chapter 3 Evaluation of the Urologic Patient
History, Physical Examination, and Urinalysis
Urologists have a unique position in medicine because their patients encompass all age groups including prenatal, pediatric, adolescent, adult, and geriatric. Because there is no medical subspecialist with similar interests, the urologist has the ability to make the initial evaluation and diagnosis and to provide medical and surgical therapy for all diseases of the genitourinary (GU) system. Historically, the diagnostic armamentarium included urinalysis, endoscopy, and intravenous pyelography. Recent advances in ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and endourology have expanded our diagnostic capabilities. Despite these advances, however, the basic approach to the patient is still dependent on taking a complete history, executing a thorough physical examination, and performing a urinalysis. These basics dictate and guide the subsequent diagnostic evaluation.
History
Overview
The medical history is the cornerstone of the evaluation of the urologic patient, and a well-taken history will frequently elucidate the probable diagnosis. However, many pitfalls can inhibit the urologist from obtaining an accurate history. The patient may be unable to describe or communicate symptoms because of anxiety, language barrier, or educational background. Therefore the urologist must be a detective and lead the patient through detailed and appropriate questioning to obtain accurate information. There are practical considerations in the art of history taking that can help to alleviate some of these difficulties. In the initial meeting, an attempt should be made to help the patient feel comfortable. During this time, the physician should project a calm, caring, and competent image that can help foster two-way communication. Impaired hearing, mental capacity, and facility with English can be assessed promptly. These difficulties are frequently overcome by having a family member present during the interview or, alternatively, by having an interpreter present.
Patients need to have sufficient time to express their problems and the reasons for seeking urologic care; the physician, however, should focus the discussion to make it as productive and informative as possible. Direct questioning can then proceed logically. The physician needs to listen carefully without distractions to obtain and interpret the clinical information provided by the patient. A complete history can be divided into the chief complaint and history of the present illness, the patient’s past medical history, and a family history. Each segment can provide significant positive and negative findings that will contribute to the overall evaluation and treatment of the patient.
Chief Complaint and Present Illness
Most urologic patients identify their symptoms as arising from the urinary tract and frequently present to the urologist for the initial evaluation. For this reason, the urologist frequently has the opportunity to act as both the primary physician and the specialist. The chief complaint must be clearly defined because it provides the initial information and clues to begin formulating the differential diagnosis. Most importantly, the chief complaint is a constant reminder to the urologist as to why the patient initially sought care. This issue must be addressed even if subsequent evaluation reveals a more serious or significant condition that requires more urgent attention. In our personal experience, a young woman presented with a chief complaint of recurrent urinary tract infections (UTIs). In the course of her evaluation, she was found to have a right adrenal mass. We subsequently focused on this problem and performed a right adrenalectomy for a benign cortical adenoma. We forgot about the woman’s original symptoms until she presented for her subsequent postoperative examination. She reminded us of her original symptoms at that time, and subsequent evaluation revealed that she had a nylon suture that had eroded into the anterior wall of her bladder from a previous abdominal vesicourethropexy performed 2 years earlier for stress urinary incontinence. Her UTIs resolved after surgical removal of the suture.
In obtaining the history of the present illness, the duration, severity, chronicity, periodicity, and degree of disability are important considerations. The patient’s symptoms need to be clarified for details and quantified for severity. Listed next are a variety of typical initial complaints. Specific questions that focus the differential diagnosis are provided.
Pain
Pain arising from the GU tract may be quite severe and is usually associated with either urinary tract obstruction or inflammation. Urinary calculi cause severe pain when they obstruct the upper urinary tract. Conversely, large, nonobstructing stones may be totally asymptomatic. Thus a 2-mm-diameter stone lodged at the ureterovesical junction may cause excruciating pain, whereas a large staghorn calculus in the renal pelvis or a bladder stone may be totally asymptomatic. Urinary retention from prostatic obstruction is also quite painful, but the diagnosis is usually obvious to the patient.
Inflammation of the GU tract is most severe when it involves the parenchyma of a GU organ. This is due to edema and distention of the capsule surrounding the organ. Thus pyelonephritis, prostatitis, and epididymitis are typically quite painful. Inflammation of the mucosa of a hollow viscus such as the bladder or urethra usually produces discomfort, but the pain is not nearly as severe.
Tumors in the GU tract usually do not cause pain unless they produce obstruction or extend beyond the primary organ to involve adjacent nerves. Thus pain associated with GU malignancies is usually a late manifestation and a sign of advanced disease.
Renal Pain
Pain of renal origin is usually located in the ipsilateral costovertebral angle just lateral to the sacrospinalis muscle and beneath the 12th rib. Pain is usually caused by acute distention of the renal capsule, generally from inflammation or obstruction. The pain may radiate across the flank anteriorly toward the upper abdomen and umbilicus and may be referred to the testis or labium. A corollary to this observation is that renal or retroperitoneal disease should be considered in the differential diagnosis of any man who complains of testicular discomfort but has a normal scrotal examination. Pain due to inflammation is usually steady, whereas pain due to obstruction fluctuates in intensity. Thus the pain produced by ureteral obstruction is typically colicky in nature and intensifies with ureteral peristalsis, at which time the pressure in the renal pelvis rises as the ureter contracts in an attempt to force urine past the point of obstruction.
Pain of renal origin may be associated with gastrointestinal symptoms because of reflex stimulation of the celiac ganglion and because of the proximity of adjacent organs (liver, pancreas, duodenum, gallbladder, and colon). Thus renal pain may be confused with pain of intraperitoneal origin; it can usually be distinguished, however, by a careful history and physical examination. Pain that is due to a perforated duodenal ulcer or pancreatitis may radiate into the back, but the site of greatest pain and tenderness is in the epigastrium. Pain of intraperitoneal origin is seldom colicky, as with obstructive renal pain. Furthermore, pain of intraperitoneal origin frequently radiates into the shoulder because of irritation of the diaphragm and phrenic nerve; this does not occur with renal pain. Typically, patients with intraperitoneal pathology prefer to lie motionless to minimize pain, whereas patients with renal pain usually are more comfortable moving around and holding the flank.
Renal pain may also be confused with pain resulting from irritation of the costal nerves, most commonly T10-T12. Such pain has a similar distribution from the costovertebral angle across the flank toward the umbilicus. However, the pain is not colicky in nature. Furthermore, the intensity of radicular pain may be altered by changing position; this is not the case with renal pain.
Ureteral Pain
Ureteral pain is usually acute and secondary to obstruction. The pain results from acute distention of the ureter and by hyperperistalsis and spasm of the smooth muscle of the ureter as it attempts to relieve the obstruction, usually produced by a stone or blood clot. The site of ureteral obstruction can often be determined by the location of the referred pain. With obstruction of the midureter, pain on the right side is referred to the right lower quadrant of the abdomen (McBurney point) and thus may simulate appendicitis; pain on the left side is referred over the left lower quadrant and resembles diverticulitis. Also, the pain may be referred to the scrotum in the male or the labium in the female. Lower ureteral obstruction frequently produces symptoms of vesical irritability including frequency, urgency, and suprapubic discomfort that may radiate along the urethra in men to the tip of the penis. Often, by taking a careful history, the astute clinician can predict the location of the obstruction. Ureteral pathology that arises slowly or produces only mild obstruction rarely causes pain. Therefore ureteral tumors and stones that cause minimal obstruction are seldom painful.
Vesical Pain
Vesical pain is usually produced either by overdistention of the bladder as a result of acute urinary retention or by inflammation. Constant suprapubic pain that is unrelated to urinary retention is seldom of urologic origin. Furthermore, patients with slowly progressive urinary obstruction and bladder distention (e.g., diabetics with a flaccid neurogenic bladder) frequently have no pain at all despite residual urine volumes over 1L.
Inflammatory conditions of the bladder usually produce intermittent suprapubic discomfort. Thus the pain in conditions such as bacterial cystitis or interstitial cystitis is usually most severe when the bladder is full and is relieved at least partially by voiding. Patients with cystitis sometimes experience sharp, stabbing suprapubic pain at the end of micturition, and this is termed strangury. Furthermore, patients with cystitis frequently experience pain referred to the distal urethra that is associated with irritative voiding symptoms such as urinary frequency and dysuria.
Prostatic Pain
Prostatic pain is usually secondary to inflammation with secondary edema and distention of the prostatic capsule. Pain of prostatic origin is poorly localized, and the patient may complain of lower abdominal, inguinal, perineal, lumbosacral, penile, and/or rectal pain. Prostatic pain is frequently associated with irritative urinary symptoms such as frequency and dysuria, and, in severe cases, marked prostatic edema may produce acute urinary retention.
Penile Pain
Pain in the flaccid penis is usually secondary to inflammation in the bladder or urethra, with referred pain that is experienced maximally at the urethral meatus. Alternatively, penile pain may be produced by paraphimosis, a condition in which the uncircumcised penile foreskin is trapped behind the glans penis, resulting in venous obstruction and painful engorgement of the glans penis (see later). Pain in the erect penis is usually due to Peyronie disease or priapism (see later).
Testicular Pain
Scrotal pain may be either primary or referred. Primary pain arises from within the scrotum and is usually secondary to acute epididymitis or torsion of the testis or testicular appendices. Because of the edema and pain associated with both acute epididymitis and testicular torsion, it is frequently difficult to distinguish these two conditions. Alternatively, scrotal pain may result from inflammation of the scrotal wall itself. This may result from a simple infected hair follicle or sebaceous cyst, but it may also be secondary to Fournier gangrene, a severe, necrotizing infection arising in the scrotum that can rapidly progress and be fatal unless promptly recognized and treated.
Chronic scrotal pain is usually related to noninflammatory conditions such as a hydrocele or a varicocele, and the pain is generally characterized as a dull, heavy sensation that does not radiate. Because the testes arise embryologically in close proximity to the kidneys, pain arising in the kidneys or retroperitoneum may be referred to the testes. Similarly, the dull pain associated with an inguinal hernia may be referred to the scrotum.
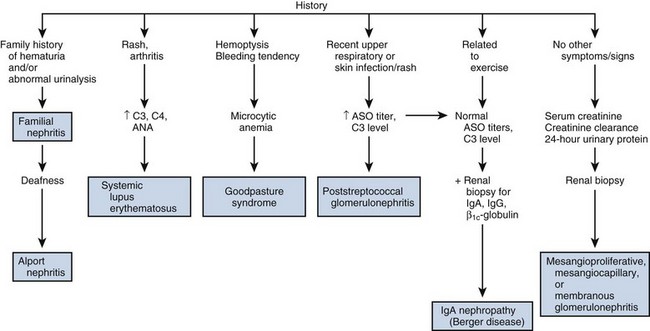
Hematuria
Hematuria is the presence of blood in the urine; greater than three red blood cells per high-power microscopic field (HPF) is significant. Patients with gross hematuria are usually frightened by the sudden onset of blood in the urine and frequently present to the emergency department for evaluation, fearing that they may be bleeding excessively. Hematuria of any degree should never be ignored and, in adults, should be regarded as a symptom of urologic malignancy until proved otherwise. In evaluating hematuria, several questions should always be asked, and the answers will enable the urologist to target the subsequent diagnostic evaluation efficiently:
Gross versus Microscopic Hematuria
The significance of gross versus microscopic hematuria is simply that the chances of identifying significant pathology increase with the degree of hematuria. Thus patients with gross hematuria usually have identifiable underlying pathology, whereas it is quite common for patients with minimal degrees of microscopic hematuria to have a negative urologic evaluation.
Timing of Hematuria
The timing of hematuria during urination frequently indicates the site of origin. Initial hematuria usually arises from the urethra; it occurs least commonly and is usually secondary to inflammation. Total hematuria is most common and indicates that the bleeding is most likely coming from the bladder or upper urinary tracts. Terminal hematuria occurs at the end of micturition and is usually secondary to inflammation in the area of the bladder neck or prostatic urethra. It occurs at the end of micturition as the bladder neck contracts, squeezing out the last amount of urine.
Association with Pain
Hematuria, although frightening, is usually not painful unless it is associated with inflammation or obstruction. Thus patients with cystitis and secondary hematuria may experience painful urinary irritative symptoms, but the pain is usually not worsened with passage of clots. More commonly, pain in association with hematuria usually results from upper urinary tract hematuria with obstruction of the ureters with clots. Passage of these clots may be associated with severe, colicky flank pain similar to that produced by a ureteral calculus, and this helps identify the source of the hematuria.
Presence of Clots
The presence of clots usually indicates a more significant degree of hematuria, and, accordingly, the probability of identifying significant urologic pathology increases.
Shape of Clots
Usually, if the patient is passing clots, they are amorphous and of bladder or prostatic urethral origin. However, the presence of vermiform (wormlike) clots, particularly if associated with flank pain, identifies the hematuria as coming from the upper urinary tract with formation of vermiform clots within the ureter.
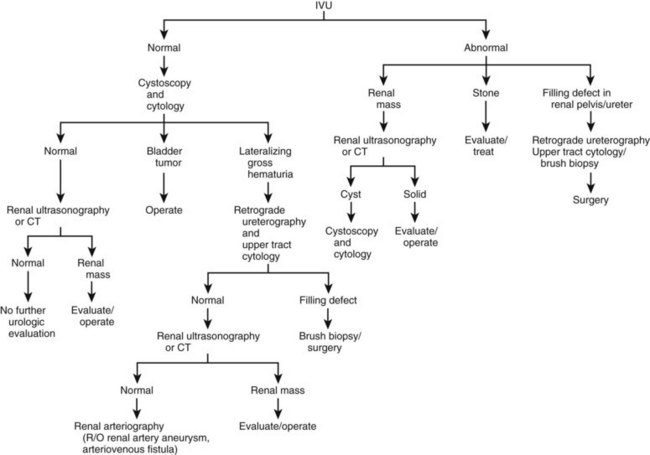
It cannot be emphasized strongly enough that hematuria, particularly in the adult, should be regarded as a symptom of malignancy until proved otherwise and demands immediate urologic examination. In a patient who presents with gross hematuria, cystoscopy should be performed as soon as possible, because frequently the source of bleeding can be readily identified. Cystoscopy will determine whether the hematuria is coming from the urethra, bladder, or upper urinary tract. In patients with gross hematuria secondary to an upper tract source, it is easy to see the jet of red urine pulsing from the involved ureteral orifice.
Although inflammatory conditions may result in hematuria, all patients with hematuria, except perhaps young women with acute bacterial hemorrhagic cystitis, should undergo urologic evaluation. Older women and men who present with hematuria and irritative voiding symptoms may have cystitis secondary to infection arising in a necrotic bladder tumor or, more commonly, flat carcinoma in situ of the bladder. The most common cause of gross hematuria in a patient older than age 50 years is bladder cancer.
