divided into two components: the centrally located medulla and the peripherally located cortex (Fig. 1–20 on the Expert Consult website![]() ). The medulla itself is composed of chromaffin cells derived from neural crest origin. These chromaffin cells are innervated directly by presynaptic sympathetic fibers traveling to the adrenal gland from the sympathetic chains. The secretion of neuroactive catecholamines by the adrenal medulla is thus under sympathetic control.
). The medulla itself is composed of chromaffin cells derived from neural crest origin. These chromaffin cells are innervated directly by presynaptic sympathetic fibers traveling to the adrenal gland from the sympathetic chains. The secretion of neuroactive catecholamines by the adrenal medulla is thus under sympathetic control.
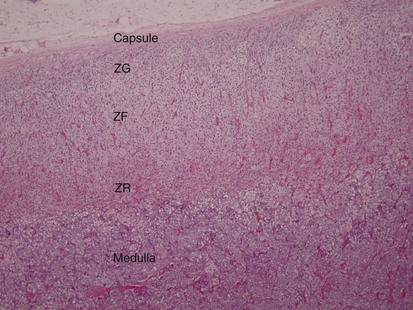
Figure 1–20 Microscopic section of the adrenal gland showing the adrenal medulla and cortex. The three cortical layers are visible: zona glomerulosa (ZG), zona fasciculata (ZF), and zona reticularis (ZR).
(Courtesy of Dr. Hossein Saboorian.)
The adrenal cortex is of mesodermal origin and makes up approximately 90% of the adrenal mass. It is composed of three layers, from external to internal: zona glomerulosa, zona fasciculata, and zona reticularis. Each layer has a different function, with the glomerulosa producing mineralocorticoids (e.g., aldosterone), the fasciculata producing glucocorticoids (e.g., cortisol), and the reticularis synthesizing sex steroids (androgens).
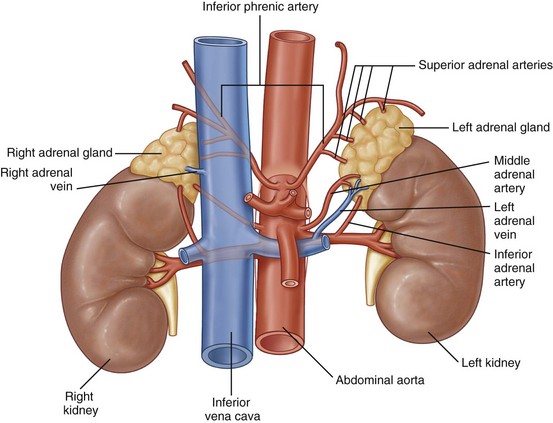
Adrenal Vessels
The arterial supply to the adrenal gland originates from three sources (Fig. 1–21). Superiorly, branches from the inferior phrenic artery feed the adrenal, whereas middle branches originate directly from the aorta. Finally, branches from the ipsilateral renal artery supply the adrenal gland. The venous drainage varies by side, although both adrenal glands are drained by a single large vein that exits anteromedially. On the left side this vein joins with the inferior phrenic vein and enters the cranial aspect of the left renal vein. On the right side, the adrenal vein enters the IVC directly on its posterolateral aspect. The lymphatic drainage of the adrenals follows the course of these veins and empties into para-aortic lymph nodes.
Kidneys
Gross and Microscopic Anatomy
The kidneys serve a number of important functions required to maintain normal human physiologic function. They are the primary organs for maintaining fluid and electrolyte balance, and they play a large role in maintaining acid-base balance. They produce renin, which plays a vital role in controlling blood pressure, and erythropoietin, which affects red blood cell production. They affect calcium metabolism, in particular calcium absorption, by converting a precursor of vitamin D to the most active form, 1,25-dihydroxyvitamin D.
Grossly, the kidneys are bilaterally paired reddish brown organs (see Figs. 1-1 and 1-22). Typically each kidney weighs 150 g in the male and 135 g in the female. The kidneys generally measure 10 to 12 cm vertically, 5 to 7 cm transversely, and 3 cm in the anteroposterior dimension. Because of compression by the liver, the right kidney tends to be somewhat shorter and wider. In children, the kidneys are relatively larger and possess more prominent fetal lobations. These lobations are present at birth and generally disappear by the first year of life, although occasionally they persist into adulthood. An additional common feature of the gross renal anatomy is a focal renal parenchymal bulge along the kidney’s lateral contour, known as a dromedary hump. This is a normal variation without pathologic significance. It is more common on the left than the right and is believed to be caused by downward pressure from the spleen or liver.
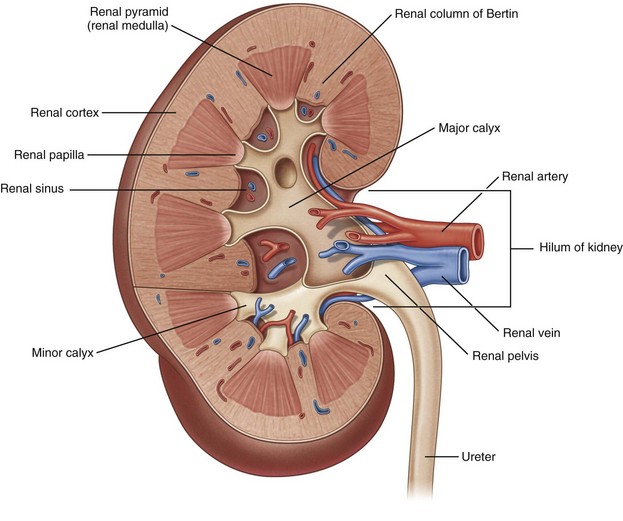
Figure 1–22 Internal structure of the kidney.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 323.)
As one proceeds centrally from the peripherally located reddish brown parenchyma of the kidney, the renal sinus is encountered. Here the vascular structures and collecting system coalesce before exiting the kidney medially. These structures are surrounded by yellow sinus fat, which provides an easily recognized landmark during renal procedures such as partial nephrectomy. At its medial border, the renal sinus narrows to form the renal hilum. It is through the hilum that the renal artery, renal vein, and renal pelvis exit the kidney and proceed to their respective destinations.