Lower Urinary Tract Symptoms
Irritative Symptoms
Frequency is one of the most common urologic symptoms. The normal adult voids five or six times per day, with a volume of approximately 300 mL with each void. Urinary frequency is due to either increased urinary output (polyuria) or decreased bladder capacity. If voiding is noted to occur in large amounts frequently, the patient has polyuria and should be evaluated for diabetes mellitus, diabetes insipidus, or excessive fluid ingestion. Causes of decreased bladder capacity include bladder outlet obstruction with decreased compliance, increased residual urine, and/or decreased functional capacity due to irritation, neurogenic bladder with increased sensitivity and decreased compliance, pressure from extrinsic sources, or anxiety. By separating irritative from obstructive symptoms, the astute clinician should be able to arrive at a proper differential diagnosis.
Nocturia is nocturnal frequency. Normally, adults arise no more than twice at night to void. As with frequency, nocturia may be secondary to increased urine output or decreased bladder capacity. Frequency during the day without nocturia is usually of psychogenic origin and related to anxiety. Nocturia without frequency may occur in the patient with congestive heart failure and peripheral edema in whom the intravascular volume and urine output increase when the patient is supine. Renal concentrating ability decreases with age; therefore urine production in the geriatric patient is increased at night, when renal blood flow is increased as a result of recumbency. In general, nocturia may be attributed to nocturnal polyuria (nocturnal urine overproduction) and/or diminished nocturnal bladder capacity (Weiss and Blaivas, 2000). Nocturia may also occur in people who drink large amounts of liquid in the evening, particularly caffeinated and alcoholic beverages, which have strong diuretic effects. In the absence of these factors, nocturia signifies a problem with bladder function secondary to urinary outlet obstruction and/or decreased bladder compliance.
Dysuria is painful urination that is usually caused by inflammation. This pain is usually not felt over the bladder but is commonly referred to the urethral meatus. Pain occurring at the start of urination may indicate urethral pathology, whereas pain occurring at the end of micturition (strangury) is usually of bladder origin. Dysuria is frequently accompanied by frequency and urgency.
Obstructive Symptoms
Decreased force of urination is usually secondary to bladder outlet obstruction and commonly results from benign prostatic hyperplasia (BPH) or a urethral stricture. In fact, except for severe degrees of obstruction, most patients are unaware of a change in the force and caliber of their urinary stream. These changes usually occur gradually and go generally unrecognized by most patients. The other obstructive symptoms noted later are more commonly recognized and are usually secondary to bladder outlet obstruction in men due to either BPH or a urethral stricture.
Urinary hesitancy refers to a delay in the start of micturition. Normally, urination begins within a second after relaxing the urinary sphincter, but it may be delayed in men with bladder outlet obstruction.
Intermittency refers to involuntary start-stopping of the urinary stream. It most commonly results from prostatic obstruction with intermittent occlusion of the urinary stream by the lateral prostatic lobes.
Postvoid dribbling refers to the terminal release of drops of urine at the end of micturition. This is secondary to a small amount of residual urine in either the bulbar or the prostatic urethra that is normally “milked back” into the bladder at the end of micturition (Stephenson and Farrar, 1977). In men with bladder outlet obstruction, this urine escapes into the bulbar urethra and leaks out at the end of micturition. Men will frequently attempt to avoid wetting their clothing by shaking the penis at the end of micturition. In fact, this is ineffective, and the problem is more readily solved by manual compression of the bulbar urethra in the perineum and blotting the urethral meatus with a tissue. Postvoid dribbling is often an early symptom of urethral obstruction related to BPH, but, in itself, seldom necessitates any further treatment.
Straining refers to the use of abdominal musculature to urinate. Normally, it is unnecessary for a man to perform a Valsalva maneuver except at the end of urination. Increased straining during micturition is a symptom of bladder outlet obstruction.
It is important for the urologist to distinguish irritative from obstructive lower urinary tract symptoms. This most frequently occurs in evaluating men with BPH. Although BPH is primarily obstructive, it produces changes in bladder compliance that result in increased irritative symptoms. In fact, men with BPH more commonly present with irritative than obstructive symptoms, and the most common presenting symptom is nocturia. The urologist must be careful not to attribute irritative symptoms to BPH unless there is documented evidence of obstruction. In general, lower urinary tract symptoms are nonspecific and may occur secondary to a wide variety of neurologic conditions, as well as to prostatic enlargement (Lepor and Machi, 1993). In this regard, two important examples are mentioned. Patients with high-grade flat carcinoma in situ of the bladder may present with urinary irritative symptoms. The urologist should be particularly aware of the diagnosis of carcinoma in situ in men who present with irritative symptoms, a history of cigarette smoking, and microscopic hematuria. In our personal experience, we cared for a 54-year-old man who presented with this history and was treated for BPH for 2 years before the diagnosis of bladder cancer was established. Once the correct diagnosis was made, the patient had developed muscle-invasive disease and required a cystectomy for cure.
The second important example is irritative symptoms resulting from neurologic disease such as cerebrovascular accidents, diabetes mellitus, and Parkinson disease. Most neurologic diseases encountered by the urologist are upper motor neuron in etiology and result in a loss of cortical inhibition of voiding with resultant decreased bladder compliance and irritative voiding symptoms. The urologist must be extremely careful to rule out underlying neurologic disease before performing surgery to relieve bladder outlet obstruction. Such surgery not only may fail to relieve the patient’s irritative symptoms but also may result in permanent urinary incontinence.
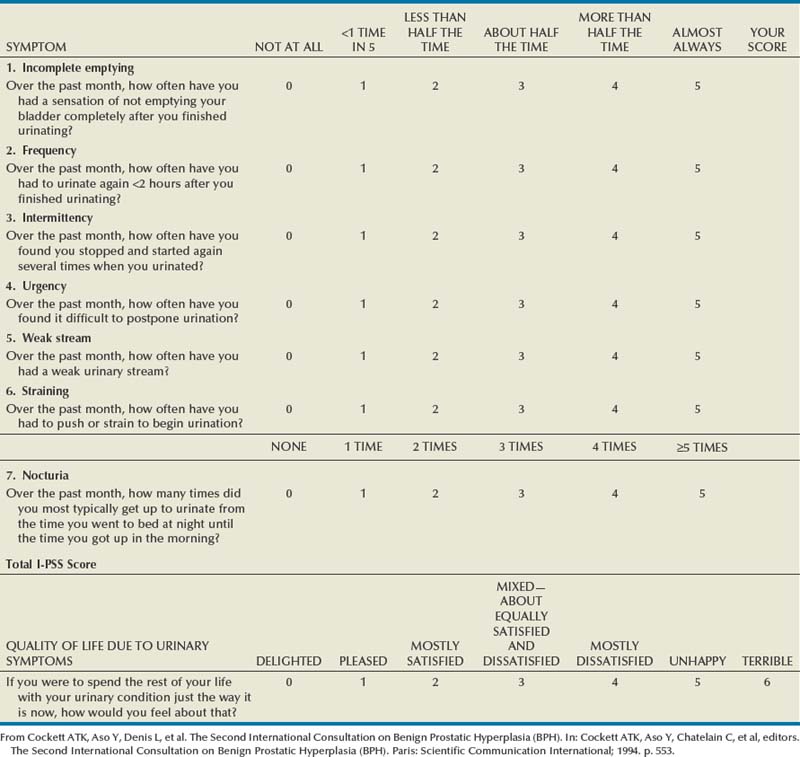
Since its introduction in 1992, the American Urological Association (AUA) symptom index has been widely used and validated as an important means of assessing men with lower urinary tract symptoms (Barry et al, 1992). The original AUA symptom score is based on the answers to seven questions concerning frequency, nocturia, weak urinary stream, hesitancy, intermittency, incomplete bladder emptying, and urgency. The International Prostate Symptom Score (I-PSS) includes these seven questions, as well as a global quality-of-life question (Table 3–1). The total symptom score ranges from 0 to 35 with scores of 0 to 7, 8 to 19, and 20 to 35 indicating mild, moderate, and severe lower urinary tract symptoms, respectively. The I-PSS is a helpful tool both in the clinical management of men with lower urinary tract symptoms and in research studies regarding the medical and surgical treatment of men with voiding dysfunction.
The use of symptom indices has limitations, and it is important for the physician to discuss the patient’s responses with him. It has been demonstrated that a grade 6 reading level is necessary to understand the I-PSS, and some patients with neurologic disorders and dementia may also have difficulty completing the symptom score (MacDiarmid et al, 1998). In addition, the symptom score and obstructive and irritative voiding symptoms are nonspecific, and the symptoms may be caused by a variety of conditions other than BPH. Similar symptom scores have been demonstrated to be present in age-matched men and women between 55 and 79 years of age (Lepor and Machi, 1993). Despite these limitations, the I-PSS is a simple adjunct in assessing men with lower urinary tract symptoms and may be used in the initial evaluation of men with lower urinary tract symptoms, as well as in the assessment of treatment response.
Incontinence
Urinary incontinence is the involuntary loss of urine. A careful history of the incontinent patient will often determine the etiology. Urinary incontinence can be subdivided into four categories.
Continuous Incontinence
Continuous incontinence is most commonly due to a urinary tract fistula that bypasses the urethral sphincter. The most common type of fistula that results in urinary incontinence is a vesicovaginal fistula usually secondary to gynecologic surgery, radiation, or obstetric trauma. Less commonly, ureterovaginal fistulas may occur from similar causes.
A second major cause of continuous incontinence is an ectopic ureter that enters either the urethra or the female genital tract. An ectopic ureter usually drains a small, dysplastic upper pole segment of kidney, and the amount of urinary leakage may be quite small. Such patients may void most of their urine normally but have a continuous amount of small urinary leakage that may be misdiagnosed for many years as a chronic vaginal discharge. In our experience, we cared for a 30-year-old woman—who had been misdiagnosed with enuresis in childhood and as having a chronic vaginal discharge in adult life—whose urinary leakage was totally corrected by surgical removal of the dysplastic, upper pole segment of her right kidney. Ectopic ureters never produce urinary incontinence in males because they always enter the bladder neck or prostatic urethra proximal to the external urethral sphincter.
Stress Incontinence
Stress incontinence refers to the sudden leakage of urine with coughing, sneezing, exercise, or other activities that increase intra-abdominal pressure. During these activities, intra-abdominal pressure rises transiently above urethral resistance, resulting in a sudden, usually small amount of urinary leakage. Stress incontinence is most common in women after childbearing or menopause and is related to a loss of anterior vaginal support and weakening of pelvic tissues. Stress incontinence is also observed in men after prostatic surgery, most commonly radical prostatectomy, in which there may be injury to the external urethral sphincter. Stress urinary incontinence is difficult to manage pharmacologically, and patients with significant stress incontinence are usually best treated surgically.
Urgency Incontinence
Urgency incontinence is the precipitous loss of urine preceded by a strong urge to void. This symptom is commonly observed in patients with cystitis, neurogenic bladder, and advanced bladder outlet obstruction with secondary loss of bladder compliance. It is important to distinguish urgency incontinence from stress incontinence for two reasons. First, urgency incontinence may result from a secondary underlying pathologic process, which should be identified; treatment of this primary problem such as infection or bladder outlet obstruction may result in resolution of urgency incontinence. Second, patients with urgency incontinence are usually not amenable to surgical correction but, rather, are more appropriately treated with pharmacologic agents that increase bladder compliance and/or increase urethral resistance.
Overflow Urinary Incontinence
Overflow urinary incontinence, often called paradoxical incontinence, is secondary to advanced urinary retention and high residual urine volumes. In these patients, the bladder is chronically distended and never empties completely. Urine may dribble out in small amounts as the bladder overflows. This is particularly likely to occur at night when the patient is less likely to inhibit urinary leakage. Overflow incontinence has been termed paradoxical incontinence because it can often be cured by relief of bladder outlet obstruction. It is, however, often difficult to make the diagnosis of overflow incontinence by history and physical examination alone, particularly in the obese patient, in whom percussion of the distended bladder may be difficult. Overflow incontinence usually develops over a considerable length of time, and patients may be totally unaware of incomplete bladder emptying. Thus any patient with significant incontinence should undergo measurement of postvoid residual urine.
Enuresis
Enuresis refers to urinary incontinence that occurs during sleep. It occurs normally in children up to 3 years of age but persists in about 15% of children at age 5 and about 1% of children at age 15 (Forsythe and Redmond, 1974). Enuresis must be distinguished from continuous incontinence, which occurs in the day and night and which, in a young girl, usually indicates the presence of an ectopic ureter. All children older than age 6 years with enuresis should undergo a urologic evaluation, although the vast majority will be found to have no significant urologic abnormality.
Sexual Dysfunction
Male sexual dysfunction is frequently used synonymously with impotence or erectile dysfunction, although impotence refers specifically to the inability to achieve and maintain an erection adequate for intercourse. Patients presenting with “impotence” should be questioned carefully to rule out other male sexual disorders including loss of libido, absence of emission, absence of orgasm, and, most commonly, premature ejaculation. Obviously, it is important to identify the precise problem before proceeding with further evaluation and treatment.
Loss of Libido
Because androgens have a major influence on sexual desire, a decrease in libido may indicate androgen deficiency arising from either pituitary or testicular dysfunction. This can be evaluated directly by measurement of serum testosterone that, if abnormal, should be further evaluated by measurement of serum gonadotropins and prolactin. Because the amount of testosterone required to maintain libido is usually less than that required for full stimulation of the prostate and seminal vesicles, patients with hypogonadism may also note decreased or absent ejaculation. Conversely, if semen volume is normal, it is unlikely that endocrine factors are responsible for loss of libido. A decrease in libido may also result from depression and a variety of medical illnesses that affect general health and well-being.
Impotence
Impotence refers specifically to the inability to achieve and maintain an erection sufficient for intercourse. A careful history will often determine whether the problem is primarily psychogenic or organic. In men with psychogenic impotence, the condition frequently develops rather quickly secondary to a precipitating event such as marital stress or change or loss of a sexual partner. In men with organic impotence, the condition usually develops more insidiously and frequently can be linked to advancing age or other underlying risk factors.
In evaluating men with impotence, it is important to determine whether the problem exists in all situations. Many men who report impotence may not be able to have intercourse with one partner but will with another. Similarly, it is important to determine whether men are able to achieve normal erections with alternative forms of sexual stimulation (e.g., masturbation, erotic videos). Finally, the patient should be asked whether he ever notes nocturnal or early morning erections. In general, patients who are able to achieve adequate erections in some situations but not others have primarily psychogenic rather than organic impotence.