Both grossly and microscopically there are two distinct components within the renal parenchyma: the medulla and the cortex. Unlike the adrenal gland, the renal medulla is not a contiguous layer. Instead, the medulla is composed of multiple, distinct, conically shaped areas noticeably darker in color than the cortex (see Fig. 1–22). These same structures are also frequently called renal pyramids, making the terms renal medulla and renal pyramid synonymous. The apex of the pyramid is the renal papilla, and each papilla is cupped by an individual minor calyx.
Key Points
Kidneys
The renal cortex is lighter in color than the medulla and not only covers the renal pyramids peripherally but also extends between the pyramids themselves. The extensions of cortex between the renal pyramids are given a special name: the columns of Bertin. These columns are significant surgically because it is through these columns that renal vessels traverse from the renal sinus to the peripheral cortex, decreasing in diameter as the columns move peripherally. It is because of this anatomy that percutaneous access to the collecting system is made through a renal pyramid into a calyx, thus avoiding the columns of Bertin and the larger vessels present within them.
Many of these gross anatomic structures can be seen on modern imaging modalities such as computed tomography (see Fig. 1–11), as well as ultrasound and magnetic resonance imaging (Fig. 1–23).
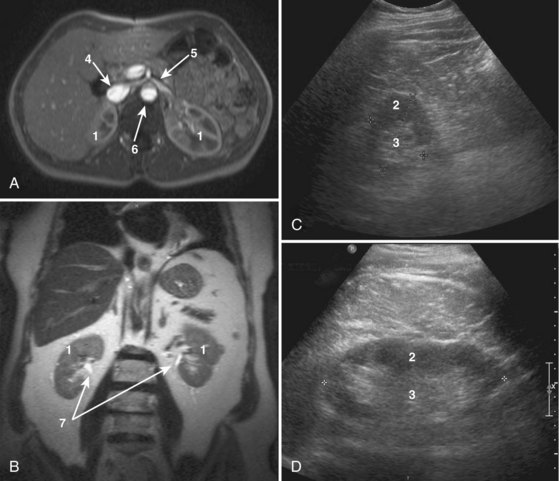
Figure 1–23 Cross-sectional imaging of the normal kidney. A, T1-weighted gadolinium-enhanced axial magnetic resonance imaging (MRI) of kidneys including the inferior vena cava, aorta, left renal vein, and superior mesenteric artery. B, Coronal T2 MRI of the kidneys. C, Transverse ultrasound imaging of the kidney. D, Sagittal ultrasound imaging of the kidney. 1, Kidney. 2, Renal cortex. 3, Renal medulla. 4, Inferior vena cava. 5, Left renal vein. 6, Aorta. 7, Renal collecting system.
Relations and Investing Fascia
Anatomic Relationships
The position of the kidney within the retroperitoneum varies greatly by side, degree of inspiration, body position, and presence of anatomic anomalies (Fig. 1–24 on the Expert Consult website![]() ). The right kidney sits 1 to 2 cm lower than the left in most individuals owing to displacement by the liver. Generally, the right kidney resides in the space between the top of the first lumbar vertebra to the bottom of the third lumbar vertebra. The left kidney occupies a more superior space from the body of the 12th thoracic vertebral body to the 3rd lumbar vertebra.
). The right kidney sits 1 to 2 cm lower than the left in most individuals owing to displacement by the liver. Generally, the right kidney resides in the space between the top of the first lumbar vertebra to the bottom of the third lumbar vertebra. The left kidney occupies a more superior space from the body of the 12th thoracic vertebral body to the 3rd lumbar vertebra.
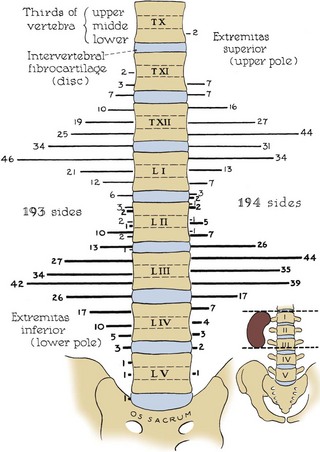
Figure 1–24 Variation among individuals in level of the kidneys relative to the spinal column. Lighter lateral lines represent upper poles of kidneys; darker lateral lines represent lower poles.
(From Anson BJ, Daseler EH. Common variations in renal anatomy, affecting blood supply, form, and topography. Surg Gynecol Obstet 1961;112:439–49.)
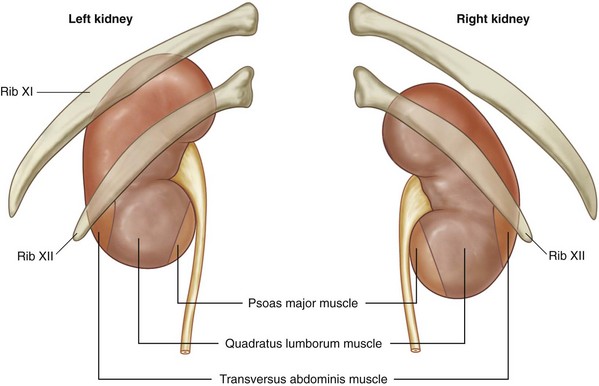
Of surgical importance are the structures surrounding the kidney (see Figs. 1-9 and 1-25). Both kidneys have similar muscular surroundings. Posteriorly, the diaphragm covers the upper third of each kidney, with the 12th rib crossing at the lower extent of the diaphragm. Also important to note for percutaneous renal procedures and flank incisions is that the pleura extends to the level of the 12th rib posteriorly. Medially the lower two thirds of the kidney lie against the psoas muscle, and laterally the quadratus lumborum and aponeurosis of the transversus abdominis muscle are encountered. The effect of the muscular relations on the kidneys is severalfold (Fig. 1–26). First, the lower pole of the kidney lies laterally and anteriorly relative to the upper pole. Second, the medial aspect of each kidney is rotated anteriorly at an angle of approximately 30 degrees. An understanding of this renal orientation is again of particular interest for percutaneous renal procedures in which kidney orientation influences access site selection.