Failure to Ejaculate
An ejaculation may result from several causes: (1) androgen deficiency, (2) sympathetic denervation, (3) pharmacologic agents, and (4) bladder neck and prostatic surgery. Androgen deficiency results in decreased secretions from the prostate and seminal vesicles causing a reduction or loss of seminal volume. Sympathectomy or extensive retroperitoneal surgery, most notably retroperitoneal lymphadenectomy for testicular cancer, may interfere with autonomic innervation of the prostate and seminal vesicles, resulting in absence of smooth muscle contraction and absence of seminal emission at time of orgasm. Pharmacologic agents, particularly α-adrenergic antagonists, may interfere with bladder neck closure at time of orgasm and result in retrograde ejaculation. Similarly, previous bladder neck or prostatic urethral surgery, most commonly transurethral resection of the prostate, may interfere with bladder neck closure, resulting in retrograde ejaculation. Finally, retrograde ejaculation may develop spontaneously in diabetic men.
Patients who complain of absence of ejaculation should be questioned regarding loss of libido or other symptoms of androgen deficiency, present medications, diabetes, and previous surgery. A careful history will usually determine the cause of this problem.
Absence of Orgasm
Anorgasmia is usually psychogenic or caused by certain medications used to treat psychiatric diseases. Sometimes, however, anorgasmia may be due to decreased penile sensation owing to impaired pudendal nerve function. Most commonly, this occurs in diabetics with peripheral neuropathy. Men who experience anorgasmia in association with decreased penile sensation should undergo vibratory testing of the penis and further neurologic evaluation as indicated.
Premature Ejaculation
Men who complain of premature ejaculation should be questioned carefully because this is obviously a subjective symptom. It is common for men to ejaculate within 2 minutes after initiation of intercourse, and many men who complain of premature ejaculation in actuality have normal sexual function with abnormal sexual expectations. However, there are men with true premature ejaculation who reach orgasm within less than 1 minute after initiation of intercourse. This problem is almost always psychogenic and best treated by a clinical psychologist or psychiatrist who specializes in treatment of this problem and other psychologic aspects of male sexual dysfunction. With counseling and appropriate modifications in sexual technique, this problem can usually be overcome. Alternatively, treatment with serotonin reuptake inhibitors such as sertraline and fluoxetine has been demonstrated to be helpful in men with premature ejaculation (Murat Basar et al, 1999).
Hematospermia
Hematospermia refers to the presence of blood in the seminal fluid. It almost always results from nonspecific inflammation of the prostate and/or seminal vesicles and resolves spontaneously, usually within several weeks. It frequently occurs after a prolonged period of sexual abstinence, and we have observed it several times in men whose wives are in the final weeks of pregnancy. Patients with hematospermia that persists beyond several weeks should undergo further urologic evaluation, because, rarely, an underlying etiology will be identified. A genital and rectal examination should be done to exclude the presence of tuberculosis, a prostate-specific antigen (PSA) and a rectal examination done to exclude prostatic carcinoma, and a urinary cytology done to exclude the possibility of transitional cell carcinoma of the prostate. It should be emphasized, however, that hematospermia almost always resolves spontaneously and rarely is associated with any significant urologic pathology.
Pneumaturia
Pneumaturia is the passage of gas in the urine. In patients who have not recently had urinary tract instrumentation or a urethral catheter placed, this is almost always due to a fistula between the intestine and the bladder. Common causes include diverticulitis, carcinoma of the sigmoid colon, and regional enteritis (Crohn disease). In rare instances, patients with diabetes mellitus may have gas-forming infections, with carbon dioxide formation from the fermentation of high concentrations of sugar in the urine.
Urethral Discharge
Urethral discharge is the most common symptom of venereal infection. A purulent discharge that is thick, profuse, and yellow to gray is typical of gonococcal urethritis; the discharge in patients with nonspecific urethritis is usually scant and watery. A bloody discharge is suggestive of carcinoma of the urethra.
Fever and Chills
Fever and chills may occur with infection anywhere in the GU tract but are most commonly observed in patients with pyelonephritis, prostatitis, or epididymitis. When associated with urinary obstruction, fever and chills may portend septicemia and necessitate emergency treatment to relieve obstruction.
Medical History
The past medical history is extremely important because it frequently provides clues to the patient’s current diagnosis. The past medical history should be obtained in an orderly and sequential manner.
Previous Medical Illnesses with Urologic Sequelae
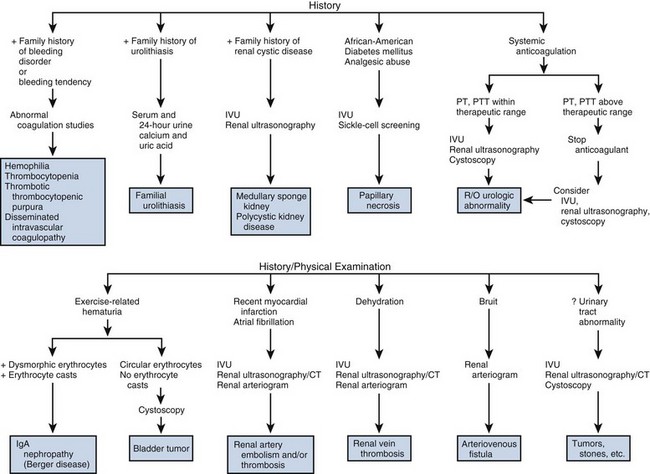
There are obviously many diseases that may affect the GU system, and it is important to listen and record the patient’s previous medical illnesses. Patients with diabetes mellitus frequently develop autonomic dysfunction that may result in impaired urinary and sexual function. A previous history of tuberculosis may be important in a patient presenting with impaired renal function, ureteral obstruction, or chronic, unexplained UTIs. Patients with hypertension have an increased risk of sexual dysfunction because they are more likely to have peripheral vascular disease and because many of the medications that are used to treat hypertension frequently cause impotence. Patients with neurologic diseases such as multiple sclerosis are also more likely to develop urinary and sexual dysfunction. In fact, 5% of patients with previously undiagnosed multiple sclerosis present with urinary symptoms as the first manifestation of the disease (Blaivas and Kaplan, 1988). As mentioned earlier, in men with bladder outlet obstruction, it is important to be aware of preexisting neurologic conditions. Surgical treatment of bladder outlet obstruction in the presence of detrusor hyperreflexia may result in increased urinary incontinence postoperatively. Finally, patients with sickle cell anemia are prone to a number of urologic conditions including papillary necrosis and erectile dysfunction secondary to recurrent priapism. There are obviously many other diseases with urologic sequelae, and it is important for the urologist to take a careful history in this regard.
Family History
It is similarly important to obtain a detailed family history because many diseases are genetic and/or familial. Examples of genetic diseases include adult polycystic kidney disease, tuberous sclerosis, von Hippel-Lindau disease, renal tubular acidosis, and cystinuria; these are but a few common and well-recognized examples.
In addition to these diseases of known genetic predisposition, there are other conditions in which the precise pattern of inheritance has not been elucidated but that clearly have a familial tendency. It is well known that individuals with a family history of urolithiasis are at increased risk for stone formation. More recently, it has been recognized that 8% to 10% of men with prostate cancer have a familial form of the disease that tends to develop about a decade earlier than the more common type of prostate cancer (Bratt, 2000). Other familial conditions are mentioned elsewhere in the text, but suffice it to state again that obtaining a careful history of previous illnesses and a family history of urologic disease can be extremely valuable in establishing the correct diagnosis.
Medications
It is similarly important to obtain an accurate and complete list of present medications because many drugs interfere with urinary and sexual function. For example, most of the antihypertensive medications interfere with erectile function, and changing antihypertensive medications can sometimes improve sexual function. Similarly, many of the psychotropic agents interfere with emission and orgasm. In our own recent experience, we cared for a man who presented with anorgasmia. He had been to several physicians without improvement in this problem. When we obtained his past medical history, he mentioned that he had been taking a psychotropic agent for transient depression for several years, and his anorgasmia resolved when this no-longer-needed medication was discontinued. The list of medications affecting urinary and sexual function is exhaustive, but, once again, each medication should be recorded and its side effects investigated to be sure that the patient’s problem is not drug related. A listing of common medications that may cause urologic side effects is presented in Table 3–2.
Table 3–2 Drugs Associated with Urologic Side Effects
| UROLOGIC SIDE EFFECTS | CLASS OF DRUGS | SPECIFIC EXAMPLES |
|---|---|---|
| Decreased libido | Antihypertensives | Hydrochlorothiazide |
| Erectile dysfunction | Propranolol | |
| Psychotropic drugs | Benzodiazepines | |
| Ejaculatory dysfunction | α-Adrenergic antagonists | Prazosin |
| Tamsulosin | ||
| α-Methyldopa | ||
| Psychotropic drugs | Phenothiazines | |
| Antidepressants | ||
| Priapism | Antipsychotics | Phenothiazines |
| Antidepressants | Trazodone | |
| Antihypertensives | Hydralazine | |
| Prazosin | ||
| Decreased spermatogenesis | Chemotherapeutic agents | Alkylating agents |
| Drugs with abuse potential | Marijuana | |
| Alcohol | ||
| Nicotine | ||
| Drugs affecting endocrine function | Antiandrogens | |
| Prostaglandins | ||
| Incontinence or impaired voiding | Direct smooth muscle stimulants | Histamine |
| Vasopressin | ||
| Others | Furosemide | |
| Valproic acid | ||
| Smooth muscle relaxants | Diazepam | |
| Striated muscle relaxants | Baclofen | |
| Urinary retention or obstructive voiding symptoms | Anticholinergic agents or musculotropic relaxants | Oxybutynin |
| Diazepam | ||
| Flavoxate | ||
| Calcium channel blockers | Nifedipine | |
| Antiparkinsonian drugs | Carbidopa | |
| Levodopa | ||
| α-Adrenergic agonists | Pseudoephedrine | |
| Phenylephrine | ||
| Antihistamines | Loratadine | |
| Diphenhydramine | ||
| Acute renal failure | Antimicrobials | Aminoglycosides |
| Penicillins | ||
| Cephalosporins | ||
| Amphotericin | ||
| Chemotherapeutic drugs | Cisplatin | |
| Others | Nonsteroidal anti-inflammatory drugs | |
| Phenytoin | ||
| Gynecomastia | Antihypertensives | Verapamil |
| Cardiac drugs | Digoxin | |
| Gastrointestinal drugs | Cimetidine | |
| Metoclopramide | ||
| Psychotropic drugs | Phenothiazines | |
| Tricyclic antidepressants | Amitriptyline | |
| Imipramine |
Previous Surgical Procedures
It is important to be aware of previous operations, particularly in a patient in whom surgery is intended. Obviously, previous operations may make subsequent ones more difficult. If the previous surgery was in a similar anatomic region, it is worthwhile to try to obtain the previous operative report. In our own experience, this small additional effort has been rewarded on numerous occasions by providing a clear explanation of the patient’s previous surgery that greatly simplified the subsequent operation. In general, it is worthwhile obtaining as much information as possible before any intended surgery because most surprises that occur in the operating room are unhappy ones.
Smoking and Alcohol Use
Cigarette smoking and consumption of alcohol are clearly linked to a number of urologic conditions. Cigarette smoking is associated with an increased risk of urothelial carcinoma, most notably bladder cancer, and it is also associated with increased peripheral vascular disease and erectile dysfunction. Chronic alcoholism may result in autonomic and peripheral neuropathy with resultant impaired urinary and sexual function. Chronic alcoholism may also impair hepatic metabolism of estrogens, resulting in decreased serum testosterone, testicular atrophy, and decreased libido.
In addition to the direct urologic effects of cigarette smoking and alcohol consumption, patients who are actively smoking or drinking at the time of surgery are at increased risk for perioperative complications. Smokers are at increased risk for both pulmonary and cardiac complications. If possible, they should discontinue smoking at least 8 weeks before surgery to optimize their pulmonary function (Warner et al, 1989). If they are unable to do this, they should at least quit smoking for 48 hours before surgery, because this will result in a significant improvement in cardiovascular function. Similarly, chronic alcoholics are at increased risk for hepatic toxicity and subsequent coagulation problems postoperatively. Furthermore, alcoholics who continue drinking up to the time of surgery may experience acute alcohol withdrawal during the postoperative period that can be life threatening. Prophylactic administration of lorazepam (Ativan) greatly reduces the potential risk of this significant complication.
Allergies
Finally, medicinal allergies should be questioned because, obviously, these medications should be avoided in future treatment of the patient. All medicinal allergies should be marked boldly on the front of the patient’s chart to avoid potential complications from inadvertent exposure to the same medications.
In summary, a careful and thorough medical history including the chief complaint and history of present illness, past medical history, and family history should be obtained in every patient. Unfortunately, time constraints often make it difficult for the physician to spend the necessary time to obtain a full history. A reasonable substitute is to have a trained nurse or other health professional see the patient first. By using a standard history form, much of the information discussed previously can be obtained in a preliminary interview. It then remains for the urologist to only fill in the blanks, have the patient elaborate on potentially relevant aspects of the past medical history, and then perform a complete physical examination.
Physical Examination
Key Points
A complete and thorough physical examination is an essential component of the evaluation of patients who present with urologic disease. Although it is tempting to become dependent on results of laboratory and radiologic tests, the physical examination often simplifies the process and allows the urologist to select the most appropriate diagnostic studies. Along with the history, the physical examination remains a key component of the diagnostic evaluation and should be performed conscientiously.
General Observations
The visual inspection of the patient provides a general overview. The skin should be inspected for evidence of jaundice or pallor. The nutritional status of the patient should be noted. Cachexia is a frequent sign of malignancy, and obesity may be a sign of underlying endocrinologic abnormalities. In this instance, one should search for the presence of truncal obesity, a “buffalo hump,” and abdominal skin striae, which are stigmata of hyperadrenocorticism. In contrast, debility and hyperpigmentation may be signs of hypoadrenocorticism. Gynecomastia may be a sign of endocrinologic disease and a possible indicator of alcoholism or previous hormonal therapy for prostate cancer. Edema of the genitalia and lower extremities may be associated with cardiac decompensation, renal failure, nephrotic syndrome, or pelvic and/or retroperitoneal lymphatic obstruction. Supraclavicular lymphadenopathy may be seen with any GU neoplasm, most commonly prostate and testis cancer; inguinal lymphadenopathy may occur secondary to carcinoma of the penis or urethra.
Kidneys
The kidneys are fist-sized organs located high in the retroperitoneum bilaterally. In the adult, the kidneys are normally difficult to palpate because of their position under the diaphragm and ribs with abundant musculature both anteriorly and posteriorly. Because of the position of the liver, the right kidney is somewhat lower than the left. In children and thin women, it may be possible to palpate the lower pole of the right kidney with deep inspiration. However, it is usually not possible to palpate either kidney in men, and the left kidney is almost always impalpable unless it is abnormally enlarged.
The best way to palpate the kidneys is with the patient in the supine position. The kidney is lifted from behind with one hand in the costovertebral angle (Fig. 3–1). On deep inspiration, the examiner’s hand is advanced firmly into the anterior abdomen just below the costal margin. At the point of maximal inspiration, the kidney may be felt as it moves downward with the diaphragm. With each inspiration, the examiner’s hand may be advanced deeper into the abdomen. Once again, it is more difficult to palpate kidneys in men because the kidneys tend to move downward less with inspiration and because they are surrounded with thicker muscular layers. In children, it is easier to palpate the kidneys because of decreased body thickness. In neonates, the kidneys can be felt quite easily by palpating the flank between the thumb anteriorly and the fingers over the costovertebral angle posteriorly.
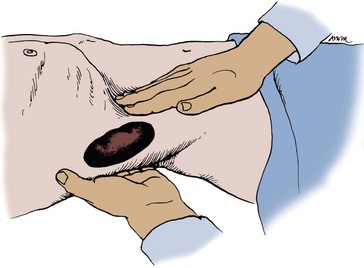
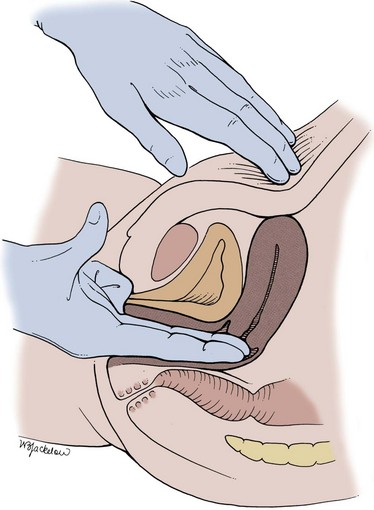
Figure 3–1 Bimanual examination of the kidney.
(From Judge RD, Zuidema GD, Fitzgerald FT, editors. Clinical diagnosis. 5th ed. Boston: Little, Brown; 1989. p. 370.)
Transillumination of the kidneys may be helpful in children younger than 1 year of age with a palpable flank mass. Such masses are frequently of renal origin. A flashlight or fiberoptic light source is positioned posteriorly against the costovertebral angle. Fluid-filled masses such as cysts or hydronephrosis produce a dull reddish glow in the anterior abdomen. Solid masses such as tumors do not transilluminate. Other diagnostic maneuvers that may be helpful in examining the kidneys are percussion and auscultation. Although renal inflammation may cause pain that is poorly localized, percussion of the costovertebral angle posteriorly more often localizes the pain and tenderness more accurately. Percussion should be done gently because in a patient with significant renal inflammation, this may be quite painful. Auscultation of the upper abdomen during deep inspiration may occasionally reveal a systolic bruit associated with renal artery stenosis or an aneurysm. A bruit may also be detected in association with a large renal arteriovenous fistula.
Every patient with flank pain should also be examined for possible nerve root irritation. The ribs should be palpated carefully to rule out a bone spur or other skeletal abnormality and to determine the point of maximal tenderness. Unlike renal pain, radiculitis usually causes hyperesthesia of the overlying skin innervated by the irritated peripheral nerve. This hypersensitivity can be elicited with a pin or by pinching the skin and fat overlying the involved area. Finally, the pain experienced during the pre-eruptive phase of herpes zoster involving any of the segments between T11 and L2 may also simulate pain of renal origin.
Bladder
A normal bladder in the adult cannot be palpated or percussed until there is at least 150 mL of urine in it. At a volume of about 500 mL, the distended bladder becomes visible in thin patients as a lower midline abdominal mass.
Percussion is better than palpation for diagnosing a distended bladder. The examiner begins by percussing immediately above the symphysis pubis and continuing cephalad until there is a change in pitch from dull to resonant. Alternatively, it may be possible in thin patients and in children to palpate the bladder by lifting the lumbar spine with one hand and pressing the other hand into the midline of the lower abdomen.
A careful bimanual examination, best done with the patient under anesthesia, is invaluable in assessing the regional extent of a bladder tumor or other pelvic mass. The bladder is palpated between the abdomen and the vagina in the female (Fig. 3–2) or the rectum in the male (Fig. 3–3). In addition to defining areas of induration, the bimanual examination allows the examiner to assess the mobility of the bladder; such information cannot be obtained by radiologic techniques such as CT and MRI, which convey static images.
Penis
If the patient has not been circumcised, the foreskin should be retracted to examine for tumor or balanoposthitis (inflammation of the prepuce and glans penis). Most penile cancers occur in uncircumcised men and arise on the prepuce or glans penis. Therefore in a patient with a bloody penile discharge in whom the foreskin cannot be withdrawn, a dorsal slit or circumcision must be performed to adequately evaluate the glans penis and urethra.
The position of the urethral meatus should be noted. It may be located proximal to the tip of the glans on the ventral surface (hypospadias) or, much less commonly, on the dorsal surface (epispadias). The penile skin should be examined for the presence of superficial vesicles compatible with herpes simplex and for ulcers that may indicate either venereal infection or tumor. The presence of venereal warts (condylomata acuminata), which appear as irregular, papillary, velvety lesions on the male genitalia, should also be noted.
The urethral meatus should be separated between the thumb and the forefinger to inspect for neoplastic or inflammatory lesions within the fossa navicularis. The dorsal shaft of the penis should be palpated for the presence of fibrotic plaques or ridges typical of Peyronie disease. Tenderness along the ventral aspect of the penis is suggestive of periurethritis, often secondary to a urethral stricture.
Scrotum and Contents
The scrotum is a loose sac containing the testes and spermatic cord structures. The scrotal wall is made up of skin and an underlying thin muscular layer. The testes are normally oval, firm, and smooth; in adults, they measure about 6 cm in length and 4 cm in width. They are suspended in the scrotum, with the right testis normally anterior to the left. The epididymis lies posterior to the testis and is palpable as a distinct ridge of tissue. The vas deferens can be palpated above each testis and feels like a piece of heavy twine.
The scrotum should be examined for dermatologic abnormalities. Because the scrotum, unlike the penis, contains both hair and sweat glands, it is a frequent site of local infection and sebaceous cysts. Hair follicles can become infected and may present as small pustules on the surface of the scrotum. These usually resolve spontaneously, but they can give rise to more significant infection, particularly in patients with reduced immunity and diabetes. Patients often become concerned about these lesions, mistaking them for testicular tumors.
The testes should be palpated gently between the fingertips of both hands. The testes normally have a firm, rubbery consistency with a smooth surface. Abnormally small testes suggest hypogonadism or an endocrinopathy such as Klinefelter disease. A firm or hard area within the testis should be considered a malignant tumor until proved otherwise. The epididymis should be palpable as a ridge posterior to each testis. Masses in the epididymis (spermatocele, cyst, and epididymitis) are almost always benign.
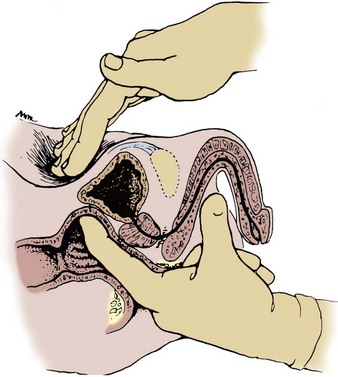
To examine for a hernia, the physician’s index finger should be inserted gently into the scrotum and invaginated into the external inguinal ring (Fig. 3–4). The scrotum should be invaginated in front of the testis, and care should be taken not to elevate the testis itself, which is quite painful. Once the external ring has been located, the physician should place the fingertips of his or her other hand over the internal inguinal ring and ask the patient to bear down (Valsalva maneuver). A hernia will be felt as a distinct bulge that descends against the tip of the index finger in the external inguinal ring as the patient bears down. Although it may be possible to distinguish a direct inguinal hernia arising through the floor of the inguinal canal from an indirect inguinal hernia prolapsing through the internal inguinal ring, this is seldom possible and of little clinical significance because the surgical approach is essentially identical for both conditions.
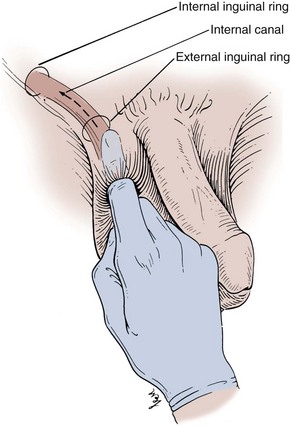
Figure 3–4 Examination of the inguinal canal.
(From Swartz MH. Textbook of physical diagnosis. Philadelphia: WB Saunders; 1989. p. 376.)
The spermatic cord is also examined with the patient in the standing position. A varicocele is a dilated, tortuous spermatic vein that becomes more obvious as the patient performs a Valsalva maneuver. The epididymis can again be palpated as a ridge of tissue running longitudinally, posterior to each testis. The testis should be palpated again between the fingers of both hands, once again taking care not to exert any pressure on the testis itself so as to avoid pain.
Transillumination is helpful in determining whether scrotal masses are solid (tumor) or cystic (hydrocele, spermatocele). A small flashlight or fiberoptic light cord is placed behind the mass. A cystic mass transilluminates easily, whereas light is not transmitted through a solid tumor.
Rectal and Prostate Examination in the Male
Digital rectal examination (DRE) should be performed in every male after age 40 years and in men of any age who present for urologic evaluation. Prostate cancer is the second most common cause of male cancer deaths after age 55 years and the most common cause of cancer deaths in men older than 70 years. Many prostate cancers can be detected in an early curable stage by DRE, and about 25% of colorectal cancers can be detected by DRE in combination with a stool guaiac test.
DRE should be performed at the end of the physical examination. It is done best with the patient standing and bent over the examining table or with the patient in the knee-chest position. In the standing position, the patient should stand with his thighs close to the examining table. The feet should be about 18 inches apart, with the knees flexed slightly. The patient should bend at the waist 90 degrees until his chest is resting on his forearms. The physician should give the patient adequate time to get in the proper position and relax as much as possible. A few reassuring words before the examination are helpful. The physician should place a glove on the examining hand and should lubricate the index finger thoroughly.
Before performing the DRE, the physician should place the palm of his other hand against the patient’s lower abdomen. This provides subtle reassurance to the patient by allowing the physician to make gentle contact with the patient before touching the anus. It also allows the physician to steady the patient and provide gentle counterpressure if the patient tries to move away as the DRE is being performed. The DRE itself begins by separating the buttocks and inspecting the anus for pathology, usually hemorrhoids, but, occasionally, an anal carcinoma or melanoma may be detected. The gloved, lubricated index finger is then inserted gently into the anus. Only one phalanx should be inserted initially to give the anus time to relax and to easily accommodate the finger. Estimation of anal sphincter tone is of great importance; a flaccid or spastic anal sphincter suggests similar changes in the urinary sphincter and may be a clue to the diagnosis of neurogenic disease. If the physician waits only a few seconds, the anal sphincter will normally relax to the degree that the finger can be advanced to the knuckle without causing pain. The index finger then sweeps over the prostate; the entire posterior surface of the gland can usually be examined if the patient is in the proper position. Normally, the prostate is about the size of a chestnut and has a consistency similar to that of the contracted thenar eminence of the thumb (with the thumb opposed to the little finger).
The index finger is extended as far as possible into the rectum, and the entire circumference is examined to detect an early rectal carcinoma. The index finger is then withdrawn gently, and the stool on the glove is transferred to a guaiac-impregnated (Hemoccult) card for determination of occult blood. Although there may be a significant incidence of false-positive and false-negative results associated with fecal occult blood testing, particularly without dietary and drug restrictions, the guaiac test is simple and inexpensive and may lead to the detection of significant gastrointestinal abnormalities (Bond, 1999). Adequate tissues, soap, and towels should be available for the patient to cleanse himself after the examination. The physician should then leave the room and allow the patient adequate time to wash and dress before concluding the consultation.
Pelvic Examination in the Female
Male urologists should always perform the female pelvic examination with a female nurse or other health care professional present. The patient should be allowed to undress in privacy and be fully draped for the procedure before the physician enters the room. The examination itself should be performed in standard lithotomy position with the patient’s legs abducted. Initially, the external genitalia and introitus should be examined, with particular attention paid to atrophic changes, erosions, ulcers, discharge, or warts, all of which may cause dysuria and pelvic discomfort. The urethral meatus should be inspected for caruncles, mucosal hyperplasia, cysts, and mucosal prolapse. The patient is then asked to perform a Valsalva maneuver and is carefully examined for a cystocele (prolapse of the bladder) or rectocele (prolapse of the rectum). The patient is then asked to cough, which may precipitate stress urinary incontinence. Palpation of the urethra is done to detect induration, which may be a sign of chronic inflammation or malignancy. Palpation may also disclose a urethral diverticulum, and palpation of a diverticulum may cause a purulent discharge from the urethra. Bimanual examination of the bladder, uterus, and adnexa should then be performed with two fingers in the vagina and the other hand on the lower abdomen (see Fig. 3–3). Any abnormality of the pelvic organs should be evaluated further with a pelvic ultrasound or CT scan.
Neurologic Examination
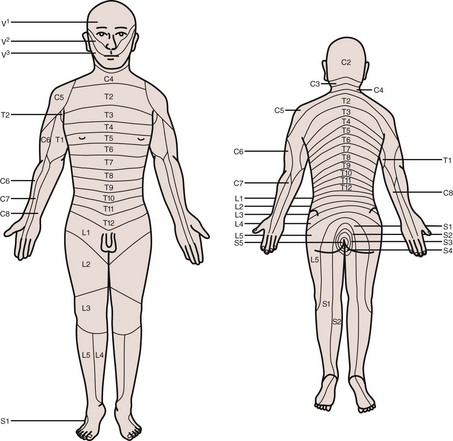
There are various clinical situations in which the neurologic examination may be helpful in evaluating urologic patients. In some cases, the level of neurologic abnormalities can be localized by the pattern of sensory deficit noted during physical examination using a dermatome map (Fig. 3–5). Sensory deficits in the penis, labia, scrotum, vagina, and perianal area generally indicate damage or injury to sacral roots or nerves. In addition to sensory examination, testing of reflexes in the genital area may also be performed. The most important of these is the bulbocavernosus reflex (BCR), which is a reflex contraction of the striated muscle of the pelvic floor that occurs in response to various stimuli in the perineum or genitalia. This reflex is most commonly tested by placing a finger in the rectum and then squeezing the glans penis or clitoris. If a Foley catheter is in place, the BCR can also be elicited by gently pulling on the catheter. If the BCR is intact, tightening of the anal sphincter should be felt and/or observed. The BCR tests the integrity of the spinal cord mediated reflex arc involving S2-S4 and may be absent in the presence of sacral cord or peripheral nerve abnormalities.