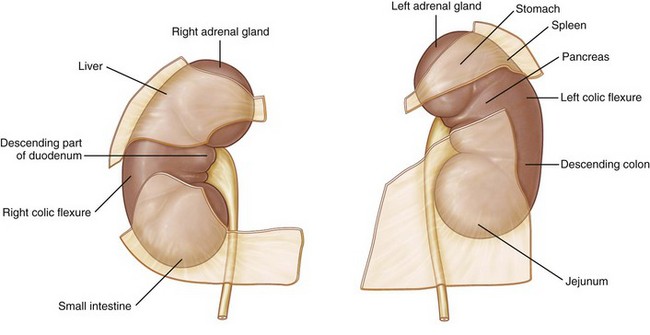
Figure 1–25 Structures related to the anterior surfaces of each kidney.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 321.)
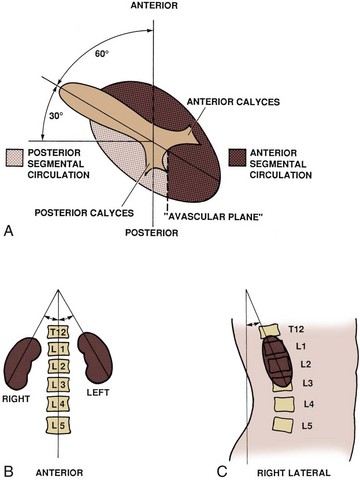
Figure 1–26 Normal rotational axes of the kidney. A, Transverse view showing approximate 30-degree anterior rotation of the left kidney from the coronal plane, relative positions of the anterior and posterior rows of calyces, and location of the relatively avascular plane separating the anterior and posterior renal circulation. B, Coronal section demonstrating slight inward tilt of the upper poles of the kidneys. C, Sagittal view showing anterior displacement of the lower pole of the right kidney.
Anteriorly, the right kidney is bordered by a number of structures (see Fig. 1–25). Cranially, the upper pole lies against the liver and is separated from the liver by the peritoneum except for the liver’s posterior bare spot. The hepatorenal ligament further attaches the right kidney to the liver because this extension of parietal peritoneum bridges the upper pole of the right kidney to the posterior liver. Also at the upper pole, the right adrenal gland is encountered. On the medial aspect, the descending duodenum is intimately related to the medial aspect of the kidney and hilar structures. Finally, on the anterior aspect of the lower pole lies the hepatic flexure of the colon.
The left kidney is bordered superiorly by the tail of the pancreas with the splenic vessels adjacent to the hilum and upper pole of the left kidney. Also cranial to the upper pole is the left adrenal gland and further superolaterally, the spleen. The splenorenal ligament attaches the left kidney to the spleen. This attachment can lead to splenic capsular tears if excessive downward pressure is applied to the left kidney. Superior to the pancreatic tail, the posterior gastric wall can overlie the kidney. Caudally, the kidney is covered by the splenic flexure of the colon.
Gerota Fascia
Interposed between the kidney and its surrounding structures is the perirenal or Gerota fascia (Figs. 1-27 through 1-29). This fascial layer encompasses the perirenal fat and kidney and encloses the kidney on three sides: superiorly, medially, and laterally. Superiorly and laterally Gerota fascia is closed, but medially it extends across the midline to fuse with the contralateral side. Inferiorly, Gerota fascia is not closed and remains an open potential space. Gerota fascia serves as an anatomic barrier to the spread of malignancy and a means of containing perinephric fluid collections. Thus perinephric fluid collections can track inferiorly into the pelvis without violating Gerota fascia.
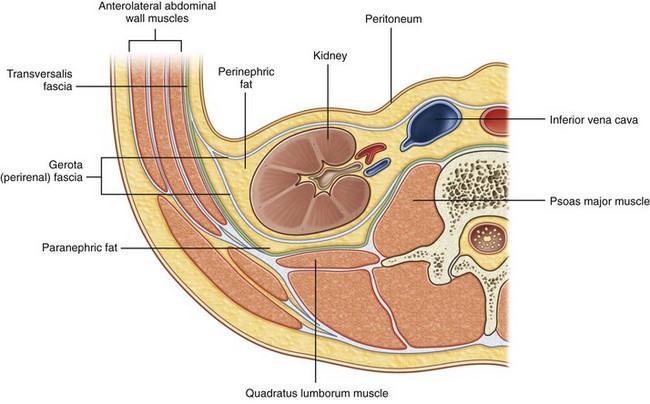
Figure 1–27 Organization of the fat and fascia surrounding the kidney.
(From Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005. p. 322.)
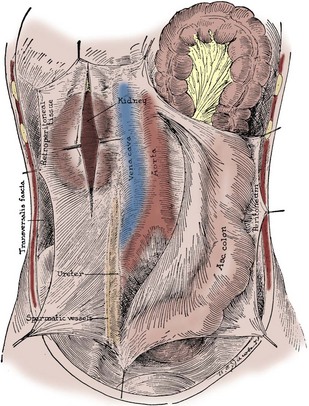
Figure 1–28 Anterior view of Gerota fascia on the right side, split over the right kidney (which it contains), and showing inferior extension enveloping the ureter and gonadal vessels. The ascending colon and overlying peritoneum have been reflected medially.
(From Tobin CE. The renal fascia and its relation to the transversalis fascia. Anat Rec 1944;89:295–311.)
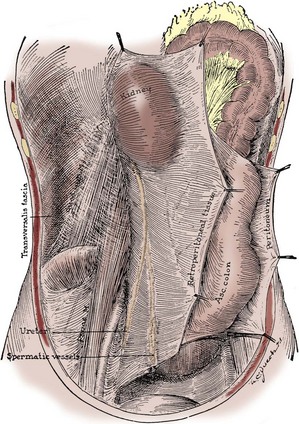
Figure 1–29 Posterior view of Gerota fascia on the right side, rotated medially with the contained kidney, ureter, and gonadal vessels, exposing the muscular posterior body wall covered by the transversalis fascia.
(From Tobin CE. The renal fascia and its relation to the transversalis fascia. Anat Rec 1944;89:295–311.)
Renal Vasculature
The renal pedicle classically consists of a single artery and a single vein that enter the kidney via the renal hilum (see Fig. 1–22). These structures branch from the aorta and inferior vena cava just below the superior mesenteric artery at the level of the second lumbar vertebra. The vein is anterior to the artery. The renal pelvis and ureter are located farther posterior to these vascular structures.