The cremasteric reflex can be elicited by lightly stroking the superior and medial thigh in a downward direction. The normal response in males is contraction of the cremasteric muscle that results in immediate elevation of the ipsilateral scrotum and testis. There is limited clinical utility for testing superficial reflexes such as the cremasteric when investigating neurologic dysfunction. However, there may be a role for testing this reflex when assessing patients with suspected testicular torsion or epididymitis. Finally, an overly active cremasteric reflex in children can lead to the mistaken diagnosis of an undescended testis in some cases.
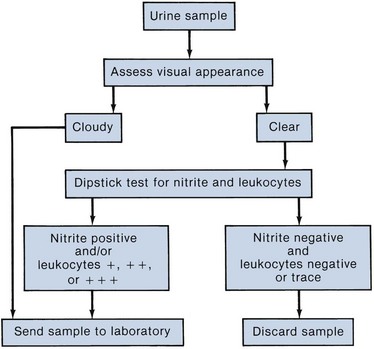
Urinalysis
The urinalysis is a fundamental test that should be performed in all urologic patients. Although, in many instances a simple dipstick urinalysis will provide the necessary information, a complete urinalysis includes both chemical and microscopic analyses.
Collection of Urinary Specimens
Males
In the male patient, a midstream urine sample is obtained. The uncircumcised male should retract the foreskin, cleanse the glans penis with antiseptic solution, and continue to retract the foreskin during voiding. The male patient begins urinating into the toilet and then places a wide-mouth sterile container under his penis to collect a midstream sample. This avoids contamination of the urine specimen with skin and urethral organisms.
In men with chronic UTIs, four aliquots of urine are obtained. These aliquots have been designated Voided Bladder 1, Voided Bladder 2, Expressed Prostatic Secretions, and Voided Bladder 3 (VB1, VB2, EPS, and VB3). The VB1 is the initial 5 to 10 mL of urine voided, whereas the VB2 is the midstream urine. The EPS is the secretions obtained after gentle prostatic massage, and the VB3 specimen is the initial 2 to 3 mL of urine obtained after prostatic massage. The value of these cultures for localization of UTIs is that the VB1 sample represents urethral flora; the VB2, bladder flora; and the EPS and VB3 samples, prostatic flora. The VB3 sample is particularly helpful when there is little or no prostatic fluid obtained by massage. To better obtain prostatic secretions, patients should be instructed to attempt to void during prostatic massage and to avoid tightening the anal sphincter and pelvic floor muscles. The four-part urine sample is particularly useful in evaluating men with suspected bacterial prostatitis (Meares and Stamey, 1968).
Females
In the female, it is more difficult to obtain a clean-catch midstream specimen. The female patient should cleanse the vulva, separate the labia, and collect a midstream specimen as described for the male patient. If infection is suspected, however, the midstream specimen is unreliable and should never be sent for culture and sensitivity. To evaluate for a possible infection in a female, a catheterized urine sample should always be obtained.
Neonates and Infants
The usual way to obtain a urine sample in a neonate or infant is to place a sterile plastic bag with an adhesive collar over the infant’s genitalia. Obviously, however, these devices may not be able to distinguish contamination from true UTI. Whenever possible, all urine samples should be examined within 1 hour of collection and plated for culture and sensitivity if indicated. If urine is allowed to stand at room temperature for longer periods of time, bacterial overgrowth may occur, the pH may change, and red and white blood cell casts may disintegrate. If it is not possible to examine the urine promptly, it should be refrigerated at 5° C.
Physical Examination of Urine
The physical examination of the urine includes an evaluation of color, turbidity, specific gravity and osmolality, and pH.
Color
The normal pale yellow color of urine is due to the presence of the pigment urochrome. Urine color varies most commonly because of concentration, but many foods, medications, metabolic products, and infection may produce abnormal urine color. This is important because many patients will seek consultation primarily because of a change in their urine color. Thus it is important for the urologist to be aware of the common causes of abnormal urine color, and these are listed in Table 3–3.
Table 3–3 Common Causes of Abnormal Urine Color
| Colorless | Very dilute urine |
| Overhydration | |
| Cloudy/milky | Phosphaturia |
| Pyuria | |
| Chyluria | |
| Red | Hematuria |
| Hemoglobinuria/myoglobinuria | |
| Anthocyanin in beets and blackberries | |
| Chronic lead and mercury poisoning | |
| Phenolphthalein (in bowel evacuants) | |
| Phenothiazines (e.g., Compazine) | |
| Rifampin | |
| Orange | Dehydration |
| Phenazopyridine (Pyridium) | |
| Sulfasalazine (Azulfidine) | |
| Yellow | Normal |
| Phenacetin | |
| Riboflavin | |
| Green-blue | Biliverdin |
| Indicanuria (tryptophan indole metabolites) | |
| Amitriptyline (Elavil) | |
| Indigo carmine | |
| Methylene blue | |
| Phenols (e.g., IV cimetidine [Tagamet], | |
| IV promethazine [Phenergan]) | |
| Resorcinol | |
| Triamterene (Dyrenium) | |
| Brown | Urobilinogen |
| Porphyria | |
| Aloe, fava beans, and rhubarb | |
| Chloroquine and primaquine | |
| Furazolidone (Furoxone) | |
| Metronidazole (Flagyl) | |
| Nitrofurantoin (Furadantin) | |
| Brown-black | Alcaptonuria (homogentisic acid) |
| Hemorrhage | |
| Melanin | |
| Tyrosinosis (hydroxyphenylpyruvic acid) | |
| Cascara, senna (laxatives) | |
| Methocarbamol (Robaxin) | |
| Methyldopa (Aldomet) | |
| Sorbitol |
From Hanno PM, Wein AJ. A clinical manual of urology. Norwalk (CT): Appleton-Century-Crofts; 1987. p. 67.
Turbidity
Freshly voided urine is clear. Cloudy urine is most commonly due to phosphaturia, a benign process in which excess phosphate crystals precipitate in an alkaline urine. Phosphaturia is intermittent and usually occurs after meals or ingestion of a large quantity of milk. Patients are otherwise asymptomatic. The diagnosis of phosphaturia can be accomplished either by acidifying the urine with acetic acid, which will result in immediate clearing, or by performing a microscopic analysis, which will reveal large amounts of amorphous phosphate crystals.
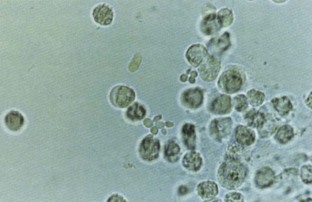
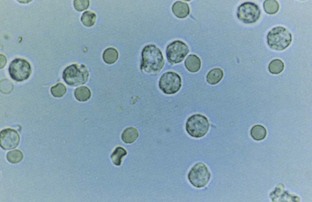
Pyuria, usually associated with a UTI, is another common cause of cloudy urine. The large numbers of white blood cells cause the urine to become turbid. Pyuria is readily distinguished from phosphaturia either by smelling the urine (infected urine has a characteristic pungent odor) or by microscopic examination, which readily distinguishes amorphous phosphate crystals from leukocytes.
Rare causes of cloudy urine include chyluria (in which there is an abnormal communication between the lymphatic system and the urinary tract resulting in lymph fluid being mixed with urine), lipiduria, hyperoxaluria, and hyperuricosuria.
Specific Gravity and Osmolality
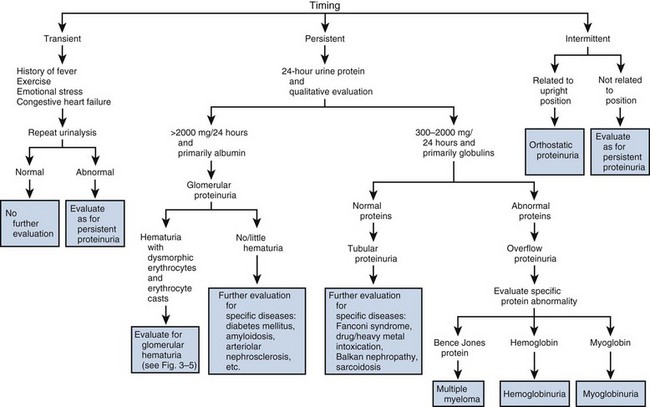
Specific gravity of urine is easily determined from a urinary dipstick and usually varies from 1.001 to 1.035. Specific gravity usually reflects the patient’s state of hydration but may also be affected by abnormal renal function, the amount of material dissolved in the urine, and a variety of other causes mentioned later. A specific gravity less than 1.008 is regarded as dilute, and a specific gravity greater than 1.020 is considered concentrated. A fixed specific gravity of 1.010 is a sign of renal insufficiency, either acute or chronic.
In general, specific gravity reflects the state of hydration but also affords some idea of renal concentrating ability. Conditions that decrease specific gravity include (1) increased fluid intake, (2) diuretics, (3) decreased renal concentrating ability, and (4) diabetes insipidus. Conditions that increase specific gravity include (1) decreased fluid intake; (2) dehydration owing to fever, sweating, vomiting, and diarrhea; (3) diabetes mellitus (glucosuria); and (4) inappropriate secretion of antidiuretic hormone. Specific gravity will also be increased above 1.035 after intravenous injection of iodinated contrast and in patients taking dextran.
Osmolality is a measure of the amount of material dissolved in the urine and usually varies between 50 and 1200 mOsm/L. Urine osmolality most commonly varies with hydration, and the same factors that affect specific gravity will also affect osmolality. Urine osmolality is a better indicator of renal function, but it cannot be measured from a dipstick and must be determined using standard laboratory techniques.
pH
Urinary pH is measured with a dipstick test strip that incorporates two colorimetric indicators, methyl red and bromothymol blue, which yield clearly distinguishable colors over the pH range from 5 to 9. Urinary pH may vary from 4.5 to 8; the average pH varies between 5.5 and 6.5. A urinary pH between 4.5 and 5.5 is considered acidic, whereas a pH between 6.5 and 8 is considered alkaline.
In general, the urinary pH reflects the pH in the serum. In patients with metabolic or respiratory acidosis, the urine is usually acidic; conversely, in patients with metabolic or respiratory alkalosis, the urine is alkaline. Renal tubular acidosis (RTA) presents an exception to this rule. In patients with both type I and II RTA, the serum is acidemic, but the urine is alkalotic because of continued loss of bicarbonate in the urine. In severe metabolic acidosis in type II RTA, the urine may become acidic, but in type I RTA, the urine is always alkaline, even with severe metabolic acidosis (Morris and Ives, 1991). Urinary pH determination is used to establish the diagnosis of RTA; inability to acidify the urine below a pH of 5.5 after administration of an acid load is diagnostic of RTA.
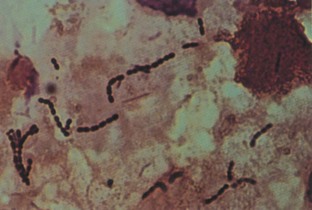
Urine pH determinations are also useful in the diagnosis and treatment of UTIs and urinary calculus disease. In patients with a presumed UTI, an alkaline urine with a pH greater than 7.5 suggests infection with a urea-splitting organism, most commonly Proteus. Urease-producing bacteria convert ammonia to ammonium ions, markedly elevating the urinary pH and causing precipitation of calcium magnesium ammonium phosphate crystals. The massive amount of crystallization may result in staghorn calculi.
Urinary pH is usually acidic in patients with uric acid and cystine lithiasis. Alkalinization of the urine is an important feature of therapy in both of these conditions, and frequent monitoring of urinary pH is necessary to ascertain adequacy of therapy.
Chemical Examination of Urine
Urine Dipsticks
Urine dipsticks provide a quick and inexpensive method for detecting abnormal substances within the urine. Dipsticks are short, plastic strips with small marker pads that are impregnated with different chemical reagents that react with abnormal substances in the urine to produce a colorimetric change. The abnormal substances commonly tested for with a dipstick include (1) blood, (2) protein, (3) glucose, (4) ketones, (5) urobilinogen and bilirubin, and (6) white blood cells.
Substances listed in Table 3–3 that produce an abnormal urine color may interfere with appropriate color development on the dipstick. In our experience, this most commonly occurs in patients taking phenazopyridine (Pyridium) for a UTI. Phenazopyridine turns the urine bright orange and makes dipstick evaluation of the urine unreliable.
Appropriate technique must be used to obtain an accurate dipstick determination. The reagent areas on the dipstick must be completely immersed in a fresh uncentrifuged urine specimen and then must be withdrawn immediately to prevent dissolution of the reagents into the urine. As the dipstick is removed from the urine specimen container, the edge of the dipstick is drawn along the rim of the container to remove excess urine. The dipstick should be held horizontally until the appropriate time for reading and then compared with the color chart. Excess urine on the dipstick or holding the dipstick in a vertical position will allow mixing of chemicals from adjacent reagent pads on the dipstick, resulting in a faulty diagnosis. False-negative results for glucose and bilirubin may be seen in the presence of elevated ascorbic acid concentrations in the urine. However, increased levels of ascorbic acid in the urine do not interfere with dipstick testing for hematuria. Highly buffered alkaline urine may cause falsely low readings for specific gravity and may lead to false-negative results for urinary protein. Other common causes of false results with dipstick testing are outdated test strips and exposure of the sticks, leading to damage to the reagents. In general, when the sticks are damaged, there will be color changes on the pads before their immersion in urine. If such color changes are noted, results with the dipstick may be inaccurate.
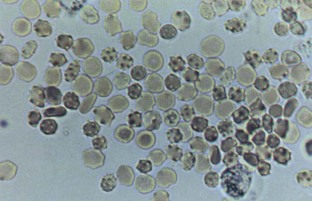
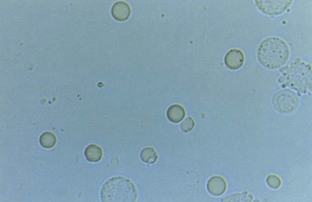
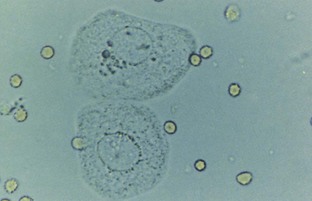
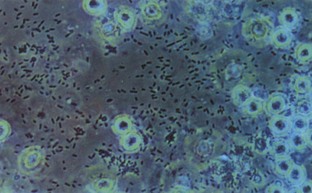
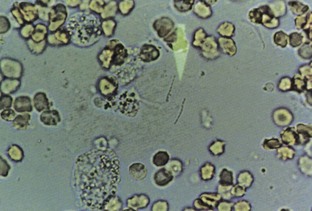
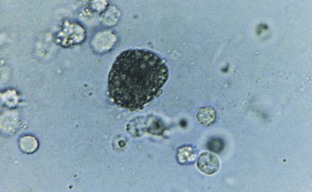
Hematuria
Normal urine should contain fewer than three red blood cells per HPF. A positive dipstick for blood in the urine indicates either hematuria, hemoglobinuria, or myoglobinuria. The chemical detection of blood in the urine is based on the peroxidase-like activity of hemoglobin. When in contact with an organic peroxidase substrate, hemoglobin catalyzes the reaction and causes subsequent oxidation of a chromogen indicator, which changes color according to the degree and amount of oxidation. The degree of color change is directly related to the amount of hemoglobin present in the urine specimen. Dipsticks frequently demonstrate both colored dots and field color change. If present, free hemoglobin and myoglobin in the urine are absorbed into the reagent pad and catalyze the reaction within the test paper, thereby producing a field change effect in color. Intact erythrocytes in the urine undergo hemolysis when they come in contact with the reagent test pad, and the localized free hemoglobin on the pad produces a corresponding dot of color change. Obviously, the greater the number of intact erythrocytes in the urine specimen, the greater the number of dots that will appear on the test paper, and a coalescence of the dots occurs when there are more than 250 erythrocytes/mL.
Hematuria can be distinguished from hemoglobinuria and myoglobinuria by microscopic examination of the centrifuged urine; the presence of a large number of erythrocytes establishes the diagnosis of hematuria. If erythrocytes are absent, examination of the serum will distinguish hemoglobinuria and myoglobinuria. A sample of blood is obtained and centrifuged. In hemoglobinuria, the supernatant will be pink. This is because free hemoglobin in the serum binds to haptoglobin, which is water insoluble and has a high molecular weight. This complex remains in the serum, causing a pink color. Free hemoglobin will appear in the urine only when all of the haptoglobin-binding sites have been saturated. In myoglobinuria, the myoglobin released from muscle is of low molecular weight and water soluble. It does not bind to haptoglobin and is therefore excreted immediately into the urine. Therefore in myoglobinuria the serum remains clear.
The sensitivity of urinary dipsticks in identifying hematuria, defined as greater than three erythrocytes/HPF of centrifuged sediment examined microscopically, is higher than 90%. Conversely, the specificity of the dipstick for hematuria compared with microscopy is somewhat lower, reflecting a higher false-positive rate with the dipstick (Shaw et al, 1985).
False-positive dipstick readings are most often due to contamination of the urine specimen with menstrual blood. Dehydration with resultant urine of high specific gravity can also yield false-positive results owing to the increased concentration of erythrocytes and hemoglobin. The normal individual excretes about 1000 erythrocytes/mL of urine, with the upper limits of normal varying from 5000 to 8000 erythrocytes/mL (Kincaid-Smith, 1982). Therefore examining urine of high specific gravity such as the first morning voided specimen increases the likelihood of a false-positive result. In addition to dehydration, another cause of false-positive results is exercise, which can increase the number of erythrocytes in the urine.
The efficacy of hematuria screening using the dipstick to identify patients with significant urologic disease is somewhat controversial. Studies in children and young adults have shown a low rate of significant disease (Woolhandler et al, 1989). In older adults, one study from the Mayo Clinic of 2000 patients with asymptomatic hematuria showed that only 0.5% had a urologic malignancy and only 1.8% developed other serious urologic diseases within 3 years after identification of the hematuria (Mohr et al, 1986). Conversely, investigators at the University of Wisconsin found that 26% of adults who had at least one positive dipstick reading for hematuria were subsequently found to have significant urologic pathology (Messing et al, 1987). Obviously, the age of the population, the completeness of the subsequent urologic evaluation, and the definition of significant disease all influence the disease rate in the group of patients with asymptomatic hematuria identified by dipstick screening. It is important to remember that, before proceeding to more complicated studies, the dipstick result should be confirmed with a microscopic examination of the centrifuged urinary sediment.
Differential Diagnosis and Evaluation of Hematuria
Hematuria may reflect either significant nephrologic or urologic disease. Hematuria of nephrologic origin is frequently associated with casts in the urine and almost always associated with significant proteinuria. Even significant hematuria of urologic origin will not elevate the protein concentration in the urine into the 100 to 300 mg/dL or 2+ to 3+ range on dipstick, and proteinuria of this magnitude almost always indicates glomerular or tubulointerstitial renal disease.
Morphologic evaluation of erythrocytes in the centrifuged urinary sediment also helps localize their site of origin. Erythrocytes arising from glomerular disease are typically dysmorphic and show a wide range of morphologic alterations. Conversely, erythrocytes arising from tubulointerstitial renal disease and of urologic origin have a uniformly round shape; these erythrocytes may or may not retain their hemoglobin (“ghost cells”), but the individual cell shape is consistently round. In individuals without significant pathology with minimal amounts of hematuria, the erythrocytes are characteristically dysmorphic but the number of cells observed is far fewer than that observed in patients with nephrologic disease. Erythrocyte morphology is more easily determined using phase contrast microscopy, but with practice this can be accomplished using a conventional light microscope (Schramek et al, 1989).
Glomerular Hematuria
Glomerular hematuria is suggested by the presence of dysmorphic erythrocytes, red blood cell casts, and proteinuria. Of those patients with glomerulonephritis proven by renal biopsy, however, about 20% will have hematuria alone without red blood cell casts or proteinuria (Fassett et al, 1982).
The glomerular disorders associated with hematuria are listed in Table 3–4. Further evaluation of patients with glomerular hematuria should begin with a thorough history. Hematuria in children and young adults, usually males, associated with low-grade fever and an erythematous rash suggests a diagnosis of immunoglobulin A (IgA) nephropathy (Berger disease). A family history of renal disease and deafness suggests familial nephritis or Alport syndrome. Hemoptysis and abnormal bleeding associated with microcytic anemia are characteristic of Goodpasture syndrome, and the presence of a rash and arthritis suggest systemic lupus erythematosus. Finally, poststreptococcal glomerulonephritis should be suspected in a child with a recent streptococcal upper respiratory tract or skin infection.
Table 3–4 Glomerular Disorders in Patients with Glomerular Hematuria
| DISORDER | PATIENTS |
|---|---|
| IgA nephropathy (Berger disease) | 30 |
| Mesangioproliferative GN | 14 |
| Focal segmental proliferative GN | 13 |
| Familial nephritis (e.g., Alport syndrome) | 11 |
| Membranous GN | 7 |
| Mesangiocapillary GN | 6 |
| Focal segmental sclerosis | 4 |
| Unclassifiable | 4 |
| Systemic lupus erythematosus | 3 |
| Postinfectious GN | 2 |
| Subacute bacterial endocarditis | 2 |
| Others | 4 |
| TOTAL | 100 |
GN, glomerulonephritis; IgA, immunoglobulin A.
Adapted from Fassett RG, Horgan BA, Mathew TH. Detection of glomerular bleeding by phase-contrast microscopy. Lancet 1982;1:1432.
Further laboratory evaluation should include measurement of serum creatinine, creatinine clearance, and, when proteinuria in the urine is 2+ or greater, a 24-hour urine protein determination. Although these tests will quantitate the specific degree of renal dysfunction, further tests are usually required to establish the specific diagnosis and particularly to determine whether the disease is due to an immune or a nonimmune etiology. Frequently, a renal biopsy is necessary to establish the precise diagnosis, and biopsies are particularly important if the result will influence subsequent treatment of the patient. Renal biopsies are extremely informative when examined by an experienced pathologist using light, immunofluorescent, and electron microscopy.
An algorithm for the evaluation of glomerular hematuria is provided in Figure 3–6.
IgA Nephropathy (Berger Disease)
IgA nephropathy, or Berger disease, is the most common cause of glomerular hematuria, accounting for about 30% of cases (Fassett et al, 1982). Therefore it is described in greater detail in this section. IgA nephropathy occurs most commonly in children and young adults, with a male predominance (Berger and Hinglais, 1968). Patients typically present with hematuria after an upper respiratory tract infection or exercise. Hematuria may be associated with a low-grade fever or rash, but most patients have no associated systemic symptoms. Gross hematuria occurs intermittently, but microscopic hematuria is a constant finding in some patients. The disease is chronic, but the prognosis in most patients is excellent. Renal function remains normal in the majority, but about 25% will subsequently develop renal insufficiency. An older age at onset, initial abnormal renal function, consistent proteinuria, and hypertension are indicators of a poor prognosis (D’Amico, 1988).
The pathologic findings in Berger disease are limited to either focal glomeruli or lobular segments of a glomerulus. The changes are proliferative and usually confined to mesangial cells (Berger and Hinglais, 1968). Renal biopsy reveals deposits of IgA, IgG, and β1c-globulin, although IgA and IgG mesangial deposits are found in other forms of glomerulonephritis as well. The role of IgA in the disease remains uncertain, although the deposits may trigger an inflammatory reaction within the glomerulus (van den Wall Bake et al, 1989). Because gross hematuria frequently follows an upper respiratory tract infection, a viral etiology has been suspected but not established. The frequent association between hematuria and exercise in this condition remains unexplained.
The clinical presentation of IgA glomerulonephritis is alarming and similar to certain systemic diseases including Schönlein-Henoch purpura, systemic lupus erythematosus, bacterial endocarditis, and Goodpasture syndrome. Therefore a careful clinical and laboratory evaluation is indicated to establish the correct diagnosis. The presence of red blood cell casts establishes the glomerular origin of the hematuria. In the absence of casts, a urologic evaluation is indicated to exclude the urinary tract as a source of bleeding and to confirm that the hematuria is arising from both kidneys. The diagnosis of IgA nephropathy is confirmed by renal biopsy demonstrating the classic deposits of immunoglobulins in mesangial cells, as described previously. Once the diagnosis has been established, repeat evaluations for hematuria are generally not indicated. Although there is no effective treatment for this condition, renal function remains stable in most patients and there are no other known long-term complications.
Nonglomerular Hematuria
Medical
Except for renal tumors, nonglomerular hematuria of renal origin is secondary to either tubulointerstitial, renovascular, or systemic disorders. The urinalysis in nonglomerular hematuria is distinguished from that of glomerular hematuria by the presence of circular erythrocytes and the absence of erythrocyte casts. Like glomerular hematuria, nonglomerular hematuria of renal origin is frequently associated with significant proteinuria, which distinguishes these nephrologic diseases from urologic diseases in which the degree of proteinuria is usually minimal, even with heavy bleeding.
As with glomerular hematuria, a careful history frequently helps establish the diagnosis. A family history of hematuria or bleeding tendency suggests the diagnosis of a blood dyscrasia, which should be investigated further. A family history of urolithiasis associated with intermittent hematuria may indicate stone disease, which should be investigated with serum and urine measurements of calcium and uric acid. A family history of renal cystic disease should prompt further radiologic evaluation for medullary sponge kidney and adult polycystic kidney disease. Papillary necrosis as a cause of hematuria should be considered in diabetics, African Americans (secondary to sickle cell disease or trait), and suspected analgesic abusers.
Medications may induce hematuria, particularly anticoagulants. Anticoagulation at normal therapeutic levels, however, does not predispose patients to hematuria. In one study, the prevalence of hematuria was 3.2% in anticoagulated patients versus 4.8% in a control group. Urologic disease was identified in 81% of patients with more than one episode of microscopic hematuria, and the cause of hematuria did not vary between groups (Culclasure et al, 1994). Thus anticoagulant therapy per se does not appear to increase the risk of hematuria unless the patient is excessively anticoagulated.
Exercise-induced hematuria is being observed with increasing frequency. It typically occurs in long-distance runners (>10 km), is usually noted at the conclusion of the run, and rapidly disappears with rest. The hematuria may be of renal or bladder origin. An increased number of dysmorphic erythrocytes have been noted in some patients, suggesting a glomerular origin. Exercise-induced hematuria may be the first sign of underlying glomerular disease such as IgA nephropathy. Conversely, cystoscopy in patients with exercise-induced hematuria frequently reveals punctate hemorrhagic lesions in the bladder, suggesting that the hematuria is of bladder origin.
Vascular disease may also result in nonglomerular hematuria. Renal artery embolism and thrombosis, arteriovenous fistulas, and renal vein thrombosis may all result in hematuria. Physical examination may reveal severe hypertension, a flank or abdominal bruit, or atrial fibrillation. In such patients, further evaluation for renal vascular disease should be undertaken.
An algorithm for the evaluation of nonglomerular hematuria is provided in Figure 3–7.
Surgical
Nonglomerular hematuria or essential hematuria includes primarily urologic rather than nephrologic diseases. Common causes of essential hematuria include urologic tumors, stones, and UTIs.
The urinalysis in both nonglomerular medical and surgical hematuria is similar in that both are characterized by circular erythrocytes and the absence of erythrocyte casts. Essential hematuria is suggested, however, by the absence of significant proteinuria usually found in nonglomerular hematuria of renal parenchymal origin. It should be remembered, however, that proteinuria is not always present in glomerular or nonglomerular renal disease.
The American Urological Association (AUA) Best Practice Policy Panel on Microscopic Hematuria has formulated practice recommendations for the detection and evaluation of asymptomatic microscopic hematuria (Grossfeld et al, 2001a, 2001b). The panel concluded that, due to the lack of specificity of urinary dipstick examination, as well as the risk and expense of evaluation, patients with a positive dipstick test should only undergo complete evaluation for hematuria if this is confirmed by the finding of 3 or more RBC/HPF on subsequent microscopic evaluation. The mainstays of evaluation, according to the panel, are voided urinary cytology, cystoscopy, and urinary tract imaging using ultrasonography, CT, and/or intravenous urography (IVU). The use of these tests in an individual patient should be based in most cases on the relative risk of significant urinary tract pathology.
An algorithm for the evaluation of essential hematuria is provided in Figure 3–8.
Proteinuria
Although healthy adults excrete 80 to 150 mg of protein in the urine daily, the qualitative detection of proteinuria in the urinalysis should raise the suspicion of underlying renal disease. Proteinuria may be the first indication of renovascular, glomerular, or tubulointerstitial renal disease, or it may represent the overflow of abnormal proteins into the urine in conditions such as multiple myeloma. Proteinuria can also occur secondary to nonrenal disorders and in response to various physiologic conditions such as strenuous exercise.
The protein concentration in the urine obviously depends on the state of hydration, but it seldom exceeds 20 mg/dL. In patients with dilute urine, however, significant proteinuria may be present at concentrations less than 20 mg/dL. Normally, urine protein is about 30% albumin, 30% serum globulins, and 40% tissue proteins, of which the major component is Tamm-Horsfall protein. This profile may be altered by conditions that affect glomerular filtration, tubular reabsorption, or excretion of urine protein, and determination of the urine protein profile by such techniques as protein electrophoresis may help determine the etiology of proteinuria.