Renal Artery
Specifically, the right renal artery leaves the aorta and progresses with a caudal slope under the IVC toward the right kidney. The left renal artery courses almost directly laterally to the left kidney. Given the rotational axis of the kidney (see Fig. 1–26), both renal arteries move posteriorly as they enter the kidney. Also, both arteries have branches to the respective adrenal gland, renal pelvis, and ureter.
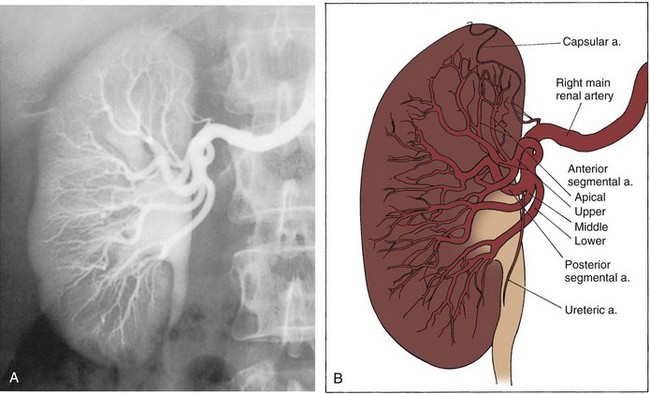
Upon approaching the kidney, the renal artery splits into four or more branches, with five being the most common. These are the renal segmental arteries (Fig. 1–30). Each segmental artery supplies a distinct portion of the kidney with no collateral circulation between them (Fig. 1–31). Thus occlusion or injury to a segmental branch will cause segmental renal infarction. Generally, the first and most constant branch is the posterior segmental branch, which separates from the renal artery before it enters the renal hilum. Typically there are four anterior branches, which from superior to inferior are apical, upper, middle, and lower. The relationship of these segmental arteries is important because the posterior segmental branch passes posterior to the renal pelvis while the others pass anterior to the renal pelvis. Ureteropelvic junction obstruction caused by a crossing vessel can occur when the posterior segmental branch passes anterior to the ureter causing occlusion. This division between the posterior and anterior segmental arteries has an additional surgical importance in that between these circulations is an avascular plane (see Figs. 1-26 and 1-31). This longitudinal plane lies just posterior to the lateral aspect of the kidney. Incision within this plane results in significantly less blood loss than outside this plane. However, there is significant variation in the location of this plane, requiring delineation before incision. This can be done with either preoperative angiography or intraoperative segmental arterial injection of a dye such as methylene blue.
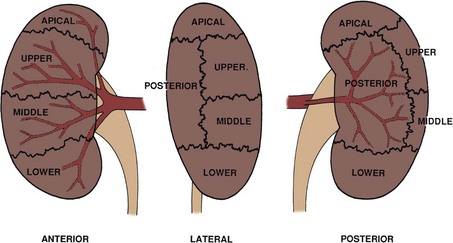
Figure 1–31 Typical segmental circulation of the right kidney, shown diagrammatically. Note that the posterior segmental artery is usually the first branch of the main renal artery and it extends behind the renal pelvis.
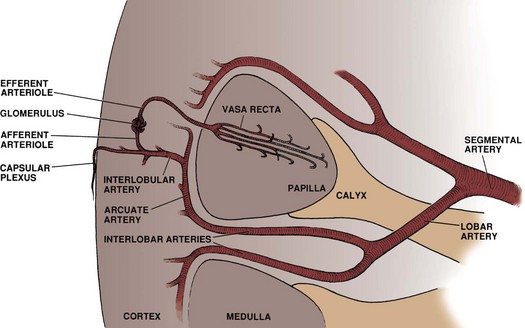
Once in the renal sinus, the segmental arteries branch into lobar arteries, which further subdivide in the renal parenchyma to form interlobar arteries (Fig. 1–32). These interlobar arteries progress peripherally within the cortical columns of Bertin, thus avoiding the renal pyramids but maintaining a close association with the minor calyceal infundibula. At the base (peripheral edge) of the renal pyramids, the interlobar arteries branch into arcuate arteries. Instead of moving peripherally, the arcuate arteries parallel the edge of the corticomedullary junction. Interlobular arteries branch off the arcuate arteries and move radially, where they eventually divide to form the afferent arteries to the glomeruli.
The 2 million glomeruli within each kidney represent the core of the renal filtration process. Each glomerulus is fed by an afferent arteriole. As blood flows through the glomerular capillaries, the urinary filtrate leaves the arterial system and is collected in the glomerular (Bowman) capsule. Blood flow leaves the glomerular capillary via the efferent arteriole and continues to one of two locations: secondary capillary networks around the urinary tubules in the cortex or descending into the renal medulla as the vasa recta.
Renal Veins
The renal venous drainage correlates closely with the arterial supply. The interlobular veins drain the postglomerular capillaries. These veins also communicate freely via a subcapsular venous plexus of stellate veins with veins in the perinephric fat. After the interlobular veins, the venous drainage progresses through the arcuate, interlobar, lobar, and segmental branches, with the course of each of these branches paralleling the respective artery. After the segmental branches, the venous drainage coalesces into three to five venous trunks that eventually combine to form the renal vein. Unlike the arterial supply, the venous drainage communicates freely through venous collars around the infundibula, providing for extensive collateral circulation in the venous drainage of the kidney (Fig. 1–33). Surgically, this is important because unlike the arterial supply, occlusion of a segmental venous branch has little effect on venous outflow.
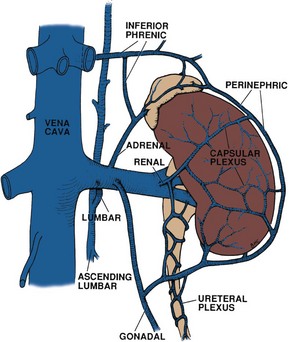
Figure 1–33 Venous drainage of the left kidney showing potentially extensive venous collateral circulation.