Pathophysiology
Most causes of proteinuria can be categorized into one of three categories: glomerular, tubular, or overflow. Glomerular proteinuria is the most common type of proteinuria and results from increased glomerular capillary permeability to protein, especially albumin. Glomerular proteinuria occurs in any of the primary glomerular diseases such as IgA nephropathy or in glomerulopathy associated with systemic illness such as diabetes mellitus. Glomerular disease should be suspected when the 24-hour urine protein excretion exceeds 1 g and is almost certain to exist when the total protein excretion exceeds 3 g.
Tubular proteinuria results from failure to reabsorb normally filtered proteins of low molecular weight such as immunoglobulins. In tubular proteinuria, the 24-hour urine protein loss seldom exceeds 2 to 3 g and the excreted proteins are of low molecular weight rather than albumin. Disorders that lead to tubular proteinuria are commonly associated with other defects of proximal tubular function such as glucosuria, aminoaciduria, phosphaturia, and uricosuria (Fanconi syndrome).
Overflow proteinuria occurs in the absence of any underlying renal disease and is due to an increased plasma concentration of abnormal immunoglobulins and other low-molecular-weight proteins. The increased serum levels of abnormal proteins result in excess glomerular filtration that exceeds tubular reabsorptive capacity. The most common cause of overflow proteinuria is multiple myeloma, in which large amounts of immunoglobulin light chains are produced and appear in the urine (Bence Jones protein).
Detection
Qualitative detection of abnormal proteinuria is most easily accomplished with a dipstick impregnated with tetrabromophenol blue dye. The color of the dye changes in response to a pH shift related to the protein content of the urine, mainly albumin, leading to the development of a blue color. Because the background of the dipstick is yellow, various shades of green will develop, and the darker the green, the greater the concentration of protein in the urine. The minimal detectable protein concentration by this method is 20 to 30 mg/dL. False-negative results can occur in alkaline urine, dilute urine, or when the primary protein present is not albumin. Nephrotic range proteinuria in excess of 1 g/24 hr, however, is seldom missed on qualitative screening. Precipitation of urinary proteins with strong acids such as 3% sulfosalicylic acid will detect proteinuria at concentrations as low as 15 mg/dL and is more sensitive at detecting other proteins and albumin. Patients whose urine is negative on dipstick but strongly positive with sulfosalicylic acid should be suspected of having multiple myeloma, and the urine should be tested further for Bence Jones protein.
If qualitative testing reveals proteinuria, this should be quantitated with a 24-hour urinary collection. Further qualitative assessment of abnormal urinary proteins can be accomplished by either protein electrophoresis or immunoassay for specific proteins. Protein electrophoresis is particularly helpful in distinguishing glomerular from tubular proteinuria. In glomerular proteinuria, albumin makes up about 70% of the total protein excreted, whereas in tubular proteinuria, the major proteins excreted are immunoglobulins with albumin making up only 10% to 20%. Immunoassay is the method of choice for detecting specific proteins such as Bence Jones protein in multiple myeloma.
Evaluation
Proteinuria should first be classified by its timing into transient, intermittent, or persistent. Transient proteinuria occurs commonly, especially in the pediatric population, and usually resolves spontaneously within a few days (Wagner et al, 1968). It may result from fever, exercise, or emotional stress. In older patients, transient proteinuria may be due to congestive heart failure. If a nonrenal cause is identified and a subsequent urinalysis is negative, no further evaluation is necessary. Obviously, if proteinuria persists, it should be evaluated further.
Proteinuria may also occur intermittently, and this is frequently related to postural change (Robinson, 1985). Proteinuria that occurs only in the upright position is a frequent cause of mild, intermittent proteinuria in young males. Total daily protein excretion seldom exceeds 1 g, and urinary protein excretion returns to normal when the patient is recumbent. Orthostatic proteinuria is thought to be secondary to increased pressure on the renal vein while standing. It resolves spontaneously in about 50% of patients and is not associated with any morbidity. Therefore if renal function is normal in patients with orthostatic proteinuria, no further evaluation is indicated.
Persistent proteinuria requires further evaluation, and most cases have a glomerular etiology. A quantitative measurement of urinary protein should be obtained through a 24-hour urine collection, and a qualitative evaluation should be obtained to determine the major proteins excreted. The findings of greater than 2 g of protein excreted per 24 hours, of which the major components are high-molecular-weight proteins such as albumin, establish the diagnosis of glomerular proteinuria. Glomerular proteinuria is the most common cause of abnormal proteinuria, especially in patients presenting with persistent proteinuria. If glomerular proteinuria is associated with hematuria characterized by dysmorphic erythrocytes and erythrocyte casts, the patient should be evaluated as outlined previously for glomerular hematuria (see Fig. 3–6). Patients with glomerular proteinuria who have no or little associated hematuria should be evaluated for other conditions, of which the most common is diabetes mellitus. Other possibilities include amyloidosis and arteriolar nephrosclerosis.
In patients in whom total protein excretion is 300 to 2000 mg/day, of which the major components are low-molecular-weight globulins, further qualitative evaluation with immunoelectrophoresis is indicated. This will determine whether the excess proteins are normal or abnormal. Identification of normal proteins establishes a diagnosis of tubular proteinuria, and further evaluation for a specific cause of tubular dysfunction is indicated.
If qualitative evaluation reveals abnormal proteins in the urine, this establishes a diagnosis of overflow proteinuria. Further evaluation should be directed to identify the specific protein abnormality. The finding of large quantities of light-chain immunoglobulins or Bence Jones protein establishes a diagnosis of multiple myeloma. Similarly, the finding of large amounts of hemoglobin or myoglobin establishes the diagnosis of hemoglobinuria or myoglobinuria.
An algorithm for the evaluation of proteinuria is provided in Figure 3–9.
Glucose and Ketones
Urine testing for glucose and ketones is useful in screening patients for diabetes mellitus. Normally, almost all the glucose filtered by the glomeruli is reabsorbed in the proximal tubules. Although small amounts of glucose may normally be excreted in the urine, these amounts are not clinically significant and are below the level of detectability with the dipstick. If, however, the amount of glucose filtered exceeds the capacity of tubular reabsorption, glucose will be excreted in the urine and detected on the dipstick. This so-called renal threshold corresponds to serum glucose of about 180 mg/dL; above this level, glucose will be detected in the urine.
Glucose detection with the urinary dipstick is based on a double sequential enzymatic reaction yielding a colorimetric change. In the first reaction, glucose in the urine reacts with glucose oxidase on the dipstick to form gluconic acid and hydrogen peroxide. In the second reaction, hydrogen peroxide reacts with peroxidase, causing oxidation of the chromogen on the dipstick, producing a color change. This double-oxidative reaction is specific for glucose, and there is no cross-reactivity with other sugars. The dipstick test becomes less sensitive as the urine increases in specific gravity and temperature.
Ketones are not normally found in the urine but will appear when the carbohydrate supplies in the body are depleted and body fat breakdown occurs. This happens most commonly in diabetic ketoacidosis but may also occur during pregnancy and after periods of starvation or rapid weight reduction. Ketones excreted include acetoacetic acid, acetone, and β-hydroxybutyric acid. With abnormal fat breakdown, ketones will appear in the urine before the serum.
Dipstick testing for ketones involves a colorimetric reaction: sodium nitroprusside on the dipstick reacts with acetoacetic acid to produce a purple color. Dipstick testing will identify acetoacetic acid at concentrations of 5 to 10 mg/dL but will not detect acetone or β-hydroxybutyric acid. Obviously, a dipstick that tests positively for glucose should also be tested for ketones, and diabetes mellitus is suggested. False-positive results, however, can occur in acidic urine of high specific gravity, in abnormally colored urine, and in urine containing levodopa metabolites, 2-mercaptoethane sulfonate sodium, and other sulfhydryl-containing compounds (Csako, 1987).
Bilirubin and Urobilinogen
Normal urine contains no bilirubin and only small amounts of urobilinogen. There are two types of bilirubin, direct (conjugated) and indirect. Direct bilirubin is made in the hepatocyte, where bilirubin is conjugated with glucuronic acid. Conjugated bilirubin has a low molecular weight, is water soluble, and normally passes from the liver into the small intestine through the bile ducts, where it is converted to urobilinogen. Therefore conjugated bilirubin does not appear in the urine except in pathologic conditions in which there is intrinsic hepatic disease or obstruction of the bile ducts.
Indirect bilirubin is of high molecular weight and bound in the serum to albumin. It is water insoluble and therefore does not appear in the urine even in pathologic conditions.
Urobilinogen is the end product of conjugated bilirubin metabolism. Conjugated bilirubin passes through the bile ducts, where it is metabolized by normal intestinal bacteria to urobilinogen. Normally, about 50% of the urobilinogen is excreted in the stool and 50% reabsorbed into the enterohepatic circulation. A small amount of absorbed urobilinogen, about 1 to 4 mg/day, will escape hepatic uptake and be excreted in the urine. Hemolysis and hepatocellular diseases that lead to increased bile pigments can result in increased urinary urobilinogen. Conversely, obstruction of the bile duct or antibiotic usage that alters intestinal flora, thereby interfering with the conversion of conjugated bilirubin to urobilinogen, will decrease urobilinogen levels in the urine. In these conditions, obviously, serum levels of conjugated bilirubin rise.
There are different dipstick reagents and methods to test for both bilirubin and urobilinogen, but the basic physiologic principle involves the binding of bilirubin or urobilinogen to a diazonium salt to produce a colorimetric reaction. False-negative results can occur in the presence of ascorbic acid, which decreases the sensitivity for detection of bilirubin. False-positive results can occur in the presence of phenazopyridine because it colors the urine orange and, similar to the colorimetric reaction for bilirubin, turns red in an acid medium.
Leukocyte Esterase and Nitrite Tests
Leukocyte esterase activity indicates the presence of white blood cells in the urine. The presence of nitrites in the urine is strongly suggestive of bacteriuria. Thus both of these tests have been used to screen patients for UTIs. Although these tests may have application in nonurologic medical practice, the most accurate method to diagnose infection is by microscopic examination of the urinary sediment to identify pyuria and subsequent urine culture. All urologists should be capable of performing and interpreting the microscopic examination of the urinary sediment. Therefore leukocyte esterase and nitrite testing are less important in a urologic practice. For purposes of completion, however, both techniques are described briefly herein.
Leukocyte esterase and nitrite testing are performed using the Chemstrip LN dipstick. Leukocyte esterase is produced by neutrophils and catalyzes the hydrolysis of an indoxyl carbonic acid ester to indoxyl (Gillenwater, 1981). The indoxyl formed oxidizes a diazonium salt chromogen on the dipstick to produce a color change. It is recommended that leukocyte esterase testing be done 5 minutes after the dipstick is immersed in the urine to allow adequate incubation (Shaw et al, 1985). The sensitivity of this test subsequently decreases with time because of lysis of the leukocytes. Leukocyte esterase testing may also be negative in the presence of infection because not all patients with bacteriuria will have significant pyuria. Therefore if one uses leukocyte esterase testing to screen patients for UTI, it should always be done in conjunction with nitrite testing for bacteriuria (Pels et al, 1989).
Other causes of false-negative results with leukocyte esterase testing include increased urinary specific gravity, glycosuria, presence of urobilinogen, medications that alter urine color, and ingestion of large amounts of ascorbic acid. The major cause of false-positive leukocyte esterase tests is specimen contamination.
Nitrites are not normally found in the urine, but many species of gram-negative bacteria can convert nitrates to nitrites. Nitrites can readily be detected in the urine because they react with the reagents on the dipstick and undergo diazotization to form a red azo dye. The specificity of the nitrite dipstick for detecting bacteriuria is higher than 90% (Pels et al, 1989). The sensitivity of the test, however, is considerably less, varying from 35% to 85%. The nitrite test is less accurate in urine specimens containing fewer than 105 organisms/mL (Kellog et al, 1987). As with leukocyte esterase testing, the major cause of false-positive nitrite testing is contamination.
It remains controversial whether dipstick testing for leukocyte esterase and nitrites can replace microscopy in screening for significant UTIs. This issue is less important to urologists, who usually have access to a microscope and who should be trained and encouraged to examine the urinary sediment. A protocol combining the visual appearance of the urine with leukocyte esterase and nitrite testing has been proposed (Fig. 3–10). It reportedly detects 95% of infected urine specimens and decreases the need for microscopy by as much as 30% (Flanagan et al, 1989). Other studies, however, have shown that dipstick testing is not an adequate replacement for microscopy (Propp et al, 1989). In summary, it has not been demonstrated conclusively that dipstick testing for UTI can replace microscopic examination of the urinary sediment. In our personal experience, we always examine the urinary sediment whenever we suspect a UTI and subsequently culture the urine when pyuria is identified.
Urinary Sediment
Obtaining and Preparing the Specimen
A clean-catch midstream urine specimen should be obtained. As described earlier, uncircumcised men should retract the prepuce and cleanse the glans penis before voiding. It is more difficult to obtain a reliable clean-catch specimen in females because of contamination with introital leukocytes and bacteria. If there is any suspicion of a UTI in a female, a catheterized urine sample should be obtained for culture and sensitivity.
If possible, the first morning urine specimen is the specimen of choice and should be examined within 1 hour. A standard procedure for preparation of the urine for microscopic examination has been described (Cushner and Copley, 1989). Ten to 15 milliliters of urine should be centrifuged for 5 minutes at 3000 rpm. The supernatant is then poured off, and the sediment is resuspended in the centrifuge tube by gently tapping the bottom of the tube. Although the remaining small amount of fluid can be poured onto a microscope slide, this usually results in excess fluid on the slide. It is better to use a small pipette to withdraw the residual fluid from the centrifuge tube and to place it directly on the microscope slide. This usually results in an ideal volume of between 0.01 and 0.02 mL of fluid deposited on the slide. The slide is then covered with a coverslip. The edge of the coverslip should be placed on the slide first to allow the drop of fluid to ascend onto the coverslip by capillary action. The coverslip is then gently placed over the drop of fluid, and this technique allows for most of the air between the drop of fluid and the coverslip to be expelled. If one simply drops the coverslip over the urine, the urine will disperse over the slide and there will be a considerable number of air bubbles that may distort the subsequent microscopic examination.
Microscopy Technique
Microscopic analysis of the urinary sediment should be performed with both low-power (×100 magnification) and high-power (×400 magnification) lenses. The use of an oil immersion lens for higher magnification is seldom, if ever, necessary. Under low power, the entire area under the coverslip should be scanned. Particular attention should be given to the edges of the coverslip, where casts and other elements tend to be concentrated. Low-power magnification is sufficient to identify erythrocytes, leukocytes, casts, cystine crystals, oval fat macrophages, and parasites such as Trichomonas vaginalis and Schistosoma hematobium.
High-power magnification is necessary to distinguish circular from dysmorphic erythrocytes, to identify other types of crystals, and, particularly, to identify bacteria and yeast. In summary, the urinary sediment should be examined microscopically for (1) cells, (2) casts, (3) crystals, (4) bacteria, (5) yeast, and (6) parasites.
Cells
Erythrocyte morphology may be determined under high-power magnification. Although phase contrast microscopy has been used for this purpose, circular (nonglomerular) erythrocytes can generally be distinguished from dysmorphic (glomerular) erythrocytes under routine brightfield high-power magnification (Figs. 3-11 to 3-15). This is assisted by adjusting the microscope condenser to its lowest aperture, thus reducing the intensity of background light. This allows one to see fine detail not evident otherwise and also creates the effect of phase microscopy because cell membranes and other sedimentary components stand out against the darkened background.
Figure 3–11 Red blood cells, both smoothly rounded and mildly crenated, typical of epithelial erythrocytes.
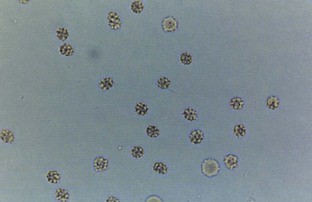
Figure 3–13 Red blood cells from a patient with interstitial cystitis. Cells were collected at cystoscopy.
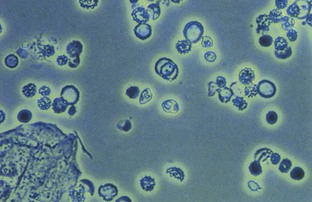
Figure 3–14 Red blood cells from a patient with Berger disease. Note variations in membranes characteristic of dysmorphic red blood cells.
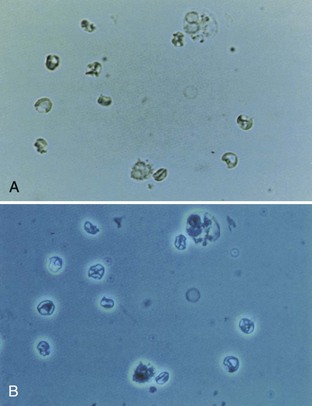
Figure 3–15 Dysmorphic red blood cells from a patient with Wegener granulomatosis. A, Brightfield illumination. B, Phase illumination. Note irregular deposits of dense cytoplasmic material around the cell membrane.
Circular erythrocytes generally have an even distribution of hemoglobin with either a round or crenated contour, whereas dysmorphic erythrocytes are irregularly shaped with minimal hemoglobin and irregular distribution of cytoplasm. Automated techniques for performing microscopic analysis to distinguish the two types of erythrocytes have been investigated but have not yet been accepted into general urologic practice and are probably unnecessary. In one study using a standard Coulter counter, microscopic analysis was found to be 97% accurate in differentiating between the two types of erythrocytes (Sayer et al, 1990). Erythrocytes may be confused with yeast or fat droplets (Fig. 3–16). Erythrocytes can be distinguished, however, because yeast will show budding and oil droplets are highly refractile.
Leukocytes can generally be identified under low power and definitively diagnosed under high-power magnification (Figs. 3-17 and 3-18; see also Fig. 3–16). It is normal to find 1 or 2 leukocytes/HPF in men and up to 5/HPF in women in whom the urine sample may be contaminated with vaginal secretions. A greater number of leukocytes generally indicates infection or inflammation in the urinary tract. It may be possible to distinguish old leukocytes, which have a characteristic small and wrinkled appearance and which are commonly found in the vaginal secretions of normal women, from fresh leukocytes, which are generally indicative of urinary tract pathology. Fresh leukocytes are generally larger and rounder, and, when the specific gravity is less than 1.019, the granules in the cytoplasm demonstrate glitter-like movement, so-called glitter cells.
Epithelial cells are commonly observed in the urinary sediment. Squamous cells are frequently detected in female urine specimens and are derived from the lower portion of the urethra, the trigone of postpubertal females, and the vagina. Squamous epithelial cells are large, have a central small nucleus about the size of an erythrocyte, and have an irregular cytoplasm with fine granularity.
Transitional epithelial cells may arise from the remainder of the urinary tract (Fig. 3–19). Transitional cells are smaller than squamous cells, have a larger nucleus, and demonstrate prominent cytoplasmic granules near the nucleus. Malignant transitional cells have altered nuclear size and morphology and can be identified with either routine Papanicolaou staining or automated flow cytometry.
Renal tubular cells are the least commonly observed epithelial cells in the urine but are most significant because their presence in the urine is always indicative of renal pathology. Renal tubular cells may be difficult to distinguish from leukocytes, but they are slightly larger.
Casts
A cast is a protein coagulum that is formed in the renal tubule and traps any tubular luminal contents within the matrix. Tamm-Horsfall mucoprotein is the basic matrix of all renal casts; it originates from tubular epithelial cells and is always present in the urine. When the casts contain only mucoproteins, they are called hyaline casts and may not have any pathologic significance. Hyaline casts may be seen in the urine after exercise or heat exposure but may also be observed in pyelonephritis or chronic renal disease.
Red blood cell casts contain entrapped erythrocytes and are diagnostic of glomerular bleeding, most likely secondary to glomerulonephritis (Figs. 3-20 and 3-21). White blood cell casts are observed in acute glomerulonephritis, acute pyelonephritis, and acute tubulointerstitial nephritis. Casts with other cellular elements, usually sloughed renal tubular epithelial cells, are indicative of nonspecific renal damage (Fig. 3–22). Granular and waxy casts result from further degeneration of cellular elements. Fatty casts are seen in nephrotic syndrome, lipiduria, and hypothyroidism.
Crystals
Identification of crystals in the urine is particularly important in patients with stone disease because it may help determine the etiology (Fig. 3–23). Although other types of crystals may be seen in normal patients, the identification of cystine crystals establishes the diagnosis of cystinuria. Crystals precipitated in acidic urine include calcium oxalate, uric acid, and cystine. Crystals precipitated in an alkaline urine include calcium phosphate and triple-phosphate (struvite) crystals. Cholesterol crystals are rarely seen in the urine and are not related to urinary pH. They occur in lipiduria and remain in droplet form.
Bacteria
Normal urine should not contain bacteria; and in a fresh uncontaminated specimen, the finding of bacteria is indicative of a UTI. Because each HPF views between 1/20,000 and 1/50,000 mL, each bacterium seen per HPF signifies a bacterial count of more than 30,000/mL. Therefore, 5 bacteria/HPF reflects colony counts of about 100,000/mL. This is the standard concentration used to establish the diagnosis of a UTI in a clean-catch specimen. This level should apply only to women, however, in whom a clean-catch specimen is frequently contaminated. The finding of any bacteria in a properly collected midstream specimen from a male should be further evaluated with a urine culture.
Under high power, it is possible to distinguish various bacteria. Gram-negative rods have a characteristic bacillary shape (Fig. 3–24), whereas streptococci can be identified by their characteristic beaded chains (Figs. 3-25 and 3-26) and staphylococci can be identified when the organisms are found in clumps (Fig. 3–27).
Yeast
The most common yeast cells found in urine are Candida albicans. The biconcave oval shape of yeast can be confused with erythrocytes and calcium oxalate crystals, but yeasts can be distinguished by their characteristic budding and hyphae (see Fig. 3–16). Yeasts are most commonly seen in the urine of patients with diabetes mellitus or as contaminants in women with vaginal candidiasis.
Parasites
Trichomonas vaginalis is a frequent cause of vaginitis in women and occasionally of urethritis in men. Trichomonads can be readily identified in a clean-catch specimen under low power (Fig. 3–28). Trichomonads are large cells with rapidly moving flagella that quickly propel the organism across the microscopic field.
Schistosoma hematobium is a urinary tract pathogen that is not found in the United States but is extremely common in countries of the Middle East and North Africa. Examination of the urine shows the characteristic parasitic ova with a terminal spike.
Expressed Prostatic Secretions
Although not strictly a component of the urinary sediment, the expressed prostatic secretions should be examined in any man suspected of having prostatitis. Normal prostatic fluid should contain few, if any, leukocytes, and the presence of a larger number or clumps of leukocytes is indicative of prostatitis. Oval fat macrophages are found in postinfection prostatic fluid (Figs. 3-29 and 3-30). Normal prostatic fluid contains numerous secretory granules that resemble but can be distinguished from leukocytes under high power because they do not have nuclei.
Summary
This chapter has detailed the basic evaluation of the urologic patient, which should include a careful history, physical examination, and urinalysis. These three basic components form the cornerstone of the urologic evaluation and should precede any subsequent diagnostic procedures. After completion of the history, physical examination, and urinalysis, the urologist should be able to establish at least a differential, if not specific, diagnosis that will allow the subsequent diagnostic evaluation and treatment to be carried out in a direct and efficient manner.
Barry MJ, Fowler FJJr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549.
Grossfeld GD, Litwin MS, Wolf JSJr, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part I. Definition, Prevalence and Etiology. Urology. 2001;57:599.
Grossfeld GD, Litwin MS, Wolf JSJr, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part II. Patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation and follow-up. Urology. 2001;57:604.
Mohr DN, Offord KP, Owen RA, Melton LJ3rd. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA. 1986;256:224.
Pels RJ, Bor DH, Woolhandler S, et al. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders: II. Bacteriuria. JAMA. 1989;262:1221.
Schramek P, Schuster FX, Georgopoulos M, et al. Value of urinary erythrocyte morphology in assessment of symptomless microhematuria. Lancet. 1989;2:1316.
Barry MJ, Fowler FJJr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148:1549.
Berger J, Hinglais N. Les depots intercapillaires d’IgA-IgG. J Urol Nephrol (Paris). 1968;74:694.
Blaivas JG, Kaplan SA. Urologic dysfunction in patients with multiple sclerosis. Semin Urol. 1988;8:159.
Bond JH. Fecal occult blood tests in occult gastrointestinal bleeding. Semin Gastrointest Dis. 1999;10:48.
Bratt O. Hereditary prostate cancer. BJU Int. 2000;85:611.
Csako G. False positive results for ketone with the drug MESNA and other free-sulfhydryl compounds. Clin Chem. 1987;33:289.
Culclasure TF, Bray VJ, Hasbargen JA. The significance of hematuria in the anticoagulated patient. Arch Intern Med. 1994;154:649.
Cushner HM, Copley JB. Back to basics: The urinalysis: a selected national survey and review. Am J Med Sci. 1989;297:193.
D’Amico G. Clinical features and natural history in adults with IgA nephropathy. Am J Kidney Dis. 1988;12:353.
Fassett RG, Horgan BA, Mathew TH. Detection of glomerular bleeding by phase-contrast microscopy. Lancet. 1982;1:1432.
Flanagan PG, Davies EA, Rooney PG, Stoot RW. Evaluation of four screening tests for bacteriuria in elderly people. Lancet. 1989;1:1117.
Forsythe WI, Redmond A. Enuresis and spontaneous cure rate: study of 1129 enuretics. Arch Dis Child. 1974;49:259.
Gillenwater JY. Detection of urinary leukocytes by Chemstrip. J Urol. 1981;125:383.
Grossfeld GD, Litwin MS, Wolf JSJr, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part I. Definition, Prevalence and Etiology. Urology. 2001;57:599.
Grossfeld GD, Litwin MS, Wolf JSJr, et al. Evaluation of asymptomatic microscopic hematuria in adults: The American Urological Association Best Practice Policy—Part II. Patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation and follow-up. Urology. 2001;57:604.
Kellog JA, Manzella JP, Shaffer SN, Schwartz BB. Clinical relevance of culture versus screens for the detection of microbial pathogens in urine specimens. Am J Med. 1987;83:739.
Kincaid-Smith P. Hematuria and exercise-related hematuria. BMJ. 1982;285:1595.
Lepor H, Machi G. Comparison of AUA symptom index in unselected males and females between fifty-five and seventy-nine years of age. Urology. 1993;42:36.
MacDiarmid SA, Goodson TC, Holmes TM, et al. An assessment of the comprehension of the American Urological Association symptom index. J Urol. 1998;159:873.
Meares EM, Stamey TA. Bacteriologic localization patterns in bacterial prostatitis and urethritis. Invest Urol. 1968;5:492.
Messing EM, Young TB, Hunt VB, et al. The significance of asymptomatic microhematuria in men 50 or more years old: findings of a home screening study using urinary dipsticks. J Urol. 1987;137:919.
Mohr DN, Offord KP, Owen RA, Melton LJ3rd. Asymptomatic microhematuria and urologic disease: a population-based study. JAMA. 1986;256:224.
Morris RC, Ives HE. Inherited disorders of the renal tubule, 4th ed. Brenner BM, Rector FCJr, editors. The kidney, vol 2. WB Saunders, Philadelphia, 1991, 1596.
Murat Basar M, Atan A, Yildiz M, et al. Comparison of sertraline to fluoxetine with regard to their efficacy and side effects in the treatment of premature ejaculation. Arch Esp Urol. 1999;52:1008.
Pels RJ, Bor DH, Woolhandler S, et al. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders: II. Bacteriuria. JAMA. 1989;262:1221.
Propp DA, Weber D, Ciesta ML. Reliability of a urine dipstick in emergency department patients. Ann Emerg Med. 1989;18:560.
Robinson RR. Clinical significance of isolated proteinuria. In: Avram MM, editor. Proteinuria. New York: Plenum Medical Book; 1985:67-82.
Sayer J, McCarthy JNP, Schmidt JD. Identification and significance of dysmorphic versus isomorphic hematuria. J Urol. 1990;143:545.
Schramek P, Schuster FX, Georgopoulos M, et al. Value of urinary erythrocyte morphology in assessment of symptomless microhematuria. Lancet. 1989;2:1316.
Shaw ST, Pan SY, Wong ET. Routine urinalysis: is the dipstick enough? JAMA. 1985;253:1956.
Stephenson TP, Farrar DJ. Urodynamic study of 15 patients with post-micturition dribble. Urology. 1977;9:404.
van den Wall Bake AW, Daha MR, van Es LA. Immunopathogenetic aspects of IgA nephropathy. Nephrologie. 1989;10:141.
Wagner MG, Smith FGJr, Tinglof BO, Cornberg E. Epidemiology of proteinuria: a study of 4807 school children. J Pediatr. 1968;73:825.
Warner MA, Offord KP, Warner ME, et al. Role of preoperative cessation of smoking and other factors in postoperative pulmonary complications: a blinded prospective study of coronary artery bypass patients. Mayo Clin Proc. 1989;64:609.
Weiss JP, Blaivas JG. Nocturia. J Urol. 2000;163:5.
Woolhandler S, Pels RJ, Bor DH. Dipstick urinalysis screening of asymptomatic adults for urinary tract disorders: I. Hematuria and proteinuria. JAMA. 1989;262:1215.