The renal vein is located directly anterior to the renal artery, although this position can vary up to 1 to 2 cm cranially or caudally relative to the artery. The right renal vein is generally 2 to 4 cm in length and enters the right lateral to posterolateral edge of the IVC. The left renal vein is typically 6 to 10 cm in length and enters the left lateral aspect of the IVC after passing posterior to the superior mesenteric artery and anterior to the aorta (Fig. 1–34 on the Expert Consult website![]() ). Compared with the right renal vein, the left renal vein enters the IVC at a slightly more cranial level and a more anterolateral location. Additionally, the left renal vein receives the left adrenal vein superiorly, lumbar vein posteriorly, and left gonadal vein inferiorly (see Fig. 1–33). The right renal vein typically does not receive any branches.
). Compared with the right renal vein, the left renal vein enters the IVC at a slightly more cranial level and a more anterolateral location. Additionally, the left renal vein receives the left adrenal vein superiorly, lumbar vein posteriorly, and left gonadal vein inferiorly (see Fig. 1–33). The right renal vein typically does not receive any branches.
Common Anatomic Variants
Anatomic variations in the renal vasculature are common, occurring in 25% to 40% of kidneys. The most common variation is supernumerary renal arteries, with up to five arteries reported. This occurs more often on the left. These additional arteries can enter through the hilum or directly into the parenchyma. Lower pole arteries on the right tend to cross anterior to the IVC, whereas lower pole arteries on either side can cross anterior to the collecting system, causing a ureteropelvic junction obstruction. When the kidney is ectopic, supernumerary arteries are even more common and their origin even more varied, with the celiac trunk, superior mesenteric artery, or iliac arteries all possible sources of ectopic renal arteries. Supernumerary veins occur as well, but this is a less common entity. The most common example is duplicate renal veins draining the right kidney via the right renal hilum. Polar veins are quite rare. Finally, the left renal vein may course behind the aorta or divide and send one limb anterior and one limb posterior to the aorta, resulting in a collar-type circumaortic formation.
Renal Lymphatics
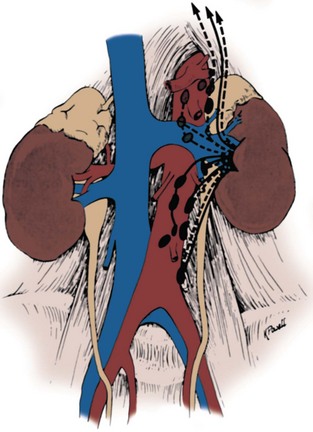
The renal lymphatics largely follow blood vessels through the columns of Bertin and then form several large lymphatic trunks within the renal sinus. As these lymphatics exit the hilum, branches from the renal capsule, perinephric tissues, renal pelvis, and upper ureter drain into these lymphatics. They then empty into lymph nodes associated with the renal vein near the renal hilum. From here, the lymphatic drainage between the two kidneys varies (Figs. 1-35 and 1-36). On the left, primary lymphatic drainage is into the left lateral para-aortic lymph nodes including nodes anterior and posterior to the aorta between the inferior mesenteric artery and the diaphragm. Occasionally, there will be additional drainage from the left kidney into the retrocrural nodes or directly into the thoracic duct above the diaphragm. On the right, drainage is into the right interaortocaval and right paracaval lymph nodes including nodes located anterior and posterior to the vena cava, from the common iliac vessels to the diaphragm. Occasionally, there will be additional drainage from the right kidney into the retrocrural nodes or the left lateral para-aortic lymph nodes.
Renal Collecting System
Microscopic Anatomy from Glomerulus to Collecting System
Microscopically, the renal collecting system originates in the renal cortex at the glomerulus as filtrate enters into Bowman capsule (Fig. 1–37 on the Expert Consult website![]() ). Together the glomerular capillary network and Bowman capsule form the renal corpuscle (malpighian corpuscle) (Fig. 1–38). The glomerular capillary network is covered by specialized epithelial cells called podocytes that, along with the capillary epithelium, form a selective barrier across which the urinary filtrate must pass. The filtrate is initially collected in Bowman capsule and then moves to the proximal convoluted tubule. The proximal tubule is composed of a thick cuboidal epithelium covered by dense microvilli. These microvilli greatly increase the surface area of the proximal tubule, allowing a large portion of the urinary filtrate to be reabsorbed in this section of the nephron.
). Together the glomerular capillary network and Bowman capsule form the renal corpuscle (malpighian corpuscle) (Fig. 1–38). The glomerular capillary network is covered by specialized epithelial cells called podocytes that, along with the capillary epithelium, form a selective barrier across which the urinary filtrate must pass. The filtrate is initially collected in Bowman capsule and then moves to the proximal convoluted tubule. The proximal tubule is composed of a thick cuboidal epithelium covered by dense microvilli. These microvilli greatly increase the surface area of the proximal tubule, allowing a large portion of the urinary filtrate to be reabsorbed in this section of the nephron.
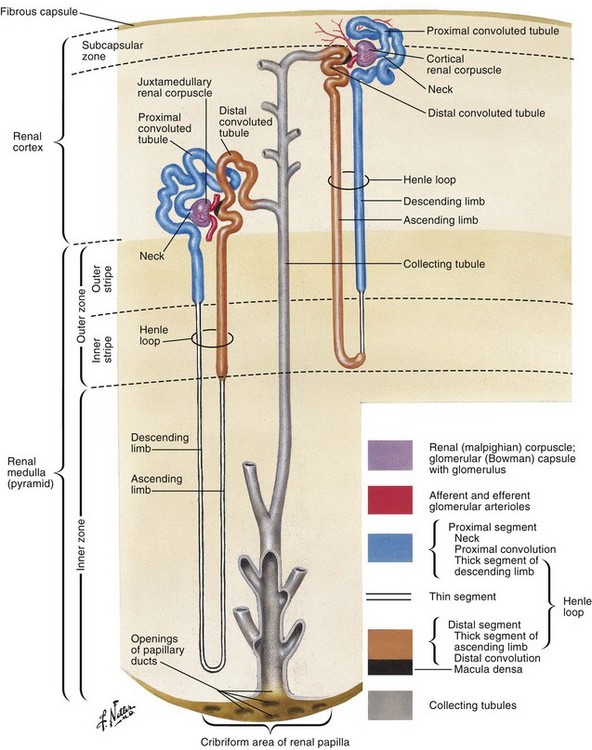
Figure 1–38 Renal nephron and collecting tubule.
(From Netter FH. Atlas of human anatomy. 4th ed. Philadelphia: Elsevier; 2006. p. 336.)

Figure 1–37 Electron micrograph of the renal corpuscle. The glomerular capillary network is enveloped by podocytes and contained within Bowman capsule. CS, capsular space of Bowman; PL, parietal layer of Bowman capsule; Po, podocyte. Arrows indicate cytoplasmic extensions from podocytes that enwrap the glomerular capillaries.
(From Kessel RG, Kardon RH. Tissues and organs: a text-atlas of scanning electron microscopy.
The proximal tubule continues deeper into the cortical tissue where it becomes the loop of Henle. The loop of Henle extends variable distances into the renal medulla. Within the renal medulla, the loop of Henle reverses course and moves back toward the periphery of the kidney. As it ascends out of the medulla the loop thickens and becomes the distal convoluted tubule. This tubule eventually returns to a position adjacent to the originating glomerulus and proximal convoluted tubule. Here the distal convoluted tubule turns once again for the interior of the kidney and becomes a collecting tubule. Collecting tubules from multiple nephrons combine into a collecting duct that extends inward through the renal medulla and eventually empties into the apex of the medullary pyramid, the renal papilla.
Renal Papillae, Calyces, and Pelvis
The renal papillae are the tip of a medullary pyramid and constitute the first gross structure of the renal collecting system. Typically, there are 7 to 9 papillae per kidney, but this number is variable, ranging from 4 to 18. The papillae are aligned in two longitudinal rows situated approximately 90 degrees from one another. There is an anterior row that owing to the orientation of the kidney faces in a lateral direction and a posterior row that extends directly posterior (see Figs. 1-26 and 1-39). Each of these papillae is cupped by a minor calyx (see Fig. 1–22). At the upper and lower poles, compound calyces are often encountered. These compound calyces are the result of renal pyramid fusion and because of their anatomy are more likely to allow reflux into the renal parenchyma (Fig. 1–40). Clinically, this can result in more severe scarring of the parenchyma overlying compound calyces.
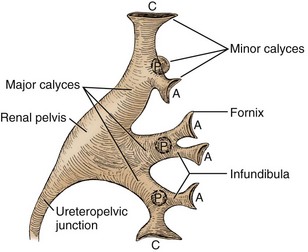
Figure 1–39 The renal collecting system (left kidney) showing major divisions into minor calyces, major calyces, and renal pelvis. A, anterior minor calyces; C, compound calyces at the renal poles; P, posterior minor calyces.
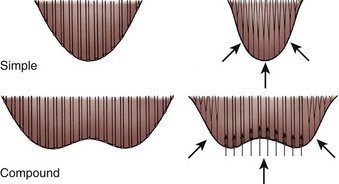
Figure 1–40 Diagram demonstrating structural and functional distinctions between simple and compound renal papillae. Back pressure causes closure of the collecting ducts in a simple papilla, effectively preventing reflux of urine into the renal parenchyma. The structure of the compound papilla allows intrarenal reflux of urine with sufficient back pressure.
After cupping an individual papilla, each minor calyx narrows to an infundibulum. Just as there is frequent variation in the number of calyces, the diameter and length of the infundibula vary greatly. Infundibuli combine to form two or three major calyceal branches. These are frequently termed the upper, middle, and lower pole calyces, and these calyces in turn combine to form the renal pelvis. The renal pelvis itself can vary greatly in size, ranging from a small intrarenal pelvis to a large predominantly extrarenal pelvis. Eventually the pelvis narrows to form the ureteropelvic junction, marking the beginning of the ureter.
On close examination, it is clear that there is significant variation in the anatomy of the renal collecting system (Figs. 1-41 through 1-43). Number of calyces, diameter of the infundibuli, and size of the renal pelvis all vary significantly among normal individuals. Even in the same individual, the renal collecting systems may be similar but are rarely identical. Because of this variation, it can be difficult to distinguish pathologic from normal on the basis of anatomy alone. Instead, it is demonstrated dysfunction that is necessary to make the diagnosis of a pathologic anatomic formation within the renal collecting system.
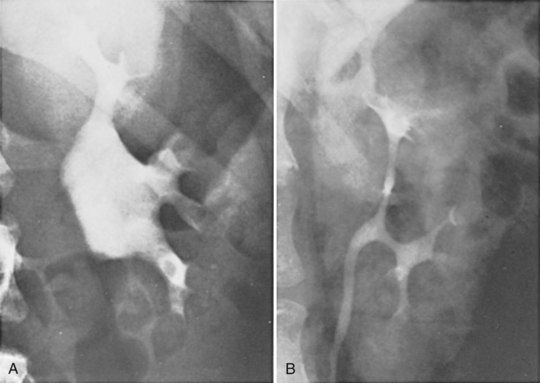
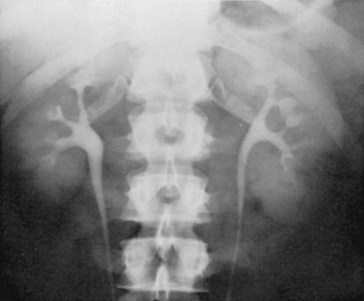
Figure 1–42 Significant variation between two normal renal pelves, demonstrated by excretory urography. A, Large, extrarenal pelvis. B, Narrow, completely intrarenal pelvis, barely larger in caliber than the ureter.
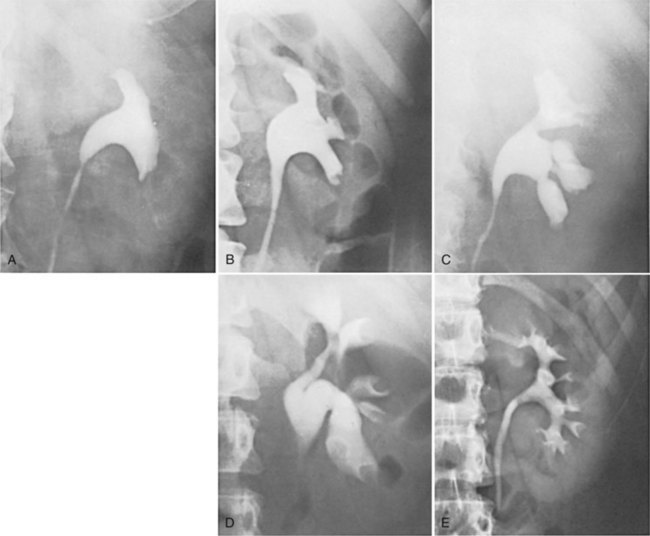
Figure 1–43 Examples of normal variations in the architecture of the renal collecting system, demonstrated by excretory urography. A, Absence of calyces. B, Minor calyces arising directly from the renal pelvis. C, Megacalyces. D, “Orchid” calyces. E, Multiple minor calyces and nearly absent renal pelvis.
Renal Innervation
Sympathetic preganglionic nerves originate from the eighth thoracic through first lumbar spinal segments and then travel to the celiac and aorticorenal ganglia. From here, postganglionic fibers travel to the kidney via the autonomic plexus surrounding the renal artery. Parasympathetic fibers originate from the vagus nerve and travel with the sympathetic fibers to the autonomic plexus along the renal artery. The primary function of the renal autonomic innervation is vasomotor, with the sympathetics inducing vasoconstriction and the parasympathetics causing vasodilation. Despite this innervation, it is important to realize that the kidney functions well even without this neurologic control, as evidenced by the successful function of transplanted kidneys.
Ureters
Key Point
Ureters
The ureters are bilateral tubular structures responsible for transporting urine from the renal pelvis to the bladder (see Fig. 1–1). They are generally 22 to 30 cm in length with a wall composed of multiple layers (Fig. 1–44). The inner layer is transitional epithelium. Next is the lamina propria. This is a connective tissue layer that along with the epithelium makes up the mucosal lining. Overlying the lamina propria is a layer of smooth muscle that is contiguous with muscle covering the renal calyces and pelvis, although in the ureter this layer is divided into an inner longitudinal and an outer circular layer. Together, these muscular layers provide the peristaltic wave that actively transports urine from the renal collecting system through the ureter to the bladder. The outermost layer is the adventitia. This thin layer surrounds the ureter and encompasses the blood vessels and lymphatics that travel along the ureter.
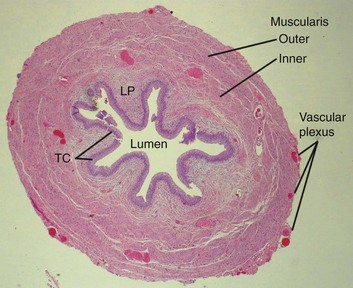
Figure 1–44 Transverse microscopic section through the ureter. Inner longitudinal layer is distinguished from outer circular and oblique muscle fibers. The rich vascular supply of the ureter is also demonstrated. LP, lamina propria; TC, transitional epithelium.
(Courtesy of Dr. Hossein Saboorian.)
Anatomic Relationships
Key to many urologic procedures is an understanding of ureteral anatomic relationships. The ureter begins at the ureteropelvic junction, which lies posterior to the renal artery and vein. It then progresses inferiorly along the anterior edge of the psoas muscle. Anteriorly, the right ureter is related to the ascending colon, cecum, colonic mesentery, and appendix. The left ureter is closely related to the descending and sigmoid colon and their accompanying mesenteries. Approximately a third of the way to the bladder the ureter is crossed anteriorly by the gonadal vessels. As it enters the pelvis the ureter crosses anterior to the iliac vessels. This crossover point is usually at the bifurcation of the common iliac into the internal and external iliac arteries, thus making this a useful landmark for pelvic procedures.
Given the proximity of the ureters to several bowel segments, malignant and inflammatory processes of the terminal ileum, appendix, right or left colon, and sigmoid colon may involve the ureter. Effects can range from microhematuria to fistula or total obstruction. Within the female pelvis, the ureters are crossed anteriorly by the uterine arteries and are closely related to the uterine cervix. This location places the ureters at risk during hysterectomy. Pathologic processes of the fallopian tube and ovary may also encroach on the ureter at the pelvic brim.
Normal Variations in Ureteral Caliber
The normal ureter is not of uniform caliber, with three distinct narrowings classically described: the ureteropelvic junction, crossing of the iliac vessels, and the ureterovesical junction (Fig. 1–45). At the ureteropelvic junction, the renal pelvis tapers into the proximal ureter. In many cases, this perceived narrowing may be more apparent than real, with no evidence of obstruction evident on radiographic or endoscopic investigation. The second region of narrowing occurs as the ureter crosses the iliac vessels. This is due to a combination of extrinsic compression of the ureter by the iliac vessels and the necessary anterior angulation of the ureter as it crosses the iliac vessels to enter into the pelvis. There is also no intrinsic change in the ureteral caliber at this location. The third site of narrowing observed in the normal ureter is the ureterovesical junction. There is a true physical restriction of the ureter as it makes the intramural passage through the bladder wall to the ureteral orifice. These three sites of ureteral narrowing are clinically significant because they are common locations for urinary calculi to lodge during passage. In addition, the angulation of the ureter, first anteriorly as it passes over the iliac vessels, then posteromedially as it enters the pelvis and courses behind the bladder, may restrict successful passage of rigid endoscopes. Appreciation of this normal angulation and the three-dimensional course of the ureter is critical for safe and successful ureteral endoscopy.
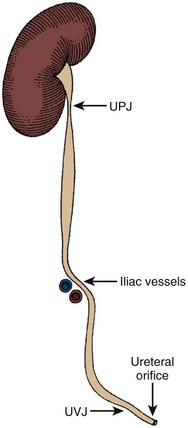
Figure 1–45 The ureter demonstrating sites of normal functional or anatomic narrowing at the ureteropelvic junction (UPJ), the iliac vessels, and the ureterovesical junction (UVJ). Note also the anterior displacement and angulation of the ureter, which occurs over the iliac vessels, as shown here diagrammatically.
Ureteral Segmentation and Nomenclature
The ureter is often arbitrarily divided into segments to assist ureteral description. The simplest system divides the ureter into the abdominal ureter extending from renal pelvis to the iliac vessels and the pelvic ureter extending from the iliac vessels to the bladder. Alternatively, the ureter can be divided into upper, middle, and lower segments (Fig. 1–46). The upper ureter extends from the renal pelvis to the upper border of the sacrum. The middle ureter comprises the segment from the upper to the lower border of the sacrum. The lower (distal or pelvic) ureter extends from the lower border of the sacrum to the bladder.
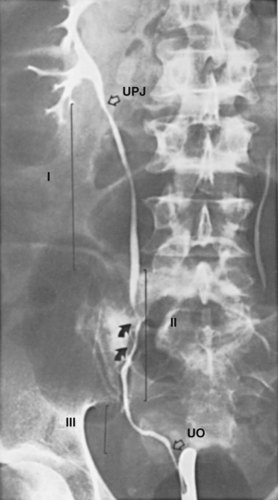
Figure 1–46 The right ureter, illustrated by retrograde injection of contrast material. UO, ureteral orifice in the bladder; UPJ, ureteropelvic junction; I, upper ureter, extending to the upper border of the sacrum; II, middle ureter, extending to the lower border of the sacrum; III, distal or lower ureter, traversing the pelvis to end in the bladder. Arrows indicate the course of the common iliac artery and vein.
Ureteral Blood Supply and Lymphatic Drainage
The ureter receives its blood supply from multiple arterial branches along its course (Fig. 1–47). Of greatest importance to the surgeon is that arterial branches to the abdominal ureter approach from a medial direction, whereas arterial branches to the pelvic ureter approach from a lateral direction. For the upper ureter these branches originate from the renal artery, gonadal artery, abdominal aorta, and common iliac artery. After entering the pelvis, additional small arterial branches to the distal ureter may arise from the internal iliac artery or its branches, especially the vesical and uterine arteries, but also from the middle rectal and vaginal arteries. After reaching the ureter, the arterial vessels course longitudinally within the periureteral adventitia in an extensive anastomosing plexus. It is this longitudinal vascularity that allows the ureter to be safely mobilized from the surrounding retroperitoneal tissues without compromising the vascular supply, provided that the periureteral adventitia is not stripped. The venous and lymphatic drainage of the ureter parallels the arterial supply. Thus ureteral lymphatic drainage varies by ureteral level. In the pelvis, ureteral lymphatics drain to internal, external, and common iliac nodes. In the abdomen the left para-aortic lymph nodes are the primary drainage site for the left ureter, whereas the abdominal portion of the right ureter is drained primarily to right paracaval and interaortocaval lymph nodes. The lymphatic drainage of the upper ureter and renal pelvis tends to join the renal lymphatics and is identical to that of the ipsilateral kidney.
Ureteral Innervation
The exact role of the ureteral autonomic input is unclear. Normal ureteral peristalsis does not require outside autonomic input but, rather, originates and is propagated from intrinsic smooth muscle pacemaker sites located in the minor calyces of the renal collecting system. The autonomic nervous system may exert some modulating effect on this process, but the exact role is unclear. The ureter receives preganglionic sympathetic input from the 10th thoracic through 2nd lumbar spinal segments. Postganglionic fibers arise from several ganglia in the aorticorenal, superior, and inferior hypogastric autonomic plexuses. Parasympathetic input is received from the 2nd through 4th sacral spinal segments.
Pain Perception and Somatic Referral
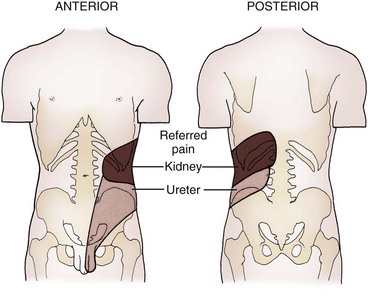
Renal pain fibers are stimulated by tension (distention) in the renal capsule, renal collecting system, or ureter. Direct mucosal irritation in the upper urinary tract may also stimulate nociceptors. Signals travel with the sympathetic nerves and result in a visceral-type pain referred to the sympathetic distribution of the kidney and ureter (eighth thoracic through second lumbar). Pain and reflex muscle spasm are typically produced over the distributions of the subcostal, iliohypogastric, ilioinguinal, and/or genitofemoral nerves, resulting in flank, groin, or scrotal (or labial) pain and hyperalgesia, depending on the location of the noxious visceral stimulus (Fig. 1–48).
Sampaio FJB. Renal anatomy: endourologic considerations. Urol Clin North Am. 2000;27:585-607.
Williams PL, Bannister LH, Berry MM, et al. Gray’s anatomy, 38th ed. New York: Churchill Livingstone; 1995.
Anson BJ, Daseler EH. Common variations in renal anatomy, affecting blood supply, form, and topography. Surg Gynecol Obstet. 1961;112:439-449.
Drake RL, Vogl W, Mitchell AWM. Gray’s anatomy for students. Philadelphia: Elsevier; 2005.
Graves FT. The anatomy of the intrarenal arteries and its application to segmental resection of the kidney. Br J Surg. 1954;42:132-139.
Hinman F, Stempen PH. Atlas of urosurgical anatomy. Philadelphia: WB Saunders; 1993.
Kaye KW, Reinke DB. Detailed caliceal anatomy for endourology. J Urol. 1984;132:1085-1088.
Parker AE. Studies on the main posterior lymph channels of the abdomen and their connections with the lymphatics of the genitourinary system. Am J Anat. 1935;56:409-443.
Pick JW, Anson BJ. The renal vascular pedicle: an anatomical study of 430 body-halves. J Urol. 1940;44:411-449.
Sampaio FJB. Renal anatomy: endourologic considerations. Urol Clin North Am. 2000;27:585-607.
Sampaio FJB, Aragão AHM. Anatomical relationship between the intrarenal arteries and the kidney collecting system. J Urol. 1990;143:679-681.
Sampaio FJB, Aragão AHM. Anatomical relationship between the renal venous arrangement and the kidney collecting system. J Urol. 1990;144:1089-1093.
Sampaio FJB, Passos MARF. Renal arteries: anatomic study for surgical and radiological practice. Surg Radiol Anat. 1992;14:113-117.
Tobin CE. The renal fascia and its relation to the transversalis fascia. Anat Rec. 1944;89:295-311.
Williams PL, Bannister LH, Berry MM, et al. Gray’s anatomy, 38th ed. New York: Churchill Livingstone; 1995.